Abstract
Purpose
The extent of glenohumeral bone loss seen in anterior shoulder dislocations plays a major role in guiding surgical management of these patients. The need for accurate and reliable preoperative assessment of bone loss on imaging studies is therefore of paramount importance to orthopedic surgeons. This article will focus on the tools that are available to clinicians for quantifying glenoid bone loss with a focus on emerging trends and research in order to describe current practices.
Recent findings
Recent evidence supports the use of 3D CT as the most optimal method for quantifying bone loss on the glenoid and humerus. New trends in the use of 3D and ZTE MRI represent exciting alternatives to CT imaging, although they are not widely used and require further investigation. Contemporary thinking surrounding the glenoid track concept and the symbiotic relationship between glenoid and humeral bone loss on shoulder stability has transformed our understanding of these lesions and has inspired a new focus of study for radiologists and orthopedist alike.
Summary
Although a number of different advanced imaging modalities are utilized to detect and quantify glenohumeral bone loss in practice, the current literature supports 3D CT imaging to provide the most reliable and accurate assessments. The emergence of the glenoid track concept for glenoid and humeral head bone loss has inspired a new area of study for researchers that presents exciting opportunities for the development of a deeper understanding of glenohumeral instability in the future. Ultimately, however, the heterogeneity of literature, which speaks to the diverse practices that exist across the world, limits any firm conclusions from being drawn.
Keywords: Glenohumeral bone loss, Bankart, Hill-Sachs, 3D CT, 3D MRI, Glenoid track
Introduction
Traumatic anterior shoulder dislocations are a known cause of glenohumeral instability. These dislocation events can cause significant osseous trauma in the form of glenoid and humeral head bone loss. The size of these Bankart and Hill-Sachs lesions, respectively, is a risk factor for recurrent instability and is critical in guiding appropriate management of these patients, which ranges from conservative management with graduated physical therapy for smaller lesions to complex reconstructive techniques, including Laterjet transfers and distal tibial allograft reconstructions for larger, more devastating defects. This treatment paradigm places heightened awareness on the need for accurate evaluation and measurement of bone loss [1, 2].
The instruments available for evaluating these deficits have evolved over the years. Historical tools like plain radiographs were unreliable and inconsistent and have given way to more precise advanced imaging techniques, including computed tomography (CT) and magnetic resonance imaging (MRI). While the methods that exist for measuring glenoid bone loss on advanced imaging have reasonable historical support in the literature, limited evidence exists for guidance of imaging of humeral head bone loss. The recent concept of the glenoid track and on-track and off-track Hill-Sachs lesions has highlighted the interconnectedness of bipolar bone loss suggesting that assessment of bone deficits in shoulder instability necessitates an evaluation of both in tandem. This review will focus on current imaging modalities used for evaluating bone loss in anterior shoulder instability with an emphasis on more contemporary approaches, in order to describe the current state of the field.
Glenoid Bone Loss: Modalities and Techniques
Historical Tools: Radiography and Arthroscopy
Prior to the emergence of advanced imaging techniques, plain radiographs represented the primary imaging tool available for scrutinizing bony anatomy and estimating glenoid injuries. Studies dating back to the 1970s were published using plain radiographs to identify glenoid bone lesions and correlate them with clinical shoulder instability. [3, 4] Other studies attempted to correlate estimates of the percentage of bone loss seen on plain radiographs with shoulder stability and need for bone augmentation with Bankart repairs. [5] While there has been some renewed interest over the past 5–10 years in the use of radiographs to quantify glenoid bone lesions due to their widespread availability, low radiation burden, and affordability, this modality remains largely a screening tool used to identify Bankart and Hill-Sachs lesions to guide advanced imaging [6–9, 10•, 11].
Intraoperative arthroscopic measurement of glenoid bone loss has also been described. These techniques use the glenoid bare spot (GBS) (Fig. 1), a critical landmark defined as the point at the center of the inferior glenoid, to measure bone loss. [12, 13] Ultimately, studies have questioned the validity of this method on the basis that the GBS is not reliably identified arthroscopically, and when it is, it is not consistently seen in the center of the inferior glenoid. [14–17] This has led to inaccuracy and inconsistency in measurements. [18, 19] Nonetheless, arthroscopic measurements of glenoid bone loss remain relevant as they continue to be used frequently in studies comparing different imaging modalities [20–22].
Fig. 1.
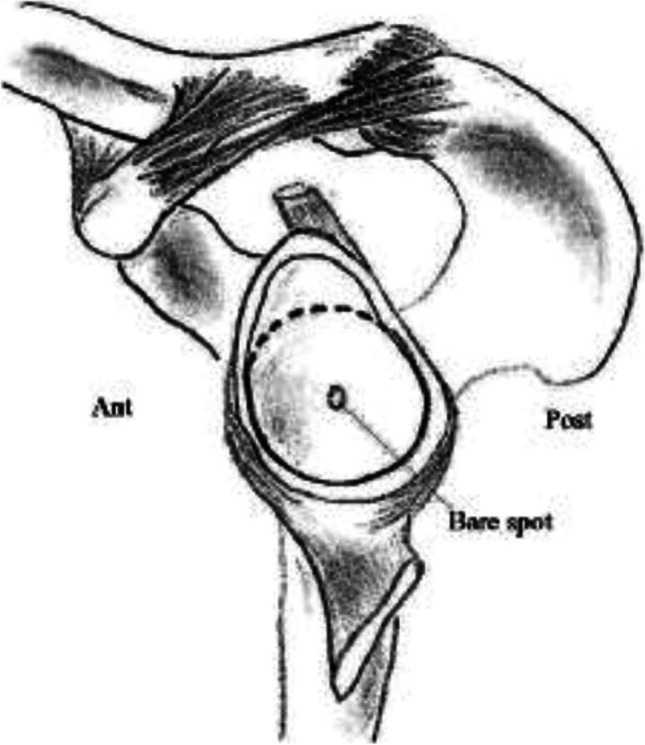
The Glenoid bare spot is located in the center of the inferior glenoid, which is estimated as a perfect circle formed from the contours of the inferior aspect of the glenoid. This landmark, which has been used to measure glenoid bone loss, has come under scrutiny for its lack of reproducibility. (From Saintmard B, Lecouvet F, Rubini A, Dubuc JE. Is the bare spot a valid landmark for glenoid evaluation in arthroscopic Bankart surgery? Acta Orthop Belg. 2009;75(6):736–42. Reprinted with permission according to BOAI open access policy)
CT Imaging
Computed tomography (CT) scan has long been the preferred and most widely utilized method for measuring glenoid bone loss. CT imaging provides excellent detail of bony anatomy allowing for clear and exact measurements of the glenoid. Several different measurement techniques for calculating glenoid bone loss have been proposed in the literature. For this review, these techniques may be classified into linear or surface area measurements [2, 10•, 23–28, 31] and statistical shape models that provide an equation to estimate bone loss. [28, 29] These approaches, which were initially designed for 2D CT, are applied to both 2D and 3D CT and MRI measurements, so a firm grasp of their underpinnings is critical to any analysis of glenoid bone loss.
Linear Models
Linear-based methods are advantageous since they are convenient and can be quickly applied using standard radiographic software. These methods of glenoid bone loss measurement typically use an “en face” view of the glenoid to measure the width of the glenoid defect, and this value is then compared to another “constant” such as the longitudinal glenoid axis or a best-fit circle [11, 30–32].
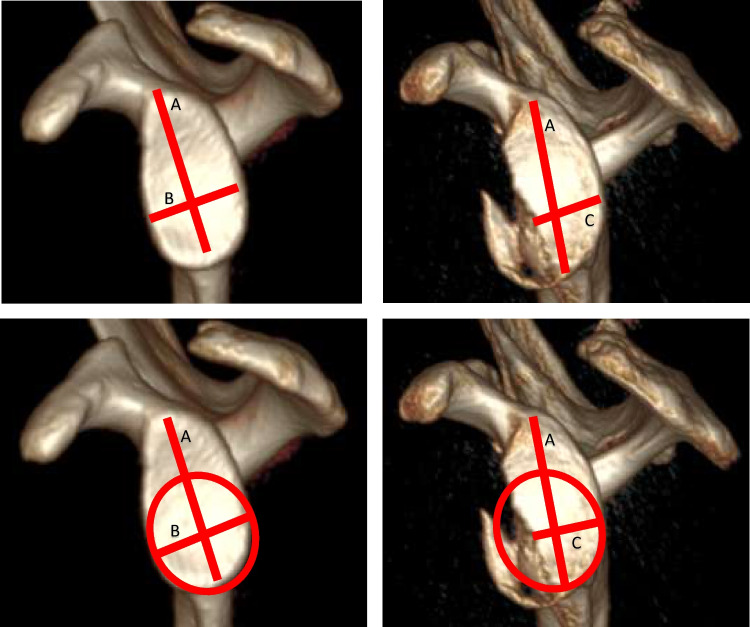
The simplest linear method in the literature involves comparisons of the glenoid width to the glenoid length. Described by Griffith et al. using 2D-CT, the “Griffith Index” describes the ratio between glenoid length (a line connecting the supraglenoid tubercle to the infraglenoid tubercle, “A”) and glenoid width (a line at the widest point of the glenoid perpendicular to the line representing glenoid length, “B”) (Fig. 2). The normal ratio (B/A) is 0.7, and an injured glenoid ratio is less than 0.7. [30] Using this method, the width-to-length (W/L) ratio and glenoid defect length, when expressed as functions of glenoid diameter, were most predictive of glenoid bone loss and recurrent instability, respectively. [30, 33] Further, validation against an arthroscopic “gold standard” showed a sensitivity and specificity of detecting glenoid bone loss with CT as 93% and 78%, respectively [19].
Fig. 2.
Examples of linear methods for measuring glenoid bone loss. In the Griffith Index (upper two images), the ratio of glenoid length:glenoid width is less in the injured glenoid C:A when compared to the uninjured glenoid B:A. In the Cheung glenoid index, a circle of best-fit on the uninjured glenoid is used as a reference for the AP width
In contrast, the vast majority of linear methods described in the literature report the comparison of the glenoid width or length to a circle of best-fit. [11, 25, 31, 32, 34, 35] The simplest iteration of these calculations is the “Glenoid Index,” described by Chuang et al. [36] Using a circle of best-fit on the uninjured glenoid as the reference for the antero-posterior glenoid width, the authors compare the ratio of the glenoid width-to-length (essentially the “Griffith Index”) between injured and uninjured sides to predict the necessity of bony repair. A number of other variations exist, all utilizing a variation of the circle of best fit for the injured glenoid. Individually, these various methods have been shown to be reproducible, though in small sample sizes with low levels of evidence [24–26, 33].
Overall, the validity and accuracy of linear-based methods of measuring glenoid bone loss remain in question. Concerns center around the notion that linear methods inaccurately approximate the inferior glenoid as a square, where reductions in glenoid width translate linearly into glenoid bone loss, as opposed to the more accurate reality of a radius-squared relationship. [37] Additionally, linear methods may underestimate defect size, especially in the case when there is bone loss in multiple planes (i.e., anterior-inferior).
Surface Area Models
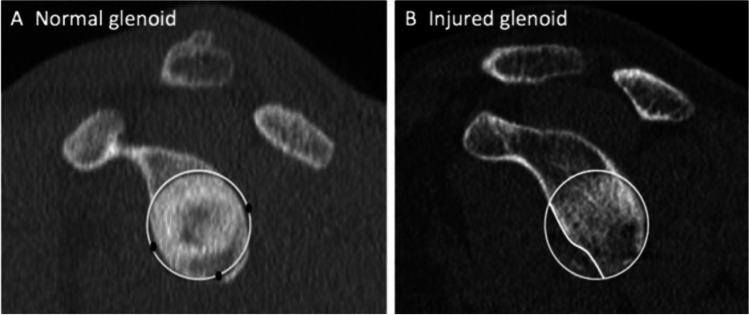
Surface area-based methods measure the area of the glenoid bony defect, which is then divided by the area of a best-fit circle, resulting in a percentage defect size. [27, 38] These techniques require specialized software that allows the user to freehand trace the glenoid defect in order to obtain its area, which has limited its widespread availability. [39] The most commonly used surface area measurement technique is the Pico method described by Baudi et al., which originally used 2D-CT to calculate bone loss as the area of the bony Bankart fragment divided by the area of the circle of best-fit (Fig. 3). [40] While this technique often uses the contralateral, uninjured glenoid as a template, the intact posterior inferior aspect of the ipsilateral glenoid can be used as a template for the glenoid best-fit circle with reasonable accuracy in cases where imaging of the contralateral shoulder is not available. This technique has the advantage of not being influenced by the position or morphologic features of glenoid bone loss, which can affect the accuracy of glenoid width or radial measurements. [23, 38, 41, 42] The PICO method and other surface area techniques have since been reproduced using other modalities, including 3D CT with good intra- and interobserver reliability [27].
Fig. 3.
In this application of the surface-based PICO method, the areas between the injured and the uninjured contralateral glenoids are compared. The defect is traced using freehand software and bone loss is presented as a percentage of the uninjured side. (from Saliken DJ, Bornes TD, Bouliane MJ, Sheps DM, Beaupre LA. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord. 2015;16:164. Reprinted with permission according to open access policy of BMC journal)
Statistical Modeling
Recent studies have queried the use of statistical and regression modeling in measurements of glenoid bone loss. These methods leverage the correlation between glenoid height and width to predict native glenoid width, which can then be used to estimate the percentage of bone loss. Owens et. al reported an MRI-based prediction of intact glenoid width based solely on the measurement of glenoid height. [29] Giles et al. validated this work using CT and similarly derived a method using glenoid height and width measurements combined with a CT-specific regression analysis to predict native glenoid width. [28] In the aforementioned studies, the developed models showed strong correlation when analyzed by sex. However, statistical modeling methods for glenoid bone loss have thus far been limited in sample size and therefore are not widely used.
Comparisonof Methods
While linear-based models are the most widely used, the literature supports the use of surface area measurements as demonstrating superior reliability to linear and statistical modeling methods. [43, 44•, 45] A laboratory study of two sawbone models of anterior and anteroinferior glenoid defects by Bois et al. found that the Pico surface area method was more accurate and reliable than linear techniques at quantifying glenoid bone loss. [46] A cadaveric study by Arenas-Miquelez et al. analyzed bone defects created in 6 fresh-frozen human shoulders using 2D and 3D CT en face images. Two linear techniques, 2 surface techniques, and 1 statistical shape model formula were subsequently used to quantify glenoid bone loss. Of the five techniques used, a surface technique was found to be superior with regard to combined consistency and accuracy for measuring glenoid bone loss. When accounting for consistency alone, the statistical shape model formula was found to be superior albeit prone to overestimation [47].
The superiority of surface area measurements was also corroborated by several clinical studies. In a study by Bakshi et. al, thirty patients with anterior shoulder instability underwent preoperative bilateral shoulder CT scans followed by three-dimensional CT reconstruction with humeral head subtraction. Glenoid bone loss was measured with the surface area and linear methods of measurement, and linear measurements were found to significantly overestimate bone loss compared to surface area measurements. [37] Similarly, a retrospective study of 125 patients with anterior glenoid bone loss found that measurements of surface area had the greatest interobserver reliability when compared to linear methods. [44•] Finally, a review of glenoid bone loss by Saliken et al. found that in 32 studies evaluating glenoid bone loss, the Pico surface area method was the most accurate and reliable method for quantifying glenoid bone loss.[48] Ultimately, the discrepancies between linear and surface area methods have been shown to have maximal discordance in the range of 15–25% bone loss, reflecting a clinically significant difference that must be reconciled in order to appropriately indicate patients for soft-tissue versus bony procedures [37].
2D vs. 3D CT
These various measurement techniques have been applied to both 2-dimensional and 3-dimensional CT images. [19, 27, 30, 36, 43, 46, 49–54] Axial 2-D CT scans are the most widely studied imaging modality and have repeatedly been shown to be helpful for the evaluation of the glenohumeral joint. [30, 50, 55] In 2D CT imaging, the acquisition of images in the coronal, sagittal, and axial planes facilitates the visualization of established landmarks for glenoid bone loss measurement. The biggest critique of 2D CT imaging stems from the difficulty obtaining a consistent en face view of the glenoid. Without multiplanar reconstruction, a 2D CT must be obtained in exactly the plane of view desired, i.e., along the axis of the glenoid surface, in order to obtain the proper measurement of its size. Any inconsistency in the version of the glenoid can significantly alter any measurements obtained ultimately limiting interobserver reliability.
3D CT with multiplanar reconstruction allows for manipulation of the CT imaging and digital subtraction of the humeral head generating an unobstructed en face view of the glenoid (Fig. 4). This modality eliminates the issues surrounding inconsistent orientation of the glenoid for measurement on 2D CT and has been shown to increase interrater agreement for the analysis of glenoid morphology and preoperative planning. [56•] As compared to 2D-CT, the benefits of 3D CT also include decreased scan duration, higher resolution imaging reconstructions, and improved cortical and topographic evaluation, though these benefits come at the expense of a higher-dose of ionizing radiation [57].
Fig. 4.
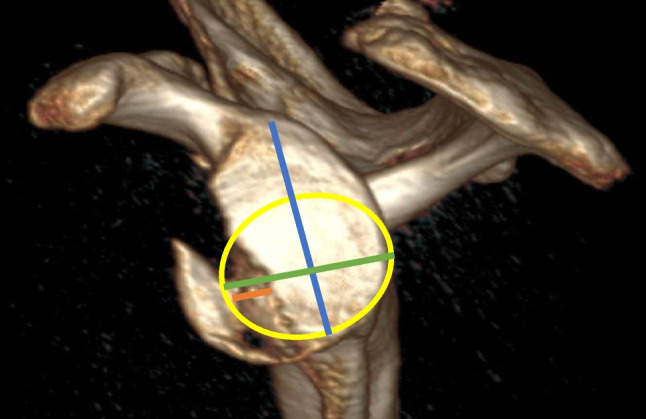
3D CT reconstructions with humeral head subtraction demonstrate an en face view of the glenoid surface
Comparison of CT Methods
The majority of current studies support the use of CT with 3D reconstruction as the method of choice for evaluation of glenoid bone loss over 2D CT. [43, 44•, 45, 46, 58, 59•, 60] There is limited evidence in the literature that 2D-CT may be comparable to 3D-CT; a study by Magarelli et al. found that 2D and 3D scans were similar in identifying the size of the glenoid defect using surface-area techniques. [55] In comparison, the study by Bois found that 2D measurements were not valid and should not be used. [46] Similarly, a study by Lacheta with 52 patients comparing 2D and 3D CT found that 3D CT scans were more accurate and had better intraobserver agreement overall compared to 2D scans. [58] In a study with 100 patients overall, Kubicka et al. found that 3D CT was more reliable with less intraobserver error compared to 2D CT. [59•] These studies further reinforce the superiority of 3D CT over 2D CT for the quantification of glenoid bone loss.
MRI Imaging
Recently, increased interest has emerged surrounding the use of magnetic resonance imaging (MRI) to perform measurements of glenoid bone loss. The introduction of 3 T (tesla) magnets has greatly improved the spatial resolution of MRI as well as its acquisition speed improving the user experience and ability to scrutinize bony detail. Critically, MRI holds a number of advantages over CT scan. For one, patients who experience traumatic shoulder dislocations will often undergo MRI imaging for evaluation of labral and other soft tissue pathology as part of standard of care. The ability to simultaneously evaluate bony sequelae without needing a second advanced imaging study would be both cost effective and efficient. Perhaps more importantly, MRI avoids radiation exposure for patients, which mitigates potential health risks.
MRI-based studies have leveraged the same techniques employed in CT imaging, largely relying on linear-based methods, to evaluate bone loss (Fig. 5). The vast majority of literature has compared MRI-based measurements to CT scan, both 2D and 3D, with overall mixed results. A number of studies have demonstrated results from MRI that are comparable to 2D CT scan in quantifying glenoid bone loss. [10•, 20, 61–63] In a series of 22 patients, Friedman et al. demonstrated moderate correlation between length-to-height ratio measurements across the glenoid in MRI and CT, although they acknowledged that further research needed to be conducted to validate the technique. [62] Sgroi et al. found similar results in their study, which compared MRI to 2D CT, AP radiographs, and West Point radiographs, suggesting MRI and CT were equivalent in terms of accuracy and reliability. [10•] In a larger study of 176 patients with anterior shoulder instability by Lee et al., MRI was found to be nearly as accurate as 2D CT, especially when using glenoid width rather than best-fit circle area to perform the calculations. [20] Moroder et al., however, found MRI to be significantly less sensitive (35%) than CT (100%) in detecting significant glenoid bone defects using a best-fit circle technique [35].
Fig. 5.
Glenoid measurements made using an en face view on T1-weighted MRI scan
While studies suggest reasonable efficacy of MRI compared to 2D CT, it performs less favorably overall when compared to 3D CT. The exception is a cadaveric study by Gyftopolous comparing MRI to 2D and 3D CT in a series of 18 specimens with glenoid bone loss using the best-fit circle method. They identified a very small (1.3% maximum) expected difference in measurements using the various techniques, although they concede there is a large learning curve for mastering the technique on MRI. [63] In contrast, Weber et al. showed that 2D MRI has decreased accuracy and worse interrater reliability for estimating glenoid bone loss when compared to 3D CT. [64] Rerko et al. illustrated that 3D CT was a superior imaging modality for assessing glenoid bone loss than 2D CT, MRI and plain radiographs. [45] Sugaya suggested that the benefits of 3D CT include vital preoperative anatomic information beyond simple degree of bone loss that makes it the most optimal study for preoperative workup of shoulder instability. [65] Nonetheless, it is relevant to note that heightened interest in the use of MRI for these bone loss measurements has led to MRI-specific formulas designed to quantify the degree of bone loss [29, 66].
3D MRI
More recently, the use of 3-Dimensional MRI has gained popularity as a new tool for evaluating glenoid bone loss in anterior shoulder instability. [21, 67–73, 74•] Similar to 3D CT scans, 3D MRI technology uses special MRI sequences and computer software programs to produce 3D renderings of the 2D MRI data. While these reconstructions use precise computer technology to improve the accuracy of the images of these 3D structures, the technological requirements and expertise required to execute it properly do currently limit its widespread use.
Early studies showed 3D MRI fared favorably in terms of accuracy when compared to bare spot arthroscopy. [21] Subsequent studies like the work by Tian et al. demonstrated the potential of 3D MRI by illustrating similar accuracy and consistency of glenoid bone loss measurement when compared to 2D CT. [72, 73] The vast majority of 3D MRI studies in this area, however, have focused on comparing its efficacy to what is considered by many to be the gold standard for glenoid bone loss measurement: 3D CT. Vopat et al. demonstrated that 3D MRI using the best fit circle technique and 3D CT were equivalent in measuring glenoid bone loss. [67] Lansdown et al. similarly confirmed the above in a study of 16 patients. They found the difference in bone loss identified on 3D MRI and 3D CT ranged from 0 to 6%, although they noted that these advanced techniques require specific software and the knowledge and experience of how to manipulate the data properly. [70] Lander et al. reaffirmed the results seen above and also performed a cost analysis demonstrating how the use of 3D MRI when eliminating the need for CT scan with secondary reconstruction could lead to a 1.67 times reduction in cost. Finally, Stillwater et al. showed that 3D MRI could not only accurately assess glenoid bone loss, but also any Hill-Sachs lesion present, which has been demonstrated to be a crucial element of overall shoulder stability [68].
Zero-Echo Time MRI
New research has investigated the use of zero-echo time MRI (ZTE) as an alternative to CT for evaluation of bone. This unique sequence setup leverages a very short T2 signal from trabecular and cortical bone which, when combined with the proton density weighting of the ZTE sequence, leads to less contrast between soft tissues. Subsequent gray-scale inversion leads to superior contrast between soft tissues and cortical bone comparable to CT scan (Fig. 6). [75] This MRI sequence has been used for enhanced imaging of the brain and skull and has been validated for the use of imaging in the hip and femoroacetabular impingement. [76, 77] Recently, these techniques have been used in the identification of glenoid pathology in shoulder instability. [78••, 79] Nevertheless, concerns remain surrounding the spatial resolution of this technique. Moreover, ZTE formatting requires skilled MRI technicians and radiologists well-versed in its use, which may limit its use on a broad level. [75] It represents an exciting new frontier in musculoskeletal imaging and osseous pathology, in particular, and will surely see continued interest in the coming years.
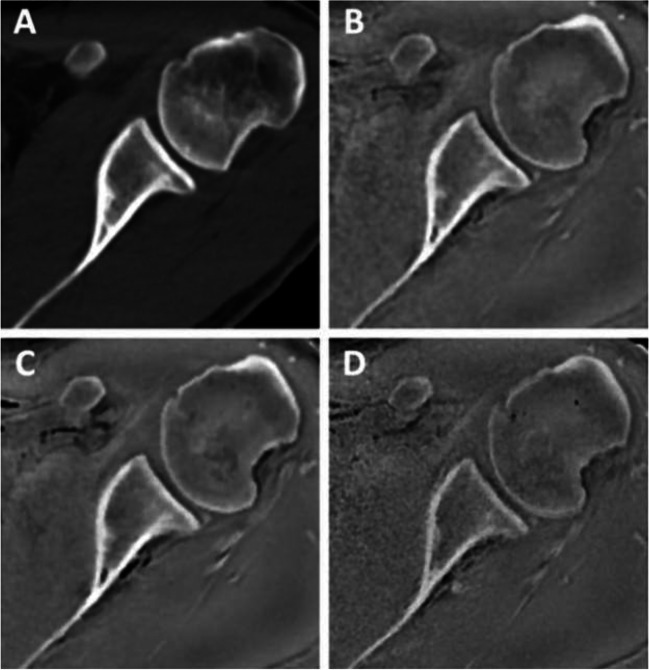
Fig. 6.
Four different axial images of the shoulder demonstrate excellent imaging of the bony structures: A CT; B ZTE MRI 1.0 mm; C ZTE MRI .8 mm; D ZTE MRI .7 mm (from de Mello RAF, Ma YJ, Ashir A, Jerban S, Hoenecke H, Carl M, et al. Three-Dimensional Zero Echo Time Magnetic Resonance Imaging Versus 3-Dimensional Computed Tomography for Glenoid Bone Assessment. Arthroscopy. 2020;36(9):2391–400. Printed with permission)
Humeral Head Bone Loss: Evaluation of the Hill-Sachs Lesion
Studies have shown that Hill-Sachs lesions are present in greater than 67% of patients who sustain anterior shoulder dislocations and may be found in up to 100% of patients with anterior shoulder instability. [80–83] Despite this, the literature on surrounding quantification of Hill-Sachs lesions and clinical correlations lags behind that which we see for glenoid bone loss. Plain radiographs are most commonly used for diagnosis, including special views such as the Stryker notch, West point, and Bernageau views, due to their affordability and usefulness as screening tools. Not surprisingly, their reliability and accuracy have been questioned, in certain cases missing more than half of clinically relevant Hill-Sachs lesions [83, 84].
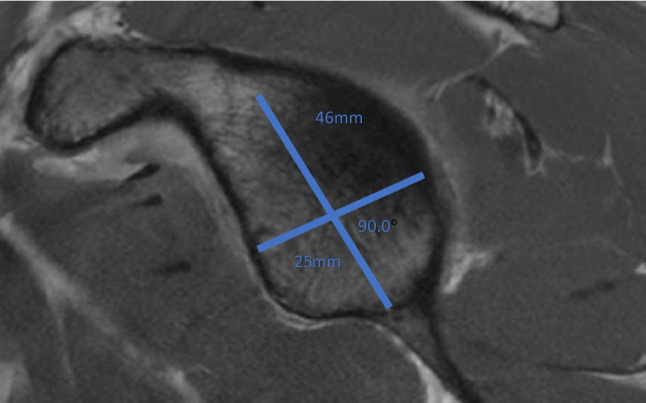
Advanced imaging modalities have allowed for more specific quantification of these lesions. The majority of studies have sought to measure lesion depth, width, and length in order to characterize the dimensions of the defects (Fig. 7). For example, a study by Cho et al. used 3D reconstructions of CT imaging to compare the size, location, and orientation of Hill-Sachs defects between engaging and non-engaging lesions. In their method, separate axial and coronal images were visualized in the slice where the lesion appeared largest. Virtual circles were drawn with the outline of the articular surface of the humeral head. Width was then defined as the distance between the ends of the lesion, and depth was the longest length between the nadir of the lesion and the outer arc of the circle. These values were then represented as a percentage of the diameter of the entire humeral head. Other angle measurements, including the Hill-Sachs angle, defined as the angle formed between a line down the longitudinal axis of the humeral shaft the axis of the deepest groove of the lesion, were also drawn in order to define the orientation and location of the defects. They showed that the size of engaging Hill-Sachs lesions was significantly larger than that of non-engaging lesions on both axial and coronal images, and lesions with larger Hill-Sachs angles were associated with engaging lesions [85].
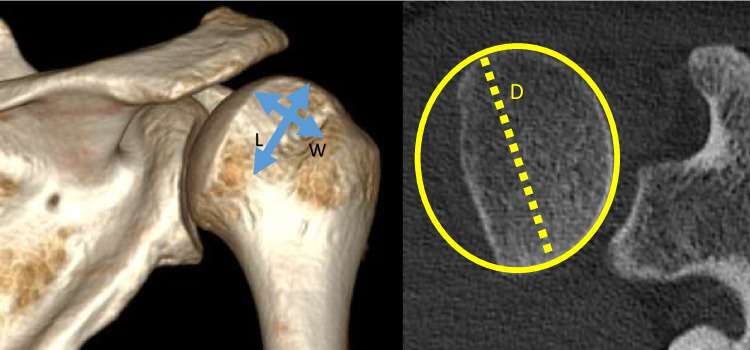
Fig. 7.
The width and depth of Hill-Sachs lesions can be measured on CT imaging. These are often presented as a percentage of the humeral head diameter (D)
While studies using a number of different imaging modalities have been published attempting to quantify humeral head bone loss, including MRI, magnetic resonance arthrography, and 2D CT, 3D CT is widely regarded as the superior imaging medium to detect and quantify the lesions. [78••, 85–91] Ultimately, further studies are needed to define a gold standard for imaging modality and measuring technique for these defects.
Bipolar Bone Loss and the Glenoid Track
While clinicians have long recognized the influence of both glenoid and humeral head bone loss on anterior shoulder instability, these two concepts were traditionally evaluated more or less independently. Only recently have we begun to appreciate the complex interplay between bony Bankart lesions and Hill-Sachs defects of the posterosuperior humeral head. [2, 92] Careful analysis of both components in tandem is necessary for any evaluation of glenohumeral instability moving forward [12, 93].
On the glenoid side, consensus agrees that anteroinferior bone loss > 25% of the inferior glenoid diameter is considered “critical” and ultimately warrants fixation with bone grafting. [94••, 95, 96] To facilitate discussion of significant humeral head bone loss, the concept of the glenoid track was defined, which is the area on the humeral head that makes contact with the glenoid with the arm in full external rotation from 0 to 60 degrees of abduction. The medial border of the glenoid track has been shown in MRI studies to be consistently located approximately 18 mm medial to the rotator cuff footprint and represents approximately 83% of the glenoid width. [97] Hill-Sachs lesions that extend beyond the borders of the glenoid track are at risk for engaging, or making contact, with the anterior rim of the glenoid with the shoulder in external rotation and abduction. However, importantly, in the setting of glenoid bone loss, the width of the glenoid track is reduced by the width of the glenoid defect. In this way, the impact of the humeral head lesion on overall stability depends on bipolar bone loss. Hill-Sachs lesions were coined as either on-track or off-track based on whether or not the lesion engages. On-track lesions do not engage, whereas off-track lesions engage [94••, 98].
The presence of an off-track Hill-Sachs lesion is a risk factor for recurrence of anterior glenohumeral instability following arthroscopic Bankart repair. [99, 100] In addition, studies have suggested that on-track versus off-track lesion status is able to better predict recurrent anterior instability over traditional glenoid bone loss measurement on MRI. [101] Ultimately, both the degree of glenoid bone loss and the on-track versus off-track status of the Hill-Sachs defect are critical in guiding surgical management and have led to the development of surgical treatment algorithms for managing bipolar bone loss in shoulder instability [94••, 102].
Role for Advanced Imaging in Evaluating On-Track versus Off-Track Lesions
Proper identification and quantification of bipolar bone loss are crucial for effective management of these patients. Both MRI and CT scan have been used to perform these measurements; however, three-dimensional CT scans appear to be the most reliable method by which to assess the on-track versus off-track status of a Hill-Sachs lesion. [89, 103–105] Regardless of modality used, many authors advocate using the contralateral glenoid to establish normal width, given that there is minimal side-to-side difference. [94••, 102, 106] The defect size (d) is then calculated as the difference between the contralateral intact glenoid width (D) minus the injured glenoid width [94••].
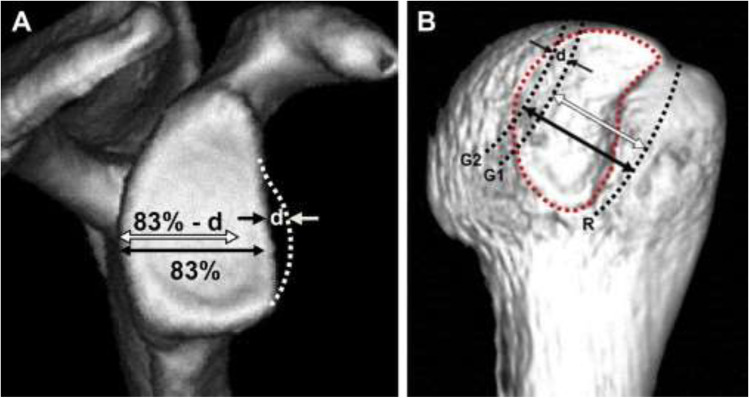
The glenoid track measures 83% of the glenoid width of the intact glenoid (D). In cases of glenoid bone loss, the size of the glenoid defect (d) is subtracted from the glenoid track, and the glenoid track measures: 0.83*D − d (Fig. 8). [94••] To determine if a lesion is on-track versus off-track, a line is drawn from the medial border of the rotator cuff footprint on the humeral head to a distance measuring 83% of the glenoid width minus the size of the bony glenoid defect, or the glenoid track. If the Hill-Sachs lesion extends medial to the medial border of the glenoid track, then the lesion is considered off-track or engaging. If the medial border of the Hill-Sachs lesion lies lateral to the medial border of the glenoid track, then the lesion is on-track and will not engage.
Fig. 8.
A The glenoid track is defined as 83% of the glenoid width less the width of the glenoid defect (83%-d). B The size of the glenoid track is reduced by the width of the glenoid defect (d). (from Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90–8. Printed with permission)
A similar method to evaluate the on-track versus off-track of a Hill-Sachs lesion is to measure the Hill-Sachs Interval. The Hill-Sachs Interval is the sum of the width of the Hill-Sachs lesion and the size of the bone bridge between the lateral border of the Hill-Sachs lesion and the medial border of the rotator cuff footprint. If the Hill-Sachs Interval is larger than the glenoid track, then the lesion is off-track, and if the Hill-Sachs Interval is smaller than the glenoid track, the lesion is on-track. [94••] Proper radiologic evaluation of the on-track or off-track status of the Hill-Sachs lesion is paramount for preoperative planning for these patients.
Conclusion
Accurate assessment of glenoid and humeral head bone loss is a crucial factor in guiding management for patients with post-traumatic shoulder instability. The literature surrounding glenoid bone loss measurement is more robust than that of the humeral head and, despite its heterogeneity, suggests that 3D CT is the most reliable and accurate tool for bone loss quantification, especially when using surface area-based methods. 3D and ZTE MRI represent exciting alternatives to CT imaging that may ultimately obviate the need for concomitant CT scan, leading to reduced health care costs and decreased radiation exposure. Widespread use of these modalities remains limited at this time due to specific software programs and technical expertise needed to execute them. Humeral head defects are similarly best detected and measured on 3D CT scan, with most techniques designed to measure the width and depth of the Hill Sachs lesions as a percentage of entire humeral head.
New research has illustrated the interconnectedness of bone defects on both the glenoid and humeral head. The concept of the glenoid track and the on-track/off-track nature of the Hill-Sachs lesion, which is intimately associated with the size of the osseous Bankart lesion, has shifted the paradigm of radiographic evaluation of anterior shoulder instability. Ultimately, further high quality research studies evaluating the specificity, sensitivity, and intra- and interobserver reliability of these different measurement techniques are needed to establish a true gold standard.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Apostolakos JM, Wright-Chisem J, Gulotta LV, Taylor SA, Dines JS. Anterior glenohumeral instability: current review with technical pearls and pitfalls of arthroscopic soft-tissue stabilization. World J Orthop. 2021;12(1):1–13. doi: 10.5312/wjo.v12.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner JW, Peebles LA, Bradley JP, Provencher MT. Anterior shoulder instability management: indications, techniques, and outcomes. Arthroscopy. 2020;36(11):2791–2793. doi: 10.1016/j.arthro.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Bernageau J, Patte D, Debeyre J, Ferrane J. Value of the glenoid profil in recurrent luxations of the shoulder. Rev Chir Orthop Reparatrice Appar Mot. 1976;62(2 suppl):142–147. [PubMed] [Google Scholar]
- 4.Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732–739. doi: 10.1016/S0749-8063(03)00684-4. [DOI] [PubMed] [Google Scholar]
- 5.Itoi E, Lee SB, Amrami KK, Wenger DE, An KN. Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography. Am J Sports Med. 2003;31(1):112–118. doi: 10.1177/03635465030310010301. [DOI] [PubMed] [Google Scholar]
- 6.Murachovsky J, Bueno RS, Nascimento LG, Almeida LH, Strose E, Castiglia MT, et al. Calculating anterior glenoid bone loss using the Bernageau profile view. Skeletal Radiol. 2012;41(10):1231–1237. doi: 10.1007/s00256-012-1439-9. [DOI] [PubMed] [Google Scholar]
- 7.Pansard E, Klouche S, Billot N, Rousselin B, Kraus TM, Bauer T, et al. Reliability and validity assessment of a glenoid bone loss measurement using the Bernageau profile view in chronic anterior shoulder instability. J Shoulder Elbow Surg. 2013;22(9):1193–1198. doi: 10.1016/j.jse.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Ikemoto RY, Nascimento LG, Bueno RS, Strose E, Almeida LH, Murachovsky J. Anterior glenoid rim erosion measured by X-ray exam: a simple way to perform the Bernageau profile view. Rev Bras Ortop. 2010;45(6):538–542. doi: 10.1590/S0102-36162010000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommaire C, Penz C, Clavert P, Klouche S, Hardy P, Kempf JF. Recurrence after arthroscopic Bankart repair: is quantitative radiological analysis of bone loss of any predictive value? Orthop Traumatol Surg Res. 2012;98(5):514–519. doi: 10.1016/j.otsr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Sgroi M, Huzurudin H, Ludwig M, Zippelius T, Reichel H, Kappe T. MRI allows accurate measurement of glenoid bone loss. Clin Orthop Relat Res. 2022;480(9):1731–1742. doi: 10.1097/CORR.0000000000002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter WR, Samim M, LaPolla FWZ, Gyftopoulos S. Imaging quantification of glenoid bone loss in patients with glenohumeral instability: a systematic review. AJR Am J Roentgenol. 2019;212(5):1096–1105. doi: 10.2214/AJR.18.20504. [DOI] [PubMed] [Google Scholar]
- 12.Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 13.Burkhart SS, Debeer JF, Tehrany AM, Parten PM. Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy. 2002;18(5):488–491. doi: 10.1053/jars.2002.32212. [DOI] [PubMed] [Google Scholar]
- 14.Kralinger F, Aigner F, Longato S, Rieger M, Wambacher M. Is the bare spot a consistent landmark for shoulder arthroscopy? A study of 20 embalmed glenoids with 3-dimensional computed tomographic reconstruction. Arthroscopy. 2006;22(4):428–432. doi: 10.1016/j.arthro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Saintmard B, Lecouvet F, Rubini A, Dubuc JE. Is the bare spot a valid landmark for glenoid evaluation in arthroscopic Bankart surgery? Acta Orthop Belg. 2009;75(6):736–742. [PubMed] [Google Scholar]
- 16.Barcia AM, Rowles DJ, Bottoni CR, Dekker TJ, Tokish JM. Glenoid bare area: arthroscopic characterization and its implications on measurement of bone loss. Arthroscopy. 2013;29(10):1671–1675. doi: 10.1016/j.arthro.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Miyatake K, Takeda Y, Fujii K, Takasago T, Iwame T. Validity of arthroscopic measurement of glenoid bone loss using the bare spot. Open Access J Sports Med. 2014;5:37–42. doi: 10.2147/OAJSM.S58748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakshi NK, Patel I, Jacobson JA, Debski RE, Sekiya JK. Comparison of 3-dimensional computed tomography-based measurement of glenoid bone loss with arthroscopic defect size estimation in patients with anterior shoulder instability. Arthroscopy. 2015;31(10):1880–1885. doi: 10.1016/j.arthro.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Griffith JF, Yung PS, Antonio GE, Tsang PH, Ahuja AT, Chan KM. CT compared with arthroscopy in quantifying glenoid bone loss. AJR Am J Roentgenol. 2007;189(6):1490–1493. doi: 10.2214/AJR.07.2473. [DOI] [PubMed] [Google Scholar]
- 20.Lee RK, Griffith JF, Tong MM, Sharma N, Yung P. Glenoid bone loss: assessment with MR imaging. Radiology. 2013;267(2):496–502. doi: 10.1148/radiol.12121681. [DOI] [PubMed] [Google Scholar]
- 21.Gyftopoulos S, Beltran LS, Yemin A, Strauss E, Meislin R, Jazrawi L, et al. Use of 3D MR reconstructions in the evaluation of glenoid bone loss: a clinical study. Skeletal Radiol. 2014;43(2):213–218. doi: 10.1007/s00256-013-1774-5. [DOI] [PubMed] [Google Scholar]
- 22.e Souza PM, Brandao BL, Brown E, Motta G, Monteiro M, Marchiori E. Recurrent anterior glenohumeral instability: the quantification of glenoid bone loss using magnetic resonance imaging. Skeletal Radiol. 2014;43(8):1085–92. doi: 10.1007/s00256-014-1894-6. [DOI] [PubMed] [Google Scholar]
- 23.Altan E, Ozbaydar MU, Tonbul M, Yalcin L. Comparison of two different measurement methods to determine glenoid bone defects: area or width? J Shoulder Elbow Surg. 2014;23(8):1215–1222. doi: 10.1016/j.jse.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Barchilon VS, Kotz E, Barchilon Ben-Av M, Glazer E, Nyska M. A simple method for quantitative evaluation of the missing area of the anterior glenoid in anterior instability of the glenohumeral joint. Skeletal Radiol. 2008;37(8):731–736. doi: 10.1007/s00256-008-0506-8. [DOI] [PubMed] [Google Scholar]
- 25.Dumont GD, Russell RD, Browne MG, Robertson WJ. Area-based determination of bone loss using the glenoid arc angle. Arthroscopy. 2012;28(7):1030–1035. doi: 10.1016/j.arthro.2012.04.147. [DOI] [PubMed] [Google Scholar]
- 26.Shaha JS, Cook JB, Song DJ, Rowles DJ, Bottoni CR, Shaha SH, et al. Redefining "critical" bone loss in shoulder instability: functional outcomes worsen with "Subcritical" bone loss. Am J Sports Med. 2015;43(7):1719–1725. doi: 10.1177/0363546515578250. [DOI] [PubMed] [Google Scholar]
- 27.Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85(5):878–884. doi: 10.2106/00004623-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Giles JW, Owens BD, Athwal GS. Estimating glenoid width for instability-related bone loss: a CT evaluation of an MRI formula. Am J Sports Med. 2015;43(7):1726–1730. doi: 10.1177/0363546515581468. [DOI] [PubMed] [Google Scholar]
- 29.Owens BD, Burns TC, Campbell SE, Svoboda SJ, Cameron KL. Simple method of glenoid bone loss calculation using ipsilateral magnetic resonance imaging. Am J Sports Med. 2013;41(3):622–624. doi: 10.1177/0363546512472325. [DOI] [PubMed] [Google Scholar]
- 30.Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423–1430. doi: 10.2214/ajr.180.5.1801423. [DOI] [PubMed] [Google Scholar]
- 31.Provencher MT, Bhatia S, Ghodadra NS, Grumet RC, Bach BR, Jr, Dewing CB, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(Suppl 2):133–151. doi: 10.2106/JBJS.J.00906. [DOI] [PubMed] [Google Scholar]
- 32.Verweij LPE, Schuit AA, Kerkhoffs G, Blankevoort L, van den Bekerom MPJ, van Deurzen DFP. Accuracy of currently available methods in quantifying anterior glenoid bone loss: controversy regarding gold standard-a systematic review. Arthroscopy. 2020;36(8):2295–313 e1. doi: 10.1016/j.arthro.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Gerber C, Nyffeler RW. Classification of glenohumeral joint instability. Clin Orthop Relat Res. 2002;400:65–76. doi: 10.1097/00003086-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Huysmans PE, Haen PS, Kidd M, Dhert WJ, Willems JW. The shape of the inferior part of the glenoid: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):759–763. doi: 10.1016/j.jse.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Moroder P, Resch H, Schnaitmann S, Hoffelner T, Tauber M. The importance of CT for the pre-operative surgical planning in recurrent anterior shoulder instability. Arch Orthop Trauma Surg. 2013;133(2):219–226. doi: 10.1007/s00402-012-1656-7. [DOI] [PubMed] [Google Scholar]
- 36.Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376–382. doi: 10.1016/j.arthro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Bakshi NK, Cibulas GA, Sekiya JK, Bedi A. A clinical comparison of linear- and surface area-based methods of measuring glenoid bone loss. Am J Sports Med. 2018;46(10):2472–2477. doi: 10.1177/0363546518783724. [DOI] [PubMed] [Google Scholar]
- 38.Baudi P, Righi P, Bolognesi D, Rivetta S, Rossi Urtoler E, Guicciardi N, et al. How to identify and calculate glenoid bone deficit. Chir Organi Mov. 2005;90(2):145–152. [PubMed] [Google Scholar]
- 39.Walter WR, Samim M, LaPolla FWZ, Gyftopoulos S. Imaging quantification of glenoid bone loss in patients with glenohumeral instability: a systematic review. AJR Am J Roentgenol. 2019;212(5):1096–105. [DOI] [PubMed]
- 40.Baudi P, Campochiaro G, Rebuzzi M, Matino G, Catani F. Assessment of bone defects in anterior shoulder instability. Joints. 2013;1(1):40–48. [PMC free article] [PubMed] [Google Scholar]
- 41.Milano G, Saccomanno MF, Magarelli N, Bonomo L. Analysis of agreement between computed tomography measurements of glenoid bone defects in anterior shoulder instability with and without comparison with the contralateral shoulder. Am J Sports Med. 2015;43(12):2918–2926. doi: 10.1177/0363546515608167. [DOI] [PubMed] [Google Scholar]
- 42.Rouleau DM, Garant-Saine L, Canet F, Sandman E, Menard J, Clement J. Measurement of combined glenoid and Hill-Sachs lesions in anterior shoulder instability. Shoulder Elbow. 2017;9(3):160–168. doi: 10.1177/1758573216681208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251–6. doi: 10.1007/s11999-012-2607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.• Min KS, Sy JW, Mannino BJ. Area measurement percentile of 3-dimensional computed tomography has the highest interobserver reliability when measuring anterior glenoid bone loss. Arthroscopy. 2023;39(6):1394–402. (Recent study demonstrating highest interobserver reliabilty when radiologists use 3D CT and area-based methods to measure glenoid bone loss.) [DOI] [PubMed]
- 45.Rerko MA, Pan X, Donaldson C, Jones GL, Bishop JY. Comparison of various imaging techniques to quantify glenoid bone loss in shoulder instability. J Shoulder Elbow Surg. 2013;22(4):528–534. doi: 10.1016/j.jse.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 46.Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569–2577. doi: 10.1177/0363546512458247. [DOI] [PubMed] [Google Scholar]
- 47.Arenas-Miquelez A, Dabirrahmani D, Sharma G, Graham PL, Appleyard R, Bokor DJ, et al. What is the most reliable method of measuring glenoid bone loss in anterior glenohumeral instability? A cadaveric study comparing different measurement techniques for glenoid bone loss. Am J Sports Med. 2021;49(13):3628–3637. doi: 10.1177/03635465211041386. [DOI] [PubMed] [Google Scholar]
- 48.Saliken DJ, Bornes TD, Bouliane MJ, Sheps DM, Beaupre LA. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord. 2015;16:164. doi: 10.1186/s12891-015-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugaya H, Moriishi J, Kanisawa I, Tsuchiya A. Arthroscopic osseous Bankart repair for chronic recurrent traumatic anterior glenohumeral instability. J Bone Joint Surg Am. 2005;87(8):1752–1760. doi: 10.2106/JBJS.D.02204. [DOI] [PubMed] [Google Scholar]
- 50.Griffith JF, Antonio GE, Yung PS, Wong EM, Yu AB, Ahuja AT, et al. Prevalence, pattern, and spectrum of glenoid bone loss in anterior shoulder dislocation: CT analysis of 218 patients. AJR Am J Roentgenol. 2008;190(5):1247–1254. doi: 10.2214/AJR.07.3009. [DOI] [PubMed] [Google Scholar]
- 51.Nofsinger C, Browning B, Burkhart SS, Pedowitz RA. Objective preoperative measurement of anterior glenoid bone loss: a pilot study of a computer-based method using unilateral 3-dimensional computed tomography. Arthroscopy. 2011;27(3):322–329. doi: 10.1016/j.arthro.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 52.De Filippo M, Castagna A, Steinbach LS, Silva M, Concari G, Pedrazzi G, et al. Reproducible noninvasive method for evaluation of glenoid bone loss by multiplanar reconstruction curved computed tomographic imaging using a cadaveric model. Arthroscopy. 2013;29(3):471–477. doi: 10.1016/j.arthro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Huijsmans PE, Haen PS, Kidd M, Dhert WJ, van der Hulst VP, Willems WJ. Quantification of a glenoid defect with three-dimensional computed tomography and magnetic resonance imaging: a cadaveric study. J Shoulder Elbow Surg. 2007;16(6):803–809. doi: 10.1016/j.jse.2007.02.115. [DOI] [PubMed] [Google Scholar]
- 54.Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85–90. doi: 10.1016/j.jse.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Magarelli N, Milano G, Baudi P, Santagada DA, Righi P, Spina V, et al. Comparison between 2D and 3D computed tomography evaluation of glenoid bone defect in unilateral anterior gleno-humeral instability. Radiol Med. 2012;117(1):102–111. doi: 10.1007/s11547-011-0712-7. [DOI] [PubMed] [Google Scholar]
- 56.Makhni EC, Tramer JS, Anderson MJJ, Levine WN. Evaluating bone loss in anterior shoulder instability. J Am Acad Orthop Surg. 2022;30(12):563–572. doi: 10.5435/JAAOS-D-22-00016. [DOI] [PubMed] [Google Scholar]
- 57.Sharifi A, Siebert MJ, Chhabra A. How to measure glenoid bone stock and version and why it is important: a practical guide. Radiographics. 2020;40(6):1671–1683. doi: 10.1148/rg.2020200008. [DOI] [PubMed] [Google Scholar]
- 58.Lacheta L, Herbst E, Voss A, Braun S, Jungmann P, Millett PJ, et al. Insufficient consensus regarding circle size and bone loss width using the ratio-"best fit circle"-method even with three-dimensional computed tomography. Knee Surg Sports Traumatol Arthrosc. 2019;27(10):3222–3229. doi: 10.1007/s00167-019-05391-9. [DOI] [PubMed] [Google Scholar]
- 59.Kubicka AM, Stefaniak J, Lubiatowski P, Dlugosz J, Dzianach M, Redman M, et al. Reliability of measurements performed on two dimensional and three dimensional computed tomography in glenoid assessment for instability. Int Orthop. 2016;40(12):2581–2588. doi: 10.1007/s00264-016-3253-9. [DOI] [PubMed] [Google Scholar]
- 60.Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577–583. doi: 10.1016/j.jse.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Stecco A, Guenzi E, Cascone T, Fabbiano F, Fornara P, Oronzo P, et al. MRI can assess glenoid bone loss after shoulder luxation: inter- and intra-individual comparison with CT. Radiol Med. 2013;118(8):1335–1343. doi: 10.1007/s11547-013-0927-x. [DOI] [PubMed] [Google Scholar]
- 62.Friedman LG, Ulloa SA, Braun DT, Saad HA, Jones MH, Miniaci AA. Glenoid bone loss measurement in recurrent shoulder dislocation: assessment of measurement agreement between CT and MRI. Orthop J Sports Med. 2014;2(9):2325967114549541. doi: 10.1177/2325967114549541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gyftopoulos S, Hasan S, Bencardino J, Mayo J, Nayyar S, Babb J, et al. Diagnostic accuracy of MRI in the measurement of glenoid bone loss. AJR Am J Roentgenol. 2012;199(4):873–878. doi: 10.2214/AJR.11.7639. [DOI] [PubMed] [Google Scholar]
- 64.Weber AE, Bolia IK, Horn A, Villacis D, Omid R, Tibone JE, et al. Glenoid bone loss in shoulder instability: superiority of three-dimensional computed tomography over two-dimensional magnetic resonance imaging using established methodology. Clin Orthop Surg. 2021;13(2):223–228. doi: 10.4055/cios20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugaya H. Techniques to evaluate glenoid bone loss. Curr Rev Musculoskelet Med. 2014;7(1):1–5. doi: 10.1007/s12178-013-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olmscheid N, Crawford SD, Dickinson C, Fajardo RS, Knake JJ, Wilcox CL, et al. Novel anterior coracoglenoid line utilizing magnetic resonance imaging (MRI) corresponds with critical glenoid bone loss. Skeletal Radiol. 2022;51(7):1433–1438. doi: 10.1007/s00256-021-03981-8. [DOI] [PubMed] [Google Scholar]
- 67.Vopat BG, Cai W, Torriani M, Vopat ML, Hemma M, Harris GJ, et al. Measurement of glenoid bone loss with 3-dimensional magnetic resonance imaging: a matched computed tomography analysis. Arthroscopy. 2018;34(12):3141–3147. doi: 10.1016/j.arthro.2018.06.050. [DOI] [PubMed] [Google Scholar]
- 68.Stillwater L, Koenig J, Maycher B, Davidson M. 3D-MR vs. 3D-CT of the shoulder in patients with glenohumeral instability. Skeletal Radiol. 2017;46(3):325–31. doi: 10.1007/s00256-016-2559-4. [DOI] [PubMed] [Google Scholar]
- 69.Lander ST, Liles JL, Kim BI, Taylor DC, Lau BC. Comparison of computed tomography and 3D magnetic resonance imaging in evaluating glenohumeral instability bone loss. J Shoulder Elbow Surg. 2022;31(11):2217–2224. doi: 10.1016/j.jse.2022.06.015. [DOI] [PubMed] [Google Scholar]
- 70.Lansdown DA, Cvetanovich GL, Verma NN, Cole BJ, Bach BR, Nicholson G, et al. Automated 3-dimensional magnetic resonance imaging allows for accurate evaluation of glenoid bone loss compared with 3-dimensional computed tomography. Arthroscopy. 2019;35(3):734–740. doi: 10.1016/j.arthro.2018.10.119. [DOI] [PubMed] [Google Scholar]
- 71.Lansdown DA, Pedoia V. Editorial commentary: Can we evaluate glenoid bone with magnetic resonance imaging? Yes, if you have the right sequence. Arthroscopy. 2020;36(9):2401–2402. doi: 10.1016/j.arthro.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 72.Ma YJ, West J, Nazaran A, Cheng X, Hoenecke H, Du J, et al. Feasibility of using an inversion-recovery ultrashort echo time (UTE) sequence for quantification of glenoid bone loss. Skeletal Radiol. 2018;47(7):973–980. doi: 10.1007/s00256-018-2898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian CY, Shang Y, Zheng ZZ. Glenoid bone lesions: comparison between 3D VIBE images in MR arthrography and nonarthrographic MSCT. J Magn Reson Imaging. 2012;36(1):231–236. doi: 10.1002/jmri.23622. [DOI] [PubMed] [Google Scholar]
- 74.Yanke AB, Shin JJ, Pearson I, Bach BR, Jr, Romeo AA, Cole BJ, et al. Three-dimensional magnetic resonance imaging quantification of glenoid bone loss is equivalent to 3-dimensional computed tomography quantification: cadaveric study. Arthroscopy. 2017;33(4):709–715. doi: 10.1016/j.arthro.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 75.Aydingoz U, Yildiz AE, Ergen FB. Zero echo time musculoskeletal MRI: technique, optimization, applications, and pitfalls. Radiographics. 2022;42(5):1398–1414. doi: 10.1148/rg.220029. [DOI] [PubMed] [Google Scholar]
- 76.Breighner RE, Bogner EA, Lee SC, Koff MF, Potter HG. Evaluation of osseous morphology of the hip using zero echo time magnetic resonance imaging. Am J Sports Med. 2019;47(14):3460–3468. doi: 10.1177/0363546519878170. [DOI] [PubMed] [Google Scholar]
- 77.Cho SB, Baek HJ, Ryu KH, Choi BH, Moon JI, Kim TB, et al. Clinical feasibility of zero TE skull MRI in patients with head trauma in comparison with CT: a single-center study. AJNR Am J Neuroradiol. 2019;40(1):109–115. doi: 10.3174/ajnr.A5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Breighner RE, Endo Y, Konin GP, Gulotta LV, Koff MF, Potter HG. Technical developments: zero echo time imaging of the shoulder: enhanced osseous detail by using MR imaging. Radiology. 2018;286(3):960–966. doi: 10.1148/radiol.2017170906. [DOI] [PubMed] [Google Scholar]
- 79.de Mello RAF, Ma YJ, Ashir A, Jerban S, Hoenecke H, Carl M, et al. Three-dimensional zero echo time magnetic resonance imaging versus 3-dimensional computed tomography for glenoid bone assessment. Arthroscopy. 2020;36(9):2391–2400. doi: 10.1016/j.arthro.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fox JA, Sanchez A, Zajac TJ, Provencher MT. Understanding the Hill-Sachs lesion in its role in patients with recurrent anterior shoulder instability. Curr Rev Musculoskelet Med. 2017;10(4):469–479. doi: 10.1007/s12178-017-9437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159–68. doi: 10.2106/00004623-198466020-00001. [DOI] [PubMed] [Google Scholar]
- 82.Welsh MF, Willing RT, Giles JW, Athwal GS, Johnson JA. A rigid body model for the assessment of glenohumeral joint mechanics: influence of osseous defects on range of motion and dislocation. J Biomech. 2016;49(4):514–519. doi: 10.1016/j.jbiomech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Yiannakopoulos CK, Mataragas E, Antonogiannakis E. A comparison of the spectrum of intra-articular lesions in acute and chronic anterior shoulder instability. Arthroscopy. 2007;23(9):985–990. doi: 10.1016/j.arthro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Charousset C, Beauthier V, Bellaiche L, Guillin R, Brassart N, Thomazeau H, et al. Can we improve radiological analysis of osseous lesions in chronic anterior shoulder instability? Orthop Traumatol Surg Res. 2010;96(8 Suppl):S88–93. doi: 10.1016/j.otsr.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Cho SH, Cho NS, Rhee YG. Preoperative analysis of the Hill-Sachs lesion in anterior shoulder instability: how to predict engagement of the lesion. Am J Sports Med. 2011;39(11):2389–2395. doi: 10.1177/0363546511398644. [DOI] [PubMed] [Google Scholar]
- 86.Ho A, Kurdziel MD, Koueiter DM, Wiater JM. Three-dimensional computed tomography measurement accuracy of varying Hill-Sachs lesion size. J Shoulder Elbow Surg. 2018;27(2):350–356. doi: 10.1016/j.jse.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 87.Ozaki R, Nakagawa S, Mizuno N, Mae T, Yoneda M. Hill-sachs lesions in shoulders with traumatic anterior instability: evaluation using computed tomography with 3-dimensional reconstruction. Am J Sports Med. 2014;42(11):2597–2605. doi: 10.1177/0363546514549543. [DOI] [PubMed] [Google Scholar]
- 88.Burns DM, Chahal J, Shahrokhi S, Henry P, Wasserstein D, Whyne C, et al. Diagnosis of engaging bipolar bone defects in the shoulder using 2-dimensional computed tomography: a cadaveric study. Am J Sports Med. 2016;44(11):2771–2777. doi: 10.1177/0363546516655797. [DOI] [PubMed] [Google Scholar]
- 89.Schneider AK, Hoy GA, Ek ET, Rotstein AH, Tate J, Taylor DM, et al. Interobserver and intraobserver variability of glenoid track measurements. J Shoulder Elbow Surg. 2017;26(4):573–579. doi: 10.1016/j.jse.2016.09.058. [DOI] [PubMed] [Google Scholar]
- 90.Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20(8):1328–1334. doi: 10.1016/j.jse.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 91.Gyftopoulos S, Yemin A, Beltran L, Babb J, Bencardino J. Engaging Hill-Sachs lesion: is there an association between this lesion and findings on MRI? AJR Am J Roentgenol. 2013;201(4):W633–W638. doi: 10.2214/AJR.12.10206. [DOI] [PubMed] [Google Scholar]
- 92.Ladd LM, Crews M, Maertz NA. Glenohumeral joint instability: a review of anatomy, xlinical presentation, and imaging. Clin Sports Med. 2021;40(4):585–599. doi: 10.1016/j.csm.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470–7. doi: 10.1302/0301-620X.89B11.18962. [DOI] [PubMed] [Google Scholar]
- 94.Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from "engaging/non-engaging" lesion to "on-track/off-track" lesion. Arthroscopy. 2014;30(1):90–98. doi: 10.1016/j.arthro.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 95.Lynch JR, Clinton JM, Dewing CB, Warme WJ, Matsen FA., 3rd Treatment of osseous defects associated with anterior shoulder instability. J Shoulder Elbow Surg. 2009;18(2):317–328. doi: 10.1016/j.jse.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto N, Itoi E. Osseous defects seen in patients with anterior shoulder instability. Clin Orthop Surg. 2015;7(4):425–429. doi: 10.4055/cios.2015.7.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Omori Y, Yamamoto N, Koishi H, Futai K, Goto A, Sugamoto K, et al. Measurement of the glenoid track in vivo as investigated by 3-dimensional motion analysis using open MRI. Am J Sports Med. 2014;42(6):1290–1295. doi: 10.1177/0363546514527406. [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649–656. doi: 10.1016/j.jse.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 99.Bracamontes-Martinez CN, Juarez-Jimenez HG, Rojas-Larios F, Sanchez-Rojas P, Calderon-Franco JA, Chavez-Garcia CR. Glenoid track "off-track" as a risk factor for recurrence of anterior glenohumeral instability in postoperative patients. Acta Ortop Mex. 2020;34(6):365–370. [PubMed] [Google Scholar]
- 100.Locher J, Wilken F, Beitzel K, Buchmann S, Longo UG, Denaro V, et al. Hill-Sachs off-track lesions as risk factor for recurrence of instability after arthroscopic bankart repair. Arthroscopy. 2016;32(10):1993–1999. doi: 10.1016/j.arthro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 101.Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918–1923. doi: 10.2106/JBJS.15.01099. [DOI] [PubMed] [Google Scholar]
- 102.Itoi E. ‘On-track’ and ‘off-track’ shoulder lesions. EFORT Open Rev. 2017;2(8):343–351. doi: 10.1302/2058-5241.2.170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clement J, Menard J, Raison M, Dumais J, Dubois L, Rouleau DM. Three-dimensional analysis of the locked position in patients with recurrent shoulder instability. J Shoulder Elbow Surg. 2017;26(3):536–543. doi: 10.1016/j.jse.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 104.Gyftopoulos S, Beltran LS, Bookman J, Rokito A. MRI evaluation of bipolar bone loss using the on-track off-track method: a feasibility study. AJR Am J Roentgenol. 2015;205(4):848–852. doi: 10.2214/AJR.14.14266. [DOI] [PubMed] [Google Scholar]
- 105.Mulleneers LIC, Van Rompaey H, Haloui B, Pouliart N. Determining on-/off-track lesions in glenohumeral dislocation using multiplanar reconstruction computed tomography is easier and more reproducible than using 3-dimensional computed tomography. Am J Sports Med. 2021;49(1):137–145. doi: 10.1177/0363546520971856. [DOI] [PubMed] [Google Scholar]
- 106.Jeske HC, Oberthaler M, Klingensmith M, Dallapozza C, Smekal V, Wambacher M, et al. Normal glenoid rim anatomy and the reliability of shoulder instability measurements based on intrasite correlation. Surg Radiol Anat. 2009;31(8):623–625. doi: 10.1007/s00276-009-0492-0. [DOI] [PubMed] [Google Scholar]