Abstract
Monitoring nitrogen utilization efficiency and soil temperature in agricultural systems for timely intervention is essential to monitor crop health, promote sustainable and precision agriculture, and reduce environmental pollution. Therefore, it is of vital significance to develop a multi-parameter sensor for effectively and accurately decoupled detection of nitrogen loss and soil temperature, which is yet to be reported. Herein, this work presents a high-performance multi-parameter sensor based on vanadium oxide ()-doped laser-induced graphene (LIG) foam to completely decouple nitrogen oxides (NOX) and temperature. By exploiting the laser-assisted synthesis, the highly porous 3D -doped LIG foam composite is readily obtained by laser scribing vanadium sulfide (V5S8)-doped block copolymer and phenolic resin self-assembled films. Compared with the intrinsic LIG, the heterojunction formed at the interface provides the sensor with a significantly enhanced response to NOX, and an ultralow limit of detection (LOD) of 3 ppb (theoretical estimate of 451 ppt) at room temperature. The sensor also exhibits a wide detection range (from 3 ppb to 5 ppm), fast response/recovery (217/650 s to 1 ppm NO2), good selectivity (ten-fold response to NO2 over other interfering gases), and stability over 16 days. Meanwhile, the sensor can accurately detect temperature over a wide linear range of 10–110°C with a small detection limit of 0.2°C. The encapsulation of the sensor with a soft membrane further allows for temperature sensing without being affected by NOX, presenting an effective strategy to decouple nitrogen loss and soil temperature for accurate soil environmental monitoring. The sensor without encapsulation but operated at elevated temperature removes the influences of ambient relative humidity and temperature variations for accurate NOX measurements. The capability to simultaneously detect ultra-low NOX concentrations and small temperature changes paves the way for the development of future multimodal electronic devices with decoupled sensing mechanisms for health monitoring and precision agriculture.
Keywords: Vanadium oxide-doped laser-induced graphene foam, multi-parameter sensor, nitrogen loss and soil temperature monitoring, precision agriculture
Graphical Abstract
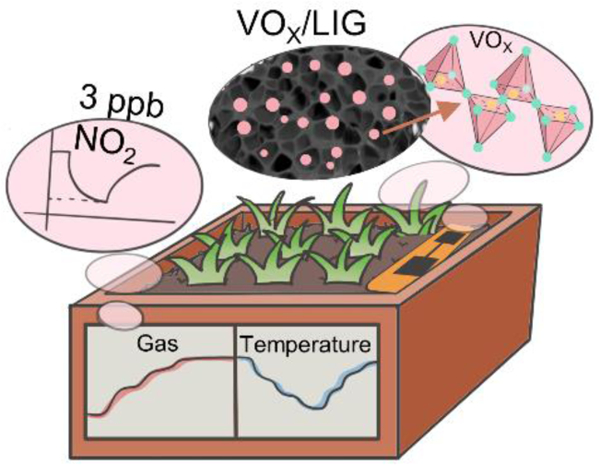
ToC text:
The highly porous 3D vanadium oxide-doped laser-induced graphene foam composite is readily obtained by laser scribing vanadium sulfide-doped block copolymer and phenolic resin self-assembled films. The resulting foam composite provides a high-performance multi-parameter sensor to completely decouple nitrogen oxides and temperature, allowing for accurate detection of nitrogen loss and soil temperature for precision agriculture.
INTRODUCTION
One key issue in traditional agricultural practices is the enormous nitrogen fertilizers applied to soil for promoted food production1, but resulting excessive nitrogen oxides (NOX including NO and NO2) emissions cause environmental pollution2–4 (e.g., photochemical smog and acid rain3). Besides, soil temperature in the range from −10 to 50°C5, 6 also influences the physical, chemical, and microbiological processes in soil, which are key to the growth of plants and crops7, 8. Continuous and real-time monitoring of soil conditions such as soil temperature and fertilizer emission will be essential to improve resource utilization, maximize agricultural yields, and minimize environmental hazards2. Therefore, there is a demanding need to develop a multi-parameter sensor to monitor NOX gas emission and soil temperature for efficient fertilization in smart or precision agriculture9, 10. Despite their paramount importance in agriculture, gas-temperature sensors with decoupled sensing mechanisms especially those prepared by low-cost and scalable fabrication methods are rarely reported11.
Sensors and sensing systems based on low-cost manufacturing approaches12, 13, 14 start to gain momentum in health monitoring and precision medicine due to their capability to collect big data from a large population and the potential to be integrated with artificial intelligence. Recent advances in gas sensors have explored various types of nanomaterials, including transition metal dichalcogenides (TMDs), metal-organic frameworks (MOFs), metal oxides, black phosphorus, as well as transition metal carbides and carbonitrides (MXene)15–18. The synthesis strategies of these nanomaterials often involve solution phase reaction, hydrothermal reaction, chemical vapor deposition, and electrodeposition19. These nanomaterials prepared by relatively complex and costly methods also need to be integrated on high-resolution interdigitated electrodes that are fabricated with photolithography in chemiresistor gas sensors20–22. Meanwhile, separately integrated heaters are commonly used to elevate the temperature for expedited gas adsorption/desorption toward real-time detection. As an alternative, the gas sensing platform based on 3D porous laser-induced graphene (LIG) by low-cost laser direct writing23 can eliminate the need for separate heaters and high-resolution interdigitated electrodes due to large specific surface area and self-heating24. The LIG has also been explored directly as a gas sensor and in many other sensors/devices23, 25–27. However, it is challenging to control the porous structure of the LIG foam when using common carbon-containing materials during laser processes. Efforts to address this issue lead to the exploitation of self-assembled block copolymer during direct laser writing28, 29 in preparing for the LIG with hierarchically porous structures30. Vanadium oxide () as a transition metal oxide exhibits excellent properties, including n-type conductivity good chemical and thermal stability, and excellent thermoelectric properties31, 32. and its composites also show excellent performance to physically adsorb and chemically interact with several gases, including nitrogen dioxide, ammonia, hydrogen, and methane, among others33–36. However, the application of and its composites is limited by complex synthesis methods, including spray pyrolysis37, hydrothermal38, sol-gel method39, electrospinning40, chemical vapor deposition41, and precipitation42. The recent report that directly synthesizes with laser43 provides inspiration and opportunities to directly laser write the foam composites. Doping metal complexes in graphene is also found to improve the gas adsorption and detection sensitivity of resulting gas sensors44, 45.
In this study, we report the one-step laser direct writing method to directly synthesize -doped 3D porous LIG foam nanocomposites by laser scribing the block copolymers (BCPs) doped with V5S8 precursor. Different from common carbon-containing materials (e.g., PI, PAI, PES, PPS) with only one monomer, the Pluronic F127-resols with a tunable mass ratio of Pluronic F127 copolymer to resols mixture in ethanol can leverage a bottom-up self-assembly process to control the resulting mesostructures and pore size distribution. The Pluronic F127-resols film can easily modulate the porous structure by the mass ratio of BCP to resins, whereas particles can be anchored on the porous LIG without aggregation46. The sensor shows excellent selectivity for NOX due to the lowest unoccupied molecular orbital (LUMO) of NOX lower than that of other gases resulting in more electrons transferred from the 47. Meanwhile, the adsorption energy of to NO2 gas molecules is greater than that of other interfering gases. The heterojunction formed at the interface significantly enhances the sensing performance of the multi-parameter sensor, allowing for the detection of the ultra-low concentration of 3 ppb NO2 and a wide range of soil temperature with high sensitivity. The sensor encapsulated by a soft membrane can block the permeation of the gas molecules to respond only to temperature variations. The accurately measured temperature can, in turn, allow the dual-modal sensor to determine the NOX emission from the soil. The proof-of-the-concept demonstration to decouple NOX emission and temperature variations from the soil presented in this work can be leveraged to design and apply multimodal devices with decoupled sensing mechanisms for precision agriculture in all-weather conditions. Integrating the sensor with data processing and wireless transmission modules further results in a remote environmental monitoring system to wirelessly detect NOX and temperatures for human health monitoring and precision agriculture.
RESULTS
Device structure and characterization
The -doped LIG sensor can be facilely obtained by laser scribing the carbon-containing material such as Pluronic F127-resols doped with a precursor such as V5S8 (Fig. 1a, Fig. S1). In brief, spin coating the V5S8-doped Pluronic F127-resols solution on a Si substrate at different speeds forms a thin film with different thicknesses (Fig. S2, S3a). After drying the thermosetting phenolic resin, applying the CO2 laser in a programmed pattern on the resulting thin film creates the -doped LIG sensor, which can be scalable for massive production (Fig. S3b). At a scanning rate of 12.7 μm/s, V5S8 in the thin film is instantaneously oxidized to, providing uniform doping of in the 3D LIG foam. The sensor capable of decoupling the NOX and temperature (Fig. 1b) allows for NOX and temperature monitoring in the soil (Fig. 1c).
Fig. 1. Schematic illustration showing.

a the fabrication, structure, b decoupling of temperature and gas, and c soil NOX gas and temperature detection.
The porous-structured LIG (Fig. 2a) doped with the laser-induced V5S8 and particles (Fig. 2b, red box) can be observed in the scanning electron microscope (SEM) image. Several different peaks in the C 1s and V 2p of XPS spectra indicate the successful formation of graphene and in the laser process (Fig. S4). The two V 2p peaks at the binding energy values of 517.60 eV and 525 eV in the spectrum correspond to V 2p3/2 and V 2p1/2, respectively (Fig. 2c). The observed binding energy values with a spin-orbit splitting of 7.4 eV are in good agreement with the V5+ oxidation state37, 48, 49. The spin-orbit splitting between V 2p3/2 and V 2p1/2 peak is 7.4 eV. The Raman spectra confirm the presence of few-layered graphene in -doped LIG (Fig. 2d) by exhibiting three prominent peaks: the D (∼1338 cm–1), G (∼1524 cm–1), and 2D (∼2673 cm–1)48. The enlarged Raman spectrum shows peaks at 142, 193, 281, 404, 695, and 991 cm–1 to confirm the formation of 50, 51. The combination of LIG and in -doped LIG abnormally shifts the peaks compared with the previously reported 51. The XRD patterns of -doped LIG show peaks at 24.0°, 30.8°, 34.3°, 36.7°, 45.4°, 47.6°, 55.8°, and 58.2° (Fig. 2e), corresponding to (201), (011), (310), (002), (112), (212), (412), and (611) (JCPDS card No. 30–0286) to confirm the formation of 52, 53. The spectra confirm the presence of , although it is difficult to distinguish from V5S8 due to the overlap in the bands54, 55. The distinct distributions of C, O, S, and V elements in the EDS spectrum in the sensing area demonstrate that V5S8 is partially transformed to during laser processing (Fig. 2f).
Fig. 2. Characterizations of laser-heated resin structures.
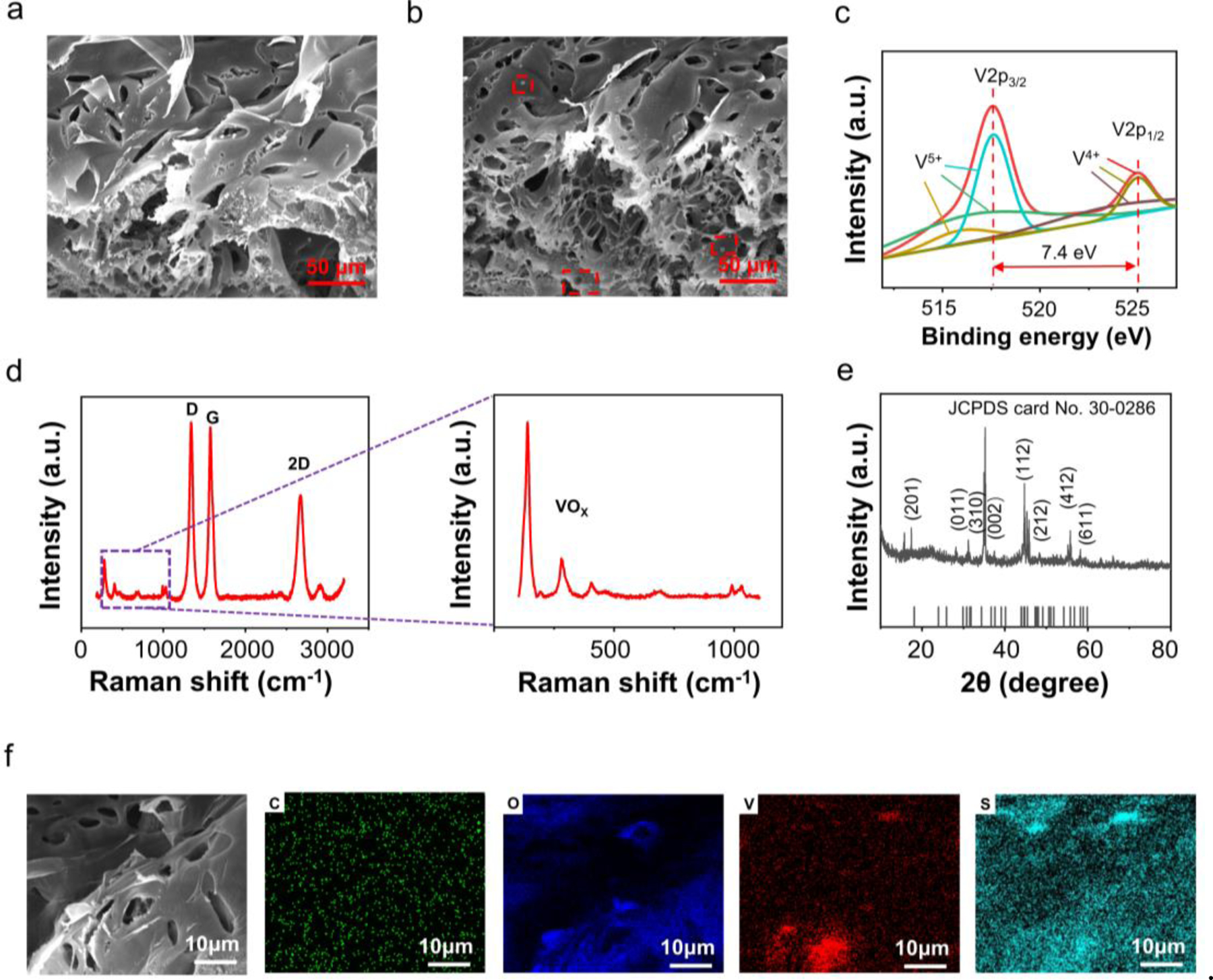
Scanning electron microscope (SEM) images of a LIG and b -doped LIG. c The narrow scan X-ray photoelectron spectrum (XPS) of V 2p regions for -doped LIG. d Raman spectrum of -doped LIG with a zoom-in shown in the inset. e XRD patterns of -doped LIG. f Energy-dispersive spectroscopy (EDS) spectrum of -doped LIG for C, O, S, and V.
Thickness effect of F127-resols hybrid film on the gas sensing performance
The film thickness modulates the laser-induced gas sensing area and gas permeation through the thickness, affecting the sensor performance. The sensor with either a small or large thickness has a low response and signal-to-noise ratio (SNR) in the gas response curve to 1 ppm NO2 at room temperature (Fig. 3a, Fig. S5, Fig. S6). On the other hand, with the increase of film thickness, the conductivity increases but gas permeability decreases56 to give a reduced gas response. The sensor with a larger thickness can provide abundant gas adsorption sites and continuous gas diffusion pathways, facilitating gas analyte-induced charge carrier generation for enhanced gas-sensing properties57. However, as the film thickness exceeds 35 μm, some structures in the resulting LIG become poor quality with uneven 3D microstructures, leading to unstable sensing performance58. Therefore, the optimal film thickness of 35 μm is selected in the following studies.
Fig. 3. Gas sensing performance characterization of -doped LIG.

a The response curves of the gas sensor based on pure LIG with different film thicknesses to 1 ppm NO2. b The comparison in the response between the gas sensors based on LIG and -doped LIG to 1 ppm NO2. c Typical response curve of the gas sensor to determine the response/recovery time. d Dynamic response test in the presence of NO2 from 1 to 5 ppm at room temperature. e Repeatability test to 0.5 ppm NO2 for five consecutive cycles. f Dynamic response test to NO2 from 300 to 700 ppb at room temperature and g its linear fit to the calibration curve (error bars from three samples). h Experimental demonstration of the ultralow limit of detection to 3 ppb NO2 at room temperature. i Selectivity test to NOX over a wide range of other gaseous molecules. (Note: results are obtained from the gas sensor based on -doped LIG unless specified otherwise).
Compared with pure sensors, the -doped sensors provide a higher response, e.g., over a two-fold increase from 1.2% to 2.8% when exposed to 1 ppm NO2 (Fig. 3b). The response/recovery time of 217/650 s to 1 ppm NO2 at room temperature is also relatively rapid (Fig. 3c). As the recovery time of 300 s is sufficient to capture the characteristics of the gas sensor, this value is selected in the following studies unless specified otherwise. As the concentration is gradually increased from 1 to 5 ppm, the continuous response curve to NO2 shows an increase from 2.5% to 5.5% (Fig. 3d), indicating a good dynamic response/recovery at room temperature. The response and recovery time also increases with the increasing gas concentration from 1 to 5 ppm (Fig. S7). The incomplete recovery comes from the short time set for rapid testing. The cycling stability of the -doped LIG sensor is confirmed by exposure to 0.5 ppm NO2 at room temperature (Fig. 3e). The consistent response and recovery of the sensor over five cycles indicate the good steady-state response of the sensor. To provide a more accurate estimation of the theoretical limit of detection (LOD), the gas sensor is exposed to NO2 with a progressively increased concentration from 300 to 700 ppb with a step size of 100 ppb, which gives a continuous response curve from 0.9%, 1.2%, 1.5%, 1.8%, to 2.1% (Fig. 3f). The linear fit of the sensor response to the NO2 gas concentration from 300 to 700 ppb yields a slope of 2.929 ppm−1 with a correlation coefficient (R2) of 0.997 (Fig. 3g). The linear fit of the gas sensor response to the lower concentration (3 ppb, 5 ppb, 7 ppb, 9 ppb, to 11 ppb) gives a slope of 8.4×10−5/ppb, which further leads to the determination of the theoretical LOD, defined as 59, as 451 ppt (Fig. S8). As it is challenging to use the static gas test setup to measure the gas concentration below 1 ppb (additionally spiked gas in the ambient environment), testing of the sensor to 3 ppb NO2 still shows a response of 0.3‰ with a signal-to-noise ratio (SNR) of 26.85 (Fig. 3h). The -doped sensor only gives a small response of 0.2%, 0.06%, 0.062%, 0.12%, 0.22%, and 0.09% to 1 ppm SO2, 100 ppm CO2, 10 ppm NH3, 100 ppm acetone, 100 ppm methanol, and 100 ppm ethanol, respectively (Fig. 3i and Fig. S9). In comparison, the significantly higher response of the sensor to NOX (e.g., 2.7%/1.1% to 1 ppm NO2/NO) highlights the excellent selectivity. The exposure of the chemiresistive sensor to an oxidizing NOX gas results in the extraction of the electrons in the valence band of the LIG to the adsorbed NOX and from to LIG47. The lowest unoccupied molecular orbital (LUMO) determines the number of transferred electrons. As the LUMO of NOX gas molecules is lower than that of other gases60, more electrons are transferred from the LIG to give a larger response and high selectivity to NOX. As a result, the -doped LIG gas sensor with an ultralow LOD and a high selectivity outperforms the other NOX gas sensors based on different nanomaterials (Tab. S1).
Effects of operating temperature from self-heating on the gas sensing performance
As the temperature is often used to modulate the gas sensing performance61–63, self-heating of the LIG is explored to modulate the temperature by changing the applied voltage during the resistance measurements (Fig. 4a) with the infrared thermal images shown in Fig. S10. As the temperature is increased from 22°C (room temperature) to 50°C, the response/recovery time decreases from 217/650 s to 88/406 s, but the response is also reduced from 2.5% to 1.3% (to 1 ppm NO2) (Fig. 4b, Fig. S11). The accelerated response/recovery at elevated temperature results from the promoted electron transfer (crossing the potential barrier), but the accelerated desorption rate of gas molecules also results in a smaller response64. This temperature-dependent behavior is consistent with the results of room-temperature NOX gas sensors in the previous literature reports65–68.
Fig. 4. Temperature effect on the sensing performance.

a Real-time response curves and b response/recovery properties of the -doped sensor to 1 ppm NO2 at different operating temperatures. c Response of the -doped sensor to 1 ppm NO2 in different humidity levels at 22 and 50°C (error bars from three samples). d Long-term stability test of the gas sensor to 1 ppm NO2 for 16 days (measurement of 2000 s in each day).
As the relative humidity (RH) level can vary in a large range in greenhouse and soil environments, it is essential to analyze its influence on the gas sensing performance69, 70. Because of the adsorption competition between NO2 and water molecules on the sensor surface in the high RH range71, the response of the sensor to 1 ppm NO2 decreases from 1.92% to 0.54% as the RH level is increased from 50% to 80% at room temperature (Fig. S12). However, the influence of RH on the NO2 response can be drastically reduced when the sensor is operated at elevated temperatures68 (e.g., a response of 1.43/1.41/1.4/1.31% in the RH of 50/60/70/80% at 50°C) (Fig. 4c, Fig. S13). The elevated temperature in the sensing area creates thermal radiation to drive the water molecules away from the region72. Additional strategies such as superhydrophobic coating can be further explored to minimize the effects of humidity73–75. The sensor is also highly stable over time, as evidenced by the almost unchanged response to 1 ppm NO2 for 16 days (Fig. 4d), demonstrating a high potential for practical applications.
Gas-sensing mechanisms and theoretical calculations
The gas sensing mechanism of the chemoresistive -doped LIG relies on the direct charge transfer between the absorbed O2 and NOX gas molecules and the sensing material. The large density of oxygen vacancy defects and dangling bonds in both LIG and allow easy adsorption of oxygen molecules onto the composite structure at room temperature. The NOX adsorbed on the surface continuously extracts electrons and extends the hole (main carriers) accumulation zone on the surface to lower the resistance, resulting in negative values in the relative changes as in the previous literature reports76. In the -doped LIG sensor, the work functions of LIG and are ca. 4.7 (WG) and 6.8 eV (WM), respectively. Due to the difference in work function, the majority charge carriers (holes and electrons) in the p-type LIG77, 78 and n-type 79 migrate across the heterojunction established at the interface forming a depletion layer by energy band bending (Fig. 5a). Heterojunction systems have been recognized to explain the enhanced gas sensing characteristics of ZnO/rGO80, ZnO/NiO81, CuO/ZnO82, CoO/SnO283, PdO-ZnO84, ZnFe2O4-ZnO85 in previous papers. The exposure of -doped LIG to NO2 traps electrons from the conduction band and further bends the energy band (Fig. 5b) to decrease the resistance. Compared with pure LIG, the -doped LIG shows enhanced carrier transfer, leading to higher conductivity86 as shown in the linear I-V curves (Fig. S14). Small changes in charge carrier concentration can also lead to great enhancement in the sensor response78. The decoration of also decreases the initial concentration of electrons in LIG, providing a larger change in NO2 gas adsorption and response per electron87.
Fig. 5.

Schematic showing band diagrams of the -doped LIG a before and b after contact. c The sensing mechanism with corresponding d band structures and DOS of -doped LIG. e Deformation charge density of NO2 adsorbed on -doped LIG. f The adsorption energy of different gas molecules adsorbed on -doped LIG.
The difference in the structure and electronic properties between pristine LIG and -doped LIG can be revealed by the density functional theory (DFT) calculations88, 89. Compared to pristine LIG, the -doped LIG with heterostructures (Fig. 5c) exhibits enhanced electronic levels near the Fermi level to elevate electron transfer (Fig. 5d) due to the decoration90–92. Deformation charge density elucidates large charge transfer between and LIG and the adsorption of NO2 on the sensor surface (Fig. 5e). The adsorption energy of gas molecules on the sensor surface can be calculated as93:
where and are the total energy of the system before and after the adsorption of gas molecules and is the energy of the isolated gas molecule. The larger negative values of correspond to the stronger interaction between the sensor surface and the gas molecule. The adsorption energy of −1.434 eV of NO2 on -doped LIG is almost 7 times of −0.219 eV on pristine LIG, indicating improved interaction between NO2 and -doped LIG89. Meanwhile, the adsorption energies of other gas molecules (e.g., NH3, SO2, CO2, and acetone) on -doped LIG are lower in magnitude than that of NO2 (Fig. 5f), which supports the highly selective detection of NO2 over interfering gas molecules.
The temperature sensing performance of the multi-parameter sensor
Due to the high electron mobility, superior thermal conductivity, and structural stability at high temperatures of LIG68, 94, the multi-parameter sensor shows a sensitive response with high repeatability (Fig. 6a) over a wide temperature range from 30 to 110°C (Fig. 6b). The fitting of the linear calibration curve gives a negative temperature coefficient and a sensitivity of 4.52×10−4°C–1 (R2 = 0.97) (Fig. 6c). Further increased linearity (R2 = 0.99) is observed in the temperature range from 30 to 50°C (relevant for soil, human body, and infant formula temperatures) (Fig. S15). The sensor also exhibits a low limit of detection of 0.2°C (Fig. 6d) and good repeatability over heating-cooling cycles (Fig. 6e, f) to monitor both subtle and large temperature changes in practical applications. The proof-of-the-concept demonstrations include the detection of formula milk temperature in the bottle for the infant (Fig. 6g) or simulated fever (Fig. 6h), with a response time of 60 s that is much shorter than that (>6 min)95 of most commercial mercurial thermometers. The above results confirm that the -doped LIG temperature sensor exhibits high sensitivity, wide detection range, fast response, and good reliability, which is suitable for dynamic temperature detection with favorable performance over the previously reported literature studies (Tab. S2).
Fig. 6. The temperature sensing performance of the multi-parameter sensor.
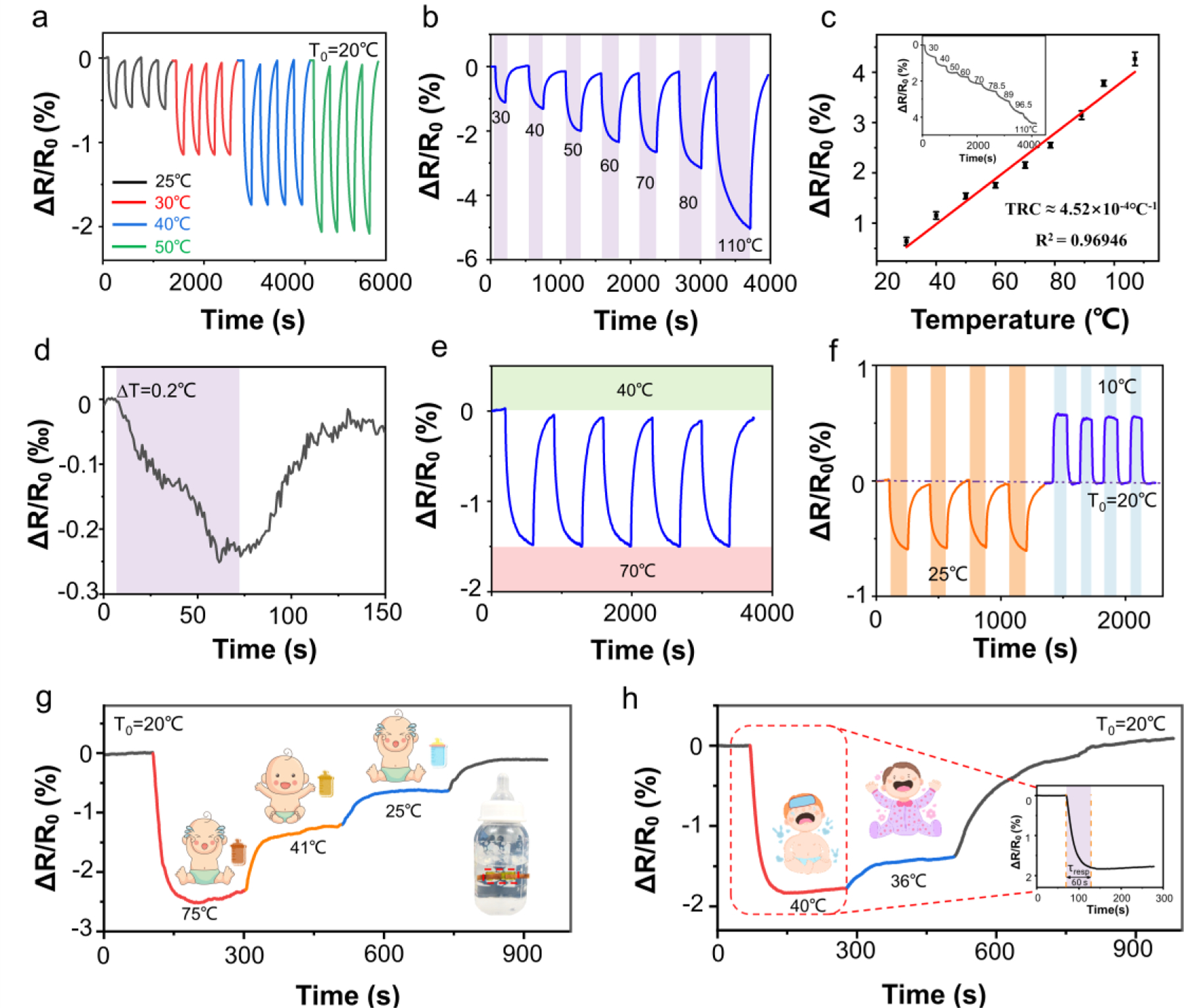
a The normalized relative resistance change (, %) of the sensor at different temperatures over multiple cycles. b The calibration curve of the sensor to temperature from 30 to 110°C and c its linear fit to determine the sensitivity (error bars from three samples) with the inset to show the resistance change during continuous heating. d The demonstration of the sensor to detect a small differential temperature change ΔT of 0.2°C. Repeatability test of the sensor e over five heating-cooling cycles between 40 and 70°C and f in various heating-cooling cycles. Dynamic response of the multi-parameter sensor to different g formula bottle and h skin temperatures (with the response curve shown in the inset).
Application of the -doped LIG multi-parameter sensor for soil monitoring
The -doped LIG sensor shows excellent sensing performance to both NOX gas and temperature, so it is imperative for the sensor to decouple gas and temperature when the two stimuli are simultaneously present in large-scale soil monitoring (Fig. 7). Therefore, the PDMS membrane that is impermeable to gas is introduced as an encapsulation layer to break the symmetry in gas and temperature response (Fig. 7a). The -doped LIG sensor encapsulated with a 10 μm-thick PDMS membrane exhibits a significantly diminished response to NO2 at room temperature (RH of 80%) (Fig. 7b, Fig. S16). Meanwhile, the rapid heat transport in the 10 μm thick PDMS membrane has minimal effect on the temperature sensing, resulting in a negligible difference with the unencapsulated sensor (Fig. 7c, Fig. S17). The encapsulated sensor can also be combined with the unencapsulated sensor operated at elevated temperature from self-heating to completely decouple the temperature and NOX gas (Fig. 7d, Fig. S18). The temperature can be accurately captured by the encapsulated sensor, whereas the NOX gas can be determined by the self-heated sensor as it removes the influence from the temperature changes, with no interference between the two input signals. In the proof-of-the-concept demonstration, the encapsulated sensor does not show any response to the NO2 gas from 1 to 3 ppm (at room temperature), which is captured by the self-heated sensor operated at 50°C (blue shaded region in Fig. 7d). Similarly, the progressively increased temperature from 22 to 50°C and then decreased back to 22°C (to 3 ppm NO2) does not cause any response in the self-heated sensor. Meanwhile, the temperature change is accurately detected by the encapsulated sensor (yellow shaded region in Fig. 7d).
Fig. 7. Demonstration of the -doped LIG sensor to decouple and temperature.

a Schematic of the encapsulated sensor to block the permeation of the gas molecules. b Response of the -doped LIG sensor with and without the encapsulation membrane to 1 ppm NO2. c Response of -doped LIG sensors with varying encapsulation thicknesses to the temperature of 30, 40, and 50°C. d Application of the encapsulated -doped LIG sensor and the unencapsulated one operated at 50°C from self-heating to completely decouple NO2 gas and temperature. The top illustrations show the changes in gas concentration and temperature.
The -doped LIG sensor capable of decoupling NOX and temperature can be applied to accurately monitor the soil temperature and NOX emission for future precision agriculture. The encapsulated sensor on the soil surface can accurately monitor the soil temperature without being affected by the NOX gas emission (Fig. 8a). By heating the soil sample in the oven to simulate overheating, the soil temperature cycled from 35°C (suitable for crop growth) to 40°C (unfavorable to crop growth) and then back to 35°C and room temperature is accurately captured by our sensor (Fig. 8b). Meanwhile, the sensor without encapsulation but operated at an elevated temperature such as 50°C from self-heating can be used to detect NOX volatilized from the soil at the specified temperature after applying urea (Fig. 8c). In the representative demonstration, the NOX gas emission from the soil sample (23 cm × 19 cm × 4 cm) fertilized with 5 g urea can be detected at a much larger response than the un-fertilized control sample after three days (Fig. 8d), which is consistent with previous literature reports7, 96. Although the sensor operated at room temperature is largely influenced by the RH (50–60% in the soil environment) to give a positive response (Fig. S19), the sensor operated at 50°C from self-heating largely eliminates the humidity effect to allow for accurate gas detection. As a result, the positive response is reduced to only 0.2‰ in unfertilized soils (RH of 50–60%) to demonstrate a very weak influence of humidity. The decoupled measurements of NOX and temperature from the multi-parameter sensor can provide accurate, real-time monitoring of the crop growth environment for smart or precision agriculture.
Fig. 8. Monitoring of soil NOX and temperature.

a Schematic diagram of excessive soil temperature and b the sensor response to suitable and over-heated soil. c Schematic diagram of urea application and d the difference in the sensor response to different fertilizer levels (operated at 50°C from self-heating).
Remote environmental monitoring system
The sensor can be integrated with data processing and wireless transmission modules to yield a remote environmental monitoring system for human health monitoring and precision agriculture (Fig. 9). The signal measured by the sensor and processed by a low pass filter to remove the noise is first digitized using a 16-bit delta-sigma modulator and then wirelessly transmitted to the smartphone via a Bluetooth module (Fig. 9a). When the real-time monitored NO2 in the local environment around the human subject exceeds the safety threshold, the system automatically triggers on a red light and send alert to the smartphone for timely protection (Fig. 9b and Video S1). Additionally, the integrated wireless monitoring system can also be used to wirelessly monitor NOX concentration after fertilization and soil temperature in the greenhouse for promoting plant growth in smart agriculture (Fig. 9c and Video S2, 3).
Fig. 9. The design and demonstration of the integrated remote environment monitoring system based on the sensor.
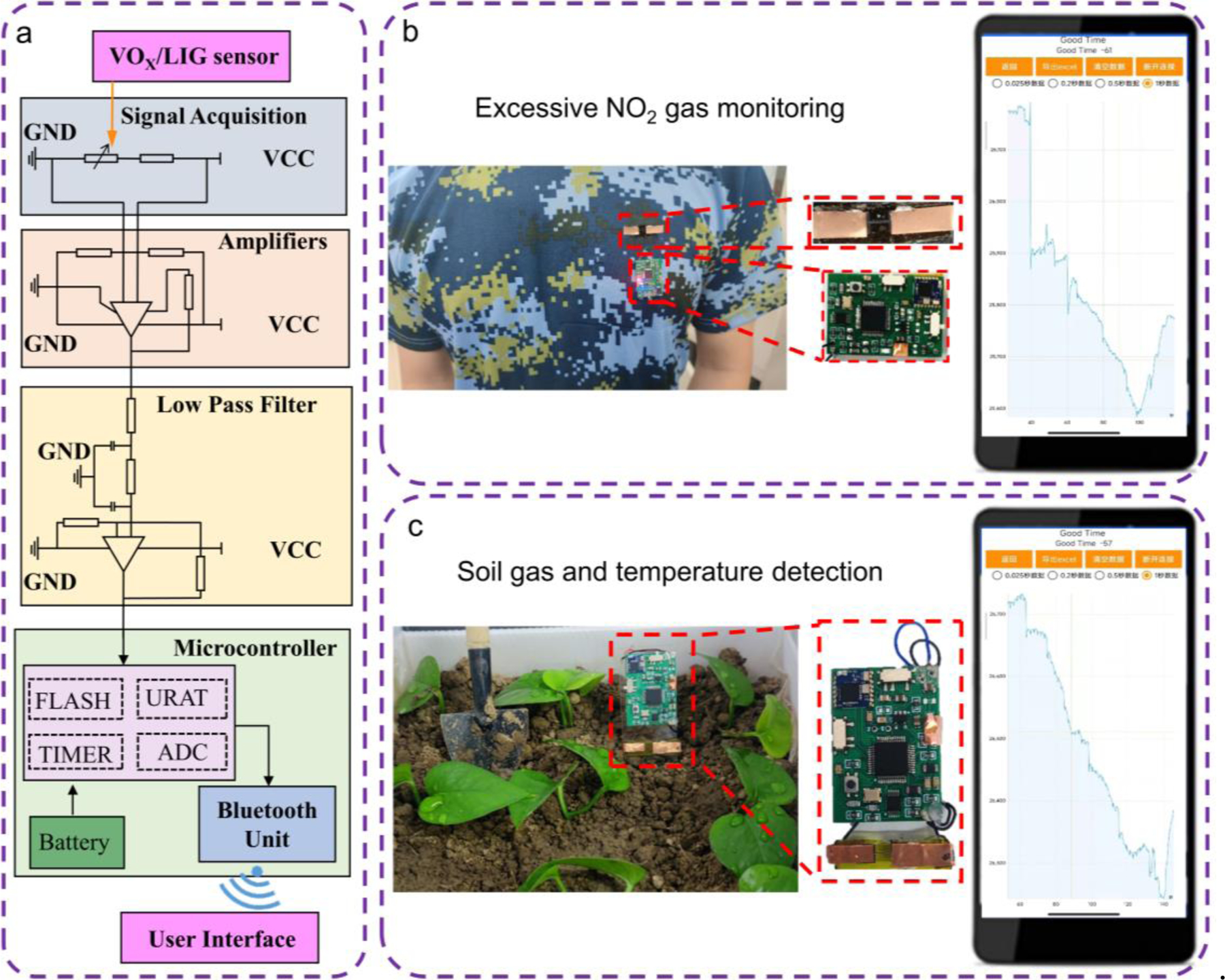
a The circuit design of the remote monitoring system for b real-time monitoring of NO2 in the local environment of the human subject and c soil gas and temperature detection for smart agriculture.
DISCUSSION
In summary, this work reports the design, fabrication, and application of -doped LIG nanocomposites to decouple NOX and temperature for soil environment monitoring. Created from a single-step laser scribing of V5S8-doped block copolymer and phenolic resin self-assembled films, the -doped LIG exhibits an ultra-low detection limit to NOX and high sensitivity/precision over a wide temperature range. Introducing a soft membrane as an encapsulation layer on the sensor blocks the permeation of the gas molecules to provide accurate temperature measurements, which further helps decouple the NOX gas from temperature. Additionally, the influence of the RH in the ambient environment can be effectively removed by operating the sensor at elevated temperatures from self-heating. The unencapsulated sensor operated at elevated temperatures also allows accurate measurements of the NOX gas without being affected by environmental temperature variations. Therefore, the combination of the encapsulated -doped LIG sensor and the unencapsulated sensor operated at elevated temperatures can completely decouple temperature and NOX without interference. The proof-of-the-concept demonstration of the multi-parameter decoupled sensors is showcased to detect nitrogen loss and soil temperature for smart agriculture. The design strategies and demonstrations from this work can also be leveraged to help create the next-generation multi-parameter stretchable sensors with decoupling sensing mechanisms.
MATERIALS AND METHODS
Preparation of V5S8-doped Pluronic F127-resols hybrid film.
The Pluronic F127-resols solution was obtained by mixing 4 g resols and 6 g Pluronic F127 copolymer in ethanol in a water bath (40°C, 4 h) (Fig. S1). Adding V5S8 to the above solution followed by stirring prepared the V5S8-doped Pluronic F127-resols solution. V5S8 were purchased from Nanjing MKNANO Tech. Co., Ltd. (www.mukenano.com). After vacuum treatment for 20 min, spin coating the solution on a silicon (Si) substrate at varying speeds (i.e., 200, 500, 850, 1000, 1500, 2000, 2500, 3000, 3500, and 4000 rpm), followed by drying in a vacuum oven at 150°C for 48 h, formed a uniform thin film (Fig. S3a).
Characterization.
Field-emission scanning electron microscopy (FESEM) (JSM 7100F, JEOL) was used to characterize the structure and morphology. X-ray photoelectron spectroscopy (XPS) was applied by ESCALAB 250 photoelectron spectrometer (Thermo Fisher Scientific, USA). Raman scattering was performed on a laser micro Raman spectrometer (Renishaw, in Via Reflex). X-ray diffraction (XRD) was obtained by a D8 Discover X-ray diffractometer.
Sensor fabrication and measurement.
The sensing region (width of 150 μm and length of 4.5 mm) with two square electrodes was directly created by scribing the V5S8-doped Pluronic F127-resols hybrid film with a CO2 laser (Universal Laser, 10.6 μm, spot size of 127 μm, and power of 9 mW) (Fig. S3b). The laser processing parameters were fixed (power of 3.0%, speed of 1%, and PPI of 500), unless specified otherwise. Copper tapes with silver paste on the square electrodes connected the sensor to the data acquisition system. The PDMS solution was prepared at room temperature with stirring using 2 g of prepolymer (a) and 0.1 g of crosslinking agent (b) in a mass ratio of 20:1. Then, the PDMS solution was spin-coated onto the sensing area of sensor at varying speeds (i.e., 6000, 7000, 8000, and 9000 rpm) and drying in a vacuum oven at 85°C for 1 h. Target gas are first collected into an aluminum foil gas collecting bag and then injected into a closed chamber for static detection. Different concentrations of NOX were prepared by diluting and fully mixing the commercial calibration gas of 50 ppm NOX with air in the chamber (volume of 5 L). The different relative humidity values in the chamber were prepared by the saturated salt solution method97. The concentration of the was obtained by injecting the needed quantity of anhydrous liquid analytes into a sealed glass container using a microliter syringe. The concentration ( in ppm) of the in the chamber was calculated using the following equation:
where , , and are the density (g ml−1), volume , and molecular weight (g mol−1) of the anhydrous liquid , is the testing temperature , and is the volume of the glass container filled with the . Demonstration of the sensor performance is provided in Video S4.
The real-time resistance was recorded by a SourceMeter (Keithley 2400) at a constant voltage of 0.05 V. The sensor response is defined as with , where is the initial resistance in air and is the resistance in the target gas. The response (or recovery) time is the time taken for the sensor response to reach 90% of the response (or recovery) at saturation in the target gas (or air).
Computational Methods.
We have employed the Vienna Ab initio Simulation Package (VASP) to perform all the spin polarized density functional theory (DFT) calculations within the generalized gradient approximation (GGA) using the Perdew-Burke-Ernzerhof (PBE) formulation. The projected augmented wave (PAW) potential was selected to describe the ionic cores and take valence electrons into account using a plane wave basis set with a kinetic energy cut-off of 520 eV. The density of k-meshs grids for Brillouin zone samping was set as . Partial occupancies of the Kohn-Sham orbitals were allowed using the Gaussian smearing method and a width of 0.05 eV. The electronic energy was considered self-consistent when the energy change was smaller than 10−6 eV. The geometry optimization was considered convergent when the force on each atom was smaller than 0.02 eV/Å.
Supplementary Material
Funding:
This work was supported by:
National Natural Science Foundation of China (51705126, 61871173)
Key Research and Development Project of Hebei Province (20271701D, 22371703D)
China Postdoctoral Science Foundation (2022M722378)
NIH (Award Nos. R21EB030140, U01DA056242, and R61HL154215)
NSF (Grant No. ECCS-1933072)
Penn State University.
Footnotes
Competing interests: Authors declare that they have no competing interests.
Data and materials availability: All data are available in the main text or the supplementary materials.
REFERENCES AND NOTES
- 1.Saud S, Wang D, Fahad S Improved nitrogen use efficiency and greenhouse gas emissions in agricultural soils as producers of biological nitrification inhibitors. Front. in Plant Sci 13, 854195 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield DE, Glazer AN, Falkowski PG The evolution and future of earth’s nitrogen cycle. Sci 330, 192–196 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Han Z, Higano Y An inventory of nitric oxide emissions from soils in China. Pollut 135, 83–90 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Li QT, Zeng W, Li YQ Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators, B 359, 131579 (2022). [Google Scholar]
- 5.Wang Y, Ge C, Garcia LC, Jenerette GD, Oikawa PY, Wang J Improved modelling of soil NOx emissions in a high temperature agricultural region: role of background emissions on NO2 trend over the US. Res. Lett 16, 084061 (2021). [Google Scholar]
- 6.Hamdi S, Moyano F, Sall S, Bernoux M, Chevallier T Synthesis analysis of the temperature sensitivity of soil respiration from laboratory studies in relation to incubation methods and soil conditions. Soil Biol. Biochem 58, 115–126 (2013). [Google Scholar]
- 7.Viets FG Soil fertility and organic matter as critical components of production systems. Agric. Ecosyst. Environ 30, 159–161 (1987). [Google Scholar]
- 8.Dong J, Steele-Dunne SC, Ochsner TE, Van de Giesen N Determining soil moisture and soil properties in vegetated areas by assimilating soil temperatures. Water Resour. Res 52, 4280–4300 (2016). [Google Scholar]
- 9.Stafford JV Implementing precision agriculture in the 21st century. J. Agric. Eng. Res 76, 267–275 (2000). [Google Scholar]
- 10.Yin H, Cao Y, Marelli B, Zeng X, Mason AJ, Cao C Soil sensors and plant wearables for smart and precision agriculture. Adv. Mater 33, 2007764 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Yang R, Zhang W, Tiwari N, Yan H, Li T, Cheng H Multimodal sensors with decoupled sensing mechanisms. Adv. Sci 9, 26, e2202470 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Wang H, Yuan W, Li Y, Gao P, Tiwari N, Chen X, Wang Z, Niu G, Cheng H Wearable pressure sensors based on MXene/Tissue papers for wireless human health monitoring. ACS Appl. Mater. Interfaces 13, 60531–60543 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Hou Z, Li G, Yu W, Xue Y, Niu G, Xin M, Yang L, Meng C, Guo S A laser-scribed wearable strain sensing system powered by an integrated rechargeable thin-film zinc-air battery for a long-time continuous healthcare monitoring. Nano Energy 107606 (2022).
- 14.Chen X, Li R, Niu G, Xin M, Xu G, Cheng H, Yang L Porous graphene foam composite-based dual-mode sensors for underwater temperature and subtle motion detection. Chem. Eng. J 444, 136631 (2022). [Google Scholar]
- 15.Koski KJ, Cui Y The new skinny in two-dimensional nanomaterials. ACS Nano 7, 3739–3743 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Kannan PK, Late DJ, Morgan H, Rout CS Recent developments in 2D layered inorganic nanomaterials for sensing. Nanoscale 7, 13293–13312 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Kim Y, Lee S, Song JG, Ko KY, Woo WJ, Lee SW, Kim H 2D transition metal dichalcogenide heterostructures for p‐and n‐type photovoltaic self‐powered gas sensor. Adv. Funct. Mater 30, 2003360 (2020). [Google Scholar]
- 18.Qin Z, Song X, Wang J, Li X, Wu C, Wang X, Zeng D Development of flexible paper substrate sensor based on 2D WS2 with S defects for room-temperature NH3 gas sensing. Appl. Surf. Sci 573, 151535 (2022). [Google Scholar]
- 19.Choi SJ, Kim ID Recent developments in 2D nanomaterials for chemiresistive-type gas sensors. Electron. Mater. Lett 14, 221–260 (2018). [Google Scholar]
- 20.Nemufulwi MI, Swart HC, Shingange K, Mhlongo GH ZnO/ZnFe2O4 heterostructure for conductometric acetone gas sensors. Sens. Actuators, B 377, 133027 (2023). [Google Scholar]
- 21.Wu K, Debliquy M, Zhang C Room temperature gas sensors based on Ce doped TiO2 nanocrystals for highly sensitive NH3 detection. Chem. Eng. J 444, 136449 (2022). [Google Scholar]
- 22.Matatagui D, López-Sánchez J, Peña A, Serrano A, Campo A, Fuente OR, Carmona N, Navarro E, Marín P, Horrillo MC Ultrasensitive NO2 gas sensor with insignificant NH3-interference based on a few-layered mesoporous graphene. Sens. Actuators, B 335, 129657 (2021). [Google Scholar]
- 23.Zhao J, Zheng C, Gao J, Gui J, Deng L, Wang Y, Xu R Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuators, B 347, 130653 (2021). [Google Scholar]
- 24.Liu J, Ji H, Lv X, Zeng C, Li H, Li F, Zhou Q Laser-induced graphene (LIG)-driven medical sensors for health monitoring and diseases diagnosis. Microchim. Acta 189, 1–14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Fei Q, Page M, Zhao G, Ling Y, Chen D, Yan Z Laser-induced graphene for bioelectronics and soft actuators. Nano Res 14, 3033–3050 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Huang X, Song W Physical and chemical sensors on the basis of Laser-induced graphene: mechanisms, applications, and perspectives. ACS Nano 15, 18708–18741 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Liu C, Yuan W, Meng C, Dutta A, Chen X, Cheng H Fully stretchable, porous MXene-graphene foam nanocomposites for energy harvesting and self-powered sensing. Nano Energy 103, 107807 (2022). [Google Scholar]
- 28.Tan KW, Wiesner U Block copolymer self-assembly directed hierarchically structured materials from nonequilibrium transient laser heating. Macromol 52, 395–409 (2019). [Google Scholar]
- 29.Tan KW, Jung B, Werner JG, Rhoades ER, Thompson MO, Wiesner U Transient laser heating induced hierarchical porous structures from block copolymer-directed self-assembly. Sci 349, 54–58 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Ji H, Meng C, Li Y, Zheng G, Chen X, Niu G, Yan J, Xue Y, Guo S Intrinsically breathable and flexible NO2 gas sensors produced by laser direct writing of self-assembled block copolymers. ACS Appl. Mater. Interfaces 14, 17818–17825 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Babar BM, Pisal KB, Mujawar SH, Patil VL, Kadam LD, Pawar UT, Patil PS Concentration modulated vanadium oxide nanostructures for NO2 gas sensing. Sens. Actuators, B 351, 130947 (2022). [Google Scholar]
- 32.Chen Y, Lian P, Feng J, Liu Y, Wang L, Liu J, Shi X Tailoring defective vanadium pentoxide/reduced graphene oxide electrodes for all-vanadium-oxide asymmetric supercapacitors. Chem. Eng. J 429, 132274 (2022). [Google Scholar]
- 33.Mounasamy V, Mani GK, Madanagurusamy S Vanadium oxide nanostructures for chemiresistive gas and vapour sensing: a review on state of the art. Microchim. Acta 187, 1–29 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Wu W, Lou Q, Wang K, Xuan C Room temperature NO2 sensing performance enhancement of VO2 (B) composited rGO structure. J. Mater. Sci.: Mater. Electron 33, 19, 15473–15482 (2022). [Google Scholar]
- 35.Liang J, Wu W, Lou Q, Wang K, Xuan C Room temperature NO2 sensing performance of Ag nanoparticles modified VO2 nanorods. J. Alloys Compd 890, 161837 (2022). [Google Scholar]
- 36.Sreedhara MB, Ghatak J, Bharath B, Rao CNR Atomic layer deposition of ultrathin crystalline epitaxial films of V2O5. ACS Appl. Mater. Interfaces 9, 3178–3185 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Mane AA, Moholkar AV Effect of film thickness on NO2 gas sensing properties of sprayed orthorhombic nanocrystalline V2O5 thin films. Appl. Surf. Sci 416, 511–520 (2017). [Google Scholar]
- 38.Pavasupree S, Suzuki Y, Kitiyanan A, Pivsa-Art S, Yoshikawa S Synthesis and characterization of vanadium oxides nanorods. J. Solid State Chem 178, 2152–2158 (2005). [Google Scholar]
- 39.Przes̈niak-Welenc M, Łapiński M, Lewandowski T, Kos̈cielska B, Wicikowski L, Sadowski W The influence of thermal conditions on V2O5 nanostructures prepared by sol-gel method. J. Nanomater 351, 130947 (2015). [Google Scholar]
- 40.Modafferi V, Trocino S, Donato A, Panzera G, Neri G Electrospun V2O5 composite fibers: synthesis, characterization and ammonia sensing properties. Thin Solid Films 548, 689–694 (2013). [Google Scholar]
- 41.Piccirillo C, Binions R, Parkin IP Synthesis and functional properties of vanadium oxides: V2O3, VO2, and V2O5 deposited on glass by aerosol‐assisted CVD. Chem. Vap. Deposition 13, 145–151 (2007). [Google Scholar]
- 42.Ng SH, Chew SY, Wang J, Wexler D, Tournayre Y, Konstantinov K, Liu HK Synthesis and electrochemical properties of V2O5 nanostructures prepared via a precipitation process for lithium-ion battery cathodes. J. Power Sources 174, 1032–1035 (2007). [Google Scholar]
- 43.Wang B, Peng R, Wang X, Yang Y, Wang E, Xin Z, Sun Y, Li C, Wu Y, Wei J, Sun J, Liu K Ultrafast, kinetically limited, ambient synthesis of vanadium dioxides through laser direct writing on ultrathin chalcogenide matrix. ACS Nano 15, 10502–10513 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Haiduk Y, Khort A Lapitskaya V, Kuznetsova T, Moskovskikh D, Savitsky A, Lapchuk N, Makhavikou M, Pankov V WO3-graphene-Cu nanocomposites for CO, NO2 and acetone gas sensors. Nano-Struct. Nano-Objects 29, 100824 (2022). [Google Scholar]
- 45.Wang C, Wang Y, Yang Z, Hu N Review of recent progress on graphene-based composite gas sensors. Ceram. Int 47, 12, 16367–16384 (2021). [Google Scholar]
- 46.Zhang C, Sha J, Fei H, Liu M, Yazdi S, Zhang J, Tour JM Single-atomic ruthenium catalytic sites on nitrogen-doped graphene for oxygen reduction reaction in acidic medium. ACS Nano 11, 6930–6941 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Zheng G, Cao Y, Meng C, Li Y, Ji H, Chen X, Niu G, Yan J, Xue Ye., Cheng H Moisture-resistant, stretchable NOX gas sensors based on laser-induced graphene for environmental monitoring and breath analysis. Microsyst. Nanoeng 8, 1, 78 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy IN, Akkinepally B, Manjunath V, Neelima G, Reddy MV, Shim J SnO2 quantum dots distributed along V2O5 nanobelts for utilization as a high-capacity storage hybrid material in Li-Ion batteries. Mol 26, 7262 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hien VX, Nhat DD, Nghi NT, Phuoc LH, Khoa CT, Vuong DD, Chien ND From vanadium powder to vanadium pentoxide rolled-up nanosheets: Hydrothermal synthesis and its ethanol sensing properties. Mater. Sci. Semicond. Process 126, 105670 (2021). [Google Scholar]
- 50.He X, Hao Y, He M, Qin X, Wang L, Yu J Stretchable thermoelectric-based self-powered dual-parameter sensors with decoupled temperature and strain sensing. ACS Appl. Mater. Interfaces 13, 60498–60507 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Mounasamy V, Mani GK, Madanagurusamy S Vanadium oxide nanostructures for chemiresistive gas and vapour sensing: a review on state of the art. Microchim. Acta 187, 253 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Ye RQ, James DK, Tour JM Laser-induced graphene: from discovery to translation. Adv. Mater 31, 1803621 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Raghu AV, Karuppanan KK, Pullithadathil B Highly sensitive, temperature-independent oxygen gas sensor based on anatase TiO2 nanoparticle grafted, 2D mixed valent VOX nanoflakelets. ACS Sens 3, 1811–1821 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Weimer MS, McCarthy RF, Emery JD, Bedzyk MJ, Sen FG, Kinaci A, Chan MKY, Hock AS, Martinson ABF Template-free vapor-phase growth of patrónite by atomic layer deposition. Chem. Mater 29, 2864–2873 (2017). [Google Scholar]
- 55.Husmann S, Budak O, Quade A, Frank A, Kruth A, Scheu C, Tolosa A, Presser V Electrospun vanadium sulfide/carbon hybrid fibers obtained via one-step thermal sulfidation for use as lithium-ion battery electrodes. J. Power Sources 450, 227674 (2020). [Google Scholar]
- 56.Gardner JW, Iskandarani MZ, Bott B Effect of electrode geometry on gas sensitivity of lead phthalocyanine thin films. Sens. Actuators, B 9, 133–142 (1992). [Google Scholar]
- 57.Gao L, Liu C, Peng Y, Deng J, Hou S, Cheng Y, Huang W, Yu J Ultrasensitive flexible NO2 gas sensors via multilayer porous polymer film. Sens. Actuators, B 368, 132113 (2022). [Google Scholar]
- 58.Lei G, Lou C, Liu X, Xie J Li Z, Zheng W, Zhang J Thin films of tungsten oxide materials for advanced gas sensors. Sens. Actuators, B 341, 129996 (2021). [Google Scholar]
- 59.Currie L Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl. Chem 67, 1699–1723 (1995). [Google Scholar]
- 60.Rad AS First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl. Surf. Sci 357, 1217–1224 (2015). [Google Scholar]
- 61.Mahdavi H, Rahbarpour S, Goldoust R, Hosseini-Golgoo SM, Jamaati H Investigating simultaneous effects of flow rate and chamber structure on the performance of metal oxide gas sensors. IEEE Sens. J 21, 21612–21621 (2021). [Google Scholar]
- 62.Rahbarpour S, Sajed S, Ghodsi N, Ghafoorifard H Operating temperature dependence of sensitivity in Ag-TiO2 Schottky type gas sensors. Mater. Res. Express 6, 085905 (2019). [Google Scholar]
- 63.Hayasaka T, Kubota Y, Liu Y, Lin L The influences of temperature, humidity, and O2 on electrical properties of graphene FETs. Sens. Actuators 285, 116–122 (2019). [Google Scholar]
- 64.Li Q, Chen D, Miao J, Lin S, Yu Z, Han Y, Yang Z, Zhi X, Cui D, An Z Ag-modified 3D reduced graphene oxide aerogel-based sensor with an embedded microheater for a fast response and high-sensitive detection of NO2. ACS Appl. Mater. Interfaces 12, 25243–25252 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Xu L, Dong B, Wang Y, Bai X, Liu Q, Song H Electrospinning preparation and room temperature gas sensing properties of porous In2O3 nanotubes and nanowires. Sens. Actuators B 147, 531–538 (2010). [Google Scholar]
- 66.Patrick DS, Govind A, Bharathi P, Mohan MK, Harish S, Archana J, Navaneethan M Hierarchical ZnO/g-C3N4 nanocomposites for enhanced NO2 gas sensing applications. Appl. Surf. Sci 609, 30, 155337 (2022). [Google Scholar]
- 67.Li J, Yang M, Li Y, Cheng X, Zhang X, Xu Y, Gao S, Zhao H, Huo L Construction of SnO2 nanoneural network by ultrasmall particles for highly selective NO2 detection at low temperature. Sens. Actuators, B 361, 15, 131703 (2022). [Google Scholar]
- 68.Yang L, Yi N, Zhu J, Cheng Z, Yin X, Zhang X, Zhu H, Cheng H Novel gas sensing platform based on a stretchable laser-induced graphene pattern with self-heating capabilities. J. Mater. Chem. A 8, 6487–6500 (2020). [Google Scholar]
- 69.Wang B, Dong X, Wang Z, Wang Y, Hou Z The humidity-induced sensitivity amplification effect in an ionization gas sensor with silicon nanostructures. IEEE Electron Device Lett 41, 908–911 (2020). [Google Scholar]
- 70.Zhu H, Li Q, Ren Y, Gao Q, Chen J, Wang N, Deng J, Xing X A new insight into cross-sensitivity to humidity of SnO2 sensor. Small 14, 1703974 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Lee CT, Wang YS High-performance room temperature NH3 gas sensors based on polyaniline-reduced graphene oxide nanocomposite sensitive membrane. Journal of Alloys and Compounds 789, 693–696 (2019). [Google Scholar]
- 72.Ghosh S, Ilango MS, Prajapati CS, Bhat N Reduction of humidity effect in WO3 thin film-based NO2 sensor using physiochemical optimization. Cryst. Res. Technol 56, 2000155 (2021). [Google Scholar]
- 73.Liu W, Si X, Chen Z, Xu L, Guo J, Wei L, Cheng G, Du Z Fabrication of a humidity-resistant formaldehyde gas sensor through layering a molecular sieve on 3D ordered macroporous SnO2 decorated with Au nanoparticles. J. Alloys Compd 919, 165788 (2022). [Google Scholar]
- 74.Liu S, Qin Y, Bai Y Highly response and humidity-resistant gas sensor based on polyaniline-functionalized Bi2MoO6 with UV activation. Electrochim. Acta 427, 140863 (2022). [Google Scholar]
- 75.Chen Z, Yu C, Bai W, Ye W, Wang J, Wei J, Wang Y, He J, Lu J Surface functionalization of ion-in-conjugation polymer sensors for humidity-independent gas detection at room temperature. Sens. Actuators, B 372, 132654 (2022). [Google Scholar]
- 76.Ogbeide O, Bae G, Yu W, Morrin E, Song Y, Song W, Li Y, Su B, An K, Hasan T Inkjet-printed rGO/binary metal oxide densor for predictive gas sensing in a mixed environment. Adv. Funct. Mater 32, 25, 2113348 (2022). [Google Scholar]
- 77.Huang M, Wang Y, Ying S, Wu Z, Liu W, Chen D, Peng C Synthesis of Cu2O-modified reduced graphene oxide for NO2 sensors. Sensors 21, 1958 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Z, Zhao B, Shen D, Tao Q, Li B, Wu R, Li B, Yang X, Li J, Song R, Zhang H, Huang Z, Zhang Z, Zhou J, Liu Y, Duan X Synthesis of ultrathin 2D nonlayered α-MnSe nanosheets, MnSe/WS2 heterojunction for high-performance photodetectors. Small Struct 2, 2100028 (2021). [Google Scholar]
- 79.Yan W, Hu M, Wang D, Li C Room temperature gas sensing properties of porous silicon/V2O5 nanorods composite. Appl. Surf. Sci 346, 216–222 (2015). [Google Scholar]
- 80.Cao P, Cai Y, Pawar D, Han S, Xu W, Fang M, Liu X, Zeng Y, Liu W, Lu Y, Zhu D Au@ZnO/rGO nanocomposite-based ultra-low detection limit highly sensitive and selective NO2 gas sensor. J. Mater. Chem C 10, 4295–4305 (2022). [Google Scholar]
- 81.Nakate UT, Ahmad R, Patil P, Wang YS, Bhat KS, Mahmoudi T, Yu YT, Suh EK, Hahn YB Improved selectivity and low concentration hydrogen gas sensor application of Pd sensitized heterojunction n-ZnO/p-NiO nanostructures. J. Alloys Compd 797, 456–464 (2019). [Google Scholar]
- 82.Han TH, Bak SY, Kim S, Lee SH, Han YJ, Yi M Decoration of CuO NWs gas sensor with ZnO NPs for improving NO2 sensing characteristics. Sensors 21, 2103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Q, Kou X, Liu C, Zhao L, Lin T, Liu F, Yang X, Lin J, Lu G Hydrothermal synthesis of hierarchical CoO/SnO2 nanostructures for ethanol gas sensor. J. Colloid Interface Sci 513, 760–766 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Majhi SM, Lee HJ, Choi HN, Cho HY, Kim JS, Lee CR, Yu YT Construction of novel hybrid PdO-ZnO p-n heterojunction nanostructures as a high-response sensor for acetaldehyde gas. CrystEngComm 21, 5084–5094 (2019). [Google Scholar]
- 85.Yang T, Yang X, Zhu M, Zhao H, Zhang M Coral-like ZnFe2O4-ZnO mesoporous heterojunction architectures: synthesis and enhanced sensing properties for triethylamine. Inorg. Chem. Front 7, 1918–1926 (2020). [Google Scholar]
- 86.Li W, Teng C, Sun Y, Cai L, Xu J, Sun M, Li X, Yang X, Xiang L, Xie D, Ren T Sprayed, scalable, wearable, and portable NO2 sensor array using fully flexible AgNPs-all-carbon nanostructures. ACS Appl. Mater. Interfaces 10, 34485–34493 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Ko WC, Kim KM, Kwon YJ, Choi H, Park JK, Jeong YK ALD-assisted synthesis of V2O5 nanoislands on SnO2 nanowires for improving NO2 sensing performance. Appl. Surf. Sci 509, 144821 (2020). [Google Scholar]
- 88.Alves APP, Koizumi R, Samanta A, Machado LD, Singh AK, Galvao DS, Silva GG, Tiwary CS, Ajayan PM One-step electrodeposited 3D-ternary composite of zirconia nanoparticles, rGO and polypyrrole with enhanced supercapacitor performance. Nano Energy 31, 225–232 (2017). [Google Scholar]
- 89.Pan Z, Zhi H, Qiu Y, Yang J, Xing L, Zhang Q, Ding X, Wang X, Xu G, Yuan H, Chen Min., Li W, Yao Y, Motta N, Liu M, Zhang Y Achieving commercial-level mass loading in ternary-doped holey graphene hydrogel electrodes for ultrahigh energy density supercapacitors. Nano Energy 46, 266–276 (2018). [Google Scholar]
- 90.Qu Y, Yang M, Chai J, Tang Z, Shao M, Kwok CT, Yang M, Wang Z, Chua D, Wang S, Lu Z, Pan H Facile synthesis of vanadium-doped Ni3S2 nanowire arrays as active electrocatalyst for hydrogen evolution reaction. ACS Appl Mater Interfaces 9, 7, 5959–5967 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Li J, Liu Z, Zhang Q, Cheng Y, Zhao B, Dai S, Wu H, Zhang K, Ding D, Wu Y, Liu M, Wang M Anion and cation substitution in transition-metal oxides nanosheets for high-performance hybrid supercapacitors. Nano Energy 57, 22–33 (2019). [Google Scholar]
- 92.Rao Y, Yuan M, Luo F, Li H, Yu J, Chen X Laser In-Situ synthesis of metallic cobalt decorated porous graphene for flexible In-Plane microsupercapacitors. J. Colloid Interface Sci 610, 775–784 (2022). [DOI] [PubMed] [Google Scholar]
- 93.Gui Y, Shi J, Yang P, Li T, Tang C, Xu L Platinum modified MoS2 monolayer for adsorption and gas sensing of SF6 decomposition products: a DFT study. High. Volt 5, 4, 454–462 (2019). [Google Scholar]
- 94.Wei W, Yi Y, Song J, Chen X, Li J Tunable graphene/nitrocellulose temperature alarm sensors. ACS Appl. Mater. Interfaces 14, 13790–13800 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khorshid L, Eser I, Zaybak A, Yapucu U Comparing mercury-in-glass, tympanic and disposable thermometers in measuring body temperature in healthy young people. J. Clin. Nurs 14, 496–500 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Sun G, Zhang Z, Xiong S, Guo X, Han Y, Wang G, Feng L, Lei Y, Li X, Yang B, Xing F, Xin M, Chen H, Li Y, Wang Z Mitigating greenhouse gas emissions and ammonia volatilization from cotton fields by integrating cover crops with reduced use of nitrogen fertilizer. Agric. Ecosyst. Environ 332, 107946 (2022). [Google Scholar]
- 97.Li T; Li L; Sun H; Xu Y; Wang X; Luo H; Liu Z; Zhang T Porous ionic membrane based flexible humidity sensor and its multifunctional applications. Adv. Sci 4, 5, 1600404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


