This cross-sectional study evaluates the characteristics of infants admitted to US intensive care units for respiratory syncytial virus (RSV) infection during the 2022 seasonal peak.
Key Points
Question
What were the clinical characteristics and outcomes of respiratory syncytial virus (RSV)–related critical illness in US infants during peak 2022 RSV transmission?
Findings
This cross-sectional surveillance study of 600 infants across 39 hospitals requiring intensive care for RSV infection found that most were delivered full-term and previously healthy. Infants aged less than 3 months and those born prematurely were at higher risk for intubation.
Meaning
These findings support the use of new preventative interventions, including long-lasting monoclonal antibodies in all infants and maternal vaccination.
Abstract
Importance
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections (LRTIs) and infant hospitalization worldwide.
Objective
To evaluate the characteristics and outcomes of RSV-related critical illness in US infants during peak 2022 RSV transmission.
Design, Setting, and Participants
This cross-sectional study used a public health prospective surveillance registry in 39 pediatric hospitals across 27 US states. Participants were infants admitted for 24 or more hours between October 17 and December 16, 2022, to a unit providing intensive care due to laboratory-confirmed RSV infection.
Exposure
Respiratory syncytial virus.
Main Outcomes and Measures
Data were captured on demographics, clinical characteristics, signs and symptoms, laboratory values, severity measures, and clinical outcomes, including receipt of noninvasive respiratory support, invasive mechanical ventilation, vasopressors or extracorporeal membrane oxygenation, and death. Mixed-effects multivariable log-binomial regression models were used to assess associations between intubation status and demographic factors, gestational age, and underlying conditions, including hospital as a random effect to account for between-site heterogeneity.
Results
The first 15 to 20 consecutive eligible infants from each site were included for a target sample size of 600. Among the 600 infants, the median (IQR) age was 2.6 (1.4-6.0) months; 361 (60.2%) were male, 169 (28.9%) were born prematurely, and 487 (81.2%) had no underlying medical conditions. Primary reasons for admission included LRTI (594 infants [99.0%]) and apnea or bradycardia (77 infants [12.8%]). Overall, 143 infants (23.8%) received invasive mechanical ventilation (median [IQR], 6.0 [4.0-10.0] days). The highest level of respiratory support for nonintubated infants was high-flow nasal cannula (243 infants [40.5%]), followed by bilevel positive airway pressure (150 infants [25.0%]) and continuous positive airway pressure (52 infants [8.7%]). Infants younger than 3 months, those born prematurely (gestational age <37 weeks), or those publicly insured were at higher risk for intubation. Four infants (0.7%) received extracorporeal membrane oxygenation, and 2 died. The median (IQR) length of hospitalization for survivors was 5 (4-10) days.
Conclusions and Relevance
In this cross-sectional study, most US infants who required intensive care for RSV LRTIs were young, healthy, and born at term. These findings highlight the need for RSV preventive interventions targeting all infants to reduce the burden of severe RSV illness.
Introduction
Respiratory syncytial virus (RSV) is the leading cause of respiratory-related hospitalizations in young children worldwide.1,2 In the US, RSV annually accounts for approximately 57 000 hospitalizations in children younger than 5 years3 and is the leading cause of hospitalizations in the first year of life4; approximately 1 in 5 RSV-positive hospitalized young children were admitted to the intensive care unit (ICU).5,6 Although most children hospitalized with RSV are previously healthy and born at term,3,5,7,8 children with a history of prematurity or certain underlying medical conditions such as congenital heart disease, neurologic or neurodevelopmental disorders, chronic lung disease, and immunocompromising conditions are at higher risk for life-threatening RSV disease.9
Palivizumab, a monoclonal antibody (IgG) targeting the RSV F protein, is given monthly by intramuscular injection during RSV season to prevent RSV-associated lower respiratory tract infection (LRTI). It has previously been the only US-licensed product for this purpose, and its use is limited to high-risk infants.10 However, the significant cost of palivizumab is a barrier to its expanded use globally. The US Food and Drug Administration has just approved a long-acting (approximately 150 days) monoclonal RSV-neutralizing antibody and a maternal vaccine for RSV prevention is under consideration.11 These products may protect both high-risk and healthy infants from medically attended RSV-associated LRTI.12,13,14,15,16 Identifying which infants are at risk for severe RSV disease is essential for assessing future clinical effectiveness and guiding product usage recommendations.
The COVID-19 pandemic disrupted typical RSV circulation patterns, leading to atypical US epidemics during 2021 and 2022.17,18 Specifically, a surge in RSV-related hospitalizations and ICU admissions among infants and young children occurred in the fall of 2022.19 Therefore, we aimed to describe the characteristics, clinical course, and outcomes, including life-threatening complications, of these US infants admitted for pediatric intensive care during this period.
Methods
Study Design
This investigation included infants (<1 year old) from 39 US pediatric hospitals representing 27 states admitted over 2 months between October 17 and December 16, 2022, coinciding with the peak of the US RSV season.19 We selected October 17 as the study start date because most participating sites had reported RSV activity by that date, which reflected national surveillance data.20 Most sites participating in the RSV Pediatric Intensive Care (RSV-PIC) registry had previously participated in US Centers for Disease Control and Prevention (CDC)–funded pediatric critical illness studies,21 and others were recruited to ensure geographic representation. Inclusion in the RSV-PIC registry required admission to the ICU or high acuity unit for 24 or more hours for RSV-related illness, symptom onset of less than 10 days before hospitalization, and evidence of laboratory-confirmed RSV before or within 72 hours after hospitalization. Infants previously included in the RSV-PIC registry and newborns never discharged after birth were excluded. To ensure geographic representation, we included the first 15 to 20 consecutive eligible infants from each participating site. Clinical characteristics, interventions, laboratory tests (including other respiratory viral testing up to 72 hours and bacterial testing up to 3 days of hospital admission), and outcomes were collected using a standardized data collection form. Three physician investigators (N.B.H., E.R.L., and A.P.C.) adjudicated bacterial coinfection.
Ethics
The Boston Children’s Hospital institutional review board (IRB) reviewed and approved the protocol. Thirty-one sites relied on Boston Children’s Hospital’s single IRB; 7 had site-specific IRB approval. A waiver of consent was granted by all respective IRBs because this was considered a minimal risk study because the data collection was through medical record reviews and included limited protected health information. This activity was reviewed by CDC and determined to meet the requirements of public health surveillance per 45 CFR §46.101(b)(4). This report adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies.22
Variables of Interest
We assessed demographic and clinical characteristics, including age, sex, race and Hispanic ethnicity, site region, underlying medical conditions, and presence of pulmonary infiltrates on chest radiograph per radiology report. Data on race and ethnicity were collected from electronic health records. Prior studies have observed racial and ethnic disparities in the severity of life-threatening RSV disease. Therefore, these data enabled the assessment of potential disparities in RSV severity, as indicated by intubation status, across different racial and ethnic groups. Infants were considered previously healthy if they had no underlying medical conditions (defined in eAppendix in Supplement 1) and were not receiving prescription medications for chronic conditions.23
We also included laboratory markers of inflammation and disease severity, including complete blood count and blood gas values, signs and symptoms, and clinical outcomes.23 To assess illness severity, we used diagnosis of pediatric acute respiratory distress syndrome (PARDS; defined in eAppendix in Supplement 1),24 the pediatric sequential organ failure assessment (pSOFA) score,25 and the pediatric logistic organ dysfunction-2 (PELOD-2) score.26,27 We captured data on receipt of high flow nasal cannula, noninvasive or invasive mechanical ventilation, vasopressors, or extracorporeal membrane oxygenation (ECMO), and death during the index hospitalization.
Statistical Analysis
Demographic characteristics, gestational age, indication for admission, and presence of underlying conditions were stratified by tracheal intubation with receipt of invasive mechanical ventilation (intubation) status. Clinical presentation (including interventions received within the first 24 hours of hospitalization) and overall clinical course and outcomes were described by age group (0-2, 3-5, and 6-11 months). Categorical variables were analyzed by Pearson χ2 test or Fisher exact test and Cochran-Armitage tests for trend for comparisons by intubation status and age group. Continuous variables were analyzed by intubation status through Kruskal-Wallis or age group through Jonckheere-Terpstra tests for trend. Two-sided P values less than .05 were considered statistically significant.
Associations between intubation status and demographic factors, gestational age, and underlying conditions were evaluated through mixed-effects multivariable log-binomial regression models to calculate prevalence ratios (appropriate for cross-sectional data), including hospital as a random effect to account for between-site heterogeneity. Where models did not converge given limited sample size, exact Poisson regression models were used. Variables associated with intubation status (α < .35) were considered potential covariates in full multivariable models if they could plausibly be associated with each exposure of interest and intubation status. Variables were retained in multivariable models if removal altered the full model effect estimate by 10% or more. Data analysis was performed in SAS version 9.4 (SAS Institute) and R version 4.2.2 (R Project for Statistical Computing).
Results
Demographic and Clinical Characteristics
Of the 600 infants in the RSV-PIC registry, 559 (93.2%) were admitted within the first month of the study period, at which time two-thirds of sites had met their enrollment target. The median (IQR) age was 2.6 (1.4-6.0) months, and 361 were male (60%); 487 infants (81%) were previously healthy, 169 (29%) were born prematurely, and nearly one-quarter were intubated (Table 1). The eFigure in Supplement 1 displays the age distribution by months, stratified by intubation status. Compared with nonintubated infants, intubated infants were younger, were more frequently delivered prematurely, and more frequently had public insurance (Table 1). Although the proportions of infants included from each census region were similar, intubation status varied by census region with more intubated infants admitted in the Midwest (53 infants [37.1%]) and South (42 infants [29.4%]). Underlying medical conditions were not higher in the intubated group, but there was a lower frequency of nonrespiratory/noncardiac disorders (Table 1 and eTable 1 in Supplement 1).
Table 1. Demographic and Clinical Characteristics of Infants Admitted to the Intensive Care or High Acuity Unit With Respiratory Syncytial Virus Infection.
| Characteristic | Infants, No. (%) | P valuea | ||
|---|---|---|---|---|
| All (N = 600) | Nonintubated (n = 457) | Intubated (n = 143) | ||
| Age, median (IQR), mo | 2.6 (1.4-6.0) | 3.1 (1.6-6.4) | 1.9 (1.0-3.2) | <.001 |
| Age group | ||||
| 0-2 mos | 323 (53.8) | 222 (48.6) | 101 (70.6) | <.001 |
| 3-5 mos | 127 (21.2) | 106 (23.2) | 21 (14.7) | |
| 6-11 mos | 150 (25.0) | 129 (28.2) | 21 (14.7) | |
| Sex | ||||
| Male | 361 (60.2) | 277 (60.6) | 84 (58.7) | .69 |
| Female | 239 (39.8) | 180 (39.4) | 59 (41.3) | |
| Race and ethnicity | ||||
| Hispanic | 135 (22.5) | 102 (22.3) | 33 (23.1) | NA |
| Non-Hispanic Asian | 13 (2.2) | 11 (2.4) | 2 (1.4) | |
| Non-Hispanic Black | 95 (15.8) | 75 (16.4) | 20 (14.0) | |
| Non-Hispanic White | 265 (44.2) | 199 (43.5) | 66 (46.2) | |
| Multiple or otherb | 30 (5.0) | 21 (4.6) | 9 (6.3) | |
| Unknown | 62 (10.3) | 49 (10.7) | 13 (9.1) | |
| SVI score, median (IQR)c | 0.50 (0.39-0.64) | 0.50 (0.40-0.64) | 0.50 (0.38-0.65) | .98 |
| Insurance | ||||
| Public | 336 (56.0) | 242 (53.0) | 94 (65.7) | .050 |
| Private | 240 (40.0) | 196 (42.9) | 44 (30.8) | |
| Self-pay | 11 (1.8) | 9 (2.0) | 2 (1.4) | |
| Other or unknown | 13 (2.2) | 10 (2.2) | 3 (2.1) | |
| Prematurityd | 169 (28.9) | 116 (26.1) | 53 (37.5) | .01 |
| Gestational age, median (IQR)e | 34.0 (32.0-35.7) | 34.0 (32.4-35.7) | 34.0 (32.0-35.4) | .91 |
| Multiple pregnancyf | 28 (4.7) | 16 (3.5) | 12 (8.4) | .18 |
| Underlying conditions | ||||
| None | 487 (81.2) | 364 (79.6) | 123 (86.0) | .07 |
| At least one | 113 (18.8) | 93 (20.4) | 20 (14.0) | |
| Nonrespiratory, noncardiac | 48 (8.0) | 46 (10.1) | 2 (1.4) | <.001 |
| Cardiac, nonrespiratory | 20 (3.3) | 15 (3.3) | 5 (3.5) | .90 |
| Respiratory | 45 (7.5) | 32 (7.0) | 13 (9.1) | .41 |
| Chronic lung disease | 22 (3.7) | 14 (3.1) | 8 (5.6) | .16 |
| Neurologic | 13 (2.2) | 9 (2.0) | 4 (2.8) | .52 |
| Trisomy 21 | 8 (1.3) | 8 (1.8) | 0 | .21 |
| Reason for admission | ||||
| LRTI | 594 (99.0) | 453 (99.1) | 141 (98.6) | .58 |
| Apnea or bradycardia | 77 (12.8) | 36 (7.9) | 41 (28.7) | <.001 |
| Cardiac arrest at home with CPR | 3 (0.5) | 1 (0.2) | 2 (1.4) | .14 |
| CNS infection | 2 (0.3) | 0 | 2 (1.4) | .06 |
| Shock requiring vasopressors | 5 (0.8) | 0 | 5 (3.5) | .001 |
| Site region | ||||
| Northeast | 146 (24.3) | 125 (27.4) | 21 (14.7) | <.001 |
| Midwest | 145 (24.2) | 92 (20.1) | 53 (37.1) | |
| South | 154 (25.7) | 112 (24.5) | 42 (29.4) | |
| West | 155 (25.8) | 128 (28.0) | 27 (18.9) | |
Abbreviations: CNS, central nervous system; CPR, cardiopulmonary resuscitation; LRTI, lower respiratory tract infection; NA, not applicable; SVI, social vulnerability index.
Fisher exact test performed if any expected cell count was less than 5.
Multiple or other race and ethnicity group included infants who were of multiple races or were classified as Native Hawaiian/Pacific Islander or American Indian/Alaskan Native.
A total of 598 observations.
Denominator was for infants with documented prematurity status (total of 585 observations).
Gestational age provided for infants born at less than 37 weeks’ gestation.
Multiple pregnancy status was collected only for 320 infants aged less than 90 days.
The primary reason for admission among most infants was LRTI (594 infants [99%]), but infants who were intubated had a higher frequency of apnea or bradycardia (77 infants [13%]) and were more frequently younger than 3 months (Table 1). Intubated infants had a higher frequency of apnea, seizures, and increased sleepiness (difficult to arouse) but lower frequency of fever (Figure 1A). Older infants had higher frequency of fever, wheezing, rapid or shallow breathing, conjunctivitis, vomiting, and diarrhea, while younger infants had higher frequency of apnea (Figure 1B).
Figure 1. Signs and Symptoms in Infants Requiring Intensive Care for Respiratory Syncytial Virus Infection.
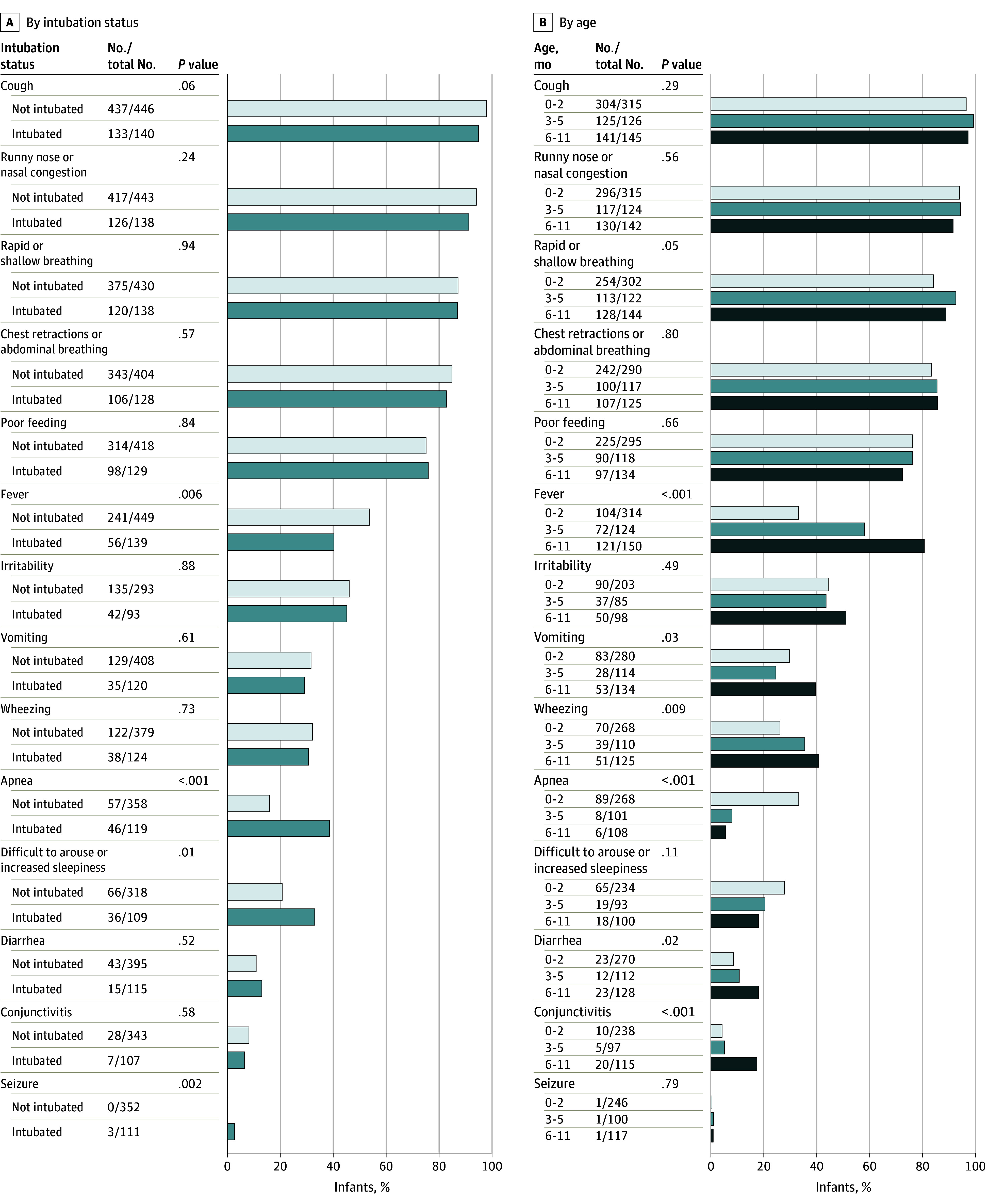
Clinical Presentation and Hospital Course
Among the 600 infants, 572 (95.3%) required oxygen support at admission (Table 2). Overall, 143 infants (24%) received invasive mechanical ventilation (median [IQR], 6.0 [4.0-10] days). Among those intubated, 101 infants (70.6%) were younger than 3 months. The highest level of respiratory support for nonintubated infants was high-flow nasal cannula (HFNC; 243 infants [40.5%]), followed by bilevel positive airway pressure (BiPAP; 150 infants [25.0%]) and continuous positive airway pressure (CPAP; 52 infants [8.7%]). The median (IQR) length of hospitalization for survivors was 5 (4-10) days. HFNC was the most common respiratory support at admission across all age groups. For infants younger than 3 months, intubation was the second most common respiratory support and for infants aged 3 months and older it was BiPAP or noninvasive ventilation. These patterns remained consistent throughout the hospital course; although the frequency of intubation in all age groups increased, the proportion remained highest for infants younger than 3 months. The median (IQR) days of invasive mechanical ventilation did not differ significantly between age groups (6 [4-10] days).
Table 2. Clinical Presentation and Course of Infants Admitted to the Intensive Care or High Acuity Unit With Respiratory Syncytial Virus Infection Stratified by Age Group in Months.
| Clinical presentation and course | Infants, No. (%) | P valuea | |||
|---|---|---|---|---|---|
| Total (N = 600) | 0-2 mos (n = 323) | 3-5 mos (n = 127) | 6-11 mos (n = 150) | ||
| Reason for admission | |||||
| LRTI | 594 (99.0) | 317 (98.1) | 127 (100.0) | 150 (100.0) | .04 |
| Apnea or bradycardia | 77 (12.8) | 67 (20.7) | 6 (4.7) | 4 (2.7) | <.001 |
| Cardiac arrest at home with CPR | 3 (0.5) | 3 (0.9) | 0 | 0 | .14 |
| CNS infection | 2 (0.3) | 2 (0.6) | 0 | 0 | .23 |
| Shock requiring vasopressors | 5 (0.8) | 3 (0.9) | 1 (0.8) | 1 (0.7) | .75 |
| Interventions within first 24 h | |||||
| Highest level of respiratory supportb | |||||
| None | 28 (4.7) | 16 (5.0) | 4 (3.1) | 8 (5.3) | .71 |
| Low-flow supplemental oxygen | 51 (8.5) | 30 (9.3) | 6 (4.7) | 15 (10.0) | .96 |
| High-flow nasal cannula oxygen | 252 (42.0) | 116 (35.9) | 71 (55.9) | 65 (43.3) | .03 |
| Continuous positive airway pressure | 52 (8.7) | 34 (10.5) | 7 (5.5) | 11 (7.3) | .18 |
| BiPAP/NIV | 114 (19.0) | 52 (16.1) | 24 (18.9) | 38 (25.3) | .02 |
| Invasive mechanical ventilation | 103 (17.2) | 75 (23.2) | 15 (11.8) | 13 (8.7) | <.001 |
| Cardiovascular support | 10 (1.7) | 6 (1.9) | 2 (1.6) | 2 (1.3) | .67 |
| Vasopressors | 6 (1.0) | 3 (0.9) | 1 (0.8) | 2 (1.3) | .72 |
| Clinical course | |||||
| Highest level of respiratory support | |||||
| None | 1 (0.2) | 1 (0.3) | 0 | 0 | >.99 |
| Low-flow supplemental oxygen | 11 (1.8) | 5 (1.5) | 1 (0.8) | 5 (3.3) | .30 |
| High-flow nasal cannula oxygen | 243 (40.5) | 114 (35.3) | 67 (52.8) | 62 (41.3) | .07 |
| Continuous positive airway pressure | 52 (8.7) | 33 (10.2) | 7 (5.5) | 12 (8.0) | .30 |
| BiPAP/NIV | 150 (25.0) | 69 (21.4) | 31 (24.4) | 50 (33.3) | .01 |
| Invasive mechanical ventilation | 143 (23.8) | 101 (31.3) | 21 (16.5) | 21 (14.0) | <.001 |
| Days ventilated, median (IQR) | 6 (4-10) | 6 (4-9) | 5 (3-10) | 7 (5-11) | .59 |
| Vasopressor-dependent shock | 27 (4.5) | 19 (5.9) | 3 (2.4) | 5 (3.3) | .15 |
| ECMO | 4 (0.7) | 2 (0.6) | 0 | 2 (1.3) | .53 |
| Days in ICU, median (IQR)c | 3 (2-7) | 4 (2-8) | 3 (2-5) | 3 (2-6) | .002 |
| Days in hospital, median (IQR)c | 5 (4-10) | 6 (4-12) | 5 (3-7) | 5 (3-8) | .001 |
| In-hospital death | 2 (0.3) | 0 | 0 | 2 (1.3) | .11 |
| Severity scores | |||||
| pSOFA score, median (IQR) | 4 (3-4) | 4 (3-5) | 3 (3-4) | 3 (3-4) | <.001 |
| PELOD-2 score, median (IQR) | 2 (1-4) | 2 (2-5) | 2 (0-4) | 0 (0-2) | <.001 |
| PARDS criteria met | 120 (20.0) | 72 (22.3) | 21 (16.5) | 27 (18.0) | .19 |
Abbreviations: BiPAP, bilevel positive airway pressure; CNS, central nervous system; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LRTI, lower respiratory tract infection; NIV, noninvasive ventilation; PARDS, pediatric acute respiratory distress syndrome; PELOD, pediatric logistic organ dysfunction; pSOFA, pediatric sequential organ failure assessment.
Fisher exact test performed if any expected cell count was less than 5.
Data displayed reflect responses to a question as to whether the infant received mechanical ventilation and/or oxygen support during the first 24 hours of hospital admission. Low-flow supplemental oxygen and invasive mechanical ventilation were also noted at admission if reported on day 1 of daily clinical variables. This information may reflect pre–pediatric intensive care unit care.
A total of 598 survivors.
Infants younger than 3 months had higher disease severity scores (ie, pSOFA and PELOD-2) and longer duration of ICU and hospital stay compared with the other age groups (Table 2). Four infants required ECMO; 2 infants died, 1 of whom was on ECMO. Details of severe clinical outcomes are provided in eTable 2 in Supplement 1.
Palivizumab Use
For 15 of the 17 infants born at less than 29 weeks’ gestation there was no documentation of receiving palivizumab despite being eligible. Three infants received palivizumab before hospitalization, none within the previous 30 days (eTable 3 in Supplement 1).
Factors Associated With Risk for Intubation
After adjusting for other factors, the risk of intubation was highest among infants younger than 3 vs 6 to 11 months old, those born prematurely (<37 weeks), and infants with public insurance compared with private (Figure 2). Five of 8 infants born at less than 30 weeks’ gestational age (62.5%) had a history of bronchopulmonary dysplasia (BPD), as did 1 of 45 infants (2.2%) born between 30 and 36 weeks’ gestational age. Although history of BPD was also associated with higher risk of intubation (adjusted prevalence ratio [aPR], 2.2; 95% CI, 1.0-4.8), this association is likely because BPD occurs exclusively in premature infants.
Figure 2. Factors Associated With Risk for Intubation in Infants Requiring Intensive Care for Respiratory Syncytial Virus Infection.
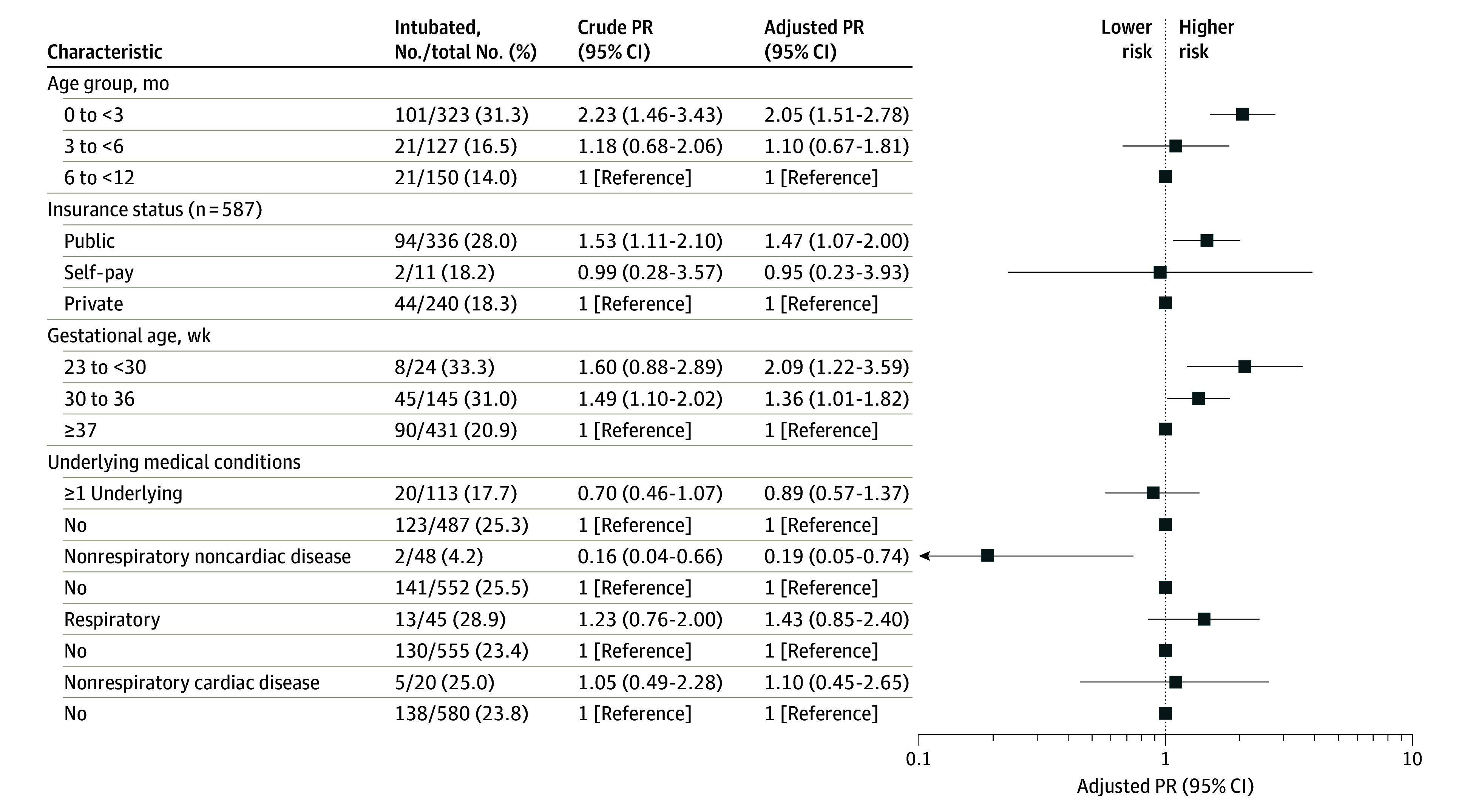
PR indicates prevalence ratio.
To determine if intubation risk was associated with high-flow nasal cannula use outside of ICU or high acuity units, we performed a post hoc analysis. During the study period, HFNC was administered in the wards of some hospitals (26 of 39 [67%]). The median proportion of patients intubated in the registry did not differ between hospitals who did and did not administer HFNC in the wards (25% vs 20%, respectively; P = .93; t17.89 = 0.08).
Bacterial Coinfection and Viral Codetection
Within 3 days of admission, 42 of 143 intubated infants (29.4%) had an endotracheal culture performed, of which 28 of 42 (66.7%) had a tracheal or lower respiratory tract bacterial coinfection; 8 of 22 infants (36.4%) tested had a urinary tract infection; the frequency of testing and positivity was low for meningitis (0 of 1) and bacteremia (2 of 11 [18.2%]) (eTable 4 in Supplement 1). The most common coinfections detected in endotracheal specimens were nontypeable Haemophilus influenzae (12 of 28 [42.9%]), Moraxella catarrhalis (9 of 28 [32.1%]), and Streptococcus pneumoniae (8 of 28 [28.6%]). The urinary tract infections were with E coli and Klebsiella spp and the bloodstream infections were Streptococcus pneumoniae and methicillin-sensitive Staphylococcus aureus.
A total of 128 children (21.3%) tested positive for at least 1 non-RSV respiratory virus. Of 556 and 554 infants who underwent SARS-CoV-2 and influenza testing (Table 3), 19 (3.4%) and 7 (1.2%) had positive results, respectively. Among the 304 infants for whom a respiratory virus panel was performed, rhinovirus/enterovirus (RV/EV) was most often detected (85 infants [28.0%]) (eTable 5 in Supplement 1).
Table 3. Viral Codetection and Bacterial Coinfection Among Infants Admitted to the Intensive Care Unit or High Acuity Unit With Respiratory Syncytial Virus Infection.
| Codetection or coinfection | Infants, No. (%) | ||
|---|---|---|---|
| All (N = 600) | Nonintubated (n = 457) | Intubated (n = 143) | |
| Viral codetectiona | |||
| SARS-CoV-2 tested | 556 (92.7) | 428 (93.7) | 128 (89.5) |
| SARS-CoV-2 detected | 19 (3.4) | 16 (3.7) | 3 (2.3) |
| Influenza tested | 554 (92.3) | 423 (92.6) | 131 (91.6) |
| Influenza detected | 7 (1.2) | 6 (1.4) | 1 (0.8) |
| Respiratory pathogen panel performedb | 304 (50.7) | 221 (48.4) | 83 (58.0) |
| Rhinovirus/enterovirus detected | 85 (28.0) | 62 (28.1) | 23 (27.7) |
| Any other virus positive | 33 (10.9) | 24 (10.9) | 9 (10.8) |
| Probable or confirmed bacterial coinfectiona | |||
| Tracheal or lower respiratory tract | 28 of 42 (66.7) | NA | 28 of 42 (66.7) |
| Blood | 2 of 11 (18.2) | 0 of 6 | 2 of 5 (40.0) |
| Urine | 8 of 22 (36.4) | 5 of 13 (38.5) | 3 of 9 (33.3) |
Abbreviation: NA, not applicable.
Viral codetections from symptom onset up to 72 hours of admission and bacterial coinfections up to 3 days of hospital admission.
Results from other respiratory virus testing, including from respiratory pathogen panels, were collected where available. Other respiratory viruses included parainfluenza (types 1, 2, 3, and 4), human metapneumovirus, adenovirus, rhinovirus/enterovirus, and seasonal coronaviruses (NL63, OC43, HKU1, and 229E).
Discussion
Our RSV-PIC registry included a nationally representative cohort of 600 RSV-confirmed infants admitted for intensive care in 39 hospitals across 27 US states during the peak 2022 RSV season. Almost one-quarter of the infants received invasive mechanical ventilation. Young infants aged less than 3 months had more severe clinical presentations and outcomes, including apnea at presentation, receipt of invasive mechanical ventilation, and longer ICU and hospital length of stays. Although having an underlying medical condition or history of prematurity is a factor associated with risk for severe RSV illness, most infants receiving ICU-level care were previously healthy and born at term. Younger and premature infants were at higher risk for intubation, but most intubated infants were born at term. Although mortality was rare, these findings emphasize the significant morbidity caused by RSV in US infants.
These infants with RSV-associated LRTI who required intermediate or intensive care during the so-called triple-demic of RSV, influenza, and SARS-CoV-2 in fall 2022 overwhelmed pediatric units across the US leading to widespread institutional ICU bed capacity challenges.28 Before the COVID-19 pandemic, the seasonality of RSV infections in temperate climates was consistent year-to-year, with cases increasing in late autumn, peaking in winter, and declining in early spring.29 However, nonpharmaceutical interventions, such as physical distancing and masking, introduced during the COVID-19 pandemic altered the circulation of RSV and other viruses, resulting in negligible RSV transmission during the 2020 to 2021 winter season.30 Simulation modeling predicted that out-of-season RSV outbreaks in 2021 and 2022 would be more intense than prepandemic outbreaks due to the expansion of the susceptible population.31 In the summer of 2021, RSV resurged out-of-season32 with reports of a markedly higher percentage of children admitted to the ICU.33 In 2022, RSV infections peaked again in October during the period where we captured data, with overall US incidence rates of RSV-associated hospitalizations roughly twice as high as at any prepandemic peak.19 In response to the RSV surge, the American Academy of Pediatrics and Children’s Hospital Association called for an emergency declaration on November 14, 2022, and the CDC issued a Health Alert Network Health Advisory.34
Although we enrolled infants aged up to 1 year, most were younger than 3 months, and this age group accounted for over 70% of intubated infants, most of whom were previously healthy. These results are consistent with previous studies in other high-resource countries. In a study of 604 Australian children (median age 4 months) with community-acquired RSV infections admitted to the ICU from 2005 to 2015, 55% of infants had no underlying medical condition and 32% required mechanical ventilation; 94% were admitted because of LRTI with a median duration of ICU stay of 3.7 days.9 In the Netherlands, of 2161 children younger than 2 years with RSV bronchiolitis from 2003 to 2016, 78% were younger than 3 months and 54% were term, but the percentage receiving invasive mechanical ventilation was higher at 72% and the median length of ICU stay was 8 days.35 Our cohort is similar to that from the US Pediatric Health Information Systems database from 2010 to 2019 in which 17% of children with bronchiolitis admitted to the ICU required invasive mechanical ventilation, with a median length of ICU stay of 2 to 3 days.6 Even though our registry included only infants with RSV, it is the most common cause of bronchiolitis.36 These studies highlight the need for prevention measures against severe RSV disease, including in young, healthy infants. In addition to preventing hospitalization associated with acute illness, preventing RSV LRTI may also change long-term outcomes potentially associated with RSV, including increased risk of developing recurrent wheezing/asthma or premature adult death.37,38
RSV is frequently codetected with other respiratory viruses, and codetection frequency depends on various factors, including age, setting, and type and timing of testing.39 In a previous study, we found that SARS-CoV-2 and influenza were associated with critical illness in children.40 However, in this cohort, neither virus was commonly detected, underscoring the role of RSV as a significant cause of severe respiratory disease in infants.28 Among the subset of infants who underwent respiratory pathogen panel testing, we found that RSV was most commonly codetected with RV/EV, as has been shown consistently in previous studies.39,41 However, the clinical significance of RV/EV codetection is unclear. In 1 study,42 clinical characteristics and outcomes of children younger than 2 years with RSV/RV codetection were largely similar to those with RSV-only detection but distinct from those with RV-only detection, which suggests a bystander role for RV when codetected with RSV. The frequency of serious bacterial infections in our cohort was fairly low. Of intubated infants that had a bacterial endotracheal culture sent within the first 3 hospitalization days, nearly two-thirds had pathogens considered true or probable infections. However, these infants comprised less than 5% of the entire RSV-PIC cohort. The role of bacterial coinfection in RSV severity remains unclear, and further investigation is needed.
Limitations
Our findings are subject to limitations. Our cohort represents only the first 15 to 20 consecutive RSV cases at each hospital as we aimed to get a representative sampling across the US, limiting the influence of single centers or regions. Thus, we did not include all cases of severe RSV admitted to the ICU during the 2-month study period, which also did not encompass the full RSV season at these centers. In addition, including only clinician-ordered, laboratory-confirmed RSV cases may have resulted in missing cases that were not tested for RSV. Furthermore, although most infants were tested for influenza and SARS-CoV-2, only half were tested with a respiratory viral panel so we are unable to systematically assess the influence of viral codetection on disease severity. Not all intubated infants were tested for bacterial coinfection and it is not possible to acquire bacterial lower respiratory samples in nonintubated patients, so the role of bacterial coinfection may be underestimated. Moreover, the proportion of intubated infants varied across regions. Although we may have selected a more severely ill cohort of infants from the hospitals that administered HFNC in the wards, our post hoc analysis did not support this hypothesis. Thus, further investigation is essential to clarify regional disparities, which could involve differences in referral practices. The study period missed the RSV peak in some states, such as Florida, precluding meeting target enrollment for that site. Infants in our cohort may not be representative of all states, which may limit generalizability. Furthermore, since we relied on medical record abstractions, data missingness is possible. We also excluded infants that were admitted to the ICU for less than 24 hours and our study is biased toward the most severe RSV infections among infants.
Conclusions
In conclusion, this surveillance registry of infants with critical RSV illness highlights that RSV causes significant morbidity in previously healthy term infants as well as those born prematurely and those with underlying conditions. Thus, prevention strategies are needed for all infants. In our study, only 2 of 17 infants eligible for palivizumab because of gestational age less than 29 weeks had documentation of receipt, highlighting potential barriers to administration and emphasizing the need to ensure that all eligible patients receive it in a timely manner. The recent experience during the COVID-19 pandemic has shown that community mitigation measures are effective in decreasing the circulation of non–COVID-19 respiratory viral pathogens, including RSV. The resurgence of RSV when these measures were lifted suggests that studies of nonpharmaceutical interventions may be important for future surges, but also underscores the need for therapeutic interventions to target all infants to reduce the overall burden of severe RSV disease. The clinical severity information in this investigation can also help guide the design of upcoming RSV prophylactic and maternal RSV vaccine effectiveness studies.
eAppendix. Definitions
eFigure. Age Distribution of Infants Requiring Intensive Care for RSV Infection
eTable 1. Underlying Medical Conditions Present in at Least 5 Critically Ill Infants With RSV, by Intubation Status
eTable 2. Clinical Characteristics of Infants Who Received ECMO or Died During Their Hospitalization
eTable 3. Clinical Characteristics of Infants Who Received Palivizumab Before Hospitalization
eTable 4. Probable or Confirmed Bacterial Coinfections Isolated Within 72 Hours of Admission Among Critically Ill Children With RSV, by Intubation Status, Underlying Medical Condition Status, and Specimen Type
eTable 5. Viral Codetections Among Critically Ill Children With RSV, by Intubation Status
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Shi T, Balsells E, Wastnedge E, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta-analysis. J Glob Health. 2015;5(2):020416. doi: 10.7189/jogh.05.020416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356-1364. doi: 10.1093/infdis/jiy056 [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588-598. doi: 10.1056/NEJMoa0804877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh M, Movva N, Jiang X, et al. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009-2019: a study of the national (nationwide) inpatient sample. J Infect Dis. 2022;226(suppl 2):S154-S163. doi: 10.1093/infdis/jiac120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics. 2020;146(1):e20193611. doi: 10.1542/peds.2019-3611 [DOI] [PubMed] [Google Scholar]
- 6.Pelletier JH, Au AK, Fuhrman D, Clark RSB, Horvat C. Trends in bronchiolitis ICU admissions and ventilation practices: 2010-2019. Pediatrics. 2021;147(6):e2020039115. doi: 10.1542/peds.2020-039115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddadin Z, Beveridge S, Fernandez K, et al. Respiratory syncytial virus disease severity in young children. Clin Infect Dis. 2021;73(11):e4384-e4391. doi: 10.1093/cid/ciaa1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rankin DA, Haddadin Z, Lipworth L, et al. Comparison of clinical presentations and burden of respiratory syncytial virus in infants across three distinct healthcare settings in Davidson County, Tennessee. Ther Adv Infect Dis. 2022;9:20499361221112171. doi: 10.1177/20499361221112171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham H, Thompson J, Wurzel D, Duke T. Ten years of severe respiratory syncytial virus infections in a tertiary paediatric intensive care unit. J Paediatr Child Health. 2020;56(1):61-67. doi: 10.1111/jpc.14491 [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):e620-e638. doi: 10.1542/peds.2014-1666 [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration . FDA approves new drug to prevent RSV in babies and toddlers. 2023. Accessed July 20, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
- 12.Rainisch G, Adhikari B, Meltzer MI, Langley G. Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants. Vaccine. 2020;38(2):251-257. doi: 10.1016/j.vaccine.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammitt LL, Dagan R, Yuan Y, et al. ; MELODY Study Group . Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837-846. doi: 10.1056/NEJMoa2110275 [DOI] [PubMed] [Google Scholar]
- 14.Simões EAF, Center KJ, Tita ATN, et al. Prefusion F protein-based respiratory syncytial virus immunization in pregnancy. N Engl J Med. 2022;386(17):1615-1626. doi: 10.1056/NEJMoa2106062 [DOI] [PubMed] [Google Scholar]
- 15.Kampmann B, Madhi SA, Munjal I, et al. ; MATISSE Study Group . Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451-1464. doi: 10.1056/NEJMoa2216480 [DOI] [PubMed] [Google Scholar]
- 16.Muller WJ, Madhi SA, Seoane Nuñez B, et al. ; MELODY Study Group . Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med. 2023;388(16):1533-1534. doi: 10.1056/NEJMc2214773 [DOI] [PubMed] [Google Scholar]
- 17.Haddadin Z, Schuster JE, Spieker AJ, et al. Acute respiratory illnesses in children in the SARS-CoV-2 pandemic: prospective multicenter study. Pediatrics. 2021;148(2):e2021051462. doi: 10.1542/peds.2021-051462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus—United States, 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72(14):355-361. doi: 10.15585/mmwr.mm7214a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . RSV-NET interactive dashboard. 2023. Accessed March 6, 2023. https://www.cdc.gov/rsv/research/rsv-net/dashboard.html
- 20.Centers for Disease Control and Prevention . The National Respiratory and Enteric Virus Surveillance System (NREVSS). 2023. Accessed March 6, 2023. https://www.cdc.gov/surveillance/nrevss/index.html
- 21.Randolph AG, Bembea MM, Cheifetz IM, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Pediatric Acute Lung Injury and Sepsis Investigators (PALISI): evolution of an investigator-initiated research network. Pediatr Crit Care Med. 2022;23(12):1056-1066. doi: 10.1097/PCC.0000000000003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800-804. doi: 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 23.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334-346. doi: 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pediatric Acute Lung Injury Consensus Conference Group . Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5):428-439. doi: 10.1097/PCC.0000000000000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. doi: 10.1001/jamapediatrics.2017.2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F; Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP) . PELOD-2: an update of the pediatric logistic organ dysfunction score. Crit Care Med. 2013;41(7):1761-1773. doi: 10.1097/CCM.0b013e31828a2bbd [DOI] [PubMed] [Google Scholar]
- 27.Olson SM, Newhams MM, Halasa NB, et al. ; Pediatric Intensive Care Influenza Investigators . Vaccine Effectiveness against life-threatening influenza illness in US children. Clin Infect Dis. 2022;75(2):230-238. doi: 10.1093/cid/ciab931 [DOI] [PubMed] [Google Scholar]
- 28.Furlow B. Triple-demic overwhelms paediatric units in US hospitals. Lancet Child Adolesc Health. 2023;7(2):86. doi: 10.1016/S2352-4642(22)00372-8 [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Hodgson D, Wang X, Atkins KE, Feikin DR, Nair H. Respiratory syncytial virus seasonality and prevention strategy planning for passive immunisation of infants in low-income and middle-income countries: a modelling study. Lancet Infect Dis. 2021;21(9):1303-1312. doi: 10.1016/S1473-3099(20)30703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuang YC, Lin KP, Wang LA, Yeh TK, Liu PY. The impact of the COVID-19 pandemic on respiratory syncytial virus infection: a narrative review. Infect Drug Resist. 2023;16:661-675. doi: 10.2147/IDR.S396434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Z, Pitzer VE, Shapiro ED, Bont LJ, Weinberger DM. Estimation of the timing and intensity of reemergence of respiratory syncytial virus following the COVID-19 pandemic in the US. JAMA Netw Open. 2021;4(12):e2141779. doi: 10.1001/jamanetworkopen.2021.41779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez A, Lively JY, Curns A, et al. ; New Vaccine Surveillance Network Collaborators . Respiratory virus surveillance among children with acute respiratory illnesses—new vaccine surveillance network, United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2022;71(40):1253-1259. doi: 10.15585/mmwr.mm7140a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics. 2021;148(3):e2021052089. doi: 10.1542/peds.2021-052089 [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention . Increased respiratory virus activity, especially among children, early in the 2022-2023 fall and winter. Updated March 6, 2023. Accessed July 12, 2023. https://emergency.cdc.gov/han/2022/han00479.asp
- 35.Linssen RS, Bem RA, Kapitein B, et al. ; PICE Study Group . Burden of respiratory syncytial virus bronchiolitis on the Dutch pediatric intensive care units. Eur J Pediatr. 2021;180(10):3141-3149. doi: 10.1007/s00431-021-04079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stempel HE, Martin ET, Kuypers J, Englund JA, Zerr DM. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009;98(1):123-126. doi: 10.1111/j.1651-2227.2008.01023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allinson JP, Chaturvedi N, Wong A, et al. Early childhood lower respiratory tract infection and premature adult death from respiratory disease in Great Britain: a national birth cohort study. Lancet. 2023;401(10383):1183-1193. doi: 10.1016/S0140-6736(23)00131-9 [DOI] [PubMed] [Google Scholar]
- 38.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9(9):731-745. doi: 10.1586/eri.11.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin G-L, Drysdale SB, Snape MD, et al. Association between disease severity and co-detection of respiratory pathogens in infants with RSV infection. medRxiv. Preprint posted online February 15, 2023. doi: 10.1101/2023.02.12.23285726 [DOI]
- 40.Halasa NB, Spieker AJ, Young CC, et al. ; Pediatric Intensive Care Influenza; Overcoming COVID-19 Investigators . Life-threatening complications of influenza vs coronavirus disease 2019 (COVID-19) in US children. Clin Infect Dis. 2023;76(3):e280-e290. doi: 10.1093/cid/ciac477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petat H, Gajdos V, Angoulvant F, et al. High frequency of viral co-detections in acute bronchiolitis. Viruses. 2021;13(6):990. doi: 10.3390/v13060990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amarin JZ, Potter M, Thota J, et al. Clinical characteristics and outcomes of children with single or co-detected rhinovirus-associated acute respiratory infection in Middle Tennessee. BMC Infect Dis. 2023;23(1):136. doi: 10.1186/s12879-023-08084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Definitions
eFigure. Age Distribution of Infants Requiring Intensive Care for RSV Infection
eTable 1. Underlying Medical Conditions Present in at Least 5 Critically Ill Infants With RSV, by Intubation Status
eTable 2. Clinical Characteristics of Infants Who Received ECMO or Died During Their Hospitalization
eTable 3. Clinical Characteristics of Infants Who Received Palivizumab Before Hospitalization
eTable 4. Probable or Confirmed Bacterial Coinfections Isolated Within 72 Hours of Admission Among Critically Ill Children With RSV, by Intubation Status, Underlying Medical Condition Status, and Specimen Type
eTable 5. Viral Codetections Among Critically Ill Children With RSV, by Intubation Status
Nonauthor Collaborators
Data Sharing Statement


