Abstract
In clinical practice, the diagnosis of breast lesions is achieved by the triple approach of the specialized surgeon, radiologist and pathologist. The recommended approach to breast lesions should always include a detailed history, along with a thorough clinical examination, mammography and/or ultrasound, as well as preoperative cytodiagnosis. In this context, fine needle aspiration cytology and core needle biopsy are the methods of choice for histological diagnosis. Herein, we aim to explain why these procedures seem to be superior compared to open biopsy and we propose a cytodiagnostic algorithm for breast lesions.
Keywords:breast, lesion, diagnosis, cytodiagnosis, algorythm.
TO THE EDITOR:
In everyday practice, the diagnosis of breast lesions is achieved by the triple approach of the specialized surgeon, radiologist and pathologist. The recommended approach to breast lesions should always include a detailed history, along with a thorough clinical examination, mammography and/or ultrasound, as well as preoperative cytodiagnosis. In this context, fine needle aspiration cytology (FNAC) and core needle biopsy (CNB) are the methods of choice for histological diagnosis. Compared to open biopsy, both procedures seem to be superior, because they not only enable a clear and reliable diagnosis, but are also minimally invasive, tolerable, easy and inexpensive. Both FNAC and CNB are important diagnostic modalities that complement each other and can even be combined in order to obtain the maximum preoperative information. In cases where the clinical and radiological assessment indicate benign or cystic lesions, FNAC is recommended, whereas for non-palpable lesions and those with microcalcifications, CNB represents a better alternative.
Fine needle aspiration cytology, with or without image guidance, may provide a rapid, cost-effective, fairly sensitive and specific diagnosis of breast lesions (1). As with tissue sections, the gained aspirates may be employed for all types of immunocytochemistry and ancillary techniques. Except for the pathologist’s experience, another prerequisite to ensure trustworthy results is, however, the presence of adequate aspirate with high cellular yields after multiple passes and excursions per pass. Notably, although cytological smears may clearly detect cancer cells, reliable prediction of malignancy, lymphovascular and/or perineural invasion, mitosis, or percentage of the ductal carcinoma in situ (DCIS) component, is virtually impossible.
The sensitivity, specificity, positive and negative predictive values of CNB are comparable, if not slightly better, with FNAC (2). Importantly, CNB seems to better assess the tissue architecture, provide more tissue material for various diagnostic tests such as fluorescence in situ hybridization (FISH), and report the prognostic parameters that FNAC does not determine. To be more accurate, this method adequately distinguishes in situ from invasive carcinoma and, in addition, we can assess prognostic indicators such as histological type and histological grade of malignancy (although often underestimated). We can also estimate predictive markers with a fair degree of reliability, especially the expression level of hormone receptors (estrogen receptor (ER) and progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2/neu) in patients who are planned to receive preoperative (neoadjuvant) chemotherapy. Nevertheless, CNB is a relatively expensive, more ”dangerous” procedure which necessitates the infrastructure for imaging and tissue processing.
To increase the diagnostic accuracy and sensitivity of the method in non-palpable lesions and especially in the case of microcalcifications, the techniques of guided, stereotactic CNB with the help of ultrasound, mammography or magnetic resonance imaging (MRI) have been developed. From the studies performed so far, it appears that vacuum assisted CNB shows better sensitivity and specificity for microcalcifications and architectural disruption (3, 4).
During the histological assessment of the lesion, the correlation of histological with imaging findings is crucial for a correct diagnosis, especially in cases of microcalcifications. With regard to histological diagnosis, the B-classification is used for CNB material, which categorizes lesions from B1 to B5. It is known that some of the lesions, which belong to category B3 in the B-classification, have an increased probability of being underestimated in CNB, but to be of higher grade in the surgical resection material. These heterogeneous groups of lesions are of “uncertain malignant potential” and vary from atypical ductal hyperplasia (ADH) or flat epithelial atypia (FEA), to lobular neoplasia (LN), papillary lesions, and radial scars (5).
Depending on the results of the CNB biopsy, the necessity or not of surgical approach to the lesion, as well as its type or extent, is determined, avoiding fast-track biopsy, which is known to have higher rates of misdiagnosis. Thus, with the current data, fast-track biopsy has no indication in undetected breast lesions and offers no additional information on positive CNB, being now limited to controlling the sentinel lymph node on already diagnosed carcinoma or, in some cases, the surgical resection margin.
The surgically removed tumor (sample) should be always oriented so that the resection margins might be correctly assessed. In case the most severe identified lesion is an in situ carcinoma of the breast, the degree of nuclear malignancy (nuclear grade), the architectural growth pattern (eg, solid), the presence or absence of necrosis, and the presence of microcalcifications should be indicated in the histological report.
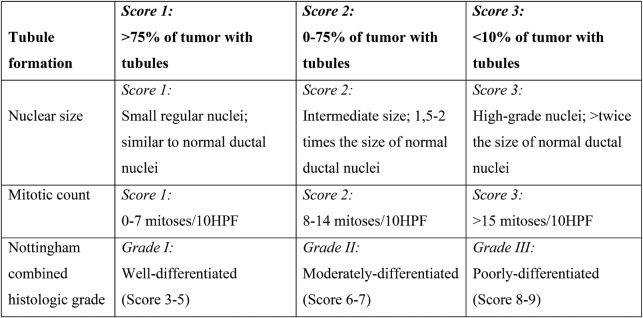
For invasive breast carcinoma, the histological report should include the size of the invasive tumor in mm, the histological type, the grade (Nottingham–Bloom–Richardson system: 1-3 (tubule formation, nuclear size, and mitotic count) (Table 1) (6), the development of one or more tumor foci, the presence of vascular infiltration, the presence of microcalcifications, the presence of concomitant DCIS, the presence of lobular neoplasia, the presence of Paget's disease of the nipple and the resection margins (distance from the nearest relevant margin in mm).
If a sentinel lymph node was removed or several axillary lymph nodes were sampled, and/or axillary lymphadenectomy was performed, the total number of lymph nodes sent for histological examination should be defined, in conjunction with the number of lymph nodes showing isolated tumor cells (ITC), micrometastasis, or metastatic infiltration (>2 mm). Eventual presence of peri-nodular extension of neoplastic cells also needs to be reported.
Together, the aforementioned aspects determine the pathological Tumor, Node, and Metastasis (pTNM) stage of the disease, which in combination with the histological grade of malignancy and the immunohistochemical expression of the molecular markers ER, PR and HER2, contribute to the identification of the prognostic American Joint Committee on Cancer (AJCC) stage (7). Of note, in cases where HER2 immunohistochemical results are inconclusive, in situ hybridization testing may be required.
Last but not least, additional immunohistochemical markers are often determined, either for diagnostic purposes (identification of a breast lesion or a specific histological type of carcinoma) or therapeutically (in cases of targeted therapy with monoclonal antibodies), including p53, Ki-67, E-Cadherin, Chromogranin, etc.
Altogether, the present work suggests an up-to-date, efficient and easily applicable diagnostic approach for breast lesions (Figure 1). Its benefits are not restricted only to its high sensitivity and specificity in the context of early breast cancer detection. Most importantly, this diagnostic approach allows for a timely exclusion of an assumed malignancy, hence significantly reducing the worries and the associated stress women with breast lesions may experience until final histological characterization of a newly identified breast tumor.
Conflict of interests: none declared.
Financial support: none declared.
TABLE 1.
Nottingham–Bloom–Richardson system
FIGURE 1.
Diagnostic algorithm for newly detected breast lesions
Contributor Information
Nikolaos GARMPIS, N.S. Christeas Laboratory of Experimental Surgery and Surgical Research, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Iason PSILOPATIS, Charité – Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt – Universität zu Berlin, Berlin, Germany.
Dimitrios DIMITROULIS, Charité – Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt – Universität zu Berlin, Berlin, Germany.
Anna GARMPI, First Department of Propedeutic Internal Medicine, Laiko General Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Konstantinos NIKOLETTOS, Obstetric and Gynecologic Clinic, Medical School, Democritus University of Thrace, Alexandroupolis, Greece.
Kleio VRETTOU, Department of Cytopathology, Sismanogleio General Hospital, Athens, Greece.
References
- 1.Mitra S, Dey P. Fine-needle aspiration and core biopsy in the diagnosis of breast lesions: A comparison and review of the literature. Cytojournal. 2016;13:18. doi: 10.4103/1742-6413.189637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti I. FNAC Versus CNB: Who wins the match in breast lesions? J Cytol. 2018;35:176–178. doi: 10.4103/JOC.JOC_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park HL, Hong J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg. 2014;3:120–127. doi: 10.3978/j.issn.2227-684X.2014.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang XC, Hu XH, Wang XR, et al. A comparison of diagnostic performance of vacuum-assisted biopsy and core needle biopsy for breast microcalcification: a systematic review and meta-analysis. Ir J Med Sci. 2018;187:999–1008. doi: 10.1007/s11845-018-1781-6. [DOI] [PubMed] [Google Scholar]
- 5.Rakha EA, Ellis IO. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol. 2007;60:1300–306. doi: 10.1136/jcp.2006.045377. [DOI] [PMC free article] [PubMed] [Google Scholar]