Abstract
Background
Envenomations by African snakes represent a high burden in the sub-Sahara region. The design and fabrication of polyspecific antivenoms with a broader effectiveness, specially tailored for its use in sub-Saharan Africa, require a better understanding of the immunological features of different Naja spp. venoms of highest medical impact in Africa; and to select the most appropriate antigen combinations to generate antivenoms of wider neutralizing scope.
Methodology/principal findings
Rabbit-derived monospecific antisera were raised against the venoms of five spitting cobras and six non-spitting cobras. The effects of immunization in the animal model were assessed, as well as the development of antibody titers, as proved by immunochemical assays and neutralization of lethal, phospholipase A2 and dermonecrotic activities. By the end of the immunization schedule, the immunized rabbits showed normal values of all hematological parameters, and no muscle tissue damage was evidenced, although alterations in aspartate aminotransferase (AST) and alkaline phosphatase (ALP) suggested a degree of hepatic damage caused mainly by spitting cobra venoms. Immunologic analyses revealed a considerable extent of cross-reactivity of monospecific antisera against heterologous venoms within the spitting and no-spitting cobras, yet some antisera showed more extensive cross-reactivity than others. The antisera with the widest coverage were those of anti-Naja ashei and anti-N. nigricollis for the spitting cobras, and anti-N. haje and anti-N. senegalensis for the non-spitting cobras.
Conclusions/significance
The methods and study design followed provide a rationale for the selection of the best combination of venoms for generating antivenoms of high cross-reactivity against cobra venoms in sub-Saharan Africa. Results suggest that venoms from N. ashei, N. nigricollis within the spitting cobras, and N. haje and N. senegalensis within the non-spitting cobras, generate antisera with a broader cross-reactivity. These experimental results should be translated to larger animal models used in antivenom elaboration to assess whether these predictions are reproduced.
Author summary
The African elapids pose a major potential to induce clinically important envenomations and severe sequelae after a snakebite accident. Within the genus Naja, different radiation events have occurred rendering the African cobras with diverse features in their venom composition. Commonly divided into spitting and non-spitting cobras, their venom is characterized as either cytotoxic or neurotoxic, respectively, based on the pathophysiology of envenomation. In this study, an experimental protocol was developed based on the generation of monospecific antisera in rabbits immunized with venoms of spitting and non-spitting African cobras. Cross-reactivity of monospecific antisera was assessed by immunochemical analyses and by neutralization of the lethal, phospholipase A2 (PLA2) and dermonecrotic activities. Results showed a large extent of intrageneric cross-reactivity by all antisera, yet few exceptions were noticed. The venoms that generated antisera with the highest cross-neutralization ability among the spitting cobras were Naja nigricollis and N. ashei, whereas for the non-spitting cobras were N. haje and N. senegalensis. These results provide evidence for the selection of the best combination of immunogens for antivenom production with the widest neutralization scope within the African cobras. Additionally, the results obtained in the rabbit model should be translated to a larger animal model used for antivenom fabrication to assess whether these predictions are confirmed.
Introduction
The African snakes with the major potential to induce clinically relevant envenomation in humans belong to the Lamprophiidae, Colubridae, Elapidae, and Viperidae families [1–2]. In turn, the most important elapids belong to the genera Dendroaspis (mambas), Hemachatus (Rinkhals), Naja (cobras), and Pseudohaje (false cobras) [2]. Within the genus Naja different adaptation events have occurred, which might have been triggered by ecological and geological events associated with the formation and expansion of the African Rift Valley. Furthermore, within the spitting cobras, at least three events have rendered the African spitting cobras as a diverse clade (i.e., the appearance and expansion of the grassland and large mammals, the volcanic activity in the western Branch of the East African Rift System, and the presence of past arid corridors from northeastern to southwestern Africa). It is likely that the spitting venom trait developed before this radiation [3].
According to the reptile database (https://reptile-database.reptarium.cz/), the Naja genus comprises 23 African species, which are commonly classified as spitting or non-spitting cobras, depending on their ability to project a venom spray from their fangs. Moreover, on the basis of the clinical characteristics of envenomations, spitting and non-spitting cobras are also classified as cytotoxic and neurotoxic cobras, respectively.
Only five spitting cobras, i.e., N. ashei (Ashe’s spitting cobra), N. katiensis (Mali cobra), N. mossambica (Mozambique spitting cobra), N. nigricincta (Barred spitting cobra) and N. nigricollis (Black-necked spitting cobra), and six non-spitting cobras, i.e., N. anchietae (Anchieta’s cobra), N. annulifera (Snouted cobra), N. haje (Egyptian cobra), N. melanoleuca (Central African forest cobra), N. nivea (Cape cobra), and N. senegalensis (Senegalese cobra) are associated with high levels of morbidity, disability, or mortality, and therefore considered by WHO as Category 1 species [2].
Cobra venoms are mainly composed of three-finger toxins (3FTxs), phospholipases A2 (PLA2s), snake venom metalloproteinases (SVMPs), cysteine-rich secretory proteins (CRISPs), and other less abundant components such as L-amino acid oxidase (LAAO), cobra venom factor (CVF), venom nerve growth factor (vNGF), and vascular endothelial growth factor (VEGF), whose relative abundance in the venom cocktail (i.e., as relative concentration percentage) varies inter- and intra-specifically [4–11]. In general, the proteins that predominate in the venoms of African cobras are 3FTxs and PLA2s, which are largely responsible for the toxicity of these venoms.
Spitting cobras have venom which, when instilled in the eyes, induces ophthalmia, blepharitis, and blindness [12,13], whereas intradermal or subcutaneous injection induces severe local necrosis, without neurotoxic manifestations [14,15]. Loss of function due to chronic ulceration, osteomyelitis, arthrodesis, hypertrophic or keloid scars, and tetanus are frequent complications of necrotic lesions produced by spitting cobras [15,16]. After several years, some scars can undergo malignant transformation to produce what is known as Marjolin’s ulcer [15].
On the other hand, neurotoxic cobras have venoms that induce progressive muscular paralysis that could involve respiratory muscles resulting in respiratory arrest and death [5,15,17]. Thus, observation and management of local tissue damage and pro- inflammatory actions (i.e., mostly caused by the abundance of cytotoxins), should be monitored along with neurotoxicity, which could lead to paralysis [5,9].
In cases of cobra envenomation, the recommended treatment is the intravenous administration of specific snake antivenoms, i.e., formulations of whole immunoglobulins, or their Fab or F(ab´)2 fragments, purified from plasma of animals immunized towards snake venoms [2]. Since the identification of the offending snake species is not always possible and the envenomation symptoms could overlap between different species, the use of polyspecific antivenoms with wide neutralization scope is usually recommended [15,18].
Nevertheless, their efficacy is limited to venoms antigenically similar to those used as immunogens to stimulate the immune response in animals used to manufacture the antivenom [5,9,18–21]. Hence, to ensure a wide coverage of neutralization without requiring the identification of the offending snake species, the use of polyspecific formulations is preferred [2,18].
The selection of venoms used as immunogen to produce polyspecific antivenoms is based on the variation/conservation of the antigenic characteristics of venoms of the medically most important snakes in the region where the antivenom is intended to be used. The selection of the most appropriate venoms for immunization should, therefore, be based on a detailed knowledge of the antigenic relatedness of venoms and the medical relevance of the species [2,5,9,18–21]. This represents a challenge in African cobra venoms, due to the abundance, diversity, and wide distribution of species [3].
In this work, we used a rabbit model to determine the antigenic relatedness between venoms of African cobra snakes classified by WHO as Category 1 species, based on the intra-species cross-reactivity between monospecific rabbit sera raised against individual spitting and non-spitting cobra venoms. This information could be useful for the rational, knowledge-based design of the most appropriate venom mixtures for the generation of pan-African antivenoms.
Materials and methods
Ethics statement
This work presents an experimental study conducted following the standard procedures of scientific ethics, including those relating to the use and care of animals. All procedures conducted in this study meet the International Guiding Principles for Biomedical Research Involving Animals [22]. All procedures involving animals were approved by the Institutional Committee for the Care and Use of Laboratory Animals of Universidad de Costa Rica (approval code CICUA 202–2020). Rabbits and mice of both sexes were obtained from the Bioterium of Clodomiro Picado Institute. Rabbits were managed in Scanbur type EC3 cages (L 823 * W 660 * H 110 mm), one rabbit per cage, while mice were handled in Tecniplast Eurostandard Type II 1264C cages (L 25 * W 40 * H 14 cm), five mice per cage. In both cases, animals were maintained at 18–24°C, 60–65% relative humidity, and a 12:12 h light-dark cycle.
Snake venoms
Venoms of adult specimens of N. anchietae (from Namibia, batch #527.002), N. annulifera (from Mozambique, batch #622.040), N. ashei (from Kenya, batch #410.191), N. haje (unknown origin, batch #222.061), N. katiensis (from Burkina Faso, batch #705.010), N. melanoleuca (unknown origin, batch #516.031), N. mossambica (from Tanzania, batch #627.002), N. nigricincta (from South Africa, batch #507.081), N. nigricollis (unknown origin, batch #616.031), N. nivea (from South Africa, batch #524.010), and N. senegalensis (from Mali, batch #805.010) were purchased from Latoxan (Portes-dès Valence, France). After collection, venom was stabilized by lyophilization and stored at -40°C. Solutions of venom were prepared immediately before use.
Reverse-phase HPLC profiling
Five milligrams of each venom were dissolved in 200 μL of 0.1% trifluoroacetic acid (TFA) and 5% acetonitrile buffer (buffer A). Insoluble material was removed by centrifugation and the proteins in the supernatant were separated by reverse-phase HPLC (RP-HPLC, HPLC system: Agilent 1100 series; Agilent Technologies), equipped with a C18 column (250 x 4.6 mm, 5 μm particle size; Agilent Technologies). The flow rate was set to 1 mL/min and the protein separation was performed with the following buffer gradient: 0% buffer B (buffer B: 95% acetonitrile, 0.1% TFA) for 5 min, followed by 0–15% B over 10 min, 15–45% B over 60 min, 45–70% B over 10 min and 70% B for 9 min [23]. Protein peaks were detected at 215 nm. The similarity in the HPLC fractions were identified by comparing the chromatograms with those previously published.
Determination of lethal activity
Groups of five mice (16–18 g; CD-1 strain; both sexes) received a subcutaneous (SC) administration of the analgesic Tramadol, at a dose of 50 mg/kg, to reduce pain during the test [24]. Fifteen minutes afterwards, mice received an intraperitoneal (IP) injection of 0.5 mL of 0.12 M NaCl, 0.04 M phosphate, pH 7.2 solution (PBS) containing different amounts of venom. The number of deaths during the following 6 h were recorded [25] and used to estimate the Median Lethal Dose (LD50; i.e., the amount of venom that results in death of 50% of the injected mice) by Probits [26]. Surviving mice were euthanized by CO2 inhalation. Results were reported as LD50 and the corresponding 95% confidence interval (95% CI).
Determination of phospholipase A2 activity
The phospholipase A2 (PLA2) activity was determined by the methodology described by Gutiérrez and coworkers [27]. Briefly, various venom doses were prepared in duplicate for each venom, and then, 100 μL of each solution was added to 1 mL of egg yolk diluted 1:5 in a solution of 0.1 M Tris, 0.01 M CaCl2 and 1% Triton X-100 (pH 8.5). The mixtures were incubated at 37°C for 30 min. Finally, the free fatty acids were extracted and titrated according to Dole [28]. The activity was expressed as μEq of fatty acid released/mg venom/minute.
Determination of dermonecrotic activity
Dermonecrotic activity was assessed as described by Rivel et al. [29]. Various amounts of venom, dissolved in 100 μL PBS, were injected by the intradermal route into groups of three mice (both sexes, CD-1, 18-20g). Injections were done in the ventral abdominal region. After 72 h, animals were euthanized by CO2 inhalation and the diameter of lesions on the inner surface of the abdominal skin was determined. The Minimum Necrotizing Dose (MND) was estimated by linear regression as the amount of venom that induces a lesion of 5 mm diameter.
Immunization of rabbits
Groups of four rabbits (both sexes, New Zealand, 2.5–3.0 kg body weight) were immunized with the venoms of single species of either spitting cobras (i.e., N. ashei, N. katiensis, N. mossambica, N. nigricincta or N. nigricollis), or non-spitting cobras (i.e., N. anchietae, N. annulifera, N. haje, N. melanoleuca, N. nivea or N. senegalensis). Immunization was performed by five SC injections, applied at two-week intervals. Total volume of injections was 2 mL and all of them contained 1 mg of venom. In the first injection, the venoms were emulsified in Complete Freund’s Adjuvant (CFA); in the second one, Incomplete Freund’s Adjuvant (IFA) was used; afterwards, in the rest of the immunization protocol, venoms were dissolved in PBS. Rabbits were monitored during immunization by a veterinarian to observe signs of toxicity. At the end of immunization, samples of 6 mL blood were collected from the ear marginal vein; 3 mL were added to EDTA as anticoagulant and used for hematological tests, while 3 mL were left to clot in order to obtain serum, which was used for serum chemistry and immunological tests. Immediately after the collection of blood, rabbits were euthanized by an overdose of anesthetic (i.e., a dose of 100 mg/kg of sodium pentobarbital, administered by the IP route).
Hematological and serum chemistry analysis
Hematological and serum chemistry analyses were conducted on each individual rabbit sample. Hematological analyses (i.e., erythrocyte, leukocyte and platelet counts, hematocrit, and hemoglobin concentration) were conducted in a Veterinary Hematology Analyzer (Exigo Eos Hematology System; Boule Diagnostics AB, Stockholm, Sweden). The following analytes were quantified in a clinical chemistry analyzer (Spin200E Automatic biochemistry analyzer; Spinreact, Barcelona, España): creatine kinase (CK), alanine aminotransferase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) were determined by the corresponding International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) methods. Urea was quantified by a modification of the Talke and Schubert method [30]; creatinine by a kinetic modification of the Jaffe colorimetric method [31]; albumin by the bromocresol green colorimetric method [32]; and total protein by the Biuret method [33].
Immune reactivity of rabbit sera by enzyme-linked immunosorbent assay (ELISA)
Polystyrene plates (Costar 9017; 96 well; flat bottom) were coated overnight at room temperature with 100 μL of PBS containing 3 μg of venom. After washing the plates five times with distilled water, 100 μL of several dilutions (from 1:1,500 to 1:40,500, dilution factor 3) of each rabbit serum sample, in PBS-2% bovine serum albumin (BSA), were added. Plates were incubated for 1 h at room temperature and washed five times. Afterwards, 100 μL of goat anti-rabbit IgG conjugated with peroxidase (Sigma-Aldrich A0545), diluted 1:5000 with PBS-2% BSA, were added to each well. Microplates were incubated for 1 h at room temperature. After a final washing step, color was developed by the addition of H2O2 and o-phenylenediamine (2 mg/mL o-phenylenediamine, 1 μL/mL hydrogen peroxide in 0.1M citrate buffer, pH 5.0). Color development was stopped by the addition of 1.0 M HCl. Absorbances at 492 nm were recorded. The relative concentration of anti-venom antibodies in the samples was calculated by interpolation of their absorbances in a calibration curve. Relative concentration was expressed as percentage, 100% corresponding to the titer of the serum raised against the homologous venom of each species. Results were expressed as mean ± SD of all rabbits in each group.
Electrophoretic analysis and western blot
Venoms (30 μg) were separated by SDS-PAGE run under non-reducing conditions using an acrylamide concentration of 12% [34]. Molecular weight markers (3 μL) were included into the gels (Thermo 26630). The gels were either stained with Coomassie Brilliant Blue R-250, decolored with water and used to display the electrophoretic profile of the venoms or transferred to a nitrocellulose membrane at 30 mAmp during 1 h. The gels used for the Western blot were later stained using Coomassie Brilliant Blue R-250 to confirm the protein transfer to the membranes. Then, the membranes were blocked with PBS-0.1% casein for 30 min. Next, membranes were incubated for 1 h with a pool of serum samples of all rabbits of each monospecific antiserum, diluted 1/500 with PBS-0.1% casein. After washing the membranes four times with PBS-0.1% casein, they were incubated for 1 h with goat anti-rabbit IgG conjugated with alkaline phosphatase, diluted 1:2000 with PBS-0.1% casein. Finally, after the last washing step, 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT) color development substrate (Sigma-Aldrich) was added, and the reaction was stopped with distilled water.
Neutralization of the lethal, PLA2, and dermonecrotic activities
The ability of pools of serum samples from all rabbits in each group to neutralize the activities of the venoms was assessed by mixing a constant challenge dose of each venom with different dilutions of the pool of each antiserum. Mixtures were incubated at 37°C for 30 min before determining the residual activity of venom by using the experimental systems described above. The challenge doses utilized were: 2 LD50s for lethal activity, for PLA2 activity a challenge dose of 3 μg for the spitting cobras and a range of 1.5–75 μg for the non-spitting cobras, and one MND for the dermonecrotic activity. In all cases, venom-only controls were included, in which venom was incubated with PBS instead of antiserum. The use of 2 LD50s as a challenge dose in the neutralization of lethality studies, instead of the usual 4–5 LD50s, is justified to increase the sensitivity of the assay, favoring the detection of cross-reactivity of antisera against venoms. For lethal activity, neutralization was expressed as the Median Effective Dose (ED50), defined as the ratio mg venom/mL antiserum at which the activity of venom was reduced to 50% [35]. For PLA2 and dermonecrotic activities, neutralization was expressed as Median Effective Dose (ED50), defined as the ratio of venom/antivenom in which the activity of venom was reduced by 50% [11]. For comparing the different antisera in terms of their ability to neutralize the effects of the lethal and dermonecrotic activities of the heterologous venoms, a value of 1 was given to antisera that had an equal or higher value of ED50 as compared to homologous antisera, and a value of 0 was assigned to antisera having a lower value of ED50 as compared to homologous antiserum. Thus, a ranked score was created assigning a summatory value to each of the antisera assessed.
Statistical analyses
In the case of lethality and its neutralization, groups having non-overlapping values of 95% CI were considered significantly different. For ELISA, PLA2 and dermonecrotic activities, the significance of the differences between mean values of groups was assessed by one-way ANOVA, followed by a Ryan-Einot-Gabriel-Welsch Range (R-E-G-W Q) in order to test all pairs of means by conforming subsets which are significantly different between them and, at the same time, excluding variables from one group to another (i.e., p-value >0.05 is considered for grouping subsets). Additionally, R-E-G-W Q test poses a good estimation power and a tight control over the Type I Error. The interpretation of this test should be addressed as variables that conform homogenous subgroups that exclude other groups of variables (i.e., either venom or antisera) at p-values >0.05 in a comparison of all pairs of means. The hematological and serum chemistry variables were assessed by one-way ANOVA, followed by a Dunnett’s t post-hoc test in order to test the pairs of means compared to a specified control group (i.e., p-value <0.05 was considered significant). Neutralization of the PLA2 and dermonecrotic activities was assessed by a Kruskal-Wallis nonparametric test. Linearity and homogeneity of variances were tested, and a p-value <0.05 was considered significant, except when otherwise specified.
Results and discussion
General characterization of Naja spp. venoms
The proteomic analyses of venoms of N. annulifera [6,8,9], N. ashei [7], N. haje [4,10], N. katiensis [10,36], N. melanoleuca [5], N. mossambica [8, 36, 37], N. nigricincta [37], N. nigricollis [10,36,38], N. nivea [21], and N. senegalensis [20] have been previously described. However, to the best of our knowledge, the complete proteomic or venom profiling analysis of N. anchietae venom has not been conducted even though this species poses a medical threat.
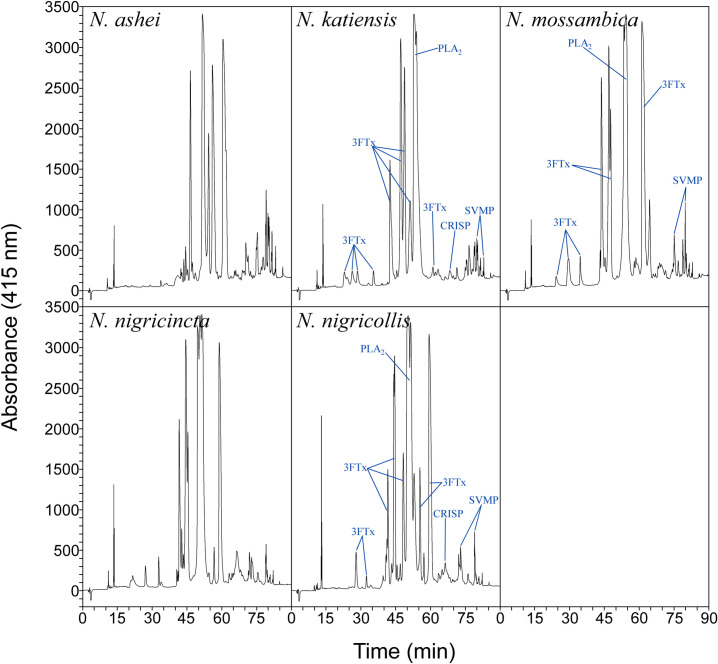
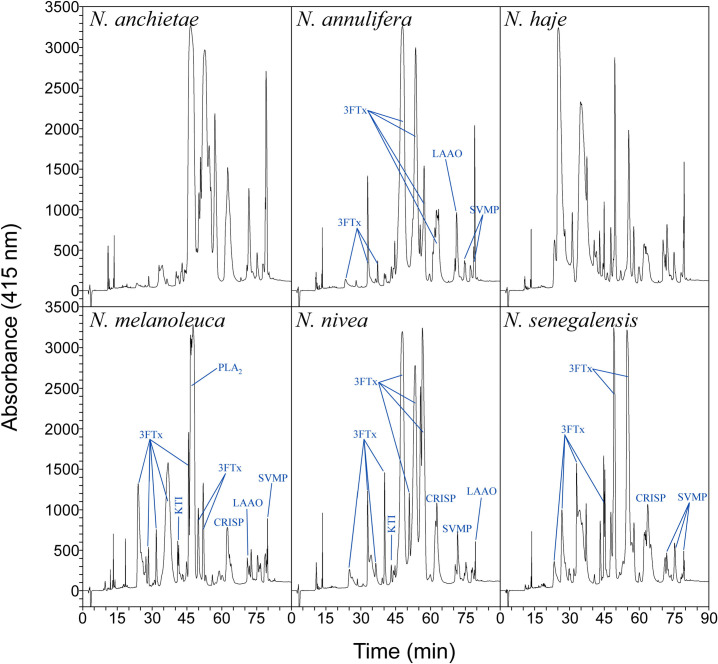
The chromatographic venom profiles of the spitting cobras (Fig 1 and S2 Data) and non-spitting cobras (Fig 2 and S3 Data) were obtained. As expected, there are intra-species variations in the relative abundance of various fractions, compared to previously published data. Nonetheless, a pattern of higher abundance of the fractions corresponding to cytotoxins and neurotoxins (i.e., 3FTxs) and PLA2s can be inferred for the spitting and non-spitting cobra venoms (S4 Data). In the case of spitting cobra venoms, cytotoxic 3FTxs and PLA2s predominate in their proteome [36] and are likely to be responsible for lethality.
Fig 1. RP-HPLC chromatograms of spitting cobra venoms.
Labeling of peaks was done based on Petras et al. [36] for the profiles of N. katiensis, N. mossambica and N. nigricollis.
Fig 2. RP-HPLC chromatograms of non-spitting cobra venoms.
Labeling of peaks was done based on Tan et al. [9] for N. annulifera, Lauridsen et al. [5] for N. melanoleuca, Tan et al. [21] for N. nivea, and Wong et al. [20] for N. senegalensis profiles.
The venoms of spitting cobras had similar LD50s, i.e., overlapping 95% CI, whereas the venoms of the non-spitting cobras showed differences with a low LD50 for N. melanoleuca and N. senegalensis, and even lower LD50 value for N. haje, the latter being the most toxic venom (Table 1 and S1 Data). The lower toxicity of venoms of the spitting cobras compared to those of some non-spitting cobras can be due to a different composition and relative concentration of neurotoxic 3FTxs and PLA2s [5,10,20,21]. The venoms of N. haje, N. melanoleuca and N. senegalensis, which have the highest toxicity, are rich in neurotoxic 3FTxs [5,10,20]. The high toxicity demonstrated by N. haje venom is associated to a severe neurotoxic syndrome, evidenced by limb paralysis, and labored respiration in mice, mainly due to the abundance of neurotoxic 3FTxs in its composition [4,10]. Toxicovenomics analysis aimed at identifying the toxins mainly responsible for lethality in these venoms is a pending task.
Table 1. Toxic activities of venoms of African Cobra snakes.
| Venom | Lethality (LD50)a | Phospholipase (PLA2)b | Dermonecrotic (MND)c |
|---|---|---|---|
| Spitting Cobras | |||
| N. ashei | 21.7 (15.8–30.5) | 158 ± 2 | 18 ± 1 |
| N. katiensis | 18.9 (13.7–24.8) | 176 ± 2 | 29 ± 6 |
| N. mossambica | 23.0 (16.1–32.6) | 156 ± 15 | 26 ± 1 |
| N. nigricincta | 21.7 (15.8–30.5) | 154 ± 2 | 14 ± 1 |
| N. nigricollis | 18.4 (10.7–26.4) | 149 ± 2 | 12 ± 1 |
| Non-spitting Cobras | |||
| N. anchietae | 69.7 (47.3–95.6) | 5 ± 0 | ND* |
| N. annulifera | 63.5 (48.5–84.6) | 4 ± 0 | ND* |
| N. haje | 1.9 (1.4–2.7) | 6 ± 1 | ND* |
| N. melanoleuca | 6.4 (4.9–8.9) | 342 ± 16 | ND* |
| N. nivea | 22.4 (17.8–28.3) | 4 ± 2 | ND* |
| N. senegalensis | 7.4 (5.7–10.1) | 7 ± 1 | ND* |
a Lethality is expressed as LD50 (95% CI) by the i.p. route, i.e., the Median Lethal Dose, defined as the amount of venom (μg) that results in the death of 50% of injected mice.
b Phospholipase A2 activity is expressed as μEq of fatty acid released per mg protein per min. PLA2 activity was determined using 3 μg of venom for the spitting cobras and a range of 1.5–75 μg for the non-spitting cobras (see Materials and Methods for details). Results are shown as mean ± SD (n = 3).
c Dermonecrotic activity is expressed as Minimum Necrotizing Dose (MND, μg/mouse, n = 3). Results are shown as mean ± SD.
*ND: No dermonecrotic activity was detected at the highest venom doses tested (20 μg for N. haje, N. melanoleuca and N. senegalensis venoms, and 80 μg for N. anchietae and N. annulifera venoms. Statistical analyses of PLA2 and MND activities are detailed in the text.
The spitting cobra venoms showed a lower PLA2 activity compared to previous works [11,36], yet no differences were observed among the five species studied (F(4; 5) = 3.963, p = 0.82). N. katiensis venom showed high PLA2 activity even though its PLA2 content is lower than that of N. nigricollis venom [10] (Table 1 and S1 Data).
The non-spitting cobra venoms had a lower PLA2 activity when compared to the spitting cobras (F(10; 11) = 340.400, p< 0.001). Moreover, differences among the six non-spitting cobra species were seen (F(5; 6) = 435.968, p< 0.001), with activity of N. melanoleuca venom being considerable higher than the rest (R-E-G-W Q post-hoc test p = 1.0) of the non-spitting cobra venoms studied. As a pattern among Naja sp venoms, PLA2 is the second most abundant protein family [5,10,11]. Regarding non-spitting cobras, the higher content of PLA2 in the venom of N. melanoleuca [5], compared to N. annulifera [9], N. nivea [21], and N. senegalensis [20] venoms, may explain the difference found in this enzymatic activity.
Only the spitting cobras induced dermonecrotic lesions in mice (Table 1 and S1 Data). The dermonecrotic activity of these venoms varied significantly (F(4; 7) = 17.982, p< 0.001), with the venom of N. nigricincta and N. nigricollis showing the highest activity (R-E-G-W Q post-hoc test p = 0.596). Since the major constituents of spitting cobra venoms are 3FTxs and PLA2, it has been demonstrated that some of these PLA2s induce cytotoxicity [39] and they may have a role in the dermonecrotic effect in murine models. Likewise, a large number of 3FTxs exhibit general cytolytic effects, being referred to as cytotoxins, causing a distinctive severe local tissue damage and necrosis without clear evidence of neurotoxicity [10,14,29,36].
Overall, the composition of the venom of spitting cobras is consistent with this toxicological profile and the high content of cytotoxic 3FTxs and PLA2s are likely responsible for the tissue necrosis that characterizes these envenomations. Spitting cobra venom contains 7–10% of SVMPs [10,11,36]. However, it is unlikely that they play a role in the pathogenesis of dermonecrosis [29]. On the other hand, despite the presence of cytotoxins and PLA2s in non-spitting cobra venoms, they did not induce necrosis in mice (Table 1 and S1 Data), suggesting that 3FTxs and PLA2s in these venoms are likely to exert a neurotoxic effect instead of cytotoxicity.
Envenomations from spitting and non-spitting cobras differ from a biogeographical standpoint [3] revealing, with the aid of proteomics and transcriptomics, significant variations in venom composition [10,36,37]. Particularly important are the variations in the cytotoxic effect between the spitting and non-spitting cobras, suggesting an alternative evolutionary path for venoms that, biologically, could provide a predominantly defensive role as well as the use of distinct niches [40]. Additionally, these differences in cobra venom phenotypes, as a result of multiple environmental and genetic interactions [8] and different behavioral responses such as predation and defensive actions (i.e., spitting, and non-spitting defensive mechanisms), may influence the clinical outcome in human envenomations.
Envenomations by African Naja sp present with a broad range of clinical manifestations, from necrosis and tissue damage to hematotoxicity and paralysis [8,15]. Different clinical presentations are the basis for the syndromic approach used in Africa to diagnose the type of envenomation and to select the appropriate antivenoms for treatment [15].
Effects induced by Naja spp. venoms in immunized rabbits
Groups of rabbits were immunized with venoms of spitting and non-spitting cobras. The first two immunization injections were administered in a water/oil emulsion (i.e., Freund’s Complete Adjuvant and Freund’s Incomplete Adjuvant), from which venoms are slowly released, reducing the tissue damage caused by the venom and enhancing the antibody response of the animals. Assuming that the antibody response developed during the initial two immunizations could reduce the toxic effects of the additional venom boosters, and to minimize the inflammation caused by Freund’s adjuvants, in the rest of the immunization scheme venoms were dissolved in PBS. Thus, it is of interest to evaluate the venom-induced toxic effects in this animal model.
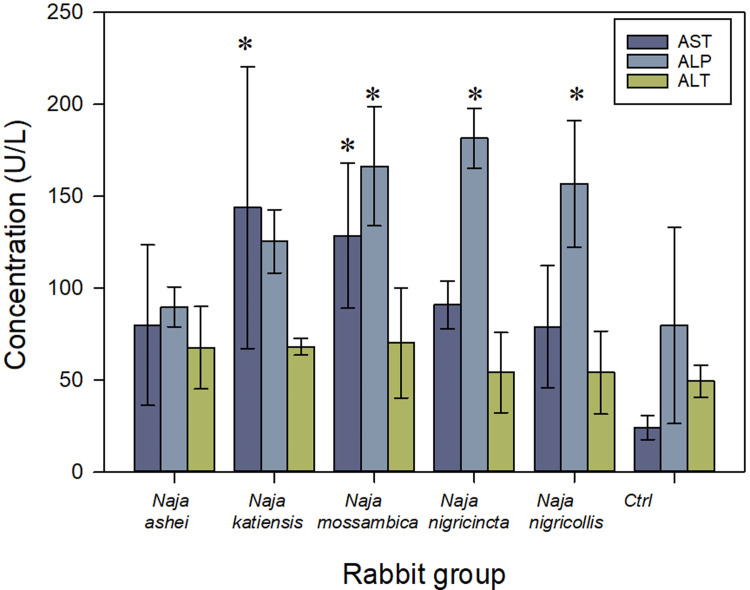
During immunization, local inflammatory reactions, at the site of injection of adjuvants and venom, were observed. By the end of the immunization schedule, the immunized rabbits showed normal values of all hematological parameters analyzed [41] (S1 Table). In addition, no muscle tissue damage was evidenced during the immunization since the CK plasma levels were within the range of the control, non-immunized rabbits [41] (S2 Table). However, the spitting cobra venoms caused some alterations in the plasma levels of AST (F(5; 18) = 4.025, p = 0.013) and ALP (F(5; 18) = 7.299, p< 0.001), but not in the plasma levels of ALT (Fig 3), when compared to control rabbits [41,42]. These alterations were specially marked in the group of rabbits immunized with N. katiensis and N. mossambica venoms (Dunnett’s t-test p = 0.003; p = 0.011, respectively) for the AST, and N. mossambica, N. nigricincta and N. nigricollis venoms (Dunnett’s t-test p = 0.004; p< 0.001; p = 0.010, respectively) for the ALP. These differences in the AST, but mainly in the ALP levels, suggest a degree of hepatic damage caused by the venom of spitting cobras during immunization. In agreement, there has been clinical evidence of hepatic damage in patients bitten by N. nigricollis [43]
Fig 3. Activity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) in serum of rabbits immunized with the venoms the spitting cobras Naja ashei, N. katiensis, N. mossambica, N. nigricincta and N. nigricollis.
Results are expressed in International Units (IU) per L and correspond to the mean ± SD of the four rabbits in each group. * p< 0.05 when compared to rabbit control groups.
Since cytotoxicity and myotoxicity are generally caused by the action of PLA2s and cytotoxic 3FTxs on several cell targets, the use of cytotoxin and PLA2 inhibitors during the immunization scheme could be an option to reduce tissue damage, a hypothesis to be evaluated in large animal models used for antivenom production.
In contrast, the non-spitting cobra venoms did not cause significant alterations in the plasma levels of the analytes studied compared with the non-immunized control group [41,42] (S2 Table). However, the plasma chemistry tests used do not detect neurotoxic alterations; therefore, a different approach should be considered when assessing the effects of the immunization scheme for non-spitting cobra venoms and other neurotoxic venoms.
Additionally, normal values of creatinine and urea in serum were found in all rabbits [41,42] (S2 Table) except for the group immunized with N. melanoleuca and N. senegalensis venoms, where the urea value was significantly different from the control group (Dunnett’s t-test p = 0.017). Likewise, normal values of total protein concentration in serum were found in all rabbits [41,42] (S2 Table) except for the groups immunized with N. anchietae and N. annulifera, where the total protein value was significantly different from the control group (Dunnett’s t-test p = 0.032; p = 0.028, respectively), suggesting that a hydric stress could have developed in these rabbits. Taking into consideration the plasma biochemistry results, it is concluded that no significant kidney injury was induced during immunization.
Our findings highlight the need to conduct analysis of local tissue and liver damage when venoms of spitting and non-spitting cobras are used in larger animal models for antivenom production. Additionally, strategies evaluating the neurotoxicity in larger animal models should be introduced, and the use of cytotoxin and PLA2 inhibitors could be added to the immunization mixture in order to minimize the extent of these pathophysiological manifestations.
Cross-reactivity and neutralization between spitting cobra venoms
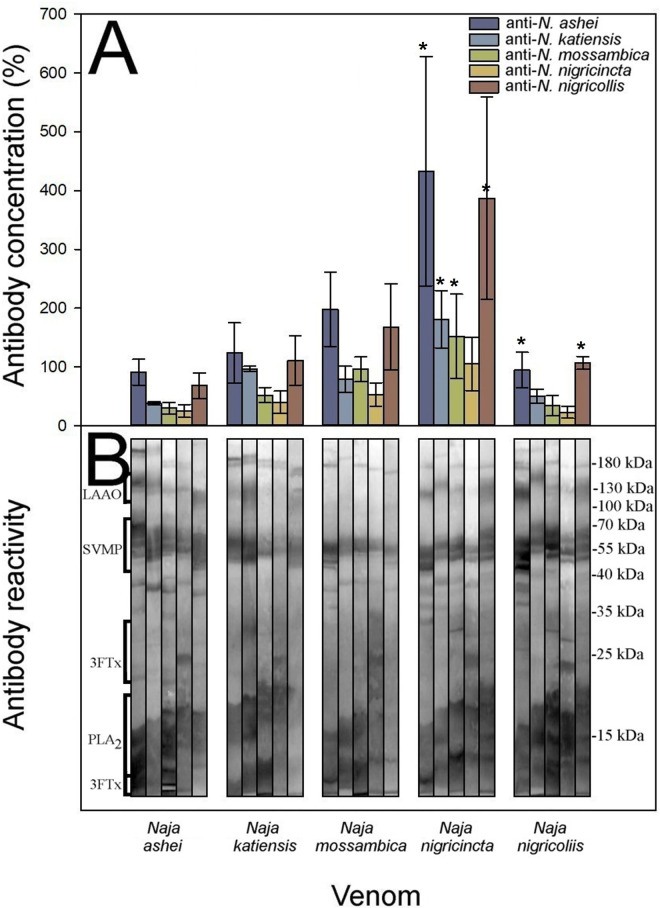
We followed the experimental protocol described by Gómez and colleagues [44] for African viperid venoms, which combines antibody titers quantified by ELISA, Western Blot, and the neutralization of toxic activities, allowing the assessment of the intrageneric cross-reactivity between monospecific rabbit sera against homologous and heterologous venoms. Cross-reactivity was noticed among all anti-Naja antisera against the spitting cobra venoms, revealing a clear antigenic venom similarity, yet there were quantitative differences between them (Fig 4 and S1 Data).
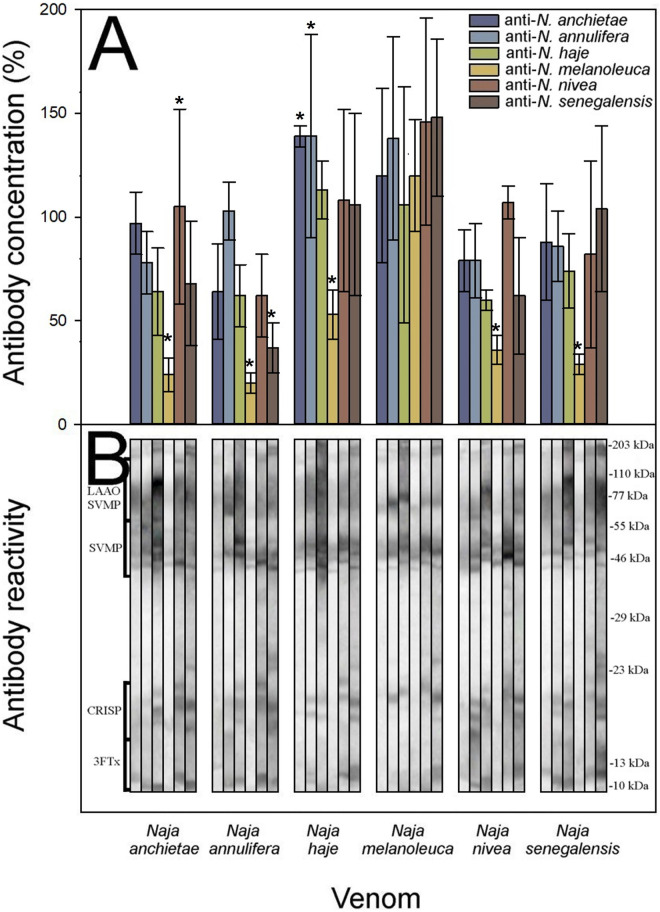
Fig 4.
Cross-reactivity between the spitting cobra venoms was determined by A) ELISA and B) Western blot. ELISA results are expressed as percentage considering as 100% the titer of serum raised against the homologous venom of each species and correspond to the mean ± SD of all rabbits in each group. Values with * are significantly different when compared to homologous antisera according to R-E-G-W Q post-hoc test.
Unexpectedly, in the spitting cobras the highest antibody responses not always were observed against the homologous venoms (Fig 4A), and all the monospecific antisera showed cross-reactivity by ELISA against the heterologous venoms (anti-N. ashei F(4; 15) = 8.543, p = 0.001; anti-N. katiensis F(4; 15) = 6.147, p = 0.004; anti-N. mossambica F(4; 15) = 6.551, p = 0.003; anti-N. nigricincta F(4; 15) = 4.218, p = 0.017; anti-N. nigricollis F(4; 15) = 5.451, p = 0.006). The anti-N. nigricincta antiserum showed a weak cross-reactivity against the heterologous venoms, yet the N. nigricincta antigen showed the highest antibody recognition by the heterologous anti-Naja antisera (R-E-G-W Q post-hoc test anti-N. ashei p = 1.0; anti-N. katiensis p = 1.0; anti-N. mossambica p = 0.956; anti-N. nigricollis p = 1.0).
The venom of N. nigricollis was recognized by the heterologous anti-N. ashei antiserum with a similar antibody titer of its homologous antiserum (R-E-G-W Q post-hoc test p = 0.944). The antisera against N. ashei and N. nigricollis showed the highest cross-reactivity against all the venoms tested (F(4; 15) = 8.543, p = 0.001; F(4; 15) = 5.451, p = 0.006, respectively; Fig 4A).
Extensive cross-reactivity was also detected by Western blot (Fig 4B). The identification of the immunoreactive protein bands was based on the molecular masses as shown in Fig 4B. Moreover, in concordance with the ELISA results, the stronger reactions seen by Western blot varied against the homologous antigens, with a similar scenario for the heterologous antigens such as SVMPs, 3FTxs, and PLA2s protein families (Fig 4B).
When the cross-neutralization of the spitting cobra venom toxic effects was analyzed, a wide neutralization of lethality by all the homologous and heterologous antisera was observed, with varying ED50 values but with a high extent of overlapping in the 95% CI (Fig 5A and S1 Data). Additionally, a general trend was observed in that the anti-N. nigricollis antiserum showed the highest neutralization efficacy against the heterologous venoms, and the anti-N. mossambica antiserum showed a lower neutralization efficacy against the heterologous venoms, although these differences were mostly non-significant.
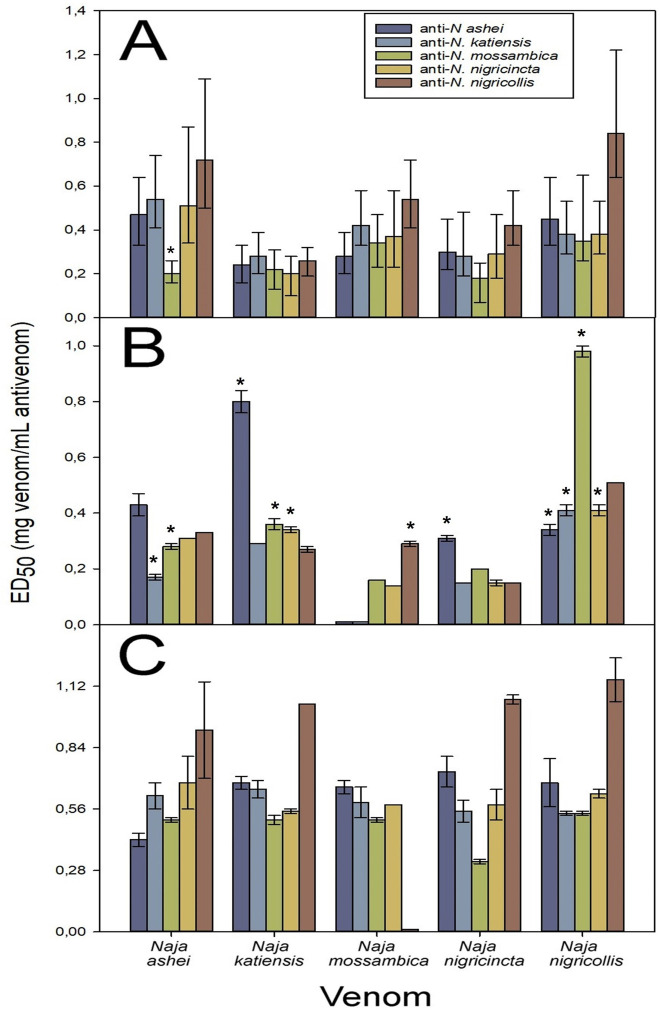
Fig 5. Cross-neutralization of the spitting cobra venom toxic effects by the antisera.
A) Neutralization of the lethal activity, B) Neutralization of the phospholipase A2 activity, C) Neutralization of the dermonecrotic activity. In the case of lethality neutralization, error bars represent the 95% CI and non-overlapping values with homologous antisera are depicted by *. In the case of PLA2 neutralization, error bars represent SD and * depict values that were significantly different from homologous antisera; while in the case of dermonecrotic neutralization, error bars represent SD.
Regarding the neutralization of the PLA2 activity, with few exceptions the antisera neutralized the homologous and heterologous venoms, albeit with different ED50 values (Kruskal-Wallis test N. ashei: p = 0.071; N. katiensis: p = 0.064; N. mossambica: p = 0.068; N. nigricincta: p = 0.065; N. nigricollis: p = 0.065). As a general trend, the anti-N. ashei and the anti-N. nigricollis antisera showed a good performance in neutralizing the PLA2 activity of the heterologous venoms when compared to the homologous antisera and, to a lesser extent, the anti-N. katiensis and the anti-N. nigricincta antiserum showed a similar behavior (Fig 5B and S1 Data). The anti-N. ashei and anti-N. katiensis antisera were unable to neutralize PLA2 activity of N. mossambica venom. The dermonecrotic activity of the spitting cobras was neutralized by the homologous and heterologous antisera with ED50 values that did not differ significantly between them (Fig 5C and S1 Data) (Kruskal-Wallis test N. ashei: p = 0.243; N. katiensis: p = 0.320; N. mossambica: p = 0.139; N. nigricincta: p = 0.308; N. nigricollis: p = 0.103). Despite the lack of significant difference, there is a trend of the anti-N. nigricollis antiserum to have a high neutralization of the dermonecrotic activity of the heterologous venoms when compared to the homologous antisera, except for N. mossambica venom where no neutralization was observed (Fig 5C). This venom was well neutralized by the rest of monospecific antivenoms.
Taken into consideration the data gathered from the neutralization of lethality and dermonecrosis, which in the case of spitting cobra venoms is a clinically relevant effect, the anti-spitting cobras’ antisera can be scored from highest to lowest cross-reactivity score (Table 2) as follows: anti-N. ashei, anti-N. nigricollis, anti-N. katiensis, anti-N. nigricincta, and anti-N. mossambica.
Table 2. Ranking of the antisera performance based on the neutralization capacity of the lethal and dermonecrotic activities of spitting and the lethal activity of the non-spitting cobra venoms.
A score system was used assigning a value of 1 to the heterologous antisera whose neutralizing capacity was equal or higher than that of the homologous antisera, and a value of 0 when the neutralizing capacity was lower than that of the homologous antiserum.
| Spitting Cobra venoms | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ED50 Lethal Activity | ED50 Dermonecrotic Activity | ||||||||||||||
| Antiserum | N. ashei | N. katiensis | N. mossambica | N. nigricincta | N. nigricollis | N. ashei | N. katiensis | N. mossambica | N. nigricincta | N. nigricollis | Total score | ||||
| Anti-N. ashei | - | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 0 | 7 | ||||
| Anti-N. katiensis | 1 | - | 1 | 1 | 0 | 1 | - | 1 | 1 | 0 | 6 | ||||
| Anti-N. mossambica | 0 | 1 | - | 1 | 1 | 1 | 0 | - | 0 | 0 | 4 | ||||
| Anti-N. nigricincta | 1 | 1 | 1 | - | 0 | 1 | 0 | 1 | - | 0 | 5 | ||||
| Anti-N. nigricollis | 1 | 1 | 1 | 1 | - | 1 | 1 | 0 | 1 | - | 7 | ||||
| Non-spitting Cobra venoms | |||||||||||||||
| ED50 Lethal Activity | |||||||||||||||
| Antiserum | N. anchietae | N. annulifera | N. haje | N. melanoleuca | N. nivea | N. senegalensis | Total score | ||||||||
| Anti-N. anchietae | - | 1 | 0 | 1 | 1 | 0 | 3 | ||||||||
| Anti-N. annulifera | 1 | - | 0 | 0 | 0 | 0 | 1 | ||||||||
| Anti-N. haje | 1 | 1 | - | 1 | 1 | 1 | 5 | ||||||||
| Anti-N. melanoleuca | 1 | 1 | 0 | - | 1 | 0 | 3 | ||||||||
| Anti-N. nivea | 1 | 1 | 0 | 0 | - | 0 | 2 | ||||||||
| Anti-N. senegalensis | 1 | 1 | 0 | 1 | 1 | - | 4 | ||||||||
Finally, the extensive cross-reactivity of the monospecific antisera generated in this work agrees with previous studies where antivenoms produced using different mixtures of Naja spp. venoms were able to neutralize the lethal, PLA2, and dermonecrotic effects of these venoms [5,9,11,19–21,36,45,46]. According to our findings, the venoms of N. ashei and N. nigricollis were well neutralized by the spitting cobras’ heterologous antisera, whereas the anti-N. ashei and the anti-N. nigricollis antisera had a better overall neutralizing capacity among the heterologous antisera compared to the other antisera, with the exception of dermonecrotic activity of N. mossambica venom (Table 2).
Cross-reactivity and neutralization between non-spitting cobra venoms
The monospecific non-spitting cobras antisera responded differently in terms of its cross-reactivity quantified by ELISA (Fig 6A and S1 Data). The venoms of N. anchietae (F(5; 15) = 4.814, p = 0.008), N. annulifera (F(5; 15) = 13.985, p< 0.001), N. haje (F(5; 15) = 3.168, p = 0.038), N. nivea (F(5; 15) = 6.973, p = 0.001), and N. senegalensis (F(5; 15) = 3.216, p = 0.036) were recognized to a different extent by the homologous and heterologous antisera. The venom of N. melanoleuca (F(5; 14) = 0.437, p = 0.815) did not show significant differences in its cross-reactivity when assessed with either homologous or heterologous antisera (Fig 6A).
Fig 6.
Cross-reactivity between the non-spitting cobra venoms was determined by A) ELISA and B) Western blot. ELISA results are expressed as percentage considering as 100% the titer of serum raised against the homologous venom of each species and correspond to the mean ± SD of all rabbits in each group. Values with * are significantly different when compared to homologous antisera according to R-E-G-W Q post-hoc test.
N. anchietae venom was poorly recognized by the anti-N. melanoleuca antiserum (R-E-G-W Q post-hoc test p = 0.051), whereas the anti-N. nivea antiserum showed a higher cross-reactivity when compared to the homologous anti-N. anchietae antiserum (R-E-G-W Q post-hoc test p = 0.302). Likewise, the venom of N. annulifera was poorly recognized by the anti-N. melanoleuca and anti-N. senegalensis antisera when compared to the homologous anti-N. annulifera antiserum (R-E-G-W Q post-hoc test p = 0.351).
The venom of N. haje was better recognized by the anti-N. anchietae and anti-N. annulifera antisera when compared to the homologous anti-N. haje antiserum (R-E-G-W Q post-hoc test p = 0.680), whilst the anti-N. melanoleuca antiserum cross-reacted poorly with N. haje venom (R-E-G-W Q post-hoc test p = 0.249) when compared to the homologous antiserum. The venom of N. melanoleuca was recognized to a similar extent by the heterologous antisera when compared to the homologous antiserum (R-E-G-W Q post-hoc test p = 0.852).
N. nivea venom showed a low cross-reactivity with the heterologous antisera, especially in the case of anti-N. melanoleuca antiserum (R-E-G-W Q post-hoc test p = 0.126) when compared to the homologous anti-N. nivea antiserum. A similar scenario was evidenced for N. senegalensis venom, with a low cross-reactivity by the heterologous antisera, especially in the case of anti-N. melanoleuca antiserum (R-E-G-W Q post-hoc test p = 0.083) when compared to the homologous antiserum. Moreover, the anti-N. melanoleuca antiserum recognized to a lesser extent N. anchietae (R-E-G-W Q post-hoc test p = 0.051), and N. annulifera (R-E-G-W Q post-hoc test p = 0.351) venoms.
Cross-reactivity was also detected by Western blot, and the identification of the immunoreactive protein bands, based on the molecular masses is shown in Fig 6B. Furthermore, in agreement with the ELISA results, the strongest reactions by Western blot were observed with heterologous antisera in some cases, with a similar scenario for heterologous antigens such as SVMP, 3FTx, PLA2 and LAAO protein families. Noteworthy, higher reactivity was observed in high molecular mass bands (SVMPs, LAAOs), as compared to the bands corresponding to PLA2s and 3FTxs. Nevertheless, a wide variation in the intensity of the immune recognition by homologous and heterologous antisera against the antigens was observed by Western blot (Fig 6B).
When the cross-neutralization of venoms of non-spitting cobra toxic effects was analyzed, there were variations in the values of ED50 regarding neutralization of lethality, with a high extent of overlapping in the 95% CI (Fig 7A and S1 Data). Particularly, the anti-N. haje antiserum showed a good neutralization profile of the heterologous non-spitting cobra venoms. However, the venom of N. haje was poorly neutralized by either the homologous or heterologous antisera (Fig 7A). Likewise, the venom of N. senegalensis was poorly neutralized by the antisera, with the exception of N. haje antiserum.
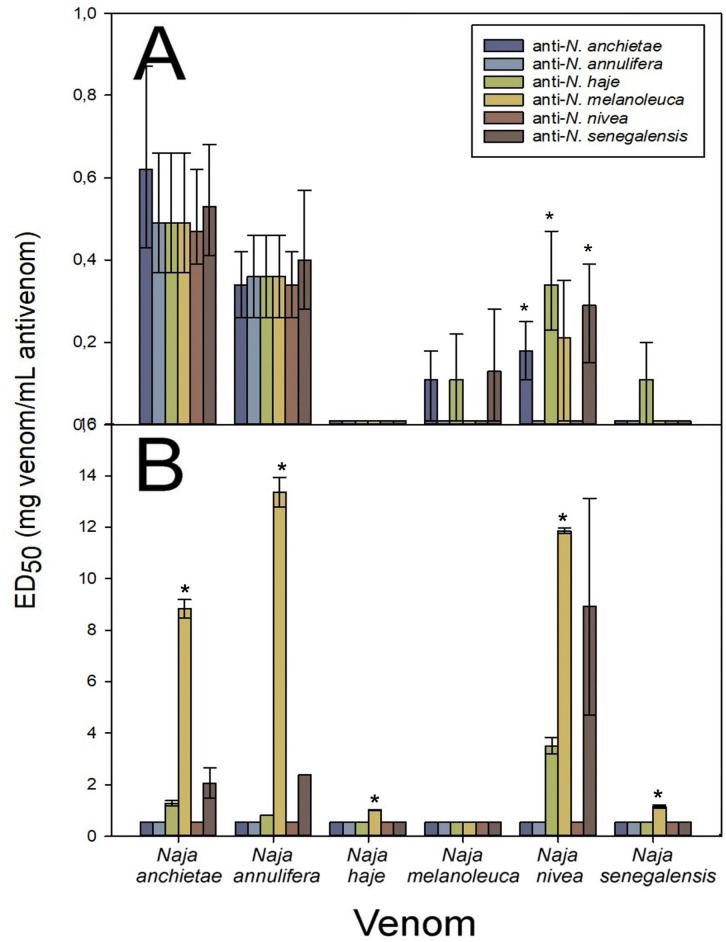
Fig 7.
Cross-neutralization of the non/spitting cobra venom toxic effects by the antisera. A) Neutralization of the lethal activity, B) Neutralization of the phospholipase A2 activity. In the lethal activity neutralization, error bars represent the 95% CI and non-overlapping values are depicted by *. In the case of PLA2 activity, error bars represent SD and * depict values that were significantly different from homologous antisera.
The neutralization of PLA2 activity by the non-spitting cobra antisera showed variable ED50 values for the homologous and heterologous venoms (Kruskal-Wallis test N. anchietae p = 0.054; N. annulifera p = 0.053; N. haje p = 0.053; N. nivea p = 0.060, N. senegalensis p = 0.053). The anti-N. melanoleuca serum showed the best PLA2 neutralization (Kruskal-Wallis test p< 0.001) except for the homologous venom (Fig 7B and S1 Data), with an overall good neutralizing capacity against PLA2 activity of the heterologous venoms. The venoms of N. haje, N. melanoleuca and N. senegalensis were poorly neutralized by the homologous and heterologous antisera.
Recently, a low content of PLA2 in the venom N. haje [4], and the incapability of detecting PLA2 in the venoms of N. annulifera [9], N. nivea [21] and N. senegalensis [20] has been described, while PLA2s comprise 13% of the proteome N. melanoleuca venom [5]. This may explain to some extent the high PLA2 activity of and the anti-PLA2 response elicited by this venom.
Considering together the data gathered from neutralization of lethality, the anti-non-spitting cobras’ antisera can be graded from highest to lowest cross-reactivity score (Table 2) as follows: anti-N. haje, anti-N. senegalensis, anti-N. anchietae, anti-N. melanoleuca, anti-N. nivea, anti-N. annulifera.
Conclusions
As a general conclusion, antisera anti-N. ashei and anti-N. nigricollis provided the highest immune coverage based on the score obtained from the lethality and dermonecrosis ED50 values for spitting cobra venoms, whereas antiserum anti-N. haje provided the highest immune coverage based on the ranked score obtained from the lethality ED50 values for non-spitting cobra venoms. Nonetheless, it is likely that the generation of antivenoms with the broadest neutralization scope will require the inclusion of venoms from additional Naja species, a hypothesis that will be assessed in future studies.
The cross-reactivity seen between monospecific anti-spitting cobra antisera suggests antigenic similarities between the toxic components of N. ashei, N. katiensis, N. mossambica, N. nigricincta and N. nigricollis venoms. Likewise, with some exceptions, cross-reactivity between monospecific anti-non-spitting cobra antisera suggests a degree of similarity between the toxic components of N. anchietae, N. annulifera, N. melanoleuca, and N. nivea venoms. However, N. haje, N. melanoleuca and N. senegalensis venoms are poorly neutralized by heterologous antisera. Insights into the basis of this phenomenon demand high-throughput technologies, such as antivenomics and toxicovenomics [5,11,47], to determine antigenic similarities between venoms and to identify the toxins responsible for overall toxicity [47].
Finally, as immunogenicity depends on the nature of the animal’s immune system selected as immunoglobulin source, the conclusions of our experiments in rabbits cannot be directly extrapolated to animal models used in antivenom production, such as horses or sheep. However, our observations can be applied to formulate rational hypotheses that can be put to experimental evaluation by immunizing horses with mixtures of several cobra venoms to produce broad neutralizing antivenoms for sub-Saharan Africa.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(RAR)
(RAR)
(XLSX)
Acknowledgments
The authors thank Jorge Gómez, Christhian Vargas, Orlando Morales, and other colleagues at Instituto Clodomiro Picado for their collaboration in the animal tests. This work was performed in partial fulfillment of the doctoral degree of Aarón Gómez at Universidad de Costa Rica.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a Wellcome Trust grant [Reference 220517/Z/20/Z] awarded to GL and JMG, and by Vicerrectoría de Investigación, Universidad de Costa Rica [projects 741-A0-804 and 741-C0-523] awarded to GL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All the authors received salary from Universidad de Costa Rica
References
- 1.Pyron RA, Burbrink FT, Colli GR, de Oca AN, Vitt LJ, Kuczynski CA, et al. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol Phylogenet Evol. 2011;58(2):329–42. doi: 10.1016/j.ympev.2010.11.006. Epub 2010 Nov 11. . [DOI] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization). Guidelines for the production, control, and regulation of snake antivenom immunoglobulins. WHO: Geneva; 2016. [Google Scholar]
- 3.Wüster W, Crookes S, Ineich I, Mané Y, Pook CE, Trape J-F, et al. The phylogeny of cobras inferred from mitochondrial DNA sequences: evolution of venom spitting and the phylogeography of the African spitting cobras (Serpentes: Elapidae: Naja nigricollis complex). Mol. Phylogen. Evol. 2007;437–453. doi: 10.1016/j.ympev.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 4.Malih I, Ahmad Rusmili MR, Tee TY, Saile R, Ghalim N, Othman I. Proteomic analysis of moroccan cobra Naja haje legionis venom using tandem mass spectrometry. J. Proteome. 2014;96:240–252, 10.1016/j.jprot.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Lauridsen LP, Laustsen AH, Lomonte B, Gutiérrez JM. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteomics. 2017;150:98–108, doi: 10.1016/j.jprot.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 6.Silva-de-Franca F, Villas-Boas IM, Serrano SMdT, Cogliati B, Chudzinski SAdA, Lopes PH, et al. Naja annulifera Snake: New insights into the venom components and pathogenesis of envenomation. PLoS Negl Trop Dis. 2019;13(1):e0007017. 10.1371/journal.pntd.0007017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hus KK, Buczkowicz J, Petrilla V, Petrillová M, Lyskowski A, Legáth J, et al. First Look at the Venom of Naja ashei. Molecules. 2018;23:609, doi: 10.3390/molecules23030609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modahl C, Roointan A, Rogers J, Currier K Mackessy SP. Interspecific and intraspecific venom enzymatic variation among cobras (Naja sp. And Ophiophagus Hannah). Comparative Biochemistry and Physiology, Part C. 2020;232:108743, 10.1016/j.cbpc.2020.108743 [DOI] [PubMed] [Google Scholar]
- 9.Tan KY, Wong KY, Tan NH, Tan CH. Quantitative Proteomics of Naja annulifera (sub-Saharan Snouted Cobra) Venom and Neutralization Activities of two Antivenoms in Africa, Elsevier B.V: 2020, 10.1016/j. ijbiomac.2020.04.173. [DOI] [PubMed] [Google Scholar]
- 10.Adamude FA, Dingwoke EJ, Abudakar MS, Ibrahim S, Mohamed G, Klein A, et al. Proteomic analysis of three medically important Nigerian Naja (Naja haje, Naja katiensis and Naja nigricollis) snake venoms. Toxicon 2021;197:24–32, 10.1016/j.toxicon.2021.03.014 [DOI] [PubMed] [Google Scholar]
- 11.Sánchez A, Segura Á, Pla D, Munuera J, Villalta M, Quesada-Bernat S, et al. Comparative venomics and preclinical efficacy evaluation of a monospecific Hemachatus antivenom towards sub-Saharan Africa cobra venoms. Journal of Proteomics. 2021;240:104196, 10.1016/j.jprot.2021.104196 [DOI] [PubMed] [Google Scholar]
- 12.Warrell DA, Ormerod LD. Snake venom ophthalmia and blindness caused by the spitting cobra (Naja nigricollis) in Nigeria. Am J Trop Med Hyg. 1976;25(3):525–9, doi: 10.4269/ajtmh.1976.25.525 . [DOI] [PubMed] [Google Scholar]
- 13.Chu ER, Weinstein SA, White J, Warrell DA. Venom ophthalmia caused by venoms of spitting elapid and other snakes: report of ten cases with review of epidemiology, clinical features, pathophysiology, and management, Toxicon. 2010;56:259–272, 10.1016/j.toxicon.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Iddon D, Theakston RDG, Ownby CL. A study of the pathogenesis of local skin necrosis induced by Naja nigricollis (spitting cobra) venom using simple histological staining techniques, Toxicon 1987;25:665–672, 10.1016/0041-0101(87)90113-9. [DOI] [PubMed] [Google Scholar]
- 15.WHO (World Health Organization). Guidelines for the prevention and clinical management of snakebite in Africa. WHO: Brazzaville; 2010.
- 16.Habib AG, Gebi UI, Onyemelukwe GC. Snake bite in Nigeria. Afr. J. Med. Med. Sci. 2001;30:171–178. [PubMed] [Google Scholar]
- 17.Warrell DA. Snake bite. Lancet. 2010;375(9708):77–88, doi: 10.1016/S0140-6736(09)61754-2. Erratum in: Lancet. 2010;375(9715):640. . [DOI] [PubMed] [Google Scholar]
- 18.León G, Sánchez L, Hernández A, Villalta M, Herrera M, Segura A, et al. Immune response towards snake venoms. Inflamm Allergy Drug Targets. 2011;10:381–98, https://doi.org.10.2174/187152811797200605 . [DOI] [PubMed] [Google Scholar]
- 19.Casasola A, Ramos-Cerillo B, de Roodt AR, Saucedo AC, Chippaux JP, Alagón A, et al. Paraspecific neutralization of the venom of African species of cobra by an equine antiserum against Naja melanoleuca: A comparative study. Toxicon 2009;53:602–608, 10.1016/j.toxicon.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Wong KY, Tan KY, Tan NH, Tan CH. A Neurotoxic Snake Venom without Phospholipase A2: Proteomics and Cross-Neutralization of the Venom from Senegalese Cobra, Naja senegalensis (Subgenus: Uraeus). Toxins (Basel). 2021;13(1):60, doi: 10.3390/toxins13010060 ; PMCID: PMC7828783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan CH, Wong KY, Huang LK, Tan KY, Tan NH, Wu WG. Snake Venomics and Antivenomics of Cape Cobra (Naja nivea) from South Africa: Insights into Venom Toxicity and Cross-Neutralization Activity. Toxins (Basel). 2022;14(12):860, doi: 10.3390/toxins14120860 ; PMCID: PMC9783313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bankowski Z& N. Howard-Jones. CIOMS (Council of International Organizations of Medical Sciences). The International Guiding Principles for Biomedical Research Involving Animals. Geneva. 1986.
- 23.Lomonte B, Calvete JJ. Strategies in ’snake venomics’ aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J Venom Anim Toxins Incl Trop Dis. 2017;28:23–6, https://doi.org.10.1186/s40409-017-0117-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera C, Bolton F, Arias AS, Harrison RA, Gutiérrez JM. Analgesic effect of morphine and tramadol in standard toxicity assays in mice injected with venom of the snake Bothrops asper. Toxicon. 2018;154:35–41, doi: 10.1016/j.toxicon.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 25.Durán G, Solano G, Gómez A, Cordero D, Sánchez A, Villalta M, et al. Assessing a 6-h endpoint observation time in the lethality neutralization assay used to evaluate the preclinical efficacy of snake antivenoms. Toxicon X. 2021;12:100087, doi: 10.1016/j.toxcx.2021.100087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finney DJ. Probit Analysis. Cambridge: Cambridge University Press. 1971. [Google Scholar]
- 27.Gutiérrez JM, Lomonte B, Chaves F, Moreno E, Cerdas L. Pharmacological activities of a toxic phospholipase and isolated from the venom of the snake Bothrops asper, Comp. Biochem. Physiol. Part C, Comp. 1986;84:159–164, 10.1016/0742-8413. [DOI] [PubMed] [Google Scholar]
- 28.Dole VP. A relation between non-esterified fatty acids in plasma and the metabolism of glucose, J. Clin. Invest. 1956;35:150–154, 10.1172/JCI103259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivel M, Solano D, Herrera M, Vargas M, Villalta M, Segura Á, et al. Pathogenesis of dermonecrosis induced by venom of the spitting cobra, Naja nigricollis: an experimental study in mice, Toxicon. 2016;119:171–179, 10.1016/j.toxicon.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Talke H, Schubert GE. Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin Wochenschr. 1965;43:174–5, https://doi.org.10.1007/BF01484513 . [DOI] [PubMed] [Google Scholar]
- 31.Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffé creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000;46(1–2):53–5. . [PubMed] [Google Scholar]
- 32.Rodkey FL. Direct spectrophotometric determination of albumin in human serum. Clin Chem. 1965;11:478–87. . [PubMed] [Google Scholar]
- 33.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the Biüret reaction. J. Biol. Chem. 1949;177:751–66. . [PubMed] [Google Scholar]
- 34.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685, https://doi.org.10.1038/227680a0 . [DOI] [PubMed] [Google Scholar]
- 35.Segura Á, Villalta M, Herrera M, León G, Harrison R, Durfa N, et al. Preclinical assessment of the efficacy of a new antivenom (EchiTAb-Plus-ICP) for the treatment of viper envenoming in sub-Saharan Africa. Toxicon. 2010;55(2–3):369–74, https://doi.org.10.1016/j.toxicon.2009.08.010 . [DOI] [PubMed] [Google Scholar]
- 36.Petras D, Sanz L, Segura Á, Herrera M, Villalta M, Solano D, et al. Snake Venomics of African Spitting Cobras: Toxin Composition and Assessment of Congeneric Cross-Reactivity of the Pan-African EchiTAb-Plus-ICP Antivenom by Antivenomics and Neutralization Approaches. J. Proteome Res. 2011;10:1266–1280, doi: 10.1021/pr101040f [DOI] [PubMed] [Google Scholar]
- 37.Katali O, Shipingana L, Nyarangó P, Pääkkönen M, Haindongo E, Rennie T, et al. Protein Identification of Venoms of the African Spitting Cobras, Naja mossambica and Naja nigricincta nigricincta. Toxins (Basel). 2020;12(8):520, doi: 10.3390/toxins12080520 ; PMCID: PMC7472217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conlon JM, Attoub S, Musale V, Leprince J, Casewell NR, Sanz L, et al. Isolation and characterization of cytotoxic and insulin-releasing components from the venom of the black-necked spitting cobra Naja nigricollis (Elapidae). Toxicon X 2020;6 100030, 10.1016/j.toxcx.2020.100030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chwetzoff S, Tsunasawa S, Sakiyama F, Ménez A. Nigexine, a phospholipase A2 from cobra venom with cytotoxic properties not related to esterase activity. Purification, amino acid sequence, and biological properties. J Biol Chem. 1989;264(22):13289–97, . [PubMed] [Google Scholar]
- 40.Panagides N, Jackson TNW, Ikonomopoulou MP, Arbuckle K, Pretzler R, Yang DC, et al. How the cobra got its flesh-eating venom: cytotoxicity as a defensive innovation and its co-evolution with hooding, aposematic marking, and spitting. Toxins. 2017;9:103, doi: 10.3390/toxins9030103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hewitt CD, Innes DJ, Savory J, Wills MR. Normal biochemical and hematological values in New Zealand white rabbits. Clin Chem. 1989;35(8):1777–9. [PubMed] [Google Scholar]
- 42.Melillo A. Rabbit Clinical Pathology. Topics in Medicine and Surgery; Journal of Exotic Pet Medicine. 2007;16(3):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warrell DA, Greenwood BM, Davidson NM, Ormerod LD, Prentice CR. Necrosis, haemorrhage and complement depletion following bites by the spitting cobra (Naja nigricollis). Q J Med. 1976. Jan;45(177):1–22. [PubMed] [Google Scholar]
- 44.Gómez A, Sánchez A, Durán G, Cordero D, Segura Á, Vargas M, et al. Intrageneric cross-reactivity of monospecific rabbit antisera against venoms of the medically most important Bitis spp. and Echis spp. African snakes. PLoS Negl Trop Dis. 2022;16(8):e0010643, 10.1371/journal.pntd.0010643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laustsen AH, Lohse B, Lomonte B, Engmark M, Gutiérrez JM. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon. 2015;104:43–45, 10.1016/j.toxicon.2015.07.334 [DOI] [PubMed] [Google Scholar]
- 46.Sánchez A, Coto J, Segura Á, Vargas M, Solano G, Herrera M, et al. Effect of geographical variation of Echis ocellatus, Naja nigricollis and Bitis arietans venoms on their neutralization by homologous and heterologous antivenoms. Toxicon. 2015;108:80–83, 10.1016/j.toxicon.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 47.Calvete JJ. Venomics: integrative venom proteomics and beyond. Biochem. J. 2017;474:611–634, 10.1042/BCJ20160577 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
(RAR)
(RAR)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.