Abstract
The parvovirus minute virus of mice NS1 protein is a multifunctional protein involved in a variety of processes during virus propagation, ranging from viral DNA replication to promoter regulation and cytotoxic action to the host cell. Since NS1 becomes phosphorylated during infection, it was proposed that the different tasks of this protein might be regulated in a coordinated manner by phosphorylation. Indeed, comparing biochemical functions of native NS1 with its dephosphorylated counterpart showed that site-specific nicking of the origin and the helicase and ATPase activities are remarkably reduced upon NS1 dephosphorylation while site-specific affinity of the protein to the origin became enhanced. As a consequence, the dephosphorylated polypeptide is deficient for initiation of DNA replication. By adding fractionated cell extracts to a kinase-free in vitro replication system, the combination of two protein components containing members of the protein kinase C (PKC) family was found to rescue the replication activity of the dephosphorylated NS1 protein upon addition of PKC cofactors. One of these components, termed HA-1, also stimulated NS1 helicase function in response to acidic lipids but not phorbol esters, indicating the involvement of atypical PKC isoforms in the modulation of this NS1 function (J. P. F. Nüesch, S. Dettwiler, R. Corbau, and J. Rommelaere, J. Virol. 72:9966–9977, 1998). The present study led to the identification of atypical PKCλ/ι as the active component of HA-1 responsible for the regulation of NS1 DNA unwinding and replicative functions. Moreover, a target PKCλ phosphorylation site was localized at S473 of NS1. By site-directed mutagenesis, we showed that this residue is essential for NS1 helicase activity but not promoter regulation, suggesting a possible modulation of NS1 functions by PKCλ phosphorylation at residue S473.
Minute virus of mice (MVMp), the prototype strain of autonomous parvoviruses, is a small icosahedral particle with a single-stranded linear DNA as a genome. This 5.1-kb DNA encodes two structural (VP) and at least four nonstructural (NS) proteins, of which NS1 is the only viral protein necessary for progeny virus production in all cell types (for reviews, see references 14 and 44). NS1 is an 83-kDa polypeptide involved in a variety of functions in the course of a virus infection. Besides trans regulation of the parvovirus P4 and P38 promoters driving the nonstructural and capsid gene expression, respectively (26, 56), NS1 influences a variety of heterologous viral and cellular promoters (34, 63), exerts a toxic action on the host cell (7, 42), and is the initiator protein for parvovirus DNA amplification (9, 12, 15, 16, 48).
Replication of the parvovirus genome occurs through the formation of a series of concatemeric duplex DNA intermediates, generated by a single-strand-copying mechanism similar to the rolling-circle replication described for bacteriophages and single-stranded plasmids (for a review, see reference 15). While conversion of the single-stranded linear genome to a covalently closed monomeric duplex is achieved in the absence of any viral proteins (5), subsequent amplification requires the activities of the major nonstructural protein NS1 (13, 17). NS1 mediates initiation of replication by site-specific nicking within the origins of replication, generating free 3′ hydroxyls, which then serve as primers for the DNA polymerase (5, 9, 12, 16, 48). At the end of this reaction, NS1 remains covalently attached to the 5′ end of the nicked strand in vivo (18, 19) and in vitro (13, 17). In addition to these initiation reactions at the left- and right-end origins, NS1 facilitates DNA polymerase-driven strand displacement synthesis by unwinding the double-stranded template in front of the replication fork (50). For these various functions, a variety of distinct biochemical activities were attributed to the viral protein. NS1 is a site- and strand-specific endonuclease (9, 12, 16, 48), binds site specifically to an [ACCA]2–3 motif (21), binds and hydrolyzes ATP, has intrinsic helicase activity (10, 65), and is able to self-associate to form oligomers (47, 54).
The multiplicity of NS1 functions requiring distinct biochemical activities of the polypeptide raised the possibility that NS1 is regulated for its different tasks in a controlled fashion during virus propagation. Such modulation of NS1 activities can be achieved through several mechanisms. The interaction of NS1 with the cofactor ATP seems to be crucial, since most of the reported NS1 functions are dependent on an intact nucleoside triphosphate (NTP)-binding domain (21, 28, 34, 35, 46–48). In addition, NS1 is able to interact directly with cellular components, such as the transcription factor SP1 (32, 37) and the novel protein SGT (23), indicating that the polypeptide might also be recruited for selected functions by cellular proteins. For a variety of proteins including simian virus 40 (SV40) large T antigen, to which NS1 has many similarities including a striking sequence homology within the helicase domain (4), posttranslational modification is a further mode of regulation (for a review, see reference 64). NS1 becomes phosphorylated at multiple serine and threonine residues in the course of a viral infection (11, 20), indicating that phosphorylation might indeed play a role in the differential modulation of NS1 activities. Using a kinase-free in vitro replication system based on plasmids containing the MVM left-end origins, we have shown that NS1 replication activity is dependent upon phosphorylation (50). The lack of replication activity of un(der)phosphorylated NS1 is most probably due to a severe reduction of nickase, helicase, and ATPase activities (49). In contrast, site-specific affinity to the cognate DNA recognition motif [ACCA]2–3 is enhanced for dephosphorylated NS1 (49). This differential effect of phosphorylation on distinct biochemical activities of NS1 is consistent with a regulation of NS1 functions by posttranslational modification.
Consecutive fractionation of HeLa cell extracts led to the identification of two protein components (termed HA-1 and HA-2) which were able, when supplied together, to phosphorylate and reactivate dephosphorylated NS1 (NS1O) for rolling-circle replication (50). The purification profile, the composition of these protein fractions, and their cofactor requirements for reactivation strongly suggested the involvement of proteins belonging to the protein kinase C (PKC) family (50). PKC consists of a class of very similar cellular kinases involved in regulatory processes such as growth control, differentiation, and transformation. For their activity, PKCs depend on cofactors such as acidic lipids, Ca2+ and diacylglycerols, and/or phorbol esters. According to their cofactor requirements, PKCs are subdivided into three groups, characterized by the presence (or absence) of motifs interacting with these cofactors (for reviews, see references 39 and 53). The selective cofactor requirement of HA-1 to achieve the rescue of NS1O helicase function suggested that the activating protein kinase(s) consisted of atypical PKC (50).
To investigate this possibility and to determine the specificity of NS1 phosphorylation and activation by PKCs in vitro, we examined PKCλ/ι as a main candidate within HA-1 regulating NS1 helicase activity. Recombinant PKCλ was produced by means of recombinant vaccinia virus expression in HeLa cells and compared to other members of the PKC family for NS1O phosphorylation and functional modulation.
MATERIALS AND METHODS
Viruses and cells.
Recombinant vaccinia viruses were constructed as previously described (46) and were propagated in monolayer cultures of BSC-40 cells, collected and purified over a sucrose cushion as described previously (38), except for the release of virus from infected cells, which was achieved by three cycles of freezing and thawing instead of sonication. A9 and BSC-40 cells were grown in Dulbecco’s modified Eagle’s medium containing 5% fetal calf serum. HeLa-S3 cells were grown in spinner bottles in the presence of 5% fetal calf serum.
Production and purification of recombinant proteins.
PKCα, PKCγ, PKCλ, and PKCζ, as well as wild-type and mutant NS1 were produced from recombinant vaccinia viruses in suspension cultures of HeLa-S3 cells and harvested 18 h postinfection (46, 49). PKC was purified from whole-cell extracts after a nuclear squeeze into the cytoplasm by using 0.3 M NaCl, while NS1 was purified from nuclear extracts (48). Dephosphorylation of NS1 protein was performed within nuclear extracts by using calf intestine alkaline phosphatase (49). All proteins were purified by means of an N-terminal His6 tag on Ni2+-NTA agarose (48). The protein preparations were analyzed by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie-blue staining and for their activities in various in vitro assays.
Plasmids.
Plasmids containing cDNAs encoding PKC isoforms were constructed as follows. PKCλ cDNA was obtained by coupled reverse transcriptase-PCR with mRNA preparations derived from mouse A9 fibroblast cells. Whole-cell RNA was prepared with hot phenol (58), and the mRNAs thereof were isolated with biotinylated oligo(dT) primers and magnetic bead-conjugated streptavidin as specified by the manufacturer (Qiagen). Based on known PKCλ sequences (2), reverse transcriptase-PCR was performed with the N-terminal primer 5′-ATACCATGGTAACACACTTTGAGCCTT-3′, containing a NcoI site (underlined), together with the C-terminal primer 5′-GAACTCGAGTCAGACACACTCTTC-3′, containing a XhoI site (underlined). This facilitated the directional cloning of PKCλ cDNA into the expression plasmid pTMHis (50), generating pTHisPKCλ. pTMHis is a derivative of pTM-1 (41), which allows the expression of N-terminal His6-tagged proteins from recombinant vaccinia viruses under the control of the bacteriophage T7 promoter and an encephalomyocarditis virus leader sequence. The entire cDNA of pTHisPKCλ has been sequenced (customized sequencing by 4-Base Lab GmbH, Reutlingen, Germany) and compared with the published PKCλ sequence (2). Besides the lack of 27 N-terminal amino acids, we changed I28 to alanine in order to generate the NcoI restriction site required for the cloning strategy. No additional changes were determined between our A9 cDNA isolates and the published PKCλ sequence. pTHisPKCγ was obtained from a human cDNA clone (33) by introducing the NcoI-XhoI fragment into pTMHis. pTHis PKCζ was constructed in two steps. An EcoRI fragment containing the human PKCζ cDNA (31) was introduced into pTMHis. The NcoI-BstEII fragment was then replaced by a PCR product obtained with the same cDNA serving as a template and the primer pair 5′-CCCACCATGGAAGGGAGCG-3′ and 5′-AAAGCCTCTTCCAGCT-3′.
Mutagenesis of S473 to alanine in the NS1 coding sequence was achieved by chimeric PCR as described previously (48), replacing the EcoRI-BstEII fragment of pTHis NS1 (48) with the PCR fragment containing the point mutation. The first PCR step was performed with the leftward primer Nu2 (5′-ATGGCCGGAAATGCTTACTCT-3′) and the rightward primer 5′-GCCTTTTTCCTTTTG-3′ and with the leftward mutagenic primer 5′-CAAAAAGGAAAAGGCGCCAAACAGATTGA-3′ (mutations are in italics) and the rightward primer TD1 (5′-GTGCTCTTTGGCAGC-3′), respectively, using pDNS5-5 as a template. In a second PCR step, the two purified PCR fragments were hybridized and further amplified with primers Nu2 and TD1.
The plasmids used as templates for in vitro replication assays were pL1-2TC and pL1-2GAA, containing the minimal active left-end MVM origin and the corresponding inactive origin, respectively (12). For P38 transactivation assays, DNA fragments containing the phosphorylation site mutant S473A as well as the nucleotide-binding-site mutants K405M and K405R (46) were cloned into pRSV-NS (61) by replacement of the EcoRV (position 385) to BstEII (position 1885) fragment in the wild-type pRSV-NS construct.
Protein extraction and fractionation by column chromatography.
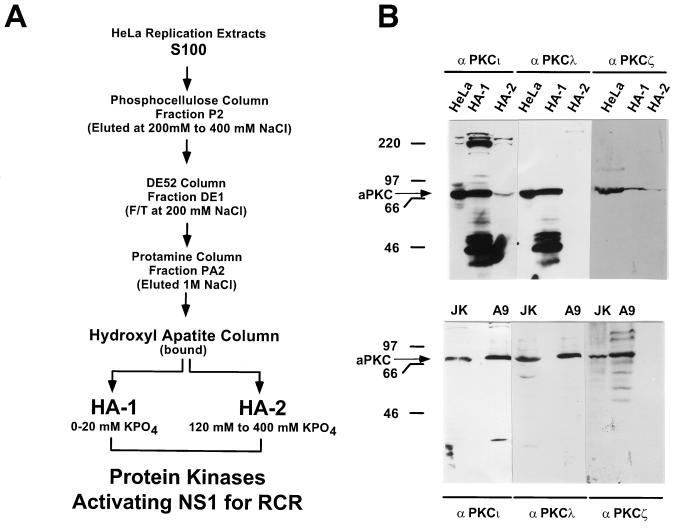
PKC-containing fractions HA-1 and HA-2, previously shown to reactivate NS1O in replication assays, were obtained from HeLa cell extracts as described in detail in reference 50 and schematized in Fig. 1A. Briefly, S100 extracts were fractionated on phosphocellulose columns to obtain P2, which eluted between 200 and 400 mM NaCl. Fraction P2 was further purified on DE52 columns. PKCs present in the flowthrough at 200 mM NaCl (fraction DE1) were concentrated by affinity chromatography on protamine chloride columns, eluted at 1 M NaCl (fraction PA2), and dialyzed against buffer B (20 mM HEPES-KOH [pH 7.5], 1 mM EDTA, 50 mM NaCl, 0.1 mM dithiothreitol [DTT], 20% sucrose, 10% glycerol). These PKC preparations were then fractionated by fast protein liquid chromatography on hydroxylapatite columns. The bound proteins, after extensive washing in buffer B without sucrose, were first eluted with buffer C (20 mM KPO4 [pH 7.5], 150 mM NaCl, 10% glycerol) to obtain HA-1. HA-2 was then obtained with a linear gradient of buffer C and buffer D (0.5 M KPO4 [pH 7.5], 150 mM NaCl, 10% glycerol) and eluted between 120 and 400 mM KPO4.
FIG. 1.
Production and analysis of NS1O-activating protein fractions HA-1 and HA-2. (A) Purification scheme for HA-1 and HA-2, the protein fractions containing kinases that are able to activate NS1O in RCR assays. HeLa replication extracts were fractionated on phosphocellulose columns. Fraction P2, eluting between 200 and 400 mM NaCl, was further purified on DE52, a strong anion-exchange column. PKCs present in the flowthrough (F/T) at 200 mM NaCl were further affinity purified on protamine chloride columns, and the bound material was fractionated by fast protein liquid chromatography on hydroxylapatite columns. HA-1 consists of the hydroxylapatite column-bound fraction which elutes at 20 mM KPO4, and HA-2 consists of the pooled fractions eluting between 120 and 400 mM KPO4 (for details, see reference 50). (B) Detection of atypical PKC isoforms in HA-1, HA-2, and whole A9 cell extracts. (Top) Protein fractions HA-1 and HA-2 were analyzed by Western blotting and revealed with monoclonal antibodies raised against the indicated atypical PKC isoforms PKCι, PKCλ, and PKCζ. The 46-kDa polypeptides revealed by PKC antibodies consist of proteolytic cleavage products, representing the catalytic domain of the respective PKC isoform (PKCm [50]) generated during the isolation procedure. Unfractionated HeLa cell extract (20 μg) served as a positive control. (Bottom) Western blot analysis of whole-cell extracts (20 μg) derived from A9 fibroblasts, a cell line derived from the natural host of MVMp and routinely used to propagate this virus. Jurkat cell extracts (10 μg) served as positive controls. The molecular mass markers and the apparent migration of atypical PKC (70 to 72 kDa) are indicated on the left.
Western blot analyses.
Protein extracts were separated by discontinuous SDS-PAGE (10% polyacrylamide), blotted on nitrocellulose membranes, and revealed with mouse primary antibodies specific for the atypical PKCι, PKCλ, or PKCζ (Transduction Laboratories) at dilutions of 1:2,500 (αPKCι and αPKCλ) or 1:200 (αPKCζ). Detection was performed with a 1:5,000 dilution of horseradish peroxidase-conjugated anti-mouse immunoglobulin Gs, using the ECL system (Amersham).
In vitro kinase reactions.
In vitro kinase reactions were performed as described previously (49), with various amounts of PKC, 100 ng of dephosphorylated NS1O, and 30 μCi of [γ-32P]ATP (3,000 mCi/mmol) in 20 μl of 20 mM HEPES-KOH (pH 7.5)–7 mM MgCl2–5 mM KCl–1 mM DTT in the presence of PKC cofactors (2 mM CaCl2 and 1 μg of l-α-phosphatidyl-l-serine [PS] per μl). After the mixtures were incubated for 30 min at 37°C, the reactions were stopped by addition of the same volume of 20 mM Tris (pH 7.5)–5 mM EDTA–0.2% SDS and heating for 30 min at 70°C. The reactions products were analyzed directly by SDS-PAGE (8% polyacrylamide) and semidry transfer onto polyvinylidene difluoride (PVDF) membranes (Millipore), allowing the subsequent proteolytic digestion of the excised membrane bound proteins.
In vivo 32P labeling and tryptic peptide analysis.
Metabolic 32P labeling of NS1 expressed from recombinant vaccinia viruses was performed 5 h after HeLa cell infection with 15 PFU each of vTF7-3 per cell and the appropriate recombinant vaccinia virus (containing the NS1 gene under control of the bacteriophage T7 promoter) per cell. The labeling conditions described for natural MVM infections of A9 cells (49), using 10−10 Ci of [32P]orthophosphate (ICN) per cell for 4 h, were applied. Cultures (107 cells) were harvested directly into RIPA buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100) containing protease and phosphatase inhibitors, and immunoprecipitations were carried out with anti-NSN (22). Immune complexes were further purified on SDS-PAGE and blotted on PVDF membranes. The band corresponding to NS1 was excised and digested with either 50 U of trypsin or 15 μg of chymotrypsin (Boehringer Mannheim) for 18 h at 37°C. The 32P-labeled peptides were then separated in two dimensions on thin-layer cellulose plates (Merck) by electrophoresis at pH 1.9 and chromatography in phosphochromatography buffer (49).
Helicase assays.
Helicase assays were performed as described previously (48) with M13-VAR as a template. Reactions were performed with 10 to 100 ng of purified NS1, incubated for 40 min at 37°C, and stopped by the addition of 0.2% SDS-2.5 mM EDTA. For reactivation experiments, titrated amounts of protein kinases were added to the reaction mixtures together with the PKC cofactors 2 mM Ca2+, 1 μg of PS per μl, or 5 nM 12-O-tetradecanoylphorbol-13-acetate (TPA). None of these PKC cofactors alone had any influence on the helicase function of native NS1P, dephosphorylated NS1, or mutant NS1 proteins serving as negative controls. Optimal reactivation of NS1O (20 ng) was achieved with 50 to 500 pg of purified PKCλ.
Replication assays.
Replication assays were carried out as described previously (50) in the presence of optimized P1-Thr providing the required cellular components, 3 U of T4 DNA polymerase, and approximately 200 ng of His-tagged vaccinia virus-produced NS1 (determined by Coomassie blue staining after SDS-PAGE). P1-Thr consists of the flowthrough fraction of 293 cell extracts purified on phosphocellulose columns relieved of endogenous serine/threonine kinases by l-Thr-affinity chromatography. This fraction contains the replication factors RPA, PCNA, and PIF. The assays were carried out in a 20-μl total volume consisting of 20 mM HEPES-KOH (pH 7.5), 5 mM MgCl2, 5 mM KCl, 1 mM DTT, 0.05 mM each dNTP, 2 mM ATP, 40 mM creatine phosphate, 1 μg of phosphocreatine kinase, 10 μCi of [α-32P]dATP (3,000 mCi/mmol), and 20 ng of the appropriate DNA template (pL1-2TC or pL1-2GAA [12]). After incubation at 37°C for 2 h, the reaction was stopped by adding 60 μl of 20 mM Tris (pH 7.5)–10 mM EDTA–0.2% SDS, and incubating the mixture at 70°C for at least 30 min. The reaction products were analyzed by agarose gel electrophoresis after immunoprecipitation with anti-NSN antiserum and digestion with HindIII.
P38 trans activation.
To measure the capacity of wild-type NS1 and mutant derivatives for trans activation of the P38 promoter, 2 × 105 A9 cells grown in monolayer cultures were cotransfected with 50 ng of the reporter plasmid pP38-Luc (63) and various amounts of the NS1 expression plasmid pRSV-NSx (x stands for the appropriate derivative of NS1). At 48 h posttransfection, the cells were harvested into lysis buffer (15 mM glyc-glycine [pH 7.8], 15 mM MgSO4, 0.4 mM EGTA, 1 mM DTT, 1% Triton X-100, 10% glycerol) and processed for measurements of luciferase activity as described previously (63). Luciferase activities are expressed relative to wild-type NS1 (100%) after subtraction of the background value in the absence of effector vector. Measurements from three independent transfection experiments were performed with various amounts of pRSV-NS1x and are given as average values with standard deviations.
RESULTS
Determination of atypical PKC isoforms present in HA-1, and production and purification of active recombinant PKCλ.
Previous investigations with a protein kinase-free in vitro replication system have shown that the replicative functions of NS1 are dependent upon the phosphorylation state of the polypeptide (49, 50). In addition, supplementation of this system with fractionated HeLa replication extracts, using consecutive preparative affinity column chromatography for purification (Fig. 1A), allowed dephosphorylated NS1 (NS1O) to become reactivated for replication activity. These investigations revealed that two separate protein components, which contained members of the PKC family (designated HA-1 and HA-2), were necessary for the rescue of NS1O (50). Further characterization of HA-1 and HA-2 revealed that only HA-1 was able to modulate NS1 helicase activity, provided that the PKC cofactor PS was supplied. This requirement for PS together with the inability of phorbol esters to stimulate the reactivation of NS1O DNA-unwinding functions, suggested the involvement of atypical members of the PKC family (50). To test this possibility and to investigate the nature of the activating protein kinase(s) within HA-1, we first analyzed protein fractions HA-1 and HA-2 for the presence of the known atypical PKC isoforms PKCι, PKCλ, and PKCζ by Western blot analyses with monoclonal antibodies raised specifically against the individual PKC isoforms. It should be mentioned that PKCι and PKCλ have a high sequence homology (2, 59), leading to cross-reactivity of the two antibodies. It is likely that PKCι is the human homologue of PKCλ and might thus have similar functions in vivo and in vitro. Unfractionated HeLa cell extracts served as positive controls. As illustrated in Fig. 1B (top panel), the HA-1 fraction proved to be highly enriched in PKCι and PKCλ compared to HA-2. In contrast, only modest amounts of PKCζ could be isolated from HeLa replication extracts (compared to the positive control), and unlike PKCι and PKCλ, PKCζ was found in significant amounts in both fractions, HA-1 and HA-2. Since only HA-1 and not HA-2 was able to rescue NS1O helicase activity (50), it is likely that PKCι and PKCλ, rather than PKCζ, consisted of the activating component in HA-1. No further information concerning the nature of the activating kinase was derived from Western blot analyses of A9 cells. All known atypical PKC isoforms were expressed in significant amounts in this natural host cell of MVMp (Fig. 1B, bottom).
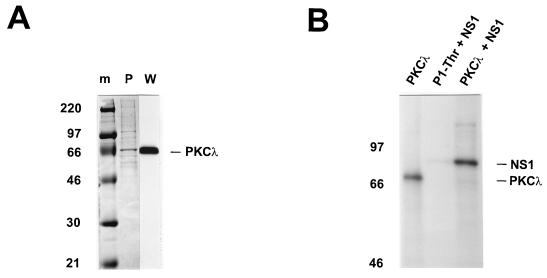
To determine the relevance of atypical PKCs to NS1 regulation, we concentrated our investigation on PKCλ. A PKCλ cDNA was obtained from A9 cells by reverse transcriptase-PCR with an N-terminal rightward primer encompassing the methionine M27 codon and a C-terminal leftward primer covering the terminal TGA (opal588) of the published sequence (2). No products were obtained with primers encompassing M1 (probably due to the unusually high GC content [>70%] within this region). Therefore, we cloned the cDNA[M27 to opal588] into the expression plasmid pTMHis1 (50), using the NcoI and XhoI restriction sites present within the primer sequences, and generated the recombinant vaccinia virus vHisPKCλ. Plasmid pTMHis1 is a derivative of pTM-1 (41) which allows recombination in vaccinia viruses and high-efficiency production of N-terminal His6-tagged proteins under the control of a bacteriophage T7 promoter and an encephalomyocarditis virus leader sequence in mammalian cells (27, 41, 50). PKCλ was produced by coinfection of vTF7-3 (27) and vHisPKCλ in HeLa-S3 cells (49), purified from whole-cell extracts over Ni2+-NTA agarose columns (Fig. 2A), and tested for activity by in vitro phosphorylation assays. Despite its modified N terminus, the purified recombinant PKCλ was active for autophosphorylation (Fig. 2B, left lane). More importantly, dephosphorylated NS1 proved to be a substrate for the recombinant PKCλ (right lane), supporting a possible role for this enzyme in NS1 regulation.
FIG. 2.
Analysis of purified recombinant PKCλ. PKCλ cDNA derived from A9 cells was cloned into pTMHis1, recombined into vaccinia virus, and expressed by coinfection with vTF7-3 in HeLa-S3 cells. The His6-tagged PKCλ was purified from whole-cell extracts on Ni2+-NTA agarose columns. (A) Purified recombinant PKCλ was analyzed by SDS-PAGE (10% polyacrylamide) and detected by Coomassie blue staining (P) or Western blotting with anti-PKCλ (W). Molecular weight markers (m) are indicated on the left, and the apparent migration of PKCλ (70 kDa) is shown on the right. (B) The phosphorylation activity of purified recombinant PKCλ was determined by in vitro kinase assays with [γ-32P]ATP in the absence (PKCλ) or in the presence (PKCλ + NS1) of dephosphorylated NS1. The products were analyzed directly by SDS-PAGE (7% polyacrylamide). P1-Thr, the “kinase-free” P1 fraction used for replication assays, served as a negative control for NS1O phosphorylation (P1-Thr + NS1).
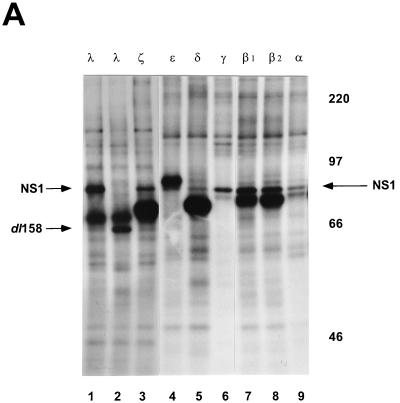
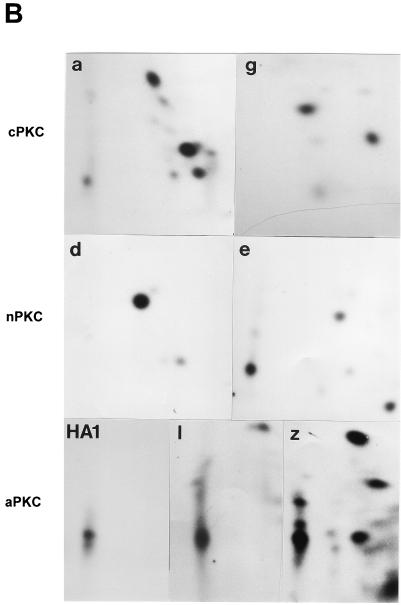
PKC phosphorylation of peptide substrates in vitro requires little specificity. A serine or threonine residue in the vicinity of a basic amino acid proved to be sufficient as a recognition sequence (53). This seems to be in contrast to the findings for NS1, a complex protein substrate comprising more than 15 PKC consensus phosphorylation sites (49). Both protein fractions HA-1 and HA-2 exerted similar PKC activity on peptide substrates and were able to phosphorylate NS1 in vitro; however, they did not activate NS1O for the same functions of NS1 (50). To characterize NS1 phosphorylation by PKC, we performed in vitro kinase assays with purified, recombinant PKC isoforms. PKCɛ, PKCδ, PKCβ1, and PKCβ2 (Panvera) were products of recombinant baculoviruses derived from insect cells, while PKCλ, PKCζ, PKCγ, and PKCα were produced in HeLa cells by recombinant vaccinia viruses. As expected from the number of consensus phosphorylation sites present in NS1, all PKC isoforms under investigation were able to phosphorylate the polypeptide (Fig. 3A). These individually 32P-labeled NS1 proteins were further analyzed for their tryptic phosphopeptide pattern by two-dimensional analyses. As illustrated in Fig. 3B, the protease cleavage of NS1 into over 70 small peptides revealed distinct phosphorylation patterns depending on the PKC isoform under investigation. These data indicate that the multiple PKC phosphorylation sites of NS1 are targets for distinct PKC isoforms in vitro, depending on the surrounding amino acids and/or the secondary structure. It should be noted that classical (PKCα, PKCγ) and novel (PKCδ, PKCɛ) PKCs each produced a unique NS1 phosphorylation pattern, while the phosphopeptide maps of atypical PKCλ and PKCζ overlapped significantly. Most interestingly, the major NS1 phosphopeptide generated by atypical PKCλ and PKCζ comigrated with the NS1 phosphopeptides produced by HA-1, the semipurified kinase fraction which was able to activate NS1O for helicase function (50). This finding further argues for the participation of atypical PKCs in the modulation of NS1 activity in vitro.
FIG. 3.
In vitro phosphorylation of NS1O by purified recombinant PKC. (A) Dephosphorylated full-length NS1O (lanes 1 and 3 to 9) and deletion mutant NS1dl158 (lane 2) were subjected to in vitro phosphorylation by incubation with the indicated PKC isoforms (lanes: 1 and 2, PKCλ; 3, PKCζ; 4, PKCɛ; 5, PKCδ; 6, PKCγ; 7, PKCβ1; 8, PKCβ2; 9, PKCα) in the presence of [γ-32P]ATP. PKCλ, PKCζ, PKCγ, and PKCα, are derived from vaccinia virus expression in HeLa cells, while PKCɛ, PKCδ, PKCβ1, and PKCβ2 (Panvera) are derivatives of baculovirus expression in insect cells. The 32P-labeled proteins were analyzed by SDS-PAGE (7% polyacrylamide), blotted onto PVDF membranes, and detected by autoradiography. The migration of NS1 (83 kDa) and NS1dl158 (65 kDa), as well as the molecular mass markers, are indicated. (B) Individually phosphorylated NS1 proteins were excised, subjected to trypsin digestion, and analyzed in two dimensions on thin-layer cellulose plates by electrophoresis at pH 1.9 and chromatography in phosphochromatography buffer. The protein kinases used were PKCα (a), PKCγ (g), PKCδ (d), PKCɛ (e), HA-1, and atypical PKC-enriched fraction from HeLa cell extract (1, PKCλ; z, PKCζ).
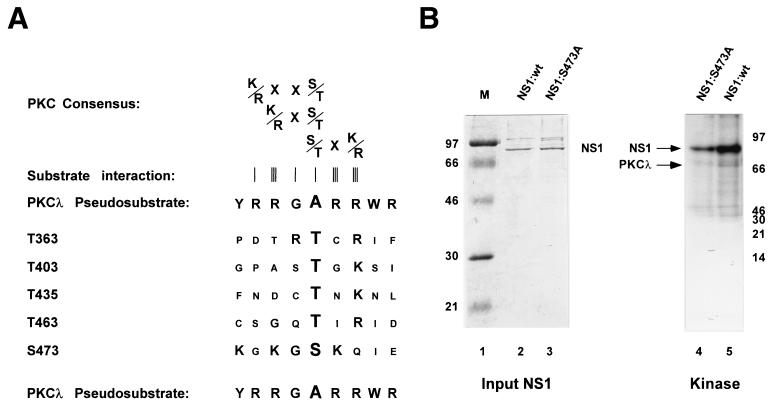
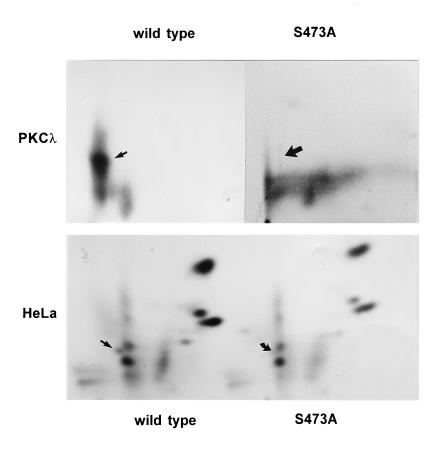
For further investigations, we attempted to identify putative target PKCλ phosphorylation sites within NS1. Previous studies have shown that the majority of phosphorylation events occurring during the replicative phase of MVM propagation are localized within the NS1 helicase domain. Moreover, a phosphopeptide located within this part of NS1 proved to be a target for HA-1 phosphorylation in vitro (11). We therefore confined the search for possible PKCλ target phosphorylation site(s) to the helicase region of NS1. As shown in Fig. 4A, there are five consensus PKC phosphorylation sites, namely, T363, T403, T435, T463, and S473, characterized by basic amino acids in the vicinity of the target serine or threonine residue (39), within this confined fragment. For further characterization of potential PKCλ phosphorylation sites, we took advantage of the regulatory properties of PKC. PKC proteins are kept in an inactive stage by a regulatory element which blocks the active site of the kinase by interacting with the substrate recognition site. This so-called pseudosubstrate site, harboring an inert alanine at the position of the target serine or threonine, resembles genuine substrates recognized by the PKC isoforms (39, 53). Assuming that charged basic amino acids, such as arginine and lysine, play a major role in substrate recognition, we compared the amino acids surrounding the candidate PKC phosphorylation sites of NS1 with the PKCλ pseudosubstrate region. Among the five putative phosphorylation sites mentioned above, S473 could be distinguished by its inclusion in a sequence containing two basic amino acids and a spacer glycine, which could be aligned with the pseudosubstrate site of PKCλ (53). Therefore, we considered S473 a likely candidate for PKCλ phosphorylation. This prediction was tested by analyses of mutant NS1 protein harboring an inert alanine at this putative target residue. His6-tagged wild-type and mutant (S473A) NS1 proteins were produced by using recombinant vaccinia viruses, dephosphorylated with calf intestine alkaline phosphatase, and purified over Ni2+-NTA agarose columns. The dephosphorylated NS1 polypeptides were quantified by Coomassie blue staining after SDS-PAGE, and subjected to in vitro phosphorylation with recombinant PKCλ. The 32P-labeled polypeptides were then analyzed by SDS-PAGE and autoradiography. As shown in Fig. 4B, when equal amounts of wild-type and mutant NS1 proteins were compared, the substitution of alanine for S473 reduced the overall in vitro PKCλ phosphorylation of NS1 over fivefold, suggesting that the mutagenesis of S473 indeed eliminated a target substrate site for this kinase. To confirm this assumption, we further analyzed the in vitro 32P-labeled wild-type and mutant NS1 proteins for their respective phosphorylation patterns. Since NS1 digestion with trypsin produced overlapping (PKCλ) phosphopeptides (45), subsequent analyses involved chymotrypsin treatments instead. As shown in Fig. 5 (top), the phosphorylation pattern of S473A could be distinguished from that of wild-type NS1 by the absence of the most prominent phosphopeptide, demonstrating that the replacement of S473 by alanine indeed eliminated a preferential target for PKCλ phosphorylation, as indicated by the comparison of overall NS1 phosphorylation (Fig. 4B). In addition, we analyzed in vivo phosphorylation of wild-type and mutant S473A NS1 proteins expressed by recombinant vaccinia viruses in HeLa cells. As with in vitro PKCλ phosphorylation, comparison of the two metabolically 32P-labeled polypeptides revealed a distinct phosphopeptide within the wild-type phosphorylation pattern which was undetectable in S473A (Fig. 5, bottom), strongly suggesting that this serine residue also serves as a substrate site for protein kinases within the natural cellular environment.
FIG. 4.
Determination of a PKCλ phosphorylation site in NS1. (A) Sequence alignment of the pseudosubstrate region of PKCλ with consensus PKC phosphorylation sites located within the helicase domain of NS1 (numbered according to the target residue). Canonical PKC consensus recognition sequences are indicated at the top, together with the tightness of their interaction with the kinase (schematized by an increasing number of vertical bars) (62). The motif around S473 represents the strongest homology (large letters) to the pseudosubstrate region of PKCλ. (B) In vitro kinase assays carried out with PKCλ and [γ-32P]ATP, using dephosphorylated NS1 as substrate. The relative amounts of input NS1 polypeptides were determined by Coomassie blue staining (left), and wild-type NS1 (lanes 2 and 5) and NS1:S473A (lanes 3 and 4) containing an amino acid replacement of the target serine at position 473 by an inert alanine were matched for their amounts in in vitro kinase assays. The reaction products were analyzed by SDS-PAGE and autoradiography (right). Migration of NS1 (83 kDa) and PKCλ (70 kDa), as well as the molecular mass markers (M), are indicated.
FIG. 5.
Phosphopeptide analyses of wild-type NS1 and mutant S473A. Wild-type NS1 and mutant NS1:S473A were phosphorylated either in vitro by incubation with PKCλ (top) or in vivo by metabolic 32P labeling through vaccinia virus expression in HeLa cells (bottom). 32P-labeled proteins were gel purified, blotted onto a PVDF membrane, subjected to chymotrypsin digestion, and analyzed in two dimensions on thin-layer cellulose plates by electrophoresis and chromatography. Phosphopeptides present in wild-type NS1 but absent in S473A are indicated by arrows.
Regulation of NS1 replication activities by PKCλ.
NS1 has been reported to be a target for a large variety of protein kinases in vitro, yet only some of them proved able to regulate the activities of the viral polypeptide (3, 49). Furthermore, the capacity for activating the DNA-unwinding functions of NS1O was found to segregate to a distinct fraction (HA-1) highly enriched for atypical PKC during consecutive purification of cell extracts (50). As shown in Fig. 3B, a striking overlap was found between the NS1 phosphopeptide pattern obtained with this “active” HA-1 protein fraction and with purified PKCλ. Taken together, these data prompted us to determine whether recombinant PKCλ was able to substitute for HA-1 phosphorylation in functional assays. At first PKCλ was tested for the rescue of NS1O DNA-unwinding activity by using standard helicase assays. Native NS1P expressed in HeLa cells served as a positive control. As a negative control, we used the mutant NS1:K405R, which harbors an amino acid substitution of the conserved lysine within the NTP-binding domain (46). As shown in Fig. 6A, in the presence of PKCλ and the cofactor PS, the helicase-impaired NS1O (lane 5) was reactivated almost to the level obtained when using native NS1 (lane 4), indicating that the recombinant PKCλ is able to phosphorylate NS1 at the correct site(s) and thus modulate NS1 replicative functions in vitro. In contrast, no helicase activity was detected when NS1:K405R was used (lane 3), demonstrating the absence of cellular helicases in both NS1 and PKCλ preparations. Moreover, these data derived from overexpression of recombinant PKCλ argue against the requirement for a PKC-interacting accessory protein, such as the recently described LIP (24), to rescue NS1O helicase function.
FIG. 6.
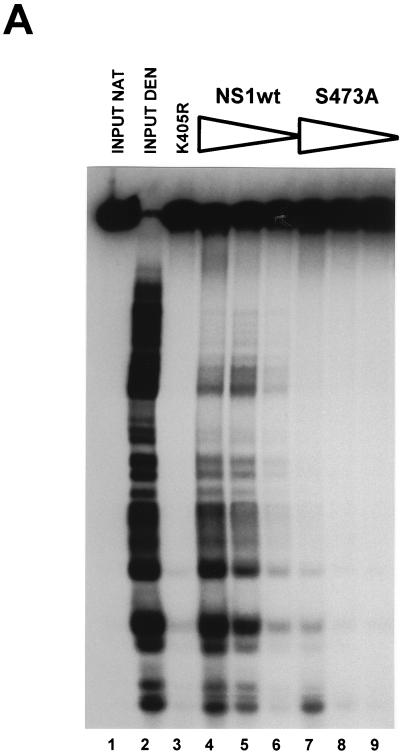
Reactivation of dephosphorylated NS1 for helicase activity. (A) Helicase assays of NS1 in the presence of ATP and M13-Var as substrate. The substrate was incubated in the presence of 20 ng of dephosphorylated NS1O (lanes 5, 6, and 7 to 11) in the presence (lanes 6 and 9 to 11) or absence (5, 8) of 500 pg of PKCλ. To stimulate PKCλ phosphorylation, PS (lanes 3 to 6, 9, and 11) or TPA (lane 10) was added to the reaction mixture. The following controls were included: input native (lane 1) and denatured (lane 2) substrate, and mutant NS1:K405R in the presence of stimulated PKCλ (lane 3), native NS1P (lane 4), and PKCλ alone (lane 7). (B) Reactivation of dephosphorylated NS1O for helicase activity in the present of stimulated PKC isoforms as described above. Input substrate native and denatured (lanes 1 and 2), the negative control mutant K405R (lane 3), native NS1P (lane 4), NS1O alone (lanes 5 and 16), NS1O in the presence of PKC (lanes 8, 10, 13, and 15), PKCλ (1 ng) (lanes 6, 7, 12, and 13), cPKC (PKCα [1 ng]) (lanes 8, 9); nPKC (PKCɛ [1 ng]) (lanes 8, 9); and PKCζ (1 ng) (lanes 14, 15) were used.
Previous investigations with HA-1 have shown that NS1O reactivation was strongly stimulated upon addition of the PKC cofactor PS. Therefore, it was of interest to determine whether recombinant PKCλ with the modified N terminus was regulated in a similar way to the endogenous protein isolated from replication extracts. To examine the cofactor requirements of PKCλ, we tested NS1O reactivation in helicase assays in the absence of cofactors and in the presence of either PS or the phorbol ester TPA (12-O-tetradecanoylphorbol-13-acetate). As previously shown for HA-1, reactivation by PKCλ was strongly stimulated upon addition of PS, while TPA, a cofactor for novel and classical PKC isoforms in vitro did not increase NS1O activity above the level obtained with unstimulated PKCλ (Fig. 6A, lanes 7 to 11). Therefore, the N-terminal modifications introduced into the recombinant PKCλ protein did not alter the function, substrate recognition, and cofactor dependency of the kinase.
The differential NS1 phosphorylation pattern generated in vitro by the various PKC isoforms (Fig. 3B), together with the failure of the PKCs present in HA-2 to reactivate NS1O for helicase activity (50), indicate that NS1 is regulated through phosphorylation by distinct PKC isoforms. To confirm this prediction, different recombinant PKC isoforms were compared for their ability to reactivate NS1O helicase function. As illustrated in Fig. 6B, besides PKCλ, only PKCζ, the other atypical PKC, was able to stimulate NS1O in helicase assays. In contrast, classical PKC, as exemplified by PKCα, and novel PKC, represented by PKCɛ, were both unable to raise NS1O helicase function to a significant extent. These results indicate that the NS1 phosphorylation site(s) modulating DNA unwinding activity is recognized efficiently by atypical PKCs but is poor a substrate for classical and novel isoforms in vitro.
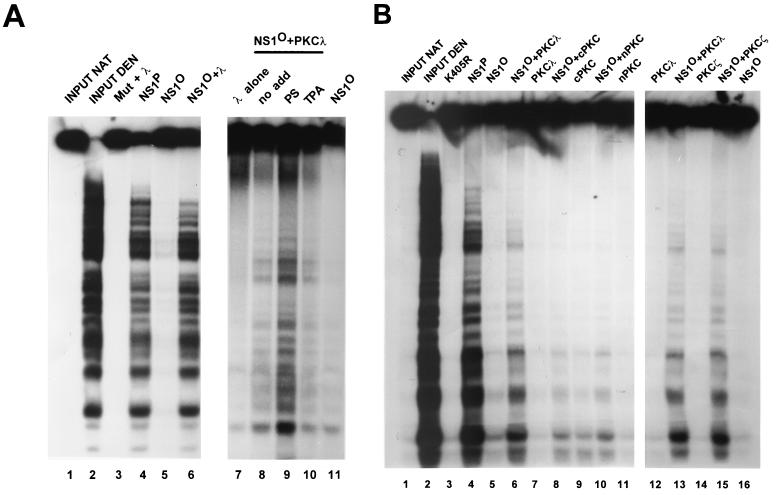
The NS1 DNA-unwinding functions appear to be required for several steps of viral DNA replication. Besides unwinding the double-stranded template in front of the replication fork to allow strand displacement synthesis by cellular polymerases, NS1 is thought to unwind the origins of replication. This local DNA unwinding of origin DNA is thought to allow site- and strand-specific nicking to occur at single-strand level, generating the free 3′ hydroxyl serving as a primer for DNA polymerases. To investigate whether PKCλ functions as an activating kinase during initiation of viral DNA replication, we performed in vitro replication assays with a kinase-free rolling-circle replication (RCR) system (50) that is based on plasmids containing the minimal left-end origins of replication (12). In a previous study we showed that dephosphorylated NS1O requires two distinct components from HeLa cell extracts (HA-1 and HA-2) to become activated for rolling-circle replication (RCR) (50). Since HA-1 could be replaced by purified PKCλ to rescue NS1O helicase activity, we determined whether the capacity of NS1O for RCR could also be up-regulated by PKCλ alone or in combination with HA-2. PS and TPA were both supplied as cofactors to achieve an optimal stimulation of PKCs. NS1P served as a positive control, while NS1:Y210F, containing an amino acid substitution for the active-site tyrosine (48), was used as a negative control. The specificity of the reaction was confirmed by using plasmid pL1-2GAA, which contains the inactive left-end origin (12), and by immunoprecipitation of the NS1-attached reaction products. As shown in Fig. 7, PKCλ or HA-2 alone was unable to activate NS1O to a significant extent above the background RCR activity. In contrast, when the protein fraction HA-2 and the recombinant PKCλ were supplied together, the dephosphorylated NS1 protein became activated to support extensive RCR. This stimulation concerned the genuine NS1 replicative function, since no activity occurred with a plasmid containing the inactive origin and since the replication products were covalently attached to NS1 as shown by immunoprecipitation. These results clearly demonstrate that recombinant PKCλ can fully substitute for the proteins present in HA-1, leading to the activation of NS1O for RCR in the presence of HA-2. The HA-2 protein constituents required, besides PKCλ, to render NS1O active for replication remain to be identified.
FIG. 7.
Reactivation of dephosphorylated NS1 in replication assays. NS1-dependent RCR of plasmids containing the left-end active (T) or inactive (G) origin was determined in a kinase-free in vitro system based on P1-Thr and T4 DNA polymerase (50). NS1O was examined either in absence of protein kinases (lanes 4 and 5) or in the presence of PKCλ (lane 6), the subcellular fraction HA-2 (lane 7), or PKCλ and HA-2 together (lanes 8 and 9), using the PKC cofactors PS and TPA. The NS1 mutant Y210F (lane 1) and native wild-type NS1P (lanes 3 and 4) served as negative and positive controls. The reaction products were analyzed by 0.8% agarose gel electrophoresis after immunoprecipitation with anti-NSN antisera, HindIII restriction digestion, and deproteination. Migration of the linearized plasmid (a), a replication intermediate produced by dephosphorylated NS1 (b), and a higher-molecular-weight species that represents replication products with displaced single-stranded tails (c) are indicated on the right.
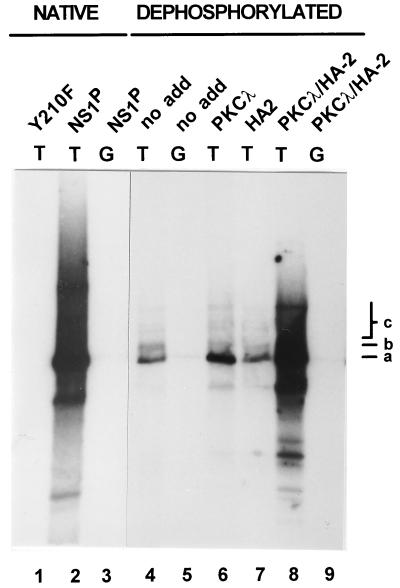
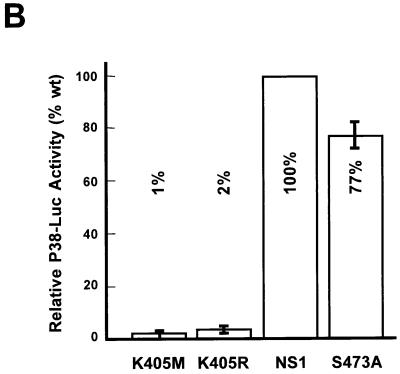
As described above, a target PKCλ phosphorylation site, S473, has been identified in NS1, which also seems to be recognized by cellular protein kinases in vivo (cf. Fig. 5), indicating that this phosphorylation site might be used for regulation of NS1 DNA-unwinding functions. To confirm this assumption, we also analyzed the NS1 mutant S473A for this property in standard helicase assays. The conservative substitution of an inert alanine for the PKCλ target residue S473 indeed drastically impaired NS1 helicase function (Fig. 8A), which could not be rescued by recombinant PKCλ (data not shown). As expected from its lack of DNA-unwinding activity, the S473A mutant was severely impaired for DNA replication, as well as site- and strand-specific nicking of double-stranded left-end substrates (data not shown). In addition, to determine whether the proposed regulation was specific for viral DNA replication or whether mutagenesis at S473 simply caused overall inactivation of the polypeptide, we analyzed this mutant for trans activation of the viral P38 promoter. A9 cells were cotransfected with the reporter plasmid pP38-Luc and the expression vector pRSV-NS containing either wild-type or mutant NS1 proteins under the control of the Rous sarcoma virus promoter. The cells were harvested 48 h posttransfection, and extracts were processed to measure luciferase activity. The nucleotide-binding-site mutants K405M and K405R served as negative controls. As shown in Fig. 8B, substitution of alanine for the PKCλ target serine residue 473 of NS1 had only limited effect on the ability of the S473A mutant to trans activate the capsid gene promoter, allowing stimulation of P38 to approximately 70% of the level achieved by wild-type NS1. With the ambiguity of site-directed mutagenesis, which does not rule out phosphorylation-independent effects of the substitutions tested, these observations strongly suggest that PKCλ phosphorylation of NS1 at serine 473 might serve as a regulatory element, priming the polypeptide for either replicative functions or transcriptional activities.
FIG. 8.
Functional analyses of mutant NS1:S473A. (A) Helicase assays were performed as described in the legend to Fig. 6 with serial 10-fold dilutions (100, 10, and 1 ng) of wild-type (lanes 4 to 6) and mutant (lanes 7 to 9) NS1:S473A with M13-Var as a substrate. Input substrate native and denatured (lanes 1 and 2) and mutant K405R (lane 3) were used. (B) Promoter P38 trans-activation assays were carried out by cotransfection of A9 cells with the reporter plasmid pP38-Luc and the expression vector pRSV-NS carrying the wild-type or mutant (K405M, K405R, or S473A, respectively) NS1 genes. Luciferase activities were measured at 48 h posttransfection. Average values from three independent transfections are presented with standard deviation bars as percentages of wild-type NS1 activity. The basal activity achieved by the reporter plasmid alone was subtracted.
DISCUSSION
The major nonstructural protein of MVM, NS1, is of crucial importance, since it is involved in many divergent processes during the course of a viral infection. NS1 activities include initiation of viral DNA replication, regulation of the viral P38 promoter driving the capsid genes, and alteration of cellular processes, which eventually lead to the death of the host cell (14, 35). The late NS1 functions causing cytotoxicity may be ascribed in part to side effects of NS1 biochemical activities necessary during virus multiplication, e.g., disregulation of cellular promoters (34), yet cell killing is directly relevant to the parvovirus life cycle since it allows the release of mature progeny virions (52, 55). If NS1 exerted its cytopathic effect(s) early in infection, virus production might be impaired. Hence, it would be favorable for efficient virus propagation if the various NS1 activities were regulated in a temporal order. NS1 is a phosphoprotein which becomes modified at multiple serine and threonine residues (20, 40, 49). The pattern of NS1 phosphopeptides varies during the course of a virus infection (11), in keeping with a possible role of phosphorylation to direct NS1 toward distinct functions. In agreement with this assumption, the initiation of viral DNA replication by NS1 has been shown to depend on phosphorylation of the viral product (50), most probably due to a deficiency of un(der)phosphorylated NS1O in site-specific nicking of the origin and helicase function (49). This is in contrast to the increased affinity of NS1O for its cognate DNA recognition motif (ACCA)2–3 (49). Since sequence-specific DNA binding is also required for other NS1 functions, such as upregulation of the P38 promoter through interaction with the tar element (8, 36, 37), it is conceivable that the protein might be selectively conditioned for specific tasks during virus production as a result of the alteration of its biochemical profile in response to phosphorylation.
The present study identifies a specific cellular protein kinase, namely, the atypical isoform of PKC (PKCλ), that is involved in the activation of unphosphorylated NS1O for DNA-unwinding activities which are necessary for initiation of DNA replication and consecutive strand displacement synthesis (48, 50, 60). Indeed, PKCλ also rescued NS1O for the initiation of RCR in vitro, provided that a second protein component, HA-2, was supplied concomitantly. The role of PKCλ in the regulation of the NS1-unwinding functions was further substantiated by the finding that an amino acid substitution for S473, a target serine for PKCλ phosphorylation in vitro, inactivated this function. Interestingly, this helicase-deficient mutant S473A retained the capacity to trans activate the viral P38 promoter, arguing for the proposed regulation of NS1 functions by differential phosphorylation of the polypeptide.
Protein phosphorylation in vitro has been described to be rather nonspecific at multiple residues that are not targeted in vivo and by protein kinases which do not influence the function of their substrates proteins apparently (39, 53). These findings also apply to NS1, which is phosphorylated by a number of protein kinases in vitro of which only some appear able to modulate NS1 activities (3, 49). This lack of in vitro specificity could be due, at least in part, to partial denaturing of the substrate protein, rendering naturally hidden phosphorylation sites accessible to the kinases. More importantly, the regulation of protein kinase activity as a function of time and location within the cell is also likely to be responsible for the restriction of phosphorylation to selected targets in vivo (25, 51, 57). This is exemplified by PKCs, which are tightly controlled for their activity not only by phosphorylation (29) and cofactors like Ca2+, acidic lipids, and/or diacylglycerols (for reviews, see references 39 and 53) but also through their intracellular compartmentalization (e.g., nuclear translocation [51]) and interaction with accessory proteins (1, 24, 30). Nevertheless, as shown by the different tryptic phosphopeptide pattern generated by individual PKC isoforms in vitro, the present study demonstrates specificity for target phosphorylation sites when presented in a complex polypeptide such as NS1. This selective choice for distinct phosphorylation sites from the pool present in NS1 is most interesting, since it indicates that the neighboring amino acids and/or the structure of the domain could determine the specificity of a target phosphorylation site for a given PKC. In addition, this study allowed us to identify a distinct kinase, PKCλ, which was able to induce the activation of NS1 DNA-unwinding functions at least in vitro. On the other hand, the identification of the NS1 phosphorylation site(s) involved in the regulation of the various activities of the polypeptide is intricate. Indeed, multiple NS1 residues are phosphorylated during a natural MVM infection in vivo (49) and the primary structure of the polypeptide comprises numerous consensus sequences that could potentially serve as targets for phosphorylation. The assignment of the regulation of an NS1 replicative function to PKCλ eventually led us to identify S473 as a possible elements in NS1 that controls distinct activities by posttranslational modification. Nevertheless, it has to be mentioned that despite the correlation of phosphorylation at S473 with the functional property of the mutant polypeptide, we cannot rule out additional or alternative modes of regulation during virus propagation.
Parvovirus-encoded proteins interact physically with various host cell factors (6, 23, 32, 37), which may contribute to the control of the viral life cycle (32, 37). This constitutes a first level at which NS proteins can be functionally regulated. The present study, demonstrating that a defined NS1 phosphorylation event performed by PKCλ correlates with the activation of NS1 helicase function, indicates that the NS1 protein could also be regulated by phosphorylation. In particular, the specificity for atypical PKCs among a group of very closely related kinases suggests that similar regulation(s) might also be found during a natural MVM infection. Moreover, the multitude of NS1 residues targeted for phosphorylation in vivo raises the possibility that other NS1 functions besides DNA-unwinding activity are modulated by phosphorylation. In particular, apparently incompatible functions of NS1 (e.g., viral DNA replication and cell death) may be dissociated as a function of time through differential phosphorylation of the viral protein. In agreement with this hypothesis, the NS1 phosphorylation pattern was found to change during progression of the parvovirus cycle (11). Thus, PKCλ phosphorylation, identified in the present work to be essential for NS1 replicative functions, starts playing a role early in infection. It is worth noting in this respect that PKCι, the human homologue of PKCλ, was shown to protect K562 cells against drug-induced apoptosis (43), while parvoviruses eventually kill hematopoietic cells through apoptosis (55). Hence, atypical PKCs may promote both parvovirus replication and cell resistance to the viral cytopathic effect, at times when the virus takes extensive advantage of the host cell machinery. The change in the NS1 phosphorylation pattern observed at later stages may be indicative of an alteration of the cellular protein kinase activity, which may favor cell killing through a new mode of NS1 and/or cellular protein phosphorylation, at times when progeny virions are ready to be released. Further investigations are required to determine whether cellular protein kinases control other NS1 tasks besides DNA replication and whether they are themselves regulated in response to the ongoing parvovirus infection.
ACKNOWLEDGMENTS
We are indebted to Bernard Moss (NIH) for making pTM-1 and the vTF7-3 virus available and to Hubert Hug (DKFZ) for providing full-length PKCα, PKCγ, and PKCζ cDNA clones. We are most grateful to Peter Tattersall and Susan Cotmore for sharing constructs used in our assays, stimulating discussions, and critical comments. We also thank Claudia Plotzky and Romuald Corbau for technical assistance.
This work was supported by the Commission of the European Communities and the German-Israeli Foundation for Scientific Research and Development.
REFERENCES
- 1.Acs P, Szallasi Z, Kazanietz M G, Blumberg P M. Differential activation of PKC isozymes by 14-3-3 protein. Biochem Biophys Res Commun. 1995;216:103–109. doi: 10.1006/bbrc.1995.2597. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto K, Mizuno K, Osada S-I, Hirai S-I, Tanuma S-I, Suzuki K, Ohno S. A new member of the third class in the protein kinase C family, PKCL, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem. 1994;269:12677–12683. [PubMed] [Google Scholar]
- 3.Astell C R, Liu Q, Harris C E, Brunstein J, Jindal H K, Tam P. Minute virus of mice cis-acting sequences required for genome replication and the role of the trans-acting viral protein, NS1. Prog Nucleic Acid Res Mol Biol. 1996;55:245–285. doi: 10.1016/s0079-6603(08)60196-8. [DOI] [PubMed] [Google Scholar]
- 4.Astell C R, Mol C D, Anderson W F. Structural and functional homology of parvovirus and papovavirus polypeptides. J Gen Virol. 1987;68:885–893. doi: 10.1099/0022-1317-68-3-885. [DOI] [PubMed] [Google Scholar]
- 5.Baldauf A, Willwand K, Mumtsidu E, Nüesch J P F, Rommelaere J. Formation of circular replicative form (RF) from single-stranded virion DNA and initiation of DNA replication at the RF 5′ telomere induced by the MVM nonstructural protein NS1. J Virol. 1997;71:971–980. doi: 10.1128/jvi.71.2.971-980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockhaus K, Plaza S, Pintel D J, Rommelaere J, Salomé N. Nonstructural proteins NS2 of minute virus of mice associate in vivo with 14-3-3 protein family members. J Virol. 1996;70:7527–7534. doi: 10.1128/jvi.70.11.7527-7534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caillet-Fauquet P, Perros M, Brandenburger A, Spagelaere P, Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990;9:2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen J, Cotmore S F, Tattersall P. Minute virus of mice transcriptional activator protein NS1 binds directly to the transactivation region of the viral P38 promoter in a strictly ATP-dependent manner. J Virol. 1995;69:5422–5430. doi: 10.1128/jvi.69.9.5422-5430.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen J, Cotmore S F, Tattersall P. A novel cellular site-specific DNA-binding protein cooperates with the viral NS1 polypeptide to initiate parvovirus DNA replication. J Virol. 1997;71:1405–1416. doi: 10.1128/jvi.71.2.1405-1416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen J, Pederson M, Aasted B, Alexandersen S. Purification and characterization of the major non-structural protein (NS1) of Aleutian mink disease parvovirus. J Virol. 1995;69:1802–1809. doi: 10.1128/jvi.69.3.1802-1809.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbau, R., J. P. F. Nüesch, N. Salomé, and J. Rommelaere. Phosphorylation of the viral non-structural protein NS1 during MVMp infection in A9 cells. Virology, in press. [DOI] [PubMed]
- 12.Cotmore S F, Tattersall P. An asymmetric nucleotide in the parvoviral 3′ hairpin directs segregation of a single active origin of DNA replication. EMBO J. 1994;13:4145–4152. doi: 10.1002/j.1460-2075.1994.tb06732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotmore S F, Nüesch J P, Tattersall P. Asymmetric resolution of a parvovirus palindrome in vitro. J Virol. 1993;67:1579–1589. doi: 10.1128/jvi.67.3.1579-1589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 15.Cotmore S F, Tattersall P. DNA replication in the autonomous parvoviruses. Semin Virol. 1995;6:271–281. [Google Scholar]
- 16.Cotmore S F, Tattersall P. High-mobility group 1/2 proteins are essential for initiating rolling circle DNA replication at a parvovirus hairpin origin. J Virol. 1998;72:8477–8484. doi: 10.1128/jvi.72.11.8477-8484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotmore S F, Nüesch J P, Tattersall P. In vitro excision and replication of 5′ telomeres of minute virus of mice DNA from cloned palindromic concatemer junctions. Virology. 1992;190:365–377. doi: 10.1016/0042-6822(92)91223-h. [DOI] [PubMed] [Google Scholar]
- 18.Cotmore S F, Tattersall P. In vivo resolution of circular plasmids containing concatemer junction fragments from minute virus of mice DNA and their subsequent replication as linear molecules. J Virol. 1992;66:420–431. doi: 10.1128/jvi.66.1.420-431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotmore S F, Tattersall P. The NS1 polypeptide of minute virus of mice is covalently attached to the 5′ termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988;62:851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotmore S F, Tattersall P. The NS1 polypeptide of the autonomous parvovirus MVM is a nuclear phosphoprotein. Virus Res. 1986;4:243–250. doi: 10.1016/0168-1702(86)90003-1. [DOI] [PubMed] [Google Scholar]
- 21.Cotmore S F, Christensen J, Nüesch J P, Tattersall P. The NS1 polypeptide of the murine parvovirus minute virus of mice binds to DNA sequences containing the motif [ACCA]2–3. J Virol. 1995;69:1652–1660. doi: 10.1128/jvi.69.3.1652-1660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotmore S F, Tattersall P. Organization of nonstructural genes of autonomous parvovirus minute virus of mice. J Virol. 1986;58:724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cziepluch C, Kordes E, Poirey R, Grewenig A, Rommelaere J, Jauniaux J-C. Identification of a novel cellular TPR containing protein, SGT, that interacts with the nonstructural protein NS1 of parvovirus H-1. J Virol. 1998;72:4149–4156. doi: 10.1128/jvi.72.5.4149-4156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz-Meco M T, Municio M M, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype lambda/iota and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–114. doi: 10.1128/mcb.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divecha N, Banfic H, Irvine R F. Inositides and the nucleus and inositides in the nucleus. Cell. 1993;74:405–407. doi: 10.1016/0092-8674(93)80041-c. [DOI] [PubMed] [Google Scholar]
- 26.Doerig C, Hirt B, Antonietti J P, Beard P. Non-structural proteins of parvovirus B19 and minute virus of mice control transcription. J Virol. 1990;64:387–396. doi: 10.1128/jvi.64.1.387-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuerst T R, Niles E G, Studier F W, Moss B. Eucaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jindal H K, Yong C B, Wilson G M, Tam P, Astell C R. Mutations in the NTP-binding motif of minute virus of mice (MVM) NS1 protein uncouple ATPase and DNA helicase functions. J Biol Chem. 1994;269:3283–3289. [PubMed] [Google Scholar]
- 29.Keranen L M, Dutil E M, Newton A C. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- 30.Klauck T M, Faux M C, Labudda K, Langeberg L K, Jaken S, Scott J D. Coordination of three signaling enzymes by AKAP 79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 31.Kochs G, Hummel R, Meyer D, Hug H, Marme D, Sarre T F. Activation and substrate specificity of the human protein kinase C a and z isoenzymes. Eur J Biochem. 1993;216:597–606. doi: 10.1111/j.1432-1033.1993.tb18179.x. [DOI] [PubMed] [Google Scholar]
- 32.Krady J K, Ward D C. Transcriptional activation by the parvovirus nonstructural protein NS-1 is mediated via a direct interaction with Sp1. Mol Cell Biol. 1995;15:524–533. doi: 10.1128/mcb.15.1.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuranami M, Powell C T, Hug H, Zeng Z, Cohen A M, Guillem J G. Differential expression of protein kinase C isoforms in human colorectal cancers. J Surg Res. 1995;58:233–239. doi: 10.1006/jsre.1995.1036. [DOI] [PubMed] [Google Scholar]
- 34.Legendre D, Rommelaere J. Terminal regions of the NS1 protein of the parvovirus minute virus of mice are involved in cytotoxicity and promoter trans-inhibition. J Virol. 1992;66:5705–5713. doi: 10.1128/jvi.66.10.5705-5713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Rhode S L., III Mutation of lysine 405 to serine in the parvovirus H-1 NS1 abolishes its functions for viral DNA replication, late promoter trans-activation, and cytotoxicity. J Virol. 1990;64:4654–4660. doi: 10.1128/jvi.64.10.4654-4660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorson C, Burger L R, Mouw M, Pintel D. Efficient transactivation of the minute virus of mice P38 promoter requires upstream binding of NS1. J Virol. 1996;70:834–842. doi: 10.1128/jvi.70.2.834-842.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorson C, Pearson J, Burger L, Pintel D J. An Sp1-binding site and TATA element are sufficient to support full transactivation by proximally bound NS1 protein of minute virus of mice. Virology. 1998;240:326–337. doi: 10.1006/viro.1997.8940. [DOI] [PubMed] [Google Scholar]
- 38.Mackett M, Smith G L, Moss B. The construction and characterization of vaccinia virus recombinants expressing foreign genes. In: Glover D M, editor. DNA cloning: a practical approach. Oxford, United Kingdom: IRL Press; 1985. [Google Scholar]
- 39.Marks F, Gschwendt M. Protein kinase C. In: Marks F, editor. Protein phosphorylation. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 1996. pp. 81–116. [Google Scholar]
- 40.Molitor T W, Joo H S, Collett M S. Identification and characterization of a porcine parvovirus nonstructural polypeptide. J Virol. 1985;55:554–559. doi: 10.1128/jvi.55.3.554-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss B, Elroy Stein O, Mizukami T, Alexander W A, Fuerst T R. Product Review. New mammalian expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 42.Mousset S, Ouadrhiri Y, Caillet Fauquet P, Rommelaere J. The cytotoxicity of the autonomous parvovirus minute virus of mice nonstructural proteins in FR3T3 rat cells depends on oncogene expression. J Virol. 1994;68:6446–6453. doi: 10.1128/jvi.68.10.6446-6453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray N R, Fields A P. Atypical protein kinase C ι protects human leukemia cells against drug-induced apaptosis. J Biol Chem. 1997;272:27521–27524. doi: 10.1074/jbc.272.44.27521. [DOI] [PubMed] [Google Scholar]
- 44.Naeger L K, Cater J, Pintel D J. The small nonstructural protein (NS2) of minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990;64:6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nüesch, J. P. Unpublished data.
- 46.Nüesch J P, Cotmore S F, Tattersall P. Expression of functional parvoviral NS1 from recombinant vaccinia virus: effects of mutations in the nucleotide-binding motif. Virology. 1992;191:406–416. doi: 10.1016/0042-6822(92)90202-z. [DOI] [PubMed] [Google Scholar]
- 47.Nüesch J P, Tattersall P. Nuclear targeting of the parvoviral replicator protein molecule NS1: evidence for self-association prior to nuclear transport. Virology. 1993;196:637–651. doi: 10.1006/viro.1993.1520. [DOI] [PubMed] [Google Scholar]
- 48.Nüesch J P, Cotmore S F, Tattersall P. Sequence motifs in the replicator protein of parvovirus MVM essential for nicking and covalent attachment to the viral origin: identification of the linking tyrosine. Virology. 1995;209:122–135. doi: 10.1006/viro.1995.1236. [DOI] [PubMed] [Google Scholar]
- 49.Nüesch J P F, Corbau R, Tattersall P, Rommelaere J. Biochemical activities of minute virus of mice nonstructural protein NS1 are modulated by the phosphorylation state of the polypeptide. J Virol. 1998;72:8002–8012. doi: 10.1128/jvi.72.10.8002-8012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nüesch J P F, Dettwiler S, Corbau R, Rommelaere J. Replicative functions of minute virus of mice NS1 protein are regulated in vitro by phosphorylation through protein kinase C. J Virol. 1998;72:9966–9977. doi: 10.1128/jvi.72.12.9966-9977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olson E N, Burgess R, Staudinger J. Protein kinase C as a transducer of nuclear signals. Cell Growth Differ. 1993;4:699–705. [PubMed] [Google Scholar]
- 52.Op de Beek A, Caillet-Fauquet P. The NS1 protein of the autonomous parvovirus minute virus of mice blocks cellular DNA replication: a consequence of lesions to the chromatin? J Virol. 1997;71:5323–5329. doi: 10.1128/jvi.71.7.5323-5329.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker P J, Dekker L V. Protein kinase C. New York, N.Y: Springer-Verlag; 1997. [Google Scholar]
- 54.Pujol A, Deleu L, Nüesch J P F, Cziepluch C, Jauniaux J-C, Rommelaere J. Inhibition of parvovirus minute virus of mice replication by a peptide involved in the oligomerization of the nonstructural protein NS1. J Virol. 1997;71:7397–7403. doi: 10.1128/jvi.71.10.7393-7403.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rayet B, Lopez-Guerrero J-A, Rommelaere J, Dinsart C. Induction of programmed cell death by parvovirus H-1 in U937 cells: connection with the tumor necrosis factor alpha signalling pathway. J Virol. 1998;72:8893–8903. doi: 10.1128/jvi.72.11.8893-8903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhode S L I, Richard S M. Characterization of the trans-activation-responsive element of the parvovirus H-1 P38 promoter. J Virol. 1987;61:2807–2815. doi: 10.1128/jvi.61.9.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ron D, Chen C H, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homologue of the beta subunit of G proteins. Proc Natl Acad Sci USA. 1994;92:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scherrer K. Isolation and sucrose gradient analysis of RNA. In: Habel K, Salszman N P, editors. Fundamental techniques in virology. New York, N.Y: Academic Press, Inc.; 1969. [Google Scholar]
- 59.Selbie L A, Schmitz-Pfeiffer C, Sheng Y, Biden T J. Molecular cloning and characterization of PKCi an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 60.Snyder R O, Im D-S, Ni T, Xiao X, Samulski R J, Muzyczka N. Features of the adeno-associated virus origin involved in substrate recognition by the viral rep protein. J Virol. 1993;67:6096–6104. doi: 10.1128/jvi.67.10.6096-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spagelaere P, Cornelis J J, Tuynder M, Rommelaere J. Lack of a detectable effect of capsid proteins on the cell-dependent activity of parvovirus MVMp promoters. Res Virol. 1994;145:5–12. doi: 10.1016/s0923-2516(07)80001-x. [DOI] [PubMed] [Google Scholar]
- 62.Uberall F, Giselbrecht S, Hellbert K, Fresser F, Bauer B, Gschwendt M, Grunicke H H, Baier G. Conventional PKC-alpha, novel PKC-epsilon and PKC-theta, but not atypical PKC-lambda are MARCKS kinases in intact NIH 3T3 fibroblasts. J Biol Chem. 1997;272:4072–4078. doi: 10.1074/jbc.272.7.4072. [DOI] [PubMed] [Google Scholar]
- 63.Vanacker J-M, Corbau R, Adelmant G, Perros M, Laudet V, Rommelaere J. Transactivation of a cellular promoter by the NS1 protein of the parvovirus minute virus of mice through a putative hormone-responsive element. J Virol. 1996;70:2369–2377. doi: 10.1128/jvi.70.4.2369-2377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weisshart K, Fanning E. Roles of phosphorylation in DNA replication. In: DePamphilis M L, editor. DNA replication in eucaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 295–330. [Google Scholar]
- 65.Wilson G M, Jindal H K, Yeung D E, Chen W, Astell C R. Expression of minute virus of mice major non-structural protein in insect cells: purification and identification of ATPase and helicase activities. Virology. 1991;185:90–98. doi: 10.1016/0042-6822(91)90757-3. [DOI] [PubMed] [Google Scholar]