Abstract
DNA and modified vaccinia virus Ankara (MVA) are vaccine vehicles suitable and safe for use in humans. Here, by using a multicytotoxic T-lymphocyte (CTL) epitope gene and a DNA prime-MVA boost vaccination regimen, high levels of CTLs specific for a single simian immunodeficiency virus (SIV) gag-derived epitope were elicited in rhesus macaques. These vaccine-induced CTLs were capable of killing SIV-infected cells in vitro. Fluorescence-activated cell sorter analysis using soluble tetrameric major histocompatibility complex-peptide complexes showed that the vaccinated animals had 1 to 5% circulating CD8+ lymphocytes specific for the vaccine epitope, frequencies comparable to those in SIV-infected monkeys. Upon intrarectal challenge with pathogenic SIVmac251, no evidence for protection was observed in at least two of the three vaccinated animals. This study does not attempt to define correlates of protective immunity nor design a protective vaccine against immunodeficiency viruses, but it demonstrates clearly that the DNA prime-MVA boost regimen is an effective protocol for induction of CTLs in macaques. It also shows that powerful tools for studying the role of CTLs in the control of SIV and human immunodeficiency virus infections are now available: epitope-based vaccines, a protocol for an effective induction of CTLs in primates, and a simple and sensitive method for quantitation of epitope-specific T cells. The advantages of the DNA prime-MVA boost regimen as well as the correlations of tetramer staining of peripheral blood lymphocytes with CTL killing in vitro and postchallenge control of viremia are discussed.
Because a safe and effective vaccine remains the best hope for controlling the human immunodeficiency virus (HIV) pandemic, the development and testing of vaccines are some of the primary objectives of AIDS research. The work presented here is a part of a long-term effort to develop an HIV vaccine with a particular emphasis on the induction of cytotoxic T lymphocytes (CTLs). This is because there is growing evidence that CTLs are an important component of the host antiviral response (9–11, 28, 37, 39, 47, 53, 58–60, 69). Identification of vaccines that induce strong CTL responses would assist in further advancing our understanding of the protective role(s) that these cells play in the fight against HIV infection. Furthermore, a reliable and effective method for CTL induction in primates would be of a great value to vaccine development in general.
Central to the evaluation of new vaccine strategies is the use of animal models. Vaccination of experimental animals permits important aspects of vaccine design and vaccination regimens to be addressed in a faster and cheaper way than human trials, and it ultimately provides a basis for a more effective planning of clinical trials. For HIV infection and AIDS, infection of nonhuman primates with immunodeficiency viruses offers a spectrum of models in terms of disease severity and difficulty in preventing virus infection. These models range from the infection of chimpanzees with HIV-1 SF2, a model in which neutralizing antibodies provide protection (6, 19, 26), to infection of rhesus macaques with simian immunodeficiency virus SIVmac, in which a role for cell-mediated immune responses in partial or complete protection is strongly supported (18, 23, 33, 42, 54). In turn, only results from clinical trials will show which of these monkey models is the closest to HIV infection of humans.
Recent advances in biotechnology have provided new tools for quantitation of the epitope-specific T-cell responses. Not long ago, detection of specific CTLs relied on standard 51Cr-release and limiting dilution assays (LDAs), which required antigen-driven expansion of T cells in vitro. These methods have tended to underestimate the numbers of CTLs. In contrast, the use of fluorochrome-labelled tetrameric major histocompatibility complex (MHC) class I-peptide complexes in fluorescence-activated cell sorter (FACS) analysis with a FACScan flow cytometer allows direct determination of MHC-peptide-specific T cells in fresh unrestimulated blood (3). This technique and the gamma interferon-based enzyme-linked immunospot (ELISPOT) assay (44) have revealed impressive T-cell responses to viral infections reflected in frequencies of virus-specific CD8+ T cells 50 to 500 times higher than that estimated originally by LDA. Thus, around 2% of circulating CD8+ T cells were HIV specific in many individuals infected with HIV (47), and up to 10% of CD8+ T cells recognized an SIV-derived epitope during persistent SIV infection of macaques (40). Here, the tetramer technology is used for the quantitation of CTL responses induced by vaccination of macaques.
The immunogen tested in this study was constructed as a string of 24 epitopes derived from HIV and SIV proteins recognized by murine, macaque, and human CTLs (32). Consequently, the same vaccines can be tested for the induction of the CTL responses in mice, monkeys, and humans. This immunogen was expressed from plasmid pTH DNA and modified virus Ankara (MVA; an attenuated vaccinia virus), i.e., vaccine vehicles that are safe and acceptable for use in humans. While both vaccines were shown to be immunogenic in mice in their own right (30–32), the most potent protocol for induction of CTLs was found to be the DNA prime-MVA boost regimen (29). This was the only protocol that fully protected mice in a malaria vaccine trial (61). Some past vaccination strategies were highly immunogenic in mice, but failed to induce comparable CTL responses in humans (72); therefore, it was important to assess the immunogenicity of the DNA prime-MVA boost regimen in nonhuman primates prior to clinical trials. Here, data are presented which demonstrate that this vaccination regimen induced high levels of SIV-specific CD8+ lymphocytes in the circulation of all immunized rhesus macaques. However, strong responses against this epitope did not protect at least two of three vaccinees against a rigorous mucosal challenge.
MATERIALS AND METHODS
Cells.
Chicken embryo fibroblasts (CEFs) were maintained in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco), 2 mM l-glutamine, and penicillin-streptomycin. B-lymphoblastoid cell lines (B-LCLs) were grown in R20 medium (RPMI 1640 supplemented with 20% FBS, 2 mM l-glutamine, and penicillin-streptomycin). All cells were cultured in a humidified incubator in 5% CO2 at 37°C.
Tissue typing of macaques by sequence-specific primer (SSP)-PCR and direct sequencing.
Genomic DNA was prepared from whole peripheral blood by using a Puregene DNA isolation kit (Gentra Systems) according to the manufacturer’s protocol, and the Mamu-A*01 alleles were identified as described previously (38). Briefly, the Mamu-A*01-alleles were amplified by PCR from 50 to 150 ng of genomic DNA by using allele-specific primers, and positive PCR products were identified on a 1% agarose gel. Positive samples were incubated with shrimp alkaline phosphatase (AP) and exonuclease I to remove unincorporated deoxynucleoside triphosphates (dNTPs) and residual single-stranded PCR primers, respectively (U.S. Biochemicals, Cleveland, Ohio) and then were heat inactivated. The enzyme-treated, heat-inactivated PCR products were sequenced with sequencing primers and the Taq FS DyeDeoxy Terminator Cycle Sequencing kit (Perkin-Elmer), and the samples were analyzed with an ABI 373 automated DNA sequencer.
Preparation of the pTH.HW DNA vaccine and immunization.
The H immunogen expressed from the multiepitope DNA and MVA vaccines (32) contained an SIV gag-derived epitope, TPYDINQML, recognized by CTLs in rhesus macaques (Macaca mulatta) in the context of the Mamu-A*01 MHC class I molecule (13, 43). However, detailed characterization of the Mamu-A*01-bound peptides revealed that the optimal binding peptide was actually CTPYDINQM (p11C, C-M) (1). Therefore, both the DNA and MVA vaccines used in this study were corrected by an insertion of nucleotide sequence 5′-GCT AGC TTC GAA TTC GCT GCC TGC into position 133 of the H gene open reading frame and were designated pTH.HW and MVA.HW, respectively. The pTH.HW DNA was prepared with the EndoFree Plasmid Maxi kit (Qiagen) according to the vendor’s protocol. Cartridges for the gene gun-mediated delivery containing gold particles coated with the pTH.HW plasmid DNA were prepared as described previously (31).
For the immunization, macaques were anesthetized, the fur was removed from the proximal area of the right hip, and the DNA-coated gold particles were delivered into the skin by using the Dermal XR particle delivery device (PowderJect Vaccines, Inc.). Eight cartridges were discharged into adjacent sites per animal per immunization, delivering a total of 8 μg of pTH.HW.
Preparation of the MVA.HW vaccine and immunization.
Recombinant MVA.HW was generated as described previously (30). The HW polyepitope gene was ligated into plasmid pMCO3, which directed the HW gene into the deletion III locus of MVA (12). Bulk stocks of the recombinant MVA were grown on primary CEFs obtained from the eggs of a specific pathogen-free flock. MVA was purified by centrifugation of cytoplasmic extracts through a 36% (wt/vol) sucrose cushion in a Beckman SW28 rotor at 13,500 rpm for 80 min. The virus stock titers were determined by immunostaining of CEF monolayers.
Anesthetized macaques were vaccinated by an intradermal (i.d.) needle injection of 5 × 108 PFU of MVA.HW in 300 μl of sterile phosphate-buffered saline (PBS) into five to six adjacent sites in the proximal region of the right hip.
Bulk CTL cultures, CTL lines, and a standard 51Cr-release assay.
Monkey peripheral blood lymphocytes (PBLs) were isolated with a Lymphoprep cushion (Nycomed Pharma As). For peptide restimulation of CTLs in vitro, 8 × 106 freshly isolated purified PBLs were incubated with 10 μM peptide in 100 μl of R20 in 5% CO2 at 37°C for 1 h and resuspended in a total of 4 ml of R20 supplemented with 25 ng of human interleukin-7 (IL-7) per ml in two 24-well-plate wells. On day 3, Lymphocult-T (Biotest AG) was added to a final concentration of 10% (vol/vol). On day 8, 5 × 106 peptide-pulsed irradiated autologous B-LCL cells were added to the cultures, followed by Lymphocult-T on day 11. Cytolytic tests were carried out on day 14. CTL lines were maintained by weekly peptide-pulsed irradiated B-LCL–Lymphocult-T restimulations.
In a 51Cr-release assay, the effector cells were diluted sequentially twofold in U-bottom wells (96-well plate; Costar) at effector/target ratios indicated in the figures. Five thousand 51Cr-labelled autologous B-LCL cells, pulsed (2 μg/ml) or unpulsed with peptide, were added to the effectors and incubated at 37°C for 6 h. Alternatively, autologous PBLs, which had been incubated for 6 days with phytohemagglutinin (PHA), were infected with SIVmacJ5 overnight, peptide pulsed or untreated, and used as targets. Spontaneous and total chromium releases were estimated from wells in which the target cells were kept in a medium alone or 5% Triton X-100, respectively. Percent specific lysis was calculated as [(sample release − spontaneous release)/(total release − spontaneous release)] × 100. The spontaneous release for all samples was below 20% of the total counts.
Preparation of soluble tetrameric MHC-peptide complexes.
DNA coding for the signal peptide-negative extracellular domains of the Mamu-A*01 MHC class I heavy chain was amplified by PCR from cDNA with the 5′ primer MamuNdeI (5′-CCT GAC TCA GAC CAT ATG GGC TCT CAC TCC ATG) and the 3′ primer 5′-G TGA TAA GCT TAA CGA TGA TTC CA CAC CAT TTT CTG TGC ATC CAG AAT ATG ATG CAG GGA TCC CTC CCA TCT CAG GGT GAG GGG C. The former primer contained a site for restriction endonuclease NdeI, and the latter included a HindIII site and coded for the peptide substrate for the biotin protein ligase enzyme BirA. The PCR product was digested with NdeI and HindIII and inserted into the same sites in the polylinker of bacterial expression vector pGMT7. The rhesus monkey gene coding for a signal peptide-negative β2-microglobulin was amplified by PCR from a cDNA clone by using primers B2MBACK (5′-TCA GAC CAT ATG TCT CGC TCC GTG GCC) and B2MFOR (5′-TCA GAC AAG CTT TTA CAT GTC TCG ATC CCA C) and likewise cloned into the NdeI and HindIII sites of pGMT7. Both chains were expressed in Escherichia coli BL-21, purified from inclusion bodies, denatured in 8 M urea, and refolded in the presence of peptide CTPYDINQM (1); biotinylated with the BirA enzyme (Avidity); and purified on fast-performance liquid chromatography and MonoQ ion-exchange columns. The amount of biotinylated refolded MHC-peptide complexes was estimated in an enzyme-linked immunosorbent assay, whereby monomeric complexes were first captured by conformation-sensitive monoclonal antibody (MAb) W6/32 and detected by AP-conjugated streptavidin (Sigma) followed by a colorimetric reagent for AP. The formation of tetrameric complexes was induced by addition of phycoerythrin (PE)-conjugated streptavidin (ExtrAvidin; Sigma) to the refolded biotinylated monomers at a molar ratio of MHC-peptide monomer–PE-streptavidin of 4:1. Streptavidin was added in 1/10 of the final amount separated by a 20-min incubation at 4°C. PE-tetrameric complexes were stored in the dark at 4°C until use.
FACS analysis.
Aliquots of Lymphoprep cushion-isolated PBLs were stored at −70°C until use. On the day of analysis, PBLs were thawed, washed once with PBA (PBS, 1% bovine serum albumin, 0.1% sodium azide), incubated with 1 μg of PE-conjugated tetrameric Mamu-A*01-Gag peptide complexes, first at 4°C for 20 min and then on ice for 5 min. One microgram of Tri-color-conjugated antihuman CD8 MAb (Caltag) was added, and this mixture was then incubated with the cells on ice for 20 min. Cells were washed twice with PBA before being fixed in formaldehyde (PBS, 1% paraformaldehyde, 2.5% FBS). Two-color cytofluorometric analysis was performed with a Becton Dickinson FACS. Voltage and compensation levels were determined by using both unstained lymphocytes for setting the background levels of fluorescence to the first log of the scale and single PE- and Tri-color-stained lymphocytes for adjusting the spectral overlap. All time points from each animals were analyzed with the same FACS settings. A gate was applied to the live lymphocytes. Data analysis and presentation were done with the CellQuest software (Becton Dickinson).
SIV challenge.
Animals were challenged intrarectally (i.r.) with 20 i.r. median infectious doses of cell supernatant of pathogenic SIVmac251 grown in simian PBLs kindly provided by A.-M. Aubertin, Université Louis Pasteur, Strasbourg, France.
Plasma RNA load.
The number of SIV genome equivalents per 1 ml of peripheral blood was determined by quantitative competitive reverse transcription (RT)-PCR method as described before (66). A calibrated amount of internal standard RNA was added to and copurified with the sample RNA to be analyzed before RNA purification to compensate for sample degradation and amplification efficiency. The limit of detection was 40 genome equivalents per ml of plasma.
Cell-associated virus load.
Freshly Lymphoprep-separated lymphocytes were cocultivated in duplicates with the human T-cell line C8166 at either a single dose of 106 cells or additionally fivefold diluted from 4 × 105 to 130 cells in duplicates. Medium and C8166 cells were replenished every 3 to 4 days, and the total culture volume was maintained at approximately 15 ml. All cultures were observed for 28 days or until a cytopathic effect was apparent. The assay limit was 1.6 infected cells per 106 PBLs. Culture results were confirmed by indirect immunofluorescent staining. Fifty percent end points were calculated by using the Kärber formula, and the results were expressed as the number of infected cells per 106 PBLs. The lack of SIV infection was confirmed by a negative nested PCR for proviral DNA.
CD4+ and CD8+ lymphocyte enumeration.
A whole-blood lysis method was used to assay PBLs for CD4+ (OKT4; Ortho Diagnostics) and CD8+ (DK25; Dako) cells (17). Briefly, heparinized blood samples were combined with a specific or isotype-matched MAb, incubated with Immunolyse (Coulter) to lyse erythrocytes, fixed with 1% formaldehyde in PBS, and analyzed on a FACScan flow cytometer (Becton Dickinson). Absolute numbers of each lymphocyte subset were determined from the differential leukocyte counts and total leukocyte counts.
Animal care.
Indian rhesus macaques were bred and immunized at the Oxford University Farm and challenged at the Centre for Applied Microbiology and Research in Porton Down in accordance with the guidelines of the Home Office Code of Practice. All procedures were carried out under general anesthesia.
RESULTS
DNA prime-MVA boost regimen induced strong CTL responses capable of killing SIV-infected PBLs.
Mamu-A*01-positive rhesus macaques Cyd, Di, and Doris were vaccinated by the DNA prime-MVA boost regimen (29) modified for primates. The animals were immunized twice i.d. with a total of 8 μg of pTH.HW DNA per immunization with the Dermal XR (formerly Accell) gene delivery device of PowderJect Vaccines, Inc. (weeks 0 and 8), followed by two i.d. needle injections of 5 × 108 PFU of MVA.HW (weeks 17 and 22) administered into the proximal area of the right hip. Both the DNA and MVA vaccines were safe and did not cause any obvious adverse reactions or discomfort.
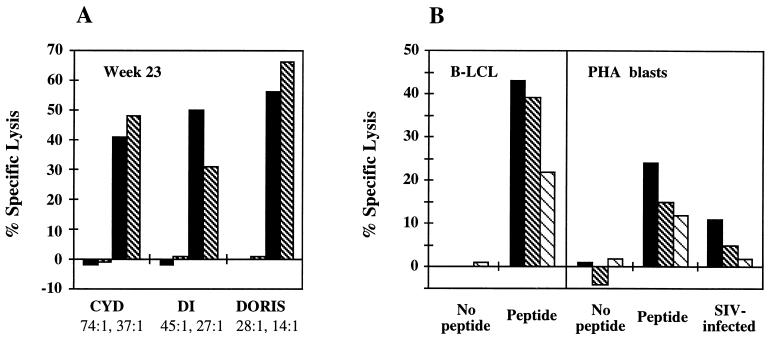
Blood from each animal was drawn at multiple time points throughout the immunization schedule. PBLs were isolated on a Lymphoprep cushion, restimulated with peptide in vitro, and tested for their cytolytic activity against peptide-pulsed autologous B-LCL cells as targets. Following the two DNA gene gun immunizations, no significant lytic activity was detected in 2-week lymphocyte cultures, although a longer peptide restimulation can result in CTL activity (2). One week after the first MVA immunization, p11C, C-M-specific CTL responses were observed only in Doris; however, all animals developed CTL responses by week 19 (not shown). CTL lines were readily established from Cyd and Doris from a bleed on week 22 that effectively lysed peptide-sensitized targets at very low effector/target ratios (not shown). The second MVA vaccination resulted in high CTL activities of cultured lymphocytes in all three animals (week 23 [Fig. 1A]), which, at least for Doris, persisted over 13 weeks (week 35 [Fig. 1B, left panel]). Moreover, the same bulk CTL cultures lysed PHA-activated SIV-infected PBLs (Fig. 1B, right panel). Because SIV infects only CD4+ T cells and the infection is never 100%, the half-lysis of SIV-infected PBLs compared to that of the peptide-pulsed targets suggested an effective recognition and killing of SIV-infected cells by vaccine-induced CTLs.
FIG. 1.
Cytolytic T-cell responses determined by a standard 51Cr-release assay. PBLs were isolated, and the bulk CTL cultures were treated in vitro with the following reagents: peptide CTPYDINQM and IL-7 on day 0, Lymphocult-T on day 3, peptide-pulsed irradiated autologous B-LCL on day 8, and Lymphocult-T on day 11. The CTL assays were carried out on day 14. (A) The restimulated effectors were incubated for 6 h with 51Cr-labelled peptide or unpulsed (the first two bars for each animal) or pulsed (the last two bars for each animal) autologous B-LCL cells at the effector/target ratios indicated below the graphs. (B) Restimulated PBLs drawn from Doris on week 35 were tested on 51Cr-labelled autologous B-LCL cells (left) or 6-day PHA-stimulated PBLs (right), which had been treated as indicated, at effector/target ratios of 54:1, 27:1, and 13:1.
DNA prime-MVA boost regimen induced high levels of CD8+ p11C, C-M-specific PBLs.
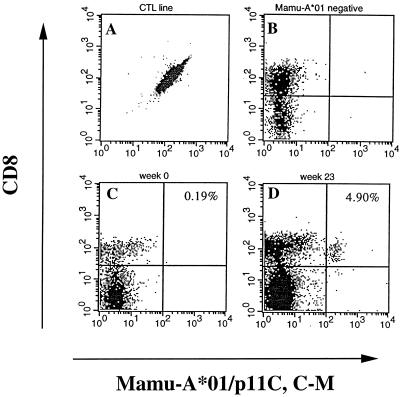
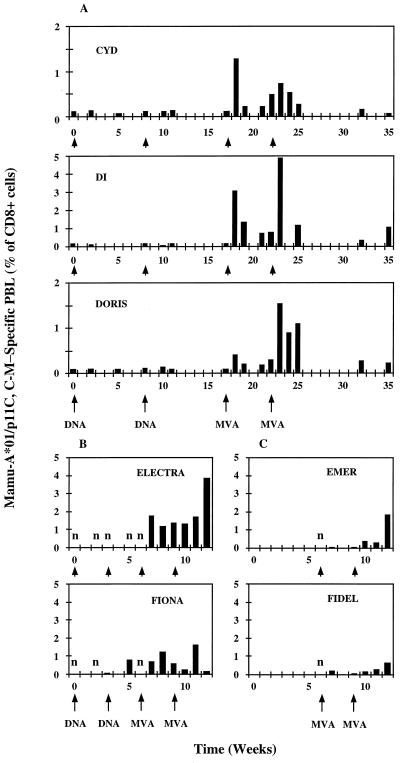
Soluble tetrameric Mamu-A*01–p11C, C-M peptide complexes were prepared as described in Materials and Methods, and their specificity was confirmed with established CTL lines and PBLs from a Mamu-A*01-negative macaque which served as respective positive and negative controls (Fig. 2). On the day of the FACScan analysis, frozen unrestimulated PBLs were analyzed for the presence of tetramer-binding CD8+ cells, and the results were presented as a percentage of CD8+ cells stained with the tetramer (i.e., CD8+ cells carrying a T-cell receptor specific for the Mamu-A*01–p11C, C-M-specific MHC-peptide complex). In these animals, DNA immunization alone did not elicit any population with above-background tetramer staining; however, the first MVA immunization stimulated frequencies ranging from 0.4 to 3% of CD8+ cells (Fig. 3A). In Di and Doris, these levels were boosted further by the second MVA vaccine up to 4.9 and 1.5%, respectively. The frequencies peaked 1 week after the MVA vaccination and decreased to one-half within approximately 1 week. A significant tetramer staining was still observed in Di and Doris 17 weeks after the last MVA immunization (week 35).
FIG. 2.
Specificity of the soluble MamuA*01–p11C, C-M tetrameric complexes. The peptide-grown CTL line (A) or frozen blood samples containing Mamu-A*01-negative rhesus macaque PBLs (B), and PBLs isolated from Di at weeks 0 (C) and 23 (D) were double stained for the CD8 marker and Mamu-A*01–p11C, C-M-specific T-cell receptors and examined by FACS.
FIG. 3.
Longitudinal analysis of vaccine-induced SIV-specific T cells. Groups of rhesus macaques were immunized with 8 μg of pTH.HW DNA with a gene gun or 5 × 108 PFU of MVA.HW i.d. at the times indicated below each graph. Frozen isolated PBL samples were thawed, double stained with PE-conjugated tetrameric MHC-peptide complexes, and human CD8-specific Tri-color-conjugated MAb for the CD8 marker, and examined by FACS. An n indicates a sample measured, but negative.
Accelerated schedule of immunization induced high levels of CD8+ p11C, C-M-specific PBLs.
The 5- to 8-week intervals between individual immunizations used in the first group of macaques were based on experiments which employed the gene gun to elicit antibody responses (20). When a faster, 3-week-interval vaccination schedule was tested, similar levels of tetramer-specific CD8+ cells were induced, although with a seemingly slower kinetics (Fig. 3B). The peak vaccine-induced lymphocyte frequencies were detected in 2 or 3 weeks rather than 1 week after the MVA immunization. One animal, Fiona, had a positive tetramer staining 2 weeks after the second DNA vaccine (Fig. 3B).
DNA vaccine primes CTL responses.
To assess whether or not the DNA vaccine primes CTL immune responses and therefore is an important part of the vaccination regimen, two animals, Emer and Fidel, were immunized with the MVA vaccine alone, and the induction of the Mamu-A*01–p11C, C-M-specific T cells in their circulation was determined by tetramer staining (Fig. 3C). The most striking observation suggesting that DNA had made a difference was that in these two animals, two MVA vaccinations were required to detect significant tetramer staining. This is similar to previously published results (63). Thus, these data, the DNA-induced tetramer staining in Fiona (Fig. 3B), and CTL lysis after a 3-week peptide restimulation in vitro (2) indicate that there was priming of CTLs by a gene gun-delivered DNA vaccine.
One out of three animals remained uninfected after a pathogenic i.r. challenge.
Animals Cyd, Di, and Doris were boosted with 5 × 108 PFU of MVA.HW on week 44 and challenged i.r. with 20 50% median infective doses of cell-free pathogenic SIVmac251 1 week later (week 45), i.e., at the peak of tetramer-determined responses, and their SIV status (Fig. 4A and B) and Mamu-A*01–p11C, C-M-specific T-cell responses (Fig. 5) were monitored at regular intervals.
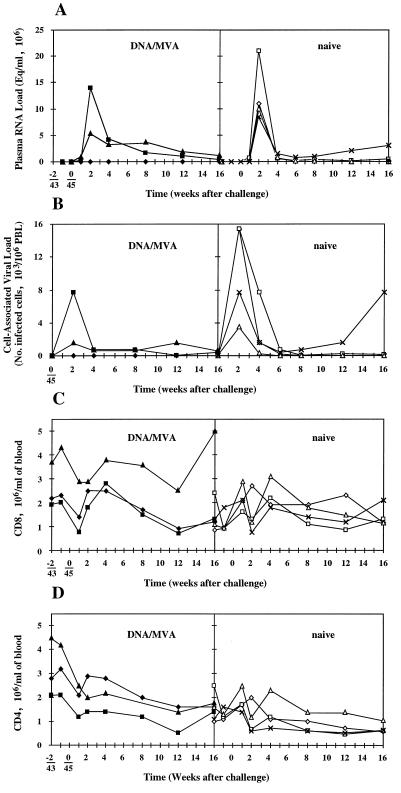
FIG. 4.
Longitudinal analysis of peripheral blood after SIV challenge. The left panels show DNA prime-MVA boost-vaccinated macaques Cyd (⧫), Di (▴), and Doris (■), and the right panels show naive macaques X23 (◊), X32 (□), and V48 (×). (A) Plasma virus loads were determined with a quantitative competitive RT-PCR. A negative result meant there were less than 40 genome equivalents (Eq) per ml of plasma. (B) Cell-associated virus loads were determined by coculturing multiple dilutions of isolated PBLs with the C8166 cell line and assessing the cytopathic effect resulting from SIV infection. A negative result meant there were less than 1.6 infected cells per 106 PBLs. (C and D) CD8+ (C) and CD4+ (D) lymphocytes were enumerated by a whole-blood lysis method whereby PBLs were first incubated with a CD8- or CD4-specific MAb followed by Immunolyse, and their counts were established from the difference between untreated and treated PBLs.
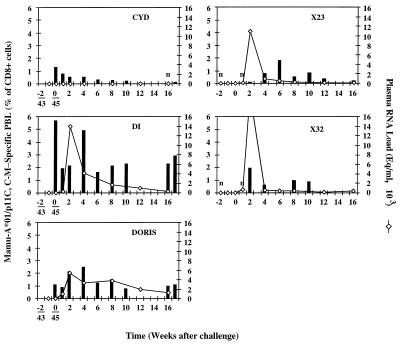
FIG. 5.
Postchallenge analysis of tetramer-specific lymphocytes. Cyd, Di, and Doris were immunized by the DNA-MVA regimen, and the X23 and X32 monkeys are two Mamu-A*01+ naive controls. Frozen isolated PBLs were double stained with fluorochrome-conjugated Mamu-A*01–p11C, C-M tetramer and a CD8-specific MAb and analyzed with a FACScan. To facilitate the correlation, the plasma virus loads from Fig. 4A were overlaid. An n indicates a sample measured, but negative. Eq, genome equivalents.
The third dose of recombinant MVA applied to the same site expanded the CD8+ Mamu-A*01–p11C, C-M-specific population to the highest levels seen during the first two MVA vaccinations (Fig. 3A and 5). Thus, any anti-MVA responses (not actually measured) did not prevent an efficient boost with the recombinant MVA-expressed immunogen. At the same time, the tetramer staining did not increase above the previous levels, so it could also be argued that following a DNA priming, the “ceiling” of MVA-elicited responses was reached with one (Cyd) or two (Di and Doris) MVA doses.
The SIV challenge infected Di and Doris, while Cyd remained SIV negative by both plasma RNA and cell-associated viral loads (Fig. 4A and B, left panels). All four naive controls became infected (Fig. 4A and B, right panels). During the first weeks following SIV challenge, absolute CD4+ and CD8+ cell counts increased transiently in all monkeys without any noticeable correlation with protection or infection (Fig. 4C and D). The postchallenge tetramer staining of uninfected Cyd’s PBLs did not show any expansion of Mamu-A*01–p11C, C-M-specific cells (Fig. 5). In contrast, the SIV challenge drove the increment in tetramer staining of PBLs of the infected Di and Doris. The temporal relationship between the Mamu-A*01–p11C, C-M-specific CD8+ cells and virus loads was consistent with the contribution of these cells to the control of the acute infection (60), after which the levels of tetramer staining decreased, but remained relatively high, presumably due to the continuous presence of replicating virus. No p11C, C-M-specific CTL escape mutants were observed in the vaccinated and infected animals in a total of 14 individually cloned PCR products derived from two independent amplifications of PBL DNA at week 16 postchallenge. Interestingly, the uninfected macaque, Cyd, showed the highest cytotoxic activity in vitro of peptide-restimulated PBLs drawn 4 weeks after the challenge (Fig. 6), which may imply the strongest long-term CTL memory among the three challenged animals.
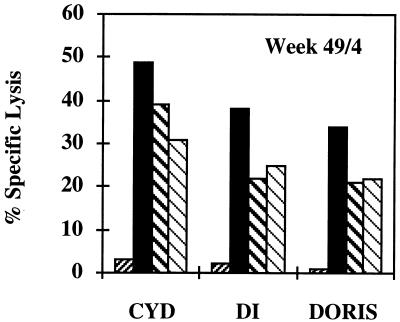
FIG. 6.
p11C, C-M-specific CTL responses 4 weeks after the SIVmac challenge (week 49). PBLs were isolated, restimulated in vitro, and tested on peptide-unpulsed (the first bar for each animal) or pulsed (the last three bars for each animal) PBLs for cytolytic activity as described in the legend to Fig. 1. The effector/target ratios were 25:1, 12:1, and 6:1.
DISCUSSION
It was demonstrated previously in mice that a particular sequence of vaccinations, DNA followed by MVA, elicited high levels of CTLs (29) and conferred complete protection against Plasmodium berghei sporozoite challenge (61). Here, immunization of macaques by using a similar DNA prime-MVA boost vaccination regimen generated strong CTL responses detected in a 51Cr-release assay after a restimulation in vitro (Fig. 1A), as well as high frequencies of peptide-specific CD8+ lymphocytes (Fig. 3) in peripheral blood.
What makes the DNA prime-MVA boost regimen efficient in induction of CTLs? The exact mechanisms are not known, but several factors may be relevant. First, priming of CTL responses by using DNA as a vaccine vehicle may allow the initial responses to be focused on the recombinant immunogen, simply because that is the only foreign protein expressed. Although recombinant poxviruses may be intrinsically more immunogenic than DNA vectors as vaccine vehicles, virus-infected cells must produce a large number of peptide epitopes derived from virus proteins and presented by MHC class I. These may compete with the recombinant immunogen for CTL immunodominance. In most previous studies, only a minority of vaccinees immunized with recombinant vaccinia virus responded to the inserted protein, although all responded to the vaccinia virus antigens (23, 27). In this regimen, DNA-primed animals have already increased frequencies of immunogen-specific CTL precursors or memory when vaccinated with MVA. Second, both the DNA and MVA vaccines were delivered into the dermis, a major immunological inductive site, in which the multiepitope immunogen is produced by keratinocytes and shed into the immediate vicinity of Langerhans cells (LCs) and/or the multiepitope gene is delivered directly into the LCs (50). Upon activation, LCs migrate to the draining lymph nodes, where they serve as the most efficient initiators and modulators of immune reactions (4). In mice, the i.d. route of immunization was more efficient than the intramuscular needle injection (61). Third, the use of MVA rather than other vaccinia virus strains or poxviruses may be relevant (61), possibly because MVA has lost several genes coding for immunomodulatory proteins, such as the soluble receptors for gamma interferon, alpha/beta interferon, tumor necrosis factor, and CC-chemokines (8). MVA also generates a strong type I interferon response, which is likely to contribute to the CTL induction (67). Fourth, both DNA and MVA mimic natural infection by expressing antigens endogenously and thus delivering them to the MHC class I processing and presentation pathway. Fifth, the expressed HW multi-CTL epitope is nontoxic to the transfected cells, which may be due to the sequestration of the polypeptide into large cytoplasmic vesicles (32). This may allow the cells to sustain the production of the immunogen over a longer time or until their destruction by the immune responses. Finally, there is now an emerging body of data suggesting that combined vaccination protocols involving different vaccine vehicles, routes, or means of antigen presentation to the immune system induce immune responses more efficiently and in some instances protected macaques against virus challenge. Thus, DNA priming followed by Env protein boosting increased antibody, but not CTL, responses and protected macaques against a nonpathogenic simian-human immunodeficiency virus strain (SHIVHXBc2) challenge (41). Sequential bimodal immunizations of monkeys with DNA and the recombinant Western Reserve (WR) strain of vaccinia virus significantly decreased virus loads after infection (21). DNA prime-recombinant fowlpox boost vaccinations held nonpathogenic HIV (36) and nonpathogenic SHIVIIIB (54) below the level of detection. In the latter study, two animals remained uninfected after a challenge with pathogenic SHIV89.6P, a protection mediated by a neutralizing antibody-independent mechanism; however, there is a possibility that protective responses could have been boosted or induced by the two previous SHIVIIIB exposures.
Mamu-A*01–p11C, C-M-specific CD8+ PBLs are functionally heterogeneous and consist mainly of two subpopulations. The first subpopulation are memory or precursor CTLs which may be fewer in number, but are able to proliferate and need to be reactivated and expanded to observe killing in vitro. Their frequencies can be determined in LDAs. The second subpopulation are the expanded CTLs, which if sufficiently high can be demonstrated by fresh killing (2). However, antigen-specific CD8+ T cells can also be “silenced” or rendered nonfunctional, but are still abundant enough to be detected by a tetramer (77). On a few occasions the present study demonstrated discordance between CTL lysis in vitro and tetramer staining. First, Cyd, Di, and Doris gave respective peptide-specific lyses of 48, 50, and 66% of total 51Cr release at week 23 (Fig. 1), while their tetramer-detected CD8+ T-lymphocyte levels were 0.7, 4.9, and 1.5% of total CD8+ PBLs, respectively (Fig. 3). Similarly, 49, 38, and 34% specific lysis at week 49 (Fig. 6) corresponded to respective 0.52, 4.90, and 2.46% tetramer-stained CD8+ PBLs (Fig. 5). A closer comparison of the immune responses in Doris at weeks 23 and 35 (Fig. 1) revealed that while the peptide-specific lysis of the same effector/target ratios decreased 1.5-fold (28:1) and 3-fold (14:1), the tetramer staining decreased 7-fold (Fig. 3A). Also, while in all animals the tetramer staining decreased at least by half between weeks 18 and 19 (Fig. 3), the peptide-specific lysis after in vitro restimulation increased (not shown). In the absence of persisting high levels of antigen due to the lack of MVA replication, the half-life of the tetramer staining was approximately 1 to 2 weeks (Fig. 3A and 5 [Cyd]). Finally, no correlation between tetramer staining and virus load could be inferred, although the numbers of animals in this study were small.
Can CTLs protect against infection with immunodeficiency viruses? Cytolytic activity cannot prevent incoming cell-free virus from infecting host cells. However, in the laboratory, CTLs killed HIV-infected cells before they produced new virions (74) and released chemokines which inhibited HIV infection (51, 68). Thus, in vivo, CTLs may be able to clear the initial small number of infected cells before HIV spreads further and establishes generalized infection (46, 73). This might explain detection of HIV-specific CTL responses in exposed, but uninfected commercial sex workers whose cells were fully susceptible to infection with HIV (56, 57), in uninfected infants born to HIV-infected mothers (59), and seronegative health care workers occupationally exposed to HIV-contaminated body fluids (49). The same SIV gag-derived p11C, C-M epitope as used in this study was shown to be immunodominant (40) and to contribute to the suppression of both the acute and chronic viremias (60) in SIV-infected monkeys. There have been two studies in which animals with vaccine-induced CTLs against the same single Gag epitope were challenged and not protected against infection (75), although a decrease in virus load was observed (45). However, there was a possibility that the levels of CTLs in the former challenge study were lower than those in the animals here due to boosting with a suboptimal 12-mer peptide, p11C (64), and protection is likely to depend on the strength of CTLs (23). Alternatively, CTLs specific for some SIV epitopes other than p11C, C-M may be more protective by analogy to the infection with lymphocytic choriomeningitis virus, in which CTLs specific for different epitopes differed in their protective capacities (24, 25). Thus, CTLs against proteins expressed early in the replication cycle (e.g., Nef, Tat, or Rev), may have a better chance to prevent infection than those against late structural proteins (e.g., Gag), although this difference is likely to be subtle.
More importantly, broader CTL responses against multiple epitopes may be critical for the success of a prophylactic HIV vaccine. Three rhesus epitopes restricted by Mamu-A*01, -A*02, and -B*01 molecules had been published (43, 70, 76) at the time of the construction of the DNA and were included in the HW multi-CTL epitope vaccines employed in this study. However, only the Mamu-A*01-restricted epitope turned out, after a correction (1), to be immunogenic in this study. Some uncertainty surrounds the Mamu-B*01-restricted epitope, because it does not fit the amino acid sequence motif currently defined for this MHC type (71). None of the three selected Mamu-A*01+ animals was Mamu-A*02+. Recently, six new epitopes and their restricting Mamu molecules have been defined, and sequence-specific primers have been designed to screen for the Mamu-A*01 (38) and several other alleles (71). These new developments increase the utility of the rhesus macaque model for testing epitope-based vaccines.
CTLs require “help” provided by CD4+ T cells for maintenance of their effector functions, and even dendritic cells may be conditioned by CD4+ cells to provide sufficient costimulatory signals to CTLs (5, 52, 62). For CTL epitope-based vaccines, such as the HW polyepitope used in this study, a long string of MHC class I-restricted epitopes will create novel helper epitopes presented by MHC class II molecules and activate CD4+ T-cell responses after vaccination. However, these CD4+ helper T cells will not be activated by incoming virus. Should a prophylactic vaccine induce HIV-specific help? Delay in CD4+ T-cell activation may slow the initial spread of HIV, because HIV replicates in activated CD4+ cells. It has been shown in HIV-infected persons that vaccination with a common recall antigen, tetanus toxoid, increased plasma virus load (48, 65). Vigorous Gag-specific CD4+ T-cell responses have been associated with the control of viremia in chronic HIV infection and may enhance CTL responses (55). Some (14, 15, 35), but not all (55), studies have found CD4+ T-cell responses in exposed seronegative individuals, which might contribute to their lack of infection (22). Comparison of protective effects by vaccination with poly-CTL epitopes and whole proteins, which contain virus-derived MHC class II epitopes, could determine how important the CD4+ T-cell responses are in prophylactic immunity (34).
In this challenge, one out of three DNA-MVA-vaccinated animals challenged 1 week after the last MVA immunization remained uninfected, while all four naive animals succumbed to infection (Fig. 4). As a part of a parallel, but separate experiment, four control macaques were immunized three times with 108 PFU of wild-type (no SIV-derived gene) MVA at 12-week intervals and challenged 2 months after the last MVA immunization. One of these animals remained uninfected (data not shown [16]). In another study, wild-type MVA immunization suppressed virus loads in animals challenged with SIV 1 month after the last MVA vaccine (7). Taken together, these results suggest that MVA may induce a nonspecific antiviral effect, the mechanism of which is currently under investigation. Although for use in humans, determination of the underlying mechanisms would be comforting, the antiviral effects of MVA are generally welcome.
In conclusion, only one out of three immunized monkeys remained uninfected after a challenge which was at the pathogenic and hard-to-protect end of the monkey model spectrum. Based on the information available, this protection could not be clearly correlated with the p11C, C-M-specific T lymphocytes in the blood at the time of the i.r. challenge. It should also be noted that the challenge dose, even though it may not have infected all animals, is likely to be much higher than the doses in natural HIV transmission. In any case, we and others believe that the high levels of vaccine-induced CTL responses in macaques merit an evaluation of the immunogenicity and, if that is satisfactory, efficacy of similar vaccines in clinical trials.
ACKNOWLEDGMENTS
We thank Eric Prieur for the preparation of CEF cells; Susan Steffen for tissue typing of rhesus macaques; Rod Dunbar and Graham Ogg for advice during the production of tetramers; Sharon Leech for assistance with virus load determination; and Sarah Wolfensohn, Maggie Lloyd, James Davys, and Mike Dennis for excellent veterinary services.
Grant support from MRC U.K. and the International AIDS Vaccine Initiative (T.H., R.V.S., and A.J.M.); MRC U.K. (T.J.B. and G.L.S.), grants RR00167, AI32426, and AI41913 (J.E.B., T.M.A., and D.I.W.); and the U.K. Department of Health (S.A.S., N.C., and M.P.C.) is fully acknowledged.
REFERENCES
- 1.Allen T M, Sidney J, del Guercio M-F, Glickman R L, Lensmeyer G L, Wiebe D A, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide-binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 2.Allen, T. M., and D. I. Watkins. Unpublished data.
- 3.Altman J, Moss P A H, Goulder P, Barouch D, McHeyzer-Williams M, Bell J I, McMichael A J, Davis M M. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–96. [Google Scholar]
- 4.Banchereau J, Steinman R M. Dendritic cell and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Help for cytotoxic T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 6.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 7.Biberfeld, G. Personal communication.
- 8.Blanchard T J, Alcami A, Andrea P, Smith G L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 9.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll M W, Moss B. E. coli beta-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. BioTechniques. 1995;19:352–356. [PubMed] [Google Scholar]
- 13.Chen Z W, Shen L, Miller M D, Ghim S H, Hughes A L, Letvin N L. Cytotoxic T lymphocytes do not appear to select for mutations in an immunodominant epitope of simian immunodeficiency virus gag. J Immunol. 1992;149:4060–4066. [PubMed] [Google Scholar]
- 14.Clerici M, Giorgi J V, Chou C C, Gudeman V K, Zack J A, Gupta P, Ho H N, Nishanian P G, Berzofsky J A, Shearer G M. Cell-mediated immune responses to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure. J Infect Dis. 1992;165:1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 15.Clerici M, Levin J M, Kessler H A, Harris A, Berzofsky J A, Landay A L, Shearer G M. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA. 1994;271:42–46. [PubMed] [Google Scholar]
- 16.Cranage, M. P., et al. Unpublished data.
- 17.Cranage M P, Sharpe S A, Whatmore A M, Polyanskaya N, Norley S, Cook N, Leech S, Dennis M J, Hall G A. In vivo resistance to simian immunodeficiency virus superinfection depends on attenuated virus dose. J Gen Virol. 1998;79:1935–1944. doi: 10.1099/0022-1317-79-8-1935. [DOI] [PubMed] [Google Scholar]
- 18.Dittmer U, Hunsmann G. Long-term non-progressive human immunodeficiency virus infection: new insights from the simian immunodeficiency virus model. J Gen Virol. 1997;78:979–984. doi: 10.1099/0022-1317-78-5-979. [DOI] [PubMed] [Google Scholar]
- 19.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:726–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 20.Fuller D H, Murphy-Corb M, Barnett S, Steimer K, Haynes J R. Enhancement of immunodeficiency virus-specific immune responses in DNA-immunized rhesus macaques. Vaccine. 1997;15:924–926. doi: 10.1016/s0264-410x(96)00271-x. [DOI] [PubMed] [Google Scholar]
- 21.Fuller D H, Simpson L, Cole K S, Clements J E, Panicali D L, Montelaro R C, Murphey-Corb M, Haynes J R. Gene gun-based nucleic acid immunization alone or in combination with recombinant vaccinia vectors suppresses virus burden in rhesus macaques challenged with a heterologous SIV. Immunol Cell Biol. 1997;75:389–396. doi: 10.1038/icb.1997.61. [DOI] [PubMed] [Google Scholar]
- 22.Furci L, Scarlatti G, Burastero S, Tambussi G, Colognesi C, Quillent C, Longhi R, Loverro P, Borgonovo B, Gaffi D, Carrow E, Malnati M, Lusso P, Siccardi A G, Lazzarin A, Beretta A. Antigen-driven C-C chemokine-mediated HIV-1 suppression by CD(+) T cells from exposed uninfected individuals expressing the wild-type CCR-5 allele. J Exp Med. 1997;186:455–460. doi: 10.1084/jem.186.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud R, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated animals. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 24.Gallimore A, Dumrese T, Hengartner H, Zinkernagel R M, Rammensee H G. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallimore A, Hombach J, Dumrese T, Rammensee H G, Zinkernagel R M, Hengartner H. A protective cytotoxic T cell response to a subdominant epitope is influenced by the stability of the MHC class I/peptide complex and the overall spectrum of viral peptides generated within infected cells. Eur J Immunol. 1998;28:3301–3311. doi: 10.1002/(SICI)1521-4141(199810)28:10<3301::AID-IMMU3301>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Girard M, Kieny M-P, Pinter A, Barre-Sinoussi F, Nara P, Kolbe H, Kusumi K, Chaput A, Reinhart T, Muchmore E, Ronco J, Kanczorek M, Gomard E, Gluckman J-P. Immunization of chimpanzee confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotch F M, Hovell R, Delchambre M, Silvera P, McMichael A J. Cytotoxic T-cell response to simian immunodeficiency virus by cynomolgus macaque monkeys immunized with recombinant vaccinia virus. AIDS. 1991;5:317–320. doi: 10.1097/00002030-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 29.Hanke T, Blanchard T J, Schneider J, Hannan C M, Becker M, Gilbert S C, Hill A V S, Smith G L, McMichael A. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine. 1998;16:439–445. doi: 10.1016/s0264-410x(97)00226-0. [DOI] [PubMed] [Google Scholar]
- 30.Hanke T, Blanchard T J, Schneider J, Ogg G S, Tan R, Becker M, Gilbert S C, Hill A V S, Smith G L, McMichael A. Immunogenicities of intravenous and intramuscular administrations of MVA-based multi-CTL epitope vaccine for HIV in mice. J Gen Virol. 1998;79:83–90. doi: 10.1099/0022-1317-79-1-83. [DOI] [PubMed] [Google Scholar]
- 31.Hanke T, Neumann V C, Blanchard T J, Becker M, Sweeney P, Hill A V S, Smith G L, McMichael A. Effective induction of HIV-specific CTL by multi-epitope DNA using a gene gun in a combined vaccination regime. Vaccine. 1999;17:589–596. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- 32.Hanke T, Schneider J, Gilbert S C, Hill A V S, McMichael A. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum: immunogenicity in mice. Vaccine. 1998;16:426–435. doi: 10.1016/s0264-410x(97)00296-x. [DOI] [PubMed] [Google Scholar]
- 33.Igarashi T, Ami Y, Yamamoto H, Shibata R, Kuwata T, Mukai R, Shinohara K, Komatsu T, Adachi A. Protection of monkeys vaccinated with vpr- and/or nef-defective simian immunodeficiency virus strain mac/human immunodeficiency virus type 1 chimeric viruses: a potential candidate live-attenuated human AIDS vaccine. J Gen Virol. 1997;78:985–989. doi: 10.1099/0022-1317-78-5-985. [DOI] [PubMed] [Google Scholar]
- 34.Kalams S A, Walker B D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaul R, Trabattoni D, Bwayo J J, Arienti D, Zagliani A, Mwangi F M, Kariuki C, Ngugi A N, MacDonald K S, Ball T B, Clerici M, Plummer F A. HIV-1 specific mucosal IgA in a cohort of HIV-1 resistant Kenyan sex workers. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kent S J, Zhao A, Best S J, Chandler J D, Boyle D B, Ramshaw I A. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knapp L A, Lehman E, Piekarczyk M S, Urvater J A, Watkins D I. A high frequency of Mamu-A*01 in the rhesus macaque detected by PCR-SSP and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 39.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. Analysis of gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys by cell staining with a tetrameric MHC class I/peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M-E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller C J, McChesney M B, Lü X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller M D, Yamamoto H, Hughes A L, Watkins D I, Letvin N L. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 44.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 45.Norley, S. Personal communication.
- 46.Nowak M A, Lloyd A L, Vasquez G M, Wiltrout T A, Wahl L M, Bischofberger N, Williams J, Kinter A, Fauci A S, Hirsch V M, Lifson J D. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J Virol. 1997;71:7518–7525. doi: 10.1128/jvi.71.10.7518-7525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 48.Ostrowski M A, Stanley S K, Justement J S, Gantt K, Goletti D, Fauci A S. Increased in vitro tetanus-induced production of HIV type 1 following in vivo immunization of HIV type 1-infected individuals with tetanus toxoid. AIDS Res Hum Retroviruses. 1997;13:473–480. doi: 10.1089/aid.1997.13.473. [DOI] [PubMed] [Google Scholar]
- 49.Pinto L A, Sullivan J, Berzofsky J A, Clerici M, Kessler H A, Landay A L, Shearer G M. Env-specific cytotoxic T lymphocytes in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J Clin Investig. 1995;96:867–876. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porgador A, Irvine K R, Iwasaki A, Barber B H, Restifo N P, Germain R N. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price D A, Sewell A K, Dong T, Tan R, Goulder P J, Rowland-Jones S L, Phillips R E. Antigen-specific release of beta-chemokines by anti-HIV-1 cytotoxic T lymphocytes. Curr Biol. 1998;8:355–358. doi: 10.1016/s0960-9822(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 52.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 53.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S-L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 56.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 57.Rowland-Jones S L, Dong T, Fowke K R, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Oyugi J, Ngugi E, Bwayo J J, MacDonald K S, McMichael A J, Plummer F A. Cytotoxic T cell responses to multiple conserved epitopes in HIV-resistant prostitutes in Nairobi. J Clin Investig. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowland-Jones S L, McMichael A. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Curr Opin Immunol. 1995;7:448–455. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 59.Rowland-Jones S L, Nixon D F, Aldhous M C, Gotch F, Ariyoshi K, Hallam N, Kroll J S, Froebel K, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 60.Schmitz J E, Kuroda M J, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reinmann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 61.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V S. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 62.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. T-cell help for cytotoxic T lymphocyte is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 63.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J, Oseroff C, Yuan L, Ruppert J, Sidney J, del Guercio M-F, Southwood S, Kubo R T, Chesnut R W, Grey H M, Chisari F V. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1995;153:5586–5592. [PubMed] [Google Scholar]
- 65.Stanley S K, Ostrowski M A, Justement J S, Gantt K, Hedayati S, Mannix M, Roche K, Schwartzentruber D J, Fox C H, Fauci A S. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 66.Ten Haaft P, Verstrepen B, Überla K, Rosenwirth B, Heeney J. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J Virol. 1998;72:10281–10285. doi: 10.1128/jvi.72.12.10281-10285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 68.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternack M S, Luster A D. Beta-chemokines are released from HIV-1-specific cytolytic T cell granules complexed to proteoglycans. Nature. 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 69.Walker B D, Chakrabarti S, Moss B, Paradis T J, Flynn T, Durno A G, Blumberg R S, Kaplan J C, Hirsch M S, Schooley R T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe N, McAdam S N, Boyson J E, Piekarczyk M S, Yasutomi Y, Watkins D I, Letvin N L. A simian immunodeficiency virus envelope V3 cytotoxic T-lymphocyte epitope in rhesus monkeys and its restricting major histocompatibility complex class I molecule Mamu-A*02. J Virol. 1994;68:6690–6696. doi: 10.1128/jvi.68.10.6690-6696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watkins, D. I., et al. Unpublished data.
- 72.Weber J, Cheisong P R, Callow D, Adams S, Patou G, Hodgkin K, Martin S, Gotch F, Kingsman A. Immunogenicity of the yeast recombinant p17/p24:Ty virus-like particles (p24-VLP) in healthy volunteers. Vaccine. 1995;13:831–834. doi: 10.1016/0264-410x(94)00061-q. [DOI] [PubMed] [Google Scholar]
- 73.Wodarz D, Lloyd A L, Jansen V A, Nowak M A. Dynamics of macrophage and T cell infection by HIV. J Ther Biol. 1999;196:101–113. doi: 10.1006/jtbi.1998.0816. [DOI] [PubMed] [Google Scholar]
- 74.Yang O O, Kalams S A, Trocha A, Cao H, Luster A, Johnson R P, Walker B D. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yasutomi Y, Koenig S, Woods R M, Madsen J, Wassef N M, Alving C R, Klein H J, Nolan T E, Boots L J, Kessler J A, Emini E A, Conley A J, Letvin N L. A vaccine-elicited, single viral epitope-specific cytotoxic T lymphocyte response does not protect against intravenous, cell-free simian immunodeficiency virus challenge. J Virol. 1995;69:2279–2284. doi: 10.1128/jvi.69.4.2279-2284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasutomi Y, McAdam S N, Boyson J E, Piekarczyk M S, Watkins D I, Letvin N L. An MHC class I B locus allele-restricted simian immunodeficiency virus envelope CTL epitope on rhesus monkeys. J Immunol. 1995;154:2516–2522. [PubMed] [Google Scholar]
- 77.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]