Abstract
In commemoration of 100 years since the discovery of glucagon, we review current knowledge about the human α cell. Alpha cells make up 30–40% of human islet endocrine cells and play a major role in regulating whole-body glucose homeostasis, largely through the direct actions of their main secretory product – glucagon – on peripheral organs. Additionally, glucagon and other secretory products of α cells, namely acetylcholine, glutamate, and GLP-1, have been shown to play an indirect role in the modulation of glucose homeostasis through autocrine and paracrine interactions within the islet. Studies of glucagon’s role as a counterregulatory hormone have revealed additional important functions of the α cell, including the regulation of multiple aspects of energy metabolism outside that of glucose. At the molecular level, human α cells are defined by expression of conserved islet-enriched transcription factors and various enriched signature genes, many of which have currently unknown cellular functions. Despite these common threads, notable heterogeneity exists amongst human α cell gene expression and function. Even greater differences are noted at the interspecies level, underscoring the importance of further study of α cell physiology in the human context. Finally, studies on α cell morphology and function in type 1 and type 2 diabetes, as well as other forms of metabolic stress, reveal a key contribution of α cell dysfunction to dysregulated glucose homeostasis in disease pathogenesis, making targeting the α cell an important focus for improving treatment.
Keywords: alpha cell, pancreatic islet, type 1 diabetes, type 2 diabetes
INTRODUCTION
Twenty-five years after the discovery of glucagon in 1923, the pancreatic α cell was identified as its source (Sutherland & Duve 1948). Since then, the α cell has gained recognition for its physiologic role in preventing life-threatening hypoglycemia via the actions of glucagon on peripheral organs, thereby opposing the peripheral effects of β cell-derived insulin on glucose homeostasis. Additionally, multiple lines of evidence have implicated the α cell in disease, most notably diabetes. Early reports revealed improvements in fasting and postprandial glucose levels following glucagon suppression in animal models of and humans with type 1 diabetes (T1D), illustrating an integral role of altered α cell function in disease-associated hyperglycemia (Gerich et al. 1974; Sakurai et al. 1974). Later studies supported this notion and implicated glucagon in the development of other clinical manifestations of diabetes, including ketosis (Raskin & Unger 1978; Lee et al. 2011). These studies and numerous others led to introduction of the “bihormonal hypothesis” by Unger and Orci in 1975, whereby excess glucagon contributes to hyperglycemia in conditions of insulin deficiency or insufficiency, and even the proposal of a glucagonocentric view of diabetes pathogenesis later in 2012 (Unger & Orci 1975; Unger & Cherrington 2012). Despite this, relatively fewer studies have been conducted with a focus on the α cell compared to the β cell (Figure 1).
Figure 1. Articles published on α cells vs. β cells.
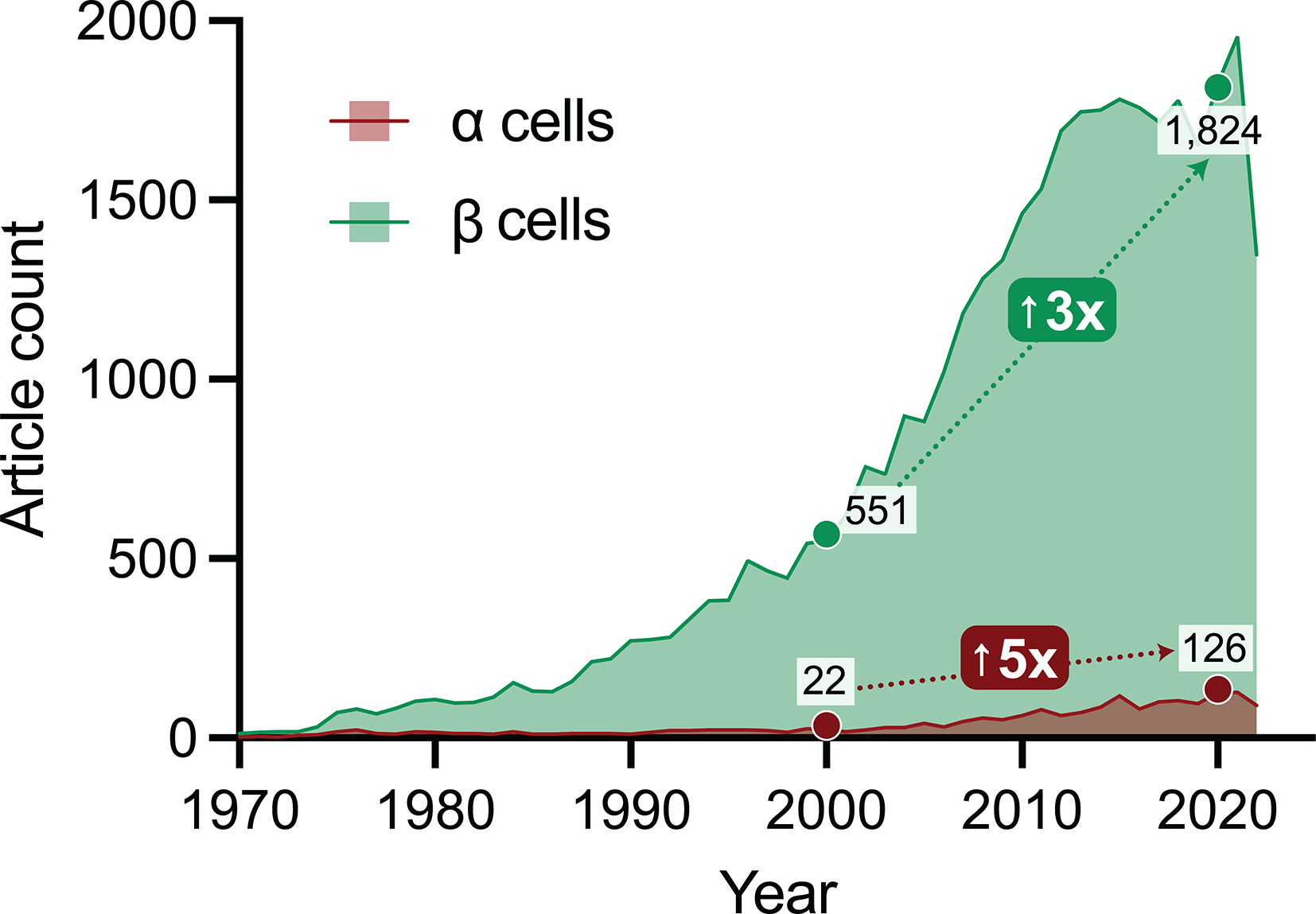
Graph shows a yearly count of research articles indexed in PubMed containing ‘alpha cell’ or ‘beta cell’ in the Title/Abstract field. Though α cells are mentioned far less frequently than β cells in paper titles and abstracts, there has been a rapid rise in research studies of both cell types in the past two decades (from 2000 to 2020, a three-fold increase for β cells and five-fold increase for α cells). The authors emphasize that this dataset represents only a small sample of α and β cell-related research but nonetheless reflects overall trends in the field.
Here, we review what is known about α cells under normal and pathophysiologic states, with a focus on the human α cell. We begin with a discussion of the healthy α cell, including cell morphology, molecular profile, and function. We summarize studies characterizing how the α cell adapts to changes in metabolic demand during pregnancy, aging, and obesity. Finally, we compare and contrast how α cell physiology is altered in T1D and type 2 diabetes (T2D). Though the focus of this review is on the human α cell, we acknowledge the limitations in studying human tissue and draw upon studies performed in animal models where appropriate, while also outlining key inter-species differences in α cell physiology. Finally, we conclude with opportunities for future studies of human α cell biology and physiology that will further our knowledge of diabetes pathophysiology leading to new strategies for therapeutic interventions.
THE HEALTHY α CELL
Islet composition and morphology
Pancreatic islets of Langerhans are heterogenous clusters of cells that work together to promote whole-body glucose homeostasis. Endocrine cells are the major cell type within the islet including β, α, δ, γ, and ϵ cells (in order of abundance) defined by their production of the hormones insulin, glucagon, somatostatin, pancreatic polypeptide (PP), and ghrelin, respectively. Additionally, multiple non-endocrine cell types are present within the islet that have been shown to play important roles in islet function, including endothelial cells, pericytes, immune cells, and nerve terminals. In the human pancreas, islet composition varies by location: the posterior head of the pancreas possesses a “PP-rich” lobe in which islets are made up of mostly γ cells at the expense of the other endocrine cell types, particularly α cells (Malaisse-Lagae et al. 1979; Stefan et al. 1983). In contrast, γ cells are sparse throughout the body and tail of the pancreas, where α and β cells are most abundant.
Endocrine cell subpopulations within human islets are spatially intermingled, forming frequent heterotypic contacts (Brelje et al. 1989; Brissova et al. 2005; Cabrera et al. 2006); however, this cellular organization varies significantly by species (see “Interspecies differences” below). The close contact between different endocrine cell types serves as a structural basis for the important paracrine interactions that occur in the human islet. In particular, α cells make up an average of 30–40% of the endocrine cell population in the human islet, depending on the individual, and receive numerous inputs from other islet endocrine cells that regulate α cell secretion (Hartig & Cox 2020). Abundant levels of β cell-derived insulin and δ cell-derived somatostatin bind to surface receptors on the α cell to decrease glucagon secretion; additionally, other secreted products, such as β cell-derived GABA, also influence α cell secretion. While much less is known about the function of γ and ϵ cells within the human islet, exposure of mouse islets to exogenous PP suggests it may also exert an inhibitory effect on glucagon release (Aragón et al. 2015). Further, α cells secrete hormones and neurotransmitters that act in a paracrine manner to regulate islet function. Secreted products of the α cell and their effects on the islet, as well as neuroendocrine receptors expressed in human α cells, are further discussed in detail later in the article. Additionally, physical contacts between cells have also been shown to influence α cell function. For example, human α cells express EphA4, which encodes the EphA4 receptor that binds the membrane-associated ephrin A5 ligand on adjacent β cells; further, signaling through ephrin receptors has been shown to modulate glucagon secretion in a juxtacrine manner (Dorrell et al. 2011; Hutchens & Piston 2015). Regarding their association with non-endocrine cell types, human α cells appear to preferentially lie directly next to vascular cells as compared with β cells (Bosco et al. 2010); in contrast, sympathetic fibers only sparsely contact endocrine cells directly, and only few parasympathetic axons seem to penetrate the human islet (Ahrén 2000; Rodriguez-Diaz et al. 2011; Dolenšek et al. 2015).
Morphologically, α cells contain secretory granules approximately 250–300 nm in diameter with an electron-dense core surrounded by a halo of relatively less dense material (Deconinck et al. 1971; Pelletier 1977) (Figure 2). The appearance of the halo is somewhat controversial, with its size and electron density varying by fixation method, but it is generally accepted as being less prominent than that of β cell secretory granules (Deconinck et al. 1971). It is possible that organelles in α and β cells exhibit ultrastructural differences, as mouse α cells display more extensive endoplasmic reticulum (ER), less prominent Golgi apparatus, and narrower mitcochondria; further studies are necessary to determine if this is the case in human islets (Pfeifer et al. 2015). Some human α cells are also ciliated, with stimulation of cilia-enriched G protein-coupled receptors (GPCRs) shown to potentiate glucagon secretion (Wu et al. 2021); however, the extent to which α cells are ciliated and the role cilia play in the integration of extracellular signals from within or outside of the islet remains to be fully determined. Moreover, lipid-rich structures, which may include both lipofuscins and lipid granules, are also present in human α cells and may increase with age and in T2D, although the role they play in α cell function is not well understood (Cnop et al. 2011; Tong et al. 2020).
Figure 2. Ultrastructure of the human α cell.
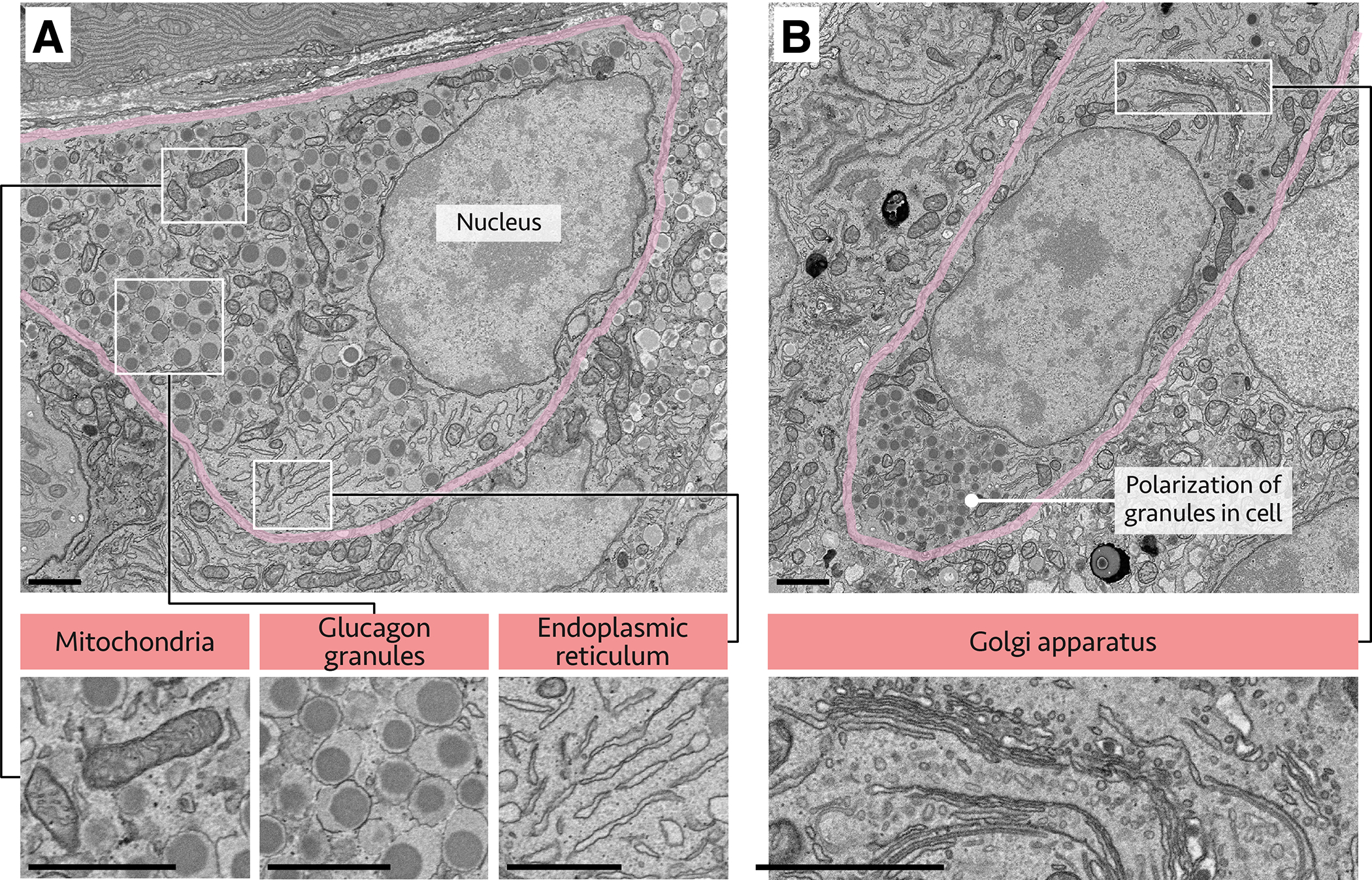
Human pancreatic tissue imaged by electron microscopy; α cells are outlined in pink, with white outlined regions shown at higher magnification below. (A) Alpha cell with annotated nucleus, mitochondria, endoplasmic reticulum, and glucagon granules. (B) Polarized α cell with granules concentrated in one pole of the cell; Golgi apparatus also annotated. Scale bars, 5 μm. Images courtesy of Rafael Arrojo e Drigo (Vanderbilt University) and Thomas Deerinck and Mark Ellisman (UCSD).
Alpha cell function
Glucagon
The main function of pancreatic α cells is to secrete glucagon, a 29 amino acid peptide hormone that is the product of posttranscriptional processing of preproglucagon (Bell et al. 1983). Preproglucagon is a 180 amino acid precursor encoded by the GCG gene that contains sequences for glucagon and glucagon-related peptides. Preproglucagon is cleaved to proglucagon in the ER, transported through the Golgi apparatus to the trans-Golgi network, and packaged into immature secretory granules, where it undergoes further processing (Guizzetti et al. 2014). Proglucagon is first cleaved into 2 major fragments: glicentin and major proglucagon fragment (MPGF) (Dey et al. 2005). Further processing within mature secretory granules occurs in a tissue-dependent manner: in pancreatic α cells, glicentin is cleaved into glucagon and glicentin-related polypeptide (GRPP) by prohormone convertase (PC) 2 (Mojsov et al. 1986; Rouillé et al. 1994). In contrast, PC1/3 is highly enriched in intestinal L cells, where MPGF is further processed into glucagon-like peptide-1 (GLP-1), GLP-2, and oxyntomodulin (Mojsov et al. 1986) (Figure 3A).
Figure 3. Glucagon gene expression and secretion in the human α cell.
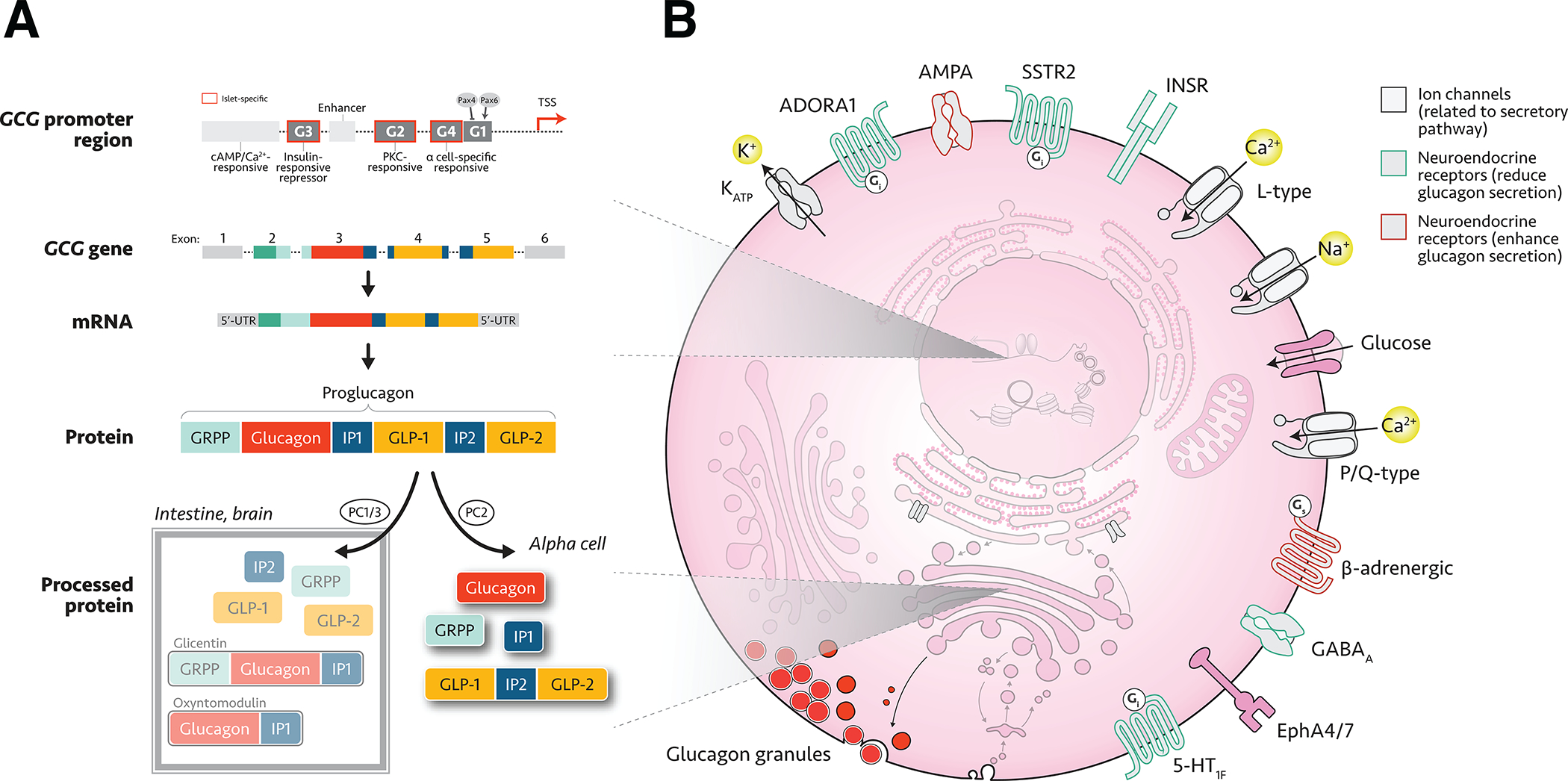
(A) Overview of expression and post-transcriptional processing of proglucagon in the α cell, also showing differential processing in other tissues (boxed inset). Abbreviations: cAMP, cyclic adenosine monophosphate; PKC, protein kinase C; TSS, transcription start site; UTR, untranslated region; GRPP, glicentin-related pancreatic polypeptide; IP, intermediate peptide; GLP, glucagon-like peptide; (B) Schematic of major human α cellular components, including neuroendocrine cell surface receptors. For effects of these receptors on α cell physiology, see Table 3. Abbreviations: K+, potassium; KATP, ATP-sensitive potassium channel; ADORA1, adenosine A1 receptor; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; SSTR2, somatostatin receptor 2; INSR, insulin receptor; Ca2+, calcium; Na+, sodium; GABA, gamma-aminobutyric acid; Eph, ephrin; 5-HT, serotonin.
The promoter for the preproglucagon gene (GCG) contains four DNA elements identified as G1 through G4, with G3 and G2 lying most upstream and G4 and G1 most proximal to the transcription start site (Figure 3A). Elements G2, G3, and G4 confer islet-specific expression (G4 shares binding sites with the insulin gene), while G1 restricts glucagon gene expression to α cells (Cordier-Bussat et al. 1995; Morel et al. 1995; Ritz-Laser et al. 1999). Element G1 is activated by PAX6 and indirectly suppressed by PAX4 (Ritz-Laser et al. 2002; Trinh et al. 2003). There are also several enhancer regions and cAMP-responsive elements within the promoter region (Philippe et al. 1988; Cordier-Bussat et al. 1995; Kieffer & Habener 1999). As single-cell assays are developing, recent studies have been able to investigate other context-specific regulators of α cell genes. In an RNAi screen using αTC1 cells, Casteels et al. identified Smndc1 as a regulator of chromatin remodeler Atrx, which maintains repressive H3K9me3 marks at the insulin gene. Although significant differences exist between this cell line and primary cells, results were recapitulated using shRNA knockdown in human α cells, strongly suggesting relevance to human α cell physiology (Casteels et al. 2022).
While less is known about the mechanisms of glucagon release compared to insulin, human α cell exocytosis relies on calcium influx through P/Q-type calcium channels as well as activity of voltage-dependent sodium channels (Ramracheya et al. 2010; Hughes et al. 2018). Studies of mouse islets suggest that synaptotagmin-7 mediates calcium-regulated exocytosis in α cells and that SNAP25 and syntaxin-1A are critical to docking, fusion, and exocytosis of secretory granules from the readily releasable and reserve pools (Gustavsson et al. 2009; Andersson et al. 2011). Glucagon secretion is modulated by blood glucose concentrations, amino acids, circulating hormones, and paracrine and autocrine signals within the islet (discussed later in “Neuroendocrine receptors”). Select aspects of glucagon production and components of α cell transduction and secretory pathways are summarized in Figure 3. For an in-depth review on mechanisms of glucagon secretion and α cell nutrient sensing, we refer to the reviews by Gao et al. and Armour et al. in this special collection.
Glucagon plays an integral role in the maintenance of glucose homeostasis, which is mediated by its impact both at the systemic and local intra-islet level. The vast majority of glucagon’s role in maintaining normoglycemia during fasting is achieved through its actions on the liver (reviewed extensively by (Jiang & Zhang 2003)). Briefly, activation of hepatic glucagon receptors (GcgRs) initiates a cAMP-dependent cascade that leads to simultaneous activation of glycogen phosphorylase and inhibition of glycogen synthase, promoting glycogenolysis and blocking glycogen synthesis, respectively. In parallel, hepatic glucagon signaling promotes gluconeogenesis by enhancing the expression of phosphoenolpyruvate carboxykinase (PEPCK), which catalyzes the rate-limiting step of the gluconeogenic pathway. Additionally, glucagon also activates the enzyme fructose 1,6-bisphosphatase (FBPase)-2, which indirectly promotes gluconeogenesis and inhibits glycolysis. Studies in rodent models demonstrate that glucagon may also regulate extrahepatic gluconeogenesis through its actions on the kidney and intestines (Mutel et al. 2011; Rahim et al. 2021). Further, glucagon may also modulate glucose homeostasis via its actions in the brain, where intracerebral glucagon promotes hyperglycemia in rodents via both cholinergic and α-adrenergic-dependent pathways (Marubashi et al. 1985; Amir 1986).
In addition to exerting systemic effects, glucagon also has an indirect impact on glucose homeostasis through its paracrine effects on islet β and δ cells. Despite the opposing whole-body actions of glucagon and insulin, multiple lines of evidence point toward the ability of intra-islet glucagon to enhance insulin secretion. Elegant studies by Rodriguez-Diaz et al. demonstrate the ability of glucagon to modulate the glycemic setpoint in human islets, as human islets exposed to a human GcgR-specific antagonist following transplantation into immunodeficient mice led to reduced human insulin production and shift in blood glucose level (Rodriguez-Diaz et al. 2018). Additionally, rodent studies demonstrate the ability of glucagon to promote insulin secretion under fed conditions via both the GcgR and GLP-1R (Svendsen et al. 2018; Capozzi et al. 2019); it remains to be seen whether this occurs in human islets. The GcgR receptor is also expressed by δ cells, where it stimulates somatostatin secretion, forming a negative feedback loop on α cell glucagon secretion (Patton et al. 1977). Finally, the GcgR is expressed by α cells, serving as a positive autocrine regulator (Ma et al. 2004).
Outside that of glucose homeostasis, other impacts of glucagon on nutrient metabolism are numerous and involve promotion of β-oxidation, inhibition of lipogenesis, and amino acid metabolism via enhanced uptake and ureagenesis in the liver (Richter et al. 2022). Multiple in vivo studies in humans implicate a role for glucagon in feeding behavior, as exogenous glucagon reduces food intake; rodent studies administering intracerebral glucagon suggest this effect of glucagon may be mediated through its actions on the hypothalamus (Schulman et al. 1957; Penick et al. 1961; Quiñones et al. 2015). Finally, glucagon may act on the kidney and heart to modulate renal excretion and cardiac function, although these aspects of glucagon physiology are less understood (Bankir et al. 2016; Petersen et al. 2018).
Other secreted factors
Beyond glucagon, the human α cell secretes several factors important for overall human islet function. Human islets are only sparsely innervated with parasympathetic fibers; instead, the α cell is a major source of intra-islet acetylcholine, which is secreted in response to α cell-specific stimuli such as low glucose (Rodriguez-Diaz et al. 2011). Acetylcholine enhances β cell secretory response to glucose in an M3 receptor-dependent manner; additionally, intra-islet acetylcholine stimulates somatostatin secretion from δ cells, providing negative feedback on insulin secretion (Molina et al. 2014).
Another neurotransmitter secreted by α cells is glutamate. Expressed exclusively in human α cells, vesicular glutamate transporter 1 (vGluT1) is necessary for glutamate secretion and has been shown to co-localize with glucagon secretory granules in rat islets (Hayashi et al. 2003; Cabrera et al. 2008). Glutamate secretion is activated by stimuli that also promote glucagon secretion, providing further evidence that the two peptides are co-secreted (Hayashi et al. 2003; Cabrera et al. 2008). In the islet, glutamate has been established as a positive autocrine regulator of α cell function. Human α cells express alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate ionotropic glutamate receptors, the activation of which leads to robust increases in glucagon secretion under low glucose conditions; in contrast, metabotropic receptor agonists have no effect on glucagon secretion, suggesting little to no expression of these receptor subtypes in human α cells, contrary to what has been reported in rat islets (Tong et al. 2002; Uehara et al. 2004; Cabrera et al. 2008; Omar-Hmeadi et al. 2020).
Alpha cells have also been implicated as a local source of glucagon-like peptide-1 (GLP-1) in the islet. A subset of human α cells express PC1/3, which alternatively cleaves the proglucagon peptide into GLP-1, GLP-2, oxyntomodulin, and glicentin (Marchetti et al. 2012; Campbell et al. 2020) (Figure 3A). Isolated human islets secrete higher levels of active GLP-1 than mouse islets, suggesting a greater impact of intra-islet GLP-1R signaling in human islets (Campbell et al. 2020). GLP-1 binds its Gs-coupled GPCR on β cells, where it potentiates glucose-stimulated insulin secretion (GSIS) in a cAMP-dependent manner. Blockade of signaling through the GLP-1R in isolated human islets significantly blunts insulin secretion, further suggesting paracrine signaling through the GLP-1R plays an important role in GSIS under normal conditions (Campbell et al. 2020; Souza et al. 2020). Studies also show that α cells may increase their capability to produce GLP-1 by upregulating PC1/3 expression under certain conditions, such as in type 2 diabetes or following exposure to GLP1-R agonists (Marchetti et al. 2012; Campbell et al. 2020; Galvin et al. 2021; Saikia et al. 2021). Despite this, the abundance of glucagon in human islets is greater than 100x that of GLP-1 as measured by liquid chromatography–mass spectrometry (LC-MS) (Galvin et al. 2021); thus, the relative contribution of glucagon and α cell-derived GLP-1 to GLP-1R signaling in human islets remains to be defined.
Molecular profile
Signature genes and identity markers
Advances in RNA-sequencing (RNA-seq) technology have enabled for the unbiased identification of α cell signature genes, a selection of which appears in Table 1 (all genes in this table appear as α cell-enriched in two or more independent studies of human islets (Wang et al. 2016; Xin et al. 2016; Enge et al. 2017; Shrestha et al. 2021; Gurp et al. 2022; Tritschler et al. 2022)). Transcriptomic profiles of human α cells share some similarities with other mammalian species and can be largely recapitulated in α-like cells derived from embryonic stem (ES) cells, though there are notable exceptions (Li et al. 2020; Sachs et al. 2020; Weng et al. 2020; Tritschler et al. 2022). While the function of many of these α cell-specific or “identity” genes has not been fully elucidated, some have been shown to have significant roles in islet function. For example, DPP4 encodes a peptidase whose role is to regulate the bioavailability of various peptides, most notably GLP-1 through cleavage into its inactive form (Deacon 2019). Given the importance of intra-islet GLP-1R signaling in insulin secretion, DPP-4 likely serves an important role in overall islet function by modulating local GLP-1 pools, as illustrated by the successful use of DPP-4 inhibitors to treat T2D. Some evidence also suggests that DPP-4 activity changes in T2D islets, which might implicate it in disease pathophysiology (Omar et al. 2014). In addition, FAP is a closely related peptidase shown to be co-expressed with DPP-4 exclusively in human α cells and while its role in α cell function is largely unknown, it may also work to influence intra-islet signaling (Busek et al. 2015). Efforts to further characterize α cell signature genes are ongoing, as evidenced by recent work by Viloria et al. to define the role of vitamin-D-binding protein (DBP; encoded by GC) in α cell function. Deletion of DBP in mice leads to smaller α cells with altered electrophysiologic properties and impaired responses to glucose, likely due to alternative functions outside that of vitamin D transport (Viloria et al. 2020). Interestingly, GC polymorphisms have been associated with altered glucose homeostasis (Viloria et al. 2022), illustrating the potential of investigations into basic α cell biology to inform our understanding of diabetes risk.
Table 1.
Select human α cell signature genes from single cell RNA-sequencing studies
| Gene | Description | Protein class | Human ES | Primate | Pig | Mouse |
|---|---|---|---|---|---|---|
|
| ||||||
| ALDH1A1 | Aldehyde dehydrogenase 1 family member A1 | Dehydrogenase | Exp | Exp | Switch | ND |
| CFC1 | Cripto, FRL-1, cryptic family 1 | Intercellular signal molecule | Exp | Exp | ND | |
| CLU | Clusterin | Intercellular signal molecule | Exp | Switch | Exp | Exp |
| CRYBA2 | Crystallin beta A2 | Structural protein | Exp | Exp | Exp | Exp |
| DPP4 | Dipeptidyl peptidase 4 | Serine protease | Exp | ND | Exp | Exp |
| F10 | Coagulation factor X | Serine protease | Exp | Switch | ND | ND |
| FAP | Fibroblast activation protein alpha | Serine protease | Exp | Exp | ND | ND |
| FXYD3 | FXYD domain-containing ion transport regulator 3 | Primary active transporter | Exp | Switch | Exp | Exp |
| FXYD5 | FXYD domain-containing ion transport regulator 5 | Primary active transporter | Exp | Switch | Exp | ND |
| GC | GC vitamin D binding protein | Transfer/carrier protein | Exp | Exp | Switch | Exp |
| GCG | Glucagon | Peptide hormone | Exp | Exp | Exp | Exp |
| GLS | Glutaminase | Hydrolase | Exp | Exp | Exp | Exp |
| HIGD1A | HIG1 hypoxia inducible domain family member 1A | Scaffold/adaptor protein | Exp | Exp | Exp | Exp |
| IRX2 | Iroquois homeobox 2 | Homeodomain transcription factor | Exp | Exp | Exp | Exp |
| KCTD12 | Potassium channel tetramerization domain containing 12 | Scaffold/adaptor protein | Exp | * | * | Exp |
| LOXL4 | Lysyl oxidase like 4 | Oxidase | Exp | ND | * | Exp |
| MUC13 | Mucin 13, cell surface associated | Transmembrane signal receptor | Exp | ND | ND | ND |
| PALLD | Palladin, cytoskeletal associated protein | Scaffold/adaptor protein | Exp | Switch | ND | Switch |
| PCSK2 | Proprotein convertase subtilisin/kexin type 2 | Serine protease | Exp | Exp | Exp | Exp |
| PLCE1 | Phospholipase C epsilon 1 | Phospholipase | Exp | ND | ND | ND |
| PLIN3 | Perilipin 3 | Scaffold/adaptor protein | Exp | ND | ND | Exp |
| RGS4 | Regulator of G protein signaling 4 | GTPase-activating protein | Exp | Exp | Exp | Exp |
| SLC7A2 | Solute carrier family 7 member 2 | Amino acid transporter | Exp | Exp | Exp | Exp |
| SMIM24 | Small integral membrane protein 24 | Transmembrane signal receptor | Exp | Exp | ND | |
| TMEM176A | Transmembrane protein 176A | Transmembrane signal receptor | Exp | Exp | Exp | Exp |
| TTR | Transthyretin | Hydrolase | Exp | Exp | Exp | Exp |
Multiple islet-enriched transcription factors (TFs) are involved in the establishment and maintenance of human α cell identity, including ARX, MAFB, and PAX6; a comprehensive list of human α cell-enriched transcripts, including several TFs, can be found in Table 2. Notably, most of these factors are conserved across species, highlighting their importance in α cell development, maturation, and/or function. For a full discussion of α cell specification and development, we refer readers to the review by Brooks and Sussel published in this collection.
Table 2.
Select human α cell identity markers from single cell RNA-sequencing studies
| Gene | Description | Protein class | Human ES | Primate | Pig | Mouse |
|---|---|---|---|---|---|---|
|
| ||||||
| ARX | Aristaless related homeobox | Homeodomain transcription factor | Exp | ND | * | Exp |
| CHGA | Chromogranin A | Intercellular signal molecule | Exp | Exp | Exp | Exp |
| CHGB | Chromogranin B | Intercellular signal molecule | Exp | Exp | Exp | Exp |
| FOXA2 | Forkhead box A2 | Winged helix/forkhead transcription factor | Exp | Exp | Exp | Exp |
| FOXO1 | Forkhead box O1 | Winged helix/forkhead transcription factor | ND | Exp | Exp | Exp |
| GATA6 | GATA binding protein 6 | DNA-binding transcription factor | ND | ND | Exp | Exp |
| ISL1 | ISL LIM homeobox 1 | Homeodomain transcription factor | Exp | Exp | Exp | Exp |
| MAFB | MAF bZIP transcription factor B | Basic leucine zipper transcription factor | Exp | ND | ND | Exp |
| NEUROD1 | Neuronal differentiation 1 | Basic helix-loop-helix transcription factor | Exp | Exp | Exp | Exp |
| NKX2–2 | NK2 homeobox 2 | Homeodomain transcription factor | ND | Exp | Exp | Exp |
| PAX6 | Paired box 6 | Homeodomain transcription factor | Exp | Exp | Exp | Exp |
| POU6F2 | POU class 6 homeobox 2 | DNA-binding transcription factor | ND | ND | Exp | Exp |
| PTPRT | Protein tyrosine phosphatase receptor type T | Protein phosphatase | ND | ND | * | Exp |
| RFX6 | Regulatory factor X6 | Winged helix/forkhead transcription factor | Exp | Exp | Exp | Exp |
| SCG5 | Secretogranin V | Intercellular signal molecule | Exp | Exp | Exp | Exp |
| SLC38A4 | Solute carrier family 38 member 4 | Amino acid transporter | Exp | Exp | Exp | Exp |
| WNT4 | Wnt family member 4 | Intercellular signal molecule | Exp | Exp | Exp | Exp |
Neuroendocrine receptors
Alpha cells respond to a variety of external signals that modulate glucagon secretion after binding to neuroendocrine receptors on the cell membrane. A summary of major neuroendocrine receptors expressed on the α cell, their subtypes, and their overall effect on glucagon secretion is summarized in Table 3.
Table 3.
Select neuroendocrine cell receptor types expressed by the α cell
| Receptor | Receptor type | Impact on glucagon secretion | References – rodent | References – human |
|---|---|---|---|---|
| Adenosine A1 receptor (ADORA1) | Gi-coupled | ↓ | (Johansson et al. 2007) | (Yip et al. 2013) |
| (Yip et al. 2013) | ||||
| Epinephrine receptors | β-adrenergic: Gs-coupled α1 adrenergic: Gq-coupled |
↑ | (Gromada et al. 1997) | (Hamilton et al. 2018) |
| (Vieira et al. 2004) | ||||
| (Hamilton et al. 2018) | ||||
| GABAA receptor | Ionotropic | ↓ | (Rorsman et al. 1989) | (Yang et al. 1994) |
| (Wendt et al. 2004) | ||||
| Glucagon receptor (GcgR) | Gs-coupled | ↑ | (Ma et al. 2004) | |
| Glutamate receptor | AMPA/kainate subtype: ionotropic | ↑ | (Weaver et al. 1996) | (Cabrera et al. 2008) |
| Insulin receptor | Tyrosine kinase | ↓ | (Maruyama et al. 1984) | (Elliott et al. 2015) |
| (Kawamori et al. 2009) | ||||
| (Elliott et al. 2015) | ||||
| Somatostatin receptor (SSTR2, predominant) |
Gi-coupled | ↓ | (Ludvigsen et al. 2003) | (Gerich et al. 1974) |
| (Kumar et al. 1999) | ||||
| (Portela-Gomes et al. 2000) | ||||
| 5-HT1F serotonin receptor | Gi-coupled | ↓ | (Almaça et al. 2016) |
Level of evidence: bold; RNA, italic; protein, underline; function
Heterogeneity
Studies at the single cell level reveal significant heterogeneity in human α cell gene expression and function. Recent single cell (sc) RNA-seq studies identified multiple α cell subclusters whose signatures vary by the level of expression of α-enriched genes including ARX, IRX2, TTR, ALDH1A1, and even GCG (Saikia et al. 2021). This is supported by work identifying two distinct populations of human α cells that varied by their abundance of granular zinc and glucagon content (Zadeh et al. 2020). Moreover, rare populations of α cells with increased expression of proliferative genes have been identified in adult human islets, which is in line with aging studies that demonstrate little change in α cell mass beyond early adulthood –see “Aging” section below (Segerstolpe et al. 2016; Fang et al. 2019). Consequently, multiple studies report heterogeneity in functional characteristics of α cells as well. Mouse α cells display a wide distribution in voltage-activated Na+ and Ca2+ currents, KATP conductance, and changes in plasma membrane Ca2+ concentrations in response to varying glucose concentrations (Huang et al. 2011; Shuai et al. 2016). In human α cells, variations in electrophysiologic properties, specifically capacitance and Na+ currents, correlate with higher expression of α cell maturity markers and secretory genes as well as downregulation of ER stress markers (Camunas-Soler et al. 2020). This observation is further supported by work from our own group, in which we showed that human α cells co-expressing the transcription factors MAFB and ARX had higher expression of genes involved in nutrient sensing and glucagon secretion, while α cells expressing neither transcription factor had upregulated stress response genes (Shrestha et al. 2021).
Inter-species differences
Multiple differences exist between species in terms of islet α cell distribution, gene expression, and some aspects of α cell physiology. Arguably the most obvious is the variability in the distribution and abundance of α cells in the islet: compared to adult human islets, mouse islets have a lower percentage of α cells that are topologically confined to the mantle of the islet along with non-β endocrine cells, whereas β cells exclusively lie within the core of the islet (Brelje et al. 1989; Brissova et al. 2005; Cabrera et al. 2006). In canine islets, this “core-mantle” arrangement of islet β and α cells is not conserved, as α cells are also observed in the islet core (Redecker et al. 1992). Non-human primate islets exhibit a reverse core-mantle structure, where α cells are mostly observed in the core and are less abundant (Wieczorek et al. 1998; Brissova et al. 2005). In pigs, α cell distribution varies at the pancreas and islet level: one lobe is characterized by a small number of α cells found primarily at the islet periphery, while the other lobe has significantly greater abundance of α cells located throughout the islet (Wieczorek et al. 1998). We refer readers to (Steiner et al. 2010) for a comprehensive review of islet morphology across a range of species.
Studies comparing gene expression in α cells across species reveal both conserved and species-specific patterns. While global patterns in gene expression are highly correlative between mouse and human α cells, a closer evaluation of α-enriched genes reveal significant variations in expression, with some genes being highly expressed in human α cells but not mouse α cells (ex. LDHA) or vice-versa (Xin et al. 2016). A recent study published by Tritshler et al. expands upon this notion by performing a cross-species RNA-sequencing analysis of human, mouse, and pig islets. Notably, only ~6% of identified human α-enriched marker genes were shared between all three species; others were either expressed but not considered marker genes, were more enriched in another endocrine cell type, or were not detected at all in α cells (Tritschler et al. 2022) (Table 1).
These highlighted inter-species differences in α cells underscore the need for validation studies in human islets to be performed whenever possible. Efforts have been made to generate stem cell-derived human α cells, which could serve as a model system for primary α cells (Rezania et al. 2011; Peterson et al. 2020). These α-like cells express canonical α cell genes such as ARX and PCSK2 and respond appropriately to glucose; however, they are not without limitations, including diminished responses to some modulators of glucagon secretion and altered gene expression compared to primary cells (Tables 1 and 2). Further, it is unknown whether these cells respond similarly to primary α cells in vivo, emphasizing the need for more comprehensive studies. The development of an immunodeficient glucagon knockout (GKO-NSG) mouse model improves upon previous challenges to study primary human α cells over time in vivo, as identical glucagon sequences in mice and humans historically prevented the detection of circulating graft-derived vs. endogenous glucagon following human islet transplantation. The GKO-NSG mice harbor an in-frame deletion of exon 3 of the Gcg gene, which corresponds to nucleotides encoding amino acids 2–29 of glucagon; this genetic strategy renders undetectable glucagon levels while preserving production of other proglucagon-derived peptides such as GLP-1 (Tellez et al. 2020). Further, transplantation of human islets ameliorates the previously reported negative effects of interrupted glucagon signaling, making this a suitable model for the study of normal human α cell physiology.
Adaptations to other physiologic states
Aging
Islet endocrine cells are long-lived, and thus may be susceptible to age-related changes in mass and/or function (Drigo et al. 2019). The vast majority of α cells are estimated to be established by 20 years of age, with little replication afterwards (Cnop et al. 2011). Studies examining the association between α cell mass and age in humans report no drastic changes in α cell mass across time (Henquin & Rahier 2011; Moin et al. 2020). However, aged human islet endocrine cells display higher rates of transcriptional noise, increased expression of stress response genes, and shortened telomeres, suggesting that age may influence cell identity and/or function (Tamura et al. 2016; Enge et al. 2017). Studies of non-human primate α cells support this, where downregulated genes in aged α cells were enriched for gene ontology terms associated with peptide secretion and multiple metabolic processes (Li et al. 2020). Similar large-scale studies have not yet been performed in human α cells, although a recent study showed that aged human β cells have altered gene expression that correlates with a decline in function (Shrestha et al. 2022), highlighting the possibility that a similar phenomenon may occur in α cells.
Pregnancy
Pregnancy represents a scenario of increased metabolic demand, where placenta-derived hormones promote peripheral insulin resistance to meet increased energy needs of the developing fetus (Catalano et al. 1993). Pregnancy in humans is associated with a rise in plasma glucagon levels during the second trimester that falls in the third; however, glucagon responses during oral glucose tolerance test are normal, suggesting minimal functional changes (Luyckx et al. 1975). Most of what we know about pregnancy-induced islet adaptations comes from rodent studies, where pregnant C57BL/6 mice show increased α cell size, fractional area per pancreatic section, and mass in late pregnancy; despite these changes, reduced secretion in response to low glucose was also observed (Quesada-Candela et al. 2020; Qiao et al. 2022). These findings were recapitulated in in vitro experiments after cultured α cells were exposed to prolactin and placental lactogen, suggesting the involvement of placental hormones in this process (Quesada-Candela et al. 2020). However, a conflicting study reported no changes to α cell area during pregnancy, underscoring the complexity of possible α cell changes (Moffett et al. 2014). Glucagon may also be required for normal pregnancy progression, as pregnancies in GcgR−/− mice show decreased fetal size, increased fetal demise, and structural placental abnormalities (Ouhilal et al. 2012). Pregnancy in mice has also been associated with altered proglucagon processing, where α cells express higher levels of GLP-1 and PC1/3, which is mirrored by an increase in pancreatic GLP-1 content (Moffett et al. 2014). Ablation of α cells during pregnancy leads to impaired glucose tolerance and reduced GSIS that is rescued by GLP-1R agonism but not glucagon, implicating an important role for α-cell derived GLP-1 in GSIS during pregnancy (Qiao et al. 2022). Of note, inter-species differences in α cell adaptations to pregnancy may exist, as pregnant C57BL/6 mice also exhibit decreased plasma glucagon in late pregnancy versus non-pregnant controls, which has not been observed in humans (Moffett et al. 2014; Quesada-Candela et al. 2020).
THE HUMAN α CELL IN T1D
T1D is characterized by autoimmune-mediated β cell destruction, resulting in a loss of β cell mass and systemic hyperglycemia. In contrast, multiple studies report normal α cell abundance, highlighting the cell-specific nature of the autoimmune process (Rahier et al. 1983; Campbell-Thompson et al. 2016; Bonnet-Serrano et al. 2018). In a small study of whole pancreata from human donors with T1D, Rahier et al. (1983) found decreased volume density of endocrine tissue in PP cell-deficient regions of the pancreas, which was further characterized by the presence of smaller islets with poorly defined borders and a higher proportion of α cells present in small clusters outside of the islets. While the overall proportion of α cells was increased, this was largely due to β cell loss; further, due to the overall reduction in pancreas weight, estimated α cell mass was similar between donors with T1D and controls (Rahier et al. 1983). These observations were largely replicated in a study of pancreata from over 70 donors with T1D and without diabetes available through the Network for Pancreatic Organ Donors with Diabetes (nPOD), which revealed no significant differences in α cell area or mass, regardless of autoantibody status or the presence of insulitis (Campbell-Thompson et al. 2016). While an additional study utilizing nPOD samples reported decreased α cell mass in T1D (Bonnet-Serrano et al. 2018), the authors attributed this finding to the significantly decreased total pancreas weight associated with T1D in the context of preserved α cell area; these discrepant findings could be due to differences in quantification and sampling methods.
In spite of preserved α cell abundance in disease, impaired glucagon secretion in T1D is well established. Individuals with T1D display similar levels of fasting glucagon as nondiabetic controls, which is inappropriate given their elevated fasting blood glucose (Unger et al. 1970; Gerich et al. 1973). In a study on glucagon responses in the fed state, carbohydrate-rich meals failed to suppress glucagon secretion in T1D participants, as compared to nondiabetic individuals that showed a robust decline in circulating glucagon (Müller et al. 1970). Additionally, those with T1D have increased plasma glucagon levels in response to infused arginine and, given their elevated blood glucose, inappropriately normal circulating glucagon following a protein-rich meal (Müller et al. 1970; Unger et al. 1970; Sherr et al. 2014). Despite the relative and/or absolute hyperglucagonemia demonstrated by these studies, individuals with T1D have impaired responses to hypoglycemia as well. While hypoglycemia normally stimulates glucagon secretion, individuals with T1D fail to respond appropriately despite preserved or even exaggerated increases in other counterregulatory hormones; this impairment appears to worsen over time (Gerich et al. 1973; Bolli et al. 1983). Together, these studies demonstrate a loss of sensitivity in glucagon response to acute changes in blood glucose in T1D.
Recent studies by our group and others have sought to further define and understand α cell dysfunction in T1D through the study of primary islets and pancreatic tissue from human donors. Primary T1D islets show multiple functional impairments in glucagon secretion, including lack of high glucose-mediated glucagon suppression, reduced amino acid-stimulated glucagon secretion, and decreased Ca2+ currents (Brissova et al. 2018; Camunas-Soler et al. 2020; Doliba et al. 2022). These functional impairments are accompanied by an altered α cell identity signature marked by decreased expression of the α-enriched TFs MAFB and ARX, upregulation of the β cell-enriched TF NKX6.1, and altered expression of genes important for α cell function (Brissova et al. 2018; Wang et al. 2019; Camunas-Soler et al. 2020).
Given the complexity of T1D pathogenesis, potential factors contributing to α cell dysfunction are numerous. A partial recovery of α cell transcriptomic profile is observed after transplantation into nondiabetic immunodeficient mice (Brissova et al. 2018), implicating a potential role for autoimmunity and/or systemic hyperglycemia in altered α cell identity state in T1D. Further, purified α cells aggregates isolated from islets from nondiabetic human donors secrete glucagon appropriately under low glucose conditions but fail to reduce their secretion in response to high glucose exposure when compared to mixed α and β cell aggregates or primary human islets, suggesting a role of β cell contact in the maintenance of α cell function (Liu et al. 2019). A recent study by Doliba et al. (2022) adds further nuance, describing that α cells from single autoantibody glutamic acid decarboxylase antibodies (GADA)+ islets also showed altered gene expression and glucagon secretory responses, despite no difference in islet composition or insulin secretion. These studies would suggest that α cell dysfunction begins prior to the onset of overt β cell loss and hyperglycemia; however, the role that loss of β-to-α cell contact and/or hyperglycemia plays in the progression of α cell dysfunction remains to be defined.
THE HUMAN α CELL IN OBESITY AND T2D
Obesity
Obesity is highly correlated with insulin resistance and is a major independent risk factor for the development of T2D. Reports of α cell abundance in obese individuals are mixed. A study focused on α cell mass in obesity conducted in Japanese individuals found that obesity had no impact on α cell area in surgically resected pancreas samples independent of diabetes status (Inaishi et al. 2016). Similarly, analysis of pancreata obtained at autopsy from a European cohort found no correlation between BMI and α cell mass (Henquin & Rahier 2011). In contrast, a study of pancreata from nondiabetic organ donors with obesity and undefined ancestry found increased α cell mass (Ellenbroek et al. 2017). These findings may be influenced by the pancreatic sampling location, as the latter study identified increased α cells in samples from the pancreatic head only (Ellenbroek et al. 2017). Nevertheless, in vivo studies of α cell function in obese individuals report fasting hyperglucagonemia despite preserved glucose-mediated glucagon suppression (Borghi et al. 1984; Bonora et al. 1990). This finding is associated with peripheral insulin resistance, as insulin sensitivity is negatively correlated with fasting glucagon level and impaired early glucagon suppression following glucose challenge (Færch et al. 2016). The pro-inflammatory nature of obesity may play a role in these changes in α cell function, as human islets upregulate proglucagon expression and glucagon secretion in vitro following exposure to IL-6, a proinflammatory cytokine that is elevated in obese individuals and associated with increased risk of developing T2D (Spranger et al. 2003; Ellingsgaard et al. 2008).
Type 2 diabetes
Alpha cell dysfunction is also a hallmark of T2D, where hyperglucagonemia is well recognized. Individuals with T2D have inappropriately normal glucagon levels in the fasted state, elevated non-fasted plasma glucagon, and delayed glucose-mediated glucagon suppression (Müller et al. 1970; Unger et al. 1970; Reaven et al. 1987; Færch et al. 2016). In a study measuring glucagon levels throughout the day, plasma glucagon was significantly elevated with blood glucose levels, highlighting the important contribution of altered α cell secretory responses to systemic hyperglycemia observed in T2D (Reaven et al. 1987). Despite these known impairments in α cell function, the literature investigating pancreatic α cell fractional area and/or mass is less consistent, with studies reporting increased (Yoon et al. 2003; Mizukami et al. 2014), decreased (Sato et al. 2015), or no difference between individuals with T2D and nondiabetic controls (Henquin & Rahier 2011; Inaishi et al. 2016). The discrepancies in those studies could result from experimental differences, including sample type (archived autopsy specimens, surgical samples, or fresh donor pancreatic tissue), study population, pancreatic sampling location, and/or quantification methods; additionally, clinical parameters such as disease duration could play a role. For example, Yoon et al. (2003) evaluated pancreatic tissue obtained from organ donors and surgically-resected pancreatic samples in a Korean cohort and found a significant increase in median islet size in patients with T2D. Additionally, islets were characterized by an overall increase in α:β cell ratio, especially in larger islets, and an increase in the relative contribution of α cells to islet area; however, the authors at least partially contributed these observations to selective loss of β cells. Similarly, a study of pancreatic autopsy samples in a Japanese cohort found increases in α cell fractional area and mass, where those with the most marked increases in α cell fractional area also had a decrease in β cell fractional area (Mizukami et al. 2014). In contrast, another study evaluating pancreatic autopsy samples in a separate Japanese cohort found decreased islet size, decreased α and β cell fractional area, and preserved α:β cell ratio (Sato et al. 2015). To further highlight the controversial nature of these findings, a third study of surgically resected samples from Japanese individuals found no effect of T2D on α cell fractional area and increased α:β cell ratio due to decreased β cell fractional area (Inaishi et al. 2016); similar observations were found in autopsy samples from a European cohort (Henquin & Rahier 2011).
At the cellular level, studies of α cells in T2D reveal functional alterations. In a study utilizing imaging techniques to measure human α cell secretion, α cells within intact islets isolated from donors with T2D exhibited a bimodal response to increasing glucose, where granule exocytosis was observed at both low and high glucose (Omar-Hmeadi et al. 2020). This is similar to the secretory profile seen in dispersed nondiabetic α cells, suggesting α cells may lose response to paracrine regulators of glucagon secretion in the T2D disease state. This was further supported by observations that α cells in islets from donors with T2D were less responsive to somatostatin and insulin-mediated inhibition (Omar-Hmeadi et al. 2020). Altered α cell response to high glucose in T2D was also observed by Dai and colleagues, who utilized Patch-seq techniques to correlate inappropriate secretory responses with altered expression of genes involved in cell fate commitment and pancreas development (Dai et al. 2021). Interestingly, high expression of α cell identity and maturity markers including ARX, ISL1, RFX6, and MAFB was highly correlated with impaired electrophysiologic profile in α cells isolated from islets from donors with T2D. Alpha cells with high ARX expression also had higher expression of progenitor genes such as NEUROD1, suggesting these seemingly more mature α cells are more susceptible to T2D-induced changes. Together, these studies provide multiple potential mechanisms for α cell dysfunction in T2D, including impaired nutrient sensing, loss of paracrine regulation, and/or acquisition of a less mature phenotype (Wang et al. 2016).
As we have discussed, α cell dysfunction is a feature of both T1D and T2D; thus, mechanisms of α cell dysfunction may either be common or disease specific. A recent study by Bosi et al (2022) addresses this question by comparing genes and pathways altered in primary α cells from donors with diabetes versus those in age-matched nondiabetic controls. Alpha cells in T2D upregulate pathways involved in defense against reactive oxygen species and strongly downregulate various glucose metabolism pathways (Segerstolpe et al. 2016; Bosi et al. 2022). In contrast, T1D α cells strongly upregulate those involved in immunity and ER stress. Interestingly, fewer differentially expressed genes are shared between T1D and T2D α cells, yet genes that overlap (e.g., ARX, MAFB, PCSK2) relate to both α cell identity and function. This would suggest that some aspects of α cell dysfunction may be disease-specific but converge on common regulators or pathways.
CONCLUSIONS
In this review, we have provided an overview of current knowledge around the human α cell. Studies in animal models have provided a strong foundation on which to base human studies and while our understanding of human α cell biology is ever evolving, there are many opportunities for future studies. For example, the vast majority of α cell genes altered in T2D have no known role in islet cell growth or function, emphasizing the need for more studies in basic α cell biology (Xin et al. 2016). Molecular studies of α cells in both T1D and T2D support the role of an intrinsic α cell defect and call for further investigation to understand disease development and progression as it relates to glucagon secretion. This is further emphasized by recent work demonstrating impairments in α cell function that precede β cell loss in GADA+ individuals, which underscore the need for molecular studies across T1D stages (Doliba et al. 2022). In particular, studies of islets from autoantibody positive individuals may reveal opportunities for new diagnostic tools for T1D and therapies for disease prevention. Additionally, comparative studies may further advance our understanding of human β cell biology, as some work suggests that the α cell is more resistant to undergoing senescence and stress-induced apoptosis than β cells in diabetes (Marroqui et al. 2015; Brawerman et al. 2022).
Given the role of α cell dysfunction in diabetes pathogenesis, targeting α cells may represent an avenue for diabetes treatment. Studies in rodents consistently demonstrate improvements in glucose homeostasis at baseline and in models of obesity, T1D, and T2D following glucagon signaling blockade (Brand et al. 1994; Gelling et al. 2003; Sloop et al. 2004; Conarello et al. 2007). However, this is limited by significant side effects, including α cell hyperplasia and hepatic steatosis, that are directly due to blocking the other biologic functions of glucagon. Despite promising results, phase I and II trials of glucagon receptor antagonists reveal similar side effects, which would need to be further characterized prior to wide-scale usage (Sammons & Lee 2015). Further understanding of normal α cell physiology and mechanisms of dysfunction in T1D and T2D may reveal additional avenues for treatment.
ACKNOWLEDGEMENTS
We thank Alvin Powers for his helpful feedback during the editing of this review. We are especially grateful to organ donors and their families, without whom many of the studies described in this review would not be possible. We thank the Alpha Cell Working Group supported by The Leona M. and Harry B. Helmsley Charitable Trust for their valuable scientific insight and discussions related to pancreatic alpha cells within. Human pancreas tissue shown in Figure 2 was provided by the Alberta Diabetes Institute IsletCore, with electron microscopy sample preparation and image acquisition by Rafael Arrojo e Drigo (Vanderbilt) and Thomas Deerinck and Mark Ellisman (UCSD). We thank Patrick MacDonald and James Lyon (Alberta Diabetes Institute IsletCore) for their efforts in human islet isolation, the Human Organ Procurement and Exchange (HOPE) program, the Trillium Gift of Life Network (TGLN) for their work in procuring human donor pancreas, and the organ donors and their families for their kind gift in support of diabetes research. Special thanks to Yan Li and Shanshan Zhang (Case Western University) for providing processed transcriptomic data from α-like ES cells that appears in Tables 1–2. The Vanderbilt Creative Data Solutions Shared Resource (RRID:SCR_022366) performed reprocessing of single cell primate islet data appearing in Tables 1–2.
FUNDING
Our work (YP, DCS, MB) discussed in this review was supported by the Human Islet Research Network (RRID:SCR_014393), the Human Pancreas Analysis Program (RRID:SCR_016202), DK112217, DK123716, DK120456, DK129469, DK135017, T32GM007347 (Vanderbilt Medical Scientist Training Program), F30DK134041, DK20593 (Vanderbilt Diabetes Research and Training Center), The Leona M. and Harry B. Helmsley Charitable Trust, and JDRF. Funding for electron microscopy (RAeD) was provided by DK120447.
Footnotes
DECLARATION OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- Ahrén B 2000. Autonomic regulation of islet hormone secretion – Implications for health and disease. Diabetologia 43 393–410. (doi: 10.1007/s001250051322) [DOI] [PubMed] [Google Scholar]
- Almaça J, Molina J, Menegaz D, Pronin AN, Tamayo A, Slepak V, Berggren P-O & Caicedo A 2016. Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells. Cell Reports 17 3281–3291. (doi: 10.1016/j.celrep.2016.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S 1986. Central glucagon-induced hyperglycemia is mediated by combined activation of the adrenal medulla and sympathetic nerve endings. Physiology & Behavior 37 563–566. (doi: 10.1016/0031-9384(86)90286-6) [DOI] [PubMed] [Google Scholar]
- Andersson SA, Pedersen MG, Vikman J & Eliasson L 2011. Glucose-dependent docking and SNARE protein-mediated exocytosis in mouse pancreatic alpha-cell. Pflügers Archiv - European Journal of Physiology 462 443–454. (doi: 10.1007/s00424-011-0979-5) [DOI] [PubMed] [Google Scholar]
- Aragón F, Karaca M, Novials A, Maldonado R, Maechler P & Rubí B 2015. Pancreatic polypeptide regulates glucagon release through PPYR1 receptors expressed in mouse and human alpha-cells. Biochimica et Biophysica Acta (BBA) - General Subjects 1850 343–351. (doi: 10.1016/j.bbagen.2014.11.005) [DOI] [PubMed] [Google Scholar]
- Bankir L, Bouby N, Blondeau B & Crambert G 2016. Glucagon actions on the kidney revisited: possible role in potassium homeostasis. American Journal of Physiology-Renal Physiology 311 F469–F486. (doi: 10.1152/ajprenal.00560.2015) [DOI] [PubMed] [Google Scholar]
- Bell GI, Sanchez-Pescador R, Laybourn PJ & Najarian RC 1983. Exon duplication and divergence in the human preproglucagon gene. Nature 304 368–371. (doi: 10.1038/304368a0) [DOI] [PubMed] [Google Scholar]
- Bolli G, Feo PD, Compagnucci P, Cartechini MG, Angeletti G, Santeusanio F, Brunetti P & Gerich JE 1983. Abnormal Glucose Counterregulation in Insulin-dependent Diabetes Mellitus: Interaction of Anti-Insulin Antibodies and Impaired Glucagon and Epinephrine Secretion. Diabetes 32 134–141. (doi: 10.2337/diab.32.2.134) [DOI] [PubMed] [Google Scholar]
- Bonnet-Serrano F, Diedisheim M, Mallone R & Larger E 2018. Decreased α-cell mass and early structural alterations of the exocrine pancreas in patients with type 1 diabetes: An analysis based on the nPOD repository. PLoS ONE 13 e0191528. (doi: 10.1371/journal.pone.0191528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E, Moghetti P, Cacciatori V, Zenere M, Tosi F, Travia D, Zoppini G, Perobelli L & Muggeo M 1990. Plasma concentrations of glucagon during hyperglycemic clamp with or without somatostatin infusion in obese subjects. Acta Diabetologia Latina 27 309–314. (doi: 10.1007/bf02580935) [DOI] [PubMed] [Google Scholar]
- Borghi VC, Wajchenberg BL & Cesar FP 1984. Plasma glucagon suppressibility after oral glucose in obese subjects with normal and impaired glucose tolerance. Metabolism 33 1068–1074. (doi: 10.1016/0026-0495(84)90089-1) [DOI] [PubMed] [Google Scholar]
- Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G & Berney T 2010. Unique Arrangement of α- and β-Cells in Human Islets of Langerhans. Diabetes 59 1202 1210. (doi: 10.2337/db09-1177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosi E, Marchetti P, Rutter GA & Eizirik DL 2022. Human alpha cell transcriptomic signatures of types 1 and 2 diabetes highlight disease-specific dysfunction pathways. IScience 25 105056. (doi: 10.1016/j.isci.2022.105056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand CL, Rolin B, JØrgensen PN, Svendsen I, Kristensen JS & Holst JJ 1994. Immunoneutralization of endogenous glucagon with monoclonal glucagon antibody normalizes hyperglycaemia in moderately streptozotocin-diabetic rats. Diabetologia 37 985–993. (doi: 10.1007/bf00400461) [DOI] [PubMed] [Google Scholar]
- Brawerman G, Ntranos V & Thompson PJ 2022. Alpha cell dysfunction in type 1 diabetes is independent of a senescence program. Frontiers in Endocrinology 13 932516. (doi: 10.3389/fendo.2022.932516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelje TC, Scharp DW & Sorenson RL 1989. Three-Dimensional Imaging of Intact Isolated Islets of Langerhans With Confocal Microscopy. Diabetes 38 808–814. (doi: 10.2337/diab.38.6.808) [DOI] [PubMed] [Google Scholar]
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM & Powers AC 2005. Assessment of Human Pancreatic Islet Architecture and Composition by Laser Scanning Confocal Microscopy. Journal of Histochemistry & Cytochemistry 53 1087–1097. (doi: 10.1369/jhc.5c6684.2005) [DOI] [PubMed] [Google Scholar]
- Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, Bottino R, Campbell-Thompson M, Aramandla R, Poffenberger G et al. 2018. α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Cell Reports 22 2667–2676. (doi: 10.1016/j.celrep.2018.02.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busek P, Hrabal P, Fric P & Sedo A 2015. Co-expression of the homologous proteases fibroblast activation protein and dipeptidyl peptidase-IV in the adult human Langerhans islets. Histochemistry and Cell Biology 143 497–504. (doi: 10.1007/s00418-014-1292-0) [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O & Caicedo A 2006. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences 103 2334–2339. (doi: 10.1073/pnas.0510790103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O, Jacques-Silva MC, Speier S, Yang S-N, Köhler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM et al. 2008. Glutamate Is a Positive Autocrine Signal for Glucagon Release. Cell Metabolism 7 545–554. (doi: 10.1016/j.cmet.2008.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, MacDonald PE, Philippaert K & Light PE 2020. Human islets contain a subpopulation of glucagon-like peptide-1 secreting α cells that is increased in type 2 diabetes. Molecular Metabolism 39 101014. (doi: 10.1016/j.molmet.2020.101014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A & Atkinson MA 2016. Insulitis and β-Cell Mass in the Natural History of Type 1 Diabetes. Diabetes 65 719–731. (doi: 10.2337/db15-0779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camunas-Soler J, Dai X-Q, Hang Y, Bautista A, Lyon J, Suzuki K, Kim SK, Quake SR & MacDonald PE 2020. Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Cell Metabolism 31 1017–1031.e4. (doi: 10.1016/j.cmet.2020.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi ME, Wait JB, Koech J, Gordon AN, Coch RW, Svendsen B, Finan B, D’Alessio DA & Campbell JE 2019. Glucagon lowers glycemia when β-cells are active. JCI Insight 4. (doi: 10.1172/jci.insight.129954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels T, Bajew S, Reiniš J, Enders L, Schuster M, Fontaine F, Müller AC, Wagner BK, Bock C & Kubicek S 2022. SMNDC1 links chromatin remodeling and splicing to regulate pancreatic hormone expression. Cell Reports 40 111288. (doi: 10.1016/j.celrep.2022.111288) [DOI] [PubMed] [Google Scholar]
- Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB & Sims EA 1993. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. American Journal of Physiology-Endocrinology and Metabolism 264 E60–E67. (doi: 10.1152/ajpendo.1993.264.1.e60) [DOI] [PubMed] [Google Scholar]
- Cnop M, Igoillo-Esteve M, Hughes SJ, Walker JN, Cnop I & Clark A 2011. Longevity of human islet α- and β-cells. Diabetes, Obesity and Metabolism 13 39–46. (doi: 10.1111/j.1463-1326.2011.01443.x) [DOI] [PubMed] [Google Scholar]
- Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L et al. 2007. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50 142–150. (doi: 10.1007/s00125-006-0481-3) [DOI] [PubMed] [Google Scholar]
- Cordier-Bussat M, Morel C & Philippe J 1995. Homologous DNA sequences and cellular factors are implicated in the control of glucagon and insulin gene expression. Molecular and Cellular Biology 15 3904–3916. (doi: 10.1128/mcb.15.7.3904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X-Q, Camunas-Soler J, Briant LJB, Santos T dos, Spigelman AF, Walker EM, Drigo RA e, Bautista A, Jones RC, Avrahami D et al. 2021. Heterogenous impairment of α cell function in type 2 diabetes is linked to cell maturation state. Cell Metabolism 34 256–268.e5. (doi: 10.1016/j.cmet.2021.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF 2019. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Frontiers in Endocrinology 10 80. (doi: 10.3389/fendo.2019.00080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deconinck JF, Potvliege PR & Gepts W 1971. The ultrastructure of the human pancreatic islets. Diabetologia 7 266–282. (doi: 10.1007/bf01211879) [DOI] [PubMed] [Google Scholar]
- Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, Carroll R & Steiner DF 2005. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, Lys70-Arg71, to Generate Glucagon. Endocrinology 146 713–727. (doi: 10.1210/en.2004-1118) [DOI] [PubMed] [Google Scholar]
- Dolenšek J, Rupnik MS & Stožer A 2015. Structural similarities and differences between the human and the mouse pancreas. Islets 7 e1024405. (doi: 10.1080/19382014.2015.1024405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doliba NM, Rozo AV, Roman J, Qin W, Traum D, Gao L, Liu J, Manduchi E, Liu C, Golson ML et al. 2022. α Cell dysfunction in islets from nondiabetic, glutamic acid decarboxylase autoantibody–positive individuals. Journal of Clinical Investigation 132 e156243. (doi: 10.1172/jci156243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Schug J, Lin CF, Canaday PS, Fox AJ, Smirnova O, Bonnah R, Streeter PR, Stoeckert CJ, Kaestner KH et al. 2011. Transcriptomes of the major human pancreatic cell types. Diabetologia 54 2832. (doi: 10.1007/s00125-011-2283-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drigo RA e, Lev-Ram V, Tyagi S, Ramachandra R, Deerinck T, Bushong E, Phan S, Orphan V, Lechene C, Ellisman MH et al. 2019. Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metabolism 30 343–351.e3. (doi: 10.1016/j.cmet.2019.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek JH, Töns HAM, Hanegraaf MAJ, Rabelink TJ, Engelse MA, Carlotti F & Koning EJP de 2017. Pancreatic α-cell mass in obesity. Diabetes, Obesity and Metabolism 19 1810–1813. (doi: 10.1111/dom.12997) [DOI] [PubMed] [Google Scholar]
- Ellingsgaard H, Ehses JA, Hammar EB, Lommel LV, Quintens R, Martens G, Kerr-Conte J, Pattou F, Berney T, Pipeleers D et al. 2008. Interleukin-6 regulates pancreatic α-cell mass expansion. Proceedings of the National Academy of Sciences 105 13163–13168. (doi: 10.1073/pnas.0801059105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AD, Ustione A & Piston DW 2015. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. American Journal of Physiology-Endocrinology and Metabolism 308 E130–E143. (doi: 10.1152/ajpendo.00344.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M, Arda HE, Mignardi M, Beausang J, Bottino R, Kim SK & Quake SR 2017. Single-Cell Analysis of Human Pancreas Reveals Transcriptional Signatures of Aging and Somatic Mutation Patterns. Cell 171 321–330.e14. (doi: 10.1016/j.cell.2017.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Færch K, Vistisen D, Pacini G, Torekov SS, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T et al. 2016. Insulin Resistance Is Accompanied by Increased Fasting Glucagon and Delayed Glucagon Suppression in Individuals With Normal and Impaired Glucose Regulation. Diabetes 65 3473–3481. (doi: 10.2337/db16-0240) [DOI] [PubMed] [Google Scholar]
- Fang Z, Weng C, Li H, Tao R, Mai W, Liu X, Lu L, Lai S, Duan Q, Alvarez C et al. 2019. Single-Cell Heterogeneity Analysis and CRISPR Screen Identify Key β-Cell-Specific Disease Genes. Cell Reports 26 3132–3144.e7. (doi: 10.1016/j.celrep.2019.02.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, Ravn P, Jermutus L, Reimann F & Gribble FM 2021. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-like Peptide-1. Journal of Proteome Research 20 4507–4517. (doi: 10.1021/acs.jproteome.1c00463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelling RW, Du XQ, Dichmann DS, Rømer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C et al. 2003. Lower blood glucose, hyperglucagonemia, and pancreatic α cell hyperplasia in glucagon receptor knockout mice. Proceedings of the National Academy of Sciences 100 1438–1443. (doi: 10.1073/pnas.0237106100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich JE, Langlois M, Noacco C, Karam JH & Forsham PH 1973. Lack of Glucagon Response to Hypoglycemia in Diabetes: Evidence for an Intrinsic Pancreatic Alpha Cell Defect. Science 182 171–173. (doi: 10.1126/science.182.4108.171) [DOI] [PubMed] [Google Scholar]
- Gerich JE, Lorenzi M, Schneider V, Karam JH, Rivier J, Guillemin R & Forsham PH 1974. Effects of Somatostatin on Plasma Glucose and Glucagon Levels in Human Diabetes Mellitus — Pathophysiologic and Therapeutic Implications. The New England Journal of Medicine 291 544–547. (doi: 10.1056/nejm197409122911102) [DOI] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding W-G, Barg S, Buschard K, Renström E & Rorsman P 1997. Adrenaline Stimulates Glucagon Secretion in Pancreatic A-Cells by Increasing the Ca2+ Current and the Number of Granules Close to the L-Type Ca2+ Channels. The Journal of General Physiology 110 217–228. (doi: 10.1085/jgp.110.3.217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti L, McGirr R & Dhanvantari S 2014. Two Dipolar α-Helices within Hormone-encoding Regions of Proglucagon Are Sorting Signals to the Regulated Secretory Pathway. Journal of Biological Chemistry 289 14968–14980. (doi: 10.1074/jbc.m114.563684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurp L van, Fodoulian L, Oropeza D, Furuyama K, Bru-Tari E, Vu AN, Kaddis JS, Rodríguez I, Thorel F & Herrera PL 2022. Generation of human islet cell type-specific identity genesets. Nature Communications 13 2020. (doi: 10.1038/s41467-022-29588-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N, Wei S, Hoang DN, Lao Y, Zhang Q, Radda GK, Rorsman P, Südhof TC & Han W 2009. Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+-induced glucagon exocytosis in pancreas. The Journal of Physiology 587 1169–1178. (doi: 10.1113/jphysiol.2008.168005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A, Zhang Q, Salehi A, Willems M, Knudsen JG, Ringgaard AK, Chapman CE, Gonzalez-Alvarez A, Surdo NC, Zaccolo M et al. 2018. Adrenaline Stimulates Glucagon Secretion by Tpc2-Dependent Ca2+ Mobilization From Acidic Stores in Pancreatic α-Cells. Diabetes 67 1128–1139. (doi: 10.2337/db17-1102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig SM & Cox AR 2020. Paracrine signaling in islet function and survival. Journal of Molecular Medicine 98 451–467. (doi: 10.1007/s00109-020-01887-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A & Moriyama Y 2003. Secretory Granule-mediated Co-secretion of L-Glutamate and Glucagon Triggers Glutamatergic Signal Transmission in Islets of Langerhans. Journal of Biological Chemistry 278 1966–1974. (doi: 10.1074/jbc.m206758200) [DOI] [PubMed] [Google Scholar]
- Henquin JC & Rahier J 2011. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia 54 1720–1725. (doi: 10.1007/s00125-011-2118-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Rupnik M & Gaisano HY 2011. Unperturbed islet α-cell function examined in mouse pancreas tissue slices. The Journal of Physiology 589 395–408. (doi: 10.1113/jphysiol.2010.200345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JW, Ustione A, Lavagnino Z & Piston DW 2018. Regulation of islet glucagon secretion: Beyond calcium. Diabetes, Obesity and Metabolism 20 127–136. (doi: 10.1111/dom.13381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens T & Piston DW 2015. EphA4 Receptor Forward Signaling Inhibits Glucagon Secretion From α-Cells. Diabetes 64 3839–3851. (doi: 10.2337/db15-0488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaishi J, Saisho Y, Sato S, Kou K, Murakami R, Watanabe Y, Kitago M, Kitagawa Y, Yamada T & Itoh H 2016. Effects of Obesity and Diabetes on α- and β-Cell Mass in Surgically Resected Human Pancreas. The Journal of Clinical Endocrinology & Metabolism 101 2874–2882. (doi: 10.1210/jc.2016-1374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G & Zhang BB 2003. Glucagon and regulation of glucose metabolism. American Journal of Physiology-Endocrinology and Metabolism 284 E671–E678. (doi: 10.1152/ajpendo.00492.2002) [DOI] [PubMed] [Google Scholar]
- Johansson SM, Salehi A, Sandström ME, Westerblad H, Lundquist I, Carlsson P-O, Fredholm BB & Katz A 2007. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochemical Pharmacology 74 1628–1635. (doi: 10.1016/j.bcp.2007.08.006) [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP & Kulkarni RN 2009. Insulin Signaling in α Cells Modulates Glucagon Secretion In Vivo. Cell Metabolism 9 350–361. (doi: 10.1016/j.cmet.2009.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ & Habener JF 1999. The Glucagon-Like Peptides. Endocrine Reviews 20 876–913. (doi: 10.1210/edrv.20.6.0385) [DOI] [PubMed] [Google Scholar]
- Kumar U, Sasi R, Suresh S, Patel A, Thangaraju M, Metrakos P, Patel SC & Patel YC 1999. Subtype-selective expression of the five somatostatin receptors (hSSTR1–5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48 77–85. (doi: 10.2337/diabetes.48.1.77) [DOI] [PubMed] [Google Scholar]
- Lee Y, Wang M-Y, Du XQ, Charron MJ & Unger RH 2011. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes 60 391–397. (doi: 10.2337/db10-0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zheng Y, Yan P, Song M, Wang S, Sun L, Liu Z, Ma S, Belmonte JCI, Chan P et al. 2020. A single-cell transcriptomic atlas of primate pancreatic islet aging. National Science Review 8 nwaa127. (doi: 10.1093/nsr/nwaa127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kin T, Ho S, Dorrell C, Campbell SR, Luo P & Chen X 2019. Abnormal regulation of glucagon secretion by human islet alpha cells in the absence of beta cells. EBioMedicine 50 306–316. (doi: 10.1016/j.ebiom.2019.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsen E, Olsson R, Stridsberg M, Janson ET & Sandler S 2003. Expression and Distribution of Somatostatin Receptor Subtypes in the Pancreatic Islets of Mice and Rats. Journal of Histochemistry & Cytochemistry 52 391–400. (doi: 10.1177/002215540405200310) [DOI] [PubMed] [Google Scholar]
- Luyckx AS, Gerard J, Gaspard U & Lefebvre PJ 1975. Plasma glucagon levels in normal women during pregnancy. Diabetologia 11 549–554. (doi: 10.1007/bf01222105) [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang Y, Gromada J, Sewing S, Berggren P-O, Buschard K, Salehi A, Vikman J, Rorsman P & Eliasson L 2004. Glucagon stimulates exocytosis in mouse and rat pancreatic alpha-cells by binding to glucagon receptors. Molecular Endocrinology (Baltimore, Md.) 19 198–212. (doi: 10.1210/me.2004-0059) [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F, Stefan Y, Cox J, Perrelet A & Orci L 1979. Identification of a lobe in the adult human pancreas rich in pancreatic polypeptide. Diabetologia 17 361–365. (doi: 10.1007/bf01236270) [DOI] [PubMed] [Google Scholar]
- Marchetti P, Lupi R, Bugliani M, Kirkpatrick CL, Sebastiani G, Grieco FA, Guerra SD, D’Aleo V, Piro S, Marselli L et al. 2012. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55 3262–3272. (doi: 10.1007/s00125-012-2716-9) [DOI] [PubMed] [Google Scholar]
- Marroqui L, Masini M, Merino B, Grieco FA, Millard I, Dubois C, Quesada I, Marchetti P, Cnop M & Eizirik DL 2015. Pancreatic α Cells are Resistant to Metabolic Stress-induced Apoptosis in Type 2 Diabetes. EBioMedicine 2 378–385. (doi: 10.1016/j.ebiom.2015.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubashi S, Tominaga M, Katagiri T, Yamatani K, Yawata Y, Hara M & Sasaki H 1985. Hyperglycaemic effect of glucagon administered intracerebroventricularly in the rat. Acta Endocrinologica 108 6–10. (doi: 10.1530/acta.0.1080006) [DOI] [PubMed] [Google Scholar]
- Maruyama H, Hisatomi A, Orci L, Grodsky GM & Unger RH 1984. Insulin within islets is a physiologic glucagon release inhibitor. Journal of Clinical Investigation 74 2296–2299. (doi: 10.1172/jci111658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami H, Takahashi K, Inaba W, Tsuboi K, Osonoi S, Yoshida T & Yagihashi S 2014. Involvement of Oxidative Stress–Induced DNA Damage, Endoplasmic Reticulum Stress, and Autophagy Deficits in the Decline of β-Cell Mass in Japanese Type 2 Diabetic Patients. Diabetes Care 37 1966–1974. (doi: 10.2337/dc13-2018) [DOI] [PubMed] [Google Scholar]
- Moffett RC, Vasu S, Thorens B, Drucker DJ & Flatt PR 2014. Incretin Receptor Null Mice Reveal Key Role of GLP-1 but Not GIP in Pancreatic Beta Cell Adaptation to Pregnancy. PLoS ONE 9 e96863. (doi: 10.1371/journal.pone.0096863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moin ASM, Cory M, Gurlo T, Saisho Y, Rizza RA, Butler PC & Butler AE 2020. Pancreatic alpha-cell mass across adult human lifespan. European Journal of Endocrinology 182 219–231. (doi: 10.1530/eje-19-0844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L & Habener JF 1986. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. Journal of Biological Chemistry 261 11880–11889. (doi: 10.1016/s0021-9258(18)67324-7) [DOI] [PubMed] [Google Scholar]
- Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren P-O & Caicedo A 2014. Control of Insulin Secretion by Cholinergic Signaling in the Human Pancreatic Islet. Diabetes 63 2714–2726. (doi: 10.2337/db13-1371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel C, Cordier-Bussat M & Philippe J 1995. The Upstream Promoter Element of the Glucagon Gene, G1, Confers Pancreatic Alpha Cell-specific Expression. Journal of Biological Chemistry 270 3046–3055. (doi: 10.1074/jbc.270.7.3046) [DOI] [PubMed] [Google Scholar]
- Müller WA, Faloona GR, Aguilar-Parada E & Unger RH 1970. Abnormal Alpha-Cell Function in Diabetes — Response to Carbohydrate and Protein Ingestion. The New England Journal of Medicine 283 109–115. (doi: 10.1056/nejm197007162830301) [DOI] [PubMed] [Google Scholar]
- Mutel E, Gautier-Stein A, Abdul-Wahed A, Amigó-Correig M, Zitoun C, Stefanutti A, Houberdon I, Tourette J-A, Mithieux G & Rajas F 2011. Control of Blood Glucose in the Absence of Hepatic Glucose Production During Prolonged Fasting in Mice. Diabetes 60 3121–3131. (doi: 10.2337/db11-0571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar BA, Liehua L, Yamada Y, Seino Y, Marchetti P & Ahrén B 2014. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia 57 1876–1883. (doi: 10.1007/s00125-014-3299-4) [DOI] [PubMed] [Google Scholar]
- Omar-Hmeadi M, Lund P-E, Gandasi NR, Tengholm A & Barg S 2020. Paracrine control of α-cell glucagon exocytosis is compromised in human type-2 diabetes. Nature Communications 11 1896. (doi: 10.1038/s41467-020-15717-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhilal S, Vuguin P, Cui L, Du X-Q, Gelling RW, Reznik SE, Russell R, Parlow AF, Karpovsky C, Santoro N et al. 2012. Hypoglycemia, hyperglucagonemia, and fetoplacental defects in glucagon receptor knockout mice: a role for glucagon action in pregnancy maintenance. American Journal of Physiology-Endocrinology and Metabolism 302 E522–E531. (doi: 10.1152/ajpendo.00420.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GS, Ipp E, Dobbs RE, Orci L, Vale W & Unger RH 1977. Pancreatic immunoreactive somatostatin release. Proceedings of the National Academy of Sciences 74 2140–2143. (doi: 10.1073/pnas.74.5.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G 1977. Identification of Four Cell Types in the Human Endocrine Pancreas By Immunoelectron Microscopy. Diabetes 26 749–756. (doi: 10.2337/diab.26.8.749) [DOI] [PubMed] [Google Scholar]
- Penick SB, Hinkle LE & Paulsen EG 1961. Depression of Food Intake Induced in Healthy Subjects by Glucagon. The New England Journal of Medicine 264 893–897. (doi: 10.1056/nejm196105042641801) [DOI] [PubMed] [Google Scholar]
- Petersen KM, Bøgevig S, Holst JJ, Knop FK & Christensen MB 2018. Hemodynamic Effects of Glucagon: A Literature Review. The Journal of Clinical Endocrinology & Metabolism 103 1804–1812. (doi: 10.1210/jc.2018-00050) [DOI] [PubMed] [Google Scholar]
- Peterson QP, Veres A, Chen L, Slama MQ, Kenty JHR, Hassoun S, Brown MR, Dou H, Duffy CD, Zhou Q et al. 2020. A method for the generation of human stem cell-derived alpha cells. Nature Communications 11 2241. (doi: 10.1038/s41467-020-16049-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer CR, Shomorony A, Aronova MA, Zhang G, Cai T, Xu H, Notkins AL & Leapman RD 2015. Quantitative analysis of mouse pancreatic islet architecture by serial block-face SEM. Journal of Structural Biology 189 44–52. (doi: 10.1016/j.jsb.2014.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J, Drucker DJ, Knepel W, Jepeal L, Misulovin Z & Habener JF 1988. Alpha-cell-specific expression of the glucagon gene is conferred to the glucagon promoter element by the interactions of DNA-binding proteins. Molecular and Cellular Biology 8 4877–4888. (doi: 10.1128/mcb.8.11.4877-4888.1988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M, Grimelius L, Öberg K & Janson ET 2000. Expression of the Five Different Somatostatin Receptor Subtypes in Endocrine Cells of the Pancreas. Applied Immunohistochemistry & Molecular Morphology 8 126–132. (doi: 10.1097/00129039-200006000-00007) [DOI] [PubMed] [Google Scholar]
- Qiao L, Saget S, Lu C, Zang T, Dzyuba B, Hay WW & Shao J 2022. The Essential Role of Pancreatic α-Cells in Maternal Metabolic Adaptation to Pregnancy. Diabetes 71 978–988. (doi: 10.2337/db21-0923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada-Candela C, Tudurí E, Marroquí L, Alonso-Magdalena P, Quesada I & Nadal Á 2020. Morphological and functional adaptations of pancreatic alpha-cells during late pregnancy in the mouse. Metabolism 102 153963. (doi: 10.1016/j.metabol.2019.153963) [DOI] [PubMed] [Google Scholar]
- Quiñones M, Al-Massadi O, Gallego R, Fernø J, Diéguez C, López M & Nogueiras R 2015. Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance. Molecular Metabolism 4 961–970. (doi: 10.1016/j.molmet.2015.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier J, Goebbels RM & Henquin JC 1983. Cellular composition of the human diabetic pancreas. Diabetologia 24 366–371. (doi: 10.1007/bf00251826) [DOI] [PubMed] [Google Scholar]
- Rahim M, Hasenour CM, Bednarski TK, Hughey CC, Wasserman DH & Young JD 2021. Multitissue 2H/13C flux analysis reveals reciprocal upregulation of renal gluconeogenesis in hepatic PEPCK-C–knockout mice. JCI Insight 6 e149278. (doi: 10.1172/jci.insight.149278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, Johnson PR, Rorsman P & Braun M 2010. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets. Diabetes 59 2198–2208. (doi: 10.2337/db09-1505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P & Unger RH 1978. Hyperglucagonemia and Its Suppression — Importance in the Metabolic Control of Diabetes. The New England Journal of Medicine 299 433–436. (doi: 10.1056/nejm197808312990901) [DOI] [PubMed] [Google Scholar]
- Reaven GM, Chen Y-DI, Golay A, Swislocki ALM & Jaspan JB 1987. Documentation of Hyperglucagonemia Throughout the Day in Nonobese and Obese Patients with Noninsulin-Dependent Diabetes Mellitus*. The Journal of Clinical Endocrinology & Metabolism 64 106–110. (doi: 10.1210/jcem-64-1-106) [DOI] [PubMed] [Google Scholar]
- Redecker P, Seipelt A, Jörns A, Bargsten G & Grube D 1992. The microanatomy of canine islets of Langerhans: implications for intra-islet regulation. Anatomy and Embryology 185 131–141. (doi: 10.1007/bf00185914) [DOI] [PubMed] [Google Scholar]
- Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL & Kieffer TJ 2011. Production of Functional Glucagon-Secreting α-Cells From Human Embryonic Stem Cells. Diabetes 60 239–247. (doi: 10.2337/db10-0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter MM, Galsgaard KD, Elmelund E, Knop FK, Suppli MP, Holst JJ, Winther-Sørensen M, Kjeldsen SAS & Albrechtsen NJW 2022. The Liver–α-Cell Axis in Health and in Disease. Diabetes 71 1852–1861. (doi: 10.2337/dbi22-0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz-Laser B, Estreicher A, Klages N, Saule S & Philippe J 1999. Pax-6 and Cdx-2/3 Interact to Activate Glucagon Gene Expression on the G1 Control Element. Journal of Biological Chemistry 274 4124–4132. (doi: 10.1074/jbc.274.7.4124) [DOI] [PubMed] [Google Scholar]
- Ritz-Laser B, Estreicher A, Gauthier BR, Mamin A, Edlund H & Philippe J 2002. The pancreatic beta-cell-specific transcription factor Pax-4 inhibits glucagon gene expression through Pax-6. Diabetologia 45 97–107. (doi: 10.1007/s125-002-8249-9) [DOI] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren P-O & Caicedo A 2011. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nature Medicine 17 888–892. (doi: 10.1038/nm.2371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Molano RD, Weitz JR, Abdulreda MH, Berman DM, Leibiger B, Leibiger IB, Kenyon NS, Ricordi C, Pileggi A et al. 2018. Paracrine Interactions within the Pancreatic Islet Determine the Glycemic Set Point. Cell Metabolism 27 549–558.e4. (doi: 10.1016/j.cmet.2018.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Berggren P-O, Bokvist K, Ericson H, Möhler H, Östenson C-G & Smith PA 1989. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341 233–236. (doi: 10.1038/341233a0) [DOI] [PubMed] [Google Scholar]
- Rouillé Y, Westermark G, Martin SK & Steiner DF 1994. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1–6 cells. Proceedings of the National Academy of Sciences 91 3242–3246. (doi: 10.1073/pnas.91.8.3242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs S, Bastidas-Ponce A, Tritschler S, Bakhti M, Böttcher A, Sánchez-Garrido MA, Tarquis-Medina M, Kleinert M, Fischer K, Jall S et al. 2020. Targeted pharmacological therapy restores β-cell function for diabetes remission. Nature Metabolism 2 192–209. (doi: 10.1038/s42255-020-0171-3) [DOI] [PubMed] [Google Scholar]
- Saikia M, Holter MM, Donahue LR, Lee IS, Zheng QC, Wise JL, Todero JE, Phuong DJ, Garibay D, Coch R et al. 2021. GLP-1 receptor signaling increases PCSK1 and β cell features in human α cells. JCI Insight 6 e141851. (doi: 10.1172/jci.insight.141851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Dobbs R & Unger RH 1974. Somatostatin-Induced Changes in Insulin and Glucagon Secretion in Normal and Diabetic Dogs. Journal of Clinical Investigation 54 1395–1402. (doi: 10.1172/jci107886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons MF & Lee ECY 2015. Recent progress in the development of small-molecule glucagon receptor antagonists. Bioorganic & Medicinal Chemistry Letters 25 4057–4064. (doi: 10.1016/j.bmcl.2015.07.092) [DOI] [PubMed] [Google Scholar]
- Sato S, Saisho Y, Inaishi J, Kou K, Murakami R, Yamada T & Itoh H 2015. Effects of Glucocorticoid Treatment on β- and α-Cell Mass in Japanese Adults With and Without Diabetes. Diabetes 64 2915–2927. (doi: 10.2337/db15-0151) [DOI] [PubMed] [Google Scholar]
- Schulman JL, Carleton JL, Whitney G & Whitehorn JC 1957. Effect of Glucagon on Food Intake and Body Weight in Man. Journal of Applied Physiology 11 419–421. (doi: 10.1152/jappl.1957.11.3.419) [DOI] [PubMed] [Google Scholar]
- Segerstolpe Å, Palasantza A, Eliasson P, Andersson E-M, Andréasson A-C, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK et al. 2016. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metabolism 24 593–607. (doi: 10.1016/j.cmet.2016.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, White NH, Arbelaez AM, Kollman C, Ruedy KJ et al. 2014. Evolution of Abnormal Plasma Glucagon Responses to Mixed-Meal Feedings in Youth With Type 1 Diabetes During the First 2 Years After Diagnosis. Diabetes Care 37 1741–1744. (doi: 10.2337/dc13-2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Saunders DC, Walker JT, Camunas-Soler J, Dai X-Q, Haliyur R, Aramandla R, Poffenberger G, Prasad N, Bottino R et al. 2021. Combinatorial transcription factor profiles predict mature and functional human islet α and β cells. JCI Insight 6 e151621. (doi: 10.1172/jci.insight.151621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Erikson G, Lyon J, Spigelman AF, Bautista A, Fox JEM, Santos C dos, Shokhirev M, Cartailler J-P, Hetzer MW et al. 2022. Aging compromises human islet beta cell function and identity by decreasing transcription factor activity and inducing ER stress. Science Advances 8 eabo3932. (doi: 10.1126/sciadv.abo3932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai H, Xu Y, Yu Q, Gylfe E & Tengholm A 2016. Fluorescent protein vectors for pancreatic islet cell identification in live-cell imaging. Pflügers Archiv - European Journal of Physiology 468 1765–1777. (doi: 10.1007/s00424-016-1864-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloop KW, Cao JX-C, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter AD et al. 2004. Hepatic and glucagon-like peptide-1–mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. Journal of Clinical Investigation 113 1571–1581. (doi: 10.1172/jci20911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza AH de, Tang J, Yadev AK, Saghafi ST, Kibbe CR, Linnemann AK, Merrins MJ & Davis DB 2020. Intra-islet GLP-1, but not CCK, is necessary for β-cell function in mouse and human islets. Scientific Reports 10 2823. (doi: 10.1038/s41598-020-59799-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H & Pfeiffer AFH 2003. Inflammatory Cytokines and the Risk to Develop Type 2 Diabetes. Diabetes 52 812–817. (doi: 10.2337/diabetes.52.3.812) [DOI] [PubMed] [Google Scholar]
- Stefan Y, Grasso S, Perrelet A & Orci L 1983. A Quantitative Immunofluorescent Study of the Endocrine Cell Populations in the Developing Human Pancreas. Diabetes 32 293–301. (doi: 10.2337/diab.32.4.293) [DOI] [PubMed] [Google Scholar]
- Steiner DJ, Kim A, Miller K & Hara M 2010. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2 135–145. (doi: 10.4161/isl.2.3.11815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland EW & Christian de Duve 1948. Origin and Distribution of the Hyperglycemic-Glycogenolytic Factor of the Pancreas. Journal of Biological Chemistry 175 663–674. (doi: 10.1016/s0021-9258(18)57183-0) [DOI] [PubMed] [Google Scholar]
- Svendsen B, Larsen O, Gabe MBN, Christiansen CB, Rosenkilde MM, Drucker DJ & Holst JJ 2018. Insulin Secretion Depends on Intra-islet Glucagon Signaling. Cell Reports 25 1127–1134.e2. (doi: 10.1016/j.celrep.2018.10.018) [DOI] [PubMed] [Google Scholar]
- Tamura Y, Izumiyama-Shimomura N, Kimbara Y, Nakamura K, Ishikawa N, Aida J, Chiba Y, Matsuda Y, Mori S, Arai T et al. 2016. Telomere attrition in beta and alpha cells with age. AGE 38 61. (doi: 10.1007/s11357-016-9923-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez K, Hang Y, Gu X, Chang CA, Stein RW & Kim SK 2020. In vivo studies of glucagon secretion by human islets transplanted in mice. Nature Metabolism 2 547–557. (doi: 10.1038/s42255-020-0213-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ouedraogo R & Kirchgessner AL 2002. Localization and function of group III metabotropic glutamate receptors in rat pancreatic islets. American Journal of Physiology-Endocrinology and Metabolism 282 E1324–E1333. (doi: 10.1152/ajpendo.00460.2001) [DOI] [PubMed] [Google Scholar]
- Tong X, Dai C, Walker JT, Nair GG, Kennedy A, Carr RM, Hebrok M, Powers AC & Stein R 2020. Lipid Droplet Accumulation in Human Pancreatic Islets Is Dependent On Both Donor Age and Health. Diabetes 69 342–354. (doi: 10.2337/db19-0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh DKY, Zhang K, Hossain M, Brubaker PL & Drucker DJ 2003. Pax-6 Activates Endogenous Proglucagon Gene Expression in the Rodent Gastrointestinal Epithelium. Diabetes 52 425–433. (doi: 10.2337/diabetes.52.2.425) [DOI] [PubMed] [Google Scholar]
- Tritschler S, Thomas M, Böttcher A, Ludwig B, Schmid J, Schubert U, Kemter E, Wolf E, Lickert H & Theis FJ 2022. A transcriptional cross species map of pancreatic islet cells. Molecular Metabolism 101595. (doi: 10.1016/j.molmet.2022.101595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, Yatsushiro S & Moriyama Y 2004. Metabotropic Glutamate Receptor Type 4 Is Involved in Autoinhibitory Cascade for Glucagon Secretion by α-Cells of Islet of Langerhans. Diabetes 53 998–1006. (doi: 10.2337/diabetes.53.4.998) [DOI] [PubMed] [Google Scholar]
- Unger RH & Cherrington AD 2012. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Journal of Clinical Investigation 122 4–12. (doi: 10.1172/jci60016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R & Orci L 1975. THE ESSENTIAL ROLE OF GLUCAGON IN THE PATHOGENESIS OF DIABETES MELLITUS. The Lancet 305 14–16. (doi: 10.1016/s0140-6736(75)92375-2) [DOI] [PubMed] [Google Scholar]
- Unger RH, Aguilar-Parada E, Müller WA & Eisentraut AM 1970. Studies of pancreatic alpha cell function in normal and diabetic subjects. Journal of Clinical Investigation 49 837–848. (doi: 10.1172/jci106297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira E, Liu Y-J & Gylfe E 2004. Involvement of α1 and β-adrenoceptors in adrenaline stimulation of the glucagon-secreting mouse α-cell. Naunyn-Schmiedeberg’s Archives of Pharmacology 369 179–183. (doi: 10.1007/s00210-003-0858-5) [DOI] [PubMed] [Google Scholar]
- Viloria K, Nasteska D, Briant LJB, Heising S, Larner DP, Fine NHF, Ashford FB, Xavier G da S, Ramos MJ, Hasib A et al. 2020. Vitamin-D-Binding Protein Contributes to the Maintenance of α Cell Function and Glucagon Secretion. Cell Reports 31 107761. (doi: 10.1016/j.celrep.2020.107761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viloria K, Hewison M & Hodson DJ 2022. Vitamin D binding protein/GC-globulin: a novel regulator of alpha cell function and glucagon secretion. The Journal of Physiology 600 1119–1133. (doi: 10.1113/jp280890) [DOI] [PubMed] [Google Scholar]
- Wang YJ, Schug J, Won K-J, Liu C, Naji A, Avrahami D, Golson ML & Kaestner KH 2016. Single-Cell Transcriptomics of the Human Endocrine Pancreas. Diabetes 65 3028–3038. (doi: 10.2337/db16-0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Traum D, Schug J, Gao L, Liu C, Consortium H, Atkinson MA, Powers AC, Feldman MD, Naji A et al. 2019. Multiplexed In Situ Imaging Mass Cytometry Analysis of the Human Endocrine Pancreas and Immune System in Type 1 Diabetes. Cell Metabolism 29 769–783.e4. (doi: 10.1016/j.cmet.2019.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CD, Yao TL, Powers AC & Verdoorn TA 1996. Differential Expression of Glutamate Receptor Subtypes in Rat Pancreatic Islets. Journal of Biological Chemistry 271 12977–12984. (doi: 10.1074/jbc.271.22.12977) [DOI] [PubMed] [Google Scholar]
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P & Braun M 2004. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes 53 1038–1045. (doi: 10.2337/diabetes.53.4.1038) [DOI] [PubMed] [Google Scholar]
- Weng C, Xi J, Li H, Cui J, Gu A, Lai S, Leskov K, Ke L, Jin F & Li Y 2020. Single-cell lineage analysis reveals extensive multimodal transcriptional control during directed beta-cell differentiation. Nature Metabolism 2 1443–1458. (doi: 10.1038/s42255-020-00314-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek G, Pospischil A & Perentes E 1998. A comparative immunohistochemical study of pancreatic islets inlaboratory animals (rats, dogs, minipigs, nonhuman primates). Experimental and Toxicologic Pathology 50 151–172. (doi: 10.1016/s0940-2993(98)80078-x) [DOI] [PubMed] [Google Scholar]
- Wu C-T, Hilgendorf KI, Bevacqua RJ, Hang Y, Demeter J, Kim SK & Jackson PK 2021. Discovery of ciliary G protein-coupled receptors regulating pancreatic islet insulin and glucagon secretion. Genes & Development 35 1243–1255. (doi: 10.1101/gad.348261.121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, Murphy AJ, Yancopoulos GD, Lin C & Gromada J 2016. RNA Sequencing of Single Human Islet Cells Reveals Type 2 Diabetes Genes. Cell Metabolism 24 608–615. (doi: 10.1016/j.cmet.2016.08.018) [DOI] [PubMed] [Google Scholar]
- Yang W, Reyes AA & Lan NC 1994. Identification of the GABAA receptor subtype mRNA in human pancreatic tissue. FEBS Letters 346 257–262. (doi: 10.1016/0014-5793(94)00485-4) [DOI] [PubMed] [Google Scholar]
- Yip L, Taylor C, Whiting CC & Fathman CG 2013. Diminished Adenosine A1 Receptor Expression in Pancreatic α-Cells May Contribute to the Pathology of Type 1 Diabetes. Diabetes 62 4208–4219. (doi: 10.2337/db13-0614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW et al. 2003. Selective β-Cell Loss and α-Cell Expansion in Patients with Type 2 Diabetes Mellitus in Korea. The Journal of Clinical Endocrinology & Metabolism 88 2300–2308. (doi: 10.1210/jc.2002-020735) [DOI] [PubMed] [Google Scholar]
- Zadeh EHG, Huang Z, Xia J, Li D, Davidson HW & Li W 2020. ZIGIR, a Granule-Specific Zn2+ Indicator, Reveals Human Islet α Cell Heterogeneity. Cell Reports 32 107904. (doi: 10.1016/j.celrep.2020.107904) [DOI] [PMC free article] [PubMed] [Google Scholar]


