Abstract
The 3′ untranslated region (3′ UTR) between the 3′ end of env and the long terminal repeat is well conserved among avian retroviruses and is essential for efficient replication. Deletion of the dr1 element within the 3′ UTR has been reported to have various effects, including reduced levels of unspliced RNA in the cytoplasm, decreased stability of unspliced RNA, decreased particle production, and decreased genomic RNA packaging. To probe the role of specific sequences within dr1 in virus replication, site-directed mutagenesis was utilized to perturb parts of the predicted secondary structure of dr1. Seven of thirteen mutations had no significant effect; the others resulted in an approximately 10- to 20-fold reduction in replication. These mutants were further characterized and found to impair cytoplasmic accumulation of unspliced RNA only slightly. Furthermore, no decreases were observed in the stability of the unspliced RNA or in the production of virus particles. Genomic RNA packaging, however, was reduced by about 10-fold. Similar amounts of particles were produced by cells containing the mutant and wild-type DNA, and all particles contained similar levels of reverse transcriptase activity. The results suggest that the region of the dr1 disrupted by the mutations plays a role in genomic RNA packaging, although that packaging may not be the only role for dr1.
The 3′ untranslated region (3′ UTR) between the 3′ end of env and the long terminal repeat (LTR) is well conserved among avian retroviruses and is essential for efficient replication. By contrast to the region in ancestral avian leukosis virus (ALV) strains, the RNA of Rous sarcoma virus (RSV) contains two copies of the 3′ UTR, which flank the oncogene src (Fig. 1A) (32). Within one copy of the 3′ UTR lie two sequences referred to as direct repeat 1 (dr1) and dr2 separated in the 3′ copy by a unique sequence (30). This repetition was probably created as part of the recombination events that led to the acquisition of src by the virus (31). The duplicated regions have subsequently diverged somewhat in sequence, although they both managed to retain function, since either copy will support efficient replication of RSV (17, 22, 24).
FIG. 1.
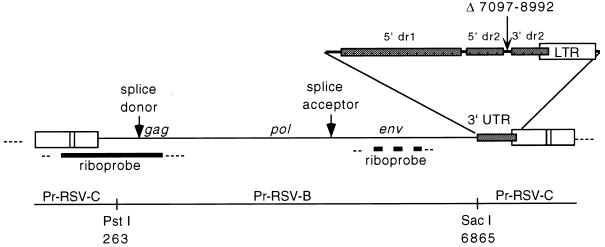
ALV provirus. Shown is the viral insert in pJON5WT, a proviral clone of a replication-competent ASLV in pBluescript SK(−). The 3′ UTR of pJON5WT was created by deletion from Prague-RSV-C of nt 7097 to 8992, which removed src and most of the sequence between src and the 3′ LTR. The riboprobe used in RNase protection assays to differentiate unspliced from spliced RNA is depicted by the solid bar. The riboprobe corresponds to nt −53 to 532 of Prague-RSV-C, spanning the splice junction at nt 398. The riboprobe used to distinguish subgroup B from B/E RNA is shown as a thick broken line and corresponds to nt 5515 to 5961 of Prague-RSV-B. The straight line depicts the regions derived from Prague-RSV-C or Prague-RSV-B. Thin dotted lines indicate the vector sequences.
All retroviruses must maintain the proper balance of spliced and unspliced RNA in the cytoplasm. In the case of simple retroviruses, the one species of spliced RNA serves as template for the translation of the Env proteins. The unspliced RNA serves as template for the translation of Gag and Gag-Pol precursor proteins and also serves as genomic RNA for packaging into progeny virions. Exactly how avian type C retroviruses export their unspliced RNA from the nucleus to the cytoplasm remains unresolved. Complex retroviruses such as human immunodeficiency virus (HIV) and human T-cell leukemia virus (HTLV) export their unspliced and partially spliced RNA from the nucleus in a manner that has been well characterized (5, 6, 10, 23). The HIV Rev (HTLV Rex) protein, translated from a fully spliced RNA, shuttles in and out of the nucleus (20), where it multimerizes on an RNA element named RRE (RxRE in HTLV) (18, 19, 27), located near the middle of env and therefore found only in RNA that has not been fully spliced (4). Rev is then able to expose its nuclear export signal, thereby facilitating the export of the bound RNA (11).
Analogous to the Rev/RRE system, simian type D retroviruses contain a nuclear export activity within their 3′ UTR (3, 33). The 3′ UTRs of Mason-Pfizer monkey virus (3) and simian retroviruses types 1 and 2 (33) function to promote the nuclear export of unspliced viral RNA from the nucleus to the cytoplasm. The sequences within the 3′ UTRs of these retroviruses responsible for this property were named “constitutive transport elements” (CTEs) (3) and are able to functionally replace the Rev/RRE system of HIV type 1 (HIV-1) (3, 26, 33).
The findings with type D viruses prompted Ogert et al. (17) to investigate the role in nuclear export of sequences within the 3′ UTR of RSV, since the region had already been shown to be required for efficient viral replication (24). These authors, using an RSV construct bearing a dr1 deletion (17) or a dr1 containing point mutations (16), showed that the 3′ UTR facilitated the accumulation of unspliced RNA in the cytoplasm. However, unlike the case for simian type D retroviruses, no nuclear retention of unspliced RNA occurred with avian retrovirus constructs lacking a dr1 element (17). The 3′ UTR of RSV functions to some degree in RNA stability, since two different dr1 deletion mutants make unspliced RNA that is degraded about twice as rapidly as is the wild type (17, 22).
Another RSV construct lacking dr1 elements but retaining src was shown to be defective in particle production, even though transcription and translation were relatively intact (22). Genomic RNA expressed from this construct was slightly less abundant in the cytoplasm compared to the wild type and was not retained in the nucleus (22). Furthermore, all three viral RNA species displayed an approximately twofold reduction in stability (22). A third ALV construct lacking a dr1 was shown to be defective in packaging its genomic RNA (24). Particle production was slightly impaired, but it paralleled the cytoplasmic level of mutant RNA, which failed to package (24).
Because of the conflicting results of several experiments with deletion constructs, we decided instead to probe the role of specific sequences within the dr1 and have therefore introduced 13 different single or tandem substitution mutations into the dr1 of an avian sarcoma/leukosis virus (ASLV) proviral construct. The present study describes 6 of those 13 mutations, each of which reduced viral replication by at least 10-fold. These mutations had little effect on the cytoplasmic accumulation of unspliced RNA or RNA stability, but they reduced packaging of viral genomic RNA by an amount comparable to the reduction in replication displayed by each of the mutants.
MATERIALS AND METHODS
Plasmids.
pJON5 plasmids contained a nonpermuted copy of an ASLV provirus in pBluescript (Fig. 1). First, a PCR fragment was generated from the pATV8 clone of Prague-RSV-C (13), adding a ClaI site immediately upstream of the 5′ LTR and ending at the PstI site at nucleotide (nt) 263. Nucleotide numbering is based on the sequenced genome of the pATV8 (21). The downstream primer also introduced a point mutation into the SacI site at nt 255. The upstream primer contained the sequence 5′-CCATCGATAATGTAGTCTTATGCAATACTCCTGTAG-3′ and the downstream primer contained the sequence 5′-CCCTGCAGTAAAGCTCCCTC-3′. pJON1 was made by ligation of this PCR fragment into pBluescript at the ClaI and PstI sites. pJON2 was created by ligation of a PstI fragment (nt 263 to 1542) from NTRE-4B (28, 29) into pJON1. pJON4 was made by cutting pJON2 with AatII (nt 1250) and SacI and then ligating in place of the excised fragment (nt 1250 to 6865) the corresponding fragment from NTRE-4B (subgroup B/E env) (28, 29). pJON5 was created by exchanging the KpnI-SacI (nt 4995 to 6865) fragment of pJON4 with the same fragment from transformation-defective Prague-RSV-B, thus resulting in a subgroup B env gene which rendered progeny virus from the clone unable to reinfect the QT6/BD cells used in the transfection experiments. Eliminating reinfection allowed for better comparison of mutant to wild-type expression. The constructs were completed by cleaving pJON5 with SacI and ligating in a SacI fragment (nt 6865 to 255) from Prague-RSV-C to provide the 3′ UTR and the 3′ LTR. These fragments contained either a wild-type or mutant 3′ UTR. This SacI fragment lacked src and the downstream copy of the dr1 (deletion of nt 7097 to 8992).
A plasmid was constructed to serve as a template for riboprobes and consisted of an EcoRI-BamHI (nt −53 to 532) viral fragment, which spans the RSV splice donor (nt 398) to differentiate between unspliced and spliced species, cloned into pBluescript (Fig. 1). The subgroup B derived env probe was constructed by inserting into pBSSK−, a PCR fragment containing nt 5515 to 5961 of the ALV env sequence. pMyc23, a plasmid serving as a transfection control and pMyc23 donor, a plasmid serving as a template for its riboprobe, were obtained from K. Beemon (14). pMyc23 contains the cellular myc gene cloned into pGEM2. pMyc23 Donor contains a NotI-BstXI fragment which is the exonic portion around the myc 5′ splice site cloned into pGEM2.
Cell culture and DNA transfection.
QT6 cells are a cell line derived from a chemically induced fibrosarcoma of Japanese quail (15). QT6 cells were grown in modified Richter’s medium containing 5% fetal calf serum (FCS). DF1 cells are immortalized chicken fibroblasts (12) and were grown in modified Richter’s medium containing 10% FCS. All transfections were done with Lipofectamine (Gibco-BRL) according to the manufacturer’s recommendations. Briefly, each 100-mm culture dish of 40% confluent cells was transfected with 10 μg of plasmid DNA for 16 h in 4 ml of OptiMEM (Gibco-BRL) medium containing 40 μl of Lipofectamine and 2.5% FCS for QT6 cells, or 5% FCS for DF1 cells. After transfection, 4 ml of modified Richter’s medium was added to the cultures. The medium contained 7.5% FCS for QT6 cells or 15% FCS for DF1 cells. The transfection efficiency was controlled by cotransfecting pMyc23 with the pJON5 plasmids. QT6 cells were harvested at 40 h, at which point medium and cells were analyzed to determine viral protein and RNA levels. DF1 cells were passaged twice weekly for 8 weeks, and reverse transcriptase (RT) assays were performed on the culture medium before each cell passage.
In experiments measuring viral RNA stability, 2 μg of actinomycin D (Gibco-BRL) per ml was added at 40 h (time zero) in fresh modified Richter’s medium with 5% FCS, and QT6 cells were harvested at 0, 6, 12, and 24 h for analysis of the viral RNA levels.
RT assays.
Production of virus was assayed by determining the amount of RT activity in the culture medium. One milliliter of culture medium was filtered through a 0.45-μm (pore-size) filter, and the virus was pelleted and incubated with 20 μl of assay buffer (50 mM Tris-Cl [pH 7.5], 60 mM NaCl, 10 mM MgCl2, 20 mM dithiothreitol, 10 mM dTTP, 5 mg of oligo(dT) per ml, 10 mg of poly(rA) per ml, 0.05% Nonidet P-40 NP-40, 0.2 μCi of [35S]dTTP) at 37°C for 1 h. The reaction was stopped by the addition of 200 μl of stop solution (0.1% Na4P2O7, 15 mM NaCl, 0.1 mg of bovine serum albumin per ml), and products were precipitated by the addition of 25 μl of 60% trichloroacetic acid (TCA). After incubation on ice for 5 min, the cocktail was filtered through nitrocellulose filters and washed three times with 6% TCA, and the filters were dried and counted in a scintillation counter.
RNA isolation, cell fractionation, and RNase protection assays.
Total cellular RNA, nuclear RNA, cytoplasmic RNA, and viral RNA were isolated by using the RNeasy Kit (Qiagen) according to the manufacturer’s recommendations. The exact formulation of Qiagen buffers RLT, RW1, and RPE is proprietary, so only approximate formulations are provided. Briefly, cellular fractionation was achieved by treating the cells with buffer RLN (50 mM Tris-Cl [pH 8.0], 140 mM NaCl, 1.5 mM MgCl2, 0.5% Nonidet P-40) for 5 min on ice, followed by centrifugation at 400 × g for 3 min at 4°C. Then, 1 ml of supernatant was used as the cytoplasmic fraction, and the pellet was washed again in RLN and used as the nuclear fraction. Cells, cell nucleus preparations, or viruses were pelleted and then disrupted with 3.8 ml of buffer RLT (ca. 4 M guanidinium thiocyanate, 10 mM Tris-Cl [pH 8.0], 1 mM EDTA) supplemented with 1% 2-mercaptoethanol. Then, 3.8 ml of 70% ethanol (or 2.8 ml of absolute ethanol for the 1-ml sample of cytoplasmic RNA) was added. Genomic DNA in the total cell and cell nucleus samples was sheared by 12 passes through a syringe with an 18-gauge needle. The mixtures were then added to the RNA-binding silica spin column and centrifuged at 14,000 × g for 1 min. The column was washed once with 7 ml of buffer RW1 (ca. 4 M guanidinium thiocyanate, 10 mM Tris-Cl [pH 8.0], 1 mM EDTA), and two times with 5 ml of buffer RPE (ca. 10 mM Tris-Cl [pH 8.0], 1 mM EDTA, 80% ethanol). RNA was then eluted in 200 μl of water, treated with DNase I, and repurified by using the RNeasy Kit prior to A260/280 determination. The virion RNA was too dilute to determine an accurate A260/280 measurement. Instead, virus was simply pelleted from identical volumes of culture medium from each transfection condition. Pelleting was done by ultracentrifugation with an SW41 rotor at 35,000 rpm for 1 h at 4°C. RNA was extracted from the virus pellets with the RNeasy kit, and the entire sample was used in the RNase protection assay.
RNase protection assays were performed on all RNA preparations and utilized [32P]rUTP-labeled antisense riboprobes generated with the Riboprobe Systems Kit (Promega) according to the manufacturer’s recommendations. The probe was synthesized at a low specific activity such that, according to the Poisson distribution, less than 2% of the probe molecules contained more than 1 [32P]rUTP. This step greatly reduced the background coming from the decay of probes that were still radioactive, since radioactive decay causes degradation of the probe. Then, 10 to 20 μg of cellular RNA, or the entire sample of virion-associated RNA, was hybridized to probe (400,000 cpm/sample) for 16 h at 50°C, followed by treatment with the RNases A (5 μg/ml) and T1 (10 U/ml) for 1 h at room temperature. Samples were then extracted with phenol-chloroform, ethanol precipitated, resuspended in 95% formamide buffer, and loaded onto a 5% polyacrylamide gel containing 8 M urea. Electrophoresis was carried out for 1 h at constant power (80 W) in 1× TBE buffer (90 mM Tris-Cl, 90 mM boric acid, 2 mM EDTA). Gels were dried onto Whatman paper and then placed against a detection screen used in conjunction with the Storm PhosphorImager (Molecular Dynamics). Bands were quantitated by using Imagequant (Molecular Dynamics) software.
Protein isolation and immunoblots.
Total cellular protein was isolated by sonicating cell pellets resuspended in phosphate-buffered saline plus pepstatin A (1 μg/ml), leupeptin (1.37 μg/ml), phenylmethylsulfonyl fluoride (100 μg/ml), and dithiothreitol (0.5 mM). The sonicate was cleared by centrifugation at 14,000 × g for 10 min and then concentrated through a microconcentrator (Amicon) with an exclusion size of 30 kDa. The concentration of the sample was determined by biuret assay, and 30 μg of total cell protein was mixed 1:1 (vol/vol) with 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (4% SDS, 20% glycerol, 120 mM Tris-Cl [pH 6.8], 0.01% bromophenol blue) and subjected to SDS–10% PAGE. Virus pellets were directly resuspended in 1× SDS-PAGE loading buffer and subjected to SDS–15% PAGE. Gels were transferred to nitrocellulose and blocked with 5% dry milk in TBS-T (25 mM Tris base, 3 mM KCl, 140 mM NaCl, 0.1% Tween-20 [pH 8.0]) for 1 h. On cellular protein blots, Pr76 Gag protein was detected by using a 1:1,000 dilution of affinity-purified rabbit anti-CA-NC antibodies, a gift of V. Vogt. On virus protein blots, p27 CA protein was detected by using a 1:1,000 dilution of rabbit anti-CA antiserum, a gift of V. Vogt. All blots were then washed in TBS-T. All blots were incubated next with a 1:30,000 dilution of an alkaline phosphatase-conjugated anti-rabbit secondary monoclonal antibody, washed three more times (5 min each time) in TBS-T, and then incubated with a fluorescent substrate for 2 min (both from the ECF Western blotting kit [Amersham]). All primary and secondary antisera were added to TBS-T containing 1% dry milk, and incubations were for 1 h at room temperature. Fluorescence was quantitated on the Storm fluorescence imager, which read blue fluorescence, and Imagequant software was used for data manipulation. Band intensities were in the linear range of the Storm imager as determined by standard curves.
RESULTS
Selection of mutants.
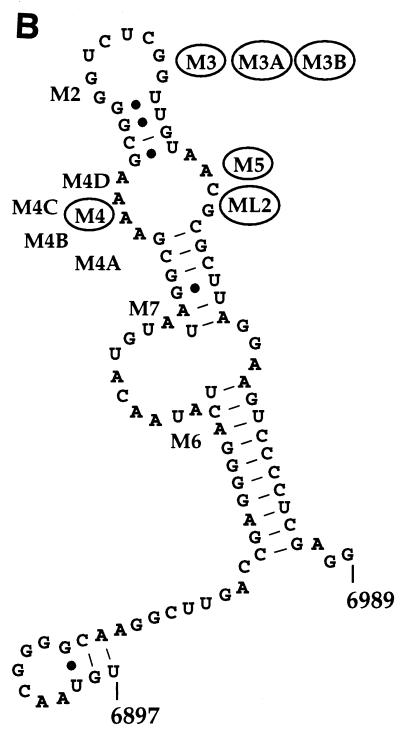
Thirteen ASLV clones, containing mutations in their 3′ UTR, were constructed by site-directed mutagenesis (Fig. 2). The rationale for making 11 of the mutations (M2, M3, M3B, M4, M4A, M4B, M4C, M4D, M5, M6, and M7) was to reduce the number of unpaired bases observed in a putative secondary structure which had been predicted by using M-fold software (Fig. 2B) (34). ML2 was intended to be a compensatory exchange of sequences from one side of a loop to the other. M3A was intended to maintain the structure but alter the sequence. The logic was to test whether maintenance of the secondary structure was critical for function and was of greater importance than sequence. As indicated in Fig. 2, seven of the mutations, intended to alter structure, replicated at wild-type levels with little or no delay in replication kinetics (data not shown). Therefore, it seems possible that specific sequences, rather than secondary structure, could play a larger role in the function of the dr1. We decided to further characterize the six mutants that failed to attain wild-type levels of replication, regardless of their effect on putative secondary structure.
FIG. 2.
Site-directed mutations in the upstream copy of dr1. The six substitution mutations shown in boldface in panel A and circled in panel B (M3, M3A, M3B, M4, M5, and ML2) resulted in at least a 10-fold reduction in viral replication compared to the wild type (Fig. 3). The remaining seven dr1 mutants failed to cause a permanent reduction in viral replication. (A) Aligned sequences of the upstream copy of dr1 from Pr-RSV-C nt 6897 to 6989 and the mutations studied here. Dashes indicate sequence identity. (B) Predicted secondary structure of dr1. The structure was predicted by using M-fold (34). The approximate locations of the mutations are indicated.
Some point mutations in the 3′ UTR of ASLV reduce viral replication.
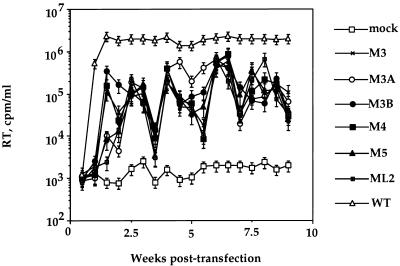
The substitution mutations selected reduced replication by 10-fold for at least 2 months of culture (Fig. 3). Replication was assayed by measuring the production of RT activity by DF1 cells transfected with each mutant provirus. All mutants displayed similarly slow increases in RT activity, eventually reaching 5 to 10% that of the wild-type virus (Fig. 3). The mutants were able to maintain this level of virus production throughout the experiment, and reversion of mutant sequences as assayed by sequencing RT-PCR-amplified fragments of virion RNA was not observed (data not shown). Almost identical results were observed when the mutant and wild-type DNAs containing a subgroup B/E env in place of the subgroup B env were transfected into QT6 cells (data not shown).
FIG. 3.
Effect of substitution mutations in dr1 on ASLV replication. The results of RT assays performed on culture medium of DF1 cells transfected with 10 μg of the indicated DNAs per ml are shown. Cells were passed when confluent (twice weekly). Samples were taken immediately before passing. Error bars indicate the standard deviation of values obtained from three separate transfections with the same DNA. The large variability in the RT activity levels of the mutants may have resulted from systemic errors in the assay, given the consistency of RT activity fluctuations among the mutants.
Point mutations in the 3′ UTR of ASLV reduce cytoplasmic accumulation of unspliced RNA only slightly.
To avoid difficulties caused by multiple rounds of infection, all of the experiments described hereafter utilized transient transfection of QT6 cells with plasmids encoding subgroup B virus. This protocol allowed for a better comparison of mutant to wild-type virus, since all viral RNA came directly from the transfected DNA and not from reinfection, which could have been occurring at different rates for each mutant tested. All studies utilizing QT6 cells were completed without passaging the cell cultures.
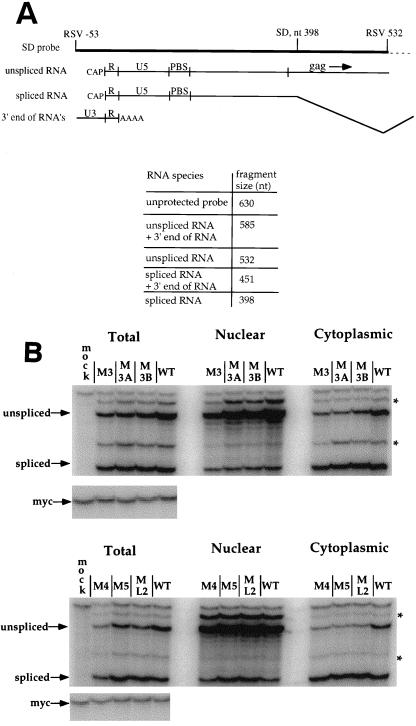
We first examined the effects of the mutations on the synthesis, splicing, and transport of spliced and unspliced viral RNA from the nucleus to the cytoplasm. In experiments with RSV constructs lacking dr1 sequences, reduced levels of unspliced viral RNA in the cytoplasm have been observed (17, 22). Cytoplasmic levels of spliced env RNA, however, are unaffected by the complete deletion of dr1 sequences (17, 22). To test whether the point mutations had an effect similar to that of the removal of dr1 sequences entirely, QT6 cells were transfected with the mutant or wild-type ASLV constructs and tested for expression 40 h later. As a control for transfection and for cellular fractionation, pMyc23 was cotransfected with the retroviral constructs, and its expression was measured in the same hybridization reaction as the viral RNA, but with a different specific riboprobe. The results of two representative RNase protection assays are shown in Fig. 4. None of the point mutations had a noticeable effect on overall RNA levels. The effect of the mutations on the ratio of unspliced to spliced RNA and their relative amounts in nuclear and cytoplasmic fractions are given in Table 1. Even though there was less unspliced viral RNA in the cytoplasm for any one mutant as compared to the wild type, all mutants also displayed a lower level of unspliced RNA in the total RNA sample. Therefore, ratios of unspliced/spliced RNA in the cytoplasm were compared to ratios of unspliced/spliced RNA in the total RNA sample to generate a second set of ratios which more accurately described the effects of the mutations on the cytoplasmic accumulation of unspliced RNA (Table 1). The differences in nuclear export of unspliced RNA between any individual mutant and the wild type (Table 1) were relatively small, up to about two-fold for M5, and cannot account for the reduction in replication shown by the RT assay (Fig. 3).
FIG. 4.
Effect of dr1 mutations on cytoplasmic accumulation of unspliced viral RNA. QT6 cells were transfected with 10 μg of the mutant DNAs in a subgroup B background to prevent reinfection. A total of 10 μg of plasmid pMyc23 was included as a transfection control. RNase protection assays were performed by using total, nuclear, and cytoplasmic RNA preparations isolated 40 h after transfection with the radiolabeled riboprobe shown in Fig. 1, as well as a riboprobe specific for myc. (A) Schematic diagram of riboprobe and protected RNA species. The thin dashed line indicates vector sequences. The sizes of the riboprobe and the protected fragments are indicated. (B) RNase protection of fractionated cellular RNA. The asterisks indicate bands that arise from protection of the probe by both ends of the same RNA molecule or by two ends from two different RNA molecules.
TABLE 1.
Ratio of unspliced/spliced viral RNAa
| Sample | Mean ratio ± SD of unspliced/spliced viral RNA:
|
|||
|---|---|---|---|---|
| Total | Nuclear | Cytoplasmic | Ratioc (% of wild type) | |
| M3 | 0.78 ± 0.05b | 3.47 ± 0.03 | 0.45 ± 0.02 | 95 |
| M3A | 0.84 ± 0.09 | 3.93 ± 0.40 | 0.31 ± 0.05 | 68 |
| M3B | 0.84 ± 0.06 | 4.25 ± 0.09 | 0.36 ± 0.02 | 81 |
| M4 | 0.69 ± 0.02 | 4.39 ± 0.03 | 0.25 ± 0.05 | 74 |
| M5 | 0.91 ± 0.01 | 4.01 ± 0.54 | 0.20 ± 0.02 | 45 |
| ML2 | 0.66 ± 0.02 | 3.68 ± 0.25 | 0.30 ± 0.01 | 93 |
| Wild type | 1.59 ± 0.40 | 5.48 ± 0.20 | 0.82 ± 0.10 | (100) |
Data from the experiment shown in Fig. 4. Bands were quantitated, and values shown were obtained by dividing the unspliced RNA by the spliced RNA values to normalize for RNA recovery. Transfection efficiency was identical in all samples, as judged by quantitating the expression of the cotransfected pMyc23 construct.
Standard deviation from five separate transfections.
Column 4 values divided by column 2 values, normalized to the wild-type ratio (0.52).
Nuclear ratios of unspliced/spliced RNA displayed the least variability among the constructs. It is notable that there was no concomitant nuclear retention of unspliced viral RNA to explain its reduction in the cytoplasm. This result is in agreement with the results of experiments with the dr1 deletion constructs (17, 22). The results with ASLV constructs are in stark contrast to the results of similar experiments with type D retroviruses, where there is an obvious nuclear retention of the unspliced RNA accounting for its absence from the cytoplasm when the 3′ UTR is mutated (3, 9, 33). It is therefore difficult to ascribe the effects of these mutations to nuclear export rather than to cytoplasmic accumulation of unspliced RNA.
The dr1 point mutations did not affect the stability of the unspliced viral RNA.
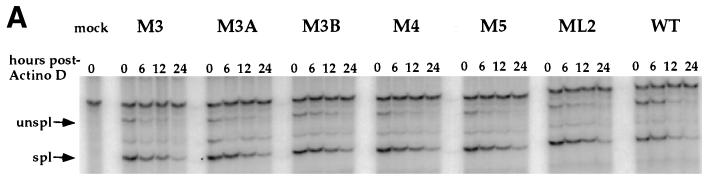
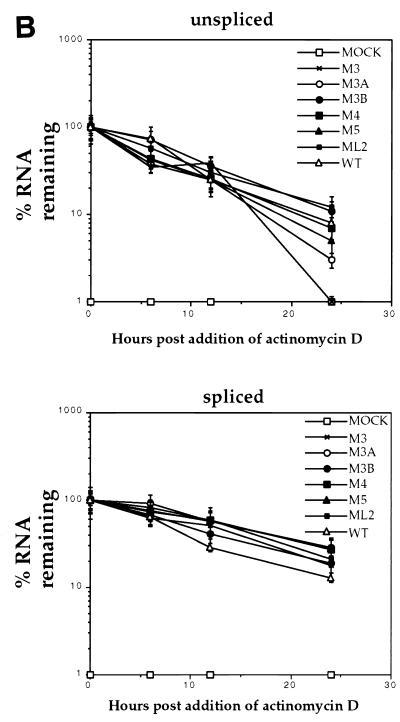
It has been reported previously that RSV proviral constructs lacking dr1 sequences express unspliced RNA which is less stable in the cytoplasm than wild-type unspliced RNA (17, 22). Since nuclear retention of unspliced RNA could not account for the reduced levels of unspliced RNA in the cytoplasm of QT6 cells transfected with mutant proviruses (Table 1), the effects of the point mutations on the stability of cytoplasmic unspliced RNA were investigated. To directly ascertain the effects of the dr1 mutations on RNA stability, QT6 cells were transfected with the mutant and wild-type constructs. Cells were treated with 2 μg of actinomycin D per ml starting 40 h posttransfection. Cytoplasmic RNA was isolated at various times and subjected to analysis by RNase protection assay (Fig. 5A). Unspliced cytoplasmic RNA from cells transfected with mutant DNAs decayed with kinetics not significantly different from the wild type (Fig. 5B). The spliced RNA’s also decayed similarly in all transfections (Fig. 5B). This result suggests that the six mutations did not decrease the stability of the unspliced message and that there must be an alternative explanation for the disappearance of unspliced RNA from the cytoplasm. As an additional control, the stability of myc RNA was measured and found to be similar to the stability of spliced env RNA (data not shown). One possible explanation for the similar stability kinetics of the wild-type and mutant unspliced RNAs is that the wild-type unspliced RNA may actually be more stable than that of the mutant but is removed from the cytoplasm more rapidly through more efficient packaging. Evidence for this explanation is discussed below.
FIG. 5.
Effect of dr1 mutations on cytoplasmic RNA stability. QT6 cells transfected with the indicated mutant DNAs were treated with 2 μg of actinomycin D per ml at 40 h posttransfection for the indicated times, when cytoplasmic RNA was harvested and subjected to analysis by RNase protection as described above. (A) Representative RNase protection assay performed as described above. (B) Amounts of unspliced viral RNAs remaining at 6, 12, and 24 h as a fraction of that amount present at the time of actinomycin D addition. Error bars indicate the standard deviations computed from three separate experiments.
ASLV dr1 mutants express amounts of Pr76 Gag protein similar to those of wild-type ASLV.
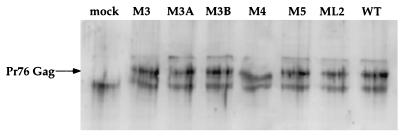
Consistent with the effect on unspliced cytoplasmic RNA, it has previously been shown that deletion of dr1 from RSV proviral constructs results in low (22) or undetectable (17) expression of Pr76 Gag protein in transfected cells. Additionally, point mutations in the downstream dr1 result in reduced expression of viral proteins in transfected cells (16).
To ascertain the efficiency of translation of unspliced viral RNA containing the mutations described in the present study, the levels of Gag in transfected cells were determined. Concentrated cell lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Gag was detected with anti RSV CA-NC antiserum (Fig. 6). No significant difference in Gag expression was observed among the lysates of cells transfected with mutant and wild-type DNA, in direct contrast to a previous report on Gag expression in dr1 point mutants (16). Therefore, the reduction in replication observed by the RT assay must be due to a defect in a replication step that occurs after the translation of Gag.
FIG. 6.
Effect of dr1 mutations on Pr76 Gag expression. Total cellular protein was isolated from QT6 cells transfected with the indicated mutant DNAs isolated at 40 h posttransfection. Then, 30 μg of protein was loaded per lane and subjected to SDS-PAGE and Western transfer. Pr76 Gag was detected with rabbit antiserum reactive to p27 CA and p7 NC. An alkaline phosphatase-conjugated secondary antibody was used in conjunction with a fluorescent substrate to detect the bands.
ASLV dr1 point mutants package genomic RNA approximately 10-fold less efficiently than wild-type ASLV.
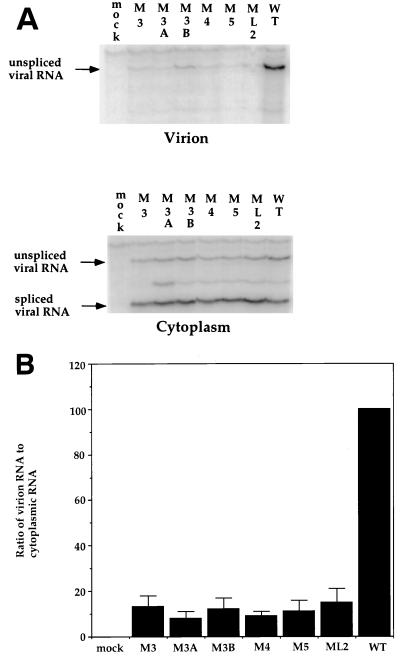
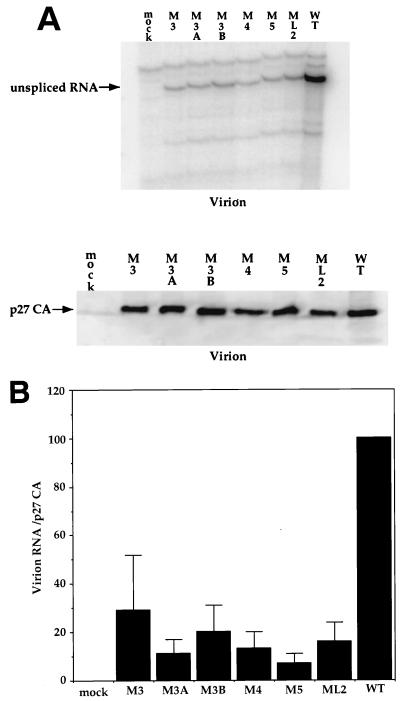
We therefore tested the ability of the six dr1 point mutations to package unspliced RNA into virions. Cell culture medium from transfected QT6 cells was filtered and split into two equal samples, and the virus was pelleted. One sample was directly resuspended in SDS-PAGE loading buffer for an analysis of the virion proteins, and the other half was subjected to RNA extraction. Both cytoplasmic RNA and viral RNA were analyzed by RNase protection (Fig. 7A). The wild-type DNA yielded about 10-fold more packaged viral RNA per unit amount of RNA in the cell cytoplasm than did any one of the six mutants (Fig. 7B). Since the difference in packaging could be due to fewer particles produced rather than the production of “empty” particles, viral genomic RNA was compared to virion-associated p27 CA protein (Fig. 8A). The wild-type DNA yielded about 10-fold more packaged viral RNA per unit amount of p27 CA protein than did any one of the six mutants (Fig. 8B). The result of this experiment strongly suggests that the reduction in packaging is due to the production of particles that are approximately 90% reduced in genomic viral RNA compared to the wild type rather than to a reduction in particle production.
FIG. 7.
Effect of dr1 mutations on the packaging of genomic RNA. Cytoplasmic RNA was harvested at 40 h posttransfection of QT6 cells, and virions were pelleted from the culture medium at 40 h posttransfection by ultracentrifugation; RNA was then isolated. The RNAs were subjected to analysis by RNase protection. (A) Representative RNase protection assays showing viral RNA levels in virions and the cytoplasm of transfected QT6 cells. (B) Ratios of virion unspliced-RNA levels to cytoplasmic RNA levels calculated from the experiments shown in panel A. Error bars indicate the standard deviations computed from five separate transfections with the same DNA.
FIG. 8.
Effect of dr1 mutations on the production of virions. Virions were pelleted from the culture medium of QT6 cells at 40 h posttransfection with the indicated DNAs. Pelleted virions were used for the preparation of RNA and were also subjected to SDS-PAGE and Western blotting, followed by detection of p27 CA with p27-specific antiserum. (A) Representative RNase protection assay and representative Western blot. (B) Ratios of virion genomic RNA to virion p27 CA protein calculated from the experiments shown in panel A. Error bars indicate the standard deviations computed from five separate experiments.
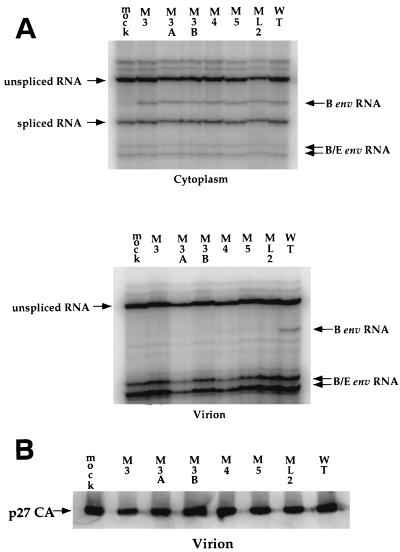
To determine whether the packaging defect was a result of a cis defect in the RNA or a trans defect in viral proteins, QT6/BD cells were transfected with a wild-type proviral construct expressing a subgroup B/E envelope and passaged twice to allow virus spread and infection of most cells. The cells were then transfected with mutant and wild-type viral constructs expressing the subgroup B envelope. This protocol resulted in a competition between the subgroup B-env-containing and the subgroup B/E-env-containing genomes for packaging. RNA was extracted from cells and virus and subjected to RNase protection by using two probes, a subgroup B env probe that spanned the region of gp85 where the two env genes differ most in sequence (7, 8) and the splice donor spanning probe (Fig. 1). Virus was also subjected to SDS-PAGE, Western transfer, and immunodetection with anti-p27 CA antiserum. The wild-type subgroup B/E genomic RNA was packaged, as was the wild-type subgroup B genomic RNA (Fig. 9A). However, the mutant subgroup B genomic RNA could not compete with the wild-type subgroup B/E genomic RNA and was therefore undetectable in virion RNA preparations (Fig. 9A). All virus preparations contained large amounts of packaged subgroup B/E genomic RNA, which resulted in two protected bands due to inefficient cleavage by RNases at a base mismatch between probe and subgroup B/E genomic RNA (Fig. 9A). p27 CA protein expression was similar in all samples (Fig. 9B). Since the defect in packaging closely resembled the reduction in replication (Fig. 3), we concluded that it was the largest contributing factor to the replication defect.
FIG. 9.
Effect of the mutations on competition for packaging of genomic RNA. QT6 cells were transfected with 10 μg of the wild-type DNA encoding the subgroup B/E env and then passaged twice. Cells were next transfected with 10 μg of the indicated DNAs encoding the subgroup B env. (A) RNA was harvested at 40 h posttransfection, and virions were pelleted from the culture medium; RNA and protein were then isolated. The RNAs were subjected to analysis by RNase protection with the two riboprobes described in Fig. 1. (B) Virions were subjected to SDS-PAGE and Western blotting, followed by detection of p27 CA with p27-specific antiserum.
DISCUSSION
The 3′ UTR is well conserved among avian retroviruses and is essential for efficient replication. However, a clear and uncontested role has not yet been ascribed to it. The dr1 element within the UTR has been shown to have a significant role in the cytoplasmic accumulation of unspliced RNA (16, 17). Another report has shown the dr1 to play a role in genomic RNA packaging (24). Still another report has concluded that the dr1 has multiple roles and that particle production is profoundly reduced when both of the dr1s are precisely deleted from an RSV construct containing src (22). The difference in results may have resulted from the use of different strains of virus, slight differences among clones of the same strain, or a variety of viral and nonviral polylinker sequences immediately flanking the different dr1 deletions and point mutations used in the different studies. Even though Ogert et al. (16) also utilized point mutations and obtained different results, their construct contained polylinker sequences flanking the mutated dr1. The extra nonviral sequence may have been sufficient to yield the different results. It should be noted, however, that cytoplasmic accumulation, as well as particle production, are necessary for packaging. Therefore, packaging could not have been assessed given the defect in cytoplasmic accumulation of unspliced RNA (16, 17) or the defect in particle production (22). In contrast to those studies, our constructs contained only viral sequences since our cloning steps used natural restriction sites found in the provirus, unlike some of the previous studies (16, 17, 24).
The results presented here suggest that the dr1 element plays a significant role in viral replication because it is necessary for the efficient packaging of viral genomic RNA into virions. Proviral DNA constructs bearing substitution mutations in this highly conserved element were also found to have a measurable and reproducible reduction in their accumulation of unspliced RNA in the cytoplasm. However, this effect was too small to account for the reduction in replication observed for all of the mutants. Also, the mutations did not directly reduce the stability of their respective transcripts compared to the wild type. Furthermore, expression of the Pr76 Gag precursor protein was found to be unaffected by the mutations. The mutations did, however, result in the production of viral particles that packaged approximately 10-fold less genomic RNA per amount of cytoplasmic unspliced RNA and per amount of virion p27 CA protein.
It has previously been reported that the dr1 element is necessary for efficient replication of ASLV (16, 17, 22, 24). The replication kinetics shown by the mutants described in this study confirm those results by examining a limited number of replication cycles in DF1 cells (Fig. 3).
Previous work has shown that deleting dr1, as well as introducing a different set of point mutations into dr1, results in decreased cytoplasmic accumulation of unspliced viral RNA (16, 17). Furthermore, dr1 was found to facilitate cytoplasmic accumulation of heterologous unspliced RNA (17). However, its role as a CTE in its natural context, a role analogous to that of simian type D retroviruses (3, 33), has been difficult to prove. Without measurable nuclear retention, it is difficult to conclude that, in the context of an RSV genome, the 3′ UTR functions as a true CTE. The point mutations we characterized here did not greatly affect the cytoplasmic accumulation of unspliced RNA, particularly compared to the same ratio for the total cellular RNA sample (Table 1). We therefore conclude that, if facilitating the cytoplasmic accumulation of unspliced RNA is a critical function of the dr1, that function was not significantly affected by our point mutations.
Recent studies examining steady-state levels of viral RNA in transfected cells treated with actinomycin D have demonstrated that deletion of dr1 results in a modest decrease in stability of the unspliced transcript (17, 22). We conducted similar experiments with constructs containing dr1 point mutations and observed no effect on the stability of unspliced cytoplasmic RNA. It should be noted, however, that the previous stability studies examined total cellular RNA, whereas we examined only the cytoplasmic fraction (17, 22). Interpretation of this result may be complicated by the reduction in genomic RNA packaging caused by the mutants. It is possible that the wild-type unspliced transcript is more stable than that of any one mutant but that the more efficient packaging of the wild-type genomic RNA leads to reduced cytoplasmic levels. This same packaging difference could also explain the similar levels of Pr76 Gag protein in cells expressing mutant and wild-type RNA (Fig. 6), despite seemingly higher levels of wild-type unspliced RNA. Even though the transfections with wild-type DNA produced higher steady-state levels of cytoplasmic unspliced RNA (Fig. 4 and 5), a higher percentage of it was encapsidated and therefore was untranslatable.
Defective packaging of the genomic RNA was the most pronounced phenotype of the six mutants tested. In contrast to the results of other investigators (16, 17, 22), the mutants presented here produced Gag protein (Fig. 6) and particles as efficiently as did the wild type (Fig. 8), demonstrating that no translation or assembly defects had arisen as a result of introducing point mutations into the dr1. The effect we observed is instead more in agreement with other reports describing the negative effects of the dr1 deletion on packaging (24) and infectious particle production (22).
Although mutations in dr1 can strongly affect packaging, the element is not absolutely required for this process. We (25) and others (2) have found that spliced RNAs containing the virus 5′ end but heterologous sequences 3′ of the splice site can be efficiently incorporated into virions. Furthermore, it has been shown that an RCAS vector containing the hygromycin gene packaged genomic RNA 10-fold less efficiently when its dr1 was deleted (1). Therefore, the role of dr1 in packaging seems to be limited to RNAs containing viral 3′ sequences. These results suggest that dr1 interacts with adjacent sequence, possibly forming a secondary or tertiary structure in conjunction with more distal sequence, to promote packaging. If the overall shape of the RNA is important for packaging, then either a positive role for the intron or a negative role for the juxtaposition of 5′ and 3′ sequences must also be considered, since env RNA is packaged inefficiently relative to genomic RNA. The presence of packaging determinants at both ends of spliced and unspliced avian retrovirus RNAs strongly suggests that different overall RNA structures are formed by both molecules. Packaging selectivity, therefore, may result from the generation of only one efficiently packageable molecule.
We cannot conclude from this study whether the dr1 element is necessary solely for efficient packaging or whether it serves other functions. The mutations characterized herein clearly inhibit packaging through the introduction of a cis defect in the genomic RNA, but these mutations spanned only about one-half of the dr1 and included only bases predicted to be unpaired in the putative secondary structure. The regions of dr1 that remained unaltered by the 13 mutations may well encode other activities.
ACKNOWLEDGMENTS
We thank Volker Vogt, Karen Beemon, and Richard Katz for helpful discussion and for providing reagents and David Lazinski, Naomi Rosenberg, Linc Sonenshein, and Elliot Androphy for helpful discussion. We thank G. Johah Rainey, Marcello Vinces, and Christopher Tipper for their assistance and Mary Bostic-Fitzgerald for preparing the manuscript.
J.M.A. was supported by training grant 5T32GM07310 and by an award from the National Cancer Institute R35CA44385 to J.M.C. J.M.C. is an American Cancer Society Research Professor.
REFERENCES
- 1.Aronoff R, Linial M L. Specificity of retroviral RNA packaging. J Virol. 1991;65:71–80. doi: 10.1128/jvi.65.1.71-80.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks J D, Yeo A, Green K, Cepeda F, Linial M L. A minimal avian retroviral packaging sequence has a complex structure. J Virol. 1998;72:6190–6194. doi: 10.1128/jvi.72.7.6190-6194.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray M, Prasad S, Dubay J W, Hunter E, Jeang K-T, Rekosh D, Hammarskljold M-L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen B. RNA-sequence-mediated gene regulation in HIV-1. Infect Agents Dis. 1994;3:68–76. [PubMed] [Google Scholar]
- 5.Cullen B R. Regulation of HIV-1 gene expression. FASEB J. 1991;5:2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- 6.Cullen B R. Mechanism of action of regulatory proteins encoded by complex retroviruses. Microbiol Rev. 1992;56:375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorner A J, Stoye J P, Coffin J M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986;45:365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 8.Dorner A J, Stoye J P, Coffin J M. Molecular basis of host range variation in avian retroviruses. J Virol. 1985;53:32–39. doi: 10.1128/jvi.53.1.32-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst R K, Bray M, Rekosh D, Hammarskjold M L. Secondary structure and mutational analysis of the Mason-Pfizer monkey virus RNA constitutive transport element. RNA. 1997;3:210–222. [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg M B, Jarrett R F, Aldovini A, Gallo R C, Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986;46:807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- 11.Fischer U, Meyer S, Teufel M, Heckel C, Luhrmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himly M, Foster D, Bottoli I, Iacovoni J, Vogt P. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- 13.Katz R A, Omer C A, Weis J H, Mitsialis A, Faras A J, Guntaka R V. Restriction endonuclease and nucleotide sequence analyses of molecularly cloned unintegrated avian tumor virus DNA: structure of large terminal repeats in circle junctions. J Virol. 1982;42:346–351. doi: 10.1128/jvi.42.1.346-351.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNally M T, Gontarek R R, Beemon K. Characterization of Rous sarcoma virus intronic sequences that negatively regulate splicing. Virology. 1991;185:99–108. doi: 10.1016/0042-6822(91)90758-4. [DOI] [PubMed] [Google Scholar]
- 15.Moscovici C, Moscovici M G, Jimenez H, Lai M M C, Hayman M J, Vogt P K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977;11:95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- 16.Ogert R A, Beemon K L. Mutational analysis of the Rous sarcoma virus dr posttranscriptional control element. J Virol. 1998;72:3407–3411. doi: 10.1128/jvi.72.4.3407-3411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogert R A, Lee L H, Beemon K L. Avian retrovirus RNA element promotes unspliced RNA accumulation in the cytoplasm. J Virol. 1996;70:3834–3843. doi: 10.1128/jvi.70.6.3834-3843.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen H S, Beidas S, Dillon P, Rosen C A, Cochrane A W. Mutational analysis of the HIV-1 Rev protein and its target sequence, the Rev responsive element. J Acquired Immune Defic Syndr. 1991;4:558–567. [PubMed] [Google Scholar]
- 19.Olsen H S, Cochrane A W, Dillon P J, Nalin C M, Rosen C A. Interaction of the human immunodeficiency virus type 1 Rev protein with a structured region in env mRNA is dependent on multimer formation mediated through a basic stretch of amino acids. Genes Dev. 1990;4:1357–1364. doi: 10.1101/gad.4.8.1357. [DOI] [PubMed] [Google Scholar]
- 20.Richard N, Iacampo S, Cochrane A. HIV-1 rev is capable of shuttling between the nucleus and cytoplasm. Virology. 1994;204:123–131. doi: 10.1006/viro.1994.1516. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz D E, Tizard R, Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983;32:853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- 22.Simpson S B, Zhang L, Craven R C, Stoltzfus C M. Rous sarcoma virus direct repeat cis elements exert effects at several points in the virus life cycle. J Virol. 1997;71:9150–9156. doi: 10.1128/jvi.71.12.9150-9156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sodroski J, Goh W C, Rosen C, Dayton A, Terwilliger E, Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986;321:412–416. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- 24.Sorge J, Ricci W, Hughes S H. cis-Acting packaging locus in the 115-nucleotide direct repeat of Rous sarcoma virus. J Virol. 1983;48:667–675. doi: 10.1128/jvi.48.3.667-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swain A, Coffin J M. Mechanism of transduction by retroviruses. Science. 1992;255:841–845. doi: 10.1126/science.1371365. [DOI] [PubMed] [Google Scholar]
- 26.Tabernero C, Zolotukhin A S, Bear J, Schneider R, Karsenty G, Felber B K. Identification of an RNA sequence within an intracisternal-A particle element able to replace Rev-mediated posttranscriptional regulation of human immunodeficiency virus type 1. J Virol. 1997;71:95–101. doi: 10.1128/jvi.71.1.95-101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiley L S, Malin M H, Tewary H K, Stockley P G, Cullen B R. Identification of a high-affinity RNA-binding site for the human immunodeficiency virus type 1 rev protein. Proc Natl Acad Sci USA. 1992;89:758–762. doi: 10.1073/pnas.89.2.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsichlis P N, Coffin J M. Recombinants between endogenous and exogenous avian tumor viruses: role of the c region and other portions of the genome in the control of replication and transformation. J Virol. 1980;33:238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsichlis P N, Conklin K F, Coffin J M. Mutant and recombinant avian retroviruses with extended host range. Proc Natl Acad Sci USA. 1980;77:536–540. doi: 10.1073/pnas.77.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Beveren C, Coffin J M, Hughes S. Appendixes. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1985. pp. 559–1222. [Google Scholar]
- 31.Weiss R, Teich N, Varmus H, Coffin J. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 32.Weiss R, Teich N, Varmus H, Coffin J. RNA tumor viruses. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1985. [Google Scholar]
- 33.Zolotukhin A S, Valentin A, Pavlakis G N, Felber B K. Continuous propagation of RRE(−) and Rev(−)RRE(−) human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J Virol. 1994;68:7944–7952. doi: 10.1128/jvi.68.12.7944-7952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuker M, Jacobson A B. “Well-determined” regions in RNA secondary structure prediction: analysis. Nucleic Acids Res. 1995;23:2791–2798. doi: 10.1093/nar/23.14.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]