Abstract
Background
Chronic shoulder dislocation has been treated by either anatomic shoulder arthroplasty (ASA) or reverse shoulder arthroplasty (RSA) with encouraging results. Although good results have been reported after both the procedures, several complications such as instability and glenoid failures have also been highlighted. The aim of this study was to aggregate the results that have been reported with the use of ASA or RSA in chronic shoulder dislocation and analyze the instability rates, complication rates, and functional outcomes.
Methods
A comprehensive search was performed in May 2020 using PubMed, EMBASE, and Cochrane Library databases. Studies that reported on the outcomes after either ASA or RSA for chronic anterior dislocation (CAD) or chronic posterior dislocation (CPD) were included in the systematic review. Methodologic quality was assessed using the Methodological Index for Nonrandomized Studies appraisal tool for observational studies.
Results
We aggregated 13 studies that included data on 128 patients with CAD and 51 patients with CPD. The combined weighted postoperative instability rate in the CAD group was significantly higher after ASA than after RSA (P = .04). There was no significant difference in the combined weighted instability rate between ASA in the CAD group and ASA in the CPD group (P = .37). The complications of RSA in CAD included glenoid base plate loosening, humeral shaft fractures, late infection, acromion fractures, and instability. The complications of the ASA in CAD and CPD included glenoid loosening and erosions, severe pain necessitating revision, severe superior migration of the head, redislocation with rupture of the cuff tendons, bone graft migration, instability, and 2 cases of neuropathies (median nerve and axillary nerve) that eventually resolved.
Conclusion
Postoperative instability was significantly more common after ASA than after RSA for chronic shoulder dislocations, but both RSA and ASA had a high complication rate in CAD. Shoulder arthroplasty improved the range of motion, functional outcomes, and pain in patients with chronic shoulder dislocation.
Keywords: Chronic shoulder dislocation, Neglected shoulder dislocation, Anatomic shoulder arthroplasty, Reverse shoulder arthroplasty, Glenoid bone defect, Posterior shoulder dislocation, Anterior shoulder dislocation
Chronic shoulder dislocation is an uncommon problem that presents unique treatment challenges. Chronic posterior dislocation may be neglected because of a missed diagnosis, but chronic anterior dislocation, although always apparent on the radiographs, has also been commonly reported in the literature.7,16 Sometimes, the problem has been left untreated, but the patients have been reported to live with limited functional abilities.3 However, often the neglected dislocation may cause severe pain and functional disabilities and may warrant some surgical treatment.3,12 Open reduction of both chronic posterior dislocation (CPD) and chronic anterior dislocation (CAD) has shown variable although encouraging results in relieving pain and improving function.2,16 But if the neglected dislocation is more than a few months old, there may be associated problems of severe humeral and glenoid bone loss which may not be amenable to open reduction techniques. Furthermore, there may be cartilage damage on the glenoid and the humeral head which may preclude any open reduction options.3 In these instances, anatomic and reverse shoulder implants have been used with varying degrees of success by several surgeons. Anatomic shoulder arthroplasty (ASA) has been performed for neglected posterior dislocation with encouraging results for more than 50 years.7 However, with the advent of the reverse shoulder arthroplasty (RSA), some surgeons suggested that neglected dislocations being a unique problem may be better treated with RSA than with ASA.14 Although good outcomes have been reported with the use of both RSA and ASA in CAD and CPD, several complications of both the procedures have also been highlighted.10,14 In particular, the most often reported complications were of postoperative instability, glenoid loosening, and severe pain, all of which decreased the overall patient satisfaction.10,22 The preferred form of shoulder arthroplasty with low complication rate and good functional results in neglected shoulder dislocations has still not been conclusively proven in the literature. Hence, we undertook a systematic review of the literature with an aim to evaluate the instability rates after RSA and ASA for CAD and CPD. We also aimed to evaluate the complications and results after RSA and ASA for CAD and CPD.
Materials and methods
The PROSPERO registration for the review protocol was pending with the registration office for several weeks and all non-COVID protocols registrations had been considerably delayed because of the current pandemic situation.
We searched the PubMed, EMBASE, and Cochrane library of medicine database with the keyword terms “chronic”, “shoulder”, “dislocation” or “neglected”, “shoulder”, “dislocation” or “locked”, “shoulder”, “dislocation”, connected with the Boolean operator “AND” as per the PRISMA guidelines11 in May 2020. We also scanned the reference lists of the most relevant original studies for additional studies. The search was made for studies published till May 2020 that reported on adult patients of age more than 18 years, in English language and with an abstract.
Search criteria
The inclusion criteria were studies reporting on patients with anterior or posterior chronic neglected shoulder dislocation (when the author states that the dislocation is either chronic or neglected or if the dislocation was unreduced for 3 weeks or more) treated by either ASA or RSA or both, reporting on the clinical outcomes, case series with more than 3 patients and minimum average follow-up of 1 year. We excluded publications with case reports of 3 or less patients, review articles, fracture dislocations, recurrent dislocations, acute dislocations, studies with only open reduction and humeral head preserving technique of treatment, and studies where it was not possible to extract data about the arthroplasty procedure.
Two authors (DS and VR) independently performed the database search and selected the studies based on the information in the abstract. Any disagreement was solved by the third author (AP). The full text of the articles was jointly screened for final inclusion based on the inclusion criteria. The methodological quality was scored using the Methodological Index for Nonrandomized Studies (MINORS) appraisal tool for observational studies.18 These criteria use 3-point scoring (0,1,2) to classify the studies on 12 points on the checklist resulting in a maximum score of 16 for noncomparative and 24 for comparative studies. The level of evidence for the studies was decided according to the criteria of Wright and Swiontkowski.25
Data extraction
We extracted the following data from the included studies in a spreadsheet:
-
1.
Study details such as author, year, and journal
-
2.
Level of evidence and type of study
-
3.
Age of the patients
-
4.
Type of dislocation (anterior or posterior) and the period of neglection
-
5.
Associated problems such as glenoid bone loss and rotator cuff tear
-
6.
Treatment method (ASA or RSA) and the postoperative follow-up period
-
7.
Complications (where the author identified them as “complications” or an event that needed revision surgery) in the postoperative follow-up
-
8.
Number of patients showing postoperative instability
-
9.
Gain in functional scores and range of motion
Statistical analysis
We meta-analyzed the data using the meta-analysis workbooks.20 The instability data from each of the papers were calculated as the event rate. Standard error and confidence intervals were calculated from the data in the papers. These data were converted to a weighted instability rate for meta-analysis. We used a random effects model (tau calculated separately for subgroups) for all subgroup meta-analysis because of the expected heterogeneity. Patients with CAD managed with either RSA or ASA were compared for their instability rates in a subgroup analysis with the random effects model. Differences between the different subgroups were calculated by analysis of variance analysis. The data on all reported complications were also extracted. Preoperative and postoperative data such as the range of motion, functional results in the form of Constant scores or American Shoulder and Elbow Surgeons score and pain scores in the form of Visual Analog Score, Likert scale score, and pain component of the Constant scores were first converted to a standardized measurement scale in the form of Hedge’s g. Then the weighted data were pooled by random sampling method. In some of the studies, the available data lacked the required dispersion variables such as the standard deviation (SD) or the standard error (SE). As a first step we tried to contact the authors of the studies to collect the missing data. When we were unable to contact the study authors, we calculated the statistics variables from the available P value of the variables, using the formula17 ; the corresponding SD was calculated by the formula . If the P value could not be found and only range was available, we attempted to calculate the SD with the formula15 .
The heterogeneity of the results within the studies was measured with I2 statistics. The I2 categories were 0%-24.9%, no heterogeneity; 25%-49.9%, low heterogeneity; 50%-74.9%, moderate heterogeneity; 75%-100%, high heterogeneity.
Sensitivity analysis was performed by leave-one-out strategy for those studies that had scores less than 10 on the MINORS scale and for those that included incomplete data. We also performed a meta regression analysis to evaluate the effect of period of neglection of the dislocation on the postoperative instability rate.
Finally, we evaluated the presence of a potential publication bias by visually analyzing the symmetry of the funnel plot and by performing the Begg and Mazumdar rank correlation test (P < .05 deemed significant bias).
Search results
The database search yielded 807 articles and abstracts. After applying the inclusion and exclusion criteria, 47 articles were selected. Finally, the full texts of 15 studies were examined for inclusion in the study. Two studies were excluded from the full-text review of 15 studies. The remaining 13 studies were included in this systematic review and qualitative analysis (Table I). Search strategy and exclusions were displayed in the PRISMA flowchart (Fig. 1).
Table I.
Summary of results of the 13 different included studies (results of Statz et al were subdivided into 2 groups).
| Sr. no | Author | Year | Study population | Mean age | Mean period of neglection (in months) | Type of dislocation | Type of intervention | No. of patients with preoperative glenoid bone defect | No. of patients with full thickness cuff | Postoperative average follow-up period (in months) | Gain in pain score/ maximum pain score | Gain in functional score | Gain in elevation | Gain in external rotation | No. of patients with postoperative instabilities | No. of postoperative complications | List of complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Raiss et al14 | 2017 | 18 | 71 | 23 | Anterior | RSA | 4 Patient had severe bone loss | 16 (10 massive tears; 6 large tears) | 42 | 7.6/15 | 33.8 | 65.3 | 15.1 | 1 | 7 | 4 Glenoid failure (1 week, 1 Month, 9 month, 2 years); 1 Instability; 1 humerus fracture; 1 late infection |
| 2 | Statz et al19 | 2016 | 9 | 62 | 7 | Anterior | RSA | 8 Patient had <25%; 4 patient had 25-50%; 3 patient had 50%-75% bone loss | 8 | 39.6 | 3/5 | NR | 63 | 57 | 0 | 4 | 1 Shaft fracture; 1 Heterotrophic ossification |
| 3 | Van Tongel et al21 | 2016 | 6 | 73 | 4.5 | Anterior | RSA | 1 patient had 90%; 1 patient had 25%; 2 had less than <12% bone loss | 3 (1 massive tear, 2 large tear) | 39 | NR | 37 | NR | NR | 0 | 0 | None |
| 4 | Werner et al22 | 2014 | 21 | 71 | 6 | Anterior | RSA | Average 45% bone loss | 6 (2 massive cuff tear; 4 massive subscapularis tear) |
58.8 | 11/15 | 51.5 | 93 | 6 | 0 | 2 | 2 Baseplate loosening (12 month and 15 month) |
| 5 | Kurowicki et al8 | 2016 | 24 | 76 | NR | Anterior | RSA | 3 Patient had > 50% bone loss | 23 | 25.5 | 6/10 | 25 | 40 | 40 | 2 | 6 | 4 Acromion type 3 fracture; 2 Instabilities |
| 6 | Statz et al19 | 2016 | 10 | 60 | 9 | Anterior | ASA | NR | 3 | 144 | 2/5 | NR | 24 | 11 | 6 | 8 | 6 Instabilities; 2 glenoid erosion |
| 7 | Flatow et al3 | 1993 | 10 | 65.1 | 29.7 | Anterior | ASA | 3 Had more than 50% bone loss | 2 large tear | 43.1 | NR | NR | 46 | 58 | 1 | 1 | 1 Anterior subluxation |
| 8 | Raiss et al13 | 2009 | 10 | 67 | NR | Anterior | ASA | 2 Patients had severe bone loss | 6 (3 supraspinatus tear and 3 subscapularis tear) | 23.8 | 7.6/15 | 42.4 | 81 | 24 | 1 | 2 | 1 Re-dislocation with rerupture of the subscapularis tendon; 1 glenoid abrasion |
| 9 | Matsoukis et al10 | 2006 | 11 | 69.3 | 24 | Anterior | ASA | 4 Patients had >30% bone loss | 5 (3 isolated supraspinatus; 1 isolated subscapularis tear; 1 massive tear) |
47.7 | 6.2/15 | 24.9 | 41.4 | 12.3 | 4 | 7 | 4 Instability; 1 Bone graft migration; 2 glenoid loosening |
| 10 | Li et al9 | 2016 | 5 | 71 | 5 | Anterior | ASA | Average 25% bone loss | None | 31.6 | NR | NR | 63 | 3 | 4 | 4 | 4 Instabilities |
| 11 | Pritchett et al12 | 1987 | 4 | 60 | 11 | Anterior | ASA | NR | NR | 30 | NR | 20 | NR | NR | 0 | 1 | 1 Axillary nerve palsy |
| 12 | Wooten et al24 | 2014 | 32 | 54 | 21.7 | Posterior | ASA | Average >45% bone loss | 3 small to medium; 1 large tear; 1 irreparable massive tear |
98.4 | 1/5 | NR | 8 | 65 | 5 | 12 | 1 Aseptic loosening; 1 Nonunion; 2 infections; 5 instabilities; 1 median neuropathy; 2 severe pain |
| 13 | Gavriilidis et al5 | 2010 | 12 | 49.8 | 14.5 | Posterior | ASA | Average >45% bone loss | 3 | 37.4 | NR | NR | 40.8 | 43.4 | 0 | 4 | 1 Severe migration of head; 2 mild migration; 1 revision of glenoid |
| 14 | Cheng et al1 | 1997 | 7 | 58 | 23 | Posterior | ASA | NR | NR | 24 | 4.2/10 | 35.5 | 32.3 | 15.4 | 1 | 1 | 1 Instability |
NR, not reported; RSA, reverse shoulder arthroplasty; ASA, anatomic shoulder arthroplasty.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. The flowchart displays the search and eligibility strategy.
Study characteristics and patient demographics
Of the 13 studies that were included in the systematic review, 12 were of level 4 evidence and 1 was of level 3 evidence (Tables I and II). Their MINORS scores ranged from 6 to 13 of a maximum possible score of 16; 7 studies scored 10 and above, whereas 6 studies scored below 10 (Table II). Ten studies were on CAD3,8, 9, 10,12, 13, 14,19,21,22 and 3 studies were on CPD1,5,24. Among the 10 studies on CAD, 4 included management by only RSA8,14,21,22 5 included management by only ASA3,9,10,12,13 and 1 included management by both RSA and ASA.19 Statz et al reported on a series of patients with CAD that were managed by ASA and RSA.19 To enable subgroup and forest plot analysis, we extracted data for both subgroups (ASA and RSA) of patients and presented them as separate studies. Data on the postoperative instability and the complications were available from all the studies, but the data on preoperative function, pain scores, and range of motion were incomplete in most of the studies. Kurowicki et al8 presented their data on RSA management of CAD and compared it with a control group of patients managed with RSA for conventional indications. Although they stated that their patients had more than 6 weeks of chronic and neglected anterior shoulder dislocation, the average period of neglection for the cohort was not stated. The cohort in the study by Raiss et al14 included 18 patients with CAD and 4 patients with CPD. Although the data on both the group of patients were not presented separately, it was possible to extract the complications and instability rate for CAD. The other study by Raiss et al13 did not report the exact period of neglection in their cohort of CAD.
Table II.
Methodological Index for Nonrandomized Studies (MINORS) scoring and level of evidence of 13 included studies.
| Sr. no | Author | Year | MINORS score (maximum score = 16) | Level of evidence |
|---|---|---|---|---|
| 1 | Raiss et al14 | 2017 | 10 | Level IV |
| 2 | Statz et al19 | 2016 | 11 | Level IV |
| 3 | Van Tongel et al21 | 2016 | 10 | Level IV |
| 4 | Werner et al22 | 2014 | 9 | Level IV |
| 5 | Kurowicki et al8 | 2016 | 13 | Level III |
| 6 | Flatow et al3 | 1993 | 6 | Level IV |
| 7 | Raiss et al13 | 2009 | 8 | Level IV |
| 8 | Matsoukis et al10 | 2006 | 9 | Level IV |
| 9 | Li et al9 | 2016 | 10 | Level IV |
| 10 | Pritchett et al12 | 1987 | 8 | Level IV |
| 11 | Wooten et al24 | 2014 | 9 | Level IV |
| 12 | Gavriilidis et al5 | 2010 | 10 | Level IV |
| 13 | Cheng et al1 | 1997 | 12 | Level IV |
The 3 studies on CPD included management by ASA only; hence, there were no RSA subgroups in CPD. The average age of the patients with CAD was 67.76 years (range, 60-76 years) and with CPD was 53.93 years (range, 49.8-58 years). There were 128 patients with CAD and 51 patients with CPD. Seventy-eight patients with CAD had been managed with RSA and 50 patients had been managed with ASA. All 51 patients with CPD had been managed with ASA. The average follow-up of all RSA patients in CAD group was 40.98 months (range, 25.5-58.8 months), of all ASA patients in the CAD group was 53.36 months (range, 23.8-144 months) and of all ASA patients in the CPD group was 53.26 months (range, 24-88.4 months). For the purpose of comparing instability rates, humeral head resurfacing, hemireplacement and total shoulder replacements were grouped under ASA. Average period of neglect for the CAD group was 13.24 months (range, 4.5-29.3 months) and for the CPD group was 19.73 months (range, 14.5-23 months).
Results
Postoperative instability
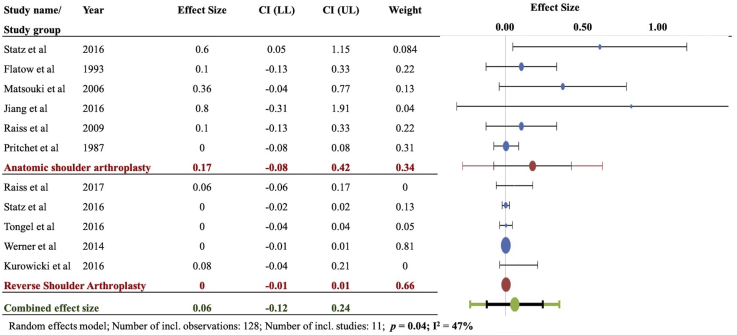
In the CAD group, the combined weighted instability rate after ASA (0.17) was significantly higher than that after RSA (0.00) (P = .04, I2 = 47%) (Fig. 2). The reported instability rates after ASA in the CAD subgroup were 6/10 in the study by Statz et al, 4/11 in that by Matsoukis et al, 1/10 in the study by Flatow et al, 4/5 in that by Li et al, 1/10 in that by Raiss et al, and 0/4 in that by Pritchet et al. The reported instability rates after RSA in the CAD subgroup were 1/18 in the study by Raiss et al, 2/24 in that by Kurowicki et al, 0/9 in that by Statz et al, 0/6 in that by Tongel et al, and 0/21 in that by Werner et al.
Figure 2.
Instability in chronic anterior dislocation (CAD): Forest plot of weighted effect size instability (number of patients showing instability/total patients) for anatomic shoulder arthroplasty (ASA) and reverse shoulder arthroplasty (RSA) in chronic anterior shoulder dislocation. Significant difference observed between ASA and RSA (P = .04). The blue ovals indicate individual weighted effect size; the red ovals indicate combined effect size for the subgroup (ASA or RSA). The green ovals indicate overall combined effect size. The dotted vertical line indicates zero effect size. The horizontal bars indicate confidence intervals of individual studies.
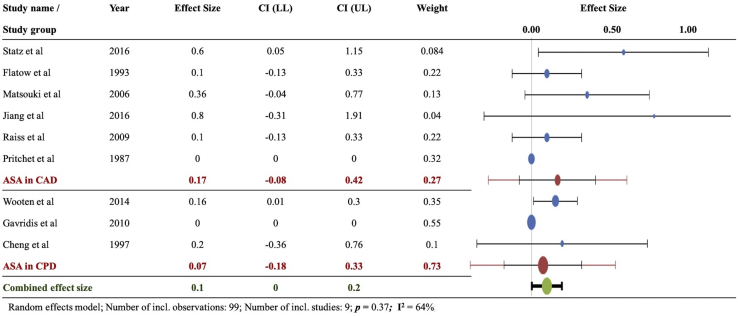
The combined weighted instability rate after ASA for CPD was 0.07. On comparison between ASA for CAD and CPD subgroups, there were no significant differences between them (P = .37, I2 = 64%) (Fig. 3). The reported instability rates after ASA for CPD were 5/32 in the study by Wooten et al, 1/10 in that by Cheng et al and 0/12 in that by Gavriilidis et al.
Figure 3.
Instability with ASA in chronic anterior dislocation (CAD) and chronic posterior dislocation (CPD): Forest plot of weighted effect size instability (number of patients showing instability/total patients) for anatomic shoulder arthroplasty (ASA) in chronic anterior dislocation and chronic posterior dislocation. No significant difference observed (P = .37). The blue ovals indicate individual weighted effect size; the red ovals indicate combined effect size for the subgroup (ASA in CAD or ASA in CPD). The green ovals indicate overall combined effect size. The dotted vertical line indicates zero effect size. The horizontal bars indicate confidence intervals of individual studies. CI (LL), confidence interval lower limit; CI (UL), confidence interval upper limit.
Postoperative complications after RSA for CAD
The complication rate in the RSA of CAD subgroup was 7/18 in the study by Raiss et al (4 glenoid failures at less than 2 years,1 instability, 1 humerus shaft fracture and 1 late infection), 4/9 in that by Statz et al (2 intraoperative humeral shaft fractures, 1 postoperative shaft fracture and 1 heterotrophic ossification), 0/6 in that by Tongel et al, 2/21 in that by Werner et al (2 glenoid baseplate loosening), and 6/24 in that by Kurowicki et al (4 type 3 acromion fractures and 2 instabilities).
Postoperative complications after ASA for CAD
The complication rate in ASA of the CAD subgroup was 8/10 in the study by Statz et al (6 instabilities; 2 glenoid erosions), 1/10 in that by Flatow et al (1 anterior subluxation), 7/11 in that by Matsoukis et al (4 instabilities, 1 bone graft migration, 2 glenoid loosening), 4/5 in that by Li et al (4 instabilities), 2/10 in that by Raiss et al (1 redislocation with rupture of the subscapularis tendon and 1 glenoid abrasion), and 1/4 in that by Pritchet et al (1 axillary nerve palsy that eventually resolved).
Postoperative complications after ASA for CPD
The complications after ASA for CPD included 1 aseptic loosening, 1 nonunion, 2 infections, 5 instabilities,1 median neuropathy (eventually resolved), 2 with severe pain in the study by Wooten et al; 1 severe head migration, 1 mild head migration, 1 revision of the glenoid in the study by Gavriilidis et al; and 1 instability in the study by Cheng et al.
Postintervention improvement
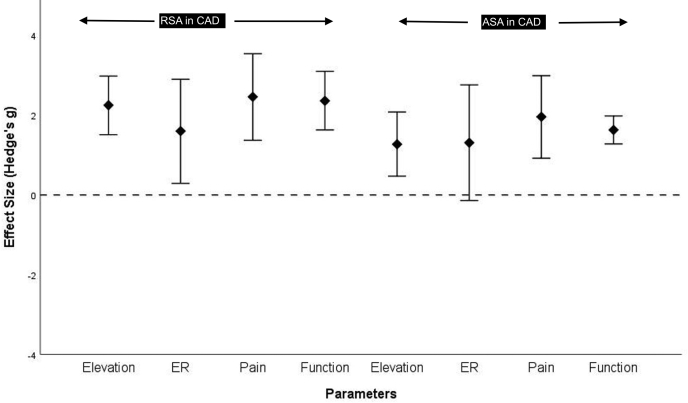
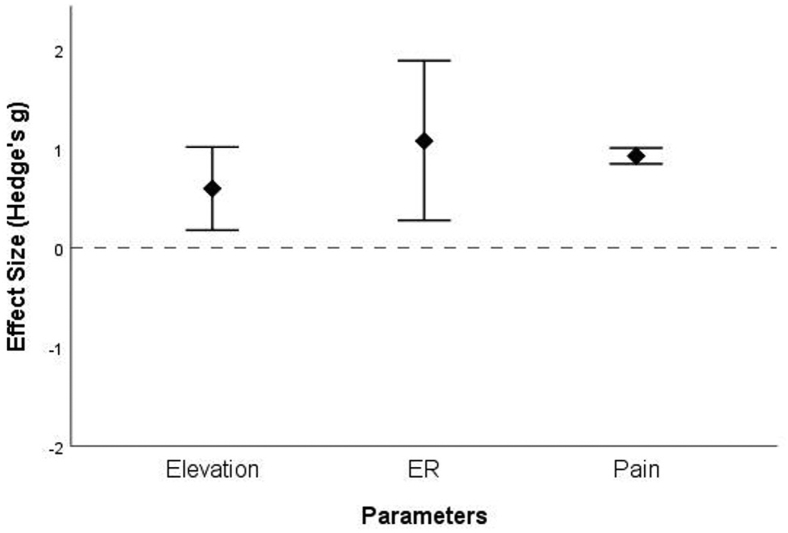
Only 6 studies provided complete preoperative and postoperative data on pain, elevation, external rotation, and functional scores. Data on internal rotation were not sufficient for pooled calculation. We pooled in the limited data, standardized it in the form of Hedge's g and calculated the postintervention improvement in the form of combined weighted effect size. Functional scores improved postintervention in the combined weighted data available from the studies by Pritchet et al, Raiss et al, and Matsoukis et al after ASA for CAD (1.63, 95% confidence interval [C.I.], 1.28 to 1.98; I2 = 0%) and from that by Raiss et al, Van Tongel et al, Werner et al, and Kurowicki et al after RSA for CAD (2.36, 95% C.I., 1.6 to 3.1; I2 = 54%) (Fig. 4). Data that were weighted and combined from the studies by Wooten et al, Gavriilidis et al, and Cheng et al showed that the elevation (0.6, 95% C.I., 0.18 to 1.0; I2 = 43%), external rotation (1.08, 95% C.I., 0.28 to 1.89; I2 = 69%), and pain (0.93, 95% C.I., 0.85 to 1.01; I2 = 0%) significantly improved after ASA in the CPD subgroup (Fig. 5). Similarly, the combined data showed improvement in elevation, external rotation, and pain after RSA in the CAD group (elevation: 2.25, 95% C.I., 1.51 to 2.98; external rotation: 1.6, 95% C.I., 0.29 to 2.9; and pain: 2.46, 95% C.I., 1.37 to 3.52 from the studies by Raiss et al, Statz et al, Werner et al and Kurowicki et al) and after ASA in the CAD group (elevation :1.27, 95% C.I., 0.47 to 2.08; external rotation: 1.31, 95% C.I., −0.14 to 2.76; and pain: 1.96, 95% C.I., 0.92 to 2.99 from the studies by Statz et al, Flatow et al, Raiss et al and Matsoukis et al) (Fig. 4).
Figure 4.
Summary of the pooled effects of reverse shoulder arthroplasty (RSA) and anterior shoulder arthroplasty (ASA) on distinct clinical parameters. Elevation; ER, external rotation; functional score (Constant score or American Shoulder and Elbow Surgeons score) and pain scores. The horizontal dotted line indicates the line of null effects (ie, no significant differences between before assessment and after assessments. The positive values indicate improvement from the baseline to postarthroplasty; negative values indicate no improvement. The diamonds represent the pooled estimates; the vertical lines correspond to the confidence interval of each estimate. As can be observed, all the vertical bars cross the null-effects line except external rotation in ASA, indicating that all the effects are significant—there are overall significant improvements in all the assessed clinical parameters.
Figure 5.
Summary of the pooled effects of anterior shoulder arthroplasty (ASA) in chronic posterior dislocation (CPD) on distinct clinical parameters. Elevation; ER, external rotation and pain scores. The horizontal dotted line indicates the line of null effects (ie, no significant differences between the before assessment and after assessments. The positive values indicate increases from the baseline to postarthroplasty; negative values indicate no improvement. The diamonds represent the pooled estimates; vertical lines correspond to the confidence interval of each estimate. As can be observed, none of the vertical bars cross the null-effects line, indicating that all the effects are significant—there are overall significant improvements in all the assessed clinical parameters.
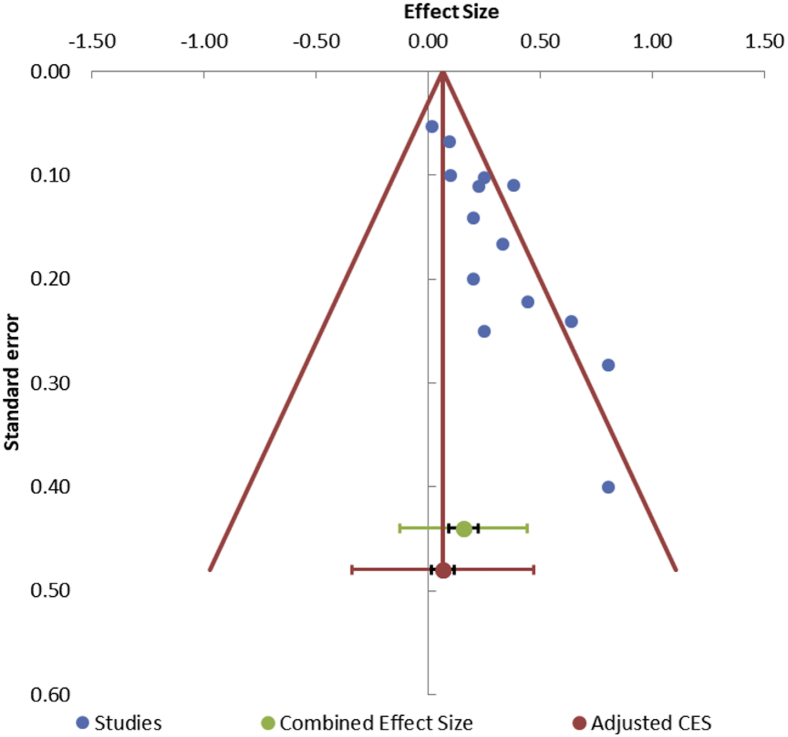
Sensitivity analysis for those studies that scored less than 10 on the MINORS scale (Table II) and for the two studies that had incompletely demarcated data8,13 showed that the exclusion of these studies did not change the results of the primary outcome. Regression analysis showed no significant effect of the period of neglect on the primary outcomes. Asymmetric distribution of the studies with funnel plot and the result (P < .001) of Begg and Mazumdar rank correlation test showed that publication bias may be present (Fig. 6).
Figure 6.
This funnel plot shows presence of publication bias: Visual inspection of the funnel plot shows that all the studies (blue dots) are distributed to one side of the combined effect size which denotes an asymmetrical funnel plot or a presence of a publication bias; Begg and Mazumdar rank correlation test = P < .05 represents statistically significant publication bias.
Discussion
Chronic shoulder dislocation, being an uncommon condition, presents several unique challenges in its management such as the presence of concomitant problems of contracted anterior soft tissues, large rotator cuff tears and severe glenoid bone loss.8,14,22 Most of the published articles on arthroplasty for CAD or CPD have been retrospective studies of few cases with level 4 evidence. Understandably, randomized trials may not be possible because of the rarity of the condition. Hence, we intended to pool in the data of multiple studies to arrive at well-meaning conclusions regarding the complications of the two most common shoulder arthroplasties (RSA and ASA) performed in the present times.
Our study found that the instability is significantly more common after anatomic arthroplasty than reverse arthroplasty for chronic anterior dislocation. This may be an important consideration while deciding on the implant of choice because the postoperative instability may be difficult to treat and may result in unsatisfactory outcomes.3,9 RSA has a lower risk of instability because it is a semiconstrained implant and may compensate for the imbalance of the surrounding soft tissues and the rotator cuff. Statz et al and Li et al found that the postoperative instability was influenced by the subscapularis removal and repair during the ASA procedure. This step is circumvented after RSA because subscapularis integrity does not contribute to the stability of RSA.6,23 We found that functional scores, pain scores, and range of movements improved after RSA and ASA. However, all the included studies in this review did not provide their preoperative data on the functional status. Although RSA had lower instability rates, both RSA and ASA showed high number of complications because RSA was associated with several complications that may be unique in CAD. A significant complication that was seen with the RSA in CAD was the glenoid base plate loosening.14 We found that some studies reported the presence of 40%-50% of anterior glenoid bone defect in CAD.14,19,22 Less than 50% support for the glenoid base plate may be a critical factor for the survival of these implants.4 Hence, a long-pegged implant along with bone graft may be required to decrease the chances of glenoid base plate failure. Other complications after RSA were that of humeral shaft fracture and acromion fracture. Humeral shaft fractures were observed both intraoperatively and postoperatively.19 The reason for the shaft fractures may be that in some cases, the anterior dislocations were neglected for several months to years. As a result, the anterior tissues may be contracted and may resist the relocation of the humerus on the glenosphere. A higher than usual force for the relocation may be needed in such a situation. The other complication of high-grade acromion fracture may also have been due to the similar pathomechanism at play in CAD. The severe and long-standing contracture found in CAD may place larger stress on the already lengthened deltoid. The desired lengthening that the surgeons may like to achieve to prevent instability and the pathomechanism of soft tissue contracture in CAD may be responsible for larger stresses and consequent fractures of the acromion after RSA. We found that ASA was also associated with several complications such as severe pain, glenoid failure, glenoid erosion, bone graft migration, and superior migration of the humeral head. The glenoid problems and severe pain may be due to eccentric forces on the humeral implant, especially because the implants were placed in excessively increased versions away from the direction of the dislocation.3,10,19 The rotator cuff that was repaired in many cases, may have eventually failed in some of these cases, leading to the superior migration.
Surprisingly, we did not find a higher incidence of neurologic problems after RSA. We found only 2 cases of neuropathies after ASA for CPD; 1 median nerve and 1 axillary nerve paresis that eventually resolved. Finally, our regression analysis revealed that the period of neglect in CAD and CPD did not influence the rate of complication and the rate of instability after intervention. This means that the complications should be anticipated irrespective of the chronicity of the shoulder dislocation. As per our study, complications after arthroplasty such as instability, glenoid loosening, severe pain, and cuff insufficiency may always be anticipated in the setting of a chronic shoulder dislocation that is left untreated beyond few months because of a combination of several predisposing and pre-existent factors such as glenoid deficiency, existing cuff tears, and medial contractures. Finally, based on our experience and our study, we can make certain recommendations for shoulder arthroplasty in the setting of a chronic shoulder dislocation. In our opinion, an RSA should be the preferred option over an ASA for the following reasons: Depending on the chronicity, the subscapularis may be severely adherent medially and to the brachial plexus. In addition, a repair of the subscapularis may also not work because of the chronic lengthening in some cases. A functioning and a stable anatomic prosthesis, in the presence of aforementioned factors, may not be reliably achieved. In addition, the status of the rest of the rotator cuff is always a concern that may affect the outcome and longevity of the implant. Reverse prosthesis may be preferred for its semiconstrained design and because the subscapularis status and rest of the rotator cuff integrity becomes less of a concern for the prosthetic stability and function. However, the glenoid management in RSA should be preplanned and more focused to prevent any baseplate-related complication. A long peg of 25 mm with an anchorage of minimum 10 mm in native glenoid bone should be planned. If the anterior glenoid deficiency reaches 50%, a humeral head graft may be used to reconstruct the glenoid and provide support to the base plate. The central peg may not always be in the center of the glenoid and should be placed in the native bone. However, in some cases the glenoid deficiency may reach 60%-80%. In these cases, an iliac crest graft may be used for reconstruction, and if the surgeon finds insufficient primary stability of the glenoid implant, a two-stage reconstruction should be performed after the bone graft heals.
The main limitations of this review are that some of the studies did not provide their complete preoperative data. In some of the studies, the measures of dispersion were not stated, and we had to calculate the confidence intervals from the available values of range. However, we used well-established formulas with the underlying assumption that there would be a uniform distribution.
Conclusion
There is a higher instability rate after ASA than after RSA for chronic shoulder dislocation. This study also showed that there are overall postintervention benefits after arthroplasty for CAD and CPD. However, some of the complications such as glenoid base plate fixation problems after RSA should be anticipated and circumvented by using long pegged baseplate and bone grafts.
Conflicts of interest
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Funding
No funding was received for the study.
Footnotes
Investigation performed at the Mumbai Shoulder Institute, Mumbai, India.
Institutional review board approval was not required for this review article.
References
- 1.Cheng S.L., Mackay M.B., Richards R.R. Treatment of locked posterior fracture-dislocations of the shoulder by total shoulder arthroplasty. J Shoulder Elbow Surg. 1997;6:11–17. doi: 10.1016/s1058-2746(97)90065-3. [DOI] [PubMed] [Google Scholar]
- 2.Delcogliano A., Caporaso A., Chiossi S., Menghi A., Cillo M., Delcogliano M. Surgical management of chronic, unreduced posterior dislocation of the shoulder. Knee Surgery. Sport Traumatol Arthrosc. 2005;13:151–155. doi: 10.1007/s00167-004-0524-6. [DOI] [PubMed] [Google Scholar]
- 3.Flatow E.L., Miller S.R., Neer C.S. Chronic anterior dislocation of the shoulder. J Shoulder Elbow Surg. 1993;2:2–10. doi: 10.1016/S1058-2746(09)80131-6. [DOI] [PubMed] [Google Scholar]
- 4.Formaini N.T., Everding N.G., Levy J.C., Santoni B.G., Nayak A.N., Wilson C., et al. The effect of glenoid bone loss on reverse shoulder arthroplasty baseplate fixation. J Shoulder Elbow Surg. 2015;24:e312–e319. doi: 10.1016/j.jse.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 5.Gavriilidis I., Magosch P., Lichtenberg S., Habermeyer P., Kircher J. Chronic locked posterior shoulder dislocation with severe head involvement. Int Orthop. 2010;34:79–84. doi: 10.1007/s00264-009-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grassi F.A., Zorzolo I. Reverse shoulder arthroplasty without subscapularis repair for the treatment of proximal humeral fractures in the elderly. Musculoskelet Surg. 2014;98(Suppl 1):5–13. doi: 10.1007/s12306-014-0321-4. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins R.J., Neers C.S., II, Pianta R.M., Mendoza F.X. Locked posterior dislocation of the shoulder. J Bone Jt Surg Am. 1987;69:9–18. [PubMed] [Google Scholar]
- 8.Kurowicki J., Triplet J.J., Momoh E., Moor M.A., Levy J.C. Reverse shoulder prosthesis in the treatment of locked anterior shoulders: a comparison with classic reverse shoulder indications. J Shoulder Elbow Surg. 2016;25:1954–1960. doi: 10.1016/j.jse.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Jiang C. The effectiveness of the Latarjet procedure in patients with chronic locked anterior shoulder dislocation a retrospective study. J Bone Jt Surg - Am. 2016;98:813–823. doi: 10.2106/JBJS.15.00832. [DOI] [PubMed] [Google Scholar]
- 10.Matsoukis J., Tabib W., Guiffault P., Mandelbaum A., Walch G., Némoz C., et al. Primary unconstrained shoulder arthroplasty in patients with a fixed anterior glenohumeral dislocation. J Bone Jt Surg - Ser A. 2006;88:547–552. doi: 10.2106/JBJS.E.00368. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pritchett J., Clark J. Prosthetic replacement for chronic unreduced dislocations of the shoulder. Clin Orthop Relat Res. 1987:89–93. [PubMed] [Google Scholar]
- 13.Raiss P., Aldinger P.R., Kasten P., Rickert M., Loew M. Humeral head resurfacing for fixed anterior glenohumeral dislocation. Int Orthop. 2009;33:451–456. doi: 10.1007/s00264-007-0487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raiss P., Edwards T.B., Bruckner T., Loew M., Zeifang F., Walch G. Reverse arthroplasty for patients with chronic locked dislocation of the shoulder (type 2 fracture sequela) J Shoulder Elbow Surg. 2017;26:279–287. doi: 10.1016/j.jse.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez A., Cox C. Improving on the Range Rule of Thumb. Hulman Undergrad Math J. 2016;13 https://scholar.rose-hulman.edu/rhumj/vol13/iss2/1 [Google Scholar]
- 16.Rowe C.R., Bertram Z. Chronic unreduced Dislocations of the Shoulder. J bone Jt surgery. 1982:494–505. [PubMed] [Google Scholar]
- 17.Sevivas N., Ferreira N., Andrade R., Moreira P., Portugal R., Alves D., et al. Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elbow Surg. 2017;26:e265–e277. doi: 10.1016/j.jse.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 18.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (Minors): Development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 19.Statz J.M., Schoch B.S., Sanchez-Sotelo J., Sperling J.W., Cofield R.H. Shoulder arthroplasty for locked anterior shoulder dislocation: a role for the reversed design. Int Orthop. 2017;41:1227–1234. doi: 10.1007/s00264-017-3450-1. [DOI] [PubMed] [Google Scholar]
- 20.Suurmond R., van Rhee H., Hak T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods. 2017;8:537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Tongel A., Claessens T., Verhofste B., De Wilde L. Reversed shoulder arthroplasty as treatment for late or ancient chronic glenohumeral dislocation. Acta Orthop Belg. 2016;82:637–642. [PubMed] [Google Scholar]
- 22.Werner B.S., Böhm D., Abdelkawi A., Gohlke F. Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg. 2014;23:1655–1661. doi: 10.1016/j.jse.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Werner B.C., Wong A.C., Mahony G.T., Craig E.V., Dines D.M., Warren R.F., et al. Clinical Outcomes After Reverse Shoulder Arthroplasty With and Without Subscapularis Repair. J Am Acad Orthop Surg. 2018;26:e114–e119. doi: 10.5435/JAAOS-D-16-00781. http://journals.lww.com/00124635-201803010-00010 [DOI] [PubMed] [Google Scholar]
- 24.Wooten C., Klika B., Schleck C.D., Harmsen W.S., Sperling J.W., Cofield R.H. Anatomic shoulder arthroplasty as treatment for locked posterior dislocation of the shoulder. J Bone Jt Surg - Ser A. 2014;96:1–6. doi: 10.2106/JBJS.L.01588. [DOI] [PubMed] [Google Scholar]
- 25.Wright J.G., Swiontkowski M.F., Heckman J.D. Introducing levels of evidence to the journal. J Bone Jt Surg AM. 2003;85:1–3. http://journals.lww.com/00004623-200301000-00001 [PubMed] [Google Scholar]