Abstract
Objective: Evaluation of sexual dimorphism is a crucial concern for anthropologists, forensic scientists, and archaeologists. Teeth have been used as an alternative tool to determine sex in forensic anthropology. However, it is necessary to use data specific to a particular population, as different populations exhibit varying degrees of sexual dimorphism. This study aimed to determine the sexual dimorphism in the buccolingual dimensions of permanent anterior teeth in the young Iranian population.
Materials and methods:A total of 100 students (50 females and 50 males) participated in the current study. A total of 1200 permanent anterior teeth were examined. The buccolingual dimension of all anterior teeth was measured using Vernier Calipers with a calibration of 0.01 mm. Data were analyzed using an independent sample T-test and paired sample T-test, and a P-value of less than 0.05 was considered statistically significant.
Results:The mean values of the buccolingual dimension of the maxillary canines on the right and left sides (13, 23) were statistically greater in males than females (P=0.04, P=0.03) and had the greatest percentage of sexual dimorphism (4.08% and 5.26%, respectively).
Conclusion:The buccolingual dimension of Iranian canines could be used as a reliable material to identify gender in forensic studies, and the degree of sexual dimorphism varies among different populations. Therefore, it is essential to use the relevant data samples for each population. It was concluded that Europeans had the greatest amount of sexual differences in the anterior teeth, while Iranians had the least amount of sexual dimorphism in the anterior teeth.
Keywords:buccolingual, dental measurement, Iranian population, sexual dimorphism.
INTRODUCTION
Sexual dimorphism in the human skeleton has been extensively studied in various populations (1-6) and is a reliable method for determining the sex (7, 8). Osteometric assessment, which involves analyzing size and shape differences (9), has been a useful technique in comparison with morphological appraisal for determining sex, and anthropologists have widely used this method (10-11).
Teeth are known to be the most durable body components, capable of withstanding high temperatures, air disasters, hurricanes, and decay for an extended period of time after other organs have deteriorated (12, 13).
Forensic odontology involves examining fragmented jaws and teeth to determine sex, and it is one of the jobs of a forensic odontologist (14-16). Sexual dimorphism in human dentition has been documented to varying degrees, and it is believed that the exploitation of food resources and environmental factors have contributed to this difference (17, 18).
Khamis and colleagues found a percentage of dimorphism in the buccolingual (BL) and mesiodistal (MD) dimensions of teeth, with BL dimensions showing greater sexual differences (19). The Y-chromosome is believed to influence the mitotic action of cells in the dental papilla and inner dental epithelium, impacting the dimension of the dentino-enamel junction. Varying degrees of dentine thickness have been observed in different populations, emphasizing the importance of collecting population-specific data (20, 21).
In some cases, when dealing with dental remains, the buccolingual dimension of the teeth may be the only available evidence. This is especially true in situations where only partial or fragmented remains are recovered. In such cases, establishing odontometric standards for the buccolingual size is crucial for identifying the sex of individuals and providing insights into their ancestry, age, and other biological characteristics. In the Iranian population, there are currently no established odontometric standards, making this research essential for forensic investigations and anthropological studies. By determining the extent of sexual dimorphism in the buccolingual size, this study will provide valuable information for identifying the sex of individuals based on dental remains.
MATERIALS AND METHODS
Participants
A total of 100 students (50 females and 50 males) aged 18 to 22 years old were recruited for the present study. The chosen age range ensured the complete eruption of all permanent canine teeth without any bone loss. Participants were selected based on specific criteria, which included the absence of fillings, caries, abrasion, attrition, abfraction, erosion, crowding, bruxism, dental disease, and dental/occlusal abnormalities.
Impression and casts
An irreversible hydrocolloid (alginate) impression material was used to obtain an impression of the teeth, which was immediately poured in dental stone to create the casts.
Measurement
The buccolingual diameters of all anterior teeth were measured using Electronic Digital Vernier Calipers with 0.01 mm resolution. Measurements were done twice by one examiner to eliminate any inter-observer error.
Data analysis
The mean and standard deviation (SD) of the buccolingual size were calculated using SPSS Software. Additionally, the percentage of sexual dimorphism was also calculated. The Independent Samples t-test was used to compare the differences between males and females in terms of buccolingual size. A p-value of less than 0.05 was considered statistically significant. Sexual dimorphism was determined by calculating the percentage difference between the sexes. The following formula was used in the present research: (male mean/female mean) - 1*100. The variance in the mean values of males and females indicates the percentage of sexual dimorphism between them. A negative value of this index suggests a larger female tooth size, while a positive value indicates a larger male tooth size. If the index value is close to zero, the difference between males and females is minimal. Therefore, a value of zero indicates no sexual dimorphism between males and females in terms of buccolingual size (22, 23).
Buccolingual dimensions
The maximum distance between the labial and lingual is called buccolingual dimensions (Figure 1).
RESULTS
Descriptive analysis of data
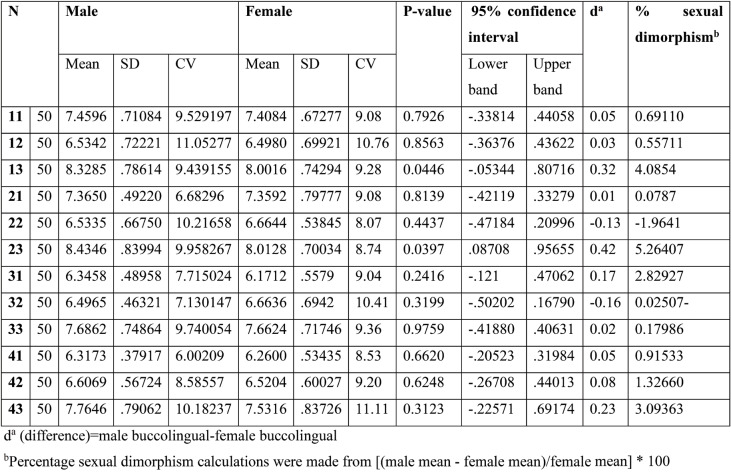
Table 1 presents the buccolingual dimensions of permanent anterior teeth. The results indicate that, with the exception of the lateral incisors (22 32), males have larger buccolingual dimensions in all tooth types in both arches. The inter-sex dimorphism ranges from 0.01 mm for the upper central incisor on the left side to 0.42 mm for the upper canines on the left side. Furthermore, a significant difference in size between males and females was found in the maxillary canine on the right and left sides (P=0.04 and P=0.03, respectively).
The most significant sexual dimorphism was observed in the dimension of the maxillary canine on the right side (5.26%), followed by the left side maxillary canine (4.08%). In contrast, the mandibular lateral incisor showed the smallest amount of sexual difference (-0.02%).
To calculate the percentage of sexual dimorphism, the following formula was used: [(male mean - female mean)/female mean] * 100
In our study, we used the simple and cost-effective linear analysis approach to measure buccolingual teeth. Results showed that women have smaller buccolingual tooth dimensions compared to men in all types of teeth in both arches, except for the left side lateral incisors. The maxillary canines were found to have the most significant sexual dimorphism, with males having larger teeth. This finding is consistent with previous studies that have also shown sexual dimorphism in canines. However, there are studies that suggest sexual dimorphism in other teeth, such as the central or lateral incisors.
Interestingly, this study found that Iranians had the greatest difference in the buccolingual size of the lower canine compared to populations from other countries such as Turkey, Malaysia, and China. Additionally, a significant difference was observed between the Greek and Iranian dentition of males regarding the lower central incisor teeth.
Overall, these findings highlight the importance of considering sexual dimorphism in dental research and practice, particularly in areas such as orthodontics and prosthodontics, where tooth size and shape play a crucial role in treatment planning and outcomes.
DISCUSSION
There are a variety of approaches to measure the buccolingual teeth, including Fourier’s analysis, linear analysis, and Moiré’s topography. Since the linear analysis method was simple and cheap to run, it was selected as the analysis approach in this study (24).
Women have smaller buccolingual tooth dimensions in comparison with men in all types of teeth in both arches except for the left side lateral incisors, which are statistically significant larger in the buccolingual dimensions of the maxillary canines.
Iranians had the greatest difference in the BL size of the lower canine with the population of three other countries, including Turkey (25), Sudanese (19), and China (26). In addition, a significant difference existed between the Greek and Iranian dentition of males regarding the lower central incisor teeth (27).
In the literature, many references have been made to teeth sexual dimorphism (28, 29), and the occurrence of a great amount of dimorphism in permanent teeth is emphasized, particularly canines (30, 31). In line with these studies, the present research has also documented the existence of sexual dimorphism in maxillary canine teeth.
The major sexual dimorphism in permanent teeth is acknowledged in certain other studies. This difference is especially found in canines, with females having smaller teeth than males (31). In referring to this issue, Garn and Lewis (32) claimed that dimorphism was mainly observed in the teeth next to the canines, while other researchers have also emphasized a noticeable sexual dimorphism in the canines (33).
However, other studies stress the manifestation of dimorphism in the remaining teeth. For instance, Acharya and Mainali (34) reported that a significant sexual dimorphism was seen in the central or lateral incisors, which was in contrast with the claim of various other researchers who believed that the least amount of sexual dimorphism was observed in the incisors (32).
Chinese researchers examined sexual dimorphism in the BL and MD diameters of permanent maxillary and mandibular teeth in a sample of Chinese Han adults and found that the BL diameter showed a greater dimorphism than the MD diameter in most of the studied teeth, including incisors, canines, premolars, and molars (39), which was contrary to the findings of Acharya and Mainali (40), and Komabayashi and Fujiwara (41).
Moreover, as shown in Table 1, the mandibular teeth were found to be less dimorphic than the maxillary teeth. This finding was in line with previous studies that have also documented a greater degree of dimorphism in the maxillary teeth than the mandibular teeth (42, 43).
In the present research we found that several of the males’ teeth were larger in size in comparison with those of females. The difference in the quantity of dentin and enamel between males and females was also studied, especially for crown dimensions (44, 45).
Sexual dimorphism has been attributed to the varying degrees of enamel by some scholars (46). Other studies have reported that the variance in the quantity of dentin is the cause of sexual dimorphism (47). Both dentin and enamel have been found to differ between males and females, with males having higher amounts of dentin and females having more enamel in their teeth (47). These differences are explained by the involvement of sex-linked genes on both the X and Y chromosomes, and the quantity of enamel and dentin in the teeth varies as a result of the role of the Y chromosomes (48); also, the effect of the Y chromosome on teeth was studied by Alvesalo (49), who found that through increasing the amount of mitotic potential of the tooth germ by the Y chromosomes, dentinogenesis was induced. Moreover, the roles of the X and Y chromosomes in teeth dimensions were studied by him by another researchers (50) and it was claimed that, despite the classical view on the dominant effect of the X chromosome on teeth size, the Y chromosome was found to have the main role in determining the teeth size through controlling the thickness of the dentin, while the X chromosome was only responsible for controlling the thickness of the enamel. Whereas, amelogenesis is believed to be stimulated and created by the X chromosomes (51).
In 2006, a study conducted at the University of Istanbul found that the main teeth sexual dimorphisms occurred in the incisors, with the greatest differences being observed in the buccolingual (BL) dimension of the maxillary and mandibular canines and the mesiodistal (MD) dimension of the mandibular canine and first premolar (52). Additionally, there was a significant difference in the BL dimension of maxillary canine teeth (13, 23) between males and females.
We used the formula of Garn and colleagues (53) to calculate the percentage of sexual difference for the BL dimensions among Iranians and to estimate sexual differences in other populations. A comparison of the sexual difference percentages in various populations for BL crown diameters is illustrated in Table 2. However, in the study by Harris and Nweeia (54) there were inaccuracies concerning male and female dimorphism calculated for lower C. The accurate values of this dimorphism are shown in Table 2. While BL crown diameters are mostly different in males and females, the amount of this dimorphism varies in different human populations. Europeans have the greatest amount of teeth sexual dimorphism compared to Iranians and Indians, who have a lower amount thereof.
Harris and Nweeia (54) claimed that sexual dimorphism in teeth dimensions did not exist among local South Americans; however, Mexican and Australian aborigines experience a considerable amount of sexual dimorphism. Although the highest difference is observed in the canine teeth, Australian aborigines are characterized by incisor dimorphism, specifically the central ones, in the BL dimensions.
The Iranian population is generally like the Asian population in terms of the amount of sexual dimorphism. All Asians are sexually less dimorphic compared to other populations. Europe and the Near East may be taken as completely separated colonies, as claimed by Richards and colleagues (55). However, this categorization fails to notice the geographical nearness of the Turkish and Greek populations. The less observed sexual dimorphism in the teeth of these populations could be due to their consumption of similar nutritional products.
In general, analysis of various patterns of teeth dimorphism among different countries reveals factors influencing it, such as the impact of the environment, genetics, and epigenetics. Disease, climate, and nutrition are among the environmental factors. In humans, the subsistence pattern has a large effect on the formation of the masticatory apparatus (25). Therefore, teeth dimensions might be influenced by eating habits. Ates and colleagues (25) reported that the higher the proportion of plants in the diet of humans, the larger the teeth become, while a meat diet results in smaller teeth.
Hanihara and Ishida (56) believe that the smaller teeth dimensions in the Western Eurasian population could be due to the smaller influence of natural selection on teeth sizes over hundreds of years. At the same time, there were some cultural variations in cooking after the introduction of agriculture in these colonies.
The impact of genes on teeth sexual dimorphism has also been emphasized in the literature (57-59). It is suggested that the variation in teeth sizes between and within human colonies can be the result of receiving these qualities from past generations. Population admixture could also play a partial role in tooth dimorphism between different populations (42). For instance, varying degrees of admixture have caused dimorphism between Mexicans and Americans from Iowa. Mexicans are genetically descendants of an admixture of mostly North American Indians (Mongoloid) and Spanish population (Caucasian), while in Iowa, the admixture is largely German, Scandinavian, and English (42).
CONCLUSION
There are variations in teeth sexual dimorphism for the BL crown size among various human populations. Therefore, every population has its own discriminant function to be used in determining the sex of human skeletal remains. It is also proven that males possess bigger teeth than females, except for the lateral incisors (22 23). While maxillary canines were found to be sexually dimorphic, with a statistically significant difference in the buccolingual dimensions, the other teeth, with sexual dimorphism, did not show any significant difference in the buccolingual dimensions.
The comparative study of teeth dimorphism between males and females in various human populations revealed that it varies among various groups. The Europeans showed the greatest amount of sexual differences in teeth in comparison with the Iranian population who showed the least amount of sexual dimorphism in teeth. Therefore, obtaining such data about a relevant population with the aim of medical diagnoses, preparation for treatment, and forensic dentistry is totally essential.
Conflicts of interest: none declared.
Financial support: none declared.
Acknowledgments: We are grateful to Tehran Azad University.
FIGURE 1.
Measurement of buccolingual diameter of anterior teeth using electronic Vernier calipers
TABLE 1.
Comparison of buccolingual dimensions of anterior teeth in males and females
TABLE 2.
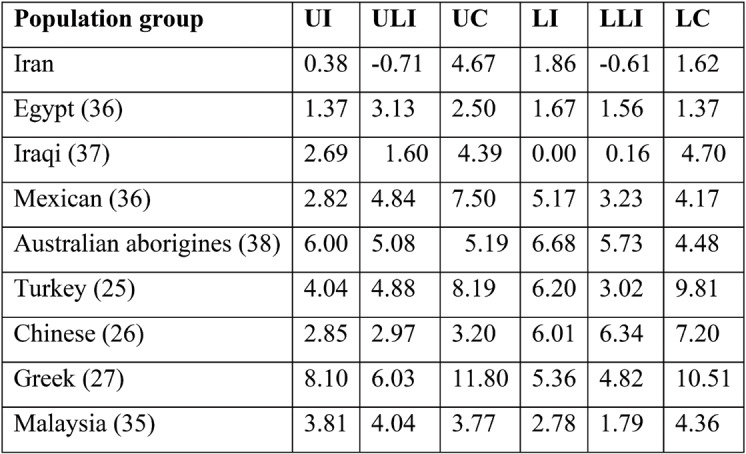
Percentage of sexual dimorphism for the buccolingual (BL) crown diameter of the anterior teeth in different population groups
Contributor Information
Mona AKAD, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania; “Elena Doamna” Clinical Hospital of Obstetrics and Gynecology, Iasi, Romania.
Razvan SOCOLOV, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania; “Elena Doamna” Clinical Hospital of Obstetrics and Gynecology, Iasi, Romania.
Roxana COVALI, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania; “Elena Doamna” Clinical Hospital of Obstetrics and Gynecology, Iasi, Romania.
Catalina Daniela STAN, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania; “Elena Doamna” Clinical Hospital of Obstetrics and Gynecology, Iasi, Romania.
Eduard CRAUCIUC, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania; “Elena Doamna” Clinical Hospital of Obstetrics and Gynecology, Iasi, Romania.
Diana POPOVICI, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania; “Elena Doamna” Clinical Hospital of Obstetrics and Gynecology, Iasi, Romania.
Cristinel Ionel STAN, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania.
Fawzy AKAD, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania.
Demetra SOCOLOV, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania.
Zeinab DAVOUDMANESH, Craniomaxillofacial Research Center, Tehran University of Medical Sciences, Tehran, Iran; Dental Material Research Center, Islamic Azad University, Dental Branch, Tehran, Iran.
Samaneh FARAJIPOUR, Dental Material Research Center, Islamic Azad University, Dental Branch, Tehran, Iran.
Nasim AZIZI, Dental Material Research Center, Islamic Azad University, Dental Branch, Tehran, Iran.
References
- 1.Koirala R, Nair R, Chalise PR. A study on sexual dimorphism of permanent maxillary canine teeth in Nepalese population. JCMC. 2021;11:3–6. [Google Scholar]
- 2.Bunn JM, Robinson, JG. A comparison of dental development in wild and captive chimpanzees. Am J Phys Anthropol. 2021;175:44–55. [Google Scholar]
- 3.Kozakiewicz A, Kozłowski T, Krajewski P. Sexual dimorphism in the modern human skeleton: A review and meta-analysis of cranial and mandibular studies. Am J Phys Anthropol. 2020;171:636–659. [Google Scholar]
- 4.Bayat M, Shariati M, Rajaeirad F, et al. Facial anthropometric norms of the young Iranian population. J Maxillofac Oral Surg. 2018;17:150–157. doi: 10.1007/s12663-016-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curate F. The Estimation of Sex of Human Skeletal Remains in the Portuguese Identified Collections: History and Prospects. Forensic Sci. 2022;2:272–286. [Google Scholar]
- 6.Bayat M, Shariati M, Rakhshan V, et al. Cephalometric risk factors of obstructive sleep apnea. Cranio. 2017;35:321–326. doi: 10.1080/08869634.2016.1239850. [DOI] [PubMed] [Google Scholar]
- 7.Kalyanpur R, Rehani B, Rudagi KB, et al. Evaluation of sexual dimorphism using mesiodistal width and intercanine width of maxillary teeth in Indian population: A pilot study. J Forensic Dent Sci. 2020;12:7–11. [Google Scholar]
- 8.Davoudmanesh Z, Shariati M, Azizi N, et al. Sexual dimorphism in permanent canine teeth and formulas for sex determination. Biomed Res India. 2017;28:2773–2777. [Google Scholar]
- 9.Rastegar Moghddam M, Davoudmanesh Z, Azizi N, et al. Prevalence and Length of the Anterior Loop of the Inferior Alveolar Nerve in Iranians. J Oral Implantol. 2017;43:333–336. doi: 10.1563/aaid-joi-D-16-00212. [DOI] [PubMed] [Google Scholar]
- 11.Menter CG, Brink JS. Sexual dimorphism in the dental and mandibular features of Australopithecus africanus. Am J Phys Anthropol. 2020;172:240–253. [Google Scholar]
- 12.Kanchan T, Krishan K. Identifying the sexual dimorphism of deciduous dentition in a paediatric South Indian population. J Forensic Leg Med. 2014;23:91. doi: 10.1016/j.jflm.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Grover N, Puri N, Singh S, Arora S. Age estimation from physiological changes of teeth: A reliable age marker? J Forensic Dent Sci. 2014;6:113–121. doi: 10.4103/0975-1475.132541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath VV, Satyanaryana A, Baghirat PV, et al. Application of the camerier’s third molar maturity index in the estimation of legal adult age in an Indian sample. J Forensic Leg Med. 2012;19:162–165. [Google Scholar]
- 15.Patterson KB, Kogan SL. Dental identification in woodbridge disaster. J Can Dent Assoc. 1971;37:275–279. [PubMed] [Google Scholar]
- 16.Chandrasekharan AR, Varshney A, Kumar A, Srinivasan SV. The role of forensic odontology in disaster victim identification: A review article. J Forensic Den Sci. 2020;12:152–157. [Google Scholar]
- 17.Smith TM, Olejniczak AJ. Dental evidence for dietary change in Europe across the Holocene. Proceed Royal Soc B. 2019;286:20182389. [Google Scholar]
- 18.Arora K. Sex determination using odontometry: A review of literature. J Forensic Dent Sci. 2019;11:1–6. [Google Scholar]
- 19.Khamis AH, Elamin F, Hassan SED, et al. Sexual dimorphism in the mesiodistal and buccolingual dimensions of permanent teeth in Sudanese population. J Forensic Dent Sci. 2020;12:24–29. [Google Scholar]
- 20.Nagamalleshwari V, Poornima P, Patil R, et al. Sexual dimorphism in permanent maxillary molars: An in vitro study. J Forensic Dent Sci. 2019;11:27–31. [Google Scholar]
- 21.Naik D, Pai ML, Doshi JJ, Devaraju D. An insight into population differences in dentin thickness of human teeth: A systematic review. J Forensic Dent Sci. 2020;12:104–110. [Google Scholar]
- 22.Gandhi N, Jain S, Kahlon H, et al. Significance of mandibular canine index in sexual dimorphism and aid in personal identification in forensic odontology. J Forensic Dent Sci. 2017;9:56–60. doi: 10.4103/jfo.jfds_15_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha R, Acharya J, Koirala S, Poudyal S. Sexual dimorphism in the permanent mandibular first molar in a Nepalese population. J Inst Med Nep. 2020;42:49–55. [Google Scholar]
- 24.Naidu D, Scott J, Ong D, Ho CT. Validity, reliability and reproducibility of three methods used to measure tooth widths for Bolton analyses. Aust Orthod J. 2009;25:97–103. [PubMed] [Google Scholar]
- 25.Ateş M, Karaman F, Işcan MY, Erdem TL. Sexual differences in Turkish dentition. Leg Med (Tokyo) 2006. [DOI] [PubMed]
- 26.Stroud JL, Buschang PH, Goaz PW. Sexual dimorphism in mesiodistal dentin and enamel thickness. Dentomaxillofac Radiol. 1994;23:169–171. doi: 10.1259/dmfr.23.3.7835519. [DOI] [PubMed] [Google Scholar]
- 27.Zorba E, Moraitis K, Manolis SK. Sexual dimorphism in permanent teeth of modern Greeks. Forensic Sci Int. 2011;210:74–81. doi: 10.1016/j.forsciint.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Flores-Mir C, Nevarez-Rodriguez A, Ramirez-Rodriguez CI. Sex determination using dental measurements: A systematic review and meta-analysis. Forensic Sci Int. 2020;308:110161. [Google Scholar]
- 29.Ruiz-Galvez ML, Paredes-Gallardo V, Gálvez-Bravo L, García-Medina B. Sexual dimorphism in permanent teeth among a Spanish population. J Forensic Odonto-Stomat. 2021;39:27–36. [Google Scholar]
- 30.Bailey SE, Lynch JM. Sexual dimorphism in dental size and shape in an archaeological population from the Late Prehispanic Andes. Am J Phys Anthropol. 2020;173:672–684. [Google Scholar]
- 31.Franco-Molina MA, Méndez-Ramírez I, Casanova-Rosado JF, Flores-Ramírez V, Sánchez-Pérez L, Maupomé G. Sexual dimorphism in dental dimensions and morphological features in a Mexican population. Arch Oral Bio. 2020;114:104695. [Google Scholar]
- 32.Garn SM, Lewis AB, Kerewsky RS. Sexual dimorphism in the buccolingual tooth diameter. J Dent Res. 1966;45:18–19. doi: 10.1177/00220345660450064301. [DOI] [PubMed] [Google Scholar]
- 33.Alvesalo L, Kari M. Sizes of deciduous teeth in 47 XYY males. Am J Hum Genet. 1977;29:486–489. [PMC free article] [PubMed] [Google Scholar]
- 34.Acharya AB, Mainali S. Sex discrimination potential of buccolingual and mesiodistal tooth dimensions. J Forensic Sci. 2008;53:790–792. doi: 10.1111/j.1556-4029.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- 35.Khamis MF, Taylor JA, Malik SN, Townsend GC. Odontometric sex variation in Malaysians with application to sex prediction. Forensic Sci Int. 2014;234:183. doi: 10.1016/j.forsciint.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Bishara SE, Ortho D, Jakobsen JR, et al. Comparisons of mesiodistal and buccolingual crown dimensions of the permanent teeth in three populations from Egypt, Mexico, and the United States. Am J Orthod Dentofacial Orthop. 1989;96:416–422. doi: 10.1016/0889-5406(89)90326-0. [DOI] [PubMed] [Google Scholar]
- 37.Ghose LJ, Baghdady V. Analysis of the Iraqi dentition: mesiodistal crown diameters of permanent teeth. J Dent Res. 1979;58:1047–1054. doi: 10.1177/00220345790580030301. [DOI] [PubMed] [Google Scholar]
- 38.Barrett MJ, Brown T, Macdonald MR. Dental observations on Australian aborigines: mesiodistal crown diameters of permanent teeth. Aust Dent J. 1963;8:150. [Google Scholar]
- 39.Liu X, LiL, LiH, et al. Sexual dimorphism in dental dimensions of Chinese Han adults. J Forensic Sci. 2020;65:902–908. [Google Scholar]
- 40.Acharya AB, Mainali S. Are dental arch dimensions and occlusion associated with body size and shape? Am J Phys Anthropol. 2007;133:698–708. [Google Scholar]
- 41.Komabayashi T, Fujiwara T. Sexual dimorphism in mesiodistal and buccolingual tooth dimensions in Japanese. J Physiol Anthropol. 2013;32 [Google Scholar]
- 42.Lunt DA. An odontometric study of medieval Danes. Acta Odontol Scand Suppl. 1969;27:1–173. [PubMed] [Google Scholar]
- 43.Moss ML, Moss-Salentijn L. Analysis of developmental processes possibly related to human dental sexual dimorphism in permanent and deciduous canines. Am J Phys Anthropol. 1977;46:407–413. doi: 10.1002/ajpa.1330460305. [DOI] [PubMed] [Google Scholar]
- 44.Uysal T, Yilmaz B, Oktem O. Sexual dimorphism in enamel and dentin thickness of permanent teeth in a Turkish population: A cone-beam computed tomography study. J Esthet Restor Dent. 2021;33:1086–1092. [Google Scholar]
- 45.Peixoto IBG, Prado FBM, Sant’Anna EF, Porto MR. Sexual dimorphism of enamel and dentin thickness in human molars: A micro-CT analysis. Arch Oral Biol. 2021123;104991 [Google Scholar]
- 46.Moosvi Z, Revie G, Manica S. Enamel thickness of human mandibular canine: A radiographic study. Bull Int Assoc Paleodont. 2022;16:221–229. [Google Scholar]
- 47.Stroud JL, Buschang PH, Goaz PW. Sexual dimorphism in mesiodistal dentin and enamel thickness. Dentomaxillofac Radiol. 1994;23:169–171. doi: 10.1259/dmfr.23.3.7835519. [DOI] [PubMed] [Google Scholar]
- 48.Alvesalo L. Human sex chromosomes in oral and craniofacial growth. Arch Oral Biol 2009 . [DOI] [PubMed]
- 49.Alvesalo L, Tammisalo E. Enamel thickness in 45, Y females’ permanent teeth. Am J Hum Genet. 1981;33:464–469. [PMC free article] [PubMed] [Google Scholar]
- 50.Alvesalo L. Sex chromosomes and human growth. A dental approach. Hum Genet. 1997;101:1–5. doi: 10.1007/s004390050575. [DOI] [PubMed] [Google Scholar]
- 52.Karaman F. Use of diagonal teeth measurements in predicting gender in a Turkish Population. J Forensic Sci. 2006;51:630–635. doi: 10.1111/j.1556-4029.2006.00133.x. [DOI] [PubMed] [Google Scholar]
- 53.Garn SM, Lewis AB, Swindler DR, Kerewsky RS. Genetic control of sexual dimorphism in tooth size. J Dent Res. 1967;46:963–972. doi: 10.1177/00220345670460055801. [DOI] [PubMed] [Google Scholar]
- 54.Harris EF, Nweeia MT. Tooth size of Ticuna Indians, Colombia, with phonetic comparisons to other Amerindians. Am J Phys Anthropol. 1980;53:81–91. doi: 10.1002/ajpa.1330530112. [DOI] [PubMed] [Google Scholar]
- 55.Richards M, Macaulay V, Hickey E, et al. Tracing european founder lineages in the near eastern mtDNA pool. Am J Hum Genet. 2000;67:1251–1276. [PMC free article] [PubMed] [Google Scholar]
- 56.Hanihara T, Ishida H. Metric dental variation of major human populations. Am J Phys Anthropol. 2005;128:287–298. doi: 10.1002/ajpa.20080. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Shi F, Zhang J, et al. Investigation of candidate genes involved in tooth crown size and shape sexual dimorphism. J Hum Gen. 2021;66:369–378. [Google Scholar]
- 58.Wang Y, Wu H, Liu J, et al. Sexual dimorphism in dental development and its association with the polymorphism of PAX9 and TBX22 genes in the Chinese population. BMC Oral Health. 2022;22:12. [Google Scholar]
- 59.Yu M, Sun H, Feng X, Cai B. Investigation of the dental sexual dimorphism in orthodontic patients and its association with genetic variants. J Orthod Sci. 2021;10:12. [Google Scholar]