Key Points
Question
What are efficacy and safety profiles associated with proton therapy vs photon therapy for esophageal cancer?
Findings
In this meta-analysis including 45 studies, proton therapy was associated with significantly reduced irradiation doses to organs at risk, and the incidence of grade 2 or higher radiation pneumonitis and pericardial effusion and grade 4 or higher lymphocytopenia. Photon therapy was associated with poor OS and PFS compared with proton therapy.
Meaning
These findings suggest that proton therapy may be more effective and safer than photon therapy for patients with esophageal cancer.
This meta-analysis evaluates whether proton therapy is associated with better efficacy and safety outcomes, including dosimetry, prognosis, and toxic effects, compared with photon therapy in patients with esophageal cancer.
Abstract
Importance
Radiotherapy plays an important role in the treatment of esophageal cancer. Proton therapy has unique physical properties and higher relative biological effectiveness. However, whether proton therapy has greater benefit than photon therapy is still unclear.
Objective
To evaluate whether proton was associated with better efficacy and safety outcomes, including dosimetric, prognosis, and toxic effects outcomes, compared with photon therapy and to evaluate the efficacy and safety of proton therapy singly.
Data Sources
A systematic search of PubMed, Embase, the Cochrane Library, Web of Science, SinoMed, and China National Knowledge Infrastructure databases was conducted for articles published through November 25, 2021, and updated to March 25, 2023.
Study Selection
For the comparison of proton and photon therapy, studies including dosimetric, prognosis, and associated toxic effects outcomes were included. The separate evaluation of proton therapy evaluated the same metrics.
Data Extraction and Synthesis
Data on study design, individual characteristics, and outcomes were extracted. If I2 was greater than 50%, the random-effects model was selected. This meta-analysis is reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Main Outcomes and Measures
The main outcomes were organs at risk (OARs) dosimetric outcomes, prognosis (overall survival [OS], progression-free survival [PFS], and objective response rate [ORR]), and radiation-related toxic effects.
Results
A total of 45 studies were included in the meta-analysis. For dosimetric analysis, proton therapy was associated with significantly reduced OARs dose. Meta-analysis showed that photon therapy was associated with poor OS (hazard ratio [HR], 1.31; 95% CI, 1.07-1.61; I2 = 11%), but no difference in PFS was observed. Subgroup analysis showed worse OS (HR, 1.42; 95% CI, 1.14-1.78; I2 = 34%) and PFS (HR, 1.48; 95% CI, 1.06-2.08; I2 = 7%) in the radical therapy group with photon therapy. The pathological complete response rate was similar between groups. Proton therapy was associated with significantly decreased grade 2 or higher radiation pneumonitis and pericardial effusion, and grade 4 or higher lymphocytopenia. Single-rate analysis of proton therapy found 89% OS and 65% PFS at 1 year, 71% OS and 56% PFS at 2 years, 63% OS and 48% PFS at 3 years, and 56% OS and 42% PFS at 5 years. The incidence of grade 2 or higher radiation esophagitis was 50%, grade 2 or higher radiation pneumonitis was 2%, grade 2 or higher pleural effusion was 4%, grade 2 or higher pericardial effusion was 3%, grade 3 or higher radiation esophagitis was 8%, and grade 4 or higher lymphocytopenia was 17%.
Conclusions and Relevance
In this meta-analysis, proton therapy was associated with reduced OARs doses and toxic effects and improved prognosis compared with photon therapy for esophageal cancer, but caution is warranted. In the future, these findings should be further validated in randomized clinical trials.
Introduction
Esophageal cancer is one of the most common malignant tumors of the digestive tract, is the sixth leading cause of cancer-related death worldwide, and represents a tremendous challenge to global health.1 As there are no obvious positive symptoms in the early stage among patients with typical progressive dysphagia or other symptoms, the tumor mostly progresses to the middle-advanced stage, with low survival rates and poor 5-year overall survival (OS) (<20%).2,3 For patients with surgically resectable disease with a local progress stage, neoadjuvant chemoradiotherapy followed by surgery is the standard treatment regimen. For patients with inoperable disease, radical concurrent chemoradiotherapy is the priority recommended treatment.1,3 Theoretically, the local control rate is related to the irradiation dose of tumor target area, and the presence of radiation-related toxic effects limits the further improvement of the prescription dose and affects the patient’s quality of life and prognosis.
The unique physical properties of proton therapy (ie, Bragg peak) allow the radiation dose with the maximum concentration on the tumor target area, thus effectively reducing the dose to organs at risk (OARs) and the incidence of radiation damage.4 In addition, the higher relative biological effectiveness of proton therapy might have the potential to improve survival outcomes further. Unfortunately, a randomized clinical trial (RCT) in patients with non–small-cell lung cancer (NSCLC) did not observe a reduction in the incidence of radiation pneumonitis (RP) with proton therapy compared with intensity-modulated radiotherapy (IMRT).5 Previous studies have also reported that proton therapy can improve local control and disease-free survival in patients with NSCLC.6,7 However, to our knowledge, there is no definitive evidence to support the efficacy and safety of proton therapy in esophageal cancer to support clinical decision-making.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. This study has been prospectively registered at PROSPERO (CRD42021293449).
Search Strategy
Electronic searches were conducted on PubMed, Embase, the Cochrane Library, Web of Science, SinoMed, and China National Knowledge Infrastructure for eligible studies published before November 25, 2021. We also conducted a forward search of included studies until March 25, 2023. The search strategy conducted in PubMed, Embase, and Cochrane combined Medical Subject Headings and free-text words. A similar search strategy was performed in Embase but transformed according to the database’s subject headings. Keywords searches were used for the remaining other databases. Moreover, the reference lists from relevant studies were manually searched for potentially eligible articles. The complete search strategies for each database are available in the eAppendix in Supplement 1).
Inclusion and Excluded Criteria
This study had 2 objectives. The first objective was to evaluate whether proton therapy was beneficial compared with photon therapy (including the dosimetric, prognosis, and toxic effects outcomes). The second objective was to assess the efficacy and safety of proton therapy using single-rate pooled analysis.
For the comparison of proton and photon therapy, inclusion criteria included for efficacy evaluation metrics were studies reporting objective response rate (ORR), OS, or progress-free survival (PFS). Safety metrics were cardiopulmonary toxic effects, myelotoxic effects, and esophageal toxic effects. For the dosimetric comparison analysis, the OARs were the lungs, heart (including substructures), spinal cord (SC), and bone marrow. If multiple proton techniques were available simultaneously in 1 study, the technique with the greatest comprehensive dose benefit to OARs was preferred. For the separate evaluation of proton therapy, the efficacy and safety evaluation metrics were same.
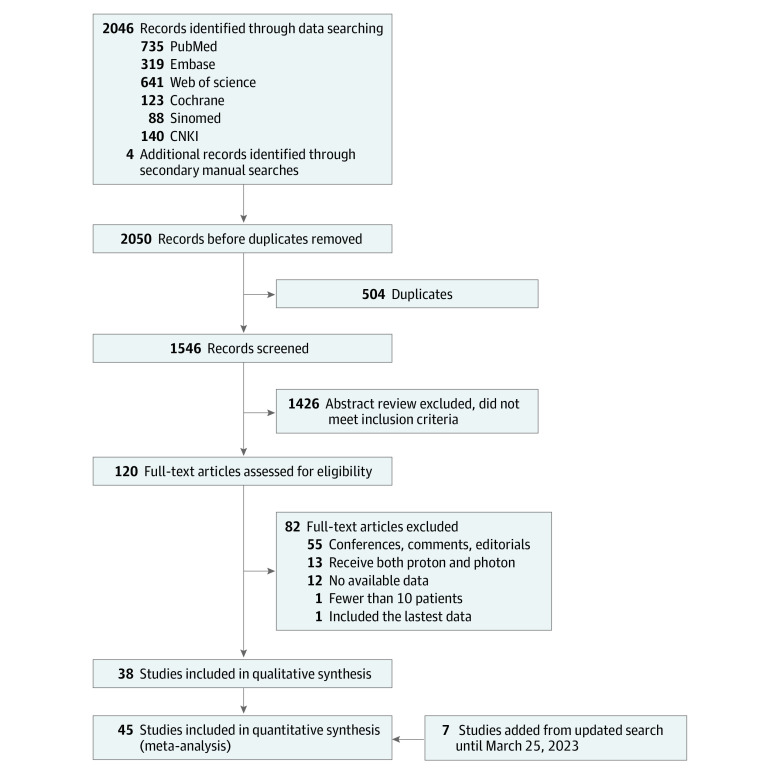
Finally, all studies had to include at least 10 patients. Studies with mixed-disease assessment were not included unless data related to esophageal cancer could be extracted separately. Additionally, studies among patients who had received radiotherapy previously were excluded, nor could patients receive proton and photon therapy simultaneously, except for salvage-therapy after recurrence. We only included the most recently published publications from the same cohort for the same metric. Editorials, letters, comments, case reports, reviews, meta-analyses, and conference abstracts were excluded (Figure 1).
Figure 1. Flowchart of the Study Screening and Evaluation Process.
Data Collection
From all included studies, we collected basic information, including author, year of publication, the number of patients, and patient characteristics, including target location, radiotherapy techniques, and prescription dose. For dosimetric comparison studies, also including OARs dose-limitation regimens, we collected the mean percentage benefit of OARs dosimetric parameters and planning quality. For efficacy and safety evaluation studies, we collected patient characteristics and also included age, study type, stage, pathology type, radiotherapy modalities (neoadjuvant or radical), chemotherapy regimens, median follow-up time, prognosis, and toxic effects. Toxic effects reported within 3 months after the end of radiotherapy were defined as acute toxic effects; all others were considered late toxic effects. If the data were only presented by graphs, Graph Digitizer software version 2.25 (GetData) was used to extract numerical values. Not all dosimetric comparison studies provided means with SDs, but if studies described median, range, or IQR, we used the methods proposed by Wan et al8 to estimate means. In terms of prognosis analysis, the OS and PFS were measured by hazard ratios (HRs) and 95% CIs that were directly reported in the included studies. When studies did not report HR but presented Kaplan-Meier survival curves instead, we obtained estimated HR from the curves by using a calculation spreadsheet developed by Tierney and colleagues.9
Quality Assessment
For quality assessments, we used the Newcastle-Ottawa Scale for non-RCT studies, Methodological Index for Nonrandomized Studies for single-group studies, and the Cochrane Collaboration tool for RCTs. Two authors then collaborated to identify eligible studies (Y.D. and T.L.), collect data (P.Z. and Y.Z.), and assess quality (M.Z. and W.T.). Any disagreement was resolved by discussion or the third reviewer (T.W. or Z.X.).
Statistical Analysis
For proton therapy single-rate analysis, we calculated the single ratios and integrated ratios with 95% CIs. Furthermore, if the distribution type of a single rate did not conform to a normal distribution, it was transformed using double-arcsine transformations to improve the reliability of the combined results. Heterogeneity was assessed by I2 statistic, with I2 greater than 50% considered significant. Random-effects models were more applicable to mitigate heterogeneity than fixed-effects models, so if I2 was greater than 50%, the random-effects model was selected. Publication biases were assessed using visual inspection of funnel plots and quantitatively assessed using Egger test (when there were ≥10 included studies). A nonparametric trim-and-fill method was also applied to minimize the influence of publication bias on the results. Sensitivity analysis was performed by deleting each study individually. All analysis were conducted in RevMan version 5.4 (Cochrane) or Stata version 17.0 (StataCorp). P values were 2-sided, and P < .05 was considered significant.
Results
A total of 45 studies met the inclusion criteria and were included in the meta-analysis.4,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53 Figure 1 describes the selection process and the reasons for excluding studies. Among the included studies, 31 studies10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,27,28,29,31,34,35,37,38,46,47,49,50,51,52 included dosimetric comparison (eTable 1 in Supplement 1), 17 studies25,26,27,28,29,30,31,32,33,34,35,36,37,38,46,48,49 assessed proton vs photon therapy (Table 1), and 10 studies4,16,39,40,41,42,43,44,45,53 were single-group studies of proton therapy (Table 2). Three studies32,33,36 used propensity score–matched analysis to control for confounding factors, and results from those analyses were used for data synthesis. Literature quality evaluations are presented in eTables 2-5 in Supplement 1.
Table 1. Description of Prognosis and Toxic Effects After Treatment With Proton or Photon Radiotherapy in Patients With Esophageal Cancer.
| Source | Patients, No. | Study Type | Characteristics (proton vs photon) | RT technology/prescription dose (proton vs photon) | Follow-up, median, mo | Outcome (proton vs photon) | Toxic effects | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proton | Photon | Age, median, y | Stage | Thoracic site | Pathological type | ||||||
| Suh et al,25 2021 | 48 | 29 | RO | 69 vs 73 | cT1-3N0M0 | Lower: 58% vs 28%a | SCC: 100% |
|
46 |
|
|
| Ebrahimi et al,26 2021 | 30 | 15 | RO | NA | NA | NA | NA |
|
NA | NA | Mean minimum ALC: 390/330/μL vs 170/μL; change in ALC: 970/1080/μL vs 1250/μL |
| Sumiya et al,27 2021 | 54 | 15 | RO | 70 | I-IV (III-IV: 41% vs 80%)a | Middle and lower: 80% vs 67% | SCC: 96% vs 93% |
|
NA |
|
|
| Lin et al,28 2020 | 46 | 61 | RCT (II) | 67 vs 67 | I-III (III: 59% vs 54%) | Lower: 83% vs 84% | AC: 91% vs 87% |
|
44.1 |
|
|
| Wang et al,29 2020 | 159 | 320 | PO | 62 | I-III | Lower: 89% | AC: 87.1% |
|
76 | NA |
|
| DeCesaris et al,30 2020 | 18 | 36 | RO | 62 | II-IV (II: 33% vs 61%)a | Middle and lower: 100% | AC: 100% |
|
25 |
|
|
| Bhangoo et al,31 2020 | 32 | 32 | RO | 71.5 vs 71.4 | T1-3N0-3M0 (T3: 63% vs 56%) | Lower: 78% vs 94% | AC: 63% vs 91%a |
|
10 vs 14 |
|
|
| Routman et al,32 2019 | 50 | 50c | RO | 66 vs 64.5 | I-IV (III-IV: 61% vs 37%)a | Lower: 88% vs 90% | AC: 88% vs 86% |
|
NA | NA | Acute: G≥4 lymphocytopenia, 24.0% vs 60.0%a |
| Shiraishi et al,33 2018 | 136 | 136c | RO | 63 vs 60 | I-IV (III-IV: 64% vs 60%) | Lower: 96% vs 97% | AC: 96% vs 98% |
|
NA | NA | Acute: G≥4 lymphocytopenia, 17.6% vs 40.4%a |
| Macomber et al,34 2018 | 16 | 39 | RO | 62 | II-III (III: 56% vs 70%) | Lower: 78% vs 84% | AC: 94% vs 76%a |
|
20 |
|
NA |
| Xi et al,35 2017 | 132 | 211 | RO | ≥67: 71% vs 38%a | I-III (III: 64% vs 67%) | Lower: 71% vs 73% | AC: 68% vs 74% |
|
44.8 vs 65.1 |
|
|
| Fang et al,36 2018 | 110 | 110c | RO | 70 vs 69 | I-IV (III-IV: 61% vs 60%) | Lower: 76.4% vs 76.4% | AC: 72% vs 76% |
|
NA |
|
Acute: G≥4 lymphocytopenia, 30.9% vs 47.3%a |
| Lin et al,37 2017 | 111 | 469 | RO | >65: 32% vs 36%/ 26% | III-IV: 64% vs 63%/64% | Lower: 98% vs 88%/95%a; | AC: 96% vs 90%/94%; |
|
NA | NA |
|
| Makishima et al,38 2015 | 25 | 19 | PO | NA | 0-III (III: 36% vs 74%)a | 88% vs 63% | SCC: 100% |
|
24 vs 20 | NA |
|
| Zhu et al,46 2021 | 246 | 500 | RO | 65 vs 62a | I-III (III: 65% vs 64%) | Lower: 88% vs 86% | NA |
|
NA | NA | Acute: G≥4 lymphocytopenia, 22.0% vs 46.2%a |
| Lin et al,48 2022 | 81 | 156 | RO | 61 | I-V (III-IV: 34% vs 66%) | NA | NA |
|
NA | NA | LT, 14.8% vs 19.9%; HET, 7.4% vs 9.6% |
| Choi et al,49 2022 | 15 | 16 | RO | Age: 62.3 vs 59.4 | T1-4N1-3M0 (T3: 73% vs 44%) | Middle and lower: 73% vs 69%; | SCC |
|
17 |
|
Acute: G≥4 lymphocytopenia, 12.5% vs 20% |
Abbreviations: AC, adenocarcinoma; ALC, absolute lymphocyte count; CCRT, concurrent chemoradiotherapy; CRT, chemoradiotherapy; CT, computed tomography; D, dimensional; F, fraction; FU, fluoropyrimidine; G, grade; HET, heart toxic effects; HT, hematological toxic effects; IMPT, intensity modulate proton therapy; LT, lung toxic effects; NA, not available or not applicable; OS, overall survival; PBT, proton beam therapy; PCE, pericardial effusion; pCR, pathologic complete response; PE, pleural effusion; PFS, progression-free survival; PO, prospective; PRT, photon radiotherapy; PSPT, passive scattering proton therapy; PTV, planning target volume; RCT, randomized clinical trial; RE, radiation esophagitis; RO, retrospective; RP, radiation pneumonitis; RT, radiotherapy; SCC, squamous cell carcinoma.
Statistically significant at P < .05.
Data obtained from graphic.
Propensity-matched analysis.
Table 2. Evaluation of Efficacy and Safety of Proton Therapy for Esophageal Cancer.
| Reference | Patients | Study type | Characteristics | Treatment Strategies | Follow-up, median, mo | Prognosis | Toxic effects | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, median, y | Stage, patients, No. | Thoracic site, patients, No. | Pathological type | |||||||
| Echeverria et al,4 2013 | 100 | RO | 66 | I, 3; II, 30; III, 51; IV, 7 | Upper, 2; middle, 16; lower, 82 | AC, 83% |
|
NA | NA | Acute: RP: G≥2, 27; G≥3, 7; G≥4, 0 |
| Hirano et al,16 2018 | 27 (37) | RO | 70 | III (100%) | Upper, 5; middle, 9; lower, 13 | SCC |
|
14.8 | 1-y OS: 91%; 1-y PFS: 41% |
|
| Parzen et al,39 2021 | 155 | PO | 68.2 | I-IV (III-IV, 54%) | NA | AC, 77% |
|
NA | NA |
|
| Ogawa et al,40 2021 | 60 (103) | RO | 69 | I, 10; II, 19; III, 31 | Upper, 15; middle, 31; lower, 14 | SCC |
|
51.5 |
|
|
| Sato et al,41 2020 | 44 (46) | RO | 70 | I, 24; II, 15; III, 2; IV, 3 | Upper, 8; middle, 26; lower, 10 | SCC |
|
31 |
|
|
| Prayongrat et al,42 2017 | 19 (32) | RO | 73 | I-IV (III-IV 52.6%) | Upper, 3; middle, 4; lower, 12 | AC, 63.2% |
|
17 |
|
|
| Zeng et al,43 2016 | 13 | PO | 70 | T3-4N0-2M0 | Lower, 11 | AC, 85% |
|
11 | OS/PFS not reached; pCR, 3 |
|
| Ishikawa et al,44 2015 | 40 | RO | 69 | I, 16; II, 9; III, 15 | Upper, 12; middle, 21; lower, 7 | NA |
|
24 |
|
|
| Lin et al,45 2012 | 62 | PO | 68 | I-IV (III-IV: 65%) | Upper, 3; middle, 11; lower, 48 | AC, 75.8% |
|
20.1 |
|
|
| Rutenburg et al,53 2023 | 17 | RO | 67.4 | T2-4N0-3M0 | Upper, 1; middle, 6; lower, 10 | AC, 88% |
|
2.1 |
|
|
Abbreviations: 5-FU, fluorouracil; AC, adenocarcinoma; CCPT, concurrent chemoproton therapy; CR, complete response; F, fraction; G, grade; HET, heart toxic effects; HT, hematological toxic effects; IMPT, intensity-modulated proton therapy; LT, lung toxic effects; OS, overall survival; PCE, pericardial effusion; pCR, pathologic complete response; PFS, progression-free survival; PO, prospectively; pPR, pathologic partial response rate; PR, partial response; PSPT, passive scattering proton therapy; PTV, planning target volume; RE, radiation esophagitis; RO, retrospective; RP, radiation pneumonitis; SCC, squamous cell carcinoma; SFUD, single-field uniform dose.
Obtained from picture.
Dosimetric Comparison Analysis
We found 25 articles10,11,12,13,14,15,16,19,20,21,22,23,24,25,28,31,35,37,38,46,47,49,50,51,52 assessing the lungs for OARS dosimetric parameters and 26 studies10,11,12,13,14,15,16,17,19,20,21,22,24,25,28,29,31,34,35,37,38,46,47,49,51,52 assessing the heart for dosimetric parameters, but some of them had no sufficient data for analysis. Pooled analysis found that proton therapy was associated with significantly reduced the mean dose to the lungs: compared with photon therapy, 24.8% less lung volume received 5 Gy or more, 12.9% less lung volume received 10 Gy or more, 6.5% less lung volume received 20 Gy or more, and 3.1% less lung volume received 30 Gy or more, with an overall mean dose reduction of 4.4 Gy. Pooled analysis showed that the proton therapy was associated with significantly reduced the mean dose to the heart: compared with photon therapy, 45.1% less heart volume received 5 Gy or more, 39.5% less heart volume received 10 Gy or more, 26.6% less heart volume received 20 Gy or more, 13.9% less heart volume received 30 Gy or more, and 7.4% less heart volume received 40 Gy or more, with an overall mean dose reduction of 9.5 Gy. In pooled analysis of cardiac substructures, results suggested that the proton therapy was associated with significantly reduced the mean doses to the left ventricle (by 10.2 Gy),13,17,20 and left anterior descending artery (by 11.2 Gy).13,17,20. Three studies11,18,21 described the bone marrow irradiation dose, although there was inconsistency in the definition of bone marrow. Pooled analysis found that proton therapy was associated with significantly reduced mean dose to the bone marrow: 10.2% less bone marrow volume received 10 Gy or more and 10.3% less bone marrow volume received 20 Gy or more, with a mean dose reduction of 2.9 Gy. Proton therapy was associated with significantly reduced the maximum dose to the SC, with a mean reduction of 6.9 Gy.11,12,14,16,20,21,22,23,24,28,51,52 All pooled analysis results are presented in eFigure 1 and eTable 6 in Supplement 1.
Most of the included studies did not show significant compromise in planning quality. Sensitivity analysis tests did not detect any changed results, indicating that the results were reliable. Publication bias tests were performed and found a publication bias analyses of volume of heart tissue receiving 30 or more Gy13,14,16,17,20,21,29,31,35,51,52 and 40 or more Gy,14,15,16,17,20,21,22,35,51,52 but there was no significant difference observed after the trim-and-fill method (eFigure 2 and eTable 6 in Supplement 1).
Efficacy and Safety of Proton vs Photon Therapy
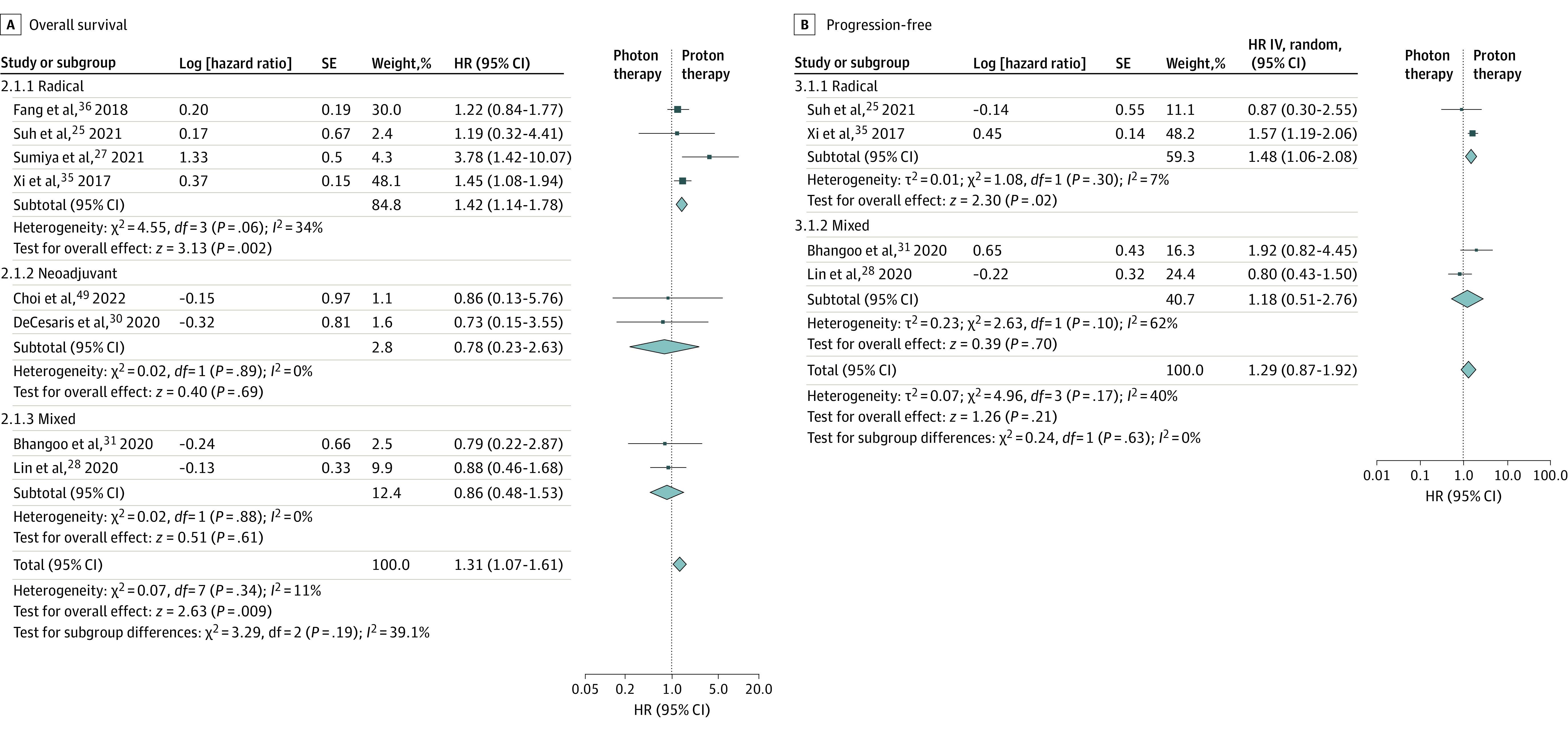
We found 8 studies25,27,28,30,31,35,36,49 that reported OS (including 510 patients receiving photon therapy and 455 patients receiving proton therapy) and 4 studies that reported PFS25,28,31,35 (including 333 patients receiving photon therapy and 258 patients receiving proton therapy). Four studies25,27,35,36 used radical therapy, 2 studies30,49 used neoadjuvant therapy, and 2 studies28,31 used mixed therapies. Pooled analysis of OS found worse OS for photon vs proton therapy for the radical group (HR, 1.42; 95% CI, 1.14-1.78, I2 = 34%), no significant difference for the neoadjuvant group (HR, 0.78; 95% CI, 0.23-2.63; I2 = 0%), and no significant difference for the mixed therapy group (HR, 0.86; 95% CI, 0.48-1.53; I2 = 0%). Overall OS was improved in the proton group compared with the photon group (HR, 1.31; 95% CI, 1.07-1.61; I2 = 11%) (Figure 2A).25,27,28,30,31,35,36,49 Pooled analysis of PFS in the photon vs proton group found worse PFS for the radical therapy group (HR, 1.48; 95% CI, 1.06-2.08; I2 = 7%) but no significant difference in the mixed therapy group (HR, 1.18; 95% CI, 0.51-2.76; I2 = 62%) or overall (HR, 1.29; 95% CI, 0.87-1.92; I2 = 40%) (Figure 2B).25,28,31,35 For ORR, only 5 studies28,30,31,34,49 reported pathological complete response (pCR), and pooled analysis found no significant difference (odds ratio [OR], 0.90; 95% CI, 0.49-1.69; I2 = 0) between proton vs photon therapy (eFigure 3 in Supplement 1). The sensitivity analysis found that the overall results changed for OS when the study by Xi et al35 was removed (HR, 1.20; 95% CI, 0.91-1.60) and for PFS when study by Lin et al28 was removed (HR, 1.55; 95% CI, 1.20-1.99).
Figure 2. Pooled Analysis of the Overall Survival and Progression-Free Survival in Photon Therapy vs Proton Therapy.
Some included studies did not distinguish toxic effects by grade or by acute vs late toxic effects, and the overall cardiopulmonary toxic effects descriptions were inconsistent and incomplete. Thus, only the commonly described toxic effects, such as RP, pleural effusion (PE), pericardial effusion (PCE), radiation esophagitis (RE), and lymphocytopenia were included in the meta-analysis. Pooled analysis of proton vs photon therapy found significant differences in odds of grade 2 or higher RP (OR, 0.40; 95% CI, 0.17-0.97; I2 = 0%), PE (OR, 0.73; 95% CI, 0.32-1.65; I2 = 35%), or PCE (OR, 0.20; 95% CI, 0.04-0.96; I2 = 44%); results were similar for grade 3 or higher RP (OR, 0.45; 95% CI, 0.12-1.67; I2 = 0%), PE (OR, 0.28; 95% CI, 0.06-1.32; I2 = 35%), and PCE (OR, 0.34; 95% CI, 0.06-2.06; I2 = 0%) .25,27,28,35,38 Pooled analysis of RE showed no significant difference between proton and photon therapy in odds of grade 2 or higher RE (OR, 1.11; 95% CI, 0.76-1.63; I2 = 0%) or for grade 3 or higher RE (OR, 0.84; 95% CI, 0.48-1.46; I2 = 0%).28,30,31,35 Pooled analysis found that proton therapy was associated with significantly lower odds of grade 4 or higher lymphocytopenia compared with photon therapy (OR, 0.35; 95% CI, 0.28-0.44; I2 = 0%).28,31,32,33,36,46,49 In contrast, the incidence of other grade 4 or higher toxic effects was too small for pooled analyses to be meaningful. Pooled analyses results are presented in eFigure 4 and eTable 7 in Supplement 1. Sensitivity analysis found that the results of grade 2 or higher RP and grade 2 or higher PCE changed after the elimination 1 of the studies, while no significant changes were observed in other toxic effects.
Efficacy and Safety of Proton Therapy
In analyses restricted to proton therapy, we found 15 studies16,25,27,28,30,31,35,36,40,41,42,44,45,49,53 that reported 1-year OS, 13 studies25,27,28,30,31,35,36,40,41,44,45,49,53 that reported 2-year OS, 11 studies25,27,28,35,36,40,41,44,45,49,53 that reported 3-year OS, and 7 studies25,27,28,35,36,40,44 that reported 5-year OS. Pooled analysis found that that the 1-year OS was 89% (95% CI, 84%-93%; I2 = 69.3%), 2-year OS was 71% (95% CI, 63%-78%; I2 = 75.5%), 3-year OS was 63% (95% CI, 53%-73%; I2 = 81.9%), and 5-year OS was 56% (95% CI, 46%-67%; I2 = 82%). In analyses of PFS for proton therapy, we found 6 studies16,25,28,31,35,40 that reported 1-year PFS, 5 studies,25,28,31,35,40 that reported 2-year PFS, 4 studies25,28,35,40 that reported 3-year PFS, and 4 studies25,28,35,40 that reported 5-year PFS. Pooled analysis found that the 1-year PFS was 65% (95% CI, 53%-77%; I2 = 83.3%), 2-year PFS was 56% (95% CI, 48%-64%; I2 = 52.1%), 3-year PFS was 48% (95% CI, 40%-56%; I2 = 45%), and 5-year PFS was 42% (95% CI, 34%-51%; I2 = 54.2%). Subgroups analysis by different treatment modalities (ie, neoadjuvant, radical, or mixed) and stage (ie, III-IV<50% or ≥50%) exhibited considerable differences in OS (eFigure 5 in Supplement 1). There may have been significant differences in PFS as well, but there were not enough data for analyses. For ORR, only 4 studies40,41,42,44 reported CR and 7 studies28,30,31,34,43,45,49 reported pCR. Pooled analysis showed that the CR was 84% (95% CI, 69%-95%; I2 = 80%) (eFigure 6 in Supplement 1) and the pCR was 31% (95% CI, 18%-44%; I2 = 56.8%) (eFigure 7 in Supplement 1). All pooled analysis results are presented in Table 3. No publication bias was detected by funnel plot analysis.
Table 3. Single-Rate Pooled Analysis of OS and PFS for Proton Therapy.
| Follow-up, y | Treatment modality | Stage | Overall | Publication bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neoadjuvant, HR (95% CI) | Radical | Mixeda | III-IV (<50%) | III-IV (≥50%) | |||||||||
| HR (95% CI) | I2, % | HR (95% CI) | I2, % | HR (95% CI) | I2, % | HR (95% CI) | I2, % | HR (95% CI) | I2, % | ||||
| OS | |||||||||||||
| 1 | 0.82 (0.66-0.94)b | 0.92 (0.85-0.97) | 78.1 | 0.84 (0.77-0.90) | 8.4 | 0.96 (0.88-1.00) | 72.1 | 0.85 (0.80-0.90) | 45.4 | 0.89 (0.84-0.93) | 69.3 | NAc | |
| 2 | 0.70 (0.52-0.85)b | 0.77 (0.67-0.85) | 79.7 | 0.58 (0.47-0.68) | 42.6 | 0.85 (0.74-0.93) | 68.8 | 0.64 (0.59-0.68) | 6.6 | 0.71 (0.63-0.78) | 75.5 | NAc | |
| 3 | NAd | 0.68 (0.55-0.80) | 87.1 | 0.50 (0.41-0.59)b | 0.78 (0.63-0.91) | 80.8 | 0.53 (0.48-0.58) | 0 | 0.63 (0.53-0.73) | 81.9 | NAc | ||
| 5 | NAe | 0.58 (0.46-0.70) | 84.6 | NAd | 0.71 (0.64-0.79) | 0 | 0.45 (0.39-0.50) | 0 | 0.56 (0.46-0.67) | 82 | Nonef | ||
| PFS | |||||||||||||
| 1 | NAe | 0.65 (0.48-0.82) | 88.9 | 0.64 (0.49-0.79) | 50.5 | NAd | 0.61 (0.53-0.68) | 45.4 | 0.65 (0.53-0.77) | 83.3 | Nonef | ||
| 2 | NAe | 0.57 (0.45-0.70) | 73.8 | 0.54 (0.43-0.65) | 0 | NAd | 0.52 (0.46-0.57) | 0 | 0.56 (0.48-0.64) | 52.1 | Nonef | ||
| 3 | NAe | 0.49 (0.38-0.60)b | NAd | NA | NAd | 0.44 (0.38-0.51) | 85.4 | 0.48 (0.40-0.56) | 45.0 | NAc | |||
| 5 | NAe | 0.43 (0.31-0.55)b | NAd | NA | NAd | 0.38 (0.32-0.44)b | 0.42 (0.34-0.51) | 54.2 | NAc | ||||
Abbreviations: NA, not applicable or not evaluable; OS, overall survival; PFS, progression-free survival.
Mixed indicates both neoadjuvant and radical treatments were used.
Included 3 studies or fewer (after double-arcsine transformations), so the I2 is not shown.
After double-arcsine transformations (did not perform publication bias test).
Represents only 1 study.
Represents no study.
Represents publication bias detected by funnel plot (because included <10 studies).
Eight studies reported grade 2 or higher RE,30,31,35,42,43,44,45,53 10 studies reported grade 2 or higher RP,4,16,27,28,35,38,40,42,43,45 4 studies reported grade 2 or higher PE,27,35,38,42 and 7 studies16,27,35,38,42,44 reported grade 2 or higher PCE for proton therapy. Pooled analysis showed that the grade 2 or higher incidence of RE was 50% (95% CI, 40%-59%; I2 = 60.8%), RP was 2% (95% CI, 0%-6%; I2 = 83.4%), PE was 4% (95% CI, 2%-7%; I2 = 0%), and PCE was 3% (95% CI, 0%-7%; I2 = 62.7%) in the proton therapy group. The included studies reporting grade 3 or higher and grade 4 or higher toxic effects occurred rarely; thus, we did not perform a pooled analysis for these, except for grade 3 or higher RE16,28,31,35,39,41,42,43,44,45,53 (incidence, 8%; 95% CI, 5%-12%; I2 = 57.1%) and grade 4 or higher lymphopenia28,31,32,33,36,39,46,49 (incidence, 17%; 95% CI, 7%-30%; I2 = 93.8%) in proton therapy (eTable 8 in Supplement 1).
Discussion
This meta-analysis is the first study to our knowledge to evaluate the efficacy and safety of proton therapy vs photon therapy for esophageal cancer; we also assessed proton therapy separately. Currently, radiotherapy for esophageal cancer remains based on photon therapy, while advanced proton therapy offers higher target conformability and low doses to OARs. Furthermore, the difference in dosimetric benefit between proton therapy and different photon techniques (eg, IMRT, volumetric modulated arc therapy) and whether the benefit translates into clinical benefit are unknown. Previous studies enrolled the patients who underwent both proton and photon therapy or did not perform a separate analysis of proton therapy.54,55,56
For the dosimetric parameters, the meta-analysis showed that proton therapy was associated with significantly reduced irradiation doses in the lungs, heart, SC, and bone marrow. Prior studies have shown that coronary and ventricular irradiation doses were associated with coronary artery disease and chronic heart failure.13,57 We performed a pooled analysis of the cardiac substructure, and we found that the mean doses to the left ventricle and left anterior descending artery were also significantly decreased. Although the bone marrow was defined inconsistently between studies, such as the thoracic vertebral body (from T1-T12 or C2-L1) or containing the ribs and sternum.11,18,27 However, no clear criteria are available. In addition, we did not distinguish proton techniques, and studies have shown that intensity-modulated proton therapy has higher target-area conformability and lower cardiopulmonary radiation dose than passive-scattering proton therapy.17,58 Gjyshi et al59 demonstrated that intensity-modulated proton therapy significantly reduced grade 3 or higher cardiopulmonary toxic effects in patients with NSCLC compared with passive-scattering proton therapy.
Meta-analysis showed that photon therapy was associated with poor OS compared with proton therapy, and the subgroup analysis showed similar results in the radical group, but these results were not observed in the mixed group. Analogously, the subgroup meta-analysis showed that photon therapy, compared with proton therapy, also was associated with poor PFS in the radical group, but no significant differences were observed in the mixed group or overall. Sensitivity analysis suggest that we need to be cautious about these results, which may be related to sample size, follow-up time, patient heterogeneity, chemotherapy regimens, and treatment modalities (radical or neoadjuvant). A 2022 meta-analysis by Zhu et al55 did not find any survival benefit from proton therapy, which may be related to the incomplete included studies and lack of subgroup analysis. Subgroup analysis revealed considerable differences in OS between different stage (III-IV<50% or ≥50%) and treatment modalities (neoadjuvant, radical, or mixed), while differences in PFS were not observed. Subgroup analysis found lower OS in the mixed group (neoadjuvant and radical therapy), which may be related to patient heterogeneity (eg, number of patients, stage, pathological type, tumor location, prescription dose), surgical regimens, timing, and whether salvage surgery was performed. Overall, both acute and late toxic effects were relatively low, except for grade 2 or higher RE, grade 2 or higher PCE, and grade 4 or higher lymphocytopenia. Carbon ion therapy has a similar dosimetric distribution with proton therapy and a higher relative biological effectiveness, but there is a paucity of data for esophageal cancer.56
This meta-analysis also found that proton therapy was associated with significantly decreased the overall incidence of grade 2 or higher RP and grade 2 or higher PCE compared with photon therapy (regardless of acute or late onset). Proton therapy was also associated with significantly reduced the incidence of acute grade 4 or higher lymphocytopenia. A 2019 study by Xu et al60 found that cardiopulmonary doses are risk factors for radiation-induced toxic effects and are independent risk factors for poor OS. RP is a common and severe toxic effect after radiotherapy in esophageal cancer. According to research results, there is heterogeneity in the distribution of lung function and inconsistent sensitivity to radiation.61 A study by Vinogradskiy et al62 showed that 4-dimensional computed tomography–based ventilation functional lung imaging-guided radiotherapy significantly reduced the incidence of grade 2 or higher RP. A study by Zhou et al63 found that using functional lung protection in esophageal cancer radiotherapy planning was associated with decreasing functional lung mean dose by 1.5 Gy and volume of lung tissue receiving 20 Gy of more by 4.7%, without compromising planning quality. A study by Ieko et al64 integrated functional lung protection into planning and showed that the proton-based functional lung–sparing planning reduced the functional lung dose more than IMRT-based functional lung–sparing planning. Previously, the low survival rate of patients with esophageal cancer and the fact that cardiotoxic effects were considered late toxic effects meant that toxic effects were not emphasized, but relatively high rates of death related to cardiotoxic effects have been reported in the long-term follow-up in patients with breast cancer and Hodgkin lymphoma.65 In recent years, various combinations have improved the prognosis of patients with esophageal cancer. Furthermore, the heart is particularly exposed to high doses, due to its anatomical location adjacent to the esophagus and the high prescription dose. Previous studies have demonstrated that older patients, lower esophageal subsite, and pre-existing cardiac disease are associated with death related to cardiotoxic effects after radiotherapy.29,66 Pericardial effusion is the most frequently observed cardiotoxic effect after radiotherapy, and it is significantly associated with the irradiated dose to the pericardium.65 However, there were insufficient data in the included studies for pooled analysis.
Previous studies have shown that bone marrow radiation dose is associated with hematologic toxic effects, especially in red bone marrow, which is highly sensitive to radiation. A meta-analysis by Zhou et al67 reported that pelvic bone marrow protection planning significantly reduced the incidence of grade 2 or higher hematologic toxic effects in cervical cancer. A 2016 study by Deek et al68 found that thoracic vertebral body dose was associated with hematological toxic effects during radiotherapy in patients with NSCLC. Other studies have shown that cardiac, aortic, spleen, and body doses are also associated with decreased grade 4 or higher lymphocytopenia, presumably associated with the blood (regarded as a moving OAR) circulating lymphocytes in it.69,70 A study by Davuluri et al71 reported that grade 4 or higher lymphocytopenia was significantly associated with OS in patients with esophageal cancer. Lymphocytopenia may be associated with an increased risk of infection and blunted antitumor immune cell response.36 In the future, we need to pay more attention to reducing the incidence of grade 4 or higher lymphocytopenia, especially when radiotherapy is combined with immunotherapy.
Limitations
There were several limitations in this study. First, there was significant heterogeneity in the OARs dosimetric analysis. The sources of heterogeneity might be patient characteristics (eg, location, planning target volume), radiotherapy modalities, and OAR dose limitations. Second, the small number of patients included in the study may be another limitation. Third, the data descriptions of the included studies were not detailed. For example, mean and SD were unavailable for dosimetric analysis, HR and 95% CI were unavailable for prognosis analysis, and toxic effects analysis did not distinguish grade and categories. The values extracted from the graphs using software might differ slightly from the actual data. Fourth, we did not pay attention to other prognosis metrics (eg, local control rate, distant metastasis–free survival), other toxic effects (eg, vomiting, fatigue), and postoperative complications. Moreover, there were not enough clinical RCTs to evaluate the efficacy and safety of proton and photon therapy.
Conclusions
This meta-analysis indicated that proton therapy was associated with significantly reduced the OARs irradiation dose, and there was a considerable difference between different photon techniques. In addition, proton therapy was associated with significantly improved prognosis and toxic effects in patients with esophageal cancer, but caution is warranted. In the future, it is still necessary to verify the benefits of proton therapy vs photon therapy for esophageal cancer in RCTs.
eAppendix. Literature Search Strategy
eFigure 1. Forest Plots of Dose-Volume Parameters for OARs With Proton vs Photon Therapy
eFigure 2. Funnel Plot of Dose-Volume Parameters for OARs With Proton vs Photon Therapy (≥10 Articles)
eFigure 3. Pooled Analysis of pCR (Proton vs Photon Therapy)
eFigure 4. Forest Plots of the Incidence of Toxic Effects in the Proton and Photon Therapy Groups (Described as OR)
eFigure 5. Example of Single-Rate Pooled Analysis of OS and PFS for Proton Therapy
eFigure 6. Single-Rate Pooled Analysis of CR (Proton Therapy)
eFigure 7. Single-Rate Pooled Analysis of pCR (Proton Therapy)
eTable 1. Detailed Comparison Information on OARs Dosimetric and Planning Quality From Proton and Photon Radiotherapy Planning
eTable 2. Results of Quality Assessment Using the Newcastle-Ottawa Scale for Non-RCT Studies (Dosimetric Parameters)
eTable 3. Results of Quality Assessment Using the Newcastle-Ottawa Scale for Non-RCT Studies (Efficacy and Safety of Proton vs Photon Therapy)
eTable 4. Results of Quality Assessment Using the Cochrane Collaboration’s Tool (RCT)
eTable 5. Results of Quality Assessment Using the MINORS (Single-Group Tests)
eTable 6. Pooled Analysis of Dose-Volume Parameters for OARs with Proton vs Photon Therapy
eTable 7. Pooled Analysis of the Incidence of Toxic Effects in the Proton and Photon Therapy Group (Described as OR)
eTable 8. Single-Rate Pooled Analysis for the Incidence of Toxic Effects in Proton Therapy
Data Sharing Statement
References
- 1.Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. doi: 10.1038/nrdp.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu D, Li G, Li H, Jia F. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(31):e7685. doi: 10.1097/MD.0000000000007685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinese Medical Doctors Association Division of Radiation Oncology Physicians . Chinese esophageal cancer radiotherapy guidelines (2020 version). Guoji Zhongliuxue Zazhi. 2020;47:641-655. doi: 10.3760/cma.j.cn371439-20201015-00095 [DOI] [Google Scholar]
- 4.Echeverria AE, McCurdy M, Castillo R, et al. Proton therapy radiation pneumonitis local dose-response in esophagus cancer patients. Radiother Oncol. 2013;106(1):124-129. doi: 10.1016/j.radonc.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao Z, Lee JJ, Komaki R, et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non–small-cell lung cancer. J Clin Oncol. 2018;36(18):1813-1822. doi: 10.1200/JCO.2017.74.0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for stage I non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(1):107-111. doi: 10.1016/j.ijrobp.2005.10.031 [DOI] [PubMed] [Google Scholar]
- 7.Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest. 1999;116(5):1313-1319. doi: 10.1378/chest.116.5.1313 [DOI] [PubMed] [Google Scholar]
- 8.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribeiro CO, Visser S, Korevaar EW, et al. Towards the clinical implementation of intensity-modulated proton therapy for thoracic indications with moderate motion: robust optimised plan evaluation by means of patient and machine specific information. Radiother Oncol. 2021;157:210-218. doi: 10.1016/j.radonc.2021.01.014 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Jabbour SK, Zhang A, Liu B, Yue NJ, Biswal NC. Proton beam therapy can achieve lower vertebral bone marrow dose than photon beam therapy during chemoradiation therapy of esophageal cancer. Med Dosim. 2021;46(3):229-235. doi: 10.1016/j.meddos.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 12.Anakotta RM, van der Laan HP, Visser S, et al. Weekly robustness evaluation of intensity-modulated proton therapy for oesophageal cancer. Radiother Oncol. 2020;151:66-72. doi: 10.1016/j.radonc.2020.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Celik E, Baus W, Baues C, et al. Volumetric modulated arc therapy versus intensity-modulated proton therapy in neoadjuvant irradiation of locally advanced oesophageal cancer. Radiat Oncol. 2020;15(1):120. doi: 10.1186/s13014-020-01570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Bhangoo RS, Sio TT, et al. Dosimetric comparison of distal esophageal carcinoma plans for patients treated with small-spot intensity-modulated proton versus volumetric-modulated arc therapies. J Appl Clin Med Phys. 2019;20(7):15-27. doi: 10.1002/acm2.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Møller DS, Alber M, Nordsmark M, Nyeng TB, Lutz CM, Hoffmann L. Validation of a robust strategy for proton spot scanning for oesophageal cancer in the presence of anatomical changes. Radiother Oncol. 2019;131:174-178. doi: 10.1016/j.radonc.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 16.Hirano Y, Onozawa M, Hojo H, et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2018;13(1):23. doi: 10.1186/s13014-018-0966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiraishi Y, Xu C, Yang J, Komaki R, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol. 2017;125(1):48-54. doi: 10.1016/j.radonc.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 18.Warren S, Hurt CN, Crosby T, Partridge M, Hawkins MA. Potential of proton therapy to reduce acute hematologic toxicity in concurrent chemoradiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2017;99(3):729-737. doi: 10.1016/j.ijrobp.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren S, Partridge M, Bolsi A, et al. An analysis of plan robustness for esophageal tumors: comparing volumetric modulated arc therapy plans and spot scanning proton planning. Int J Radiat Oncol Biol Phys. 2016;95(1):199-207. doi: 10.1016/j.ijrobp.2016.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling TC, Slater JM, Nookala P, et al. Analysis of intensity-modulated radiation therapy (IMRT), proton and 3D conformal radiotherapy (3D-CRT) for reducing perioperative cardiopulmonary complications in esophageal cancer patients. Cancers (Basel). 2014;6(4):2356-2368. doi: 10.3390/cancers6042356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81(5):1336-1342. doi: 10.1016/j.ijrobp.2010.07.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2008;72(1):278-287. doi: 10.1016/j.ijrobp.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu X, Yin Y, Chen Q, et al. Dosimetric comparison between proton therapy and x-ray intensity-modulated radiotherapy for cervical esophageal cancer. Chin J Radiat Oncol. 2011;20(3):226-229. doi: 10.3760/cma.j.issn.1004-4221.2011.03.016 [DOI] [Google Scholar]
- 24.Wang J, Palmer M, Bilton SD, et al. Comparing proton beam to intensity modulated radiation therapy planning in esophageal cancer. Int J Part Ther. 2015;1(4):866-877. doi: 10.14338/IJPT-14-00018.1 [DOI] [Google Scholar]
- 25.Suh YG, Bayasgalan U, Kim HT, et al. Photon versus proton beam therapy for T1-3 squamous cell carcinoma of the thoracic esophagus without lymph node metastasis. Front Oncol. 2021;11:699172. doi: 10.3389/fonc.2021.699172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebrahimi S, Lim G, Liu A, et al. Radiation-induced lymphopenia risks of photon versus proton therapy for esophageal cancer patients. Int J Part Ther. 2021;8(2):17-27. doi: 10.14338/IJPT-20-00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumiya T, Ishikawa H, Hiroshima Y, et al. The impact of lymphopenia during chemoradiotherapy using photons or protons on the clinical outcomes of esophageal cancer patients. J Radiat Res. 2021;rrab094. doi: 10.1093/jrr/rrab094 [DOI] [PubMed] [Google Scholar]
- 28.Lin SH, Hobbs BP, Verma V, et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J Clin Oncol. 2020;38(14):1569-1579. doi: 10.1200/JCO.19.02503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Palaskas NL, Yusuf SW, et al. Incidence and onset of severe cardiac events after radiotherapy for esophageal cancer. J Thorac Oncol. 2020;15(10):1682-1690. doi: 10.1016/j.jtho.2020.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeCesaris CM, Berger M, Choi JI, et al. Pathologic complete response (pCR) rates and outcomes after neoadjuvant chemoradiotherapy with proton or photon radiation for adenocarcinomas of the esophagus and gastroesophageal junction. J Gastrointest Oncol. 2020;11(4):663-673. doi: 10.21037/jgo-20-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhangoo RS, DeWees TA, Yu NY, et al. Acute toxicities and short-term patient outcomes after intensity-modulated proton beam radiation therapy or intensity-modulated photon radiation therapy for esophageal carcinoma: a Mayo Clinic experience. Adv Radiat Oncol. 2020;5(5):871-879. doi: 10.1016/j.adro.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Routman DM, Garant A, Lester SC, et al. A Comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv Radiat Oncol. 2019;4(1):63-69. doi: 10.1016/j.adro.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiraishi Y, Fang P, Xu C, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128(1):154-160. doi: 10.1016/j.radonc.2017.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macomber MW, Bowen SR, Gopan O, et al. Heart dose and outcomes in radiation treatment for esophageal cancer. Cureus. 2018;10(3):e2378. doi: 10.7759/cureus.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xi M, Xu C, Liao Z, et al. Comparative outcomes after definitive chemoradiotherapy using proton beam therapy versus intensity modulated radiation therapy for esophageal cancer: a retrospective, single-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99(3):667-676. doi: 10.1016/j.ijrobp.2017.06.2450 [DOI] [PubMed] [Google Scholar]
- 36.Fang P, Shiraishi Y, Verma V, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Part Ther. 2018;4(3):23-32. doi: 10.14338/IJPT-17-00033.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol. 2017;123(3):376-381. doi: 10.1016/j.radonc.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 38.Makishima H, Ishikawa H, Terunuma T, et al. Comparison of adverse effects of proton and x-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J Radiat Res. 2015;56(3):568-576. doi: 10.1093/jrr/rrv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parzen JS, Chuong MD, Chang J, et al. Dosimetry and acute toxicity profile of patients with esophageal cancer treated with proton beam radiation therapy: outcomes from the Proton Collaborative Group REG001-09 Trial. Adv Radiat Oncol. 2021;6(5):100751. doi: 10.1016/j.adro.2021.100751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa K, Ishikawa H, Hisakura K, et al. Retrospective analysis of neoadjuvant chemotherapy followed by surgery versus definitive chemoradiotherapy with proton beam for locally advanced esophageal squamous cell carcinoma. Int J Clin Oncol. 2021;26(10):1856-1863. doi: 10.1007/s10147-021-01981-1 [DOI] [PubMed] [Google Scholar]
- 41.Sato D, Motegi A, Kadota T, et al. Therapeutic results of proton beam therapy with concurrent chemotherapy for cT1 esophageal cancer and salvage endoscopic therapy for local recurrence. Esophagus. 2020;17(3):305-311. doi: 10.1007/s10388-020-00715-y [DOI] [PubMed] [Google Scholar]
- 42.Prayongrat A, Xu C, Li H, Lin SH. Clinical outcomes of intensity modulated proton therapy and concurrent chemotherapy in esophageal carcinoma: a single institutional experience. Adv Radiat Oncol. 2017;2(3):301-307. doi: 10.1016/j.adro.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng YC, Vyas S, Dang Q, et al. Proton therapy posterior beam approach with pencil beam scanning for esophageal cancer: Clinical outcome, dosimetry, and feasibility. [Article in German]. Strahlenther Onkol. 2016;192(12):913-921. doi: 10.1007/s00066-016-1034-4 [DOI] [PubMed] [Google Scholar]
- 44.Ishikawa H, Hashimoto T, Moriwaki T, et al. Proton beam therapy combined with concurrent chemotherapy for esophageal cancer. Anticancer Res. 2015;35(3):1757-1762. [PubMed] [Google Scholar]
- 45.Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83(3):e345-e351. doi: 10.1016/j.ijrobp.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu C, Mohan R, Lin SH, et al. Identifying individualized risk profiles for radiotherapy-induced lymphopenia among patients with esophageal cancer using machine learning. JCO Clin Cancer Inform. 2021;5:1044-1053. doi: 10.1200/CCI.21.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oonsiri S, Kitpanit S, Kannarunimit D, Chakkabat C, Lertbutsayanukul C, Prayongrat A. Comparison of intensity modulated proton therapy beam configurations for treating thoracic esophageal cancer. Phys Imaging Radiat Oncol. 2022;22:51-56. doi: 10.1016/j.phro.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin SH, Liao K, Lei X, et al. Health care resource utilization for esophageal cancer using proton versus photon radiation therapy. Int J Part Ther. 2022;9(1):18-27. doi: 10.14338/IJPT-22-00001.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi JH, Lee JM, Kim MS, et al. A comparative analysis of photon versus proton beam therapy in neoadjuvant concurrent chemoradiotherapy for intrathoracic squamous cell carcinoma of the esophagus at a single institute. Cancers (Basel). 2022;14(8):2033. doi: 10.3390/cancers14082033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato T, Ono T, Narita Y, Komori S, Murakami M. Dose-volume comparison of intensity modulated proton therapy and volumetric modulated arc therapy for cervical esophageal cancer. Med Dosim. 2022;47(3):216-221. doi: 10.1016/j.meddos.2022.02.009 [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Fan B, Sun T, et al. The feasibility of dose escalation using intensity-modulated radiotherapy (IMRT) and intensity-modulated proton therapy (IMPT) with FDG PET/CT guided in esophageal cancer. J Cancer Res Ther. 2022;18(5):1261-1267. doi: 10.4103/jcrt.jcrt_382_22 [DOI] [PubMed] [Google Scholar]
- 52.Cui Y, Pan Y, Li Z, et al. Dosimetric analysis and biological evaluation between proton radiotherapy and photon radiotherapy for the long target of total esophageal squamous cell carcinoma. Front Oncol. 2022;12:954187. doi: 10.3389/fonc.2022.954187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutenberg MS, Hoppe BS, Starr JS, et al. Proton therapy with concurrent chemotherapy for thoracic esophageal cancer: toxicity, disease control, and survival outcomes. Int J Part Ther. 2022;9(3):18-29. doi: 10.14338/IJPT-22-00021.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholas O, Prosser S, Mortensen HR, Radhakrishna G, Hawkins MA, Gwynne SH. The promise of proton beam therapy for oesophageal cancer: a systematic review of dosimetric and clinical outcomes. Clin Oncol (R Coll Radiol). 2021;33(8):e339-e358. doi: 10.1016/j.clon.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 55.Zhu X, Li D, Shanzhou Q, et al. Efficacy and safety of proton therapy and photon therapy for esophageal cancer: a meta-analysis. J Mod Med Health. 2022;38(6):964-969. doi: 10.3969/j.issn.1009-5519.2022.06.014 [DOI] [Google Scholar]
- 56.Lin LL, Cai HY, Liu Y, Li ZH. The efficacy and safety of proton, carbon ion and TOMO radiotherapy for esophageal cancer: a systematic review and meta-analysis. Asian J Surg. 2022;45(6):1311-1312. doi: 10.1016/j.asjsur.2022.01.079 [DOI] [PubMed] [Google Scholar]
- 57.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 58.Mohan R, Grosshans D. Proton therapy—present and future. Adv Drug Deliv Rev. 2017;109:26-44. doi: 10.1016/j.addr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gjyshi O, Xu T, Elhammali A, et al. Toxicity and survival after intensity-modulated proton therapy versus passive scattering proton therapy for NSCLC. J Thorac Oncol. 2021;16(2):269-277. doi: 10.1016/j.jtho.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu C, Guo L, Liao Z, et al. Heart and lung doses are independent predictors of overall survival in esophageal cancer after chemoradiotherapy. Clin Transl Radiat Oncol. 2019;17:17-23. doi: 10.1016/j.ctro.2019.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou PX, Zhang SX. Functional lung imaging in thoracic tumor radiotherapy: application and progress. Front Oncol. 2022;12:908345. doi: 10.3389/fonc.2022.908345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vinogradskiy Y, Castillo R, Castillo E, et al. Results of a multi-institutional phase 2 clinical trial for 4DCT-ventilation functional avoidance thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2022;112(4):986-995. doi: 10.1016/j.ijrobp.2021.10.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou P, Wang R, Yu H, et al. 4DCT ventilation function image-based functional lung protection for esophageal cancer radiotherapy. Strahlenther Onkol. 2023;199(5):445-455. doi: 10.1007/s00066-022-02012-2 [DOI] [PubMed] [Google Scholar]
- 64.Ieko Y, Kadoya N, Kanai T, et al. The impact of 4DCT-ventilation imaging-guided proton therapy on stereotactic body radiotherapy for lung cancer. Radiol Phys Technol. 2020;13(3):230-237. doi: 10.1007/s12194-020-00572-5 [DOI] [PubMed] [Google Scholar]
- 65.Beukema JC, van Luijk P, Widder J, Langendijk JA, Muijs CT. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol. 2015;114(1):85-90. doi: 10.1016/j.radonc.2014.11.037 [DOI] [PubMed] [Google Scholar]
- 66.Gharzai L, Verma V, Denniston KA, Bhirud AR, Bennion NR, Lin C. Radiation therapy and cardiac death in long-term survivors of esophageal cancer: an analysis of the Surveillance, Epidemiology, and End Result Database. PLoS One. 2016;11(7):e0158916. doi: 10.1371/journal.pone.0158916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou P, Zhang Y, Luo S, Zhang S. Pelvic bone marrow sparing radiotherapy for cervical cancer: a systematic review and meta-analysis. Radiother Oncol. 2021;165:103-118. doi: 10.1016/j.radonc.2021.10.015 [DOI] [PubMed] [Google Scholar]
- 68.Deek MP, Benenati B, Kim S, et al. Thoracic vertebral body irradiation contributes to acute hematologic toxicity during chemoradiation therapy for non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;94(1):147-154. doi: 10.1016/j.ijrobp.2015.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang E, Deng M, Egleston B, et al. Dose to heart, spine, aorta, and body predict for severe lymphopenia and poor survival in patients undergoing chemoradiation for esophageal cancer. Int J Radiat Oncol Biol Phys. 2019;105(1):E206-E207. doi: 10.1016/j.ijrobp.2019.06.2041 [DOI] [Google Scholar]
- 70.Chadha AS, Liu G, Chen HC, et al. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int J Radiat Oncol Biol Phys. 2017;97(2):323-332. doi: 10.1016/j.ijrobp.2016.10.046 [DOI] [PubMed] [Google Scholar]
- 71.Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):128-135. doi: 10.1016/j.ijrobp.2017.05.037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Literature Search Strategy
eFigure 1. Forest Plots of Dose-Volume Parameters for OARs With Proton vs Photon Therapy
eFigure 2. Funnel Plot of Dose-Volume Parameters for OARs With Proton vs Photon Therapy (≥10 Articles)
eFigure 3. Pooled Analysis of pCR (Proton vs Photon Therapy)
eFigure 4. Forest Plots of the Incidence of Toxic Effects in the Proton and Photon Therapy Groups (Described as OR)
eFigure 5. Example of Single-Rate Pooled Analysis of OS and PFS for Proton Therapy
eFigure 6. Single-Rate Pooled Analysis of CR (Proton Therapy)
eFigure 7. Single-Rate Pooled Analysis of pCR (Proton Therapy)
eTable 1. Detailed Comparison Information on OARs Dosimetric and Planning Quality From Proton and Photon Radiotherapy Planning
eTable 2. Results of Quality Assessment Using the Newcastle-Ottawa Scale for Non-RCT Studies (Dosimetric Parameters)
eTable 3. Results of Quality Assessment Using the Newcastle-Ottawa Scale for Non-RCT Studies (Efficacy and Safety of Proton vs Photon Therapy)
eTable 4. Results of Quality Assessment Using the Cochrane Collaboration’s Tool (RCT)
eTable 5. Results of Quality Assessment Using the MINORS (Single-Group Tests)
eTable 6. Pooled Analysis of Dose-Volume Parameters for OARs with Proton vs Photon Therapy
eTable 7. Pooled Analysis of the Incidence of Toxic Effects in the Proton and Photon Therapy Group (Described as OR)
eTable 8. Single-Rate Pooled Analysis for the Incidence of Toxic Effects in Proton Therapy
Data Sharing Statement