Abstract
Background
Achondroplasia is the most common form of skeletal dysplasia. Recent advances in therapeutic options have highlighted the need for understanding the burden and treatment landscape of the condition. This systematic literature review (SLR) aimed to identify health-related quality of life (HRQoL)/utilities, healthcare resource use (HCRU), costs, efficacy, safety and economic evaluation data in achondroplasia and to identify gaps in the research.
Methods
Searches of MEDLINE, Embase, the University of York Centre for Reviews and Dissemination (CRD), the Cochrane Library and grey literature were performed. Articles were screened against pre-specified eligibility criteria by two individuals and study quality was assessed using published checklists. Additional targeted searches were conducted to identify management guidelines.
Results
Fifty-nine unique studies were included. Results demonstrated a substantial HRQoL and HCRU/cost-related burden of achondroplasia on affected individuals and their families throughout their lifetimes, particularly in emotional wellbeing and hospitalisation costs and resource use. Vosoritide, growth hormone (GH) and limb lengthening all conferred benefits for height or growth velocity; however, the long-term effects of GH therapy were unclear, data for vosoritide were from a limited number of studies, and limb lengthening was associated with complications. Included management guidelines varied widely in their scope, with the first global effort to standardise achondroplasia management represented by the International Achondroplasia Consensus Statement published at the end of 2021. Current evidence gaps include a lack of utility and cost-effectiveness data for achondroplasia and its treatments.
Conclusions
This SLR provides a comprehensive overview of the current burden and treatment landscape for achondroplasia, along with areas where evidence is lacking. This review should be updated as new evidence becomes available on emerging therapies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02549-3.
Keywords: Achondroplasia, Disease overview, Dwarfism, Growth hormone, Limb lengthening, Short stature, Vosoritide
Key Summary Points
| Recent advances in therapeutic options have highlighted the need for understanding the burden and treatment landscape of achondroplasia. |
| This SLR included 59 studies reporting clinical or economic outcomes related to the burden of achondroplasia for patients and their caregivers. |
| Treatment options for achondroplasia have historically been limited; however, evidence for new therapies is emerging. |
| Current published literature likely underestimates the true burden of achondroplasia in terms of HRQoL and costs. |
| There is a need for further research to inform best practice for the management of achondroplasia, which should aim to relieve clinical, humanistic and economic burden. |
Introduction
Achondroplasia is the most common form of skeletal dysplasia [1]. It is a rare genetic disease with an estimated prevalence of approximately 1:25,000 live births and affects 250,000 people worldwide [2, 3]. The condition is caused by a recurrent gain-of-function pathogenic variant of the fibroblast growth factor receptor 3 (FGFR3) gene [4, 5]. In addition to extreme short stature (height for a patient’s age that is > 5 standard deviations below the mean) [6], clinical features include rhizomelic limb shortening, macrocephaly, frontal bossing, depressed nasal bridge, relatively small chest and midfacial retrusion [7]. These characteristics typically present at birth or in early childhood [8]. Consequently, achondroplasia is usually diagnosed prenatally or in early infancy [7].
Individuals with achondroplasia may suffer from a range of serious and debilitating complications over the course of their lifetime [9]. Foramen magnum stenosis (the narrowing of the opening at the base of the skull) is considered to be the most severe complication. It can result in compression of the brain stem and spinal cord and lead to sudden death unless patients undergo timely surgical decompression [7, 10]. Other common serious orthopaedic complications include spinal deformities (kyphosis/lordosis and spinal stenosis) and tibial bowing (genu varum) that can lead to pain and limited mobility [5]. Individuals may also experience respiratory problems, leading to sleep disordered breathing, upper airway obstruction and ear, nose and throat (ENT) complications and dental malocclusion, amongst other complications [5, 10–12]. Evidence suggests that achondroplasia also incurs an increased risk of premature death and the average life expectancy is approximately 10 years lower than for the general population [13–15]. In addition to the high clinical burden of disease, available data indicate that achondroplasia is associated with detrimental impacts on physical and mental health-related quality of life (HRQoL) [16–18].
Historically, management of achondroplasia has been largely symptomatic. Surgical interventions aim to improve specific complications, including decompression surgeries for foramen magnum or spinal stenosis, tonsillectomy or adenoidectomy for obstructive sleep apnoea and tympanostomy tube insertion for otitis media [19–22]. Surgical limb lengthening has been investigated in studies since as early as the 1930s and aims to improve individuals’ height and proportionality [23]. However, in practice, use of limb lengthening varies by geography and can be associated with high treatment burden and severe complications [7]. Furthermore, procedures are only performed on long bones, such as the femur or tibia [23], and therefore do not help complications related to other bone types. Despite a clear unmet need, pharmacological therapy options have been previously limited. Until recently, only growth hormone (GH) therapy was indicated for the treatment of achondroplasia and is only approved for use in Japan [24]. Moreover, the long-term efficacy of GH for achondroplasia continues to be debated [25]. In 2021, vosoritide (Voxzogo®), a C-type natriuretic peptide (CNP) analogue, was approved for use in children with achondroplasia in the European Union, US and Brazil [26–28]. Several other therapies are in development, including infigratinib, an FGFR1-3 inhibitor, TA-46 (Recifercept), an FGFR3 decoy, and Transcon-CNP, a CNP [29, 30].
At this critical point with the development and arrival of new therapies, there is a need to comprehensively understand the burden and treatment landscape for achondroplasia, including treatment outcomes and the economic impact of therapies. However, a contemporary and comprehensive overview of the existing evidence base is lacking. Aiming to address this, a systematic literature review (SLR) was conducted to provide an overview of current evidence on the burden and treatment of achondroplasia based on a series of systematic, comprehensive searches of the literature, and to highlight current gaps in the literature.
Methods
The SLR was conducted and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [31]. Systematic literature searches were conducted in August 2020 and updated in June 2021 in accordance with a pre-specified protocol to identify HRQoL/utilities, healthcare resource use (HCRU) and costs in achondroplasia and efficacy, safety and economic evaluations of potential therapies. Where it was judged that there was limited evidence specific to achondroplasia, the searches were expanded to include other forms of short stature. Additional targeted searches were conducted from June 2021 to identify relevant clinical management guidelines.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Identification of Evidence
Electronic English database searches were conducted from database inception in MEDLINE, Embase, the University of York Centre for Reviews and Dissemination (CRD), the Cochrane Library and the International Health Technology Assessment Database (HTAD). These were supplemented by targeted searches of Latin American (Literatura Latino-Americana e do Caribe em Ciências da Saúde), French (Littérature Scientifique en Santé), German (CrescNet.org) and Japanese (医中誌 [Ichushi] Web) databases. The MEDLINE databases and Embase were searched via the Ovid SP platform (available via paid subscription). The other databases searched were freely accessible. In addition, searches of clinical trial registries; health technology assessment (HTA) body websites; economic websites; bibliographies and conference proceedings since 2018 were conducted. Searched congresses included the Annual Genetics Meetings, International Society for Pharmacoeconomics and Outcomes Research (Europe and International Meetings), Endocrine Society Conferences, European Society for Paediatric Endocrinology and International Conference on Children’s Bone Health.
To identify treatment and clinical management guidelines for achondroplasia and other short stature conditions, targeted searches were performed in Google, PubMed, the International Guidelines Library, Evidence Search, GuidelineCentral.com, Das Portal der wissenschaftlichen Medizin, Agenzia Nazionale per i Servizi Sanitari Regionali and Haute Autorité de Santé.
Full details of all literature searches, including search strategies, are presented in Supplementary Appendix 2.
Selection of Studies and Data Extraction
Articles were included if they met pre-defined eligibility criteria based on the Population(s), Intervention(s), Comparator(s) and Outcome(s) (PICO) framework (Supplementary Table 1).
Studies were required to be primary research articles (in any language) reporting on a relevant outcome (including HRQoL/utilities, caregiver quality of life [QoL], HCRU/cost, efficacy, safety, economic evaluations). Studies reporting HRQoL, utility, HCRU or cost outcomes could include both children and/or adults to account for the lifetime impacts of achondroplasia. Studies reporting clinical outcomes (efficacy or safety) were limited to paediatric individuals with achondroplasia that received any pharmacological intervention or surgical limb lengthening. Management guidelines were required to report at least one recommendation relevant to the management of achondroplasia or another short stature condition.
Titles, abstracts and relevant full texts were screened against the eligibility criteria by two independent reviewers. Results from the English databases searches were dual reviewed with any discrepancies between the two reviewers discussed and resolved, arbitrated by a third independent reviewer if necessary. Review of the supplementary databases, grey literature sources and guidelines was conducted by a single reviewer with a second reviewer providing input in cases of uncertainty. All included records were confirmed by a second reviewer. Key information from each included study, including study characteristics, patient characteristics and outcomes, was extracted into a pre-specified data extraction grid by a single individual. A second individual independently verified the extracted information.
Changes to Protocol
Caregiver quality of life was not included as an outcome of interest until the update to the searches; therefore, evidence from the original searches was re-screened to ensure all relevant articles were identified. Articles from the Japanese database, Ichushi Web, were not extracted because of the availability of substantial evidence from other sources.
Quality Assessment
Different quality assessment tools were employed, based on study design, and were completed by one individual and verified by a second independent individual. The quality of randomised clinical trials (RCTs) was assessed using the tool developed by the University of York CRD, as recommended by the National Institute for Health and Care Excellence (NICE) [32]. Interventional non-RCTs and observational studies were assessed using the Downs and Black checklist [33]. Quality assessments of HCRU/cost and HRQoL/utility studies were not conducted as no validated quality assessment tool exists to the authors’ knowledge.
Results
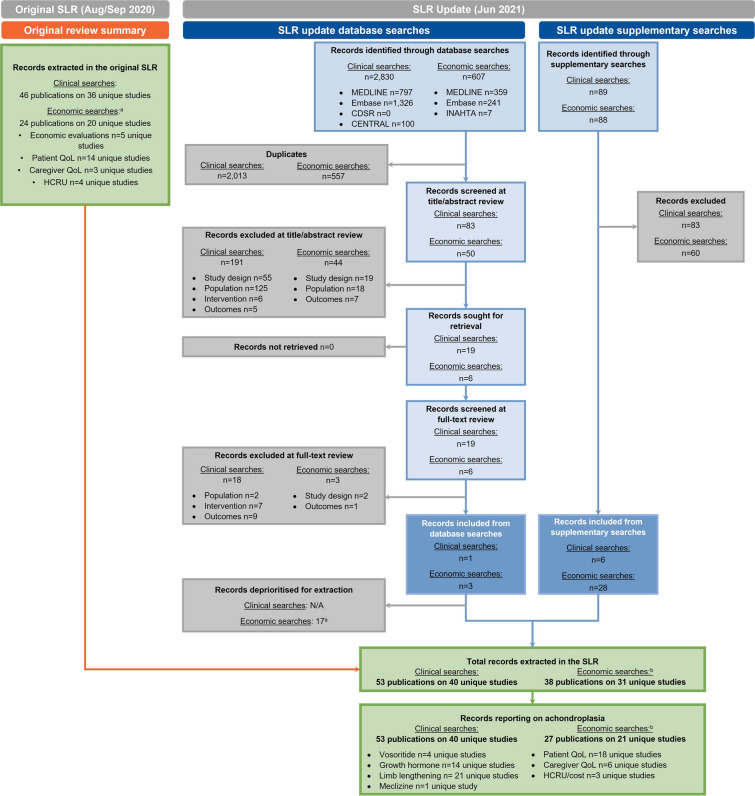
The number of studies included at each stage of the SLR across all outcomes is presented in a PRISMA flow diagram (Fig. 1). This article focuses on the studies that reported outcomes specifically for achondroplasia. Fifty-nine unique studies were included (40 from the clinical searches and 21 from the economic searches, with two studies identified in both streams). The geographic spread of included studies is presented in Fig. 2. Study details are summarised in Tables 1, 2, 3, 4 and 5.
Fig. 1.
PRISMA flow diagram of studies included in the SLR. PRISMA diagram reporting flow of studies included in the SLR. In total, 59 unique studies were included across both streams (two studies were included in both the clinical and economic searches [36, 38]). aSome records identified in the economic searches were included in multiple evidence streams (i.e., both patient QoL and costs). bStudies reporting outcomes for forms of short stature other than achondroplasia are not included in this article. CDSR Cochrane Database of Systematic Reviews, CENTRAL Cochrane Central Register of Controlled Trials, HCRU healthcare resource use, INAHTA International Network of Agencies for Health Technology Assessment, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, QoL quality of life, SLR systematic literature review
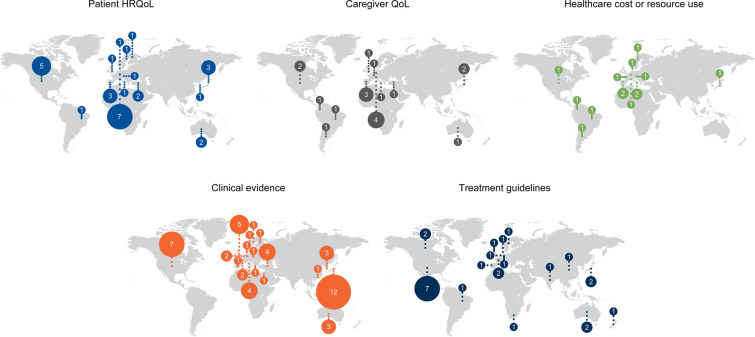
Fig. 2.
Geographical spread of studies included in the review. Geographic spread of studies reporting different outcomes in the literature review. Bubble size scaled to represent number of studies. International studies are recorded in multiple countries, aligned with their participating centres. Patient HRQoL: Germany, n = 7; US, n = 5; Japan and Spain, n = 3; Australia and Turkey, n = 2; Austria, Brazil, Denmark, Finland, Italy, South Korea, Sweden and UK, n = 1. Caregiver QoL: Germany, n = 4; Spain, n = 3; Japan and US, n = 2; Argentina, Australia, Brazil, Colombia, France, Italy, Turkey and UK, n = 1. Healthcare cost and resource use: Italy and Spain, n = 2; Argentina, Austria, Brazil, Colombia, Denmark, France, Germany, Japan, Sweden and US, n = 1. Clinical evidence: Japan, n = 12; US, n = 7; UK, n = 5; Italy and Turkey, n = 4; Australia and South Korea, n = 3; France and Germany, n = 2; Denmark, Finland, Greece, Hong Kong, Israel, Norway, Poland, Spain and Sweden, n = 1. Treatment guidelines: US, n = 7; Australia, Canada, France and Japan, n = 2; Brazil, China, Denmark, Germany, India, New Zealand, Portugal, South Africa, Sweden, The Netherlands and UK, n = 1
Table 1.
Characteristics and results of included patient HRQoL studies
| Study name | Country | Sample size | Intervention or prior treatment | Tool, unit | QoL at baseline (BL) | QoL post-intervention (PI) | ||
|---|---|---|---|---|---|---|---|---|
| Self-reported | Parent/caregiver-reported | Self-reported | Parent/caregiver-reported | |||||
| Ireland 2011 [48] | Australia | 35 parents of children aged 3–12 | None | WeeFIM-II, mean (SD) | - |
3 years: 51.14 (13.34) 5 years: 86.67 (15.11) 7 years: 95.44 (11.84) |
- | - |
| Study 111-301 [36] | Australia; Germany; Japan; Spain; Turkey; US; UK | 121 patients aged 5–17 | Vosoritide 15 μg/kg (n = 60) or placebo (n = 61) | QoLISSY, median (IQR) |
Vosoritide (n = 30): 66.84 (52.08–77.09) PBO (n = 36): 66.50 (57.12–77.51) |
Vosoritide (n = 60): 56.25 (47.25–68.40) PBO (n = 61): 58.33 (39.59–70.54) |
CfB at Week 52 Vosoritide (n = 26): 0.69 (− 4.17 to 8.34) PBO (n = 37): 1.39 (− 7.64 to 9.38) |
CfB at Week 52 Vosoritide (n = 57): − 1.73 (− 6.94 to 7.29) PBO (n = 60): 1.22 (− 3.82 to 11.64) |
| PedsQL, median (IQR) |
Vosoritide (n = 28): 74.46 (65.76–84.24) PBO (n = 35): 73.91 (66.30–89.77) |
Vosoritide (n = 59): 71.74 (58.70–84.78) PBO (n = 59): 73.86 (59.78–84.78) |
CfB at Week 52 Vosoritide (n = 25): 1.09 (− 6.68 to 8.70) PBO (n = 33): 0.00 (− 10.87 to 6.52) |
CfB at Week 52 Vosoritide (n = 56): − 0.54 (− 7.61 to 7.62) PBO (n = 57): 2.96 (− 5.43 to 9.78) |
||||
| WeeFIM, mean (SD) |
Vosoritide (n = 57): 109.82 (13.56) PBO (n = 60): 110.57 (13.71) |
– |
CfB at Week 52 Vosoritide (n = 54): 2.31 (8.01) PBO (n = 59): 1.86 (10.03) |
– | ||||
| LIAISE [44, 122] | Austria; Germany; Italy; Spain; Sweden; Denmark | 186 patients aged 5–84 | Limb lengthening | EQ-5D-5L utility, mean |
Adults (n = 74): 0.7 Reference population: 0.9 |
– | – | – |
| EQ-5D-5L VAS, mean |
Adults (n = 74): 73.9 Reference population: 80.1 |
– | – | – | ||||
| NHP, mean (SD) | Adults (n = 74): 16.0 (18.9) | – | – | – | ||||
| BPI-SF, % patients |
Adults (n = 72) ≥ 1 pain site: 70.3 ≥ 3 pain sites: 41.9 |
– | – | – | ||||
| QoLISSY, mean (SD) | Children/adolescents (n = 67) or parents (n = 108): 58.0 (21.8) | – | – | |||||
| PedsQL, mean (SD) | n = 105: 69.3 (16.3) | – | – | - | ||||
| WeeFIM, mean (SD) | Not specified (n = 104): 112.7 (13.3) | – | – | |||||
| APPT, % patients |
Adolescents (n = 50) ≥ 1 pain site: 58.6 ≥ 3 pain sites: 32.9 |
– | – | – | ||||
| Cervan (2008) [43] | Brazil | 22 patients aged 15–54 | None | WHOQOL-BREF, mean (SD) |
ACH (n = 22) Male: 77.2 (6.4) Female: 69.6 (11.3) Male: p vs. controls: 0.761 Female: p vs. controls: 0.077 Controls (n = NR) Male: 76.0 (10.6) Female: 76.8 (8.3) |
– | – | – |
| Finnish Skeletal Dysplasia Register [42] | Finland | 8 adults aged 16–54 | None | 15D: adults, mean utility score | Adults (n = 8), age- and sex-standardised: 0.911 | – | – | – |
| BKMF 2016 [40] | Germany |
58 children aged 8–18 56 parents |
Self-help intervention |
QoLISSY, BL: mean (SD) PI: MD (SD, 95% CI) |
Participants (n = 44): 58.56 (17.16) Non-participants (n = 13): 53.04 (17.07) |
Participants (n = 41): 51.78 (20.34) p vs. children: NR Non-participants (n = 13): 48.16 (15.60) p vs. children: NR |
Participants: 4.10 (2.04; 2.44–9.60) p vs. non-participants: 0.040 |
Parent-reported: − 1.92 (2.11; − 9.59 to -2.44) p vs. children: 0.001 |
| BKMF and UKE collaboration 2017 [34] | Germany | 80 patients aged 8–29 | Patient education and intervention program |
QoLISSY, BL: mean (SD) PI: MD (SE, 95% CI) |
All self-reported (n = 61): 60.52 (18.53) Children (n = 45): 49.95 (22.23) Young adults (n = 16): 69.52 (12.84) p vs. children: < 0.001 |
Parent-reported (n = 44): 47.44 (18.39) p vs. self-report: 0.008 |
Participants: 5.33 (1.55; 2.25–8.41) Non-participants: − 2.88 (2.68; − 8.22 to 2.45) p vs. participants: 0.009 |
– |
| Rohenkohl (2015) [41, 52] | Germany | 89 children aged 8–17 | None | KIDSCREEN-10, mean (SD) |
Patients with ACH (n = 89): 78.42 (10.94) p vs. participants without ACH: 0.55 Participants without ACH (n = NR): 77.73 (13.22) |
Parent-reported (n = 63): 72.76 (10.58) | – | - |
| SDQ, mean (SD) |
Patients with ACH (n = 89): 9.12 (5.18); p vs. patients without ACH: 0.035 Participants without ACH (n = NR): 10.3 (5.2) |
– | – | – | ||||
| QoLISSY, mean (SD) |
Patients with ACH (n = 89): 60.52 (18.53) p vs. parent-reported: < 0.001 |
Parent-reported (n = 63): 48.39 (18.08) | – | – | ||||
| DISABKIDS, mean (SD) |
Patients with ACH (n = 89): 74.01 (16.07) p vs. parents: NR |
Parent-reported (n = 63): 68.00 (15.61) | – | – | ||||
| Witt 2019 (APLES study) [45] | Germany | 47 children aged 5–14 | None | PedsQL, mean (SD) |
Self-reported (n = 47): 73.76 (18.04) Reference value: 83.84 |
Parent-reported (n = 73): 63.70 (15.83) p vs. self-reported: ≤ 0.01 Reference value: 82.70 |
– | – |
| Bloemeke (2019) (APLES study) [35, 46] | Germany; Spain | 88 aged 5–14 | None | APLES, mean (SD) |
Self-reported (n = 87): 70.55 (12.24) p vs. parent-reported: ≤ 0.01 |
Parent-reported (n = 132): 60.57 (11.54) | – | – |
| Matsushita (2019) [47] | Japan | 184 patients aged 10–67 | None | SF-36, mean (SD) |
PCS Patients 100–139 cm (n = 130): 38.08 (17.20) Patients 140–159 cm (n = 45): 49.42 (12.77) MCS Patients 100–139 cm: 53.65 (10.66) Patients 140–159 cm: 52.47 (11.86) |
– | – | – |
| Nishimura (2014) [18] | Japan | 73 children aged 8–18 | None | Short stature-related experience scales,a range in overall averages across items |
Total (n = 73): − 0.2 to 1.3 M (n = 30): − 0.1 to 1.4 F (n = 43): − 0.5 to 1.2 |
– | – | – |
| Kim (2012) [37, 123] | South Korea | 34 patients aged 6–20 | Limb lengthening (tibial/femoral) | AAOS lower limb score, mean (SD) | – | – | 17.27 (8.16); p vs. non-participants: 0.645 | - |
| SF-36, mean (SD) | – | – | 52.77 (17.43); p vs. non-participants: 0.3078 | - | ||||
| Rosenberg self-esteem scale, mean (SD) | – | – | 22.1 (2.5); p vs. non-participants: < 0.001 | - | ||||
| Batibay (2020) [38] | Turkey | 49 patients aged 11–18 | Limb lengthening (tibial/femoral) | PedsQL, mean (SD) | – | – | All (n = 49): 80.80 (4.48; range 73–90); p vs. controls: 0.701 | - |
| Alade (2013) [51] | US | 361 patients, mean age 35 | None | BPI, mean (SD) |
Severity (n = 88): 2.9 (1.8) Interference (n = 88): 3.0 (2.7) 64.1% of 153 respondents experienced “more than everyday pain” |
– | – | – |
| Bleck scale, % |
Adults with ACH: 13.0 Children with ACH: 2.7 p vs. adults: NR |
– | – | – | ||||
| Gollust (2003) [50, 124] | US | 189 adults, mean age 40.5 | None | Ferrans and Powers Quality of Life Index, mean (SD) |
Adults with ACH (n = 189): − 14.083 (3.248) |
– | – | – |
| Mahomed (1998) [39] | US | 473 adults aged 18–90 | Surgery in 298/437 participants | SF-36, mean |
PCS Patients without surgery (n = NR): 45.9–48.7 MCS Patients without surgery: 49.5–50.2 |
– |
PCS Patients with surgery (n = NR): 27.5–47.1; p vs. patients without surgery: NR MCS Patients with surgery: 39.3–52.5; p vs. patients without surgery: NR |
– |
| Yonko (20210 [49] | US | 25 adults aged 19–66 | One patient had undergone prior surgical limb lengthening | SF-36, mean (SD) |
PCS All (n = 25): 36.9 (14.8) F (n = 15): 35.2 (13.4) M (n = 10): 39.5 (17.2); p vs. F patients: 0.238 MCS All (n = 25): 38.9 (15.4) F (n = 15): 42.3 (15.5) M (n = 10): 33.7 (14.5); p vs. F patients: 0.062 |
– | – | – |
15D 15-dimensional measure of health-related quality of life, 16D 16-dimensional measure of health-related quality of life, AAOS American Academy of Orthopaedic Surgeons, ADL activities of daily living, APLES Achondroplasia Personal Life Experience Scale, APPT Adolescent Pediatric Pain Tool, AZL Academic Hospital in Leiden, BKMF Bundesverband Kleinwüchsige Menschen und ihre Familien, BL baseline, BPI(-SF) Brief Pain Inventory (-Short Form), CfB change from baseline, CHH cartilage-hair hypoplasia, DD diastrophic dysplasia, EQ-5D-5L EuroQol 5-Dimension 5-Level, F female, M male, MCS mental component summary, MD mean difference, NHP Nottingham Health Profile, NR not reported, PBO placebo, PCS physical component summary, PedsQL Pediatric Quality of Life Inventory, PI post-intervention, QoL quality of life, QoLISSY Quality of Life in Short Stature Youth, RCS role component summary, SD standard deviation, SDQ Strengths and Difficulties Questionnaire, SF-36 Short Form 36, TACQOL-S TNO-AZL Children’s Quality of Life Short Stature Module, TNO Netherlands Organization for Applied Scientific Research, UK United Kingdom, UKE University Medical Center Hamburg Eppendorf, US United States, VAS visual analogue scale; vs, versus, WeeFIM(-II) Functional Independence Measure for Children, WHOQOL-BREF World Health Organization Quality of Life: Brief Version
aConsisting of items from the TNO TACQOL-S and additional questions derived from publications on short stature
Table 2.
Characteristics and results of included caregiver QoL studies
| Study name | Country | Sample size | Scale details, unit | Intervention or prior treatment | Key QoL findings |
|---|---|---|---|---|---|
| Study 111-301 [36] | Australia; Germany; Japan; Spain; Turkey; US; UK | 121 | QoLISSY ‘effects on parents’ subscale, median (IQR) | Vosoritide 15 μg/kg (n = 60) or placebo (n = 61) |
At baseline Vosoritide (n = 60): 60.00 (46.25–72.50) Placebo (n = 61): 62.50 (40.00–77.50) CfB at week 52 (MD) Vosoritide (n = 57): − 2.50 (− 10.00 to 10.00) Placebo (n = 60): 0.00 (− 7.50 to 15.00) |
| BKMF 2016 [40] | Germany | 56 | QoLISSY (German version) ‘effects on parents’ subscale, mean (SD) | Self-help intervention |
At baseline Parents of participants: 62.73 (19.67) Parents of non-participants: 62.24 (21.98); p vs. participants: 0.117 At follow-up Not reported |
| Rohenkohl (2015) [41, 52] | Germany | 63 | QoLISSY (German version) ‘effects on parents’ subscale, mean (SD) | None | Parents: 62.75 (19.85) |
| Witt (2019) (APLES study) [45] | Germany | 73 | SF-8, mean (SD) | None |
Parent SF-8 PCS: 50.50 (8.49); p vs. German reference population 0.85 Parent SF-8 MCS: 46.51 (10.22); p vs. German reference population ≤ 0.01 |
| Baratela (2021) [54] | Japan; Europe (Spain, France, Italy); Latin America (Brazil, Argentina, Colombia) | 660 | NR | None apparent | Over 50% of caregivers reported impacted emotional wellbeing; < 40% were offered social/psychological support |
| Pfeiffer (2020) [53] | Spain; US | 36 | APEM, % | None apparent |
Parent-reported (% with impact or issue) Managing child’s medical care treatment: 92 Impacts on parent emotional wellbeing: 100 Impacts on parent physical wellbeing: 28 Limit social/other activities: 28 Strain on family: 56 Work/productivity issues: 78 Expert-reported (% with impact or issue) Managing child’s medical care treatment: 86 Impacts on parent emotional wellbeing: 100 Impacts on parent physical wellbeing: 0 Limit social/other activities: 14 Strain on family: 86 Work/productivity issues: 57 |
APEM Achondroplasia Parent Experience Measure, MCS Mental Component Score, MD mean or median difference, NR not reported, PCS Physical Component Score, QoL quality of life, QoLISSY Quality of Life in Short Stature Youth, SF-8 Short Form 8, UK United Kingdom, US United States
Table 3.
Characteristics and results of included cost and resource use studies
| Study name | Setting | Population | Direct costs reported | Resource use reported |
|---|---|---|---|---|
| Achondroplasia | ||||
| LIAISE [56, 57] | Germany, Spain, Italy, Sweden, Austria, Denmark | Children and adults with achondroplasia | None | Length of stay; frequency of specialist visits; inpatient/outpatient visits per patient; medications and supporting therapies per patient; proportion |
| Baratela (2021) [24] | Japan, Europe (Spain, France, Italy) and Latin America (Brazil, Argentina, Colombia) | Caregivers of patients with achondroplasia | None | Proportion of patients with primary physician visits every 6 months; frequency of primary physician appointments |
| Chen (2021) [55] | US | Adults and children with achondroplasia (N = 1985) |
Cost year: 2017, USD Total cost of hospitalisation; total inpatient costs; primary payer (insurance) |
Length of stay |
US United States, USD US dollar
Table 4.
Characteristics of included clinical studies
| Study name | Country | Sample size | Study design | Study duration | Intervention | Comparator(s) | Reported outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Height or change in heighta | AGV | Bone morphology | Adverse events | |||||||
| Vosoritide | ||||||||||
| Study 111-301 (NCT03197766) [36] | Australia; Germany; Japan; Spain; Turkey; UK; US | 121 | Double-blind phase 3 RCT | 52 weeks | Vosoritide, 15.0 µg/kg daily (n = 60) | Vosoritide placebo, daily (n = 61) | ✓ | ✓ | ✓ | |
| Study 111-302 (NCT03424018) [60] | 119 | Open-label phase 3 extension study | + 52 weeks (up to 2 years for study − 301 and − 302) | Vosoritide, 15.0 µg/kg daily (n = 119) | NA | ✓ | ✓ | ✓ | ||
| Study 111-202 (NCT02055157) [58] | US; Australia; France; UK | 35 | Non-randomised dose-escalation phase 2 trial | 24 months |
Cohort 1 (n = 8): Vosoritide 2.5 µg/kg once-daily during first 6 months then increased to 7.5 µg/kg then 15.0 µg/kg based on safety and efficacy data Cohort 2 (n = 8): Vosoritide 7.5 µg/kg once-daily during first 6 months; then increased to 15.0 µg/kg based on safety and efficacy data Cohort 3 (n = 10): Vosoritide 15.0 µg/kg once-daily Cohort 4 (n = 9): Vosoritide 30.0 µg/kg once-daily |
NA | ✓ | ✓ | ✓ | ✓ |
| Study 111-205 (NCT02724228) [59] | 30 | Open-label phase 2 extension study | + 36 months (up to 60 months for study − 202 and − 205) | Patients continued on same stable dose of vosoritide as they were upon completion of Study 111-202 | NA | ✓ | ✓ | ✓ | ||
| Growth hormone | ||||||||||
| Stamoyannou (1997) [66] | Greece | 15 | Single arm trial | 2 years | GH 1 IU/kg/week | NA | ✓ | ✓ | ✓ | ✓ |
| Weber (1996) [91] | Italy | 6 | Single arm trial | 18 months | rhGH 0.1 IU/kg/day | NA | ✓ | ✓ | ||
| Seino (2000) [61] | Japan | 145 | Open-label RCT | 4 years | GH, 0.33 (1.0 IU) mg/kg/week | GH, 0.17 (0.5 IU) mg/kg/week | ✓ | ✓ | ✓ | |
| Kanazawa (2003) [62] | Japan | 73 | Single arm trial | 1 year | GH 0.35 mg/kg/week | NA | ✓ | ✓ | ✓ | |
| Kubota (2016) [125] | Japan | 16 | Single arm trial | 4 years, 11 months | GH 0.35 mg/kg/week | NA | ||||
| Nishi (1993) [126] | Japan | 6 | Single arm trial | 4 years | GH 0.5 IU/kg/week | NA | ✓ | ✓ | ||
| Tanaka (1998) [88] | Japan | 42 | Single arm trial | 3 years | GH 1.0 or 0.5 IU/kg/week | NA | ✓ | ✓ | ||
| Tanaka (2003) [64, 88] | Japan | 11 | Single arm trial | 3 years | GH 0.5 IU/kg/week or 1.0 IU/kg/week | NA | ✓ | ✓ | ✓ | |
| Yamate (1993) [89] | Japan | 22 | Single arm trial | 6 months | rhGH 1 IU/kg/week | NA | ✓ | |||
| Harada (2017) [68] | Japan | 22 | Retrospective cohort study | NR | rhGH 0.05 mg/kg/dayb | NA | ✓ | ✓ | ||
| Hertel (2005) [65] | Sweden; Norway; Finland; Denmark; Germany | 35 | Open-label RCT | 5 years | GH, 0.033 (0.1 IU) mg/kg/week | GH, 0.067 (0.2 IU) mg/kg/week | ✓ | ✓ | ✓ | |
| Ramaswami (1999) [63] | UK | 35 | Single arm trial | 6 years | GH median dose 30 (15.8–40.0) U/m2/week | NA | ✓ | ✓ | ||
| Shohat (1996) [90] | US | 11 | Single arm trial | 2 years | rhGH 0.04 mg/kg/day | NA | ✓ | ✓ | ✓ | |
| NCGS Database [67] | US | 14 | Retrospective cohort study | 1 year | GH, mean 0.306 mg/kg/week | NA | ✓ | ✓ | ||
| Limb lengthening | ||||||||||
| Edwards 1994 [78] | Australia | 10 | Single arm trial | 5 years | Tibial and femoral lengthening | NA | ✓ | ✓ | ||
| Prevot (1997) [72] | France | 12 | Retrospective cohort study | NR | Lower and upper extremity lengthening | NA | ✓ | ✓ | ||
| Cheng (2002) [93] | Hong Kong | 7 | Single arm trial | NR | Lower limb lengthening | NA | ||||
| Ganel (1996) [81] | Israel | 12 | Retrospective cohort study | NR | Femur or tibia lengthening | NA | ✓ | |||
| De Bastani (1996) [71] | Italy | 25 | Retrospective cohort study | NR | Lower limb lengthening | NA | ✓ | ✓ | ||
| Peretti (1995) [69] | Italy | 22 | Retrospective cohort study | NR | Lower limb lengthening | NA | ✓ | |||
| Aldegheri (1999) [74] | Italy | 29 | Retrospective cohort study | 5 years 11 months | Tibial lengthening | NA | ✓ | ✓ | ||
| Kadono (2018) [77] | Japan | 6 | Single arm trial | NR | Tibial limb lengthening | NA | ✓ | ✓ | ✓ | |
| Nakano-Mastsuoka (2017) [87] | Japan | 54 | Retrospective cohort study | 16 years 1 month | Humeral lengthening | NA | ✓ | ✓ | ||
| Shadi (2007) [79] | Poland | 5 | Single arm trial | 3 years | Humeral lengthening | NA | ✓ | ✓ | ||
| Song (2012) [82, 83] | South Korea | 35 | Retrospective case–control | 5 years | Bilateral tibial lengtheningc | Observation only | ✓ | ✓ | ||
| Song (2020) [84] | South Korea | 36 | Retrospective cohort study | NR | Bilateral tibial lengthening | NA | ✓ | ✓ | ||
| Devmurari (2010) [75] | South Korea | 14 | Retrospective cohort study | NR | Femoral lengthening | NA | ✓ | |||
| Kocaoğlu (2014) [76] | Turkey | 22 | Single arm trial | 8 years 11 months | Lower limb lengthening | NA | ✓ | ✓ | ||
| Batibay (2020) [38] | Turkey | 49 | Retrospective case–control | NR | Bilateral femur and tibial lengthening | NA | ✓ | ✓ | ||
| Balci (2015) [85] | Turkey | 18 | Retrospective case series | 12 years | Bilateral humeral lengthening | NA | ✓ | ✓ | ||
| Bridgman (1993) [80] | UK | 7 | Retrospective cohort study | 6 years | Lower limb lengthening | NA | ✓ | |||
| Donaldson (2015) [70] | UK | 10 | Retrospective cohort study | 15 years | Lower limb lengthening | NA | ✓ | ✓ | ||
| Griffith (2006) [94] | US | 2 | Retrospective cohort study | NR | Two limb lengthenings of the same bone | NA | ✓ | |||
| Price (1989) [73] | US | 3 | Retrospective case series | NR | Bilateral tibial and femoral lengthening | NA | ✓ | ✓ | ||
| Morrison (2020) [86] | US | 9 | Retrospective case series | 19 years | Humeral lengthening | NA | ✓ | ✓ | ||
| Meclizine | ||||||||||
| Kitoh (2020) [92] | Japan | 12 | Non-randomised 2-arm trial | 4 months | Meclizine 25 mg once daily | Meclizine 25 mg twice daily | ✓ | |||
AGV annualised growth velocity, GH growth hormone, NA not applicable, NR not reported, rhGH recombinant human growth hormone, US United States, UK United Kingdom
a Includes standing height, limb length etc.
b15 patients also received lower limb lengthening
aAdditionally, 12 patients underwent femoral lengthening
Table 5.
Summary of included management guidelines
| Country | Organisation and year of publication | Condition | Guidance category | Intervention/management strategy of recommendation |
|---|---|---|---|---|
| Achondroplasia | ||||
| US | Skeletal Dysplasia Management Consortium 2020 [103] | Achondroplasia | Management | Polysomnography; foramen magnum decompression; MRI; patient history and physical exam; CT scans; MRI |
| American Academy of Pediatrics 2020 [100] | Achondroplasia | Management | Growth and developmental measurements; neurological evaluation; neuroimaging; monitoring; audiological evaluation; physical evaluation; motor development evaluation; polysomnography; expert consultation; physical therapy; speech evaluation; medical evaluation; pain evaluation | |
| Skeletal Dysplasia Management Consortium 2016 [102] | Skeletal dysplasia; achondroplasia | Management | Patient history and clinical exam; polysomnography; MRI; audiological evaluation; management; adenoidectomy and/or tonsillectomy; monitoring; specialised dental and orthodontic care; imaging and/or evaluation of the larynx | |
| Australia | The Sydney Children's Hospital Network 2021 [106] | Achondroplasia | Management | Physiotherapy |
| France | OSCAR—French Rare Diseases Healthcare Network 2017 [99] | Achondroplasia | Management | Expert consultation; Clinical evaluation; monitoring; MRI; polysomnography; audiological evaluation; physiotherapy |
| Japan | Guidelines Development Committee 2020 [104] | Achondroplasia | Treatment and management | Foramen magnum decompression; shunt surgery; non-invasive positive pressure ventilation; surgical treatment (tonsillectomy or adenoidectomy); spinal decompression; leg lengthening surgery |
| International | Skeletal Dysplasia Management Consortium 2021 [110] | Skeletal dysplasia; achondroplasia; hypochondroplasia | Management | Surgical decompression; neuromonitoring; flexion/extension plain radiographs; advanced imaging; physical exam; prophylactic C1–C2 fusion; repeated evaluation of patients for thoracolumbar kyphosis; stabilisation of thoracolumbar kyphosis via surgery; respiratory function monitoring; brace or cast treatment; surgical techniques that preserve spine growth; monitoring |
| International Achondroplasia Consensus Statement Group [109] | Achondroplasia | Diagnosis, treatment and management | Diagnostics; prenatal care; multi-disciplinary care; foramen magnum stenosis; spinal stenosis; sleep apnoea; motor development, helping aids and assistive devices; lifelong care; psychosocial health; GH; limb lengthening; audiological assessment; orthodontics; pain management; diet and exercise; importance of patient advocacy groups | |
| Other short stature conditions | ||||
| International | Growth Hormone Research Society 2019 [108] | GH deficiency; non-GH deficiency indications | Treatment and management | Recombinant hGH; alternative treatments to recombinant hGH |
| US and Canada | Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society 2016 [107] | GH deficiency; idiopathic short stature; primary IGF-1 deficiency | Treatment, management, and follow-up |
GH (for GH deficiency and idiopathic short stature) IGF-1 treatment (for primary IGF-1 deficiency) |
| US | Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee 2003 [101] | GH deficiency; Turner syndrome; SGA; Prader-Willi syndrome; idiopathic short stature; patients receiving GH | Treatment, management, and follow-up | GH |
| South Africa | Paediatric and Adolescent Endocrine and Diabetes Society of South Africa 2009 [105] | GH deficiency; Turner syndrome; Prader-Willi syndrome; SGA; idiopathic short stature | Treatment | GH |
| Wales | All Wales Clinical Biochemistry Audit Group 2004 [98] | GH deficiency | Treatment and follow-up | GH |
CT computerised tomography, IGF-1 Insulin-like growth factor 1, GH growth hormone, hGH human growth hormone, MRI magnetic resonance imaging, NR not reported, SGA small for gestational age, US, United States
Burden of Short Stature Conditions
HRQoL and Utilities
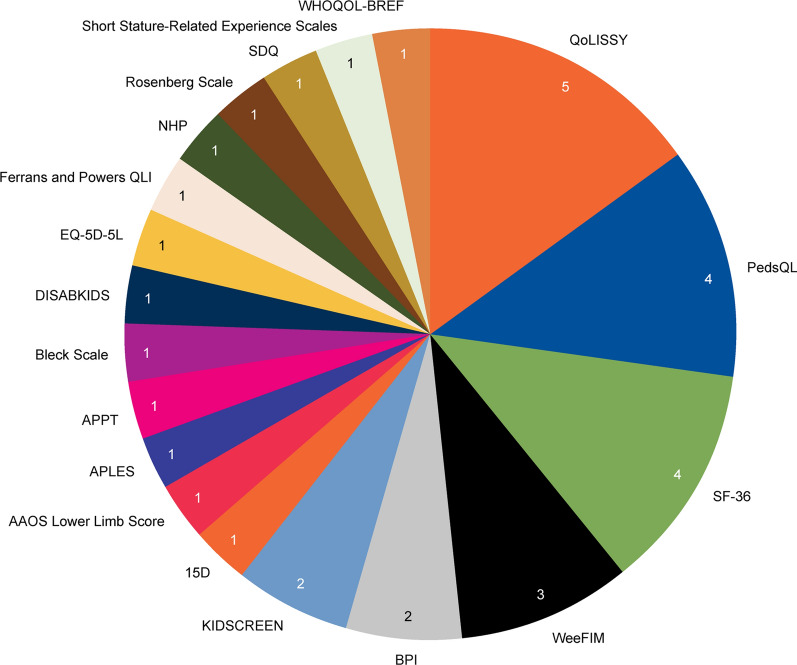
Eighteen studies reported HRQoL outcomes for individuals with achondroplasia, of which 13 were conducted in a European setting (Fig. 2). The majority of studies included children only (n = 8) or a mixed population of children and adults (n = 7), with three measuring HRQoL in adults only (Table 1). Nineteen different instruments were used to elicit HRQoL data. The most commonly used was the Quality of Life in Short Statured Youth (QoLISSY) questionnaire, used in five studies. This was followed by the Pediatric Quality of Life Inventory (PedsQL) and the 36-Item Short Form Health Survey (SF-36), each used in four studies (Fig. 3). HRQoL was self-reported in 10 studies, caregiver-reported in one study, and both in seven studies. Utilities were assessed in two studies, one using the EQ-5D-5L scale and one using the 15-dimensional (15D) validated generic self-assessment instruments of HRQoL to measure utility indexes.
Fig. 3.
HRQoL scales used in the included studies. HRQoL scales used to measure HRQoL in achondroplasia. Nineteen different instruments were used to elicit HRQoL data. The most commonly used scale was the QoLISSY questionnaire (n = 5 studies), followed by PedsQL and the SF-36 (n = 4 studies), WeeFIM (n = 3 studies), BPI and KIDSCREEN (n = 2 studies). Other scales were used in one study each, including two studies that assessed utilities (via EQ-5D and 15D scales). The figure does not tally with the number of included studies (N = 18) because of some studies using multiple QoL scales. 15D 15-dimensional measure of health-related quality of life, AAOS American Academy of Orthopaedic Surgeons, APLES Achondroplasia Personal Life Experience Scale, APPT Adolescent Pediatric Pain Tool, BPI Brief Pain Inventory, EQ-5D-5L EuroQol 5-Dimension 5-Level, NHP Nottingham Health Profile, PedsQL Pediatric Quality of Life Inventory, QLI Quality of Life Index, QoLISSY Quality of Life in Short Stature Youth, SDQ Strengths and Difficulties Questionnaire, SF-36 Short Form 36, WeeFIM Functional Independence Measure for Children, WHOQOL-BREF World Health Organization Quality of Life: Brief Version
Two of the measured tools were condition-specific (QoLISSY and the Achondroplasia Personal Life Experience Scale [APLES]); the others were generic scales. QoLISSY is a tool that is scored from 0–100 (higher score indicates better HRQoL). It contains 22 items covering physical, social and emotional HRQoL, 10 items covering additional aspects of coping, four items covering general attitude to body height and additional items in the parent version covering child’s future and impact on parents [34]. APLES is an instrument that was developed based on the International Classification of Functioning-Children and Youth Version. It contains 21 items covering self-perception, friends, recreation, school and physical domains [35].
Six studies reported HRQoL data relating to an intervention for achondroplasia. The interventions included vosoritide (n = 1) [36], limb lengthening (n = 2) [37, 38], surgical procedures (not limited to limb lengthening) (n = 1) [39] and self-help/education seminars (n = 2) [34, 40]. Only the self-help and patient education interventions resulted in demonstrable benefits to patients’ HRQoL compared with scores for non-participants [34, 40]. Both studies were based in Germany, recruited participants from patient organisations and measured HRQoL using QoLISSY (details aforementioned). Both studies investigated self-help seminars that were designed following focus group discussions and a questionnaire for patients and their parents. They included eight modules covering physical, emotional, social and coping domains. In Rohenkhol 2016, across 58 children aged 8–17 years, mean (± SD) QoLISSY scores for participants after the intervention significantly increased by 5.12 (± 1.75; p = 0.003). This differed significantly from scores of 13 non-participants, which decreased by 2.94 (± 3.36) (p = 0.040). Similar increases were reported across all QoL domains, but the largest was social (+ 7.26), followed by physical (+ 6.52) then emotional (+ 5.01). The same study also compared patient- and parent-reported scores, finding that children reported a significantly more positive change in QoL compared to their parents (+ 4.10 vs. − 1.92; p = 0.001). Parents rated the change in emotional QoL as the worst, at − 4.27 [40]. Very similar findings were reported in Witt 2017, which included patients with achondroplasia aged 18–28. The total QoLISSY scores (reported by 61 patients and 44 parents) increased by 5.33 (± 1.55) in those that participated, whereas there was a reduction of 2.88 (± 2.68) for those who did not participate (p = 0.009). Similar gains were seen across, social, emotional and attitudes domains (+ 6.51, + 5.99 and + 5.20, respectively) [34]. The study also compared patients’ and parents’ perspectives (unrelated to any intervention) and found that patients rated their HRQoL significantly higher across all subscales of QoLISSY than their parents. Prior to intervention, the authors found that clinical, sociodemographic and psychosocial variables explained 49% of the variance of the QoLISSY total score, with attitudes towards body height identified as the most relevant predictor for HRQoL. It should be noted that both studies were conducted by the same research group and are therefore likely to have included overlapping patients.
A further 12 studies reported HRQoL unrelated to treatment. Four studies compared QoL in participants with and without achondroplasia [41–44], four studies compared patient- and caregiver/parent-reported values [41, 44–46], three compared subgroups of patients with achondroplasia (different height groups [47], age groups [48] and sexes [49]) and three studies made no comparisons [18, 50, 51]. In the four studies that compared caregiver/parent- and patient-reported values, where the age of patients ranged from 5–17 years, HRQoL was consistently judged to be lower by caregivers than patients, across all HRQoL scales [41, 44–46]. This difference was significant in the three studies that reported results of statistical testing [35, 41, 45, 46]. One of these studies used a condition-specific HRQoL tool, APLES, and parents rated their child’s HRQoL significantly lower than the self-reported value for total score and across all domains apart from “interaction with others” [35]. Where scores for patients with achondroplasia were compared to those without achondroplasia, HRQoL was consistently lower in achondroplasia in both children and adult populations [41–44]. In an Australian study that compared WeeFIM-II parent-reported scores for a population of children with achondroplasia aged 3–12 years, HRQoL was reported to increase with increasing age of the child [48]. In Matsushita 2019, a Japanese study that compared SF-36 scores for two height groups of children and adults (aged 10–67 years), mean physical component summary (PCS) scores were higher for those in the 140–159 cm group compared with those in the 100–139 cm group (49.42 ± 12.77 vs. 38.08 ± 17.20; p value not reported). However, mean mental component summary (MCS) scores were similar in both groups (52.47 ± 11.86 vs. 53.65 ± 10.66), indicating that there may be a stronger association between height and physical aspects of QoL than mental aspects [47]. Finally, in a US-based study of 25 adults aged 19–66 with achondroplasia, the mean self-reported PCS SF-36 score was non-significantly lower in female than male patients (35.2 ± 13.4 vs. 39.5 ± 17.2; p = 0.238) while the MCS score was non-significantly higher in female than male patients (42.3 ± 15.5 vs. 33.7 ± 14.5; p = 0.062) [49].
Five studies measured HRQoL using more than one separate tool [34, 36, 41, 44, 51, 52]. However, none of the studies aimed to compare tools in terms of their suitability for assessing HRQoL in achondroplasia. Instead, the same trends were reported across different tools. For example, the multinational LIAISE study found associations between physical domain and height Z-score in QoLISSY, and mobility and Z-score in WeeFIM [44]. In one study that reported results from two generic tools (KIDSCREEN: comprised of 10 items that assess general and subjective health and wellbeing; DISABKIDS: comprised of 10 items that assess the impact of chronic health conditions and two items that measure the impact of treatment) and one condition-specific tool (QoLISSY: details aforementioned), the authors concluded that QoLISSY was a reliable and valid tool to measure HRQoL in achondroplasia, based on the Cronbach’s alpha statistic and correlations with KIDSCREEN dimensions. However, they did not specifically comment on the suitability of this tool compared with the others [34, 41, 52].
Limited utility data were identified. One study, conducted in Finland, included a small number of adult participants (n = 8) with achondroplasia for which 15D utility values were measured and reported separately to other conditions [42]. Mean score was marginally lower in adults with achondroplasia compared with the control population (0.911 vs. 0.929). A second article (a conference abstract) reported EQ-5D-5L utility index scores for 74 adults with achondroplasia in the multinational Lifetime Impact of Achondroplasia Study in Europe (LIAISE) study [44]. The mean utility index score was 0.7 for adults with achondroplasia compared with 0.9 for the reference population.
Quality of Life of Caregivers for Individuals with Achondroplasia
Five out of six studies reporting caregiver QoL included parents of children or adolescents with achondroplasia; the other included any caregivers (Table 2). One study used the 8-Item Short Form Health Survey (SF-8) [45] and one used the Achondroplasia Parent Experience Measure (APEM) [53]. A third reported descriptive data on the personal impact on carers of children with achondroplasia rather than measuring caregiver burden using a specific QoL instrument [54]. Three studies reported the ‘Effect on parents’ subscale in the parent-report version of the QoLISSY questionnaire [36, 40, 52].
In the three studies where it was specifically evaluated, the QoL of parents of children with achondroplasia was detrimentally impacted [45, 53, 54]. In Witt 2019, a German cross-sectional study in 73 parents, parents reported significantly worse mental health compared with a reference population (mean SF-8 score 46.51 vs. 53.25; p ≤ 0.01), while physical health was not affected (mean score 50.50 vs. 50.30; p = 0.85) [45]. In two studies, caregiver emotional wellbeing was reportedly negatively impacted by achondroplasia [53, 54]. The impact of interventions on parent HRQoL was only reported in the vosoritide pivotal phase 3 trial 111-301, with a decrease of 2.50 on the QoLISSY parents subscale. However the confidence intervals were wide ranging [36].
Healthcare Costs and Resource Use
Three studies published as congress abstracts reported costs or HCRU data in achondroplasia [54–56] (Table 3). One study was US-based and reported hospital-related costs and length of stay [55]; the other two studies were international and reported HCRU.
The US-based study used 2017 data from the National Inpatient Sample and estimated total hospitalisation-associated costs for all patients with achondroplasia at approximately $40 million [55]. Notably, costs were equally contributed by adults and children ($19.7 million for adults, $19.9 million for children). Total mean per-patient inpatients costs were $19,959 (95% confidence interval [CI] $16,801–$23,118), an increase of $7789 for people with achondroplasia compared to the general population [55]. Average hospital length of stay was 6.8 days (5.7–8.0), an increase of 2.2 days compared to the general population. These data were published via a conference abstract and further details, such as main drivers of total costs, were not reported.
The multinational LIAISE study reported HCRU data based on a retrospective review of medical records from 186 patients aged 5–84 years over a minimum of five years [56, 57]. Data were stratified by age group and included inpatient admissions per patient per year (mean 2.5 [range 1.5–3.1]), medications reported per patient per year (mean 7.2 [range 4.4–14.7]) and number of different specialist visits per year (mean 3.7 [range 1.7–6.0]) [57]. Some HCRU categories appeared to be associated with age. For example, mean duration of stay per inpatient visit ranged from 3.7–6.7 days in the 0–5 to 21–30 age groups and from 11.7–21.0 in the 31–40 to 51–60 age groups. Meanwhile, the frequency of annual specialist visits was higher for age groups 0–5 and 11–15 (25.7 and 29.1, respectively, vs. a range of 3.0–11.3 for other age groups). However, no statistical testing for significance was conducted. An update from this study reported on surgical procedures and healthcare practitioner visits, as well as inpatient and outpatient stays, in the same period. Of 186 patients, 72.0% had undergone ≥ 1 surgical procedures [56].
A multinational cross-sectional survey of 660 parents/caregivers (Baratela 2021) found that, excluding Japan where GH is standard care treatment, two thirds of children with achondroplasia had a primary care visit every six months, which would often involve travel of > 60 miles to attend. Similar to findings from LIAISE, the frequency of visits was reported to decrease with increasing age (> 1 visit per year for > 90% of 0–2 year olds vs. 41–71% of 12–18 year olds) [54].
Treatment of Achondroplasia
Efficacy and Safety of Achondroplasia Treatments
Forty unique studies (three RCTs, two extension studies, 17 non-randomised trials and 18 observational studies) reported on efficacy and/or safety of potential therapy options for achondroplasia. The majority of studies reported on either GH (n = 14) or limb lengthening (n = 21), while two trials, each with an extension study, investigated vosoritide, and one exploratory phase 1a trial investigated meclizine (Table 4). Most studies were conducted in the Asia-Pacific region (n = 18), followed by Europe (n = 13) (Fig. 2). Sample size was generally small, with 95% of the included studies including <100 patients and approximately 25% with a patient population <10. The most frequently reported clinical outcomes across all studies were change in standing height (n = 20) and growth velocity (n = 14).
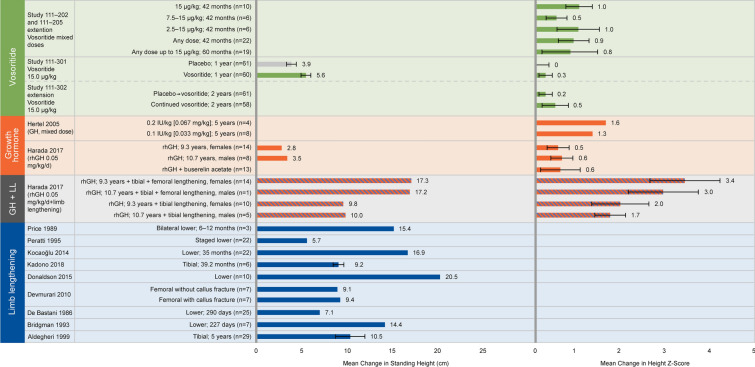
Change in Height
A favourable effect on change in height was reported for all identified interventions (Fig. 4). Four clinical studies investigated vosoritide in children with achondroplasia aged ≥5 years [36, 58–60]. In Study 111-301, a placebo-controlled RCT, patients receiving 15.0 μg/kg/day vosoritide for one year achieved a least-squares (LS) mean change in height Z-score of 0.27 (95% CI 0.18–0.36; p < 0.0001 vs. placebo) [36]. The mean change in standing height for the treated vs. untreated patients was 5.59 ± 1.06 vs. 3.93 ± 1.08 cm. The extension phase of the study (Study 111-302) demonstrated that benefits were sustained after two years of vosoritide treatment [60], with differences in LS mean change from baseline height of 3.34 cm (95% CI 2.76–3.93) and + 0.44 (95% CI 0.25–0.63) in height Z-score for vosoritide-treated vs. untreated patients. In Study 111-202, an open-label phase 2 study, and its extension, Study 111-205, children achieved an increase in mean standing height Z-score by 30, 42 or 60 months of treatment compared to baseline, with mean increase in height Z-score of 0.78 (± 0.70) after 60 months of treatment [58, 59].
Fig. 4.
Mean change in height reported across studies. Legend: Mean change in A standing height, B height Z-score from baseline following intervention. A favourable effect on change in height was reported for all ifentified inteventions. However, outcomes were reported over different time periods for the therapies. For vosoritide, outcomes were measured at 2–5 years; for GH, outcomes were measured at 5–10.7 years; for limb lengthening, outcomes were measured from 6 months to 5 years. Included studies: Study 111-202 [58] and extension Study 111-205 [59]; Study 111-301 [36] and extension Study 111-302 [60]; Hertel [65]; Harada [68]; Price [73]; Peretti [69]; Kocaoğlu [76]; Kadono [77]; Donaldson [70]; Devmurari [75]; De Bastini [71]; Bridgman [80]; Aldegheri [74]. GH growth hormone, LL limb lengthening, rhGH recombinant human growth hormone
Eight studies measured standing height in response to GH therapy over a time period of up to 10.7 years, with a significant favourable impact on standing height Z-score reported by five studies [36, 61–64]. This ranged in an increase from baseline in standing height Z-score of +0.2 after one year of treatment [62] to +1.6 after five years of treatment [65]. The other three studies also reported positive changes in height Z-score following GH but did not report whether they were statistically significant [65–67]. One study reported overall change in height following GH. Mean change in standing height was +2.8 cm after 9.3 (±2.5) years in females (p < 0.06 vs. baseline) and +3.5 cm after 10.7 (±4) years in males (p < 0.05 vs. baseline) [68].
Reported increase in standing height following limb-lengthening surgery varied considerably across 9 studies, from a mean of 5.7 [69, 70] to 20.5 cm [70], likely because of the use of different surgical procedures and population characteristics, such as age, in different studies. The studies included sample sizes of 3–29 patients at starting ages of 5–16.7 years (mean age at start of treatment was not reported in two studies) [69–77]. The mean age at the start of treatment in the study reporting the greatest mean increase in standing height (20.5 cm) was 7.8 years [70]. Patients in five of these studies had undergone tibial and femoral limb-lengthening surgery [69–71, 73, 76], and in three studies patients had undergone tibial lengthening alone [74, 75] [77]. One study did not clearly report procedures [72]. One study reported mean change in standing height at three separate time points after consecutive surgical procedures, demonstrating a cumulative effect with total increases of 5.7 cm after first tibial lengthening (n = 14), 6.5 cm after subsequent femoral lengthening (n = 8) and 8.7 cm after second tibial lengthening (n = 6) [69]. Ten limb-lengthening studies reported outcomes that were related to growth but not change in height, including change in tibial and femoral length separately, extent of elongation and change in arm span (data not shown) [38, 78–87].
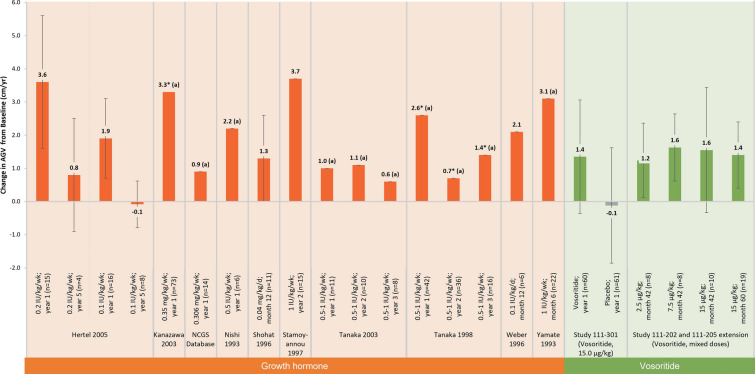
Annualised Growth Velocity (AGV)
Out of 12 GH studies that reported AGV, four reported statistically significant increases following GH compared to baseline [61–63, 88]. The greatest significant increase after one year of GH treatment was reported by Kanazawa 2003 (from 3.9 cm/year at baseline to 7.2 cm/year) [62, 89]. The smallest increase was from 3.9 cm/year at baseline to 4.6 cm/year after two years of GH in Tanaka 1998 [88]. A further six studies compared AGV following GH to AGV at baseline, but without testing for statistical significance [64–66, 89–91] (Fig. 5). In three GH studies that measured AGV at different time points, a trend towards tachyphylaxis was observed [64, 65, 88]. An RCT assessed two doses (low-dose and high-dose) of GH after 1 and 5 years of treatment. For both dose groups, mean change in growth velocity from baseline was lower after 5 years than after one year (low-dose group: 1.9 ± 1.2 cm/year at 1 year, − 0.08 ± 0.7 at 5 years; high-dose group: 3.6 ± 2.0 at 1 year, 0.8 ± 1.7 at 5 years) [65]. Similar findings were reported in two single-arm trials, with lower mean growth velocity after three years of treatment compared to one year [64, 88]. Again, statistical significance between the different time points was not assessed.
Fig. 5.
Mean change in AGV reported across studies. Mean change in AGV from baseline following intervention for GH and vosoritide studies, reported at different follow-up time points. Two GH studies reported a significant increase in AGV. A further two GH studies, Seino 2000 [61] and Ramaswami 1999 [63], did not explicitly report change in AGV from baseline, only that it was significantly higher following one year of GH therapy and therefore are not included in the figure. In three GH studies that measured AGV at different time points, a trend towards tachyphylaxis was observed. In vosoritide studies, a positive increase in AGV was observed, though statistical significance was not reported. Included studies: Hertel [65]; Kanazawa [62]; NCGS Database [67]; Nishi [126]; Shohat [90]; Stamoyannou [66]; Tanaka [64]; Tanaka [88]; Weber [91]; Yamate [89]; Study 111-301 [36]; Study 111-202 [58] and extension Study 111-205 [59]. *Statistically significant change from baseline; achange in AGV derived from calculation based on information reported in the study. AGV annualised growth velocity, GH growth hormone, rhGH recombinant human growth hormone
In vosoritide studies, a positive increase in AGV was observed. In the phase 2 studies, daily vosoritide treatment at a dose of 15 µg/kg resulted in sustained increases in AGV for up to 60 months (Fig. 5) [58, 59]. The benefit of vosoritide was further demonstrated by the results of the phase 3 randomized placebo-controlled trial (111-301), which showed a statistically significant improvement in AGV of 1.57 cm/year after 52 weeks compared to placebo [36]; furthermore, this AGV improvement was sustained after two years in the extension Study 111-302 (data not shown) [60].
Only two studies reported growth velocity in relation to limb lengthening. The first was a single arm trial that reported distraction rates ranging from 0.5–1.5 mm/day during tibial limb lengthening [77]. The second was a retrospective case-control study that presented change in growth velocity for patients following bilateral tibial lengthening. A statistically significant difference in growth velocity was not detected after one year (p = 0.53) but a decrease in mean growth rate of 59.5% was detected after two years (p = 0.03) for patients undergoing surgery (n = 23) compared with those under observation only (n = 12) [83].
Bone Morphology
In vosoritide studies, bone age was reported to have progressed normally [36, 58], indicating that vosoritide does not lead to premature bone ageing among children with achondroplasia. Findings were inconsistent in GH studies. After 2 years of GH therapy in one study, bone-to-chronological-age ratio was reported to decrease moderately, from 0.93 ± 0.13 to 0.90 ± 0.10, a positive result although statistical significance was not reported [66]. Another study found that GH therapy decreased mean bone mineral density (BMD) Z-score, though the effect appeared to lessen over time (baseline: 1.1; year 1: −0.6 ± 1.1; year 2: −0.21 ± 1.6; year 3: 0.04 ± 1.02) [64].
Adverse Events (AEs)
Nine out of 14 GH studies reported AEs. Six studies reported that no AEs occurred. However, it was not clear whether only serious AEs were considered and therefore mild events were not reported. In the remaining three studies, sleep apnoea, kidney failure and advancement of bone age (n = 2) were the only AEs observed [65, 66, 91]. In a phase 1a safety study on meclizine, no serious AEs were reported. Four out of six children experienced a low-grade AE in the group receiving one 25 mg tablet per day in the fasted state, while one out of six children experienced a low-grade AE in the group receiving two 25 mg tablets per day in the fed state [92]. As this study was only conducted over a 7-day period, longer follow-up would be needed to evaluate the reliability of this finding. Over studies 111-301 and 111-302 and their extensions, 98–100% of patients receiving vosoritide experienced an AE, most commonly injection site reaction and injection site erythema, at 73–86% and 68–86%, respectively [36, 58–60]. Most AEs were mild, with a low proportion of serious AEs in both studies (5% in the treatment arm of the RCT; 7% in the placebo arm; 11% in the dosing/extension study). AEs were reported in 15 of 21 limb-lengthening studies, of which all were related to the surgical procedure. Most commonly reported were fractures and pin site/tract infections, and AEs related to soft tissue/nerve damage were also common [38, 70–74, 76–79, 84–87, 93, 94].
Cost-Effectiveness Evidence of Treatments for Achondroplasia
No economic evaluations were identified for treatments for achondroplasia, highlighting the unmet need for studies exploring the cost-effectiveness of therapy options. In economic evaluations investigating therapies for other short stature conditions, drivers of cost-effectiveness results included dose of GH [95, 96] and utility values associated with height Z-scores and post-treatment quality-adjusted life years (QALYs) [97].
Quality of Treatment-Related Evidence Base
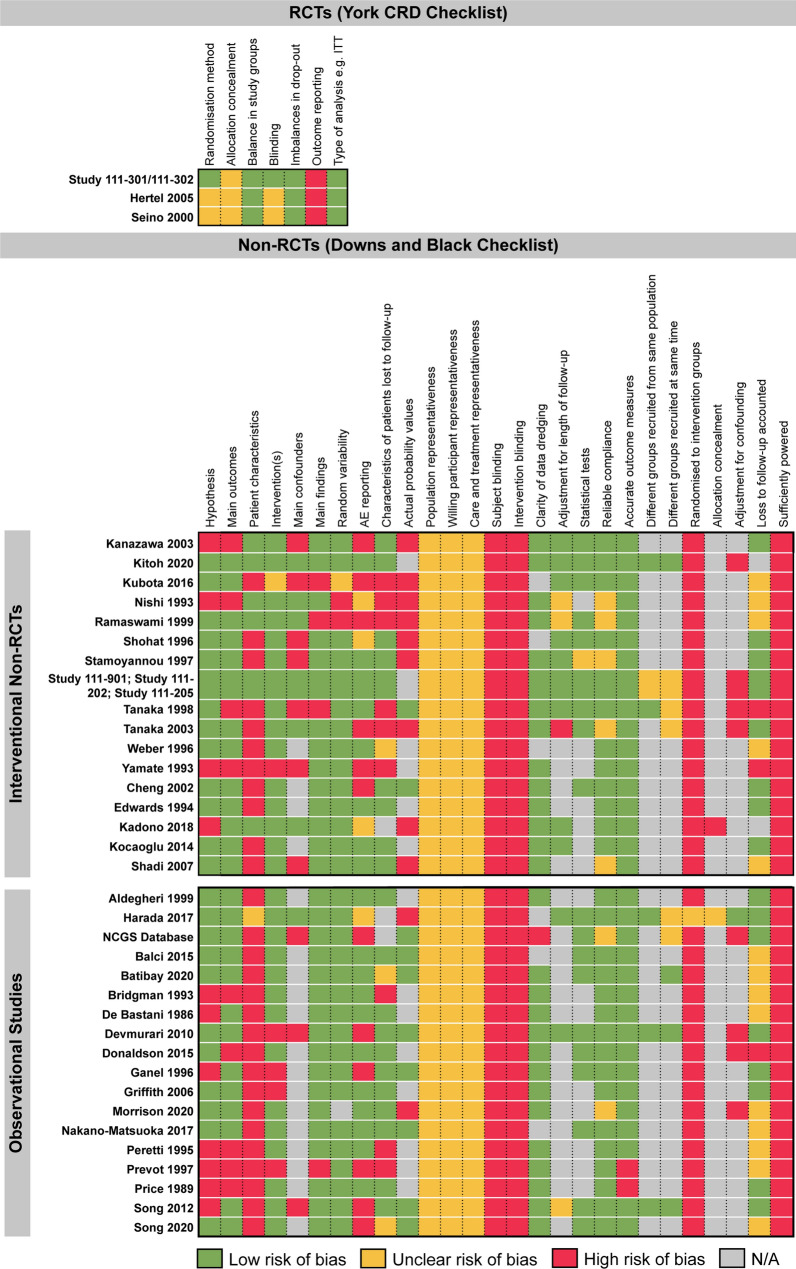
Across the clinical evidence base, only three of 40 studies were randomised, and of these, only one was placebo controlled [36, 61, 65]. The randomised studies were generally of high quality, with all three using intention-to-treat analysis, reporting similar baseline characteristics between arms and none reporting any unexpected drop-outs (Fig. 6). However, method of randomisation was not described in two RCTs [61, 65], and none provided details allocation concealment. Of the 17 interventional non-RCTs, the majority clearly described the measured outcomes, stated the objectives and provided estimates of the random variability in outcome data. However, in all 17 studies, the representativeness of patients to the entire population of children with achondroplasia from which they were recruited was unclear, as the studies did not report the proportion of the source population from which patients were derived. Of the 18 observational studies, 11 stated the objectives clearly, 13 described the main outcomes to be measured, and 14 clearly described the intervention of interest. However, the characteristics of patients was not described clearly by any study, highlighting a particular weakness in this area.
Fig. 6.
Summary of quality assessments. Summary of quality assessment scoring for different study designs reporting clinical evidence. RCTs were assessed using the York CRD tool [32]; non-randomised interventional studies and observational studies were assessed using the Downs and Black checklist [33]. Separate quality assessments were not performed for the extension studies of Study 111-301 and 111–202. All three RCTs used ITT analysis and reported similar baseline characteristics between arms; however, none provided details on allocation concealment. Of the 17 interventional non-RCTs, the majority clearly described the measured outcomes, stated the objectives and provided estimates of the random variability in outcome data. However, the representativeness of patients to the entire population of children with achondroplasia from which they were recruited was unclear. Of the 18 observational studies, 11 stated the objectives clearly, 13 described the main outcomes to be measured, and 14 clearly described the intervention of interest. However, the characteristics of patients were not described clearly by any study. AE adverse event, CRD Centre fpr Reviews and Dissemination, ITT intention to treat, NA not applicable, RCT randomised controlled trial
Treatment Guidelines for Achondroplasia and Short Stature Conditions
Thirteen guidelines on the management of achondroplasia and/or other short stature conditions were identified from targeted searches (Table 5). Nine of the 13 included guidelines were from the perspective of a single country, of which two had European perspectives (Wales [98], France [99]). Four had a US perspective [100–103]; one Japanese [104], one South African [105], and one Australian [106]. Four guidelines had an international perspective [107–110]. Common themes reported across multiple guidelines included monitoring and clinical evaluation, surgery and GH treatment. Monitoring was often recommended for specific complications, such as respiratory function monitoring in patients with thoracic spinal deformity. In achondroplasia, the Skeletal Dysplasia Management Consortium recommended a comprehensive history and physical examination be performed every two months to screen for foramen magnum stenosis (FMS) [102]. Furthermore, the Guidelines Development Committee for Achondroplasia from the Japanese Society for Pediatric Endocrinology strongly recommended foramen magnum decompression for managing spinal cord compression due to FMS [104]. GH recommendations for conditions such as growth hormone deficiency (GHD) included monitoring serum levels, measuring growth velocity and considering dose increases and reductions in various subgroups [101, 107, 108].
There was little consensus between the older guidelines, largely as they did not examine the same short stature conditions, use the same data collection methods or focus on the same aspects of treatment and management. However, a set of international management guidelines on achondroplasia by Savarirayan and colleagues were published in November 2021 [109]. These guidelines were developed by a group of 55 international experts using a modified Delphi process and provide consensus statements on many aspects of achondroplasia management and treatment across patients’ lifespan.
Key consensus statements from these guidelines are summarised in Fig. 7.
Fig. 7.
Key consensus statement from International Management Guidelines on Achondroplasia. GH growth hormone
These statements align with the results from the clinical SLR, where it was found that there is limited evidence for the long-term efficacy of GH and its effect on body proportion ratios, which may be a more meaningful outcome for some patients. Given the recent approval of vosoritide, the therapy is expected to feature in more detail in future guidelines [26, 27].
Discussion
Overview
Overall, 59 studies were identified in this SLR reporting on a range of outcomes relating to the burden and treatment of achondroplasia.
In some areas, there was a clear direction in findings. For example, the HRQoL results support that achondroplasia is associated with substantial burden on affected individuals and their families throughout their lifetime. HRQoL was consistently reported to be lower in individuals with achondroplasia compared to a population without achondroplasia, in line with previous findings [111]. Domains that were most often associated with worse HRQoL were physical or mobility related and an association between short stature and decreased HRQoL was identified. However, in studies that measured HRQoL following pharmacological or surgical interventions (a main aim of which is to increase height), no significant improvements in HRQoL were reported. Meanwhile, in two studies that investigated self-help interventions with a psychosocial support element, HRQoL was reported to improve following treatment [45, 46]. While only reported by a small number of studies with small sample sizes, this finding highlights that interventions specifically tailored to patient’s needs may be more successful in improving HRQoL than those that focus on a single element only. It also supports the need for multidisciplinary treatment options including a component for psychosocial support, given the rare and complex nature of achondroplasia. This is further supported by a qualitative study that was published after the date of this SLR’s searches [112], which found that impacts of achondroplasia are multifaceted. Difficulties in performing activities of daily living, bullying or unwanted attention, and negative effects on self-esteem were noted as key challenges for individuals with achondroplasia [112].
Notably, only two of the tools used to elicit HRQoL were condition specific (QoLISSY and APLES). No studies aimed to directly compare the suitability of different tools; therefore, conclusions on the most appropriate scale for measuring HRQoL in achondroplasia cannot be drawn. However, QoLISSY was found to be a reliable and well-validated tool and has the added benefit of items covering QoL of parents/caregivers.
Disparities were consistently reported between HRQoL as assessed by patients with achondroplasia vs. their parents or caregivers, with HRQoL judged to be significantly lower by parents/caregivers (usually across all domains). Interestingly, this is in contrast to findings from other short stature conditions reported by a 2021 SLR, whereby the majority of studies (four out of six) demonstrated good agreement between child- and parent-reported QoL. This could suggest that this issue is more prevalent in achondroplasia than in other short stature conditions [113]. A 2016 study conducted as part of the retest phase of the QoLISSY project assessed levels of agreement between child and parent reports of both generic and condition-specific HRQoL and found higher discrepancies for generic tools compared with condition-specific tools [114]. It also found that the extent of discrepancies was more influenced by family and social relationships, such as parent-child relationships, compared with clinical or sociodemographic factors. For example, a poorer parent-child relationship (as perceived by the parent) was a predictor of larger discrepancy in scoring of generic HRQoL. Furthermore, higher parental burden was significantly associated with parent underrating of condition-specific HRQoL. In this SLR, the burden of achondroplasia on parents was demonstrated in that QoL of parents/caregivers, particularly in domains related to emotional wellbeing, was reported to be adversely affected [41–45, 53, 54]. In a 2022 study, caregivers were concerned about obtaining appropriate medical care, alongside financial, relational and emotional challenges [112].
In addition to burden on HRQoL, achondroplasia has a significant economic burden on healthcare systems, with one US study finding all achondroplasia hospitalisations cost approximately $40 million in 2017 and that individuals with achondroplasia spent 2.2 days longer in hospital than patients without the condition [55]. The same study reported that costs were contributed to equally by children and adults, highlighting the impact of the condition beyond childhood and over the course of the lifetime. Furthermore, wider socioeconomic factors, such as employment, income and education level, are also likely impacted in individuals with achondroplasia. For example, studies have demonstrated that achondroplasia negatively impacts children’s participation in school [115, 116], with further studies finding that adults have lower annual income and less education compared with their unaffected first-degree relatives [50]. These factors, along with other indirect costs, should also be considered when estimating the true economic burden of the condition.
Findings in the clinical evidence base were less consistent. Nineteen studies on pharmacological interventions and 21 on limb-lengthening surgery, of varying methodological quality, were identified in the SLR. Key outcomes were change in height and AGV. On the whole, vosoritide and GH therapy conferred benefits for height or growth velocity, but the longer-term effects of GH were unclear because of evidence of trends of a waning effect over time [109]. The efficacy of vosoritide was found to be maintained for up to five years, suggestive of cumulative benefits of this newly approved therapy [58, 117]; however, this finding was only reported in two trials and two extension studies. Up to now, use of vosoritide has not been part of standard clinical practice, but this may be expected to change in the near future because of its recent approval and promising clinical data. While investigated in a large number of studies, the clinical evidence for limb lengthening was mixed; for example, AEs related to the surgical procedure were commonly reported. Indeed, limb lengthening remains a controversial procedure in practice, with varied uptake in different countries [23]. Meclizine was only investigated in a phase 1a exploratory trial, with no efficacy outcomes reported, and there are currently no plans for a phase 2 trial [118]. Therefore, comparisons between this and other interventions cannot be drawn for efficacy outcomes [92]. Alongside the pharmacological agents identified by the SLR, there are several ongoing pre-clinical studies of other drug therapies, including infigratinib, TA-46 (Recifercept) and Transcon-CNP. Clinical trials investigating these agents will likely be published in the future, adding to the evidence base on safety and efficacy [29].
Most guideline recommendations identified in this review were management based and suggested monitoring and clinical evaluations, such as measuring growth and development and examining patient history, often to identify various complications common in patients with short stature [102, 104]. The recently published International Consensus Statement on the management of achondroplasia provides consensus statements on limb lengthening and GH amongst other aspects of achondroplasia management and represents the first global effort to standardise care for individuals with achondroplasia [109]. These guidelines highlight that there is still an unmet need for treatment options in achondroplasia, as limb lengthening and GH treatments have potential problems. Recently published management recommendations from Latin America highlight that achondroplasia-associated comorbidities are not limited to orthopaedic-related concerns; thus, there is a requirement for multidisciplinary teams for effective treatment of achondroplasia [119].
Gaps in the Evidence
This SLR identified several gaps in the current literature. There is a substantial lack of utility data for achondroplasia, identified in only two studies, both in adults [42, 44]. With treatments most commonly indicated for children, this emphasises the difficulty in accurately estimating inputs for economic modelling relating to the condition and the need for further research in this area. Furthermore, while total height may be increased by all identified interventions, based on current evidence it is unclear whether they confer benefits to individuals’ QoL or functioning. HCRU/cost data are also very limited, with only one study reporting cost data [55] and three studies reporting resource use specifically for achondroplasia [54–56]. In addition, the identified study considered costs from a healthcare payer perspective and did not provide a breakdown of individual cost components. As such, drivers of total costs and out-of-pocket costs to patients and caregivers are currently unknown, which limits the extent to which the full societal impact of achondroplasia cannot be estimated.
Furthermore, information on which factors have the highest impact on HRQoL and costs/HCRU is substantially lacking. Indeed, there is currently no evidence on the cost-effectiveness of interventions for achondroplasia. At present, published economic evaluations only investigate GH therapy in forms of short stature other than achondroplasia.
Strengths
There are a number of strengths to the methodology and results of this work. The SLR used systematic methods in line with the Cochrane Handbook for Systematic Reviews of Interventions to conduct an exhaustive search of the literature, identifying evidence relevant to the review objectives [120]. Furthermore, articles published at any date in any language were eligible for inclusion and were not restricted by study design in the economic searches. The majority of evidence identified by the economic searches was published in the last five years, providing a contemporary perspective on economic evidence in achondroplasia. The randomised trials included were generally of high quality, and the interventional non-RCTs and observational studies included in the clinical searches were of moderate quality, with a lack in description of the patient population limiting this.
Limitations
However, there are also some limitations. First, a substantial proportion of the included clinical evidence (primarily for limb lengthening) was published more than 20 years ago and therefore may not accurately reflect current clinical practice. In addition, a number of studies reported outcomes in mixed populations of children and adults, where data relating to adults with the condition may not be directly applicable to children. Furthermore, due to the nature of the rare condition, some sample sizes in included HRQoL studies were small (< 100 participants), which may limit the reliability of findings. These studies were also often only conducted in one country and used heterogeneous measures to assess HRQoL. Larger, multinational studies could help elucidate more concrete conclusions on the impact of achondroplasia on HRQoL. Additionally, further research is needed to better quantify the impact of achondroplasia on caregivers to build on current research that indicates that it impacts caregivers’ emotional wellbeing. Finally, the authors are aware of one study reporting on staged upper and lower limb lengthening (Leiva-Gea et al., 2020) that was not captured in the systematic database searches because of a lack of study design text word or indexing terms in the published record [121]. However, the findings of this study are in line with those included in this SLR and do not change the review’s conclusions.
Conclusion
This SLR provides a comprehensive and contemporary overview of the published evidence related to the burden and treatment of achondroplasia, along with current recommendations for management.
Overall, the results highlight that achondroplasia confers substantial burden in terms of patient HRQoL, burden on caregivers and economic burden on healthcare systems and individuals. The published data likely even underestimate the true burden of the condition given the need to consider lifetime impacts on patients and their families and lack of reporting on specific cost categories or productivity losses based on education and employment. Treatment options for achondroplasia are currently limited. Of the four interventions identified in this SLR, vosoritide and meclizine have a mechanism of action that targets the underlying cause of achondroplasia; however, research is still in early stages for meclizine with only one small study identified by this SLR. Vosoritide, GH and limb lengthening all have some benefit for height and growth velocity; however, the long-term effects of GH are unclear and limb lengthening is associated with a risk of complications. Current best practice for disease management is lifelong multidisciplinary care with a high risk of invasive procedures over the lifetime.
Based on currently available data, perhaps the biggest challenge currently facing the field is that it is not possible to evaluate the benefit of treatment options in relation to HRQoL or economic burden because of very limited reporting of factors that have the highest influence on both outcomes. There is a clear unmet need for studies such as economic evaluations that consider all relevant inputs for assessing the burden of achondroplasia. With the development of pharmacological treatment options that target the underlying cause of achondroplasia, there is a need to build on the emerging evidence to further inform best practice for the management of achondroplasia such that the clinical, humanistic and economic burden on patients and their families can be alleviated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was sponsored by BioMarin Pharmaceutical Inc. The Rapid Service and Open Access Fees were funded by BioMarin Pharmaceutical Inc. Support for third-party writing assistance for this article, provided by Faye Saville, Costello Medical, UK, was funded by BioMarin Pharmaceuticals in accordance with Good Publication Practice (GPP3) guidelines.
Medical Writing and Editorial Assistance
The authors acknowledge Faye Saville, MSci, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Author Contributions
Substantial contributions to study conception and design: Molly Murton, Emma Drane, Danielle Goff-Leggett, Renée Shediac, Jamie O’Hara, Melita Irving, Thomas Butt; substantial contributions to analysis and interpretation of the data: Molly Murton, Emma Drane, Danielle Goff-Leggett, Renée Shediac, Jamie O’Hara, Melita Irving, Thomas Butt; drafting the article or revising it critically for important intellectual content: Molly Murton, Emma Drane, Danielle Goff-Leggett, Renée Shediac, Jamie O’Hara, Melita Irving, Thomas Butt; final approval of the version of the article to be published: Molly Murton, Emma Drane, Danielle Goff-Leggett, Renée Shediac, Jamie O’Hara, Melita Irving, Thomas Butt.
Disclosures
Melita Irving has received honoraria for consultancy services from BioMarin, QED Therapeutics, Sanofi, Ascendis, Alexion, Kyowa Kirin, Innoskel, Novo Nordisk; Thomas Butt and Renée Shediac are employees and shareholders of BioMarin; Molly Murton, Emma Drane and Danielle Goff-Leggett are employees of Costello Medical. Jamie O’Hara has no conflicts of interest to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The MEDLINE databases and Embase were searched via the Ovid SP platform (available via paid subscription). The other databases searched were freely accessible.
References
- 1.Wright MJ, Irving MD. Clinical management of achondroplasia. Arch Dis Child. 2012;97(2):129. doi: 10.1136/adc.2010.189092. [DOI] [PubMed] [Google Scholar]
- 2.Horton WA, Hall JG, Hecht JT. Achondroplasia. The Lancet. 2007;370(9582):162–172. doi: 10.1016/S0140-6736(07)61090-3. [DOI] [PubMed] [Google Scholar]
- 3.Foreman PK, van Kessel F, van Hoorn R, van den Bosch J, Shediac R, Landis S. Birth prevalence of achondroplasia: A systematic literature review and meta-analysis. Am J Med Genet A. 2020;182(10):2297–2316. doi: 10.1002/ajmg.a.61787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baujat G, Legeai-Mallet L, Finidori G, Cormier-Daire V, Le Merrer M. Achondroplasia. Best Pract Res Clin Rheumatol. 2008;22(1):3–18. doi: 10.1016/j.berh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Ireland PJ, Pacey V, Zankl A, Edwards P, Johnston LM, Savarirayan R. Optimal management of complications associated with achondroplasia. Appl Clin Genet. 2014;7:117–125. doi: 10.2147/TACG.S51485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nehme AM, Riseborough EJ, Tredwell SJ. Skeletal growth and development of the achondroplastic dwarf. Clin Orthop Relat Res. 1976;116:8–23. [PubMed] [Google Scholar]
- 7.Pauli RM. Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis. 2019;14(1):1. doi: 10.1186/s13023-018-0972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okenfuss E, Moghaddam B, Avins AL. Natural history of achondroplasia: A retrospective review of longitudinal clinical data. Am J Med Genet A. 2020;182(11):2540–2551. doi: 10.1002/ajmg.a.61825. [DOI] [PubMed] [Google Scholar]
- 9.Hoover-Fong J, Cheung MS, Fano V, Hagenas L, Hecht JT, Ireland P, et al. Lifetime impact of achondroplasia: current evidence and perspectives on the natural history. Bone. 2021;146:115872. doi: 10.1016/j.bone.2021.115872. [DOI] [PubMed] [Google Scholar]
- 10.Hunter AG, Bankier A, Rogers JG, Sillence D, Scott CI. Medical complications of achondroplasia: a multicentre patient review. J Med Genet. 1998;35(9):705–712. doi: 10.1136/jmg.35.9.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaffanello M, Cantalupo G, Piacentini G, Gasperi E, Nosetti L, Cavarzere P, et al. Sleep disordered breathing in children with achondroplasia. World Journal of Pediatrics. 2017;13(1):8–14. doi: 10.1007/s12519-016-0051-9. [DOI] [PubMed] [Google Scholar]
- 12.Ednick M, Tinkle BT, Phromchairak J, Egelhoff J, Amin R, Simakajornboon N. Sleep-related respiratory abnormalities and arousal pattern in achondroplasia during early infancy. J Pediatr. 2009;155(4):510–515. doi: 10.1016/j.jpeds.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Hecht JT, Francomano CA, Horton WA, Annegers JF. Mortality in achondroplasia. Am J Hum Genet. 1987;41(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- 14.Hashmi SS, Gamble C, Hoover-Fong J, Alade AY, Pauli RM, Modaff P, et al. Multicenter study of mortality in achondroplasia. Am J Med Genet A. 2018;176(11):2359–2364. doi: 10.1002/ajmg.a.40528. [DOI] [PubMed] [Google Scholar]
- 15.Wynn J, King TM, Gambello MJ, Waller DK, Hecht JT. Mortality in achondroplasia study: a 42-year follow-up. Am J Med Genet A. 2007;143A(21):2502–2511. doi: 10.1002/ajmg.a.31919. [DOI] [PubMed] [Google Scholar]
- 16.Fernández Arregui. Social stigmatization in achondroplasia. Department of Social and Organizational Psychology; 2010. https://www.fundacionalpe.org/images/alpe/library/social_stigmatization_english.pdf. Accessed Feb 2021.
- 17.Jennings SE, Ditro CP, Bober MB, Mackenzie WG, Rogers KJ, Conway L, et al. Prevalence of mental health conditions and pain in adults with skeletal dysplasia. Qual Life Res. 2019;28(6):1457–1464. doi: 10.1007/s11136-019-02102-2. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura N, Hanaki K. Psychosocial profiles of children with achondroplasia in terms of their short stature-related stress: a nationwide survey in Japan. J Clin Nurs. 2014;23(21–22):3045–3056. doi: 10.1111/jocn.12531. [DOI] [PubMed] [Google Scholar]
- 19.The International Society for Pediatric Neurosurgery. Foramen Magnum Decompression in Achondroplasia in Children. 2020. Available at: https://www.ispn.guide/spinal-diseases-and-anomalies-in-children/metabolic-bone-disease-in-children-the-craniospinal-effects/management-of-metabolic-bone-disease-in-children/the-operation-for-metabolic-bone-disease-in-children/foramen-magnum-decompression-in-achondroplasia-in-children/. Accessed Mar 2021.
- 20.National Health Service. Lumbar decompression surgery. https://www.nhs.uk/conditions/lumbar-decompression-surgery/what-happens/. Accessed Nov 2020.
- 21.National Health Service. Adenoidectomy. https://www.nhs.uk/conditions/adenoids-and-adenoidectomy/. Accessed Nov 2020.
- 22.National Health Service. Glue ear. https://www.nhs.uk/conditions/glue-ear/. Accessed Nov 2020.
- 23.Hosny GA. Limb lengthening history, evolution, complications and current concepts. J Orthop Traumatol. 2020;21(1):3. doi: 10.1186/s10195-019-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yorifuji T, Higuchi S, Kawakita R. Growth hormone treatment for achondroplasia. Pediatr Endocrinol Rev. 2018;16(Suppl 1):123–128. doi: 10.17458/per.vol16.2018.yhk.ghachondroplasia. [DOI] [PubMed] [Google Scholar]
- 25.Miccoli M, Bertelloni S, Massart F. Height outcome of recombinant human growth hormone treatment in achondroplasia children: a meta-analysis. Hormone Res Paediatr. 2016;86(1):27–34. doi: 10.1159/000446958. [DOI] [PubMed] [Google Scholar]
- 26.BioMarin. European Commission Approves BioMarin's VOXZOGO® (vosoritide) for the Treatment of Children with Achondroplasia from Age 2 Until Growth Plates Close. Available at https://investors.biomarin.com/2021-08-27-European-Commission-Approves-BioMarins-VOXZOGO-R-vosoritide-for-the-Treatment-of-Children-with-Achondroplasia-from-Age-2-Until-Growth-Plates-Close. Accessed Oct 2021.
- 27.FDA. FDA Approves First Drug to Improve Growth in Children with Most Common Form of Dwarfism. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-improve-growth-children-most-common-form-dwarfism. Accessed Dec 2021.
- 28.Anvisa. Voxzogo (vosoritida): novo registro. 2021. https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/novos-medicamentos-e-indicacoes/voxzogo-vosoritida-novo-registro. Accessed Dec 2021.
- 29.DelveInsight. Achondroplasia-Pipeline Insight. 2021.
- 30.Breinholt VM, Rasmussen CE, Mygind PH, Kjelgaard-Hansen M, Faltinger F, Bernhard A, et al. TransCon CNP, a sustained-release C-type natriuretic peptide prodrug, a potentially safe and efficacious new therapeutic modality for the treatment of comorbidities associated with fibroblast growth factor receptor 3-related skeletal dysplasias. J Pharmacol Exp Ther. 2019;370(3):459–471. doi: 10.1124/jpet.119.258251. [DOI] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 32.Centre for Reviews and Dissemination . Systematic Reviews: CRD's guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2008. [Google Scholar]
- 33.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witt S, Rohenkohl A, Bullinger M, Sommer R, Kahrs S, Klingebiel K-H, et al. Understanding, assessing and improving health-related quality of life of young people with achondroplasia—a collaboration between a patient organization and academic medicine. Pediatr Endocrinol Rev. 2017;15(Suppl 1):109. doi: 10.17458/per.vol15.2017.wrm.improvinghealthrelatedquality. [DOI] [PubMed] [Google Scholar]
- 35.Bloemeke J, Sommer R, Witt S, Dabs M, Badia FJ, Bullinger M, et al. Piloting and psychometric properties of a patient-reported outcome instrument for young people with achondroplasia based on the International Classification of Functioning Disability and Health: the Achondroplasia Personal Life Experience Scale (APLES) Disabil Rehabil. 2019;41(15):1815–1825. doi: 10.1080/09638288.2018.1447028. [DOI] [PubMed] [Google Scholar]
- 36.Savarirayan R, Tofts L, Irving M, Wilcox W, Bacino CA, Hoover-Fong J, et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomised, double-blind, phase 3, placebo-controlled, multicentre trial. The Lancet. 2020;396(10252):684–692. doi: 10.1016/S0140-6736(20)31541-5. [DOI] [PubMed] [Google Scholar]
- 37.Kim S-J, Balce GC, Agashe MV, Song S-H, Song H-R. Is bilateral lower limb lengthening appropriate for achondroplasia?: midterm analysis of the complications and quality of life. Clin Orthop Rel Res. 2012;470(2):616–621. doi: 10.1007/s11999-011-1983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batibay SG, Balci HI, Bayram S, Chodza M, Goksoy S, Hurmeydan OM, et al. Quality of life evaluation following limb lengthening surgery in patients with achondroplasia. Indian J Orthop. 2020;54(Suppl 1):39–46. doi: 10.1007/s43465-020-00127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahomed NN, Spellmann M, Goldberg MJ. Functional health status of adults with achondroplasia. Am J Med Genet. 1998;78(1):30–35. doi: 10.1002/(SICI)1096-8628(19980616)78:1<30::AID-AJMG7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 40.Rohenkohl A, Sommer R, Kahrs S, Bullinger M, Klingebiel K, Quitmann J. Evaluation of a self-help supported counseling concept for children and adolescents with disproportional Short stature. Klin Padiatr. 2016;228(1):17–23. doi: 10.1055/s-0035-1564106. [DOI] [PubMed] [Google Scholar]
- 41.Rohenkohl AC, Sommer R, Bestges S, Kahrs S, Klingebiel K-H, Bullinger M, et al. Living with achondroplasia-how do young persons with disproportional short stature rate their quality of life and which factors are associated with quality of life? Zeitschrift fur Kinder-und Jugendpsychiatrie und Psychotherapie. 2015;43(6):433–441. doi: 10.1024/1422-4917/a000385. [DOI] [PubMed] [Google Scholar]
- 42.Apajasalo M, Sintonen H, Rautonen J, Kaitila I. Health-related quality of life of patients with genetic skeletal dysplasias. Eur J Pediatr. 1998;157(2):114–121. doi: 10.1007/s004310050781. [DOI] [PubMed] [Google Scholar]
- 43.Cervan MP, Silva MCPd, Lima RLdO, Costa RFd. Comparative study of quality of life level between achondroplasics and non-achondroplasics subjects. Jornal Brasileiro de Psiquiatria. 2008;57(2):105–11.
- 44.Maghnie M, Semler O, Guillen-Navarro E, Wiesel A, Allegri AEM, Selicorni A, et al. Health-related quality of life (HRQoL) in achondroplasia: findings from a multinational, observational study. Mol Genet Metab. 2021;132:S127–S128. doi: 10.1016/S1096-7192(21)00280-8. [DOI] [Google Scholar]
- 45.Witt S, Kolb B, Bloemeke J, Mohnike K, Bullinger M, Quitmann J. Quality of life of children with achondroplasia and their parents-a German cross-sectional study. Orphanet J Rare Dis. 2019;14(1):1–9. doi: 10.1186/s13023-019-1171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloemeke J, Sommer R, Witt S, Bullinger M, Nordon C, Badia FJ, et al. Cross-cultural selection and validation of instruments to assess patient-reported outcomes in children and adolescents with achondroplasia. Qual Life Res. 2019;28(9):2553–2563. doi: 10.1007/s11136-019-02210-z. [DOI] [PubMed] [Google Scholar]
- 47.Matsushita M, Kitoh H, Mishima K, Yamashita S, Haga N, Fujiwara S, et al. Physical, mental, and social problems of adolescent and adult patients with achondroplasia. Calcif Tissue Int. 2019;104(4):364–372. doi: 10.1007/s00223-019-00518-z. [DOI] [PubMed] [Google Scholar]
- 48.Ireland PJ, Mcgill J, Zankl A, Ware RS, Pacey V, Ault J, et al. Functional performance in young Australian children with achondroplasia. Dev Med Child Neurol. 2011;53(10):944–950. doi: 10.1111/j.1469-8749.2011.04050.x. [DOI] [PubMed] [Google Scholar]
- 49.Yonko EA, Emanuel JS, Carter EM, Raggio CL. Quality of life in adults with achondroplasia in the United States. Am J Med Genet A. 2021;185(3):695–701. doi: 10.1002/ajmg.a.62018. [DOI] [PubMed] [Google Scholar]
- 50.Gollust SE, Thompson RE, Gooding HC, Biesecker BB. Living with achondroplasia in an average-sized world: An assessment of quality of life. Am J Med Genet A. 2003;120(4):447–458. doi: 10.1002/ajmg.a.20127. [DOI] [PubMed] [Google Scholar]
- 51.Alade Y, Tunkel D, Schulze K, McGready J, Jallo G, Ain M, et al. Cross-sectional assessment of pain and physical function in skeletal dysplasia patients. Clin Genet. 2013;84(3):237–243. doi: 10.1111/cge.12045. [DOI] [PubMed] [Google Scholar]
- 52.Rohenkohl A, Bullinger M, Quitmann J. Quality of life in children, adolescents, and young adults with achondroplasia. Der Orthopade. 2015;44(3):212–218. doi: 10.1007/s00132-014-3020-9. [DOI] [PubMed] [Google Scholar]
- 53.Pfeiffer KM, Brod M, Smith A, Gianettoni J, Viuff D, Ota S, et al. Assessing the impacts of having a child with achondroplasia on parent well-being. Qual Life Res. 2020;30(1):203–215. doi: 10.1007/s11136-020-02594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baratela W, Alves I, Pan W, Pimenta JM, Roberts C, Sessa M, et al. Achondroplasia caregiver survey-a global perspective on diagnostic pathways, healthcare management and personal impact from carers of children with achondroplasia. Mol Genet Metab. 2021;132:S100. doi: 10.1016/S1096-7192(21)00234-1. [DOI] [Google Scholar]
- 55.Chen E, Yan T, Chang E, Broder M, Tarbox M, Abrahamson LA, et al. PMS8 National Burden of achondroplasia in adults and children: an analysis of the national inpatient sample. Value in Health. 2021;24:S139. doi: 10.1016/j.jval.2021.04.685. [DOI] [Google Scholar]
- 56.Maghnie M, Semler O, Guillen-Navarro E, Wiesel A, Allegri AEM, Selicorni A, et al., editors. Lifetime impact of achondroplasia in Europe (LIAISE): findings from a multinational observational study. In: Molecular Genetics and Metabolism; 2021.
- 57.Pan W, editor Lifetime impact of achondroplasia on health-related quality of life (HR-QoL) and healthcare resource use: Interim results from a multinational study. In: The 2020 ACMG Annual Clinical Genetics Meeting; 2020.
- 58.Savarirayan R, Irving M, Bacino CA, Bostwick B, Charrow J, Cormier-Daire V, et al. C-type natriuretic peptide analogue therapy in children with achondroplasia. N Engl J Med. 2019;381(1):25–35. doi: 10.1056/NEJMoa1813446. [DOI] [PubMed] [Google Scholar]
- 59.Hoover-Fong J, Dickson PI, Harmatz P, Larimore K, Jayaram K, Labed AH, et al. Vosoritide for children with achondroplasia: a 60-month update from an ongoing phase 2 clinical trial. Mol Genet Metab. 2021;132:S101. doi: 10.1016/S1096-7192(21)00236-5. [DOI] [Google Scholar]
- 60.Savarirayan R, Tofts L, Irving M, Wilcox W, Bacino CA, Hoover-Fong J, et al. Persistent and stable growth promoting effects of vosoritide in children with achondroplasia for up to 2 years: results from the ongoing phase 3 extension study. J Endocr Soc. 2021;5(Supplement_1):A670–A671. doi: 10.1210/jendso/bvab048.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seino Y, Yamanaka Y, Shinohara M, Ikegami S, Koike M, Miyazawa M, et al. Growth hormone therapy in achondroplasia. Horm Res. 2000;53(Suppl 3):53–56. doi: 10.1159/000023534. [DOI] [PubMed] [Google Scholar]
- 62.Kanazawa H, Tanaka H, Inoue M, Yamanaka Y, Namba N, Seino Y. Efficacy of growth hormone therapy for patients with skeletal dysplasia. J Bone Miner Metab. 2003;21(5):307–310. doi: 10.1007/s00774-003-0425-7. [DOI] [PubMed] [Google Scholar]
- 63.Ramaswami U, Rumsby G, Spoudeas HA, Hindmarsh PC, Brook CGD. Treatment of achondroplasia with growth hormone: Six years of experience. Pediatr Res. 1999;46(4):435–439. doi: 10.1203/00006450-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka N, Katsumata N, Horikawa R, Tanaka T. The comparison of the effects of short-term growth hormone treatment in patients with achondroplasia and with hypochondroplasia. Endocr J. 2003;50(1):69–75. doi: 10.1507/endocrj.50.69. [DOI] [PubMed] [Google Scholar]
- 65.Hertel NT, Eklof O, Ivarsson S, Aronson S, Westphal O, Sipila I, et al. Growth hormone treatment in 35 prepubertal children with achondroplasia: a five-year dose-response trial. Acta Paediatr. 2005;94(10):1402–1410. doi: 10.1080/08035250510039982. [DOI] [PubMed] [Google Scholar]
- 66.Stamoyannou L, Karachaliou F, Neou P, Papataxiarchou K, Pistevos G, Bartsocas C. Growth and growth hormone therapy in children with achondroplasia: a two-year experience. Am J Med Genet. 1997;72(1):71–76. doi: 10.1002/(SICI)1096-8628(19971003)72:1<71::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 67.Key LL, Jr, Gross AJ. Response to growth hormone in children with chondrodysplasia. J Pediatr. 1996;128(5 II):S14–S17. doi: 10.1016/S0022-3476(96)70004-5. [DOI] [PubMed] [Google Scholar]
- 68.Harada D, Namba N, Hanioka Y, Ueyama K, Sakamoto N, Nakano Y, et al. Final adult height in long-term growth hormone-treated achondroplasia patients. Eur J Pediatr. 2017;176(7):873–879. doi: 10.1007/s00431-017-2923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peretti G, Memeo A, Paronzini A, Marzorati S. Staged lengthening in the prevention of dwarfism in achondroplastic children: a preliminary report. J Pediatr Orthop B. 1995;4(1):58–64. doi: 10.1097/01202412-199504010-00009. [DOI] [PubMed] [Google Scholar]
- 70.Donaldson J, Aftab S, Bradish C. Achondroplasia and limb lengthening: results in a UK cohort and review of the literature. J Orthop. 2015;12(1):31–34. doi: 10.1016/j.jor.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Bastiani G, Aldegheri R, Renzi Brivio L, Trivella G. Chondrodiatasis-controlled symmetrical distraction of the epiphyseal plate Limb lengthening in children. J Bone Jt Surg Br Vol. 1986;68(4):550–556. doi: 10.1302/0301-620X.68B4.3733829. [DOI] [PubMed] [Google Scholar]
- 72.Prevot J, Fockens W. Pathological fractures following bone lengthening. [French] Chirurgie-Memoires de l'Academie de Chirurgie. 1997;122(2):92–93. [Google Scholar]
- 73.Price CT. Limb lengthening for achondroplasia: early experience. J Pediatr Orthop. 1989;9(5):512–515. doi: 10.1097/01241398-198909010-00002. [DOI] [PubMed] [Google Scholar]
- 74.Aldegheri R. Distraction osteogenesis for lengthening of the tibia in patients who have limb-length discrepancy or short stature. J Bone Jt Surg Am Vol. 1999;81(5):624–634. doi: 10.2106/00004623-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Devmurari KN, Song HR, Modi HN, Venkatesh KP, Ju KS, Song SH. Callus features of regenerate fracture cases in femoral lengthening in achondroplasia. Skeletal Radiol. 2010;39(9):897–903. doi: 10.1007/s00256-009-0742-6. [DOI] [PubMed] [Google Scholar]
- 76.Kocaoğlu M, Bilen FE, Dikmen G, Balci HI, Eralp L. Simultaneous bilateral lengthening of femora and tibiae in achondroplastic patients. Acta Orthop Traumatolog Turcica. 2014;48(2):157–163. doi: 10.3944/AOTT.2014.3274. [DOI] [PubMed] [Google Scholar]
- 77.Kadono I, Kitoh H, Mishima K, Matsushita M, Sato K, Kako M, et al. Changes in the range of motion of the lower limb joints during extensive tibial lengthening in achondroplasia. J Pediatr Orthop B. 2018;27(6):535–540. doi: 10.1097/BPB.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 78.Edwards DJ, Bickerstaff DB, Bell MJ. Periosteal stripping in achondroplastic children. Little effect on limb length in 10 cases. Acta Orthop Scand. 1994;65(3):333–334. doi: 10.3109/17453679408995464. [DOI] [PubMed] [Google Scholar]
- 79.Shadi M, Koczewski P. Humeral lengthening with a monolateral external fixator in achondroplasia. [Polish] Pediatr Endocrinol Diabetes Metab. 2007;13(3):121–124. [PubMed] [Google Scholar]
- 80.Bridgman S, Bennet G, Evans G, Stirling J. Leg lengthening. J R Coll Surg Edinb. 1993;38(2):101–104. [PubMed] [Google Scholar]
- 81.Ganel A, Horoszowski H. Limb lengthening in children with achondroplasia: differences based on gender. Clin Orthop Relat Res (1976-2007) 1996;332:179–183. doi: 10.1097/00003086-199611000-00024. [DOI] [PubMed] [Google Scholar]
- 82.Song SH, Agashe MV, Huh YJ, Hwang SY, Song HR. Physeal growth arrest after tibial lengthening in achondroplasia: 23 children followed to skeletal maturity. Acta Orthop. 2012;83(3):282–287. doi: 10.3109/17453674.2012.678802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song SH, Kim SE, Agashe MV, Lee H, Refai MA, Park YE, et al. Growth disturbance after lengthening of the lower limb and quantitative assessment of physeal closure in skeletally immature patients with achondroplasia. J Bone Jt Surg Br. 2012;94(4):556–563. doi: 10.1302/0301-620X.94B4.28375. [DOI] [PubMed] [Google Scholar]
- 84.Song MH, Kim TJ, Chang AS, Song HR. Wire bending development and progression during Ilizarov system tibial lengthening in skeletally immature patients with achondroplasia. J Orthop Transl. 2020;25:73–79. [Google Scholar]
- 85.Balci HI, Kocaoglu M, Sen C, Eralp L, Batibay SG, Bilsel K. Bilateral humeral lengthening in achondroplasia with unilateral external fixators: is it safe and does it improve daily life? Bone Jt J. 2015;97(11):1577–1581. doi: 10.1302/0301-620X.97B11.36037. [DOI] [PubMed] [Google Scholar]
- 86.Morrison SG, Georgiadis AG, Dahl MT. Lengthening of the Humerus using a motorized lengthening nail: a retrospective comparative series. J Pediatr Orthop. 2020;40(6):E479–E486. doi: 10.1097/BPO.0000000000001453. [DOI] [PubMed] [Google Scholar]
- 87.Nakano-Matsuoka N, Fukiage K, Harada Y, Kashiwagi N, Futami T. The prevalence of the complications and their associated factors in humeral lengthening for achondroplasia: retrospective study of 54 cases. J Pediatr Orthop B. 2017;26(6):519–525. doi: 10.1097/BPB.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka H, Kubo T, Yamate T, Ono T, Kanzaki S, Seino Y. Effect of growth hormone therapy in children with achondroplasia: growth pattern, hypothalamic-pituitary function, and genotype. Eur J Endocrinol. 1998;138(3):275–280. doi: 10.1530/eje.0.1380275. [DOI] [PubMed] [Google Scholar]
- 89.Yamate T, Kanzaki S, Tanaka H, Kubo T, Moriwake T, Inoue M, et al. Growth hormone (GH) treatment in achondroplasia. J Pediatr Endocrinol Metab. 1993;6(1):45–52. doi: 10.1515/JPEM.1993.6.1.45. [DOI] [PubMed] [Google Scholar]
- 90.Shohat M, Tick D, Barakat S, Bu X, Melmed S, Rimoin DL. Short-term recombinant human growth hormone treatment increases growth rate in achondroplasia. J Clin Endocrinol Metab. 1996;81(11):4033–4037. doi: 10.1210/jcem.81.11.8923856. [DOI] [PubMed] [Google Scholar]
- 91.Weber G, Prinster C, Meneghel M, Russo F, Mora S, Puzzovio M, et al. Human growth hormone treatment in prepubertal children with achondroplasia. Am J Med Genet. 1996;61(4):396–400. doi: 10.1002/(SICI)1096-8628(19960202)61:4<396::AID-AJMG17>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 92.Kitoh H, Matsushita M, Mishima K, Nagata T, Kamiya Y, Ueda K, et al. Pharmacokinetics and safety after once and twice a day doses of meclizine hydrochloride administered to children with achondroplasia. PLoS ONE. 2020;15(4 (no pagination)):e0229639. doi: 10.1371/journal.pone.0229639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng JCY, Maffulli N, Sher A, Ng BKW, Ng E. Bone mineralization gradient at the callotasis site. J Orthop Sci. 2002;7(3):331–340. doi: 10.1007/s007760200056. [DOI] [PubMed] [Google Scholar]
- 94.Griffith SI, McCarthy JJ, Davidson RS. Comparison of the complication rates between first and second (repeated) lengthening in the same limb segment. J Pediatric Orthop. 2006;26(4):534–536. doi: 10.1097/01.bpo.0000226275.70706.d5. [DOI] [PubMed] [Google Scholar]
- 95.Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al. Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation. Health Technol Assess. 2002;6(19):1–96. doi: 10.3310/hta6190. [DOI] [PubMed] [Google Scholar]
- 96.Takeda A, Cooper K, Bird A, Baxter L, Gospodarevskaya E, Frampton GK, et al. Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation. Health Technol Assess. 2010;14(42):1–209. doi: 10.3310/hta14420. [DOI] [PubMed] [Google Scholar]
- 97.Christensen T, Fidler C, Bentley A, Djurhuus C. The cost-effectiveness of somatropin treatment for short children born small for gestational age (SGA) and children with growth hormone deficiency (GHD) in Sweden. J Med Econ. 2010;13(1):168–178. doi: 10.3111/13696991003652248. [DOI] [PubMed] [Google Scholar]
- 98.Evans C, Gregory JW. The investigation of short stature: a survey of practice in Wales and suggested practical guidelines. J Clin Pathol. 2004;57(2):126–130. doi: 10.1136/jcp.2002.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doctors of the coordinating site (Necker) Center of Reference Constitutional Bone Diseases (CR MOC). Achondroplasie. OSCAR-French Rare Diseases Healthcare Network 2017. 2017. Available at: http://www.filiere-oscarfr.
- 100.Hoover-Fong J, Scott CI, Jones MC. Health supervision for people with achondroplasia. Pediatrics. 2020;145(6):771–783. doi: 10.1542/peds.2020-1010. [DOI] [PubMed] [Google Scholar]
- 101.Wilson TA, Rose SR, Cohen P, Rogol AD, Backeljauw P, Brown R, et al. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003;143(4):415–421. doi: 10.1067/S0022-3476(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 102.White KK, Bompadre V, Goldberg MJ, Bober MB, Campbell JW, Cho TJ, et al. Best practices in the evaluation and treatment of foramen magnum stenosis in achondroplasia during infancy. Am J Med Genet A. 2016;170(1):42–51. doi: 10.1002/ajmg.a.37394. [DOI] [PubMed] [Google Scholar]
- 103.White KK, Bober MB, Cho T-J, Goldberg MJ, Hoover-Fong J, Irving M, et al. Best practice guidelines for management of spinal disorders in skeletal dysplasia. Orphanet J Rare Dis. 2020;15(1):1–11. doi: 10.1186/s13023-020-01415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kubota T, Adachi M, Kitaoka T, Hasegawa K, Ohata Y, Fujiwara M, et al. Clinical practice guidelines for achondroplasia. Clin Pediatr Endocrinol. 2020;29(1):25–42. doi: 10.1297/cpe.29.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Segal D. Guideline for using growth hormone in paediatric patients in South Africa: treatment of growth hormone deficiency and other growth disorders: guideline. S Afr Med J. 2009;99(3):187–195. [PubMed] [Google Scholar]
- 106.The Sydney Children's Hospital Network. Achondroplasia: practice guidelines for allied health professionals practice guideline. 2021. https://www.schn.health.nsw.gov.au/_policies/pdf/2016-235.pdf. Accessed Nov 2021.
- 107.Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Hormone Res Paediatr. 2016;86(6):361–397. doi: 10.1159/000452150. [DOI] [PubMed] [Google Scholar]
- 108.Collett-Solberg PF, Ambler G, Backeljauw PF, Bidlingmaier M, Biller BM, Boguszewski MC, et al. Diagnosis, genetics, and therapy of short stature in children: a growth hormone research society international perspective. Hormone Res Paediatr. 2019;92(1):1–14. doi: 10.1159/000502231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savarirayan R, Ireland P, Irving M, Thompson D, Alves I, Baratela WAR, et al. International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nat Rev Endocrinol. 2022;18(3):173–189. doi: 10.1038/s41574-021-00595-x. [DOI] [PubMed] [Google Scholar]
- 110.Savarirayan R, Tunkel DE, Sterni LM, Bober MB, Cho T-J, Goldberg MJ, et al. Best practice guidelines in managing the craniofacial aspects of skeletal dysplasia. Orphanet J Rare Dis. 2021;16(1):1–13. doi: 10.1186/s13023-021-01678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Constantinides C, Landis SH, Jarrett J, Quinn J, Ireland PJ. Quality of life, physical functioning, and psychosocial function among patients with achondroplasia: a targeted literature review. Disabil Rehabil. 2021;44:6166–6178. doi: 10.1080/09638288.2021.1963853. [DOI] [PubMed] [Google Scholar]
- 112.Shediac R, Moshkovich O, Gerould H, Ballinger R, Williams A, Bellenger MA, et al. Experiences of children and adolescents living with achondroplasia and their caregivers. Mol Genet Genom Med. 2022;10:e1891. doi: 10.1002/mgg3.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Backeljauw P, Cappa M, Kiess W, Law L, Cookson C, Sert C, et al. Impact of short stature on quality of life: A systematic literature review. Growth Hormon IGF Res. 2021;57:101392. doi: 10.1016/j.ghir.2021.101392. [DOI] [PubMed] [Google Scholar]
- 114.Quitmann J, Rohenkohl A, Sommer R, Bullinger M, Silva N. Explaining parent-child (dis) agreement in generic and short stature-specific health-related quality of life reports: do family and social relationships matter? Health Qual Life Outcomes. 2016;14(1):1–12. doi: 10.1186/s12955-016-0553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pfeiffer KM, Brod M, Smith A, Gianettoni J, Viuff D, Ota S, et al. Assessing physical symptoms, daily functioning, and well-being in children with achondroplasia. Am J Med Genet A. 2021;185(1):33–45. doi: 10.1002/ajmg.a.61903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pfeiffer KM, Brod M, Smith A, Viuff D, Ota S, Charlton RW, et al. Functioning and well-being in older children and adolescents with achondroplasia: a qualitative study. Am J Med Genet Part A. 2021;188(2):454–462. doi: 10.1002/ajmg.a.62534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Savarirayan R, Tofts L, Irving M, Wilcox WR, Bacino CA, Hoover-Fong J, et al. Safe and persistent growth-promoting effects of vosoritide in children with achondroplasia: 2-year results from an open-label, phase 3 extension study. Genet Med. 2021;23(12):2443–2447. doi: 10.1038/s41436-021-01287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wrobel W, Pach E, Ben-Skowronek I. Advantages and disadvantages of different treatment methods in achondroplasia: a review. Int J Mol Sci. 2021;22(11):5573. doi: 10.3390/ijms22115573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Llerena J, Kim C, Fano V, Rosselli P, Collett-Solberg PF, de Medeiros PFV, et al. Achondroplasia in Latin America: practical recommendations for the multidisciplinary care of pediatric patients. BMC Pediatr. 2022;22(1):1–13. doi: 10.1186/s12887-022-03505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Higgins JP. Cochrane handbook for systematic reviews of interventions version 5.0. 1. The Cochrane Collaboration. 2008. Available at: https://training.cochrane.org/handbook/current. Accessed Aug 2020.
- 121.Leiva-Gea A, Delgado-Rufino FB, Queipo-de-Llano A, Mariscal-Lara J, Lombardo-Torre M, Luna-González F. Staged upper and lower limb lengthening performing bilateral simultaneous surgery of the femur and tibia in achondroplastic patients. Arch Orthop Trauma Surg. 2020;140:1665–1676. doi: 10.1007/s00402-020-03360-3. [DOI] [PubMed] [Google Scholar]
- 122.Maghnie M, Semler O, Guillen-Navarro E, Selicorni A, Heath KE, Haeusler G, et al. Lifetime impact of achondroplasia study in Europe (LIAISE): findings from a multinational observational study. Orphanet J Rare Dis. 2023;18(1):56. doi: 10.1186/s13023-023-02652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim S, Agashe M, Song S, Choi H, Lee H, Song HR. Comparison between upper and lower limb lengthening in patients with achondroplasia: a retrospective study. J Bone Jt Surg Br. 2012;94(1):128–133. doi: 10.1302/0301-620X.94B1.27567. [DOI] [PubMed] [Google Scholar]
- 124.Gollust SE, Thompson RE, Gooding HC, Biesecker BB. Living with achondroplasia: attitudes toward population screening and correlation with quality of life. Prenatal Diagn Publ Aff Int Soc Prenatal Diagn. 2003;23(12):1003–1008. doi: 10.1002/pd.743. [DOI] [PubMed] [Google Scholar]
- 125.Kubota T, Wang W, Miura K, Nakayama H, Yamamoto K, Fujiwara M, et al. Serum NT-proCNP levels increased after initiation of GH treatment in patients with achondroplasia/hypochondroplasia. Clin Endocrinol. 2016;84(6):845–850. doi: 10.1111/cen.13025. [DOI] [PubMed] [Google Scholar]
- 126.Nishi Y, Kajiyama M, Miyagawa S, Fujiwara M, Hamamoto K. Growth hormone therapy in achondroplasia. Acta Endocrinol. 1993;128(5):394–396. doi: 10.1530/acta.0.1280394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.