Abstract
Objective:
Assess associations of social determinants of health (SDoH) using area deprivation index (ADI), race/ethnicity and insurance type with textbook outcomes (TO).
Background:
Individual- and contextual-level SDoH affect health outcomes, but only one SDoH level is usually included.
Methods:
Three healthcare system cohort study using National Surgical Quality Improvement Program (2013–2019) linked with ADI risk-adjusted for frailty, case status, and operative stress examining TO/TO components (unplanned reoperations, complications, mortality, emergency department/observation stays, and readmissions).
Results:
Cohort (34,251 cases) mean age 58.3 [SD = 16.0], 54.8% females, 14.1% Hispanics, 11.6% Non-Hispanic Blacks, 21.6% with ADI >85, and 81.8% TO. Racial and ethnic minorities, non-private insurance, and ADI >85 patients had increased odds of urgent/emergent surgeries (adjusted odds ratios [aORs] range: 1.17–2.83, all P < 0.001). Non-Hispanic Black patients, ADI >85 and non-Private insurances had lower TO odds (aORs range: 0.55–0.93, all P < 0.04), but ADI >85 lost significance after including case status. Urgent/emergent versus elective had lower TO odds (aOR = 0.51, P < 0.001). ADI >85 patients had higher complication and mortality odds. Estimated reduction in TO probability was 9.9% (95% confidence interval [CI] = 7.2%–12.6%) for urgent/emergent cases, 7.0% (95% CI = 4.6%–9.3%) for Medicaid, and 1.6% (95% CI = 0.2%–3.0%) for non-Hispanic Black patients. TO probability difference for lowest-risk (White-Private-ADI <85-elective) to highest-risk (Black-Medicaid-ADI >85-urgent/emergent) was 29.8% for very frail patients.
Conclusion:
Multilevel SDoH had independent effects on TO, predominately affecting outcomes through increased rates/odds of urgent/emergent surgeries driving complications and worse outcomes. Lowest-risk versus highest-risk scenarios demonstrated the magnitude of intersecting SDoH variables. Combination of insurance type and ADI should be used to identify high-risk patients to redesign care pathways to improve outcomes. Risk adjustment including contextual neighborhood deprivation and patient-level SDoH could reduce unintended consequences of value-based programs.
Keywords: social risk factors, surgical outcomes, vulnerable populations, safety-net hospitals
Mini-Abstract
Retrospective cohort study (34,251 cases) examined multilevel Social Determinants of Health on Textbook Outcomes (TO). Area deprivation index (ADI) > 85, non-private insurance and non-Hispanic Black patients had lower TO odds, adjusting for urgent/emergent cases eliminated the ADI > 85 association. Non-private insurance and ADI > 85 patients had more urgent/emergent cases, increasing odds of complications/readmissions.
INTRODUCTION
Social determinants of health (SDoH) are the conditions of places in which people live, work, and play.1 SDoH are a multilevel construct, comprised of both individual- and contextual-level factors as highlighted in the newly released Centers for Medicare and Medicaid Services Framework for Health Equity.1 Both individual- and contextual-level SDoH contribute to inequitable social conditions,2 which directly and indirectly affect health,3 life expectancy, and outcomes.4,5 Individual SDoH such as low income may result in poorer living conditions and fewer opportunities to prioritize health and access to healthcare. Contextual SDoH such as area deprivation may exacerbate or create challenges by limiting access to healthy food options, clean and safe living conditions or physical access to care.6 For surgical outcomes specifically, socially determined barriers or delays to care may result in patients preoperatively presenting with more acute conditions,7–9 requiring urgent or emergent surgeries,7–9 and postoperatively facing more difficulties paying for medications, caring for surgical wounds or obtaining time off from work for recovery. Furthermore, value-based medicine programs have disproportionately affected safety-net hospitals (SNH) serving populations with disadvantaged SDoH,10,11 unintentionally exacerbating health disparities. The Hospital Readmission Reduction Program and other pay for performance programs have penalized SNH for worse outcomes,10,12,13 further limiting resources to treat disadvantaged populations and potentially shifting resources from higher to lower safety-net burden hospitals.14
Many studies using large administrative databases classifying hospitals as high or low safety-net burden found that high-burden hospitals have higher complication rates.15–17 Administrative databases lack detailed data on patient risk factors and outcomes.18–20 Variations in assigning ICD-9/10 codes across institutions further adds to inaccuracies.19 Area deprivation scores are absent or included at the county or the ZIP code level, which is too large of an area to accurately assess patients at the neighborhood level. For surgical patients, cases are mostly classified as elective or emergent; however, urgent surgeries have worse outcomes than elective cases21 and are more prevalent in vulnerable populations.16 Finally, while administrative database studies are “generalizable,” comparisons between high-burden and low-burden healthcare systems cannot determine whether differences are due to patient risk factors or quality of care leading to speculation that these studies measured differences in patient populations rather than quality of care.7
While individual- and contextual-level SDoH affect health outcomes,3,22,23 risk adjustment is challenging as few of these factors are collected in Electronic Health Records (EHR).24 Contextual-level SDoH measures include area-level socioeconomic status (SES) variables (eg, median income; percent poverty) or indices such as the area deprivation index (ADI),25 social vulnerability index,26 or the distressed community index.27 Indices using patient ZIP codes or counties showed mixed effects on outcomes23,26,28–30 likely due to the lack of granularity.22,31 To address these limitations, the National Academy of Medicine suggested using the more granular “Block Group”-level ADI4 and Centers for Medicare & Medicaid Services (CMS) are considering adding ADI to risk adjustment.2 High ADI scores signify higher neighborhood deprivation and were associated with increased readmissions for colorectal surgeries.22 Similarly, low ADI for Medicare beneficiaries was associated with improved postoperative outcomes; however, patients identified as White compared to Black disproportionately benefitted.32 Other studies found disparities in diagnosis, treatment, and survival for patients with pancreatic cancer33,34 and breast cancer35 which were associated with residential racial segregation which is a root cause of disadvantaged SDoH.36 However, because the effect of individual- and contextual-level SDoH on outcomes may vary by outcome type, composite measures such as “Textbook Outcomes” (TO) often provide more comprehensive assessments of surgical outcomes than single variables.30,37,38
We designed this study using high-quality, nurse-abstracted data enriched with EHR/billing data and linked with ADI to examine these associations in a cohort of patients from three academic healthcare systems. To our knowledge, no publications have examined the associations between postoperative TO, block group ADI and patient-level social risk factors (eg, insurance type, race/ethnicity as a social construct39). We hypothesize that ADI, insurance type, and race/ethnicity have independent effects on TO. This study is especially timely as attention to SDoH has increased over the past decade,24 including the introduction of legislature to address health disparities through SDoH.40
MATERIALS AND METHODS
Study Population and Data
This study included patients undergoing inpatient procedures in the 2013–2019 American College of Surgeons National Surgical Quality Improvement Program (NSQIP), from three academic healthcare systems following STROBE Reporting Guidelines. NSQIP variables were used for retrospective cohort identification, as well as providing standardized definitions of preoperative risk factors and complications.41 Working within each site, locally identified NSQIP data were merged with multiple data sources including EHR and the ADI25 at each site. Patient self-reported race and ethnicity were derived from NSQIP variables and EHR. After finalizing the data linkage, local data were deidentified before merging the combined data for analyses. The Institutional Review Boards of the University of Pittsburgh Healthcare System (UPMC) and University of North Carolina agreed to rely on the University of Texas Health San Antonio Institutional Review Board, which approved this study with a waiver of informed consent.
Area Deprivation Index
Area deprivation index (ADI) is a composite measure of 17 education, employment, housing-quality, and poverty measures from the American Community Survey at the block group-level. ADI identifies patients living in disadvantaged neighborhoods,25,42 ranking block groups from 1 to 100, with higher values indicating higher levels of socioeconomic deprivation.25 Patients’ home addresses were geocoded to the block group-level using ArcGIS 10.7 desktop version to assign 2018 ADI scores. Post office box addresses were excluded. We defined “Highly deprived” neighborhoods as ADI >85 representing the top 15th percentile of deprivation. Sensitivity analyses examined ADI >75 as the highest-risk quartile of deprivation.
Estimating Patient Frailty/Premorbid Conditions
The risk analysis index (RAI) assesses frailty using variables available in NSQIP. It has been validated previously in multiple datasets43–45 and renders a score ranging from 0 to 81 categorized as robust (≤20), normal (21–29), frail (30–39), and very frail (≥40).43 RAI is used as a single variable estimate of patient-level variability that overcomes barriers to model fit encountered by less parsimonious models.46–49
Expanded Operative Stress Score Assignment and Case Status
The operative stress score (OSS) estimates surgical-induced physiologic stress of procedures across surgical specialties based on CPT codes by assigning a score ranging from 1 to 5, with 1 and 5 representing very low and very high physiological stress, respectively. Similar to the RAI, the OSS is a single variable estimate of procedure-level variability, overcoming barriers to model fit encountered with less parsimonious models. We used the expanded OSS48 with 2343 CPT codes, providing improved case coverage for nonmajority male populations compared to the original OSS.47 We used the 5 levels of the expanded OSS rather than individual CPT codes to alleviate problems with model convergence encountered when a diversity of less common procedures are included to better represent the range of surgeries performed at hospitals. After excluding cases without an expanded OSS assigned to the principal CPT code, OSS was assigned using the highest score for all available procedures within each case.48
Case status was determined from NSQIP variables with urgent cases being defined as “no” responses to elective and emergency variables.21 Given their unplanned status, urgent and emergent surgeries were combined and compared to elective cases.
Unplanned Reoperations, Clavien-Dindo IV, and Mortality 30-day Complications
We used the NSQIP REOPERATION variable to define unplanned reoperations. Clavien-Dindo classifies complications based on their treatments50; Level IV complications were approximated using the NSQIP variables of postoperative septic shock, postoperative dialysis, pulmonary embolus, myocardial infarction, cardiac arrest, prolonged ventilation, reintubation, or stroke‚ as previously reported.48,51 Mortality occurring within 30 days from the ‚date of surgery was determined using NSQIP variables and EHR augmented by state mortality and Social Security Death Master File data.
30-Day Emergency Department Visits/Observation Stays and Readmissions
NSQIP only tracks patients for 30 days after surgery and contains 30-day readmission variables from the date of surgery. We merged NSQIP data with EHR to determine readmissions and emergency department visits/observation stays (EDOS) within 30 days of discharge from the index procedure’s hospitalization, to be consistent with Medicare’s Hospital Readmission Reduction Program definition of 30-day readmissions.
TO Composite Variable
TO is a composite variable, increasingly used as a quality metric, which uses various definitions to define optimal postoperative outcomes as the absence of undesirable outcomes such as complications. Lower odds of TO are associated with worse outcomes while higher odds of TO are associated with better outcomes.52,53 Multiple TO definitions have been used in surgical oncology.30,37,38 We defined TO as surgeries not having any of the following undesirable events: (1) unplanned reoperations, (2) Clavien-Dindo IV (CDIV), (3) mortality, (4) EDOS, and (5) readmissions. Complications (reoperations, CDIV, and mortality) were tracked for 30 days after the date of surgery, while EDOS and readmissions were tracked for 30 days after the date of hospital discharge.
Insurance Type
Identified, local NSQIP data were merged with local EHR and managerial accounting data to determine insurance type. Insurance type was categorized by encounter billing data supplemented with EHR data and defined as (1) Private including Tricare and Workers’ Compensation, (2) Medicare, (3) Medicaid, and (4) Uninsured including self-pay and county indigent care programs. “Other” insurance type included encounters charged to the Department of Corrections, liability insurance, Veterans Affairs, unknown/missing and other state payor types; these cases were excluded.
Study Outcomes
Our primary outcome was the differential associations of race/ethnicity, ADI, and insurance type with TO for the index surgery adjusted for RAI, OSS, and case status with secondary analyses of the components of TO. Subanalyses assessed preoperative presentation acuity using urgent/emergent cases.
Management of Missing Variables
Cases were excluded due to missing (1) expanded OSS coverage of principal CPT code, (2) ADI assignment secondary to group quarters or low population and housing,25 (3) case status, 4) race/ethnicity, (5) insurance type, and (6) variables used to calculate the RAI. NSQIP stopped collecting cognitive decline variables after 2012. We treated this missing variable as not having any cognitive decline for all patients in the calculation of RAI, as previously reported.45 Comparison of RAI models before and after 2012 showed that missing cognitive decline variable, because of its rare occurrences, had minimal effects on model discrimination and calibration.45
Statistical Analysis
Categorical data were summarized using counts and percentages and continuous data using mean and standard deviation (SD). Chi-square tests and F-tests were used to test for differences between groups for categorical and continuous variables.
We used nested random intercept logistic regression models to assess the association between race/ethnicity, ADI and insurance type with TO and the 5 components of TO adjusting for RAI, OSS, and case status. The random effect was used to account for clustering within each healthcare system. We calculated marginal effects from these final models (a predicted increase or decrease in the probability of an outcome associated with each predictor variable). To illustrate the magnitude of intersecting risks, we calculated predicted probabilities for TO for lowest- and highest-risk patient scenarios based upon the odds ratios from the final nested TO model for the race/ethnicity, ADI, insurance and case status groups versus the reference group. We additionally performed secondary analyses using random intercept logistic regression models to assess the association between SDoH and case status. We conducted sensitivity analyses using ADI >75 as a cutoff using a broader categorization of deprivation and adding age groups 18–44, 45–64, and ≥65 years as predictor variables. Analyses were performed using R 4.1.1 (R Project for Statistical Computing).
RESULTS
Population Characteristics
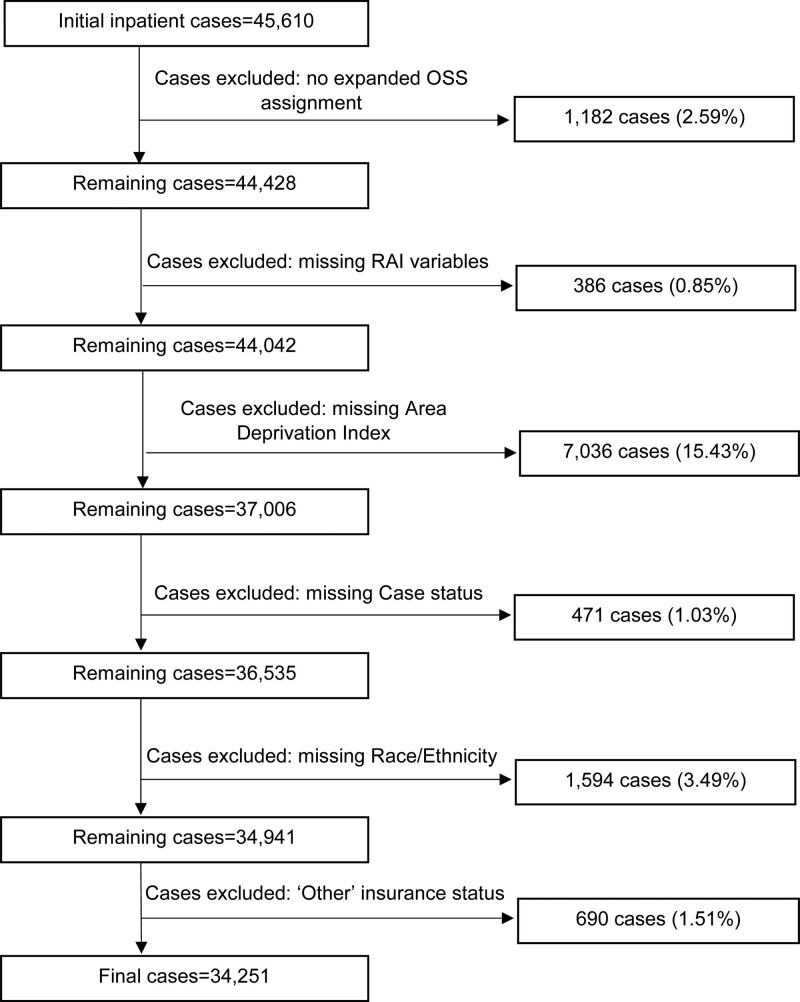
The cohort included 34,251 inpatient cases present in the 2013–2019 NSQIP (Fig. 1). Clinical site burden (Supplemental Table 1, http://links.lww.com/AOSO/A202) was determined by the percent of uninsured patients at each site as high (35.5%), medium (6.9%) and low (0.7%). Most cases (Table 1) were performed on Non-Hispanic White patients (72.6%), followed by Hispanic (14.1%) and Non-Hispanic Black patients (11.6%). Patients had a mean (SD) age of 58.3 (16.0) years, slightly more than half were females (54.8%) and 21.6% of the cohort lived in highly deprived neighborhoods (ADI>85).
FIGURE 1.
Flow diagram of study cohort. National Surgery Quality Improvement Program inpatient cases from 2013 to 2019. Cases were excluded for having no expanded OSS assignment for the principal CPT code, missing variables used to calculate the RAI, missing home address used to assign an ADI/or ADI not assigned to the block group, missing case status, missing race/ethnicity and categorized as “other” insurance type. “Other” insurance status was defined as including encounters charged to the Department of Corrections, liability insurance, Veterans Administration, unknown/missing, and other state payor types. Cases lacking an expanded OSS assignment for the principal CPT code were excluded to avoid erroneously assigning a lower stress OSS based upon additional procedures that were performed. For example, a principal CPT code for a highly stressful procedure not assigned an expanded OSS could be assigned an OSS1 if the additional CPT codes contained any procedure with an expanded OSS rating.
TABLE 1.
Patient Characteristics and Clinical Outcomes by Insurance Type
| Overall | Private | Medicare | Medicaid | Uninsured | P | |
|---|---|---|---|---|---|---|
| Number (%)* | 34,251 | 13,546 (39.5) | 14,907 (43.5) | 2826 (8.3) | 2972 (8.7) | |
| Age [mean (SD)] | 58.3 (16.0) | 50.4 (12.6) | 70.0 (11.56) (11.55) | 46.7 (13.6) | 47.2 (12.4) | <0.001 |
| Sex (Female) | 18,760 (54.8) | 7991 (59.0) | 7677 (51.5) | 1577 (55.8) | 1515 (51.0) | <0.001 |
| Race/ethnicity | <0.001 | |||||
| Black, non-Hispanic | 3982 (11.6) | 1429 (10.5) | 1654 (11.1) | 636 (22.5) | 263 (8.8) | |
| Hispanic, any race | 4816 (14.1) | 1012 (7.5) | 1078 (7.2) | 844 (29.9) | 1882 (63.3) | |
| Multiracial, non-Hispanic | 573 (1.7) | 277 (2.0) | 125 (0.8) | 97 (3.4) | 74 (2.5) | |
| White, non-Hispanic | 24,880 (72.6) | 10,828 (79.9) | 12,050 (80.8) | 1249 (44.2) | 753 (25.3) | |
| RAI | <0.001 | |||||
| Robust (≤20) | 18,571 (54.2) | 10,509 (77.6) | 3369 (22.6) | 2182 (77.2) | 2511 (84.5) | |
| Normal (21–29) | 11,593 (33.8) | 2159 (15.9) | 8667 (58.1) | 418 (14.8) | 349 (11.7) | |
| Frail (30–39) | 3657 (10.7) | 842 (6.2) | 2533 (17.0) | 192 (6.8) | 90 (3.0) | |
| Very frail (≥40) | 430 (1.3) | 36 (0.3) | 338 (2.3) | 34 (1.2) | 22 (0.7) | |
| ADI >85 (highly deprived) | 7408 (21.6) | 2190 (16.2) | 3050 (20.5) | 1065 (37.7) | 1103 (37.1) | <0.001 |
| Clinical sites† | <0.001 | |||||
| High-burden | 6225 (18.2) | 1446 (10.7) | 1603 (10.8) | 967 (34.2) | 2209 (74.3) | |
| Medium-burden | 8991 (26.3) | 3802 (28.1) | 3519 (23.6) | 1047 (37.0) | 623 (21.0) | |
| Low-burden | 19,035 (55.6) | 8298 (61.3) | 9785 (65.6) | 812 (28.7) | 140 (4.7) | |
| Case status | <0.001 | |||||
| Elective | 22,871 (66.8) | 10,389 (76.7) | 9588 (64.3) | 1595 (56.4) | 1299 (43.7) | |
| Urgent | 7762 (22.7) | 2071 (15.3) | 3530 (23.7) | 871 (30.8) | 1290 (43.4) | |
| Emergent | 3618 (10.6) | 1086 (8.0) | 1789 (12.0) | 360 (12.7) | 383 (12.9) | |
| Expanded OSS (surgical-induced physiologic stress) | <0.001 | |||||
| OSS1 (very low) | 339 (1.0) | 107 (0.8) | 107 (0.7) | 51 (1.8) | 74 (2.5) | |
| OSS2 (low) | 7050 (20.6) | 2760 (20.4) | 2735 (18.3) | 688 (24.3) | 867 (29.2) | |
| OSS3 (moderate) | 19,611 (57.3) | 7985 (58.9) | 8465 (56.8) | 1563 (55.3) | 1598 (53.8) | |
| OSS4 (high) | 6197 (18.1) | 2373 (17.5) | 2963 (19.9) | 461 (16.3) | 400 (13.5) | |
| OSS5 (very high) | 1054 (3.1) | 321 (2.4) | 637 (4.3) | 63 (2.2) | 33 (1.1) | |
| 30-day complications‡ | ||||||
| Reoperation | 2090 (6.1) | 658 (4.9) | 1040 (7.0) | 213 (7.5) | 179 (6.0) | <0.001 |
| CDIV | 1783 (5.2) | 372 (2.7) | 1105 (7.4) | 179 (6.3) | 127 (4.3) | <0.001 |
| Mortality | 747 (2.2) | 125 (0.9) | 544 (3.6) | 57 (2.0) | 21 (0.7) | <0.001 |
| 30-day EDOS§ | 1,914 (5.6) | 580 (4.3) | 717 (4.8) | 269 (9.5) | 348 (11.7) | <0.001 |
| 30-day readmissions§ Readmissionsd | 1628 (4.8) | 406 (3.0) | 620 (4.2) | 267 (9.4) | 335 (11.3) | <0.001 |
| TO | 28,015 (81.8) | 11,815 (87.2) | 11,902 (79.8) | 2093 (74.1) | 2205 (74.2) | <0.001 |
Bolded P-values indicate significance at the P < .05 level.
*Percent calculation by row, the rest of the percent calculations were by column.
†Burden level categorized based upon the proportion of uninsured patients at each clinical site.
‡Complications defined as 30 days from date of the index surgery.
§EDOS and Readmissions defined as 30 days from date of hospital discharge of the index surgery; EDOS and Readmissions were evaluated independently; membership in 1 group does not exclude a case from membership in the other.
Increased Odds of Urgent/Emergent Cases Among Racial and Ethnic Minorities, Non-Private Insurance and ADI >85
Patients from the racial and ethnic minority groups of Black, non-Hispanic (adjusted odds ratio [aOR] = 1.34, 95% CI = 1.23–1.45, P < 0.001; Table 2), Hispanic, any race (aOR = 1.36, 95% CI = 1.23–1.50, P < 0.001) and Multiracial, non-Hispanic (aOR = 1.33, 95% CI = 1.09–1.62, P = 0.004) had higher odds of undergoing urgent/emergent cases versus patients identified as White. Patients living in highly deprived areas (aOR = 1.17, 95% CI = 1.10–1.24, P < 0.001) had higher odds of undergoing urgent/emergent versus elective procedures. Medicare (aOR = 1.56, 95% CI = 1.47–1.66, P < 0.001), Medicaid (aOR = 2.36, 95% CI = 2.15–2.59, P < 0.001), and Uninsured (aOR = 2.83, 95% CI = 2.56–3.12, P < 0.001) groups had higher odds of urgent/emergent procedures versus Private. Marginal effects for the probability of undergoing an urgent/emergent procedure were highest for very frail patients (37.1%), Uninsured (21.5%), and Medicaid (17.5%) patients.
TABLE 2.
Urgent/Emergent Case Status Adjusted for Race/Ethnicity, Frailty, ADI, and Insurance Type
| Urgent/Emergent | ||||||
|---|---|---|---|---|---|---|
| Predictors | aOR | 95% CI | P | aΔU/E (%) | 95% CI (%) | P |
| Race/ethnicity (Ref = White, non-Hispanic) | ||||||
| Black, non-Hispanic | 1.336 | 1.232–1.450 | <0.001 | 5.8 | 3.8–7.8 | <0.001 |
| Hispanic, any race | 1.357 | 1.227–1.501 | <0.001 | 6.1 | 3.8–8.5 | <0.001 |
| Multiracial, non-Hispanic | 1.332 | 1.094–1.621 | 0.004 | 5.7 | 1.5–9.9 | 0.007 |
| RAI (Ref = normal 21–29) | ||||||
| Robust (≤20) | 0.903 | 0.849–0.961 | 0.001 | −2.0 | −3.2 to −0.7 | 0.003 |
| Frail (30-39) | 1.597 | 1.475–1.729 | <0.001 | 9.7 | 7.5–11.9 | <0.001 |
| Very frail (≥40) | 5.861 | 4.652–7.383 | <0.001 | 37.1 | 32.7–41.5 | <0.001 |
| ADI >85 (Ref = ADI ≤85) | 1.168 | 1.100–1.239 | <0.001 | 3.1 | 1.7–4.4 | <0.001 |
| Insurance (Ref = Private) | ||||||
| Medicare | 1.561 | 1.466–1.661 | <0.001 | 8.6 | 6.2–11.0 | <0.001 |
| Medicaid | 2.361 | 2.150–2.593 | <0.001 | 17.5 | 13.7–21.3 | <0.001 |
| Uninsured | 2.829 | 2.561–3.124 | <0.001 | 21.5 | 17.5–25.6 | <0.001 |
Bolded P-values indicate significance at the P < .05 level.
aΔU/E indicates adjusted change in the probability of Urgent/Emergent case status; Ref, reference value.
Decreased Odds of TO (Worse Outcomes) Among Black Non-Hispanic Patients, Non-Private Insurance, and ADI >85
Nested models controlling for frailty and operative stress demonstrated that Black Non-Hispanic, ADI>85 and non-Private insurances were associated with lower odds of TO (Table 3, M1 and M2), but ADI was not significant after adding urgent/emergent cases (Table 3, M3). Odds of TO decreased from Uninsured (aOR = 0.87, 95% CI = 0.78–0.97, P = 0.015) to Medicare (aOR = 0.74, 95% CI = 0.68–0.80, P < 0.001), and Medicaid (aOR = 0.61, 95% CI = 0.55–0.68, P < 0.001, Table 3 M3) versus Private. Estimated probability differences of TO were −9.9% (95% CI = −12.6% to −7.2%) for urgent/emergent cases, −7.0% (95% CI = −9.3% to −4.6%) for Medicaid, and −1.6% (95% CI = −3.0% to −0.2%) for Black, non-Hispanic patients (Table 3 Marginal Effects M3). Using M3 with moderate-stress procedures (OSS3), the probabilities of TO were estimated for the lowest-risk and highest-risk groups stratified by frailty (Table 4). As frailty increased, the difference between lowest and highest risk increased. For instance, very frail patients at lowest-risk (White, non-Hispanic, Private, ADI ≤85, elective surgery) versus highest-risk (Black, non-Hispanic, Medicaid, ADI >85, urgent/emergent surgery) had a TO probability of 77.3% and 47.6%, respectively, a difference of 29.8%.
TABLE 3.
TO and Marginal Effects on Textbook Outcomes using 3 Nested Models (M1–M3) Adjusted for Race/Ethnicity, RAI*, OSS†, ADI, Insurance, and Case Status.
| TO M1 | TO M2 | TO M3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | aOR | 95% CI | P | aOR | 95% CI | P | aOR | 95% CI | P |
| Race/Ethnicity (Ref = White, non-Hispanic) | |||||||||
| Black, non-Hispanic | 0.832 | 0.758–0.914 | <0.001 | 0.872 | 0.794–0.958 | 0.004 | 0.894 | 0.813–0.982 | 0.020 |
| Hispanic, any race | 0.924 | 0.829–1.031 | 0.157 | 0.974 | 0.872–1.088 | 0.637 | 1.011 | 0.904–1.131 | 0.848 |
| Multiracial, non-Hispanic | 0.855 | 0.687–1.063 | 0.159 | 0.866 | 0.695–1.079 | 0.200 | 0.887 | 0.710–1.107 | 0.290 |
| ADI >85 (Ref = ADI ≤85) | 0.888 | 0.828–0.952 | 0.001 | 0.927 | 0.865–0.995 | 0.035 | 0.950 | 0.885–1.019 | 0.153 |
| Insurance (Ref = Private) | |||||||||
| Medicare | 0.697 | 0.647–0.752 | <0.001 | 0.738 | 0.684–0.796 | <0.001 | |||
| Medicaid | 0.550 | 0.495–0.611 | <0.001 | 0.614 | 0.552–0.683 | <0.001 | |||
| Uninsured | 0.756 | 0.676–0.845 | <0.001 | 0.868 | 0.775–0.973 | 0.015 | |||
| Urgent/emergent (Ref = Elective) | 0.511 | 0.480–0.544 | <0.001 | ||||||
| Marginal Effects | |||||||||
| TO M1 | TO M2 | TO M3 | |||||||
| Predictors | aΔTO (%) | 95% CI | P | aΔTO (%) | 95% CI | P | aΔTO (%) | 95% CI | P |
| Race/ethnicity (Ref = White, non-Hispanic) | |||||||||
| Black, non-Hispanic | −2.7 | −4.3 to −1.1 | 0.001 | −2.0 | −3.5 to −0.5 | 0.010 | −1.6 | −3.0 to −0.2 | 0.028 |
| Hispanic, any race | −1.1 | −2.7 to 0.5 | 0.172 | −0.4 | −1.9 to 1.2 | 0.640 | 0.1 | −1.4 to 1.7 | 0.848 |
| Multiracial, non-Hispanic | −2.3 | −5.6 to 1.1 | 0.186 | −2.1 | −5.4 to 1.3 | 0.224 | −1.7 | −5.0 to 1.6 | 0.308 |
| ADI >85 (Ref = ADI ≤85) |
−1.7 | −2.8 to −0.6 | 0.003 | −1.1 | −2.1 to −0.0 | 0.046 | −0.7 | −1.7 to 0.3 | 0.163 |
| Insurance (Ref = Private) | |||||||||
| Medicare | −4.9 | −6.7 to −3.1 | <0.001 | −4.1 | −5.6 to −2.6 | <0.001 | |||
| Medicaid | −8.7 | −11.7 to −5.7 | <0.001 | −7.0 | −9.3 to −4.6 | <0.001 | |||
| Uninsured | −3.7 | −5.6 to −1.8 | <0.001 | −1.8 | −3.4 to −0.2 | 0.024 | |||
| Urgent/emergent (Ref = elective) | −9.9 | −12.6 to −7.2 | <0.001 | ||||||
Bolded P-values indicate significance at the P < .05 level.
To defines an optimal outcome, lower odds of TO are associated with worse outcomes and higher odds of TO are associated with better outcomes.
*Adjustments for frailty by the RAI not shown.
†Adjustments for operative stress by the expanded OSS not shown.
aΔTO indicates adjusted change in the absolute probability of TO; Ref, reference value.
TABLE 4.
Lowest-Risk Versus Highest-Risk Scenarios on Probability of TO Based Upon Table 3 M3 Stratified by Frailty
| TO Probability | TO Probability | TO Probability Difference | |
|---|---|---|---|
| Frailty | Lowest-Risk White, non-Hispanic Private Insurance ADI ≤ 85 Elective surgery OSS3 (moderate) % |
Highest-Risk Black, non-Hispanic Medicaid ADI>85 Urgent/emergent surgery OSS3 (moderate) % |
Lowest-Highest Risk % |
| RAI | |||
| Robust (≤20) | 89.6 | 69.6 | 20.0 |
| Normal (21–29) | 87.7 | 65.5 | 22.2 |
| Frail (30–39) | 84.4 | 59.1 | 25.4 |
| Very frail (≥40) | 77.3 | 47.6 | 29.8 |
| TO probability difference Robust-Very frail (%) | 12.3 | 22.0 |
Bolded P-values indicate significance at the P < .05 level.
Third nested model (M3) from Table 3 used to estimate probabilities using the most common (57.3%) moderate stress surgery group (OSS3). Higher probabilities of TO are associated with better outcomes.
Associations Differ Across TO Components: Increased Odds (Worse Outcomes) of CDIV and Mortality for ADI >85 and EDOS and Readmissions for non-Private Insurance
Urgent/emergent versus elective cases had higher odds of reoperation, CDIV, and mortality (Table 5). Patients from highly deprived areas had higher odds of CDIV complications (aOR = 1.20, 95% CI = 1.07–1.35, P = 0.002) and mortality (aOR = 1.29, 95% CI = 1.07–1.54, P = 0.007). Compared to Private, Medicare patients had higher odds of unplanned reoperations, CDIV and mortality, while Medicaid patients had higher odds of unplanned reoperations and CDIV complications. Uninsured patients were younger, had lower frailty/RAI scores, and underwent lower stress surgeries (Supplemental Table 2, http://links.lww.com/AOSO/A202), had lower odds of mortality (aOR = 0.57, 95% CI = 0.35–0.93, P = 0.024) versus Private.
TABLE 5.
Complication*, EDOS,† and Readmission† Components of TO Adjusted for Race/Ethnicity, RAI‡, OSS§, ADI, Insurance, and Case Status
| Reoperation | CDIV | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | aOR | 95% CI | P | aOR | 95% CI | P | aOR | 95% CI | P |
| Race/ethnicity (Ref = White, non-Hispanic) | |||||||||
| Black, non-Hispanic | 1.137 | 0.982–1.317 | 0.085 | 1.087 | 0.920–1.285 | 0.327 | 0.868 | 0.671–1.123 | 0.282 |
| Hispanic, any race | 0.902 | 0.746–1.091 | 0.289 | 0.737 | 0.605–0.897 | 0.002 | 0.708 | 0.542–0.925 | 0.012 |
| Multiracial, non-Hispanic | 0.971 | 0.666–1.416 | 0.880 | 0.739 | 0.453–1.203 | 0.224 | 1.044 | 0.525–2.073 | 0.903 |
| ADI >85 (Ref = ADI ≤85) | 1.036 | 0.928–1.158 | 0.529 | 1.202 | 1.068–1.353 | 0.002 | 1.285 | 1.071–1.541 | 0.007 |
| Insurance (Ref = Private) | |||||||||
| Medicare | 1.190 | 1.058–1.338 | 0.004 | 1.685 | 1.469–1.932 | <0.001 | 1.473 | 1.183–1.835 | 0.001 |
| Medicaid | 1.395 | 1.177–1.653 | <0.001 | 1.575 | 1.292–1.919 | <0.001 | 1.402 | 0.999–1.966 | 0.050 |
| Uninsured | 1.000 | 0.823–1.216 | 0.998 | 0.829 | 0.659–1.044 | 0.111 | 0.567 | 0.347–0.927 | 0.024 |
| Urgent/emergent (Ref = Elective) |
1.662 | 1.509–1.831 | <0.001 | 3.517 | 3.157–3.919 | <0.001 | 8.711 | 7.180–10.567 | <0.001 |
| EDOS | Readmissions | ||||||||
| Predictors | aOR | 95% CI | P | aOR | 95% CI | P | |||
| Race/ethnicity (Ref = White, non-Hispanic) | |||||||||
| Black, non-Hispanic | 1.155 | 0.989–1.349 | 0.069 | 1.010 | 0.846–1.207 | 0.909 | |||
| Hispanic, any race | 1.245 | 1.055–1.468 | 0.009 | 0.971 | 0.831–1.135 | 0.711 | |||
| Multiracial, non-Hispanic | 1.147 | 0.810–1.624 | 0.439 | 1.263 | 0.894–1.783 | 0.185 | |||
| ADI >85 (Ref = ADI ≤85) | 1.013 | 0.905–1.135 | 0.820 | 0.948 | 0.837–1.074 | 0.403 | |||
| Insurance (Ref = Private) | |||||||||
| Medicare | 1.227 | 1.079–1.395 | 0.002 | 1.296 | 1.120–1.500 | 0.001 | |||
| Medicaid | 1.691 | 1.439–1.986 | <0.001 | 1.717 | 1.444–2.042 | <0.001 | |||
| Uninsured | 1.523 | 1.294–1.792 | <0.001 | 1.249 | 1.054–1.479 | 0.010 | |||
| Urgent/emergent (Ref = Elective) |
1.006 | 0.905–1.119 | 0.909 | 1.207 | 1.074–1.355 | 0.002 | |||
Bolded P-values indicate significance at the P < .05 level.
Ref indicates reference value.
*Defined as 30 days from date of the index surgery.
†Defined as 30 days from date of hospital discharge of the index surgery.
‡Adjustments for frailty by the RAI not shown.
§Adjustments for operative stress by the expanded OSS not shown.
Hispanic patients had higher odds of EDOS versus White, non-Hispanic patients (Table 5). Patients with non-Private insurance had higher odds of EDOS and readmissions. Urgent/emergent versus elective procedures had higher odds of readmissions (aOR = 1.21, 95% CI = 1.07–1.36, P = 0.002). Reoperation and CDIV complications were added as predictor variables for EDOS and readmission (Supplemental Table 3, http://links.lww.com/AOSO/A202). Reoperations were associated with higher odds of EDOS (aOR = 1.63, 95% CI = 1.38–1.93, P < 0.001) and readmissions (aOR = 4.63, 95% CI = 4.00–5.36, P < 0.001), while CDIV complications only increased the odds of readmissions (aOR = 1.38, 95% CI = 1.15–1.66, P < 0.001).
Sensitivity Analysis With ADI >75 and Adding Age as a Predictor Variable
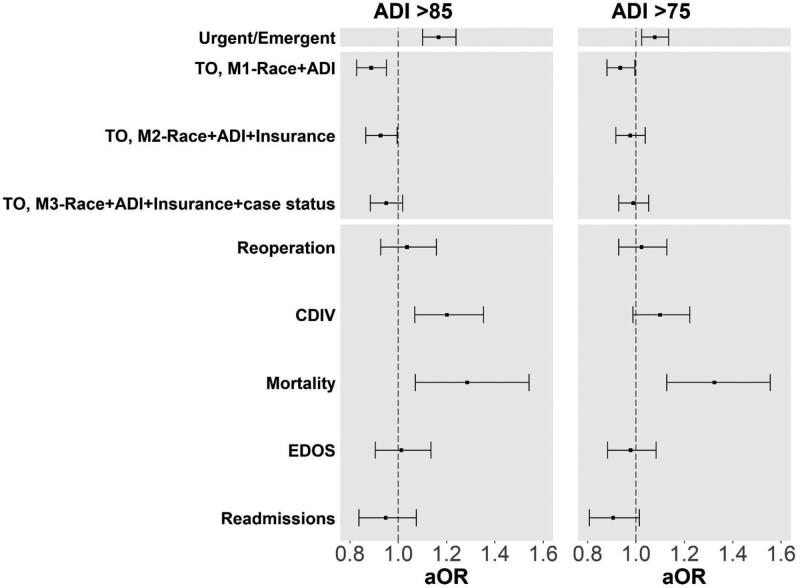
We expanded the definition of highly deprived to include patients living in the 25% most deprived block groups (Fig. 2). Regardless of cutoff, patients from highly deprived neighborhoods had higher odds of undergoing urgent/emergent cases. Similarly, patients with ADI >75 and ADI >85 had lower odds of TO after adjusting for race/ethnicity, operative stress, and frailty. ADI >85, but not ADI >75, continued to be significant after adjusting for insurance type (Table 3 M2). For TO components, ADI was a significant predictor of mortality regardless of which cutoff was used.
FIGURE 2.
Case status, TO and TO components with a sensitivity analysis using ADI >75 as a cutoff point. Forest plot of Tables 2, 3, and 5 (left) with a sensitivity analysis using the ADI >75 as a cutoff point (right). Using different ADI cutoffs resulted in 7408 (21.6%) and 12,340 (36.0%) of the cohort being classified as highly deprived for ADI >85 and ADI >75, respectively. Urgent/emergent cases (Table 2) were adjusted for race/ethnicity RAI/frailty, and insurance type. TO nested models (Table 3) were adjusted for RAI and expanded operative stress score in addition to the variables listed in models 1–3 (M1–M3). TO components (Table 5) were adjusted for RAI, expanded operative stress score, race/ethnicity, insurance type, and urgent/emergent case status (M3 model variables).
Although RAI/frailty included age, we added age as a separate predictor (Supplemental Figure 1, http://links.lww.com/AOSO/A202). ADI >85 continued to be significantly associated with TO in M1 which included race/ethnicity, but not in M2 which included insurance type. The addition of age did not alter the results for the TO components.
DISCUSSION
This study examined independent associations between TO and (1) contextual-level SDoH measured by ADI and (2) individual-level SDoH using race/ethnicity as a social construct39 and insurance type. ADI, race/ethnicity, and insurance type were each independently associated with worse outcomes as demonstrated by lower odds of TO but the association with ADI lost significance after adjusting for urgent/emergent cases. Associations with the TO components varied by social risk factor but were predominantly associated with urgent/emergent cases, suggesting that increased urgent/emergent surgeries among patients from deprived neighborhoods lead to increased complications and lower odds of achieving TO. The current study lays the groundwork for healthcare systems to use their own data to understand their populations. This roadmap can assist healthcare systems explore patterns in outcomes that may identify quality improvement projects tailored to their unique populations.
A strength of this study is the use of high-quality, nurse-abstracted NSQIP data from 3 healthcare systems enriched with EHR data and linked to the granular ADI, as recommended by the National Academy of Medicine.4 Using multiple sources provides a more complete dataset for the patients, overcoming many of the known limitations of administrative data. Administrative data uses ICD-9/10 codes, which vary across healthcare systems and often cannot distinguish between preoperative risk factors (eg, preoperative pneumonia or urinary tract infections) from postoperative complications. NSQIP is an audited clinical registry using strict definitions, enabling more accurate identification of preoperative risk factors and complications.18,19 Furthermore, we differentiated urgent from elective cases in contrast to many administrative database studies.16,54 Although other studies found that outcomes are related to race, income and insurance, this study is the first to combine these factors into a single model, explores their combined impact, and suggests that each SDoH acts independently to thwart positive surgical outcomes.
Prior surgery research studied isolated associations with either insurance type54,55 or neighborhood deprivation.22,42 This is one of the first studies to simultaneously examine the granular, block group ADI, insurance type, and race/ethnicity on TO, a composite outcome. Although insurance type emerged as a more powerful and consistent factor in TO than ADI, ADI was still associated with CDIV complications and mortality for patients with insurance. Best practices should include both insurance type and ADI in risk adjustment. Future studies should evaluate the changing impact of ADI stratified by insurance type.
The importance of including both granular neighborhood deprivation and individual social risk factors was further illustrated by a study of pediatric asthma patients that showed ADI was associated with increased hospitalizations, modified by insurance type.56 Alternatively, chemotherapy completion and mortality among breast cancer patients showed associations with insurance type but not with ZIP code-level neighborhood SES.23 Area measures only estimate individual SES and neighborhood conditions, potentially confounding analyses,31 especially when the area/population is large.57 Therefore, we used block group ADI to better approximate the patient’s neighborhood; this granular measure demonstrated lower odds of TO (Table 3). ADI remained a significant predictor of CDIV complications and mortality, even after controlling for urgent/emergent surgeries (Table 5).
Cutoffs defining deprivation for ADI and other indices vary.26,42 ADI >85, but not ADI >75, remained significantly associated with TO after including insurance. However, significant associations between ADI and TO components were unaffected by the categorization of deprivation, demonstrating that outcomes can vary with the definition of deprivation used. Neighborhood deprivation and insurance are both independently associated with TO, and both need to be considered in evaluating risk for poor outcomes. However, measuring neighborhood deprivation has nuances that depend on the granularity of the data (block versus ZIP code level) and the cutoff values used. Based on these nuances, the relationship between neighborhood deprivation and TO and its components may be altered. Health systems should establish cutoffs predictive of their populations, to identify patients from deprived neighborhoods and potentially improve their outcomes by providing additional services to improve access to care and postoperative follow up.
We chose TO as our primary outcome because composite measures often provide more comprehensive assessments of surgical outcomes than single variables.30,37,38 Our data demonstrate the utility of this approach as Black, non-Hispanic versus White, non-Hispanic patients had lower odds of TO but were similar for all TO components. Thus, identification of healthcare disparities may be improved by composite variables secondary to the additive effects of each component.
Our data demonstrated increased rates/odds of urgent/emergent surgery among patients that (1) were minorities, (2) were living in highly deprived neighborhoods for both ADI cutoffs, and (3) had non-Private insurance. Urgent/emergent cases were associated with lower/worse odds of TO and higher/worse odds of all TO components except EDOS. ADI >85 was associated with lower odds of TO but was no longer significant after adjusting for urgent/emergent cases, suggesting that the increased rates/odds of urgent/emergent surgeries in patients living in highly deprived neighborhoods was a driving factor in their worse outcomes. Prior studies support our findings; urgent21 and emergent surgeries54,58 were associated with increased complications. Urgent surgeries generally occur in the context of unplanned hospitalizations and were more prevalent in all 4 insurance types compared to emergency cases. However, urgent procedures were lowest in patients with Private insurance (15.3%) and highest in patients with no insurance (43.4%). Grouping urgent and elective cases together may produce disproportionately high complication rates in patient populations with higher rates of urgent surgeries. Risk adjustment should differentiate urgent from elective cases.
Consistent with the growing literature,56,59 contextual-level SDoH (eg, neighborhood deprivation) are distinct from individual-level SDoH (eg, insurance) yet are tightly interrelated. A novel aspect of this study is that we estimated the reduction in TO probability of 9.9% for urgent/emergent cases, 7.0% for Medicaid, and 1.6% for non-Hispanic Black patients. We used these probability differences to show lowest- versus highest-risk differences demonstrating the clinical significance of including multilevel SDoH and the intersection and clustering of these SDoH variables. Specifically, disparities associated with race/ethnicity, insurance type, and neighborhood deprivation are long-term effects of residential racial segregation.34,36 We quantified the absolute difference between the lowest- and highest-risk scenarios to illustrate how the risks of race, insurance type, and neighborhood deprivation compound in populations exposed to multiple social risk factors. Very frail patients at lowest-risk (White, non-Hispanic, Private, ADI ≤ 85, elective surgery) versus highest-risk (Black, non-Hispanic, Medicaid, ADI >85, urgent/emergent surgery) had a TO probability of 77.3% and 47.6%, respectively, a difference of 29.8%, showing the magnitude of intersecting risks. Robust, normal and frail lowest- and highest-risk patients had 20.0%, 22.2%, 25.4% differences in TO probability, respectively. These disparities have eluded recognition and precise definition because the risk factors are unavailable in NSQIP without the novel linkage to ADI and insurance type used here. Further research is needed to develop and evaluate strategies for alleviating these disparities. As a first step, we encourage NSQIP to begin collecting more robust preoperative variables assessing individual- and contextual-level SDoH.
Racial and ethnic minorities, uninsured and Medicaid patients as well as patients living in high poverty26,32 or segregated areas have worse outcomes.34 However, these factors are not included in risk adjustment placing SNH serving higher proportions of patients from disadvantaged backgrounds at greater risk of being penalized for worse outcomes.10,11,60–62 Our findings are in line with these concerns and show the need to include social-risk adjustment in value-based programs. While CMS has assessed the landscape of available contextual SDoH for risk adjustment,2 clear actions have not yet been outlined. Continued efforts by policy makers are needed to address this issue.
Limitations
This was a cross-sectional analysis showing associations rather than causal relationships. It is also possible that alternate, unmeasured clinical and social risk factors could confound our findings. The data are derived from three diverse healthcare systems which may restrict generalizability. However, this study has no equivalent published in the literature, and given that the data are derived from three large academic health centers, the study can affect the national debate regarding SDoH in public policy and risk adjustment. Finally, our NSQIP cohort was a sample of major surgeries performed at 3 healthcare systems; it did not include all procedures during the study period. Our data only included inpatient surgeries, limiting generalizability to outpatient procedures.
CONCLUSION
Neighborhood deprivation and insurance type had independent effects on TO. However, after adjusting for increased urgent/emergent cases, the association with insurance type remained significant, but not neighborhood deprivation. This suggests that neighborhood deprivation may affect outcomes predominately through increased rates/odds of patients undergoing urgent/emergent surgeries further driving complications and worse outcomes. Lowest-risk versus highest-risk scenarios demonstrated the magnitude of intersecting SDoH variables. Our results show that the combination of insurance type and ADI can be useful for identifying high-risk patients, enabling healthcare systems to focus scarce resources on vulnerable populations. Outcomes might be improved by redesigning care pathways that identify and alleviate SDoH. The methods in this study can serve as a roadmap for healthcare systems to use their own data to better understand their unique populations. Better inclusion of multilevel SDoH, including neighborhood deprivation in risk adjustment, could reduce the unintended consequences of value-based medicine programs in decreasing resources to SNH.
Acknowledgments
The authors thank Bill Ross, BS, Kellie M. Walters, MPH, and our informatics and data analyst teams on U01TR002393 for their contributions to this work.
P.K.S., J.K., M.A.J., and L.S.M. had full access to the deidentified NSQIP data and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: P.K.S., S.S., M.A.J., D.E.H., and J.K. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: S.S., J.K., M.A.J., and P.K.S. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: J.K., M.A.J., and C.-P.W. Obtained funding: P.K.S., D.E.H., K.B.S., L.S.K., B.B.B., S.S., and C.-P.W. Administrative, technical, or material support: All authors. Supervision: P.K.S. S.S, J.K. and M.A.J. are co-first authors, these authors contributed equally to the contents of this study.
Supplementary Material
Footnotes
Published online 21 February 2023
S.S., J.K., and M.A.J. are co-first authors, and these authors contributed equally to the contents of this study.
This research was supported by grant U01TR002393 (J.K. M.A.J., D.E.H., K.B.S., L.S.K., C.-P.W., H.-D.S., S.S., and P.K.S.), from the National Center for Advancing Translational Sciences and the Office of the Director, NIH and Clinical Translational Science Awards UL1TR002489 [NC Translational and Clinical Science (NC TraCS), University of North Carolina], UL1TR001857 (University of Pittsburgh) and UL1TR002645 (University of Texas Health San Antonio) from the National Center for Advancing Translational Sciences, NIH and P30AG044271 from the National Institute for Aging, NIH (P.K.S. and B.B.B.).
Disclosure: D.E.H. reported receiving grants from the National Institutes of Health and Veterans administration during the conduct of this study; he also reported a consulting relationship with FutureAssure, LLC. K.B.S. reported receiving grant funding from the National Institutes of Health. L.S.K. reported receiving royalties from Springer, Wolters-Klower, and McGraw-Hill. P.K.S. reported receiving grants from the National Institutes of Health and Veterans Health Administration and salary support from Texas A&M School of Medicine, South Texas Veterans Health Care System, and the University of Texas Health San Antonio during the conduct of the study. S.S. reports receiving grants from the National Institutes of Health and Veterans Health Administration. The other authors declare that they have nothing to disclose. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The opinions expressed here are those of the authors and do not necessarily reflect the position of the United States government.
The dataset for this article contains data from the (1) American College of Surgeons National Surgical Quality Improvement Program and (2) electronic health records from multiple institutions cannot be shared without authorization from the American College of Surgeons and the University of Texas Health San Antonio, University Health, University of Pittsburgh Medical Center and University of North Carolina.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.CMS Office of Minority Health. CMS Framework for Health Equity 2022-2032. 2022. Available at: https://www.cms.gov/files/document/cms-framework-health-equity.pdf. Accessed August 8, 2022.
- 2.Breslau J, Martin L, Timbie J, et al. Landscape of Area-Level Deprivation Measures and Other Approaches to Account for Social Risk and Social Determinants of Health in Health Care Payments. ASPE; 2022. [Google Scholar]
- 3.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 4.NASEM. Accounting for Social Risk Factors in Medicare Payment National Academies Press, National Academies of Sciences, Engineering, and Medicine; 2017. [PubMed] [Google Scholar]
- 5.NASEM. The Growing Gap in Life Expectancy by Income, Implications for Federal Programs and Policy Responses. National Academies Press, National Academies of Sciences, Engineering and Medicine; 2015. [PubMed] [Google Scholar]
- 6.Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97:407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon RC, Kim J, Schmidt S, et al. Association of insurance type with inpatient surgery 30-day complications and costs. J Surg Res. 2022;282:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs M, Kim J, Tetley J, et al. Costs of failure to achieve textbook outcomes: association of insurance type with outcomes and cumulative costs for inpatient surgeries. J Am Coll Surg. 2023;236(2):352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tetley J, Jacobs M, Kim J, et al. Association of insurance type with colorectal surgery outcomes and costs at a safety-net hospital—a retrospective observational study. Ann Surg Open. 2022;3:e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favini N, Hockenberry JM, Gilman M, et al. Comparative trends in payment adjustments between safety-net and other hospitals since the introduction of the hospital readmission reduction program and value-based purchasing. JAMA. 2017;317:1578–1580. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa JF, Wang DE, Jha AK. Characteristics of hospitals receiving the largest penalties by US pay-for-performance programmes. BMJ Qual Saf. 2016;25:898–900. [DOI] [PubMed] [Google Scholar]
- 12.Chee TT, Ryan AM, Wasfy JH, et al. Current state of value-based purchasing programs. Circulation. 2016;133:2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zogg CK, Thumma JR, Ryan AM, et al. Medicare’s hospital acquired condition reduction program disproportionately affects minority-serving hospitals: variation by race, socioeconomic status, and disproportionate share hospital payment receipt. Ann Surg. 2020;271:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khullar D, Schpero WL, Bond AM, et al. Association between patient social risk and physician performance scores in the first year of the merit-based incentive payment system. JAMA. 2020;324:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Hoyler MM, White RS, et al. Hospital safety-net burden is associated with increased inpatient mortality and perioperative complications after colectomy. J Surg Res. 2021;259:24–33. [DOI] [PubMed] [Google Scholar]
- 16.Hoehn RS, Wima K, Vestal MA, et al. Effect of hospital safety-net burden on cost and outcomes after surgery. JAMA Surg. 2016;151:120–128. [DOI] [PubMed] [Google Scholar]
- 17.Rosero EB, Modrall JG, Joshi GP. Failure to rescue after major abdominal surgery: the role of hospital safety net burden. Am J Surg. 2020;220:1023–1030. [DOI] [PubMed] [Google Scholar]
- 18.Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256:973–981. [DOI] [PubMed] [Google Scholar]
- 19.Lawson EH, Zingmond DS, Hall BL, et al. Comparison between clinical registry and medicare claims data on the classification of hospital quality of surgical care. Ann Surg. 2015;261:290–296. [DOI] [PubMed] [Google Scholar]
- 20.Jollis JG, Ancukiewicz M, DeLong ER, et al. Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med. 1993;119:844–850. [DOI] [PubMed] [Google Scholar]
- 21.Mullen MG, Michaels AD, Mehaffey JH, et al. Risk associated with complications and mortality after urgent surgery vs elective and emergency surgery: implications for defining “quality” and reporting outcomes for urgent surgery. JAMA Surg. 2017;152:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghirimoldi FM, Schmidt S, Simon RC, et al. Association of socioeconomic area deprivation index with hospital readmissions after colon and rectal surgery. J Gastrointest Surg. 2020;25:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obeng-Gyasi S, O’Neill A, Zhao F. Impact of insurance and neighborhood socioeconomic status on clinical outcomes in therapeutic clinical trials for breast cancer. Cancer Med. 2021;10:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhavsar NA, Gao A, Phelan M, et al. Value of neighborhood socioeconomic status in predicting risk of outcomes in studies that use electronic health record data. JAMA Network Open. 2018;1:e182716–e182716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med. 2018;378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz A, Hyer JM, Tsilimigras D, et al. The impact of social vulnerability subthemes on postoperative outcomes differs by racial/ethnic minority status. Am J Surg. 2021;223:353–359. [DOI] [PubMed] [Google Scholar]
- 27.Mehaffey JH, Hawkins RB, Charles EJ, et al. Socioeconomic “distressed communities index” improves surgical risk-adjustment. Ann Surg. 2020;271:470–474. [DOI] [PubMed] [Google Scholar]
- 28.Eapen ZJ, McCoy LA, Fonarow GC, et al. Utility of socioeconomic status in predicting 30-day outcomes after heart failure hospitalization. Circ Heart Fail. 2015;8:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernheim SM, Parzynski CS, Horwitz L, et al. Accounting for patients’ socioeconomic status does not change hospital readmission rates. Health Aff (Millwood). 2016;35:1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz A, Dalmacy D, Herbert C, et al. Association of county-level racial diversity and likelihood of a textbook outcome following pancreas surgery. Ann Surg Oncol. 2021;28:8076–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryere J, Pornet C, Copin N, et al. Assessment of the ecological bias of seven aggregate social deprivation indices. BMC Public Health. 2017;17:86–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz A, Valbuena VSM, Dimick JB, et al. Association of neighborhood deprivation, race, and postoperative outcomes: improvement in neighborhood deprivation is associated with worsening surgical disparities. [published online ahead of print July 7, 2022] Ann Surg. doi: 10.1097/SLA.0000000000005475. [DOI] [PubMed] [Google Scholar]
- 33.Blanco BA, Poulson M, Kenzik KM, et al. The impact of residential segregation on pancreatic cancer diagnosis, treatment, and mortality. Ann Surg Oncol. 2021;28:3147–3155. [DOI] [PubMed] [Google Scholar]
- 34.Poulson MR, Papageorge MV, LaRaja AS, et al. Socioeconomic mediation of racial segregation in pancreatic cancer treatment and outcome disparities. Ann Surg. Online ahead of print July 15, 2022. [DOI] [PubMed] [Google Scholar]
- 35.Poulson MR, Beaulieu-Jones BR, Kenzik KM, et al. Residential racial segregation and disparities in breast cancer presentation, treatment, and survival. Ann Surg. 2021;273:3–9. [DOI] [PubMed] [Google Scholar]
- 36.The Petrie-Flom Center Staff. Structural racism: the root cause of the social determinants of health. Bill of Health: Examining the Intersection of Health Law, Biotechnology, and Bioethics. 2020. Available at: https://blog.petrieflom.law.harvard.edu/2020/09/22/structural-racism-social-determinant-of-health/. Accessed April 11, 2022. [Google Scholar]
- 37.Bolger JC, Al Azzawi M, Whooley J, et al. Surgery by a minimally invasive approach is associated with improved textbook outcomes in oesophageal and gastric cancer. Eur J Surg Oncol. 2021;47:2332–2339. [DOI] [PubMed] [Google Scholar]
- 38.Hyer JM, Tsilimigras DI, Diaz A, et al. High social vulnerability and “textbook outcomes” after cancer operation. J Am Coll Surg. 2021;232:351–359. [DOI] [PubMed] [Google Scholar]
- 39.Ford CL, Harawa NT. A new conceptualization of ethnicity for social epidemiologic and health equity research. Soc Sci Med (1982). 2010;71:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Congress.gov. H.R.7585 - 117th Congress (2021-2022): Health Equity and Accountability Act of 2022. June 29, 2022. Available at: http://www.congress.gov/. Accessed November 8, 2022.
- 41.Shiloach M, Frencher SK, Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American college of surgeons national surgical quality improvement program. J Am Coll Surg. 2010;210:6–16. [DOI] [PubMed] [Google Scholar]
- 42.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arya S, Varley P, Youk A, et al. Recalibration and external validation of the risk analysis index: a surgical frailty assessment tool. Ann Surg. 2019;272:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothenberg KA, George EL, Trickey AW, et al. Assessment of the risk analysis index for prediction of mortality, major complications, and length of stay in patients who underwent vascular surgery. Ann Vasc Surg. 2020;66:442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinall MC, Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2019;155:e194620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinall MC, Jr, Youk A, Massarweh NN, et al. Association of preoperative frailty and operative stress with mortality after elective vs emergency surgery. JAMA Netw Open. 2020;3:e2010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Q, Kim J, Hall DE, et al. Association of frailty and the expanded operative stress score with preoperative acute serious conditions, complications and mortality in males compared to females: a retrospective observational study. Ann Surg. 2023;277(2):e294–e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George EL, Massarweh NN, Youk A, et al. Comparing veterans affairs and private sector perioperative outcomes after noncardiac surgery. JAMA Surg. 2022;157:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sood A, Meyer CP, Abdollah F, et al. Minimally invasive surgery and its impact on 30-day postoperative complications, unplanned readmissions and mortality. Br J Surg. 2017;104:1372–1381. [DOI] [PubMed] [Google Scholar]
- 52.Merath K, Chen Q, Bagante F, et al. A Multi-institutional international analysis of textbook outcomes among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma. JAMA Surg. 2019;154:e190571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolfschoten NE, Kievit J, Gooiker GA, et al. Focusing on desired outcomes of care after colon cancer resections; hospital variations in “textbook outcome”. Eur J Surg Oncol. 2013;39:156–163. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz DA, Hui X, Schneider EB, et al. Worse outcomes among uninsured general surgery patients: does the need for an emergency operation explain these disparities? Surgery. 2014;156:345–351. [DOI] [PubMed] [Google Scholar]
- 55.Dasenbrock HH, Wolinsky JP, Sciubba DM, et al. The impact of insurance status on outcomes after surgery for spinal metastases. Cancer. 2012;118:4833–4841. [DOI] [PubMed] [Google Scholar]
- 56.Nkoy FL, Stone BL, Knighton AJ, et al. Neighborhood deprivation and childhood asthma outcomes, accounting for insurance coverage. Hosp Pediatr. 2018;8:59–67. [DOI] [PubMed] [Google Scholar]
- 57.Adams J, Ryan V, White M. How accurate are townsend deprivation scores as predictors of self-reported health? A comparison with individual level data. J Public Health (Oxf). 2005;27:101–106. [DOI] [PubMed] [Google Scholar]
- 58.Havens JM, Peetz AB, Do WS, et al. The excess morbidity and mortality of emergency general surgery. J Trauma Acute Care Surg. 2015;78:306–311. [DOI] [PubMed] [Google Scholar]
- 59.Meeker JR, Canelón SP, Bai R, et al. Individual-level and neighborhood-level risk factors for severe maternal morbidity. Obstetr Gynecol. 2021;137:847–854. [DOI] [PubMed] [Google Scholar]
- 60.Shih T, Ryan AM, Gonzalez AA, et al. Medicare’s hospital readmissions reduction program in surgery may disproportionately affect minority-serving hospitals. Ann Surg. 2015;261:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salerno AM, Horwitz LI, Kwon JY, et al. Trends in readmission rates for safety net hospitals and non-safety net hospitals in the era of the US Hospital Readmission Reduction Program: a retrospective time series analysis using Medicare administrative claims data from 2008 to 2015. BMJ Open. 2017;7:e016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson MP, Waters TM, Kaplan CM, et al. Most hospitals received annual penalties for excess readmissions, but some fared better than others. Health Aff (Millwood). 2017;36:893–901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.