Key Points
Question
What is the median door-in-door-out time for interhospital transfer of patients with stroke, and what patient and hospital-level factors are associated with door-in-door-out time?
Findings
In this retrospective US registry–based study that included 108 913 patients with acute stroke requiring interhospital transfer from 1925 hospitals, the median door-in-door-out time was 174 minutes. Age 80 years or older, female sex, Black race, and Hispanic ethnicity were significantly associated with longer door-in-door-out times, whereas emergency medical services prenotification, severe stroke, and ischemic stroke eligible for endovascular therapy were significantly associated with shorter times.
Meaning
This US registry–based study evaluated door-in-door-out times for patients with acute stroke requiring interhospital transfer and identified disparities and health system factors that could be possible targets for quality improvement initiatives.
Abstract
Importance
Treatments for time-sensitive acute stroke are not available at every hospital, often requiring interhospital transfer. Current guidelines recommend hospitals achieve a door-in-door-out time of no more than 120 minutes at the transferring emergency department (ED).
Objective
To evaluate door-in-door-out times for acute stroke transfers in the American Heart Association Get With The Guidelines-Stroke registry and to identify patient and hospital factors associated with door-in-door-out times.
Design, Setting, and Participants
US registry–based, retrospective study of patients with ischemic or hemorrhagic stroke from January 2019 through December 2021 who were transferred from the ED at registry-affiliated hospitals to other acute care hospitals.
Exposure
Patient- and hospital-level characteristics.
Main Outcomes and Measures
The primary outcome was the door-in-door-out time (time of transfer out minus time of arrival to the transferring ED) as a continuous variable and a categorical variable (≤120 minutes, >120 minutes). Generalized estimating equation (GEE) regression models were used to identify patient and hospital-level characteristics associated with door-in-door-out time overall and in subgroups of patients with hemorrhagic stroke, acute ischemic stroke eligible for endovascular therapy, and acute ischemic stroke transferred for reasons other than endovascular therapy.
Results
Among 108 913 patients (mean [SD] age, 66.7 [15.2] years; 71.7% non-Hispanic White; 50.6% male) transferred from 1925 hospitals, 67 235 had acute ischemic stroke and 41 678 had hemorrhagic stroke. Overall, the median door-in-door-out time was 174 minutes (IQR, 116-276 minutes): 29 741 patients (27.3%) had a door-in-door-out time of 120 minutes or less. The factors significantly associated with longer median times were age 80 years or older (vs 18-59 years; 14.9 minutes, 95% CI, 12.3 to 17.5 minutes), female sex (5.2 minutes; 95% CI, 3.6 to 6.9 minutes), non-Hispanic Black vs non-Hispanic White (8.2 minutes, 95% CI, 5.7 to 10.8 minutes), and Hispanic ethnicity vs non-Hispanic White (5.4 minutes, 95% CI, 1.8 to 9.0 minutes). The following were significantly associated with shorter median door-in-door-out time: emergency medical services prenotification (−20.1 minutes; 95% CI, −22.1 to −18.1 minutes), National Institutes of Health Stroke Scale (NIHSS) score exceeding 12 vs a score of 0 to 1 (−66.7 minutes; 95% CI, −68.7 to −64.7 minutes), and patients with acute ischemic stroke eligible for endovascular therapy vs the hemorrhagic stroke subgroup (−16.8 minutes; 95% CI, −21.0 to −12.7 minutes). Among patients with acute ischemic stroke eligible for endovascular therapy, female sex, Black race, and Hispanic ethnicity were associated with a significantly higher door-in-door-out time, whereas emergency medical services prenotification, intravenous thrombolysis, and a higher NIHSS score were associated with significantly lower door-in-door-out times.
Conclusions and Relevance
In this US registry–based study of interhospital transfer for acute stroke, the median door-in-door-out time was 174 minutes, which is longer than current recommendations for acute stroke transfer. Disparities and modifiable health system factors associated with longer door-in-door-out times are suitable targets for quality improvement initiatives.
This retrospective study involving patients with acute stroke requiring transfers from hospitals in the Get With the Guidelines–Stroke registry assesses patient and hospital factors associated with door-in-door-out times.
Introduction
Interhospital transfer is often required to ensure efficient access to time–dependent acute ischemic stroke therapies including intravenous (IV) thrombolysis and endovascular therapy.1,2 Additionally, patients with hemorrhagic stroke or acute ischemic stroke can be transferred for neurosurgical or neurocritical care services. Analyses of nationwide data suggested that 13% of acute ischemic stroke admissions from January 2010 to March 2014 involved an interhospital transfer3 with even higher rates for patients requiring endovascular therapy between 2012 and 2017.4 Because stroke treatments are exquisitely time-sensitive,1,2 the Joint Commission5 and Brain Attack Coalition6 recommend that the time from “arrival to discharge” for transfer from an emergency department (ED)–door-in-door-out time be less than 120 minutes for stroke transfers. However, for patients with acute ischemic stroke who are eligible for endovascular therapy, door-in-door-out times are often prolonged,4,7 leading to worse clinical outcomes.3,4,8,9
Data are limited on door-in-door-out times in clinical practice with incomplete knowledge about factors associated with interhospital transfer.7,10,11 Understanding existing disparities, as well as potentially modifiable factors associated with door-in-door-out times, could help hospitals redesign interhospital transfer processes to reduce delays.7 Additionally, establishing a national baseline for door-in-door-out times could help inform targets for future interhospital transfer interventions in acute stroke. The objective of this study was to characterize door-in-door-out times for acute stroke transfers in a large, contemporary sample of US hospitals participating in the Get With the Guidelines–Stroke registry and to identify patient and hospital factors associated with door-in-door-out times.
Methods
Data were obtained from the Get With The Guidelines–Stroke registry, an ongoing, national database for voluntary quality improvement maintained by American Heart Association/American Stroke Association (AHA/ASA). This database, which is representative of the US Medicare ischemic stroke population,12 is used by more than 2000 hospitals and has more than 9 million patient records.3,13,14 Participating hospitals received human research approval to enroll patients without individual consent under the Common Rule15 or a waiver of authorization and exemption by its institutional review board (IRB). Advarra, the IRB for the AHA, determined this study was exempt from oversight. This study follows Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)16 guidelines for observational studies.
Study Population
Reporting of door-in-door-out times to the Joint Commission began in January 2019.5 We included patients who had acute ischemic stroke or hemorrhagic stroke between January 2019 and December 2021 who were (1) not admitted at the transferring hospital and (2) transferred from the ED to another acute care hospital. These data were obtained from the transferring hospital. Additionally, patients were excluded if they (1) had transient ischemic attack (TIA), (2) were transferred from a comprehensive stroke center or another acute care hospital because such transfers do not represent typical interhospital care pathways, (3) had door-in-door-out times that were negative values or exceeded 3 days, or (4) had a stroke type listed as “not otherwise specified” (Figure 1).
Figure 1. Study Population.
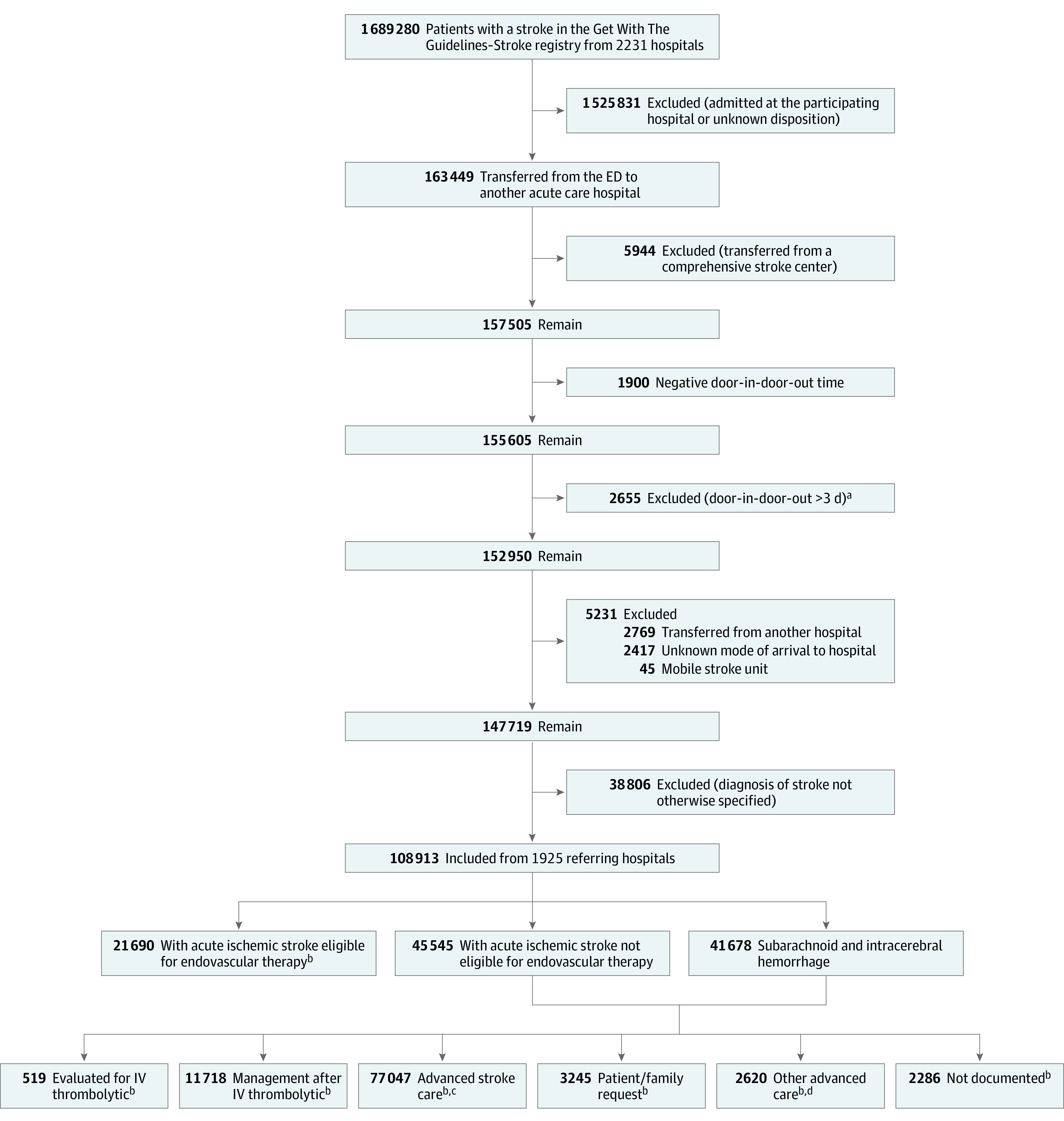
aDoor-in-door-out times exceeding 3 days were excluded based on an assessment of the distribution of the time and an empiric interpretation that such outliers would likely represent atypical reasons for transfer such as family request, bed capacity constraints, or insurance (out of network) factors rather than clinical factors, although some excluded may have had clinical reasons for their transfer.
bParticipating hospitals were allowed to report more than 1 transfer reason from the 7 prespecified options
cAdvanced stroke care includes neurocritical care and surgical or other time-critical therapy.
dOther advanced care includes critical care not related to stroke.
ED indicates emergency department; IV, intravenous.
Outcomes
The primary outcome was door-in-door-out time (arrival time to transfer out of the ED) as continuous and categorical variables (≤120 vs 120 minutes).
Prespecified Covariates
Patient demographic and clinical characteristics, as well as arrival, hospital, and geographic factors contained in the registry, were included in the analysis. Patient-level demographics included age, sex, race and ethnicity, and health insurance. Patient demographics, including fixed categories for race and ethnicity were extracted from the medical record for inclusion in the registry. These variables were included as racial and sex disparities in other aspects of stroke identification and care have been well described in the literature.17,18,19 Patient risk factors included history of hypertension, diabetes, dyslipidemia, atrial fibrillation, prior stroke, prior TIA, prosthetic heart valve, coronary artery disease or myocardial infarction, carotid artery stenosis, peripheral vascular disease, heart failure, and smoking.
Arrival covariates included mode of arrival to ED (eg, use of emergency medical services [EMS] to the transferring hospital vs private mode of arrival), EMS prenotification (defined as advanced notification of suspected stroke by EMS technicians),20 after-hours arrival (defined during the weekend [Saturday or Sunday] or before 7 am or after 6 pm on Monday-Friday),21 and presentation during the COVID-19 pandemic (before or after March 11, 2020).22 Clinical covariates included stroke type (hemorrhagic stroke or acute ischemic stroke), National Institutes of Health Stroke Scale (NIHSS) scores stratified by quartiles, and IV thrombolysis administration at the transferring hospital. Imaging tests performed and large vessel occlusion visualization were also evaluated for inclusion in the analysis; however, both were ultimately excluded due to high rates of missingness.
Transferring hospital characteristics included geographic location (rural or urban), annual volume of thrombolysis, teaching status, mean daily hospital census, and primary stroke center certification level (vs acute stroke–ready and noncertified hospitals).
Statistical Analysis
Descriptive statistics were performed for the overall cohort and stratified by prespecified stroke subgroups: (1) patients with hemorrhagic stroke; (2) patients with acute ischemic stroke transferred for endovascular therapy consideration; and (3) patients with acute ischemic stroke transferred for reasons other than endovascular therapy consideration. These prespecified groupings were chosen to reflect current door-in-door-out performance measures recognized by the Joint Commission.23 Median door-in-door-out times were calculated for each US state. Absolute standardized differences (SD) compared characteristics between door-in-door-out time categories (≤120 vs >120 minutes). This approach was used given the large sample size,24,25 wherein an absolute SD of more than 10% indicates a practical important difference in covariates between the door-in-door-out time groups.
Primary Analysis
Multivariable regression models were estimated to identify patient and hospital characteristics associated with the door-in-door-out time. Generalized estimating equations (GEE) were used to account for within-hospital clustering, a method used by previous studies conducted using Get With The Guidelines data.4,26 Door-in-door-out time as a continuous outcome was log-transformed to account for the rightward skew of the data. The association between covariates and door-in-door-out times and the corresponding 95% CIs were calculated27 using GEE median and logistic regression models for the overall cohort and each stroke subgroup adjusting for the prespecified covariates noted above. For GEE regression models, an intercept was calculated for each model, representing the median door-in-door-out time for each model with all patient and hospital characteristics set as the reference category. Analysis outputs from these models are reported as minutes greater than or less than the intercept (median) door-in-door-out time. The odds ratios in the logistic regression models represent the odds of door-in-door-out time longer than 120 minutes relative to the reference category for each variable adjusted for all other patient and hospital characteristics. Patients with missing NIHSS values were not included in the primary analysis. Given their degree of missingness, vascular imaging and large vessel occlusion visualization were not included in any models. All statistical tests were 2-sided using the nominal type I error rate α = .05.
Secondary Analysis
To account for missingness, we conducted 2 imputation methods for GEE models. First, in a rule-based imputation approach, missing values for insurance status were assigned to Medicare for patients 65 years or older, an approach used by other Get With The Guidelines studies.28 The missing values in all other categorical covariates were imputed to the most frequent category and the GEE models were then fit on the imputed data sets.4 Second, we used multiple imputations by chained equations (eMethods in Supplement 1).
Post Hoc Exploratory Analysis
We performed additional analyses using variables derived post hoc, including the last time the patient was known to be well to arrival time and various hospital-level variables including (1) stroke transfer volume, (2) telestroke utilization, (3) hospitals’ proportions of patients in our sample with Medicaid or self-pay insurance, and (4) tenecteplase utilization. The imputed and exploratory models are further described in the eMethods section of Supplement 1.
All statistical analyses were performed on the AHA Precision Medicine Platform using statistical software available on the platform including SAS Studio, version 9.4, and R version 4.2.0. The R package geepack was used to estimate the GEE models.29
Results
The baseline characteristics for the overall cohort and the subgroups are provided in Table 1. Among 108 913 patients (mean [SD] age, 66.7 [15.2] years; 71.7% White; 50.6% male) transferred from 1925 hospitals, 67 235 patients had acute ischemic stroke and 41 678 patients had hemorrhagic stroke. The most common reasons for transfer were advanced stroke care (70.7%), evaluation for endovascular therapy (20.3%), and IV thrombolysis management (10.8%). Most patients presented to teaching hospitals (62.9%), in urban areas (68.5%), and during the COVID-19 pandemic (59.6%). EMS prenotification was used in 43.9% overall patients and in 62.7% of patients with acute ischemic stroke eligible for endovascular therapy. Most covariates had low missing values (<10%), with the exception of NIHSS score (21.4%), insurance status (27.5%), whether vascular imaging was obtained (49.8%), and large vessel occlusion visualization (58.2%). The median NIHSS score was 7 (IQR, 2-15). Vascular imaging was obtained from 38 318 of 67 235 patients (57.0%) with acute ischemic stroke, and of those 21 849 (32.5%) had a large vessel occlusion visualized.
Table 1. Characteristics of the Patients With Stroke and by Stroke Type.
| No. (%) of patients | ||||
|---|---|---|---|---|
| Overall (N = 108 913) | Acute ischemic stroke | Hemorrhagic stroke (n = 41 678) | ||
| Endovascular therapy eligible (n = 21 690) | Other (n = 45 545) | |||
| Demographics | ||||
| Age, y | ||||
| 18-≤59 | 33 271 (30.5) | 5291 (24.4) | 13 444 (29.5) | 14 536 (34.9) |
| 60-≤69 | 25 496 (23.4) | 5083 (23.4) | 11 167 (24.5) | 9246 (22.2) |
| 70-≤79 | 26 282 (24.1) | 5461 (25.2) | 11 224 (24.6) | 9597 (23.0) |
| 80-≤110 | 23 864 (21.9) | 5855 (27.0) | 9710 (21.3) | 8299 (19.9) |
| Sex, No. | 10 8852 | 21 680 | 45 511 | 41 661 |
| Female | 53 808 (49.4) | 10 746 (49.6) | 21 850 (48.0) | 21 212 (50.9) |
| Male | 55 044 (50.6) | 10 934 (50.4) | 23 661 (52.0) | 20 449 (49.1) |
| Race/ethnicity, No. | 108 827 | 21 656 | 45 514 | 41 657 |
| Black or African American non-Hispanic | 15 917 (14.6) | 3222 (14.9) | 6487 (14.3) | 6208 (14.9) |
| Hispanic | 7070 (6.5) | 1306 (6.0) | 2441 (5.4) | 3323 (8.0) |
| Other non-Hispanica | 7798 (7.2) | 1573 (7.3) | 2575 (5.7) | 3650 (8.8) |
| White non-Hispanic | 78 042 (71.7) | 15 555 (71.8) | 34 011 (74.7) | 28 476 (68.4) |
| Insurance, No.b | 78 975 | 16 550 | 33 184 | 29 241 |
| Medicaid | 3364 (4.3) | 570 (3.4) | 1442 (4.3) | 1352 (4.6) |
| Medicare | 61 947 (78.4) | 13 483 (81.5) | 26 407 (79.6) | 22 057 (75.4) |
| Private, VA, CHAMPUS, or other | 10 431 (13.2) | 1866 (11.3) | 4220 (12.7) | 4345 (14.9) |
| Self-pay or none | 2568 (3.3) | 475 (2.9) | 893 (2.7) | 1200 (4.1) |
| Not determined | 665 (0.8) | 156 (0.9) | 222 (0.7) | 287 (1.0) |
| Medical history, No. | 108 215 | 21 488 | 45 399 | 41 328 |
| Hypertension | 68 685 (63.5) | 14 028 (65.3) | 30 221 (66.6) | 24 436 (59.1) |
| Dyslipidemia | 37 810 (34.97) | 7997 (37.2) | 17 310 (38.1) | 12 503 (30.3) |
| Diabetes | 27 228 (25.2) | 5405 (25.2) | 13 493 (29.7) | 8330 (20.2) |
| Prior stroke | 20 456 (18.9) | 3872 (18.0) | 10 017 (22.1) | 6567 (15.9) |
| CAD or MI | 18 050 (16.7) | 4045 (18.8) | 8630 (19.0) | 5375 (13.0) |
| Smoking | 16 001 (14.8) | 3082 (14.3) | 7746 (17.1) | 5173 (12.5) |
| Atrial fibrillation | 15 376 (14.2) | 4557 (21.2) | 6003 (13.2) | 4816 (11.7) |
| Heart failure | 7385 (6.8) | 1999 (9.3) | 3210 (7.1) | 2176 (5.3) |
| Prior TIA | 6183 (5.7) | 1198 (5.6) | 3365 (7.4) | 1620 (3.9) |
| Peripheral vascular disease | 2266 (2.1) | 520 (2.4) | 1065 (2.3) | 681 (1.6) |
| Carotid artery stenosis | 2080 (1.9) | 511 (2.4) | 1040 (2.3) | 529 (1.3) |
| Prosthetic heart valve | 961 (0.9) | 246 (1.1) | 364 (0.8) | 351 (0.8) |
| Arrival and clinical data | ||||
| Arrival mode, No. | 107 370 | 21 456 | 44 962 | 40 952 |
| Private | 36 719 (34.2) | 3884 (18.1) | 18 808 (41.8) | 14 027 (34.3) |
| EMS no prenotification | 23 555 (21.9) | 4113 (19.2) | 8643 (19.2) | 10 799 (26.4) |
| EMS prenotification | 47 096 (43.9) | 13 459 (62.7) | 17 511 (38.9) | 16 126 (39.4) |
| During pandemic | 64 941 (59.6) | 13 336 (61.5) | 26 902 (59.1) | 24 703 (59.3) |
| NIH Stroke Scale score, No.c | 85 597 | 21 158 | 41 456 | 22 983 |
| 0-1 | 16 574 (19.4) | 1312 (6.2) | 7798 (18.8) | 7464 (32.5) |
| 2-4 | 17 789 (20.8) | 2572 (12.2) | 11 228 (27.1) | 3989 (17.4) |
| 5-12 | 24 006 (28.0) | 6346 (30.0) | 12 888 (31.1) | 4772 (20.8) |
| >12 | 27 228 (31.8) | 10 928 (51.6) | 9542 (23.0) | 6758 (29.4) |
| Missing | 23 316 (21.4) | 532 (2.5) | 4089 (9.0) | 18 695 (44.9) |
| Median (IQR) | 7 (2-15) | 13 (6-20) | 5 (2-12) | 5 (1-15) |
| IV thrombolytic initiated | NA | 9201 (42.4) | 15 066 (33.1) | NA |
| Imaging characteristics | ||||
| Vascular imaging performed | 38 323 (35.2) | 16 436 (75.8) | 21 882 (48.0) | 5 (<1) |
| Vascular imaging data missingd | 54 259 (49.8) | 2824 (13.0) | 9795 (21.5) | 41 640 (99.9) |
| Large vessel occlusion | 21 849 (20.1) | 14 359 (66.2) | 7490 (16.4) | 0 |
| Data missing | 63 346 (58.2) | 4593 (21.2) | 17 076 (37.5) | 41 677 (100.0) |
| Transferring hospital characteristics | ||||
| Primary stroke center | ||||
| Yes | 46 488 (42.7) | 11 676 (53.8) | 15 066 (33.1) | 19 746 (47.4) |
| Noe | 62 425 (57.3) | 10 014 (46.2) | 30 479 (66.9) | 21 932 (52.6) |
| Location, No. | 107 556 | 21 405 | 44 901 | 41 250 |
| Rural | 33 932 (31.5) | 4366 (20.4) | 18 602 (41.4) | 10 964 (26.6) |
| Urban | 73 624 (68.5) | 17 039 (79.6) | 26 299 (58.6) | 30 286 (73.4) |
| IV thrombolytic cases per y, No. | 108 164 | 21 666 | 44 963 | 41 535 |
| 0-9 | 32 012 (29.6) | 3777 (17.4) | 16 994 (37.8) | 11 241 (27.1) |
| 10-19 | 37 995 (35.1) | 7505 (34.6) | 14 921 (33.2) | 15 569 (37.5) |
| 20-29 | 24 275 (22.4) | 6532 (30.1) | 8302 (18.5) | 9441 (22.7) |
| 30-126 | 13 882 (12.8) | 3852 (17.8) | 4746 (10.6) | 5284 (12.7) |
| Teaching status, No. | 99 664 | 20 047 | 41 376 | 38 241 |
| Teaching hospital | 62 681 (62.9) | 14 754 (73.6) | 22 382 (54.1) | 25 545 (66.8) |
| Daily hospital census, No. | 99 664 | 20 047 | 41 376 | 38 241 |
| 0-99 | 52 526 (52.7) | 6981 (34.8) | 26 441 (63.9) | 19 104 (50.0) |
| 100-199 | 32 230 (32.3) | 8209 (40.9) | 10 494 (25.4) | 13 527 (35.4) |
| ≥200 | 14 908 (15.0) | 4857 (24.2) | 4441 (10.7) | 5610 (14.7) |
Abbreviations: CAD, coronary artery disease; CHAMPUS, Civilian Health and Medical Program of the Uniformed Services; EMS, emergency medical services; EVT, endovascular therapy; IV, intravenous; MI, myocardial infarction; NA, not applicable; NIH, National Institutes of Health; TIA, transient ischemic attack; VA, Veterans Affairs.
Other includes American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, or unable to determine, extracted from the medical record for inclusion in the registry.
Extracted from the medical record for inclusion in the registry. Patients with both Medicaid and Medicare were assigned to Medicare.
Score ranges from 0 to 42 (higher scores indicate greater severity).
Imaging that was not performed or not reported at the transferring hospital.
Included acute stroke ready and noncertified hospitals.
Median door-in-door-out times were 174 minutes (IQR, 116-276 minutes) overall; 132 minutes (IQR, 97-189 minutes) among patients with acute ischemic stroke eligible for endovascular therapy; 178 minutes (IQR, 119-275 minutes), with hemorrhagic stroke; and 201 minutes (IQR, 129-319 minutes) with acute ischemic stroke-other. The median door-in-door-out times by state in the overall cohort and by stroke subgroup are depicted in Figure 2.
Figure 2. Median Door-in-Door-out Times by State.
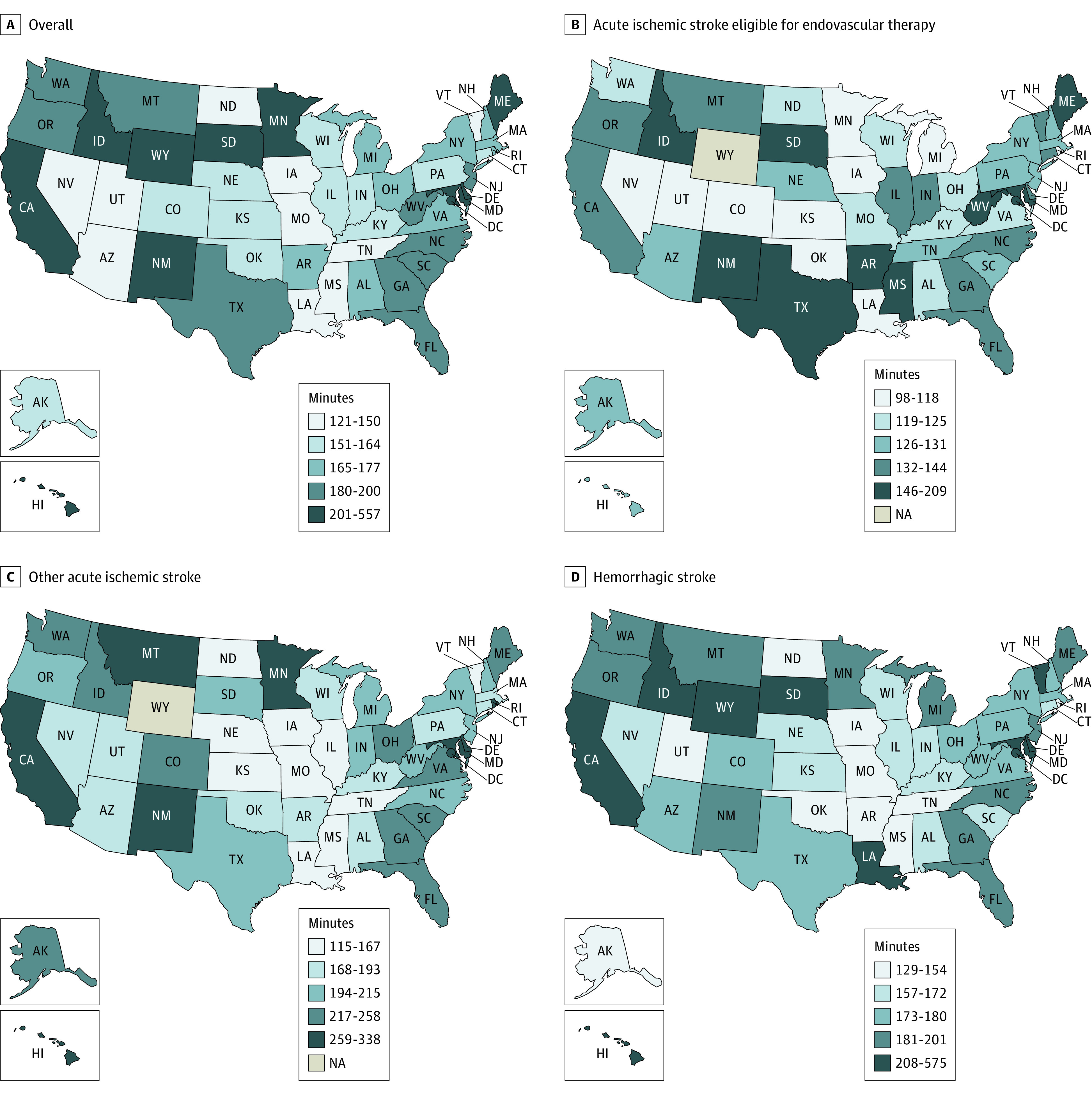
NA indicates not applicable (Wyoming did not have any patients with acute ischemic stroke transferred who met the study’s inclusion criteria).
Table 2 compares characteristics between door-in-door-out time groups (dichotomized by ≤120 vs >120 minutes). Door-in-door-out time of 120 minutes or less was achieved in 27.3% overall. Patients with the following characteristics were significantly more likely to have door-in-door-out time exceeding 120 minutes than those with times of 120 minutes or less: Black race (15.3% vs 12.8%); Hispanic ethnicity (7.0% vs 5.1%); pandemic period (61.0% vs 55.8%); and magnetic resonance imaging (MRI) performed (4.8% vs 0.8%). The following characteristics were significantly more frequent in the door-in-door-out time of 120 minutes or less group: White race (74.6% vs 70.6%), EMS prenotification (57.3% vs 38.8%), and NIHSS score exceeding 12 (39.2% vs 19.7%).
Table 2. Patient, Arrival, and Clinical and Hospital Characteristics Stratified by Door-in-Door-out Time.
| No. (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 108 913) | Acute ischemic stroke | Hemorrhagic stroke (n = 41 678) | ||||||||||
| Endovascular therapy eligible (n = 21 690) | Other (n = 45 545) | |||||||||||
| Minutes | Absolute SD | Minutes | Absolute SD | Minutes | Absolute SD | Minutes | Absolute SD | |||||
| ≤120 (n = 29 741) | >120 (n = 79 172) | ≤120 (n = 9241) | >120 (n = 12 449) | ≤120 (n = 9806) | >120 (n = 35 739) | ≤120 (n = 10 694) | >120 (n = 30 984) | |||||
| Demographics | ||||||||||||
| Age, y | ||||||||||||
| 18-≤59 | 8594 (28.9) | 24 677 (31.2) | 5.0 | 2172 (23.5) | 3119 (25.1) | 3.6 | 2820 (28.8) | 10 624 (29.7) | 3.6 | 3602 (33.7) | 10 934 (35.3) | 7.0 |
| 60-≤69 | 7122 (23.9) | 18 374 (23.2) | 2189 (23.7) | 2894 (23.2) | 2393 (24.4) | 8774 (24.6) | 2540 (23.8) | 6706 (21.6) | ||||
| 70-≤79 | 7305 (24.6) | 18 977 (24.0) | 2351 (25.4) | 3110 (25.0) | 2393 (24.4) | 8831 (24.7) | 2561 (23.9) | 7036 (22.7) | ||||
| 80-≤110 | 6720 (22.6) | 17 144 (21.7) | 2529 (27.4) | 3326 (26.7) | 2200 (22.4) | 7510 (21.0) | 1991 (18.6) | 6308 (20.4) | ||||
| Sex, No. | 29 727 | 79 125 | 9239 | 12 441 | 9799 | 35 712 | 10 689 | 30 972 | ||||
| Female | 14 325 (48.2) | 39 483 (49.9) | 3.4 | 4577 (49.5) | 6169 (49.6) | 0.1 | 4653 (47.5) | 17 197 (48.2) | 1.3 | 5095 (47.7) | 16 117 (52.0) | 8.8 |
| Male | 15 402 (51.8) | 39 642 (50.1) | 4662 (50.5) | 6272 (50.4) | 5146 (52.5) | 18 515 (51.8) | 5594 (52.3) | 14 855 (48.0) | ||||
| Race/ethnicity, No. | 29 717 | 79 110 | 9225 | 12 431 | 9801 | 35 713 | 10 691 | 30 966 | ||||
| Black or African American non-Hispanic | 3818 (12.8) | 12 099 (15.3) | 11.1 | 1220 (13.2) | 2002 (16.1) | 10.5 | 1175 (12.0) | 5312 (14.9) | 14.0 | 1423 (13.3) | 4785 (15.5) | 11.1 |
| Hispanic | 1530 (5.1) | 5540 (7.0) | 488 (5.3) | 818 (6.6) | 366 (3.7) | 2075 (5.8) | 676 (6.3) | 2647 (8.5) | ||||
| Other non-Hispanica | 2193 (7.4) | 5605 (7.1) | 713 (7.7) | 860 (6.9) | 516 (5.3) | 2059 (5.8) | 964 (9.0) | 2686 (8.7) | ||||
| White non-Hispanic | 22 176 (74.6) | 55 866 (70.6) | 6804 (73.8) | 8751 (70.4) | 7744 (79.0) | 26 267 (73.6) | 7628 (71.3) | 20 848 (67.3) | ||||
| Insurance, No.b | 22 067 | 56 908 | 7106 | 9444 | 7335 | 25 849 | 7626 | 21 615 | ||||
| Medicaid | 861 (3.9) | 2503 (4.4) | 3.9 | 216 (2.3) | 354 (2.8) | 4.5 | 328 (3.3) | 1114 (3.1) | 7.2 | 317 (3.0) | 1035 (3.3) | 6.3 |
| Medicare | 17 227 (78.1) | 44 720 (78.6) | 5810 (62.9) | 7673 (61.6) | 5726 (58.4) | 20 681 (57.9) | 5691 (53.2) | 16 366 (52.8) | ||||
| Private, VA, CHAMPUS, or other | 2994 (13.6) | 7437 (13.1) | 822 (8.9) | 1044 (8.4) | 998 (10.2) | 3222 (9.0) | 1174 (11.0) | 3171 (10.2) | ||||
| Self-pay or none | 796 (3.6) | 1772 (3.1) | 192 (2.1) | 283 (2.3) | 232 (2.4) | 661 (1.8) | 372 (3.5) | 828 (2.7) | ||||
| Not determined | 189 (0.9) | 476 (0.8) | 66 (0.7) | 90 (0.7) | 51 (0.5) | 171 (0.5) | 72 (0.7) | 215 (0.7) | ||||
| Medical history, No. | 29 528 | 78 687 | 9144 | 12 344 | 9762 | 35 637 | 10 622 | 30 706 | ||||
| Hypertension | 18 574 (62.9) | 50 111 (63.7) | 1.6 | 5833 (63.1) | 8195 (65.8) | 5.8 | 6364 (64.9) | 23 857 (66.8) | 4.6 | 6377 (59.6) | 18 059 (58.3) | 3.5 |
| Dyslipidemia | 10 135 (34.3) | 27 675 (35.2) | 1.8 | 2107 (22.8) | 3298 (26.5) | 8.7 | 3610 (36.8) | 13 700 (38.3) | 4.0 | 3097 (29.0) | 9406 (30.4) | 4.1 |
| Diabetes | 6667 (22.6) | 20 561 (26.1) | 8.3 | 3428 (37.1) | 4569 (36.7) | 2.3 | 2546 (26.0) | 10 947 (30.6) | 10.6 | 2014 (18.8) | 6316 (20.4) | 4.8 |
| Prior stroke | 5018 (17.0) | 15 438 (19.6) | 6.8 | 2089 (22.6) | 2468 (19.8) | 7.2 | 1941 (19.8) | 8076 (22.6) | 7.3 | 1602 (15.0) | 4965 (16.0) | 3.9 |
| CAD or MI | 4867 (16.5) | 13 183 (16.8) | 0.7 | 1475 (16.0) | 2397 (19.3) | 8.8 | 1807 (18.4) | 6823 (19.1) | 3.2 | 1345 (12.6) | 4030 (13.0) | 2.9 |
| Smoking | 4009 (13.6) | 11 992 (15.2) | 4.7 | 510 (5.5) | 688 (5.5) | 2.1 | 1555 (15.9) | 6191 (17.3) | 4.7 | 1219 (11.4) | 3954 (12.8) | 5.0 |
| Atrial fibrillation | 4737 (16.0) | 10 639 (13.5) | 7.1 | 101 (1.1) | 145 (1.2) | 2.2 | 1502 (15.3) | 4501 (12.6) | 8.4 | 1146 (10.7) | 3670 (11.8) | 4.4 |
| Heart failure | 1981 (6.7) | 5404 (6.9) | 0.6 | 1715 (18.6) | 2330 (18.7) | 2.2 | 694 (7.1) | 25 16 (7.0) | 2.7 | 442 (4.1) | 1734 (5.6) | 7.3 |
| Prior TIA | 1642 (5.6) | 4541 (5.8) | 0.9 | 196 (2.1) | 315 (2.5) | 3.4 | 721 (7.4) | 2644 (7.4) | 2.7 | 411 (3.8) | 1209 (3.9) | 2.6 |
| Peripheral vascular disease | 549 (1.9) | 1717 (2.2) | 2.3 | 214 (2.3) | 306 (2.5) | 2.3 | 184 (1.9) | 881 (2.5) | 4.8 | 151 (1.4) | 530 (1.7) | 3.5 |
| Carotid artery stenosis | 522 (1.8) | 1558 (2.0) | 1.6 | 845 (9.1) | 1154 (9.3) | 2.2 | 203 (2.1) | 837 (2.3) | 3.3 | 123 (1.2) | 406 (1.3) | 2.9 |
| Prosthetic heart valve | 263 (0.9) | 698 (0.9) | <0.1 | 1235 (13.4) | 1847 (14.8) | 4.7 | 77 (0.8) | 287 (0.8) | 2.7 | 85 (0.8) | 266 (0.9) | 2.6 |
| Arrival and clinical data | ||||||||||||
| Arrival mode, No. | 29 309 | 78 061 | 9126 | 12 330 | 9662 | 35 300 | 10 521 | 30 431 | ||||
| Private | 7128 (24.3) | 29 591 (37.9) | 38.4 | 1247 (13.7) | 2637 (21.4) | 26.6 | 3109 (32.2) | 15 699 (44.5) | 33.3 | 2772 (26.3) | 11 255 (37.0) | 34.0 |
| EMS no prenotification | 5385 (18.4) | 18 170 (23.3) | 1506 (16.5) | 2607 (21.1) | 1564 (16.2) | 7079 (20.1) | 2315 (22.0) | 8484 (27.9) | ||||
| EMS prenotification | 16 796 (57.3) | 30 300 (38.8) | 6373 (69.8) | 7086 (57.5) | 4989 (51.6) | 12 522 (35.5) | 5434 (51.6) | 10 692 (35.1) | ||||
| During pandemic | 16 607 (55.8) | 48 334 (61.0) | 10.6 | 5626 (60.9) | 7710 (61.9) | 2.2 | 5123 (52.2) | 21 779 (60.9) | 17.6 | 5858 (54.8) | 18 845 (60.8) | 12.3 |
| NIH Stroke Scale scorec | 25 847 | 59 750 | 9099 | 12 059 | 9350 | 32 106 | 7398 | 15 585 | ||||
| 0-1 | 2293 (8.9) | 14 281 (23.9) | 54.6 | 244 (2.7) | 1068 (8.6) | 39.0 | 652 (7.0) | 7146 (22.3) | 58.6 | 1397 (18.9) | 6067 (38.9) | 47.9 |
| 2-4 | 3940 (15.2) | 13 849 (23.2) | 754 (8.3) | 1818 (14.6) | 1919 (20.5) | 9309 (29.0) | 1267 (17.1) | 2722 (17.5) | ||||
| 5-12 | 7959 (30.8) | 16 047 (26.9) | 2648 (29.1) | 3698 (29.7) | 3308 (35.4) | 9580 (29.8) | 2003 (27.1) | 2769 (17.8) | ||||
| >12 | 11 655 (45.1) | 15 573 (26.1) | 5453 (59.9) | 5475 (44.0) | 3471 (37.1) | 6071 (18.9) | 2731 (36.9) | 4027 (25.8) | ||||
| Mean (SD) score | 12.38 (9.01) | 8.45 (8.96) | 43.8 | 14.93 (8.03) | 12.42 (8.92) | 29.5 | 10.89 (8.20) | 7.07 (7.55) | 48.4 | 11.14 (10.34) | 8.22 (10.60) | 27.8 |
| Imaging characteristics, No. | 26 968 | 72 015 | 8477 | 11 569 | 8910 | 32 555 | 9581 | 27 891 | ||||
| Computed tomography | 26 943 (99.9) | 71 080 (98.7) | 14.5 | 8474 (100.0) | 11 518 (99.6) | 8.3 | 8898 (99.9) | 31 873 (97.9) | 18.8 | 9571 (99.9) | 27 689 (99.3) | 9.7 |
| MRI | 220 (0.8) | 3465 (4.8) | 24.3 | 70 (0.8) | 380 (3.3) | 17.4 | 99 (1.1) | 2427 (7.5) | 31.7 | 51 (0.5) | 658 (2.4) | 15.3 |
| Vascular imaging | 10 392 (34.9) | 27 931 (35.3) | 9.3 | 6671 (72.2) | 9765 (78.4) | 17.3 | 3721 (37.9) | 18 161 (50.8) | 27.9 | 0 | 5 (<1) | 2.8 |
| Missing datad | 14 177 (47.7) | 40 082 (50.6) | 1257 (13.6) | 1567 (12.6) | 2230 (22.7) | 7565 (21.2) | 10 690 (100.0) | 30950 (99.9) | ||||
| Large vessel occlusion | 8101 (27.2) | 13 748 (17.4) | 27 | 6098 (66.0) | 8261 (66.4) | 4.6 | 2003 (20.4) | 5487 (15.4) | 20.3 | 1 (<1) | 0 | 1.4 |
| Missing data | 16 751 (56.3) | 46 595 (58.9) | 2037 (22.0) | 2556 (20.5) | 4021 (41.0) | 13 055 (36.5) | 10 693 (100) | 30 984 (100) | ||||
| Transferring hospital characteristics | ||||||||||||
| Primary stroke center | ||||||||||||
| Yes | 13 232 (44.5) | 33 256 (42.0) | 5.0 | 5164 (55.9) | 6512 (52.3) | 7.2 | 3237 (33.0) | 11 829 (33.1) | 0.2 | 4831 (45.2) | 14 915 (48.1) | 5.9 |
| Noe | 16 509 (55.5) | 45 916 (58.0) | 4077 (44.1) | 5937 (47.7) | 6569 (67.0) | 23 910 (66.9) | 5863 (54.8) | 16 069 (51.9) | ||||
| Location, No. | 29 333 | 78 223 | 9072 | 12 333 | 9683 | 35 218 | 10 578 | 30 672 | ||||
| Rural | 9594 (32.7) | 24 338 (31.1) | 3.4 | 1579 (17.4) | 2787 (22.6) | 13.0 | 4592 (47.4) | 14 010 (39.8) | 15.5 | 3423 (32.4) | 7541 (24.6) | 17.3 |
| Urban | 19 739 (67.3) | 53 885 (68.9) | 7493 (82.6) | 9546 (77.4) | 5091 (52.6) | 21 208 (60.2) | 7155 (67.6) | 23 131 (75.4) | ||||
| IV thrombolytic cases per y, No. | 29 602 | 78 562 | 9237 | 12 429 | 9711 | 35 252 | 10 654 | 30 881 | ||||
| 0-9 | 8011 (27.1) | 24 001 (30.6) | 8.0 | 1443 (15.6) | 2334 (18.8) | 12.3 | 3398 (35.0) | 13 596 (38.6) | 7.9 | 3170 (29.8) | 8071 (26.1) | 16.6 |
| 10-19 | 10 653 (36.0) | 27 342 (34.8) | 3058 (33.1) | 4447 (35.8) | 3331 (34.3) | 11 590 (32.9) | 4264 (40.0) | 11 305 (36.6) | ||||
| 20-29 | 7057 (23.8) | 17 218 (21.9) | 2999 (32.5) | 3533 (28.4) | 1848 (19.0) | 6454 (18.3) | 2210 (20.7) | 7231 (23.4) | ||||
| 30-126 | 3881 (13.1) | 10 001 (12.7) | 1737 (18.8) | 2115 (17.0) | 1134 (11.7) | 3612 (10.2) | 1010 (9.5) | 4274 (13.8) | ||||
| Teaching status, No. | 27 290 | 72 374 | 8484 | 11 563 | 9021 | 32 355 | 9785 | 28 456 | ||||
| Teaching hospital | 17 181 (63.0) | 45 500 (62.9) | 0.2 | 6309 (74.4) | 8445 (73.0) | 3.0 | 4826 (53.5) | 17 556 (54.3) | 1.5 | 6046 (61.8) | 19 499 (68.5) | 14.2 |
| Daily census, No. | 27 290 | 72 374 | 8484 | 11 563 | 9021 | 32 355 | 9785 | 28 456 | ||||
| 0-99 | 14 392 (52.7) | 38 134 (52.7) | 0.5 | 2860 (33.7) | 4121 (35.6) | 7.4 | 5862 (65.0) | 20 579 (63.6) | 3.1 | 5670 (57.9) | 13 434 (47.2) | 24.2 |
| 100-199 | 8790 (32.2) | 23 440 (32.4) | 3415 (40.3) | 4794 (41.5) | 2239 (24.8) | 8255 (25.5) | 3136 (32.0) | 10 391 (36.5) | ||||
| ≥200 | 4108 (15.1) | 10 800 (14.9) | 2209 (26.0) | 2648 (22.9) | 920 (10.2) | 3521 (10.9) | 979 (10.0) | 4631 (16.3) | ||||
Abbreviations: CAD, coronary artery disease; CHAMPUS, Civilian Health and Medical Program of the Uniformed Services; EMS, emergency medical services; IV, intravenous; MI, myocardial infarction; MRI, magnetic resonance imaging; NIH, National Institute of Health; SD, standardized differences; TIA, transient ischemic attack; VA, Veterans Affairs.
Other includes American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, or unable to determine, extracted from the medical record for inclusion in the registry.
Extracted from the medical record for inclusion in the registry. Patients with both Medicaid and Medicare were assigned to Medicare.
Score range from 0 to 42 (higher scores indicate greater severity).
Imaging not performed or not reported at the transferring hospital.
Included acute stroke–ready and noncertified hospitals.
Table 3 presents median door-in-door-out times using GEE regression models overall and by stroke subgroup. In the overall model, the following were significantly associated with longer door-in-door-out times: age 80 years or older vs those aged 18 through 59 years (14.90 minutes; 95% CI, 12.32-17.47 minutes), female sex (5.21 minutes; 95% CI, 3.55-6.86 minutes), Black non-Hispanic vs White non-Hispanic (8.21 minutes; 95% CI, 5.67-10.75 minutes), Hispanic vs White non-Hispanic (5.37 minutes; 95% CI, 1.77-8.97 minutes), acute ischemic stroke–other stroke type vs hemorrhagic stroke type (46.98 minutes; 95% CI, 42.24-51.72 minutes), urban location (14.30 minutes; 95% CI, 7.34-21.25 minutes), pandemic period (16.13 minutes; 95% CI, 13.84-18.43 minutes), hospital daily census of 200 or more patients vs 0 to 99 (26.06 minutes; 95% CI, 14.84-37.28 minutes), and annual IV thrombolysis volume of 30 to 126 vs 0 to 9 (27.09 minutes; 95% CI, 13.31-40.87 minutes).
Table 3. Generalized Estimating Equations Regression Results for the Overall Cohort and Stroke Subgroups.
| Minutes (95% CI) | ||||
|---|---|---|---|---|
| Overall (N = 74 083) | Acute ischemic stroke | Hemorrhagic (n = 19 890) | ||
| Eligible for endovascular therapy (n = 18 481) | Other (n = 35 698) | |||
| Intercepta | 185.26 (177.43 to 193.08) | 206.85 (193.48 to 220.21) | 244.60 (232.60 to 256.61) | 168.58 (158.14 to 179.02) |
| Demographics | ||||
| Age, y | ||||
| 18-≤59 | [Reference] | [Reference] | [Reference] | [Reference] |
| 60-≤69 | 1.24 (−0.86 to 3.33) | −1.28 (−5.26 to 2.69) | 0.33 (−3.51 to 4.16) | 2.38 (−1.50 to 6.27) |
| 70-≤79 | 5.56 (3.38 to 7.74) | 2.18 (−1.96 to 6.31) | 4.12 (0.12 to 8.12) | 10.08 (5.69 to 14.46) |
| 80-≤110 | 14.90 (12.32 to 17.47) | 12.29 (7.78 to 16.79) | 8.61 (4.02 to 13.21) | 30.77 (24.70 to 36.84) |
| Sex | ||||
| Male | [Reference] | [Reference] | [Reference] | [Reference] |
| Female | 5.21 (3.55 to 6.86) | 4.16 (1.26 to 7.07) | 6.92 (3.97 to 9.88) | 6.47 (3.46 to 9.49) |
| Race and ethnicity | ||||
| Black or African American non-Hispanic | 8.21 (5.67 to 10.75) | 12.36 (7.46 to 17.26) | 12.56 (7.73 to 17.39) | 2.51 (−2.14 to 7.15) |
| Hispanic | 5.37 (1.77 to 8.97) | 11.20 (4.69 to 17.71) | 7.00 (−0.09 to 14.09) | 5.63 (0.80 to 12.05) |
| Other non-Hispanicb | 0.22 (−2.85 to 3.28) | 3.01 (−2.84 to 8.87) | 4.38 (−1.89 to 10.64) | −5.82 (−11.00 to −0.65) |
| White non-Hispanic | [Reference] | [Reference] | [Reference] | [Reference] |
| Medical history and prior medications | ||||
| Hypertension | 0.31 (−1.38 to 2.00) | 3.72 (0.57 to 6.88) | 0.53 (−2.46 to 3.52) | −2.57 (−5.80 to 0.65) |
| Dyslipidemia | 0.14 (−1.65 to 1.93) | −1.88 (−4.79 to 1.04) | 0.54 (−2.79 to 3.88) | 1.09 (−2.55 to 4.73) |
| Diabetes | 8.93 (6.96 to 10.90) | 7.22 (3.82 to 10.61) | 8.73 (5.24 to 12.22) | 10.23 (6.18 to 14.27) |
| Prior stroke | 12.66 (10.46 to 14.86) | 9.48 (5.47 to 13.49) | 10.26 (6.80 to 13.72) | 15.83 (11.13 to 20.53) |
| CAD/prior MI | 1.62 (−0.54 to 3.78) | 1.90 (−1.85 to 5.66) | 3.46 (−0.33 to 7.25) | 2.00 (−2.91 to 6.92) |
| Smoking | 6.70 (4.35 to 9.05) | 6.31 (1.77 to 10.85) | 7.02 (2.92 to 11.13) | 3.34 (−1.21 to 7.89) |
| Atrial fibrillation | −4.04 (−6.24 to −1.83) | −6.77 (−10.16 to −3.39) | −8.99 (−13.27 to −4.71) | 1.74 (−3.43 to 6.90) |
| Heart failure | 4.71 (1.68 to 7.73) | 2.26 (−2.48 to 7.00) | 7.73 (2.01 to 13.46) | 13.18 (5.51 to 20.86) |
| Prior TIA | −0.46 (−3.62 to 2.69) | −0.49 (−6.46 to 5.48) | −1.30 (−6.28 to 3.68) | 2.75 (−4.86 to 10.35) |
| Peripheral vascular disease | 5.50 (−0.28 to 11.28) | 3.39 (−6.52 to 13.30) | 4.53 (−5.01 to 14.06) | 3.95 (−9.07 to 16.96) |
| Carotid artery stenosis | 4.65 (−1.01 to 10.32) | 2.33 (−6.98 to 11.64) | 2.57 (−6.74 to 11.89) | 11.60 (−3.77 to 26.98) |
| Prosthetic heart valve | 0.26 (−6.66 to 7.19) | −2.46 (−13.25 to 8.34) | −7.53 (−23.34 to 8.28) | 5.71 (−8.53 to 19.94) |
| Prior antithrombotic medication | −4.27 (−6.08 to −2.47) | 0.05 (−3.28 to 3.37) | −2.91 (−5.93 to 0.11) | −3.63 (−7.36 to 0.10) |
| Arrival and clinical data | ||||
| Stroke subtype | ||||
| Hemorrhagic stroke | [Reference] | NA | NA | NA |
| Acute ischemic stroke | ||||
| Endovascular therapy eligible | −16.83 (−21.01 to −12.66) | NA | NA | NA |
| Other | 46.98 (42.24 to 51.72) | NA | NA | NA |
| Received IV Thrombolytic | NA | −15.30 (−18.24 to −12.36) | −59.64 (−63.48 to −55.80) | NA |
| NIH Stroke Scale scorec | ||||
| 0-1 | [Reference] | [Reference] | [Reference] | [Reference] |
| 2-4 | −33.07 (−35.35 to −30.79) | −36.80 (−44.03 to −29.57) | −35.47 (−39.30 to −31.64) | −31.47 (−34.90 to −28.04) |
| 5-12 | −53.95 (−56.03 to −51.87) | −64.79 (−70.53 to −59.05) | −57.27 (−61.08 to −53.46) | −47.07 (−50.20 to −43.94) |
| >12 | −66.68 (−68.67 to −64.68) | −77.98 (−83.57 to −72.38) | −84.84 (−88.50 to −81.18) | −46.22 (−49.48 to −42.96) |
| Arrival mode and time | ||||
| Private arrival mode | [Reference] | [Reference] | [Reference] | [Reference] |
| EMS no prenotification | 1.22 (−1.32 to 3.77) | 7.26 (1.68 to 12.83) | 5.23 (0.76 to 9.70) | −6.80 (−10.91 to −2.68) |
| EMS prenotification | −20.12 (−22.12 to −18.13) | −15.38 (−19.52 to −11.23) | −18.36 (−21.71 to −15.01) | −22.95 (−26.36 to −19.54) |
| After hours | −2.78 (−4.43 to −1.13) | 10.95 (7.92 to 13.97) | −7.83 (−10.75 to −4.92) | −5.35 (−8.21 to −2.48) |
| During pandemic | 16.13 (13.84 to 18.43) | 3.21 (−0.21 to 6.62) | 27.68 (23.64 to 31.71) | 16.10 (12.49 to 19.71) |
| Transferring hospital characteristics | ||||
| Primary stroke center | 4.97 (−0.96 to 10.90) | −0.78 (−7.51 to 5.96) | 6.19 (−2.90 to 15.28) | 9.75 (1.81 to 17.69) |
| Location | ||||
| Rural | [Reference] | [Reference] | [Reference] | [Reference] |
| Urban | 14.30 (7.34 to 21.25) | −9.63 (−17.33 to −1.93) | 24.22 (13.42 to 35.03) | 14.19 (5.35 to 23.02) |
| Annual thrombolysis volume | ||||
| 0-9 | [Reference] | [Reference] | [Reference] | [Reference] |
| 10-19 | 5.94 (−1.15 to 13.04) | 2.40 (−6.58 to 11.39) | 5.00 (−5.35 to 15.35) | 6.06 (−2.90 to 15.03) |
| 20-29 | 7.79 (−1.43 to 17.01) | −3.42 (−13.76 to 6.92) | 11.95 (−2.49 to 26.39) | 14.32 (2.29 to 26.35) |
| 30-126 | 27.09 (13.31 to 40.87) | −2.68 (−14.89 to 9.53) | 22.82 (2.39 to 43.24) | 33.79 (15.23 to 52.35) |
| Teaching status | ||||
| Nonteaching | [Reference] | [Reference] | [Reference] | [Reference] |
| Teaching | −3.20 (−8.97 to 2.58) | 0.75 (−6.68 to 8.19) | −1.56 (−10.47 to 7.35) | −5.66 (−13.06 to 1.73) |
| Daily census | ||||
| 0-99 | [Reference] | [Reference] | [Reference] | [Reference] |
| 100-199 | 9.88 (2.63 to 17.12) | 10.05 (1.70 to 18.41) | 1.52 (−8.95 to 11.99) | 23.20 (12.99 to 33.42) |
| ≥200 | 26.06 (14.84 to 37.28) | 7.22 (−3.85 to 18.29) | 6.31 (−9.40 to 22.03) | 58.53 (40.44 to 76.61) |
Abbreviations: CAD, coronary artery disease; EMS, emergency medical services; IV, intravenous; MI, myocardial infarction; NA, not applicable; NIH, National Institute of Health; TIA, transient ischemic attack.
The intercept represents the median door-in-door-out time for each model with all patient and hospital characteristics set as the reference category. Analysis outputs from these models are reported as minutes greater or less than the intercept (median door-in-door-out time).
Other includes American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, or unable to determine; extracted from the medical record for inclusion in the registry.
Score ranges from 0-42 (higher scores indicate greater severity).
The following were significantly associated with shorter median door-in-door-out times: acute ischemic stroke eligible for endovascular therapy vs hemorrhagic stroke subgroup (−16.83 minutes; 95% CI, −21.01 to −12.66 minutes), NIHSS score exceeding 12 vs 0 to 1 (−66.68 minutes; 95% CI, −68.67 to −64.68 minutes), and EMS prenotification (−20.12 minutes; 95% CI, −22.12 to −18.13 minutes).
Significantly longer median door-in-door-out times were associated with the following factors in patients with acute ischemic stroke eligible for endovascular therapy: age 80 years or older vs 18 through 59 years (12.29 minutes; 95% CI, 7.78-16.79 minutes), female sex (4.16 minutes; 95% CI, 1.26-7.07 minutes), Black non-Hispanic vs White non-Hispanic (12.36 minutes; 95% CI, 7.46-17.26 minutes), Hispanic vs White non-Hispanic (11.20 minutes; 95% CI, 4.69-17.71 minutes), and admitted after hours (10.95 minutes; 95% CI, 7.92-13.97 minutes). The following characteristics were associated with significantly shorter median door-in-door-out times: IV thrombolysis administration (−15.30 minutes; 95% CI, −18.24 to −12.36 minutes), NIHSS score exceeding 12 vs 0 to 1 (−77.98 minutes; 95% CI, −83.57 to −72.32 minutes), urban location (−9.63 minutes; 95% CI, −17.33 to −1.93 minutes), and EMS prenotification (−15.38 minutes; 95% CI, −19.5 to −11.23 minutes).
GEE logistic regression (eTable 1 in Supplement 2), rule-based imputation (eTable 2 in Supplement 2), and multiple imputation (eTable 3 in Supplement 3) models yielded overall similar results to the above (eResults in Supplement 1). Notably, with imputations, the point estimate of the association between Black race or Hispanic ethnicity and door-in-door-out times was somewhat attenuated. In post hoc exploratory analyses, increasing time from the last time a patient was known to be well to ED arrival was significantly associated with increasing door-in-door-out times and higher volume of stroke transfers per hospital was significantly associated with decreasing door-in-door-out times (eTable 4 in Supplement).
Discussion
In this study of patients with acute stroke transferred from hospitals participating in the Get With The Guidelines–Stroke registry, the overall median door-in-door-out time was 174 minutes. Several patient and hospital factors, including age, female sex, Black race, Hispanic ethnicity, stroke severity, stroke type or reason for transfer, and EMS prenotification were associated with door-in-door-out times.
For the subgroup with acute ischemic stroke eligible for endovascular therapy, the median door-in-door-out time was 132 minutes, the fastest door-in-door-out times that may be explained by the clearly efficacious and time-dependent nature of endovascular therapy and existence of established protocols for screening, identification, and rapid transfer.3,8 It is well established in the literature that decreased time to reperfusion can increase the likelihood of good clinical outcomes2,30; thus, the current study provides contemporary national door-in-door-out times that could serve as a baseline for future broad-scale quality improvement interventions.
In 2013, the Brain Attack Coalition recommended a door-in-door-out time of 120 minutes or less for acute stroke–ready hospitals, hospitals capable of initiating acute stroke care before transferring appropriate patients for definitive care.6 In this study, only 27.3% of patients had a door-in-door-out time of 120 minutes or less, suggesting that current median door-in-door-out times exceed this recommended target.
A possible explanation for why the hemorrhagic stroke subgroup had faster door-in-door-out times than the acute ischemic stroke–other subgroup is that guidelines recommend emergency transfer of patients with hemorrhagic stroke from community hospitals to centers with dedicated stroke expertise,31,32 significantly streamlining the transfer algorithm for such patients. It is also likely that additional workup and treatment of acute ischemic stroke, including computed tomographic (CT) angiogram as well as IV thrombolysis, adds substantial time7 or impedes transfer.
From a systems and quality improvement standpoint, EMS prenotification, not merely EMS mode of arrival, was significantly associated with shorter door-in-door-out times in the overall cohort and in the subgroup with acute ischemic stroke eligible for endovascular therapy. EMS prenotification has been previously associated with reduced door-to-needle times in acute ischemic stroke thrombolysis.33 The data from this study suggest that EMS prenotification may also be helpful for expediting door-in-door-out times.
This study also found sex and race and ethnic disparities in door-in-door-out times. Black race and Hispanic ethnicity were both significantly associated with longer door-in-door-out times. Racial disparities in stroke care and outcomes are well described and constitute a major public health concern.34 More specifically, Black patients and patients with low-income may be less likely to receive endovascular therapy17; however, this has been improving over time.18 Although limited access to advanced care may be one explanation, it may also be a multifactorial problem with contributions from structural racism and other factors at the hospital and systems levels as well.35 Additionally, female sex was significantly associated with prolonged door-in-door-out time. Prior literature has shown that female patients are less likely to receive certain stroke care benchmarks, such as door-to-CT time of 25 minutes or less.19 Controlling for insurance status partially mitigated these disparities, but further study is warranted to ascertain the underlying causes and to ultimately implement system redesigns to achieve health equity.
There was substantial geographic variation in overall door-in-door-out times by state and each stroke subgroup. Urban hospital location was significantly associated with prolonged door-in-door-out times in the overall cohort compared with a rural location. However, the direction of this association was not uniform across subgroups. Comparing the subgroup with acute ischemic stroke eligible for endovascular therapy with the hemorrhagic stroke subgroup, the former was associated with faster times in urban locations whereas the latter with slower times in urban locations. Prior literature has shown that patients with hemorrhagic stroke hospitalized at rural hospitals had twice the odds of mortality than those in urban hospitals,36 and thus ED clinicians in rural areas may be quicker to transfer such patients than clinicians in urban areas. Another study found that patients with acute ischemic stroke undergoing endovascular therapy from rural areas had worse functional outcomes than those from urban areas, an association hypothesized to be due to longer times to reperfusion in rural vs urban patients.37 Indeed, a rural location was significantly associated with increased door-in-door-out time among patients with acute ischemic stroke eligible for endovascular therapy, although time to reperfusion was not assessed in this study. Reducing geographic variation in care is a worthy goal for future quality improvement initiatives.
During the COVID-19 pandemic, the median door-in-door-out time in the overall stroke cohort was 16 minutes greater than it was prior to the pandemic. The COVID-19 pandemic was associated with a transient decline in overall stroke hospitalizations, interhospital transfer for acute interventions, IV thrombolysis,38 and endovascular therapy.39 In-hospital delays in care, particularly with acute stroke treatments such as IV thrombolysis and endovascular therapy, were demonstrated in some cases.40 However, patients admitted with stroke during the COVID-19 pandemic had a higher probability of having a large vessel occlusion warranting endovascular therapy, higher in-hospital mortality, and higher baseline NIHSS scores,41 potentially related to the postulated unique pathophysiology in COVID-19–associated stroke,42 as well as higher thresholds for patients presenting to medical attention during the pandemic.43 Further delineation of the specific factors leading to time delays in acute stroke treatment and transfer during the COVID-19 pandemic is imperative to optimize care delivery during future health system emergencies.
Limitations
This study has several limitations. First, missing or incomplete data are a limitation of this data set. Hospitals sending patients for transfer may be more likely to have missing or incomplete data. Of note, the NIHSS score was missing in 21.4% of the study sample. The data set also included a large proportion of patients with hemorrhagic strokes, and NIHSS score is not typically recorded for these patients. The missingness of the NIHSS score may not be random; a previous study of the Get With The Guidelines–Stroke data found that documentation of NIHSS scores was higher in patients who arrived by ambulance, arrived soon after onset, and were treated at primary stroke centers.44 Nearly half of patients had missing variables related to vascular imaging. These missing data on imaging and other procedural steps make understanding the root causes of interhospital transfer delays challenging.
Second, there is inherent selection bias because hospitals participating in the Get With The Guidelines–Stroke registry have exhibited an interest in tracking and improving stroke care. The majority of the patients in this study were transferred from teaching hospitals in urban areas, which is typical of participating hospitals12 but differs from a prior study reporting US nationwide interhospital transfer for acute ischemic stroke and TIA.45 Together, these factors may somewhat limit the generalizability of the study results, although, overall, the registry has been shown to be accurate46 and representative of the national Medicare stroke population.12
Third, the self-report of transfer indication (eg, for endovascular therapy) may be erroneous. Fourth, although EMS prenotification was found to be a key process step associated with door-in-door-out time, there was a lack of information on EMS systems (eg, public vs private). Fifth, some potential determinants of door-in-door-out time were not considered in this analysis, including: distance to comprehensive stroke center and bed availability (previous studies have shown this was a rate-limiting issue for care especially in the early pandemic)47; simultaneous vs consecutive vascular imaging; and utilization of automated artificial intelligence imaging software for CT angiogram/CT perfusion. Several additional hospital-level variables were derived and included in eTable 4 in Supplement 2; however, given that these variables were not present within the original Get With The Guidelines data set, these results should be treated as exploratory and confirmed with future research.
Sixth, although the analysis included a covariate to assess the impact of the pandemic on door-in-door-out times, the dichotomized variable may not sufficiently capture the temporal effects of COVID-19 in EMS availability, hospital capacity, and bed availability, which impacted interhospital transfer. Seventh, the current study did not evaluate for an association between door-in-door-out times and clinical outcomes, a crucial future area of study.
Conclusions
In this US registry-based study of patients who required interhospital transfer for acute stroke, the median door-in-door-out time was 174 minutes, which was longer than current recommendations for acute stroke transfer. Disparities and modifiable health system factors associated with longer door-in-door-out times are suitable targets for quality improvement initiatives.
eMethods
eResults
eReferences
eTable 1. GEE Logistic Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
eTable 2. Rule-Based Imputed GEE Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
eTable 3. Multiple Imputations GEE Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
eTable 4. Exploratory GEE Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
Data Sharing Statement
References
- 1.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480-2488. doi: 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 3.Ali SF, Fonarow G, Liang L, et al. Rates, characteristics, and outcomes of patients transferred to specialized stroke centers for advanced care. Circ Cardiovasc Qual Outcomes. 2018;11(9):e003359. doi: 10.1161/CIRCOUTCOMES.116.003359 [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Xian Y, Sheng S, et al. Use, temporal trends, and outcomes of endovascular therapy after interhospital transfer in the United States. Circulation. 2019;139(13):1568-1577. doi: 10.1161/CIRCULATIONAHA.118.036509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroke outpatient. In: Specifications Manual for Joint Commission National Quality Measure (v2021B); 2021. Accessed January 31, 2023. https://manual.jointcommission.org/releases/TJC2021B/MIF0391.html
- 6.Alberts MJ, Wechsler LR, Jensen MEL, et al. Formation and function of acute stroke-ready hospitals within a stroke system of care recommendations from the Brain Attack Coalition. Stroke. 2013;44(12):3382-3393. doi: 10.1161/STROKEAHA.113.002285 [DOI] [PubMed] [Google Scholar]
- 7.Prabhakaran S, Khorzad R, Parnianpour Z, et al. Door-in-door-out process times at primary stroke centers in Chicago. Ann Emerg Med. 2021;78(5):674-681. doi: 10.1016/j.annemergmed.2021.06.018 [DOI] [PubMed] [Google Scholar]
- 8.Rinaldo L, Brinjikji W, McCutcheon BA, et al. Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J Neurointerv Surg. 2017;9(12):1166-1172. doi: 10.1136/neurintsurg-2016-012824 [DOI] [PubMed] [Google Scholar]
- 9.McTaggart RA, Moldovan K, Oliver LA, et al. Door-in-door-out time at primary stroke centers may predict outcome for emergent large vessel occlusion patients. Stroke. 2018;49(12):2969-2974. doi: 10.1161/STROKEAHA.118.021936 [DOI] [PubMed] [Google Scholar]
- 10.Zachrison KS, Onnela JP, Hernandez A, et al. Ischemic stroke transfer patterns in the northeast United States. J Stroke Cerebrovasc Dis. 2019;28(2):295-304. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.048 [DOI] [PubMed] [Google Scholar]
- 11.Holl JL, Khorzad R, Zobel R, et al. Risk assessment of the door-in-door-out process at primary stroke centers for patients with acute stroke requiring transfer to comprehensive stroke centers. J Am Heart Assoc. 2021;10(18):e021803. doi: 10.1161/JAHA.121.021803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves MJ, Fonarow GC, Smith EE, et al. Representativeness of the Get With The Guidelines-Stroke Registry: comparison of patient and hospital characteristics among Medicare beneficiaries hospitalized with ischemic stroke. Stroke. 2012;43(1):44-49. doi: 10.1161/STROKEAHA.111.626978 [DOI] [PubMed] [Google Scholar]
- 13.Smaha LA; American Heart Association . The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148(5)(suppl):S46-S48. doi: 10.1016/j.ahj.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 14.Schwamm LH, Fonarow GC, Reeves MJ, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119(1):107-115. doi: 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 15.Protection of human subjects: revised Common Rule. Agency for Healthcare Research and Quality. Accessed May 2, 2023. https://www.ahrq.gov/funding/policies/human-subjects/index.html
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta AM, Fifi JT, Shoirah H, et al. Racial and socioeconomic disparities in the use and outcomes of endovascular thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol. 2021;42(9):1576-1583. doi: 10.3174/ajnr.A7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheriff F, Xu H, Maud A, et al. Temporal trends in racial and ethnic disparities in endovascular therapy in acute ischemic stroke. J Am Heart Assoc. 2022;11(6):e023212. doi: 10.1161/JAHA.121.023212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polineni SP, Perez EJ, Wang K, et al. ; Florida Stroke Registry . Sex and race-ethnic disparities in door-to-CT time in acute ischemic stroke: the Florida stroke registry. J Am Heart Assoc. 2021;10(7):e017543. doi: 10.1161/JAHA.120.017543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CB, Peterson ED, Smith EE, et al. Emergency medical service hospital prenotification is associated with improved evaluation and treatment of acute ischemic stroke. Circ Cardiovasc Qual Outcomes. 2012;5(4):514-522. doi: 10.1161/CIRCOUTCOMES.112.965210 [DOI] [PubMed] [Google Scholar]
- 21.Jahan R, Saver JL, Schwamm LH, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322(3):252-263. doi: 10.1001/jama.2019.8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acute stroke ready outpatient. In: Specifications Manual for Joint Commission National Quality Measures (v2021A); 2021. Accessed May 7, 2023. https://manual.jointcommission.org/releases/TJC2021A/MIF0347.html
- 24.Xiong Y, Gu H, Zhao XQ, et al. Clinical characteristics and in-hospital outcomes of varying definitions of minor stroke: from a large-scale nation-wide longitudinal registry. Stroke. 2021;52(4):1253-1258. doi: 10.1161/STROKEAHA.120.031329 [DOI] [PubMed] [Google Scholar]
- 25.Mamdani M, Sykora K, Li P, et al. Reader’s guide to critical appraisal of cohort studies: 2, assessing potential for confounding. BMJ. 2005;330(7497):960-962. doi: 10.1136/bmj.330.7497.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ader J, Wu J, Fonarow GC, et al. Hospital distance, socioeconomic status, and timely treatment of ischemic stroke. Neurology. 2019;93(8):e747-e757. doi: 10.1212/WNL.0000000000007963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62(2):143-148. doi: 10.1016/j.jclinepi.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romano JG, Smith EE, Liang L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the Get With the Guidelines-Stroke registry. JAMA Neurol. 2015;72(4):423-431. doi: 10.1001/jamaneurol.2014.4354 [DOI] [PubMed] [Google Scholar]
- 29.Højsgaard S, Halekoh U, Yan J. The R Package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1-11. doi: 10.18637/jss.v015.i02 [DOI] [Google Scholar]
- 30.Fransen PSS, Berkhemer OA, Lingsma HF, et al. ; Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands Investigators . Time to reperfusion and treatment effect for acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2016;73(2):190-196. doi: 10.1001/jamaneurol.2015.3886 [DOI] [PubMed] [Google Scholar]
- 31.Hemphill JC III, Greenberg SM, Anderson CS, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060. doi: 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 32.Bako AT, Bambhroliya A, Meeks J, et al. National trends in transfer of patients with primary intracerebral hemorrhage: an analysis of 12-year nationwide data. J Stroke Cerebrovasc Dis. 2021;30(12):106116. doi: 10.1016/j.jstrokecerebrovasdis.2021.106116 [DOI] [PubMed] [Google Scholar]
- 33.Kamal N, Smith EE, Jeerakathil T, Hill MD. Thrombolysis: improving door-to-needle times for ischemic stroke treatment—a narrative review. Int J Stroke. 2018;13(3):268-276. doi: 10.1177/1747493017743060 [DOI] [PubMed] [Google Scholar]
- 34.Levine DA, Duncan PW, Nguyen-Huynh MN, Ogedegbe OG. Interventions targeting racial/ethnic disparities in stroke prevention and treatment. Stroke. 2020;51(11):3425-3432. doi: 10.1161/STROKEAHA.120.030427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zachrison KS, Cross D. Racial disparities in endovascular thrombectomy: it’s more than just access. Stroke. 2022;53(3):864-866. doi: 10.1161/STROKEAHA.121.037921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otite FO, Akano EO, Akintoye E, et al. Rural-urban disparities in intracerebral hemorrhage mortality in the USA: preliminary findings from the national inpatient sample. Neurocrit Care. 2020;32(3):715-724. doi: 10.1007/s12028-020-00950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luchowski P, Szmygin M, Wojczal J, et al. Stroke patients from rural areas have lower chances for long-term good clinical outcome after mechanical thrombectomy. Clin Neurol Neurosurg. 2021;206:106687. doi: 10.1016/j.clineuro.2021.106687 [DOI] [PubMed] [Google Scholar]
- 38.Nogueira RG, Qureshi MM, Abdalkader M, et al. ; SVIN COVID-19 Global Stroke Registry; SVIN COVID-19 Global Stroke Registry . Global impact of COVID-19 on stroke care and IV thrombolysis. Neurology. 2021;96(23):e2824-e2838. doi: 10.1212/WNL.0000000000011885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yavagal DR, Saini V, Inoa V, et al. ; MT2020 Global Executive Committee . International survey of mechanical thrombectomy stroke systems of care during COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2021;30(8):105806. doi: 10.1016/j.jstrokecerebrovasdis.2021.105806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsanos AH, de Sa Boasquevisque D, Al-Qarni MA, et al. In-hospital delays for acute stroke treatment delivery during the COVID-19 pandemic. Can J Neurol Sci. 2021;48(1):59-65. doi: 10.1017/cjn.2020.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsanos AH, Palaiodimou L, Zand R, et al. Changes in stroke hospital care during the COVID-19 pandemic: a systematic review and meta-analysis. Stroke. 2021;52(11):3651-3660. doi: 10.1161/STROKEAHA.121.034601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamm B, Huang D, Royan R, Lee J, Marquez J, Desai M. Pathomechanisms and treatment implications for stroke in COVID-19: a review of the literature. Life (Basel). 2022;12(2):207. doi: 10.3390/life12020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jasne AS, Chojecka P, Maran I, et al. Stroke code presentations, interventions, and outcomes before and during the COVID-19 pandemic. Stroke. 2020;51(9):2664-2673. doi: 10.1161/STR.0000000000000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves MJ, Smith EE, Fonarow GC, et al. Variation and trends in the documentation of National Institutes of Health stroke scale in GWTG-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2015;8(6)(suppl 3):S90-S98. doi: 10.1161/CIRCOUTCOMES.115.001775 [DOI] [PubMed] [Google Scholar]
- 45.George BP, Doyle SJ, Albert GP, et al. Interfacility transfers for US ischemic stroke and TIA, 2006-2014. Neurology. 2018;90(18):e1561-e1569. doi: 10.1212/WNL.0000000000005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J. 2012;163(3):392-398, 398.e1. doi: 10.1016/j.ahj.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 47.Kerleroux B, Fabacher T, Bricout N, et al. ; SFNR, the ETIS registry, and the JENI-Research Collaborative . Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak: decreased activity, and increased care delays. Stroke. 2020;51(7):2012-2017. doi: 10.1161/STROKEAHA.120.030373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eResults
eReferences
eTable 1. GEE Logistic Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
eTable 2. Rule-Based Imputed GEE Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
eTable 3. Multiple Imputations GEE Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
eTable 4. Exploratory GEE Regression Results for the Overall, AIS-EVT Eligible, AIS-Other, and Hemorrhagic Groups
Data Sharing Statement


