Abstract
Introduction
Severe obesity is often present with non-alcoholic fatty liver disease (NAFLD) and obstructive sleep apnea (OSA). Emerging researches suggest OSA plays an important role in NAFLD development and progression while the relationship between OSA and NAFLD is still conflicting. The interaction of OSA and NAFLD should be further evaluated as obesity surges. The purpose of this study was to assess the prevalence of OSA and NAFLD in patients with obesity undergoing bariatric surgery and evaluate the association between OSA and severity of NAFLD.
Methods
141 patients with severe obesity undergoing preoperative polysomnography and intraoperative liver biopsy during bariatric surgery were investigated. Clinical, anthropometric variables, liver enzymes, fasting blood glucose, fasting serum insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) were measured. The severity of NAFLD was assessed by degree of steatosis, ballooning, intralobular inflammation, and NAFLD activity score. The diagnosis and severity assessment of OSA was based on an apnea/hypopnea index (AHI).
Results
OSA was diagnosed in 127 (90.07%), NAFLD in 124 (87.94%), and non-alcoholic steatohepatitis in 72 (51.06%) patients. There was a statistical difference in BMI, waist circumstance, neck circumstance, high-density lipoprotein cholesterol, fasting insulin, and HOMA-IR among the three groups divided by the severity of AHI. In addition, the distribution of hepatic steatosis grades among the three groups was statistically different (p = 0.025). AHI was significantly associated with HOMA-IR and hepatic steatosis when assessing the association between OSA parameters and liver histology in NAFLD (p < 0.05). Patients with steatosis of grades 1–3 had significantly elevated aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transferase, triglycerides, fasting insulin, fasting glucose, HOMA-IR, and AHI compared with the patients with steatosis of grade 0. In a multivariable logistic analysis, the positive association between AHI and hepatic steatosis attenuated after adjusting for HOMA-IR.
Conclusion
Prevalence of OSA and NAFLD was high in patients with obesity eligible for bariatric procedures. HOMA-IR, but not AHI, was an independent risk factor for hepatic steatosis in this population.
Keywords: Hepatic steatosis, Obstructive sleep apnea, Insulin resistance, Obesity
Introduction
In parallel to the rapid transitions of social, economic, and environmental, especially the dramatical changes of lifestyle factors such as dietary habits and physical activity, obesity has become a major public health issue in China. In the most recent national surveys, more than half of Chinese adults are overweight or obese according to the ethnic-specific standards [1]. Global prevalence of non-alcoholic fatty liver disease (NAFLD) has reached 25% with obesity epidemic [2]. NAFLD is a multi-system metabolic disease, which is closely related to obesity, diabetes, atherosclerosis, and cardiovascular disease [3]. Non-alcoholic steatohepatitis (NASH), an inflammatory form of NAFLD, can progress to cirrhosis and even require liver transplantation [4]. Although the mechanisms of pathogenesis for NAFLD remain enigmatic, a multiple-hit hypothesis including lipotoxicity, oxidative stress, insulin resistance, genetic and epigenetic factors, and gut microbiota is the widespread theory for the progression of NAFLD [5].
Like NAFLD, obstructive sleep apnea (OSA), a common sleep-disordered breathing disease characterized by apnea and hypopnea caused by narrowing of the pharynx during sleep, has become a growing health problem worldwide and its prevalence in obese individuals exceeds 40% [6]. The pathogenesis of OSA is a clinical syndrome characterized by chronic intermittent hypoxia and sleep fragmentation, accompanied by a series of physiological changes such as hypoxemia and hypercapnia [7].
OSA can cause multi-system damage and is also associated with metabolic syndrome, such as dyslipidemia, type 2 diabetes mellitus (T2DM), hypertension, and insulin resistance, which influence the progression of NAFLD [8, 9]. A growing number of studies from epidemiological data [10, 11], intermittent hypoxia animal studies [12], and clinical intervention studies [13] suggest that OSA, as an independent risk factor for insulin resistance, may contribute to the progression of hepatic steatosis [14].
Patients with OSA often present with insulin resistance, which is associated with the development and progression of NAFLD [15]. However, the relationship between OSA, NAFLD, and insulin resistance is unclear and requires further research. Furthermore, NAFLD and OSA are both strongly impacted by race [16, 17], while similar study from China is lacking. The primary objective of this study was to investigate the effect of OSA on liver enzymes and NAFLD histological components such as steatosis, hepatocyte ballooning, and lobular inflammation as well as NAFLD activity score (NAS) in Chinese patients with severe obesity.
Materials and Methods
Study Population
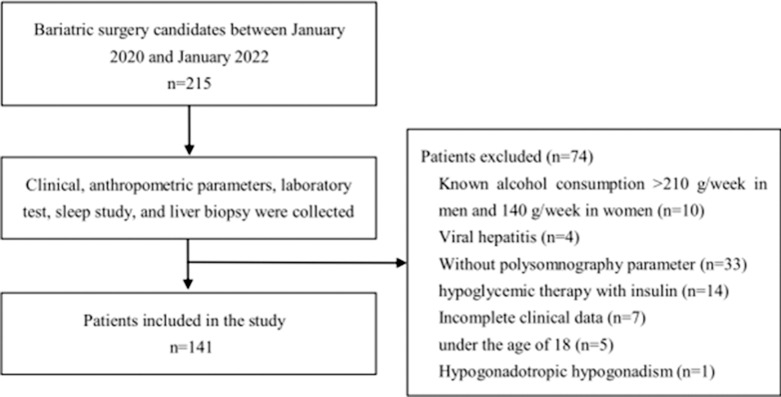
This study included 141 consecutive patients who underwent bariatric surgery at Tianjin Medical University General Hospital between January 2020 and January 2022. Severely obese Chinese patients with body mass index (BMI) ≥32.5 kg/m2 or BMI ≥27.5 kg/m2 with obesity-related comorbidities were evaluated by a multidisciplinary team. Bariatric surgery is considered for those unable to lose weight with diet, behavior modification, or medication therapy [18]. Electronic medical records were retrospectively reviewed to determine that these patients underwent polysomnography (PSG) prior to surgery and intraoperative wedge liver biopsy. Clinical data were collected including age, sex, BMI, disease duration. A detailed history was obtained including history of alcohol use, T2DM, hypertension, or dyslipidemia. Exclusion criteria included alcohol intake greater than 210 g/week in men and 140 g/week in women, hepatotoxic drug application; concomitant liver disease with other definite etiologies, such as autoimmune hepatitis, Wilson disease, hemochromatosis, or alpha-1 antitrypsin deficiency; and malignant disease. Due to the effect of exogenous insulin on homeostasis model assessment of insulin resistance (HOMA-IR) values, we excluded patients receiving insulin therapy. All patients were tested for hepatitis B and C virus markers, and the results were negative. Figure 1 shows the flowchart of identification of study population.
Fig. 1.
Flowchart of eligible and available patients in the study.
The diagnosis of T2DM was based on the World Health Organization (WHO) criteria, or known diagnosis of diabetes. Hypertension was diagnosed by a blood pressure >130/85 mm Hg or ongoing treatment for hypertension. Hyperlipidemia was defined as a total cholesterol concentration of ≥5.17 mmol/L, a triglyceride (TG) concentration of ≥1.7 mmol/L, a low-density lipoprotein cholesterol (LDL-C) concentration of >3.37 mmol/L, a high-density lipoprotein cholesterol (HDL-C) lipoprotein<1.03 mmol/L in men or 1.29 mmol/L in women, or a history of hyperlipidemia [19].
Anthropometric and Biochemical Measurements
BMI was calculated as weight (in kilograms) divided by height (in meters squared). Neck circumference was measured from the upper edge of the seventh cervical vertebra at the back of the neck (the most protruding part of the back of the neck when looking down) to the front of the pomum adami. Waist circumference was measured at the girth of the midpoint line between the lowest point of the rib and the upper border of the iliac crest.
Blood samples for estimation of biochemical parameters were collected between 08:00 and 10:00 a.m. after a 12-h overnight fasting period. Laboratory tests included serum glucose, serum insulin, aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, gamma glutamyltransferase (GGT), and fasting lipid profile including total cholesterol, TG, HDL-C, and LDL-C. In addition to biochemical indicators, hepatitis B and C serologies were performed concurrently. The HOMA-IR was calculated as fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5 [20].
Polysomnography
All patients were referred for overnight PSG before bariatric surgery, and the reports were published according to standard [21]. Apnea/hypopnea index (AHI) was defined as the total number of respiratory events (included apnea plus hypopnea) divided by sleep time (ev/h). Oxygen saturation index (ODI) was defined as the number of times the oxygen saturation decreased by ≥4% per hour of sleep time. Other polysomnographic parameters were also recorded, including the lowest O2 saturation (LaSO2) and the percentage of total sleep time with oxygen saturation <90% (T90%). The diagnosis of OSA is based on clinical manifestations and AHI ≥5 events/hour. According to AHI thresholds, the severity of OSA was classified as follows: no apnea (AHI <5.0 ev/h); mild apnea (AHI 5.0–14.9 ev/h); moderate apnea (AHI 15.0–30.0 ev/h); and severe apnea (AHI >30.0 ev/h) [22].
Liver Histology
All patients signed consent for liver biopsy and underwent intraoperative liver biopsy during bariatric surgery. Fresh liver biopsy specimens were fixed in 10% neutral buffered formalin and stained with hematoxylin-eosin. Liver biopsy histologic evaluation was interpreted by a single experienced pathologist in a blinded fashion, including grades of steatosis, inflammation, cellular ballooning: (1) steatosis: grade 0 (<5%); grade 1 (5%–33%); grade 2 (34%–66%); grade 3 (>66%); (2) lobular inflammation (necrotic foci counted under ×20 microscope): grade 0, none; grade 1 (<2 foci); grade 2 (2–4 foci); grade 3 (>4 foci); (3) hepatocyte ballooning: grade 0, none; grade 1, rare; grade 2, common. The NAFLD activity score. AS is the sum of scores for steatosis, lobular inflammation, and hepatocyte ballooning, ranging from 0 to 8. NAFLD was defined as the presence of grade 1 or more significant steatosis. NASH was defined as the grade 1 of steatosis plus hepatocyte ballooning and lobular inflammation according to the American Association for the Study of Liver Diseases (AASLD)-recommended liver pathological grade [23].
Statistical Analysis
Statistical analysis was performed using version 25.0 of IBM SPSS Statistics for Windows. All descriptive data were tested for normal distribution before statistical analysis. Data with normal distribution are expressed as mean ± standard, and data with non-normal distribution are expressed as median (interquartile range). In addition, categorical data are expressed as proportion (percentages). For comparisons between groups of continuous variables following a normal distribution, one-way ANOVA was performed first, followed by post hoc comparisons. For non-normally distributed variables, the Kruskal-Wallis H test (K) was performed to compare multiple groups. Categorical variables were analyzed by χ2 test or Fisher’s exact test. Spearman’s correlation test was used to investigate the correlation between variables. Predictor variables that were significant in the univariate analysis at p < 0.1 were included in the final multivariate model. Multivariate logistic regression was then used to identify independent predictors of hepatic steatosis. Histological assessments were performed as follows: hepatic steatosis (grade ≥1), ballooning (grade ≥1), lobular inflammation (graded ≥1), which were analyzed as dichotomous variables. A p value of < 0.05 was considered statistically significant.
Study Approval
The study was approved by the Tianjin Medical University General Hospital Institutional Review Board (approval number IRB2020-YX-029-01) with wavier of individual patient consent, and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. We did not obtain informed consent in the present study because we used an electronic data set compiled from medical records from the Department of Endocrinology and Metabolism, which does not contain personally identifiable information except for the date of birth.
Results
Baseline Characteristics
The characteristics of 141 research subjects are presented in Table 1. Of the 141 patients studied, 111 (78.23%) were female. The average age was 30.81 ± 6.83 years with an average BMI of 39.77 ± 7.05 kg/m2. Age, BMI, and waist circumference were significantly higher in males compared to the females. Diabetes was diagnosed in 49 (34.75%) patients, arterial hypertension in 44 (31.21%) patients, and 124 (87.94%) presented with dyslipidemia. In this study population, there were no differences in the prevalence rates of type 2 diabetes between the men and women, and the prevalence of hypertension in male was significantly higher than that in female (p = 0.039), whereas dyslipidemia was more common in female than in male (p = 0.002). Except for the ALT and GGT, there was no significant difference between male and female subjects for other metabolic parameters.
Table 1.
General characteristics of the whole cohort and stratified by gender
| Variables | Total, n = 141 | Male, n = 30 | Female, n = 111 | p value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 30.81±6.83 | 28.30±6.68 | 31.49±6.74 | 0.023* |
| BMI, kg/m2 | 39.77±7.05 | 43.54±7.03 | 38.75±6.74 | 0.001* |
| Waist circumference, cm | 115.00 (106.00–128.50) | 131.00 (118.50–143.25) | 112.00 (103.00–122.00) | 0.000* |
| Neck circumference, cm | 42.00 (39.00–45.00) | 46.00 (44.00–48.00) | 41.00 (39.00–43.00) | 0.000* |
| Current smoking, n (%) | 17 (12.06) | 10 (33.33) | 7 (6.30) | 0.000* |
| Comorbidity, n (%) | ||||
| Diabetes | 49 (34.75) | 9 (30.00) | 40 (36.03) | 0.538 |
| Arterial hypertension, | 44 (31.21) | 14 (46.67) | 30 (27.03) | 0.039* |
| Dyslipidemia | 124 (87.94) | 21 (70.00) | 103 (92.79) | 0.002* |
| OSA (AHI ≥5 ev/h) | 127 (90.07) | 29 (96.67) | 98 (88.29) | 0.301 |
| Sleep study characteristics | ||||
| AHI | 19.30 (11.20–48.15) | 58.20 (18.93–90.28) | 17.40 (10.00–35.70) | 0.000* |
| ODI | 16.60 (9.40–41.10) | 48.15 (19.08–87.75) | 14.90 (9.00–29.30) | 0.000* |
| T90% | 0.30 (0.00–6.00) | 5.20 (0.23–24.08) | 0.10 (0.00–2.90) | 0.001* |
| LaSO2 | 83.00 (74.00–88.00) | 76.50 (69.75–85.25) | 85.00 (79.00–88.00) | 0.002* |
| Average SpO2 | 95.00 (93.00–96.00) | 93.00 (92.00–96.00) | 95.00 (94.00–96.00) | 0.003* |
| Biochemical characteristics | ||||
| ALT, U/L | 41.00 (27.50–78.50) | 57.50 (38.50–83.00) | 39.00 (24.00–77.00) | 0.020* |
| AST, U/L | 26.00 (18.00–45.00) | 31.50 (24.00–45.00) | 23.00 (17.00–46.00) | 0.085 |
| ALKP, U/L | 69.00 (60.00–82.00) | 74.00 (67.00–83.50) | 69.00 (58.00–81.00) | 0.122 |
| GGT, U/L | 36.00 (24.50–56.50) | 52.00 (41.00–75.75) | 31.00 (22.00–52.00) | 0.000* |
| TC, mmol/L | 4.96 (4.46–5.47) | 4.95 (4.27–5.43) | 4.96 (4.53–5.58) | 0.429 |
| TG, mmol/L | 1.81 (1.44–2.50) | 1.85 (1.41–2.39) | 1.81 (1.44–2.51) | 0.761 |
| HDL-C, mmol/L | 1.06 (0.96–1.22) | 1.00 (0.89–1.14) | 1.08 (0.97–1.23) | 0.072 |
| LDL-C, mmol/L | 3.26±0.74 | 3.27±0.91 | 3.25±0.69 | 0.893 |
| Fasting glucose, mmol/L | 5.49 (4.95–6.71) | 5.33 (4.86–6.53) | 5.50 (4.96–6.74) | 0.346 |
| Fasting insulin, μIU/mL | 22.60 (16.15–32.25) | 23.50 (17.73–33.63) | 22.30 (15.80–31.80) | 0.636 |
| HOMA-IR | 5.93 (4.25–7.64) | 5.99 (3.92–7.65) | 5.88 (4.27–7.67) | 0.936 |
BMI, body mass index; OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; T90%, the percentage of total sleep time spent with SpO2 <90; LaSO2, lowest O2 saturation; average SpO2, average O2 saturation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALKP, alkaline phosphatase; GGT, gamma glutamyl transferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance.
*p < 0.05.
Liver Histological Findings
Table 2 shows the histological findings from the hepatic biopsies of the 141 patients studied. NAFLD was diagnosed in 124 (87.94%), and NASH in 72 (51.06%) of the patients studied. The prevalence of NAFLD and NASH was not statistically different between men and women. The patients with steatosis with grade from 0 to 3 were distributed as follows: grade 0: 17 (12.06%), grade 1: 36 (25.53%), grade 2: 49 (34.75%), grade 3: 39 (27.66%); hepatocyte ballooning: grade 0: 8 (5.67%), grade 1: 95 (67.38%), grade 2: 38 (26.95%); lobular inflammation: grade 0: 61 (43.26%), grade 1: 35 (24.82%), grade 2: 26 (18.44%), grade 3: 19 (13.48%). NAS was significantly higher in male compared with the female (p = 0.018). However, there was no difference between male and female in the distribution of lobular inflammation and hepatocyte ballooning.
Table 2.
Histological findings of the hepatic biopsies from the 141 obese patients studied
| Variables | Total, n = 141 | Male, n = 30 | Female, n = 111 | p value |
|---|---|---|---|---|
| NAFLD, n (%) | 124 (87.94) | 29 (96.67) | 95 (85.59) | 0.122 |
| NASH, n (%) | 72 (51.06) | 19 (63.33) | 53 (47.75) | 0.130 |
| Steatosis, n (%) | 0.057 | |||
| Grade 0 | 17 (12.06) | 1 (3.33) | 16 (14.41) | |
| Grade 1 | 36 (25.53) | 9 (30.00) | 27 (24.32) | |
| Grade 2 | 49 (34.75) | 7 (23.33) | 42 (37.84) | |
| Grade 3 | 39 (27.66) | 13 (43.33) | 26 (23.42) | |
| Hepatocyte ballooning, n (%) | 0.778 | |||
| Grade 0 | 8 (5.67) | 1 (3.33) | 7 (6.31) | |
| Grade 1 | 95 (67.38) | 20 (66.67) | 75 (67.57) | |
| Grade 2 | 38 (26.95) | 9 (30.00) | 29 (26.13) | |
| Lobular inflammation | 0.109 | |||
| Grade 0, n (%) | 61 (43.26) | 9 (30.00) | 52 (46.85) | |
| Grade 1, n (%) | 35 (24.82) | 6 (20.00) | 29 (26.13) | |
| Grade 2, n (%) | 26 (18.44) | 8 (26.67) | 18 (16.22) | |
| Grade 3, n (%) | 19 (13.48) | 7 (23.33) | 12 (10.81) | |
| NAS | 4.00 (3.00–5.00) | 5.00 (3.00–6.25) | 4.00 (3.00–5.00) | 0.018* |
NAFLD, non-alcoholic fatty liver disease; NAFL, non-alcoholic hepatic steatosis; NASH, non-alcoholic steatohepatitis; NAS, NAFLD activity score.
*p < 0.05.
PSG Evaluation and Characteristics of Patients Based on AHI Severity
As expected, OSA was diagnosed in 127 (90.07%) research subjects (Table 1). Men had higher AHI, ODI, and T90% than women, whereas the men presented with lower LaSO2 and average SpO2 than women. The prevalence of OSA did not differ between the men and the women.
Regarding AHI, 50 (35.46%) of the cohort had AHI <15, 37 (26.24%) with AHI 15–30, 54 (38.30%) with AHI >30. The clinical and biochemical characteristics of three groups are shown in Table 3. The three groups did not differ in terms of age, and the prevalence of diabetes, arterial hypertension, dyslipidemia, and smoking. Increasing AHI level was associated with higher BMI, WC, and NC. Except for HDL-C, fasting insulin, and HOMA-IR, there were no significant differences in other biochemical characteristics among the three groups.
Table 3.
Characteristics of the participants according to AHI
| Variables | AHI <15, n = 50 | AHI15-30, n = 37 | AHI >30, n = 54 | p value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Gender, n (%) | ||||
| Male | 5 (10.00) | 6 (16.22) | 19 (35.19) | 0.005* |
| Female | 45 (90.00) | 31 (83.78) | 35 (64.81) | |
| Age, years | 29.88±6.17 | 30.46±7.68 | 31.91±6.77 | 0.301 |
| BMI, kg/m2 | 36.33±6.46 | 40.37±7.76 | 42.53±7.76 | 0.000* |
| Waist circumference, cm | 107.00 (101.00–115.75) | 120.00 (111.50–129.50) | 123.50 (110.75–135.25) | 0.000* |
| Neck circumference, cm | 40.00 (37.75–43.00) | 41.50 (40.00–43.00) | 44.00 (41.00–47.00) | 0.000* |
| Smoking, n (%) | 4 (8.00) | 3 (8.11) | 10 (18.52) | 0.329 |
| Comorbidity, n (%) | ||||
| Diabetes | 17 (34.00) | 10 (27.03) | 22 (40.74) | 0.398 |
| Arterial hypertension | 14 (28.00) | 9 (24.32) | 21 (38.89) | 0.281 |
| Dyslipidemia | 44 (88.00) | 33 (89.19) | 47 (87.04) | 0.953 |
| Biochemical characteristics | ||||
| ALT, U/L | 38.50 (26.75–66.50) | 44.00 (29.00–90.00) | 52.00 (27.75–75.00) | 0.432 |
| AST, U/L | 23.00 (17.00–35.25) | 27.00 (17.00–48.50) | 27.50 (18.75–51.00) | 0.370 |
| ALKP, U/L | 70.00 (57.00–80.25) | 69.00 (62.50–82.50) | 69.00 (61.00–83.00) | 0.853 |
| GGT, U/L | 31.00 (22.25–54.00) | 41.00 (27.00–50.00) | 44.00 (24.50–66.50) | 0.362 |
| TC, mmol/L | 4.89 (4.42–5.50) | 4.96 (4.51–5.33) | 5.04 (4.47–5.58) | 0.968 |
| TG, mmol/L | 1.66 (1.42–2.30) | 2.05 (1.55–2.91) | 1.81 (1.43–2.47) | 0.203 |
| HDL-C, mmol/L | 1.09 (0.99–1.26) | 1.04 (0.91–1.14) | 1.04 (0.92–1.17) | 0.029* |
| LDL-C, mmol/L | 3.15±0.63 | 3.34±0.75 | 3.30±0.82 | 0.441 |
| Fasting glucose, mmol/L | 5.36 (4.89–6.77) | 5.36 (4.90–6.62) | 5.79 (5.08–6.75) | 0.516 |
| Fasting insulin, µIU/mL | 18.50 (14.03–24.48) | 27.10 (17.85–33.80) | 25.10 (17.90–33.63) | 0.006* |
| HOMA-IR | 4.85 (3.10–6.96) | 6.39 (4.76–8.34) | 6.31 (4.69–9.00) | 0.021* |
AHI, apnea-hypopnea index; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALKP, alkaline phosphatase; GGT, gamma glutamyl transferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance.
*p < 0.05.
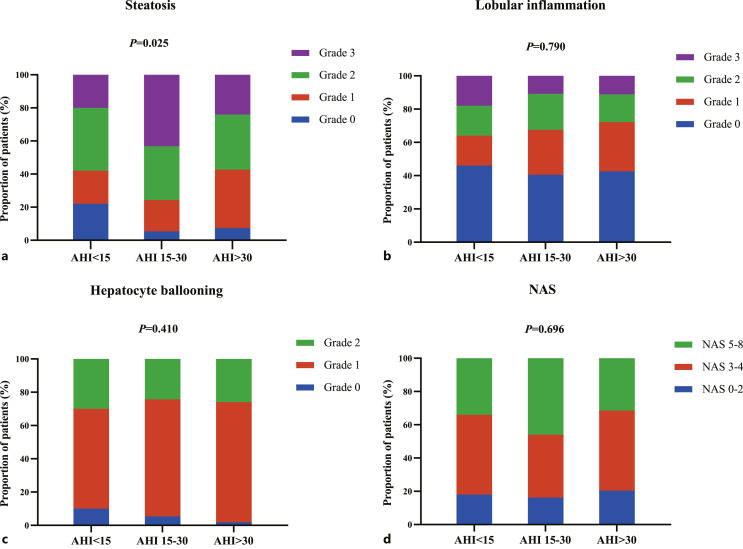
The prevalence of NASH was 42.00% in group with AHI <15, 59.46% in group with AHI 15–30, 53.70% in group with AHI >30. However, there was no statistical difference in the prevalence of NASH and NAS among the three groups (p = 0.242 and 0.696, respectively). The distribution of patients in different grades of steatosis, hepatocyte ballooning, lobular inflammation, and NAS depending on the severity of AHI is provided in Figure 2. Hepatic histopathology findings demonstrated that no significant differences in hepatocyte ballooning and lobular inflammation (p = 0.410 and 0.790, respectively) were detected, while the results showed a significant difference in steatosis among the three groups divided based on AHI severity (p = 0.025). The details of proportion in different groups depending on severity of AHI are provided in online supplementary Table 1 (for all online suppl. material, see https//doi.org/10.1159/000528789).
Fig. 2.
Proportion of patients in different grades of hepatic histopathology and NAS depending on severity of AHI. a Steatosis represented as grade 0, grade 1, grade 2, grade 3. b Lobular inflammation represented as grade 0, grade 1, grade 2, grade 3. c Hepatocyte ballooning represented as grade 0, grade 1, grade 2. d NAS presented as 0–2, 3–4, 5–8. AHI, apnea-hypopnea index; NAS, non-alcoholic fatty liver activity score.
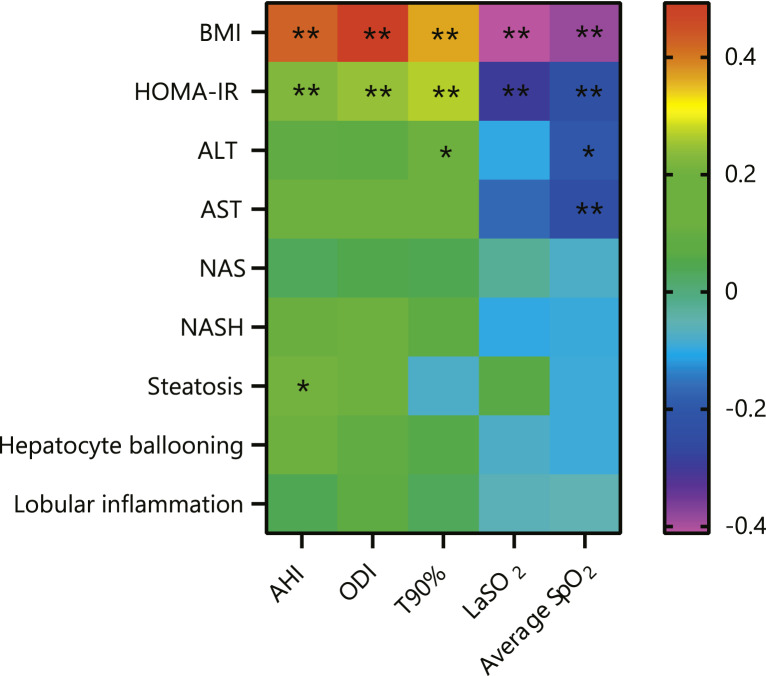
Correlation Analysis of OSA and NAFLD Parameters
To evaluate the relationship of OSA and NAFLD, Spearman correlation analysis was performed on PSG parameters (AHI, ODI, T90%, LaSO2, average SpO2) with BMI, HOMA-IR, liver enzymes, NAS, steatosis, ballooning, and intralobular inflammation, respectively (online suppl. Table 2), and is displayed via heatmap in Figure 3. The BMI presented a positive statistically significant correlation with AHI (rs = 0.425, p = 0.000), ODI (rs = 0.492, p = 0.000), and T90% (rs = 0.365, p = 0.000), but a negative correlation with LaSO2 (rs = −0.412, p = 0.000) and average SpO2 (rs = −0.387, p = 0.000). The HOMA-IR showed a positive, statistically significant, but weak correlation with AHI (rs = 0.225, p = 0.007), ODI (rs = 0.246, p = 0.003), and T90% (rs = 0.268, p = 0.001), but a negative correlation with LaSO2 (rs = −0.294, p = 0.000) and average SpO2 (rs = −0.234, p = 0.005). The weak correlation also occurred between the ALT and average SpO2 (rs = −0.199, p = 0.018), AST and average SpO2 (rs = −0.243, p = 0.004), AST and T90% (rs = 0.197, p = 0.019).
Fig. 3.
Relationship between study sleep variables and BMI, HOMA-IR, and NAFLD-related variables. Heat map showing Spearman correlation between study sleep variables (AHI, ODI, T90%, LaSO2, average SpO2) and BMI, HOMA-IR, ALT, AST, NAS, NASH, steatosis, hepatocyte ballooning, and lobular inflammation. *p < 0.05, **p < 0.01. NAFLD, non-alcoholic fatty liver disease; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; T90%: the percentage of total sleep time spent with SpO2 <90; LaSO2, lowest O2 saturation; average SpO2, average O2 saturation; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA-IR, homeostasis model assessment of insulin resistance; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis.
In addition, to study the relationship between the OSA parameters and liver histology in NAFLD, correlation tests were also conducted among the polysomnographic parameters and histological findings and NAS. Only AHI presented a positive, statistically significant, but weak correlation with the presence of steatosis (rs = 0.209, p = 0.013).
Characteristics of Patients according to the Grade of Steatosis
To clarify the relationship between OSA severity and hepatocyte steatosis, the patients were divided into two groups according to the grade of steatosis: grade 0 and grades 1–3. Table 4 presents the clinical characteristics of the participant, and subjects with steatosis of grades 1–3 had significantly higher AST, ALT, GGT, TG, fasting glucose, fasting insulin, HOMA-IR, and AHI compared with the patients with steatosis of grade 0. Patients with steatosis have lower levels of HDL-C than that without steatosis (p = 0.045). There was no significant difference between two groups for demographic, anthropometric, other metabolic, and polysomnographic parameters.
Table 4.
Characteristics of the participants according to the grade of steatosis
| Variables | Steatosis (grade 0), n = 17 | Steatosis (grade 1–3), n = 124 | p value |
|---|---|---|---|
| Clinical characteristics | |||
| Gender, n (%) | |||
| Male | 1 (5.88) | 29 (23.39) | 0.122 |
| Female | 16 (94.12) | 95 (76.61) | |
| Age, years | 29.24±5.96 | 31.02±6.94 | 0.313 |
| BMI, kg/m2 | 38.47±8.34 | 39.94±6.88 | 0.420 |
| Waist circumference, cm | 109.00 (101.50–124.50) | 117.00 (108.00–128.75) | 0.139 |
| Neck circumference, cm | 42.00 (38.00–45.00) | 42.00 (40.00–45.00) | 0.583 |
| Smoking, n (%) | 0 (0.00) | 17 (13.71) | 0.085 |
| Comorbidity, n (%) | |||
| Diabetes | 5 (29.41) | 44 (35.48) | 0.622 |
| Arterial hypertension | 7 (41.18) | 37 (29.84) | 0.344 |
| Dyslipidemia | 13 (76.47) | 111 (89.52) | 0.127 |
| OSA (AHI ≥5 ev/h) | 14 (82.35) | 113 (91.13) | 0.378 |
| Sleep study characteristics | |||
| AHI | 8.40 (6.50–28.90) | 20.90 (12.75–51.10) | 0.014* |
| ODI | 11.00 (4.15–28.45) | 17.65 (9.68–42.20) | 0.089 |
| T90%, n (%) | 0.10 (0.00–2.90) | 0.30 (0.00–6.48) | 0.366 |
| LaSO2, n (%) | 86.00 (73.00–89.00) | 83.00 (74.25–88.00) | 0.470 |
| Average SpO2, n (%) | 95.00 (94.00–97.00) | 95.00 (93.00–96.00) | 0.277 |
| Biochemical characteristics | |||
| ALT, U/L | 20.00 (12.25–29.50) | 49.50 (32.00–83.00) | 0.000* |
| AST, U/L | 17.00 (14.00–18.50) | 27.50 (19.00–48.50) | 0.000* |
| ALKP, U/L | 73.00 (67.50–85.50) | 69.00 (60.00–81.75) | 0.257 |
| GGT, U/L | 19.00 (16.50–38.50) | 41.00 (25.25–60.50) | 0.001* |
| TC, mmol/L | 4.66 (4.37–5.35) | 4.97 (4.48–5.53) | 0.317 |
| TG, mmol/L | 1.55 (1.08–1.77) | 1.86 (1.47–2.72) | 0.001* |
| HDL-C, mmol/L | 1.17 (1.04–1.27) | 1.05 (0.94–1.19) | 0.045* |
| LDL-C, mmol/L | 3.08±0.76 | 3.28±0.74 | 0.284 |
| Fasting glucose, mmol/L | 5.15 (4.70–5.62) | 5.61 (4.98–6.97) | 0.011* |
| Fasting insulin, µIU/mL | 16.00 (12.15–25.90) | 22.85 (17.53–33.10) | 0.016* |
| HOMA-IR | 4.42 (2.49–6.29) | 6.07 (4.39–8.36) | 0.005* |
BMI, body mass index; OSA, obstructive sleep apnea; AHI, apnea-hypopnea index; ODI, oxygen desaturation index; T90%, the percentage of total sleep time spent with SpO2 <90; LaSO2, lowest O2 saturation; average SpO2, average O2 saturation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALKP, alkaline phosphatase; GGT, gamma glutamyl transferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance.
*p < 0.05.
Multivariate Logistic Regression Model of Hepatocyte Steatosis
Hepatic steatosis (grade ≥1) was analyzed as dichotomous variables. In multivariate logistic regression analysis (shown in Table 5), HOMA-IR was significantly associated with steatosis after adjusting for age, sex, neck circumference, BMI, and smoking (model 1) (OR, 1.666; 95% CI, 1.187–2.340; p = 0.003). Moreover, adjusting for diabetes, hypertension, and dyslipidemia (model 2) did not alter the association between HOMA-IR and steatosis (OR, 1.625; 95% CI, 1.138–2.321; p = 0.008). On the basis of model 2, hepatocyte ballooning and intralobular inflammation were further adjusted, and the association was still statistically significant (OR, 1.663; 95% CI, 1.146–2.413; p = 0.007). More importantly, the association still existed after adjusting for AHI (OR, 1.671; 95% CI, 1.148–2.434; p = 0.007), suggesting an independent effect of insulin resistance on steatosis.
Table 5.
Multivariate logistics regression analyses of the association between steatosis and HOMA-IR
| OR | 95% CI | p value | |
|---|---|---|---|
| Model 1 | 1.666 | 1.187–2.340 | 0.003* |
| Model 2 | 1.625 | 1.138–2.321 | 0.008* |
| Model 3 | 1.663 | 1.146–2.413 | 0.007* |
| Model 4 | 1.671 | 1.148–2.434 | 0.007* |
Model 1: adjusted for age, gender, neck circumference, BMI, smoking.
Model 2: adjusted for variables in model 1 and also adjusted for diabetes, hypertension, and dyslipidemia.
Model 3: adjusted for variables in model 2 and also adjusted for hepatocyte ballooning and lobular inflammation.
Model 4: adjusted for variables in model 3 and also adjusted for AHI.
*p < 0.05.
Discussion
These findings demonstrate a high prevalence of OSA and NAFLD coexistence among patients with severe obesity that underwent bariatric surgery. The relationship between OSA and NAFLD is unclear, we observed an association between AHI and insulin resistance, and insulin resistance was an independent risk factor for hepatic steatosis.
The prevalence of OSA was 90.07% in our study, which was consistent with other studies that have shown a prevalence of more than 70% in the severe obese and bariatric surgery population [24–28]. In addition, the BMI presented a positive statistically significant correlation with AHI, ODI, and T90%, but a negative correlation with LaSO2 and average SpO2. Our findings support the view of increased prevalence of OSA that is directly related to increased BMI [29]. Furthermore, the prevalence of NAFLD and NASH patients in our study is consistent with those found by others in bariatric surgery candidates [24, 30]. This highlighted the importance of screening patients with OSA and NAFLD in patients with severe obesity, especially for the bariatric surgery candidates.
The results of existing researches on the relationship between OSA and NAFLD are controversial. Jin S et al. [11] conducted a systematic literature review between January 2007 and April 2017, and 9 studies including 2,272 participants were assessed for inclusion. Meta-regression analysis revealed that OSA was remarkably associated with severity of steatosis, hepatocyte ballooning, intralobular inflammation, and fibrosis, but not NAS. In addition, parallel result was implied by Mishra P et al. that OSA is associated not only with NASH, but also with pathological components of NASH such as steatosis, inflammation, and fibrosis. These studies suggest that OSA may play a major role in the occurrence and progression of NAFLD.
Unfortunately, correlation between OSA parameters with NASH and the NAS has not been demonstrated in the present study. The results of two studies conducted by Jouet et al. [31] and Ulitsky et al. [32] failed to confirm an association between OSA and NASH, which support our results. The study by Daltro C et al. [28] evaluated 40 patients with obesity submitted to bariatric surgery and analyzed the association between OSA and the features of NAFLD including severity of steatosis, intralobular inflammation, hepatocyte ballooning, and fibrosis from the intraoperative liver biopsies. Consistent with our findings, they demonstrated that OSA was associated with insulin resistance, not NASH severity.
Although clinical studies have demonstrated a relationship between OSA and NAFLD, the study on the association between OSA parameters and liver histology in NAFLD, including steatosis, hepatocyte ballooning, and lobular inflammation, is limited. Schwenger et al. found the AHI correlated with liver inflammation in a single-center cross-sectional study of 61 patients undergoing bariatric surgery [24], while our study found that AHI is associated with steatosis in correlation analysis.
So far, related studies have reveled conflicting results and further researches are warranted. Liver pathology data for this study were obtained from intraoperative wedge biopsies during bariatric surgery. Liver biopsy methods include needle biopsy and wedge biopsy. Needle biopsy provides a sample of liver parenchyma, while wedge biopsy provides a “wedge” of liver tissue removed from its surface. They are much larger, but are generally not optimal for assessment of liver fibrosis and/or inflammation [33]. Since this study used wedge-shaped liver biopsy, it may have influenced the analysis of the relationship between OSA and lobular inflammation to some extent.
It is relevant to especially emphasize that the definition of NASH has changed over time, and the currently accepted pathological diagnostic criteria require the presence of >5% steatosis, hepatocyte ballooning, and inflammation [34]. However, most studies used NAS ≥5 as the diagnostic criteria for NASH or did not consider hepatocyte ballooning as a necessary condition for the diagnosis of NASH. This histological definition of NASH may lead to severe misclassification, resulting in patients with simple steatosis being misclassified as NASH, and is not recommended [35]. Therefore, we speculate that the conflicting correlation between OSA and NASH may also be affected by the different definition criteria of NASH in related studies. In our study, we defined NASH as the presence of steatosis, hepatocyte ballooning, and intralobular inflammation, and simultaneously assessed NAFLD activity with NAS rather than defined NASH.
In addition, different populations included in clinical studies may also affect the association between OSA and NAFLD. The impact of the metabolic syndrome on the severity of NAFLD is beyond doubt [36]. Peter Benotti et al. [37] found that the association between OSA and NAFLD depended on the presence of metabolic syndrome. They demonstrated that the association of OSA with lobular inflammation and fibrosis was only present in the absence of metabolic syndrome. However, in the metabolic syndrome cohort, the effect of hypoxia appeared to be overwhelmed by the strong effect of metabolic syndrome on NAFLD severity. The majority of bariatric surgery candidates included in this study had metabolic syndrome, affecting the relationship between OSA and NAFLD.
However, the underlying mechanism of the relationship between OSA and NAFLD remains a mystery. Although the pathogenesis of NAFLD remains unclear, the “two-hit” hypothesis is a prevalent theory for the progression of NAFLD [38]. Insulin resistance promotes the accumulation of massive intrahepatic lipids in the liver, leading to the “first-hit.” In contrast to the previous “two-hit” theory, increasing evidence favors the importance of the “multiple-hit” theory in the pathogenesis of NAFLD: including lipotoxicity, oxidative stress, insulin resistance, gut microbiota, dysfunctional mitochondria, and genetic and epigenetic factors [5]. Both the “two-hit” theory and the “multiple-hit” theory affirm the importance of insulin resistance in the progression of NAFLD. Our data demonstrate strong correlations between insulin resistance and steatosis in patients with severe obesity. At multivariable analysis, we demonstrated that insulin resistance was an independent risk factor for hepatic steatosis. The present results support the idea that insulin resistance plays a crucial role in the pathogenesis of NAFLD.
Coincidentally, recent studies have shown an association between insulin resistance and OSA [39–41]. The mechanisms involved are the release of catecholamines due to microarousal, followed by activation of the hypothalamic-pituitary-adrenal axis to increase the production of cortisol, both of which are antagonists of insulin [42]. In addition, epidemiologic data [10, 43] and an interventional study [13] both indicate that OSA as an independent factor of insulin resistance predisposes patients to progression of hepatic steatosis [14]. But this may not actually be the case, in our study, the HOMA-IR showed a statistically significant, but weak correlation with the markers of the severity of OSA, such as AHI, ODI, and T90%. Besides, OSA not only causes insulin resistance, but also exacerbates NAFLD through chronic intermittent hypoxia [44]. OSA disrupts the balance of oxidants and antioxidants, resulting in dyslipidemia, elevated circulating free fatty acid levels, and lipid peroxidation, which are involved in the NAFLD [8].
It is generally known that insulin resistance and fatty acid metabolism disorders can lead to hepatic steatosis, and insulin resistance is also an independent risk factor for OSA [25]. Previous studies of OSA and NAFLD interactions had emphasized potential importance of insulin resistance, which is worth exploring when studying this association. Therefore, it is speculated that OSA may contribute to increased insulin resistance, which in turn increases hepatic steatosis and then leads to the progression of NAFLD [44]. The above hypothesis was confirmed by Daltro C et al. [28], and a positive correlation between HOMA-IR and steatosis severity was observed in their study, suggesting that OSA-induced hepatic steatosis may be mediated by exacerbating insulin resistance. This finding is consistent with our results.
In our study, we investigated the association between OSA and NAFLD in Chinese patients with severe obesity. Moreover, intraoperative liver biopsy was conducted in all study subjects and the severity of steatosis, intralobular inflammation, and ballooning were analyzed. In addition, this study selected Chinese obese people as the study population and provided evidence of different races for OSA and NAFLD. It is undeniable that there are still some limitations in this study. First, this study used a cross-sectional design and cause-and-effect conclusions could not be made. Second, sample size was limited due to the invasive nature of liver biopsies and the availability of PSG. Third, the results of this study were obtained in severe obese population, who may not be representative of the entire NAFLD patient population. Furthermore, obesity itself is a cause of NAFLD, leading to a strong confounding variable. Another major limitation of the present study was the lack of histological data on hepatic fibrosis, thus making it impossible to assess the effect of OSA on fibrosis.
Conclusion
In conclusion, the present study suggests that NAFLD was associated with the insulin resistance but not the severity of OSA in severe obese populations. All studies were observational and prospective studies were lacking to better assess this association. To better explore the potential therapeutic implications and disease prevention progress, future research should focus on the specific mechanisms of insulin resistance in the progression of OSA and NAFLD.
Acknowledgments
The authors are appreciative and acknowledge the important contributions made by the pathological staff, surgical staff, bariatric clinic nurses, operating room nurses, and study participants from the Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital.
Statement of Ethics
The study was approved by the Tianjin Medical University General Hospital Institutional Review Board (approval number IRB2020-YX-029-01) with wavier of individual patient consent and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of Interest Statement
The authors declared no conflict of interest.
Funding Sources
This study was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK- 030A) and Tianjin Health Science and Technology Project (TJWJ2022MS002).
Author Contributions
C.Z., S.T., and J.X. designed research, collected clinical data, and wrote the manuscript. Y.Z. was involved in clinical data collection and revision of the paper. L.S., J.Z., and M.L. designed the study, and revised and edited the manuscript paper. L.D. was accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK- 030A) and Tianjin Health Science and Technology Project (TJWJ2022MS002).
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
References
- 1. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–92. 10.1016/S2213-8587(21)00045-0. [DOI] [PubMed] [Google Scholar]
- 2. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–24. 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–86. 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 4. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–83. 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 5. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism. 2016;65(8):1038–48. 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 6. Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14(5):417–31. 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 7. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–400. 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 8. Patterson RE, Kalavalapalli S, Williams CM, Nautiyal M, Mathew JT, Martinez J, et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am J Physiol Endocrinol Metab. 2016;310(7):E484–94. 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Méndez-Lucas A, et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53(6):1080–92. 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the sleep heart health study. Am J Epidemiol. 2004;160(6):521–30. 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 11. Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):841–51. 10.1007/s11325-018-1625-7. [DOI] [PubMed] [Google Scholar]
- 12. Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pamidi S, Wroblewski K, Stepien M, Sharif-Sidi K, Kilkus J, Whitmore H, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am J Respir Crit Care Med. 2015;192(1):96–105. 10.1164/rccm.201408-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815–25. 10.1007/s11695-013-0981-4. [DOI] [PubMed] [Google Scholar]
- 15. Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J Gastroenterol. 2020;26(20):2669–81. 10.3748/wjg.v26.i20.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9(6):419–36. 10.1016/j.smrv.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17. Estes C, Chan HL, Chien RN, Chuang WL, Fung J, Goh GB, et al. Modelling NAFLD disease burden in four Asian regions-2019-2030. Aliment Pharmacol Ther. 2020;51(8):801–11. 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Wang C, Zhu S, Zhang P, Liang H. Guidelines for the surgical treatment of obesity and type 2 diabetes in China (2019 edition). Chin J Pract Surg. 2019;39:301–6. [Google Scholar]
- 19. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–80. 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21. Hori T, Sugita Y, Koga E, Shirakawa S, Inoue K, Uchida S, et al. Proposed supplements and amendments to “A Manual of standardized terminology, Techniques and scoring System for sleep Stages of human subjects”, the rechtschaffen & kales (1968) standard. Psychiatry Clin Neurosci. 2001;55(3):305–10. 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- 22. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 23. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24. Schwenger KJ, Ghorbani Y, Li C, Fischer SE, Jackson TD, Okrainec A, et al. Obstructive sleep apnea and non-alcoholic fatty liver disease in obese patients undergoing bariatric surgery. Obes Surg. 2020;30(7):2572–8. 10.1007/s11695-020-04514-3. [DOI] [PubMed] [Google Scholar]
- 25. Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179(3):228–34. 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee YH, Johan A, Wong KK, Edwards N, Sullivan C. Prevalence and risk factors for obstructive sleep apnea in a multiethnic population of patients presenting for bariatric surgery in Singapore. Sleep Med. 2009;10(2):226–32. 10.1016/j.sleep.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27. Sareli AE, Cantor CR, Williams NN, Korus G, Raper SE, Pien G, et al. Obstructive sleep apnea in patients undergoing bariatric surgery: a tertiary center experience. Obes Surg. 2011;21(3):316–27. 10.1007/s11695-009-9928-1. [DOI] [PubMed] [Google Scholar]
- 28. Daltro C, Cotrim HP, Alves E, de Freitas LA, Araújo L, Boente L, et al. Nonalcoholic fatty liver disease associated with obstructive sleep apnea: just a coincidence. Obes Surg. 2010;20(11):1536–43. 10.1007/s11695-010-0212-1. [DOI] [PubMed] [Google Scholar]
- 29. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 30. Tai CM, Yu ML, Tu HP, Huang CK, Hwang JC, Chuang WL. Derivation and validation of a scoring system for predicting nonalcoholic steatohepatitis in Taiwanese patients with severe obesity. Surg Obes Relat Dis. 2017;13(4):686–92. 10.1016/j.soard.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 31. Jouët P, Sabaté JM, Maillard D, Msika S, Mechler C, Ledoux S, et al. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg. 2007;17(4):478–85. 10.1007/s11695-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 32. Ulitsky A, Ananthakrishnan AN, Komorowski R, Wallace J, Surapaneni SN, Franco J, et al. A noninvasive clinical scoring model predicts risk of nonalcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2010;20(6):685–91. 10.1007/s11695-010-0118-y. [DOI] [PubMed] [Google Scholar]
- 33. Padoin AV, Mottin CC, Moretto M, Berleze D, Kupski C, Glock L, et al. A comparison of wedge and needle hepatic biopsy in open bariatric surgery. Obes Surg. 2006;16(2):178–82. 10.1381/096089206775565159. [DOI] [PubMed] [Google Scholar]
- 34. Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–53. 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network CRN . Nonalcoholic Fatty Liver Disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–20. 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–73. 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 37. Benotti P, Wood GC, Argyropoulos G, Pack A, Keenan BT, Gao X, et al. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity. 2016;24(4):871–7. 10.1002/oby.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party. Hepatology. 1998;27:1463–6. 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 39. Morgenstern M, Wang J, Beatty N, Batemarco T, Sica AL, Greenberg H. Obstructive sleep apnea: an unexpected cause of insulin resistance and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):187–204. 10.1016/j.ecl.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 40. Song SO, He K, Narla RR, Kang HG, Ryu HU, Boyko EJ. Metabolic consequences of obstructive sleep apnea especially pertaining to diabetes mellitus and insulin sensitivity. Diabetes Metab J. 2019;43(2):144–55. 10.4093/dmj.2018.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mesarwi O, Polak J, Jun J, Polotsky VY. Sleep disorders and the development of insulin resistance and obesity. Endocrinol Metab Clin North Am. 2013;42(3):617–34. 10.1016/j.ecl.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–8. 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 43. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31. 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 44. Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, et al. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45(4):1007–13. 10.1002/hep.21593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.