Abstract
1.1. Background:
Inflammatory Bowel Disease (IBD) are the manifestation of overzealous dys-regulated immune response in the intestinal tract, directed primarily against the indigenous microbes combined with defective functioning of anti-inflammatory pathways. Finding a trustable lead to predicting de novo Crohn’s Disease (CD) prior to performing “pouch surgery”, Restorative Proctocolectomy (RPC) with Ileal Pouch-Anal Anastomosis (IPAA) for UC and/or Indeterminate Colitis (IC) is clinically important and remains debatable. De novo CD is a subsequent long-term postoperative complication in IBD patients with Ulcerative Colitis (UC) undergoing IPAA. Herewith we discuss this understanding in laboratory-based basic science research, with its molecular application as a possible corner stone tool for clinical progress and success in the IBD Clinic. Crypt Paneth cell (PCs) secreted enteroendocrine alpha-defensin 5 (DEFA5)” if developed properly is likely to solve diagnostic and prognostic difficulty in IBD Clinics. DEFA5 has shown the ability to differentiate the predominant subtypes of colonic IBD (CC vs. UC) at first endoscopy biopsy, avoiding diagnosis delay prior to colectomy. In addition, DEFA5 accurately circumvents indeterminate colitis (IC) patients into accurate IBD subtype (UC or CC). Further, DEFA5 can be used in selecting CC patients that may have positive outcomes after IPAA surgery [1]. Furthermore, likewise, DEFA5 can predict UC patients likely to have positive or poor outcome, e.g. those patients that are likely to transform/ convert and adhere to de novo Crohn’s after IPAA can be picked up in endoscopy biopsy before surgery.
1.2. Aim:
To assessed comprehensive state-of-the-art understanding domains on the de novo Crohn’s disease subsequent to IPAA surgery for ulcerative colitis.
1.3. Methods:
A literature search based on preferred reporting items for over-review and meta-analysis protocols (PRISMA-P) was performed. A comprehensive current search of PubMed, MEDLINE, CINAHL, Embase, Google® search engine and Cochrane Database of collected reviews was performed from January 1990 through December 2018. The search consists of retrospective studies and case reports of reporting postoperative de novo CD incidence and adverse events. Secondary and hand/manual searches of reference lists, other studies cross-indexed by authors, reviews, commentaries, books and meeting abstracts were also performed. Studies were included only if the diagnosis of de novo CD was established clinically and histologically based on inflammation of afferent limb(s) or perianal disease. The search excluded non-English language and non-human studies as well as editorials.
1.4. Results:
Published data on de novo CD developing after RPC with IPAA are still limited. A total of three hundred and sixty-five (#365) patients in 13 publications reported de novo CD after a median follow-up of 66 (range: 3–236) months. All patients were diagnosed with clinically active pouch CD during follow-up surveillance after IPAA for UC or IC. A de novo CD diagnosis depended on either inflammation in the mucosa involving the small intestine proximal to the ileal pouch any time after IPAA surgery and/or when perianal complications developed after closure of a temporary diverting loop ileostomy. Successful management is facilitated by co-operation within a multidisciplinary team of gastroenterologists and colorectal surgeons and closely involving the patient in therapeutic decisions. Awareness of symptoms leads to timely consultation, diagnosis, treatment and restoration of intestinal continuity.
1.5. Conclusion:
The nature history and risk of de novo CD after IPAA for UC remains debatable. Chronic pouchitis and/or pouch failure often precedes a diagnosis of de novo CD. A successful management is facilitated by a triad cooperation between gastroenterologists, colorectal surgeons and the patient.
Keywords: Ulcerative colitis, Proctocolectomy, Ileal pouch-anal anastomosis, De novo Crohn’s Disease, Transformation, Conversion, Change of diagnosis, Human alpha-defensin 5
5. Introduction
Restorative Proctocolectomy (RPC) with Ileal Pouch-Anal Anastomosis (IPAA) is indicated in approximately 20% to 30% as the current recommended surgical standard procedure for curative treatment for suitable patients with medically refractory UC and IC predicted as UC [1–3]. Ulcerative colitis and IC designated UC generally, demonstrate acceptable functional results after RPC with IPAA [4, 5], but approximately 5 to 10 per cent of IPAA patients are subsequently diagnosed with de novo CD, leading to increased morbidity and rates of pouch failure [1, 5–9]. In preparation, prior to RPC with IPAA surgery patients must be well-informed, counseled and guided about de novo CD, given the fact that many patients view it as a curative surgery [10]. De novo CD is one of the most devastating complications of IPAA, which ultimately increases the risk for pouch malfunctions [11–15]. Restorative proctocolectomy with IPAA is usually contraindicated in patients with authentic colonic CD, also known as Crohn’s Colitis (CC), because of high risks and vulnerability for disease recurrence, fistulas, abscesses, and strictures which may lead to a significant higher incidence of pouch failure and pouch excision [16–18]. However, in highly selected patients with CC, RPC with IPAA has been indicated with positive outcomes [7, 19, 20]. Therefore, validation of the diagnostic potential using DEFA5, a newly found reliable marker to identify CC patients (Crohn’s-like clinically but have lower DEFA5 levels) as well as IC patients who are potential candidates for this sphincter-preserving operation, is recommended. While RPC with IPAA is acceptable for patients with UC [2] or IC predicted as UC [21, 22], it should not be the first option for treating patients with CC. Identifying robust biomarkers predicting de novo CD among patients with UC and IC is clinically relevant and would improve surgical decision making. This underscores the critical need for predictive and precision medicine that can optimize diagnosis and disease management, provide more cost-effective strategies, and minimize the risk of adverse practices. Our experience in patients that were identified with pathologic features associated with UC-like subgroup of CC [PMID: 25278708] and those whose diagnoses clinically changed from UC to de novo CD [1] after IPAA surgery, all showed prominent DEFA5 staining compared to those whose diagnosis remained unchanged. This is an indication that DEFA5, if well developed, could be used as a tool to predict UC patients likely to transform and convert and adhere to de novo CD prior to IPAA surgery. In this review of a field of vision narrative, we summarize the current status of prediction of de novo CD risk, clinical course, investigations, and response to treatment based on clinical case presentations. We also discuss concisely the potential and limitations of the currently used strategies.
6. Materials and Methods
A literature review was performed according to the preferred reporting items for over-review and meta-analysis protocols (PRISMA-P) guidelines [23]. A search of comprehensive reporting of UC-IPAA postoperative de novo CD incidence, prevalence and adverse events was performed. Publications regarding de novo CD in IPAA patients following proctocolectomy surgery for UC between January 1990 and December 2018 were searched. PubMed, MEDLINE, EMBASE (Excerpta Medica database), CINAHL, the Cochrane library, Web of Science, and Google® were searched using the following terms: ulcerative colitis, total colectomy, proctocolectomy, restorative proctocolectomy, ileal pouch-anal anastomosis, de novo CD. Secondary and hand/manual searches of reference lists, other studies cross-indexed by authors, reviews, commentaries, books and meeting abstracts were also performed.
7. Results
Published data on de novo CD (pouch Crohn’s disease) developing after RPC with IPAA for UC and IC are presented in (Table 1). A total of 5042 IPAA patients were followed and ultimately 407 (8%) patients diagnosed with de novo CD are reported in the English literature to date (95% confidence interval (CI), 6.1–15.4%) after a median follow-up of 66 months (range: 3–236). All patients were diagnosed with clinically active pouch CD during follow-up surveillance after IPAA for UC or IC. Disease distinguishing traits significantly influenced pouch retention. The interval from pouch construction, increased incidence of nonblood diarrhea, disease fistulation, and location were used as prognostic indicators when de novo CD was diagnosed.
Table 1:
Depicts the thirteen full-text publications included in Overview of de novo Crohn’s disease Incidence. Full publications included in Overview of de novo Crohn’s disease Incidence
| Study Authors | Publication Year | Study Period | Number of IPAA Patients in Studies | Number of De novo CD | De novo CD Incidence in % |
|---|---|---|---|---|---|
| Goldstein et al. [27] | 1997 | 1981–1995 | 74 | 8 | 10.8 |
| Peyregne et al. [28] | 2000 | 1985–1997 | 43 | 4 | 9.3 |
| Rossi et al. [29] | 2002 | 1989–2000 | 68 | 4 | 5.9 |
| Melton et al. [30] | 2010 | 1983–2007 | 2814 | 87 | 3.1 |
| Haveran et al. [31] | 2011 | 1990–2009 | 382 | 32 | 8.3 |
| Coukos et al. [32] | 2012 | 2006–2010 | 142 | 21 | 14.8 |
| Tyler et al. [33] | 2012 | N/A-2007 | 399 | 50 | 12.5 |
| Ahmed et al. [8] | 2016 | 1992–2014 | 199 | 42 | 21 |
| Zaghiyan et al. [15] | 2016 | 1997–2007 | 237 | 40 | 16.9 |
| Diederen et al. [34] | 2017 | 2000–2015 | 303 | 14 | 4.6 |
| Lightner et al. [16] | 2017 | 1982–2016 | Unreported | 35 | N/A |
| Yanai et al. [35] | 2017 | 1981–2013 | 253 | 54 | 21.3 |
| Shamah et al. [26] | 2018 | 1960–2015 | 128 | 16 | 12.5 |
| Total | 1997–2018 | 58 Years | 5042 | 407 | 3.1–21.3% (mean 11.75) |
De novo CD diagnosis depended on either inflammation in the mucosa involving the small intestine proximal to the ileal pouch any time after IPAA surgery and/or when perianal complications developed more than 3 months after closure of a temporary diverting loop ileostomy. Time to diagnosis of de novo CD was widely defined as the time period from the closure of a diverting loop ileostomy. Alternatively, diagnoses associated to inflammatory or anatomic disorders of the ileal pouch afferent limb or efferent limb obstruction, pouch or anastomotic stricture, pouchitis, cuffitis, anal sphincter dysfunction, or de novo CD were evaluated using reported history, physical examination, pouch endoscopy and histologic interpretation. When there was a lack of findings and/or concern for alternative diagnoses (i.e., pouch leak, abscess or fistula), additional evaluation with imaging or examination under general anesthesia was reported as widely performed.
Treatment of de novo CD:
A firm diagnosis de novo CD often difficult to distinguish from that of chronic antibiotic resistant pouchitis or technical complications related to pouch surgery. Making an accurate diagnosis of de novo CD vs. other postoperative complications is important for, both, decisions regarding medical management and pouch excision vs. reconstruction. Most patients diagnosed with de novo CD are not considered for pouch reconstructive surgery [24]. There is very limited data available on the medical management of de novo CD and the quality of the studies are oftentimes execrable. Successful management is facilitated by co-operation within a multidisciplinary team of gastroenterologists and colorectal surgeons; and most importantly, closely involving the patient in therapeutic decisions with a person-centered approach and health coaching. Awareness of symptoms leads to timely consultation, diagnosis, treatment and restoration of intestinal continuity. The adapted representative data from Scandinavian [24], on cumulative proportions of patients undergoing surgery and time to surgery is shown in (figure 1). Surgical endoscopy interventions are depicted in (Figure 2A and B). Endoscopic balloon dilatation is an efficacious and safe alternative to surgical resection of a strictured de novo CD. About 52% of patients at 5-year follow-up require either no additional dilatation or only one additional dilation, where-as 36% required surgical resection.
Figure 1:
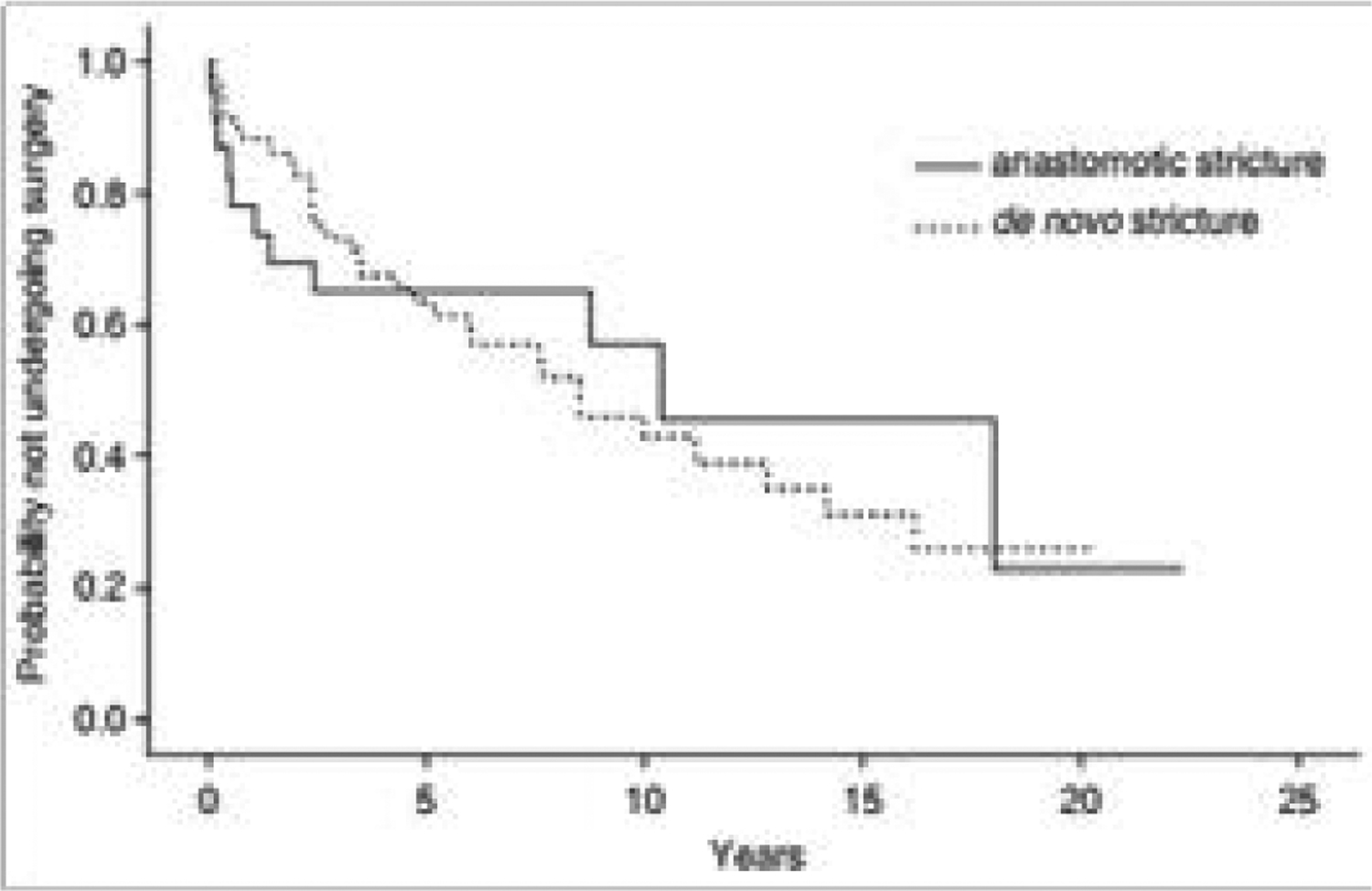
Kaplan-Meier estimated plot showing probability of surgery-free survival in relation to time after index dilatation in patients with anastomotic or de novo strictures (p = 0.86). This analysis is restricted to 83 patients having repeated dilatations only for strictures causing symptoms of bowel obstruction. Adapted with permission from “Endoscopic dilatation is an efficacious and safe treatment of intestinal strictures in Crohn’s disease”, by Gustavsson et al., Aliment Pharmacol Ther 2012;36:151–158 [24].
Figure 2:
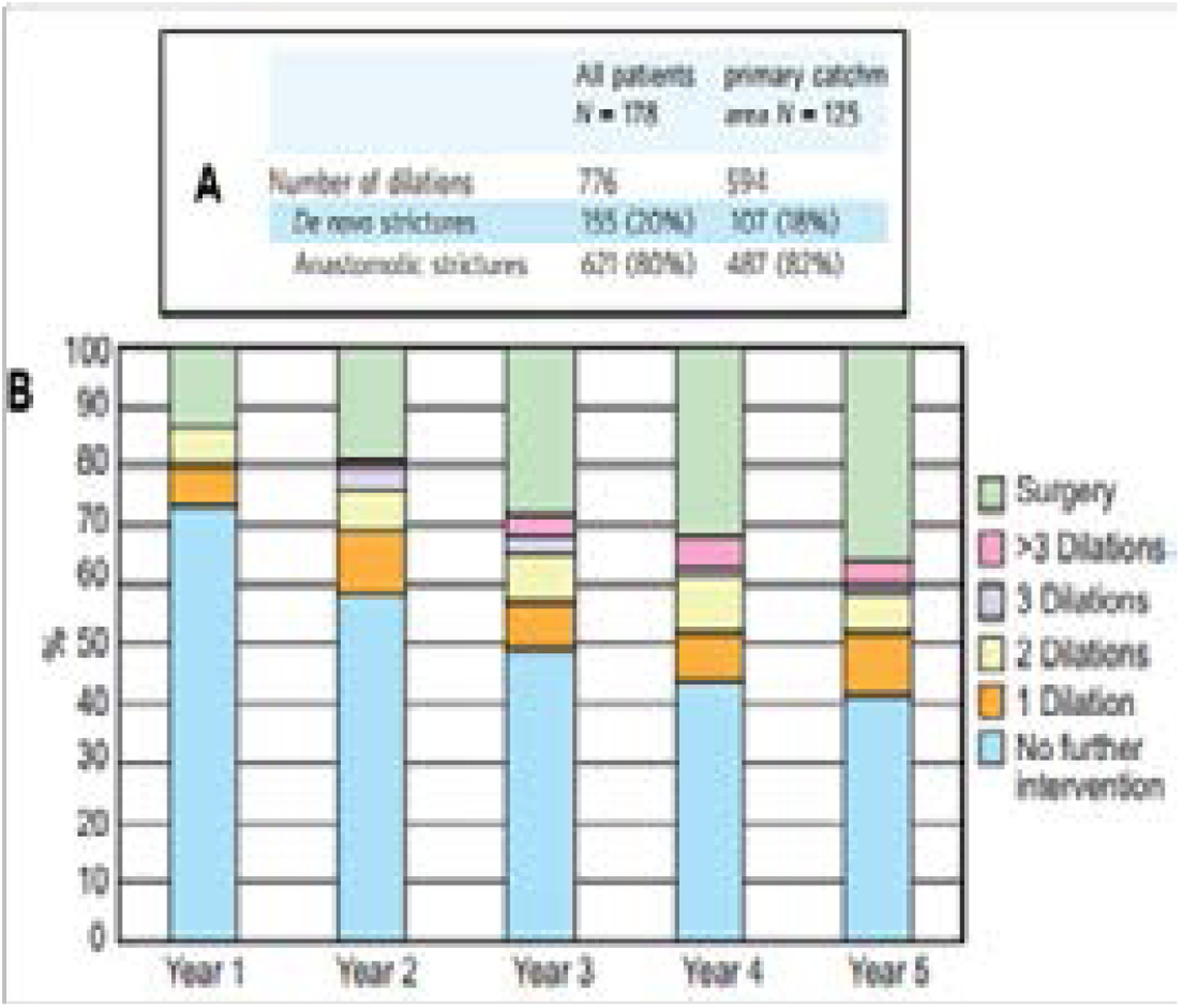
Kaplan-Meier estimated plot showing probability of surgery-free survival in relation to time after index dilatation in patients with anastomotic or F strictures (p = 0.86). This analysis is restricted to 83 patients having repeated dilatations only for strictures causing symptoms of bowel obstruction. Adapted with permission from “Endoscopic dilatation is an efficacious and safe treatment of intestinal strictures in Crohn’s disease”, by Gustavsson et al., Aliment Pharmacol Ther 2012;36:151–158 [24].
7.1. Limitations:
According to the articles reviewed, the retrospective nature of these studies and Referral bias were limitations.
8. Discussion
Apprehension of the true incidence of the authentic de novo CD and standardizing the diagnostic criteria for this condition remain critical goals in improving the care of patients undergoing IPAA for the management of IBD. The incidence and prevalence of de novo CD after RPC with IPAA are addressed in many studies [8,14,15,25–27]. Out of the 13 projects herewith included, 12 studies included patients with UC and/or IC following IPAA and longitudinal analysis of patients who ultimately developed de novo CD [10,17,28–36]. A typical representative presentational result of de novo CD after RPC with IPAA is depicted by the Kaplan Meier in (figure 3) [25]. The estimated 10-year pouch retention rate in patients with a fistula at the time of de novo CD diagnosis was 38%; significantly lower than those without fistulae (44% vs. 76%, p = 0.004). Further, (figure 4) is representative data of early vs. late diagnosis of de novo CD [25]. The 10-year pouch retention rate significantly improved in patients with a late diagnosis of de novo CD (63% vs. 39%, p = 0.012). De novo CD leads to increased morbidity [27] and higher incidence of pouch failure [8,14,15,26]. Long-term observational studies of prospectively followed IPAA patients in a cohort from Cedar-Sinai Medical Center in Los Angeles, California [17], have found a higher rate of such pouch complications and pouch excision. Murrell at al., reported similar risk of de novo CD after IPAA after a median follow-up of 26 months [37]. Because de novo CD may develop over a longer follow-up period, the group evaluated the previously published IPAA cohort after the initial analysis (at 26 months) and showed an increase in new de novo CD by 19 per-cent during a median follow-up period of 69 months (range: 3–236) [17].
Figure 3:
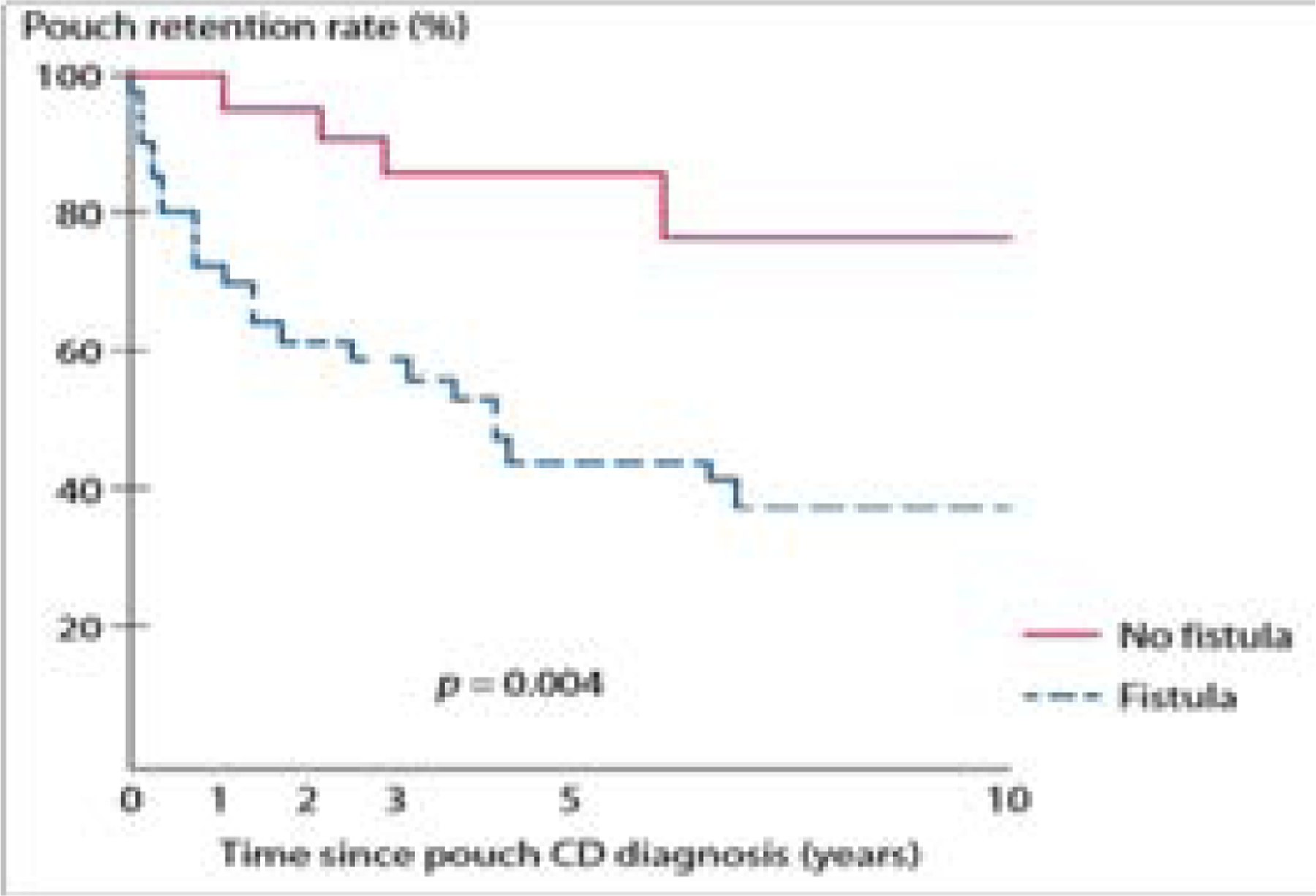
Kaplan-Meier estimated pouch survival in patients developing fistulas vs. without fistulas at the time of diagnosis of de novo Crohn’s disease. Adapted with permission from “Do clinical characteristics of de novo pouch Crohn’s disease after restorative proctocolectomy affect ileal pouch retention?” by Gu et al., Dis Colon Rectum 2014;57:76–82 [25].
Figure 4:
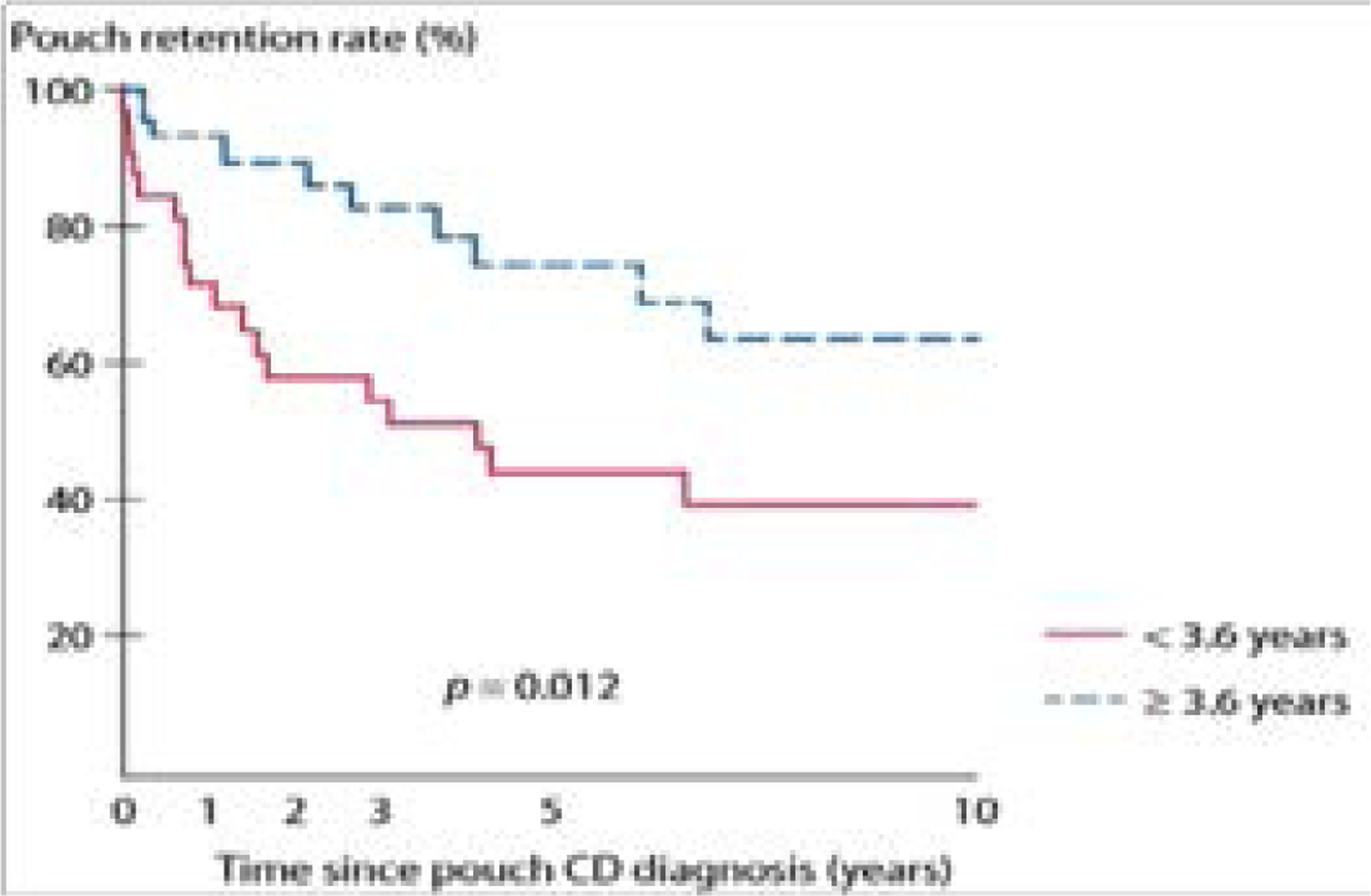
Kaplan-Meier estimated pouch survival in patients with different time interval from IPAA to de novo Crohn’s disease. Adapted with permission from “Do clinical characteristics of de novo pouch Crohn’s disease after restorative proctocolectomy affect ileal pouch retention? by Gu et al., Dis Colon Rectum 2014;57:76–82 [25].
The studies highlighted the importance of distinguishing preoperative IBD (UV vs. CC) from postoperative IC patients when the diagnostic classification for these two diseases is inconclusive [1,37]. Based on The Working Party of the Montreal World Congress of Gastroenterology classification [38], maintained the preoperative and postoperative diagnostic classification [17], thereby overcoming several limitations of other studies that only used the postoperative diagnosis [8,11,12,39–44] of IC or UC when comparing outcomes after IPAA.
8.1. Is de novo CD after IPAA for UC a misdiagnosis of Crohn’s colitis?
Admittedly unknown, there are 3 possible causes that attempts to explain the development of de novo CD after IPAA for UC and IC: (i) authentic CD that was not evident prior to colectomy surgery, (ii) aberrated entity due to novel immunopathogenesis ingredient factors of the ileal pouch (antigen-antibody reaction against mucosal resistance), and (iii) a natural “transformation” of UC to de novo CD [9,45,46]. This last explains the “myth “of de novo CD; which lies on the observation that subsets of surgically untreated patients who are suffering from UC after initial definitive diagnosis work-up later develop features of CD allowing to change in original diagnosis [45]. These patients are thought to be “misdiagnosed” [1]. This statement may not be wholly true given the fact that there is a possibility that these patients with authentic UC are “transformed”. Transformation occur because of an altered microbiome ecology in the ileal pouch [47], and immune environment [48], and subsequently the patient actually “converts”. Since there is evidenced overlap between clinical characteristics, epidemiological features, and disease associated genes/ genetic loci stratified by pathogenetic pathways and by disease location [49–51], it is possible that the authentic UC adhere to de novo CD within a given individual.
Pathway analyses highlighted differential gene expression with an up-regulation of Innate Immune Pathways (IIP) in CC and an up-regulation of Adaptive Immune Pathways (AIP) in UC [52].
In a recent report, PC specific peptide “Human alpha-defensin 5 (DEFA5)” differentiates the predominant subtypes of colonic IBD (CC vs. UC) [1]. DEFA5 accurately identifies CC or UC phenotype among IC patients with a positive predictive value of 96 percent [1]. Furthermore, DEFA5 can be used in selecting certain CC and UC patients that may have positive or negative outcomes after IPAA [1]. The distinction between UC and CC among patients with IC is of utmost importance when determining a patient’s candidacy for RPC and IPAA surgery [2,53,54].
8.2. Designating de novo Crohn’s disease at initial endoscopy biopsy
The overarching scientific premise is that UC patients likely to have positive or poor outcome, especially those that may transform/convert and adhere to de novo CD after IPAA based on Paneth cells (PCs) can be categorized using DEFA5 expression during the first colonic mucosal endoscopy biopsy [55]. The significance is that we can anticipate patients with potential poorer outcomes from IPAA for de novo Crohn’s disease. Both observational and analytical data show that in colectomy specimens with an IC patient based on pathology criteria, the diagnosis can be resolved by quantitative IHC staining for DEFA5 that predicts the actual, later diagnosis (CD vs. UC) as it becomes clinically apparent [1]. Admittedly, the diagnosis based on clinical criteria were correct for most IC patients, but the diagnoses were significantly delayed (7–14 years). Quantifiable DEFA5 staining can clearly predict/ -classify the natural pathogenesis of IC to CC. The data in the retrospective cohort does specify classification in the initial (earliest) tissue specimen (pre-colectomy colonoscopy biopsy specimens that lead to the initial diagnosis of an IBD colitis). Whether DEFA5 is merely an association or an actual driver of the de novo Crohn’s activity (or a marker for a mechanism of accelerated or refractory disease) remains to be elucidated. An important question is whether these observations are due primarily, or possibly secondary, to inflammation. The increase in the colonic DEFA5 expression in CC patients correlated with the fecal calprotectin level (r = 0.481, p = 0.02) but not with the local histological inflammatory scores, suggesting that the general level of intestinal inflammation might trigger colonic Paneth cell metaplasia which is an additional protective mechanism in colonic inflammation [56,57]. It is noteworthy that clinical phenotype is the only clinical parameter that seems to be stable over time and does not change with disease progression (such as stenosis or fistulation) [58].
The etiopathogenesis of IBD is multifactorial and interplayed (e.g. immunological, genetic and environmental factors etc.) [59]. The action of dysregulation of PCs in IBD has very important mechanistic implications because it leads to dysregulation of the secretion of DEFA5 and other antimicrobial peptides which are vital in the checks and balances regulation of gut microbes and homeostasis. Understanding specific signaling pathways of the colonic immunological system is therefore important. Perminow et al. has described “defective Paneth Cell-mediated host defense in ileal CD” [57]. They also demonstrate disparity of DEFA5 between small vs. large bowel CD and correlated this with fecal calprotectin levels. In the study, they describe a specific decrease of DEFA5 levels and TCF-4 mRNA expression in ileal CD. This decrease was seen to be independent of inflammation, whereas inflammation seemed to induce PC metaplasia in the colon [57]. The data extended the hypothesis of the role of antimicrobial host defense in CD patients. Similar observations were reported. [60]. These studies report the mechanisms of intestine luminal immune response, with nothing about the diagnostic accuracy and/or predictive subsequent de novo CD in colonic biopsies prior to surgery with RPC and IPAA. We have demonstrated the detection of DEFA5 in patient tissues and sera as a specific, and more reliable, diagnostic tool to distinguish CC from UC. With this tool, we have validated that misdiagnoses of IBD as IC cases will be circumvented and appropriate care can be provided to the hitherto IC classified patients [1]. We also anticipate that data from this study will provide a strong basis to explore the possibility of DEFA5 and/or disease specific cytokines as a potential therapeutic target for CC. Florian Kuhn et al. describes surgical principals in the treatment of UC [61]. We published this information 10 years earlier and agree [2]. However, there is no information on the diagnosis accuracy or predictive subsequent de novo CD. We have demonstrated, in real patients, the diagnostic accuracy and subsequent predictive de novo CD prior to surgery with RPC and IPAA [1].
9. Article Highlights
9.1. Background:
The de novo CD is one of the most common long-term complication of IPAA and a leading cause of pouch failure and pouch excision or permanent diversion. So, it is desirable to find preoperative clinical characteristics that can predict eventual outcome of patients with UC prior to IPAA who would develop de novo CD. This will allow patient personalized surveillances.
9.2. Motivation:
Aimed at identifying a unique biomarker that efficiently distinguish CC from UC among IC patient cohorts (at first endoscopy biopsy). The use of DEFA5 like a marker that can select certain UC (and CC) patients who may have positive or negative outcomes after IPAA, would be critically important for choosing the correct surgical strategy, avoiding to subject patients with CC, to a harmful surgical treatment.
9.3. Methods:
We performed literature search based on PRISMA-P, between 1990 and 2018.
9.4. Results:
DEFA5 bioassay is a predictive and diagnostic test specific for de novo CD and CC. Patients who develop de novo CD are preoperatively thought to have a “misdiagnosed” CC. This claim remains skeptical given the fact that there is a possibility that these patients had a truly authentic UC but are “transformed” and convert to adhere to de novo CD. Natural transformation occurs because of an altered microbiome ecology in the ileal pouch, and immune environment, and subsequently the patient actually “converts”.
9.5. Conclusions:
DEFA5 has shown the ability to delineate CC and UC phenotype and circumvents indeterminate Colitis (IC) into accurate authentic UC or CC. DEFA5 may be used to predict de novo CD prior to and can select CC patients who are likely to have positive OUTCOME after IPAA surgery. DEFA5, is detectable in circulating human blood.
9.6. Perspective:
DEFA5 bioassays, if successfully developed may provide an important translational accessory for improved IBD diagnosis, initial assessment prior to surgical intervention and more importantly, prescription of disease subtype appropriate treatment options. Future endeavors will AIM to determine the molecular basis of stem cell differentiation in the crypt of CC patients, and if disease severity depend on the levels of secreted DEFA5 and/or specific pro-inflammatory cytokines.
4. Core tip:
Due to lack of specificity, applicable robust preoperative risk factor(s) predicting de novo Crohn’s Disease (CD) have to date not been identified. Appreciating the incidence of de novo CD and bring into conformity with standard diagnostic criteria remains critical challenge and goals in the surgical care of patients with ulcerative colitis (UC) and/or Indeterminate Colitis (IC) indicated Restorative Proctocolectomy (RPC) and Ileal Pouch-Anal Anastomosis (IPAA). Approximately 15% of patients diagnosed with UC and another 15% of IC patients who are surgically treated with RPC and IPAA resolve with an ultimate painstaking devastating complication, the de novo CD, which often cause significant morbidity and pouch failure. Several of these patients may not respond favorably to conversional conservative medical treatment, ultimately requiring surgical revision or permanent fecal diversion. These patients are preoperatively thought to have a “misdiagnosed” Crohn’s Colitis (CC). This claim remains skeptical given the fact that there is a possibility that these patients had a truly authentic UC but are “transformed” and convert to adhere to de novo CD. Natural transformation occurs because of an altered microbiome ecology in the ileal pouch, and immune environment, and subsequently the patient actually “converts”. In certain cases, authentic CD are not evident prior to proctocolectomy surgery and/or gradually manifest/acquire an aberrated novel immunopathogenesis ingredient factors of the ileal pouch (antigen-antibody reaction against the mucosal resistance) of individual patients. Crypt Paneth cell (PC) secreted antimicrobial peptide, Human alpha-defensin 5 (DEFA5 or HD) has shown the ability to delineate UC from CC and that circumvents IC into actual diagnosis of UC or CC at initial clinic visit endoscopy biopsy procedures, a realizable potential that might subdue diagnostic delay in the IBD Clinic. Furthermore, DEFA5 denotes UC patients prior to colectomy likely to transform and convert to de novo CD after IPAA surgery. We also propose that DEFA5 may permit selection of certain subtype of CC patients who are likely to have a positive outcome following IPAA surgery. Promising data are underway.
Acknowledgments
The authors wish to thank Dr. George Bates, M.D. for providing invaluable suggestions and comments. The authors thank the Meharry Office for Scientific Editing and Publications for scientific editing support (NIH/S21MD000104).
Funding
NIH/NIDDK-R21DK095186; VICTR-CTSA-1UL1RR024975-01; NIH/NCATS VICTR- 2UL1TR000445-06; NIH/NCI-3U54CA091408-09S1;NIH/NCI-3U54CA091408-09S2; U54MD007593-09, U54CA09148-08 and 5U54MD007586 (The RCMI Program in Health Disparities Research at Meharry Medical College)
Abbreviations:
- IBD
Inflammatory Bowel Disease
- UC
Ulcerative colitis
- CD
Crohn’s disease
- CC
Crohn’s colitis
- IC
Indeterminate colitis
- MOOSE
meta-analyses of observational studies
- RPR
Restorative proctocolectomy
- IPAA
Ileal pouch-anal anastomosis
- PRISMA-P
Preferred reporting items for systematic review and meta-analysis Protocols
- MEDLINE
Medical Literature Analysis and retrieval system online
- EMBASE
Excerpta Medica database
- CINAHL
Cumulative index of Nursing and Allied Health Literature
- SCFAs
Short-chain fatty acids
- NOD2
Nucleotide-binding oligomerization domain-containing protein 2
- TNF-α
Tumor necrosis factor-alpha
- DEFA5
Human Alpha-defensin 5 (Paneth cell specific)
- PC
Paneth cell
References
- 1.Williams AD, Korolkova OY, Sakwe AM, Geiger TM, James SD, Muldoon RL, et al. Human alpha defensin 5 is a candidate biomarker to delineate inflammatory bowel disease. PLoS One. 2017; 12(8):e0179710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M’Koma AE, Wise PE, Muldoon RL, Schwartz DA, Washington MK, Herline AJ. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis. 2007; 22: 1143–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes EL, Kochar B, Jessup HR, Herfarth HH. The Incidence and Definition of Crohn’s Disease of the Pouch: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2019; 25: 1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Annals of surgery. 1995; 222: 120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meagher AP, Farouk R, Dozois RR, Kelly KA, Pemberton JH. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998; 85: 800–3. [DOI] [PubMed] [Google Scholar]
- 6.Lightner AL, Mathis KL, Dozois EJ, Hahnsloser D, Loftus EV, Raffals LE, et al. Results at Up to 30 Years After Ileal Pouch-Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm Bowel Dis. 2017; 23:781–90. [DOI] [PubMed] [Google Scholar]
- 7.Le Q, Melmed G, Dubinsky M, McGovern D, Vasiliauskas EA, Murrell Z, et al. Surgical outcome of ileal pouch-anal anastomosis when used intentionally for well-defined Crohn’s disease. Inflamm Bowel Dis. 2013; 19:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CJ, Maclean AR, Cohen Z, Macrae HM, O’Connor BI, McLeod RS. Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum. 2005; 48: 1542–9. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed S, Melmed G, McGovern D, Robbins LA, Shih D, Vasiliauskas E, et al. Nonbloody Diarrhea but Not Significant Weight Loss at Diagnosis Is Associated with the Development of Denovo Crohn’s Disease After Ileal Pouch-anal Anastomosis for Ulcerative Colitis. Inflamm Bowel Dis. 2016; 22: 654–61. [DOI] [PubMed] [Google Scholar]
- 10.Shamah S, Schneider J, Korelitz BI. High Incidence of Recurrent Crohn’s Disease Following Colectomy for Ulcerative Colitis Revealed with Long Follow-Up. Dig Dis Sci. 2018; 63: 446–51. [DOI] [PubMed] [Google Scholar]
- 11.Dayton MT, Larsen KR, Christiansen DD. Similar functional results and complications after ileal pouch anal anastomosis in patients with indeterminate vs ulcerative colitis. Archives of surgery. 2002; 137: 690–4. [DOI] [PubMed] [Google Scholar]
- 12.Alexander F, Sarigol S, DiFiore J, Stallion A, Cotman K, Clark H, et al. Fate of the pouch in 151 pediatric patients after ileal pouch anal anastomosis. J Pediatr Surg. 2003; 38: 78–82. [DOI] [PubMed] [Google Scholar]
- 13.Braveman JM, Schoetz DJ, Marcello PW, Roberts PL, Coller JA, Murray JJ, et al. The fate of the ileal pouch in patients developing Crohn’s disease. Dis Colon Rectum. 2004; 47: 1613–9. [DOI] [PubMed] [Google Scholar]
- 14.Tulchinsky H, Hawley PR, Nicholls J. Long-term failure after restorative proctocolectomy for ulcerative colitis. Ann Surg. 2003; 238: 229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahnloser D, Pemberton JH, Wolff BG, Larson DR, Crownhart BS, Dozois RR. Results at up to 20 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Br J Surgy. 2007; 94: 333–40. [DOI] [PubMed] [Google Scholar]
- 16.Shen B Crohn’s disease of the ileal pouch: reality, diagnosis, and management. Inflamm Bowel Dis. 2009; 15: 284–94. [DOI] [PubMed] [Google Scholar]
- 17.Zaghiyan K, Kaminski JP, Barmparas G, Fleshner P. De novo Crohn’s Disease after Ileal Pouch-Anal Anastomosis for Ulcerative Colitis and Inflammatory Bowel Disease Unclassified: Long-Term Follow-Up of a Prospective Inflammatory Bowel Disease Registry. Am Surg. 2016; 82: 977–81. [PubMed] [Google Scholar]
- 18.Lightner AL, Dattani S, Dozois EJ, Moncrief SB, Pemberton JH, Mathis KL. Pouch excision: indications and outcomes. Colorectal disease. 2017; 19: 912–6. [DOI] [PubMed] [Google Scholar]
- 19.Shen B, Patel S, Lian L. Natural history of Crohn’s disease in patients who underwent intentional restorative proctocolectomy with ileal pouch-anal anastomosis. Aliment Pharmacol Ther. 2010; 31: 745–53. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wu B, Shen B. Diagnosis and differential diagnosis of Crohn’s disease of the ileal pouch. Curr Gastroenterol Rep. 2012; 14: 406–13. [DOI] [PubMed] [Google Scholar]
- 21.Koslowski MJ, Kubler I, Chamaillard M, Schaeffeler E, Reinisch W, Wang G, et al. Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn’s disease. PLoS One. 2009; 4: e4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011; 9: 356–68. [DOI] [PubMed] [Google Scholar]
- 23.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015; 350: g7647. [DOI] [PubMed] [Google Scholar]
- 24.Gustavsson A, Magnuson A, Blomberg B, Andersson M, Halfvarson J, Tysk C. Endoscopic dilation is an efficacious and safe treatment of intestinal strictures in Crohn’s disease. Aliment Pharmacol Ther. 2012; 36: 151–8. [DOI] [PubMed] [Google Scholar]
- 25.Gu J, Stocchi L, Kiran RP, Shen B, Remzi FH. Do clinical characteristics of de pouch Crohn’s disease after restorative proctocolectomy affect ileal pouch retention? Dis Colon Rectum. 2014; 57: 76–82. [DOI] [PubMed] [Google Scholar]
- 26.Tekkis PP, Heriot AG, Smith O, Smith JJ, Windsor AC, Nicholls RJ. Long-term outcomes of restorative proctocolectomy for Crohn’s disease and indeterminate colitis. Colorectal disease. 2005; 7: 218–23. [DOI] [PubMed] [Google Scholar]
- 27.Shen B, Fazio VW, Remzi FH, Delaney CP, Bennett AE, Achkar J, et al. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomoses. Am J Gastroenterol. 2005; 100: 93–101. [DOI] [PubMed] [Google Scholar]
- 28.NS, Sanford WW, Bodzin JH. Crohn’s-like complications in patients with Goldstein ulcerative colitis after total proctocolectomy and ileal pouch-anal anastomosis. Am J Surg Pathol. 1997; 21: 1343–53. [DOI] [PubMed] [Google Scholar]
- 29.Peyregne V, Francois Y, Gilly FN, Descos JL, Flourie B, Vignal J. Outcome of ileal pouch after secondary diagnosis of Crohn’s disease. Int J Colorectal Dis. 2000; 15: 49–53. [DOI] [PubMed] [Google Scholar]
- 30.Rossi HL, Brand MI, Saclarides TJ. Anal complications after restorative proctocolectomy (J-pouch). Am Surg. 2002; 68: 628–30. [PubMed] [Google Scholar]
- 31.Melton GB, Kiran RP, Fazio VW, He J, Shen B, Goldblum JR, et al. Do preoperative factors predict subsequent diagnosis of Crohn’s disease after ileal pouch-anal anastomosis for ulcerative or indeterminate colitis? Colorectal Dis. 2010; 12: 1026–32. [DOI] [PubMed] [Google Scholar]
- 32.Haveran LA, Sehgal R, Poritz LS, McKenna KJ, Stewart DB, Koltun WA. Infliximab and/or azathioprine in the treatment of Crohn’s disease-like complications after IPAA. Dis Colon Rectum. 2011; 54: 15–20. [DOI] [PubMed] [Google Scholar]
- 33.Coukos JA, Howard LA, Weinberg JM, Becker JM, Stucchi AF, Farraye FA. ASCA IgG and CBir antibodies are associated with the development of Crohn’s disease and fistulae following ileal pouch-anal anastomosis. Dig Dis Sci. 2012; 57: 1544–53. [DOI] [PubMed] [Google Scholar]
- 34.Tyler AD, Milgrom R, Xu W, Stempak JM, Steinhart AH, McLeod RS, et al. Antimicrobial antibodies are associated with a Crohn’s disease-like phenotype after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2012; 10: 507–12. [DOI] [PubMed] [Google Scholar]
- 35.Diederen K, Sahami SS, Tabbers MM, Benninga MA, Kindermann A, Tanis PJ, et al. Outcome after restorative proctocolectomy and ileal pouch-anal anastomosis in children and adults. Br J Surg. 2017; 104: 1640–7. [DOI] [PubMed] [Google Scholar]
- 36.Yanai H, Ben-Shachar S, Mlynarsky L, Godny L, Leshno M, Tulchinsky H, et al. The outcome of ulcerative colitis patients undergoing pouch surgery is determined by pre-surgical factors. Aliment Pharma col Ther. 2017; 46: 508–15. [DOI] [PubMed] [Google Scholar]
- 37.Murrell ZA, Melmed GY, Ippoliti A, Vasiliauskas EA, Dubinsky M, Targan SR, et al. A prospective evaluation of the long-term outcome of ileal pouch-anal anastomosis in patients with inflammatory bowel disease-unclassified and indeterminate colitis. Dis Colon Rectum. 2009; 52: 872–8. [DOI] [PubMed] [Google Scholar]
- 38.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Towards an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19: 5A–36A. [DOI] [PubMed] [Google Scholar]
- 39.Koltun WA, Schoetz DJ, Roberts PL, Murray JJ, Coller JA, Veidenheimer MC. Indeterminate colitis predisposes to perineal complications after ileal pouch-anal anastomosis. Dis Colon Rectum. 1991; 34: 857–60. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson KG, Owen DA, Wankling G. Restorative proctocolectomy and indeterminate colitis. Am J Surg. 1994; 167: 516–8. [DOI] [PubMed] [Google Scholar]
- 41.Bodzin JH, Klein SN, Priest SG. Ileoproctostomy is preferred over ileo-anal pull-through in patients with indeterminate colitis. Am Surg. 1995; 61: 590–3. [PubMed] [Google Scholar]
- 42.Yu CS, Pemberton JH, Larson D. Ileal pouch-anal anastomosis in patients with indeterminate colitis: longterm results. Dis Colon Rectum 2000; 43: 1487–96. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre PB, Pemberton JH, Wolff BG, Dozois RR, Beart RW. Indeterminate colitis. Long-term outcome in patients after ileal pouch-anal anastomosis. Dis Colon Rectum. 1995; 38: 51–4. [DOI] [PubMed] [Google Scholar]
- 44.Pishori T, Dinnewitzer A, Zmora O, Oberwalder M, Hajjar L, Cotman K, et al. Outcome of patients with indeterminate colitis undergoing a double-stapled ileal pouch anal anastomosis. Dis Colon Rectum. 2004; 47: 717–21. [DOI] [PubMed] [Google Scholar]
- 45.Moss AC, Cheifetz AS. How often is a diagnosis of ulcerative colitis changed to Crohn’s disease and vice versa? Inflamm Bowel Dis. 2008; 14: S155–6. [DOI] [PubMed] [Google Scholar]
- 46.Melmed GY, Elashoff R, Chen GC, Nastaskin I, Papadakis KA, Vasiliauskas EA, et al. Predicting a change in diagnosis from ulcerative colitis to Crohn’s disease: a nested, casecontrol study. Clin Gastroenterol Hepatol. 2007; 5: 602–8. [DOI] [PubMed] [Google Scholar]
- 47.Raffals LH. Understanding the pouch microbiota: an evolving story. Dig Dis Sci. 2012; 57: 2734–6. [DOI] [PubMed] [Google Scholar]
- 48.Scarpa M, Grillo A, Scarpa M, Brun P, Castoro C, Pozza A, et al. Innate immune environment in ileal pouch mucosa: alpha5 defensin up-regulation as predictor of chronic/relapsing pouchitis. J Gastrointest Surg. 2012; 16: 188–201. [DOI] [PubMed] [Google Scholar]
- 49.Waterman M, Xu W, Stempak JM, Milgrom R, Bernstein CN, Griffiths AM, et al. Distinct and overlapping genetic loci in Crohn’s disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis. 2011; 17: 1936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cunliffe RN, Rose FR, Keyte J, Abberley L, Chan WC, Mahida YR. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut. 2001; 48: 176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wehkamp J, Schwind B, Herrlinger KR, Baxmann S, Schmidt K, Duchrow M, et al. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig Dis Sci. 2002; 47: 1349–55. [DOI] [PubMed] [Google Scholar]
- 52.Fabregat A, Sidiropoulos K, Viteri G, Forner O, Marin-Garcia P, Arnau V, et al. Reactome pathway analysis: a high-performance in-memory approach. BMC Bioinformatics. 2017; 18: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen B, Remzi FH, Brzezinski A, Lopez R, Bennett AE, Lavery IC, et al. Risk factors for pouch failure in patients with different phenotypes of Crohn’s disease of the pouch. Inflamm Bowel Dis. 2008; 14: 942–8. [DOI] [PubMed] [Google Scholar]
- 54.Shen B, Remzi FH, Lavery IC, Lashner BA, Fazio VW. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clinical gastroenterology and Hepatology. 2008; 6: 145–58. [DOI] [PubMed] [Google Scholar]
- 55.Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn’s disease. Mucosal Immunol. 2008; 1: S67–74. [DOI] [PubMed] [Google Scholar]
- 56.Ballard BR, M’Koma AE. Paneth Cell Physiology and Pathophysiology in Inflammatory Bowel Disease. In: Gazouli M, Theodoropoulos G (eds) Digestive System Diseases. Stem Cell Biology and Regenerative Medicine. Human Press Cham. Switzerland: Springer. 2019: 165–80. [Google Scholar]
- 57.Perminow G, Beisner J, Koslowski M, Lyckander LG, Stange E, Vatn MH, et al. Defective paneth cell mediated host defense in pediatric ileal Crohn’s disease. Am J Gastroenterol. 2010; 105: 452–9. [DOI] [PubMed] [Google Scholar]
- 58.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001; 49:777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.M’Koma AE. The Multifactorial Etiopathogeneses Interplay of Inflammatory Bowel Disease: An Overview. Gastrointest Disord. 2018; 1: 75–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fahlgren A, Hammarstrom S, Danielsson A, Hammarstrom ML. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol. 2003; 131: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhn F, Klar E. Surgical Principles in the Treatment of Ulcerative Colitis. Viszeralmedizin. 2015; 31: 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


