Abstract
Introduction
Infections are a major problem after left ventricular assist device (LVAD) implantation that affects morbidity, mortality, and the quality of life. Obesity often increases the risk for infection. In the cohort of LVAD patients, it is unknown if obesity affects the immunological parameters involved in viral defense. Therefore, this study investigated whether overweight or obesity affects immunological parameters such as CD8+ T cells and natural killer (NK) cells.
Methods
Immune cell subsets of CD8+ T cells and NK cells were compared between normal-weight (BMI 18.5–24.9 kg/m2, n = 17), pre-obese (BMI 25.0–29.9 kg/m2, n = 24), and obese (BMI ≥30 kg/m2, n = 27) patients. Cell subsets and cytokine serum levels were quantified prior to LVAD implantation and at 3, 6, and 12 months after LVAD implantation.
Results
At the end of the first postoperative year, obese patients (31.8% ± 2.1%) had a lower proportion of CD8+ T cells than normal-weight patients (42.4% ± 4.1%; p = 0.04), and the percentage of CD8+ T cells was negatively correlated with BMI (p = 0.03; r = −0.329). The proportion of circulating NK cells increased after LVAD implantation patients in normal-weight (p = 0.01) and obese patients (p < 0.01). Patients with pre-obesity showed a delayed increase (p < 0.01) 12 months after LVAD implantation. Further, obese patients showed an increase in the percentage of CD57+ NK cells after 6 and 12 months (p = 0.01) of treatment, higher proportions of CD56bright NK cells (p = 0.01), and lower proportions of CD56dim/neg NK cells (p = 0.03) 3 months after LVAD implantation than normal-weight patients. The proportion of CD56bright NK cells positively correlated with BMI (p < 0.01, r = 0.403) 1 year after LVAD implantation.
Conclusions
This study documented that obesity affects CD8+ T cells and subsets of NK cells in patients with LVAD in the first year after LVAD implantation. Lower proportions of CD8+ T cells and CD56dim/neg NK cells and higher proportion of CD56bright NK cells were detected in obese but not in pre-obese and normal-weight LVAD patients during the first year after LVAD implantation. The induced immunological imbalance and phenotypic changes of T and NK cells may influence viral and bacterial immunoreactivity.
Keywords: Obesity, Left ventricular assist device, Natural killer cells, T cells, Body mass index
Introduction
Implantation of a left ventricular assist device (LVAD) is an evolving treatment option for patients with end-stage heart failure and is performed in approximately 3,500 patients per year worldwide [1]. The percentage of LVAD patients with pre-obesity and obesity is high because obesity is a risk factor for heart failure [2]. Several studies have indicated increased infection rates after LVAD implantation in obese patients [3–5]. Thus, obesity seems to affect the immune system in patients with LVAD. However, the extent of immunological changes in relation to body mass index (BMI) in patients with LVAD has not been described in detail.
In general, obesity induces a pro-inflammatory state up to systemic inflammation [6], which affects the T-cell hemostasis and cytokine production [7]. Several studies have found altered counts of CD4+ and CD8+ T cells, cytokines such as interleukin (IL)-17, IL-22, interferon (IFN)-γ and tumor necrosis factor (TNF)-α [8–11], as well as immune activation [12] in obese patients, compared with nonobese patients. Furthermore, effects on natural killer (NK) cells have been reported, ranging from reduced NK cell frequencies to decreased degranulation and cytotoxic capabilities [6, 13]. In addition, senescence of immune cells is promoted by overweight and obesity [14, 15]. In the present study, we aimed to investigate whether overweight or obesity affects immunological parameters such as CD8+ T cells and NK cells involved in antiviral defense of patients with LVAD in the first year after LVAD implantation.
Materials and Methods
Study Groups and Clinical Characteristics
This study was approved by the Ethics Committee of the Medical Faculty of the University of Leipzig, Germany (ID: 225/17-ek), and was performed according to the guidelines of the Declaration of Helsinki (2013). Written informed consent was obtained from all patients before study initiation.
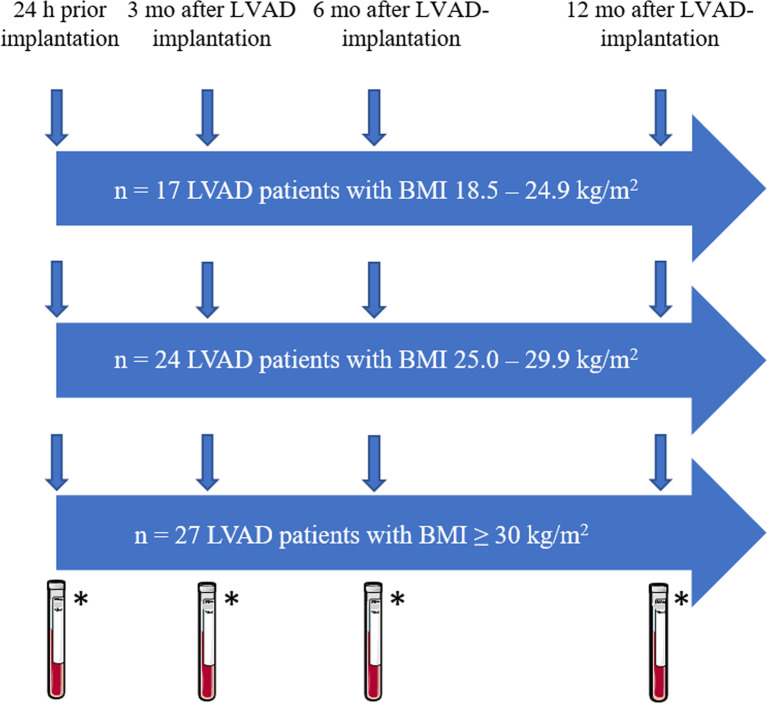
The study comprised 68 patients who underwent an LVAD implantation between September 2018 and January 2021. Immunological parameters, including CD8+ T cells and NK cells, were compared between the groups within the first postoperative year. The patients were divided into three groups based on their BMI at the time of LVAD implantation (normal weight: BMI 18.5–24.9 kg/m2, n = 17; pre-obesity: BMI 25.0–29.9 kg/m2, n = 24; obesity: BMI ≥30 kg/m2, n = 27). Citrated blood and serum were obtained at four different time points as shown in Figure 1: prior to LVAD implantation and at 3 months (1st follow-up [FU]), 6 months (2nd FU), and 12 months (3rd FU) after LVAD implantation.
Fig. 1.
Study design. BMI, body mass index; LVAD, left ventricular assist device; mo, months; *blood withdrawal for flow cytometric analyses and serum collection for cytokine measurements.
Patient-specific and clinical data included all primary and secondary diagnoses in addition to age, sex, date of birth, and BMI. Furthermore, intolerance to medicines and food, immunodeficiencies, and infectious diseases that may have been present up to 6 weeks before LVAD implantation were documented. Current and any pre-/perioperative medication, nicotine or alcohol abuse, and complications during and after LVAD implantation were recorded. During the postoperative course, the occurrence and type of infections were observed in a 1-year and a 2-year FU. Furthermore, the differential blood count and inflammatory marker C-reactive protein were included in the observation at all four measurement time points within the first year after LVAD implantation. Citrated blood and serum were obtained from 10 healthy participants and analyzed for comparison.
Blood Sampling
After peripheral blood withdrawal, citrated blood samples were analyzed using flow cytometry. Sera were centrifuged at 2,000 g at room temperature (RT) for 10 min, aliquoted, and frozen at −20°C until analysis.
Flow Cytometry
Citrated blood samples were used to determine the proportion of the following immunological cell populations: total CD3+ T cells and their proportion of CD8+ T cells as well as their degree of terminal differentiation/senescence (CD57) and activation (CD25), total NK cells and the proportion of their subpopulations (CD56bright and CD56dim NK cells), and the degree of terminal differentiation (CD57). In brief, samples were incubated with different antibody panels for 20 min at RT: panel A: CD57-APC, CD56-FITC, CD16-APC, and CD3-PerCP/Cy5.5; panel B: CD57-APC, CD8-FITC, CD25-PE-Cy7, and CD3-PerCP/Cy5.5. In panel A, NK cells were identified as CD16+CD56+ cells and their subpopulations as CD56dimCD16+, CD56brightCD16−, and CD56brightCD16dim cells. Additionally, we determined the degree of differentiation among NK cells based on the expression of CD57. Panel B was used to capture CD3+ and CD8+ T cells and the expression of CD57 and CD25. The antibodies were purchased from Becton Dickinson (BD, Franklin Lakes, NJ, USA) and BioLegend (San Diego, CA, USA). Following antibody incubation, erythrocytes were lysed with 2 mL FACS lysing solution (BD Biosciences) for 10 min. After centrifugation at 300 g for 5 min at RT, the cells were washed with 4 mL phosphate-buffered saline, followed by additional centrifugation. The cells were fixed by adding 500 μL of 1% formalin-phosphate-buffered saline before analysis. Flow cytometric analysis was performed using a BD LSR II cytometer with FACS-Diva 2.0 software version 6.1.3 (BD Biosciences). Standardization of the instrument was performed by weekly measurements of Cytometer Setup and Tracking Beads (BD Biosciences). In general, 100,000 events were recorded in each panel.
Quantification of Cytokines
The cytokines IL-2, IL-10, and IFN-γ were quantified in the serum samples using the Bio-Plex Pro Human Screening Panel 5plx EXP (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions. For assay analysis, a Luminex® 200 device and Luminex XPonent® software version 3.1 (Luminex, Austin, TX, USA) were used. TNF-α in serum samples was quantified using the ELISA Max Deluxe Set Human TNF-α (BioLegend).
Statistics
Data were collected and evaluated using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). Statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Data are presented as mean ± standard error of the mean for continuous variables and as the number (percent) for categorical variables. Group comparisons of ordinal data were performed using the χ2 test for frequencies greater than 5 or using Fisher’s exact test for frequencies lower than or equal to 5. One-way analysis of variance was performed to compare metric data. Levene’s test was used to test the homogeneity of variance. In cases of equal variances, the analysis of variance test was carried out at the global level using Scheffé post hoc tests. For variance inequality, the Welch test and Dunnett-T3 post hoc tests were applied. Mauchly’s tests were used to test for sphericity to observe of differences between the groups over time. Within-subject effects and within-subject contrast tests were used to relate the differences between and within the groups, respectively, to the preoperative measurement. Furthermore, the Pearson’s correlation coefficient of different cellular parameters with BMI at the end of the first year post-LVAD implantation was determined. The significance level was set at p ≤ 0.05.
Results
Patient Characteristics and Clinical Data
Three BMI groups which categorized the body weight of the patients with LVAD as normal weight (BMI 18.5–24.9 kg/m2, n = 17), pre-obesity (BMI 25.0–29.9 kg/m2, n = 24), and obesity (BMI ≥30 kg/m2, n = 27) according to the WHO criteria were compared. In all groups, the patients were predominantly male (normal weight: 88.2%; pre-obesity: 79.2%; obesity: 85.2%; Table 1). The groups were comparable in terms of age at the time of LVAD implantation, sex, underlying etiology of heart failure, and treatment strategy. The choice of the LVAD device differed between the groups (p = 0.05). Most obese patients received the HeartMate 3™, while one-third of normal-weight patients received the HVAD™. There were no significant differences in the comorbidities at the time of LVAD implantation. Pre-obese patients had significantly fewer implantations of a cardioverter-defibrillator or cardiac resynchronization therapies (54.2%) than patients with normal weight (82.4%) or obesity (85.2%; p = 0.04). Left ventricular ejection fraction was severely restricted (below 30%) in all three groups. Infectious diseases and nicotine and alcohol consumption were comparable among patients in the 6 weeks before LVAD implantation.
Table 1.
Demographic and clinical characteristics of patients prior to LVAD implantation
| Normal weight (n = 17) | Pre-obesity (n = 24) | Obesity (n = 27) | p value | |
|---|---|---|---|---|
| Age at implantation, years | 64.0±1.7 | 60.0±1.8 | 57.4±2.0 | 0.06 |
| Male gender, n (%) | 15 (88.2) | 19 (79.2) | 23 (85.2) | 0.77 |
| BMI, kg/m2 | 22.0±0.6 | 27.5±0.3 | 35.7±0.8 | <0.01 |
| Etiology, n (%) | ||||
| ICM | 10 (58.8) | 14 (58.3) | 10 (37.0) | 0.22 |
| DCM | 7 (41.2) | 10 (41.7) | 17 (63.0) | |
| NYHA classification, n (%) | ||||
| Class II | 1 (9.1) | 0 (0) | 0 (0) | 0.06 |
| Class III | 6 (35.3) | 9 (37.5) | 18 (66.7) | |
| Class IV | 9 (52.9) | 15 (62.5) | 9 (33.3) | |
| Indication of LVAD, n (%) | ||||
| Bridge to transplant | 1 (5.9) | 5 (20.8) | 3 (11.1) | 0.76 |
| Bridge to decision | 8 (47.1) | 10 (41.7) | 13 (48.1) | |
| Destination therapy | 8 (47.1) | 9 (37.5) | 11 (40.7) | |
| LVAD device, n (%) | ||||
| HeartMate 3™ | 12 (70.6) | 21 (87.5) | 26 (96.3) | 0.05 |
| HVAD™ | 5 (29.4) | 3 (12.5) | 1 (3.7) | |
| Comorbidities, n (%) | ||||
| Arterial hypertension | 16 (94.1) | 21 (87.5) | 24 (88.9) | 0.89 |
| Hyperlipoproteinemia | 9 (52.9) | 10 (41.7) | 19 (70.4) | 0.12 |
| Diabetes mellitus type 2 | 6 (35.3) | 13 (54.2) | 11 (40.7) | 0.44 |
| Chronic kidney disease | 9 (52.9) | 14 (58.3) | 21 (77.8) | 0.17 |
| Hypothyroidism | 0 (0) | 5 (20.8) | 4 (14.8) | 0.13 |
| Chronic inflammatory disease | 1 (5.9) | 1 (4.2) | 1 (3.7) | 1 |
| COPD/bronchial asthma | 3 (17.6) | 0 (0) | 2 (7.4) | 0.08 |
| Presence of CRT/ICD, n (%) | 14 (82.4) | 13 (54.2) | 23 (85.2) | 0.04 |
| Prior valve surgery, n (%) | 4 (23.5) | 4 (16.7) | 9 (33.3) | 0.40 |
| Prior CVA, n (%) | 3 (17.6) | 4 (16.7) | 3 (11.1) | 0.75 |
| Prior malign disease, n (%) | 4 (23.5) | 3 (12.5) | 1 (3.7) | 0.13 |
| History of chemotherapy/radiation, n (%) | 1 (5.9) | 2 (8.3) | 0 (0) | 0.34 |
| Infectious diseasesa, n (%) | 4 (23.5) | 6 (25.0) | 5 (18.5) | 0.87 |
| Intolerancesb, n (%) | 2 (11.8) | 6 (25.0) | 5 (18.5) | 0.91 |
| History of drug abuse, n (%) | 0 (0) | 1 (4.2) | 1 (3.7) | 1 |
| Nicotine consumption, n (%) | ||||
| Current nicotine abuse | 4 (23.5) | 4 (16.7) | 7 (25.9) | 0.08 |
| Former nicotine abuse | 9 (52.9) | 9 (37.5) | 17 (63.0) | |
| Nonsmoker | 4 (23.5) | 8 (33.3) | 1 (3.7) | |
| Not specified | 0 (0) | 3 (12.5) | 2 (7.4) | |
| Alcohol consumption, n (%) | ||||
| Current alcohol abuse | 3 (17.6) | 2 (8.3) | 2 (7.4) | 0.63 |
| Former alcohol abuse | 3 (17.6) | 6 (25.0) | 3 (11.1) | |
| No alcohol abuse | 10 (58.8) | 13 (54.2) | 16 (59.3) | |
| Not specified | 1 (5.9) | 3 (12.5) | 6 (22.2) | |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; CVA, cerebrovascular accident; DCM, dilatative cardiomyopathy; HTx, heart transplantation; ICD, implantable cardioverter defibrillator; ICM, ischemic cardiomyopathy; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
aFrom 6 weeks prior to LVAD implantation.
bIncludes intolerances to medicines, foods, insect venoms.
Patients’ medications were recorded for 6 weeks prior to LVAD implantation to document immunosuppressive or immunomodulating factors that could potentially bias the measurement of the immune parameters (online suppl. Table 1; see online suppl. material at https://doi.org/10.1159/000530174). Immunosuppressive drugs comprised glucocorticoids. Additionally, the drugs included were angiotensin-converting enzyme inhibitors/angiotensin 1-receptor antagonists, platelet agglutination inhibitors, beta-blockers, aldosterone antagonists, diuretics, statins, antiarrhythmics, anticoagulants, medications for chronic obstructive pulmonary disease, bronchial asthma, and antibiotics. There were no significant differences between the groups regarding co-medication before LVAD implantation. The ventilation time (normal weight: 57 h ± 112 h; pre-obesity: 73 h ± 158 h; obesity: 62 h ± 135 h; p = 0.93) as well as the proportion of patients with invasive (normal weight: 6%; pre-obesity: 0%; obesity: 0%; p = 0.25) and noninvasive ventilation (normal weight: 0%; pre-obesity: 8%; obesity: 0%; p = 0.18) prior to LVAD implantation was comparable between the study groups.
LVAD-Specific, LVAD-Related, and Non-LVAD Infections
Newly occurring infections after LVAD implantation were categorized into LVAD-specific, LVAD-related, and non-LVAD. There were no significant differences in the overall incidence between the groups in the first year post-LVAD implantation (LVAD-specific infections: p = 0.26; LVAD-related infections: p = 0.58; non-LVAD infections: p = 0.64) (Table 2). Among LVAD-specific infections, the driveline exit site was predominant. Pocket, pump, or cannula infections did not occur in the study cohort. Among non-LVAD infections, a significantly higher incidence of pulmonary infections was observed in normal-weight patients (normal weight: 35.3%; pre-obesity: 8.3%; obesity: 3.7%; p = 0.01).
Table 2.
Occurrence of infections within 12 months following LVAD implantation in normal-weight, pre-obese, and obese patients
| Normal weight (n = 17) | Pre-obesity (n = 24) | Obesity (n = 27) | p value | |
|---|---|---|---|---|
| LVAD-specific infections, n (%) | ||||
| Percutaneous DL infection | 2 (11.8) | 7 (29.2) | 5 (18.5) | 0.41 |
| LVAD-related infections, n (%) | ||||
| Infectious endocarditis | 1 (5.9) | 0 (0) | 1 (3.7) | 0.72 |
| Pathogens detected in BC | 1 (5.9) | 4 (16.7) | 3 (11.1) | 0.65 |
| Non-LVAD infections, n (%) | ||||
| Pulmonary infections | 6 (35.3) | 2 (8.3) | 1 (3.7) | 0.01 |
| Urinary infections | 1 (5.9) | 3 (12.5) | 5 (18.5) | 0.54 |
| Clostridium difficile infections | 1 (5.9) | 0 (0) | 2 (7.4) | 0.46 |
| Pathogens detected in BC | 0 (0) | 3 (12.5) | 5 (18.5) | 0.18 |
| Other | 0 (0) | 1 (4.2) | 1 (3.7) | 1 |
| Isolated bacteria in BC, n (%) | ||||
| Gram positive | 1 (5.9) | 6 (25.0) | 5 (18.5) | 0.29 |
| Gram negative | 0 (0) | 2 (8.3) | 1 (3.7) | 0.61 |
| Isolated bacteria at DL, n (%) | ||||
| Gram positive | 2 (11.8) | 4 (16.7) | 4 (14.8) | 1 |
| Gram negative | 2 (11.8) | 6 (25.0) | 4 (14.8) | 0.56 |
| Infections per patient | 0.9±0.2 | 0.8±0.3 | 1.0±0.2 | 0.82 |
| Time to first infection event, mo | 0.6±0.2 | 0.7±0.2 | 0.9±0.2 | 0.66 |
BC, blood culture; BMI, body mass index; DL, driveline; LVAD, left ventricular assist device.
The 2-year FU revealed statistically comparable rates of LVAD-specific (p = 0.24), LVAD-related (p = 0.33), and non-LVAD infections (p = 0.13). However, the absolute frequencies showed a tendency to a higher risk for infection in pre-obese and obese patients than in normal-weight patients for LVAD-specific (normal weight: 12.5%, pre-obese: 36.8%, obese: 32.0%) and LVAD-related (normal weight: 6.3%, pre-obese: 15.8%, obese: 24.0%) infections (data not shown).
Flow Cytometric Analysis
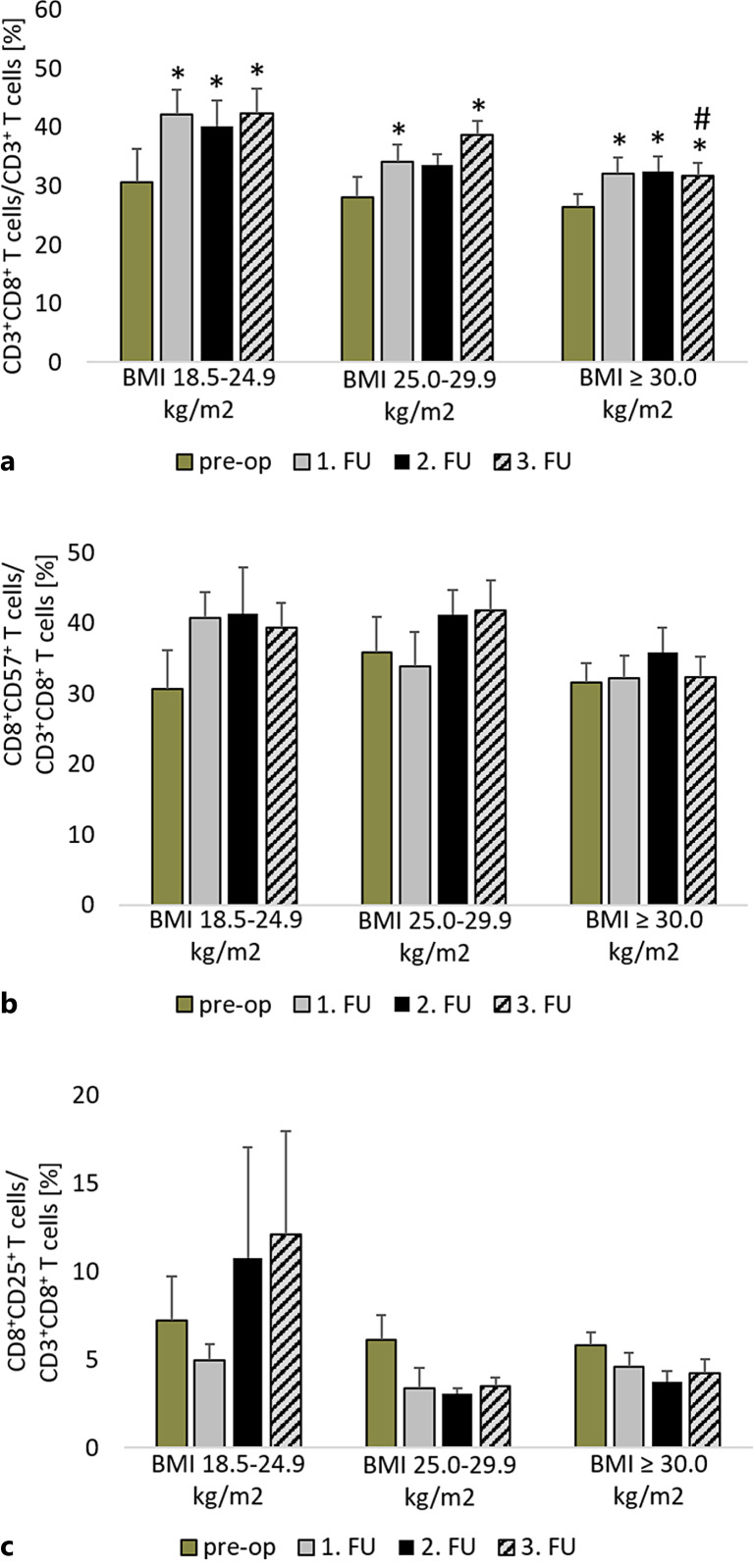
After LVAD implantation, there was a significant increase in the proportion of CD8+ T cells among the CD3+ T cells (normal weight: p < 0.01; pre-obesity: p < 0.01; obesity: p < 0.02) (shown in Fig. 2a). At the end of the first postoperative year, obese patients (31.8% ± 2.1%) showed a significantly lower proportion of CD8+ T cells than normal-weight patients (42.4% ± 4.1%; p = 0.04). Furthermore, the percentage of CD8+ T cells was negatively correlated with BMI (p = 0.03; r = −0.329) 1 year after LVAD implantation.
Fig. 2.
Comparison of the proportion of CD8+ T cells in all CD3+ T cells (a), CD8+CD57+ T cells in all CD8+ T cells (b), CD8+CD25+ T cells in all CD8+ T cells (c) before LVAD implantation and in the FU period in normal-weight, pre-obese, and obese patients. *p ≤ 0.05 compared to the pre-operative value of the same group. #p ≤ 0.05 compared to normal-weight patients in the same period. Measurement dates comprise the time prior to LVAD implantation (pre-op), after 3 months (1st FU), after 6 months (2nd FU), and after 12 months (3rd FU). BMI, body mass index; CD, cluster of differentiation; FU, follow-up.
Terminal differentiation of CD8+ T cells was examined using CD57 expression. The reference range for this analysis is approximately 20% [16]. The proportions of CD57 were above this range in all groups before and after LVAD implantation (shown in Fig. 2b) and did not change over time (normal weight: p = 0.06; pre-obesity: p = 0.09; obesity: p = 0.27). There were no significant differences between the groups in the preoperative and FU periods (pre-op: p = 0.69; 1st FU: p = 0.32; 2nd FU: p = 0.58; 3rd FU: p = 0.14).
Additionally, the state of activation was examined by measuring CD25 expression. There were no detectable differences in CD8+ T cell activation measured by CD25 expression between the groups at any time point (pre-op: p = 0.86; 1st FU: p = 0.50; 2nd FU: p = 0.35; 3rd FU: p = 0.30) (shown in Fig. 2c). LVAD implantation did not affect the activation of CD8+ T cells (normal weight: p = 0.54; pre-obesity: p = 0.10; obesity: p = 0.21).
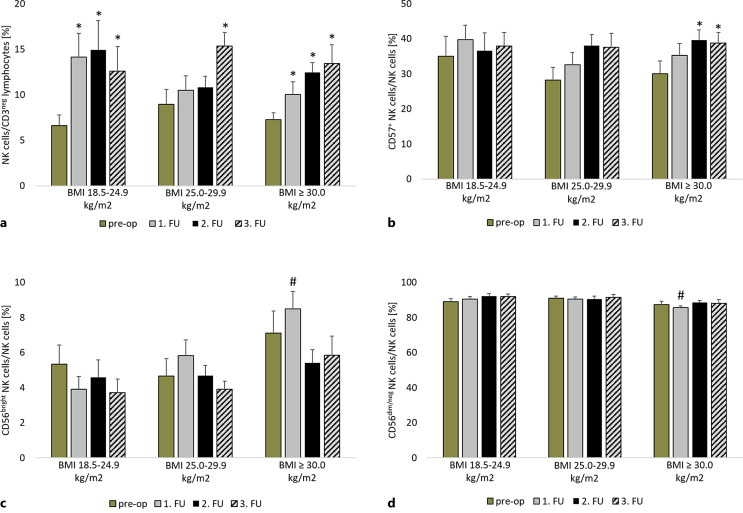
In addition to CD8+ T cells, NK cells and their subpopulations were quantified and showed no significant differences in the proportion of total NK cells between the groups in the preoperative and FU periods (pre-op: p = 0.53; 1st FU: p = 0.26; 2nd FU: p = 0.41; 3rd FU: p = 0.65) (shown in Fig. 3a). The proportion of circulating NK cells increased during the observation period. This increase was significant in normal-weight (p = 0.01) and obese patients (p < 0.01) during the FU period. Patients with pre-obesity showed a significant increase (p < 0.01) in the proportion of NK cells 12 months after LVAD implantation.
Fig. 3.
Comparison of the proportion of natural killer cells (NK cells) in all CD3− lymphocytes (a), CD57+ NK cells (b), CD56bright NK cells (c), CD56dim/neg NK cells (d) in all NK cells before LVAD implantation and in the FU period in normal-weight, pre-obese, and obese patients. *p ≤ 0.05 compared to the pre-operative value of the same group. #p ≤ 0.05 compared to normal-weight patients in the same period. Measurement dates comprise the time prior to LVAD implantation (pre-op), after 3 months (1st FU), after 6 months (2nd FU), and after 12 months (3rd FU). BMI, body mass index; CD, cluster of differentiation; FU, follow-up.
The reference range for the expression of CD57 in NK cells is approximately 60% [16]. Flow cytometric analysis revealed that the expression level of CD57 was below this reference range in all groups at all measurement time points (shown in Fig. 3b). The proportion of CD57+ NK cells (pre-op: p = 0.53; 1st FU: p = 0.42; 2nd FU: p = 0.84; 3rd FU: p = 0.96) was comparable between groups. Obese patients showed a significant increase in the percentage of CD57+ NK cells after 6 and 12 months (p = 0.01) of treatment.
CD56bright and CD56dim/neg were analyzed as subpopulations of circulating NK cells. Preoperatively, the proportions of CD56bright (p = 0.28) and CD56dim/neg NK cells (p = 0.29) were comparable between the groups (shown in Fig. 3c). Obese patients showed a significantly higher proportion of CD56bright NK cells 3 months after LVAD implantation (8.5% ± 1.0%) than normal-weight patients (3.9% ± 0.7; p = 0.01). There was no change in the proportion of CD56bright NK cells within the groups after LVAD implantation (normal weight: p = 0.37; pre-obesity: p = 0.18; obesity: p = 0.06), but the proportion of CD56bright NK cells positively correlated with BMI (p < 0.01, r = 0.403) 1 year after LVAD implantation.
Decreased proportions of CD56dim/neg NK cells in obese patients at 3 months after LVAD implantation compared with normal-weight and pre-obese patients (normal weight: 90.5% ± 1.4%; pre-obesity: 90.4% ± 1.2%; obesity: 85.5% ± 1.1%; pto normal weight = 0.03, pto pre-obesity = 0.02) were observed. No significant changes within the groups (normal weight: p = 0.21; pre-obesity: p = 0.86; obesity: p = 0.26) (shown in Fig. 3d) were observed.
Cytokine Measurement
Lastly, serum cytokine levels of IFN-γ, IL-2, IL-10, and TNF-α were determined by multiplexing or ELISA. Although IL-10 and TNF-α levels appeared to be elevated in overweight or obese compared to normal-weight LVAD patients before and after implantation, variants within the groups were too high to yield statistical significance (Table 3).
Table 3.
Serum levels of T-cell- and NK-cell-related cytokines within 12 months following LVAD implantation in normal-weight, pre-obese, and obese patients
| Normal weight (n = 17) | Pre-obesity (n = 24) | Obesity (n = 27) | p value | |
|---|---|---|---|---|
| IFN-γ, pg/mL | ||||
| Pre-OP | 0.11±0.25 | 1.02±3.66 | 0.09±0.28 | 0.63 |
| 1st FU | 0.00±0.00 | 1.69±6.00 | 0.00±0.00 | 0.36 |
| 2nd FU | 0.05±0.18 | 6.70±21.00 | 0.16±0.38 | 0.41 |
| 3rd FU | 0.00±0.00 | 0.74±2.56 | 0.05±0.21 | 0.38 |
| IL-2, pg/mL | ||||
| Pre-OP | 0.09±0.32 | 0.15±0.39 | 0.09±0.28 | 0.87 |
| 1st FU | 0.00±0.00 | 0.07±0.29 | 0.20±0.60 | 0.47 |
| 2nd FU | 0.09±0.32 | 0.00±0.00 | 0.08±0.30 | 0.68 |
| 3rd FU | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 1 |
| IL-10, pg/mL | ||||
| Pre-OP | 0.00±0.00 | 0.39±1.25 | 0.69±1.17 | 0.23 |
| 1st FU | 0.00±0.00 | 0.05±0.18 | 0.46±1.09 | 0.16 |
| 2nd FU | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 1 |
| 3rd FU | 0.00±0.00 | 0.00±0.00 | 0.54±2.09 | 0.49 |
| TNF-α, pg/mL | ||||
| Pre-OP | 0.22±0.39 | 0.56±0.87 | 0.70±1.02 | 0.06 |
| 1st FU | 0.62±1.01 | 0.82±1.01 | 1.09±2.28 | 0.69 |
| 2nd FU | 0.42±0.65 | 0.53±0.77 | 0.87±1.59 | 0.49 |
| 3rd FU | 0.25±0.63 | 0.82±1.28 | 0.70±1.58 | 0.43 |
FU, follow-up; IFN-γ, interferon-γ; IL-2/10, interleukin-2/10; pre-OP, preoperative; TNF-α, tumor necrosis factor-α.
Discussion
The present study documented that obesity affects CD8+ T cells and subsets of NK cells in patients with LVAD in the first year after LVAD implantation. Lower percentages of CD8+ T cells and CD56dim/neg NK cells, and higher percentage of CD56bright NK cells, were detected in obese but not in pre-obese and normal-weight LVAD patients during the first year after LVAD implantation. The induced immunological imbalance and phenotypic changes of T and NK cells may influence viral and bacterial immunoreactivity and therefore, lay the foundation for increased rates of infections in these patients.
An immunological imbalance was shown for cytotoxic CD8+ T cells that were lower in obese than in normal-weight patients at the end of the first post-operative year. This finding was supported by a negative correlation between the percentage of CD8+ T cells and BMI at 1 year after LVAD implantation which has been stated previously for a non-LVAD study cohort by El-Hafez et al. [8]. Dysregulation of T-cell homeostasis and a lower percentage of CD8+ T cells are known to limit antiviral immunological defense [7], which could affect the complication rate and survival after LVAD implantation. For example, a limited antiviral defense may lead to cytomegalovirus reactivation in patients with LVAD. Cytomegalovirus reactivation after LVAD implantation is rare but is associated with gastrointestinal bleeding in these patients [17, 18].
The expression of the activation marker CD25 and the terminal differentiation marker CD57 in CD8+ T cells was comparable between the study groups. CD57 expression on T cells is considered a surrogate marker for the replicative senescence of T cells [19]. Investigation of the senescence phenotype, particularly in the CD8+ T-cell compartment, indicates an immune risk profile that can predict morbidity and mortality [20]. Furthermore, the CD57 phenotype has been linked to the cytolytic function of CD8+ T cells [21]. According to our results, it can be concluded that CD8+ T-cell activation and senescence did not differ in overweight and adipose patients with LVAD.
Analysis of cytokines related to CD8+ T-cell functionality revealed comparability between LVAD patients with different BMIs. Both the pro-inflammatory cytokine IL-2 which is responsible for T-cell activation as well as for the proliferation of NK cells [22, 23] or the anti-inflammatory cytokine IL-10 that suppresses cytotoxic T-cell responses and has been reported to negatively correlate with the BMI [24, 25] were investigated. TNF-α, a cytokine released by adipocytes and immune cells in response to chronic inflammation [26], showed higher levels in overweight or obese compared to normal-weight LVAD patients before and after implantation, but the differences were without statistical significance. Because TNF-α is one of the most common cytokines that elevates following cardiac stress or heart failure [27], increased levels of this pro-inflammatory cytokine can be detected prior to LVAD implantation.
The proportion of circulating NK cells increased during the first year after LVAD implantation and was comparable between normal-weight, pre-obese, and obese patients with LVAD. However, the phenotype of NK cells differed, and obese patients showed a higher proportion of CD56bright NK cells and a lower proportion of CD56dim/neg NK cells at 3 months after LVAD implantation compared with normal-weight patients. Additionally, the proportion of CD56bright NK cells was positively correlated with BMI 1 year after LVAD implantation. Our data on LVAD patients correlated with that obtained in a non-LVAD cohort reporting an increased number of CD56bright NK cells in obese patients compared with normal-weight patients [28]. CD56bright NK cells are less cytotoxic than CD56dim/neg NK cells but show enhanced production of TNF-α and IFN-γ [29]. It has been hypothesized that the shift to a higher percentage of CD56bright NK cells may be induced by obesity-related metabolites leading to impaired NK-cell cytotoxicity in obese patients [28]. This hypothesis underpins the observation that obese patients are more susceptible to infections than normal-weight patients [28, 30]. Significantly higher expression of IFN-γ was not detected in pre-obese and obese patients in our study, which can be explained by the measurement in serum and not by intracellular measurements. An impaired NK-cell phenotype in overweight patients, as reported by Naujoks et al. [31], was not observed in our study.
The optimal treatment for the immunological shift in obese patients with LVAD seems to be body weight reduction by dietary intervention, physical activity, and bariatric surgery [30, 31]. However, body weight reduction can be difficult to achieve in obese patients with LVAD because of restrictions in activity due to the device and a lack of knowledge about healthy nutrition. Furthermore, whether body weight reduction reverses immunological changes is controversial. While some groups reported successful reactivation of NK-cell functionality [13, 31] after weight loss, others did not find an effect on T-cell response and metabolism [32].
Although our study did not show differences between the rates of LVAD-specific, LVAD-related, and non-LVAD infections, we detected immunological differences related to antiviral defense in patients with LVAD. The median time between LVAD implantation and the first detection of LVAD-specific and -related infections ranged from 3 to 7 months [33, 34]. However, statistically significant BMI-related infection rates were detected 2 years after LVAD implantation [4, 35, 36]. Thus, a 2-year FU of infection rates is recommended to improve data interpretation.
A higher incidence of pulmonary infections in normal-weight patients compared to obese patients was observed in our study. This finding is in contrast to studies reporting that obesity diminished the immune defense against pulmonary infections [37, 38]. Thus, other factors increasing the risk for pulmonary infections should be taken into account. Therefore, the total duration of ventilation in the peri- and postoperative period and the incidence of preimplant ventilation were compared because these parameters have been associated with higher rates of infection in LVAD patients [39]. Neither the ventilation time nor the incidence of preimplant invasive and noninvasive ventilation differed between the study groups and could explain the higher incidence of pulmonary infections in normal-weight patients.
The choice of LVAD device differed significantly between the groups. The majority of obese patients received the HeartMate 3™, while one-third of normal-weight patients received the HVAD™. It is known that the choice of the LVAD type is associated with different rates of bleeding complications, but differences in infection rates have not been reported for the HeartMate 3™ and HVAD™ devices. Therefore, we did not assume bias in the immunological parameters of the LVAD type.
The limitation of our study is that data on the hip-to-waist ratio prior to LVAD implantation were missing. This parameter could serve as a reliable marker for adipose classification in patients. To avoid bias in the study results due to misclassification of the patients because of heart failure-induced edema, the weight and classification of the patients after LVAD implantation were checked. Weight reduction was observed in approximately 15% of the patients but did not lead to a change in the BMI classification and therefore, a change in the study group.
Conclusions
Our study documented changes in immunological parameters involved in the antiviral defense of patients in the first year after LVAD implantation. These changes involve the CD8+ T-cell compartment and NK-cell subsets and are related to BMI. These estimated changes may help understand the increased risk of infection in obese patients with LVAD.
Statement of Ethics
This study protocol was reviewed and approved by Ethics Committee of the Medical Faculty of the University of Leipzig (approval number ID: 225/17-ek) and was performed according to the guidelines of the Declaration of Helsinki (2013). Written informed consent was obtained from all patients before study initiation.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Eva Katharina Messer received a Kaltenbach doctoral fellowship of the German Heart Foundation.
Author Contributions
Design and conception: E.K.M., A.L.M., K.K., M.A.B., and M.T.D.; data acquisition: E.K.M., K.K., F.S., J.H., and L.H.; data analysis: E.K.M., K.K., M.-T.D., and L.H.; data interpretation: E.K.M., K.K., S.E., Do.Sc., K.J., and Di.Sa.; and supervision: M.A.B. and M.T.D. All authors drafted the article, made critical revisions, and approved the final version.
Funding Statement
Eva Katharina Messer received a Kaltenbach doctoral fellowship of the German Heart Foundation.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Goldstein DJ, Meyns B, Xie R, Cowger J, Pettit S, Nakatani T, et al. Third annual report from the ISHLT mechanically assisted circulatory support registry: a comparison of centrifugal and axial continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2019;38(4):352–63. 10.1016/j.healun.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 2. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–13. 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3. Yost G, Coyle L, Gallagher C, Graney N, Siemeck R, Tatooles A, et al. The impact of extreme obesity on outcomes after left ventricular assist device implantation. J Thorac Dis. 2017;9(11):4441–6. 10.21037/jtd.2017.10.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khan MS, Yuzefpolskaya M, Memon MM, Usman MS, Yamani N, Garan AR, et al. Outcomes associated with obesity in patients undergoing left ventricular assist device implantation: a systematic review and Meta-analysis. ASAIO J. 2020;66(4):401–8. 10.1097/MAT.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 5. Forest SJ, Xie R, Kirklin JK, Cowger J, Xia Y, Dipchand AI, et al. Impact of body mass index on adverse events after implantation of left ventricular assist devices: an IMACS registry analysis. J Heart Lung Transplant. 2018;37(10):1207–17. 10.1016/j.healun.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Shea D, Hogan AE. Dysregulation of natural killer cells in obesity. Cancers. 2019;11(4):573. 10.3390/cancers11040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Touch S, Clément K, André S. T cell populations and functions are altered in human obesity and type 2 diabetes. Curr Diab Rep. 2017;17(9):81. 10.1007/s11892-017-0900-5. [DOI] [PubMed] [Google Scholar]
- 8. El-Hafez HA, Abd El-Azi SM. Association between immune competence and metabolic parameters in obesity. Trends Med Res. 2012;7(2):53–61. 10.3923/tmr.2012.53.61. [DOI] [Google Scholar]
- 9. Womack J, Tien PC, Feldman J, Shin JH, Fennie K, Anastos K, et al. Obesity and immune cell counts in women. Metabolism. 2007;56(7):998–1004. 10.1016/j.metabol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao R, Tang D, Yi S, Li W, Wu C, Lu Y, et al. Elevated peripheral frequencies of Th22 cells: a novel potent participant in obesity and type 2 diabetes. PLoS One. 2014;9(1):e85770. 10.1371/journal.pone.0085770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes. 2009;33(1):151–6. 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 12. Ringseis R, Eder K, Mooren FC, Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015;21:58–68. [PubMed] [Google Scholar]
- 13. Bähr I, Spielmann J, Quandt D, Kielstein H. Obesity-associated alterations of natural killer cells and immunosurveillance of cancer. Front Immunol. 2020;11:245. 10.3389/fimmu.2020.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pangrazzi L, Naismith E, Miggitsch C, Carmona Arana JA, Keller M, Grubeck-Loebenstein B, et al. The impact of body mass index on adaptive immune cells in the human bone marrow. Immun Ageing. 2020;17:15. 10.1186/s12979-020-00186-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ilavská S, Horváthová M, Szabová M, Nemessányi T, Jahnová E, Tulinská J, et al. Association between the human immune response and body mass index. Hum Immunol. 2012;73:480–5. 10.1016/j.humimm.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 16. Kared H, Martelli S, Ng TP, Pender SL, Larbi A. CD57 in human natural killer cells and T-lymphocytes. Cancer Immunol Immunother. 2016;65(4):441–52. 10.1007/s00262-016-1803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sandkovsky U, Florescu DF, Um JY, Raichlin E, Lowes BD, Kapalis M, et al. Cytomegalovirus reactivation and colitis after left ventricular assist device placement. Int J Infect Dis. 2013;17(5):e348–51. 10.1016/j.ijid.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 18. Pfau P, Rothstein RD. Cytomegalovirus cecal ulcer in a patient awaiting cardiac transplantation. Am J Gastroenterol. 1996;91(11):2435–6. [PubMed] [Google Scholar]
- 19. Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169(4):1984–92. 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 20. Spielmann G, Johnston CA, O'Connor DP, Foreyt JP, Simpson RJ. Excess body mass is associated with T cell differentiation indicative of immune ageing in children. Clin Exp Immunol. 2014;176(2):246–54. 10.1111/cei.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MRet al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 23. Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang JS, Chang CC, Chien E, Lin SS, Cheng-Shiuan T, Bai CH, et al. Association between interleukin 1β and interleukin 10 concentrations: a cross-sectional study in young adolescents in Taiwan. BMC Pediatr. 2013;13:123. 10.1186/1471-2431-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charles BA, Doumatey A, Huang H, Zhou J, Chen G, Shriner D, et al. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. J Clin Endocrinol Metab. 2011;96(12):E2018–22. 10.1210/jc.2011-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alzamil H. Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J Obes. 2020;2020:5076858. 10.1155/2020/5076858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schumacher SM, Naga Prasad SV. Tumor necrosis factor-α in heart failure: an updated review. Curr Cardiol Rep. 2018;20(11):117. 10.1007/s11886-018-1067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bähr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res. 2018;66(2):234–44. 10.1007/s12026-018-8989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tobin LM, Mavinkurve M, Carolan E, Kinlen D, O'Brien EC, Little MA, et al. NK cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight. 2017;2(24):e94939. 10.1172/jci.insight.94939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Milner JJ, Beck MA. Micronutrients, immunology and inflammation: the impact of obesity on the immune response to infection. Pro Nutr Soc. 2012;71(2):298–306. 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naujoks W, Quandt D, Hauffe A, Kielstein H, Bähr I, Spielmann J. Characterization of surface receptor expression and cytotoxicity of human NK cells and NK cell subsets in overweight and obese humans. Front Immunol. 2020;11:573200. 10.3389/fimmu.2020.573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rebeles J, Green WD, Alwarawrah Y, Nichols AG, Eisner W, Danzaki K, et al. Obesity-induced changes in T-cell metabolism are associated with impaired memory T-cell response to influenza and are not reversed with weight loss. J Infect Dis. 2019;219(10):1652–61. 10.1093/infdis/jiy700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blanco-Guzman MO, Wang X, Vader JM, Olsen MA, Dubberke ER. Epidemiology of left ventricular assist device infections: findings from a large nonregistry cohort. Clin Infect Dis. 2021;72(2):190–7. 10.1093/cid/ciaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahal A, Ruch Y, Meyer N, Perrier S, Minh TH, Schneider C, et al. Left ventricular assist device-associated infections: incidence and risk factors. J Thorac Dis. 2020;12(5):2654–62. 10.21037/jtd.2020.03.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akay MH, Nathan SS, Radovancevic R, Poglajen G, Jezovnik MK, Candelaria IN, et al. Obesity is associated with driveline infection of left ventricular assist devices. ASAIO J. 2019;65(7):678–82. 10.1097/MAT.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 36. Mano A, Kilic A, Lampert BC, Smith SA, Whitson B, Hasan AK. Impact of change in body mass index on outcomes after left ventricular assist device implantation in obese patients. ASAIO J. 2019;65(7):668–73. 10.1097/MAT.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 37. Mancuso P. Obesity and respiratory infections: does excess adiposity weigh down host defense? Pulm Pharmacol Ther. 2013;26(4):412–9. 10.1016/j.pupt.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandez C, Manuel A. Obesity, respiratory disease and pulmonary infections. Ann Res Hosp. 2017;1:1. 10.21037/arh.2017.08.06. [DOI] [Google Scholar]
- 39. Miller PE, Caraballo C, Ravindra NG, Mezzacappa C, McCullough M, Gruen J, et al. Clinical implications of respiratory failure in patients receiving durable left ventricular assist devices for end-stage heart failure. Circ Heart Fail. 2019;12(11):e006369. 10.1161/CIRCHEARTFAILURE.119.006369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.