Abstract
Objectives:Inflammatory bowel diseases (IBD) have been associated with multiple environmental factors, including diet. A dietary pattern characterized by low fiber content, high fat content and high carbohydrate content has been linked to the development of IBD. The objective of the current investigation is to examine the potential link between dietary patterns and the occurrence of IBD and to investigate whether there are any differences in relation to the type of IBD and specific food groups.
Material and methods:We conducted an observational retrospective comparative study using three cohorts: 89 Crohn’s disease (CD) patients, 40 ulcerative colitis (UC) patients and 64 healthy subjects. All participants underwent structured interviews and were required to complete a questionnaire regarding their dietary habits either prior to the onset of IBD or within the last year for control subjects.
Results:A higher proportion of CD patients reported a higher rate of salt intake (71.9% vs. 53.1%, p-value = 0.043), sweetened beverages (38.2% vs. 17.2%, p-value=0.022), processed meat (66.3% vs. 40.6%, p-value=0.007), fatty meat (50.6% vs. 28.1%, p-value=0.021), fried foods (47.2% vs. 9.4%, p-value<0.001) and mayonnaise (21.3% vs. 6.2%, p-value=0.032) and a lower intake of nuts and seeds (20.2% vs. 43.8%, p-value=0.004) and yogurt (23.6% vs. 43.8%, p-value=0.030) compared to healthy subjects. Compared to controls, in the UC group there was a higher consumption of salt (85% vs. 53.1%, p-value=0.003), sweetened beverages (47.5% vs. 17.2%, p-value=0.005), fatty meat (55% vs. 28.1%, p-value=0.025) and fried foods (55% vs. 9.4%, p-value<0.001) and a lower intake of nuts and seeds (10% vs. 43.8%, p-value=0.001).
Conclusion:Diet patterns before the onset of the disease are similar in patients with Crohn's disease and patients with ulcerative colitis: increased consumption of sweetened beverages, processed and fatty meat, fried food, salt, store-bought ice cream, and mayonnaise, and decreased intake of seeds, nuts, and yogurt.
Keywords:Crohn's disease, ulcerative colitis, diet.
INTRODUCTION
Inflammatory bowel diseases (IBD), which primarily comprise Crohn's disease (CD) and ulcerative colitis (UC), are characterized by a persistent inflammation of the gastrointestinal tract (1-3). The underlying cause of this inflammation is attributed to an aberrant immune response resulting from the interplay between environmental factors and the commensal gut microbiota in affected individuals (4-6).
In developed nations, IBD, including CD and UC, gained recognition after the industrial revolution. The incidence of IBD sharply increased during the 20th century, especially in industrialized countries such as the US, Canada, and Western Europe (3, 7, 8).
There is evidence to suggest that the risk of developing IBD is higher in the first-generation offspring who relocate from areas with a low prevalence of the condition to areas with a high prevalence. This finding indicates that the development of IBD is influenced by both environmental factors and genetic predisposition (1, 3).
Inflammatory bowel diseases (IBD) have been associated with multiple environmental factors including smoking, hygiene, antibiotics, oral contraceptive pills (OCPs), non-steroid anti-inflammatory drugs (NSAIDs), diet, breastfeeding, vitamin D, stress, air pollution, and microorganisms. However, the exact mechanism underlying the contribution of these factors to the development of IBD remains unclear, and several risk factors lack consistent evidence across studies (7, 9-11).
A dietary pattern characterized by low fiber content, high fat content and high carbohydrate content has been linked to the development of IBD. This association is thought to be mediated through the influence of the diet on insulin resistance, gut permeability, proinflammatory signaling, and gut microbiota dysbiosis (12-16).
The objective of the current investigation is to examine the potential link between dietary patterns before the onset of IBD and disease occurrence and to investigate whether differences exist in relation to the type of IBD and specific food groups.
MATERIALS AND METHODS
Study design and patients
The present retrospective case-control study selected 193 participants, including 129 IBD patients who were consecutively monitored in the Gastroenterology Outpatient Unit of Fundeni Clinical Institute, Bucharest, Romania, between October 2021 and May 2022, and 64 healthy controls who were matched for age and sex distribution with the case group. The control group comprised 50% of hospital employees and 50% were referred by local general practitioners in close proximity to the hospital.
Our study enrolled IBD patients who met the inclusion criteria of being over 18 years old and having a disease duration of at least six months, while those with concurrent Clostridium difficile or Cytomegalovirus (CMV) colitis, complete oral intake suppression, exclusive parenteral/enteral nutrition, other gastrointestinal diseases, including cancer and infectious diseases, and those who declined to participate in the study were excluded.
During the study, all participants were subjected to an interview and asked to complete a dietary questionnaire that encompassed their current and past eating habits (including diet prior to the onset of disease) as depicted in Table 1. The entire process was supervised by a team member, and each participant provided informed consent before being enrolled in the study
All study participants provided informed consent and voluntarily agreed to participate in the study. To gather patients' medical information, such as discharge papers and laboratory results, the hospital's informatics system was accessed. The study protocol was approved by the Ethics Committee of Fundeni Clinical Institute, Bucharest, Romania.
Variables
Tables 2 and 3 summarize the demographic information of the study population, including age, sex, disease type (Crohn's disease/ulcerative colitis), disease classification according to the Montreal classification, and disease severity. The Crohn's disease activity index (CDAI) and Mayo scores were used to evaluate disease severity for Crohn's disease and ulcerative colitis, respectively.
The study recorded several diet-related variables, including the weekly consumption of fish, processed meat, seeds and nuts, as well as the daily portions of vegetables, fruits and high-grain cereals. The consumption frequency of several foods was also observed, such as fried foods, commercial ice cream, mayonnaise, cheese, yogurt, fruit yogurt, butter, fatty meat, margarine, and chips/nachos/other snacks. Sugar-sweetened beverages consumption ranged from less than one liter per day to more than one liter per day.
Statistical analysis
Data analysis was conducted using the statistical software SPSS (version 20.0, IBM Corporation, Armonk, NY, USA). Quantitative variables with a parametric distribution were presented as mean and standard deviation, while those with a non-parametric distribution were presented as median with minimum and maximum. Differences between the three groups were analyzed using one-way ANOVA, followed by Scheffé Post Hoc Test for multiple comparisons due to the unequal groups. A p-value of less than 0.05 was considered statistically significant.
The sample size was determined based on a statistical power of 80% and an alpha error of 5% to detect an odds ratio of at least 3, assuming a risk factor prevalence of 62% (daily sugar consumption based on data from the Romanian National Institute of Public Health). Using Win- Pepi 11.65, the estimated sample size was 116 patients for the study group and 58 for the control group (17).
RESULTS
A. Crohn’s disease group
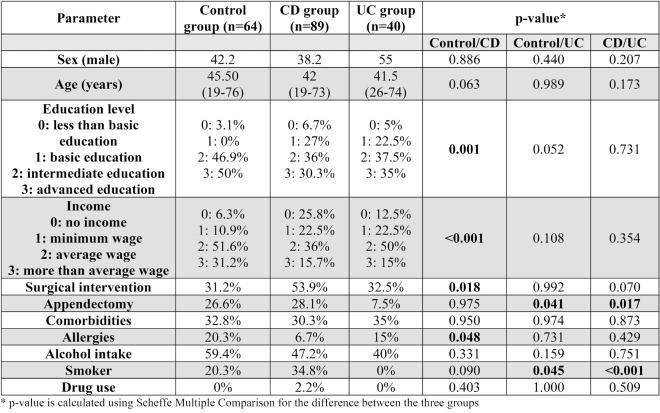
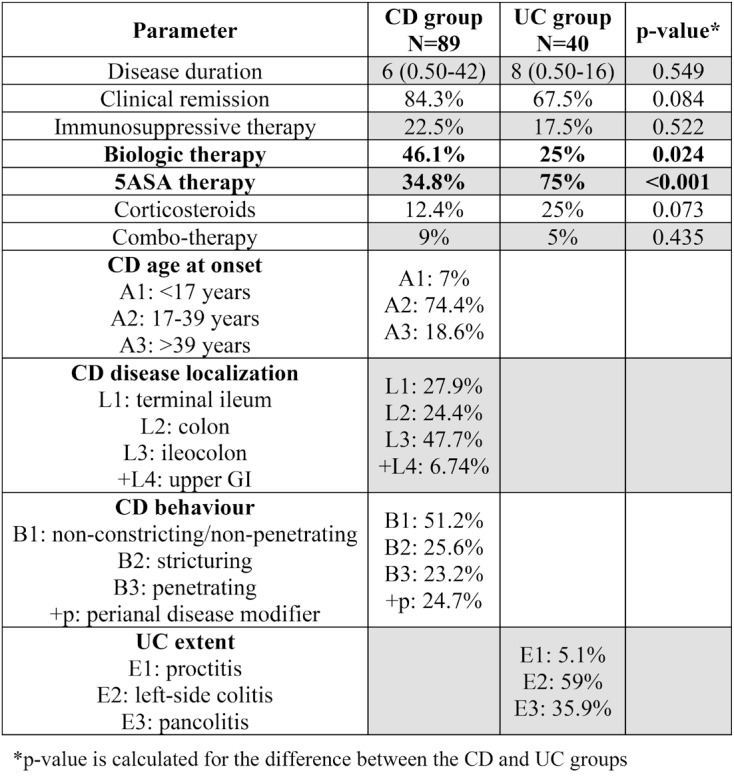
The male gender was less prevalent (38.2%) in this cohort, with a median age of 42 years and a median disease duration of six years. Approximately one third of patients had an advanced education degree and 15% had an income above the average level. Disease onset occurred most frequently between 17 and 40 years of age, with the ileocolonic location and the B1 phenotype being the most common. Perianal disease was present in 24.7% of patients, with 53.9% undergoing surgery and 28.1% appendectomy. Comorbidities were present in 30.3% of patients, 34.8% were active smokers, 2.2% had a history of substance use disorder, 6.7% had documented allergies, and 47.2% were regular alcohol users. The majority of patients received biologic therapy (46.1%) (Tables 2 and 3).
B. Ulcerative colitis group
Males represented 55% of the UC group, with a median age of 41.5 years and a median duration of the disease of eight years. About one third had an advanced education degree, and 15% earned a more than average wage. Most patients had left colonic disease extension (59%), and 32.5% had a surgical intervention. None were active smokers or had a history of substance use disorder; 35% had other comorbidities, 15% had documented allergies, and 40% were regular alcohol users. Also, 75% of patients were treated with 5ASA (Tables 2 and 3).
C. Control group
The control cohort had 42.2% males with a median age of 45.5 years. Half of them had an advanced education degree and one third had a higher than average income. About a quarter of them underwent an appendectomy, 32.8% had other comorbidities, 20.3% were active smokers, 20.3% had documented allergies, and 59.4% were regular alcohol users (Table 2).
Comparison of the three groups
No significant differences were observed between groups in terms of age or gender. However, there were significant differences in the prevalence of appendectomy, allergies and smoking status between groups. The appendectomy status differed between groups as follows: 28.1% in the CD group, 26.6% in the control group and 7.5% in the UC group (p-value =0.012). Using multiple comparison analysis, we obtained a p-value of 0.041 between UC and control group and a p-value of 0.017 between UC and CD group. Allergies were more frequent in control group (20.3%) compared to CD group (6.7%) (p-value=0.048). The smoking status was also different between groups: 34.8% among CD subjects, 20.3% among controls and 0% reported among UC patients (p-value <0.001) (Table 2).
As for the treatment options, there were statistically significant differences between CD and UC groups. Three quarters of UC patients were on 5ASA compared to one third in CD group (p-value <0.001), while almost half of CD patients were on biologic therapy compared to only a quarter of UC patients (p-value=0.024). There were 84% patients in clinical remission in the CD arm compared to 67% in the UC arm (see Table 3).
Dietary factors analysis
Comparison between CD and control subjects
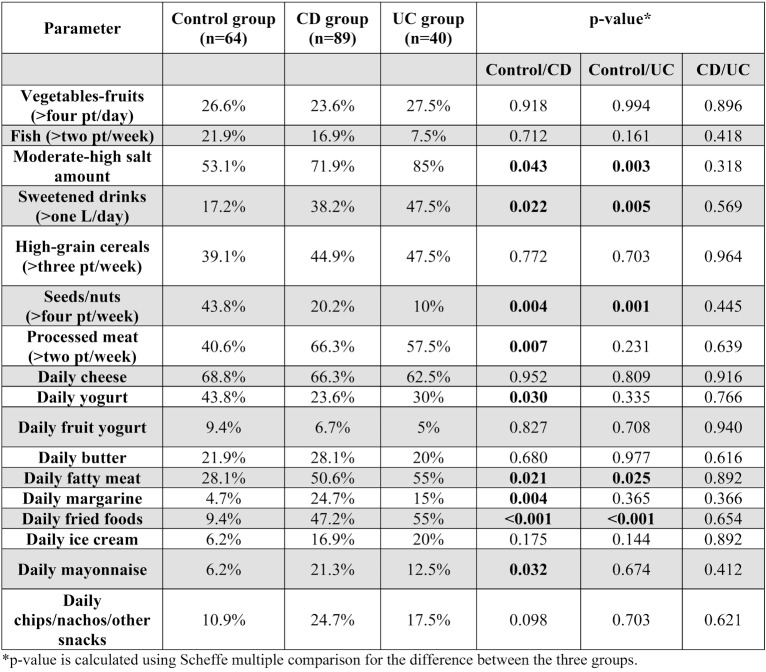
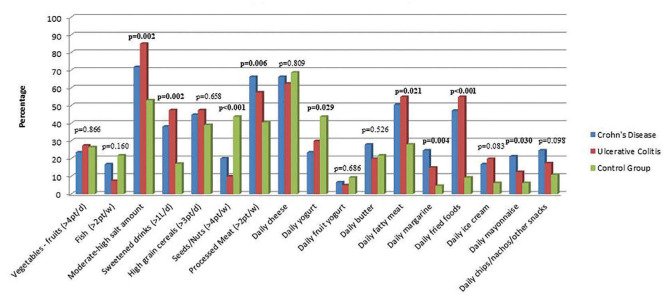
Concerning diet composition before the disease onset, there were several differences between groups. Compared to the control group, the CD group had a higher rate of salt intake (71.9% vs. 53.1%, p-value=0.043), sweets and sweetened beverages (38.2% vs. 17.2%, p-value=0.022), processed meat (66.3% vs. 40.6%, p-value= 0.007), fatty meat (50.6% vs. 28.1%, p-value= 0.021), fried foods (47.2% vs. 9.4%, p-value< 0.001) and mayonnaise (21.3% vs. 6.2%, p-value=0.032). There was also a significantly lower intake of nuts and seeds (20.2% vs. 43.8%, p-value=0.004) and yogurt (23.6% vs. 43.8%, p-value=0.030). There were no statistically significant differences identified in the consumption of vegetables-fruits, fish, high grain cereals, cheese, butter, margarine, commercial ice cream and chips/nachos/other snacks between the two examined groups (Table 4, Figure 1).
Comparison between UC and control subjects
Compared to controls, in the UC group there was a higher consumption of salt (85% vs. 53.1%, p-value=0.003), sweets and sweetened beverages (47.5% vs. 17.2%, p-value=0.005), fatty meat (55% vs. 28.1%, p-value=0.025) and fried foods (55% vs. 9.4%, p-value<0.001). Meanwhile, the intake of nuts and seeds was lower in the UC group (10% vs. 43.8%, p-value=0.001) (Table 4, Figure1).
There were no statistically significant differences between UC and CD groups, no matter the amount or food category (Table 4, Figure 1).
DISCUSSION
The development and progression of IBD can be notably influenced by diet. However, despite a growing interest in this area among researchers, no universally applicable dietary approach for the management of IBD currently exists. As a result, patients with IBD often display a heightened interest in dietary recommendations, specifically with regard to foods to consume or avoid. Against the backdrop of increasing IBD prevalence in Eastern Europe attributed to the adoption of westernized lifestyles, we aimed to undertake a comparative analysis of dietary habits among patients with UC, those with CD and healthy controls.
Statistical analysis of our study failed to uncover any significant inter-group differences concerning the consumption of vegetables, fruits or fish. The proportions of vegetable and fruit intake among the control, CD and UC groups were 26.6%, 23.6%, and 27.5%, respectively. Meanwhile, fish consumption was observed in 21.9%, 16.9%, and 7.5% of the control, CD and UC groups, respectively. Nevertheless, it is noteworthy that the control group's fish intake was thrice that of the UC group.
Racine et al (8) reported that individuals with a high intake of sugar, sugary drinks, and sweets along with a low intake of vegetables and fruits had an increased risk of developing UC. Conversely, no increase in risk was observed in those with a high intake of sugar, sugary drinks, and sweets combined with a high intake of vegetables and fruits. The EpiCom cohort, a population-based prospective inception cohort, included 1182 IBD patients from 31 European nations, comprising 444 CD patients, 627 UC patients, and 111 IBD unclassified patients. At the time of diagnosis, patients completed an 87-item questionnaire about environmental factors. Results from the study indicate that Eastern European CD and UC patients had a significantly higher sugar intake compared to their Western European counterparts, but lower fiber intake (p<0.01) (18). These observations are consistent with the results obtained in our study, where we found that healthy participants reported a consumption of sweetened beverages that was three times lower than that reported by UC subjects and two times lower than that reported by CD patients.
There were no significant differences in the consumption of high grain cereals between IBD patients and the control group (39.1% vs. 44.9% vs. 47.5%). However, the control group reported higher consumption of nuts and seeds compared to CD and UC groups (43.8% vs. 20.2% vs. 10%). These findings are not consistent with prior research that demonstrates the favorable effects of fiber intake, specifically for ulcerative colitis (19, 20). A Danish study among one pediatric population upheld these findings for ulcerative colitis (OR = 0.3, CI: 0.1-0.8) (21).
With respect to the consumption of dairy products, the control group exhibited a statistically significant higher intake of yogurt as compared to the CD group (43.8% vs. 23.6%, p-value= 0.030); however, the intake of cheese and butter was similar between groups.
Narula et al (15) conducted a recent study on the association between the consumption of highly processed meals and the incidence of inflammatory bowel disease (IBD) in participants from the PURE (Prospective Urban Rural Epidemiology) cohort, consisting of individuals from 21 countries. The study included 467 IBD incident cases, with a median follow-up period of 9.7 years. Results showed that subjects who consumed one to four servings of highly processed meals had a significantly higher risk of developing IBD compared to those who did not consume such meals. In this study, no significant association was found between the incidence of IBD and the consumption of white meat, unprocessed red meat, dairy products, starchy foods, fruits, vegetables, and legumes. This suggests that the risk of developing IBD may be more closely related to the processing method or ultra-processing degree of the food, rather than the food items themselves.
Our study aligns with previous findings on the impact of highly processed meals on IBD. Although our study has a retrospective design, we observed statistically significant differences in the consumption of processed meat between healthy individuals and those with CD (9.4% vs. 47.2%, p-value <0.001) or UC (9.4% vs. 55%, p-value <0.001). The consumption of processed meat has been associated with the presence of compounds that act as detergents, potentially disrupting the intestinal microbiota and compromising the gut barrier function, ultimately contributing to inflammation (22, 23).
Additionally, our study identified a difference in the consumption of fatty meat between healthy individuals (28.1%) and those with CD (50.6%) or UC (55%).
Red meat has been linked to an increased risk of IBD due to the presence of linoleic acid, which can give rise to pro-inflammatory mediators such as leukotrienes and prostaglandins upon metabolism (24-26). In a meta-analysis conducted in 2015, which included nine studies, it was found that individuals who consumed meat frequently had a significantly higher risk of developing IBD compared to those who consumed it infrequently or not at all (pooled RR:1.5, 95% CI:1.15-1.95) (27). Research has demonstrated that the consumption of unsaturated omega-3 fatty acids has an anti-inflammatory effect and is associated with a decreased risk of developing UC (28).
Apart from processed meats, foods with high levels of food additives include margarine, store-bought mayonnaise, ice cream, chips, and similar products. In our study, we observed significant differences in the consumption of margarine (4.7% in control subjects versus 24.7% in CD patients, p-value=0.004) and store-bought mayonnaise (6.2% in control subjects versus 21.3% in CD patients, p-value=0.032). Our study revealed that salt intake was significantly higher in the IBD cohort as compared to healthy subjects, with 71.9% of CD patients and 85% of UC patients consuming higher amounts of salt in their diet, in contrast to 53.1% of healthy subjects.
The principal advantage of this investigation is the ability to compare healthy individuals with subgroups of IBD, namely UC and CD, thereby allowing for the identification of statistically significant differences that cannot be generalized to every disease subtype. The examination relied on a comprehensive dietary questionnaire that effectively assessed the food intake patterns of each participant, making it applicable to the general population. However, the study is not without limitations, including its retrospective nature and the relatively small sample size, despite achieving adequate statistical power. As a recommendation, these encouraging results require verification in larger, randomized, controlled clinical trials.
CONCLUSION
The results of the present study indicate significant differences in dietary patterns between healthy individuals and those with IBD. Going further, we highlighted the fact that the diet patterns before the onset of the disease (according to the retrospective questionnaire) did not differ significantly between patients with Crohn's disease and those with ulcerative colitis (increased consumption of sweetened beverages, processed and high-fat meat, fried food, salt, store-bought ice cream, and mayonnaise; decreased intake of seeds, nuts, and yogurt). This suggests that the same type of anti-inflammatory diet can be applied in the two types of the disease (low in saturated fats, emulsifiers, red and processed meats) in order to obtain and maintain the remission of the disease.
Conflicts of interest: none declared.
Financial support: none declared.
TABLE 1.
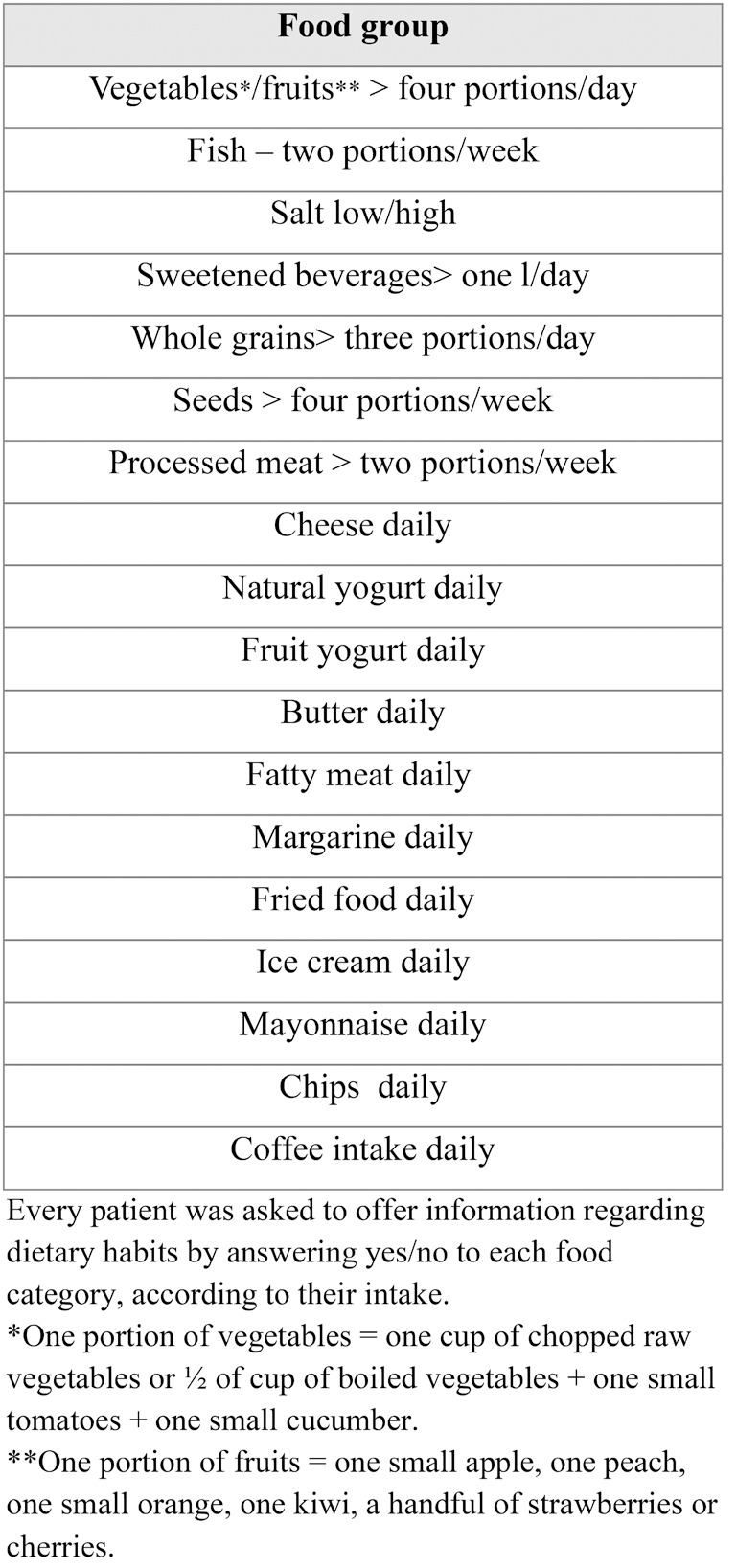
Information regarding patients’ dietary pattern before IBD onset
TABLE 2.
A comparison of the three-group characteristics
TABLE 3.
IBD patients – general characteristics
TABLE 4.
Dietary patterns in CD, UC and control groups
FIGURE 1.
Dietary pattern and consumption amount
Contributor Information
Carmen Monica PREDA, ”Carol Davila” Gastroenterology & Hepatology Department, Fundeni Clinical Institute, Bucharest, Romania.
Doina ISTRATESCU, Department of Gastroenterology, Fundeni Clinical Institute, Bucharest, Romania.
Maria NITESCU, ”Carol Davila” University of Medicine and Pharmacy, “Prof. Dr. Matei Bals” National Institute for Infectious Diseases, Bucharest, Romania.
Teodora MANUC, ”Carol Davila” Gastroenterology & Hepatology Department, Fundeni Clinical Institute, Bucharest, Romania.
Mircea MANUC, ”Carol Davila” Gastroenterology & Hepatology Department, Fundeni Clinical Institute, Bucharest, Romania.
Tudor STROIE, ”Carol Davila” Gastroenterology & Hepatology Department, Fundeni Clinical Institute, Bucharest, Romania.
Mihai CATRINOIU, ”Carol Davila” Gastroenterology & Hepatology Department, Fundeni Clinical Institute, Bucharest, Romania.
Cristian TIERANU, Elias Emergency Hospital, Gastroenterology & Hepatology Department, Bucharest, Romania.
Corina Gabriela MEIANU, ”Carol Davila” Gastroenterology & Hepatology Department, Fundeni Clinical Institute, Bucharest, Romania.
Letitia TUGUI, Department of Gastroenterology, Fundeni Clinical Institute, Bucharest, Romania.
Cosmin Alexandru CIORA, Department of Gastroenterology, Fundeni Clinical Institute, Bucharest, Romania.
Edouard LOUIS, Department of Gastroenterology, University Hospital CHU Liège, Belgium.
Mircea DICULESCU, ”Carol Davila” Gastroenterology & Hepatology Department, Fundeni Clinical Institute, Bucharest, Romania.
References
- 1.Kuenzig ME, Fung SG, Marderfeld L, et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology. 2022;162:1147–1159. doi: 10.1053/j.gastro.2021.12.282. [DOI] [PubMed] [Google Scholar]
- 2.Manuc TE, Manuc MM, Diculescu MM. Recent insights into the molecular pathogenesis of Crohn's disease: a review of emerging therapeutic targets. Clin Exp Gastroenterol. 2016;9:59–70. doi: 10.2147/CEG.S53381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 4.Carmen-Monica P, Doina I. Etiology of Ulcerative Colitis. In: Ulcerative Colitis.Partha, P, Ed. IntechOpen: Rijeka. 2022, p Ch. 1.
- 5.Derwa Y, Gracie, DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389–400. doi: 10.1111/apt.14203. [DOI] [PubMed] [Google Scholar]
- 6.Levine A, Rhodes JM, Lindsay JO, et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1381–1392. doi: 10.1016/j.cgh.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Preda CM, Manuc T, Istratescu D, et al. Environmental Factors in Romanian and Belgian Patients with Inflammatory Bowel Disease - a Retrospective Comparative Study. Maedica (Bucur) 2019. [DOI] [PMC free article] [PubMed]
- 8.Racine A, Carbonnel F, Chan SS, et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm Bowel Dis. 2016;22:345–354. doi: 10.1097/MIB.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 9.Preda CM, Manuc T, Chifulescu A, et al. Diet as an environmental trigger in inflammatory bowel disease: a retrospective comparative study in two European cohorts. Rev Esp Enferm Dig. 2020;112:440–447. doi: 10.17235/reed.2020.6552/2019. [DOI] [PubMed] [Google Scholar]
- 10.Schnabel L, Buscail C, Sabate JM, et al. Association Between Ultra-Processed Food Consumption and Functional Gastrointestinal Disorders: Results From the French NutriNet-Santé Cohort. Am J Gastroenterol. 2018;113:1217–1228. doi: 10.1038/s41395-018-0137-1. [DOI] [PubMed] [Google Scholar]
- 11.Suskind DL, Lee D, Kim YM, et al. The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn's Disease: A Randomized Diet Controlled Trial. Nutrients. 2020;12:3749. doi: 10.3390/nu12123749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bischoff SC, Bager P, Escher J, et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2023;42:352–379. doi: 10.1016/j.clnu.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Ananthakrishnan AN, Khalili H, Song M, et al. High School Diet and Risk of Crohn's Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2015;21:2311–2319. doi: 10.1097/MIB.0000000000000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chassaing B, Compher C, Bonhomme B, et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology. 2022;162:743–756. doi: 10.1053/j.gastro.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narula N, Wong ECL, Dehghan M, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ. 2021;374:n1554. doi: 10.1136/bmj.n1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitescu M, Istratescu D, Preda CM, et al. Role of an Exclusion Diet (Reduced Disaccharides, Saturated Fats, Emulsifiers, Red and Ultraprocessed Meats) in Maintaining the Remission of Chronic Inflammatory Bowel Diseases in Adults. Medicina(Kaunas) 2023. [DOI] [PMC free article] [PubMed]
- 17.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen V, Chan S, Luben R, et al. Fibre intake and the development of inflammatory bowel disease: A European prospective multi-centre cohort study (EPIC-IBD). J Crohns Colitis. 2018;12:129–136. doi: 10.1093/ecco-jcc/jjx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amarapurkar AD, Amarapurkar DN, Rathi P, et al. Risk factors for inflammatory bowel disease: A prospective multi-center study. Indian J Gastroenterol. 2018;37:189–195. doi: 10.1007/s12664-018-0850-0. [DOI] [PubMed] [Google Scholar]
- 20.Niewiadomski O, Studd C, Wilson J, et al. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern Med J. 2016;46:669–676. doi: 10.1111/imj.13094. [DOI] [PubMed] [Google Scholar]
- 21.Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease - a population based study 2007-2009. J Crohns Colitis. 2013;7:79–88. doi: 10.1016/j.crohns.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Abegunde AT, Muhammad BH, Bhatti O, Ali T. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J Gastroenterol. 2016;22:6296–6317. doi: 10.3748/wjg.v22.i27.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414–1427. doi: 10.1136/gutjnl-2016-313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:112–125. doi: 10.4292/wjgpt.v7.i1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogler G, Zeitz J, Biedermann L. The Search for Causative Environmental Factors in Inflammatory Bowel Disease. Dig Dis 2016;34(Suppl 1) [DOI] [PubMed]
- 26.Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160:29–44. doi: 10.1016/j.trsl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Ge J, Han TJ, Liu J, et al. Meat intake and risk of inflammatory bowel disease: A meta-analysis. Turk J Gastroenterol. 2015;26:492–497. doi: 10.5152/tjg.2015.0106. [DOI] [PubMed] [Google Scholar]
- 28.John S, Luben R, Shrestha SS, et al. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:602–606. doi: 10.1097/MEG.0b013e3283352d05. [DOI] [PubMed] [Google Scholar]