Abstract
Objective
To evaluate the effect of protracted low dose, low dose rate exposure to ionising radiation on the risk of cancer.
Design
Multinational cohort study.
Setting
Cohorts of workers in the nuclear industry in France, the UK, and the US included in a major update to the International Nuclear Workers Study (INWORKS).
Participants
309 932 workers with individual monitoring data for external exposure to ionising radiation and a total follow-up of 10.7 million person years.
Main outcome measures
Estimates of excess relative rate per gray (Gy) of radiation dose for mortality from cancer.
Results
The study included 103 553 deaths, of which 28 089 were due to solid cancers. The estimated rate of mortality due to solid cancer increased with cumulative dose by 52% (90% confidence interval 27% to 77%) per Gy, lagged by 10 years. Restricting the analysis to the low cumulative dose range (0-100 mGy) approximately doubled the estimate of association (and increased the width of its confidence interval), as did restricting the analysis to workers hired in the more recent years of operations when estimates of occupational external penetrating radiation dose were recorded more accurately. Exclusion of deaths from lung cancer and pleural cancer had a modest effect on the estimated magnitude of association, providing indirect evidence that the association was not substantially confounded by smoking or occupational exposure to asbestos.
Conclusions
This major update to INWORKS provides a direct estimate of the association between protracted low dose exposure to ionising radiation and solid cancer mortality based on some of the world’s most informative cohorts of radiation workers. The summary estimate of excess relative rate solid cancer mortality per Gy is larger than estimates currently informing radiation protection, and some evidence suggests a steeper slope for the dose-response association in the low dose range than over the full dose range. These results can help to strengthen radiation protection, especially for low dose exposures that are of primary interest in contemporary medical, occupational, and environmental settings.
Introduction
Unlike many carcinogens, which have been reduced or removed once recognised, the public’s exposure to ionising radiation has increased in recent decades.1 2 3 In the US, for example, the average person’s annual effective dose doubled between 1985 and 2006 and has remained elevated since,4 primarily owing to increases in exposure to ionising radiation from medical imaging procedures (whereas the average radiation worker’s annual occupational dose remained relatively constant over that period).5 6 7 Understanding of associations between low dose and low dose rate radiation exposures and cancer informs decisions about medical and commercial uses of ionising radiation, as well as decisions about exposure limits for members of the public and people working with ionising radiation.
The study of Japanese survivors of the atomic bombs serves as the primary basis for the quantitative risk estimates used in radiation protection.8 9 Although that study concerns a high dose rate setting, findings from it inform contemporary assessments for low dose and low dose rate radiation exposures.10 11 12 The International Nuclear Workers Study (INWORKS) was undertaken to provide a large scale international assessment of mortality risks from protracted low dose, low dose rate ionising radiation exposures.13 INWORKS pools cohorts of nuclear workers monitored with radiation badges in France, the UK, and the US, countries that have assembled some of the largest and most informative cohorts of nuclear workers in the world.14 15 16 17 18 Here, we report on a major update of analyses of associations between radiation dose and mortality due to solid cancers in INWORKS, with follow-up extended by 10 or more years in each country.
Methods
INWORKS was established to provide a basis for deriving quantitative estimates of the association between protracted low dose, low dose rate exposure to ionising radiation and mortality. INWORKS builds on the work done for the International Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry by taking advantage of data from the most informative cohorts involved in that study.19 Criteria for selection of the study cohorts included completeness and quality of data, start of facility operations, and exposure primarily to high energy, low linear energy transfer penetrating radiations.13 Data came from three major French employers (Commissariat à l’Energie Atomique et aux énergies alternatives, Orano, and Electricité de France), from the UK National Registry for Radiation Workers (which includes information provided by major employers of nuclear workers including the Atomic Weapons Establishment, British Nuclear Fuels, UK Atomic Energy Authority, British Energy Generation, Magnox Electric, and the Ministry of Defence, among others), and from the US Department of Energy’s Hanford site, Savannah River site, Oak Ridge National Laboratory, and Idaho National Laboratory, as well as from the Portsmouth Naval Shipyard. To be included, workers must have the information needed for linkages with vital records (that is, individual identifiers and date of birth) and must have been employed in the nuclear industry for at least one year and monitored for external radiation with personal dosimeters.13
In all countries, institutional review boards determined that documentation of informed consent was not necessary for this records based study. In France, information on workers came from existing records, with no direct contact with participants, and the institutional review board waived requirements for individual informed consent. In the UK, workers could refuse to participate in the National Registry for Radiation Workers, although less than 1% did. In the US, information on workers came from existing records, with no direct contact with participants, and the institutional review board waived requirements for informed consent.
We derived individual annual estimates of whole body dose primarily due to external exposure to penetrating radiation in the form of photons from personal occupational exposure monitoring data.20 21 22 Unless otherwise stated, any reference to dose in this paper implies absorbed dose to the colon expressed in gray (Gy). We derived the estimated colon dose to facilitate comparison with analyses of associations between radiation dose and solid cancer done in other major cohorts.23 24 Film dosimeters with one or two elements (that is, filters, often made of lead, tin, or cadmium) were commonly used for personal dosimetry beginning in the 1940s.20 Multi-element dosimeters were implemented in most mixed activity facilities by the late 1950s to account for mixed field irradiation and allow for better estimation of dose over a wider range of photon energies.20 Thermoluminescent dosimeters largely replaced the film badge beginning in the 1970s.20 Administrative practices also changed over time; the frequency of dosimeter exchange was greater (for example, weekly or biweekly) before around 1965 and lesser (for example, monthly or quarterly) thereafter.20 Portsmouth Naval Shipyard’s dose information was not available after 1996; however, few people in that study cohort were still working after 1996. We did not add recorded estimates of doses from tritium intakes or neutron exposures to recorded dose from exposure to external photon radiation.22 We used available records of estimated neutron doses, which were recorded in a unit of measure for equivalent dose (that is, rem or Sv), only to construct categories of neutron monitoring status: whether a worker had a positive recorded neutron dose, and, if so, whether their recorded neutron dose ever exceeded 10% of their total external radiation dose of record.20 22 25
Available measures of incorporated radionuclides included bioassay results, indication of confirmed uptake (for example, fraction of a body burden or annual limit on intake), or an assigned committed dose. We used available records or workstation-exposure matrix information (for France) to categorise workers on the basis of indication of a known or suspected internal contamination (we identified French and US workers with a known or suspected uptake, as well as UK workers who were known to have been monitored for internal exposure).20 22
We ascertained vital status through 2012, 2014, and 2016 for the UK, French, and US cohorts, respectively, through linkage with national and regional death registries, employers’ records, tax records, and social security administration records. We abstracted information on underlying cause of death from death certificates and coded it according to the ICD (international classification of diseases) revision in effect at the time of death. We examined all cancer related mortality (ICD-9 codes 140-208) because radiation induced cancers could occur at most, if not all, sites after whole body exposure to ionising radiation and because death certificate data could be more accurate for identifying all cancers as a group than for identifying specific types of cancer. We examined solid cancer (ICD-9 codes 140-199) as a primary outcome of interest and an outcome typically examined in studies of the effects of low dose radiation. We also examined the association between radiation dose and solid cancer excluding lung cancer (ICD-9 code 162), because the exclusion of lung cancer is an indirect method to evaluate concerns about confounding by smoking; solid cancer excluding cancers of the oral cavity and pharynx, oesophagus, stomach, colon, rectum, liver, gallbladder, pancreas, nasal cavity, larynx, lung, cervix, ovary, bladder, kidney, and ureter (ICD-9 codes 140-151, 153-154.1, 154.8-157, 160-162, 180, 183, and 188-189), which constitute a larger group of smoking related cancers26; chronic obstructive pulmonary disease (ICD-9 codes 490-492, and 496), because this outcome is strongly associated with tobacco smoking but not known to be associated with low dose ionising radiation, providing an indirect method to assess concerns about confounding by smoking27; solid cancer excluding cancers of the lung, liver, and bone (ICD-9 codes 155, 162, and 170), which are three organs that may receive substantial doses in cases of incorporated plutonium24 28 29; and solid cancer excluding cancers of the lung and pleura (ICD-9 codes 162 and 163), to assess concerns about potential bias due to occupational exposure to asbestos. Supplementary table A provides additional details on the ICD codes that define each outcome category.
A person entered the study on the date of first dosimetric monitoring or one year after the date of first employment, whichever was later. The national death registry in France provides individual information on causes of death only from 1968 onwards, so French workers entered follow-up on 1 January 1968 or later. For the UK cohort, start of follow-up for workers first employed before 1955 was defined as 1 January 1955 owing to indications that follow-up information before that date may not be complete.30 31 A person exited the study on the earliest of the date of death, date lost to follow-up, or end of follow-up for vital status ascertainment.
Statistical methods
The statistical methods used were similar to those used in previous international studies of nuclear workers.18 We quantified radiation dose-mortality associations by using a stratum specific model for mortality rates, Ik, of the form Ik=exp(αk)(1+βZ), where k indexes strata, Z is the cumulative dose in Gy, and β is excess relative rate (the relative rate minus 1) per Gy.32 33 34 The excess relative rate is expressed as a proportional increase in the rate over baseline, per unit dose, where a value of 0 indicates no radiation associated increase in the mortality rate. Models were fitted using Poisson regression methods for analysis of mortality rates, incorporating person time at risk as the rate denominator.35 We adjusted estimates of excess relative rate per Gy, through stratification, for the effects of country, attained age (in 5 year intervals), sex, year of birth (in 10 year intervals), socioeconomic status (French, US, and UK workers employed by the Atomic Energy Authority and Atomic Weapons Establishment classified into five categories on the basis of job title: professional and technical workers, administrative staff, skilled workers, unskilled workers, and uncertain; other UK workers classified into two broader categories of non-industrial and industrial employees), duration of employment or radiation work (in 10 year intervals), and neutron monitoring status. Information on country, age, sex, and year of birth was complete; we included workers with missing information on job classification (<1% of workers were missing such information) in the category of uncertain socioeconomic status. We identified our adjustment set of covariates on the basis of substantive knowledge and consideration of causal structures facilitated by reference to directed acyclic graphs (supplementary figure A).36 37 38 To allow for a minimal induction and latency period between exposure and death, cumulative doses were lagged by 10 years; we chose a 10 year lag a priori, and it facilitates comparison of results with our previous analysis of these data as well as with some other studies of solid cancer mortality among nuclear workers.18 19 39 40
We did sensitivity analyses in which cumulative doses were lagged five years, 15 years, or 20 years, cumulative doses were restricted to the lower dose range, workers with a positive neutron dose were excluded, workers flagged for internal contamination or monitoring were excluded, and regression model adjustment was made for workers flagged for internal contamination or monitoring. We compared results obtained under alternative lags with respect to goodness of model fit.41 We examined the dose-response association visually by fitting a regression model with indicator variables for categories of cumulative dose (that is, a piecewise constant model for the association) and plotting the resultant relative rate estimates against category specific mean dose values (noting that reported estimates of excess relative rate per Gy were derived from regression models fitted to the full data tabulation). To formally assess departure from linearity in the effect of cumulative dose, we fitted a model that also included a quadratic function of cumulative dose, and we also fitted a linear exponential model of the form Ik=exp(αk)(1+βZ)exp(δZ); we evaluated the improvement in model goodness of fit by using a likelihood ratio test statistic. To evaluate the influence of a single country on overall results, analyses excluded one country at a time, and we fitted a model with a product term between country and dose, allowing heterogeneity to be assessed on the basis of the likelihood ratio test. We derived an estimate of between country heterogeneity in association by using the method of DerSimonian and Laird for random effects.42 43 To assess the effect of inaccuracies in dose estimates for workers employed in the early years of nuclear industry operations, we excluded workers hired before 1958 and before 1965; we chose these dates because they represent the years at various facilities when dosimetry improved owing to changes in dosimeter technology and administrative practice.22 25
We report likelihood based 90% confidence intervals for estimates of the excess relative rate per Gy, a common approach in radiation epidemiological studies in which the objective is to evaluate whether an increased risk of cancer exists after exposure to radiation; this facilitates comparison of the precision of our estimated associations with findings reported in other important epidemiological studies of populations exposed to radiation.19 39 40 44 45 46 47 We report the change in deviance on inclusion of a term in the regression model as a likelihood ratio test statistic along with its associated P value, which provides a continuous measure of the fit of the model to the data (that is, compatibility between the observed data and the model used to compute the statistic).48 We interpret the P value as a continuous measure rather than limiting interpretation to dichotomisation of the P value at a threshold for declaring significance (such as 0.05). We fitted models by using conditional Poisson regression with primary control for confounding obtained by stratification, implemented in the SAS software package (version 9.4).49
Patient and public involvement statement
No patients were involved in setting the research question, the outcome measures, or the design and implementation of the study. The nuclear sites at which workers were employed were restricted, we lacked permissions to engage directly with employees, and the study involves large number of workers employed in the past. However, discussions with workers helped to motivate our study analyses and consideration of study limitations.
Results
The study included 309 932 workers and encompassed 10.7 million person years of follow-up (table 1). The study cohort included 40 445 women. We followed the average worker to nearly 70 years of age; among these workers we observed 103 553 deaths by the end of follow-up, of which 31 009 deaths were due to cancer and 28 089 deaths were due to solid cancer. Less than 2% of decedents had a missing or unknown underlying cause of death, and less than 2% of workers emigrated or were otherwise lost to follow-up for vital status ascertainment.
Table 1.
Characteristics of cohorts included in INWORKS: nuclear workers in France, UK, and US, 1944-2016
| Characteristic | France | UK | US | INWORKS |
|---|---|---|---|---|
| Calendar years of follow-up | 1968-2014 | 1955-2012 | 1944-2016 | 1944-2016 |
| Workers | 60 697 | 147 872 | 101 363 | 309 932 |
| Person years (millions): | 2.08 | 4.67 | 3.98 | 10.72 |
| Men | 1.80 | 4.27 | 3.17 | 9.24 |
| Women | 0.28 | 0.40 | 0.81 | 1.48 |
| Deaths (all causes): | 12 270 | 39 933 | 51 350 | 103 553 |
| All cancer | 4885 | 12 556 | 13 568 | 31 009 |
| Solid cancer | 4446 | 11 574 | 12 069 | 28 089 |
| Solid cancer other than lung | 3317 | 8308 | 8198 | 19 823 |
| Chronic obstructive pulmonary disease | 133 | 1545 | 2527 | 4205 |
| Average duration of follow-up (years) | 34.2 | 31.6 | 39.3 | 34.6 |
| Average age at end of follow-up (years) | 64.8 | 62.5 | 71.4 | 65.9 |
| Average individual cumulative dose (mGy) | 12.9 | 20.19 | 16.8 | 17.7 |
| Average individual cumulative dose to colon* (mGy) | 17.8 | 22.75 | 20.1 | 20.9 |
Among workers whose estimated dose was >0.
The excess relative rate was 0.53 (90% confidence interval 0.30 to 0.77) per Gy for all cancer mortality and 0.52 (0.27 to 0.77) per Gy for solid cancer mortality (table 2). Our a priori 10 year lag assumption was reasonably well supported by the data (supplementary table B). The estimated association between radiation dose and solid cancer was slightly smaller in magnitude and poorer in model goodness of fit under a five year lag assumption than under a 10 year lag assumption. The estimated association between radiation dose and solid cancer was similar in magnitude and poorer in model goodness of fit under a 20 year lag assumption than under a 10 year lag assumption (supplementary table B). Under a 15 year lag assumption, the estimated association between radiation dose and solid cancer was slightly larger in magnitude and had slightly better model goodness of fit than under a 10 year lag assumption (supplementary table B).
Table 2.
Estimates of excess relative rate (ERR) per Gy for death due to specific outcome categories in INWORKS
| Category | Deaths | ERR per Gy* (90% CI) |
|---|---|---|
| All cancer | 31 009 | 0.53 (0.30 to 0.77) |
| Solid cancer | 28 089 | 0.52 (0.27 to 0.77) |
| Solid cancer other than lung | 19 823 | 0.46 (0.18 to 0.76) |
| Chronic obstructive pulmonary disease | 4205 | 0.12 (−0.43 to 0.68) |
10 year lag assumption.
CI: confidence interval.
Strata: country, age, sex, birth cohort, socioeconomic status, duration employed, neutron monitoring status.
To evaluate the impact of data from each country on the summary estimate for the pooled data, we excluded countries from the INWORKS cohort one at a time. The estimate for the association between cumulative dose under a 10 year lag and solid cancer mortality was 0.47 (0.22 to 0.73) per Gy when we excluded France, 0.41 (0.04 to 0.80) per Gy when we excluded the UK, and 0.66 (0.35 to 1.00) per Gy when we excluded the US from INWORKS. We observed minimal evidence of heterogeneity in the estimated associations by country on the basis of a statistical test (likelihood ratio test=2.3, df=2; P=0.31). A random effects model suggested modest between country variance (τ2=0.01; Q statistic for heterogeneity=2.3, df=2; P=0.31), with 16% of the overall variation in study results being due to between study heterogeneity.
The association between cumulative dose, lagged 10 years, and solid cancer mortality was reasonably well described by a linear model (fig 1); inclusion of a parameter describing the linear association between cumulative dose and solid cancer contributed substantially to model goodness of fit (supplementary table B). The addition of a parameter for the square of cumulative dose led to only a modest improvement in model goodness of fit compared with the linear model (likelihood ratio test =2.51, df=1; P=0.11), suggesting some downward curvature (that is, a negative estimated coefficient for the quadratic term). The addition of a parameter for an exponential term in the model led to a modest improvement in model goodness of fit for a linear-exponential model compared with the linear model (likelihood ratio test =3.17, df=1; P=0.08), again suggesting some downward curvature. To assess the trend over the lower cumulative dose range, we estimated associations between cumulative dose and solid cancer mortality over restricted ranges of 0-400 mGy cumulative dose (excess relative rate 0.63 (0.34 to 0.92) per Gy), 0-200 mGy cumulative dose (0.97 (0.55 to 1.39) per Gy), 0-100 mGy cumulative dose (1.12 (0.45 to 1.80) per Gy), 0-50 mGy cumulative dose (1.38 (0.20 to 2.60) per Gy), and 0-20 mGy cumulative dose (1.30 (−1.33 to 4.06) per Gy) (supplementary table C). Over the restricted range of 0-200 mGy cumulative dose, the association between cumulative dose and solid cancer mortality was well described by a linear model, and the addition of a parameter for the square of cumulative dose led to minimal improvement in model goodness of fit compared with the linear model (likelihood ratio test=0.54, df=1; P=0.46).
Fig 1.
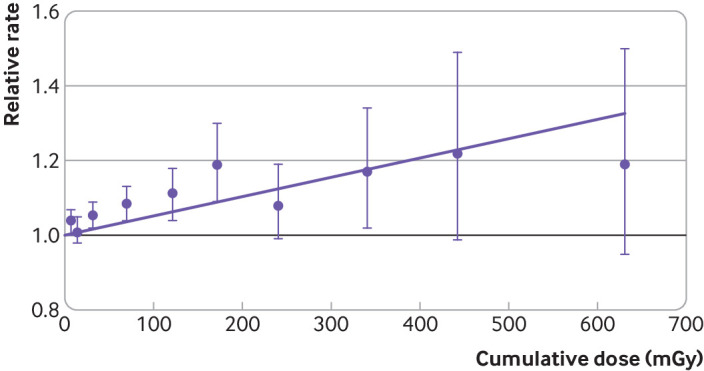
Relative rate of mortality due to solid cancer by categories of cumulative colon dose, lagged 10 years in INWORKS. Bars indicate 90% confidence intervals, and purple line depicts fitted linear model for change in excess relative rate of solid cancer mortality with dose. Strata: country, age, sex, birth cohort, socioeconomic status, duration employed, neutron monitoring status
To indirectly assess potential confounding by smoking, we estimated the association between cumulative radiation dose and solid cancers other than lung cancer (excess relative rate 0.46 (0.18 to 0.76) per Gy) (table 2). The association between cumulative radiation dose and solid cancers other than lung cancer was reasonably well described by a linear model (supplementary figure B); neither the addition of a parameter for the square of cumulative dose (likelihood ratio test=0.24, df=1; P=0.62) nor the addition of a parameter in a linear-exponential model led to substantial improvement in model goodness of fit compared with the linear model (likelihood ratio test=0.26, df=1; P=0.61). We also estimated the association between cumulative radiation dose and solid cancer excluding a broader group of smoking related cancers (excess relative rate 0.52 (0.10 to 0.99) per Gy, based on 8889 deaths). We examined the association between cumulative radiation dose and chronic obstructive pulmonary disease, an outcome strongly associated with tobacco smoking but not known to be associated with low dose ionising radiation; we observed minimal evidence of association between cumulative radiation dose and chronic obstructive pulmonary disease (excess relative rate 0.12 (−0.43 to 0.68) per Gy) (table 2). To indirectly assess potential confounding by asbestos, we estimated the association between radiation dose and solid cancers other than lung cancer and pleural cancer (excess relative rate 0.43 (0.15 to 0.73) per Gy, based on 19 550 deaths).
To address concerns about potential inaccuracies in dose estimation in the early years of operations, we examined the association between cumulative radiation dose and solid cancer mortality restricted to the 238 639 workers hired in 1958 or later (excess relative rate 1.22 (0.74 to 1.72) per Gy) and restricted to the 189 386 workers hired in 1965 or later (1.44 (0.65 to 2.32) per Gy) (supplementary table D). For comparison, we examined the association among workers who were hired before 1958 (excess relative rate 0.20 (−0.07 to 0.49) per Gy). Similarly to analyses of the full cohort, we observed evidence of downward curvature in the association between cumulative dose and solid cancer mortality in the analyses restricted to workers hired in 1965 or later (change in deviance on addition of a parameter for the square of cumulative dose was 2.68, df=1; P=0.10, and change in deviance on addition of a parameter for an exponential term in the model was 5.39, df=1; P=0.02). In analyses restricted to workers hired in these more contemporary periods, estimated associations between cumulative radiation dose and solid cancers other than lung cancer were similar in magnitude to estimates of association for solid cancer; neither the addition of a parameter for the square of cumulative dose (likelihood ratio test=0.08, df=1; P=0.78) nor the addition of a parameter in a linear-exponential model led to substantial improvement in model goodness of fit compared to the linear model (likelihood ratio test=0.17, df=1; P=0.68). In analyses restricted to workers hired in these more contemporary periods, we observed minimal evidence of association between radiation dose and chronic obstructive pulmonary disease (supplementary table D).
Because our primary interest is in the effect of external exposure to penetrating photons, we examined results in analyses restricted to the 84% of workers (9.05 million person years and 23 410 deaths due to solid cancer) who were never flagged for incorporated radionuclides or internal monitoring (excess relative rate 0.82 (0.46 to 1.22) per Gy). For comparison, we examined results among workers who were flagged for incorporated radionuclides or internal monitoring (excess relative rate 0.21 (−0.11 to 0.56) per Gy) (supplementary table E). We found negligible evidence of curvature in the association between cumulative dose and solid cancer mortality in analyses restricted to workers who were never flagged for incorporated radionuclides or internal monitoring (change in deviance on addition of a parameter for the square of cumulative dose=0.39, df=1; P=0.53), nor in analyses restricted to workers who were flagged for incorporated radionuclides or internal monitoring (change in deviance on addition of a parameter for the square of cumulative dose=1.02, df=1; P=0.31). We also estimated the association between cumulative radiation dose and solid cancers other than lung, liver, and bone cancer among workers who had no reported internal deposition (excess relative rate 0.81 (0.36 to 1.28) per Gy, based on 15 943 deaths). In addition, in the full cohort, we estimated the association between cumulative radiation dose and solid cancer after further adjusting for indication of incorporated radionuclides or internal monitoring (excess relative rate 0.52 (0.26 to 0.78) per Gy).
Because of concerns about measurement of exposure to neutrons, we examined results in analyses restricted to the 9.45 million person years and 24 213 deaths due to solid cancer observed among workers who had no reported neutron dose (excess relative rate 0.55 (0.23 to 0.90) per Gy). For comparison, we examined results among workers who had recorded neutron dose (supplementary table F).
We assessed the sensitivity of results to adjustment for socioeconomic status, duration of employment, and neutron monitoring, by fitting a simpler model that adjusted only for country, age, sex, and birth cohort. The estimated association between cumulative radiation dose and deaths due to solid cancer (excess relative rate 0.49 (0.30 to 0.69) per Gy) was similar in magnitude to that obtained from the fully adjusted model, with somewhat greater precision in analyses using the simpler adjustment set of covariates. In a separate sensitivity analysis, we restricted the analysis to men, among whom most of the collective dose and cancer deaths were accrued; the estimated association between cumulative dose under a 10 year lag and solid cancer was 0.52 (0.28 to 0.77) per Gy, based on 27 115 deaths).
Discussion
This study, which involved a major update to an international cohort mortality study of radiation dosimeter monitored workers, reports evidence of an increase in the excess relative rate of solid cancer mortality with increasing cumulative exposure to ionising radiation at the low dose rates typically encountered by French, UK, and US nuclear workers. The study provides evidence in support of a linear association between protracted low dose external exposure to ionising radiation and solid cancer mortality. Although some evidence suggests a steeper slope for the dose-response association at lower doses than over the full dose range (supplementary table C), a linear model offers a simple summarisation of the association with reasonable fit to the observed data (fig 1).
INWORKS draws on a large international collaboration to assemble records for radiation monitored workers and follow them over time to study cause specific mortality in relation to dose. With this updated follow-up, the magnitudes of estimates of association are similar to the values reported in the previous analysis (supplementary table G).18 However, this analysis encompasses a more than 50% increase in the number of solid cancer deaths compared with the previous analysis,18 and it consequently affords improved precision in these estimates of association (supplementary table G). Notably, the study provides one of the most informative assessments to date on the magnitude of the radiation dose-solid cancer association in the low dose region, a key concern for contemporary radiation protection. The study provides evidence for a positive association between radiation dose and solid cancer mortality in the 0-100 mGy and 0-50 mGy cumulative dose ranges (supplementary table C). For comparison, previous analyses of the Life Span Study of Japanese atomic bomb survivors have explored the minimal dose level at which a significant association is observed between radiation dose and solid cancer mortality and reported a range of approximately 0-150 mGy (based on follow-up information for that study through 2003).50 Of course, estimates of association obtained in analyses restricted to these lower dose ranges are less precise than those obtained in an unrestricted analysis (supplementary table C); however, analyses restricted to these lower dose ranges directly relate to the radiation protection community’s interest in epidemiological evidence of a radiation dose-cancer association at low doses (for example, ≤100 mGy).51 Restricting analyses to information at these lower dose ranges showed that the estimated excess relative rate per Gy for solid cancer mortality in the unrestricted analysis (table 2) was smaller in magnitude than the estimate obtained on restricting the analysis to the lower dose ranges, indicative of attenuation of the association at the highest cumulative exposure levels. For people interested only in the exposure-response relation in the low cumulative exposure range, a linear trend estimate obtained in analyses restricted to a lower cumulative exposure range may be appealing as it is not influenced by any attenuation at higher exposure levels.
Comparison with other studies
Analyses of cancer in the Life Span Study of the Japanese atomic bomb survivors serve as the primary quantitative basis for the calculation of radiation detriment in systems of radiological protection.52 The study of Japanese atomic bomb survivors is challenging as a basis for assessing contemporary concerns about radiation protection because many atomic bomb survivors were exposed to acute high doses of radiation, and selective survival after the atomic bombings, as well as wartime conscription of healthy adults out of the cities before the bombings, mean that the study members are a select subset of the pre-war population. For the purposes of radiation protection, people often assume that low dose rate exposures pose less carcinogenic hazard than the high dose rate exposures experienced by the Japanese atomic bomb survivors.9 However, persistent concerns about effects of low dose radiation exposures have motivated a wide range of research activities, including epidemiological studies of workers in the nuclear industry.51 53 Our study does not find evidence of reduced risk per unit dose for solid cancer among workers typically exposed to radiation at low dose rates. The estimated association between radiation and solid cancer mortality in INWORKS (excess relative rate 0.52 (90% confidence interval 0.27 to 0.77) per Gy) is larger than, albeit statistically compatible with, an estimate from a mortality analysis of male survivors of the Japanese atomic bomb exposed at ages 20-60 years (excess relative rate 0.32 (95% confidence interval 0.01 to 0.50) per Sv).19 53
The coherence of findings from INWORKS with those derived from other contemporary epidemiological studies of low dose radiation (mean doses <100 mGy) also contributes to an overall evaluation of the study findings.54 55 56 57 A recent meta-analysis of studies of mortality in populations exposed to low doses of radiation, including the previous INWORKS analysis, found that the meta-analytic summary estimate of excess relative rate per Gy for solid cancer mortality was very close to the INWORKS study summary estimate, and also compatible with estimates derived from the Japanese Life Span Study.57 However, when considering studies of higher doses, an important exception was the study of workers employed in the Soviet programme for plutonium production at the Mayak facilities in the southern Urals, which reported an excess relative rate for solid cancer per Gy that was three to four times lower than the our INWORKS summary estimate and the summary estimate derived from the Life Span Study of the Japanese atomic bomb survivors.57 Given its size and the high magnitude of doses, the Mayak study exerted substantial influence on meta-analytic estimates of the excess relative rate for solid cancer per Gy that included higher dose studies.57 The reasons for differences between the Mayak study and INWORKS are unclear, but in the early years of operation at the Mayak facilities many workers were highly exposed with substantial uncertainty about their internal and external radiation doses.28 57 Analyses of mortality among French nuclear workers showed a positive association between estimated colon dose and solid cancer mortality (excess relative rate 0.69 (95% confidence interval −0.28 to 1.77) per Gy)58; we note that INWORKS includes a sizable fraction of this cohort. Analyses of mortality among US nuclear workers showed a positive association between cumulative dose and solid cancer mortality (excess relative rate 0.19 (95% confidence interval −0.10 to 0.52) per Gy), which was of larger magnitude among workers first hired after 196059; again, we note the overlap between this cohort and INWORKS. Analyses of cancer incidence among workers in the UK National Registry for Radiation Workers (UK NRRW) showed a positive association between external dose and solid cancer incidence (excess relative rate 0.20 (95% confidence interval −0.00 to 0.43) per Sv), although a linear model seemed to overestimate risk at higher doses, such that a linear-exponential model fitted the data better than a linear model, with the linear component of the model yielding an excess relative rate per Sv of 1.14 (0.30 to 2.36).60 Among workers in that cohort exposed to only external radiation, the estimated excess relative rate per Sv (0.52, 0.11 to 0.96) was more clearly linear, and in analyses of solid cancer incidence other than lung the estimated excess relative rate per Sv was also more clearly linear (noting that INWORKS includes a sizable fraction of the workers in the UKNRRW cohort). In contrast to analyses of the UK NRRW, our analyses of INWORKS adjusted the recorded dose to account for bias in historical dosimeter response and attenuation, taking the estimated colon dose as the quantity of interest, but we still observed some downward curvature. Analyses of radiation-mortality associations in INWORKS using recorded photon dose as the dose metric, rather than adjusted estimates of colon dose, yielded estimates of association of somewhat lower magnitude but similar goodness of model fit to estimates obtained in analyses using the estimated colon dose (supplementary table H). As this study shows, large scale studies of nuclear worker such as INWORKS, as well as studies of Mayak workers and the US Million Person Study,28 61 provide important information to support the radiological protection system.
Strengths and limitations of study
This study draws on the previous work done to characterise the performance of the various radiation dosimeters used in France, the UK, and the US over the study period and to account for differences between countries and over time in dosimeter performance. The performances of a variety of types of dosimeters were evaluated,21 and panels of experts were convened to characterise workplace conditions, monitoring routines, photon energies, and exposure geometries over the study period. A database of correction factors was developed to account for the influence of geometries of exposure, energies of photons, and other sources of bias and uncertainty in radiation dose estimates.20 22 For these INWORKS analyses, we adjusted the recorded dose to account for bias in historical dosimeter response and attenuation, taking the estimated colon dose as the quantity of interest.22 Despite those efforts, concerns have been expressed that errors in radiation dose estimates for workers employed in the early years of the industry’s operations could lead to biased estimates of radiation dose-cancer mortality associations.62 63 64 Workers employed in the earliest years of the industry were often monitored with open window or single element personal film badge dosimeters, and film badges were exchanged on a relatively frequent basis.20 22 65 Consequently, exposure measurement error related to personal dosimeter technology, monitoring practices, and historical records, particularly in the early years of operation, has received attention.20 63 65
We report analyses restricted to workers hired in more recent periods, showing that our overall results were not driven solely by information contributed by workers employed in the earliest years of the industry. To the contrary, after exclusion of workers hired in the earliest years of operations our estimate of the excess relative rate per Gy for solid cancer was larger than the estimate derived from analysis of the full cohort (supplementary table D). The results obtained in analyses of the full INWORKS cohort are of interest in comparison with our early report (supplementary table G); however, among contemporary workers with presumably higher quality dosimetry information, the linear estimate of the radiation dose-solid cancer mortality association was larger than the overall summary estimate of association (supplementary table D). Improvements over time in radiation dosimetry should lead to more accurate dose estimates and to estimates of radiation risk that are less susceptible to bias due to exposure measurement error in analyses restricted to workers employed in more contemporary periods. Of course, comparisons of the magnitudes of summary radiation risk estimates between subgroups should be viewed with caution because subgroups may have different distributions of modifying factors (such as time since exposure)17; in this paper, we have not focused on assessment of such modifiers. Nevertheless, our estimates of radiation risks among the more contemporary workers (supplementary table D) should be of interest because exposures and work conditions among these workers are more indicative of the current experience. Interestingly, although downward curvature in a radiation dose-response model may be induced when highly exposed workers are subject to more measurement error than those with lower exposure,66 67 68 evidence of downward curvature in our study persisted in analyses restricted to more recent hires. This suggests that errors in external dose estimates are unlikely to fully explain the attenuation of the dose-response association at the highest doses. Of course, some measurement error persists in contemporary dose estimates; however, modern dosimetry systems tend to produce individual dose estimates with markedly less error than earlier dosimetry systems, and our assessment of the dosimeters used in this more contemporary period indicate high levels of accuracy and comparability in performance of dosimeters used in all three countries.20 22
The workplace spectra encountered by nuclear workers (predominantly photons of energies between 100 and 3000 kiloelectron volt) have been suggested to be more effective at causing cancer than the spectra encountered by survivors of the nuclear bomb (predominantly in the 2000-5000 kiloelectron volt range).20 22 69 Although attention to the adequacy of radiation protection standards in settings involving low energy photons is warranted,70 a relatively small fraction of absorbed doses from external exposures in INWORKS was due to lower energy (<250 kiloelectron volt) photons,20 which is the range at which the evidence of increased biological effectiveness is greatest.70 71 Moreover, the spectra encountered by workers in our study is presumably directly relevant for contemporary radiation protection in occupational, and many medical, settings.
Although INWORKS lacks individual level data on several potentially important confounding factors, including cigarette smoking, we were able to indirectly assess confounding by smoking. For example, after exclusion of lung cancers from the group of solid cancers we observed evidence of a positive dose-response association similar in magnitude to that observed for all solid cancers (table 2). Such a pattern is contrary to what would be expected if substantial confounding by smoking existed, as is the minimal evidence of association between radiation dose and chronic obstructive pulmonary disease, an outcome strongly associated with smoking (table 2).72 Figure 1 and supplementary figure B help to inform interpretation of the effect of lung cancer on the association between cumulative dose and solid cancer. At the highest category of cumulative dose, a linear model for the association fits somewhat better after exclusion of lung cancers from the group of solid cancers. Such attenuation at high exposure levels, not unusual in mortality studies in industrial cohorts, could suggest negative confounding (at the highest cumulative dose levels) by a lung carcinogen, exposure dependent effect modification, or selection bias.66 67 68 Because we do not have individual level data on smoking, we cannot empirically answer questions about modification of the effect of radiation by smoking. Similarly, we observed little evidence that exposure to asbestos substantially confounds the association between cumulative radiation dose and solid cancer mortality in this study population: after exclusion of lung and pleural cancers from the group of solid cancers, we observed a dose-response association similar in magnitude to that for all solid cancers. Exclusion of workers flagged for internal radionuclide monitoring resulted in a larger estimate of excess relative rate per Gy of solid cancer than an analysis without such exclusion and reduced evidence of downward curvature in the association between cumulative dose and solid cancer mortality, suggesting that attenuation of the dose-response association at higher doses may be associated with factors related to internal radionuclide monitoring status. After exclusion of deaths due to lung, liver, and bone cancers (sites that may receive substantial doses in cases of incorporated plutonium), the estimate of excess relative rate per Gy remained similar in magnitude. Further investigation of the influence of internal monitoring, period of hire, and dose range is warranted. A relatively small proportion of workers were judged to be substantially exposed to neutrons20; our primary analyses adjusted for an indicator of potential for substantial exposure to neutrons, while acknowledging the potential for underestimated or missed doses from neutrons of some energies, particularly in early period of operations. An expert group of dosimetrists recommended flagging workers with substantial neutron doses but not incorporating these into organ dose estimates owing to limitations of historical neutron dosimetry and between country differences in methods.22 In a sensitivity analysis, we observed that among workers who had no reported neutron dose, the estimated association between colon dose and mortality due to solid cancer was similar to the estimate obtained for the whole cohort after adjustment for neutron monitoring status.
This analysis focused on the broad category of mortality due to all solid cancers, a commonly examined outcome of interest for assessment of radiation risk. The results provide one simple summarisation of radiation associated excess cancer mortality. Of course, site specific cancer risk estimates also are of interest and inform understanding of variation in radiation-cancer associations between cancer sites14; however, in studies that rely on death certificate information, the specificity of the death certificate as a tool for ascertaining cancer occurrence is often better for a broad category (such as solid cancer) than for narrow disease specific categories. Moreover, in epidemiological studies of low dose radiation, regression model estimates for cancer site specific outcomes are often unstable (reflecting small numbers of radiation related excess cases). In the past, we have illustrated the use of a hierarchical regression approach to stabilise site specific estimates,14 but this paper focuses on all solid cancers combined. Further examination of the association between radiation dose and lung cancer mortality in future site specific analyses should help to further inform interpretation of the overall solid cancer mortality associations. Although our results directly relate to relatively contemporary French, UK, and US nuclear workers, variation over time and between populations in the distribution of cancers by site may influence a population summary estimate of excess relative rate per Gy for all solid cancers, as discussed, for example, with regards to interpretation of findings from the Japanese Life Span Study.73
Studies of worker include a group of people who tend to be healthier than the general population (that is, they must be fit enough to secure employment),74 75 and long term workers tend to be healthier than short term workers, which can lead to a “healthy worker survivor” bias that may obscure or distort estimates of the harmful effects of protracted occupational exposures.36 76 77 78 Attenuation of the slope of an occupational exposure-response association at high cumulative exposure levels could arise because long term workers tend to have lower disease rates than short term workers and their cumulative exposures tend to be higher than the cumulative exposures accrued by short term workers. Interestingly, we observed less evidence of such attenuation in analyses that excluded lung cancer from the group of solid cancers, which could suggest bias that disproportionately masks the effect of exposure to radiation on lung cancer mortality at the highest cumulative doses (thereby leading to evidence of downward curvature). Despite such limitations, our study provides direct estimates of radiation risks among relatively contemporary working age adults in the French, UK, and US nuclear industries; as such, the results of INWORKS offer a useful complement to findings derived from the study of Japanese atomic bomb survivors.
Conclusions
INWORKS is unusual in its international scope, and the study benefits from decades of work by researchers in France,46 79 the UK,31 80 81 and the US,82 83 84 85 as well as in international collaborations,20 21 22 39 65 86 to assemble these data, achieve the high level of completeness of information, and support these analyses by critical assessments of the quality of information and methods supporting this study. The results of this major update of INWORKS should help to inform deliberations of radiation protection organisations, such as the International Commission on Radiological Protection, regarding risk assessment in settings of low dose and low dose rate radiation exposures, particularly with regards to evidence supportive of assumptions about the magnitude of the excess relative rate per Gy and linearity of the association between protracted relatively low dose and low dose rate exposures and solid cancer mortality.9
What is already known on this topic
Ionising radiation is an established cause of cancer
The primary quantitative basis for radiation protection standards comes from studies of people exposed to acute, high doses of ionising radiation
What this study adds
The results of an updated study of nuclear workers in France, the UK, and the US suggest a linear increase in the relative rate of cancer with increasing exposure to radiation
Some evidence suggested a steeper slope for the dose-response association at lower doses than over the full dose range
The risk per unit of radiation dose for solid cancer was larger in analyses restricted to the low dose range (0-100 mGy) and to workers hired in the more recent years of operations
Acknowledgments
The construction of the French cohort was realised by the Institut de Radioprotection et de Sûreté Nucléaire (IRSN) with partial funding from Orano and Electricité de France (EDF). The IRSN thanks all people from the French Alternative Energies and Atomic Energy Commission (CEA), Orano, and EDF who cooperated in the elaboration of the French cohort. The United Kingdom Health Security Agency thanks all of the organisations and individuals participating in the UK’s National Registry for Radiation Workers for their cooperation, and the National Registry for Radiation Workers’ steering group for its continued support.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: DBR conceived the study. DBR, KL, DL, MG, RH, KKR, SB, AK, MKSB, and ITC developed the research questions and designed the study. KL and DL worked on provision of the French data; KKR, SB, and RDD worked on provision of the US data; MG and RH worked on provision of the UK data. MM was responsible for data management and processing as well as some analyses. ITC was responsible for the dosimetry. DBR did the statistical analysis and produced the initial draft of the manuscript, which was revised and approved by all authors. DBR is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Funding: This work was partly funded by the US National Cancer Institute (R01CA242852). The French cohort was coordinated by IRSN, with part funding from Orano and Electricité de France. The US cohort was coordinated by the US National Institute for Occupational Safety and Health. The UK cohort was coordinated by the UK Health Security Agency, which operates the UK’s National Registry for Radiation Workers. The sponsors had no role in the study design, the data analysis and interpretation, or the writing of the report.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the US National Cancer Institute, Orano, the French Alternative Energies and Atomic Energy Commission, and Electricité de France; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Plain language messages about the results will be shared with the appropriate offices at the US National Institute for Occupational Safety and Health, the French Institut de Radioprotection et de Sûreté Nucléaire, the UK Health Security Agency, and the International Agency for Research on Cancer; these organisations engage with employers, workers’ representatives, members of the public (for example, via social media feeds), and advocacy groups. Some of the lead researchers (DBR, DL) will also contact expert and advisory bodies (International Commission on Radiological Protection, National Council on Radiation Protection and Measurements, United Nations Scientific Committee on the Effects of Atomic Radiation), of which they are already part. Organisations such as the International Agency for Research on Cancer rely on publications such as this one to inform their patient and public facing materials on websites such as iarc.who.int.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved by the International Agency for Research on Cancer’s ethical review committee (No 11-09 and later amendments), relevant ethical committees of the participating countries, and the ethical review committee of the University of North Carolina at Chapel Hill.
Data availability statement
For reasons of ethics and permissions from different agencies, the data are maintained at the International Agency for Research on Cancer (Lyon, France) and cannot be made available outside of the agency.
References
- 1. International Agency for Research on Cancer . A review of human carcinogens. Part D: Radiation. International Agency for Research on Cancer, 2012. [Google Scholar]
- 2. International Agency for Research on Cancer . Ionizing Radiation, Part 1: X- and Gamma-Radiation, and Neutrons. IARC Press, 2000. [Google Scholar]
- 3. United Nations . Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2008 report to the General Assembly, with scientific annexes. United Nations, 2010. [Google Scholar]
- 4. United Nations Scientific Committee on the Effects of Atomic Radiation . Sources, Effects and Risks of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation 2020/2021 Report to the General Assembly, with Scientific Annexes. United Nations, 2022. [Google Scholar]
- 5. Institute of Medicine . Breast Cancer and the Environment: A Life Course Approach. Institute of Medicine, the National Academies, 2012. [Google Scholar]
- 6. National Council on Radiation Protection and Measurements . Medical radiation exposure of patients in the United States: recommendations of the National Council on Radiation Protection and Measurements. NCRP report no 184. National Council on Radiation Protection and Measurements, 2019. [Google Scholar]
- 7. Villoing D, Yoder RC, Passmore C, Bernier MO, Kitahara CM, Multicenter Study of Recorded Occupational Radiation Badge Doses in Nuclear Medicine . A U.S. Radiology 2018;287:676-82. 10.1148/radiol.2018171138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Research Council, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation . Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. The National Academies Press, 2006. [PubMed] [Google Scholar]
- 9. International Commission on Radiological Protection . The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1-332. [DOI] [PubMed] [Google Scholar]
- 10. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 11. Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 2004;363:345-51. 10.1016/S0140-6736(04)15433-0 [DOI] [PubMed] [Google Scholar]
- 12. Herzog P, Rieger CT. Risk of cancer from diagnostic X-rays. Lancet 2004;363:340-1. 10.1016/S0140-6736(04)15470-6 [DOI] [PubMed] [Google Scholar]
- 13. Hamra GB, Richardson DB, Cardis E, et al. Cohort Profile: The International Nuclear Workers Study (INWORKS). Int J Epidemiol 2016;45:693-9. 10.1093/ije/dyv122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson DB, Cardis E, Daniels RD, et al. Site-specific Solid Cancer Mortality After Exposure to Ionizing Radiation: A Cohort Study of Workers (INWORKS). Epidemiology 2018;29:31-40. 10.1097/EDE.0000000000000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2015;2:e276-81. 10.1016/S2352-3026(15)00094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillies M, Richardson DB, Cardis E, et al. Mortality from Circulatory Diseases and other Non-Cancer Outcomes among Nuclear Workers in France, the United Kingdom and the United States (INWORKS). Radiat Res 2017;188:276-90. 10.1667/RR14608.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daniels RD, Bertke SJ, Richardson DB, et al. Examining temporal effects on cancer risk in the international nuclear workers’ study. Int J Cancer 2017;140:1260-9. 10.1002/ijc.30544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015;351:h5359. 10.1136/bmj.h5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ 2005;331:77. 10.1136/bmj.38499.599861.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thierry-Chef I, Marshall M, Fix JJ, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: study of errors in dosimetry. Radiat Res 2007;167:380-95. 10.1667/RR0552.1 [DOI] [PubMed] [Google Scholar]
- 21. Thierry-Chef I, Pernicka F, Marshall M, Cardis E, Andreo P. Study of a selection of 10 historical types of dosemeter: variation of the response to Hp(10) with photon energy and geometry of exposure. Radiat Prot Dosimetry 2002;102:101-13. 10.1093/oxfordjournals.rpd.a006078 [DOI] [PubMed] [Google Scholar]
- 22. Thierry-Chef I, Richardson DB, Daniels RD, et al. INWORKS Consortium . Dose Estimation for a Study of Nuclear Workers in France, the United Kingdom and the United States of America: Methods for the International Nuclear Workers Study (INWORKS). Radiat Res 2015;183:632-42. 10.1667/RR14006.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grant EJ, Brenner A, Sugiyama H, et al. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958-2009. Radiat Res 2017;187:513-37. 10.1667/RR14492.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sokolnikov M, Preston D, Gilbert E, Schonfeld S, Koshurnikova N. Radiation effects on mortality from solid cancers other than lung, liver, and bone cancer in the Mayak worker cohort: 1948-2008. PLoS One 2015;10:e0117784. 10.1371/journal.pone.0117784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilbert ES, Fix JJ. Accounting for bias in dose estimates in analyses of data from nuclear worker mortality studies. Health Phys 1995;68:650-60. 10.1097/00004032-199505000-00004 [DOI] [PubMed] [Google Scholar]
- 26. International Agency for Research on Cancer . A review of human carcinogens. E. Personal habits and indoor combustions. International Agency for Research on Cancer, 2012. [Google Scholar]
- 27. Richardson DB, Laurier D, Schubauer-Berigan MK, Tchetgen Tchetgen E, Cole SR. Assessment and indirect adjustment for confounding by smoking in cohort studies using relative hazards models. Am J Epidemiol 2014;180:933-40. 10.1093/aje/kwu211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sokolnikov M, Preston D, Stram DO. Mortality from solid cancers other than lung, liver, and bone in relation to external dose among plutonium and non-plutonium workers in the Mayak Worker Cohort. Radiat Environ Biophys 2017;56:121-5. 10.1007/s00411-016-0670-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sokolnikov ME, Gilbert ES, Preston DL, et al. Lung, liver and bone cancer mortality in Mayak workers. Int J Cancer 2008;123:905-11. 10.1002/ijc.23581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kendall GM, Muirhead CR, MacGibbon BH, et al. Mortality and occupational exposure to radiation: first analysis of the National Registry for Radiation Workers. BMJ 1992;304:220-5. 10.1136/bmj.304.6821.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haylock RGE, Gillies M, Hunter N, Zhang W, Phillipson M. Cancer mortality and incidence following external occupational radiation exposure: an update of the 3rd analysis of the UK national registry for radiation workers. Br J Cancer 2018;119:631-7. 10.1038/s41416-018-0184-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas D. General relative risk models for survival time and matched case-control analysis. Biometrics 1981;37:673-86 10.2307/2530149. [DOI] [Google Scholar]
- 33. Breslow NE, Lubin JH, Marek P, et al. Multiplicative Models and Cohort Analysis. J Am Stat Assoc 1983;78:1-12 10.1080/01621459.1983.10477915. [DOI] [Google Scholar]
- 34. Barlow WE. General Relative Risk Models in Stratified Epidemiologic Studies. Appl Stat 1985;34:246-57 10.2307/2347470. [DOI] [Google Scholar]
- 35. Frome EL. The analysis of rates using Poisson regression models. Biometrics 1983;39:665-74. 10.2307/2531094 [DOI] [PubMed] [Google Scholar]
- 36. Schubauer-Berigan MK, Berrington de Gonzalez A, Cardis E, et al. Evaluation of Confounding and Selection Bias in Epidemiological Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. J Natl Cancer Inst Monogr 2020;2020:133-53. 10.1093/jncimonographs/lgaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37-48. 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 38. Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002;155:176-84. 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- 39. Cardis E, Gilbert ES, Carpenter L, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res 1995;142:117-32. 10.2307/3579020 [DOI] [PubMed] [Google Scholar]
- 40. Muirhead CR, O’Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009;100:206-12. 10.1038/sj.bjc.6604825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richardson DB, Cole SR, Chu H, Langholz B. Lagging exposure information in cumulative exposure-response analyses. Am J Epidemiol 2011;174:1416-22. 10.1093/aje/kwr260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 43. Little MP, Azizova TV, Bazyka D, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012;120:1503-11. 10.1289/ehp.1204982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Preston DL, Kato H, Kopecky K, Fujita S. Studies of the mortality of A-bomb survivors. 8. Cancer mortality, 1950-1982. Radiat Res 1987;111:151-78. 10.2307/3577030 [DOI] [PubMed] [Google Scholar]
- 45. Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res 2007;168:1-64. 10.1667/RR0763.1 [DOI] [PubMed] [Google Scholar]
- 46. Metz-Flamant C, Laurent O, Samson E, et al. Mortality associated with chronic external radiation exposure in the French combined cohort of nuclear workers. Occup Environ Med 2013;70:630-8. 10.1136/oemed-2012-101149 [DOI] [PubMed] [Google Scholar]
- 47. Gilbert ES, Cragle DL, Wiggs LD. Updated analyses of combined mortality data for workers at the Hanford Site, Oak Ridge National Laboratory, and Rocky Flats Weapons Plant. Radiat Res 1993;136:408-21. 10.2307/3578555 [DOI] [PubMed] [Google Scholar]
- 48. Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 2016;31:337-50. 10.1007/s10654-016-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richardson DB, Langholz B. Background stratified Poisson regression analysis of cohort data. Radiat Environ Biophys 2012;51:15-22. 10.1007/s00411-011-0394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cologne J, Preston DL, Grant EJ, Cullings HM, Ozasa K. Effect of follow-up period on minimal-significant dose in the atomic-bomb survivor studies. Radiat Environ Biophys 2018;57:83-8. 10.1007/s00411-017-0720-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berrington de Gonzalez A, Daniels RD, Cardis E, et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Rationale and Framework for the Monograph and Overview of Eligible Studies. J Natl Cancer Inst Monogr 2020;2020:97-113. 10.1093/jncimonographs/lgaa009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cléro E, Vaillant L, Hamada N, et al. History of radiation detriment and its calculation methodology used in ICRP Publication 103. J Radiol Prot 2019;39:R19-36. 10.1088/1361-6498/ab294a [DOI] [PubMed] [Google Scholar]
- 53. Leuraud K, Richardson DB, Cardis E, et al. Risk of cancer associated with low-dose radiation exposure: comparison of results between the INWORKS nuclear workers study and the A-bomb survivors study. Radiat Environ Biophys 2021;60:23-39. 10.1007/s00411-020-00890-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. National Council on Radiation Protection and Measurements . NCRP 2018 Implications of Recent Epidemiologic Studies for the Linear Nonthreshold Model and Radiation Protection, Commentary No. 27. National Council on Radiation Protection and Measurements, 2018. [Google Scholar]
- 55. Rühm W, Laurier D, Wakeford R. Cancer risk following low doses of ionising radiation - Current epidemiological evidence and implications for radiological protection. Mutat Res Genet Toxicol Environ Mutagen 2022;873:503436. 10.1016/j.mrgentox.2021.503436 [DOI] [PubMed] [Google Scholar]
- 56. Hauptmann M, Daniels RD, Cardis E, et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. J Natl Cancer Inst Monogr 2020;2020:188-200. 10.1093/jncimonographs/lgaa010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shore R, Walsh L, Azizova T, et al. Risk of solid cancer in low dose-rate radiation epidemiological studies and the dose-rate effectiveness factor. Int J Radiat Biol 2017;93:1064-78. [DOI] [PubMed] [Google Scholar]
- 58. Laurent O, Samson E, Caër-Lorho S, Fournier L, Laurier D, Leuraud K. Updated Mortality Analysis of SELTINE, the French Cohort of Nuclear Workers, 1968-2014. Cancers (Basel) 2022;15:79. 10.3390/cancers15010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kelly-Reif K, Bertke SJ, Daniels RD, Richardson DB, Schubauer-Berigan MK. Ionizing radiation and solid cancer mortality among US nuclear facility workers. Int J Epidemiol 2023;dyad075. 10.1093/ije/dyad075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hunter N, Haylock RGE, Gillies M, Zhang W. Extended analysis of solid cancer incidence among the Nuclear Industry Workers in the UK: 1955-2011. Radiat Res 2022;198:1-17. 10.1667/RADE-20-00269.1 [DOI] [PubMed] [Google Scholar]
- 61. Boice JD, Jr, Quinn B, Al-Nabulsi I, et al. A million persons, a million dreams: a vision for a national center of radiation epidemiology and biology. Int J Radiat Biol 2022;98:795-821. 10.1080/09553002.2021.1988183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wakeford R. The growing importance of radiation worker studies. Br J Cancer 2018;119:527-9. 10.1038/s41416-018-0134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wakeford R. Overview of epidemiological studies of nuclear workers: opportunities, expectations, and limitations. J Radiol Prot 2021;41. 10.1088/1361-6498/ac0df4 [DOI] [PubMed] [Google Scholar]
- 64. Mitchell TJ, Ostrouchov G, Frome EL, Kerr GD. A method for estimating occupational radiation dose to individuals, using weekly dosimetry data. Radiat Res 1997;147:195-207. 10.2307/3579421 [DOI] [PubMed] [Google Scholar]
- 65. Gilbert ES, Thierry-Chef I, Cardis E, Fix JJ, Marshall M. External dose estimation for nuclear worker studies. Radiat Res 2006;166:168-73. 10.1667/RR3126.1 [DOI] [PubMed] [Google Scholar]
- 66. Reeves GK, Cox DR, Darby SC, Whitley E. Some aspects of measurement error in explanatory variables for continuous and binary regression models. Stat Med 1998;17:2157-77. [DOI] [PubMed] [Google Scholar]
- 67.Uncertainties in Radiation Dosimetry and their Impact on Dose-Response Analyses. In: Ron E, Hoffman FO, eds. Proceedings of a workshop held September 3-5, 1997 in Bethesda, Maryland: National Cancer Institute; US Department of Health and Human Services; Public Health Service 1997. [Google Scholar]
- 68. Stayner L, Steenland K, Dosemeci M, Hertz-Picciotto I. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health 2003;29:317-24. 10.5271/sjweh.737 [DOI] [PubMed] [Google Scholar]
- 69. Cullings HM, Fujita S, Funamoto S, Grant EJ, Kerr GD, Preston DL. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res 2006;166:219-54. 10.1667/RR3546.1 [DOI] [PubMed] [Google Scholar]
- 70. National Council on Radiation Protection and Measurements . Evaluation of the relative effectiveness of low-energy photons and electrons in inducing cancer in humans. National Council on Radiation Protection and Measurements, 2018. [Google Scholar]
- 71. Kocher DC, Apostoaei AI, Hoffman FO. Radiation effectiveness factors for use in calculating probability of causation of radiogenic cancers. Health Phys 2005;89:3-32. 10.1097/01.HP.0000154172.48895.45 [DOI] [PubMed] [Google Scholar]
- 72. Richardson DB. Occupational exposures and lung cancer: adjustment for unmeasured confounding by smoking. Epidemiology 2010;21:181-6. 10.1097/EDE.0b013e3181c6f7d9 [DOI] [PubMed] [Google Scholar]
- 73. Brenner AV, Preston DL, Sakata R, et al. Comparison of All Solid Cancer Mortality and Incidence Dose-Response in the Life Span Study of Atomic Bomb Survivors, 1958-2009. Radiat Res 2022;197:491-508. 10.1667/RADE-21-00059.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McMichael AJ. Standardized mortality ratios and the “healthy worker effect”: Scratching beneath the surface. J Occup Med 1976;18:165-8. 10.1097/00043764-197603000-00009 [DOI] [PubMed] [Google Scholar]
- 75. Wilcosky T, Wing S. The healthy worker effect. Selection of workers and work forces. Scand J Work Environ Health 1987;13:70-2. 10.5271/sjweh.2078 [DOI] [PubMed] [Google Scholar]
- 76. Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology 1994;5:189-96. 10.1097/00001648-199403000-00009 [DOI] [PubMed] [Google Scholar]
- 77. Robins J. A New Approach To Casual Inference In Mortality Studies With A Sustained Exposure Period-Application To Control Of The Healthy Worker Survivor Effect. Math Model 1986;7:1393-512 10.1016/0270-0255(86)90088-6. [DOI] [Google Scholar]
- 78. McGeoghegan D. Healthy worker effect. J Radiol Prot 2001;21:179. 10.1088/0952-4746/21/2/101 [DOI] [PubMed] [Google Scholar]
- 79. Telle-Lamberton M, Bergot D, Gagneau M, et al. Cancer mortality among French Atomic Energy Commission workers. Am J Ind Med 2004;45:34-44. 10.1002/ajim.10306 [DOI] [PubMed] [Google Scholar]
- 80. Muirhead CR, Goodill AA, Haylock RG, et al. Occupational radiation exposure and mortality: second analysis of the National Registry for Radiation Workers. J Radiol Prot 1999;19:3-26. 10.1088/0952-4746/19/1/002 [DOI] [PubMed] [Google Scholar]
- 81. Atkinson WD, Law DV, Bromley KJ, Inskip HM. Mortality of employees of the United Kingdom Atomic Energy Authority, 1946-97. Occup Environ Med 2004;61:577-85. 10.1136/oem.2003.012443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Checkoway H, Mathew RM, Shy CM, et al. Radiation, work experience, and cause specific mortality among workers at an energy research laboratory. Br J Ind Med 1985;42:525-33. 10.1136/oem.42.8.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Watkins J, Cragle D, Frome E, et al. Collection, Validation, and Treatment of Data for a Mortality Study of Nuclear Industry Workers. Appl Occup Environ Hyg 1997;12:195-205 10.1080/1047322X.1997.10389488. [DOI] [Google Scholar]
- 84. Gilbert E. Some computer simulations based on the linear relative risk model. Pacific Northwest Laboratory, 1991. 10.2172/6040130. [DOI] [Google Scholar]
- 85. Fix JJ, Gilbert ES, Baumgartner WV. An assessment of bias and uncertainty in recorded dose from external sources of radiation for workers at the Hanford site. Pacific Northwest Laboratory, 1994. 10.2172/10177505. [DOI] [Google Scholar]
- 86. Gilbert ES. Methods of analyzing mortality of workers exposed to low levels of ionizing radiation. Pacific Northwest Laboratories, Battelle Memorial Institute, 1977. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
For reasons of ethics and permissions from different agencies, the data are maintained at the International Agency for Research on Cancer (Lyon, France) and cannot be made available outside of the agency.


