Abstract
Background
Dislocation is a major complication of revision THA after two-stage exchange for periprosthetic joint infection (PJI). The likelihood of dislocation can be particularly high if megaprosthetic proximal femoral replacement (PFR) has been performed during a second-stage reimplantation. Dual-mobility acetabular components are an established way of reducing the instability risk in revision THA; however, the likelihood of dislocation for dual-mobility reconstructions in the setting of a two-stage PFR has not been studied systematically, although patients with these reconstructions might be at an increased risk.
Questions/purposes
(1) What is the risk of dislocation and revision for dislocation in patients who underwent PFR with a dual-mobility acetabular component as part of two-stage exchange for hip PJI? (2) What is the risk of all-cause implant revision and what other procedures were performed (apart from revision for a dislocation) in these patients? (3) What potential patient-related and procedure-related factors are associated with dislocation?
Methods
This was a retrospective study from a single academic center including procedures performed between 2010 and 2017. During the study period, 220 patients underwent two-stage revision for chronic hip PJI. Two-stage revision was the approach of choice for chronic infections, and we did not perform single-stage revisions for this indication during the study period. Thirty-three percent (73 of 220) of patients underwent second-stage reconstruction with a single-design, modular, megaprosthetic PFR because of femoral bone loss, using a cemented stem. A cemented dual-mobility cup was the approach of choice for acetabular reconstruction in the presence of a PFR; however, 4% (three of 73) were reconstructed with a bipolar hemiarthroplasty to salvage an infected saddle prosthesis, leaving 70 patients with a dual-mobility acetabular component and a PFR (84% [59 of 70]) or total femoral replacement (16% [11 of 70]). We used two similar designs of an unconstrained cemented dual-mobility cup during the study period. The median (interquartile range) patient age was 73 years (63 to 79 years), and 60% (42 of 70) of patients were women. The mean follow-up period was 50 ± 25 months with a minimum follow-up of 24 months for patients who did not undergo revision surgery or died (during the study period, 10% [seven of 70] died before 2 years). We recorded patient-related and surgery-related details from the electronic patient records and investigated all revision procedures performed until December 2021. Patients who underwent closed reduction for dislocation were included. Radiographic measurements of cup positioning were performed using supine AP radiographs obtained within the first 2 weeks after surgery using an established digital method. We calculated the risk for revision and dislocation using a competing-risk analysis with death as a competing event, providing 95% confidence intervals. Differences in dislocation and revision risks were assessed with Fine and Gray models providing subhazard ratios. All p values were two sided and the p value for significance was set at 0.05.
Results
The risk of dislocation (using a competing-risks survivorship estimator) was 17% (95% CI 9% to 32%) at 5 years, and the risk of revision for dislocation was 12% (95% CI 5% to 24%) at 5 years among patients treated with dual-mobility acetabular components as part of a two-stage revision for PJI of the hip. The risk of all-cause implant revision (using a competing-risk estimator, except for dislocation) was 20% (95% CI 12% to 33%) after 5 years. Twenty-three percent (16 of 70) of patients underwent revision surgery for reinfection and 3% (two of 70) of patients underwent stem exchange for a traumatic periprosthetic fracture. No patients underwent revision for aseptic loosening. We found no differences in patient-related and procedure-related factors or acetabular component positioning for patients with dislocation with the numbers available; however, patients with total femoral replacements had a higher likelihood of dislocation (subhazard ratio 3.9 [95% CI 1.1 to 13.3]; p = 0.03) and revision for a dislocation (subhazard ratio 4.4 [95% CI 1 to 18.5]; p = 0.04) than those who received PFR.
Conclusion
Although dual-mobility bearings might be an intuitive potential choice to reduce the dislocation risk in revision THA, there is a considerable dislocation risk for PFR after two-stage surgery for PJI, particularly in patients with total femoral replacements. Although the use of an additional constraint might appear tempting, published results vary tremendously, and future studies should compare the performance of tripolar constrained implants to that of unconstrained dual-mobility cups in patients with PFR to reduce the risk of instability.
Level of Evidence
Level III, therapeutic study.
Introduction
Periprosthetic joint infection (PJI) is a rare but dreaded complication after total arthroplasty that occurs in approximately 1% to 2% of patients [15]. However, the PJI rate can be much greater in revision arthroplasty, reaching as high as 50% in complicated, multiply revised patients with a history of infection [16]. The increase in the number of patients undergoing primary and revision arthroplasty suggests that the number of associated revision procedures will rise [14]. A common surgical approach for chronic hip PJI is a two-stage exchange arthroplasty with first-stage resection arthroplasty and removal of all foreign materials, debridement of the surrounding soft tissue, and vigorous lavage. Consequently, an intermediate cement spacer construct is placed, and systemic antibiotics are administered. After antibiotic treatment, second-stage reimplantation with a revision implant is performed; infection can be controlled in approximately 80% to 90% of patients [22].
A frequent issue during staged hip reconstruction for PJI is bone loss because of the need for an aggressive debridement during the first stage of the surgery and the need to remove femoral stems, which can lead to additional bone loss, particularly for revision stems [3, 4, 7]. In patients with severe bone loss of the proximal femur, megaprosthetic proximal femoral replacement (PFR) using modular off-the-shelf implants is a viable choice to restore bone continuity [3, 8, 13]. However, one of the main complications of PFR is dislocation caused by the loss of the abductor muscle insertion [26]. Furthermore, patients who have undergone a two-stage exchange for infection have already undergone debridement and resection of the hip’s periarticular tissue envelope, and they are considered at increased risk for dislocation after second-stage revision THA. A recent study [19] found that 10% of 512 patients with a two-stage hip exchange for PJI experienced dislocations and concluded that dislocation remains a major concern in two-stage revision THA.
Therefore, it would be reasonable to assume that patients who undergo two-stage revision THA for PJI using a PFR have a particularly high likelihood of dislocation.
One potential approach to diminish the dislocation risk in revision THA is to use dual-mobility acetabular components, which have been proven to be successful in preserving hip stability, even in high-risk cases [9]. One study [25] found that in 216 patients who underwent revision THAs and were considered particularly prone to instability because of severe bone or soft tissue deficiencies and underwent reconstruction with a cemented dual-mobility cup, 11% still experienced dislocations. However, there were few details about the type of femoral reconstruction in that study. Therefore, although a higher risk can be assumed, it is unclear what likelihood of dislocation can be expected in a presumed high-risk situation of a two-stage reconstruction for PJI using a PFR [1], and to our knowledge, there are no studies that systematically assess this issue in revision THA.
We therefore asked: (1) What is the risk of dislocation and revision for dislocation in patients who underwent PFR with a dual-mobility acetabular component as part of two-stage exchange for hip PJI? (2) What is the risk of all-cause implant revision and what other procedures were performed (apart from revision for a dislocation) in these patients? (3) What potential patient-related and procedure-related factors are associated with dislocation?
Patients and Methods
Study Design and Setting
In this retrospective, comparative study, we investigated all patients who underwent a two-stage exchange for chronic hip PJI at one academic general orthopaedic referral center for revision arthroplasty between 2010 and 2017.
Patients
We identified 220 patients with a two-stage revision. Two-stage revision was the approach of choice to manage all chronic infections during the study period, and we did not perform any single-stage exchange procedures for chronic PJI. We identified 220 patients in this query, 33% of whom (73 of 220) underwent second-stage reimplantation using a PFR or total femoral replacement (TFR) with a modular megaprosthesis (MUTARS, Implantcast). Megaprosthetic PFR was performed in all patients with full bone loss of the greater and lesser trochanter, and all femoral stems were cemented. Three patients underwent bipolar hemiarthroplasty as a salvage of a saddle prosthesis and were excluded, leaving 70 patients with a dual-mobility acetabular bearing and a PFR (84% [59 of 70]) or TFR (16% [11 of 70]) for further analysis (Fig. 1).
Fig. 1.
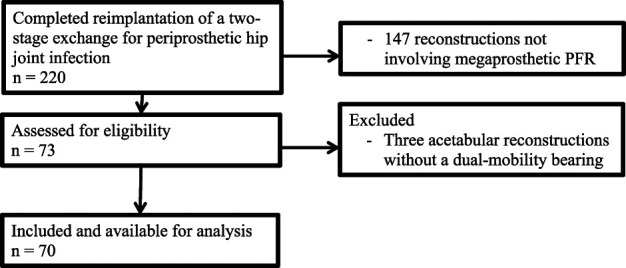
This flow diagram highlights the inclusion and exclusion criteria for this study.
All patients had a minimum follow-up of 24 months with a mean follow-up period of 50 ± 25 months. Patients who reached one of the study endpoints (dislocation or revision surgery) before 2 years and those who died before 2 years without reaching an endpoint were included. Twenty-seven percent (19 of 70) of patients died of unrelated cause after a mean time of 37 ± 28 months after second-stage surgery.
Descriptive Data
Sixty percent (42 of 70) of patients were women and the mean age at revision surgery was 73 ± 6 years. These patients had undergone a mean of 5 ± 2 previous revision THA procedures and 2 ± 0.8 previous operations for PJI (Table 1).
Table 1.
Patient demographics
| Variable | Value |
| Age in years | 73 ± 6 |
| CCI | 4 ± 1.5 |
| ASA | 3 ± 1 |
| BMI in kg/m2 | 26 ± 4 |
| Number of previous revision hip arthroplasties | 5 ± 2 |
| Number of previous operations for infection | 2 ± 0.8 |
| Length of the femoral component in mm | 200 ± 86 |
Data are presented as the mean ± SD. CCI = Charlson comorbidity index; ASA = American Society of Anesthesiologists score.
Treatment Approaches and Diagnosis of Infection
Assuming an increased dislocation risk, the general approach for acetabular reconstruction in patients undergoing PFR or TFR was to use one of two cemented, nonconstrained, dual-mobility cups with a similar design (Avantage, Biomet, until 2012 [n = 23] and Ecofit 2M, Implantcast, from 2013 onward [n = 47]) and, if needed, porous metal wedges (10% [seven of 70]) or cage-based reconstructions (34% [24 of 70]) for more extensive defects. Both designs feature an asymmetric design with 10° asymmetry for increased head coverage. The median (interquartile range) diameter of the acetabular component was 53 mm (IQR 50 to 56 mm), the median diameter of the dual-mobility head was 46 mm (IQR 44 to 50 mm), and the median femoral head size was 28 mm (IQR 28 to 32 mm). A synthetic mesh was not used in any patient. The MUTARS features a 35-mm offset in the femoral components that can be varied with different neck lengths using different heads with 3.5-mm intervals. The median neck length used was 3.5 mm (IQR 0 to 3.5 mm). We used 0-mm heads in 24% of patients (17 of 70).
Beginning in 2011, the diagnosis of PJI was based on the criteria of the Musculoskeletal Infection Society [21], and before 2011, the diagnosis was based on the Centers for Disease Control criteria using clinical, laboratory, and microbiological findings [12]. An infection was considered chronic if the last surgery was more than 6 weeks prior, if patients had symptoms for that period, or if a fistula was present. During the first-stage surgery, all infected tissue was debrided or resected, a minimum of three tissue samples were taken for microbiology culture, and an antibiotic-loaded handmade articulating spacer was inserted. These were manufactured using 6-mm titanium rods that were bent to re-create leg length and femoral offset. During the study period, polymethyl methacrylate cement (Copal, Heraeus Medical) was used, and antibiotics were added depending on antibiotic resistance. For sensitive organisms, 1 g of clindamycin and 1 g of gentamicin was added, whereas for gram-positive resistant organisms, 2 g of vancomycin per 40 g of cement was added. For gram-negative infections, 2 to 4 g of meropenem was added per 40 g of cement. All patients underwent a minimum of 6 weeks of combined systemic intravenous and oral antibiotics.
Second-stage reimplantation was planned if all wounds had healed and serum infection parameters had reduced. Systemic antibiotic therapy was continued for 2 weeks after reimplantation if the wound healed without problems and long-term culture results were negative. Long-term suppression therapy was not used during the study period, and eradication of infection was defined by the Delphi consensus criteria requiring healed wounds, no further surgeries for infection, and the absence of PJI-related mortality [6].
Postoperatively, patients were given hip precautions for 6 weeks, with no flexion greater than 90° and no adduction more than 0°. Dislocation was managed with closed reduction under general anesthesia and postoperative abduction splinting or a hip orthosis for 12 weeks. If closed reduction was not successful, open reduction was performed and the position of the modular component was evaluated. If soft tissues had elongated, a longer head or modular component was implanted to achieve greater soft tissue tension and stability. If necessary, the modular component rotation was modified.
We obtained data from patients’ electronic medical records, including demographics, surgical details, and microbiology (Table 2). Furthermore, cup positioning was determined on AP supine pelvic radiographs that were obtained during the hospital stay. Cup version and inclination were measured using Trauma CAD digital planning software (Brainlab) [17, 20]. The median cup inclination was 38° (IQR 34° to 42°), and the median anteversion was 17° (IQR 4° to 23°).
Table 2.
Microbiological findings at first-stage explantation
| Culture microbiology | Percentage (n) of patients (n = 70) |
| CoNS | 26 (18) |
| Staphylococcus aureus | 11 (8) |
| Culture-negative | 17 (12) |
| Streptococci | 1 (1) |
| Gram-negative | 14 (10) |
| Polymicrobial | 33 (23) |
| Other | 7 (5) |
Data are presented as % (n). CoNS = coagulase-negative Staphylococcus.
Primary and Secondary Study Outcomes
Our primary study goal was to assess the percentage of patients who were treated for dislocation and the risk of revision surgery for that reason. To address this question, we accessed patient records and operating room records to identify those who underwent closed reduction or had revision surgery for a dislocation.
Our secondary study goals were to determine the overall risk of implant revisions for reasons other than a dislocation as well as to identify factors that are potentially associated with a greater risk of dislocation or revision. For this purpose, patient records and operating room records were investigated, potentially associated patient-related and procedure-related factors were extracted, and an increase or decrease in risk was calculated with the statistical methods described below.
Ethical Approval
Ethical approval for this study was obtained from the Ethikkommision der Ärztekammer Westfalen-Lippe und der Westfaelischen-Wilhelms Universitaet Münster (reference number 2019-650-F-s). The study was performed in accordance with the ethical standard of the 1964 Declaration of Helsinki.
Statistical Analysis
Frequencies were analyzed and are given as the absolute percentage and number. All numerical variables were tested for normality using the Kolmogorov-Smirnov test. Considering normality of the tested variables, the mean and standard deviation are presented, and comparative testing was done using a t-test. For nonparametric distributions, we present the median with a 25% to 75% IQR, which we compared using the Mann-Whitney U test. Categorical variables were compared using crosstables and the chi-square test. The risk of dislocation, revision for dislocation, and all-cause implant revision were evaluated using a competing-risk approach with death as a competing event. This was done using the command stcrprep in Stata (Stata Corp). Fine and Gray models with death as a competing event were used to calculate differences in the dislocation and revision risk between TFR and PFR, along with subhazard ratios. All values are given with corresponding 95% confidence intervals. All p values were two-sided, and the p value for significance was set at 0.05.
Results
Dislocation Risk and Risk of Revision for Dislocation
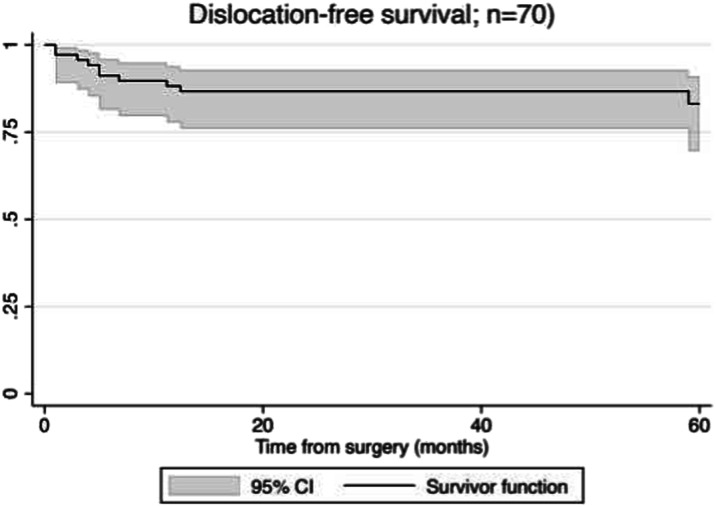
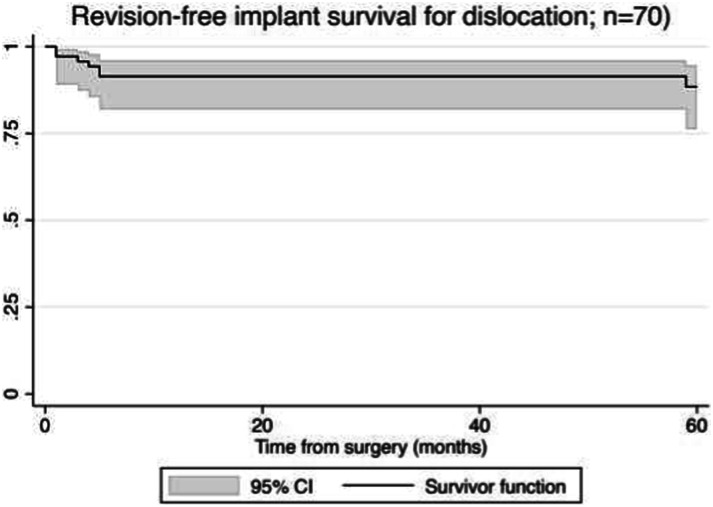
The risk of dislocation (using a competing-risks survivorship estimator) was 17% (95% CI 9% to 32%) at 5 years (Fig. 2), and the risk of revision for dislocation (again, using a competing-risks survivorship estimator) was 12% (95% CI 5% to 24%) at 5 years (Fig. 3) among patients treated with dual-mobility acetabular components as part of a two-stage revision for PJI of the hip. All patients who underwent revision underwent exchange of the femoral head and dual-mobility inlay as well as the modular proximal femur component. One patient underwent cup revision for a late dislocation 73 months after second-stage reimplantation. There were no intraprosthetic dislocations of the femoral head and dual-mobility inlay.
Fig. 2.
This competing-risk survival curve shows dislocation-free survival.
Fig. 3.
This competing-risk survival curve shows revision-free survival for implant dislocation.
Risk of All-cause Implant Revision and Revision Procedures
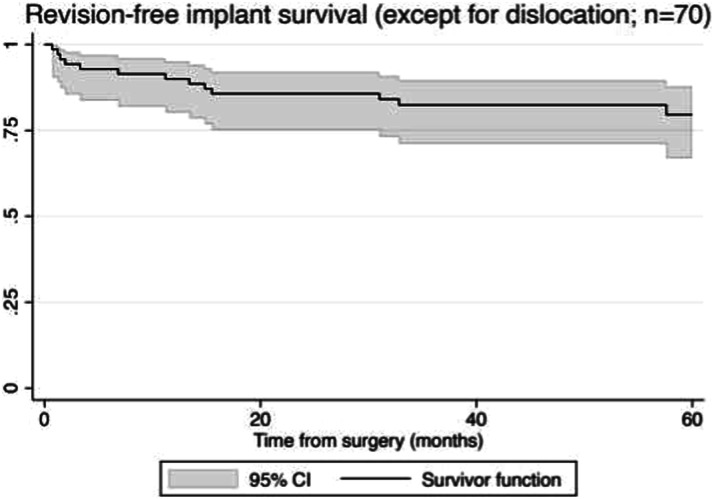
The risk of all-cause implant revision (using a competing-risk estimator, except for dislocation) was 20% (95% CI 12% to 33%) after 5 years (Fig. 4). A total of 23% (16 of 70) of patients underwent revision surgery for reinfection, and 3% (two of 70) of patients underwent stem exchange for a traumatic periprosthetic fracture. No patients underwent revision for aseptic loosening. In three patients with infection, implant salvage was attempted with single-stage debridement, component exchange, and irrigation followed by antibiotics; however, this was unsuccessful in all of these patients. All patients who experienced a reinfection ultimately underwent repeat implant removal.
Fig. 4.
This competing-risk survival curve shows revision-free implant survival, excluding dislocations.
Potential Patient-related and Procedure-related Factors Associated With Dislocation
Patients with TFR had a higher risk of dislocation than those with PFR (subhazard ratio 3.9 [95% CI 1.1 to 13.3]; p = 0.03). Likewise, the risk of revision for dislocation was higher in patients with a TFR than in patients with a PFR (subhazard ratio 4.4 [95% CI 1 to 18.5]; p = 0.04). With the numbers available, there was no difference in inclination or anteversion between patients with dislocation and those without (40° versus 37°; p = 0.52 and 15° versus 18°; p = 0.31). Furthermore, there was no difference in the median size of acetabular components (53 versus 52 mm; p = 0.61), dual-mobility head (46 versus 45 mm; p = 0.25) or femoral head size (28 versus 28 mm; p = 0.39), or head length (0 versus 3.5 mm; p = 0.19). There was no difference in the mean BMI (26 kg/m2 versus 28 kg/m2; p = 0.2), mean age (72 versus 73 years; p = 0.55), mean comorbidity score (4 versus 4; p = 0.75), mean proximal femur reconstruction length (160 versus 170 mm; p = 0.56), or mean number of previous revision arthroplasties (3 versus 3; p = 0.69) between patients with dislocation and those without.
Discussion
Dual-mobility acetabular components are an option to reduce the dislocation risk in revision THA. Given that the likelihood of dislocation is higher in patients who undergo megaprosthetic PFR and those who undergo second-stage reimplantation surgery as part of a two-stage exchange for PJI, it appears intuitive for surgeons to consider the use of a dual-mobility bearing in such cases. However, in this study, we found a dislocation risk of 17% after 5 years in these patients; the 5-year probability of revision for dislocation was 12%. The dislocation risk and risk of revision for a dislocation were alarmingly high in patients who underwent TFR compared with those who underwent PFR in this study (subhazard ratio 3.9 and 4.4, respectively). These findings can help surgeons in the decision-making process when such complex reconstructions are being considered and to adequately counsel affected patients. Although the use of additional constraint (liners or cups) might appear tempting, published results vary tremendously [5, 25] and it is unclear whether constrained liners or cups will reduce the risk of instability in patients with a PFR or TFR after two-stage exchange.
Limitations
First, this was a retrospective analysis that relied on follow-up data; patients might have undergone revision surgery elsewhere, unknown to us. We tried to mitigate this effect by defining a minimum follow-up period. However, no patients who did not reach one of the study endpoints were lost before 2 years. Additionally, because these complex procedures are usually performed in specialized institutions in our country, it is likely that many patients are referred to the center that performed that surgery when there are complications. Nonetheless, the reported revision risk should be considered a low-end estimate. Although the calculated CIs are somewhat broad at 5 years considering the limited number of patients and events, the upper boundary of the 95% CI for dislocation risk was greater than 30% and the risk of revision was almost 25%.
Second, although we could identify TFRs as a potential risk factor for dislocation, other potential risk factors might not have been detected in the analysis because of the limited number of patients available and the limited number of patients presenting with a certain risk factor. These sparse data bias should caution readers when assessing potential influencing factors for revision surgery. Larger studies are needed to account for other relevant factors. Third, although patients were followed for more than 4 years, there are no routine functional scores available retrospectively for this cohort. Although it appears desirable to determine whether functional outcome potentially differs in patients who had dislocations, the numbers available are still small, and this aspect must be addressed in future studies. Finally, although this study used similar designs of unconstrained dual-mobility cups that were commonly used in our region at the time of surgery, our results cannot be generalized to different implant types or components with an additional constraint.
Dislocation Risk and Risk of Revision for Dislocation
The risk of dislocation and revision for dislocation was high in this study, as would to some extent be expected in patients with infection and severe bone loss. We were nonetheless surprised by how high the risk was, despite the routine use of dual-mobility cups that were expected to mitigate that risk. The dislocation risk in PFR has been investigated in previous studies. One study investigated 57 patients who underwent PFR for infection as part of a single-stage exchange; those patients had an 8.8% dislocation risk compared with a matched group without PFR who had a 10.5% dislocation risk [2]. However, 95% of patients in the group with PFR had a constrained liner or a cemented dual-mobility cup compared with only 37% in the control group. Additionally, that study [2] did not further discuss the dislocation risk in the group with a dual-mobility cup. In addition, the type of femoral reconstruction varied because not all proximal femoral defects were reconstructed with a megaprosthetic implant. Therefore, although it is difficult to compare the results from the present study, nonetheless, their results [2] should be appreciated as support for an increased use of constraint or dual-mobility cups in the setting of revision THA for infection to avoid instability. Another recent study investigated 46 patients with PFR, 19 of whom underwent reconstruction for second-stage reimplantation in a two-stage exchange procedure for PJI [23]. Those authors found no dislocations when dual-mobility cups were used. However, this was only the case for two patients who underwent PFR after PJI and six patients in the entire cohort. Considering the relatively small number of patients with reconstruction for PJI in their study, it is unclear whether the differences in dislocation risk might be biased, and surgeons should be aware that even with a dual-mobility articulation, the dislocation risk might be high in patients with PFR after surgery for PJI.
Based on our findings, the use of constrained liners or tripolar constrained may be considered when performing revision THA deemed to be at high risk of dislocation. These implants were used in our department in the past but were abandoned because of the perception that the revision frequency and risk of recurrent instability was high, although this has not been reviewed systematically [7]. Although this perceived risk of complications does not replace a comparative study design that cannot be provided with the setting of our study, there are other studies that investigated constrained acetabular components in revision THA. One study that included 49 patients with recurrent dislocations (including 25 patients with PJI and some with previous dual-mobility reconstructions) who were perceived to be at high risk and underwent revision THA using a constrained liner found a risk of revision of 41%; 31% of the study population experienced recurrent instability [25]. Although the authors noted a high rate of mechanical complications associated with the bipolar component they used, they found no dislocations in the subgroup treated for PJI. Similarly, a recent study on 52 patients treated with a constrained tripolar liner for revision THA including 13 patients with PJI found that 16% of the constrained liners underwent revision, mostly for recurrent dislocation [5]. Although both author groups mentioned the complexity of the patients they included and cautioned against attributing the revision risk to the implant alone, it remains questionable whether a constrained liner would lead to a greatly reduced risk of instability or revision in patients with proximal femoral bone loss and compromised soft tissues, as we saw in the patients in our study. However, considering that the cited studies only used descriptive statistics and the included patients had heterogeneous indications, we await prospective studies with an adequate statistical design before making stricter recommendations about what kinds of articulations to use in this setting.
Risk of All-cause Implant Revision and Revision Procedures
Although the dislocation risk was high in our study, the predominant reason for revision was infection in 23% of patients. This is comparable to a study that investigated 40 megaprosthetic hip reconstructions after surgery for PJI and reported that 17% developed reinfection [3]. The slight difference between their study and ours may have been a function of antibiotic suppression, which those authors favored. Nonetheless, the reinfection risk of PFRs, particularly if performed after PJI, can be as high as 47%, as noted by another study [23]. These studies noted that surgical options to manage these challenging situations can be limited, and usually older patients, who often have many comorbid conditions, may either decline further interventions or be deemed unfit for further operations. Consequently, surgeons should strongly consider suppressive antibiotics or evaluate other ways to reduce the infection risk in these patients.
Potential Patient-related and Procedure-related Factors Associated With Dislocation
Although identifying potential dislocation risk factors is difficult and our study should be interpreted with caution considering the potential sources of bias that come with limited patient numbers, nearly half of the patients in this study who had a TFR experienced a dislocation. Although TFRs are infrequently performed, it is assumed that with expanding indications for megaprosthetic reconstructions, the use of TFR might increase as well [10]. Most publications on TFR are limited to small case series; however, in a multi-institutional study [10] that included 166 patients treated over a 34-year period, mainly for oncologic indications, the authors noted that 33% of patients among 61 TFRs performed as a revision underwent early rerevision, with infection as the most common reason for revision. Contrary to the present study’s findings, instability was not the cause of any of the revision TFRs. We can only speculate on the reasons for this, because it would be intuitive to assume that TFR complications would partly mirror the ones encountered in PFR, but it is possible that there are some methodologic differences. Considering the multicenter approach of the aforementioned study [10], it is unclear whether they investigated the type of acetabular reconstruction and whether they included closed reduction without additional surgery as an event. The absence of instability might therefore be explained by the potentially high percentage of hemiarthroplasty reconstructions with the addition of synthetic mesh soft tissue reconstructions reported by the same group for oncologic PFR [11], which is a favored approach to reduce instability in oncologic PFR and TFR [18, 24]. Considering the heterogeneity among patients treated with this approach and the limited number of studies available, it is unclear whether dual-mobility bearings are sufficient to mitigate the dislocation risk in patients with a TFR. Surgeons should be aware of the potentially high dislocation risk in revision THA with a TFR and should explore possible ways to mitigate this risk.
Conclusion
Although dual-mobility bearings might be a sensible choice to reduce the dislocation risk in revision THA, there is a considerable dislocation risk for PFR after two-stage surgery for PJI. Additionally, revision procedures for recurrent infection must be expected. Surgeons should explore additional means of increasing hip stability, particularly in patients with TFRs, in whom we found the risk of dislocation to be especially severe. Although the use of an additional constraint might appear tempting, published results vary tremendously, and future studies should investigate the performance of (tripolar) constrained implants compared with that of unconstrained dual-mobility cups in patients with PFR to potentially reduce the risk of instability.
Footnotes
One or more of the authors (CT, JS, BM) certifies receipt of personal payments or benefits, during the study period, in an amount of less than USD 10,000 from Implantcast GmbH, unrelated to this work. One of the authors (GG) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Implantcast GmbH; this author also holds a patent for a silver coating for metallic endoprosthesis.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the Ethikkommision der Ärztekammer Westfalen-Lippe und der Westfaelischen-Wilhelms Universitaet Münster (reference number 2019-650-F-s).
The last two authors contributed equally to this manuscript.
Contributor Information
Jan Schwarze, Email: jan.schwarze@ukmuenster.de.
Maria Anna Smolle, Email: maria.smolle@medunigraz.at.
Jan Pützler, Email: jan.puetzler@ukmuenster.de.
Burkhard Moellenbeck, Email: burkhard.moellenbeck@ukmuenster.de.
Kristian Nikolaus Schneider, Email: kristian.schneider@ukmuenster.de.
Martin Schulze, Email: martin.schulze@ukmuenster.de.
Sebastian Klingebiel, Email: sebastian.klingebiel@ukmuenster.de.
Georg Gosheger, Email: gerog.gosheger@ukmuenster.de.
References
- 1.Abdel MP. CORR insights(R): what is the dislocation and revision rate of dual-mobility cups used in complex revision THAs? Clin Orthop Relat Res. 2021;479:286-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelaziz H, Schroder M, Shum Tien C, et al. Resection of the proximal femur during one-stage revision for infected hip arthroplasty: risk factors and effectiveness. Bone Joint J. 2021;103:1678-1685. [DOI] [PubMed] [Google Scholar]
- 3.Alvand A, Grammatopoulos G, de Vos F, et al. Clinical outcome of massive endoprostheses used for managing periprosthetic joint infections of the hip and knee. J Arthroplasty. 2018;33:829-834. [DOI] [PubMed] [Google Scholar]
- 4.Corona PS, Vicente M, Lalanza M, Amat C, Carrera L. Use of modular megaprosthesis in managing chronic end-stage periprosthetic hip and knee infections: is there an increase in relapse rate? Eur J Orthop Surg Traumatol. 2018;28:627-636. [DOI] [PubMed] [Google Scholar]
- 5.Derksen A, Kluge M, Wirries N, et al. Constrained tripolar liner in patients with high risk of dislocation - analysis of incidence and risk of failure. J Orthop. 2021;25:288-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471:2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieckmann R, Schmidt-Braekling T, Gosheger G, Theil C, Hardes J, Moellenbeck B. Two stage revision with a proximal femur replacement. BMC Musculoskelet Disord. 2019;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grammatopoulos G, Alvand A, Martin H, Whitwell D, Taylor A, Gibbons CLMH. Five-year outcome of proximal femoral endoprosthetic arthroplasty for non-tumour indications. Bone Joint J. 2016;98:1463-1470. [DOI] [PubMed] [Google Scholar]
- 9.Hartzler MA, Abdel MP, Sculco PK, Taunton MJ, Pagnano MW. Otto Aufranc Award: dual-mobility constructs in revision THA reduced dislocation, rerevision, and reoperation compared with large femoral heads. Clin Orthop Relat Res. 2018;476:293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson ER, Keeney BJ, Husson EG, et al. Nonmechanical revision indications portend certain limb-salvage failure following total femoral replacement. J Bone Joint Surg Am. 2020;102:1511-1520. [DOI] [PubMed] [Google Scholar]
- 11.Henderson ER, Keeney BJ, Pala E, et al. The stability of the hip after the use of a proximal femoral endoprosthesis for oncological indications: analysis of variables relating to the patient and the surgical technique. Bone Joint J. 2017;99:531-537. [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-332. [DOI] [PubMed] [Google Scholar]
- 13.Korim MT, Esler CN, Ashford RU. Systematic review of proximal femoral arthroplasty for non-neoplastic conditions. J Arthroplasty. 2014;29:2117-2121. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J Arthroplasty. 2018;33:3238-3245. [DOI] [PubMed] [Google Scholar]
- 16.Leitner L, Posch F, Amerstorfer F, Sadoghi P, Leithner A, Glehr M. The dark side of arthroplasty: competing risk analysis of failed hip and knee arthroplasty with periprosthetic joint infection. J Arthroplasty. 2020;35:2601-2606. [DOI] [PubMed] [Google Scholar]
- 17.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217-220. [PubMed] [Google Scholar]
- 18.Lex JR, Evans S, Parry MC, Jeys L, Stevenson JD. Acetabular complications are the most common cause for revision surgery following proximal femoral endoprosthetic replacement: what is the best bearing option in the primary and revision setting? Bone Joint J. 2021;103:1633-1640. [DOI] [PubMed] [Google Scholar]
- 19.McAlister IP, Perry KI, Mara KC, Hanssen AD, Berry DJ, Abdel MP. Two-stage revision of total hip arthroplasty for infection Is associated with a high rate of dislocation. J Bone Joint Surg Am. 2019;101:322-329. [DOI] [PubMed] [Google Scholar]
- 20.Nho JH, Lee YK, Kim HJ, Ha YCH, Suh YS, Koo KH. Reliability and validity of measuring version of the acetabular component. J Bone Joint Surg Br. 2012;94:32-36. [DOI] [PubMed] [Google Scholar]
- 21.Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petis SM, Abdel MP, Perry KI, Mabry TM, Hanssen AD, Berry DJ. Long-term results of a 2-stage exchange protocol for periprosthetic joint infection following total hip arthroplasty in 164 hips. J Bone Joint Surg Am. 2019;101:74-84. [DOI] [PubMed] [Google Scholar]
- 23.Strony J, Sukhonthamarn K, Tan TL, Parvizi J, Brown SA, Nazarian DG. Worse outcomes are associated with proximal femoral replacement following periprosthetic joint infection. J Arthroplasty. 2022;37:559-564. [DOI] [PubMed] [Google Scholar]
- 24.Theil C, Mollenbeck B, Gosheger G, et al. Acetabular erosion after bipolar hemiarthroplasty in proximal femoral replacement for malignant bone tumors. J Arthroplasty. 2019;34:2692-2697. [DOI] [PubMed] [Google Scholar]
- 25.Unter Ecker N, Kocaoglu H, Zahar A, Haasper C, Gehrke T, Citak M. What is the dislocation and revision rate of dual-mobility cups used in complex revision THAs? Clin Orthop Relat Res. 2021;479:280-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viste A, Perry KI, Taunton MJ, Hanssen AD, Abdel MP. Proximal femoral replacement in contemporary revision total hip arthroplasty for severe femoral bone loss: a review of outcomes. Bone Joint J. 2017;99:325-329. [DOI] [PubMed] [Google Scholar]