Abstract
Background
Gay and bisexual men using HIV pre-exposure prophylaxis (PrEP) are at increased risk for sexually transmissible infections. Hepatitis C virus (HCV) risk among PrEP users is less clear. We explored HCV prevalence and incidence among cohorts of gay and bisexual men using PrEP and sources of heterogeneity across studies.
Methods
This was a systematic review and meta-analysis of open-label PrEP studies to April 2022 reporting HCV prevalence at baseline or incidence during follow-up among gay and bisexual men using PrEP. Pooled prevalence and incidence estimates were calculated using random-effects meta-analysis, and subgroup analyses were performed by study- and country-level characteristics, including availability of HCV direct-acting antiviral (DAA) therapy at time of study.
Results
Twenty-four studies from 9 countries were included, with a total sample of 24 733 gay and bisexual men. Pooled HCV antibody baseline prevalence was 0.97% (95% CI, 0.63%–1.31%), and pooled HCV RNA baseline prevalence was 0.38% (95% CI, 0.19%–0.56%). Among 19 studies reporting HCV incidence, incidence ranged from 0.0 to 2.93/100 person-years (py); the pooled estimate was 0.83/100py (95% CI, 0.55–1.11). HCV incidence was higher in 12 studies that began follow-up before broad DAA availability (1.27/100py) than in 8 studies that began follow-up after broad DAA availability (0.34/100py) and higher in studies in Europe compared with North America and Australia.
Conclusions
Early reports of high HCV incidence among PrEP-using cohorts likely reflect enrollment of individuals based on specific risk-based eligibility criteria for smaller studies and enrollment before DAA scale-up. In contexts where both DAAs and PrEP have been implemented at scale, studies report lower HCV incidence. PrEP-specific HCV testing guidelines should be guided by local epidemiology.
Keywords: Hepatitis C, HIV, gay and bisexual men, men who have sex with men, pre-exposure prophylaxis
Global guidelines on hepatitis C treatment and prevention highlight gay and bisexual men and other men who have sex with men as a priority population [1]. Among gay and bisexual men globally, those living with HIV have been historically overrepresented in hepatitis C diagnoses [2, 3], a result of intersecting behavioral and demographic risk factors [4] and driven further by specific and more concentrated sexual networks constituted of gay and bisexual men with HIV [5]. The availability of highly efficacious direct-acting antiviral (DAA) treatments for hepatitis C virus (HCV) in many countries from early 2014 has galvanized hepatitis C elimination efforts globally, leading to ambitious elimination targets [6, 7]. Widespread uptake of DAA treatment has been associated with rapid declines in population-level hepatitis C viremia and incidence among gay and bisexual men with HIV in multiple settings, including in Australia [8] and Europe [9, 10]. However, alongside the development and approval of DAA treatments for HCV, biomedical advances in HIV prevention, including treatment as prevention (TasP) [11] and HIV pre-exposure prophylaxis (PrEP) [12], have been associated with increases in condomless sex among gay and bisexual men [13, 14], as well as an increases in bacterial STIs [15]. While the impact of PrEP implementation on HCV transmission among HIV-negative gay and bisexual men is less documented, the influence of PrEP on condom use and sexual networks, specifically increased rates of sero-different sex among gay and bisexual men [13], has raised concerns for the potential bridging of HCV transmission networks between gay and bisexual men with HIV and HIV-negative gay and bisexual men [16, 17].
While hepatitis C elimination strategies are underpinned by testing and treating populations at ongoing risk for hepatitis C [8, 18], including gay and bisexual men, national guidelines on how often PrEP users should be screened for HCV vary considerably, from every 3 to 12 months for all gay and bisexual men using PrEP [19–21] to only in the presence of ongoing risk factors (eg, injection drug use) [22], with some countries, such as the United Kingdom, having no specific guidelines for HCV screening in PrEP users [23]. Early clinical studies that reported relatively high baseline risk of hepatitis C among gay and bisexual men taking PrEP were likely biased toward individuals with specific risk characteristics, as early PrEP demonstration studies often had specific risk-based enrollment criteria (eg, recent condomless anal sex with casual partners or recent STI diagnosis [24]). Participants in these studies may not reflect wider populations of PrEP users following widespread PrEP implementation or their HCV risk in the context of widespread DAA availability.
Estimates of pooled hepatitis C incidence among gay and bisexual men using PrEP have varied across reviews [25, 26], and in the context of declining hepatitis C incidence among gay and bisexual men with HIV following DAA implementation [8, 10, 27], none have investigated heterogeneity in hepatitis C risk among PrEP users relative to DAA availability at the time of study. In this systematic review and meta-analysis of hepatitis C among gay and bisexual men using HIV PrEP, we aimed to provide updated estimates of hepatitis C prevalence and incidence among PrEP users globally and examine rates of hepatitis C incidence among PrEP cohorts across study- and country-level characteristics, including the availability of DAA treatments for hepatitis C at the time of PrEP rollout.
METHODS
A protocol for this review was registered prospectively (PROSPERO registration number 2020 CRD42020179455).
Eligibility Criteria
Studies were included if they reported data on hepatitis C prevalence or incidence among gay and bisexual men using HIV PrEP, inclusive of daily or on-demand/event-driven PrEP. We included prospective observational cohort studies, open-label 1-armed trials, and nonblinded randomized controlled trials (ie, participants were aware they were using PrEP).
Outcomes
To be included, studies must have reported 1 of the below primary outcomes:
hepatitis C antibody prevalence—point estimate of hepatitis C antibody positivity at PrEP initiation or study baseline;
hepatitis C RNA prevalence—point estimate of hepatitis C RNA positivity/viremia at PrEP initiation or study baseline (among all participants);
hepatitis C incidence—incidence rate per 100 person-years of PrEP use of hepatitis C (primary and re-infection) or cumulative incidence of hepatitis C during PrEP use.
Search Strategy
We searched the following databases on April 20, 2022: Medline and EMBASE (using OVID) and PubMed. Search strings included medical subject headings and free text relating to the following (see Supplementary Materials 1 for full search strings):
MSM (men who have sex with men, gay and bisexual men, gay men);
pre-exposure prophylaxis (PrEP, Truvada, tenofovir, TDF, emtricitabine);
hepatitis C (HCV, hepatitis C virus).
We also conducted manual searches of relevant international HIV and viral hepatitis conferences (Supplementary Materials 1).
Abstracts and titles were screened independently by 2 reviewers (M.T. and B.H.). For studies that reported at least 1 outcome, full texts were obtained and assessed to confirm eligibility. Where multiple publications or conference abstracts reported data from the same cohort or study, the most recent citation or the citation with the most complete data for the relevant outcomes was included. Where 2 citations reported data from the same study but reported different outcomes (eg, HCV antibody prevalence and HCV RNA prevalence), both were included.
Data Extraction
Data were extracted and assessed independently by 2 reviewers using a standardized form to collate the following study characteristics and outcomes where reported:
study design;
location of study;
date of start and end of study follow-up;
sample size (number included in hepatitis C outcomes);
participant demographics (including the proportion classified as MSM or transgender women, age, ethnicity);
-
hepatitis C behavioral risk characteristics of the cohort and/or hepatitis C cases (if reported):
sexual behavior (eg, number of partners, condom use, group sex, fisting); and/or
drug use (eg, chemsex drug use, injecting drug use);
-
primary outcome measures:
for prevalence and incidence outcomes, numerator and denominator data were extracted separately where available; where unavailable, reported prevalence rates or incidence rates were extracted;
for antibody prevalence calculations (where numerator and denominator were reported), the numerator was taken as the number of participants who tested positive for HCV antibodies at baseline and the denominator was taken as the number of participants tested for HCV antibodies at baseline;
for RNA prevalence calculations (where numerator and denominator were reported), the numerator was taken as the number of participants who tested positive for HCV RNA at baseline and the denominator was the number of participants tested for either HCV antibody or RNA at baseline;
for hepatitis C incidence calculations (where number of infections and person-time at risk were reported), the incidence rate was taken as the number of new hepatitis C infections (including primary and re-infections) divided by the number of person-years accrued.
Any disagreements were resolved by consensus, and study authors were contacted via email a maximum of 2 times to obtain missing data or further information where needed.
Study Setting and DAA Availability
The rate of hepatitis C transmission among PrEP users is likely linked to community-level hepatitis C viremia among the wider gay and bisexual men population at the time of PrEP implementation. To explore the potential effect of the timing of DAA availability (and impact of this on hepatitis C prevalence) on hepatitis C incidence among PrEP users, we searched PubMed, national policy documents, and other gray literature as applicable to record when DAAs became broadly available in each respective jurisdiction of the included studies (data sources and results in Supplementary Table 1). Each study was categorized according to the broad availability of DAA treatments to gay and bisexual men during the study follow-up in the respective country; studies were categorized as
study initiated before broad DAA availability (limited or no access to subsidized DAAs, or restrictions on DAA prescribing based on liver disease stage or substance use, at the time of PrEP study follow-up initiation); or
study initiated after broad DAA availability (DAAs available with no restrictions based on liver disease stage or substance use at the time of PrEP study follow-up initiation).
Statistical Analysis
Random-effects meta-analysis was used to calculate pooled estimates for hepatitis C prevalence (antibody positivity and RNA positivity separately) at PrEP initiation/study baseline and incidence during follow-up. To estimate pooled hepatitis C prevalence, a double arcsine transformation was performed in order to constrain confidence intervals between 0.0 and 1.0, and the metaprop command in Stata was used to cumulate prevalence estimates [28]. For hepatitis C incidence, we extracted the reported number of incident infections and person-time-at-risk from each study and calculated incidence rates per 100 person-years and confidence intervals using the the exact chi-square method to allow for upper confidence intervals for 0 rates to be calculated [29] and used the metan Stata command to cumulate incidence estimates [30]. The inverse-variance method was used to weight studies in pooled estimates. Statistical heterogeneity between studies was assessed by calculating I2 and χ2 statistics, with a χ2 significance level of .10 and I2 >50% considered a moderate to high level of heterogeneity [31].
Subgroup analyses were performed to identify sources of heterogeneity between studies by stratifying studies by country, availability of DAAs relative to PrEP rollout, and sample size (number of participants contributing to the estimate of the respective outcome). Sample size (dichotomized into <500 or ≥500 participants as the median sample size was 485) was included as PrEP studies with a smaller recruitment capacity may have prioritized enrollment of individuals reporting greater HIV-related risk behaviors. Due to heterogeneity in reported sexual and drug use behavior measures across studies, a meta-analysis by behavioral outcomes was not feasible. As such, we report a narrative review of behavioral outcomes. All statistical analyses were performed using Stata software (version 15.1 for Windows; StataCorp, College Station, TX, USA).
Risk of Bias Assessment
A modified Newcastle-Ottawa Scale (Supplementary Materials 2) [32] was used to assess the risk of bias in the included studies. Risk of bias in individual studies was assessed based on sample representativeness of the population of gay and bisexual men who use PrEP, evidence for confirmation of outcome (new hepatitis C infection), and adequate follow-up time. Bias was classified using a numerical scale from 0 to 2 for each criterion, with a maximum total score of 8. A score of ≥7 was classified as low risk of bias.
RESULTS
Search Results and Included Studies
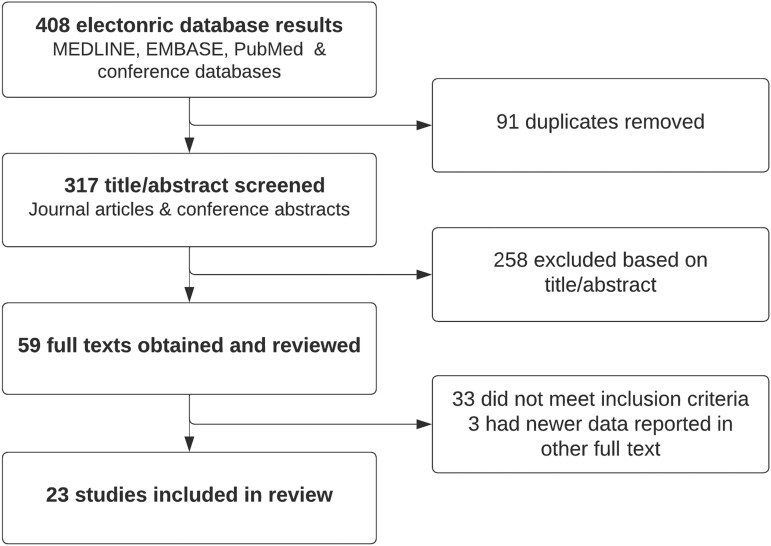
The electronic database search resulted in a total of 408 citations, of which 91 were duplicates, leaving 317 unique citations (Figure 1). A total of 23 studies met inclusion criteria and were included in the review. The characteristics of the included studies and the outcomes they report are shown in Table 1. Studies were from 9 countries and included a total of 24 733 gay and bisexual men using PrEP. Two publications eported different outcomes from the same cohort; Reyniers et al. reported hepatitis C prevalence, and Vuylesteke et al. reported incidence from the Belgian PrEP study. One study (Harney et al.) included data from 2 other studies (Amin et al. and Cornelisse et al.) as part of a national-level presentation of data from Australia and was excluded from meta-analyses and the total sample calculation due to the determination of person-time in this study (hepatitis C incidence rates calculations for PrEP users in this study potentially included person-time during periods of non-PrEP use). Two studies [27, 33] reported changes in annual rates of hepatitis C incidence among large cohorts of PrEP users over time (Supplementary Results 1).
Figure 1.
Search results and screening process.
Table 1.
Characteristics of Included Studies
| Study | Project/Study/Clinic | Study Type | Start Date | End Date | Country (State/Province) | Cohort Size* | Cohort Population | Included in Pooled Estimates | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HCV Ab Prevalence | HCV RNA Prevalence | HCV Incidence | ||||||||
| Aloysius et al. 2017 [34] | InterPrEP | Prospective cohort | Feb-16 | Mar-17 | UK | 573 | 100% MSM 75% daily PrEP |
… | … | Yes |
| Amin et al. 2021 a [35] | EPIC-NSW Studya | Prospective cohort Demonstration study |
Mar-16 | Apr-19 | Australia (New South Wales) | 8658a | 98.5% male 91.8% identify gay 6.7% identify bisexual |
Yes | Yes | Yes |
| Ayerdi Aguirrebengoa et al. 2021 [36] | Centre Sanitario Sandoval | Retrospective descriptive | Jan-17 | Jan-19 | Spain | 110 | 98.2% MSM 1.8% TGW |
Yes | … | Yes |
| Cornelisse et al. 2020a [37] | PrEPX Studya | Prospective cohort Demonstration study |
Jun-16 | Mar-18 | Australia (Victoria) | 3202a | 99.1% male 98.7% gay or bisexual |
Yes | Yes | Yes |
| Cotte et al. 2018 [38] | French Dat'AIDS cohort | Prospective cohort | Jan-16 | May-17 | France | 903 | 100% MSM | Yes | Yes | Yes |
| Desai et al. 2020 [39] | PROUD | Open-label deferred RCT | Nov-12 | Oct-16 | UK | 544 | 100% MSM | Yes | Yes | Yes |
| Gras et al. 2020 [40] | ANRS IPERGAY PrEP | RCT & open-label trial | Feb-12 | Jun-16 | France | 429 | 100% MSM | … | … | Yes |
| Hamed et al. 2018 [41] | Clinic Network in Newark | Clinic-based prospective cohort | May-16 | Mar-18 | USA (New Jersey) | 74 | 74% male 40% MSM 45% reported HIV-positive partners (eligibility inc. MSM, PWID, HIV-positive partner) |
… | Yes | … |
| Harney et al. 2021a [27] | ACCESS surveillance networka | Retrospective multiclinic analysis | Jan-16 | Dec-19 | Australia | 23 373a | 100% MSM (algorithm based on self-report and rectal swab history) | … | … | … |
| Hassan et al. 2019 [42] | CCTG 595 PATH-PrEP | 2 prospective cohorts | Feb-13 | Jul-16 | USA (California) | 599 | 99.7% MSM 0.3% TGF |
Yes | … | Yes |
| Hoornenborg et al. 2020 [43, 44] | Amsterdam PrEP project | Prospective cohort | Aug-15 | Sep-18 | Netherlands | 350 | 99.4% MSM 0.6% TGF |
Yes | Yes | Yes |
| Lalley-Chareczko et al. 2018 [45] | Philadelphia FIGHT clinic | Prospective cohort | NR | NR | USA (Pennsylvania) | 50 | 90% MSM 10% TGF |
… | … | Yes |
| Mikati et al. 2018 [46] | NYC Sexual Health Clinics | Retrospective clinic audit | Sep-16 | Dec-19 | USA (New York) | 381 | 100% MSM | Yes | Yes | … |
| Molina et al. 2019 [47] | ANRS PREVENIR | Open-label RCT | May-17 | Oct-18 | France | 3067 | 98.5% MSM | … | … | Yes |
| Nguyen et al. 2018 [48] | Clinique l'Actuel | … | Jan-10 | Jan-15 | Canada (Quebec) | 109 | 100% MSM | … | … | Yes |
| Noret et al. 2018 [49] | Saint-Louis Hospital | Prospective cohort | Nov-15 | Apr-17 | France | 1049 | 99.4% MSM 0.3% TGW |
… | Yes | Yes |
| Peĉavar et al. 2021 [50] | Demonstration study | Prospective cohort Demonstration study |
Aug-18 | Oct-20 | Slovenia | 74 | 100% MSM | Yes | … | Yes |
| Ramiere et al. 2019 [33] | Lyon University Hospital | Retrospective clinic audit | Jan-14 | Dec-17 | France | NR | 100% MSM | … | … | Yes |
| Reyniers et al. 2018b [51] | Belgian PrEP studyb | Cross-sectional analysis from prospective cohort | Oct-15 | NR | Belgium | 200c | 98.5% MSM 1.5% TGW |
… | Yes | … |
| Tabatabavakili et al. 2022 [52] | University HIV Prevention Clinic | Retrospective clinic audit | Oct-12 | Sep-19 | Canada (Ontario) | 109 | 100% MSM | Yes | Yes | Yes |
| Thompson et al. 2022 [53] | BC PrEP Program | Prospective cohort | Jan-18 | Aug-19 | Canada (British Columbia) | 3967 | 98.5% MSM | Yes | Yes | Yes |
| Volk et al. 2015 [54] | Kaiser Permanente SF MC | Retrospective clinic audit | Feb-11 | Dec-14 | USA (California) | 485 | 100% MSM | … | … | Yes |
| Vuylesteke et al. 2019b [55] | Belgian PrEP studyb | Prospective cohort | Oct-15 | Jan-18 | Belgium | 200c | 98.5% MSM 1.5% TGW |
… | … | Yes |
Abbreviations: Ab, antibody; DAA, direct-acting antiviral; HCV, hepatitis C virus; MSM, men who have sex with men; NR, not reported; PrEP, pre-exposure prophylaxis; PWID, people who inject drugs; RCT, randomized controlled trial; TGW, transgender women.
Harney et al. includes PrEP users captured in Cornelisse et al. and Amin et al. and is excluded from pooled estimates and total sample calculation.
These 2 studies report data from the same cohort; Reyniers et al. reports prevalence and Vuylesteke et al. reports incidence.
*N represented those eligible for HCV analyses if reported; otherwise represents full PrEP cohort at baseline.
Hepatitis C Prevalence at PrEP Initiation
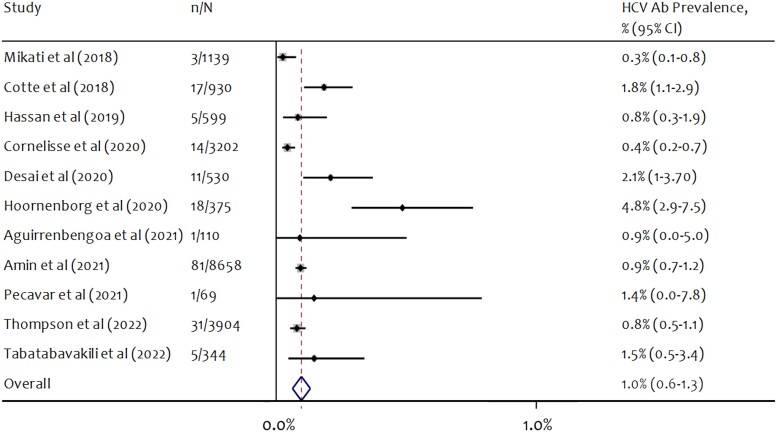
Eleven studies reported HCV antibody prevalence at study baseline. Antibody prevalence ranged from 0.26% to 4.80% across studies (Figure 2). The pooled estimate of hepatitis C antibody prevalence was 0.97% (95% CI, 0.63%–1.31%), and heterogeneity was high across studies (I2 = 78.3%; P < .001) (Table 2). Six PrEP studies that reported hepatitis C antibody positivity at baseline began follow-up before broad availability of DAA treatments in the respective country or jurisdiction. Among these 6 PrEP studies, the pooled estimate of antibody positivity was 1.75% (95% CI, 0.93%–2.56%). Among the 5 studies where follow-up began after broad availability of DAA treatments, the pooled estimate of antibody positivity was 0.62% (95% CI, 0.32%–0.92%). Pooled antibody prevalence was greater in studies with <500 participants (2.08%; 95% CI, 0.46%–3.71%) compared with studies with ≥500 participants (0.81%; 95% CI, 0.50%–1.13%). Antibody prevalence was greatest (pooled prevalence >1%) in studies from the Netherlands, the United Kingdom, France, and Slovenia (Table 2).
Figure 2.
Forest plot of random-effects meta-analysis of HCV antibody prevalence at baseline among gay and bisexual men using PrEP. n = number of participants positive for HCV antibodies at study baseline; N = number of participants tested for HCV antibodies. Abbreviations: HCV, hepatitis C virus; PrEP, pre-exposure prophylaxis.
Table 2.
Pooled Estimates of HCV Antibody and RNA Prevalence Among Gay and Bisexual Men Using PrEP
| Variable | No. of Studies | Pooled Estimate (95% CI), % |
Heterogeneity χ2 Test (I2, %) |
|---|---|---|---|
| HCV antibody prevalence, % | |||
| Overall | 11 | 0.97 (0.63–1.31) | P < .001 (78.3) |
| By DAA availability | |||
| Study follow-up started before broad DAA availability | 6 | 1.75 (0.93–2.56) | P = .016 (64.3) |
| Study follow-up started after broad DAA availability | 5 | 0.62 (0.32–0.92) | P = .001 (78.7) |
| Sample size | |||
| <500 | 4 | 2.08 (0.46–3.71) | P = .034 (65.4) |
| ≥500 | 7 | 0.81 (0.50–1.13) | P < .001 (80.4) |
| Country | |||
| USA | 2 | 0.35 (0.07–0.62) | P = .216 (34.5) |
| UK | 1 | 2.08 (1.04–3.68) | … |
| France | 1 | 1.83 (1.07–2.91) | … |
| Canada | 2 | 0.82 (0.53–1.10) | P = .380 (0.0) |
| Netherlands | 1 | 4.80 (2.87–7.48) | … |
| Australia | 2 | 0.72 (0.56–0.87) | P = .003 (89.0) |
| Spain | 1 | 0.91 (0.02–4.96) | … |
| Benin | 1 | 1.45 (0.04–7.81) | … |
| HCV RNA prevalence, % | |||
| Overall | 11 | 0.38 (0.19–0.56) | P < .001 (72.4) |
| By DAA availability | |||
| Study follow-up started before broad DAA availability | 6 | 0.97 (0.38–1.55) | P = .005 (69.5) |
| Study follow-up started after broad DAA availability | 5 | 0.23 (0.09–0.38) | P = .014 (67.9) |
| Sample size | |||
| <500 | 4 | 2.23 (0.76–3.70) | P = .071 (57.3) |
| ≥500 | 7 | 0.28 (0.15–0.41) | P = .017 (61.0) |
| Country | |||
| USA | 2 | 0.09 (0.00–0.27) | … |
| UK | 1 | 0.57 (0.12–1.65) | … |
| France | 2 | 0.48 (0.17–0.79) | … |
| Canada | 2 | 0.19 (0.06–0.32) | … |
| Belgium | 1 | 1.50 (0.31–4.32) | … |
| Netherlands | 1 | 4.00 (2.26–6.51) | … |
| Australia | 2 | 0.33 (0.23–0.43) | … |
See Supplementary Figures 1–6 for forest plots for HCV prevalence by study characteristics.
Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; PrEP, pre-exposure prophylaxis.
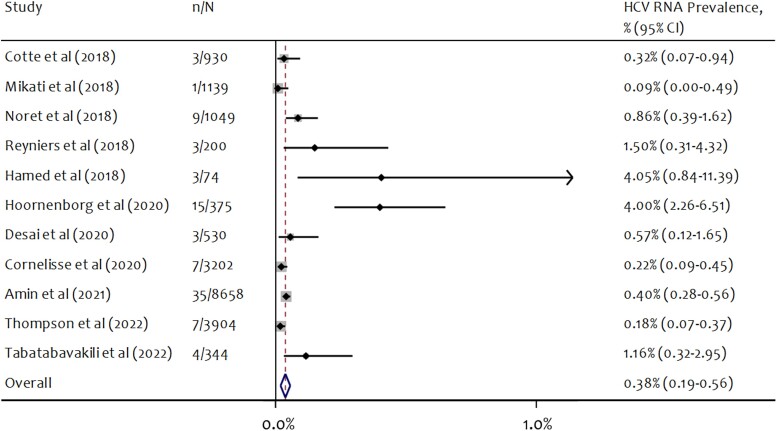
Eleven studies reported HCV RNA prevalence at study baseline. HCV RNA prevalence ranged from 0.09% to 4.05% across studies (Figure 3). The pooled estimate of hepatitis C RNA prevalence was 0.38% (95% CI, 0.19%–0.56%), and heterogeneity was high (I2 = 72.4%; P < .001) (Table 2). Six PrEP studies that reported HCV RNA positivity at baseline began follow-up before broad availability of DAA treatments in the respective country or jurisdiction. Among these 6 studies, the pooled estimate of RNA positivity was 0.97% (95% CI, 0.38%–1.55%). Among the 5 studies that started follow-up after broad availability of DAA treatments, the pooled estimate of RNA positivity was 0.23% (95% CI, 0.09%–0.38%). Pooled RNA prevalence was greater in studies with <500 participants (2.23%; 95% CI, 0.76%–3.70%) compared with in studies with ≥500 participants (0.28%; 95% CI, 0.15%–0.41%). RNA prevalence was greatest (pooled prevalence >1%) in studies from the Netherlands and Belgium (Table 2). See Supplementary Figures 1–6 for forest plots for HCV prevalence by study characteristics.
Figure 3.
Forest plot of random-effects meta-analysis of HCV RNA prevalence at baseline among gay and bisexual men using PrEP. n = number of participants positive for HCV RNA at study baseline; N = number of participants tested for HCV antibodies and/or RNA. The denominator for RNA prevalence is the number of study participants tested for HCV (either antibody or RNA) at baseline, as many study protocols only tested participants for RNA if they were antibody positive. Abbreviations: HCV, hepatitis C virus; PrEP, pre-exposure prophylaxis.
Hepatitis C Incidence During PrEP Use
Nineteen studies reported hepatitis C incidence. In these studies, there were a total of 180 incident hepatitis C infections over a cumulative total of 28 429 person-years of PrEP use. Hepatitis C incidence ranged from 0.0 to 2.93 per 100 person-years across studies (Figure 2). The weighted pooled estimate of hepatitis C incidence from random-effects meta-analysis was 0.83 (95% CI, 0.55–1.11) per 100 person-years. Heterogeneity was high across studies (I2 = 81.7%; P < .001) (Table 3).
Table 3.
Pooled Estimates of HCV Incidence Among Gay and Bisexual Men Using PrEP
| Variable | No. of Studies | Pooled Estimate (95% CI) |
Heterogeneity χ2 Test (I2, %) |
|---|---|---|---|
| HCV incidence (rate/100 person-y) | |||
| Overall | 19 | 0.83 (0.55–1.11) | P < .001 (81.7) |
| By DAA availability | |||
| Study follow-up started before broad DAA availability | 12 | 1.27 (0.69–1.86) | P < .001 (81.8) |
| Study follow-up started after broad DAA availability | 6 | 0.34 (0.14–0.55) | P = .009 (65.1) |
| Sample size | |||
| <500 | 11 | 1.37 (0.85–1.88) | P = .120 (34.9) |
| ≥500 | 8 | 0.54 (0.26–0.81) | P < .001 (87.2) |
| Country | |||
| USA | 3 | 0.04 (0.00–0.34) | P = .520 (0.0) |
| UK | 2 | 1.39 (0.00–2.87) | P = .034 (77.8) |
| France | 5 | 1.17 (0.74–1.60) | P = .025 (64.1) |
| Canada | 3 | 0.29 (0.13–0.46) | P = .787 (0.0) |
| Belgium | 1 | 2.93 (1.53–5.64) | |
| Netherlands | 1 | 2.30 (1.39–3.79) | |
| Australia | 2 | 0.23 (0.06–0.40) | P = .185 (43.0) |
| Spain | 1 | 1.93 (0.52–4.93) | |
See Supplementary Figure 7 for forest plot showing HCV incidence by sample size.
Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; PrEP, pre-exposure prophylaxis.
Hepatitis C Incidence by DAA Availability
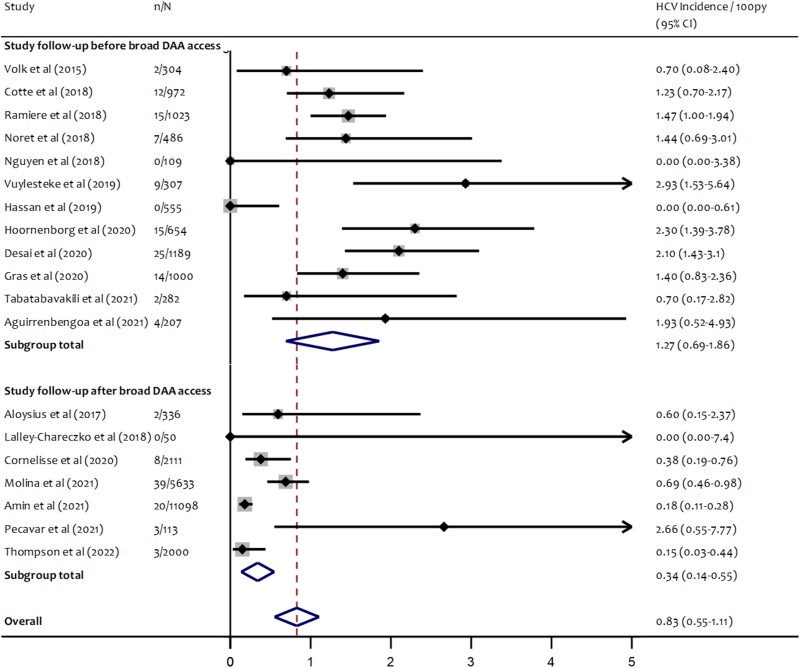
Twelve PrEP studies began follow-up before broad availability of DAA treatments (ie, DAA treatments became widely available during or after cessation of follow-up). In these 12 studies, the pooled estimate of hepatitis C incidence was 1.27/100 person years (95% CI, 0.69–1.86); heterogeneity remained high (I2 = 81.8%; P < .001). Seven PrEP studies reporting hepatitis C incidence began follow-up after broad availability of DAA treatments in the respective country or jurisdiction. Among these 7 studies, less heterogeneity was detected (I2 = 65%; P = .009), and the pooled estimate of hepatitis C incidence was 0.34/100 person-years (95% CI, 0.12–.53) (Figure 4).
Figure 4.
Forest plot of random-effects meta-analysis of HCV incidence among gay and bisexual men using PrEP by DAA availability in respective countries/jurisdictions at time of study initiation. n = number of participants diagnosed with incident HCV infection during study follow-up; N = total number of person-years of follow-up. Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; PrEP, pre-exposure prophylaxis.
Hepatitis C Incidence by Sample Size
Among 11 studies that included <500 participants, heterogeneity was low (I2 = 33.9%; P = .120), and the pooled estimate of hepatitis C incidence was 1.37/100 person-years (95% CI, 0.85–1.88). Among the 8 PrEP studies with ≥500 participants, the pooled estimate of hepatitis C incidence was lower at 0.54/100 person-years (95% CI, 0.26–0.81); heterogeneity was high (I2 = 87.2%; P < .001) (Supplementary Figure 7).
Hepatitis C Incidence by Country
In subgroup analyses by country, heterogeneity was low across studies within each country except for the 2 studies from the United Kingdom (I2 = 77.8%) and the 5 studies from France (I2 = 64.1%). Incidence among studies in Australia, the United States, and Canada was lower compared with studies from European countries (Table 3).
Behavioral Data
Heterogeneity in reporting of sexual and drug-related behaviors across studies precluded meta-analysis by hepatitis C risk behavior. Supplementary Table 2 summarizes sexual and drug use–related behaviors reported among study participants and, where reported in studies, behaviors associated with hepatitis C diagnosis. Studies reported different sexual behavior indicators, including recent condomless intercourse, receptive/insertive condomless intercourse, number of casual partners, reporting HIV-positive partners, group sex, sex at sex-on-premises venues, and fisting. Many studies also reported recent and lifetime injection drug use (IDU), as well as engagement in chemsex, for which definitions varied. See Supplementary Results 2 for a narrative synthesis of behavioral data.
Risk of Bias
Fourteen of the 23 studies were considered at low risk of bias (score ≥7) when graded using the modified Newcastle Ottawa Scale for cohort studies (Supplementary Table 3). The main biases identified were representativeness of the cohort (ie, smaller studies that recruited participants with specific risk criteria), confirmation of the outcome (ie, where details of antibody/RNA testing protocols were not reported), and adequacy of follow-up (ie, where reported mean/median follow-up for incidence calculations was <6 months).
DISCUSSION
In this review of hepatitis C among gay and bisexual men using PrEP, pooled estimates for hepatitis C incidence were lower than previously reported in other meta-analyses [25, 26], due largely to the inclusion of more recent and larger studies reporting lower rates of hepatitis C. Ours is the first review to explore the difference in hepatitis C incidence by respective country- and state-level availability of hepatitis C DAA treatments at the time of PrEP initiation. Pooled hepatitis C baseline prevalence and incidence among PrEP studies that initiated follow-up after broad access to DAAs became available were lower than in studies that initiated follow-up during periods of limited or no DAA access. Hepatitis C incidence was also lower in non-European studies and studies that enrolled large numbers of gay and bisexual men and implemented PrEP at scale.
The observed levels of heterogeneity in baseline hepatitis C prevalence across studies included in this review are likely reflective of both HCV prevalence within gay and bisexual men populations at the time of study enrollment and risk-based enrollment criteria of specific studies. Further, individuals who elected to participate in early PrEP trials, or “early adopters” of PrEP, likely represent individuals with a higher hepatitis C risk profile, including behavioral characteristics not necessarily included in study eligibility criteria. As with hepatitis C incidence, HCV RNA positivity was also lower in studies where DAAs were available at the time of enrollment and in larger studies and those undertaken outside of Europe. However, while RNA positivity and hepatitis C incidence were ∼4-fold lower in post-DAA studies, HCV antibody positivity was only ∼2.7-fold lower in post-DAA studies. This suggests that the enrollment of individuals with a lower risk profile (ie, fewer individuals with previous HCV exposure) may not fully explain the lower incidence observed in post-DAA studies. The lower incidence in post-DAA studies may reflect lower community-level hepatitis C viremia due to DAA implementation. Surveillance data from Australia suggest that uptake of DAA treatment among gay and bisexual men coinfected with hepatitis C and HIV led to rapid declines in both community-level hepatitis C viremia and new diagnoses of hepatitis C [8]. It is also possible that early reports of high hepatitis C incidence from early PrEP trials may have led to increased awareness of hepatitis C transmission among clinicians and PrEP users, and subsequent increases in testing and HCV treatment uptake.
Several previous reviews have explored hepatitis C among gay and bisexual men. A systematic review and meta-analysis of PrEP studies reporting prevalence and incidence of sexually transmissible infections, including hepatitis C, included 4 studies that reported HCV prevalence and 8 studies that reported hepatitis C incidence up to November 2018 [26]. In this meta-analysis, prevalence of HCV was 2.0%, and incidence was 0.43/100PY. Heterogeneity in incidence across studies was very high (I2 = 87%; P < .001); however, sources of heterogeneity were not explored beyond country income level. A more recent review (to October 2019) included studies that reported hepatitis C prevalence and incidence among gay and bisexual men (HIV-positive and HIV-negative). In this review, 4 studies reporting hepatitis C incidence among gay and bisexual men using PrEP were included; the pooled estimate of 1.48/100PY among gay and bisexual men using PrEP was similar to the hepatitis C incidence among studies of gay and bisexual men with HIV, suggesting that hepatitis C risk among gay and bisexual men enrolled in earlier PrEP studies was comparable to that of gay and bisexual men with HIV [25]. Our review highlights that previous pooled estimates of hepatitis C incidence among gay and bisexual men using PrEP may not be reflective of current hepatitis C among gay and bisexual men using PrEP in all settings, in the context of later widespread access to PrEP, or in the post-DAA era.
While PrEP users may represent a subgroup of HIV-negative individuals who report higher rates of behavior associated with hepatitis C risk, PrEP users remain highly engaged in clinical care and testing. Given declining trends in hepatitis C among gay and bisexual men in countries where DAAs are widely accessible, the impact of PrEP rollout and associated changes in behavior and sexual networks on hepatitis C elimination efforts may be offset by coinciding DAA availability in these countries. Consistent with findings previously reported for other sexually transmitted infections [56], modeling outcomes suggest that a decline in hepatitis C could be seen among gay and bisexual men in the context of PrEP scale-up via increased rates of HCV screening and treatment, even with moderate to high levels of reduced condom use [23]. However, people often transition in and out of PrEP care, and, in many settings, rates of PrEP discontinuation among gay and bisexual men are high, with high cost and inaccessibility to care being common reasons for discontinuation [57]. Such structural barriers to PrEP use may also impact efforts to diagnose and treat hepatitis C infections among gay and bisexual men through reduced PrEP-related hepatitis C screening. Further, in settings where DAAs have not been widely rolled out yet or are not subsidised, high prevalence of hepatitis C among gay and bisexual men with HIV may contribute to growing transmission among HIV-negative gay and bisexual men through increased rates of sero-different sex.
Current World Health Organiation (WHO) guidelines recommend hepatitis C testing among key populations, including gay and bisexual men, with specific recommendations for 3–6-monthly testing for gay and bisexual men with HIV and people with a cured or resolved infection for reinfection [1]; guidelines on how often HIV-negative gay and bisexual men, including PrEP users, should be tested for primary HCV infection vary internationally [1, 58]. Australian guidelines recommend testing annually for hepatitis C for all gay and bisexual men using PrEP or living with HIV, regardless of the presence of drug- or sexual-related risk behavior [21]. Previous findings from Australia show that PrEP users are not homogenous in terms of STI risk [59], and this review suggests that PrEP use alone may not be a strong indicator of hepatitis C risk. Where testing constraints exist, testing guidelines should be centered on the presence of specific risk factors that remain strong indicators of hepatitis C risk and should be informed by local epidemiological contexts. However, it should be acknowledged that the efficiency of risk-based screening is dependent on clinicians being able to accurately identify risk, which may not be feasible during limited clinical interactions with competing priorities. Hepatitis C antibody testing is relatively cheap, and in countries with developed models of care it can be easily added to routine PrEP monitoring tests. In such settings, universal screening of PrEP users for primary infection is likely to be cost-effective. While there are few data on rates of hepatitis C reinfection among gay and bisexual men using PrEP, high rates of reinfection have been observed in gay and bisexual men with HIV [60], and as such, PrEP users treated for HCV should be regularly tested for reinfection, in line with WHO guidelines [1]. Hepatitis C self-testing is likely to be acceptable to key populations, including gay and bisexual men [61], and could be offered with HIV self-testing as an additional method for increasing screening during periods of risk for PrEP users [62].
We did not find any eligible studies of hepatitis C among gay and bisexual men using PrEP in Southeast Asia or Africa, 2 regions identified as having the highest pooled HCV prevalence in a previous review of studies of gay and bisexual men (5.0% and 5.8% pooled prevalence in Southeast Asia and Africa, respectively) [25]. Another recent review restricted to studies in Asia reported a pooled HCV prevalence of 5.2% among HIV-negative gay and bisexual men, with the highest prevalence detected in studies from Indonesia and Vietnam [63]. While PrEP programs, clinical trials, and demonstration projects have been implemented in 10 countries in Asia and >18 countries in Africa [64], the lack of available data on hepatitis C incidence among PrEP users may hinder appropriate responses and informed testing guidelines for hepatitis C among PrEP users in these regions.
Limitations
There are several limitations of our review that should be acknowledged. First, heterogeneity in reported sexual and drug use behaviors precluded a subgroup analysis disaggregated by prevalence of HCV-related risk factors. While some studies reported behaviors associated with hepatitis C diagnosis, many reported behaviors at baseline, which may not reflect behaviors associated with hepatitis C acquisition during periods of PrEP use. Second, not all studies reported adherence to PrEP, and we cannot be sure that all individuals included in pooled estimates were current PrEP users. Third, while we used date of DAA availability extracted from the published literature and national policy documents, it is likely that in some settings DAAs were accessible through clinical trials or special access programs. Further, some of the included PrEP studies spanned long periods of time, and we were not able to disaggregate hepatitis C incidence rates by year for studies with longer follow-up periods. This may impact the validity of our subgroup analysis by DAA availability. Finally, testing protocols in studies differed, and many studies did not report testing frequency. Studies with more frequent testing may be more likely to capture infections that may have been missed among patients lost to follow-up in studies with less frequent testing.
CONCLUSIONS
Early reports of high hepatitis C incidence among cohorts of gay and bisexual men using PrEP likely reflect specific risk-based eligibility criteria of smaller PrEP studies, which enrolled participants at a time when hepatitis C DAA treatment had not been fully scaled-up. More recent studies in settings where both DAAs and PrEP have been implemented at scale report lower hepatitis C incidence among PrEP users. PrEP-specific HCV testing guidelines should be guided by local epidemiological contexts and consider the cost-effectiveness of universal HCV screening among PrEP users at a time when HCV prevalence and incidence among PrEP users are declining. Continued surveillance of hepatitis C transmission among gay and bisexual men using PrEP alongside availability of harm reduction measures will be vital to maintain low prevalence in this population.
Supplementary Material
Acknowledgments
Patient consent. This study only included a secondary data analysis and, as a result, did not require patient consent or ethics approval.
Financial support. M.W.T. and B.L.H. were supported by postgraduate scholarships from the National Health and Medical Research Council. Burnet Institute acknowledges support from the Victorian Government Operational Infrastructure Fund. E.J.W., M.E.H., J.S.D., and M.A.S. acknowledge fellowship support from the National Health and Medical Research Council.
Contributor Information
Michael W Traeger, Burnet Institute, Melbourne, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Brendan L Harney, Burnet Institute, Melbourne, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia; Department of Infectious Diseases, The Alfred and Monash University, Melbourne, Australia.
Rachel Sacks-Davis, Burnet Institute, Melbourne, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Daniela K van Santen, Burnet Institute, Melbourne, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia; Department of Infectious Diseases, Research and Prevention, Public Health Service of Amsterdam, Amsterdam, the Netherlands; Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Vincent J Cornelisse, Department of Infectious Diseases, The Alfred and Monash University, Melbourne, Australia; Kirketon Road Centre, Sydney, Australia; The Kirby Institute, UNSW Sydney, Sydney, Australia.
Edwina J Wright, Burnet Institute, Melbourne, Australia; Department of Infectious Diseases, The Alfred and Monash University, Melbourne, Australia.
Margaret E Hellard, Burnet Institute, Melbourne, Australia; Department of Infectious Diseases, The Alfred and Monash University, Melbourne, Australia; Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, Australia.
Joseph S Doyle, Burnet Institute, Melbourne, Australia; Department of Infectious Diseases, The Alfred and Monash University, Melbourne, Australia.
Mark A Stoové, Burnet Institute, Melbourne, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. World Health Organization . Consolidated Guidelines on HIV, Viral Hepatitis and STI Prevention, Diagnosis, Treatment and Care for Key Populations. World Health Organization; 2022. [PubMed] [Google Scholar]
- 2. Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS 2015; 29:2335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan AE, Perlman DC, Neurer J, Smith DJ, Des Jarlais DC, Hagan H. Prevalence of hepatitis C virus infection among HIV+ men who have sex with men: a systematic review and meta-analysis. Int J STD AIDS 2017; 28:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newsum AM, Matser A, Schinkel J, et al. Incidence of HCV reinfection among HIV-positive MSM and its association with sexual risk behavior: a longitudinal analysis. Clin Infect Dis 2021; 73:460–7. [DOI] [PubMed] [Google Scholar]
- 5. Bartlett SR, Applegate TL, Jacka BP, et al. A latent class approach to identify multi-risk profiles associated with phylogenetic clustering of recent hepatitis C virus infection in Australia and New Zealand from 2004 to 2015. J Int AIDS Soc 2019; 22:e25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Global Health Sector Strategy on Viral Hepatitis 2016–2021. World Health Organization; 2016. [Google Scholar]
- 7. World Health Organization . Interim Guidance for Country Validation of Viral Hepatitis Elimination. World Health Organization; 2021. [Google Scholar]
- 8. Doyle JS, van Santen DK, Iser D, et al. Microelimination of hepatitis C among people with human immunodeficiency virus coinfection: declining incidence and prevalence accompanying a multicenter treatment scale-up trial. Clin Infect Dis 2021; 73:e2164–72. [DOI] [PubMed] [Google Scholar]
- 9. Garvey LJ, Cooke GS, Smith C, et al. Decline in hepatitis C virus (HCV) incidence in men who have sex with men living with human immunodeficiency virus: progress to HCV microelimination in the United Kingdom? Clin Infect Dis 2021; 72:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boerekamps A, van den Berk GE, Lauw FN, et al. Declining hepatitis C virus (HCV) incidence in Dutch human immunodeficiency virus-positive men who have sex with men after unrestricted access to HCV therapy. Clin Infect Dis 2018; 66:1360–5. [DOI] [PubMed] [Google Scholar]
- 11. Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV 2018; 5:e438–47. [DOI] [PubMed] [Google Scholar]
- 12. Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018; 67:676–86. [DOI] [PubMed] [Google Scholar]
- 14. Holt M, Lea T, Mao L, et al. Community-level changes in condom use and uptake of HIV pre-exposure prophylaxis by gay and bisexual men in Melbourne and Sydney, Australia: results of repeated behavioural surveillance in 2013–17. Lancet HIV 2018; 5:e448–56. [DOI] [PubMed] [Google Scholar]
- 15. Traeger MW, Cornelisse VJ, Asselin J, et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 2019; 321:1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradshaw D, Vasylyeva TI, Davis C, et al. Transmission of hepatitis C virus in HIV-positive and PrEP-using MSM in England. J Viral Hepat 2020; 27:721–30. [DOI] [PubMed] [Google Scholar]
- 17. Charre C, Cotte L, Kramer R, et al. Hepatitis C virus spread from HIV-positive to HIV-negative men who have sex with men. PLoS One 2018; 13:e0190340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030. World Health Organization; 2022. [Google Scholar]
- 19. The Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine. National PrEP Guidelines Update. Prevent HIV by Prescribing PrEP. The Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine; 2021. [Google Scholar]
- 20. Nederlandse Vereniging van HIV Behandelaren. HIV pre-expositie profylaxe (PrEP) richtlijn Nederland 2019. 2019. Available at: https://nvhb.nl/wp-content/uploads/2019/04/PrEP-richtlijn-Nederland-versie-2-dd-15-april-2019.pdf. Accessed August 31, 2022.
- 21. STIGMA . Sexually Transmitted Infection Testing Guidelines for Men who Have Sex With Men 2019. STIGMA; 2019. [Google Scholar]
- 22. Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC recommendations for hepatitis C screening among adults—United States, 2020. MMWR Recomm Rep 2020; 69:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macgregor L, Desai M, Martin NK, et al. Scaling up screening and treatment for elimination of hepatitis C among men who have sex with men in the era of HIV pre-exposure prophylaxis. EClinicalMedicine 2020; 19:100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoornenborg E, Achterbergh RC, van der Loeff MFS, et al. Men who have sex with men more often chose daily than event-driven use of pre-exposure prophylaxis: baseline analysis of a demonstration study in Amsterdam. J Int AIDS Soc 2018; 21:e25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin F, Dore GJ, Matthews G, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021; 6:39–56. [DOI] [PubMed] [Google Scholar]
- 26. Ong JJ, Baggaley RC, Wi TE, et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis. JAMA Netw Open 2019; 2:e1917134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harney BL, Sacks-Davis R, van Santen DK, et al. The incidence of hepatitis C among gay, bisexual, and other men who have sex with men in Australia, 2009–2019. Clin Infect Dis 2021; 74:1804–11. [DOI] [PubMed] [Google Scholar]
- 28. Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hahn GJ, Meeker WQ. Statistical Intervals: A Guide for Practitioners. John Wiley & Sons, Inc; 1991. [Google Scholar]
- 30. StataCorp . Stata: Meta-Analysis Reference Manual—Release 18. Statistical Software. StataCorp LLC; 2023. [Google Scholar]
- 31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 5, 2013.
- 33. Ramière C, Charre C, Miailhes P, et al. Patterns of hepatitis C virus transmission in human immunodeficiency virus (HIV)-infected and HIV-negative men who have sex with men. Clin Infect Dis 2019; 69:2127–35. [DOI] [PubMed] [Google Scholar]
- 34. Aloysius I, Savage A, Zdravkov J, et al. InterPrEP. Internet-based pre-exposure prophylaxis with generic tenofovir DF/emtricitabine in London: an analysis of outcomes in 641 patients. J Virus Erad 2017; 3:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amin J, Vaccher S, Templeton DJ, et al. Low prior exposure and incidence of hepatitis C in HIV negative gay and bisexual men taking PrEP: findings from the EPIC-NSW prospective implementation study. Clin Infect Dis 2022; 75:1497–502. [DOI] [PubMed] [Google Scholar]
- 36. Ayerdi Aguirrebengoa O, Vera García M, Arias Ramírez D, et al. Low use of condom and high STI incidence among men who have sex with men in PrEP programs. PLoS One 2021; 16:e0245925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cornelisse VJ, Traeger MW, Wright EJ, et al. Low incidence of hepatitis C among a cohort of HIV-negative gay and bisexual men using HIV pre-exposure prophylaxis (PrEP) in Melbourne, Australia, and the contribution of sexual transmission. J Acquir Immune Defic Syndr 2021; 87:1011–5. [DOI] [PubMed] [Google Scholar]
- 38. Cotte L, Cua E, Reynes J, et al. Hepatitis C virus incidence in HIV-infected and in preexposure prophylaxis (PrEP)-using men having sex with men. Liver Int. 2018. doi: 10.1111/liv.13922. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39. Desai M, White E, Vora N, et al. High incidence of hepatitis C virus infection observed in the PROUD study of HIV pre-exposure prophylaxis. J Viral Hepat 2020; 27:852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gras J, Mahjoub N, Charreau I, et al. Early diagnosis and risk factors of acute hepatitis C in high-risk MSM on preexposure prophylaxis. AIDS 2020; 34:47–52. [DOI] [PubMed] [Google Scholar]
- 41. Hamed A, Saling C, Brown MS, et al. Incidence of sexually transmitted infections (STIs) in patients on pre-exposure prophylaxis (PrEP). Open Forum Infect Dis 2018; 5(Suppl_1):S462. [Google Scholar]
- 42. Hassan A, Agustin HGS, Burke L, et al. Low incidence and prevalence of hepatitis C in two cohorts of HIV pre-exposure prophylaxis adherence interventions in men who have sex with men in Southern California. J Viral Hepat 2022; 29:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoornenborg E, Coyer L, Boyd A, et al. High incidence of HCV in HIV-negative men who have sex with men using pre-exposure prophylaxis. J Hepatol 2020; 72:855–64. [DOI] [PubMed] [Google Scholar]
- 44. Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS 2017; 31:1603–10. [DOI] [PubMed] [Google Scholar]
- 45. Lalley-Chareczko L, Clark D, Conyngham C, et al. Delivery of TDF/FTC for pre-exposure prophylaxis to prevent HIV-1 acquisition in young adult men who have sex with men and transgender women of color using a urine adherence assay. J Acquir Immune Defic Syndr 2018; 79:173–8. [DOI] [PubMed] [Google Scholar]
- 46. Mikati T, Jamison K, Borges C, Daskalakis D. Low prevalence of hepatitis C virus among New York City MSM initiating PrEP and PEP, 2016–2017 at NYC sexual health clinics. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI); March 4-7, 2018; Boston, MA. [Google Scholar]
- 47. Molina J-M, Ghosn J, Algarte-Genin M, et al. Incidence of HIV-infection with daily or on-demand PrEP with TDF/FTC in Paris area. Update from the ANRSPrevenir study. Paper presented at: 10th IAS Conference on HIV Science; July 21-24, 2019; Mexico. [Google Scholar]
- 48. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS 2018; 32:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS 2018; 32:2161–9. [DOI] [PubMed] [Google Scholar]
- 50. Pecavar B, Kokosar Ulcar B, Kordis M, et al. Pre-exposure prophylaxis for HIV with oral tenofovir disoproxil fumarate/emtricitabine in men who have sex with men: Slovenian National Demonstration Project. Int J STD AIDS 2021; 32:1060–5. [DOI] [PubMed] [Google Scholar]
- 51. Reyniers T, Nostlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr 2018; 79:186–94. [DOI] [PubMed] [Google Scholar]
- 52. Tabatabavakili S, Cerocchi O, Hansen BE, Bogoch DI, Feld JJ. Incidence of hepatitis C virus infections among users of HIV pre-exposure prophylaxis in a large academic centre in Toronto, Canada. Hepatology 2019; 70:182A. [Google Scholar]
- 53. Thompson KA, Blank G, Toy J, et al. Prevalence and incidence of hepatitis C infection amongst men who have sex with men in a population-based pre-exposure prophylaxis program in British Columbia, Canada. Liver Int 2022; 42:1528–35. [DOI] [PubMed] [Google Scholar]
- 54. Volk JE, Marcus JL, Phengrasamy T, Hare CB. Incident hepatitis C virus infections among users of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis 2015; 60:1728–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behaviour and STI incidence. J Int AIDS Soc 2019; 22:e25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis 2017; 65:712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang J, Li C, Xu J, et al. Discontinuation, suboptimal adherence, and reinitiation of oral HIV pre-exposure prophylaxis: a global systematic review and meta-analysis. Lancet HIV 2022; 9:e254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. World Health Organization . Guidelines on Hepatitis B and C Testing. World Health Organization;2017. [Google Scholar]
- 59. Traeger MW, Murphy D, Ryan KE, et al. Latent class analysis of sexual behaviours and attitudes to sexually transmitted infections among gay and bisexual men using PrEP. AIDS Behav 2021; 26:1808–20. [DOI] [PubMed] [Google Scholar]
- 60. Hosseini-Hooshyar S, Hajarizadeh B, Bajis S, et al. Risk of hepatitis C reinfection following successful therapy among people living with HIV: a global systematic review, meta-analysis, and meta-regression. Lancet HIV 2022; 9:e414–27. [DOI] [PubMed] [Google Scholar]
- 61. Fajardo E, Watson V, Kumwenda M, et al. Usability and acceptability of oral-based HCV self-testing among key populations: a mixed-methods evaluation in Tbilisi, Georgia. BMC Infect Dis 2022; 22:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. World Health Organization . Recommendations and Guidance on Hepatitis C Virus Self-Testing. World Health Organization;2021. [PubMed] [Google Scholar]
- 63. Zheng Y, Ying M, Zhou Y, Lin Y, Ren J, Wu J. Global burden and changing trend of hepatitis C virus infection in HIV-positive and HIV-negative MSM: a systematic review and meta-analysis. Front Med (Lausanne) 2021; 8:774793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. PrEPMAP . Status of PrEP by country. 2022. Available at: https://www.prepmap.org/prep_status_by_country. Accessed November 19, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.