Executive summary
Traumatic brain injury (TBI) has the highest incidence of all common neurological disorders, and poses a substantial public health burden. TBI is increasingly documented not only as an acute condition but also as a chronic disease with long-term consequences, including an increased risk of late-onset neurodegeneration. The first Lancet Neurology Commission on TBI, published in 2017, called for a concerted effort to tackle the global health problem posed by TBI. Since then, funding agencies have supported research both in high-income countries (HICs) and in low-income and middle-income countries (LMICs). In November 2020, the World Health Assembly, the decision-making body of WHO, passed resolution WHA73.10 for global actions on epilepsy and other neurological disorders, and WHO launched the Decade for Action on Road Safety plan in 2021. New knowledge has been generated by large observational studies, including those conducted under the umbrella of the International Traumatic Brain Injury Research (InTBIR) initiative, established as a collaboration of funding agencies in 2011. InTBIR has also provided a huge stimulus to collaborative research in TBI and has facilitated participation of global partners. The return on investment has been high, but many needs of patients with TBI remain unaddressed. This update to the 2017 Commission presents advances and discusses persisting and new challenges in prevention, clinical care, and research.
In LMICs, the occurrence of TBI is driven by road traffic incidents, often involving vulnerable road users such as motorcyclists and pedestrians. In HICs, most TBI is caused by falls, particularly in older people (aged ≥65 years), who often have comorbidities. Risk factors such as frailty and alcohol misuse provide opportunities for targeted prevention actions. Little evidence exists to inform treatment of older patients, who have been commonly excluded from past clinical trials—consequently, appropriate evidence is urgently required. Although increasing age is associated with worse outcomes from TBI, age should not dictate limitations in therapy. However, patients injured by low-energy falls (who are mostly older people) are about 50% less likely to receive critical care or emergency interventions, compared with those injured by high-energy mechanisms, such as road traffic incidents.
Mild TBI, defined as a Glasgow Coma sum score of 13–15, comprises most of the TBI cases (over 90%) presenting to hospital. Around 50% of adult patients with mild TBI presenting to hospital do not recover to pre-TBI levels of health by 6 months after their injury. Fewer than 10% of patients discharged after presenting to an emergency department for TBI in Europe currently receive follow-up. Structured follow-up after mild TBI should be considered good practice, and urgent research is needed to identify which patients with mild TBI are at risk for incomplete recovery.
The selection of patients for CT is an important triage decision in mild TBI since it allows early identification of lesions that can trigger hospital admission or life-saving surgery. Current decision making for deciding on CT is inefficient, with 90–95% of scanned patients showing no intracranial injury but being subjected to radiation risks. InTBIR studies have shown that measurement of blood-based biomarkers adds value to previously proposed clinical decision rules, holding the potential to improve efficiency while reducing radiation exposure. Increased concentrations of biomarkers in the blood of patients with a normal presentation CT scan suggest structural brain damage, which is seen on MR scanning in up to 30% of patients with mild TBI. Advanced MRI, including diffusion tensor imaging and volumetric analyses, can identify additional injuries not detectable by visual inspection of standard clinical MR images. Thus, the absence of CT abnormalities does not exclude structural damage—an observation relevant to litigation procedures, to management of mild TBI, and when CT scans are insufficient to explain the severity of the clinical condition.
Although blood-based protein biomarkers have been shown to have important roles in the evaluation of TBI, most available assays are for research use only. To date, there is only one vendor of such assays with regulatory clearance in Europe and the USA with an indication to rule out the need for CT imaging for patients with suspected TBI. Regulatory clearance is provided for a combination of biomarkers, although evidence is accumulating that a single biomarker can perform as well as a combination. Additional biomarkers and more clinical-use platforms are on the horizon, but cross-platform harmonisation of results is needed. Health-care efficiency would benefit from diversity in providers.
In the intensive care setting, automated analysis of blood pressure and intracranial pressure with calculation of derived parameters can help individualise management of TBI. Interest in the identification of subgroups of patients who might benefit more from some specific therapeutic approaches than others represents a welcome shift towards precision medicine. Comparative-effectiveness research to identify best practice has delivered on expectations for providing evidence in support of best practices, both in adult and paediatric patients with TBI.
Progress has also been made in improving outcome assessment after TBI. Key instruments have been translated into up to 20 languages and linguistically validated, and are now internationally available for clinical and research use. TBI affects multiple domains of functioning, and outcomes are affected by personal characteristics and life-course events, consistent with a multifactorial bio-psycho-socio-ecological model of TBI, as presented in the US National Academies of Sciences, Engineering, and Medicine (NASEM) 2022 report. Multidimensional assessment is desirable and might be best based on measurement of global functional impairment. More work is required to develop and implement recommendations for multidimensional assessment. Prediction of outcome is relevant to patients and their families, and can facilitate the benchmarking of quality of care. InTBIR studies have identified new building blocks (eg, blood biomarkers and quantitative CT analysis) to refine existing prognostic models. Further improvement in prognostication could come from MRI, genetics, and the integration of dynamic changes in patient status after presentation.
Neurotrauma researchers traditionally seek translation of their research findings through publications, clinical guidelines, and industry collaborations. However, to effectively impact clinical care and outcome, interactions are also needed with research funders, regulators, and policy makers, and partnership with patient organisations. Such interactions are increasingly taking place, with exemplars including interactions with the All Party Parliamentary Group on Acquired Brain Injury in the UK, the production of the NASEM report in the USA, and interactions with the US Food and Drug Administration. More interactions should be encouraged, and future discussions with regulators should include debates around consent from patients with acute mental incapacity and data sharing. Data sharing is strongly advocated by funding agencies. From January 2023, the US National Institutes of Health will require upload of research data into public repositories, but the EU requires data controllers to safeguard data security and privacy regulation. The tension between open data-sharing and adherence to privacy regulation could be resolved by cross-dataset analyses on federated platforms, with the data remaining at their original safe location. Tools already exist for conventional statistical analyses on federated platforms, however federated machine learning requires further development. Support for further development of federated platforms, and neuroinformatics more generally, should be a priority.
This update to the 2017 Commission presents new insights and challenges across a range of topics around TBI: epidemiology and prevention (section 1); system of care (section 2); clinical management (section 3); characterisation of TBI (section 4); outcome assessment (section 5); prognosis (Section 6); and new directions for acquiring and implementing evidence (section 7). Table 1 summarises key messages from this Commission and proposes recommendations for the way forward to advance research and clinical management of TBI.
Introduction
The first Lancet Neurology Commission on traumatic brain injury (TBI),1 published in 2017, provided a comprehensive resource for subsequent research, clinical care, and policy development. The Commission did more than just collate data; it provided an integrated picture of TBI in 2017, identified gaps in knowledge, and presented recommendations to improve clinical care and research from the perspectives of policymakers, clinicians, and researchers. This resource provided the foundation for a substantial body of subsequent research, informed the strategies of major funding organisations (such as the EU and the US National Institutes of Health [NIH]), and was used to brief legislators and inform policy.2
The 2017 Commission documented that TBI was estimated to remain one of the top three causes of injury-related death and disability up to 2030. Overall, 50 million–60 million people have a TBI each year, costing the global economy around US$400 billion annually. Of all common neurological disorders, TBI has the highest incidence and poses a substantial public health burden (figure 1). In Europe, more than 2 million people are admitted to hospital each year because of TBI, and about 82 000 die.3 Care for all severities of TBI was noted to be inconsistent across centres, regions, and countries, both for acute and post-acute care. The 2017 Commission recognised that the substantial between-centre variability in treatment and outcome in TBI offered unique opportunities for comparative-effectiveness research to improve the strength of evidence. Methods for diagnosis and classification of patients with TBI were noted to be insufficient to permit targeting of current and new therapies to the needs of individual patients. The Commission underlined the need for multidimensional outcome constructs that quantify the overall burden of disability from TBI, to guide improved clinical management, and to support high-quality research.
Figure 1: Global incidence and prevalence of traumatic brain injury compared with other common neurological diseases.
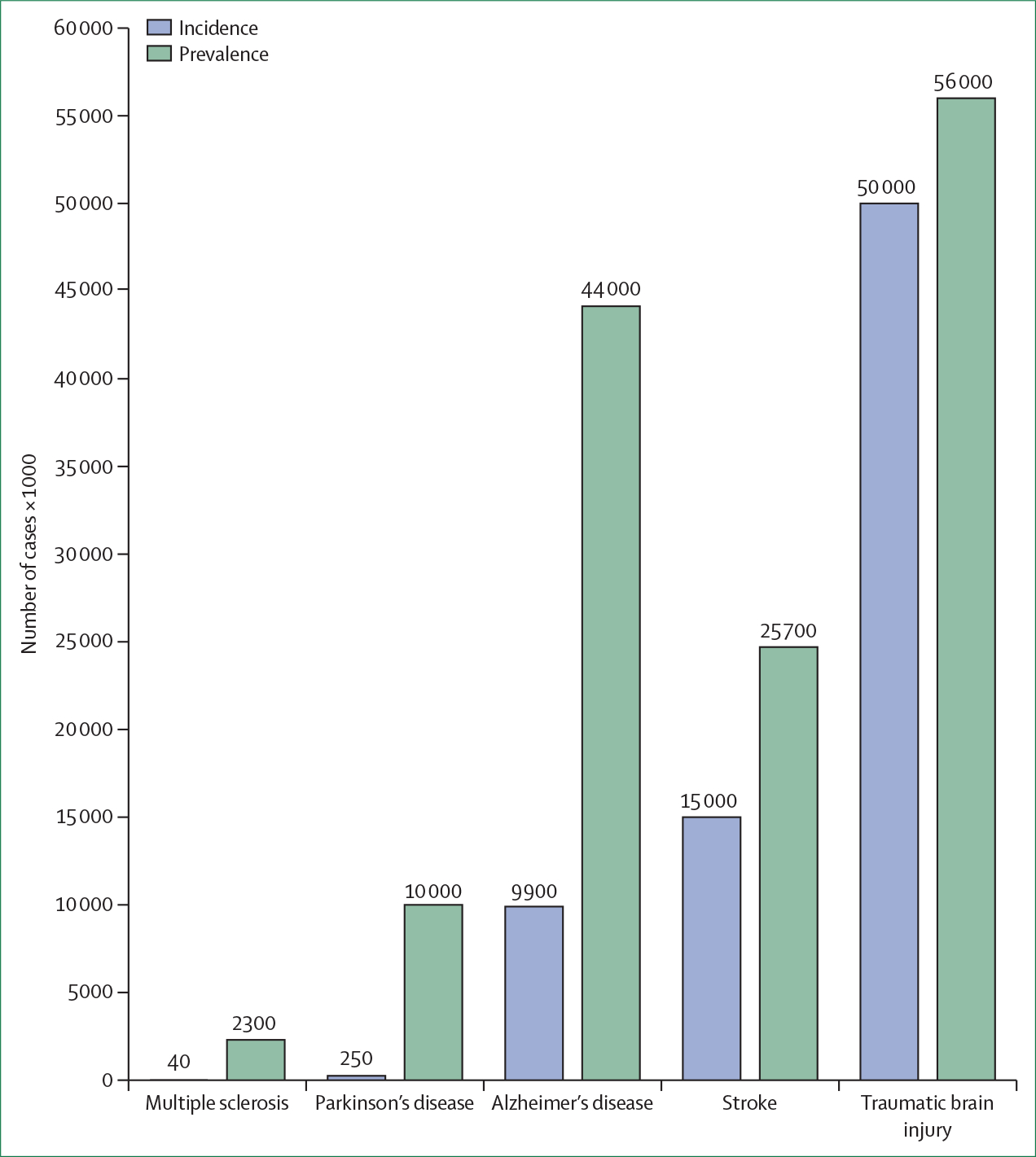
Data are from multiple sources. Incidence is quantified as the number of cases per year, and prevalence as the number of cases at a given time point. The numbers provided are best estimates. However, it should be recognised that data collection and reporting are inconsistent across different parts of the world, and that data reported for the various diseases do not always reflect exactly the same time period. Modified from a draft provided by Carl Long, NeuroTrauma Sciences.
Since publication of the 2017 Commission, much has changed in the field of TBI. Studies have provided new data on epidemiology and casemix of TBI in the hospital setting, and new insights regarding the effects of systems of care on TBI management and outcome. Clinical care has been informed by the results of a substantial body of research since that Commission, much of which was supported by the International TBI Research (InTBIR) initiative, a coalition of major funding bodies that came together in 2011 to support neurotrauma research.4,5 Although there have been advances in the characterisation of TBI with the use of advanced neuroimaging, blood biomarkers, and genomics, these advances have not yet been fully translated into clinical care. However, there is increasing evidence that these advances will facilitate identification of patients with TBI who share specific disease mechanisms, treatment response characteristics, or prognosis, thus providing a basis for individualised management. Progress has also occurred in the prediction and characterisation of outcome following TBI, and although these advances are still being developed in research settings, their clinical application will likely occur over the next few years. Challenges remain, particularly in low-income and middle-income countries (LMICs), relating to prevention of TBI, access to care, and provision of clinical guidelines that can be implemented in resource-limited contexts. It is also crucial that we ensure equitable integration of researchers from LMICs in neurotrauma research. Disparities in care provision have also been identified in high-income countries (HICs). In the research context, developments both within the TBI field and insights into novel approaches to trial design from the COVID-19 pandemic have highlighted exciting new approaches and opportunities for generating evidence to support clinical care. Many of these new approaches are dependent on collaborative research and data-sharing, a process that can be constrained by regulatory requirements and facilitated by novel data analysis techniques.
This 2022 update on the Commission for TBI describes advances along with attendant challenges and opportunities, and provides a staging post in ongoing efforts to improve clinical care and outcomes.
Section 1: Epidemiology and prevention of TBI
The first Lancet Neurology Commission on TBI1 highlighted the huge public health burden posed by TBI. Reported population-based incidence rates in New Zealand and North America were between 811 and 979 per 100 000 people per year and hospital discharge rates in the EU were 287·2 per 100 000 people per year, with substantial variation between Member States. We highlighted how methodological variations confound comparisons of TBI epidemiology patterns between regions, countries, and continents, and emphasised the need for standardised epidemiological monitoring and international consensus on definitions and approaches. Since then, the Global Burden of Diseases, Injuries, and Risk Factors (GBD) study has provided estimates of global TBI incidence rates using a standardised approach.6 CENTER-TBI and TRACK-TBI—two large-scale, real-world observational studies on TBI of all severities—have provided insight into TBI-related disability and characteristics of patients currently presenting to hospital. The COVID-19 pandemic has had clear effects on both TBI incidence and presenting causes of injury. In this section, we discuss these new findings on the incidence, mechanisms, and burden of TBI. We additionally focus on four subpopulations of increasing relevance: older people, children and adolescents, criminal offenders, and sports participants, also highlighting specific targets for prevention and ongoing prevention initiatives.
Incidence and mechanisms
The age-adjusted incidence of all severities of TBI from epidemiological population-based studies published between 2015 and 2020 ranged between 476 per 100 000 individuals in South Korea7 to 787 per 100 000 individuals in the USA.8 However, these incidence studies might still underestimate the true extent of the problem. A Canadian study of concussion9 (a subset of mild TBI) revealed a higher annual incidence rate of 1153 per 100 000 individuals. Small regional or single-centre studies reported a decrease in TBI incidence during periods of COVID-19 lockdowns, reflecting decreased mobility, reduced participation in sports and recreational activities, and possibly reluctance to seek medical treatment for milder injuries.10,11 A few cohort studies reported12,13 an increase in suspected head trauma in children and gunshot wounds to the head. Although there has been an increase in intimate partner violence during the pandemic,14,15 there are no specific data on TBI in this context.
For the pre-COVID-19 era, GBD reported a worldwide age-standardised TBI incidence of 369 per 100 000 people (95% CI 331–412) in 2016.6 Updated rates for 2019 are 346 per 100 000 people (298–401).16 These rates are lower than previously reported in population-based studies and closer to those reported for hospital admissions. A likely explanation is that GBD mainly accesses data from hospital presentations and uses an indirect approach to capturing TBI incidence involving identification of external causes of injury and linking the nature of the most severe injury to the external cause. Therefore, TBI might not be captured in cases with severe extracranial injuries.
Assessing temporal trends and national variations in TBI incidence is complex and confounded by methodological diversity. We previously found a ten-fold difference in the reported incidence of hospital admissions for TBI between countries in Europe,3 probably reflecting nation-specific differences in data capture and reporting methods. A major strength of the GBD approach is that it uses a standardised approach across all global regions and calculates age-adjusted incidence rates, enabling cross-country comparisons. Nevertheless, GBD also reported substantial between-country differences in incidence rates. A common denominator in both approaches is the use of hospital International Classification of Diseases (ICD) injury coding for data extraction. Inconsistencies within and between hospitals in ICD coding might be a main cause of the large variations observed. These considerations highlight a crucial need to standardise conduct and reporting of incidence studies. The main injury mechanisms causing TBI are falls, road traffic incidents, and violence. GBD reported that, overall, their relative contributions have remained stable between 1990 and 2019, with falls being the most common cause (52% in 1990 and 54% in 2019). These average numbers might, however, mask shifts within regions. Global modelling suggests that the incidence of TBI in LMICs is significantly higher than in HICs,6,17 and is mainly driven by road traffic collisions, particularly those involving motorcyclists. These findings were confirmed in the Global Neurotrauma Outcomes Study,18 showing a clear relationship between the mechanism of injury and the UN’s Human Development Index (a composite index of life expectancy, education, and per person income indicators, which is used to rank countries into four tiers of human development: low, medium, high, and very high; figure 2).
Figure 2: Between-country variations in mechanism of traumatic brain injury according to the Human Development Index.
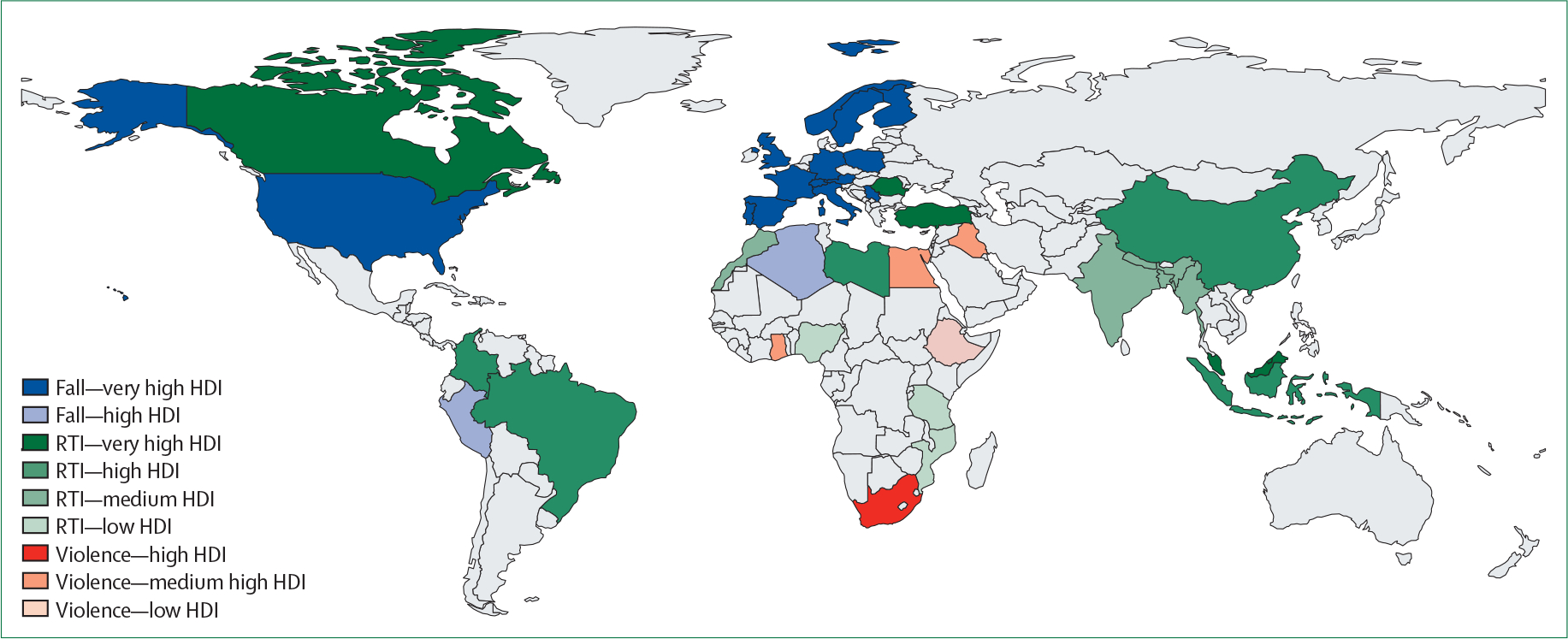
Figure modified from Clark et al with permission.18 HDI=Human Development Index. RTI=road traffic incident.
By contrast with findings in LMICs, the CENTER-TBI registry,19 which mainly collected data from HICs, reported that 12 127 (56%) of 21 681 patients with TBI were injured by falls, of whom 71% had a low-energy (ground-level) fall. Compared with 13 059 patients injured by high-energy transfer, those injured through low-energy falls were older (median 74 years [IQR 56–84] vs 42 years [25–60]), and more often female (50% [95% CI 48–51] vs 32% [31–34]). The CENTER-TBI Core study20 reported alcohol involvement in 28% of incidental falls versus 17% in road traffic incidents. Although these findings illustrate the success of traffic-related alcohol prevention campaigns, they also emphasise the importance of campaigns to prevent fall-related injuries (panel 1). Alcohol and cannabis abuse were particularly prominent in violence-related TBI (64% and 15%, respectively).
Panel 1: Targets for prevention and ongoing prevention actions for traumatic brain injury.
Targets for prevention identified in the Lancet Neurology Commissions on traumatic brain injury (TBI)
Road traffic safety: of particular relevance to low-income and middle-income countries (LMICs).
Older people: fall prevention, including campaigns to increase awareness of increased risk with excessive alcohol use; address frailty.
Children and adolescents: targeted prevention with a particular focus on car safety, traffic education, and protection of juvenile sporters; early intervention and support to prevent violent head trauma.
Criminal offenders: implementation of rehabilitative justice systems; provision of special considerations to support offenders who have had TBI or intimate partner violence, or both.
Sports: implementation of measures to mitigate risks in contact sports; development of a consensus on sideline assessment protocols across different sports and uniform return-to-play guidelines; improvement of design and mandated use of helmets in individual sports, such as horse-riding and cycling.
Ongoing prevention actions
WHO Decade for Action on Road Safety in 2021
The initiative aims to reduce traffic related deaths and injuries by at least 50% by 2030, with clear recommendations for safer traffic systems, measures needed to implement these systems, and allocation of key responsibilities for such implementation.
US Centers for Disease Control and Prevention (CDC): Older adult Fall Prevention
In the USA, falls result in around 3 million hospital attendances, 800 000 hospitalisations, and 34 000 deaths each year―most commonly for hip fracture or TBI―with total medical costs that exceed US $50 billion. Recognition of this burden has led to comprehensive recommendations for both health professionals and the lay public for measures to prevent falls.
CDC STEADI Initiative (Stopping Elderly Accidents, Deaths and Injuries) for health-care providers
This initiative describes a coordinated approach for health-care providers to implement guidelines for fall prevention, and includes three core elements:
Screen patients for risk of falls,
Assess modifiable risk factors, and
Intervene to reduce risk by using effective clinical and community strategies.
UK National Health Service (NHS) guidance. Falls: applying All Our Health
This guidance document for health professionals and the lay public covers the multifactorial causes of falls, estimates their costs to the NHS, and suggests strategies for mitigation.
Sport-related concussion
Rugby union: introduction of law changes around tackle height and related sanctions for foul play under the Head Contact Process.21,22
Ice hockey: removal of body checking at youth-participation level to reduce concussion risk.23
Soccer: introduction of restrictions to heading training from youth to professional levels by several national associations.
Various sports: deployment of mouthguard sensors during training and match-play that gather head kinematic data around head impacts and injury to inform risk-reduction measures.
The burden of TBI
The true consequences of TBI go beyond the dynamics of their occurrence or fatality, and are better reflected in measures of disease burden—ie, years of lost life (YLLs) and years lived with disability (YLDs). GBD reports that TBI was a cause of 8·1 million YLDs in 2016. YLLs due to TBI have been reported for selected countries or population groups. European data captured from 16 countries suggests that each TBI death is associated with about 24 YLLs. This extrapolates to a pooled age-standardised rate of about 160 YLLs per 100 000.24 Few studies have estimated both the fatal and non-fatal burden of TBI, quantified as disability-adjusted life years (DALYs). In New Zealand, estimates revealed 20 300 DALYs attributable to TBI, accounting for 27% of total injury-related health loss and 2·4% of DALYs from all causes. A total of 71% of TBI DALYs resulted from fatal TBI.25 More studies on the disease burden of TBI are required.
TBI in older adults
The frequency of hospital admissions for TBI is highest in older people (aged ≥65 years), followed by children and adolescents (figure 3).3 Rates of TBI in older people are rising and exceed population growth in some countries.26 Relative to their younger counterparts, older adults more often sustain TBI from falls and have more severe cognitive and functional impairments,27 and might be at greater risk for post-recovery functional decline.28 However, psychological outcomes can be better, perhaps because older individuals have had more opportunity to develop coping skills, achieve life goals, and might have less pressure to resume economic productivity.29
Figure 3: Estimated frequency of hospital discharges and deaths in cases of traumatic brain injury by age group in Europe.
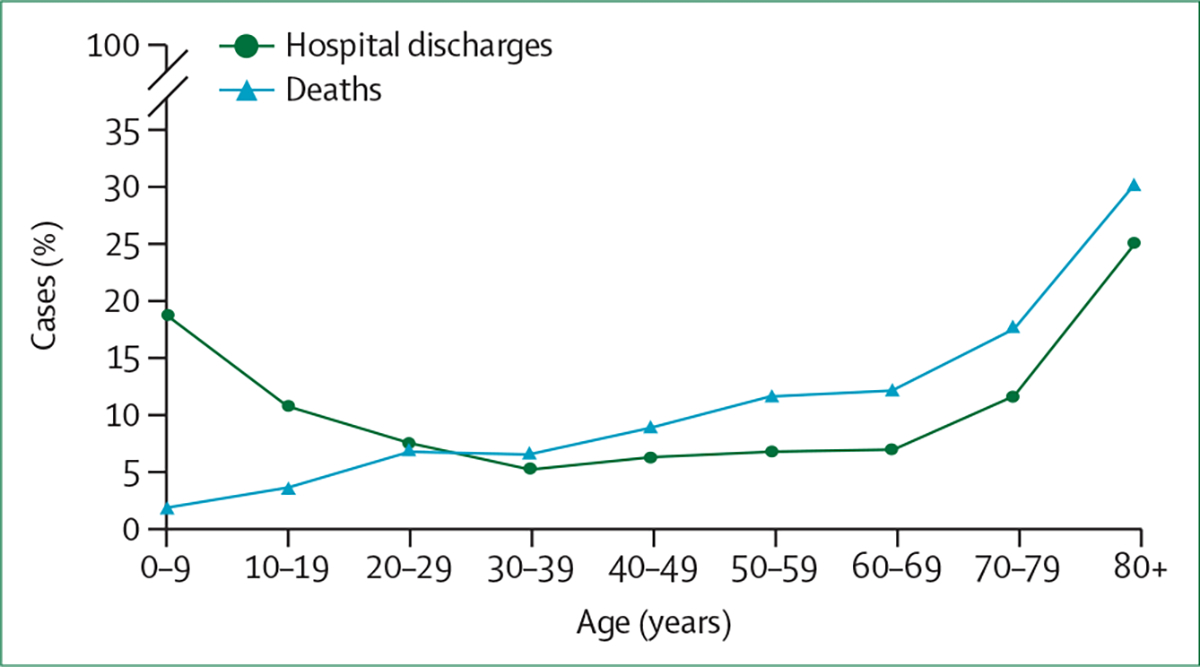
Figure created using data from Majdan et al.3
Consistent with epidemiological studies in HICs, the proportion of older patients (ie, aged ≥65 years) enrolled in CENTER-TBI was high (28% in the Core study, 38% in the registry), but lower in TRACK-TBI (12%). Nevertheless, the CDC report that older adults account for 43·9% of all TBI-related hospital admissions in the USA.30 In the China registry,31 older patients accounted for 2500 (18·3%) of 13 627 enrolled participants. The increasing number of older patients with TBI is of direct relevance to policy makers and clinicians. Most previous clinical trials excluded patients older than 65 years. Consequently, very little—if any—evidence exists to inform the clinical management of older patients. TBI in older adults can have a distinct pathophysiology and injury severity indices used in younger adults might be less appropriate in older people.32 Additionally, older patients often have comorbidities requiring multiple medications. Together with age-related physical and cognitive decline, the presence of comorbidities can complicate acute and long-term management, rehabilitation care needs, and outcome measurement.
The association between age and outcome is partly indirect; risk, mechanism, and type of injury, as well as recovery potential, are intricately linked to the concept of frailty. Frailty is a consequence of cumulative decline in physiological systems across a lifetime. It reflects, as a state of vulnerability, the poor resolution of homoeostasis after a stressor event (eg, TBI), resulting in increased risk of poor health outcomes. CENTER-TBI developed a novel TBI-specific frailty measurement index using a cumulative deficit approach.33 The overall median frailty index score in CENTER-TBI was 0·07 (IQR 0·03–0·15), with a median score of 0·17 (0·08–0·27) in older adults aged at least 65 years. Higher frailty scores were significantly associated with unfavourable outcome. External validation on data from TRACK-TBI supported the robustness of these findings. Evidence that TBI in older adults is biologically distinct and recognition of the relevance of frailty underscore the need for research to inform acute and long-term care in older adults.
TBI in children and adolescents
The paediatric and adolescent age group (age range 0–19 years) has the second-highest incidence of hospital admissions for TBI.34 Approximately 345 children or adolescents per 100 000 are admitted to hospitals in the EU per year, and about 3 per 100 000 die as a consequence of a TBI, resulting in around 184 YLLs per 100 00034 individuals. In the USA, approximately 1 million–2 million children and adolescents have a mild TBI annually, and youths with a previous concussion have a four-fold risk of having a recurrent concussion.35 Moreover, children and young people (aged 5–18 years) have a significantly increased risk of mental health issues, psychiatric hospitalisation, and self-harm after TBI compared with those after an orthopedic injury.36 Although the paediatric and adolescent age group shows the lowest TBI mortality overall (about 5% of all TBI deaths), the burden of these deaths is substantial: about 3000 TBI-related deaths occurring in the EU each year result in nearly 200 000 YLLs.24 These findings suggest that targeted prevention of TBI in this group could result in substantial gains in quality of life and in decrease of YLLs (panel 1).
Violence, crime, and TBI
Violence is an important cause of TBI; and in turn, having had a TBI can predispose an individual to violent behaviour and criminal offending. Violence is the third-most common cause of TBI. In the CENTER-TBI Core and TRACK-TBI studies, 6·7% (293/4388 and 171/2537, respectively) of injuries were caused by violence. In the CENTER-TBI China registry, which collected data only for patients admitted to hospital, 1714 (13%) of 13 138 had a TBI resulting from violence.37 Populations in precarious circumstances are especially at risk for violence-related TBI—eg, communities affected by conflict (including migrants and refugees) and indigenous populations who are socially disadvantaged.38,39 The prevalence of TBI within prison populations is also high: up to 64%40 of male inmates and 78% of female inmates have a history of TBI. Intimate partner violence is the most frequently reported cause41 of previous TBI in incarcerated women, with many experiencing their first injury in childhood or adolescence. When compared with other causes of TBI due to interpersonal violence, intimate partner violence far more commonly affects women than men (75% vs 5%), more often results in severe TBI (27% vs 5%), and is associated with nearly three times the mortality (14% vs 5%).42 Young people with TBI in the criminal justice system often did not have appropriate parenting support, and were excluded from school or exposed to gang violence, or both. Without appropriate support, TBI in incarcerated individuals is associated with poor engagement in rehabilitation and re-conviction. In the UK, Brain Injury Link Workers enable neuro-rehabilitation practices in some prisons, with promising outcomes—an initiative that deserves broader implementation and validation.
TBI can lead to impaired social communication and behavioural dysregulation associated with an increased risk of crime, especially reactive violence in response to a perceived threat.43 Epidemiological studies from various high-income jurisdictions (eg, Sweden, Canada, and Australia) indicate an approximate 2–3 fold increased risk of serious crime in individuals after a TBI compared with non-injured controls. The UN General Comment (no 24) urges States to implement rehabilitative justice systems, rather than focusing on a punitive approach.44 England and Wales in the UK have developed guidelines for sentencing adults with mental disorders, development disorders, or neurological impairments.45 These examples reflect a need for a holistic approach uniting health, education, social care, and justice systems, to strengthen preventative and proactive measures (panel 1).
Sport-related TBI
Sport-related TBI has received considerable media attention. There is increasing awareness of the risk of adverse brain-health consequences caused by TBI and repetitive head impacts in sport.46–48 A retrospective cohort study49 compared mortality from neurodegenerative disease in 7676 former professional soccer players with that of 23 028 matched controls from the general population, and found that mortality from neurodegenerative disease was around three times higher in former soccer players compared with controls (subhazard ratio 3·45; 95% CI 2·11–5·62; p<0·001). However, mortality from other common diseases (eg, ischaemic heart disease and lung cancer) was lower in the former soccer players.
Several studies report on the incidence of sport-related concussions, but establishing comparative risks within and between sports is challenging. Reportedly, the risk of sport-related concussion is highest among collision sports, particularly rugby and American football.50,51 In CENTER-TBI, sports and recreational activities were reported as a cause of TBI in 312 (7%) of 4509 cases. Of these, horse-riding (19%), skiing or snowboarding (16%), cycling (11%), and soccer (11%) were the main activities involved. In TRACK-TBI, 218 (8·7%) of 2520 injuries occurred during sport and recreational activities. Of note, these data reflect a selected cohort of patients with injuries motivating hospital attendance and are not representative of the overall risk of sport-associated TBI. The community-based BIONIC study52 in New Zealand collected data over a 12-month period for 1369 patients with TBI across all ages in a population of 173 205 people, and reported that 21% of injuries were related to sports and recreational activities.
Substantial advances in the immediate management and rehabilitation of sport-related concussion in the past decade have resulted in increased detection and notable decreases of same-season repeat concussions (see section 2).53–55 Various global sports organisations have developed initiatives to better understand risk factors for sport-related concussion and to implement measures to mitigate risks. For example, a review of match data from the professional rugby union showed that around half of sport-related concussions occur during the tackle, mostly involving the player making the tackle.56 Leveraging this information, World Rugby have embarked on targeted initiatives to address the risk of concussion associated with tackling57 (panel 1). Data from the CARE Consortium study indicate that concussion risk and head-impact exposures are highest among American college footballers in preseason and in practice, triggering targeted initiatives to reduce the risk for these athletes.58,59 However, there remains a need for continued data collection to inform public health policy and practice changes designed to maximise athlete health and safety. Large, multicentre, prospective surveillance projects are ongoing (appendix p 3), and will provide further insight on the risks of sport-related TBI. Current initiatives are mainly directed at rugby, American football, and soccer, but the CENTER-TBI and TRACK-TBI data suggest the need for an additional focus on individual sports, such as horse-riding and cycling, where simple preventive measures (eg, improved helmet design and use) can make a substantial difference.60,61
Main messages and recommendations
Main messages
Worldwide, TBI is a leading cause of injury-related death and disability, with devastating effects on patients and their families.
Wide variations exist in global estimates of TBI incidence and in reported incidence, prevalence, and mortality rates between regions and countries. Variations in approaches to data capture and interpretation probably contribute to these variations, confounding comparisons.
More than 90% of patients presenting to hospital with TBI have mild TBI, but there is little evidence to inform treatment of patients with mild TBI.
In HICs, older patients (≥65 years) who are mostly injured by falls account for 30–40% of hospital admissions for TBI. Frailty and alcohol abuse contribute to falls causing TBI in older people.
People in LMICs are disproportionately affected by TBI, with most injuries caused by road traffic incidents. There are substantial disparities in care, with little infrastructure for emergency pre-hospital care and very little access to post-acute care.
Although there is a strong focus on the risk of sport-related concussion and repetitive head impacts in team sports, most patients seen in hospital with sport-related concussion have sustained the injury during individual sports or recreational activities.
TBI and criminal offending are closely and bidirectionally related. TBI associated with intimate partner violence affects women more commonly and is associated with worse outcomes compared with other interpersonal violence.
Recommendations
Continue concerted efforts to address this vast global health problem and focus on better prevention, improved access to care, and promotion of clinical research to improve treatment standards.
Develop a uniform process for data capture and reporting of TBI epidemiology that is agreed upon by governments and institutions.
Increase public health interest and establish a research focus on mild TBI.
Target fall prevention for older people in HICs and make preparations for subsequent implementation of successful strategies in LMICs as demographics change.
Deliver on implementation of Road Safety goals, described in WHO’s Decade for Action on Road Safety plan launched in 2021. Improve emergency pre-hospital care and develop an infrastructure for post-acute care.
Continue education and public pressure on governing bodies to ensure consistent implementation of safe-play rules that are compliant with guidelines. Reinforce preventive measures in individualised sports, such as cycling and horse-riding.
Implement rehabilitative justice systems; provide special considerations to support criminal offenders who have had a TBI. Develop and implement initiatives to recognise, reduce, and manage intimate partner violence.
Section 2: Systems of care for TBI
In the 2017 Commission on TBI, we highlighted inconsistencies in access to acute and post-acute care between centres, regions, and countries, and reported substantial variations in systems and quality of care. We recommended that measures to improve systems of care for patients with TBI, ensuring continuity of care, should be high on policy agendas. In this section, we present new data on persisting variations in care, identify disparities in care provision, and discuss their implications for health policy, differentiated for the pre-hospital setting, the emergency departments, and post-acute care. Figure 4 presents a summary overview of advances and challenges in care for TBI along the trauma chain. We additionally provide a discussion on the management of sport-related concussions, a field in which substantial progress has been made towards protecting the brains of athletes, and on the specific persisting challenges of TBI care in LMICs. By contrast with the first Commission, we do not discuss cost-effectiveness, as few new data are available.
Figure 4: Advances and remaining challenges in the provision of health care for people with traumatic brain injury along the trauma chain.
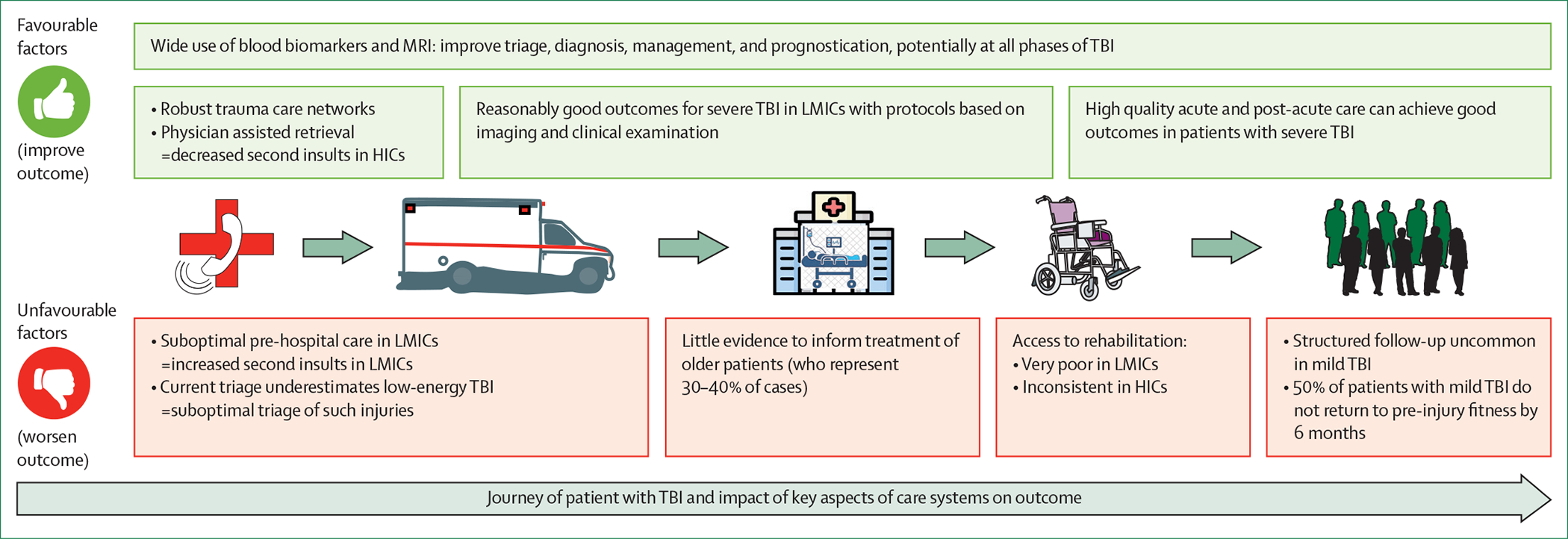
Continuity of care along the chain of trauma health care is of paramount importance to achieve good outcomes. If pre-hospital care is inadequate, secondary damage might be so severe that outcome will be poor, no matter how good the in-hospital treatment might be. Conversely, benefits accrued from excellent in-hospital treatment might be lost if they are not consolidated by good post-acute care. Note that many challenges relate to transitions across the links of the trauma chain. TBI=traumatic brain injury. HICs=high-income countries. LMICs=low-income and middle-income countries.
Pre-hospital care
The pre-hospital phase (eg, care at the scene of the incident and during transport to hospital) is a time of high risk for hypoxia, hypotension, and expanding intracranial mass lesions. Emergency medical services implement resuscitative interventions to prevent secondary brain injuries and decide whether the patient requires specialist care in a regional trauma centre. Such specialist care might require prolonged transportation from a closer non-specialist hospital. These time-critical assessments are complicated by other causes of impaired consciousness in injured patients, including extracranial injury, metabolic derangement, intoxication, or pre-existing chronic neurological impairments.
The CENTER TBI core study collected detailed data on current pre-hospital practices in Europe. In patients with moderate or severe TBI,62 pre-hospital hypoxia (in 64 [5·5%] of 1160 individuals) and hypotension (in 124 [10·6%] of 1160) were less common than previously reported in the IMPACT studies63 (hypoxia in 1150 [20·3%] of 5661 individuals, and hypotension in 1211 [18·3%] of 6629). Secondary insults were associated with major extracranial injuries (odds ratio [OR] 3·6, 95% CI 2·6–5·0 for hypotension and 4·4, 2·9–6·7 for hypoxia). Pre-hospital intubation was associated with better functional outcome in patients with higher abbreviated injury scores in the thoracic and abdominal regions (p=0·009 and p=0·02, respectively). There was substantial variation between countries and centres in all aspects of pre-hospital care (at scene interventions, time spent at scene, and en route), which were not sufficiently explained by patient characteristics and did not clearly translate to differences in outcome.62,64 The greatest driver of longer on-scene time was intubation (mean increase 8·3 min, 95% CI 5·6–11·1). Substantial variation was observed in secondary referrals, with a median OR of 1·69 between countries.64,65 Of 1347 patients with moderate or severe TBI, 195 (14·5%) were admitted after secondary referral, and presented more often with CT abnormalities than patients who were admitted directly: mass lesions (52% vs 34%), midline shift (54% vs 36%), and acute subdural haematoma (77% vs 65%)—reflecting the main reasons for secondary referral. Secondary referral was not significantly associated with functional outcome (adjusted OR 1·07, 95% CI 0·78–1·69), or with survival at discharge (OR 1·05, 0·58–1·90).
These results appear to contrast with the evidence provided in the 2017 Commission on TBI,1 which supported direct transport of more severely injured patients to regional trauma centres. We recognise that CENTER-TBI did not capture information on patients who stayed in regional hospitals and that this limitation might have confounded results. Our interpretation is that, within a system of care that embeds appropriate and rapid transfer following initial presentation to a regional hospital, the outcome is similar to that observed in patients directly transported to a trauma centre. Current triage tools,66,67 such as the Field Triage Decision Scheme established by the American College of Surgeons Committee on Trauma,68 for identifying patients requiring direct (prolonged) primary transportation to a regional trauma centre are heavily weighted towards patients with high-energy injury mechanisms.69 Older patients with intracranial injury often go undetected by current triage tools because they often have a discordantly high GCS at the scene of the incident in relation to the severity of intracranial injury shown on subsequent neuroimaging,70 and typically have had a low-energy injury through falling. The potential severity of such injuries should not be underestimated, and any decrease in conscious level should motivate transport to a trauma centre where neurosurgical treatment is available. Nevertheless, the data from CENTER-TBI, showing similar outcomes in patients directly or secondarily transferred, indicate that most patients with a stable and high GCS can be safely initially assessed at the emergency department of the closest hospital, and then referred to a specialist hospital, if required, according to neuroimaging findings.
Emergency department
On arrival to an emergency department, the role of emergency physicians and nurses is to continue efforts to minimise the risk of secondary brain injury while unravelling the diagnostic conundrum of whether the patient’s condition is due to TBI or other injuries and illnesses. Prioritisation is a main feature in this approach, following the principle of treat first what kills first. Evidence-based guidance and protocolised approaches according to the principles of Advanced Trauma Life Support71 are the main pillars in this phase of care72 to ensure a systematic and structured approach. Intubation is recommended in the pre-hospital guidelines of the Brain Trauma Foundation73 for all patients with a GCS of 8 or less. Evidence from CENTER-TBI shows that in-hospital intubation had a significant beneficial effect on functional outcome in patients with GCS of 10 or lower (p=0·01).74 In combination with the findings from CENTER-TBI in the pre-hospital setting, these results suggest that major extracranial injury should drive the decision to intubate in the pre-hospital setting, and that indications for intubation in-hospital should be broadened to also include patients with a GCS of 9 or 10. Large, international, multicentre, randomised trials have examined the efficacy of tranexamic acid as part of haemorrhage control in injured patients. Although there is significant benefit of tranexamic acid in patients with trauma with clinically significant extracranial bleeding, including those who also have TBI,75 the evidence in isolated TBI is still under debate.76,77
Additionally, in the emergency-department setting, the main focus is on patients with high-energy injuries and on those with severe TBI. CENTER-TBI found disparities in care for patients injured by low-energy mechanisms: compared with 13 059 patients injured by high-energy transfer, those injured through low-energy falls had similar rates of CT brain scan abnormalities and in-hospital mortality, but were 50% less likely to receive critical care or emergency interventions.19 From a perspective of systems of care, a major decision made in the emergency department phase relates to triaging for CT scanning. A strategy of imaging all patients with a head injury would guarantee not missing any clinically relevant structural damage, but would be costly, expose many patients to unnecessary radiation, and increase crowding in the emergency department. Clinical decision rules have been developed to select patients for CT scanning. Examples include the New Orleans criteria, the Canadian CT head rule,78 the UK National Institute for Health and Care Excellence (NICE) guideline for head injury, and the CT in Head Injury Patients rule.79 These clinical decision rules substantially reduce the number of CT scans but are still inefficient, as 90–95% of scans performed show no intracranial injury.80 The assessment of certain protein-based blood biomarkers has the potential to improve the performance of these rules. In 2013, the Scandinavian Neurotrauma guidelines81 incorporated S100 calcium-binding protein B (S100B) to reduce CT scan usage in low-risk patients with mild TBI and reported that, even with incomplete implementation, the approach saved €39 per patient82 managed. A systematic review supported the clinical use of S100B for detecting intracranial abnormalities,83 and the ALERT trial84 provided evidence in favour of using glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1). Based on these data, the GFAP and UCH-L1 tandem biomarker-based plasma test has been cleared by the FDA to rule out the need for CT imaging in patients with mild TBI.85 The same test recently obtained a CE mark in the EU, indicating conformity with European health and safety standards.86
TRACK-TBI, reporting on 1359 patients with GCS 3–15, found that GFAP showed better diagnostic performance for predicting intracranial abnormalities than did S100B.87 The regulatory landscape for the diagnostic use of blood-based biomarkers is presented in detail in the appendix (p 4). One company has received regulatory clearance for a point-of-care device. Use of such devices will permit faster turnover times in the emergency department, and will also facilitate application in out-of-hospital settings. Regulatory clearance is, however, restricted to a specific combination of biomarkers (GFAP and UCH-L1). Data currently available suggest that there is no clear diagnostic benefit from combinations of biomarkers, as GFAP in isolation performs as well as all biomarkers combined. The clinical utility of biomarkers will depend on their added value compared with clinical decision rules, and this added value has insufficiently been addressed in previous studies. One study88 published in 2022 explored the value of adding GFAP and UCH-L1 to three clinical decision rules (the New Orleans criteria, the Canadian CT head rule, and the National Emergency X-ray Utilisation Study) in a cohort of 349 patients with mild TBI (GCS 13–15) presenting to the emergency department within 4 h of injury. At predefined cutoff thresholds, GFAP outperformed UCH-L1 (area under the receiver operating characteristic curve [AUC] 0·83, 95% CI 0·73–0·93 vs 0·72, 0·61–0·82) for predicting CT abnormalities. Adding continuous GFAP to the clinical decision rules improved the AUC, particularly for the Canadian CT head rule (to 0·88, 95% CI 0·81–0·95). A limitation of this study, however, was the low number of events (CT positivity in 23 [7%] of 349 individuals). Stronger evidence in support of adding biomarkers to clinical decision rules was provided by CENTER-TBI. Six biomarkers (S100B, GFAP, UCH-L1, neuron-specific enolase [NSE], neurofilament light [NfL], and total tau) were analysed in 1889 patients with mild TBI (CT positivity in 874 [46%] of 1889) and their diagnostic accuracy for predicting CT abnormalities compared with four clinical decision rules (the Canadian CT head rule, the CT in Head injury Patients, NICE, and New Orleans criteria). GFAP outperformed other biomarkers and all clinical decision rules in predicting CT abnormalities (AUC for GFAP 0·85, 95% CI 0·83–0·87 vs 0·71–0·74 for the multivariable models containing components of the rules). Combining GFAP with the rule components marginally increased their discriminative ability to AUC (0·86–0·87 [95% CI 0·85–0·88]). The addition of other biomarkers did not provide added value over GFAP. A limitation of CENTER-TBI was that most blood samples were not obtained within the first few hours after injury (median sampling time 11·8 h, IQR 5·4–18·6). However, results were consistent when modelling was used to estimate biomarker concentrations 2 h after injury. These results support the development of novel clinical decision rules, combining GFAP with clinical characteristics. Clinical implementation will require robust assay platforms for clinical use, and further validation studies are needed, in broader populations with early sampling, to determine cost-effectiveness.
Post-acute care
The frequent occurrence of impairments in functioning and participation in daily life activities after TBI (see section 5) highlights the need for continued rehabilitation efforts. The US National Institute for Disability, Independent Living, and Rehabilitation Research has funded the TBI Model Systems of Care since 1987, a clinical care and research infrastructure network that enrols individuals with moderate to severe TBI at the time of in-patient rehabilitation and follows them up at 1, 2, and 5 years, and every 5 years thereafter.89 The Monash Epworth Rehabilitation Research Centre in Australia has led a similarly designed study investigating rehabilitation outcomes up to 30 years after a TBI.90 Both studies have shown that early and continuous rehabilitation can consolidate the progress gained in the acute clinical phase, and reduce the length of stay in hospital and socioeconomic burden. Individuals who experience discontinuity of care between the acute and post-acute phase have poorer functional outcomes and satisfaction with care than those who receive a continuous chain of rehabilitation.91,92 Moreover, fragmented systems of care can cause patients and caregivers to feel unsupported and uncertain about how to access resources and negotiate care transitions.93 Despite broad recognition among clinicians of the needs for appropriate post-acute care, CENTER-TBI, which reported on 1206 individuals with moderate to severe disability at 6 months after injury, showed that 90% reported rehabilitation needs, but only 30% received in-patient rehabilitation and 15% received out-patient rehabilitation.94
At the milder end of the TBI spectrum, where inpatient rehabilitation needs are different, the need for post-discharge rehabilitation is even more neglected. Results from CENTER-TBI showed that only a fifth of individuals with mild TBI who had persisting symptoms received outpatient rehabilitation at 6 months post-injury.95 Both CENTER-TBI and TRACK-TBI found further deficiencies in care with regard to the discharge policy from the emergency department. Provider profiling in CENTER-TBI showed that 90% of centres do not routinely schedule a follow-up appointment for patients with TBI discharged home from the emergency department, and around 50% do not follow patients up after discharging them from the ward.96 The CENTER-TBI Core study data show that only 26% of patients discharged from an emergency department received written information and 6% received a follow-up appointment in hospital. In TRACK-TBI, fewer than half of the participants received educational material at discharge from the emergency department and saw a health-care provider within 3 months after mild TBI.97 Yet both studies showed that 30% of patients discharged from an emergency department did not attain full recovery by 6 months.
Taken together, the evidence suggests that access to rehabilitation and structured follow-up care following TBI remains suboptimal and underlines the need for increased knowledge about TBI consequences, the assessment of functional impairments and corresponding rehabilitation needs, and referrals to rehabilitation.
Sport-related concussions
Triage decisions play a prominent part in the management of confirmed and suspected sport-related concussion, regarding removal from play, treatment considerations, and decisions on return to play. Broad consensus exists among international experts and global sports organisations that, at all levels of sport, any athlete with clear signs or symptoms of concussion—so-called red flags—should be immediately removed from play.98,99 Exclusion of a possible sport-related concussion is more challenging. Although various sideline assessment tools and protocols have been developed to aid in concussion recognition,100–102 no perfect sideline assessments tools exist for its diagnosis or, importantly, its exclusion, with most tools showing substantial observer variability.100 Assessments of symptoms and multimodality testing protocols have the highest sensitivity and specificity for concussion detection,103,104 and are incorporated into the widely used Sport Concussion Assessment Tool,105 elements of which are included in the extensive, multimodal Head Injury Assessment protocol.106 Although several global consensus statements endorse recommendations of the rugby union based around the notion of ‘if in doubt, sit them out’, there is remarkable variability in how these recommendations are translated to clinical practice.107 An editorial in the British Journal of Sports Medicine raised a red flag towards the professional soccer association Fédération Internationale de Football Association because of their variable policies that might compromise athlete care, and made a strong plea for adoption of standards introduced into other fields of sport.108 We suggest that efforts should be made to operationalise consensus statements on the management of sport-related concussion to increase consistency of sideline assessment protocols across sports.
Sport-related concussion rehabilitation and follow-up protocols use a multidimensional approach to monitoring symptom resolution and return to functional baseline before resuming return-to-play progression, with return to normal life prioritised (eg, education and work) before returning to sport.55,98 Typically, return-to-play protocols begin with low-intensity exercise, gradually progressing to sport-specific contact activities, with a growing trend toward early subthreshold exercise and domain-specific rehabilitation. These protocols are mainly pragmatically oriented, while research studies using advanced blood and neuroimaging biomarkers continue to improve our understanding of sport-related concussion.
Continued investment in research remains crucial to determining factors that might contribute to potential adverse brain-health outcomes associated with sport-related concussion and repetitive head impacts. In parallel, efforts to reduce the incidence of concussion and head impact exposure in athletes should continue (see section 1).58
Challenges in LMICs
The 2019 GBD study109 estimated that almost 90% of the 4 million global deaths due to injuries occurred in LMICs, with autopsy studies suggesting that TBI is responsible for a substantial proportion of these deaths.110 Increasing industrialisation and changing demographics in LMICs are associated with an epidemiological shift from communicable, maternal, neonatal, and nutritional diseases towards non-communicable diseases and injuries, with a predicted increasing burden of injuries in LMICs over the coming years.109,111,112 Quality of care and outcomes of TBI vary throughout the world. In the 2017 Commission on TBI, we reported a 3·3 times difference in the odds of unfavourable outcome between centres at the extremes of the outcome range (2·5th vs 97·5th percentiles) in the IMPACT studies, but this difference increased to a 6·6 times difference on analysis of data from the CRASH trial, which included patients from LMICs. The Global Neurotrauma Outcomes Study,18 an observational cohort study of 1635 patients across 57 countries receiving emergency neurosurgery for TBI, found significant differences in management and short-term mortality between countries with different levels of human development index (HDI). Patients in countries with medium and low levels of HDI had temporal delays to surgery as well as limited access to CT scanning, intensive care, and intracranial pressure monitoring.
An absence of beds in an intensive care unit (ICU) often results in severe TBI being managed in the emergency department or wards with less monitoring, physiological support, or medical attention. ICU beds are generally allocated on a first-come, first-serve basis in the absence of triage support (often for ethical reasons). Management and outcomes from this large group of patients with severe TBI managed outside the ICU are completely unstudied. The most crucial limitations to care are not the absence of advanced technology, but the availability of ICU beds, basic physiological support devices (eg, bedside monitors, ventilators, etc), and access to CT imaging.
On comparison of data collected in the context of CENTER-TBI between India and Europe, we found large differences in the provision of pre-hospital and post-acute care: despite a similar distribution of injury severity classified by the GCS, 89·6% of patients received emergency care at the scene of incident in Europe versus only 5·8% in India. In Europe, 16·3% were discharged from hospital to a rehabilitation facility versus 0·4% in India. Such differences highlight the need for systems-wide approaches to improving TBI care in LMICs,113–115 as well as clinical practice guidelines tailored to the resources available116 (see section 3). Various initiatives have been developed over the past 5 years in this direction at global institutional, governmental, and investigator-driven levels (panel 2). Global institutional and governmental initiatives appropriately have a main focus on road traffic safety, whereas investigator-driven initiatives are more directed at improving care provision and stimulating research. Investigator-led initiatives have been hugely successful in involving clinicians and researchers from LMICs in neurotrauma research, in developing advocacy initiatives, and in implementing educational activities and protocols to improve the care for patients with TBI in LMICs (see appendix, p 6 for key accomplishments). Some unpublished studies from LMICs in Latin America have shown that protocolised care (eg, the CREVICE protocol:117 see section 3) in the absence of intracranial pressure monitoring can produce 6-month outcomes superior to those predicted by the CRASH prognostic model for low-income countries (LICs), and similar to those predicted for HICs by the IMPACT and CRASH models (Chesnut R, personal communication). Improving prevention, advancing care, and stimulating research in LMIC settings are urgent unmet needs. Current efforts should be strengthened and new efforts developed.
Panel 2: Initiatives specifically in low-income and middle-income countries to decrease the incidence of traumatic brain injury, improve the care for patients, and stimulate collaborative research.
Global institutional and Governmental initiatives with a primary aim on road traffic safety
WHO Decade for Action on Road Safety plan
The initiative, launched in 2021, aims to reduce traffic-related deaths and injuries by at least 50% by 2030. It was implemented following the adoption of resolution A/RES/74/299 “Improving global road safety” by the UN General Assembly.
The Federation Internationale de l’Automobile Action for Road Safety
This campaign was launched in support of the UN Decade of Action for Road Safety and involves four key priorities: advocacy at the highest levels, action by clubs on the ground, involvement of the motor sport community, and campaigns and partnerships.
The National Highways Authority India (NHAI)
NHAI is seeking bids for providing free emergency clinical care for automotive incidents occurring on highways connecting the Delhi–Mumbai–Chennai, Chennai–Kolkata, Kolkata–Agra, and Agra–Delhi corridors (the so-called golden quadrilateral).
The World Bank Road Safety Project in Bangladesh
The project will pilot comprehensive road safety measures, including improved engineering designs, signing and marking, pedestrian facilities, speed enforcement, and emergency care on two major highways in Bangladesh.
Guide to implementation of the Toward Zero Deaths (TZD) national strategy on highway safety (2022)
The TZD national strategy, initiated in 2014, previously had a high level focus on national leadership and direction, with details of implementation in the USA left to individual states. However, the persistent number of traffic fatalities led to publication of this report, which provides guidance to states and other highway safety stakeholders to advance the implementation of the TZD national strategy through programmes, tools, and techniques.
Investigator-led initiatives to advance the care for traumatic brain injury (TBI) in low-income and middle-income countries (LMICs) and to stimulate collaborative research
UK National Institute for Health and Care Research Global Health Research Group on Neurotrauma18
Its overarching mission is to improve global neurotrauma care. Four main themes are identified: mapping TBI care, understanding TBI care, innovation in TBI, and measuring and nurturing research capacity. A total of 57 countries are involved in the collaboration.18
The US National Institutes of Health and Fogarty-International-Research-Institute-funded Global Neurotrauma Research Group
The main focus is on Spanish-speaking countries in Central and South America, with a central aim to build capacity and implement and test protocols for TBI management in LMICs.
Main messages and recommendations
Main messages
Disparities in care exist in HICs and relate to: older people injured by low-energy mechanisms (falls), access to rehabilitation for patients with moderate-to-severe TBI, and follow-up in patients with mild TBI.
Current triage tools used in emergency settings are heavily focused on high-energy injuries and might insufficiently identify the severity of brain injury resulting from low-energy mechanisms.
Blood-based biomarkers, particularly GFAP, provide added value to clinical decision rules for selecting patients with mild TBI for CT scanning. However, few assay platforms have been approved for clinical use and substantial variability exists between platforms.
Implementation of safe-play protocols to protect participants in sports from acute and long-term adverse effects of brain injury is highly variable across different team sports.
Recommendations
Address disparities through close collaboration between policymakers and clinicians. Approaches to consider include: critical appraisal of triage tools used in emergency settings, involvement of rehabilitation services at an early stage of the in-hospital treatment for TBI, and establishment of structured follow-up after mild TBI as good practice.
Critical appraisal of triage tools used in emergency settings is needed.
Develop and seek regulatory approval for robust clinical use platforms. Establish cross-platform harmonisation of assay results, avoiding unnecessary use of combinations of biomarkers.
Operationalise consensus statements on the management of sport-related concussion to increase consistency of sideline assessment and return-to-play protocols across sports.
Section 3: Clinical management of TBI
Patients with moderate-to-severe TBI are a minority (around 10%) of all patients with TBI presenting to hospital, with the remainder (around 90%) having mild TBI (GCS 13–15). However, although the proportion of patients with moderate-to-severe TBI is smaller, many patients are admitted to the ICU and receive acute inpatient therapeutic interventions, and more severe injury results in the greatest burden of death and disability for individual patients. The first 2017 Commission emphasised the scarce evidence supporting the management in patients with severe TBI; highlighted substantial between-centre variances in practice; and showed that physiological management targets were based on population averages and took little account of heterogeneity in pathophysiology and therapy responsiveness.
In this section, we describe how casemix and clinical practice have evolved since the 2017 Commission for patients with TBI admitted to an ICU, summarise how emerging evidence and insights have refined and individualised current management in HICs, and discuss advances and persisting challenges in LMICs. Section 4 provides greater detail regarding future prospects for identifying subgroups of patients who might benefit from specific therapeutic approaches.
The current picture of TBI in the ICU
Data from over a decade ago had led to the perception that most patients with TBI in an ICU are young and have severe TBI (GCS ≤8). Even at the time of the 2017 Commission, there were emerging data suggesting that the demographics of TBI were changing, and these insights have been confirmed by large observational studies (see section 1). In CENTER-TBI,20 the median age in the ICU stratum was 49 years (IQR 29–65), compared with a median 30 years (21–45) in the IMPACT studies,118 and 26% of patients were older than 65 years. This change in demographics is important, as age can impair physiological reserve, and many older patients are on treatments for comorbidities, which can modulate disease course and outcome.119,120 Fewer than half of patients admitted to ICU for TBI in HICs have severe TBI and more than a third have mild TBI (GCS 13–15). ICU admission of patients with mild TBI is often prompted by factors other than TBI severity, in particular major extracranial injuries (abbreviated injury scale ≥3 in any extracranial body part), which were seen in 55% of ICU admissions with TBI.121 These extracranial injuries typically involved the thorax (35%), spine (18%), and extremities (17%), and often required surgical interventions (29%). However, other patients with mild TBI were admitted to an ICU because of a perceived risk for clinical and neurological deterioration. Although some of these admissions might have been appropriately prudent, others represent costly over-triage, estimated at 17% of cases in a US study.122
TBI as a systemic disease
Systemic organ dysfunction in TBI can result from extracranial injury, but TBI itself, and therapies for intracranial hypertension, also contribute. Systemic complications include respiratory failure (see later in this section), renal failure,123 adrenal insufficiency,124 myocardial injury,125 and potentially multiple organ dysfunction.126 Acute kidney injury occurs in about 12% of patients with TBI in an ICU and is associated with worse outcome. Osmotic therapy and hypernatremia are important modifiable risk factors (hazard ratios 2·08 and 1·88, respectively), attention to which could reduce the incidence of acute kidney injury.123 These data underline the importance of general ICU management in addition to the specific management of TBI, and further establish TBI as a systemic condition.127,128
Individualising clinical targets for ICU management
TBI still does not have interventions targeting disease mechanisms; management in the ICU remains focused on physiological targets, including intracranial pressure and cerebral perfusion pressure (ie, the difference between mean arterial pressure and intracranial pressure), and in some centres, multimodality monitoring (see later in this section and section 4).129,130 The BEST TRIP randomised trial, published before the last Commission in 2017, recruited 324 patients with severe TBI from South American centres, and did not show any benefit of a protocol based on intracranial pressure monitoring when compared with management based on clinical examination and serial imaging.131 However, the more recent SYNAPSE-ICU study, published in 2021, undertook comparative-effectiveness analysis of intracranial pressure monitoring in 146 ICUs across the world including 1287 patients with TBI, and showed that the use of intracranial pressure monitoring was associated with increased therapy intensity, lower mortality, and better functional outcome at 6 months.132 Understanding these apparently contrasting results requires further exploration, but the differences could be related to the settings where the studies were conducted.
The harm associated with intracranial pressure elevation is unlikely to be uniform across patients and over time. Although existing guidelines suggest treating an intracranial pressure that is greater than 22 mm Hg and keeping cerebral perfusion pressure between 60 and 70 mm Hg,133 these thresholds are not absolute. CENTER-TBI confirmed earlier research134 showing that both intensity and duration of intracranial pressure insults are associated with poorer outcomes,135 and identified an intracranial pressure treatment threshold of 18 mm Hg (± 4 mm Hg), consistent with other evidence for treatment thresholds of less than 20 mm Hg.136,137 This concept of so-called intracranial pressure dose, that integrates both the intensity and duration of an intracranial pressure event, is increasingly gaining recognition. Moreover, the evolution of intracranial pressure over time should be taken into consideration to guide management decisions. Tolerance of intracranial pressure insults is reduced by impaired cerebral autoregulation (ie, the ability to maintain cerebral blood flow in the face of changing cerebral perfusion pressure), and assessment of autoregulatory status is increasingly used to titrate cerebral perfusion pressure targets (see also section 4 and appendix p 14).
Characterisation of ongoing pathophysiology can be facilitated by the monitoring of partial pressure of brain tissue oxygen (PbtO2) and cerebral metabolism using microdialysis. Observational studies show that low PbtO2,138–140 elevated lactate-to-pyruvate ratio, and low brain tissue glucose concentrations141 are associated with a poor outcome after severe TBI, but evidence for targeting these parameters to improve outcomes is still accumulating. The phase 2 study BOOST-2,142 done in 119 patients with severe TBI, showed that management incorporating monitoring of PbtO2 in addition to intracranial pressure reduced brain tissue hypoxia, and might improve TBI outcomes. Three ongoing phase 3 randomised trials comparing management with or without PbtO2 monitoring will recruit a total of 2274 patients with severe TBI and should provide definitive answers (details provided in appendix p 8). Cerebral microdialysis is less widely used than PbtO₂ monitoring, despite promising data from prospective observational studies and small randomised trials suggesting that metabolic derangements detected by cerebral microdialysis are associated with worse TBI outcomes.143 A fundamental limitation of these monitors is their focal nature, which only indirectly measures a heterogeneous and diffuse pathophysiology.144–146
Managing TBI and suspected raised intracranial pressure in settings with few resources
A major finding from the BEST TRIP trial131 was the achievement of satisfactory outcomes from TBI despite resource limitations. In settings with few resources, the management approach typically involves more physician input, using more frequent clinical examinations and CT scanning than in high-income settings because there is less monitoring capacity and a smaller role is afforded to nurses in guiding patient care. This greater physician involvement might increase the clinical detection of neurological changes, reinforcing the crucial value of on-site intensivists in non-monitored TBI care. In the BEST TRIP trial, patients in the control group were treated according to a protocol based on imaging and clinical examination (ICE). This standardised approach in combination with high physician involvement is likely to have contributed to the satisfactory outcomes observed.
Following the BEST TRIP trial, a prospective two-phase NIH-funded study (R01-NS-058302) investigated the efficacy of the ICE protocol outside of a trial setting. In the phase 1 of the study, outcomes from a new group of centres in resource-limited settings not using set protocols were compared with those from a group of the original BEST TRIP investigators using the ICE protocol. Subsequently, a consensus conference comprising the investigators and other clinicians developed a more comprehensive version of the ICE protocol—the Consensus-Revised ICE (CREVICE) protocol,117 which was then prospectively tested in both groups. Preliminary analysis of the findings showed that protocolised care is superior to non-protocolised care.147
CREVICE filled in many gaps in the ICE protocol, including formalising the decision-tree leading to the diagnosis of suspected intracranial hypertension (panel 3). In one study,148 investigators examined the correlation of these criteria with an intracranial pressure greater than 22 mm Hg in the BEST TRIP trial monitored group and found a sensitivity of 93·9% and a specificity of 42·4%. This approach will treat most intracranial hypertension cases but there might be overtreatment of patients with subthreshold intracranial pressure.
Panel 3: Diagnosis of suspected intracranial hypertension.
Intracranial hypertension is suspected and treatment is recommended in the presence of one of the following major or two of the following minor criteria:
Major criteria
Compressed cisterns (CT classification of Marshall diffuse injury III; see appendix p 12)
Midline shift greater than 5 mm (Marshall diffuse injury IV)
Non-evacuated mass lesion
Minor criteria
Glasgow Coma sum motor score of 4 or less
Pupillary asymmetry
Abnormal pupillary reactivity
CT classification of Marshall diffuse injury II (ie, basal cisterns are present with midline shift 0–5 mm or a high-density or mixed-density lesion of 25 cm3 or less, or both)
Managing monitor-documented intracranial hypertension involves intervention in direct response to supra-threshold values. By contrast, treating suspected intracranial hypertension involves scheduled or non-reflex interventions (eg, periodic hypertonic agent infusions). This so-called tranquility approach contrasts with the crisis-management approach for monitored patients. As treatment cannot be predicated on quantitative intracranial pressure values, it can be difficult to decide when treatment for suspected intracranial hypertension can be tapered. The CREVICE consensus conference addressed this challenge by creating a heatmap describing their aggregate treatment-related practice tendencies for weaning treatment based on duration of acceptable ICE evaluations117 (figure 5). The uncertainty (yellow fields) apparent in this heatmap reveals the importance of developing better indicators for tapering treatment. A detailed flow chart for weaning of intracranial pressure-directed therapy for suspected intracranial hypertension is presented in the appendix (p 7). Non-invasive methods of estimating intracranial pressure, such as measurement of optic nerve sheath diameter,149 could help to guide decisions to initiate, escalate, and wean treatment for suspected intracranial hypertension, possibly decreasing the inefficiencies of non-monitored management (eg, overtreatment, increased length of stay, more decompressions, etc). CT imaging is central to the CREVICE approach. However, in many low-resource settings the patient and their family bear the imaging costs, limiting CT usage. Additionally, availability and functional status of CT devices in LMIC settings are often problematic. Unrestricted access to at least a few CT studies should be considered crucial to effective implementation of the CREVICE protocol. What to do in settings without CT access remains an important unresolved question.
Figure 5: Consensus-derived matrix for de-escalation of therapy in suspected intracranial hypertension.
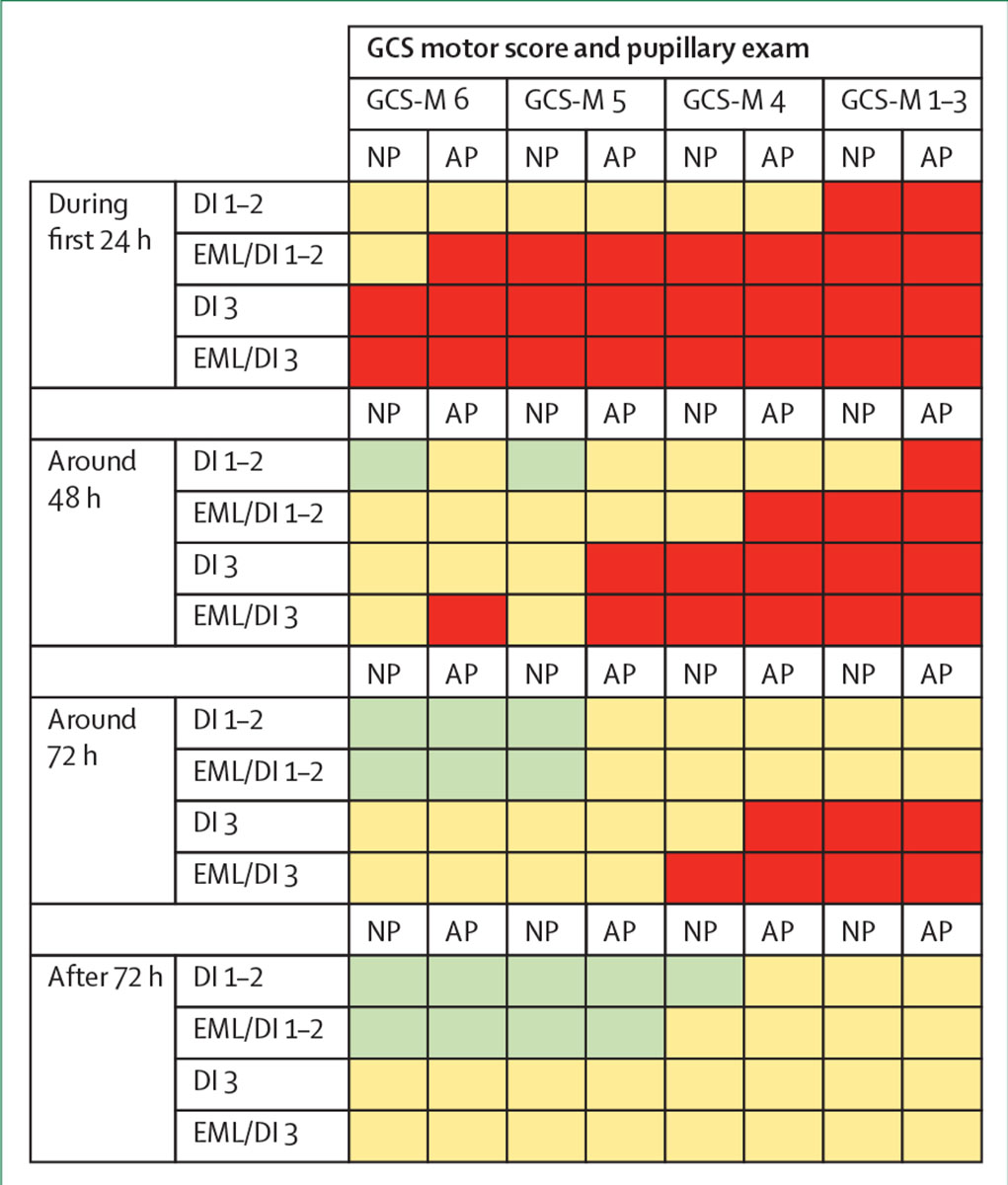
This decision-support heatmap matrix represents tendencies to wean ongoing treatment for intracranial pressure on the basis of the most recent Marshall CT scan classification and clinical status exam (GCS motor score and pupillary exam) in patients who have been stable for 24, 48, 72, or more than 72 h. Green cells in the table indicate a decision to initiate weaning; red cells indicate a decision to continue treatment; and yellow cells indicate an indeterminate situation, where further consideration is needed (modified with permission from Chesnut et al).117 GCS=Glasgow Coma Scale. NP=normal pupils. AP=abnormal pupils, without worsening since injury. DI=diffuse injury (graded by the Marshall CT classification—see appendix p 12). EML=evacuated mass lesion.
Overall, even if the standard in high-resource settings is monitoring intracranial pressure in severe cases, evidence is accumulating that attentive teams of intensivists and neurosurgeons can achieve a reasonably good outcome from severe TBI in low-resources settings using institutional protocols that include imaging and clinical examination (eg, ICE and CREVICE). However, good post-acute care would appear essential to consolidate these benefits. These considerations should direct allocation of available finances within trauma centres in low-resource settings and the funding of research aimed at improving the outcome from severe TBI where it is most common, but also the most under-resourced.
Frameworks for care: the role of rigorous evidence-based recommendations versus expert consensus
Over the past two decades, several guidelines have been produced for the management of TBI, and an analysis in 2021 suggested that their use was associated with improved patient outcomes.150 However, increasing rigour in grading evidence resulted in fewer strong recommendations, loss of clinical appeal, and reduced support for decision making.151 This paradox illustrates the realities of clinical practice when strong evidence is unavailable or insufficient. Consensus-based efforts help to bridge this gap (see panel 4). In general, deviations from guidelines are discouraged, but some deviations might represent individualisation of care, driving better outcomes. Consequently, guidelines are best thought of as frameworks for care, rather than prescriptive instructions for management. However, analysis of the use of more intensive (and potentially hazardous) intracranial pressure therapies in CENTER-TBI suggests substantial practice variation between centres, and many clinicians use more hazardous therapies before exhausting safer options.156 To remain current, guidelines must be continuously updated to reflect evolving evidence: living guidelines (in parallel with living systematic reviews)157 could be one way to address this need. Transforming guidelines into a so-called living document will, however, require long-term support by an authoritative organisation or funding body.
Panel 4: Established and recent guidelines for the intensive care unit management of traumatic brain injury.
Brain Trauma Foundation Guidelines
Trauma Quality Improvement Program guidelines of the American College of Surgeons (2015)153
Present best practices in the management of traumatic brain injury, including recommendations for physiological targets. This document is scheduled for an update in 2023.
The Seattle International Brain Injury Consensus Conference
Used expert consensus to integrate existing treatments into management algorithms:
Consensus REVised Imaging and Clinical Examination guidelines
Present a useful approach for managing suspected intracranial hypertension based on imaging and clinical observation in settings where there are few facilities for monitoring intracranial pressure and cerebral perfusion pressure (2020)117
New evidence and insights regarding critical care management
Ventilatory management and tracheostomy
Tracheal intubation and mechanical ventilation can be necessary in TBI because of extracranial injuries, airway compromise, depressed consciousness, or to manipulate carbon dioxide (PaCO₂) for intracranial pressure management. However, both TBI and mechanical ventilation can potentially worsen pulmonary injury,158 and positive pressure ventilation has complex and multifactorial interactions with intracranial dynamics.159,160 Optimal ventilation strategies in TBI remain variable and are under investigation.161 CENTER-TBI showed substantial between-centre variability in PaCO₂ concentrations, and nearly two thirds of patients without intracranial hypertension had PaCO₂ concentrations below 4·7 kPa (35 mm Hg) for most of the time. Although such hyperventilation was not clearly associated with poorer outcome, concerns about inducing brain hypoxia by hypocapnia remain.162 We suggest that hyperventilation might be indicated in patients with raised intracranial pressure due to vasodilation and hyperaemia (identified, for example, by PbtO₂ monitoring), but not in patients with raised intracranial pressure from other causes, and this concept should be further explored.
Although CENTER-TBI showed that in-hospital intubation had a significant beneficial effect on outcome in patients with a GCS of 10 or lower (p=0·01;74 see also section 2), 20% of mechanically ventilated patients developed ventilator-associated pneumonia, which prolonged ICU stay but did not affect TBI outcome.163 Tracheostomy was required in 31% of ventilated patients with TBI, but there was considerable inter-centre variation in its use (mean OR 2·2) and timing. Late tracheostomy (undertaken >7 days post injury) was associated with a longer mean ICU stay (49 vs 39 days; p=0·003) and greater odds of a worse outcome (OR 1·69, 95% CI 1·07–2·67; p=0·018).164 However, these and other165,166 data do not show causality—supporting the case for a robust randomised trial of tracheostomy timing. A randomised trial on this topic has been conducted in the area of stroke and did not show a benefit of an early tracheostomy.167
Fluid and haemodynamic management
Haemodynamic management in TBI usually depends on cerebral perfusion pressure targets, and includes fluid therapy and vasoactive drugs, neither of which are addressed in current guidelines.133 Comparative-effectiveness research analysis in CENTER-TBI showed that higher positive mean daily fluid balances were associated with worse clinical outcomes, both in patient-level and hospital-level analyses after accounting for confounders.168 Furthermore, despite slightly more favourable prognostic characteristic in centres with lower-than-median fluid balances, more patients had cardiac output monitoring, which might have contributed to more restrictive fluid administration. However, cardiac output is routinely monitored in less than 20% of patients with a TBI in an ICU. It would be important to investigate implementation of comprehensive haemodynamic monitoring to optimise management and assess effects on outcome.
Venous thromboembolism prophylaxis
Pharmacological prophylaxis of venous thromboembolism must balance the risk of venous thromboembolism against concerns that treatment might precipitate or worsen intracranial bleeding.169 CENTER-TBI reported substantial inter-centre variation in policies for pharmacological venous thromboembolism prophylaxis,170 unaccounted for by casemix differences. The use of pharmacological prophylaxis was moderately associated with more favourable outcome (OR 1·4, 95% CI 1·1–1·7, and OR 1·5, 1·1–2·0, in multivariable and propensity-adjusted analyses, respectively). However, a retrospective analysis of data from patients with TBI who were managed surgically, collected between 2012 and 2016 from US trauma centres, showed that prophylaxis delay was associated with increased venous thromboembolism (adjusted OR 1·08, 95% CI 1·04–1·12), but decreased the risk of repeated neurosurgery (adjusted OR 0·72, 0·59–0·88 per day of delay in pharmacological venous thromboembolism prophylaxis).171 These persisting uncertainties regarding optimal dose, timing, and selection of patients for pharmaceutical thromboprophylaxis following TBI172 have resulted in a call for a large randomised trial on this topic.
Coagulopathy and its management
About 20% of patients with isolated TBI show abnormalities on conventional tests of haemostasis,120 and these are associated with greater progression of intracranial haematomas,119 higher mortality, and worse functional outcomes, compared with patients with a normal coagulation profile.120 Advanced testing of platelet function, fibrinolysis, and viscoelastic testing can better characterise haemostatic defects than conventional tests,173 but current management remains largely based on conventional laboratory parameters.174
Surgical management
Timely evacuation of an expanding traumatic intracranial haematoma in a patient with deteriorating consciousness is lifesaving. However, many patients present with a stable low-level or high-level of consciousness. Uncertainty exists, particularly in patients with an acute subdural haematoma or a traumatic intracerebral haematoma, on indications and timing of surgery, reflected in large practice variations (figure 6). In CENTER-TBI, comparative-effectiveness research analyses in patients with an acute subdural haematoma showed that centre preference for an acute surgical strategy compared with that of initial conservative treatment was not significantly associated with better outcome (OR 0·92, 95% CI 0·77–1·09).175 However, 11% of patients in the initial conservative group required delayed surgery (at a median of 19·1 h, IQR 8·1–84·6). These data suggest that, where surgical equipoise exists, early evacuation of acute subdural haematoma does not lead to a better outcome compared with a strategy favouring initial conservative treatment.
Figure 6: Between-centre differences in surgery in acute traumatic brain injury.
(A) Acute surgery in acute subdural haematoma, (B) primary decompressive craniectomy in acute subdural haematoma, and (C) early surgery in traumatic intracerebral haematoma. A logistic random-effects model, adjusted for predefined confounders, was used to estimate the acute surgery preference per centre, with corresponding 95% CIs. The MOR reflects the between-centre variation. An MOR equal to 1 represents no variation; the larger the MOR, the larger the variation. The proportion of patients with an acute subdural haematoma undergoing acute surgery ranged from 7% to 52% (IQR 13–35) between centres, with an MOR of 1·84, suggesting an almost two-fold difference in the likelihood of an identical patient receiving surgery in different centres. Furthermore, the type of surgery for acute subdural haematoma varied between centres: the proportion of primary decompressive craniectomies (as opposed to craniotomies) ranged from 6% to 67% (IQR 12–26), with an adjusted MOR for primary decompressive craniectomies of 2·68 (p<0·0001). Of 367 patients with a large traumatic intracerebral haematoma, the proportion who received acute surgery ranged from 13% to 48%, with an MOR of 1·39 (p=0·27). ASDH=acute subdural haematoma. MOR=median odds ratio. TICH=traumatic intracerebral haematoma.
For patients with a large traumatic intracerebral haematoma (n=367), comparative-effectiveness research analysis showed no overall clear benefit of early surgery (OR 1·05, 95% CI 0·63–1·73). However, subgroups with moderate TBI (GCS 9–12) or isolated traumatic intracerebral haematoma had a better outcome (OR 1·50, 95% CI 1·10–1·98 and 1·84, 1·27–2·53, respectively) after early surgery than with initiative conservative management. Conversely, conservative management in patients with mild TBI and in those with a smaller traumatic intracerebral haematoma (<33 cc) was associated with better outcome (OR 0·61, 95% CI 0·36–0·94 and 0·75, 0·48–1·00, respectively). These results are consistent with findings of the STITCH(Trauma) trial,176 which randomly assigned 170 patients with TBI with traumatic intraparenchymal haemorrhage to conservative management or early surgery. The study findings were inconclusive because of low recruitment, leading to premature termination by the funder. Thus, there seems to be no uniform effect of surgery in acute subdural haematoma and traumatic intracranial haemorrhage. Meta-analyses of data from CENTER-TBI, TRACK-TBI, and STITCH(Trauma) could provide more definitive evidence to inform guidelines.
There is also uncertainty regarding the benefits of decompressive craniectomy, a procedure that aims to mitigate the effects of raised intracranial pressure by removing part of the skull. Decompressive craniectomy can be a primary procedure (combined with evacuation of a haematoma) or a secondary procedure (to decompress the brain). In a population of patients with TBI in the ICU, who were recruited to CENTER-TBI and the harmonised Australian OzENTER study, decompressive craniectomy was performed in 320 (13·7%) of 2336 patients; craniectomy was performed as a primary procedure in 81% of these 320 patients.177 Previous trials have investigated the effectiveness of decompressive craniectomy as a secondary procedure and suggest that it provides no benefit when used relatively early,178 but that it might improve survival with acceptable functional outcome as a rescue therapy for refractory intracranial hypertension.179,180 Until recently, no evidence was available to support the use of decompressive craniectomy as a primary procedure. RESCUE-ASDH, a multicentre randomised trial comparing craniotomy versus decompressive craniectomy for patients undergoing evacuation of a traumatic acute subdural haematoma has completed recruitment of 462 individuals, and is undergoing analysis (Kolias A, personal communication). Results are awaited and will hopefully provide support or refute the efficacy of primary decompressive craniectomy. In LMICs across many parts of the world, decompressive craniectomy is performed even more frequently than in HICs. In the China CENTER registry, decompressive craniectomy was performed also mostly as a primary procedure in 1354 (48%) of 2804 patients with severe TBI, compared with 222 (20·8%) of 1068 with severe TBI in CENTER-TBI.37 In the BEST TRIP trial, conducted in Bolivia and Ecuador, decompressive craniectomy was performed in 93 (29%) of 323 patients,131 and similar rates (28%) were seen in all groups of the intracranial pressure/CREVICE study (Chesnut R, personal communication). In LMICs, use of decompressive craniectomy appears to be often determined by resource limitations, including ICU beds being unavailable. This perceived third indication for decompression is completely unstudied, and indications for and results from such common practice are unknown.
Post-acute care
Long-term outcome studies completed over the past 10 years make clear that TBI symptoms can persist or worsen over time, and more than 50% of patients with moderate to severe TBI receiving in-hospital rehabilitation have a decline in daily function or die within 5 years of injury, suggesting that TBI should be viewed as a chronic disease. Between 1 and 5 years after injury, decline occurs as frequently as improvement. Despite these data, multidisciplinary rehabilitation is underused after TBI, with many individuals reporting unmet rehabilitation needs and long-term service use below the prevalence of morbidity.94,181 A review by the National Academies of Sciences, Engineering, and Medicine182 published in 2022 concluded that TBI care in the USA frequently fails to meet the needs of individuals, families, and communities affected by this condition, despite the substantial burden it poses. Similar findings were reported by CENTER-TBI (see section 2), which found large inconsistencies in provision of rehabilitation services between centres, regions, and countries. Although existing evidence suggests that rehabilitation is more effective when started acutely and offered continuously,183 only approximately a third of patients reporting cognitive and psychological impairments received interventions in these domains.94,184 Significant negative predictors for receipt of rehabilitation were pre-injury unemployment (OR 0·80, 95% CI 0·67–0·95), living in central or eastern Europe (0·42, 0·20–0·87), admission to a hospital ward (0·47, 0·37–0·59; reference: admission to ICU), or direct discharge from the emergency room (0·24,94,184 0·17–0·33). These and other data185,186 suggest that rehabilitation referral is not only driven by clinical needs, but also by demographic, social, and organisational factors, raising issues regarding equitable access to appropriate rehabilitation care. In the USA, provision of follow-up care appears to be affected by social factors such as insurance coverage and race.187
There is also a huge need to address long-term problems such as fatigue and sleep disturbance; cognitive impairments affecting memory, attention, and executive function; behavioural problems; and anxiety and depression; and provide services to facilitate return to work, study, and driving, all of which are significantly affected for at least 5–10 years post-injury. There is a need for the development, evaluation, and implementation of treatment protocols for these problems that are adapted for TBI.188 International guidelines for the treatment of cognitive and communication impairments following TBI, including post-treatment amnesia, originally published by the INCOG group in 2014, have been updated in 2022, but there is a need for many more large-scale controlled studies to validate interventions.189 Taken together, there is an extensive and pressing need for improving post-acute care in the long-term perspective after TBI.
Understanding TBI outcome in the ICU population
The overall 6-month mortality for ICU admissions in CENTER-TBI was 21%, the rate of unfavourable outcome 43%, and only 16% made a complete recovery (Glasgow Outcome Scale-Extended [GOSE] 8).121 Between-centre variations in 6-month outcome (median odds ratio [MOR] 1·2) were substantially less compared with previous studies (MOR 3·3).190 This finding signals that treatment standards have probably improved over time, and that high-quality care might be more important than specific interventions.
Interpretation of mortality rates might be confounded by current policies related to withdrawal of life-sustaining measures, a topic not addressed in the 2017 Commission. Yet, most deaths (54%–70%) in patients with very severe TBI result from such decisions.191,192 CENTER-TBI reported that withdrawal of life-sustaining measures occurred in 86% of patients who died after TBI, ranging from no deaths in some centres to almost 100% in others,193 mirroring previous data from Canada (rates of 45%–87%).191 Although withdrawal of life-sustaining measures is appropriate in many situations, there has been concern that such decisions194,195 are not always equitable,196 and can lead to a self-fulfilling prophecy of death,197 a particular concern when the withdrawal is performed too early. In CENTER-TBI, withdrawal of life-sustaining measures was instituted within 72 h of the TBI in almost half the patients who died after decisions made about withdrawal of life-sustaining measures. These decisions were primarily made in patients with severe TBI affecting brainstem reflexes. However, some patients with very severe brain injuries can achieve favourable outcomes over time. TRACK-TBI followed 271 patients with severe TBI and found that 52% were able to function independently at home by 12 months, with 19% reporting no disability.198 78% of patients in a vegetative state at 2 weeks recovered consciousness by 12 months, and 25% regained orientation. These figures from TRACK-TBI are consistent with past data from the TBI Models Systems in the USA, where 68% of those with a GCS of 3 in the emergency department regained consciousness during in-patient rehabilitation, and 21% of survivors were able to live independently following discharge.199 Acute severe impairment does therefore not universally imply poor functional outcome. Although delaying withdrawal of life-sustaining measures could lead to prolonged suffering and impose unnecessary burden on patients, caregivers, and clinicians, instituting it too early can sometimes result in the death of patients who might otherwise have eventually achieved an outcome that was acceptable200 for their families. Clinicians should be aware of uncertainties surrounding prognosis201 and avoid premature decisions on withdrawal of life-sustaining measures when prognosis is uncertain.202,203
Main messages and recommendations
Main messages
Older patients often have comorbidities, but very little evidence exists to inform their treatment.
Guidelines for the management of TBI are best thought of as frameworks for care. To remain current, guidelines must be continuously updated to reflect evolving evidence.
In low-resource settings, in the absence of facilities for monitoring intracranial pressure, a reasonably good outcome from severe TBI can be obtained using institutional protocols that include imaging and clinical examinations.
Substantial between-centre differences exist for the acute surgical management of patients with traumatic intracranial haematoma.
Access to rehabilitation services is inconsistent and there is an absence of protocols for treating long-term problems.
Withdrawal of life-sustaining measures is common in patients who die after TBI, but—if undertaken prematurely—can lead to self-fulfilling prophecies and increase the risk for unnecessary deaths.
Recommendations
Stimulate a research focus on older patients with TBI.
Guidelines should be transformed into a living document that will require long-term support by an authoritative organisation or funding body.
In low-resource settings, funding is needed for research aimed at improving the outcomes from severe TBI, where it is most common, but also most under-resourced.
Meta-analyses across studies are required to provide stronger evidence to inform which patients are most likely to benefit from early surgery.
Access to rehabilitation services needs to be improved, and evidence-based treatments for long-term problems—including fatigue, and cognitive and behavioural changes—developed.
Clinicians should be aware of uncertainties surrounding prognosis and avoid premature decisions on withdrawing life-sustaining measures (eg, within 72 h after injury) when prognosis is uncertain.
Section 4: Characterisation of TBI—the path to precision medicine
The 2017 Commission on TBI made the case for precision medicine in the ICU setting—ie, targeting a specific treatment to a subset of patients, with a common biological basis of disease, who are most likely to benefit from these approaches. The Commission highlighted the fact that interventions that might be beneficial in some patients can also result in harms in others, and there has been limited success in matching therapies to patients. We also identified the clinical heterogeneity and complexity of TBI as key barriers that limited our ability to target patients for existing therapies, or to effectively show the benefit of new therapies that might be efficacious only in a subset of patients. We described how this clinical heterogeneity was underpinned by widely varied pathophysiological processes, which are present to variable extents in different patients, at different stages in the same patient, and in different parts of the brain in a single patient at a given time. Response to therapy, disease course, and outcome can also be substantially affected by variations in intrinsic host biology, comorbidities, and pre-TBI therapies. We recognised that better tools were needed to characterise the severity and type of acute TBI than those provided by available clinical classification systems, such as the GCS, or CT classification schemes in isolation.
In this section, we report on advances in improved characterisation, achieved by incorporation of clinical variables beyond the GCS, as well as advanced neuroimaging, multimodality monitoring, blood biomarkers, and genomics. These advances, in some instances, have identified key subsets of patients who would not have been recognised using conventional tools (eg, detection of severe diffuse axonal injury by MRI which would have been missed by CT). In other instances, such as with the use of multimodality physiological monitoring, we are closer to achieving individualised approaches by titrating the choice and intensity of (often hazardous) treatments, not just between patients, but over time in individual patients, while taking account of pre-injury comorbidities, extracranial injuries, and crucially, the choices expressed by patients and families about acceptable outcomes.
Clinical characterisation and classification
The GCS remains the main instrument for classifying the severity of TBI as mild (GCS 13–15), moderate (GCS 9–12), or severe (GCS ≤8). However, it does not capture the specific pathoanatomical features or pathophysiology in individual patients, and is confounded by factors such as drug and alcohol use, medications, and tracheal intubation.20,204 The duration of post-traumatic amnesia is widely used as an indicator of injury severity and reported to be a strong predictor of outcome in survivors of TBI.205,206 However, accurate determination of the duration of post-traumatic amnesia is not possible early after injury in patients who are still in post-traumatic amnesia, and post-traumatic amnesia is therefore more relevant to rehabilitation settings. Additional clinical variables (see also section 2) are important modulators of TBI course and outcome. Major extracranial injury, often associated with high-energy trauma, is known to influence the course, complications, management, and outcome of TBI.207 However, a recent analysis from CENTER-TBI published in 202119 has also drawn attention to the risks of substantial intracranial injury even from low-energy mechanisms, particularly in older patients, which might be underestimated by the initial GCS and, in the context of a comorbid cohort, result in poor outcomes.19 For mild TBI, neck pain, early post-concussional symptoms, and mental health can increase the risk of incomplete recovery.208,209
There is also a need to identify specific patient groups that might benefit from individual treatments.210–212 CENTER-TBI and TRACK-TBI applied unsupervised clustering methods to identify such groups or disease endotypes (ie, a subtype of a health condition that is defined by a distinct functional or pathobiological mechanism, first described in the context of asthma). CENTER-TBI identified GCS, extracranial injuries, and mechanism of injury as important distinguishing factors for subgroups of patients with TBI across all injury severities.213 Clustering of biomarkers revealed two subgroups of patients with TBI who showed differences in injury severity.214 In the subgroup of patients admitted to the ICU, six early endotypes could be distinguished by GCS and degree of metabolic derangement, primarily described by pH, lactate, SpO2, pCO2, blood glucose, creatinine, and body temperature.215 A US study216 identified potential endotypes using mainly haematological and coagulation factors, such as platelet count, haemoglobin, haematocrit, prothrombin time, international normalised ratio, and glucose. Among patients with mild TBI, TRACK-TBI identified five subgroups distinguished by demographic factors, CT characteristics, blood pressure, substance use, fall injuries, administration of intravenous fluids, and history of neurological and psychiatric disease.211,217 These advanced analysis tools suggest that there are clinical subgroups with potential clinical and therapeutic relevance and that require further investigation. An early demonstration of this approach comes from a secondary analysis of the PAMPer randomised trial of pre-hospital thawed plasma in high risk trauma patients, which showed that the overall benefit demonstrated in the trial was confined to patients who had TBI, and was specifically seen in an endotypic subgroup characterised by multiomic analysis.212
Neuroimaging
Results of CT and MRI inform treatment decisions and have prognostic significance. Imaging common data elements, originally developed by a multidisciplinary task force of the US National Institutes of Health-National Institute of Neurological Disorders and Stroke (NIH-NINDS), has now been well validated (appendix p 9),218–220 and improve reporting consistency compared with data extraction from local radiology reports.218 Basic, or core, common data elements appear to have the most prognostic value,221 whereas supplementary or advanced and emerging imaging common data elements have less consistent relevance to outcome. Common data elements also permit the creation of large neuroimaging databases, which facilitate large-scale meta-analyses.217,222 The frequency of co-occurrences of CT abnormalities, assessed according to the common data elements, was explored in the CENTER-TBI (n=4087) and TRACK-TBI (n=2670) studies (figure 7). CT abnormalities were present in CENTER-TBI in 1345 (49 %) of 2744 patients with mild TBI and in 1112 (93%) of 1193 with moderate to severe TBI, and in TRACK-TBI in 789 (38%) of 2104 patients with mild TBI and in 470 (90%) of 520 with moderate to severe TBI.
Figure 7: UpSet plot of pathoanatomic common data elements reported on early CT, by traumatic brain injury severity.
(A) Data are from the CENTER-TBI study. (B) Data are from theTRACK-TBI study. Of a potential of 17 imaging CDEs, 14 were included in the analysis. Ventricular compression, mixed density subdural haematoma, and penetrating injuries were excluded as these were either not reported or not included in TRACK-TBI. The vertical bar graphs depict the absolute frequencies of lesion combinations. Combinations with fewer than ten occurrences and CDEs that appeared exclusively in such infrequent combinations are not shown. The horizontal bar graphs depict the absolute frequency of each CDE in the cohort. Traumatic subarachnoid haemorrhage, skull fracture, intraparenchymal haemorrhage (haematoma or contusion), and acute subdural haematoma were the most frequently occurring abnormalities in both studies. Although there are some differences in co-occurrence of abnormalities, five of the top six combinations in each study are consistent. Differences in co-occurrence were probably affected by differences in casemix. However, cisternal compression was less frequently scored in TRACK-TBI and oedema or ischaemia were more frequently scored than in CENTER-TBI, which might reflect differences in reporting. In both studies, co-occurrence of abnormalities was dependent on the severity of the initial injury. CDE=common data elements. TBI=traumatic brain injury. NA=not applicable. SAH=subarachnoid haemorrhage. IPH=intraparenchymal haemorrhage. ASDH=acute subdural haematoma. IVH=intraventricular haemorrhage. EDH=epidural haematoma. DAI=diffuse axonal injury. TAI=traumatic axonal injury.
Various CT classification schemes exist (appendix p 12) and have been evaluated in terms of their prognostic value.223 The Helsinki CT score and the Stockholm CT score seem to be more accurate in predicting outcome (AUC 0·75–0·80), but are more complex to score than the Rotterdam CT score and the Marshall CT classification. Although developed in adult TBI cohorts, these classification systems also perform well in evaluating paediatric TBI.224 –226 A role is emerging for quantitative CT in diagnosing and following up TBI, an application facilitated by artificial-intelligence-based automated image interpretation.227–229 The use of new approaches to analyse existing data is as important as the availability of new imaging modalities. For example, one study217 showed three distinct CT-based clusters in mild TBI: presence of contusion and subarachnoid haemorrhage or subdural haematoma, or both; intraventricular or petechial haemorrhage, or both; and epidural haematoma. This result was largely reproduced in CENTER-TBI and related to outcome at 1-year post-TBI.
Major advances have resulted from the application of MRI to TBI, with a role for identifying injuries that are not visible on CT imaging. Both CENTER-TBI and TRACK-TBI reported structural traumatic abnormalities on MRI, performed at 2–3 weeks after injury, in approximately 30% of patients with mild TBI who had a normal CT on presentation.20,230 Advanced MRI techniques, such as diffusion tensor imaging and susceptibility weighted imaging (both of which are now routinely available on clinical scanners) are more sensitive at detecting superficial contusions, traumatic axonal injury,231 and traumatic vascular injury232—additionally, traumatic axonal injury and traumatic vascular injury are now recognised as distinct entities with pathophysiological and outcome impact. Quantitative assessments of MRI, such as volumetric analyses,233,234 reductions in fractional anisotropy, and increases in mean diffusivity can identify injury not detectable by visual inspection of MR images. Such injuries can be particularly relevant in mild TBI, because fractional anisotropy and diffusivity abnormalities identify patients who are likely to have persistent post-concussional symptoms and long-term disability.235–237 Crucially, the timing of imaging is important, and imaging within 3 days of a TBI might be more sensitive than imaging in the second week.236 Quantitative diffusion tensor imaging has strong predictive value in people more severe TBI, in whom diffuse axonal injury might represent the substrate for disorders of consciousness.238 Functional MRI239 and MR spectroscopy240 might add additional sensitivity, but these are currently still making the transition from research techniques to clinical tools. In the chronic phase of TBI, there is increasing interest in using MRI to detect global and regional loss of brain volume, which could represent accelerated brain ageing.241–243 Such late volume loss is related to, and can be predicted by, brain injury biomarkers up to a decade after TBI.243,244
Body fluid biomarkers
Protein biomarkers
Among the technologies to improve phenotyping of TBI, blood biomarkers are the closest to being implementated into routine clinical care. Building on past results that primarily focused on S100B, a quartet of new brain injury biomarkers (GFAP, NfL, UCH-L1, and tau) have been evaluated in several studies of TBI.83 Biomarkers perform well in predicting CT and MRI positivity, outperforming clinical decision rules (see section 2). The combination of GFAP and UCH-L1 has received FDA clearance in the context of aiding in the identification of CT-detectable brain lesions within 12 h of mild TBI (initial GCS 13–15).87,245 GFAP on its own has emerged as an excellent brain injury biomarker for prediction of CT positivity246,247 and MRI positivity in CT-negative individuals.230 However, a direct comparison of GFAP against S100B is confounded by the more rapid kinetics of S100B and by the fact that samples in some of the larger studies were variably analysed in plasma or serum, and sometimes obtained relatively late—possibly outside the timeframe that would be relevant to selecting patients for CT. Initial expectations that patterns of biomarker elevation might differentiate focal from diffuse injury248,249 have not been confirmed, and some data suggest that biomarkers correlate more with the burden of injury than with different pathoanatomical types of TBI.214 In addition to diagnosing CT positivity, biomarkers have been shown to prognosticate late outcomes in large cohorts of patients (see also section 6).244,247,250 CENTER-TBI reported the highest incremental value for UCH-L1, S100B, NfL, and total tau for predicting mortality or unfavourable outcome.251 When compared with more severe TBI, biomarkers were less predictive of outcomes in mild TBI and of incomplete recovery overall. TRACK-TBI likewise reported lower incremental value of GFAP and UCH-L1 for predicting incomplete recovery, when compared with the prediction of mortality or unfavourable outcome.252 The use of several different platforms means that substantial cross-platform calibration and regulatory approval will be required before quantitative thresholds can come into routine clinical use.253,254 However, when applied to outcome prognostication, the relative merits of discrete thresholds versus a continuous scale of biomarker concentrations remains an open question.
Other circulating biomarkers (such as amyloid beta species, phospho-tau, heart fatty acid binding protein, and IL-10)255–257 are also in the process of investigation as prognostic biomarkers. Integrated characterisation of the innate and adaptive host response might be able to separate subsets of patients with different inflammatory endotypes,212,258 or to detect the presence of autoantibodies against brain and extracranial antigens,259 leading to the rational selection of patients for anti-inflammatory and immunomodulatory therapies. Subacute and chronic trends of biomarker concentrations are increasingly being investigated as markers of disease evolution and chronicity in TBI. S100B has been used by some centres to detect disease evolution and second insults in patients with more severe TBI,260 and emerging evidence shows that similar insights might be provided by secondary rises in GFAP and other biomarkers, both in mild TBI261 and in more severe injury.260 However, the biomarkers of interest in TBI have very different apparent half-lives (ranging from hours for S100B to weeks for NfL) and expected times to peak concentrations following a single insult, and it is important that any assessment of their performance accounts for these factors.260,261 Beyond the acute phase, NfL values correlate with (and predict) accelerated brain volume loss, functional trajectories, and cognitive performance for months to years after TBI.243,244
Metabolomics and microRNAs
TBI is characterised by a mismatch between the requirement and production of metabolic energy in the brain—referred to as an energy crisis—and by metabolic dysregulation, both of which are reflected in mass spectrometry-based analysis of serum. Such analyses reveal varied and complex alterations in the metabolome, including in the lipidome (ie, the characterisation of molecular lipids) and the glycome (ie, the characterisation of glycan structures). Specific metabolic signatures have been shown to be associated with a diagnosis of TBI, imaging findings on CT262,263 and MRI,264 injury severity,262,265 and patient outcomes.262,265–267 These findings extend across the severity spectrum of TBI, including patients with mild TBI268 and sport-related concussion.269 Deciphering the striking diversity of the metabolome is an important goal of precision medicine in TBI. Future large-scale prospective studies with broad analytical coverage are warranted. There is also emerging interest in the measurement in blood and saliva of microRNAs, the amounts of which are rapidly altered in the brain in experimental TBI.270–272
Genomics
A range of methods can be used to obtain evidence on the effect of the patient’s genetics on TBI outcome. Rare but highly penetrant variants in genes such as CACNA1A cause life-threatening brain swelling in response to a trivial head injury.273 Multiple candidate gene-association studies274,275 have investigated whether more common variants in different genes might influence TBI outcomes. The choice of genetic variants in these studies has been driven by a-priori hypotheses regarding TBI pathophysiology. However, candidate gene studies have recognised shortcomings,276 including low genetic coverage, selection bias, cryptic population stratification, high false-positive rates, publication bias, and poor replication—many of which are overcome by genome-wide association studies. One large genome-wide association study of TBI outcome in around 5000 patients277 estimated that 26% of outcome variance in TBI might be heritable, well within the range seen in common neurological diseases with recognised genetic associations. Although no genome-wide association study hits reached genome-wide significance, several achieved statistical thresholds that merit further investigation. However, with one exception, this study failed to replicate any hits from past candidate gene studies, including the previously reported association of poor outcomes with APOE ε4 carriage.274 The one successful replication was for variations in MBL2, the gene for mannose-binding lectin, which has previously been associated with significant variations in the host inflammatory response and TBI outcome.278
The failure to replicate other past candidate gene hits might be because previous reports were false-positives, but at least some of these past studies examined outcomes other than GOSE—such as cognition and psychological health, which require further investigation. However, perhaps the most important role of candidate gene studies is the understanding of disease biology and identifying enriched populations of patients for trials and treatment with novel or repurposed agents.279,280 For example, one study280 showed that variation in two genes, ABCC8 (SUR1) and TRPM4, contributed to progression of intracerebral haemorrhage after severe TBI, and the addition of genetic information significantly improved prediction of haemorrhage progression when compared with baseline models. Such results are important because they reflect biology in experimental models and clinical studies, and can provide a basis for mechanistic enrichment in developing trials of SUR-1 antagonists.
Physiological monitoring
Substantial advances have been made towards precision medicine approaches based on physiological monitoring. In patients with more severe TBI, tolerance of intracranial pressure dose (see section 3) is reduced by impaired autoregulation, which is more common with diffuse intracranial injury.281–284 Autoregulatory status can be assessed by evaluating intracranial pressure responses to induced or spontaneous variations in arterial blood pressure (appendix p 14). Variations in autoregulatory status are commonly quantified by the pressure reactivity index (ie, a correlation coefficient between intracranial pressure and cerebral perfusion pressure), which shows strong independent associations with 6-month outcomes.137,281,285–288 The parabolic relationship between cerebral perfusion pressure and the pressure reactivity index allows the derivation of a personalised optimal cerebral perfusion pressure and clinically actionable thresholds.289–295 The COGITATE phase 2 trial295 explored the feasibility and safety of targeting a personalised optimal cerebral perfusion pressure, and phase 3 trials are now in planning.
Intracranial pressure monitoring is increasingly being supplemented by PbtO2 monitoring157 and (less frequently) by microdialysis.141 Comprehensive metabolic profiling, along with assessment of autoregulation, provides a more nuanced picture of cerebrovascular physiology in TBI, particularly when intracranial pressure thresholds are close to treatment thresholds. Abnormal brain biochemistry can prompt aggressive treatment of intracranial pressure even when intracranial pressure is not markedly elevated, or in other cases can allow clinicians to withhold such treatment escalation if biochemistry is not deranged. The latter approach is particularly important when considering de-escalation of therapy, or in withholding interventions when patients are particularly vulnerable to their side effects (eg, vasopressors for cerebral perfusion pressure augmentation in patients with pre-existing coronary artery disease or injury). Commonly accepted thresholds for different monitoring modalities (appendix p 18) can be used to inform management strategies.129
Data integration for individualised management
The preceding parts of this section describe the rich range of data that are available for better characterisation of TBI. Newer blood biomarkers, such as GFAP, can help to refine decisions about which patients with mild TBI receive a CT, or require an MRI if CT is normal, and the combination of biomarkers, quantitative CT, and MRI can help to identify patients who are at risk of persistent symptoms and require more frequent follow up. In more severe TBI, discordantly high biomarker concentrations in the presence of a CT with little or no abnormality might change patient management pathways—leading to ICU admission, and suggesting greater care with physiological stability, or consideration for early MRI to detect CT-occult diffuse axonal injury and informing discussion with families about prognosis and therapy choices. Identification of patients at risk of refractory intracranial hypertension at an early stage would be desirable, both to calibrate expectations of progress and to select patients for trials of novel therapies to lower intracranial pressure.296,297 However, current clinical, imaging, and monitoring variables explain less than 10% of the variation in the need for second-tier intracranial pressure therapies.152
Much work is still required to identify group differences in underlying pathophysiology or disease mechanisms. Advanced analyses of the inflammatory host response might identify patients with other inflammatory and host responses,212,258 and facilitate creation of enriched populations or endotypes for anti-inflammatory therapies.212,298–300 Genetic variations that associate with disease course or outcome could have prognostic significance, and might identify key pathobiological pathways as therapeutic targets for novel drug development. However, such endotypic subgroups will necessarily be of a smaller size than the overall cohort, and large sample sizes and well characterised phenotypes are needed to draw statistically robust inferences, and to ensure that rare (but still biologically relevant) genetic variations are not missed. Collection of such large datasets would be facilitated if funders required that all clinical research studies—both observational and interventional—should bank blood for genomic analysis alongside collection of common data element-based data at presentation and outcome.
As highlighted in the 2017 Commission, identification of meaningful subgroups (ie, endotypes) for targeting therapeutic approaches warrants the application of machine-learning techniques and artificial intelligence, given the size and complexity of available databases. Some progress in this direction has been made, but most studies to date have been exploratory301 or had a specific focus on prognostic modelling.302 A substantial stimulus is needed to implement neuroinformatics to support clinical decision-making in the field of TBI.
Main messages and recommendations
Main messages
Substantial advances have been made towards individualised management, with improved characterisation and understanding of disease processes in TBI, but we are not yet sufficiently able to match acute care therapies to subgroups of patients.
Identification of meaningful subgroups (endotypes) for targeting therapeutic approaches warrants the application of machine-learning techniques and artificial intelligence.
A normal CT scan on presentation after TBI does not mean the absence of structural traumatic abnormalities. MRI and blood biomarker measurement can have a major role for identifying inuries that are not visible on CT imaging.
Current intracranial pressure thresholds for treatment neglect the harmful effects of more modest and prolonged elevation of intracranial pressure (intracranial pressure dose) and the modulation of such harm by variations in cerebrovascular physiology and brain metabolism.
Approximately 26% of outcome variance in TBI might be heritable, in line with the range seen in common neurological diseases with recognised genetic associations.
Recommendations
Research needs to aim to identify subgroups of patients who would be most likely to benefit from specific interventions.
A substantial stimulus is needed to implement neuroinformatics to support clinical decision-making in the field of TBI.
Lawyers involved in litigation procedures should be aware that absence of CT abnormalities does not mean absence of TBI. Research should be stimulated to determine which patients with a normal CT scan would benefit from MRI.
Consider initiation of intracranial pressure therapy for intracranial pressure at lower thresholds, and autoregulatory status and brain chemistry as ancillary guides to titrate therapy.
Research should be stimulated to better understand the effects of genetic variation on disease biology and how this variation might allow identification of enriched populations of patients for trials and treatment with novel or repurposed agents. Establishment of the required large datasets could be stimulated if funders would encourage all clinical research studies to bank blood for genomic analysis alongside common data element-based data collection at presentation and outcome.
Section 5: Assessment of TBI outcome
In the 2017 Commission on TBI in 2017, we highlighted how trauma to the brain affects multiple outcome domains and suggested that multidimensional constructs are needed to better quantify the consequences of TBI. Since then, various studies, including CENTER-TBI and TRACK-TBI, have produced a body of empirical data to inform the development of multidimensional approaches. In this section, we present these data with an additional focus on the relevance of post-traumatic stress disorder (PTSD) in civilian TBI, on outcome after mild TBI, on sex and gender differences in outcome, and on the relation of TBI to late dementia.
A holistic approach to outcome after TBI
There are inherent challenges to studying the associations of a remote exposure (ie, TBI) with outcomes of interest, particularly as the interval between exposure and outcome increases. Effects of the TBI are overlaid upon personal characteristics, such as age, preinjury mental health, coping skills, and the influence of other life course events, such as litigation and access to health care. Disentangling these relationships requires the study of large and heterogeneous cohorts.303 Interdisciplinary collaboration, including neurology, psychology, psychiatry, and sociology is needed to improve the understanding of the chronic consequences of TBI.304 This process would be facilitated by the use of modern psychometric methods305 (eg, to define endpoints), advanced statistical approaches (such as latent profile analysis to identify neurobehavioural phenotypes),306 and life-course epidemiological methods that consider TBI in the context of other environmental and genetic exposures. This approach reflects the increasing recognition by clinicians and policy makers of the relevance of a multifactorial bio-psycho-socio-ecological model to TBI, as enunciated in a report of the National Academies of Science, Engineering and Medicine in the USA182 (figure 8).
Figure 8: Outcomes after a traumatic brain injury: the bio-psycho-socio-ecological model.
The pyramid represents how BPSE factors capture individual differences that can substantially affect the outcome trajectory after a traumatic brain injury, in some cases leading to a better outcome and in others leading to a worse than expected outcome. B=biological (eg, brain injury severity, host response, and genetics); P=psychological (eg, coping skills and mental health); S=social (eg, social support and employment); and E=ecological (eg, health-care systems). BPSE=bio-psycho-socio-ecological.
Standardisation of outcome assessments
The introduction and widespread adoption of common data elements (see also Section 7) for assessing outcomes307 has enabled a crucial infrastructure for TBI outcome research. The common data elements initiative, coordinated by NIH-NINDS, aims to standardise data collection and coding by recommending the most relevant outcome instruments for use in TBI. However, challenges have become apparent following their implementation into research. Differing conventions across TBI subpopulations (eg, paediatric vs adult or sport-related vs non-sport-related TBI) have led to distinct common data elements even for similar outcomes. Impediments to global adoption include an absence of validated translations of instruments originally developed in English and of cross-cultural norms, and copyright and proprietary issues. Outcome instruments, designated as core common data elements (ie, those recommended across studies) should be freely available to facilitate widespread use, including in settings with few resources, but some of these instruments are proprietary. Linguistic validation and psychometric evaluation of 11 outcome instruments in up to 20 languages was performed in the context of CENTER-TBI.308 Psychometric and validity analyses showed satisfactory to excellent reliability of all instruments, and showed that correlations between measures were consistent across languages.309 These translations provide a solid basis for multinational TBI research and practice, and are available on the CENTER-TBI website.
Particular subgroups will require specific assessment approaches—eg, most outcomes in the common data elements cannot be completed for patients with disorders of consciousness. In this population of patients, the Coma Recovery Scale–Revised has been recommended by international guidelines.310–312 The incorporation of common data elements into paediatric TBI research requires unique considerations. Children have individual differences in abilities and capacities to comprehend and respond to patient-reported questionnaires, and thus parents or caregivers will often act as an interlocutor. During childhood, attention, language, executive skills, and psycho-social functioning rapidly change. Age-specific norms need to be established in TBI and non-TBI control groups with and without pre-existing comorbid conditions (eg, attention-deficit hyperactivity disorder, anxiety, and depression). Future directions for the common data element initiative on outcomes should focus on the frequency and effects of impairments to determine which elements have practical utility, on decreasing redundancy among measures, and on identifying those elements that are most responsive in specific TBI subgroups (see also section 7).
Standardisation and imputation of outcome instruments
Standardisation of the administration and scoring of outcome common data elements is more complex than might appear. Many measures exist in more than one version or have variations in administration. Studies thus need to develop a manual of operations and implement training for investigators to ensure consistency; methods should be shared to promote transparency and reproducibility.
Differences in scoring the GOSE have been found between studies in Europe and the USA.313 In Europe, the GOSE rating has typically encompassed all disabilities related to injury (GOSE-ALL) including extracranial injuries; however, in the USA studies have commonly focused on the effects of brain injury (GOSE-TBI), excluding disability from other sources.314 Both approaches have pros and cons: when compared with GOSE-ALL, GOSE-TBI might more specifically relate to the consequences of TBI (rather than extracranial injury). However, GOSE-TBI might be confounded by the subjectivity and expertise of the assessor. Differences also exist between adult and paediatric versions of GOSE: the paediatric GOSE adopts a contrary convention for numbering the scale, with 8 corresponding to being dead and 1 to complete recovery.315 These examples illustrate the challenges of harmonising outcomes and highlight the need for deep harmonisation, going beyond the simple alignment of coding of variables and ensuring that, for example, instrument versions, methods of administration, and interpretation of scores are similar before performing meta-analyses across studies (see section 7). Recommendations for scoring the GOSE are provided in a CENTER-TBI and TRACK-TBI publication.316
Satisfactory completion rates for outcome instruments are crucial to guard against bias, maintain statistical power, and increase generalisability. Analysis of all data (eg, using imputation) is preferred over selection of complete cases. Historically, the last observation carry forward method has been accepted by regulators, but this approach is problematic and can underestimate current functioning due to ongoing recovery.317 Alternative approaches that take account of trajectories of outcomes over time should be considered. Single imputation of the GOSE based on modelling data at one or more timepoints was successfully implemented in CENTER-TBI,317 and propensity weighting methods were applied in other studies.314 The TBI research field has begun adopting such techniques, but there is a need for consensus on approaches and their extension to assessments other than functional outcome.
Towards a multidimensional outcome assessment
The Evidence-Based Clinical Outcome assessment Platform, a systematic approach for ascertaining the suitability of a TBI outcome for a specific purpose and population of interest (ie, context of use)318 provides some guidance for the selection of TBI endpoints. However, there are many gaps in the psychometrics available for TBI outcomes. A primary motivation for implementing multidimensional outcome assessment is to obtain greater detail than that provided by global scales such as the GOSE. Although the GOSE tends to be the assessment that most commonly captures impairment (figure 9), other instruments provide better characterisation of impairments, particularly in patients with mild TBI, and scores on individual instruments might be the only evidence of impairment (see panel 5). Structural modelling from TRACK-TBI indicated that mental-health-related symptoms can be reconfigured to reflect core dimensions of psychopathology underlying diverse symptoms assessed across different common data elements,322 such as internalising factors (eg, depression, anxiety, and fear), and somatic symptoms (eg, sleep disturbances, pain, and physical complaints). Conversely, data from single instruments, such as the Rivermead Post-Concussion Symptoms Questionnaire, can be treated as unidimensional, that is measuring a general factor (severity of symptoms), or can be decomposed into subfactors reflecting different types of symptoms (emotional, cognitive, and visual). In the latter case it is employed as a multidimensional scale. Treating instruments as unidimensional (ie, reflecting a single construct or attribute) offers simplicity, whereas encompassing the multidimensional structure of single common data elements provides insight into the breadth of clinical sequelae after TBI. The overall structure of TBI outcomes, however, still remains to be elucidated, and better understanding of their architecture will inform advances in methods and guidelines for outcome assessment.
Figure 9: UpSet plots of impaired scores on outcomes from (A) the CENTER-TBI study and (B) the TRACK-TBI study.
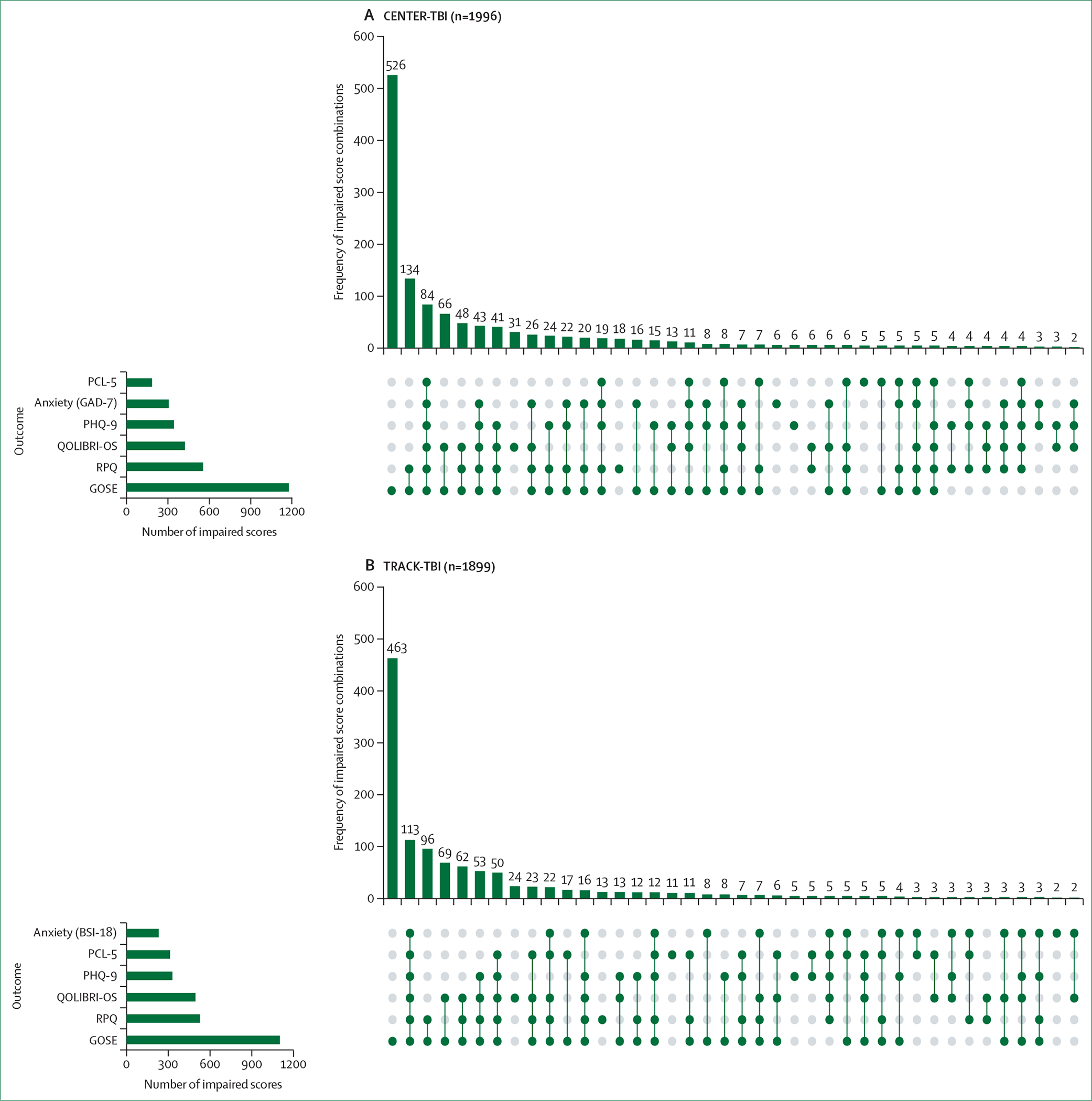
Horizontal bars depict the frequencies of impairment on individual assessments; vertical bar scores depict the frequencies of profiles of impairment across all assessments (group sizes fewer than ten are not shown). Both samples include all severities of traumatic brain injury and cases with complete data on all six outcome measures at the 6-month follow-up. Impairment was defined as a Glasgow Outcome Scale-Extended score of less than 8, a Rivermead Post Concussion Symptoms Questionnaire score of at least 16, a Quality of Life After Brain Injury-Overall Scale score of less than 52, a Patient Health Questionnaire-9 Depression Scale score of more than 9, a Generalized Anxiety Disorder-7 score of more than 7, an 18-item Brief Symptom Inventory Anxiety Scale T score of at least 63, and a Post-traumatic Stress Disorder Checklist for DSM-5 score of at least 33. In both datasets, the GOSE is the outcome on which impaired scores are most frequent, followed by the Rivermead Post Concussion Symptoms Questionnaire, the Quality of Life After Brain Injury-Overall Scale, and mental health scales. Heterogenous combinations of impairment are apparent, with a similar order of clinical outcome profiles across studies. The top panel showing data for CENTER-TBI is modified with permission from Wilson et al.319 PCL-5=Post-traumatic Stress Disorder Checklist for DSM-5. GAD-7=Generalized Anxiety Disorder-7. PHQ-9=Patient Health Questionnaire-9. QOLIBRI-OS=Quality of Life After Brain Injury-Overall Scale. RPQ=Rivermead Post Concussion Symptoms Questionnaire. GOSE=Glasgow Outcome Scale-Extended. BSI-18=18-item Brief Symptom Inventory.
Panel 5: The relevance of multidimensional outcome assessment in mild traumatic brain injury309,314,315,320,321.
Outcome and recovery after mild traumatic brain injury (TBI) are affected by a combination of variables (see also section 6), including acute injury characteristics, the type and extent of TBI pathology, concomitant polytrauma, demographic characteristic (eg, age and sex), and psychosocial factors (eg, premorbid or coexisting psychological health problems). Global functional outcome measures do not have the precision and detail needed for characterising the heterogeneous impairments found after mild TBI. These measures should be complemented by a multidimensional outcome-assessment approach providing more sensitive and comprehensive measurement, which can guide TBI systems of care and improve clinical trial endpoints. As findings from the CENTER-TBI and TRACK-TBI studies show, recovery is often slow or incomplete after mild TBI, and thus timely multidimensional outcome evaluation and therapy should be offered when possible.
Comparison of the GOSE score with cognitive, mental health, and quality of life outcomes indicates that cognitive impairment most strongly differentiates between patients with moderate and severe disability, whereas adverse mental health outcomes differentiate between patients at the upper levels of functional outcome (between good recovery and moderate disability).323 These findings suggest that patients in different disability categories require different approaches to outcome assessment, and that the choice of instruments can be guided by the rating on the GOSE. For example, the assessment strategy appropriate for individuals who are severely disabled is different from that used to identify possible impairment in patients reporting good recovery but persisting symptoms.319 One example of the need for targeting assessment is mild TBI (panel 5). An alternative approach to incorporating multiple outcomes is the creation of composite endpoints, and some progress has been made in this direction. The Brief Test of Adult Cognition by Telephone is feasible in individuals with moderate to severe TBI,324 and was shown to be essentially unidimensional (ie providing a measure of general cognitive function) using data from TRACK-TBI and an overall composite score was derived, thus simplifying its use in analyses.325 Different methodological approaches have been summarised and their theoretical strengths and weaknesses discussed.305 Rich datasets should be leveraged to empirically evaluate composite endpoints derived by different methodological approaches or from different measures. Both multidimensional approaches and composites need to be validated for qualification by regulatory bodies as a primary endpoint for relevant contexts of use.
PTSD and TBI
For many years it was thought that individuals with a TBI seldom develop PTSD, as they often have amnesia for the event. This is a misconception—extensive evidence shows that military personnel repeatedly exposed to blast explosions or combat injuries show high rates of PTSD.326 Since the 2017 Commission, PTSD is now increasingly recognised as being prevalent also in civilians with TBI. Two systematic reviews found prevalence rates for PTSD respectively of 16·3% across all TBI severities,327 and of 13·8% following mild TBI and 11·8% following moderate to severe TBI328 at 6 months after injury. A similar rate was found in CENTER-TBI, with 13·5% across all severities.329 In TRACK-TBI, the rate at 6 months of probable PTSD for individuals with mild TBI was somewhat higher, at 19·2%.330 Risk factors for PTSD after mild TBI include lower education, antecedent psychiatric disorder, and injury resulting from violence. Individuals with comorbid PTSD and mild TBI reported lower quality of life, were more likely to be admitted to the hospital and use rehabilitation care, and have lower return-to-work rates compared with individuals with mild TBI only.95 A screening tool, such as the PTSD Checklist for the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) can identify individuals at risk for PTSD to initiate adequate and timely intervention. Currently, the risk for PTSD and its co-occurrence with TBI is likely to be high in the people of Ukraine, both in military and civilian populations (panel 6).
Panel 6: The Russian invasion of Ukraine: high risk for traumatic brain injury and post-traumatic stress disorder.
The risk for post-traumatic stress disorder (PTSD)—and its co-occurrence with traumatic brain injury (TBI)—is likely to be high in the people of Ukraine, both in military and civilian populations. Extensive studies on blast TBI (TBI caused by explosions) and PTSD have been conducted in the USA on military personnel and veterans who were involved in the Iraq and Afghanistan conflicts. Blast TBI was considered the signature injury of these conflicts, with reports showing that around 20% of veterans returning from deployment had a single or multiple blast TBI injuries.326 Reported prevalence rates of PTSD in veterans were between 11% and 20%, but were much higher in veterans who had had a blast TBI, with reported rates between 33% and 65%.331 In the initial phases of the Iraq and Afghanistan conflicts, substantial delays of up to 1 year or more occurred between the events and definitive diagnosis.332–334 We know that PTSD is also common after civilian TBI, with reported rates between 12% and 19%.330 These issues are now being reprised in the Russian invasion of Ukraine, with risks being experienced not just by military personnel, but also civilians who are victims of artillery and rocket attacks. Additionally, experts are recognising a third category of individuals who are at particularly high risk of PTSD—those who are directly involved in the conflict but are not professional soldiers, termed civilian combatants—who often do not have the preparation and training of military personnel.335
We echo alerts from PTSD experts to the imminent mental health problems that are emerging across these populations at risk, and support their calls for addressing this issue as soon as the situation permits. We emphasise the coexistence of PTSD with TBI and the overlap in presentation (and possibly even biology)336 additionally underlines the need to consider both diagnoses in individuals who present with either. To our knowledge, no studies have yet been implemented in Ukraine to document the frequency of blast TBI and PTSD.
We also call for awareness of the risk for TBI, PTSD, and related mental health problems in refugees, and suggest that this should be urgently addressed both by screening people at risk and by implementing surveillance studies. The EU Agency for Asylum provides guidance and practical tools to Member States to help screen asylum seekers for vulnerability, and is also operationally assisting some Member States with this task through the deployment of vulnerability experts on the ground. However, there is a difference in procedures between applicants for international protection (ie, asylum seekers) and beneficiaries of temporary protection (eg, refugees from Ukraine). Although temporary protection beneficiaries already benefit from protection status with provision of the same rights and access to services as national citizens, including health care, vulnerability screening is not mandated as in the case of asylum seekers. Consequently, there is no systematic and common approach across Member States for screening or for provision of medical and psychological care. Even if screening for vulnerability is performed, this does not specifically focus on identifying PTSD in combination with TBI. We suggest that refugees from Ukraine who have been exposed to explosions, physical insults, or psychological stressors of warfare, are screened for both TBI and PTSD (as well as for major depression, which frequently accompanies both disorders).337 To not do so in patients with a diagnosis of TBI might miss a treatable psychological health condition. Conversely, if PTSD is diagnosed, not detecting concomitant TBI might result in suboptimal treatment and overlook other TBI-related complications. Screening alone is not sufficient, and the principles of identification, assessment, and referral should be followed to allow appropriate intervention.
Mild TBI
More than 90% of all TBI cases are classified as mild, based on a GCS of 13–15. A common perception is that almost all such patients will make a full recovery, but this is not the case. TRACK-TBI reported that 53% (95% CI 49–56) of patients with mild TBI had functional limitations at 12 months after injury.314 Common impairments included reduced work capacity, problems with social function, family disruption, and other disabling symptoms. CENTER-TBI reported that 1215 (51%) of 2374 patients with mild TBI had a GOSE score of less than 8 at 6 months after injury.338 The concept of incomplete recovery was further explored in a cohort of 1612 patients with mild TBI in whom other measures were available, and 63% of patients showed impairments on one or more instruments. These data illustrate that the persisting effects of mild TBI are common and well outside the conventional perception of mild. A major research challenge is to identify patients at risk for incomplete recovery early after mild TBI to facilitate timely intervention to improve outcome.
Sex and gender differences
Sex and gender, as factors representing biological and sociocultural human characteristics, respectively, interact with injury cause, injury severity, age, and outcome in complex ways. TBI is more common in young males, who also sustain on average more severe injuries than their female counterparts. However, compared with older men, there is a higher incidence of TBI due to falls in older women and a growing awareness of intimate partner violence as a cause of mild TBI in women. The risk for concussion is higher50,339 among female athletes when compared with male athletes, with evidence of longer recovery times.340 The association between sex and outcome is less clear. Studies in moderate to severe TBI, reporting on survival or functional status, generally show similar outcomes in men and women, or better outcomes in women.341 Studies in mild TBI, collecting data on self-reported cognitive and psychological symptoms, mostly report worse outcomes in women.342,343 In CENTER-TBI, women reported more severe post-concussion and mental health symptoms, and poorer health-related quality of life (HRQOL). 6 months after mild TBI (but not after moderate to severe TBI), women also had a higher likelihood of worse GOSE scores.344 The outcome differences after mild TBI could not be explained by differences in care pathways or sociodemographic characteristics, and were only partly mediated by psychiatric history.345 TRACK-TBI reported more PTSD symptoms in women, and a greater TBI-related symptom burden.342 Importantly, the effect of gender on TBI-related symptoms seen in people with TBI was not present in an orthopaedic trauma control sample. The interactions between sex, gender, and outcomes might also be related to hormonal and genetic responses to injury.341 A detailed discussion on endocrine dysfunction after TBI is presented in the appendix (p 20).
Relation to late neurodegenerative disease
TBI is recognised as a potentially modifiable risk factor for neurodegenerative disease.46,346,347 Estimates suggest that at least 3% of dementia cases in the general population are attributable to TBI,346 and there is growing evidence of a higher risk among former elite-level contact sports athletes.49,348–350 Nevertheless, the associated clinical presentations are heterogeneous and remain poorly defined,351,352 partly because of the complex interrelationship between the acute injury and the evolving pathological processes contributing to progressive decline.352 Emerging descriptions of TBI-related neurodegeneration document multiple proteinopathies, typically associated with wider neurodegenerative disease,353–355 in addition to the more TBI-specific pathology of chronic traumatic encephalopathy.356
Unlike many widely studied neurodegenerative disorders for which disease onset is typically unknown,357 exposure to TBI or repetitive head impacts, or both, can be identified and studied prospectively. The Late Effects of TBI study, initiated in 2015 and longitudinal follow-up initiated in 2019, is a longitudinal prospective brain donor programme that aims to characterise chronic post-traumatic neuropathology and to identify in-vivo biomarkers of post-traumatic neurodegeneration.358 In sports medicine, progress has been made toward strategies for early detection of disease, including the use of PET imaging359,360 in at-risk athletes, but the ability to predict late neurological health problems in former athletes remains uncertain. Studies in individuals with a history of repeated TBI or head impacts present unique opportunities to identify neuropathological signatures, neurobehavioral features (such as cognitive impairment and mental health problems), and novel biomarkers of adverse brain health, with direct relevance to understanding both neurodegeneration following TBI and neurodegenerative disease.103,361
Main messages and recommendations
Main messages
Around 50% of patients with mild TBI presenting to hospital do not recover to pre-TBI levels of health and wellbeing by 6 months after injury.
Outcome in women after TBI is poorer compared with men.
PTSD occurs in 13–19% of civilians with TBI.
There is little understanding of individual differences in recovery after TBI.
Age-specific norms are absent for many outcome instruments.
Although around 3% of dementia cases in the general population are attributable to TBI, and there is growing evidence of higher risk of dementia among former elite-level contact sports athletes, the associated clinical presentations are heterogeneous and remain poorly defined.
Recommendations
Care pathways should be implemented to ensure the structured follow-up of patients with mild TBI, and research should be stimulated to identify patients with mild TBI at high risk for incomplete recovery, which would allow timely evaluation and treatment.
Research should be facilitated to help explain sex and gender differences and inform intervention strategies.
Awareness of the risk of PTSD after TBI needs to be increased, and implementation of routine screening procedures considered.
Research on multifactorial influences on outcome should be stimulated.
Research should be stimulated to develop age-specific and population-specific standards for outcome instruments commonly used in TBI.
Studies in individuals with a history of repeated TBI or head impacts to identify neuropathological signatures, neurobehavioral characteristics, and novel biomarkers of adverse brain health should be stimulated.
Section 6: Prognosis in TBI
Prognosis is an essential element of medicine. Realistic counselling on expected outcomes is of utmost relevance to patients and their relatives. Major applications of prognostic analysis are at the level of the individual patient. Moreover, group-level application can be used for calculating a baseline risk as a reference for evaluating quality of care, and for stratification and covariate adjustment in clinical trials.362 Prognostic models provide risk estimates from multiple patient and disease characteristics that are based on systematic analysis of empirical data. Robust and externally validated models exist for moderate and severe TBI, but not for mild TBI. The 2017 Commission on TBI concluded that (1) existing models for moderate to severe TBI require refinement, (2) there is a need for further development and validation of models in mild TBI, (3) models should be developed to predict outcome beyond mortality and GOSE scores, and (4) a need was identified to develop a set of quality indicators in TBI. In this section we describe the advances towards accomplishing these four goals.
Prognostic models in moderate to severe TBI
Most prognostic models have been developed for patients with moderate to severe TBI. A systematic review363 identified 58 papers describing the development, validation, or extension of 67 different multivariable prognostic models for functional outcome in these patient groups, published between 2006 and 2018. There were 149 external validations of prognostic models. The International Mission on Prognosis and Analysis of Clinical trials in Traumatic brain injury (IMPACT)364 and Corticoid Randomisation After Significant Head injury (CRASH)365 prognostic models were developed on the largest cohorts (8509 and 10 008 individuals, respectively) and have been most often externally validated (56 and 24 times, respectively). IMPACT developed three models and CRASH two models (table 2). Overall, these models showed good discrimination (ie, the ability of a prediction model to separate subjects with and without the outcome of interest; AUCs around 0·8). However, calibration (ie, how well observed outcomes relate to a predicted outcome) was variable and partly influenced by selection of the validation population (casemix). More complex models showed slightly better discrimination compared with the IMPACT Core model (including age, GCS motor score, and pupillary reactivity) and basic CRASH models (age, GCS total score, pupillary reactivity, and major extracranial injury). No competing models are available that have withstood the test of multiple external validations. The IMPACT and CRASH models remain the most widely used in clinical TBI research. However, the IMPACT models were developed on data accrued 25–38 years ago (before the implementation of guidelines) and the CRASH models on data collected around 20 years ago. The validity of model predictions in current practice might therefore be under question. Both CENTER-TBI366 and TRACK-TBI assessed the performance of these models on their contemporary data, collected between 2014 and 2020, and, as with the systematic review, found good discrimination (table 2), although mortality was lower than expected (figure 10).
Table 2:
External validation of the IMPACT and CRASH prognostic models on data from the CENTER-TBI and TRACK-TBI studies
| IMPACT models |
CRASH models |
||||
|---|---|---|---|---|---|
| Core* | Extended† | Lab‡ | Basic§ | CT¶ | |
|
| |||||
| CENTER-TBI | |||||
| Mortality | 0.81 (0.79–0.84; n=1173) | 0.85 (0.82–0.87; n=1030) | 0.85 (0.82–0.87; n=1006) | 0.86 (0.83–0.88; n=1742) | 0.88 (0.86–0.90; n=1542) |
| Unfavourable outcome | 0.77 (0.74–0.80; n=1173) | 0.80 (0.78–0.83; n=1030) | 0.81 (0.78–0.84; n=1006) | 0.82 (0.80–0.84; n=1742) | 0.84 (0.82–0.86; n=1542) |
| TRACK-TBI | |||||
| Mortality | 0.79 (0.74–0.84; n=441) | 0.84 (0.79–0.88; n=406) | 0.81 (0.76–0.86; n=406) | 0.88 (0.83–0.91; n=841) | 0.91 (0.87–0.94; n=841) |
| Unfavourable outcome | 0.77 (0.72–0.81; n=441) | 0.82 (0.77–0.85; n=406) | 0.82 (0.78–0.85; n=406) | 0.86 (0.83–0.89; n=841) | 0.88 (0.85–0.91; n=841) |
Data are discriminatory performance (expressed as AUC with 95% CIs) of the models. Validation cohorts were selected to be consistent with the development populations. For validation of the IMPACT models, patients with moderate to severe TBI (GCS ≤ 12) aged at least 14 years in CENTER-TBI and at least 17 years in TRACK-TBI were selected. For validation of the CRASH models, patients with a GCS of less than 15 aged more than 15 years in CENTER-TBI and at least 17 years in TRACK-TBI were selected. AUCs cannot be directly compared between the IMPACT and CRASH validations, as they are based on different selections of patients. AUC=area under the receiver operating characteristic curve. GCS=Glasgow Coma sum score.
IMPACT Core model: age, GCS motor score, and pupillary reactivity.
IMPACT extended model: core predictors plus hypoxia, hypotension, Marshall CT classification, presence of traumatic subarachnoid haemorrhage, and presence of epidural haematoma.
IMPACT Lab model: predictors extended model plus glucose and haemoglobin concentrations from samples obtained at presentation.
CRASH basic model: age, GCS total score, pupillary reactivity, and major extracranial injury.
CRASH CT model: basic model plus petechial haemorrhages, obliteration of third ventricle or basal cisterns, presence of traumatic subarachnoid haemorrhage, midline shift of more than 5mm, and non-evacuated haematoma.
Figure 10: Calibration plots for external validation of the IMPACT lab models for mortality and unfavorable outcome on data from the CENTER-TBI and TRACK-TBI studies.
The correspondence between observed outcomes and predicted risks is shown as LOESS smoothed curves with 95% CI. The red line is the reference with perfect agreement (calibration intercept 0, slope 1). The distribution of predicted risks is shown at the bottom of each graph, stratified by outcome (1 vs 0). LOESS=Locally Estimated Scatterplot Smoothing.
The calibration plots in figure 10 reflect comparisons between observed and expected outcomes, which can inform benchmarking of quality of care. At the group level, the rate of unfavourable outcome (GOSE <5) was 55%, similar to that predicted by the IMPACT core prognostic model (observed–expected ratio 1·06, 95% CI 0·97–1·14), but mortality was lower than expected (observed–expected ratio 0·70, 95% CI 0·62–0·76). These findings were replicated in an analysis of 441 adult patients from TRACK-TBI, and showed that over time, mortality decreased but that came at a cost of more survivors with severe disability at 6 months. These analyses are robust, as they are adjusted for differences in casemix by the model, but we noted that comparison of contemporary data with historical series is problematic (appendix p 22). The good discrimination of the IMPACT and CRASH models shows that they are still valuable for current-day research. The lower-than-expected mortality, however, indicates a need for updating the models.367 Moreover, the IMPACT models only explain 35% of variance in outcome. This could be increased by extending the models, either by adding new predictors or by adding dynamic information on current predictors as it becomes available over time (dynamic modelling). Data from CENTER-TBI show that adding UCH-L1 to the IMPACT and CRASH models substantially increased the percentage of variability in the outcome that is explained by the predictors (expressed as R2) for predicting mortality: by 12·5% (95% CI 7·3–17·8) when added to IMPACT Core and by 9·2% (5·6–13 2) when added to CRASH (figure 11).
Figure 11: Absolute incremental value (delta R2) of biomarkers when added to the (left) IMPACT Core and (right) CRASH basic models for predicting mortality and unfavourable outcome.
For IMPACT, we selected patients with moderate to severe TBI (Glasgow Coma sum score ≤12; n=737) and for CRASH, patients with a Glasgow Coma sum score of less than 15, thus also including patients with mild TBI (n=1083). Six biomarkers are considered separately, in combination (Comb: GFAP plus UCH-L1), and altogether (All). The vertical lines depict the 95% CIs around the estimates. The explained variance (R2) for the models without biomarkers was as follows: IMPACT Core: 30·7% [95% CI 23·5–37·7] for mortality and 22·6% [15·6–29·1] for unfavourable outcome; CRASH basic: 35·2% [95% CI 28·8–41·8] for mortality and 33·8% [28·4–39·7] for unfavourable outcome. UCH-L1, S100B, and total tau have the greatest incremental value. Combinations of biomarkers appear to have little added value. S100B=S100 calcium-binding protein B. UCH-L1=ubiquitin C-terminal hydrolase L1. GFAP=glial fibrillary acidic protein. NSE=neuron-specific enolase. NfL=neuro-filament light chain. Comb=combination.
The aim in developing the IMPACT and CRASH models was to establish a baseline prognostic risk before enrolment of a patient in a clinical trial. Hence, they are static models using only characteristics available upon admission. In the 1970s, one study368 showed that prognostic estimates become more accurate when adding information obtained on days 2–3 and days 4–7 after injury. Since then, however, very few studies have attempted dynamic modelling. The systematic review cited above reported 12 extensions in five studies, including the APACHE II score, intracranial pressure, and vital parameters obtained within the first 24 h. More recently, autoregulatory indices have shown potential in terms of prognostication (see section 4). The logic of dynamic prediction is already intuitively used in clinical practice, where experienced clinicians constantly re-estimate a patient’s prognosis considering new information.
Despite the broad acceptance of prognostic models in TBI research, their use in clinical practice is low. Barriers to clinical implementation include broad CIs around prognostic estimates and the fact that most models are based on predictors assessed on presentation, whereas clinicians typically consider the clinical course after admission. Following updates and extensions of existing models, efforts are needed to facilitate broader implementation by educating clinicians on their relevance for clinical practice (eg, for provision of reliable expectations to patients and their relatives, and for benchmarking quality of care).
Prognostic models for functional outcome in mild TBI
Few prognostic models have been developed for mild TBI.209,365,369 We identified five methodologically sound models for the GOSE, reported in three studies. The best known are the CRASH (for GCS ≤14) and the UPFRONT models. Published models contain different predictors to those on moderate to severe TBI and include, besides age, GCS, extra-cranial injuries, and alcohol intoxication, sex and gender, education, and pre-injury mental health. Broadly speaking, prognosis in mild TBI is driven to a greater extent by what the patient brings to the injury (eg, pre-injury comorbidities and mental health) than in moderate and severe TBI. This observation was confirmed in the CENTER-TBI and TRACK-TBI studies, which found pre-injury health (including mental health) and sociodemographic characteristics (eg, education, employment, sex, and race and ethnicity) to be prominent predictors of outcome in mild TBI. Injury severity, however, remained one of the strongest predictors of GOSE, showing that what injury brings to the patient (eg, injury severity) is also relevant for global outcome after mild TBI. Validation of existing models for predicting the GOSE in CENTER-TBI data showed poor performance, with discrimination varying between 0·66 and 0·79, and highly variable calibration. Broader issues in prediction after mild TBI are whether the traditional split of GOSE into unfavourable versus favourable outcome as commonly used for moderate and severe TBI, is appropriate, and whether models should be based only on acute symptoms. After mild TBI, few patients have unfavourable outcome as defined by a GOSE of less than 5 (11% in CENTER-TBI). Some publications370 suggest considering a split between lower and upper good recovery (eg, GOSE <8 vs GOSE=8, best defined as incomplete recovery) or the use of GOSE as an ordinal variable (1–8) as most appropriate for predictive modelling in mild TBI. The percentage of patients with incomplete recovery 6 months after mild TBI was substantial in the UPFRONT study (44%),209 in CENTER-TBI (51%),20 and in TRACK-TBI (60%).314 The UPFRONT model found that including symptoms recorded at 2 weeks post-injury (dynamic modelling) increased discrimination from an AUC of 0·69 to 0·77, and the explained variance (Nagelkerke R2) from 14% to 27%. Also in CENTER-TBI, post-injury symptoms, assessed in a subset of 640 patients with mild TBI discharged from an emergency department, showed incremental value for the prediction of functional outcome following mild TBI, increasing the AUC from 0·63 to 0·74 and Nagelkerke R2 from 7% to 21%. Whereas post-injury symptoms increase model performance, other categories of predictors showed inconsistent or minor incremental predictive ability. In CENTER-TBI, some blood-based biomarkers (eg, NfL) showed associations with the GOSE, but did not substantially improve prediction of incomplete recovery. The role of CT abnormalities is uncertain: in the UPFRONT study, CT abnormality was not a predictor of incomplete recovery, but in both CENTER-TBI and TRACK-TBI some CT and MRI findings were predictive of the GOSE.217,236,371 Other studies have shown a strong association between the duration of post-traumatic amnesia and outcome.205
Predicting outcomes beyond the GOSE
Outcome after TBI is multidimensional (see section 5). Despite the value of the GOSE for describing functional outcome, it lacks detail in characterising the heterogeneous nature of impairments, particularly those resulting from mild TBI. Global functional outcome measures should be complemented by domain-specific instruments, providing more comprehensive assessment. Persisting post-concussion symptoms, HRQOL, depression, and PTSD have been analysed in the prognostic context,372,330 mostly in patients with mild TBI. We identified three prognostic models for predicting persisting post-concussion symptoms,209,369,373 but each of these studies used different approaches to defining these symptoms.338 Models developed by two different groups that included symptoms measured early after injury, in addition to sociodemographic and medical variables, reported good performance in predicting persisting post-concussion symptoms (AUC 0·75 and 0·76, respectively).208,209 New models for predicting persisting post-concussion symptoms were developed in CENTER-TBI in a cohort of 1605 patients with complete outcome assessment at 6 months. The Core model explained only 4% of variance; extending this model with other variables available at admission increased the explanation of variance to 9%–12%, and adding information obtained at 2–3 weeks in a cohort of 476 patients, for whom this information was available, increased the explained variance from 6% to 37%. CENTER-TBI further explored prediction of HRQOL in 2666 adult patients who had completed the SF36 version 2 and QOLIBRI questionnaires at 6 months after injury and found that medical and injury related characteristics were more important for the prediction of the physical component summary score, whereas patient-related characteristics were more important for prediction of the mental component summary score and the QOLIBRI following TBI. However, the proportion of variance explained (R2) was relatively low (19% for the physical component score of SF36, 9% for mental component score, and 13% for the QOLIBRI). Inclusion of HRQOL assessments at 2–3 weeks after injury, which were available in 436 patients, substantially increased the R2 to 37% for the SF-PCS, to 36% for the SF-MCS, and to 48% for QOLIBRI.
Development of quality indicators
Measurement of quality of care can guide quality improvement initiatives and benchmarking—ie, with feedback on performance, between-centre discussions on policies, and opportunities to study best practice. Improvement of care and outcomes through quality measurement requires valid quality indicators, which are measurable aspects of quality of care. Quality indicators have been developed in other clinical areas, for example in sepsis, stroke, and in children with TBI, but are absent for general use in TBI. CENTER-TBI developed a set of quality indicators specific to TBI and explored benchmarking quality of care by comparing observed to predicted outcomes. Quality indicators were developed in an extensive Delphi process and consisted of 17 structure indicators, 16 process indicators, and nine outcome indicators.374 The indicators were subsequently validated using data from 2006 adult patients enrolled to the ICU stratum of CENTER-TBI.375 26 of the initial 42 indicators could be validated in the CENTER-TBI data. The other 16 indicators related to organisational aspects, which could not be evaluated on patient data. Overall, nine structure and five process indicators showed potential for quality improvement purposes for patients with TBI in the ICU (appendix p 23). Other indicators might, however, have value in other settings, for example time until CT scan for mild TBI in the emergency department. The indicator set developed in CENTER-TBI is an important first step towards benchmarking and quality improvement programmes for patients with TBI, but requires further validation and refinement.
Main messages and recommendations
Main messages
Despite the broad acceptance of prognostic models in TBI research, they are not often used in clinical practice. This discrepancy might be partly due to the low precision of prediction in individual patients.
Prognostic models have been developed and extensively validated for moderate and severe TBI. No well-validated models exist for mild TBI, nor do models exist that are applicable across all ranges of TBI severity.
Robust prognostic models exist for moderate to severe TBI, but these only account for 35% of the variance in outcome.
Prognostic models for mild TBI are less developed than those for moderate to severe TBI, and their performance can be enhanced by including information obtained at 2–3 weeks after injury.
Incomplete recovery in mild TBI (GOSE <8) represents a prognostic endpoint with clinical, research, and societal relevance. Models for predicting post-concussion symptoms after mild TBI have used different approaches to defining post-concussion symptoms.
Quality indicators developed for TBI are restricted to the ICU setting and are not yet ready for translation into clinical practice.
Recommendations
Efforts are needed to facilitate broader implementation of prognostic models by educating clinicians on their relevance for clinical practice. Clinical acceptance and use of prognostic models would be facilitated by greater precision of prediction for individual patients.
Initiatives should be stimulated to develop models applicable across the range of TBI severity. Availability of such models would constitute a huge step forward and facilitate implementation into clinical practice.
Research should be stimulated to update and extend these models, either by adding new predictors (eg, biomarkers) or by adding dynamic information on current predictors as it becomes available over time (dynamic modelling).
Researchers developing prognostic models for mild TBI should focus on dynamic modelling by including information obtained in the first few weeks after injury.
Initiatives should be stimulated to develop a consensus on the definitions of outcomes, such as persistent post-concussion symptoms, to support the development of clinically relevant prognostic models for mild TBI.
Research should be stimulated to refine, validate, and implement quality indicators for TBI.
Section 7: New directions for acquiring and implementing evidence
Introduction
The 2017 Commission on TBI in 2017 highlighted the need for internationalisation of the common data elements to ensure standardised data collection, the opportunities for comparative-effectiveness research, and the need for globally coordinated research efforts. In this section, we discuss accomplishments towards these aims and identify new challenges. We particularly focus on consent procedures, data sharing in the context of current privacy regulation, novel approaches to evidence generation, and international collaboration.
Study approvals, consent procedures, and the regulatory environment
In May 2018, the General Data Protection Regulation (GDPR: 2016/679) entered into force in the EU and, although broadly addressing privacy and human rights laws, contains provisions also for health research. Specifically, health data are designated as a special category of data, requiring explicit consent for collection and processing. Exemptions include general epidemiological studies serving a public health purpose. Consent is restricted to the aims of the study and the use of data outside this context requires specific consent. GDPR recognises the issue of absence of mental capacity to provide consent, and specific provisions are made in the case of children. However, no provisions are included relevant to patients with an acute absence of capacity or to emergency situations. In TBI (and in other acute neurological diseases), there is both an absence of capacity and an emergency situation. Although conducted before GDPR entered into force, the experiences of CENTER-TBI are relevant to informing regulatory policy. The protocol for CENTER-TBI was evaluated by institutional review boards of 66 centres in 18 European countries, of which 14 considered the study to be observational and two (France and Hungary) considered the study to be interventional, because the study involved blood sampling and outcome assessments that would not be part of clinical routine. Two countries (the Netherlands and Serbia) considered it as both observational and interventional.376 A primary institutional review board assessment was conducted centrally in 11 (61%) of 18 countries and locally in seven (39%) of 18 countries. Although central review is intended to be directly applicable to all national centres, local institutional review board approval was required in six (55%) of 11 countries with central procedures. Local institutional review board approval is generally considered a feasibility check, but full review was often conducted, extending the median duration of final IRB approval in centres participating in CENTER-TBI to 114 days (IQR 75–224 days), with a range from 1 to 535 days. This substantial variation reflects an absence of clear directions in European countries, especially in national legislation, and can adversely affect efficiency of multinational clinical research on TBI. We suggest that the EU should develop and implement directives that regulate central and additional local approval.
Obtaining consent for patients with possible acute mental incapacity poses challenges. In CENTER-TBI, informed consent from the patient was obtained for 805 (95%) of 844 patients enrolled in the emergency room stratum and for 1266 (83%) of 1517 in the admission stratum.377 These patients all had mild TBI and were considered to have sufficient mental capacity to provide consent. Nevertheless, formal documentation of mental capacity, for example by administering the Galveston Orientation and Amnesia test, should be considered before requesting consent. This capacity was documented in TRACK-TBI, where 2216 (87%) of 2550 patients with mild TBI provided consent themselves. In patients with reduced mental capacity, alternatives in CENTER-TBI included proxy consent (eg, by a family member) and deferred consent. Proxy consent was applied in 1377 (64%) of 2137 patients and deferred consent in 334 (16%) of 2137 patients.377 A relative disadvantage of proxy consent is that relatives might not be available soon after injury or might be too overwhelmed to provide valid proxy consent.378 Deferred consent was used only in around a third of centres where it was considered valid, but it might be the preferred option in observational studies in which risks to patients are minimal or absent. In TRACK-TBI, alternative consent procedures for patients with reduced mental capacity included proxy consent by a legally authorised representative or a waiver of consent (at approved institutions). Waiver of consent was used in 327 (10%) of 3284 patients in the total cohort. Proxy consent was the most frequently used alternative in patients with moderate to severe TBI (470 [70%] of 674).
In January 2022, the clinical trials regulation (536/2014) entered into force in the EU and the clinical trials information system was implemented by the European Medicines Agency. To our knowledge, it does not apply to observational studies. The concern is that this more complex regulation might be inappropriately applied to studies such as CENTER-TBI, since two EU nations classified it as an interventional study. A uniform regulatory definition of what should be considered an interventional and an observational study in the EU is needed. In the US, NIH classifies essentially all prospective human studies as trials, and from Jan 25, 2023, all data will need to be deposited in an approved repository.
Internationalisation of common data elements
Standardisation of data collection and data coding received a strong impetus with the publication of the first version of the common data elements for TBI in 2010.379 The current iteration of the common data elements (version 2.0; panel 7) was published in 2012, but since then various issues have arisen.
Panel 7: Classification of traumatic brain injury common data elements.
Core elements
A small set of 29 data elements that are relevant to all traumatic brain injury (TBI) clinical studies.
Basic elements
Data elements beyond the core elements, which are recommended for studies of:
Concussion or mild TBI (n=114*)
Acute hospitalisation (n=105*)
Rehabilitation for moderate or severe TBI (n=143*)
Epidemiology (n=110*)
Supplementary elements
Additional data elements for which inclusion depends upon the particulars of the study.
*These numbers are inflated as various common data elements might relate to one variable. For example, the paediatric Glasgow Outcome Scale-Extended contains 17 CDEs.
Absence of evidence-based criteria for common data element designation has led to proliferation of the number of elements, especially in the supplemental category, and redundancy across element domains. Importantly, common data elements cannot be universally used becacuse of a variety of factors including legal and regulatory issues, semantic or cultural considerations, inconsistent or duplicative categorisation of data elements, and idiosyncratic modes of administration. For example, several core common data elements do not meet GDPR standards as they include potential patient identifiers (eg, date of birth). The core elements of race and ethnicity and the basic elements of educational level of the patient and caregiver are US-centric, and many outcome common data elements are available only in the English language or are copyrighted (see section 5).222 To address these and other issues raised by investigators across the world, NIH-NINDS is planning to convene a work group in 2023, which will be tasked to advise on updating the TBI common data elements to version 3.0. This update is long overdue but can now be informed by the empirical experience in the CENTER-TBI and TRACK-TBI studies to determine which elements have most practical utility, to decrease redundancy, and to identify outcome common data elements that are most responsive in specific TBI subgroups. The version 3.0 update is anticipated to improve global utility of TBI common data elements, but broad international input will be essential to transform the elements to global standards.
Data collection and curation
Data collection according to the common data elements should permit cross-validation of study results33,217 and facilitate meta-analyses across multiple studies to answer research questions that would be difficult to address with single studies alone. However, meta-analysis across CENTER-TBI and TRACK-TBI has taught us that simple alignment of data coding is insufficient and that a deeper level of harmonisation is required, which we designate as deep harmonisation (appendix p 24). For example, outcome data such as the GOSE can be collected inclusive or exclusive of the influence of extra-cranial injuries on function, and interpretation of a given GOSE score is possible only with knowledge of how data were collected (see section 5). Detailed documentation of meta-data is essential and procedures to ensure data quality should run from design through data collection to analysis. Post-processing of data is important to facilitate analyses. For example, the imputation of missing baseline covariates380 or outcome timepoints317 after TBI is statistically challenging,381,382 but predicates the reproducibility of all subsequent analyses. These procedures all belong to the process of data curation, which should involve both domain experts and data scientists. The aim of data curation is to create a final dataset that is well documented and as complete and free from errors as possible. This goal is already demanding in HICs with adequate resources (detailed discussion provided in appendix p 24), but becomes even more challenging in LMICs (panel 8). Data curation in CENTER-TBI and TRACK-TBI was much more complex and time consuming than initially anticipated, and motivated the development of consensus guidelines for Data Acquisition, Quality and Curation for Observational Research Designs391 as a framework for robust data curation applicable beyond TBI. Costs involved in data curation and deep harmonisation in preparation of data sharing are substantial and have been estimated to amount to 15–20% of the study budget.392 Few funding mechanisms exist to support work to make data reusable, and this should be an area for further development of data science policy.
Panel 8: Challenges of data collection in low-income and middle-income countries.
The substantial burden of traumatic brain injury (TBI) in low-income and middle-income countries (LMICs) represents both a clinical need and a research opportunity. However, resource limitations affect medical record keeping,383 civil registration systems, and clinical research, making accurate determinations of TBI hospital admissions and deaths challenging.384,385 Researchers in LMICs cite insufficient funding, incentives, training, and institutional support as barriers to conducting high-quality research,386 with patient follow-up being logistically challenging.387 Considerable heterogeneity exists within LMICs, which, despite the described limitations, include several centres of excellence that have also provided unique and valuable contributions to scientific knowledge.131 Understanding these varied systems of TBI care requires mixed-methods approaches388 to data collection, and more recognition by peers and policy makers of the value of qualitative research.389 Both clinical outcome instruments and qualitative methods require adaptation to account for linguistic and cultural heterogeneity. Finally, it is crucial that LMIC researchers are fully involved in scientific aspects of research, rather than just providing samples for what has been termed “helicopter research”.390
Data sharing and privacy regulation
Data curation and provision of meta-data are essential for data sharing and their reuse, adhering to the FAIR principles: Findable, Accessible, Interoperable, and Reusable.393,394 Although the original study investigators are responsible for ensuring the FAIR principles of interoperability and reusability, the good use of data should be considered a joint responsibility of both the original investigators and researchers reusing the data. This joint responsibility would also be in line with GDPR, which stipulates that the data controller (ie, original investigators or sponsor) remains responsible for what is done with the data. Moreover, insufficient knowledge of study design can lead to erroneous interpretation of findings. For example, in CENTER-TBI a researcher reported substantial between-centre variation in admission rates for mild TBI, ranging from 0 to 100%. This variation could, however, be explained by the fact that some centres only enrolled patients in the emergency room stratum (never admitted to hospital) and others only to the admission stratum (always admitted). Interactions between researchers seeking to access the data and the original investigators should be strongly encouraged and mechanisms developed to cover the costs involved.
Data sharing provides opportunities for other researchers to confirm previous findings or to address new research questions and, most importantly, facilitates meta-analysis across studies. Policymakers strongly support data sharing, as this approach optimises the returns from invested public funding. Meta-analyses can be performed on pooled study results or on individual patient data. The former approach is common in systematic reviews, where larger sample sizes permit greater precision than individual studies. Meta-analysis of individual patient data offers greater opportunities compared with meta-analysis of pooled study results: new study questions can be addressed and heterogeneity between studies can facilitate comparative-effectiveness research. Pooled analysis of individual patient data (one-step process) has been successful in TBI, as shown by the IMPACT studies, for which initially data from eight randomised controlled trials and three observational studies were pooled and analysed centrally.395 However, the regulatory environment has become more stringent since these data were collected over 25 years ago, raising barriers to direct pooling of data across institutions and countries, even among willing partners.396–398 An alternative to pooling of data is to perform federated analysis (ie, centralised analysis with individual-level data remaining on local servers). In this approach, the data remain at their original location and a platform is constructed for decentralised coanalysis. Data federation strikes a balance between protection of patient privacy399,400 and public health interests by bringing the analysis to the data, instead of the data to the analysis. This approach allows a two-stage meta-analytical process (which has a similar statistical interpretation as a one-stage meta-analysis: see appendix p 27), and other decentralised analyses. Tools already exist for implementation of standard statistical analyses on federated platforms and implementation of machine learning approaches are under development. CENTER-TBI is now in the process of implementing a platform for federated analysis, allowing external researchers to access and analyse the data without any need for transfer of the data. Federated analyses offer novel venues to augment and accelerate TBI research worldwide and are potentially applicable to conditions beyond TBI.
Comparative-effectiveness research
In the 2017 Commission on TBI, we identified a particular potential for observational comparative-effectiveness research in the field of TBI, and suggested that evidence from high-quality observational studies could be as valuable as that from randomised trials because of their greater generalisability. CENTER-TBI has extensively applied observational comparative-effectiveness research to identify effective treatments for TBI by leveraging existing variation in treatment and outcomes between centres and countries. Large variations in different treatment approaches were observed (see section 3), but variation in outcome in the ICU stratum (median OR 1·2 [median OR represents the relative odds of the outcome in two randomly sampled centres])121 was smaller than in previous studies on moderate and severe TBI (median OR 3·3; figure 12).190 This finding does not necessarily preclude meaningful analysis, as different treatment preferences might affect an outcome in different directions.401
Figure 12: Caterpillar plots of between-centre differences in interventions and outcomes in the CENTER-TBI study.
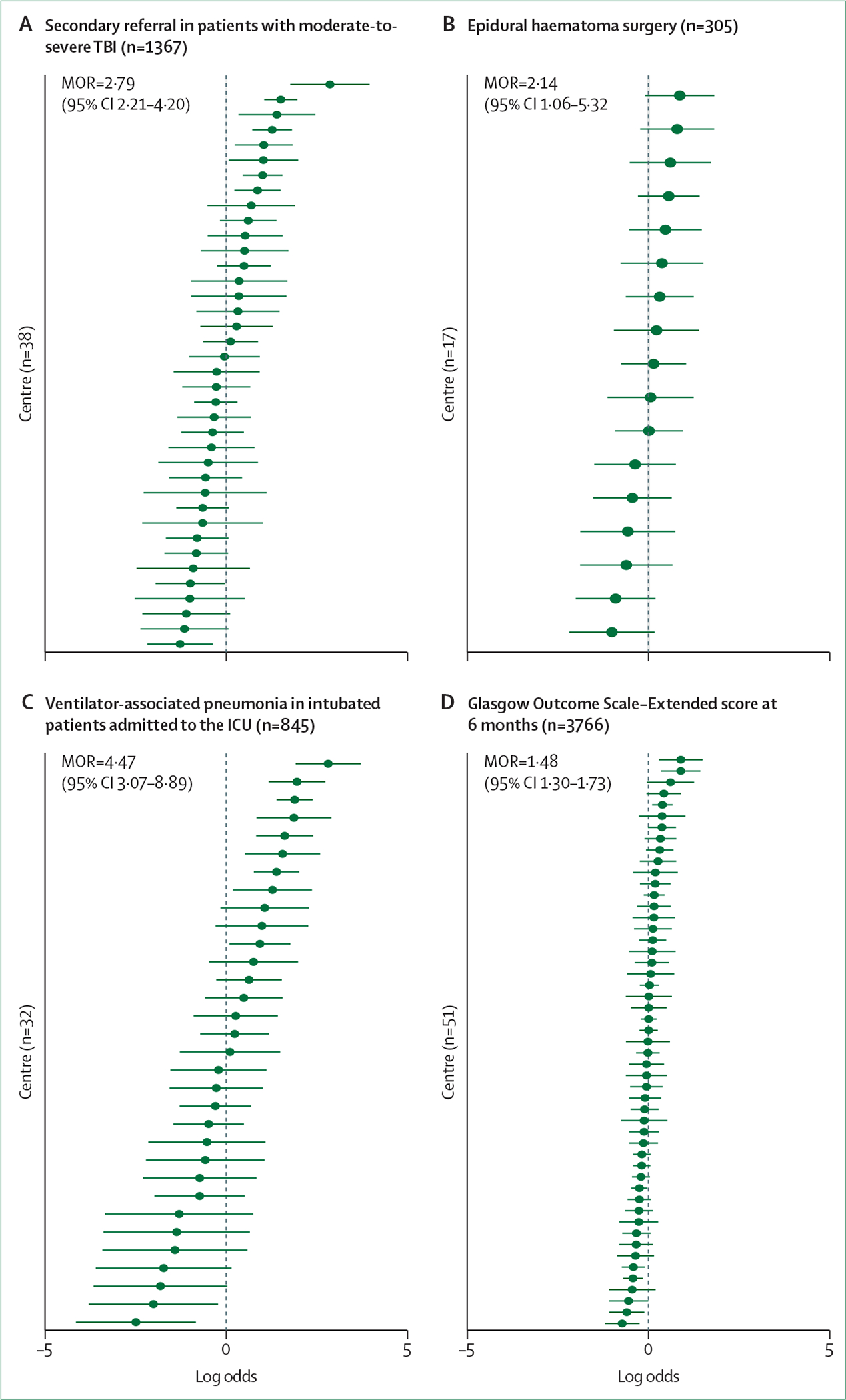
(A) Secondary versus primary referral to a specialised neuro-trauma centre in patients with moderate-to-severe traumatic brain injury. (B) Epidural haematoma surgery during hospitalisation in patients with an epidural haematoma on the admission CT. (C) Ventilator-associated pneumonia in intubated patients admitted to the ICU. (D) Glasgow Outcome Scale–Extended score at 6 months (higher scores on the ordinal scale). To estimate centre-specific effects, a random-effect regression model was created for each intervention and outcome. Each model was adjusted for casemix severity using variables from the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) Lab model: age, Glasgow Coma Scale–motor score, pupillary response, hypoxia, hypotension, Marshall CT classification, presence of traumatic subarachnoid haemorrhage, presence of epidural haematoma (omitted in the model for epidural haematoma surgery), and first glucose and first haemoglobin measurements from blood samples obtained on presentation. Multiple imputation was used for missing values of adjustment variables. For each intervention and outcome, only centres that enrolled at least ten patients from the target subgroup (eg, patients with an epidural haematoma in [B], intubated ICU patients in [C]) were included. As such, the number of centres on the y-axis differs between plots. Centres are ordered by their estimated random effect. For (A), (B), (C), (D), the origin of the x-axis represents the overall log-odds of experiencing the intervention and outcome for all patients from all centres for each respective plot. Random effects of more than 0 mean an increased likelihood and less than 0 mean a decreased likelihood of experiencing the intervention and outcome for a given centre. The MOR is a summary measure of the overall between-centre variation and is interpreted as the odds ratio of experiencing the intervention and outcome for the same patient, when comparing two randomly selected centres. An MOR of 1 indicates no between-centre differences, and larger MORs indicate higher variation. The MORs 95% CIs were derived from the profile likelihood CIs of the random effect SDs. ICU=intensive care unit. TBI=traumatic brain injury. MOR=median odds ratio.
A particular challenge to drawing causal inferences from observational comparative-effectiveness research analysis is the risk for confounding by indication. Broadly speaking, two mitigation approaches can be differentiated: adjustment-based methods (eg, propensity scores and covariate adjustment) and so-called quasi-experimental designs (eg, an instrumental variable approach).402 Both approaches were used in CENTER-TBI. We identified best practices in, for example, fluid management in the ICU and surgery for acute subdural haematoma (see section 3). The ADAPT study successfully applied comparative-effectiveness research to the analysis of the relative effectiveness of hypertonic saline and mannitol for managing intracranial pressure and cerebral perfusion pressure in paediatric patients with severe TBI.403 Nevertheless, residual confounding might remain and was likely in the exploration of benefits of monitoring intracranial pressure by a ventricular catheter (allowing for drainage of CSF) versus by an intraparenchymal sensor.404 A clear comparative-effectiveness research analysis plan before data collection, which considers all variables that are likely to interact with treatment effects, can ensure that all relevant data are collected and used to minimise residual confounding.
Causal interpretation of observational comparative-effectiveness research studies depends on methodological rigour, which can make interpretation and scientific review complex. CENTER-TBI had substantial problems during the peer-review process of multiple submissions for publication. These problems suggest that comparative-effectiveness research is not yet considered mainstream and highlight the need for reviewers with sufficient understanding of such research methods to judge their quality and to recognise when comparative-effectiveness research is an appropriate approach to answer a specific question.
CENTER-TBI and ADAPT403,405 have shown that observational comparative-effectiveness research in TBI meets expectations. Nevertheless, challenges were encountered and lessons were learned (panel 9). The strength of evidence resulting from well-conducted comparative-effectiveness research studies can be considered high. Such studies, using robust methodology showing results that are mechanistically plausible, can be causally interpreted. They can inform clinical practice408 and can complement randomised trials, specifically in situations for which there is no clinical equipoise or the standard treatment is difficult to define.
Panel 9: Comparative effectiveness research analyses: challenges and recommended solutions based on the experience from the CENTER-TBI study.
Post-hoc study question
Problem: insufficient parameters to adjust for differences between treatment groups for a specific question.
Potential solution: pre-specification of study question and collection of all variables that are likely to relate to the selection of treatments.
Confounding by indication
Problem: residual confounding.
Potential solution: application of different approaches to analysis with different assumptions; for example, instrumental variable approach and propensity matching.
Small between-centre differences in outcome
Problem: decreases likelihood of identifying best practices.
Potential solution: none.
Peer-reviewers of submitted manuscripts insufficiently familiar with comparative-effectiveness research
Problem: multiple revisions or rejection from journals.
Potential solution: improved understanding of comparative effectiveness research methods among peers.
Strength of comparative-effectiveness research results undervalued compared with those from randomised trials
Problem: low interest and poor understanding of comparative-effectiveness research.
Potential solution: emphasise the relevance of real-world data. The COVID-19 pandemic has accelerated the implementation and acceptance of new research paradigms and increased awareness of the relevance of real-world data and real-world evidence.406,407
Novel approaches to randomised trials
Although observational comparative-effectiveness research is increasingly recognised as relevant to the field of TBI, randomised trials remain the standard for determining efficacy of interventions. Historical controls (ie, patients from cohorts in previous studies) are accepted by regulatory authorities in rare diseases with small but homogeneous patient populations, but TBI is not rare and patients are highly heterogeneous. A discussion on the use of cohort studies is included in the appendix (p 29). The classic design of a randomised trial involves strict enrolment criteria and randomised, blinded allocation of patients to two treatment groups. Randomised trials are costly and labour intensive. Means to make the conduct of these trials more efficient are needed. For these reasons, interest in non-classic designs is increasing with lessons learned from other fields. We discuss various approaches below.
Platform trial design
Platform trials,409–413 used with great effectiveness in the COVID-19 pandemic to identify effective treatments, provide infrastructures to study several interventions in a specific patient population simultaneously, avoiding multiple independent trials. Interventions with similar mechanisms of action can form a specific domain or category of treatment. Patients can be randomly assigned in more than one domain, but to only one intervention of a specific domain. The approach also allows addition of new domains, or interventions within domains, when novel interventions emerge or research priorities change. Although their applicability in TBI remains untested, platform designs might enhance the efficiency of trials in this population.
Adaptive design
The long interval between intervention and final outcome assessment in TBI trials poses challenges for determining efficacy of an acute intervention and increases costs. Trial efficiency can potentially be increased by using adaptive designs, in which some features of the design can be dropped (eg, study drugs that show early futility) or other study agents inserted (ie, adapted as part of the trial design). Criteria for such changes need to be pre-specified and require interim analyses by an independent statistical team and decisions following a-priori determined thresholds (superiority, inferiority, or futility). By necessity, an adaptive design involves repeated interim analyses, incrementing the risk of a type 1 error. Bayesian statistics have been successfully used to mitigate this risk.409,414 Implementing an adaptive design in TBI trials would, however, involve the use of early endpoints. To date, such early endpoints, which need to be related to final outcome, are exploratory in TBI. Work published in 2022 suggests that blood-based biomarkers and advanced MRI imaging, captured in the acute postinjury phase, are associated with patient outcome (see section 4),251,252,415 suggesting that these might serve as endpoints for proof of concept that a drug is hitting a target in an adaptive design. The TRACK-TBI Network is designing a phase 2 adaptive platform randomised control trial using a master protocol to test three drugs against placebo. With the adaptive design, the investigators aim to discover early utility or futility using biomarker endpoints along with functional, cognitive, and symptom measures. The potential of an adaptive randomisation design is also being explored in the context of the HOBIT trial on hyperoxia.416
Pragmatic mega-trials
Pragmatic mega-trials recruit large patient numbers, use simple data collection forms, and target small treatment effects that apply across the population of patients studied or in identifiable subgroups with adequate sample size following stratified randomisation to deliver statistical power. To date, they have been one of the more successful approaches in TBI. Examples of pragmatic mega-trials in TBI are in the appendix (p 30). Mega-trials can detect small treatment effects that apply to an unselected population of TBI patients, but questions have been raised about their clinical relevance.417 Furthermore, their pragmatic design and limited data collections often make identification of discrete treatment-responsive subgroups impossible. Past mega-trials have all tested relatively inexpensive drugs already approved for other indications, and would appear less appropriate for complex interventions or for new drugs or interventions, for which safety monitoring is more relevant.
International collaborations
The formation of the InTBIR initiative, established in 2011 initially as a collaboration of funding agencies,5 formed a major step forward towards international collaborations to support innovative study design. 10 years after inception, the InTBIR initiative has become an international open science ecosystem in clinical TBI research. Major studies conducted under the auspices of InTBIR included CENTER-TBI, TRACK-TBI, CREACTIVE, and ADAPT, and several other multicentre studies and trials. CENTER-TBI evolved from a primarily European-based project to a global study with linked data collections in Australia, China, and India. Since its inception, the operating model of InTBIR has evolved, and it is now a collaborative consortium of neurotrauma researchers, with funding bodies having a less directive role in its function than previously. Models of collaborative research networks, such as the Canadian Traumatic Brain Injury consortium, could be emulated globally with InTBIR. This new phase of InTBIR, which is still in evolution, promises to provide a platform for international and transdisciplinary collaboration, with a new tranche of collaborative studies in development. These studies will include projects conducted in Australia in the context of the Mission for TBI, announced by the Australian Government in 2019 to dedicate grant funding of 50 million Australian dollars over a 10-year period. The Mission for TBI aims to accelerate Australian-led TBI research to develop and deliver innovative and effective treatments that improve health outcomes, in partnership with people with TBI and carers. Global collaborations are needed with researchers from LMICs because the greatest burden of TBI occurs in these countries. An example is the NIHR Global Health Research Group on Neurotrauma, which is already showing high productivity18 (see also section 2).
Interactions with regulators, research funders, and policy makers
Neurotrauma researchers have traditionally pursued academic outputs and sought translation of their findings through clinical guidelines (usually in conjunction with professional bodies) and new diagnostic and therapeutic interventions (usually through collaboration with industry partners). However, it has become increasingly obvious that to affect clinical care and outcomes, there is also a need to interact with regulators (appendix p 31), policy makers, and research funders. The release of the 2017 Commission in the European Parliament exemplified such engagement, and provided an ongoing stimulus for researchers to pursue engagement outside the academic bubble. Panel 10 lists three examples of such outputs that have materialised since the Commission was published.
Panel 10: Examples of regulatory and policy engagement in traumatic brain injury research.
The UK All Party Parliamentary Group on Acquired Brain Injury (APPG ABI), a non-partisan group of UK members of parliament, was launched in 2017. All UK members of parliament were provided with a copy of the 2017 Commission on traumatic brain injury (TBI), and the APPG, chaired by member of parliament Chris Bryant, obtained input from a range of experts in brain injury, in a process facilitated by the UK Acquired Brain Injury Forum (UKABIF), an organisation that advocates for patients with acute brain injury. In 2018, the APPG launched the Time for Change report based on these deliberations, which addressed issues in neurorehabilitation, education, the criminal justice system, sport-related concussion, and welfare benefits. This report and testimonies from the APPG ABI have informed parliamentary debates and the development of government policy in the area, and these procedures have become recognised as a model for progress.2
The TRACK-TBI study group has engaged with regulators and US policy makers. TRACK-TBI has used each of the Critical Path for Innovation Meetings (CPIM), submission of letters of support for promising biomarker candidates, obtained qualification of medical device development tools (MDDTs), and convened public consensus conferences with participation from the US Food and Drug Administration to develop and disseminate evidentiary standards aimed at improving efficiency and achieving success in drug and device development. For example, in 2019, approval as an MDDT was provided for an OsiriX Common Data Element package, which assists health-care providers, such as neuroradiologists, to better identify eligible patients for enrolment in mild TBI clinical trials.
TRACK-TBI leadership has had extensive engagement with the US National Academies of Sciences, Engineering, and Medicine (NASEM). In 2020, concerned about the under-studied, high burden, and complex health issues attendant to TBI, NASEM convened a Committee on Accelerating Progress in Traumatic Brain Injury Research and Care. Composed of a broad membership of stakeholders, the Committee, including several clinical and research experts from TRACK-TBI, analysed barriers and opportunities to improve TBI research and the currently fractured care systems from the perspectives of researchers, public institutions, industry, and importantly, patients and caregivers. The Committee’s report,182 released in 2022, presents a roadmap to guide the field over the next decade.
Main messages and recommendations
Main messages
TBI is often characterised by both an acute loss of mental capacity of patients to provide informed consent for participation in research and by an emergency situation. Although GDPR recognises the issue of absence of capacity to provide consent, no provisions are included that are relevant to patients with acute lack of capacity or to emergency situations.
Standardisation of clinical data collection, based on the TBI common data elements, provides a common language for research, but the common data elements need to be made internationally applicable when aiming for global standardisation of clinical data collection. There is substantial redundancy and duplication in current common data elements.
Data sharing and analyses across different datasets do not necessarily require data transfer and can be done on a federated platform. Use of a federated platform facilitates broad use of data, while reducing the risks for violation of data security and facilitating privacy regulation.
Costs of data curation and deep harmonisation in preparation for sharing research data are under-estimated and can amount to up to 20% of a study budget.
Comparative-effectiveness research has delivered on expectations by identifying best practices and providing strong evidence in support of treatments, but its interpretation can be complex, contributing to a perception that the strength of results from such research is lower than that of results from randomised clinical trials.
Recommendations
Regulatory guidance should be developed. Consider mandating that researchers document objective proof of mental capacity in the study participant, who might have temporarily lost capacity, before seeking informed consent.
NIH-NINDS, as the coordinating agency for the TBI common data elements, needs to ensure appropriate international input when developing the next update of the common data elements to version 3.0. Updates to and refinement of TBI common data elements should be informed by the pragmatic experience of large-scale studies conducted under the InTBIR initiative.
Support is required for the development of platforms for federated analysis, particularly for the development and implementation of machine-learning techniques on such platforms.
For completed studies, mechanisms should be developed to facilitate the maintenance of the data and provision of guidance to external researchers wishing to analyse the data. For new studies, inclusion of an appropriate budget to prepare data for sharing should be foreseen in the grant award.
Educational and outreach efforts are needed to improve the understanding of comparative-effectiveness research methods and their interpretation.
Conclusions: Looking backward and looking forward
This update provides an overview of progress since the publication of the 2017 Commission on TBI in 2017 and outlines remaining challenges. Tables 3 and 4 summarise key messages and recommendations from the original Commission, the progress made over the past 5 years in addressing these, and persisting and new challenges identified as part of this update. Table 3 contains key messages of particular relevance to policymakers, whereas table 4 contains key messages mainly directed at clinical management and research.
Table 3:
Summary overview of progress and outstanding issues, based on the key messages of particular relevance to policymakers and recommendations presented in the 2017 Commission
| Original recommendation | Progress since the 2017 Commission | New and persisting challenges | Work for next decade | |
|---|---|---|---|---|
|
| ||||
| Worldwide, TBI is a leading cause of injury-related death and disability, with a devastating effect on patients and their families | Concerted efforts to address this vast global health problem should focus on policies aimed at reducing the burden and effects of TBI, through better prevention, improved access to care, and promotion of clinical research to improve treatment standards | Increased attention to road safety in LMICs: WHO’s Decade for Action on Road Safety launched in 2021. Substantial support for TBI research from funding agencies, including the European Commission, NIH-NINDS and the Department of Defence in the USA, NHMRC in Australia, and the MRC in the UK. No clear evidence of change in mortality or disability outcomes from TBI, despite advances in care and research. However, these advances are likely to have a latent period before they have an effect on these key statistics | LMICs are disproportionately affected by TBI. Populations at increased risk include children, older people, and criminal offenders. Adverse brain health consequences caused by TBI and repetitive head impacts in sport | Deliver on implementation of Road Safety goals of 50% reduction in traffic-related injuries and deaths by 2030. Target prevention and intervention actions to populations at increased risk. Harmonise safe-play rules across team sports and increase prevention focus for individual sports |
| Coordinated research efforts on a global basis are needed to address the growing public health problem of TBI | A commitment of governmental and non-governmental funding bodies, as well as industrial partners, is needed to foster global collaborations and to establish national and international biorepositories and databases that can facilitate future TBI research | Substantial success with large collaborative studies in InTBIR—large bodies of research with valuable databases, image repositories, and biobanks. Some follow-up funding, but this is less integrated. Clear evidence of engagement with policy makers and regulators. New TBI funding initiatives (eg, Mission TBI in Australia) and engagement of global partners in LMICs (eg,NIHR Global Neurotrauma Initiative) | There is no structured follow-up funding to support maintenance of the databases and to facilitate meta-analyses across the datasets | Exploiting the results and (perhaps more importantly) the networks of these studies will require collaborations supported by ongoing funding. Discussions are underway—both between individual study partners and globally through InTBIR. Continue to engage with policy makers and funders, but further involvement of patient advocacy organisations is also needed. Encourage governments and funders to integrate efforts—‘how’ is at least as important as ‘how much’ |
| In LMICs, the incidence of TBI due to traffic incidents is increasing, while in HICs, TBI increasingly affects elderly people, mostly resulting from falls; however, methodological variations confound comparisons of epidemiological patterns of TBI between regions, countries, and continents | An international consensus is needed on definitions and standardised epidemiological monitoring of TBI to allow accurate measurement of incidence, prevalence, and mortality, and comparison of rates of access to community, hospital, and residential care | Still no accepted framework for epidemiological data collection and reporting, with resulting wide variations in global estimates of TBI incidence (2017 Commission estimate of 50 million–60 million per year; vs Global Burden of Disease study estimate of 24 million–30 million per year) without objective evidence of which is correct | Inconsistencies within and between hospitals in ICD coding could contribute to the large variations observed | There is a need for an accepted process for data capture and reporting of TBI epidemiology, agreed upon by governments and institutions |
| TBI results in substantial health care and societal costs | More effective strategies for TBI prevention are urgently needed and could deliver cost savings that help to fund research and improve access to health care for TBI | Most ongoing prevention actions are directed at road traffic safety. Only a few new studies on the cost burden of TBI have been published since the 2017 Commission | Increased recognition of the relevance of frailty and alcohol abuse as factors contributing to falls causing TBI in older people | Target fall prevention for older people in HICs. Need to develop fall prevention strategies more universally, and as demographics in LMICs change, ensure implementation also in these countries. Develop a research focus on the cost burden of TBI and cost-effectiveness of diagnostics (including biomarkers) and treatment |
| Access to health care is often inconsistent between centres, regions, and countries, especially for acute and post-acute care | Health-care policies should aim to improve access to acute and post-acute care to reduce the effects of TBI on patients, families, and society | No clear improvement in access to acute and post-acute care. Some improvements in engagement of LMIC partners in Global Health Research in TBI and development of protocols for acute TBI management suitable for deployment in resource-limited settings. Epidemiology of TBI might have changed during the COVID-19 pandemic. Reports of the effects of COVID-19 on TBI care and outcome are variable | Disparities in care identified in high-income countries for: older people injured by low-energy mechanisms (falls), who are less often taken to a trauma centre, receive less critical care, and fewer neurosurgical interventions compared with patients injured by high-energy mechanisms. Access to rehabilitation for patients with moderate to severe TBI. Absence of structured follow-up for mild TBI, despite more than 50% of patients do not recover to pre-TBI levels of health and wellbeing by 6 months after injury. Near-total absence of adequate pre-hospital and post-acute care in LMICs | Reappraisal of triage tools used in emergency settings, aiming to decrease the current strong focus on high-energy mechanisms. Involve rehabilitation services at an early stage of the in-hospital treatment for TBI. All patients discharged from hospital after mild TBI should be scheduled for follow-up. Develop and implement health-care policies in LMICs to correct this disparity. Need to monitor the effects of COVID-19 on TBI care and address these if clinically significant. |
TBI=traumatic brain injury. LMICs=low-income and middle-income countries. NIH-NINDS=US National Institutes of Health-National Institute of Neurological Disorders and Stroke. NHMRC=Australian National Health and Medical Research Council. MRC=UK Medical Research Council. InTBIR=International Traumatic Brain Injury Research. NIHR=UK National Institute for Health and Care Research. HICs=high-income countries. ICD=International Classification of Diseases.
Table 4:
Summary overview of progress and outstanding issues, based on the key messages mainly directed at clinical management and research and recommendations presented in the first Commission
| Original recommendation | Progress since the 2017 Commission | New and persisting challenges | Work for next decade | |
|---|---|---|---|---|
|
| ||||
| Second or subsequent concussions that occur before recovery from an initial concussion can be associated with more severe symptoms and more prolonged recovery than a single injury of similar severity | Any risk of an early second injury after even a mild TBI should be avoided; professional sporting organisations should set an example for children and amateur athletes by immediately removing from play anyone with a suspected concussion | Increasing visibility of dangers of concussion and most professional sporting bodies have (or are moving towards having) good concussion protocols. Substantial new knowledge on sport-related concussion from very large observational studies | Implementation of protocols is still incomplete across different team sports, and isolated poor practice is still visible during high-profile televised events. Although there is a strong focus on the risk of sport-related concussion and repetitive head impacts in team sports, most patients seen in hospital with sport-related concussion have sustained the injury during individual sports or recreational activities | Need to ensure broad adoption of sport-related concussion protocols. Continue education and public pressure on governing bodies to ensure consistent practice that is compliant with guidelines. Re-enforce preventive measures in individualised sports such as cycling and horse-riding |
| Methods of diagnosis and classification of patients with TBI are insufficient to permit targeting of current and new therapies to the needs of individual patients | Research is needed to improve the precision of diagnosis, classification, and characterisation of TBI using multidomain approaches | There have been substantial gains from a large body of research reported in the past 5 years, much of it from large collaborative studies, incorporating advanced neuroimaging and blood biomarker data. Approaches to individualised titration of ICU care using multimodality monitoring are now being tested in clinical trials. Insights from genomic medicine and measurement of host inflammatory response are less developed, but datasets are available for exploitation | Identification of groups of patients who would be most likely to benefit from specific interventions. Use of blood-based biomarkers is on the verge of a breakthrough for diagnostic and prognostic use in TBI, but implementation in practice is adversely affected by an absence of clinical-use assays and cross-platform harmonisation | Use advanced analytic techniques, including artificial intelligence approaches to derive clinically relevant insights. Integration of new data sources to identify patient endotypes for trials of existing and new therapies. Translate these research advances into real-world approaches to individualised management of patients |
| Evidence underpinning guidelines for medical, surgical, and rehabilitation interventions for TBI is weak | Robust evidence is needed to inform guidelines on medical, surgical, and rehabilitation interventions, and hence improve outcomes for patients with TBI | Evidence from randomised clinical trials available in some areas (mainly surgical), and additional trials are now underway or in preparation. Comparative-effectiveness research is making increasing contributions. New generation of expert consensus-based guidance to supplement rigorous evidence-based guidelines | Although comparative-effectiveness research analyses have proven to provide strong evidence, the methodology is complex and often insufficiently understood or appreciated | Continue to seek highest quality of evidence (from randomised controlled trials) wherever possible, but understand the opportunities and limitations of comparative-effectiveness research and expert consensus. Consider platform trials and adaptive designs to accelerate evidence generation. Better publicise the potential of comparative-effectiveness research analyses |
| Substantial between-centre variability in treatment and outcome in TBI offers unique opportunities for comparative-effectiveness research to improve the strength of evidence | Comparative-effectiveness research should be supported to identify best practices and to improve the level of evidence for systems of care and diagnostic and therapeutic interventions | Between-centre variability in practice confirmed in large observational studies, and comparative-effectiveness research analyses have permitted identification of best practices (eg, fluid management, hyperosmolar therapy, thrombosis prophylaxis, and surgical approaches) | Outcome variance between centres is less substantial than expected. Some scepticism experienced from peer-reviewers during the publication process | Better quantitation of opportunities and limitations of comparative-effectiveness research approaches—we need to understand when a result from comparative-effectiveness research is the best evidence available and when it should serve to set a hypothesis as a prelude to a formal randomised clinical trial |
| Trauma disturbs the brain in complex ways, affecting multiple outcome domains | Multidimensional outcome constructs that quantify the overall burden of disability from TBI need to be developed and validated to guide improved clinical management and support high-quality research | Translation and linguistic validation of outcome instruments, previously only available in English, into multiple languages. New tools to understand and compensate for missingness of outcome data. Better understanding of how different outcome assessments (eg, GOSE, quality of life, mental health, and cognition) fit together and affect the overall outcome | Uncertainty if multidimensional assessment should focus on the use of multiple instruments or on development of a composite outcome score | Implement systems of outcome assessment that integrate different instruments, understand the benefits and limitations of such approaches, and then find ways to refine them |
| TBI might represent an important modifiable risk factor for epilepsy, stroke, and late-life neurodegenerative diseases | Studies are needed in children and adults to better understand links between TBI of all severities and an increased risk of later neurological diseases | Further data on epidemiological links between repeated concussions and late neurodegeneration, supported by a few studies examining medium term outcomes of acute TBI over a decade. Large collaborative efforts ongoing to collate late neuropathology data for TBI-related neurodegeneration WHO initiative on epilepsy in the context of other diseases (including TBI); and guidelines on TBI-related pituitary dysfunction | Absence of early clinical criteria to determine the onset of late-life neurodegenerative diseases | Continue to look for and correct treatable late consequences of TBI. Need definitive studies with a long follow-up to address the long-term consequences of TBI. Combine post-mortem neuropathology with ante-mortem molecular imaging to understand the mechanisms of TBI-related neurodegeneration |
| A validated set of quality indicators is essential for the benchmarking of quality of care, but none exists for TBI | Efforts are needed to develop a set of quality indicators for TBI that includes structure, process, and outcome metrics | Some progress with development of quality indicators | Quality indicators developed are restricted to the ICU setting. Not ready yet for translation into practice | Refine, validate, and implement quality indicators |
TBI=traumatic brain injury. ICU=intensive care unit. GOSE=Glasgow Outcome Scale-Extended.
There has clearly been progress in the past 5 years, mostly due to the collaborative efforts triggered by the InTBIR initiative. However, many of these advances have yet to achieve routine clinical implementation and major issues persist in the care of patients with TBI in LMICs. Substantial progress has been made in incorporating novel clinical variables, blood biomarkers, and neuroimaging into patient characterisation, and implementation of these advances into clinical practice is currently underway. However, additional progress is needed in terms of achieving precision medicine approaches to TBI management. Although further evidence is needed, these approaches might include incorporation of molecular and genomic information into clinical practice, assisted by new approaches to data analysis. Finally, engagement with funders, policy makers, and regulators, and closer partnership with patient representative groups could address important strategic issues in TBI research and delivery of care.
The final columns of tables 3 and 4 suggest a work plan to address challenges over the next decade. We hope that a following update of the Commission on TBI in 5–10 years might document further progress in all of these areas, including TBI prevention, clinical management, and patient outcomes.
Supplementary Material
Table 1:
Key messages and recommendations
| Key messages | Recommendations | |
|---|---|---|
|
| ||
| Section 1 | Worldwide, TBI is a leading cause of injury-related death and disability, with devastating effects on patients and their families | Continue concerted efforts to address this vast global health problem and focus on better prevention, improved access to care, and promotion of clinical research to improve treatment standards |
| Section 1 | More than 90% of patients presenting to hospital with TBI have so-called mild TBI (GCS 13–15), but evidence to inform treatment of patients with mild TBI is scarce | Increase public health interest and establish a research focus on mild TBI |
| Section 1 | In HICs, older patients (≥65 years) who are mostly injured by falls account for 30–40% of hospital admissions for TBI. Frailty and alcohol abuse contribute to falls causing TBI in older people | Target fall prevention for older citizens in HICs |
| Sections 1, 2 | People in LMICs are disproportionately affected by TBI, with most injuries caused by road traffic incidents. Substantial disparities in care exist, with little infrastructure available for emergency pre-hospital care and very little access to post-acute care | Deliver on implementation of road safety goals, described in WHO’s Decade for Action on Road Safety plan launched in 2021. Improve emergency pre-hospital care and develop an infrastructure for post-acute care |
| Section 2 | Disparities in care also exist in HICs and relate to: older people injured by low-energy mechanisms (falls); access to rehabilitation for patients with moderate to severe TBI (GCS ≤12); and follow-up in patients with mild TBI | Address disparities through close collaboration between policymakers and clinicians; Approaches to consider include: critical appraisal of triage tools used in emergency settings; involvement of rehabilitation services at an early stage of the in-hospital treatment for TBI; and establishment of structured follow-up after mild TBI as good practice |
| Sections 2, 4 | Use of blood-based biomarkers is on the verge of a breakthrough for diagnostic and prognostic use in TBI, but few assay platforms have been approved for clinical use and substantial variability exists between platforms | Stimulate the development, validation, and approval of clinical-use platforms, and facilitate cross-platform harmonisation of biomarker assays |
| Section 3 | Older patients often have co-morbidities, but very little evidence exists to inform their treatment | Stimulate a research focus on older patients with TBI |
| Section 3 | Access to rehabilitation services is inconsistent and no protocols for treating long-term problems exist | Improve access to rehabilitation services and develop evidence-based treatments for long-term problems—including fatigue, and cognitive and behavioural changes |
| Section 4 | Substantial advances have been made towards individualised management with improved characterisation and understanding of disease processes in TBI, but physicians are not yet sufficiently able to match therapies to subgroups of patients | Stimulate research to identify subgroups of patients who would be most likely to benefit from specific interventions |
| Section 5 | Around 50% of patients with mild TBI presenting to hospital do not recover to pre-TBI levels of health and wellbeing by 6 months after injury | Implement care pathways to ensure structured follow-up of patients with mild TBI, and stimulate research to identify patients with mild TBI at high risk for incomplete recovery, which would allow timely evaluation and treatment |
| Section 5 | Outcome in women after TBI is poorer than in men | Facilitate research to help explain this sex and gender difference and inform intervention strategies |
| Section 6 | Prognostic models have been developed and extensively validated for moderate and severe TBI. No well-validated models exist for mild TBI, nor do models exist that are applicable across all ranges of TBI severity | Initiatives should be stimulated to develop models applicable across the range of TBI severity. Availability of such models would constitute a huge step forward and facilitate implementation into clinical practice |
| Section 6 | Quality indicators developed for TBI are restricted to the ICU setting and are not yet ready for translation into practice | Research should be stimulated to refine, validate, and implement quality indicators for TBI |
| Section 7 | Costs of data curation and deep harmonisation in preparation for sharing research data are underestimated and can amount to up to 20% of a study budget | For completed studies, mechanisms should be developed to facilitate maintenance of the data and to provide guidance to external researchers wishing to analyse the data. For new studies, inclusion of an appropriate budget to prepare data for sharing should be foreseen in the grant award |
| Section 7 | TBI is often characterised by both an acute mental incapacity of patients to provide informed consent for participation in research and an emergency situation. Although GDPR recognises the issue of absence of capacity to provide consent, no provisions are included that are relevant to patients with an acute absence of capacity or to emergency situations | Develop better regulatory guidance. Consider mandating that researchers obtain objective proof of mental capacity of the study participant, who might have been temporarily mentally incapacitated, before requesting informed consent. |
| Section 7 | Data sharing and analyses across different datasets do not necessarily require data transfer and can be done on a federated platform. Use of a federated platform facilitates broad use of data and reduces the risks for violation of data security and privacy regulation | Support is required for the development of platforms for federated analysis, particularly for development and implementation of machine-learning techniques on such platforms |
TBI=traumatic brain injury. HICs=high-income countries. LMICs=low-income and middle-income countries. GCS=Glasgow Coma sum score. ICU=intensive care unit. GDPR=general data protection regulation.
Acknowledgments
We are enormously grateful to our patients with traumatic brain injury—the Commission’s most important stakeholders, who have shared their experiences with us and taught us so much. We are deeply indebted to all researchers and study personnel not included in the author list or in the Group contributor list, who contributed to the data collection for the studies reported in this Commission. We acknowledge the support provided by the funding agencies joined in the InTBIR initiative: the Canadian Institutes of Health Research, the European Commission, the US National Institutes of Health-National Institute of Neurological Disorders and Stroke, One Mind, and the US Department of Defense. The Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) project received additional support from NeuroTrauma Sciences, USA; the Hannelore Kohl Stiftung, Germany; and Integra Life Sciences, USA. Synergistic funding for the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study was obtained through the TBI Endpoints Development award from the US Department of Defense. Additionally, DKM was supported through a Senior Investigator Award and funding for the Cambridge Biomedical Research Centre from the UK National Institute for Health and Care Research.
Footnotes
Declaration of interests
No funding was provided specifically for this Commission paper; however, most authors are involved in the International Initiative for Traumatic Brain Injury Research (InTBIR) as a scientific participant or an investigator. This Commission would not have been possible without the indirect facilitation provided by the InTBIR network. AIRM declares consulting fees from PresSura Neuro, Integra Life Sciences, and NeuroTrauma Sciences. DKM reports research support, and educational and consulting fees from Lantmannen AB, GlaxoSmithKline, Calico, PresSura Neuro, NeuroTrauma Sciences, and Integra Neurosciences. GTM declares grants from the US National Institutes of Health-National Institute of Neurological Disorders and Stroke (grant U01NS086090), the US Department of Defense (grant W81XWH-14-2-0176, grant W81XWH-18-2-0042, and contract W81XWH-15-9-0001). MC reports licensing fees for ICM+ software from Cambridge Enterprise and was an honorary (unpaid) director for Medicam. PS reports licensing fees for ICM+ software from Cambridge Enterprise. MBS has in the past 3 years received consulting income from Acadia Pharmaceuticals, Aptinyx, atai Life Sciences, Boehringer Ingelheim, Bionomics, BioXcel Therapeutics, Clexio, Eisai, EmpowerPharm, Engrail Therapeutics, Janssen, Jazz Pharmaceuticals, and Roche/Genentech. MBS also has stock options in Oxeia Biopharmaceuticals and EpiVario and is paid for editorial work on Depression and Anxiety (Editor-in-Chief), Biological Psychiatry (Deputy Editor), and UpToDate (Co-Editor-in-Chief for Psychiatry). KKWW holds stock options in Gryphon Bio. All other authors declare no competing interests.
Contributor Information
Andrew I R Maas, Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium.
David K Menon, Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Geoffrey T Manley, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Mathew Abrams, International Neuroinformatics Coordinating Facility, Karolinska Institutet, Stockholm, Sweden.
Cecilia Åkerlund, Department of Physiology and Pharmacology, Section of Perioperative Medicine and Intensive Care, Karolinska Institutet, Stockholm, Sweden.
Nada Andelic, Division of Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway.
Marcel Aries, Department of Intensive Care, Maastricht UMC, Maastricht, Netherlands.
Tom Bashford, Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Michael J Bell, Critical Care Medicine, Neurological Surgery and Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Yelena G Bodien, Department of Neurology and Department of Physical Medicine and Rehabilitation, Harvard Medical School, Boston, MA, USA.
Benjamin L Brett, Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA.
András Büki, Department of Neurosurgery, Faculty of Medicine and Health Örebro University, Örebro, Sweden; Department of Neurosurgery, Medical School; ELKH-PTE Clinical Neuroscience MR Research Group; and Neurotrauma Research Group, Janos Szentagothai Research Centre, University of Pecs, Pecs, Hungary.
Randall M Chesnut, Department of Neurological Surgery and Department of Orthopaedics and Sports Medicine, University of Washington, Harborview Medical Center, Seattle, WA, USA.
Giuseppe Citerio, School of Medicine and Surgery, Universita Milano Bicocca, Milan, Italy; NeuroIntensive Care, San Gerardo Hospital, Azienda Socio Sanitaria Territoriale (ASST) Monza, Monza, Italy.
David Clark, Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Betony Clasby, Department of Sociological Studies, University of Sheffield, Sheffield, UK.
D Jamie Cooper, School of Public Health and Preventive Medicine, Monash University and The Alfred Hospital, Melbourne, VIC, Australia.
Endre Czeiter, Department of Neurosurgery, Medical School; ELKH-PTE Clinical Neuroscience MR Research Group; and Neurotrauma Research Group, Janos Szentagothai Research Centre, University of Pecs, Pecs, Hungary.
Marek Czosnyka, Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Kristen Dams-O’Connor, Department of Rehabilitation and Human Performance and Department of Neurology, Brain Injury Research Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Véronique De Keyser, Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium.
Ramon Diaz-Arrastia, Department of Neurology and Center for Brain Injury and Repair, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Ari Ercole, Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Thomas A van Essen, Department of Neurosurgery, Leiden University Medical Center, Leiden, Netherlands; Department of Neurosurgery, Medical Center Haaglanden, The Hague, Netherlands.
Éanna Falvey, College of Medicine and Health, University College Cork, Cork, Ireland.
Adam R Ferguson, Brain and Spinal Injury Center, Department of Neurological Surgery, Weill Institute for Neurosciences, University of California San Francisco and San Francisco Veterans Affairs Healthcare System, San Francisco, CA, USA.
Anthony Figaji, Division of Neurosurgery and Neuroscience Institute, University of Cape Town, Cape Town, South Africa.
Melinda Fitzgerald, Curtin Health Innovation Research Institute, Curtin University, Bentley, WA, Australia; Perron Institute for Neurological and Translational Sciences, Nedlands, WA, Australia.
Brandon Foreman, Department of Neurology and Rehabilitation Medicine, University of Cincinnati Gardner Neuroscience Institute, University of Cincinnati, Cincinnati, OH, USA.
Dashiell Gantner, School of Public Health and Preventive Medicine, Monash University and The Alfred Hospital, Melbourne, VIC, Australia.
Guoyi Gao, Department of Neurosurgery, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine.
Joseph Giacino, Department of Physical Medicine and Rehabilitation, Harvard Medical School and Spaulding Rehabilitation Hospital, Charlestown, MA, USA.
Benjamin Gravesteijn, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Fabian Guiza, Department and Laboratory of Intensive Care Medicine, University Hospitals Leuven and KU Leuven, Leuven, Belgium.
Deepak Gupta, Department of Neurosurgery, Neurosciences Centre and JPN Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, India.
Mark Gurnell, Metabolic Research Laboratories, Institute of Metabolic Science, University of Cambridge, Cambridge, UK.
Juanita A Haagsma, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Flora M Hammond, Department of Physical Medicine and Rehabilitation, Indiana University School of Medicine, Rehabilitation Hospital of Indiana, Indianapolis, IN, USA.
Gregory Hawryluk, Section of Neurosurgery, GB1, Health Sciences Centre, University of Manitoba, Winnipeg, MB, Canada.
Peter Hutchinson, Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Mathieu van der Jagt, Department of Intensive Care, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Sonia Jain, Biostatistics Research Center, Herbert Wertheim School of Public Health, University of California, San Diego, CA, USA.
Swati Jain, Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Ji-yao Jiang, Department of Neurosurgery, Shanghai Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Hope Kent, Department of Psychology, University of Exeter, Exeter, UK.
Angelos Kolias, Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Erwin J O Kompanje, Department of Intensive Care, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Fiona Lecky, Centre for Urgent and Emergency Care Research, Health Services Research Section, School of Health and Related Research, University of Sheffield, Sheffield, UK.
Hester F Lingsma, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Marc Maegele, Cologne-Merheim Medical Center, Department of Trauma and Orthopedic Surgery, Witten/Herdecke University, Cologne, Germany.
Marek Majdan, Institute for Global Health and Epidemiology, Department of Public Health, Faculty of Health Sciences and Social Work, Trnava University, Trnava, Slovakia.
Amy Markowitz, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Michael McCrea, Department of Neurosurgery and Neurology, Medical College of Wisconsin, Milwaukee, WI, USA.
Geert Meyfroidt, Department and Laboratory of Intensive Care Medicine, University Hospitals Leuven and KU Leuven, Leuven, Belgium.
Ana Mikolić, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Stefania Mondello, Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, Messina, Italy.
Pratik Mukherjee, Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, USA.
David Nelson, Section for Anesthesiology and Intensive Care, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden.
Lindsay D Nelson, Department of Neurosurgery and Neurology, Medical College of Wisconsin, Milwaukee, WI, USA.
Virginia Newcombe, Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
David Okonkwo, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA, USA.
Matej Orešič, School of Medical Sciences, Örebro University, Örebro, Sweden.
Wilco Peul, Department of Neurosurgery, Leiden University Medical Center, Leiden, Netherlands.
Dana Pisică, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands; Department of Neurosurgery, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Suzanne Polinder, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Jennie Ponsford, Monash-Epworth Rehabilitation Research Centre, Turner Institute for Brain and Mental Health, School of Psychological Sciences, Monash University, Melbourne, VIC, Australia.
Louis Puybasset, Department of Anesthesiology and Intensive Care, APHP, Sorbonne Université, Hôpital Pitié-Salpêtrière, Paris, France.
Rahul Raj, Department of Neurosurgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Chiara Robba, Department of Anaesthesia and Intensive Care, Policlinico San Martino IRCCS for Oncology and Neuroscience, Genova, Italy, and Dipartimento di Scienze Chirurgiche e Diagnostiche, University of Genoa, Italy.
Cecilie Røe, Division of Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway.
Jonathan Rosand, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA.
Peter Schueler, ICON, Drug Development Neurosciences, Langen, Germany.
David J Sharp, Department of Brain Sciences, Imperial College London, London, UK.
Peter Smielewski, Brain Physics Lab, Division of Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Murray B Stein, Department of Psychiatry and Department of Family Medicine and Public Health, UCSD School of Medicine, La Jolla, CA, USA.
Nicole von Steinbüchel, Institute of Medical Psychology and Medical Sociology, University Medical Center Goettingen, Goettingen, Germany.
William Stewart, Department of Neuropathology, Queen Elizabeth University Hospital and University of Glasgow, Glasgow, UK.
Ewout W Steyerberg, Department of Biomedical Data Sciences Leiden University Medical Center, Leiden, Netherlands.
Nino Stocchetti, Department of Pathophysiology and Transplantation, Milan University, and Neuroscience ICU, Fondazione IRCCS Ca Granda Ospedale Maggiore Policlinico, Milan, Italy.
Nancy Temkin, Departments of Neurological Surgery, and Biostatistics, University of Washington, Seattle, WA, USA.
Olli Tenovuo, Department of Rehabilitation and Brain Trauma, Turku University Hospital, and Department of Neurology, University of Turku, Turku, Finland.
Alice Theadom, National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, Auckland University of Technology, Auckland, New Zealand.
Ilias Thomas, School of Medical Sciences, Örebro University, Örebro, Sweden.
Abel Torres Espin, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Alexis F Turgeon, Department of Anesthesiology and Critical Care Medicine, Division of Critical Care Medicine, Université Laval, CHU de Québec-Université Laval Research Center, Québec City, QC, Canada.
Andreas Unterberg, Department of Neurosurgery, Heidelberg University Hospital, Heidelberg, Germany.
Dominique Van Praag, Departments of Clinical Psychology and Neurosurgery, Antwerp University Hospital, and University of Antwerp, Edegem, Belgium.
Ernest van Veen, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
Jan Verheyden, Icometrix, Leuven, Belgium.
Thijs Vande Vyvere, Department of Radiology, Faculty of Medicine and Health Sciences, Department of Rehabilitation Sciences (MOVANT), Antwerp University Hospital, and University of Antwerp, Edegem, Belgium.
Kevin K W Wang, Department of Psychiatry, University of Florida, Gainesville, FL, USA.
Eveline J A Wiegers, Department of Public Health, Erasmus MC University Medical Center Rotterdam, Rotterdam, Netherlands.
W Huw Williams, Centre for Clinical Neuropsychology Research, Department of Psychology, University of Exeter, Exeter, UK.
Lindsay Wilson, Division of Psychology, University of Stirling, Stirling, UK.
Stephen R Wisniewski, University of Pittsburgh Graduate School of Public Health, Pittsburgh, Pennsylvania, USA.
Alexander Younsi, Department of Neurosurgery, Heidelberg University Hospital, Heidelberg, Germany.
John K Yue, Department of Neurological Surgery, University of California, San Francisco, CA, USA.
Esther L Yuh, Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, USA.
Frederick A Zeiler, Departments of Surgery, Human Anatomy and Cell Science, and Biomedical Engineering, Rady Faculty of Health Sciences and Price Faculty of Engineering, University of Manitoba, Winnipeg, MB, Canada.
Marina Zeldovich, Institute of Medical Psychology and Medical Sociology, University Medical Center Goettingen, Goettingen, Germany.
Roger Zemek, Departments of Pediatrics and Emergency Medicine, University of Ottawa, Children’s Hospital of Eastern Ontario, ON, Canada.
References
- 1.Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017; 16: 987–1048. [DOI] [PubMed] [Google Scholar]
- 2.Menon DK, Bryant C. Time for change in acquired brain injury. Lancet Neurol 2019; 18: 28. [DOI] [PubMed] [Google Scholar]
- 3.Majdan M, Plancikova D, Brazinova A, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health 2016; 1: e76–83. [DOI] [PubMed] [Google Scholar]
- 4.Bell MJ, Kochanek PM. International traumatic brain injury research: an annus mirabilis? Lancet Neurol 2019; 18: 904–05. [DOI] [PubMed] [Google Scholar]
- 5.Tosetti P, Hicks RR, Theriault E, Phillips A, Koroshetz W, Draghia-Akli R. Toward an international initiative for traumatic brain injury research. J Neurotrauma 2013; 30: 1211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James SL, Theadom A, Ellenbogen RG, et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H-K, Leigh J-H, Lee YS, et al. Decreasing incidence and mortality in traumatic brain injury in Korea, 2008–2017: a population-based longitudinal study. Int J Environ Res Public Health 2020; 17: 6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ 2017; 66: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer L, Levy C, Bayley M. Increasing incidence of concussion: true epidemic or better recognition? J Head Trauma Rehabil 2020; 35: E60–66. [DOI] [PubMed] [Google Scholar]
- 10.Lester A, Leach P, Zaben M. The impact of the COVID-19 pandemic on traumatic brain injury management: lessons learned over the first year. World Neurosurg 2021; 156: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reitzle L, Schmidt C, Färber F, et al. Perceived access to health care services and relevance of telemedicine during the COVID-19 pandemic in Germany. Int J Environ Res Public Health 2021; 18: 7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa JM, Boddu J, Kader M, et al. The effects of lockdown during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic on neurotrauma-related hospital admissions. World Neurosurg 2021; 146: e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lãzãrescu AM, Benichi S, Blauwblomme T, et al. Abusive head trauma in infants during the COVID-19 pandemic in the Paris metropolitan area. JAMA Netw Open 2022; 5: e2226182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viero A, Barbara G, Montisci M, Kustermann K, Cattaneo C. Violence against women in the COVID-19 pandemic: a review of the literature and a call for shared strategies to tackle health and social emergencies. Forensic Sci Int 2021; 319: 110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brink J, Cullen P, Beek K, Peters SAE. Intimate partner violence during the COVID-19 pandemic in Western and Southern European countries. Eur J Public Health 2021; 31: 1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global Health Data Exchange. https://ghdx.healthdata.org/gbd-results-tool (accessed Aug 9, 2022).
- 17.Dewan MC, Rattani A, Gupta S, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg 2018; 130: 1–18. [DOI] [PubMed] [Google Scholar]
- 18.Clark D, Joannides A, Adeleye AO, et al. Casemix, management, and mortality of patients receiving emergency neurosurgery for traumatic brain injury in the Global Neurotrauma Outcomes Study: a prospective observational cohort study. Lancet Neurol 2022; 21: 438–49. [DOI] [PubMed] [Google Scholar]
- 19.Lecky FE, Otesile O, Marincowitz C, et al. The burden of traumatic brain injury from low-energy falls among patients from 18 countries in the CENTER-TBI registry: a comparative cohort study. PLoS Med 2021; 18: e1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steyerberg EW, Wiegers E, Sewalt C, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol 2019; 18: 923–34. [DOI] [PubMed] [Google Scholar]
- 21.Stokes KA, Locke D, Roberts S, et al. Does reducing the height of the tackle through law change in elite men’s rugby union (the championship, England) reduce the incidence of concussion? A controlled study in 126 games. Br J Sports Med 2021; 55: 220–25. [DOI] [PubMed] [Google Scholar]
- 22.Tierney G, Weaving D, Tooby J, et al. Quantifying head acceleration exposure via instrumented mouthguards (iMG): a validity and feasibility study protocol to inform iMG suitability for the TaCKLE project. BMJ Open Sport Exerc Med 2021; 7: e001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black AM, Hagel BE, Palacios-Derflingher L, Schneider KJ, Emery CA. The risk of injury associated with body checking among Pee Wee ice hockey players: an evaluation of Hockey Canada’s national body checking policy change. Br J Sports Med 2017; 51: 1767–72. [DOI] [PubMed] [Google Scholar]
- 24.Majdan M, Plancikova D, Maas A, et al. Years of life lost due to traumatic brain injury in Europe: a cross-sectional analysis of 16 countries. PLoS Med 2017; 14: e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Te Ao B, Tobias M, Ameratunga S, et al. Burden of traumatic brain injury in New Zealand: incidence, prevalence and disability-adjusted life years. Neuroepidemiology 2015; 44: 255–61. [DOI] [PubMed] [Google Scholar]
- 26.Dams-O’Connor K, Cuthbert JP, Whyte J, Corrigan JD, Faul M, Harrison-Felix C. Traumatic brain injury among older adults at level I and II trauma centers. J Neurotrauma 2013; 30: 2001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner RC, Dams-O’Connor K, Morrissey MR, Manley GT. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma 2018; 35: 889–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dams-O’Connor K, Ketchum JM, Cuthbert JP, et al. Functional outcome trajectories following inpatient rehabilitation for TBI in the United States: a NIDILRR TBIMS and CDC interagency collaboration. J Head Trauma Rehabil 2020; 35: 127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senathi-Raja D, Ponsford J, Schönberger M. The association of age and time postinjury with long-term emotional outcome following traumatic brain injury. J Head Trauma Rehabil 2010; 25: 330–38. [DOI] [PubMed] [Google Scholar]
- 30.Waltzman D, Haarbauer-Krupa J, Womack LS. Traumatic brain injury in older adults—a public health perspective. JAMA Neurol 2022; 79: 437–38; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C, Lang L, He Z, et al. Epidemiological characteristics of older patients with traumatic brain injury in China. J Neurotrauma 2022; 39: 850–59. [DOI] [PubMed] [Google Scholar]
- 32.Kehoe A, Smith JE, Bouamra O, Edwards A, Yates D, Lecky F. Older patients with traumatic brain injury present with a higher GCS score than younger patients for a given severity of injury. Emerg Med J 2016; 33: 381–85. [DOI] [PubMed] [Google Scholar]
- 33.Galimberti S, Graziano F, Maas AIR, et al. Effect of frailty on 6-month outcome after traumatic brain injury: a multicenter cohort study with external validation. Lancet Neurol 2022; 21: 153–62. [DOI] [PubMed] [Google Scholar]
- 34.Majdan M, Melichova J, Plancikova D, et al. Burden of traumatic brain injuries in children and adolescents in Europe: hospital discharges, deaths and years of life lost. Children 2022; 9: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Ierssel J, Osmond M, Hamid J, Sampson M, Zemek R. What is the risk of recurrent concussion in children and adolescents aged 5–18 years? A systematic review and meta-analysis. Br J Sports Med 2021; 55: 663–69. [DOI] [PubMed] [Google Scholar]
- 36.Ledoux A-A, Webster RJ, Clarke AE, et al. Risk of mental health problems in children and youths following concussion. JAMA Netw Open 2022; 5: e221235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao G, Wu X, Feng J, et al. Clinical characteristics and outcomes in patients with traumatic brain injury in China: a prospective, multicentre, longitudinal, observational study. Lancet Neurol 2020; 19: 670–77. [DOI] [PubMed] [Google Scholar]
- 38.Esterman A, Thompson F, Fitts M, et al. Incidence of emergency department presentations for traumatic brain injury in Indigenous and non-Indigenous residents aged 15–64 over the 9-year period 2007–2015 in North Queensland, Australia. Inj Epidemiol 2018; 5: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salaheen Z, Moghaddamjou A, Fehlings M. Neurotrauma in indigenous populations of Canada—challenges and opportunities at a global level: a scoping review. World Neurosurg 2022; published online Aug 1: S1878-8750(22)01059-2. 10.1016/j.wneu.2022.07.108. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell T, Theadom A, du Preez E. Prevalence of traumatic brain injury in a male adult prison population and its association with the offence type. Neuroepidemiology 2017; 48: 164–70. [DOI] [PubMed] [Google Scholar]
- 41.McMillan TM, Aslam H, Crowe E, Seddon E, Barry SJE. Associations between significant head injury and persisting disability and violent crime in women in prison in Scotland, UK: a cross-sectional study. Lancet Psychiatry 2021; 8: 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabbe BJ, Braaf S, Cameron PA, Berecki-Gisolf J. Epidemiology and 6- and 12-month outcomes of intimate partner violence and other violence-related traumatic brain injury in major trauma: a population-based trauma registry study. J Head Trauma Rehabil 2022; 37: E1–9. [DOI] [PubMed] [Google Scholar]
- 43.Williams WH, Chitsabesan P, Fazel S, et al. Traumatic brain injury: a potential cause of violent crime? Lancet Psychiatry 2018; 5: 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.UN Committee on the Rights of the Child. Children’s rights in juvenile justice. General comment no 24 (2019), replacing general comment no 10 (2007). https://www.ohchr.org/Documents/HRBodies/CRC/GC24/GeneralComment24.pdf (accessed Aug 9, 2022).
- 45.UK Sentencing Council. Sentencing offenders with mental disorders, developmental disorders, or neurological impairments, 2020. https://www.sentencingcouncil.org.uk/overarching-guides/magistrates-court/item/sentencing-offenders-with-mental-disorders-developmental-disorders-or-neurological-impairments/ (accessed Aug 9, 2022). [DOI] [PubMed]
- 46.Wilson L, Stewart W, Dams-O’Connor K, et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 2017; 16: 813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walton SR, Kerr ZY, Mannix R, et al. Subjective concerns regarding the effects of sport-related concussion on long-term brain health among former NFL players: an NFL-LONG study. Sports Med 2022; 52: 1189–203. [DOI] [PubMed] [Google Scholar]
- 48.Walton SR, Brett BL, Chandran A, et al. Mild cognitive impairment and dementia reported by former professional football players over 50 yr of age: an NFL-LONG study. Med Sci Sports Exerc 2022; 54: 424–31. [DOI] [PubMed] [Google Scholar]
- 49.Mackay DF, Russell ER, Stewart K, MacLean JA, Pell JP, Stewart W. Neurodegenerative disease mortality among former professional soccer players. N Engl J Med 2019; 381: 1801–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theadom A, Mahon S, Hume P, et al. Incidence of sports-related traumatic brain injury of all severities: a systematic review. Neuroepidemiology 2020; 54: 192–99. [DOI] [PubMed] [Google Scholar]
- 51.Van Pelt KL, Puetz T, Swallow J, Lapointe AP, Broglio SP. Data-driven risk classification of concussion rates: a systematic review and meta-analysis. Sports Med 2021; 51: 1227–44. [DOI] [PubMed] [Google Scholar]
- 52.Theadom A, Starkey NJ, Dowell T, et al. Sports-related brain injury in the general population: an epidemiological study. J Sci Med Sport 2014; 17: 591–96. [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Comstock RD, Yi H, Harvey HH, Xun P. New and recurrent concussions in high-school athletes before and after traumatic brain injury laws, 2005–2016. Am J Public Health 2017; 107: 1916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCrea M, Broglio S, McAllister T, et al. Return to play and risk of repeat concussion in collegiate football players: comparative analysis from the NCAA Concussion Study (1999–2001) and CARE Consortium (2014–2017). Br J Sports Med 2020; 54: 102–09. [DOI] [PubMed] [Google Scholar]
- 55.McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 2017; 51: 838–47. [DOI] [PubMed] [Google Scholar]
- 56.Tucker R, Raftery M, Kemp S, et al. Risk factors for head injury events in professional rugby union: a video analysis of 464 head injury events to inform proposed injury prevention strategies. Br J Sports Med 2017; 51: 1152–57. [DOI] [PubMed] [Google Scholar]
- 57.Raftery M, Tucker R, Falvey ÉC. Getting tough on concussion: how welfare-driven law change may improve player safety—a rugby union experience. Br J Sports Med 2020; 55: 527–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCrea MA, Shah A, Duma S, et al. Opportunities for prevention of concussion and repetitive head impact exposure in college football players: a Concussion Assessment, Research, and Education (CARE) consortium study. JAMA Neurol 2021; 78: 346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mack CD, Solomon G, Covassin T, et al. Epidemiology of concussion in the National Football League, 2015–2019. Sport Heal A Multidiscip Approach 2021; 13: 423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dodds N, Johnson R, Walton B, et al. Evaluating the impact of cycle helmet use on severe traumatic brain injury and death in a national cohort of over 11000 pedal cyclists: a retrospective study from the NHS England Trauma Audit and Research Network dataset. BMJ Open 2019; 9: e027845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker CE, Martin P, Wilson MH, Ghajari M, Sharp DJ. The relationship between road traffic collision dynamics and traumatic brain injury pathology. Brain Commun 2022; 4: fcac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gravesteijn BY, Sewalt CA, Stocchetti N, et al. Prehospital management of traumatic brain injury across Europe: a CENTER-TBI Study. Prehosp Emerg Care 2021; 25: 629–43. [DOI] [PubMed] [Google Scholar]
- 63.McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007; 24: 287–93. [DOI] [PubMed] [Google Scholar]
- 64.Sewalt CA, Gravesteijn BY, Menon D, et al. Primary versus early secondary referral to a specialized neurotrauma center in patients with moderate/severe traumatic brain injury: a CENTER TBI study. Scand J Trauma Resusc Emerg Med 2021; 29: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006; 60: 290–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gianola S, Castellini G, Biffi A, et al. Accuracy of pre-hospital triage tools for major trauma: a systematic review with meta-analysis and net clinical benefit. World J Emerg Surg 2021; 16: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhaumik S, Hannun M, Dymond C, et al. Prehospital triage tools across the world: a scoping review of the published literature. Scand J Trauma Resusc Emerg Med 2022; 30: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.American College of Surgeons Committee on Trauma. Resources for optimal care of the injured patient 2014. Chicago: The Committee, 2014. https://www.facs.org/media/yu0laoqz/resources-for-optimal-care.pdf (accessed Aug 9, 2022).
- 69.Fuller G, Pandor A, Essat M, et al. Diagnostic accuracy of prehospital triage tools for identifying major trauma in elderly injured patients: a systematic review. J Trauma Acute Care Surg 2021; 90: 403–12. [DOI] [PubMed] [Google Scholar]
- 70.Lecky FE, Russell W, McClelland G, et al. Bypassing nearest hospital for more distant neuroscience care in head-injured adults with suspected traumatic brain injury: findings of the head injury transportation straight to neurosurgery (HITS-NS) pilot cluster randomised trial. BMJ Open 2017; 7: e016355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henry S ATLS Advanced Trauma Life Support 10th edn student course manual. ACS American College of Surgeons, 2018. https://www.academia.edu/39781997/Student_Course_Manual_ATLS_Advanced_Trauma_Life_Support (accessed Aug 9, 2022). [Google Scholar]
- 72.Moran CG, Lecky F, Bouamra O, et al. Changing the system—major trauma patients and their outcomes in the NHS (England) 2008–17. EClinicalMedicine 2018; 2–3: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury, 2nd edn. Prehospital Emerg care 2008; 12 (suppl 1): S1–52. [DOI] [PubMed] [Google Scholar]
- 74.Gravesteijn BY, Sewalt CA, Nieboer D, et al. Tracheal intubation in traumatic brain injury: a multicentre prospective observational study. Br J Anaesth 2020; 125: 505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.CRASH-2 collaborators, Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011; 377: 1096–101. [DOI] [PubMed] [Google Scholar]
- 76.CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet 2019; 394: 1713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rowell SE, Meier EN, McKnight B, et al. Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA 2020; 324: 961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT head rule for patients with minor head injury. Lancet 2001; 357: 1391–96. [DOI] [PubMed] [Google Scholar]
- 79.Smits M, Dippel DWJ, Steyerberg EW, et al. Predicting intracranial traumatic findings on computed tomography in patients with minor head injury: the CHIP prediction rule. Ann Intern Med 2007; 146: 397–405. [DOI] [PubMed] [Google Scholar]
- 80.Smits M, Dippel DWJ, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA 2005; 294: 1519–25. [DOI] [PubMed] [Google Scholar]
- 81.Undén J, Ingebrigtsen T, Romner B. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med 2013; 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calcagnile O, Anell A, Undén J. The addition of S100B to guidelines for management of mild head injury is potentially cost saving. BMC Neurol 2016; 16: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mondello S, Sorinola A, Czeiter E, et al. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: a living systematic review and meta-analysis. J Neurotrauma 2021; 38: 1086–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bazarian JJ, Biberthaler P, Welch RD, et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol 2018; 17: 782–89. [DOI] [PubMed] [Google Scholar]
- 85.US Food Drug Administration. FDA authorizes marketing of first blood test to aid in the evaluation of concussion in adults. FDA news release, Feb 13, 2018. https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-blood-test-aid-evaluation-concussion-adults (accessed Aug 9, 2022).
- 86.Abbott Diagnostics. Brain injury test achieves CE mark. Labmate Online, 2021. https://www.labmate-online.com/news/laboratory-products/3/abbott-diagnostics/brain-injury-test-achieves-ce-mark/56875 (accessed Aug 9, 2022).
- 87.Okonkwo DO, Puffer RC, Puccio AM, et al. Point-of-care platform blood biomarker testing of glial fibrillary acidic protein versus s100 calcium-binding protein B for prediction of traumatic brain injuries: a transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma 2020; 37: 2460–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papa L, Ladde JG, O’Brien JF, et al. Evaluation of glial and neuronal blood biomarkers compared with clinical decision rules in assessing the need for computed tomography in patients with mild traumatic brain injury. JAMA Netw Open 2022; 5: e221302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dijkers MP, Marwitz JH, Harrison-Felix C. Thirty years of national institute on disability, independent living, and rehabilitation research traumatic brain injury model systems center research—an update. J Head Trauma Rehabil 2018; 33: 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ponsford J, Harrison-Felix C, Ketchum JM, Spitz G, Miller AC, Corrigan JD. Outcomes 1 and 2 years after moderate to severe traumatic brain injury: an international comparative study. Arch Phys Med Rehabil 2021; 102: 371–77. [DOI] [PubMed] [Google Scholar]
- 91.Andelic N, Bautz-Holter E, Ronning P, et al. Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J Neurotrauma 2012; 29: 66–74. [DOI] [PubMed] [Google Scholar]
- 92.Tverdal CB, Howe EI, Røe C, et al. Traumatic brain injury: patient experience and satisfaction with discharge from trauma hospital. J Rehabil Med 2018; 50: 505–13. [DOI] [PubMed] [Google Scholar]
- 93.Abrahamson V, Jensen J, Springett K, Sakel M. Experiences of patients with traumatic brain injury and their carers during transition from in-patient rehabilitation to the community: a qualitative study. Disabil Rehabil 2017; 39: 1683–94. [DOI] [PubMed] [Google Scholar]
- 94.Andelic N, Røe C, Tenovuo O, et al. Unmet rehabilitation needs after traumatic brain injury across europe: results from the CENTER-TBI Study. J Clin Med 2021; 10: 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Vlegel M, Polinder S, Mikolic A, et al. The association of post-concussion and post-traumatic stress disorder symptoms with health-related quality of life, health care use and return-to-work after mild traumatic brain injury. J Clin Med 2021; 10: 2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foks KA, Cnossen MC, Dippel DWJ, et al. Management of mild traumatic brain injury at the emergency department and hospital admission in Europe: a survey of 71 neurotrauma centers participating in the CENTER-TBI Study. J Neurotrauma 2017; 34: 2529–35. [DOI] [PubMed] [Google Scholar]
- 97.Seabury SA, Gaudette É, Goldman DP, et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: results from the TRACK-TBI study. JAMA Netw Open 2018; 1: e180210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med 2019; 53: 213–25. [DOI] [PubMed] [Google Scholar]
- 99.Patricios JS, Ardern CL, Hislop MD, et al. Implementation of the 2017 Berlin Concussion in Sport Group Consensus Statement in contact and collision sports: a joint position statement from 11 national and international sports organisations. Br J Sports Med 2018; 52: 635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harmon KG, Whelan BM, Aukerman DF, et al. Diagnostic accuracy and reliability of sideline concussion evaluation: a prospective, case-controlled study in college athletes comparing newer tools and established tests. Br J Sports Med 2022; 56: 144–50. [DOI] [PubMed] [Google Scholar]
- 101.Davis GA, Makdissi M, Bloomfield P, et al. Concussion guidelines in national and international professional and elite sports. Neurosurgery 2020; 87: 418–25. [DOI] [PubMed] [Google Scholar]
- 102.Ellenbogen RG, Batjer H, Cardenas J, et al. National Football League head, neck and spine committee’s concussion diagnosis and management protocol: 2017–18 season. Br J Sports Med 2018; 52: 894–902. [DOI] [PubMed] [Google Scholar]
- 103.Garcia GP, Broglio SP, Lavieri MS, McCrea M, McAllister T. Quantifying the value of multidimensional assessment models for acute concussion: an analysis of data from the NCAA-DoD care consortium. Sports Med 2018; 48: 1739–49. [DOI] [PubMed] [Google Scholar]
- 104.Garcia GP, Yang J, Lavieri MS, McAllister TW, McCrea MA, Broglio SP. Optimizing components of the sport concussion assessment tool for acute concussion assessment. Neurosurgery 2020; 87: 971–81. [DOI] [PubMed] [Google Scholar]
- 105.Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edn (SCAT5): background and rationale. Br J Sports Med 2017; 51: 848–50. [DOI] [PubMed] [Google Scholar]
- 106.Raftery M, Falvey ÉC. Rugby’s implementation lessons: the importance of a ‘compliance wedge’ to support successful implementation for injury prevention. Br J Sports Med 2022; 56: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yeo PC, Yeo EQY, Probert J, Sim SHS, Sirisena D. A systematic review and qualitative analysis of concussion knowledge amongst sports coaches and match officials. J Sports Sci Med 2020; 19: 65–77. [PMC free article] [PubMed] [Google Scholar]
- 108.Gouttebarge V, Goedhart EA, Orhant E, Patricios J. Avoiding a red card: recommendations for a consistent standard of concussion management in professional football (soccer). Br J Sports Med 2022; 56: 308–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pfeifer R, Teuben M, Andruszkow H, Barkatali BM, Pape HC. Mortality patterns in patients with multiple trauma: a systematic review of autopsy studies. PLoS One 2016; 11: e0148844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.GBD 2013 DALYs and HALE Collaborators, Murray CJL, Barber RM, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015; 386: 2145–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clark D, Joannides A, Ibrahim Abdallah O, et al. Global Neurotrauma Outcomes Study (GNOS) collaborative. Management and outcomes following emergency surgery for traumatic brain injury—a multi-centre, international, prospective cohort study (the Global Neurotrauma Outcomes Study). Int J Surg Protoc 2020; 20: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bashford T, Clarkson PJ, Menon DK, Hutchinson PJA. Unpicking the Gordian knot: a systems approach to traumatic brain injury care in low-income and middle-income countries. BMJ Glob Health 2018; 3: e000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bashford T, Joannides A, Phuyal K, et al. Nuancing the need for speed: temporal health system strengthening in low-income countries. BMJ Glob Health 2019; 4: e001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Corley J, Barthélemy EJ, Lepard J, et al. Comprehensive policy recommendations for head and spine injury care in low- and middle-income countries. World Neurosurg 2019; 132: 434–36. [DOI] [PubMed] [Google Scholar]
- 116.Rubiano AM, Vera DS, Montenegro JH, et al. Recommendations of the Colombian consensus committee for the management of traumatic brain injury in prehospital, emergency department, surgery, and intensive care (beyond one option for treatment of traumatic brain injury: a stratified protocol [BOOTStraP]). J Neurosci Rural Pract 2020; 11: 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chesnut RM, Temkin N, Videtta W, et al. Consensus-based management protocol (CREVICE protocol) for the treatment of severe traumatic brain injury based on imaging and clinical examination for use when intracranial pressure monitoring is not employed. J Neurotrauma 2020; 37: 1291–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mushkudiani NA, Engel DC, Steyerberg EW, et al. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007; 24: 259–69. [DOI] [PubMed] [Google Scholar]
- 119.Mathieu F, Güting H, Gravesteijn B, et al. Impact of antithrombotic agents on radiological lesion progression in acute traumatic brain injury: a CENTER-TBI propensity-matched cohort analysis. J Neurotrauma 2020; 37: 2069–80. [DOI] [PubMed] [Google Scholar]
- 120.Böhm JK, Güting H, Thorn S, et al. Global characterisation of coagulopathy in isolated traumatic brain injury (iTBI): a CENTER-TBI analysis. Neurocrit Care 2021; 35: 184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huijben JA, Wiegers EJA, Lingsma HF, et al. Changing care pathways and between-center practice variations in intensive care for traumatic brain injury across Europe: a CENTER-TBI analysis. Intensive Care Med 2020; 46: 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonow RH, Quistberg A, Rivara FP, Vavilala MS. Intensive care unit admission patterns for mild traumatic brain injury in the USA. Neurocrit Care 2019; 30: 157–70. [DOI] [PubMed] [Google Scholar]
- 123.Robba C, Banzato E, Rebora P, et al. Acute kidney injury in traumatic brain injury patients: results from the collaborative European neurotrauma effectiveness research in traumatic brain injury study. Crit Care Med 2021; 49: 112–26. [DOI] [PubMed] [Google Scholar]
- 124.Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med 2005; 33: 2358–66. [DOI] [PubMed] [Google Scholar]
- 125.Krishnamoorthy V, Manley GT, Jain S, et al. Incidence and clinical impact of myocardial injury following traumatic brain injury: a pilot TRACK-TBI study. J Neurosurg Anesthesiol 2022; 34: 233–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krishnamoorthy V, Temkin N, Barber J, et al. Association of early multiple organ dysfunction with clinical and functional outcomes over the year following traumatic brain injury: a transforming research and clinical knowledge in traumatic brain injury study. Crit Care Med 2021; 49: 1769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Robba C, Bonatti G, Pelosi P, Citerio G. Extracranial complications after traumatic brain injury: targeting the brain and the body. Curr Opin Crit Care 2020; 26: 137–46. [DOI] [PubMed] [Google Scholar]
- 128.McDonald SJ, Sharkey JM, Sun M, et al. Beyond the brain: peripheral interactions after traumatic brain injury. J Neurotrauma 2020; 37: 770–81. [DOI] [PubMed] [Google Scholar]
- 129.Meyfroidt G, Bouzat P, Casaer MP, et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med 2022; 48: 649–66. [DOI] [PubMed] [Google Scholar]
- 130.Hawryluk GWJ, Citerio G, Hutchinson P, et al. Intracranial pressure: current perspectives on physiology and monitoring. Intensive Care Med 2022; published online July 11. 10.1007/s00134-022-06786-y. [DOI] [PubMed] [Google Scholar]
- 131.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 2012; 367: 2471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Robba C, Graziano F, Rebora P, et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): an international, prospective observational cohort study. Lancet Neurol 2021; 20: 548–58. [DOI] [PubMed] [Google Scholar]
- 133.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edn. Neurosurgery 2017; 80: 6–15. [DOI] [PubMed] [Google Scholar]
- 134.Güiza F, Depreitere B, Piper I, et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med 2015; 41: 1067–76. [DOI] [PubMed] [Google Scholar]
- 135.Donnelly J, Güiza F, Depreitere B, Meyfroidt G, Czosnyka M, Smielewski P. Visualising the pressure-time burden of elevated intracranial pressure after severe traumatic brain injury: a retrospective confirmatory study. Br J Anaesth 2021; 126: e15–17. [DOI] [PubMed] [Google Scholar]
- 136.Hawryluk GWJ, Nielson JL, Huie JR, et al. Analysis of normal high-frequency intracranial pressure values and treatment threshold in neurocritical care patients: insights into normal values and a potential treatment threshold. JAMA Neurol 2020; 77: 1150–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Åkerlund CA, Donnelly J, Zeiler FA, et al. Impact of duration and magnitude of raised intracranial pressure on outcome after severe traumatic brain injury: a CENTER-TBI high-resolution group study. PLoS One 2020; 15: e0243427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Santbrink H, Maas AI, Avezaat CJ. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery 1996; 38: 21–31. [DOI] [PubMed] [Google Scholar]
- 139.Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue pO₂ to outcome after severe head injury. Crit Care Med 1998; 26: 1576–81. [DOI] [PubMed] [Google Scholar]
- 140.Chang JJJ, Youn TS, Benson D, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med 2009; 37: 283–90. [DOI] [PubMed] [Google Scholar]
- 141.Hutchinson PJ, Jalloh I, Helmy A, et al. Consensus statement from the 2014 international microdialysis forum. Intensive Care Med 2015; 41: 1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med 2017; 45: 1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeiler FA, Thelin EP, Helmy A, Czosnyka M, Hutchinson PJA, Menon DK. A systematic review of cerebral microdialysis and outcomes in TBI: relationships to patient functional outcome, neurophysiologic measures, and tissue outcome. Acta Neurochir (Wien) 2017; 159: 2245–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Veenith TV, Carter EL, Geeraerts T, et al. Pathophysiologic mechanisms of cerebral ischemia and diffusion hypoxia in traumatic brain injury. JAMA Neurol 2016; 73: 542–50. [DOI] [PubMed] [Google Scholar]
- 145.Launey Y, Fryer TD, Hong YT, et al. Spatial and temporal pattern of ischemia and abnormal vascular function following traumatic brain injury. JAMA Neurol 2020; 77: 339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hermanides J, Hong YT, Trivedi M, et al. Metabolic derangements are associated with impaired glucose delivery following traumatic brain injury. Brain 2021; 144: 3492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chesnut R, Temkin N, Videtta W. Testing the impact of protocolized care of severe traumatic brain injury patients without intracranial pressure monitoring: the ICE protocol. Neurosurgery (in press; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alali AS, Temkin N, Barber J, et al. A clinical decision rule to predict intracranial hypertension in severe traumatic brain injury. J Neurosurg 2018; 131: 612–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Robba C, Donnelly J, Cardim D, et al. Optic nerve sheath diameter ultrasonography at admission as a predictor of intracranial hypertension in traumatic brain injured patients: a prospective observational study. J Neurosurg 2019; 132: 1279–85. [DOI] [PubMed] [Google Scholar]
- 150.Hawryluk GWJ, Ghajar J. Evolution and impact of the Brain Trauma Foundation guidelines. Neurosurgery 2021; 89: 1148–56. [DOI] [PubMed] [Google Scholar]
- 151.Volovici V, Steyerberg EW, Cnossen MC, et al. Evolution of evidence and guideline recommendations for the medical management of severe traumatic brain injury. J Neurotrauma 2019; 36: 3183–89. [DOI] [PubMed] [Google Scholar]
- 152.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery 2006; 58 (suppl 3): S25–46. [DOI] [PubMed] [Google Scholar]
- 153.American College of Neurosurgeons. ACS TQIP: best practices in the management of traumatic brain injury, 2015. https://www.facs.org/media/mkej5u3b/tbi_guidelines.pdf (accessed Aug 6, 2022).
- 154.Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus conference (SIBICC). Intensive Care Med 2019; 45: 1783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 2020; 46: 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huijben JA, Dixit A, Stocchetti N, et al. Use and impact of high intensity treatments in patients with traumatic brain injury across Europe: a CENTER-TBI analysis. Crit Care 2021; 25: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Synnot A, Gruen RL, Menon D, et al. A new approach to evidence synthesis in traumatic brain injury: a living systematic review. J Neurotrauma 2021; 38: 1069–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ziaka M, Exadaktylos A. Brain-lung interactions and mechanical ventilation in patients with isolated brain injury. Crit Care 2021; 25: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen H, Menon DK, Kavanagh BP. Impact of altered airway pressure on intracranial pressure, perfusion, and oxygenation: a narrative review. Crit Care Med 2019; 47: 254–63. [DOI] [PubMed] [Google Scholar]
- 160.Li HP, Lin YN, Cheng ZH, Qu W, Zhang L, Li QY. Intracranial-to-central venous pressure gap predicts the responsiveness of intracranial pressure to PEEP in patients with traumatic brain injury: a prospective cohort study. BMC Neurol 2020; 20: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Robba C, Citerio G, Taccone FS, et al. Multicentre observational study on practice of ventilation in brain injured patients: the VENTIBRAIN study protocol. BMJ Open 2021; 11: e047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Citerio G, Robba C, Rebora P, et al. Management of arterial partial pressure of carbon dioxide in the first week after traumatic brain injury: results from the CENTER-TBI study. Intensive Care Med 2021; 47: 961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Robba C, Rebora P, Banzato E, et al. Incidence, risk factors, and effects on outcome of ventilator-associated pneumonia in patients with traumatic brain injury: analysis of a large, multicenter, prospective, observational longitudinal study. Chest 2020; 158: 2292–303. [DOI] [PubMed] [Google Scholar]
- 164.Robba C, Galimberti S, Graziano F, et al. Tracheostomy practice and timing in traumatic brain-injured patients: a CENTER-TBI study. Intensive Care Med 2020; 46: 983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marra A, Vargas M, Buonanno P, Iacovazzo C, Coviello A, Servillo G. Early vs late tracheostomy in patients with traumatic brain injury: systematic review and meta-analysis. J Clin Med 2021; 10: 3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McCredie VA, Alali AS, Scales DC, et al. Effect of early versus late tracheostomy or prolonged intubation in critically ill patients with acute brain injury: a systematic review and meta-analysis. Neurocrit Care 2017; 26: 14–25. [DOI] [PubMed] [Google Scholar]
- 167.Bösel J, Niesen W-D, Salih F, et al. Effect of early vs standard approach to tracheostomy on functional outcome at 6 months among patients with severe stroke receiving mechanical ventilation: the SETPOINT2 randomized clinical trial. JAMA 2022; 327: 1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wiegers EJA, Lingsma HF, Huijben JA, et al. Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): a prospective, multicentre, comparative-effectiveness study. Lancet Neurol 2021; 20: 627–38. [DOI] [PubMed] [Google Scholar]
- 169.Skrifvars MB, Bailey M, Presneill J, et al. Venous thromboembolic events in critically ill traumatic brain injury patients. Intensive Care Med 2017; 43: 419–28. [DOI] [PubMed] [Google Scholar]
- 170.Huijben JA, Pisica D, Ceyisakar I, et al. Pharmaceutical venous thrombosis prophylaxis in critically ill traumatic brain injury patients. Neurotrauma Reports 2022; 2: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Byrne JP, Witiw CD, Schuster JM, et al. Association of venous thromboembolism prophylaxis after neurosurgical intervention for traumatic brain injury with thromboembolic complications, repeated neurosurgery, and mortality. JAMA Surg 2022; 157: e215794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu VM, Alvi MA, Rovin RA, Kasper EM. Clinical outcomes following early versus late pharmacologic thromboprophylaxis in patients with traumatic intracranial hemorrhage: a systematic review and meta-analysis. Neurosurg Rev 2020; 43: 861–72. [DOI] [PubMed] [Google Scholar]
- 173.Maegele M. Coagulopathy and progression of intracranial hemorrhage in traumatic brain injury: mechanisms, impact, and therapeutic considerations. Neurosurgery 2021; 89: 954–66. [DOI] [PubMed] [Google Scholar]
- 174.Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edn. Crit Care 2019; 23: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.van Essen TA, Lingsma HF, Pisică D, et al. Surgery versus conservative treatment for traumatic acute subdural haematoma: a prospective, multicentre, observational, comparative-effectiveness study. Lancet Neurol 2022; 21: 620–31. [DOI] [PubMed] [Google Scholar]
- 176.Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage (STITCH[Trauma]): the first randomized trial. J Neurotrauma 2015; 32: 1312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gantner D, Wiegers E, Bragge P, et al. Decompressive craniectomy practice following traumatic brain injury in comparison with randomized trials: harmonized, multi-center cohort studies in Europe, the United Kingdom, and Australia. J Neurotrauma 2022; 39: 860–69. [DOI] [PubMed] [Google Scholar]
- 178.Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011; 364: 1493–502. [DOI] [PubMed] [Google Scholar]
- 179.Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 2016; 375: 1119–30. [DOI] [PubMed] [Google Scholar]
- 180.Kolias AG, Adams H, Timofeev IS, et al. Evaluation of outcomes among patients with traumatic intracranial hypertension treated with decompressive craniectomy vs standard medical care at 24 months: a secondary analysis of the RESCUEicp randomized clinical trial. JAMA Neurol 2022; 79: 664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Andelic N, Forslund MV, Perrin PB, et al. Long-term follow-up of use of therapy services for patients with moderate-to-severe traumatic brain injury. J Rehabil Med 2020; 52: jrm00034. [DOI] [PubMed] [Google Scholar]
- 182.National Academies of Sciences Engineering and Medicine. Traumatic brain injury: a roadmap for accelerating progress. National Academies of Sciences Engineering and Medicine Consensus Study Report, 2022. https://nap.nationalacademies.org/catalog/25394/traumatic-brain-injury-a-roadmap-for-accelerating-progress (accessed Aug 8, 2022). [PubMed] [Google Scholar]
- 183.Andelic N, Ye J, Tornas S, et al. Cost-effectiveness analysis of an early-initiated, continuous chain of rehabilitation after severe traumatic brain injury. J Neurotrauma 2014; 31: 1313–20. [DOI] [PubMed] [Google Scholar]
- 184.Jacob L, Cogné M, Tenovuo O, et al. Predictors of access to rehabilitation in the year following traumatic brain injury: a European prospective and multicenter study. Neurorehabil Neural Repair 2020; 34: 814–30. [DOI] [PubMed] [Google Scholar]
- 185.Jourdan C, Bayen E, Vallat-Azouvi C, et al. Late functional changes post-severe traumatic brain injury are related to community reentry support: results from the PariS-TBI Cohort. J Head Trauma Rehabil 2017; 32: E26–34. [DOI] [PubMed] [Google Scholar]
- 186.Cullen N Canadian healthcare perspective in traumatic brain injury rehabilitation. J Head Trauma Rehabil 2007; 22: 214–20. [DOI] [PubMed] [Google Scholar]
- 187.Haines KL, Nguyen BP, Vatsaas C, Alger A, Brooks K, Agarwal SK. Socioeconomic status affects outcomes after severity-stratified traumatic brain injury. J Surg Res 2019; 235: 131–40. [DOI] [PubMed] [Google Scholar]
- 188.Ponsford JL, Downing MG, Olver J, et al. Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J Neurotrauma 2014; 31: 64–77. [DOI] [PubMed] [Google Scholar]
- 189.Bayley M, Teasell R, Velikonja D, et al. INCOG 2.0 guidelines for cognitive rehabilitation following traumatic brain injury: what’s changed from 2014 to now? Head Trauma Rehabil (in press; ). [DOI] [PubMed] [Google Scholar]
- 190.Lingsma HF, Roozenbeek B, Li B, et al. Large between-center differences in outcome after moderate and severe traumatic brain injury in the international mission on prognosis and clinical trial design in traumatic brain injury (IMPACT) study. Neurosurgery 2011; 68: 601–07. [DOI] [PubMed] [Google Scholar]
- 191.Turgeon AF, Lauzier F, Simard J-F, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ 2011; 183: 1581–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Williamson T, Ryser MD, Ubel PA, et al. Withdrawal of life-supporting treatment in severe traumatic brain injury. JAMA Surg 2020; 155: 723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.van Veen E, van der Jagt M, Citerio G, et al. Occurrence and timing of withdrawal of life-sustaining measures in traumatic brain injury patients: a CENTER-TBI study. Intensive Care Med 2021; 47: 1115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Curtis JR, Vincent J-L. Ethics and end-of-life care for adults in the intensive care unit. Lancet 2010; 376: 1347–53. [DOI] [PubMed] [Google Scholar]
- 195.Kompanje EJO, Piers RD, Benoit DD. Causes and consequences of disproportionate care in intensive care medicine. Curr Opin Crit Care 2013; 19: 630–35. [DOI] [PubMed] [Google Scholar]
- 196.Williamson TL, Adil SM, Shalita C, et al. Palliative care consultations in patients with severe traumatic brain injury: who receives palliative care consultations and what does that mean for utilization? Neurocrit Care 2022; 36: 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocrit Care 2013; 19: 347–63. [DOI] [PubMed] [Google Scholar]
- 198.McCrea MA, Giacino JT, Barber J, et al. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol 2021; 78: 982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma 2012; 29: 59–65. [DOI] [PubMed] [Google Scholar]
- 200.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016; 102: 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lazaridis C Withdrawal of life-sustaining treatments in perceived devastating brain injury: the key role of uncertainty. Neurocrit Care 2019; 30: 33–41. [DOI] [PubMed] [Google Scholar]
- 202.Harvey D, Butler J, Groves J, et al. Management of perceived devastating brain injury after hospital admission: a consensus statement from stakeholder professional organizations. Br J Anaesth 2018; 120: 138–45. [DOI] [PubMed] [Google Scholar]
- 203.Souter MJ, Blissitt PA, Blosser S, et al. Recommendations for the critical care management of devastating brain injury: prognostication, psychosocial, and ethical management: a position statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care 2015; 23: 4–13. [DOI] [PubMed] [Google Scholar]
- 204.Tenovuo O, Diaz-Arrastia R, Goldstein LE, Sharp DJ, van der Naalt J, Zasler ND. Assessing the severity of traumatic brain injury—time for a change? J Clin Med 2021; 10: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Walker WC, Stromberg KA, Marwitz JH, et al. Predicting long-term global outcome after traumatic brain injury: development of a practical prognostic tool using the traumatic brain injury model systems national database. J Neurotrauma 2018; 35: 1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ponsford JL, Spitz G, McKenzie D. Using post-traumatic amnesia to predict outcome after traumatic brain injury. J Neurotrauma 2016; 33: 997–1004. [DOI] [PubMed] [Google Scholar]
- 207.Carroll EL, Manktelow AE, Outtrim JG, et al. Influence of concomitant extracranial injury on functional and cognitive recovery from mild versus moderateto severe traumatic brain injury. J Head Trauma Rehabil 2020; 35: E513–23. [DOI] [PubMed] [Google Scholar]
- 208.Cnossen MC, van der Naalt J, Spikman JM, et al. Prediction of persistent post-concussion symptoms after mild traumatic brain injury. J Neurotrauma 2018; 35: 2691–98. [DOI] [PubMed] [Google Scholar]
- 209.van der Naalt J, Timmerman ME, de Koning ME, et al. Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol 2017; 16: 532–40. [DOI] [PubMed] [Google Scholar]
- 210.Saatman KE, Duhaime A-C, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma 2008; 25: 719–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Si B, Dumkrieger G, Wu T, et al. Sub-classifying patients with mild traumatic brain injury: a clustering approach based on baseline clinical characteristics and 90-day and 180-day outcomes. PLoS One 2018; 13: e0198741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wu J, Vodovotz Y, Abdelhamid S, Guyette FX, et al. Multi-omic analysis in injured humans: patterns align with outcomes and treatment responses. Cell Rep Med 2021; 2: 100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gravesteijn BY, Sewalt CA, Ercole A, et al. Toward a new multidimensional classification of traumatic brain injury: a collaborative European neurotrauma effectiveness research for traumatic brain injury study. J Neurotrauma 2020; 37: 1002–10. [DOI] [PubMed] [Google Scholar]
- 214.Whitehouse DP, Monteiro M, Czeiter E, et al. Relationship of admission blood proteomic biomarkers levels to lesion type and lesion burden in traumatic brain injury: a CENTER-TBI study. EBioMedicine 2022; 75: 103777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Åkerlund CAI, Holst A, Stocchetti N, et al. Clustering identifies endotypes of traumatic brain injury in an intensive care cohort: a CENTER-TBI study. Crit Care 2022; 26: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Folweiler KA, Sandsmark DK, Diaz-Arrastia R, Cohen AS, Masino AJ. Unsupervised machine learning reveals novel traumatic brain injury patient phenotypes with distinct acute injury profiles and long-term outcomes. J Neurotrauma 2020; 37: 1431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yuh EL, Jain S, Sun X, et al. Pathological computed tomography features associated with adverse outcomes after mild traumatic brain injury: a TRACK-TBI study with external validation in CENTER-TBI. JAMA Neurol 2021; 78: 1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vande Vyvere T, Wilms G, Claes L, et al. Central versus local radiological reading of acute computed tomography characteristics in multi-center traumatic brain injury research. J Neurotrauma 2019; 36: 1080–92. [DOI] [PubMed] [Google Scholar]
- 219.Rincon SP, Mukherjee P, Levin HS, et al. Interrater reliability of national institutes of health traumatic brain injury imaging common data elements for brain magnetic resonance imaging in mild traumatic brain injury. J Neurotrauma 2021; 38: 2831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Harburg L, McCormack E, Kenney K, et al. Reliability of the NINDS common data elements cranial tomography (CT) rating variables for traumatic brain injury (TBI). Brain Inj 2017; 31: 174–84. [DOI] [PubMed] [Google Scholar]
- 221.Vande Vyvere T, De La Rosa E, Wilms G, et al. Prognostic validation of the NINDS common data elements for the radiologic reporting of acute traumatic brain injuries: a CENTER-TBI study. J Neurotrauma 2020; 37: 1269–82. [DOI] [PubMed] [Google Scholar]
- 222.Meeuws S, Yue JK, Huijben JA, et al. Common data elements: critical assessment of harmonization between current multi-center traumatic brain injury studies. J Neurotrauma 2020; 37: 1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Thelin EP, Nelson DW, Vehviläinen J, et al. Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: an observational, multicenter study. PLoS Med 2017; 14: e1002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hale AT, Stonko DP, Brown A, et al. Machine-learning analysis outperforms conventional statistical models and CT classification systems in predicting 6-month outcomes in pediatric patients sustaining traumatic brain injury. Neurosurg Focus 2018; 45: E2. [DOI] [PubMed] [Google Scholar]
- 225.Mikkonen ED, Skrifvars MB, Reinikainen M, et al. Validation of prognostic models in intensive care unit-treated pediatric traumatic brain injury patients. J Neurosurg Pediatr 2019; 24: 330–37. [DOI] [PubMed] [Google Scholar]
- 226.Liesemer K, Riva-Cambrin J, Bennett KS, et al. Use of Rotterdam CT scores for mortality risk stratification in children with traumatic brain injury. Pediatr Crit Care Med 2014; 15: 554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Monteiro M, Newcombe VFJ, Mathieu F, et al. Multiclass semantic segmentation and quantification of traumatic brain injury lesions on head CT using deep learning: an algorithm development and multicentre validation study. Lancet Digit Health 2020; 2: e314–22. [DOI] [PubMed] [Google Scholar]
- 228.Jain S, Vyvere TV, Terzopoulos V, et al. Automatic quantification of computed tomography features in acute traumatic brain injury. J Neurotrauma 2019; 36: 1794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kuo W, Häne C, Mukherjee P, Malik J, Yuh EL. Expert-level detection of acute intracranial hemorrhage on head computed tomography using deep learning. Proc Natl Acad Sci USA 2019; 116: 22737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yue JK, Yuh EL, Korley FK, et al. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: a prospective multicentre study. Lancet Neurol 2019; 18: 953–61. [DOI] [PubMed] [Google Scholar]
- 231.Amyot F, Arciniegas DB, Brazaitis MP, et al. A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. J Neurotrauma 2015; 32: 1693–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sandsmark DK, Bashir A, Wellington CL, Diaz-Arrastia R. Cerebral microvascular injury: a potentially treatable endophenotype of traumatic brain injury-induced neurodegeneration. Neuron 2019; 103: 367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stein MB, Yuh E, Jain S, et al. Smaller regional brain volumes predict posttraumatic stress disorder at 3 months after mild traumatic brain injury. Biol Psychiatry Cogn Neurosci Neuroimaging 2021; 6: 352–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Diamond BR, Donald CLM, Frau-Pascual A, et al. Optimizing the accuracy of cortical volumetric analysis in traumatic brain injury. MethodsX 2020; 7: 100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yuh EL, Cooper SR, Mukherjee P, et al. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma 2014; 31: 1457–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Richter S, Winzeck S, Kornaropoulos EN, et al. Neuroanatomical substrates and symptoms associated with magnetic resonance imaging of patients with mild traumatic brain injury. JAMA Netw Open 2021; 4: e210994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Palacios EM, Yuh EL, Mac Donald CL, et al. Diffusion tensor imaging reveals elevated diffusivity of white matter microstructure that is independently associated with long-term outcome after mild traumatic brain injury: a TRACK-TBI study. J Neurotrauma 2022; published online July 18. 10.1089/neu.2021.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Puybasset L, Perlbarg V, Unrug J, et al. Prognostic value of global deep white matter DTI metrics for 1-year outcome prediction in ICU traumatic brain injury patients: an MRI-COMA and CENTER-TBI combined study. Intensive Care Med 2022; 48: 201–12. [DOI] [PubMed] [Google Scholar]
- 239.Palacios EM, Yuh EL, Chang Y-S, et al. Resting-state functional connectivity alterations associated with six-month outcomes in mild traumatic brain injury. J Neurotrauma 2017; 34: 1546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bartnik-Olson BL, Alger JR, Babikian T, et al. The clinical utility of proton magnetic resonance spectroscopy in traumatic brain injury: recommendations from the ENIGMA MRS working group. Brain Imaging Behav 2021; 15: 504–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cole JH, Leech R, Sharp DJ. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol 2015; 77: 571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dennis EL, Taylor BA, Newsome MR, et al. Advanced brain age in deployment-related traumatic brain injury: a LIMBIC-CENC neuroimaging study. Brain Inj 2022; 36: 662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Newcombe VFJ, Ashton NJ, Posti JP, et al. Post-acute blood biomarkers and disease progression in traumatic brain injury. Brain 2022; 145: 2064–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Graham NSN, Zimmerman KA, Moro F, et al. Axonal marker neurofilament light predicts long-term outcomes and progressive neurodegeneration after traumatic brain injury. Sci Transl Med 2021; 13: eabg9922. [DOI] [PubMed] [Google Scholar]
- 245.Wang KKW, Kobeissy FH, Shakkour Z, Tyndall JA. Thorough overview of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein as tandem biomarkers recently cleared by US Food and Drug Administration for the evaluation of intracranial injuries among patients with traumatic brain injury. Acute Med Surg 2021; 8: e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Papa L, Silvestri S, Brophy GM, et al. GFAP out-performs S100β in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J Neurotrauma 2014; 31: 1815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Czeiter E, Amrein K, Gravesteijn BY, et al. Blood biomarkers on admission in acute traumatic brain injury: relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine 2020; 56: 102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mondello S, Jeromin A, Buki A, et al. Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J Neurotrauma 2012; 29: 1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mondello S, Papa L, Buki A, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care 2011; 15: R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hossain I, Mohammadian M, Takala RSK, et al. Early levels of glial fibrillary acidic protein and neurofilament light protein in predicting the outcome of mild traumatic brain injury. J Neurotrauma 2019; 36: 1551–60. [DOI] [PubMed] [Google Scholar]
- 251.Retel Helmrich I, Czeiter E, Amrein K, et al. Incremental prognostic value of acute serum biomarkers for functional outcome following TBI: an observational cohort study from CENTER-TBI. Lancet Neurol 2022; 21: 792–802. [DOI] [PubMed] [Google Scholar]
- 252.Korley F, Jain S, Sun X, et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 levels for predicting functional recovery in the track-tbi cohort: an observational cohort study. Lancet Neurol 2022; 21: 803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sarkis GA, Zhu T, Yang Z, et al. Characterization and standardization of multiassay platforms for four commonly studied traumatic brain injury protein biomarkers: a TBI endpoints development study. Biomarkers Med 2021; 15: 1721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wang KK, Munoz Pareja JC, Mondello S, et al. Blood-based traumatic brain injury biomarkers—clinical utilities and regulatory pathways in the United States, Europe and Canada. Expert Rev Mol Diagn 2021; 21: 1303–21. [DOI] [PubMed] [Google Scholar]
- 255.Hossain I, Mohammadian M, Takala RSK, et al. Admission levels of total tau and β-amyloid isoforms 1–40 and 1–42 in predicting the outcome of mild traumatic brain injury. Front Neurol 2020; 11: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lagerstedt L, Azurmendi L, Tenovuo O, et al. Interleukin 10 and heart fatty acid-binding protein as early outcome predictors in patients with traumatic brain injury. Front Neurol 2020; 11: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Posti JP, Takala RSK, Lagerstedt L, et al. Correlation of blood biomarkers and biomarker panels with traumatic findings on computed tomography after traumatic brain injury. J Neurotrauma 2019; 36: 2178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Huie JR, Diaz-Arrastia R, Yue JK, et al. Testing a multivariate proteomic panel for traumatic brain injury biomarker discovery: a TRACK-TBI pilot study. J Neurotrauma 2019; 36: 100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Needham EJ, Stoevesandt O, Thelin EP, et al. Complex autoantibody responses occur following moderate to severe traumatic brain injury. J Immunol 2021; 207: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Thelin EP, Zeiler FA, Ercole A, et al. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front Neurol 2017; 8: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hier DB, Obafemi-Ajayi T, Thimgan MS, et al. Blood biomarkers for mild traumatic brain injury: a selective review of unresolved issues. Biomark Res 2021; 9: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Thomas I, Dickens AM, Posti JP, et al. Serum metabolome associated with severity of acute traumatic brain injury. Nat Commun 2022; 13: 2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dickens AM, Posti JP, Takala RSK, et al. Serum metabolites associated with computed tomography findings after traumatic brain injury. J Neurotrauma 2018; 35: 2673–83. [DOI] [PubMed] [Google Scholar]
- 264.Thomas I, Dickens AM, Posti JP, et al. Integrative analysis of circulating metabolite profiles and magnetic resonance imaging metrics in patients with traumatic brain injury. Int J Mol Sci 2020; 21: 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Orešič M, Posti JP, Kamstrup-Nielsen MH, et al. Human serum metabolites associate with severity and patient outcomes in traumatic brain injury. EBioMedicine 2016; 12: 118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mondello S, Sandner V, Goli M, et al. Exploring serum glycome patterns after moderate to severe traumatic brain injury: a prospective pilot study. EClinicalMedicine 2022; 50: 101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Thomas I, Dickens AM, Posti JP, et al. Serum metabolome associated with severity of acute traumatic brain injury. Nat Commun 2022; 13: 2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jeter CB, Hergenroeder GW, Ward NH 3rd, Moore AN, Dash PK. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma 2013; 30: 671–79. [DOI] [PubMed] [Google Scholar]
- 269.Miller MR, Robinson M, Bartha R, et al. Concussion acutely decreases plasma glycerophospholipids in adolescent male athletes. J Neurotrauma 2021; 38: 1608–14. [DOI] [PubMed] [Google Scholar]
- 270.Devoto C, Lai C, Qu B-X, et al. Exosomal microRNAs in military personnel with mild traumatic brain injury: preliminary results from the chronic effects of neurotrauma consortium biomarker discovery project. J Neurotrauma 2020; 37: 2482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Di Pietro V, Yakoub KM, Scarpa U, Di Pietro C, Belli A. MicroRNA signature of traumatic brain injury: from the biomarker discovery to the point-of-care. Front Neurol 2018; 9: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Porteny J, Tovar E, Lin S, Anwar A, Osier N. Salivary biomarkers as indicators of TBI diagnosis and prognosis: a systematic review. Mol Diagn Ther 2022; 26: 169–87. [DOI] [PubMed] [Google Scholar]
- 273.Kors EE, Terwindt GM, Vermeulen FL, et al. Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol 2001; 49: 753–60. [DOI] [PubMed] [Google Scholar]
- 274.McFadyen CA, Zeiler FA, Newcombe V, et al. Apolipoprotein E4 polymorphism and outcomes from traumatic brain injury: a living systematic review and meta-analysis. J Neurotrauma 2021; 38: 1124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zeiler FA, McFadyen C, Newcombe VFJ, et al. Genetic influences on patient-oriented outcomes in traumatic brain injury: a living systematic review of non-apolipoprotein e single-nucleotide polymorphisms. J Neurotrauma 2021; 38: 1107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Duncan LE, Ostacher M, Ballon J. How genome-wide association studies (GWAS) made traditional candidate gene studies obsolete. Neuropsychopharmacology 2019; 44: 1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kals M, Kunzmann K, Parodi L, et al. A genome-wide association study of outcome from traumatic brain injury. EBioMedicine 2022; 77: 103933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Osthoff M, Walder B, Delhumeau C, Trendelenburg M, Turck N. Association of lectin pathway protein levels and genetic variants early after injury with outcomes after severe traumatic brain injury: a prospective cohort study. J Neurotrauma 2017; 34: 2560–66. [DOI] [PubMed] [Google Scholar]
- 279.Joy MT, Ben Assayag E, Shabashov-Stone D, et al. CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell 2019; 176: 1143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jha RM, Zusman BE, Puccio AM, et al. Genetic variants associated with intraparenchymal hemorrhage progression after traumatic brain injury. JAMA Netw Open 2021; 4: e2116839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zeiler FA, Ercole A, Cabeleira M, et al. Descriptive analysis of low versus elevated intracranial pressure on cerebral physiology in adult traumatic brain injury: a CENTER-TBI exploratory study. Acta Neurochir (Wien) 2020; 162: 2695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zeiler FA, Donnelly J, Nourallah B, et al. Intracranial and extracranial injury burden as drivers of impaired cerebrovascular reactivity in traumatic brain injury. J Neurotrauma 2018; 35: 1569–77. [DOI] [PubMed] [Google Scholar]
- 283.Zeiler FA, Mathieu F, Monteiro M, et al. Diffuse intracranial injury patterns are associated with impaired cerebrovascular reactivity in adult traumatic brain injury: a CENTER-TBI validation study. J Neurotrauma 2020; 37: 1597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zeiler FA, Mathieu F, Monteiro M, et al. Systemic markers of injury and injury response are not associated with impaired cerebrovascular reactivity in adult traumatic brain injury: a Collaborative European Neurotrauma Effectiveness Research In Traumatic Brain Injury (CENTER-TBI) study. J Neurotrauma 2021; 38: 870–78. [DOI] [PubMed] [Google Scholar]
- 285.Zeiler FA, Ercole A, Cabeleira M, et al. Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: a CENTER-TBI study. Acta Neurochir 2019; 161: 1217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zeiler FA, Ercole A, Beqiri E, et al. Association between cerebrovascular reactivity monitoring and mortality is preserved when adjusting for baseline admission characteristics in adult traumatic brain injury: a CENTER-TBI study. J Neurotrauma 2020; 37: 1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zeiler FA, Ercole A, Beqiri E, et al. Cerebrovascular reactivity is not associated with therapeutic intensity in adult traumatic brain injury: a CENTER-TBI analysis. Acta Neurochir 2019; 161: 1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 1997; 41: 11–17. [DOI] [PubMed] [Google Scholar]
- 289.Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002; 30: 733–38. [DOI] [PubMed] [Google Scholar]
- 290.Aries MJH, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 2012; 40: 2456–63. [DOI] [PubMed] [Google Scholar]
- 291.Zeiler FA, Ercole A, Cabeleira M, et al. Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult traumatic brain injury: a Collaborative European Neurotrauma Effectiveness Research In Traumatic Brain Injury (CENTER-TBI) study. J Neurotrauma 2019; 36: 1505–17. [DOI] [PubMed] [Google Scholar]
- 292.Zeiler F, Aries M, Czosnyka M, Smielewski P. Cerebral autoregulation monitoring in traumatic brain injury: an overview of recent advances in personalized medicine. J Neurotrauma 2022; published online July 6. 10.1089/neu.2022.0217. [DOI] [PubMed] [Google Scholar]
- 293.Depreitere B, Güiza F, Van den Berghe G, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg 2014; 120: 1451–57. [DOI] [PubMed] [Google Scholar]
- 294.Riemann L, Beqiri E, Smielewski P, et al. Low-resolution pressure reactivity index and its derived optimal cerebral perfusion pressure in adult traumatic brain injury: a CENTER-TBI study. Crit Care 2020; 24: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tas J, Beqiri E, van Kaam RC, et al. Targeting autoregulation-guided cerebral perfusion pressure after traumatic brain injury (COGiTATE): a feasibility randomized controlled clinical trial. J Neurotrauma 2021; 38: 2790–800. [DOI] [PubMed] [Google Scholar]
- 296.Jha RM, Bell J, Citerio G, et al. Role of sulfonylurea receptor 1 and glibenclamide in traumatic brain injury: a review of the evidence. Int J Mol Sci 2020; 21: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gatzinsky K, Johansson E, Jennische E, Oshalim M, Lange S. Elevated intracranial pressure after head trauma can be suppressed by antisecretory factor-a pilot study. Acta Neurochir 2020; 162: 1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gradisek P, Carrara G, Antiga L, et al. Prognostic value of a combination of circulating biomarkers in critically ill patients with traumatic brain injury: results from the European CREACTIVE study. J Neurotrauma 2021; 38: 2667–76. [DOI] [PubMed] [Google Scholar]
- 299.Vijapur SM, Vaughan LE, Awan N, DiSanto D, McKernan GP, Wagner AK. Treelet transform analysis to identify clusters of systemic inflammatory variance in a population with moderate-to-severe traumatic brain injury. Brain Behav Immun 2021; 95: 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lee S, Hwang H, Yamal J-M, et al. IMPACT probability of poor outcome and plasma cytokine concentrations are associated with multiple organ dysfunction syndrome following traumatic brain injury. J Neurosurg 2019; 131: 1931–37. [DOI] [PubMed] [Google Scholar]
- 301.Nielson JL, Cooper SR, Yue JK, et al. Uncovering precision phenotype-biomarker associations in traumatic brain injury using topological data analysis. PLoS One 2017; 12: e0169490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Abujaber A, Fadlalla A, Gammoh D, Abdelrahman H, Mollazehi M, El-Menyar A. Prediction of in-hospital mortality in patients with post traumatic brain injury using National Trauma Registry and Machine Learning Approach. Scand J Trauma Resusc Emerg Med 2020; 28: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Djimeu EW, Houndolo D-G. Power calculation for causal inference in social science: sample size and minimum detectable effect determination. J Dev Effect 2016; 8: 508–27. [Google Scholar]
- 304.Haarbauer-Krupa J, Pugh MJ, Prager EM, Harmon N, Wolfe J, Yaffe K. Epidemiology of chronic effects of traumatic brain injury. J Neurotrauma 2021; 38: 3235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Silverberg ND, Crane PK, Dams-O’Connor K, et al. Developing a cognition endpoint for traumatic brain injury clinical trials. J Neurotrauma 2017; 34: 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Brett BL, Kramer MD, Whyte J, et al. Latent profile analysis of neuropsychiatric symptoms and cognitive function of adults 2 weeks after traumatic brain injury: findings from the TRACK-TBI study. JAMA Netw Open 2021; 4: e213467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Thurmond VA, Hicks R, Gleason T, et al. Advancing integrated research in psychological health and traumatic brain injury: common data elements. Arch Phys Med Rehabil 2010; 91: 1633–36. [DOI] [PubMed] [Google Scholar]
- 308.von Steinbuechel N, Rauen K, Krenz U, et al. Translation and linguistic validation of outcome instruments for traumatic brain injury research and clinical practice: a step-by-step approach within the observational CENTER-TBI study. J Clin Med 2021; 10: 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Steinbuechel NV, Rauen K, Bockhop F, et al. Psychometric characteristics of the patient-reported outcome measures applied in the CENTER-TBI study. J Clin Med 2021; 10: 2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018; 91: 450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol 2020; 27: 741–56. [DOI] [PubMed] [Google Scholar]
- 312.Royal College of Physicians. Prolonged disorders of consciousness national clinical guidelines London, 2020. https://www.rcplondon.ac.uk/guidelines-policy/prolonged-disorders-consciousness-following-sudden-onset-brain-injury-national-clinical-guidelines (accessed Aug 9, 2022).
- 313.Bodien YG, McCrea M, Dikmen S, et al. Optimizing outcome assessment in multicenter TBI trials: perspectives from TRACK-TBI and the TBI endpoints development initiative. J Head Trauma Rehabil 2018; 33: 147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nelson LD, Temkin NR, Dikmen S, et al. Recovery after mild traumatic brain injury in patients presenting to US Level I trauma centers: a Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study. JAMA Neurol 2019; 76: 1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Nelson LD, Ranson J, Ferguson AR, et al. Validating multidimensional outcome assessment using the traumatic brain injury common data elements: an analysis of the TRACK-TBI pilot study sample. J Neurotrauma 2017; 34: 3158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wilson L, Boase K, Nelson LD, et al. A manual for the Glasgow Outcome Scale-Extended interview. J Neurotrauma 2021; 38: 2435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kunzmann K, Wernisch L, Richardson S, et al. Imputation of ordinal outcomes: a comparison of approaches in traumatic brain injury. J Neurotrauma 2021; 38: 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Christoforou AN, Armstrong MJ, Bergin MJG, et al. An evidence-based methodology for systematic evaluation of clinical outcome assessment measures for traumatic brain injury. PLoS One 2020; 15: e0242811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wilson L, Horton L, Polinder S, et al. Tailoring multidimensional outcomes to level of functional recovery after traumatic brain injury. J Neurotrauma 2022; published online June 24. 10.1089/neu.2022.0013. [DOI] [PubMed] [Google Scholar]
- 320.Voormolen DC, Zeldovich M, Haagsma JA, et al. Outcomes after complicated and uncomplicated mild traumatic brain injury at three-and six-months post-injury: results from the CENTER-TBI study. J Clin Med 2020; 9: 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Nelson LD, Brett BL, Magnus BE, et al. Functional status examination yields higher measurement precision of functional limitations after traumatic injury than the glasgow outcome scale-extended: a preliminary study. J Neurotrauma 2020; 37: 675–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Nelson LD, Kramer MD, Joyner KJ, et al. Relationship between transdiagnostic dimensions of psychopathology and traumatic brain injury (TBI): a TRACK-TBI study. J Abnorm Psychol 2021; 130: 423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wilson L, Horton L, Kunzmann K, et al. Understanding the relationship between cognitive performance and function in daily life after traumatic brain injury. J Neurol Neurosurg Psychiatry 2021; 92: 407–17. [DOI] [PubMed] [Google Scholar]
- 324.Dams-O’Connor K, Sy KTL, Landau A, et al. The feasibility of telephone-administered cognitive testing in individuals 1 and 2 years after inpatient rehabilitation for traumatic brain injury. J Neurotrauma 2018; 35: 1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Nelson LD, Barber JK, Temkin NR, et al. Validity of the brief test of adult cognition by telephone in level 1 trauma center patients six months post-traumatic brain injury: a TRACK-TBI study. J Neurotrauma 2021; 38: 1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Stein MB, Kessler RC, Heeringa SG, et al. Prospective longitudinal evaluation of the effect of deployment-acquired traumatic brain injury on posttraumatic stress and related disorders: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Am J Psychiatry 2015; 172: 1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Iljazi A, Ashina H, Al-Khazali HM, et al. Post-traumatic stress disorder after traumatic brain injury-a systematic review and meta-analysis. Neurol Sci 2020; 41: 2737–46. [DOI] [PubMed] [Google Scholar]
- 328.Van Praag DLG, Cnossen MC, Polinder S, Wilson L, Maas AIR. Post-traumatic stress disorder after civilian traumatic brain injury: a systematic review and meta-analysis of prevalence rates. J Neurotrauma 2019; 36: 3220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Van Praag DLG, Wouters K, Van Den Eede F, et al. Neurocognitive correlates of probable posttraumatic stress disorder following traumatic brain injury. Brain and Spine 2022; 2: 100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Stein MB, Jain S, Giacino JT, et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: a TRACK-TBI study. JAMA Psychiatry 2019; 76: 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM. Posttraumatic stress disorder mediates the relationship between mild traumatic brain injury and health and psychosocial functioning in veterans of Operations Enduring Freedom and Iraqi Freedom. J Nerv Ment Dis 2009; 197: 748–53. [DOI] [PubMed] [Google Scholar]
- 332.Agimi Y, Regasa LE, Ivins B, Malik S, Helmick K, Marion D. Role of Department of Defense policies in identifying traumatic brain injuries among deployed US service members, 2001–2016. Am J Public Health 2018; 108: 683–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Escolas SM, Luton M, Ferdosi H, Chavez BD, Engel SD. Traumatic brain injuries: unreported and untreated in an army population. Mil Med 2020; 185 (suppl 1): 154–60. [DOI] [PubMed] [Google Scholar]
- 334.Silver JM, McAllister TW, Arciniegas DB. Textbook of traumatic brain injury, third edn. American Psychiatric Association Publishing; 2018; 13: 10.1176/appi.books.9781615372645 (accessed Aug 9, 2022). [DOI] [Google Scholar]
- 335.Bryant RA, Schnurr PP, Pedlar D. Addressing the mental health needs of civilian combatants in Ukraine. Lancet Psychiatry 2022; 9: 346–47. [DOI] [PubMed] [Google Scholar]
- 336.Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry 2009; 166: 768–76. [DOI] [PubMed] [Google Scholar]
- 337.Howlett JR, Nelson LD, Stein MB. Mental health consequences of traumatic brain injury. Biol Psychiatry 2022; 91: 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Mikolić A, Polinder S, Steyerberg EW, et al. Prediction of global functional outcome and post-concussive symptoms after mild traumatic brain injury: external validation of prognostic models in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. J Neurotrauma 2021; 38: 196–209. [DOI] [PubMed] [Google Scholar]
- 339.Bretzin AC, Covassin T, Wiebe DJ, Stewart W. Association of sex with adolescent soccer concussion incidence and characteristics. JAMA Netw Open 2021; 4: e218191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Master CL, Katz BP, Arbogast KB, et al. Differences in sport-related concussion for female and male athletes in comparable collegiate sports: a study from the NCAA-DoD Concussion Assessment, Research and Education (CARE) consortium. Br J Sports Med 2021; 55: 1387–94. [DOI] [PubMed] [Google Scholar]
- 341.Gupte R, Brooks W, Vukas R, Pierce J, Harris J. Sex differences in traumatic brain injury: what we know and what we should know. J Neurotrauma 2019; 36: 3063–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Levin HS, Temkin NR, Barber J, et al. Association of sex and age with mild traumatic brain injury-related symptoms: a TRACK-TBI study. JAMA Netw Open 2021; 4: e213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yue JK, Levin HS, Suen CG, et al. Age and sex-mediated differences in six-month outcomes after mild traumatic brain injury in young adults: a TRACK-TBI study. Neurol Res 2019; 41: 609–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Mikolić A, van Klaveren D, Groeniger JO, et al. Differences between men and women in treatment and outcome after traumatic brain injury. J Neurotrauma 2021; 38: 235–51. [DOI] [PubMed] [Google Scholar]
- 345.Mikolic A, Groeniger JO, Zeldovich M, et al. Explaining outcome differences between men and women following mild traumatic brain injury. J Neurotrauma 2021; 38: 3315–31. [DOI] [PubMed] [Google Scholar]
- 346.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Brett BL, Gardner RC, Godbout J, Dams-O’Connor K, Keene CD. Traumatic brain injury and risk of neurodegenerative disorder. Biol Psychiatry 2022; 91: 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology 2012; 79: 1970–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Russell ER, Mackay DF, Stewart K, MacLean JA, Pell JP, Stewart W. Association of field position and career length with risk of neurodegenerative disease in male former professional soccer players. JAMA Neurol 2021; 78: 1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Daneshvar DH, Mez J, Alosco ML, et al. Incidence of and mortality from amyotrophic lateral sclerosis in National Football League athletes. JAMA Netw Open 2021; 4: e2138801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Graham NS, Sharp DJ. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry 2019; 90: 1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Katz DI, Bernick C, Dodick DW, et al. National Institute of Neurological Disorders and Stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology 2021; 96: 848–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013; 9: 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Johnson VE, Stewart W, Arena JD, Smith DH. Traumatic brain injury as a trigger of neurodegeneration. Adv Neurobiol 2017; 15: 383–400. [DOI] [PubMed] [Google Scholar]
- 355.Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol 2019; 138: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bieniek KF, Cairns NJ, Crary JF, et al. The second NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2021; 80: 210–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ritchie K, Ritchie CW, Yaffe K, Skoog I, Scarmeas N. Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimers Dement 2015; 1: 122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Edlow BL, Keene CD, Perl DP, et al. Multimodal characterization of the late effects of traumatic brain injury: a methodological overview of the late effects of traumatic brain injury project. J Neurotrauma 2018; 35: 1604–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mantyh WG, Spina S, Lee A, et al. Tau positron emission tomographic findings in a former US football player with pathologically confirmed chronic traumatic encephalopathy. JAMA Neurol 2020; 77: 517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Stern RA, Adler CH, Chen K, et al. Tau positron-emission tomography in former National Football League players. N Engl J Med 2019; 380: 1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Klein AP, Tetzlaff JE, Bonis JM, et al. Prevalence of potentially clinically significant magnetic resonance imaging findings in athletes with and without sport-related concussion. J Neurotrauma 2019; 36: 1776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AI. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol 2010; 9: 543–54. [DOI] [PubMed] [Google Scholar]
- 363.Dijkland SA, Foks KA, Polinder S, et al. Prognosis in moderate and severe traumatic brain injury: a systematic review of contemporary models and validation studies. J Neurotrauma 2020; 37: 1–13. [DOI] [PubMed] [Google Scholar]
- 364.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008; 5: e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 2008; 336: 425–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Dijkland SA, Helmrich IRAR, Nieboer D, et al. Outcome prediction after moderate and severe traumatic brain injury: external validation of two established prognostic models in 1742 European patients. J Neurotrauma 2021; 38: 1377–88. [DOI] [PubMed] [Google Scholar]
- 367.Steyerberg EW, Borsboom GJJM, van Houwelingen HC, Eijkemans MJ, Habbema JD. Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med 2004; 23: 2567–86. [DOI] [PubMed] [Google Scholar]
- 368.Jennett B, Teasdale G, Braakman R, Minderhoud J, Heiden J, Kurze T. Prognosis of patients with severe head injury. Neurosurgery 1979; 4: 283–89. [DOI] [PubMed] [Google Scholar]
- 369.Jacobs B, Beems T, Stulemeijer M, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma 2010; 27: 655–68. [DOI] [PubMed] [Google Scholar]
- 370.Lingsma HF, Yue JK, Maas AIR, et al. Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the TRACK-TBI pilot study. J Neurotrauma 2015; 32: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Yuh EL, Mukherjee P, Lingsma HF, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013; 73: 224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Helmrich IRAR, van Klaveren D, Dijkland SA, et al. Development of prognostic models for health-related quality of life following traumatic brain injury. Qual Life Res 2022; 31: 451–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Cnossen MC, Winkler EA, Yue JK, et al. Development of a prediction model for post-concussive symptoms following mild traumatic brain injury: a TRACK-TBI pilot study. J Neurotrauma 2017; 34: 2396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Huijben JA, Wiegers EJA, de Keizer NF, et al. Development of a quality indicator set to measure and improve quality of ICU care for patients with traumatic brain injury. Crit Care 2019; 23: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Huijben JA, Wiegers EJA, Ercole A, et al. Quality indicators for patients with traumatic brain injury in European intensive care units: a CENTER-TBI study. Crit Care 2020; 24: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Timmers M, van Dijck JTJM, van Wijk RPJ, et al. How do 66 European institutional review boards approve one protocol for an international prospective observational study on traumatic brain injury? Experiences from the CENTER-TBI study. BMC Med Ethics 2020; 21: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.van Wijk RPJ, van Dijck JTJM, Timmers M, et al. Informed consent procedures in patients with an acute inability to provide informed consent: policy and practice in the CENTER-TBI study. J Crit Care 2020; 59: 6–15. [DOI] [PubMed] [Google Scholar]
- 378.Shepherd V, Hood K, Sheehan M, Griffith R, Wood F. ‘It’s a tough decision’: a qualitative study of proxy decision-making for research involving adults who lack capacity to consent in UK. Age Ageing 2019; 48: 903–09. [DOI] [PubMed] [Google Scholar]
- 379.Maas AI, Harrison-Felix CL, Menon D, et al. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch Phys Med Rehabil 2010; 91: 1641–49. [DOI] [PubMed] [Google Scholar]
- 380.Ercole A, Dixit A, Nelson DW, et al. Imputation strategies for missing baseline neurological assessment covariates after traumatic brain injury: a CENTER-TBI study. PLoS One 2021; 16: e0253425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Gravesteijn BY, Sewalt CA, Venema E, et al. Missing data in prediction research: a five-step approach for multiple imputation, illustrated in the CENTER-TBI study. J Neurotrauma 2021; 38: 1842–57. [DOI] [PubMed] [Google Scholar]
- 382.Nielson JL, Cooper SR, Seabury SA, et al. Statistical guidelines for handling missing data in traumatic brain injury clinical research. J Neurotrauma 2021; 38: 2530–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Silvestre E, Wood F. Health Information Systems: analysis of country-level strategies, indicators, and resources, 2019. https://www.measureevaluation.org/resources/publications/tr-18-289.html (accessed Aug 9, 2022).
- 384.Mikkelsen L, Phillips DE, AbouZahr C, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet 2015; 386: 1395–406. [DOI] [PubMed] [Google Scholar]
- 385.Tropeano MP, Spaggiari R, Ileyassoff H, et al. A comparison of publication to TBI burden ratio of low- and middle-income countries versus high-income countries: how can we improve worldwide care of TBI? Neurosurg Focus 2019; 47: E5. [DOI] [PubMed] [Google Scholar]
- 386.Whiffin CJ, Smith BG, Esene IN, et al. Neurosurgeons’ experiences of conducting and disseminating clinical research in low-income and middle-income countries: a reflexive thematic analysis. BMJ Open 2021; 11: e051806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Smith BG, Whiffin CJ, Esene IN, et al. Neurotrauma clinicians’ perspectives on the contextual challenges associated with long-term follow-up following traumatic brain injury in low-income and middle-income countries: a qualitative study protocol. BMJ Open 2021; 11: e041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Kohler K, Nwe Myint PP, Wynn S, et al. Systems approach to improving traumatic brain injury care in Myanmar: a mixed-methods study from lived experience to discrete event simulation. BMJ Open 2022; 12: e059935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Whiffin CJ, Smith BG, Selveindran SM, et al. The value and potential of qualitative research methods in neurosurgery. World Neurosurg 2022; 161: 441–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Adame F Meaningful collaborations can end ‘helicopter research’. Nature 2021; published online June 29. 10.1038/d41586-021-01795-1. [DOI] [PubMed] [Google Scholar]
- 391.Ercole A, Brinck V, George P, et al. Guidelines for data acquisition, quality and curation for observational research designs (DAQCORD). J Clin Transl Sci 2020; 4: 354–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Maas AIR, Ercole A, De Keyser V, Menon DK, Steyerberg EW. Opportunities and challenges in high-quality contemporary data collection in traumatic brain injury: the CENTER-TBI experience. Neurocrit Care 2022; published online March 18. 10.1007/s12028-022-01471-w. [DOI] [PubMed] [Google Scholar]
- 393.Huie JR, Chou A, Torres-Espin A, et al. FAIR data reuse in traumatic brain injury: exploring inflammation and age as moderators of recovery in the TRACK-TBI pilot. Front Neurol 2021; 12: 768735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Wilkinson MD, Dumontier M, Aalbersberg IJJ, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data 2016; 3: 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Maas AIR, Murray GD, Roozenbeek B, et al. Advancing care for traumatic brain injury: findings from the IMPACT studies and perspectives on future research. Lancet Neurol 2013; 12: 1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.European Society of Human Genetics. Balancing data protection and research needs in the age of the GDPR. ScienceDaily, June 17, 2019. www.sciencedaily.com/releases/2019/06/190617100942.htm (accessed Aug 9, 2022). [Google Scholar]
- 397.Phillips M International data-sharing norms: from the OECD to the General Data Protection Regulation (GDPR). Hum Genet 2018; 137: 575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Kalkman S, Mostert M, Gerlinger C, van Delden JJM, van Thiel GJMW. Responsible data sharing in international health research: a systematic review of principles and norms. BMC Med Ethics 2019; 20: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Dayan I, Roth HR, Zhong A, et al. Federated learning for predicting clinical outcomes in patients with COVID-19. Nat Med 2021; 27: 1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Rieke N, Hancox J, Li W, et al. The future of digital health with federated learning. NPJ Digit Med 2020; 3: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Ceyisakar IE, Huijben JA, Maas AIR, et al. Can we cluster ICU treatment strategies for traumatic brain injury by hospital treatment preferences? Neurocrit Care 2022; 36: 846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Cnossen MC, van Essen TA, Ceyisakar IE, et al. Adjusting for confounding by indication in observational studies: a case study in traumatic brain injury. Clin Epidemiol 2018; 10: 841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kochanek PM, Adelson PD, Rosario BL, et al. Comparison of intracranial pressure measurements before and after hypertonic saline or mannitol treatment in children with severe traumatic brain injury. JAMA Netw Open 2022; 5: e220891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Volovici V, Pisică D, Gravesteijn BY, et al. Comparative effectiveness of intracranial hypertension management guided by ventricular versus intraparenchymal pressure monitoring: a CENTER-TBI study. Acta Neurochir 2022; 164: 1693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Bell MJ, Rosario BL, Kochanek PM, et al. Comparative effectiveness of diversion of cerebrospinal fluid for children with severe traumatic brain injury. JAMA Netw Open 2022; 5: e2220969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Kadakia KT, Krumholz HM. Designing cures 2.0—from corridors to cornerstones. N Engl J Med 2022; 386: 1677–79. [DOI] [PubMed] [Google Scholar]
- 407.Concato J, Corrigan-Curay J. Real-world evidence—where are we now? N Engl J Med 2022; 386: 1680–82. [DOI] [PubMed] [Google Scholar]
- 408.Manley GT, Lingsma HF, Maas AIR. ADAPTing to a new era of comparative-effectiveness research in traumatic brain injury—generating evidence from observational data. JAMA Netw Open 2022; 5: e220899. [DOI] [PubMed] [Google Scholar]
- 409.Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) study. Rationale and design. Ann Am Thorac Soc 2020; 17: 879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Estcourt LJ, Turgeon AF, McQuilten ZK, et al. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA 2021; 326: 1690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.ATTACC investigators, ACTIV-4a investigators, REMAP-CAP investigators, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med 2021; 385: 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med 2021; 384: 1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results. N Engl J Med 2021; 384: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Park JJH, Detry MA, Murthy S, Guyatt G, Mills EJ. How to use and interpret the results of a platform trial: users’ guide to the medical literature. JAMA 2022; 327: 67–74. [DOI] [PubMed] [Google Scholar]
- 415.Palacios EM, Owen JP, Yuh EL, et al. The evolution of white matter microstructural changes after mild traumatic brain injury: a longitudinal DTI and NODDI study. Sci Adv 2020; 6: eaaz6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Gajewski BJ, Berry SM, Barsan WG, et al. Hyperbaric oxygen brain injury treatment (HOBIT) trial: a multifactor design with response adaptive randomization and longitudinal modeling. Pharm Stat 2016; 15: 396–404. [DOI] [PubMed] [Google Scholar]
- 417.Maas AIR, Steyerberg EW, Citerio G. Tranexamic acid in traumatic brain injury: systematic review and meta-analysis trumps a large clinical trial? Intensive Care Med 2021; 47: 74–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


