Summary
Nitrogen oxides (NOx) emissions carry pernicious consequences on air quality and human health, prompting an upsurge of interest in eliminating them from the atmosphere. The electrochemical NOx reduction reaction (NOxRR) is among the promising techniques for NOx removal and potential conversion into valuable chemical feedstock with high conversion efficiency while benefiting energy conservation. However, developing efficient and stable electrocatalysts for NOxRR remains an arduous challenge. This review provides a comprehensive survey of recent advancements in NOxRR, encompassing the underlying fundamentals of the reaction mechanism and rationale behind the design of electrocatalysts using computational modeling and experimental efforts. The potential utilization of NOxRR in a Zn-NOx battery is also explored as a proof of concept for concurrent NOx abatement, NH3 synthesis, and decarbonizing energy generation. Despite significant strides in this domain, several hurdles still need to be resolved in developing efficient and long-lasting electrocatalysts for NOx reduction. These possible means are necessary to augment the catalytic activity and electrocatalyst selectivity and surmount the challenges of catalyst deactivation and corrosion. Furthermore, sustained research and development of NOxRR could offer a promising solution to the urgent issue of NOx pollution, culminating in a cleaner and healthier environment.
Subject areas: Catalysis, Electrochemical energy storage, Electrochemical energy conversion, Energy sustainability
Graphical abstract
Catalysis; Electrochemical energy storage; Electrochemical energy conversion; Energy sustainability
Introduction
Air pollution remains a grave concern that plagues the world, as millions of individuals succumb to premature deaths each year due to inhalation of toxic air.1 The World Health Organization (WHO) has reported that an estimated 7 million people meet their untimely demise yearly due to air pollution, with over 90% of these fatalities transpiring in low- and middle-income countries.2,3 While the sources of air pollution vary, they predominantly stem from industrial activities, transportation, power generation, and residential heating and cooking.2 Particulate matter (PM), nitrogen oxides (NOx), sulfur oxides (SOx), and volatile organic compounds (VOCs) rank among the most noxious air pollutants, imperiling human health and the environment.4,5
NOx is one of the most significant contributors to air pollution, and it has been linked to respiratory and cardiovascular diseases, lung cancer, and stroke.4,5,6 The impact of air pollution particularly concerns vulnerable groups such as children, elderly individuals, and those with underlying health conditions.7 Moreover, it has significant environmental consequences, as likely as acid rain, ozone depletion, and climate change. In the US, transportation is responsible for the largest share of NOx emissions, contributing approximately 36% of the total emissions in 2017.8 Meanwhile, power generation and industrial activities are responsible for 23% and 17% of total NOx emissions, respectively.9,10 Globally, the International Energy Agency (IEA) estimated that in 2020, NOx emissions from the power sector and transportation reached 14.6 million and 13.7 million metric tons, respectively. As such, developing effective strategies to reduce NOx emissions from these sources is crucial. Governments and various institutions across the globe are making concerted efforts to curb NOx emissions by enforcing more stringent regulations on emissions from automobiles and power plants while encouraging the adoption of cleaner energy technologies.10,11,12 Nevertheless, NOx emissions continue to substantially threaten air quality and the health of living beings, calling for more intensive efforts to mitigate their impact on human health and the environment.9,13 Researchers have explored various methods and technologies to obviate the deleterious effects of NOx pollution and abate NOx emissions. Among them, selective catalytic reduction (SCR) is the most widely used in stationary applications, selective noncatalytic reduction (SNCR) is applied in specific situations, and other technologies are still being explored (e.g., electrochemical NOx reduction).
NH3 is a highly sought-after chemical compound for its role as a crucial feedstock in the production of fertilizers.14 This is due to its nitrogen content, which is essential for the growth of plants. As a matter of fact, it is the most widely utilized chemical worldwide for fertilizer production.14,15 The current global demand for NH3 as a chemical feedstock exceeds 150 million tons per year, and this demand is expected to grow in the coming years in response to the increasing need for food and enhanced crop yields.16 In addition to global agriculture for nitrogen-based fertilizer use, a solid incentive to deploy NH3 for chemical and energy industries is expected to grow at different scales. Due to its relatively high H2 content, NH3 can be directly combusted as likely end-use H2 or used as a feedstock in manufacturing. Given the anticipated future demand, alternative NH3 synthesis is an emerging area to pursue. At present, the Haber-Bosch process, first developed in the early 20th century, is the predominant method used to produce NH3. This process entails reacting highly purified N2 gas with H2 gas obtained from natural gas or other hydrocarbons in the presence of a catalyst under high pressure (10–30 MPa) and temperature (400–500°C).17,18,19 The resultant product, NH3, is subsequently collected and purified. However, this process is not without its flaws, such as its considerable energy consumption and emission of greenhouse gases like CO2 during production to perform the reaction properly; the best available techniques consume energy of about 27.4–31.8 GJ tNH3-.20 The development of more sustainable methods for NH3 production is, therefore, crucial to ensure a reliable and sustainable supply of this essential chemical feedstock.
The electrochemical reduction of NOx, hereafter known as NOxRR, presents an innovative and environmentally friendly opportunity for the elimination of NOx pollutants and sustainable NH3 production.21 This technology can be performed at ambient pressure and room temperature, making it an ideal approach for scaling up and industrial applications.22 The lower bond energy and polar nature of NO compared to N2 (N=O 607 kJ mol−1, N≡N 941 kJ mol−1 at 25°C) results in lower activation energy and more energy-efficient electrolysis than its nitrogen reduction reaction (NRR) counterpart.23,24,25 Moreover, the technology has demonstrated high selectivity toward NH3 production and provides precise control over reaction conditions, allowing for a finely tuned NH3 production rate. It can also be powered using renewable sources of electricity, such as photovoltaic (PV) technology, which enhances an additional layer of environmental friendliness, making it a reliable and sustainable solution for mitigating NOx emissions and reducing the overall carbon footprint of the process.21 The integration of these technologies also has immense potential for reducing NOx emissions while promoting sustainable development. The deployment of such systems can also contribute to overall grid stability and reduce reliance on traditional energy sources, thereby enabling the use of renewable energy.
The conversion of NOx through electrochemical reduction is a complicated process that includes several reactions and intermediate steps involving the transfer of both protons and electrons, leading to the formation of stable end products.26 However, this process is often fraught with challenges as it tends to produce residual nitrogen-containing compounds such as NO2, N2, N2O, and N2H4, which not only impede the overall efficiency of the reaction but also contribute to environmental pollution.23,27,28,29,30,31 The slow reaction kinetics resulting from gaseous NO’s low solubility in aqueous electrolytes and the concurrent hydrogen evolution reaction (HER) pose fundamental obstacles to NOxRR.29,32 Regrettably, electrocatalytic NOxRR techniques suffer from relatively low NH3 yield and selectivity. Moreover, the design of high-performing and durable electrocatalysts for NOxRR still presents significant challenges. Catalyst deactivation, corrosion, and electrode fouling are other issues that can reduce the efficiency and lifespan of electrocatalysts, especially in corrosive environments and highly acidic electrolytes.31,33 Despite the intricate kinetics and complexity involved in the electrochemical reduction of NOx, recent years have witnessed significant strides in the development of efficient and cost-effective electrochemical NOxRR systems. Numerous electrocatalytic materials, including single-atom catalysts (SACs), metal oxides, metal sulfides, and metal phosphides, have been computationally and experimentally explored for their ability to catalyze this reaction.23,24,32,34,35 Given the rapid acceleration of research progress in the field of NOxRR, a comprehensive review that provides a panoramic overview of the current state of research and directs future efforts is imperative yet nonexistent to our knowledge. Thus, this review aims to present an up-to-date and comprehensive discussion, spanning from elucidating the underlying mechanisms of the NOxRR to conducting a thorough theoretical screening through computational simulations. We also summarize the cutting-edge developments in various electrocatalysts and gas diffusion layers (GDE) for NOxRR for the scale-up study. More importantly, we highlight the proof-of-concept of the Zn-NOx battery using NOxRR electrocatalysts as the cathode materials. It is more attractive to develop the Zn-NOx battery as a promising “3-in-1” strategy: reduce NOx emission, produce NH3, and generate electrical energy. Finally, we examine the current challenges and provide a perspective on future efforts to optimize electrochemical NOxRR. We anticipate that this review will inspire significant research and essential advancements in the field of electrocatalysts for emerging NOxRR.
Existing technologies for NOx mitigation
Selective catalytic reduction
Selective catalytic reduction (SCR) is the most widespread state-of-the-art technology for NOx mitigation and is deemed the most efficacious technology that harnesses a catalyst to transmute NOx into nitrogen (N2) and water vapor (H2O) at an appropriate temperature range of 200°C–400°C, resulting in a NOx emissions decline of up to 90%.36,37,38 A reductant, commonly anhydrous ammonia (NH3), aqueous ammonia (NH4OH), or a urea (CO(NH2)2) solution, is infused into a stream of flue or exhaust gas and is reacted upon a catalyst.39,40,41,42 As the reaction progresses, N2 and carbon dioxide (CO2) are generated, if urea is utilized. SCR systems necessitate a dependable source of NH3 or other reducing agents, which might entail supplementary expenses for conveyance and storage, causing energy consumption.37,42 Furthermore, the implementation of SCR catalysts is frequently hindered by the inclusion of precious metals, such as Pt, Pd, Ir, or Ru, which restrains its usage in industrial scale applications.38 An unsatisfactory design or maintenance of SCR systems may cause unreacted NH3 to be vented into the atmosphere, posing a significant hazard to public health and the environment.43 The catalyst employed in SCR systems may be contaminated over time due to contact with pollutants, such as sulfur, reducing its potency. Therefore, although SCR technology is remarkably effective in mitigating NOx emissions, it is crucial to assess its advantages versus the costs and potential shortcomings.
Selective non-catalytic reduction
Flue gases are often generated in the industrial processes with noticeable particles and reactive volatiles, which lead to the deactivation of a bulky unit of catalyst beds over time, raising overall operating and capital costs.44 With the long-life expectancies of industrial plants, technology to mitigate their pollution ought to be retrofitted within a lifetime.45 When the catalysts are found non-viable for NOx mitigation, the SNCR process is one of the relevant options.46 In the SNCR, NOx is reduced to N2 with NH3 or, alternatively, CO(NH2)2, ammonia salts, or cyanuric acid at high temperatures ranging from 900°C to 1000°C.46 Unlike SCR, the SNCR takes place without the need for a catalyst. The reported SNCR efficiencies are 30–70% depending upon several factors, including temperature, NOx level, reagent-flue gas mixing, NH3-NOx ratio, and reaction time, but efficiencies as high as 80% are also achievable.47 SNCR has fewer capital costs than SCR as the simplicity of the process only requires reagent storage and injection equipment, there is no need for a catalytic bed, and it is easier to retrofit, which is likely suited to be adopted by major industries in developing countries.47 Operating costs, however, are comparable to the SCR. Some issues correspond with the unreacted NH3 (otherwise known as NH3 slips), formation of NH4HSO4, and N2O.47 The NH3 slip and N2O formation can be resolved with careful control of operating parameters. The NH4HSO4 may corrode and plug equipment.47 Despite the prevalence of the SNCR process thus far, some general disadvantages make the technology less appealing in meeting the context of stringent emission standards and, therefore, developing alternatives to mitigate NOx emissions is of great interest.
Fundamentals of electrocatalytic NOx reduction reaction mechanism in aqueous electrolytes
The previously reported NOx mitigation technologies have been increasingly under pressure because of various specific challenges. Alternative technologies have been explored in an attempt to substitute or improve upon current traditional mitigation facilities. The NOxRR is amongst prospective means that challenge the status quo. Although NOxRR is still in its infant development, the prospects of this concept are promising owing to the fuel cell development for energy generation that has generally accelerated and the reported results on NOxRR that compare favorably with solid oxide fuel cells.48
NOx capture
To date, research on electrochemical NOx reduction is almost exclusively performed on the benchtop scale.49 The control and reduction of NOx emissions usually implement particular emission control technologies and practices involving the emission treatments at the sources (e.g., industrial stacks or exhaust systems); amongst the essential considerations is therefore to capture NOx from its source prior to NOxRR operation in aqueous electrolytes. Adopted from the production of N2-containing compounds from the air (so-called N2 fixation), there are pioneering developed industrial methods reported as thermal and non-thermal plasma. A published work used thermal plasma arc discharge with a temperature >3000°C to activate air, followed by quenching with water to synthesize nitric acid. The major disadvantage of this process for N2 fixation is definitely its higher energy requirements.49,50 Non-thermal plasma is rather more advantageous to generate high energy electrons with temperatures several orders of magnitudes more elevated than the surrounding gas due to an ionized gas composed of a range of species (including electrons, ions, radicals, molecular fragments) at various energy levels.54 This process is applicable for several applications ranging from rapid synthesis to surface modification of electrode materials.51,52,53 It is likely to create high-energy species that can activate atmospheric dinitrogen molecules while keeping the reaction temperature and energy consumption for NOx generation lower than the thermal plasmas in small-scale reactors, which are even much lower than the Haber-Bosch process.54 According to this ground, the non-thermal plasma with much-improved rate and energy efficiency is preferable for the facile NOx capture in the scalable NOxRR as it was reported to be potential for NOx fixation into ammonium at very low cell potentials whilst obtaining considerable ammonium yields and rate.54
As collective efforts to mitigate NOx pollution, NOx capture technologies are in place as an emerging area to explore NOx to NH3. As the relevant studies are undergoing, novel findings that offer long-term technological and environmental benefits are open areas to fill and very much looked forward to. Being typical early stage technologies, NOxRR may face immaturity and cost-inefficiency at its first development, but technological advancements, growing markets, and accumulated experience will undoubtedly aid its further development.
Electrocatalytic NOx reduction reaction mechanism in aqueous electrolytes
Exploring the fundamental mechanism of electrochemical NOx reduction is an indispensable step toward developing efficient electrocatalysts and achieving high conversion yields. However, the NOxRR faces significant challenges due to the involvement of multiple intermediate species, making it difficult to predict the reaction pathway and the stability of the products. NOx comprises a mixture of N2O, NO, NO2, N2O3, N2O4, and N2O5, with NO and NO2 accounting for 90% and 5%, respectively. While most NOx reacts easily with liquid water, NO and NO2 exhibit low solubility in water. Consequently, the electrochemical reduction process of NOx becomes exceedingly intricate, involving multiple proton-electron coupling reactions and the formation of stable products and intermediates. The focus is placed on the electroreduction of NO and NO2 species to simplify the reaction. NO2, being water-soluble, is readily transformed into nitrites (NO2−) and nitrates (NO3−) under reduction conditions, as indicated in Equations 1 and 2, respectively23,27,28,29,30:
| NO2 + H2O → NO3- + 2H+ + e- E = −0.78 V vs. RHE | (Equation 1) |
| NO2 + H+ + e-→ NO2- + H+ E = 1.07 V vs. RHE | (Equation 2) |
Upon further reduction of NO3−, a plethora of intermediates can be generated, including but not limited to NH3, NO2−, hydroxylamine (NH2OH), NO, and N2O. The specific intermediates formed are contingent on the potential observed during electrochemical reduction, highlighting the complexity of the reaction process.
| 2NO3- + 12H+ + 10e-→ N2 + 6H2O E = 1.25 V vs. RHE | (Equation 3) |
| 2NO3- + 10H+ + 8e-→ N2O + 5H2O E = 1.12 V vs. RHE | (Equation 4) |
| NO3- + 4H+ + 3e-→ NO + 2H2O E = 0.96 V vs. RHE | (Equation 5) |
| NO3- + 2H+ + 2e-→ NO2- + H2O E = 0.94 V vs. RHE | (Equation 6) |
| NO3- + 9H+ + 8e-→ NH3 + 3H2O E = 0.88 V vs. RHE | (Equation 7) |
| NO3- + 7H+ + 6e-→ NH2OH + 2H2O E = 0.73 V vs. RHE | (Equation 8) |
Similarly, NO2− reduction will proceed through the following reactions:
| 2NO2- + 8H+ + 6e-→ N2 + 4H2O E = 1.45 V vs. RHE | (Equation 9) |
| 2NO2- + 6H+ + 4e-→ N2O + 3H2O E = 1.29 V vs. RHE | (Equation 10) |
| NO2- + 2H+ + e-→ NO + H2O E = 1.00 V vs. RHE | (Equation 11) |
| NO2- + 7H+ + 6e-→ NH3 + 2H2O E = 0.86 V vs. RHE | (Equation 12) |
| NO2- + 5H+ + 4e-→ NH2OH + H2O E = 0.62 V vs. RHE | (Equation 13) |
Amidst the plethora of electrochemical studies, several have demonstrated that NO reduction, under varying reaction conditions, tends to traverse four distinct routes:
| 2NO + 4H+ + 4e-→ N2 + 2H2O E = 1.68 V vs. RHE | (Equation 14) |
| 2NO + 2H+ + 2e-→ N2O + H2O E = 1.59 V vs. RHE | (Equation 15) |
| NO + 5H+ + 5e-→ NH3 + H2O E = 0.71 V vs. RHE | (Equation 16) |
| 2NO + 3H+ + 3e-→ NH2OH E = 0.38 V vs. RHE | (Equation 17) |
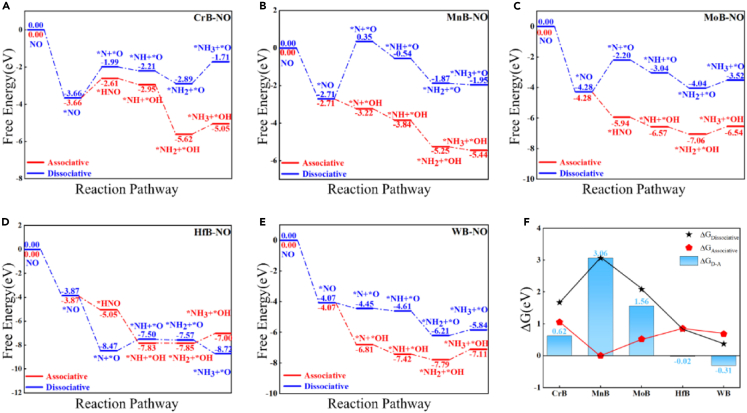
The associative and dissociative mechanisms of NRR typically describe the formal reaction pathways of electrochemical NOxRR. He et al.54 utilized density functional theory (DFT) calculations with 2D transition metal borides (MBenes), such as CrB, MnB, MoB, HfB, and WB, as model catalysts to elucidate the associative and dissociative mechanisms of NOxRR. The hydrogenation of adsorbed NO molecules on the surface of MBenes can generally follow two different pathways: associative and dissociative. In the dissociative pathway, N-O bond activation and breaking occur immediately after the adsorption process, inducing the formation of isolated N and O atoms on the MBenes surface, which will be subsequently converted into NH3 and H2O upon the addition of H+ + e- as depicted in Figure 1. When producing NH3 via the associative pathway, the N-O bond is fully activated but not broken when a single NO molecule is adsorbed on the catalyst surface. The subsequent hydrogenation process of the NO molecule involves a selective transfer of hydrogen and electrons into either the N or O atom, producing different reaction intermediates. Hence, it is essential to calculate the adsorption barrier and determine the binding site of the H proton to confirm the intermediates, such as ∗NOH or ∗NHO, since the proton binding site in each step will govern the reaction pathways.54
Figure 1.
The associative and dissociative mechanisms of NOxRR
Free-energy diagrams of NH3 synthesis via associative and dissociative pathways after NO adsorption on (A) CrB, (B) MnB, (C) MoB, (D) HfB, and (E) WB.
(F) The energy limit of several substrates in the hydrogenation of NO molecules to NH3 via dissociative and associative mechanisms. The ΔGD-A in the figure shows the free energy difference of the rate-limiting steps associated with dissociative and associative mechanisms. Reprinted from.54 Copyright Elsevier, 2022.
Catalyst designs for electrochemical NOx reduction reaction
The design of efficient and durable electrocatalysts for NOx reduction involves two distinct approaches: computational screening and experimental testing. Computational screening, also known as rational design, involves computer simulations to predict the properties of potential catalysts and select the most promising candidates for experimental validation. This approach is based on the principle that a catalyst’s electronic and geometric properties determine its activity and selectivity in a reaction and, by manipulating these properties, one can design more efficient and selective electrocatalysts for NOx reduction.
Experimental testing, on the other hand, includes synthesizing and testing various electrocatalysts using a range of characterization and performance evaluation techniques to determine their effectiveness in NOx reduction. This approach can provide critical insights into the real-world performance of catalysts, such as their stability, durability, and ability to operate under varying conditions. Recently, some groups of researchers have focused on high-throughput screening (HTS) techniques to discover the most promising electrocatalysts at unprecedented times. These techniques involve using automated equipment to rapidly test a large number of samples in parallel, allowing for the efficient evaluation of potential catalysts.
Both computational screening and experimental testing play an essential role in developing efficient and durable electrocatalysts for NOx reduction. For instance, they can design an effective catalyst by searching the appropriate active site, the region on the catalyst surface where the reaction occurs. The active site must have the appropriate chemical properties to facilitate the desired reaction, and the size and shape of the active site can also influence the catalyst’s performance. In addition to the active site, the overall structure, including the presence of defects or impurities on the catalyst’s surface, can also contribute to its performance, such as reaction rate and catalyst yield. By combining these approaches, researchers can design and test a wide range of potential catalysts quickly and cost-effectively, discovering novel and effective electrocatalysts that can help address the urgent problem of NOx pollution.
Computational design of electrocatalytic NOx reduction catalyst
Efficient catalyst design is crucial for the practical development of electrochemical NOx reduction. In the past, countless catalysts were specifically designed with improved performances. Still, computational design has become an important tool for accelerating the discovery of new catalysts, understanding the fundamental mechanisms, and identifying active sites on catalyst surfaces. With the advances in computational abilities, researchers can now design and screen potential catalysts using HTS, reducing the time and cost of traditional trial-and-error experiments.
In the emerging field of NOxRR, researchers are now mostly screening single- and biatom catalysts along with supporting 2D materials using a range of powerful tools, including DFT, molecular dynamics (MD), and HTS methods, as shown in Figure 2. These simulation tools allow researchers to predict the behavior of materials at the atomic scale and molecular level, and to explore their catalytic properties. The outputs of these simulations include highly optimized catalysts with improved activity and selectivity, identification of limiting potential, exploration of reaction pathways (such as distal or alternating), and active sites on the catalyst surface that facilitate catalytic reactions. This information is invaluable in designing and developing new catalysts as it provides insights into the underlying mechanisms of catalytic reactions, ultimately leading to the development of more efficient and sustainable chemical processes. With the continuous advancement of computational simulation techniques, the possibilities for developing new and highly efficient catalytic materials are limitless.
Figure 2.
Workflows of NOx electroreduction catalysts screening via computational simulations
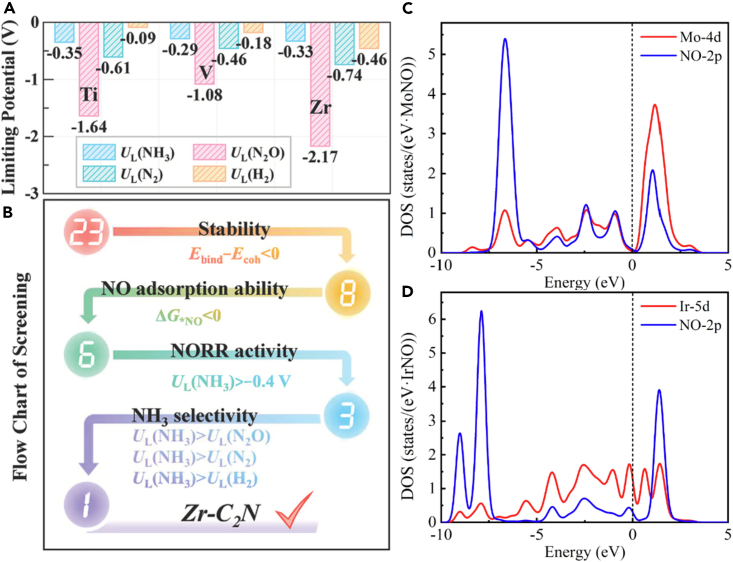
The primary obstacle in NOxRR is to fine-tune the product selectivity of the catalysts toward NH3. To overcome this issue, a methodical screening approach for effective SACs has been suggested utilizing first-principles calculations. SACs have garnered significant attention due to their high metal utilization efficiency and exceptional catalytic activity. Through a study involving 23 transition metals (ranging from Ti to Au) embedded in monolayer N-doped graphene (C2N), it was discovered that six TM-C2N could efficiently adsorb and activate the NO molecule due to the “donation/back donation” mechanism between the TM atom and NO.55 When considering the NOxRR activity toward NH3, Ti-, V-, and Zr-C2N display elevated activity levels with less negative limiting potentials of −0.35, −0.29, and −0.33 V, respectively, as shown in Figure 3A. Ultimately, Zr-C2N is identified as a promising NOxRR catalyst due to its ability to suppress the formation of byproducts such as N2O, N2, and H2 (Figure 3B). The use of the same supporting materials revealed that V2/Cr2/Mn2/Mo2-C2N exhibited the most significant potential in catalyzing NO, with remarkable stability, activity, and selectivity. Moreover, the results showed that the NH3 synthesis through NOxRR had a low kinetic barrier when utilizing V2/Cr2/Mn2/Mo2-C2N.56 Upon analysis, it was found that a substantial charge transfer takes place between NO and TM2-C2N, thus providing additional evidence for the “back donation/donation” mechanism of NO adsorption on TM2-C2N. The process involves a series of five hydrogenation steps, in which NO + 5H+ + 5e-→ NH3 + H2O. Moreover, three potential pathways for the direct conversion of NO-to-NH3 exist: N-end, O-end, and side-on pathways. The adsorption patterns of NO molecules determine which pathway is utilized. As such, this reveals that the method by which NO molecules are adsorbed can greatly impact the conversion efficiency of NO-to-NH3.
Figure 3.
Several promising NOxRR electrocatalysts
(A) Limiting potentials of Ti-, V-, and Zr-C2N, (B) Zr-C2N as promising NOxRR electrocatalysts, adapted from55 with permission, Copyright John Wiley & Sons, Inc. 2021., and (C and D) orbital hybridization between Mo-4d or Ir-5d and N-2p. Reproduced with permission from,58 Copyright Elsevier 2022.
The substitution of supporting materials with B-defected boron phosphide (BP) monolayers led to the discovery of Ti@BP, V@BP, Cu@BP, and Au@BP as the most favorable catalysts.57 In the event of nitrogen doping into BP monolayers, Mo- and Ir-anchored N-doped BP were identified as promising electrocatalysts for NOxRR, showcasing limiting potentials of −0.10 V and −0.06 V, respectively.58 These catalysts exhibited significantly greater or at least similar rate constants compared to the exceptional Pt(111) surface. According to electronic analysis, the Mo-4d or Ir-5d orbitals could effectively hybridize with the NO-2p orbitals, providing adequate activation of the adsorbed NO species (Figures 3C and 3D). On the surface of Pt(100), Ti, Cr, Co, and Ni SACs demonstrated remarkable NOxRR catalytic activity.59 Specifically, the Ni-Pt combination displays exceptional activity and selectivity for NOxRR in NH3 synthesis. Furthermore, the Ti/Co/Ni-Pt combination exhibits higher selectivity for NOxRR than the HER. Of the 27 evaluated TM@MoS2 catalysts (from 3days to 5days period), 19 were found to be both thermodynamically stable and practically feasible in the context of the defective MoS2 monolayer. Of these catalysts, 13 showed high NOxRR activity toward NH3, and six favored the production of NH2OH. It is noteworthy that the TM@MoS2 catalysts demonstrated efficacy in suppressing HER and byproducts (N2O/N2). Five of the evaluated catalysts (TM = Ni, V, Cr, Nb, Ti) displayed exceptionally low limiting potentials (UL = −0.18 to 0 V) in producing NH3, while two (TM = Ag and Pt) generated NH2OH with a UL of 0 V.60 Co- and Rh-HAB (hexaaminobenzene) MOFs were investigated as potential electrocatalysts for NOxRR by Huang et al.61 Computational simulations showed that both MOFs had moderate NO adsorption strength to the substrate, making them promising candidates for NOxRR catalytic activity. Furthermore, the calculations demonstrated that the Co-HAB MOF was the most effective electrocatalyst for NOxRR, capable of generating NH3 at a low NO coverage with a limiting potential of −0.26 V.
The design of bilayer single-atom catalysts (BSACs) with adjustable surface chemistry has been suggested as a universal approach to heterogeneous catalysis.62 Heterogeneous BSACs have exhibited a propensity for augmenting the capture and activation of NOx, which is critical for electrochemical NH3 synthesis. The inversion symmetry breaking inherent in these BSACs engenders a built-in electric field around the active sites that can be tuned to optimize the catalytic performance for NOxRR, including encompassing NOx adsorption and activation, limiting potential, selectivity, and reaction pathway. After subjecting a range of homonuclear M2-CmNn-GN BSACs and their heterogeneous counterparts to high-throughput calculations, three systems, specifically TiV-N4, NbV-N4, and GaV-N4-GN, were determined to be effective in suppressing competitive HER while exhibiting limiting potentials of −0.32, −0.20, and −0.25 V, respectively.
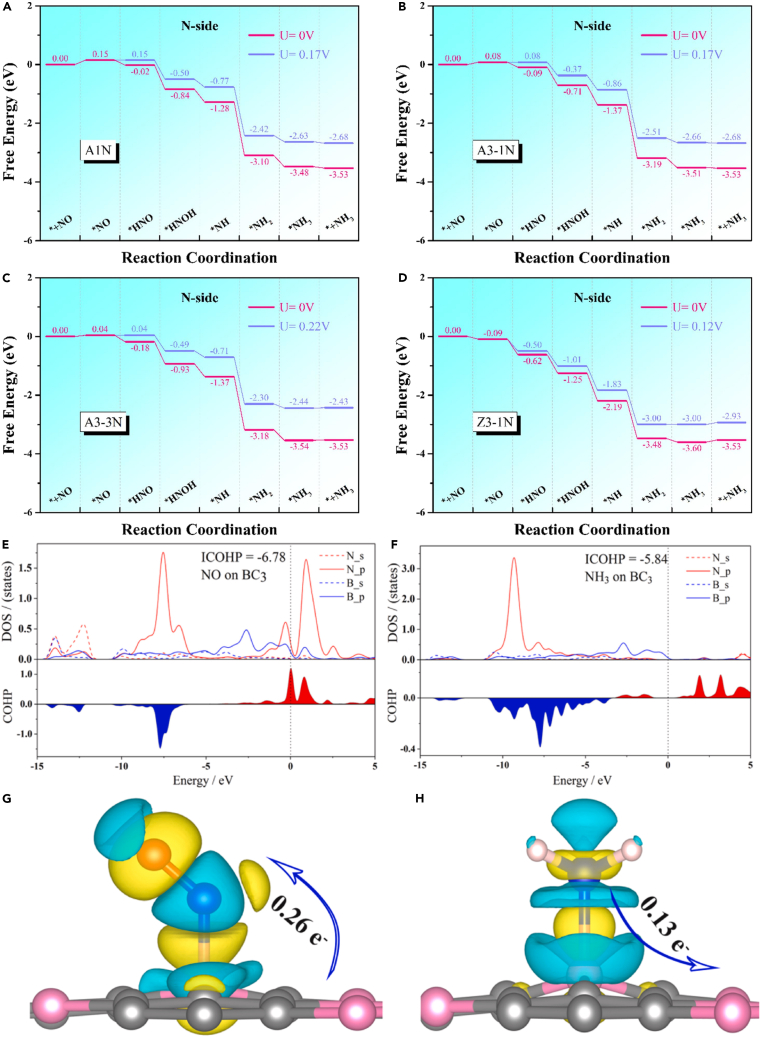
2D materials can serve as a supporting platform and a standalone electrocatalyst for activating and hydrogenating NOx molecules. To this end, researchers proposed a metal-free NOxRR catalyst design using hexagonal boron nitride-graphene (hBN-graphene) heterostructures with a Ccenter-CN2 configuration.63 The modified hBN-graphene interface resulted in impressive NOxRR activity, achieving a low limiting potential of −0.22 V. In addition, the hBN-graphene heterostructures effectively inhibited HER and showed considerable thermal stability according to ab-initio MD simulations. By assessing the catalytic activity of NOxRR through two general reaction pathways, the N-side pathway emerges as the preferred route over the O-side pathway. In the O-side pathway, the rate-determining steps involve the formation of H2O and the first hydrogenation of NO, while the initial hydrogenation of NO and subsequent hydrogenation steps are the pivotal steps in the N-side pathway. The first hydrogenation step demonstrates a better thermodynamic preference for the N atom, underscoring the N-side pathway’s preference for NOxRR on the modified hBN-graphene heterostructures. The armchair structures exhibit exceptional NOxRR catalytic activity, reaching a sufficiently low limiting potential of about −0.2 V. However, despite the low energy barrier of the first hydrogenation step in structure Z3-1N, it cannot catalyze the step ∗NH2 → ∗NH3 efficiently.63 Figures 4A–4D displays the overall NOxRR of hBN-graphene.
Figure 4.
NOxRR activity of using NO on hBN-graphene and BC3
(A–D) Gibbs free energy diagrams representing the NOxRR of four structures (A1N, A3-1N, A3-3N, and Z3-1N) are displayed using gaseous NO as the reference potential, with the preferred pathway illustrated under zero (pink) and limiting potential (blue) conditions.
(E and F) Representation of the PDOS of NO and NH3 adsorbed on the BC3 surface, along with ICOHP analysis of the relevant regions. The bonded states are highlighted in blue, while the anti-bonded states are represented in red.
(G and H) Graphical depictions of the charge difference density, with areas of electron accumulation in yellow and electron loss in blue. The iso-surface value is 0.005 e−/Bohr.3 Reproduced with permission from63 and.64 Copyright Elsevier 2022.
Recently, a study highlighted the exceptional catalytic activity of BC3 2D materials, producing an astonishingly low limiting potential of −0.29/-0.11 V.64 The three reaction pathways, including NO(g) + 5H+ + 5e-→ NH3 (g) + H2O, were observed, and the mechanism behind NO activation and NH3 adsorption/desorption was explained by examining the density of states and ICOHP of adsorbed NO and NH3 on the BC3 surface. Figure 4E illustrates that the NO intermediate gets attached to the B site, where the p orbital electrons of N and B atoms have significant involvement. The opposing bonding state suggests that the N-B bond formation is hindered due to the strong interaction between N and B. The electron cloud of the B atom interacts with NO, leading to the formation of the N-B bond. Figure 4G demonstrates an increment in the electron density, leading to better interaction between NO and BC3. A single N atom active site is produced when the proton-electron pairs attack the O atom in NO. Figure 4F reveals that the s and p orbitals of the B atom interact with the p orbital of the N atom, responsible for the adsorption of NH3 on the BC3 surface. Figure 4H depicts a reduction in the electron density of the N atom, while an increase in the electron density is observed in the area around the B atom. By conducting a Bader charge analysis, it was discovered that the BC3 surface undergoes an electron transfer of 0.13 e-.64
Another 2D material, so-called MXenes, also showed a promising NOxRR electrocatalytic activity with a limiting potential of −1.09 V (bare Ti2C) to −0.08 V (Ti2CS2) and −0.14 V (Ti2CO2).65 Although no experimental effort has been attempted for these emerging 2D materials, MXenes have shown electrochemical reduction activity and stability for CO2RR.66 In conclusion, the advancements in computational simulation and theoretical calculations have led to the discovery of a wide range of promising electrocatalysts. These findings shed light on the underlying mechanisms of catalysis and offer a practical and efficient means of designing new and improved materials for energy conversion and storage applications.
Catalyst design by experimentation
The performance of electrocatalysts to convert NOx molecules into NH3 strongly depends on their composition and structure. While the intrinsic composition of electrocatalysts will determine the adsorption behavior of NOx molecules and thus affect reaction pathways, the structures of catalysts are intended to boost efficiency and product selectivity. In this section, we discuss recent advances in the development of electrocatalysts for NOx electrochemical reduction, emphasizing catalyst compositions and various engineering strategies.
Single-atom catalysts (SACs)
SACs are an emerging group of catalysts composed of individual metal atoms attached to a supporting material. Due to their near-complete atomic utilization and excellent catalytic performance, SACs have become increasingly popular.67,68 In 2011, Zhang et al. conducted the first research on SACs, where single Pt atoms were synthesized and anchored on iron oxide. They observed that SACs exhibited exceptional stability and catalytic activity, particularly in the oxidation of CO.69 Since then, a series of SACs have been reported, which include noble and non-noble metals. SACs have additional advantages over conventional nanoparticle-based catalysts due to their sizes, structural effects, and strong metal-support interactions. The coordination environment of the metal atoms in SACs also plays a critical role in determining their catalytic properties.70 Recent research has focused on the promising applications of SACs in the NOxRR.
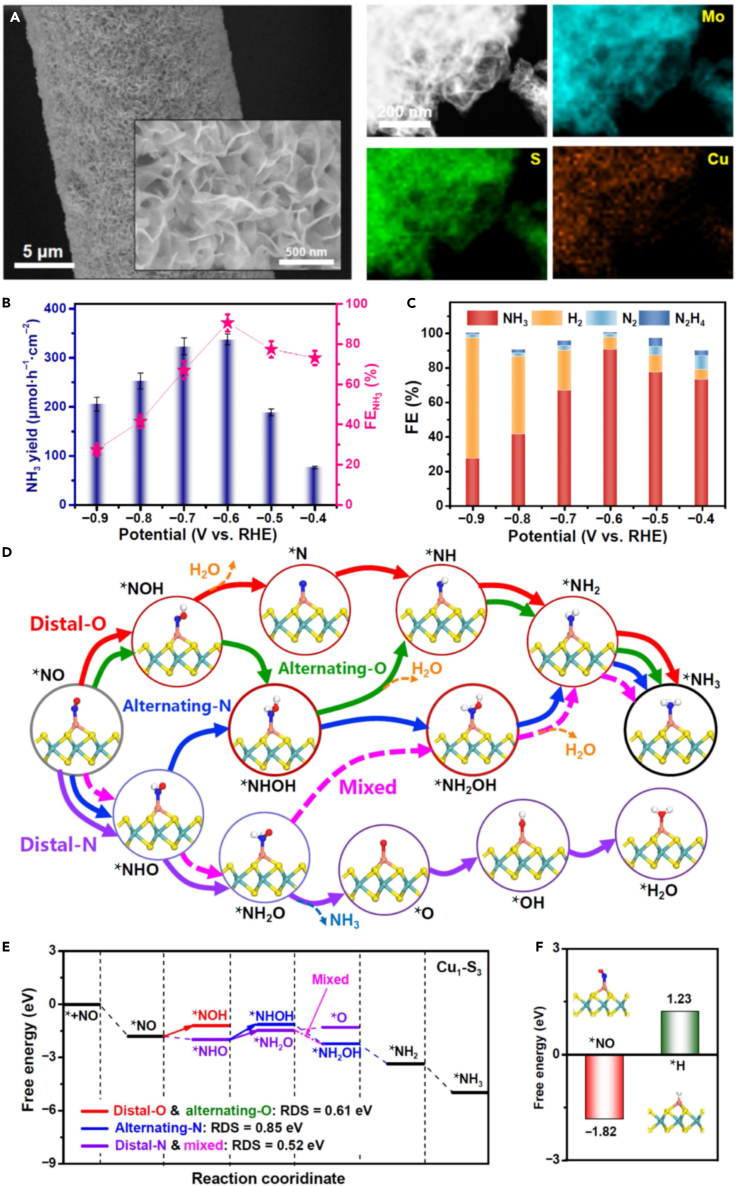
Researchers have synthesized a group of electrocatalysts by combining individual metal atoms (Al, Mn, Fe, Cu, and Nb) with B,N co-doped carbon nanotubes.71 These catalysts have shown remarkable efficiency for ambient electrosynthesis of NH3, with the single-atomic-site Nb catalyst generating an NH3 yield rate of 8.2 × 10−8 mol cm−2 s−1. This exceeds the efficiency of the best-known NRR catalysts by a hundred times and nearly meets the US Department of Energy (DOE) target. The superior performance of the Nb catalyst has been attributed to the presence of single Nb-B2N2 sites in it. Theoretical computations have suggested that these sites enable NO adsorption and reduce the energy barrier of the potential-determining step. A remarkable record in electrochemical NH3 synthesis rate was established using commercially available Cu electrodes, with 517.1 μmol h−1 cm−2 achieved at −0.9 V vs. RHE, accompanied by an FE (Faradaic efficiency) of 93.5%.26 Furthermore, single-atomic Cu anchored on MoS2 nanosheets (SEM and EDS mapping) is shown in Figure 5A. At −0.6 V vs. RHE, this catalyst exhibited outstanding performance, achieving an NH3 yield rate of 337.5 μmol h−1 cm−2 and an FE of 90.6%, surpassing nearly all previously reported NOxRR catalysts. (Figures 5B and 5C).72 By utilizing a combination of DFT, MD simulations, and ab initio MD (AIMD) simulations, it was discovered that the activation and hydrogenation of NO were achieved through a mixed pathway (distal and alternating) with Cu-anchored MoS2 catalysts. This simultaneous retardation of proton coverage led to high activity and selectivity for the NOxRR at the atomic level. (Figures 5D–5F). The NOxRR process has been successfully achieved using Cu/porous–TiO2 binary catalysts.73 The utilization of porous TiO2 with ultrafine Cu nanoparticles on its surface can provide greater surface areas and channels for superior NO adsorption capacity while suppressing the agglomeration of Cu nanoparticles. The electrocatalytic performance of the Cu/P–TiO2 electrode was remarkable, resulting in an NH3 yield of 207.1 μmol h−1 mg−1 and an FE of 86.5% at −0.3 V vs. RHE in 0.1 M K2SO4.73
Figure 5.
Single-atomic Cu anchored on MoS2 nanosheets
(A) SEM images and the corresponding EDS mapping of Cu/MoS2.
(B) NH3 yields and FE of Cu1/MoS2 at various potentials.
(C) FEs of different products after bulk electrolysis for an hour.
(D) Schematic of different NOxRR pathways.
(E) Free energy diagrams of NOxRR pathways on Cu1/MoS2.
(F) Binding free energies of ∗NO and ∗H on Cu1-S3. Adapted from72, Copyright Springer Nature 2022.
Other promising SACs are bismuth (Bi) and gold (Au).74 Bi possessed high adsorption energy for hydrogen; thus, the competing HER can be suppressed. However, its inferior electrical conductivity due to its semiconductor nature limits its electrocatalytic activity. To overcome this limitation, Bi nanoparticles anchored in carbon nanosheet (Bi@C) have been developed, exhibiting an impressive NH3 yield of 93.7 μmol h−1 mgcat.−1 and a high FE of 93.0% in 0.1 M Na2SO4 electrolyte.75 At a potential of −0.65 V, Bi powder yielded NH3 via NO reduction at a rate of 6 ± 2 × 102 pmol s−1 cm−2, while the use of Au/C resulted in an NH3 production rate of 8.1 ± 0.9 × 102 pmol s−1 cm−2 at −0.30 V.74 Co SACs are highly active and durable in NOxRR when anchored on MoS2 (Co/MoS2).76 Co/MoS2 exhibited excellent NOxRR performances, selectively absorbed NO molecules, and promoted the hydrogenation energetics of the NOxRR process, leading to a high NH3 yield of 217.6 μmol h−1 cm−2 and an NH3-FE of 87.7% at −0.5 V vs. RHE. Recent studies suggested that W single atoms could efficiently activate nitrogenous molecules, indicating the potential of W-SACs in NOxRR. A single-atom W confined in MoO3–x amorphous nanosheets (W/MoO3–x) was designed. W/MoO3–x had the highest FE of 91.2% and NH3 yield rate of 308.6 μmol h−1 cm–2.77 Using other catalysts, such as p-block Sb,78 Ir,79 and In80 anchored in amorphous a-MoO3 yield an NH3 rate of 273.5 μmol h−1 cm−2, 438.8 μmol h−1 cm−2, and 242.6 μmol h−1 cm−2 at −0.6 V vs. RHE, respectively. The isolated Sb and Ir atoms in O vacancies of amorphous MoO3 powerfully activated and hydrogenated NO with a near-zero energy barrier. The isolated Sb atoms anchored in Sb2S3 have also shown selective NOxRR with high yield and FE.81
In conclusion, SACs have shown great promise for diverse electrolytic reactions due to their unique properties of high atomic utilization and low-coordination environment. SACs should be anchored on supported materials to prevent the aggregation process. Amorphous substrates with abundant vacancies and dangling bonds can provide confined spaces to anchor metal atoms and induce a strong metal–substrate interaction, making them an attractive platform for SACs. Table 1 shows the recent progress of SACs for electrocatalytic NOxRR.
Table 1.
Electrocatalytic NOxRR performance of SACs, TMDs, carbon-based, and metal alloys electrocatalysts
| Catalysts | Electrolyte, membrane, cell | NH3 yield | FE (%), potential vs. RHE | By product | Reference |
|---|---|---|---|---|---|
| Binanodendrites | 0.1 M Na2SO4, H-type cell, Nafion 117 | 70.2 μmol h−1 mgcat.−1 | 89.2% at −0.5 V | N2, N2H4, H2 | Lin, et al.92 |
| Cu | 0.1 M NaOH +0.9 M NaClO4, gas-fed three-compartment flow cell, Nafion 117 | – | 83.5% at 0.08 V | N2, N2O, NH2OH | Ko, et al.93 |
| Co | 44.7% at −0.03 V | ||||
| Pt | 22.2% at 0.04 V | ||||
| Cu foam | 0.25 M Li2SO4, H-type cell, Nafion 115 | 517.1 μmol h−1 cm−2 | 93.5% at −0.9 V | N2, N2O, H2 | Long, et al.26 |
| Ir | 0.5 M Na2SO4, H-type cell, Nafion 117 | 438.8 μmol h−1 cm−2 | 92.3% at −0.47 V | H2, N2H4, N2O | Chen, et al.79 |
| Co | 0.5 M Na2SO4, H-type cell, Nafion 117 | 217.6 μmol h−1 cm−2 | 87.7% at −0.5 V | H2, N2H4, N2 | Li, et al.76 |
| BiNPs@carbon NS | 0.1 M Na2SO4 + 0.05 mM Fe(II)EDTA, H-type cell, Nafion 117 | 93.7 μmol h−1 mgcat.−1 | 93.0% at −0.4 V | H2 | Liu, et al.75 |
| Ru nanosheets | 0.5 M Na2SO4, H-type cell, Nafion 117 | 45.0 μmol h−1 mgcat.−1 | 66.0% at −0.2 V | H2 | Li, et al.94 |
| MoS2 nanosheet on graphite felt (MoS2/GF) | 0.1 M HCl, H-type cell, Nafion 117 | 99.6 μmol cm−2 h−1 | 76.6% at −0.7 V | N2H4 | , et al.34 |
| Cu/MoS2 | 0.5 M Na2SO4, H-type cell, Nafion 117 | 337.5 μmol h−1 cm−2 | 90.6% at −0.6 V | – | Chen, et al.72 |
| P-doped MoS2 | 0.1 M [C4mpyr][eFAP], H-type cell, Nafion 211 | 22.8 μmol h−1 mgcat.−1 | 69.0% at −0.6 V | – | Wei, et al.95 |
| Fe-doped MoS2−x | 0.5 M Na2SO4, H-type cell, Nafion 117 | 288.2 μmol h−1 cm−2 | 82.5% at − 0.6 V | H2, N2H4, N2 | Chen, et al.96 |
| Honeycomb carbon nanofiber (CNFs) | 0.2 M Na2SO4, H-type cell, Nafion 117 | 22.4 μmol h−1 cm−2 | 88.8% at −0.6 V | N2H4, H2 | Ouyang, et al.86 |
| Au/rGO | 0.5 M Na2SO4, three-electrodes cell | 14.6 μmol h−1 cm−2 | 65.2% at −0.1 V | H2 | Xiong, et al.25 |
| Epoxy-functionalized carbon | 0.1 M PBS +0.2 M Na2SO3, H-type cell, Nafion 117 | – | 83.3% at −1.1 V vs. SCE | N2, N2O | Sharif, et al.88 |
| Nb-supported B, N co-doped CNT | 0.1 M HCl, three-channel flow cell, Nafion 117 | 2952.0 μmol h−1 cm−2 | 77.0% at −0.6 V | – | Peng, et al.71 |
| g-C3N4 nanosheets | 0.1 M PBS, H-type cell, Nafion 117 | 30.7 μmol h−1 cm−2 | 45.6% at −0.4 V | H2 | Li, et al.97 |
| Cu-Ti hollow fibers | 0.05 M H2 SO4, H-type cell, Nafion 117 |
400.0 μmol cm−2 h−1 | 90.0% at −0.6 V | H2, N2O, N2 | Shi, et al.89 |
| CuFe | 0.1 M PBS, H-type cell, Nafion 117 | 100 μmol h−1 cm−2 | 90.6% at −0.8 V | – | Hao, et al.29 |
| PdCu | 0.5 M NaOH, three-electrode cell | – | 24.1% at −0.9 V | N2H4, N2, H2 | Soto-Hernández, et al.24 |
| Cu1-xRux | 0.5 M Na2SO4, H-type cell, Nafion 117 | 17.7 μmol h−1 cm−2 | 64.9% at −1.1 V | – | Soto-Hernández, et al.24 |
| RuGa | 0.1 M K2SO4, H-type cell, Nafion 117 | 320.6 μmol h−1 mgcat.−1 | 72.3% at −0.2 V | – | Zhang, et al.91 |
Transition metal dichalcogenides
Nanostructures based on transition metal dichalcogenides (TMDs) have recently emerged as appealing electrocatalysts for various electrocatalytic reactions, including NOxRR, owing to their excellent properties. Despite their surface-active site on their 2D structures,82 TMDs have barely been investigated as efficient noble metals and SACs compared to other materials. A detailed discussion on the synthesis and properties of TMDs can be found elsewhere.83 To clarify their catalytic activity and product selectivity, a thorough evaluation of some representative 1T′ phase single layer TMDs such as MoS2, MoSe2, MoTe2, TaTe2, and WTe2 was conducted using first-principle calculations.84 Investigating the reaction pathway is critical to designing the most efficient TMDs catalysts. In 1T′-TMDs, NO molecules are adsorbed at a sulfur vacancy site and favorably undergo two hydrogeneration pathways, leading to the formation of NH3. A possible reaction pathway under low coverage is consistent with the reaction ∗NO → ∗NOH → ∗N → ∗NH → ∗NH2 → ∗NH3. Under high NO coverage, 1T′-TMDs yield N2O or N2 via different pathways.84 To suppress the competing HER, surface-defected 1T′–MoS2, 1T′–MoSe2, 1T′–MoTe2, 1T′–WTe2, and 1T′–TaTe2 catalysts with S and Se vacancies are introduced, as the Gibbs free adsorption energies of H are much weaker than those of ∗NO in a defected surface.84 The high catalytic activity of these catalysts for NOxRR arises from a large amount of activated NO at the ∗NO, where the electron is accumulated on ∗NO, and the depleted regions are in adjacent metals.
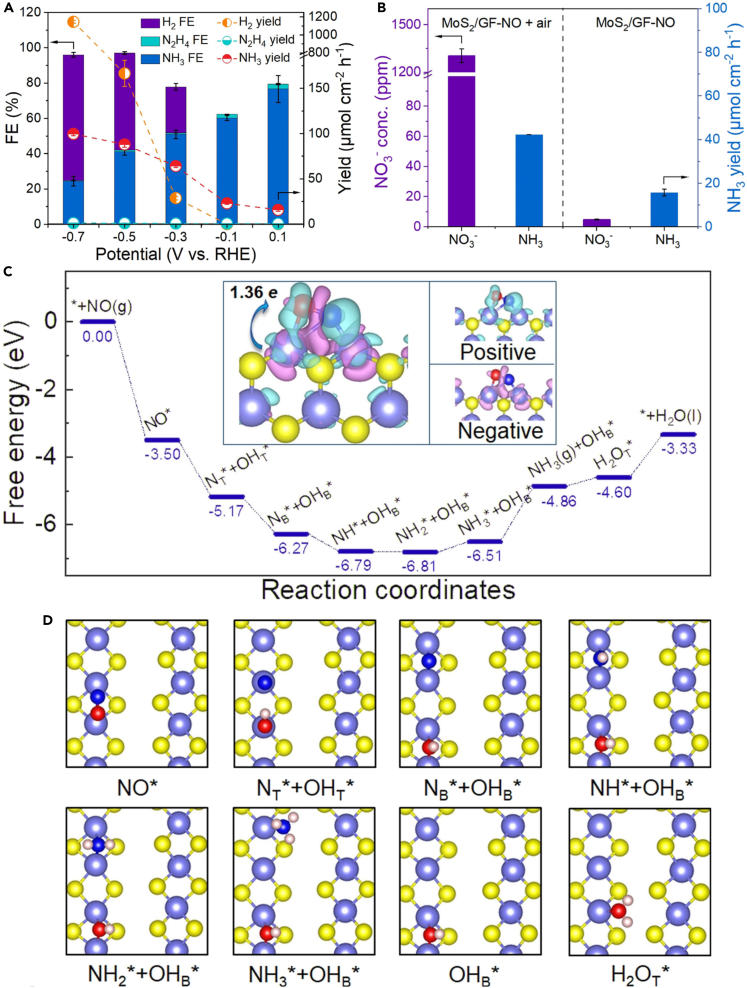
Experimental results support the excellent performance of TMDs toward highly selective electrochemical NO reduction. For instance, MoS2 nanosheets were grown on graphite felt (GF) to construct 3D MoS2/GF nanoarchitectures. Under acidic conditions (0.1 M HCl), MoS2/GF achieves an FE of 76.6% and up to 99.6 μmol cm−2 h−1 of NH3 yield. The NH3 yield remained almost unchanged after repeated cycles, indicating the excellent electrochemical long-term stability of MoS2/GF (Figures 6A and 6B). The DFT calculation has provided an intuitive insight into the catalytic mechanism. According to the calculation, NO molecules tend to adsorb side-on at the Mo-Mo bridge (B) site, which has a stronger bond strength than H on the positively charged metal sites, thus suggesting the potential suppression of competing HER. The surface-terminated Mo atoms may also effectively catalyze and activate NOx molecules, as seen in the Mo2C case.85 Figures 6C and 6D shows the NOxRR pathway to NH3 involving Mo2C/GF. Furthermore, the MoS2/GF can be used as a cathode in a Zn-NO battery device, delivering a power density of 1.04 mW cm−2 with 24.2 μmol h−1 mgcat.−1 NH3 yield. This presents an opportunity to tackle NO pollutants more sustainably by removing NO while generating electricity and chemical feedstocks. Table 1 shows the recent progress of TMDs for electrocatalytic NOxRR.
Figure 6.
MoS2 nanosheets on graphite felt (GF) to construct 3D MoS2/GF nanoarchitectures
(A) The distribution of products for MoS2/GF at different potentials.
(B) The NH3 yield and the concentration of NO3− for MoS2/GF after 1 h of electrolysis in gas-tight and open chambers.
(C and D) The free energy landscape for NOxRR on MoS2(101) and corresponding atomic structures of the intermediates. The local density of states (DOS) of the adsorbed NO and its bonded Mo atoms is also shown. The insets in (C) represent charge density differences for the adsorbed NO, where cyan and red regions indicate electron accumulation and loss, respectively. The topmost atomic layer of the substrate and the adsorbed intermediates are shown in (D) for clarity, with Mo represented by light blue spheres, S by yellow spheres, N by dark blue spheres, O by red spheres, and H by pink spheres. Reproduced with permission from.34 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2021.
Carbon-based electrocatalysts
The creation of catalysts devoid of noble metals that can drive the NOxRR reaction would open up the possibility for broader-scale utilization of NH3 synthesis via electrochemical NO reduction. Carbon-based materials have proven to be highly effective electrocatalysts and supported in a wide range of electrocatalytic applications, including N2 reduction (NRR), O2 reduction (ORR), and CO2 reduction (CO2RR), owing to their exceptional electrical conductivity, electrochemical stability, and corrosion resistance. Among the many advantages of carbon-based catalysts is the abundance of carbon, which is readily available in various forms of hydrocarbon compounds from biomass waste. This makes producing carbon-based catalysts from biomass an attractive and sustainable approach. Additionally, due to the diverse range of carbon allotropes, carbon-based catalysts can be systematically designed into various shapes, including 0D (carbon nano- or quantum dots), 1D (carbon nanofibers), 2D (rGO, GO), and 3D monoliths.
Carbon nanofibers, through peroxidation and carbonization processes, can be transformed from a smooth surface into a structure resembling a honeycomb with interconnected and open nanocavities.86 As a result, the specific surface area of the carbon nanofiber catalyst is increased, allowing for a high NH3 yield of 22.4 μmol h−1 cm−2 with an FE of up to 88.3%. DFT calculations suggest that the most active centers for NO adsorption and activation are –OH groups.
In the 2D form, rGO/Au nanocomposites could yield NH3 of 14.6 μmol h−1 cm−2 with an FE of 65.2% at neutral pH, which was further increased to 98.3% at acidic conditions (pH = 1).25 Moreover, 2D boron carbide (B2.6C) grown on a Ti/TiO2 electrode showed remarkable NOxRR activity coupled with long-term durability toward NH3 synthesis and Zn-NO battery.87 It produced a yield of 216.4 μmol h−1 cm−2 and an FE of 87.6% as nanocatalysts for NO electroreduction, and a power density of 1.7 mW cm−2 with an NH3 yield of 66.2 μmol h−1 cm−2 as Zn-NO battery device. DFT calculations have shown that the bonding between B and C atoms transfers electrons to NO orbitals, thus allowing NO to be activated and reduced with less energy requirement (1.48 eV).
Finally, 3D monolithic carbon with abundant hierarchical nano- and mesopore structures obtained from carbonization and CO2-activation of biomass have demonstrated excellent efficiency toward electrochemical NOx reduction, achieving an ∼83.3% FE under ambient conditions (neutral pH and room temperature) as well as excellent stability up to 168 h.88 The recent progress of electrocatalytic NOxRR performance of carbon-based electrocatalysts is shown in Table 1.
Metal alloys
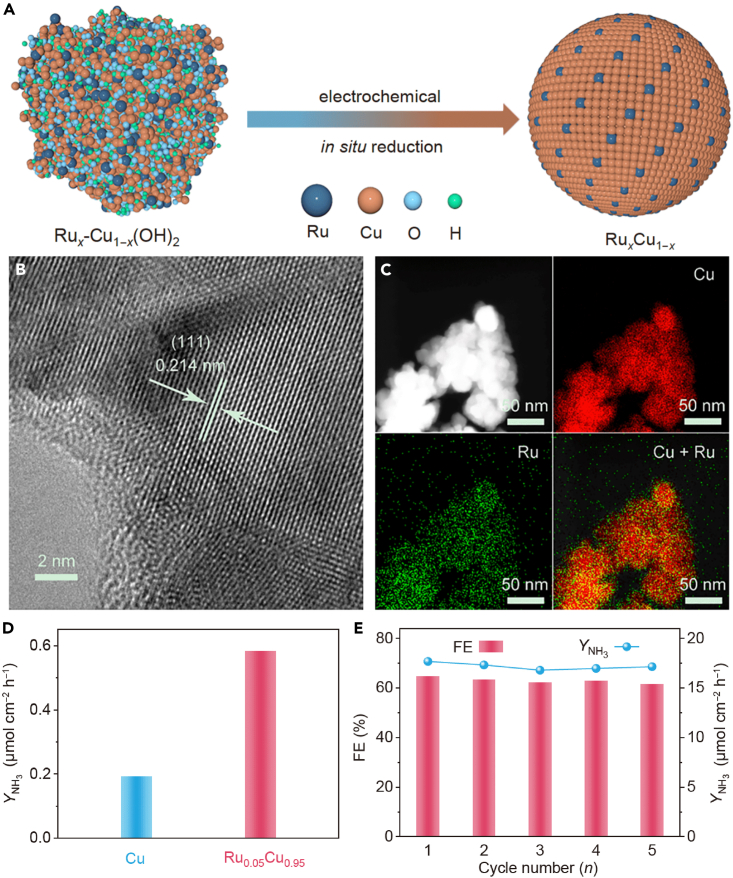
As previously discussed, recent studies have shown promising results in the electrochemical reduction of NO using SACs consisting of mono-metallic elements such as Ir, Pt, Rh, Ru, Pd, Cu, Ni, Ag, and Au. However, achieving low-cost production while maintaining (or even enhancing) the catalytic activity of metal nanocatalysts remains challenging. Cu is a benchmark NOxRR catalyst due to its low-cost fabrication, but it suffers from low selectivity. One strategy to improve its selectivity is to alloy Cu with other metallic elements. Cu has been alloyed with Ti,89 Fe,29 Pd,24 Ru,90 and Ga,91 all of which have significantly enhanced the conversion of NOx molecules to NH3. For example, adding Pd atoms to Cu to fabricate PdCu nanoalloy catalysts significantly suppressed the competing HER, facilitating the transfer of protons to NOx molecules to drive the NOxRR.24 PdCu catalysts exhibited six times higher catalytic activity than pristine Cu or Pd. Similarly, adding a small amount of Ru to Cu nanoparticles could improve electrocatalytic performance for NH3 synthesis (FE: 64.9%, NH3 yield: 17.7 μmol h−1 cmcat.−2), surpassing that of pristine Cu (FE: 33.0%, NH3 yield: 5.73 μmol h−1 cmcat.−2).90 The RuxCu1-x nanoalloy was fabricated using an in situ electrochemical reduction conversion approach. Using differential electrochemical mass spectrometry (DEMS), it was discovered that the electronic transfer from Ru to Cu resulted in a downshift of Cu d-band center, facilitating the rate-limiting hydrogenation step and reducing NH3 desorption energy, which should lead to high NH3 yield (17.7 μmol h−1 cm−2) and FE (64.9% at −1.1 V) as depicted in Figure 7.90 Furthermore, when Cu was alloyed with Fe, the resulting CuFe nanoalloy showed promise as a highly effective and versatile catalyst for the NOx-based pollutants (NO, NO2, NO2−, and NO3−) electroreduction to NH3.29 The presence of Cu and Fe atoms in the nanoalloy led to a synergistic effect that lowered the free energy and promoted the hydrogenation process, as revealed by experimental and theoretical investigations. The low-cost CuFe catalyst alloy can treat water and atmosphere pollutants and convert them into valuable chemicals.
Figure 7.
The RuxCu1-x nanoalloy using an in situ electrochemical reduction conversion approach
(A) A graphical scheme for synthesizing RuxCu1–x.
(B) HRTEM image of Ru0.05Cu0.95.
(C) HAADF-STEM image and the corresponding EDX mapping images of Ru0.05Cu0.95.
(D) NH3 rate over Cu and Ru0.05Cu0.95 after ECSA normalization.
(E) The FE of NH3 production and corresponding yield rate measured during the recycling tests of NOxRR over Ru0.05Cu0.95 at a potential of −1.1 V vs. Ag/AgCl. Reprinted with permission from90, Copyright Springer Nature, Inc. 2021.
Despite exhibiting remarkable NOxRR activity, developing metal alloy electrocatalysts for electrochemical NOx reduction necessitates meticulous consideration of various challenges and a concerted effort to overcome them. This is because, compared to noble metal catalysts such as Pt, the electrocatalytic activity of metal alloy electrocatalysts for NOx reduction is typically lower. Consequently, higher overpotentials are required to attain the desired reduction reaction, which can lead to reduced energy efficiency and amplified electrode wear. Additionally, the reaction environment in electrochemical NOx reduction can be acutely harsh, characterized by acidic or alkaline conditions, high temperatures, and high current densities. Consequently, metal alloy electrocatalysts must exhibit stability under such conditions to maintain their catalytic activity over time. Furthermore, the cost of metal alloy electrocatalysts can be a significant factor in the commercial viability of electrochemical NOx reduction technology. While they are generally less expensive than noble metal catalysts, their lower FE necessitates higher energy consumption. Table 1 highlights the groundbreaking advances in the electrocatalytic performance of metal alloys electrocatalysts.
Metal oxides
Metal oxides have been renowned for their resilience against harsh environments owing to their chemical stability. Numerous studies have demonstrated that NH3 selectivity exhibits superior performance in acidic conditions compared to neutral or basic ones, making it reasonable to explore the potential of metal oxides for NOxRR applications. However, the inert surface and low conductivity of pristine metal oxides have hindered their practical applications. Instead, surface defective metal oxides are energetically more favorable for electrocatalytic applications.
Oxygen vacancies (Vo) are a prevalent type of structural defect in metal oxides, which can significantly impact their electronic and catalytic properties. Therefore, designing metal oxides with an increased density of oxygen defects could be a valuable strategy for optimizing NOxRR for NH3 synthesis. For instance, CuO nanocatalysts have been designed with Vo using flame spray pyrolysis (FSP) and mild plasma treatment, resulting in increased Vo amounts linearly correlated to plasma treatment duration.22 The O1s core level spectra analysis via XPS showed the occurrence of Vo with a binding energy of 531.5 eV, whereas EPR measurements revealed the production of ionic superoxide species that resulted from the combination of O2 molecules and Vo. Defective CuO achieved an NH3 yield of 520 μmol h−1 cm−2 at an operating cell voltage of 2.2 V, with over ten operating hours of electrochemical stability in a flow electrolyzer. Undoubtedly, NOx molecules are captured and activated at the Vo site by bonding with surface Cu atoms and oxygen ions, effectively lowering the binding energy to a remarkable −1.87 eV. The subsequent reduction reaction unequivocally demonstrates that the free energy of most intermediates is significantly lower on the CuO surface with Vo than on the pristine surface.
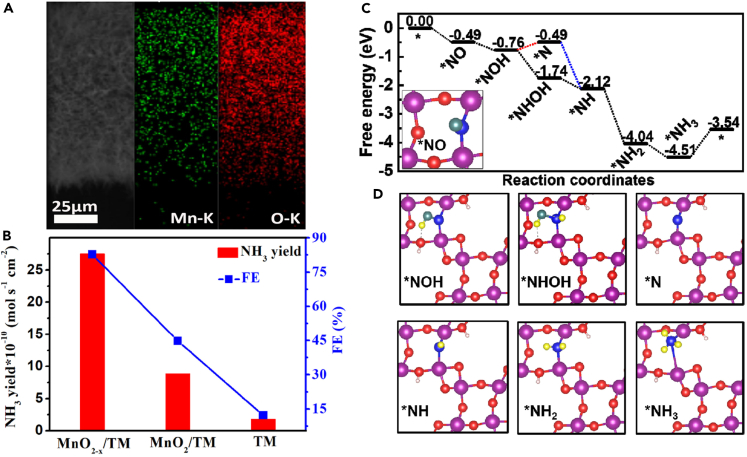
MnO2 nanoarrays grown on Ti mesh (MnO2-x NA/TM) have also demonstrated improved NOxRR performance through the Vo strategy.32 Under the same conditions (0.2 M Na2SO4), the catalysts achieved a notable NH3 yield of 9.9 μmol h−1 cm−2 with an FE of 82.8%, clearly exceeding the yield and FE of the pristine MnO2 counterpart (3.2 μmol h−1 cm−2, 44.8%). This remarkable performance can be attributed to the stronger interaction between NO and Mn atoms induced by the Vo —the less interaction between H and MnO2 results in high NOxRR selectivity. Additionally, NOxRR preferably occurs through an alternating pathway on the MnO2-x(211) surface, with no endothermic steps observed along the reaction pathway. The Ti mesh substrate can also accommodate the growth of other metal oxide nanoarrays, such as NiO nanoarray (NiO NA/TM) catalysts. Such catalysts are highly active and selective for NOxRR, achieving an NH3 yield of 125.3 μmol h−1 cm−2 and an FE of up to 90.0% (see Figure 8).98 Metal oxides of distinct morphology have been investigated as potential electrocatalysts for NOx reduction. For instance, Fe2O3 in the form of nanorods has demonstrated exceptional performance, achieving an NH3 yield of 78.0 μmol h−1 cm−2 and an FE of 86.7% at −0.4 V.99 Meanwhile, TiO2 nanotubes have exhibited a remarkably high FE of 89.0% at −0.8 V and NH3 yield of 25.0 μmol h−1 mgcat.−1.100 Nonetheless, both electrocatalysts generate undesirable byproducts such as H2 and N2H4. Despite the low conductivity limitation of metal oxides, their defective form has outperformed most pristine surface metal oxides. Notwithstanding their attractive cost and abundance, metal oxide electrocatalysts pose significant challenges in the context of electrochemical NOx reduction. Foremost, metal oxide electrocatalysts often exhibit low electrocatalytic activity, leading to diminished energy efficiency and heightened electrode wear. Furthermore, the selectivity of reduction reactions involving metal oxide electrocatalysts can be challenging to control, resulting in the unwanted formation of products. Table 2 illustrates the latest developments in the electrocatalytic performance of metal oxide electrocatalysts for NOxRR.
Figure 8.
MnO2 nanoarrays on Ti mesh (MnO2-x NA/TM) for improving NOxRR performance through the Vo strategy
(A and B) SEM and its corresponding EDX elemental mapping images of MnO2-x nanoarrays grown on Ti mesh (MnO2-x NA/TM) and their (B) corresponding data on NH3 yields and FEs with different electrodes after bulk electrolysis for an hour at −0.7 V.
(C and D) Free energy diagrams of the NOxRR on MnO2-x(211) along the distal and alternating pathways and (D) the corresponding structures of reaction intermediates. Reprinted with permission from32, Copyright Elsevier Inc 2022.
Table 2.
Electrocatalytic NOxRR performance of metal oxides, metal phosphides, metal sulfides, metal nitrides, and metal borides
| Catalysts | Electrolyte, membrane, cell | NH3 yield | FE (%), potential vs. RHE | By product | Reference |
|---|---|---|---|---|---|
| MnO2 nanoarray | 0.2 M Na2SO4, H-type cell, Nafion 117 | 9.9 μmol h−1 cm−2 | 82.8% at −0.7 V | H2 | Li, et al.32 |
| NiO nanosheet array | 0.1 M Na2SO4, H-type cell, Nafion 117 | 125.3 μmol h−1 cm−2 | 90% at −0.6 V | – | Liu, et al.98 |
| CuO | 0.05 M KNO3 + 0.05 M H2SO4, H-type cell, Nafion 117 | 334.0 μmol h−1 cm−2 | 45.0% at −0.7 V | – | Daiyan, et al.22 |
| TiO2−x | 0.2 M PBS, H-type cell, Nafion 211 | 72.5 μmol h−1 cm−2 | 92.5% at −0.6 V | N2, H2 | Li, et al.108 |
| W/MoO3–x | 0.5 M Na2SO4, H-type cell, Nafion 117 | 308.6 μmol h−1 cm−2 | 91.2% at −0.5 V | H2, N2H4, N2 | Chen, et al.77 |
| Fe2O3 nanorods | 0.1 M NaOH, H-type cell, Nafion 117 | 78.0 μmol h−1 cm−2 | 86.7% at −0.4 V | H2, N2H4 | Liang, et al.99 |
| P-doped TiO2 nanotubes | 0.1 M [C4mpyr][eFAP], H-type cell, Nafion 211 | 25.0 μmol h−1 mgcat.−1 | 89.0% at −0.8 V | H2 | Zhang, et al.100 |
| Cu NPs/TiO2 | 0.1 M K2SO4, H-type cell, Nafion 117 |
207.1 μmol h−1 mg−1 | 86.5% at −0.3 V | N2H4 | Chen, et al.73 |
| Ni2P nanoarray | 0.1 M HCl, H-type cell, Nafion 117 | 33.5 μmol h−1 cm−2 | 76.9% at −0.2 V | N2H4, H2 | Mou, et al.105 |
| FeP nanorod array | 0.2 M PBS, three-electrodes cell | 14.6 μmol h−1 cm−2 | 88.5% at −0.2 V | – | Liang, et al.35 |
| CoP nanowires | 1.0 M NaOH and 2 mM NaNO2, H-type cell, Nafion 117 | 22.4 μmol h−1 cm−2 | 91.6% at −0.3 V | – | Zhang, et al.104 |
| CoS nanosheet | 0.52 M Na2SO4, H-type cell, Nafion 117 | 44.7 μmol h−1 cm−2 | 53.6% at −0.4 V | H2, N2H4 | Zhang, et al.107 |
| SnS2−x catalyst | 0.5 M Na2SO4, H-type cell, Nafion 117 | 78.6 μmol h−1 cm−2 | 90.3% at −0.7 V | H2, N2H4, N2 | Li, et al.109 |
| MoC nanocrystal | 0.1 M HCl, H-type cell, Nafion 211 | 79.4 ± 0.9 μmol h−1 cm−2 | 89.0% at −0.8 V | – | Meng, et al.110 |
| Nanoporous VN | 0.1 M HCl, H-type cell, Nafion 211 | 302.4 μmol h−1 mgcat.−1 | 85.0% at −0.6 V | – | Qi, et al.111 |
| Amorphous NiB2 | 0.5 M Na2SO4, H-type cell, Nafion 117 | 167.1 μmol h−1 cm−2 | 89.6% at −0.4 V | H2 | Chen, et al.112 |
Metal phosphides
Metal phosphides have emerged as an up-and-coming class of electrocatalysts due to their superior conductivity and remarkable stability compared to other transition metal materials. Multifunctional active sites, adjustable structures, and superior stability are the distinguishing characteristics of metal phosphides, which contribute to their electrocatalytic performance comparable to Pt-based materials. Their metal conductor properties and nearly spherical triangular prism element structure offer higher crystal surface exposure, providing more active sites for electrocatalytic processes. The impressive electrocatalytic activity of metal phosphides electrocatalysts is linked to the intrinsic properties of active sites, electron transfer capability, and the number of accessible active sites. In metal phosphides, the phosphorus atoms act as proton acceptors, while the metal atoms serve as hydride acceptors, both of which are crucial active centers. Metal phosphides have proven to be highly versatile electrocatalysts for a variety of reactions, including the HER, OER, ORR, HOR, CO2RR, NRR, and, most recently, NOxRR.101,102,103
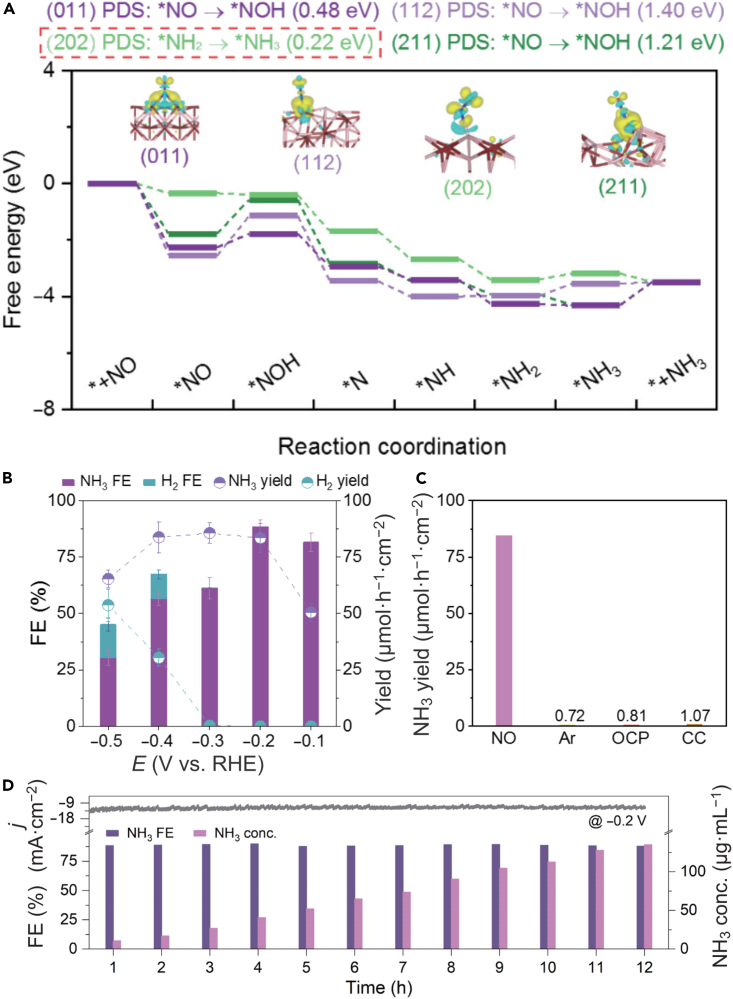
In their quest for highly efficient and stable electrocatalysts, researchers have developed cobalt phosphide (CoP) nanowire arrays that are self-supported on carbon cloth for use in the electrocatalytic NOx− reduction of under ambient conditions in an alkaline NaOH solution.104 At −0.4 V vs. RHE, the CoP demonstrated a remarkable NH3 yield rate of 18.6 μmol h−1 cm−2 and an FE of 65.0%, maintaining its activity even after ten cycles of electrocatalysis. The exceptional CoP performance is attributed to the charge separation effect within the material, where the Co(δ+) → P(δ-) mechanism plays a significant role. It significantly enhances the hydrogenation of NOx− and produces NH3 selectively by facilitating the adsorption of NOx− and H atoms on the Co and P sites. Similarly, the use of a nanosheet array of Ni2P grown on carbon paper (Ni2P/CP) has demonstrated the provision of a highly selective electrocatalyst for the hydrogenation of NOx to NH3, exhibiting a large NH3 output of 33.5 μmol h−1 cm−2 and an FE of up to 76.9% at −0.2 V vs. RHE.105 Based on theoretical calculations, the Ni2P(111) surface was found to facilitate NO adsorption and activation via an “acceptance-donation” mechanism, with ∗NO hydrogenation to ∗NOH identified as the potential-determining step. Researchers have also investigated the feasibility of using FeP electrocatalyst, which demonstrated a low onset potential of −0.014 V and impressive FE of 88.5% with a good generation rate of NH3 (85.6 μmol h−1 cm−2) for the stable electrochemical reduction of NO during an uninterrupted 12-h electrolytic process, as shown in Figures 9B–9D.35 The computer simulation indicated that the adsorbed NO molecules obtained 0.39 e− transferred from the FeP(202) facet (inset in Figure 9A), which showed the electron acceptance and donation phenomenon. The formation of ∗NH3 from ∗NH2 was identified as the determining factor for the electrocatalytic activity of the FeP(202) facet. Table 2 illustrates the recent advances in the electrocatalytic performance of metal phosphides electrocatalysts for NOxRR.
Figure 9.
The feasibility of using FeP electrocatalyst for NOxRR
(A) Free energy diagrams illustrating the electrocatalytic NOxRR mechanisms occurring on FeP(011), (112), (202), and (211) surfaces, with insets depicting the charge redistribution resulting from NO adsorption. The FeP(202) surface was found to have the lowest free energy barrier for the formation of ∗NH3, indicating that the formation of ∗NH3 from ∗NH2 on this surface was the potential-determining step.
(B and C) Yields and FEs of NH3 and H2 obtained on FeP/CC and (C) NH3 yields obtained using various electrodes under different conditions after bulk electrolysis for an hour.
(D) The long-term stability tests of FeP/CC for NH3 generation. Reproduced with permission from35, Copyright Springer Nature 2022.
To assess the potential of nitrogen-doped BP monolayer-supported transition-metal atoms (MN3/BP) as NOxRR electrocatalysts, the study conducted a systematic first-principles calculation of 29 transition-metal atoms. The results showed that MoN3/BP and IrN3/BP are the most promising electrocatalysts for NH3 synthesis, with limiting potentials of −0.10 V and −0.06 V, respectively, comparable to the excellent Pt(111) surface.57 The electronic analysis revealed that adsorbed NO species could be activated by hybridizing Mo-4d or Ir-5d orbitals with NO-2p orbitals. Notably, MoN3/BP and IrN3/BP exhibit high thermal stability and can be conveniently synthesized utilizing MoCl3 and IrCl3 as precursors. Computational simulations have reinforced the potential of metal phosphides as efficient and robust electrocatalysts for NOxRR and shed light on the reaction mechanism underlying the electroreduction of NO to NH3 under ambient conditions. The supporting BP monolayer was surprisingly an active NOxRR electrocatalyst, as it was recently revealed.106 BP monolayers showed FE of 83.3% at −0.7 V vs. RHE with an NH3 yield rate of 96.6 μmol h−1 cm−2. This finding opens a new avenue for metal-free NOxRR electrocatalysts.
Metal sulfides, metal nitrides, and metal borides
The investigation into metal sulfides, metal nitrides, and metal borides electrocatalysts for the electrochemical reduction of NOx is a relatively underexplored research area compared to other similar electrocatalytic applications like HER, OER, ORR, and NRR. This could be attributed to various factors, including the intricacy of NOx reduction, the dearth of suitable metal sulfides, metal nitrides, and metal borides catalysts, and the arduous experimental conditions required to study the electrochemical reduction of NOx. Nevertheless, the potential advantages of utilizing these electrocatalysts for NOx reduction make it a promising field of research, which could pave the way to develop more efficient and sustainable methods for mitigating NOx emissions.
To our knowledge, the CoS nanosheet is the only member of the metal sulfides family examined for electrocatalytic NOx reduction applications107 —the CoS nanosheets with sulfur vacancies (CoS1–x) have been identified as effective electrocatalysts. In 0.2 M Na2SO4 electrolyte, CoS1–x has demonstrated a considerably higher NH3 yield rate (44.7 μmol h−1 cm−2) and FE (53.6%) than the pristine CoS counterpart (27.0 μmol cm−2 h−1; 36.7%) at a voltage of −0.4 V vs. RHE.107
In order to investigate the NO reduction mechanism over CoS1-x, DFT was employed. Focusing on the (100) facet of CoS1–x, computational models for CoS(100) and CoS1–x(100) crystal facets were created, and it was found that the NO molecule was adsorbed on both facets due to strong interactions between O atoms in the NO molecule and Co atoms in CoS1–x. In addition, the investigation demonstrated that the initial hydrogenation of NO was the potential determining step (PDS) of NOxRR on CoS(100) and CoS1–x(100) facets, with corresponding Gibbs free energy barriers of 0.42 and 0.19 eV,107 respectively. Thus, this study provides crucial information on the possible utilization of transition-metal sulfides with sulfur vacancies for electrochemical NOx reduction to NH3.
In the case of metal nitrides, a development has emerged as a nanoporous VN film. This film has been engineered to serve as a NOxRR electrocatalyst with exceptional selectivity and stability. It achieved a remarkable FE of 85%, accompanied by an NH3 yield rate of 302.4 μmol h−1 mgcat.−1) at −0.6 V vs. RHE. Moreover, this catalyst demonstrated unparalleled durability, showcasing an outstanding level of sustained activity over a substantial period of 40 h, and maintained negligible current density and minimal decay in the NH3 yield rate throughout this extended duration. Meanwhile, amorphous NiB2 catalysts achieved FE of 89.6% with a high NH3 yield rate at an impressive value of 167.1 μmol h−1 cm−2. It has been unveiled that the Ni sites within NiB2 play a pivotal role as the primary active centers. These centers can optimize the binding energy of the crucial ∗NHO intermediate, reducing energy barriers. Additionally, they exhibit a higher adsorption affinity toward NO than H2O/H species. This unique characteristic significantly contributes to facilitating active and selective NORR, enhancing the overall efficiency of the process. Table 2 summarizes the recent advances in the electrocatalytic performance of metal sulfides, metal nitrides, and metal borides electrocatalysts for NOxRR.
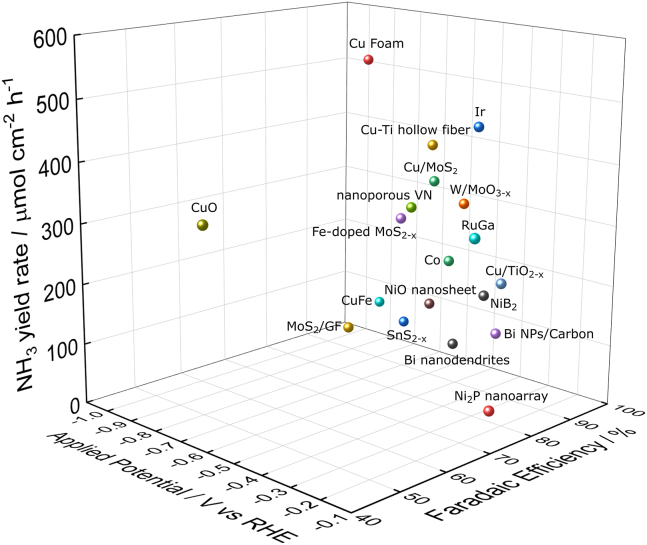
Overall, there has been extensive exploration of various electrocatalysts for NOxRR. Some have demonstrated high NH3 yield but require operation at high voltage, resulting in substantial electrical energy consumption and, subsequently, high NH3 prices. Conversely, although high FE is observed when operated at low voltage, the NH3 yield rate may not meet industrial requirements. In this context, researchers need to develop highly active catalysts that exhibit both high FE at low operating voltage and satisfactory NH3 yield rates. We present the comprehensive performance metrics (NH3 yield rate, FE, and operating voltage) of selected catalysts in Figure 10.
Figure 10.
Overall performances of selected NOxRR electrocatalysts
Beyond electrocatalysts design
While much attention has been focused on electrocatalyst design for NOxRR, there is a need to go beyond this and explore other factors that can enhance the electrochemical performance of NOxRR. This includes modifications to the electrolyte and gas diffusion electrode (GDE) design, which can significantly impact the reaction efficiency. By noting these aspects, it may be possible to develop more efficient and sustainable methods for NOxRR, which could ultimately lead to a reduction in NOx emissions. This chapter will explore various strategies and techniques beyond electrocatalyst design for enhancing the electrochemical NOxRR process. We will discuss the modification of electrolytes to improve reaction kinetics and the design of GDEs to optimize mass transfer. Additionally, we will delve into the proof-of-concept of Zn-NO batteries and the integration of highly active electrocatalysts as cathode materials into the battery architecture for improved performance. This chapter aims to provide a comprehensive overview of the latest developments and challenges in NOxRR enhancement beyond electrocatalyst design.
Electrolytes modification
Utilizing NOx gas directly from the atmosphere presents a new opportunity for transforming waste into valuable chemicals. However, capturing and concentrating NOx from polluted air demands sophisticated infrastructure, which may hinder the practical application of this approach. Additionally, the inadequate solubility of NOx gas in aqueous electrolytes, predominantly the NO molecule, hinders the mass transfer and creates an unfavorable surrounding for the electrochemical conversion of NOx. Overcoming these challenges has become a major focus of research in the field of electrochemical reduction of NOx. This section will discuss the latest advancements in developing deliberately designed electrolytes aimed at fully optimizing NOxRR.
Producing NH3 via NOxRR offers significant advantages compared to NRR and Haber-Bosch methods, which rely on inert N2 as a resource. However, achieving high conversion rates of NOx through electroreduction is limited by the intrinsically low solubility of NO (∼1.94 mM at 25°C) in water. In electrochemical reduction processes like NRR and CO2RR, the choice of electrolytes as gas-dissolving media is critical. For example, electrolyte design has enabled high selectivity in the electroreduction of CO2 into targeted chemicals. Similarly, to achieve higher conversion rates and improved selectivity in electrocatalytic NOxRR, strategic control over the gas solubility and fine-tuning the electrochemical processes occurring at the interface of the electrode and electrolyte are vital. With a meticulous approach, the performance of electrocatalytic reactions can be significantly enhanced, leading to remarkable gains in efficiency and productivity.
EDTA−Fe2+ metal complex (EFeMC) is a well-known compound that can capture NOx rapidly. The ligands within EFeMC provide a favorable environment to prevent Fe precipitation, a major adsorption site for NO, and to stabilize the adsorbed-NO on a metal complex (NO@EFeMC). It is expected that EFeMC increases the solubility of NO in aqueous electrolytes. Kim et al.113 have recently unveiled a critical contribution of EFeMC in the selective reduction of NO for efficient NH3 production. The electrochemical reduction of NO in phosphate-buffered saline (PBS) electrolyte with the presence of EFeMC additives is diffusion-controlled because charges are taken from the electrode via an outer-sphere electron transfer of the Helmholtz plane where the NO@EFeMC species exist. This has led to nearly 100% efficiency for NH3 with a current density of 50 mA cm−2 at −0.165 V vs. RHE and negligible catalytic degradation for up to 120 h. Without EFeMC additives (or only PBS), the N2O molecule was a dominant product at low overpotential, indicating that N−N dimerization (N2O formation) favorably occurs in a more accessible free NO molecules environment, in line with the previous report.114 Such dimerization is less possible in PBS electrolytes containing EFeMC additive because the accessibility to free NO molecules is unavailable. However, the conversion efficiency decreased in a highly concentrated NO gas due to its desorption from EFeMC that may react with other species, such as NH3OH+, forming an N2O molecule. From a complementary computational study, the EDTA ligand is involved in the electron transfer process, providing favorable reaction-free energy and stabilizing the rate-limiting ∗HNO intermediate through H-bond formation. Ferrous-based electrolyte such as ferric chelate has been used to enhance NO conversion yield of 14.6 μmol h−1 cm−2 at −0.1 V with FE of 65.2%.25 The wide choice of additives will allow for tailoring the electrolytes toward the most favorable conditions for the electrochemical reduction of NOx to NH3.
Ferrous chelate can also be a promising electrolyte to drive the NOxRR because it acts as an effective electrolyte for NOx absorption in the NOxRR process based on the brown-ring reaction. Ferrous chelate can be fully reduced at a lower onset potential on rGO (reduced graphene oxide) electrode, allowing continuous NOx reduction. Additionally, ferrous chelate can be regenerated for reuse, making it a sustainable option for NOxRR.25 The application of ferrous chelate circumvents the influence of EDTA on NH3 detection and facilitates the two-phase transition from FeⅡ(NO)Cit to NH3. By reducing ferrous iron by coordination reaction, the potential associated with the HER and NOxRR is lowered, enabling selective activation of coordinated NOx in the ferrous electrolyte by the Au catalyst while preventing the reduction of ferrous to iron under relevant potentials.
Despite being a good capturer for NOx, the presence of metal complexes as electrolyte additives may need to be more friendly for producing high-purity NH3. Alternatively, Krzywda et al.89 performed electrochemical reduction of NOx in different electrolytes (H2SO4, HClO4, and Na2SO4) without additives in an attempt to solve the low solubility and limited mass transfers of the NOx. They found that NH3 and H2 were preferably formed in an acidic environment, while N2O and N2 existed in copious quantities at neutral electrolytes. In acidic electrolytes, the occurrence of HER is high because the requirement of the water dissociation step is fulfilled, which allows the transformation of NH4+ to NH3 during the scan direction toward positive potential. In neutral electrolytes, HER is kinetically sluggish and suppressed, thus increasing the chance of NO with OH− interaction with subsequent formation of N2O, N2, or NO2−/NO3− species. This work showed a positive indication that electrochemical reduction of NOx could be performed in additives-free electrolytes.
Design of gas diffusion electrode (GDE)
Besides the importance of electrolyte choice, electrode configuration and design may overcome the limitation of NOx. A GDE is an electrode that allows a gas to diffuse through it and react with a liquid electrolyte. The GDE consists of three distinctive layers, namely the gas diffusion layer (GDL), catalyst layer (CL), and microporous layer (MPL), which serves as a barrier between the GDL and CL, and are essential components in the construction of the electrode.115 The GDL allows the gas to diffuse uniformly and provides electrical conductivity, while the CL contains the active catalyst particles that facilitate the electrochemical reaction. The MPL enhances the gas transport and prevents the electrolyte from flooding the GDL. GDEs have been utilized for various electrochemical processes, such as CO2RR and NRR. Cheon et al.116 presented a new approach to converting NO to NH3 using a GDE. The reaction mechanism involves several steps, including (1) NO gas diffuses through the GDE and reaches the catalyst layer, where it is adsorbed and reduced to nitroxyl (HNO) by electrons from the external circuit; (2) HNO reacts with water to form nitrous acid (HNO2) and hydroxide ions (OH−); (3) HNO2 is further reduced to NO2−and protons (H+); (4) NO2− reacts with water to form nitric acid (HNO3) and OH−; (5) HNO3 is reduced to NH3 and O2 at the catalyst surface; and (6) NH3 dissolves in the electrolyte and diffuses out of the GDE, while O2 gas bubbles out of the electrode. This method offers a more convenient and cost-effective means of handling and storing NO, as dilute NO (0.1%) can be used instead of concentrated NO (10%). The GDE also provides improved mass transport and reaction kinetics for NO electroreduction, leading to high production rates of NH3 (up to 2.4 mmol h−1 cm−2) with high selectivity (up to 95%).
Cu foam is a conventional GDE for electrochemical reduction of N2 or NOx, which gives a high NH3 production rate with a decent FE> 90.0%. However, Cu foam electrodes are not sustained in acidic conditions, which impacts their long-standing use. Correspondingly, researchers developed a Cu-Ti hollow fiber electrode as a non-traditional electrode geometry to combat NOx mass transport limitations and enhance electrode stability in low pH environments.89 In a feed gas containing 10% of NO, NH3 production rates of 400 μmol h−1 cm−2 with FE of almost 90.0% were achieved in neutral electrolytes at −0.6 V vs. RHE, improving the practical ability of the flow cell system configuration. It will also be relevant when seeing NOx waste from the combustion process as a NOx source. However, the high NH3 production rate and FE emphasized in this work can still not compete with the state-of-the-art HB process, which requires 10 kWh kg−1 of NH3. This work has paved the way for using flow-through hollow fiber electrode configuration and low concentration of NO feeding while maintaining a high conversion rate regardless.
While GDE is critical for scaling up NOx electrocatalysis, the catalyst requirements may differ from those of h-type electrochemical cells. In a typical GDE, the flow of electrolytes containing saturated NOx needs to be continuously supplied to the catholyte to promote NOx reduction. In this regard, the mechanical shear may corrode and diminish the catalyst layer after some time. Therefore, a strong attachment between catalysts or the catalyst and current collector is necessary to prevent a decrease in the catalyst amount during the extended operating hours of catholyte flows. Additionally, the catalysts should rapidly capture and activate the NOx molecules in the catholyte due to this flow, ensuring effective conversion into NH3.
NOxRR electrocatalysts as cathode materials in Zn-NO batteries
The Zn-NO battery is a redox flow battery that employs Zn and NO as the active redox species and is operated in an H-type configuration. Within this arrangement, the cathodes, containing catalysts, are located in a cathodic electrolyte, which could either be a neutral or acidic solution. Meanwhile, an anodic electrolyte with a polished zinc plate is immersed in 1 M KOH or NaOH. A bipolar membrane separates these two electrolytes. The Zn-NO battery has gained significant attention due to its potential for high energy density, cost-effectiveness, and eco-friendliness. It is a proof-of-concept of achieving three goals at once, including NO elimination, sustainable NH3 production, and electrical energy output. The performance of Zn-NO is characterized by two parameters; power density (mW cm−2) and NH3 yield (μmol h−1 mgcat.−1) it delivers. This subchapter will discuss the mechanisms of storage and conversion, recent developments in cathode materials, and the limitations and challenges associated with Zn-NO batteries. The storage and conversion mechanisms of the batteries rely on reversible redox reactions between Zn and NO, and the electrochemical processes taking place at each electrode can be elucidated as follows during the charging-discharging cycle:
| Cathode: NO + 5H+ + 5e-→ NH3 + H2O | (Equation 18) |
| Anode: Zn + 2OH– → ZnO + H2O + 2e- | (Equation 19) |
| Overall: 5Zn + 2NO + 3H2O → 5ZnO + 2NH3 | (Equation 20) |
The materials used in Zn-NO batteries are critical for their performance and durability. The negative half-cell typically uses a Zn-based electroactive material at which, so far, only a polished Zn plate has been reported. The positive half-cell typically uses a NOxRR catalyst to reduce NOx to NH3, as shown in Figure 11. These materials must be carefully chosen to ensure high efficiency, stability, and safety in the Zn-NO system.
Figure 11.
Zn-NOx battery structures and working mechanism
Several previously discussed electrocatalysts have been employed as cathode materials in Zn-NO batteries, and some have demonstrated a remarkably long-lasting performance. These electrocatalysts have been observed to play a crucial role in improving the efficiency and stability of the battery by enhancing the conversion of nitrate ions into nitrite ions. This has resulted in better performance and increased durability of the batteries, making them more practical for various applications. The performances of NOxRR electrocatalyst cathode in Zn-NO battery are compiled in Table 3.
Table 3.
Performance of the NOxRR electrocatalysts as a cathode in Zn-NO battery
| Electrocatalysts | Power density | NH3 yield | Reference |
|---|---|---|---|
| MoC | 1.80 mW cm−2 | 57.5 μmol h−1 mgcat.−1 at 0.5 mA cm−2 | Meng, et al.110 |
| TiO2−x | 0.84 mW cm−2 | 14.2 μmol h−1 cm−2 | Li, et al.108 |
| NiO | 0.88 mW cm−2 | 13.4 μmol h−1 cm−2 | Liu, et al.98 |
| MoS2 nanosheet | 1.04 mW cm−2 | 24.2 μmol h−1 mgcat.−1 | Zhang, et al.34 |
| Ni2P | 1.53 mW cm−2 | 3.7 μmol h−1 mgcat.−1 | Mou, et al.105 |
| Binanoparticles/carbon nanosheet | 2.35 mW cm−2 | 20.9 μmol h−1 mgcat.−1 | Liu, et al.75 |
| Binanodendrites | 2.33 mW cm−2 | 5.0 μmol h−1 cm−2 | Lin, et al.92 |
| NiFe layered double hydroxide | 1.80 mW cm−2 | 32.0 μmol h−1 cm−2 | Meng, et al.117 |
| Single atomic Ce | 3.40 mW cm−2 | 18.2 μmol h−1 mgcat.−1 | Zhang, et al.118 |
| Fe2O3 nanorod | 1.18 mW cm−2 | 8.5 μmol h−1 mgcat.−1 | Liang, et al.99 |
| CoS1–x nanosheet | 2.06 mW cm−2 | 87.8 μmol h−1 mgcat.−1 | Zhang, et al.107 |
| CoP nanoarray | 0.496 mW cm−2 | 16.8 μmol h−1 mgcat.−1 | Liang, et al.119 |
| Hexagonal Co nanosheets | 4.66 mW cm−2 | 14.6 μmol h−1 mgcat.−1 | Wang, et al.120 |
There are generally several limitations and challenges associated with Zn-NO batteries. One of the main challenges is the low solubility of NO in most electrolytes, which limits the amount of NO that can be stored in the positive half-cell and reduces the overall energy density of the battery. Another challenge is the low conductivity of NO, which can reduce the rate at which the redox reactions occur and limit the battery’s power density. Additionally, the corrosion of Zn and the instability of NO under certain conditions can also affect the performance and durability of Zn-NO batteries. For example, the exposure of Zn to O2 or water can cause corrosion, while the exposure of NO to water or high temperatures can cause decomposition. These issues must be carefully addressed to ensure the long-term stability and reliability of Zn-NO batteries. Overall, Zn-NO batteries have the potential to provide a high-energy, low-cost, and environmentally friendly alternative to conventional batteries. However, further research is needed to overcome the challenges associated with their storage and conversion mechanisms, materials, and stability.
Conclusion and outlook
In summation, this review offers an extensive overview of the recent advancements in the application of electrochemical reduction for toxic NOx gas elimination. Electrochemical reduction presents an attractive option for NOx remediation in the air, as it boasts exceptional properties such as high efficiency, lack of secondary pollutants, and environmental friendliness. The triumph of electrochemical NOx reduction hinges on the quality of electrode materials employed. Therefore, a diverse range of electrocatalysts, including SACs, 2D materials, metal oxides, metal phosphides, molecular catalysts, etc., have been extensively studied, both computationally and experimentally, for their remarkable electrochemical performance and cost-effectiveness. Extensive progress has been made in exploring electrolyte additives and GDE concerning NOx electroreduction. The potential of NOx electroreduction catalysts as a Zn-NOx battery cathode has additionally proven crucial in NOx abatement, environmentally friendly NH3 production, and electricity generation. Nevertheless, unresolved challenges, as listed below, necessitate further research and development.
-
(1)
The reduction of NOx has been the subject of extensive research; however, the detailed mechanism is still uncertain due to the complex multiple electron-proton transfers and instability of intermediates. Although DFT calculations and MD simulations are valuable tools, they may only partially replicate the complex and dynamic electrochemical reaction process under realistic circumstances. To establish an optimal electrochemical system, developing a range of in situ electrochemical characterization methods is crucial to clarify the reaction intermediates, reaction pathways, final products, and structure-activity relationships (SARs).
-
(2)
Most NOx research centers on electrochemical reduction, where NH3 selectivity can reach nearly 80%. Other methods, such as photocatalysis, thermal catalysis, and photoelectrochemical processes, may prove promising for NOx treatment in the future. These methods offer the potential for cost-effectiveness due to the direct utilization of almost infinite solar energy to drive catalytic processes. For example, solar water splitting and CO2 conversion into fuels have been successfully tested. Therefore, there is an opportunity for solar conversion of NOx into NH3.
-
(3)
Metallic electrodes, mainly in the single-atom form, are the backbone of the electrochemical reduction of NOx, owing to their nearly full atomic utilization and remarkable catalytic activity resulting from a low-coordination surrounding and a greater number of accessible active sites. Nonetheless, these electrocatalysts are susceptible to oxidative dissolution, surface poisoning, and leaching, which can diminish their electrochemical activity and pose environmental hazards. Additionally, typical experiments only evaluate their performance over a few hours, which needs to be improved for industrial application or to compete with the current Haber-Bosch process. Hence, developing highly stable electrocatalysts capable of maintaining their performance for more than 1000 h is essential to realize the goal of transforming waste into valuable resources. Apart from stability, the cathode materials’ activity and selectivity should also be emphasized more in practical applications.
-
(4)
The conundrum of NOx solubility in aqueous solutions calls for an immediate resolution. One viable approach to this predicament involves the exploration of electrolytes, such as ionic liquids, which potentially enhance the solubility of NOx and augment the ionic conductivity of the electrochemical system as a whole. In addition, optimizing the GDE should be pursued with fervor to enable the continuous generation of NH3 from NOx. Further research is needed to fully explore these techniques’ potential and develop innovative solutions to tackle the challenges of NOx solubility in aqueous solutions.
-
(5)
Enhancing the efficiency of NOx mass transfer and maximizing NOx concentration on the catalyst surface are critical aspects of the structural design of a GDE-type electrolyzer. These optimizations enable the attainment of industrial-grade current density and superior electroreduction performance. However, to allow the large-scale application of NOx electrocatalysts in the future, it is essential to improve their stability. This necessitates the development of catalyst materials with enhanced performance, long-term stability, and reduced cost. Additionally, addressing challenges such as electrolyte flooding and inorganic salt crystallization in GDE systems is crucial. To overcome these obstacles, explore superhydrophobic materials and utilize emerging ionic liquid-based electrolytes. By tackling these issues, it becomes possible further to optimize the performance and durability of GDE-type electrolyzers, paving the way for their widespread implementation.
-
(6)
The selectivity of certain NOxRR catalysts may remain relatively low, resulting in less-than-optimal energy efficiency, often below 50%. While there is some value in the by-products of H2 and N2H4, their actual usefulness depends on various factors, including scale and geographic location. Unfortunately, NOxRR typically requires more negative potentials than normal HER (at equivalent partial current densities). Consequently, the production of H2 products during NOxRR comes at a significantly higher energy cost than in practical water electrolysis.
-
(7)
The utilization of NOx electrocatalysts in the cathode of a Zn-NOx battery represents a remarkable demonstration of the synergy between the eradication of NOx, NH3 synthesis, and electricity generation. Nevertheless, the implementation of this technology faces hurdles, such as the non-rechargeable nature of Zn-NOx, attributed to the limited activity of NOx catalysts to undergo the OER during the charging process. To surmount this challenge, efforts should be directed toward exploring innovative cathode materials with bifunctionality for both NOxRR and OER so that Zn-NOx can be charged and discharged.
-
(8)
As the generic system works at aqueous conditions, the environmental concerns induced by ammonium in the solution should be strictly considered. Ammonium has various effects depending on the specific circumstances and the system’s chemistry. The primary concern is that ammonium is potentially toxic and difficult to handle once converted to nitrate (NO3−) and nitrite (NO2−). Therefore, the operating parameters should be valued carefully to prevent the associated issues and ensure proper operations.
-
(9)
When evaluating the feasibility and sustainability of the electrochemical reduction of NOx, life cycle assessment can help quantify the environmental impact of the entire process, from raw material extraction to end-of-life disposal, and identify areas for improvement. Techno-economic analysis, on the other hand, can evaluate the economic viability of the process and identify ways to reduce production costs. By noting both environmental and economic factors, the development of electrochemical NOx reduction technologies can be optimized for maximum sustainability and commercialization potential.
Acknowledgments
A.H. acknowledges the financial support from The Indonesia Endowment Funds for Education and National Research and Innovation Agency under Riset dan Inovasi untuk Indonesia Maju (RIIM) with grant number 82/II.7/HK/2022. V.N.A. acknowledges support from the Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for a Research Activity Start-up (No. 21K21329). Z.W.S. acknowledges the Agency for Science, Technology and Research (Central Research Fund Award).
Declaration of interests
The authors state that there are no financial interests or personal relationships that could have potentially affected the work reported in this paper.
Contributor Information
Angga Hermawan, Email: angga.hermawan@brin.go.id.
Zhi Wei Seh, Email: sehzw@imre.a-star.edu.sg.
References
- 1.Brunekreef B., Holgate S.T. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 2.Lelieveld J., Evans J.S., Fnais M., Giannadaki D., Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 3.India State-Level Disease Burden Initiative Air Pollution Collaborators. Dey S., Gupta T., Dhaliwal R.S., Brauer M., Cohen A.J., Stanaway J.D., Beig G., Joshi T.K., Aggarwal A.N., et al. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: the Global Burden of Disease Study 2017. Lancet Planet. Health. 2019;3:e26–e39. doi: 10.1016/S2542-5196(18)30261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallero D. Fundamentals of Air Pollution. Elsevier; 2014. The Science of Air Pollution; pp. 43–81. [DOI] [Google Scholar]
- 5.Bai X., Chen H., Oliver B.G. The health effects of traffic-related air pollution: A review focused the health effects of going green. Chemosphere. 2022;289:133082. doi: 10.1016/j.chemosphere.2021.133082. [DOI] [PubMed] [Google Scholar]
- 6.Kampa M., Castanas E. Human health effects of air pollution. Environ. Pollut. 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Williams P.B., Alexis N., Barnes C., Bernstein I.L., Bernstein J.A., Nel A., Peden D., Diaz-Sanchez D., Tarlo S.M., Bernstein J.A. Health effects of air pollution. J. Allergy Clin. Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Choma E.F., Evans J.S., Gómez-Ibáñez J.A., Di Q., Schwartz J.D., Hammitt J.K., Spengler J.D. Health benefits of decreases in on-road transportation emissions in the United States from 2008 to 2017. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2107402118. e2107402118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masera K., Hossain A.K. Advancement of biodiesel fuel quality and NOx emission control techniques. Renew. Sustain. Energy Rev. 2023;178:113235. doi: 10.1016/j.rser.2023.113235. [DOI] [Google Scholar]
- 10.Mohd Shafie S.H., Mahmud M. Urban Air Pollutant from Motor Vehicle Emissions in Kuala Lumpur, Malaysia. Aerosol Air Qual. Res. 2020;20:2793–2804. doi: 10.4209/aaqr.2020.02.0074. [DOI] [Google Scholar]
- 11.Sarfraz M. 2021. Green Technologies to Combat Air Pollution; pp. 143–161. [DOI] [Google Scholar]
- 12.Wang S., Hao J. Air quality management in China: Issues, challenges, and options. J. Environ. Sci. 2012;24:2–13. doi: 10.1016/S1001-0742(11)60724-9. [DOI] [PubMed] [Google Scholar]
- 13.Xu W., Xie Y., Xia D., Ji L., Huang G. Visualizing invisible NOx emissions and remodeling policy requirements within bidirectional supply-demand control. J. Clean. Prod. 2022;380:134915. doi: 10.1016/j.jclepro.2022.134915. [DOI] [Google Scholar]
- 14.Erisman J.W., Sutton M.A., Galloway J., Klimont Z., Winiwarter W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008;1:636–639. doi: 10.1038/ngeo325. [DOI] [Google Scholar]
- 15.Klerke A., Christensen C.H., Nørskov J.K., Vegge T. Ammonia for hydrogen storage: challenges and opportunities. J. Mater. Chem. 2008;18:2304. doi: 10.1039/b720020j. [DOI] [Google Scholar]
- 16.Kitano M., Inoue Y., Yamazaki Y., Hayashi F., Kanbara S., Matsuishi S., Yokoyama T., Kim S.-W., Hara M., Hosono H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012;4:934–940. doi: 10.1038/nchem.1476. [DOI] [PubMed] [Google Scholar]
- 17.Amrillah T., Hermawan A., Alviani V.N., Seh Z.W., Yin S. MXenes and their derivatives as nitrogen reduction reaction catalysts: recent progress and perspectives. Mater. Today Energy. 2021;22:100864. doi: 10.1016/j.mtener.2021.100864. [DOI] [Google Scholar]
- 18.Guo X., Du H., Qu F., Li J. Recent progress in electrocatalytic nitrogen reduction. J. Mater. Chem. 2019;7:3531–3543. doi: 10.1039/C8TA11201K. [DOI] [Google Scholar]
- 19.Foster S.L., Bakovic S.I.P., Duda R.D., Maheshwari S., Milton R.D., Minteer S.D., Janik M.J., Renner J.N., Greenlee L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018;1:490–500. doi: 10.1038/s41929-018-0092-7. [DOI] [Google Scholar]
- 20.Smith C., Hill A.K., Torrente-Murciano L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020;13:331–344. doi: 10.1039/C9EE02873K. [DOI] [Google Scholar]
- 21.Hermawan A., Amrillah T., Alviani V.N., Raharjo J., Seh Z.W., Tsuchiya N. Upcycling air pollutants to fuels and chemicals via electrochemical reduction technology. J. Environ. Manag. 2023;334:117477. doi: 10.1016/j.jenvman.2023.117477. [DOI] [PubMed] [Google Scholar]
- 22.Daiyan R., Tran-Phu T., Kumar P., Iputera K., Tong Z., Leverett J., Khan M.H.A., Asghar Esmailpour A., Jalili A., Lim M., et al. Nitrate reduction to ammonium: From CuO defect engineering to waste NOx-to-NH3 economic feasibility. Energy Environ. Sci. 2021;14:3588–3598. doi: 10.1039/d1ee00594d. [DOI] [Google Scholar]
- 23.Wan H., Bagger A., Rossmeisl J. Electrochemical Nitric Oxide Reduction on Metal Surfaces. Angew Chem. Int. Ed. Engl. 2021;60:21966–21972. doi: 10.1002/anie.202108575. [DOI] [PubMed] [Google Scholar]
- 24.Soto-Hernández J., Santiago-Ramirez C.R., Ramirez-Meneses E., Luna-Trujillo M., Wang J.A., Lartundo-Rojas L., Manzo-Robledo A. Electrochemical reduction of NOx species at the interface of nanostructured Pd and PdCu catalysts in alkaline conditions. Appl. Catal. B Environ. 2019;259:118048. doi: 10.1016/j.apcatb.2019.118048. [DOI] [Google Scholar]
- 25.Xiong Y., Li Y., Wan S., Yu Y., Zhang S., Zhong Q. Ferrous-based electrolyte for simultaneous NO absorption and electroreduction to NH3 using Au/rGO electrode. J. Hazard Mater. 2022;430:128451. doi: 10.1016/j.jhazmat.2022.128451. [DOI] [PubMed] [Google Scholar]
- 26.Long J., Chen S., Zhang Y., Guo C., Fu X., Deng D., Xiao J. Direct Electrochemical Ammonia Synthesis from Nitric Oxide. Angew Chem. Int. Ed. Engl. 2020;59:9711–9718. doi: 10.1002/anie.202002337. [DOI] [PubMed] [Google Scholar]
- 27.Lin C.-Y., Hung W.-T., Wu C.-T., Ho K.-C. Electrochemical reduction of NO2 at a Pt/membrane electrode—Application to amperometric NO2 sensing. Sensor. Actuator. B Chem. 2009;136:32–38. doi: 10.1016/j.snb.2008.10.049. [DOI] [Google Scholar]
- 28.KUWABATA S., NAKAGAWA M. Electrochemical Reduction of NO, NO2−, and NO3− using Prussian Blue Film-coated Electrode. Electrochemistry. 2002;70:855–859. doi: 10.5796/electrochemistry.70.855. [DOI] [Google Scholar]
- 29.Hao R., Tian L., Wang C., Wang L., Liu Y., Wang G., Li W., Ozin G.A. Pollution to solution: A universal electrocatalyst for reduction of all NOx-based species to NH3. Chem Catal. 2022;2:622–638. doi: 10.1016/j.checat.2022.01.022. [DOI] [Google Scholar]
- 30.Merino L., Örnemark U., Toldrá F. Analysis of nitrite and nitrate in foods: overview of chemical, regulatory and analytical aspects. Adv. Food Nutr. Res. 2017;81:65–107. doi: 10.1016/bs.afnr.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Kim D.H., Ringe S., Kim H., Kim S., Kim B., Bae G., Oh H.-S., Jaouen F., Kim W., Kim H., Choi C.H. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 2021;12:1856. doi: 10.1038/s41467-021-22147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z., Ma Z., Liang J., Ren Y., Li T., Xu S., Liu Q., Li N., Tang B., Liu Y., et al. MnO2 nanoarray with oxygen vacancies: An efficient catalyst for NO electroreduction to NH3 at ambient conditions. Mater. Today Phys. 2022;22:100586. doi: 10.1016/j.mtphys.2021.100586. [DOI] [Google Scholar]
- 33.Foral M.J., Langer S.H. Sulfur coverage effects on the reduction of dilute nitric oxide at platinum black gas diffusion electrodes. Electrochim. Acta. 1991;36:299–307. doi: 10.1016/0013-4686(91)85253-4. [DOI] [Google Scholar]
- 34.Zhang L., Liang J., Wang Y., Mou T., Lin Y., Yue L., Li T., Liu Q., Luo Y., Li N., et al. High-Performance Electrochemical NO Reduction into NH 3 by MoS 2 Nanosheet. Angew Chem. Int. Ed. Engl. 2021;60:25263–25268. doi: 10.1002/anie.202110879. [DOI] [PubMed] [Google Scholar]
- 35.Liang J., Zhou Q., Mou T., Chen H., Yue L., Luo Y., Liu Q., Hamdy M.S., Alshehri A.A., Gong F., Sun X. FeP nanorod array: A high-efficiency catalyst for electroreduction of NO to NH3 under ambient conditions. Nano Res. 2022;15:4008–4013. doi: 10.1007/s12274-022-4174-0. [DOI] [Google Scholar]
- 36.Andana T., Rappé K.G., Gao F., Szanyi J., Pereira-Hernandez X., Wang Y. Recent advances in hybrid metal oxide–zeolite catalysts for low-temperature selective catalytic reduction of NOx by ammonia. Appl. Catal. B Environ. 2021;291:120054. doi: 10.1016/j.apcatb.2021.120054. [DOI] [Google Scholar]
- 37.Zhao S., Luo C., Tang B., Chen L., Xiang M., Dai J., Li H., Sun Z. Research progress on selective catalytic reduction (SCR) catalysts for NO removal from coal-fired flue gas. Int. J. Surg. Case Rep. 2022;97:107432. doi: 10.1016/j.fuproc.2022.107432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan Y., Liu Y., Lv Q., Wang B., Che D. Review on the selective catalytic reduction of NO with H2 by using novel catalysts. J. Environ. Chem. Eng. 2021;9:106770. doi: 10.1016/j.jece.2021.106770. [DOI] [Google Scholar]
- 39.Kim H.-S., Kasipandi S., Kim J., Kang S.-H., Kim J.-H., Ryu J.-H., Bae J.-W. Current Catalyst Technology of Selective Catalytic Reduction (SCR) for NOx Removal in South Korea. Catalysts. 2020;10:52. doi: 10.3390/catal10010052. [DOI] [Google Scholar]
- 40.Wang Z.y., Guo R.t., Shi X., Liu X.y., Qin H., Liu Y.z., Duan C.p., Guo D.y., Pan W.g. The superior performance of CoMnOx catalyst with ball-flowerlike structure for low-temperature selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2020;381:122753. doi: 10.1016/j.cej.2019.122753. [DOI] [Google Scholar]
- 41.D’Agostino C., Chansai S., Gladden L.F., Hardacre C. Correlating the strength of reducing agent adsorption with Ag/Al2O3 catalyst performances in selective catalytic reduction (SCR) of NOx. Catal. Today. 2022;384–386:274–278. doi: 10.1016/j.cattod.2021.01.013. [DOI] [Google Scholar]
- 42.Pappas D.K., Boningari T., Boolchand P., Smirniotis P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature Selective Catalytic Reduction (SCR) of NO x by NH 3. J. Catal. 2016;334:1–13. doi: 10.1016/j.jcat.2015.11.013. [DOI] [Google Scholar]
- 43.Guan B., Zhan R., Lin H., Huang Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014;66:395–414. doi: 10.1016/j.applthermaleng.2014.02.021. [DOI] [Google Scholar]
- 44.Bartholomew C.H. Mechanisms of catalyst deactivation. Appl. Catal. Gen. 2001;212:17–60. doi: 10.1016/S0926-860X(00)00843-7. [DOI] [Google Scholar]
- 45.Papageorgaki S., Reklaitis G.V. Retrofitting a general multipurpose batch chemical plant. Ind. Eng. Chem. Res. 1993;32:345–362. doi: 10.1021/ie00014a012. [DOI] [Google Scholar]
- 46.Alves L., Holz L.I., Fernandes C., Ribeirinha P., Mendes D., Fagg D.P., Mendes A. A comprehensive review of NOx and N2O mitigation from industrial streams. Renew. Sustain. Energy Rev. 2022;155:111916. doi: 10.1016/j.rser.2021.111916. [DOI] [Google Scholar]
- 47.Radojevic M. Reduction of nitrogen oxides in flue gases. Environ. Pollut. 1998;102:685–689. doi: 10.1016/S0269-7491(98)80099-7. [DOI] [Google Scholar]
- 48.Li T., Rabuni M.F., Kleiminger L., Wang B., Kelsall G.H., Hartley U.W., Li K. A highly-robust solid oxide fuel cell (SOFC): simultaneous greenhouse gas treatment and clean energy generation. Energy Environ. Sci. 2016;9:3682–3686. doi: 10.1039/C6EE02562E. [DOI] [Google Scholar]
- 49.Sun J., Alam D., Daiyan R., Masood H., Zhang T., Zhou R., Cullen P.J., Lovell E.C., Jalili A.R., Amal R., Amal R. A hybrid plasma electrocatalytic process for sustainable ammonia production. Energy Environ. Sci. 2021;14:865–872. doi: 10.1039/D0EE03769A. [DOI] [Google Scholar]
- 50.Cherkasov N., Ibhadon A.O., Fitzpatrick P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. Process Intensif. 2015;90:24–33. doi: 10.1016/j.cep.2015.02.004. [DOI] [Google Scholar]
- 51.Ouyang B., Zhang Y., Xia X., Rawat R.S., Fan H.J. A brief review on plasma for synthesis and processing of electrode materials. Mater. Today Nano. 2018;3:28–47. doi: 10.1016/j.mtnano.2018.11.002. [DOI] [Google Scholar]
- 52.Liang H., Ming F., Alshareef H.N. Applications of Plasma in Energy Conversion and Storage Materials. Adv. Energy Mater. 2018;8:1801804. doi: 10.1002/aenm.201801804. [DOI] [Google Scholar]
- 53.Zhang Y., Rawat R.S., Fan H.J. Plasma for Rapid Conversion Reactions and Surface Modification of Electrode Materials. Small Methods. 2017;1:1700164. doi: 10.1002/smtd.201700164. [DOI] [Google Scholar]
- 54.He C., Wang J., Fu L., Zhao C., Huo J. Associative vs. dissociative mechanism: Electrocatalysis of nitric oxide to ammonia. Chin. Chem. Lett. 2022;33:1051–1057. doi: 10.1016/j.cclet.2021.09.009. [DOI] [Google Scholar]
- 55.Niu H., Zhang Z., Wang X., Wan X., Kuai C., Guo Y. A Feasible Strategy for Identifying Single-Atom Catalysts Toward Electrochemical NO-to-NH 3 Conversion. Small. 2021;17:2102396. doi: 10.1002/smll.202102396. [DOI] [PubMed] [Google Scholar]
- 56.Zang Y., Wu Q., Wang S., Huang B., Dai Y., Ma Y. High-Throughput Screening of Efficient Biatom Catalysts Based on Monolayer Carbon Nitride for the Nitric Oxide Reduction Reaction. J. Phys. Chem. Lett. 2022;13:527–535. doi: 10.1021/acs.jpclett.1c03938. [DOI] [PubMed] [Google Scholar]
- 57.Liu S., Xing G., Liu J.y. Computational screening of single-atom catalysts for direct electrochemical NH3 synthesis from NO on defective boron phosphide monolayer. Appl. Surf. Sci. 2023;611:155764. doi: 10.1016/j.apsusc.2022.155764. [DOI] [Google Scholar]
- 58.Wu J., Yu Y.-X. A theoretical descriptor for screening efficient NO reduction electrocatalysts from transition-metal atoms on N-doped BP monolayer. J. Colloid Interface Sci. 2022;623:432–444. doi: 10.1016/j.jcis.2022.05.034. [DOI] [PubMed] [Google Scholar]
- 59.Shi P., Pang D., Zhang Z., Lin L., He C. Transition metals embedded Pt (1 0 0) surface as an electrocatalysts for NO reduction reaction: A first-principles study. Appl. Surf. Sci. 2023;619:156744. doi: 10.1016/j.apsusc.2023.156744. [DOI] [Google Scholar]
- 60.Tursun M., Wu C. Single Transition Metal Atoms Anchored on Defective MoS 2 Monolayers for the Electrocatalytic Reduction of Nitric Oxide into Ammonia and Hydroxylamine. Inorg. Chem. 2022;61:17448–17458. doi: 10.1021/acs.inorgchem.2c02247. [DOI] [PubMed] [Google Scholar]
- 61.Huang B., Chen B., Zhu G., Peng J., Zhang P., Qian Y., Li N. Electrochemical Ammonia Synthesis via NO Reduction on 2D-MOF. ChemPhysChem. 2022;23:e202100785. doi: 10.1002/cphc.202100785. [DOI] [PubMed] [Google Scholar]
- 62.Lv X., Mou T., Li J., Kou L., Frauenheim T. Tunable Surface Chemistry in Heterogeneous Bilayer Single-Atom Catalysts for Electrocatalytic NO x Reduction to Ammonia. Adv. Funct. Mater. 2022;32:2201262. doi: 10.1002/adfm.202201262. [DOI] [Google Scholar]
- 63.Zhou Q., Gong F., Xie Y., Xia D., Hu Z., Wang S., Liu L., Xiao R. A general strategy for designing metal-free catalysts for highly-efficient nitric oxide reduction to ammonia. Fuel. 2022;310:122442. doi: 10.1016/j.fuel.2021.122442. [DOI] [Google Scholar]
- 64.He C., Yang H., Xi M., Fu L., Huo J., Zhao C. Efficient electrocatalytic reduction of NO to ammonia on BC3 nanosheets. Environ. Res. 2022;212:113479. doi: 10.1016/j.envres.2022.113479. [DOI] [PubMed] [Google Scholar]
- 65.Zhao S., Li Y., Guo Z., Tang C., Sa B., Miao N., Zhou J., Sun Z. Breaking scaling relations in nitric oxide reduction by surface functionalization of MXenes. J. Mater. Chem. 2022;10:25201–25211. doi: 10.1039/D2TA06354A. [DOI] [Google Scholar]
- 66.Amrillah T., Supandi A.R., Puspasari V., Hermawan A., Seh Z.W. MXene-Based Photocatalysts and Electrocatalysts for CO2 Conversion to Chemicals. Trans. Tianjin Univ. 2022;28:307–322. doi: 10.1007/s12209-022-00328-9. [DOI] [Google Scholar]
- 67.Zhao Y., Zheng L., Jiang D., Xia W., Xu X., Yamauchi Y., Ge J., Tang J. Nanoengineering Metal–Organic Framework-Based Materials for Use in Electrochemical CO2 Reduction Reactions. Small. 2021;17:20065900–e2006618. doi: 10.1002/smll.202006590. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Wang D., Li Y. Rational Design of Single-Atom Site Electrocatalysts: From Theoretical Understandings to Practical Applications. Adv. Mater. 2021;33:2008151. doi: 10.1002/adma.202008151. [DOI] [PubMed] [Google Scholar]
- 69.Qiao B., Wang A., Yang X., Allard L.F., Jiang Z., Cui Y., Liu J., Li J., Zhang T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011;3:634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- 70.Su J., Ge R., Dong Y., Hao F., Chen L. Recent progress in single-atom electrocatalysts: concept, synthesis, and applications in clean energy conversion. J. Mater. Chem. 2018;6:14025–14042. doi: 10.1039/C8TA04064H. [DOI] [Google Scholar]
- 71.Peng X., Mi Y., Bao H., Liu Y., Qi D., Qiu Y., Zhuo L., Zhao S., Sun J., Tang X., et al. Ambient electrosynthesis of ammonia with efficient denitration. Nano Energy. 2020;78:105321. doi: 10.1016/j.nanoen.2020.105321. [DOI] [Google Scholar]
- 72.Chen K., Zhang G., Li X., Zhao X., Chu K. Electrochemical NO reduction to NH3 on Cu single atom catalyst. Nano Res. 2022;16:5857–5863. doi: 10.1007/s12274-023-5384-9. [DOI] [Google Scholar]
- 73.Chen L., Sun W., Xu Z., Hao M., Li B., Liu X., Ma J., Wang L., Li C., Wang W. Ultrafine Cu nanoparticles decorated porous TiO2 for high-efficient electrocatalytic reduction of NO to synthesize NH3. Ceram. Int. 2022;48:21151–21161. doi: 10.1016/j.ceramint.2022.04.006. [DOI] [Google Scholar]
- 74.Choi J., Du H.-L., Nguyen C.K., Suryanto B.H.R., Simonov A.N., MacFarlane D.R. Electroreduction of Nitrates, Nitrites, and Gaseous Nitrogen Oxides: A Potential Source of Ammonia in Dinitrogen Reduction Studies. ACS Energy Lett. 2020;5:2095–2097. doi: 10.1021/acsenergylett.0c00924. [DOI] [Google Scholar]
- 75.Liu Q., Lin Y., Yue L., Liang J., Zhang L., Li T., Luo Y., Liu M., You J., Alshehri A.A., et al. Bi nanoparticles/carbon nanosheet composite: A high-efficiency electrocatalyst for NO reduction to NH3. Nano Res. 2022;15:5032–5037. doi: 10.1007/s12274-022-4283-9. [DOI] [Google Scholar]
- 76.Li X., Chen K., Lu X., Ma D., Chu K. Atomically dispersed Co catalyst for electrocatalytic NO reduction to NH3. Chem. Eng. J. 2023;454:140333. doi: 10.1016/j.cej.2022.140333. [DOI] [Google Scholar]
- 77.Chen K., Wang J., Zhang H., Ma D., Chu K. Self-Tandem Electrocatalytic NO Reduction to NH 3 on a W Single-Atom Catalyst. Nano Lett. 2023;23:1735–1742. doi: 10.1021/acs.nanolett.2c04444. [DOI] [PubMed] [Google Scholar]
- 78.Chen K., Zhang Y., Xiang J., Zhao X., Li X., Chu K. p-Block Antimony Single-Atom Catalysts for Nitric Oxide Electroreduction to Ammonia. ACS Energy Lett. 2023;8:1281–1288. doi: 10.1021/acsenergylett.2c02882. [DOI] [Google Scholar]
- 79.Chen K., Wang G., Guo Y., Ma D., Chu K. Iridium single-atom catalyst for highly efficient NO electroreduction to NH3. Nano Res. 2023 doi: 10.1007/s12274-023-5556-7. [DOI] [Google Scholar]
- 80.Chen K., Zhang N., Wang F., Kang J., Chu K. Main-group indium single-atom catalysts for electrocatalytic NO reduction to NH 3. J. Mater. Chem. 2023;11:6814–6819. doi: 10.1039/D3TA00606A. [DOI] [Google Scholar]
- 81.Chen K., Zhang Y., Du W., Guo Y., Chu K. Atomically isolated and unsaturated Sb sites created on Sb 2 S 3 for highly selective NO electroreduction to NH 3. Inorg. Chem. Front. 2023;10:2708–2715. doi: 10.1039/D3QI00268C. [DOI] [Google Scholar]
- 82.Hermawan A., Septiani N.L.W., Taufik A., Yuliarto B., Suyatman, Yin S. Advanced Strategies to Improve Performances of Molybdenum-Based Gas Sensors. Nano-Micro Lett. 2021;13:207. doi: 10.1007/s40820-021-00724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manzeli S., Ovchinnikov D., Pasquier D., Yazyev O.V., Kis A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017;2:17033. doi: 10.1038/natrevmats.2017.33. [DOI] [Google Scholar]
- 84.Tursun M., Wu C. NO Electroreduction by Transition Metal Dichalcogenides with Chalcogen Vacancies. Chemelectrochem. 2021;8:3113–3122. doi: 10.1002/celc.202100790. [DOI] [Google Scholar]
- 85.Chen K., Shen P., Zhang N., Ma D., Chu K. Electrocatalytic NO Reduction to NH 3 on Mo 2 C Nanosheets. Inorg. Chem. 2023;62:653–658. doi: 10.1021/acs.inorgchem.2c03714. [DOI] [PubMed] [Google Scholar]
- 86.Ouyang L., Zhou Q., Liang J., Zhang L., Yue L., Li Z., Li J., Luo Y., Liu Q., Li N., et al. High-efficiency NO electroreduction to NH3 over honeycomb carbon nanofiber at ambient conditions. J. Colloid Interface Sci. 2022;616:261–267. doi: 10.1016/j.jcis.2022.02.074. [DOI] [PubMed] [Google Scholar]
- 87.Liang J., Liu P., Li Q., Li T., Yue L., Luo Y., Liu Q., Li N., Tang B., Alshehri A.A., et al. Amorphous Boron Carbide on Titanium Dioxide Nanobelt Arrays for High-Efficiency Electrocatalytic NO Reduction to NH 3. Angew Chem. Int. Ed. Engl. 2022;61:2022020877–e202202095. doi: 10.1002/anie.202202087. [DOI] [PubMed] [Google Scholar]
- 88.Sharif H.M.A., Li T., Mahmood N., Ahmad M., Xu J., Mahmood A., Djellabi R., Yang B. Thermally activated epoxy-functionalized carbon as an electrocatalyst for efficient NOx reduction. Carbon N. Y. 2021;182:516–524. doi: 10.1016/j.carbon.2021.06.042. [DOI] [Google Scholar]
- 89.Krzywda P.M., Paradelo Rodríguez A., Benes N.E., Mei B.T., Mul G. Effect of Electrolyte and Electrode Configuration on Cu-Catalyzed Nitric Oxide Reduction to Ammonia. Chemelectrochem. 2022;9:1–7. doi: 10.1002/celc.202101273. [DOI] [Google Scholar]
- 90.Shi J., Wang C., Yang R., Chen F., Meng N., Yu Y., Zhang B. Promoting nitric oxide electroreduction to ammonia over electron-rich Cu modulated by Ru doping. Sci. China Chem. 2021;64:1493–1497. doi: 10.1007/s11426-021-1073-5. [DOI] [Google Scholar]
- 91.Zhang H., Li Y., Cheng C., Zhou J., Yin P., Wu H., Liang Z., Zhang J., Yun Q., Wang A.L., et al. Isolated Electron-Rich Ruthenium Atoms in Intermetallic Compounds for Boosting Electrochemical Nitric Oxide Reduction to Ammonia. Angew Chem. Int. Ed. Engl. 2023;62:e202213351. doi: 10.1002/anie.202213351. [DOI] [PubMed] [Google Scholar]
- 92.Lin Y., Liang J., Li H., Zhang L., Mou T., Li T., Yue L., Ji Y., Liu Q., Luo Y., et al. Bi nanodendrites for highly efficient electrocatalytic NO reduction to NH3 at ambient conditions. Mater. Today Phys. 2022;22:100611. doi: 10.1016/j.mtphys.2022.100611. [DOI] [Google Scholar]
- 93.Ko B.H., Hasa B., Shin H., Zhao Y., Jiao F. Electrochemical Reduction of Gaseous Nitrogen Oxides on Transition Metals at Ambient Conditions. J. Am. Chem. Soc. 2022;144:1258–1266. doi: 10.1021/jacs.1c10535. [DOI] [PubMed] [Google Scholar]
- 94.Li X., Gao Y., Li Y., Li Y., Liu H., Yang Z., Wu H., Hu Y. Electrocatalytic Reduction of Low-Concentration Nitric Oxide into Ammonia over Ru Nanosheets. ACS Energy Lett. 2022;300:1187–1195. doi: 10.1021/acsenergylett.2c00207. [DOI] [Google Scholar]
- 95.Wei T., Bao H., Wang X., Zhang S., Liu Q., Luo J., Liu X. Ionic Liquid-Assisted Electrocatalytic NO Reduction to NH 3 by P-Doped MoS 2. ChemCatChem. 2023;15 doi: 10.1002/cctc.202201411. [DOI] [Google Scholar]
- 96.Chen K., Wang J., Kang J., Lu X., Zhao X., Chu K. Atomically Fe-doped MoS2−x with Fe-Mo dual sites for efficient electrocatalytic NO reduction to NH3. Appl. Catal. B Environ. 2023;324:122241. doi: 10.1016/j.apcatb.2022.122241. [DOI] [Google Scholar]
- 97.Li K., Shi Z., Wang L., Wang W., Liu Y., Cheng H., Yang Y., Zhang L. Efficient electrochemical NO reduction to NH3 over metal-free g-C3N4 nanosheets and the role of interface microenvironment. J. Hazard Mater. 2023;448:130890. doi: 10.1016/j.jhazmat.2023.130890. [DOI] [PubMed] [Google Scholar]
- 98.Liu P., Liang J., Wang J., Zhang L., Li J., Yue L., Ren Y., Li T., Luo Y., Li N., et al. High-performance NH 3 production via NO electroreduction over a NiO nanosheet array. Chem. Commun. 2021;57:13562–13565. doi: 10.1039/D1CC06113E. [DOI] [PubMed] [Google Scholar]
- 99.Liang J., Chen H., Mou T., Zhang L., Lin Y., Yue L., Luo Y., Liu Q., Li N., Alshehri A.A., et al. Coupling denitrification and ammonia synthesis via selective electrochemical reduction of nitric oxide over Fe 2 O 3 nanorods. J. Mater. Chem. 2022;10:6454–6462. doi: 10.1039/D2TA00744D. [DOI] [Google Scholar]
- 100.Zhang S., Liu Q., Tang X., Zhou Z., Fan T., You Y., Zhang Q., Zhang S., Luo J., Liu X. Electrocatalytic reduction of NO to NH3 in ionic liquids by P-doped TiO2 nanotubes. Front. Chem. Sci. Eng. 2023;17:726–734. doi: 10.1007/s11705-022-2274-8. [DOI] [Google Scholar]
- 101.Li X., Xing W., Hu T., Luo K., Wang J., Tang W. Recent advances in transition-metal phosphide electrocatalysts: Synthetic approach, improvement strategies and environmental applications. Coord. Chem. Rev. 2022;473:214811. doi: 10.1016/j.ccr.2022.214811. [DOI] [Google Scholar]
- 102.Pu Z., Liu T., Amiinu I.S., Cheng R., Wang P., Zhang C., Ji P., Hu W., Liu J., Mu S. Transition-Metal Phosphides: Activity Origin, Energy-Related Electrocatalysis Applications, and Synthetic Strategies. Adv. Funct. Mater. 2020;30:2004009. doi: 10.1002/adfm.202004009. [DOI] [Google Scholar]
- 103.Shi Y., Li M., Yu Y., Zhang B. Recent advances in nanostructured transition metal phosphides: synthesis and energy-related applications. Energy Environ. Sci. 2020;13:4564–4582. doi: 10.1039/D0EE02577A. [DOI] [Google Scholar]
- 104.Zhang H., Wang G., Wang C., Liu Y., Yang Y., Wang C., Jiang W., Fu L., Xu J. CoP nanowires on carbon cloth for electrocatalytic NOx− reduction to ammonia. J. Electroanal. Chem. 2022;910:116171. doi: 10.1016/j.jelechem.2022.116171. [DOI] [Google Scholar]
- 105.Mou T., Liang J., Ma Z., Zhang L., Lin Y., Li T., Liu Q., Luo Y., Liu Y., Gao S., et al. High-efficiency electrohydrogenation of nitric oxide to ammonia on a Ni 2 P nanoarray under ambient conditions. J. Mater. Chem. 2021;9:24268–24275. doi: 10.1039/D1TA07455E. [DOI] [Google Scholar]
- 106.Zhang G., Wan Y., Zhao H., Guo Y., Chu K. A metal-free catalyst for electrocatalytic NO reduction to NH 3. Dalton Trans. 2023;52:6248–6253. doi: 10.1039/D3DT00994G. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L., Zhou Q., Liang J., Yue L., Li T., Luo Y., Liu Q., Li N., Tang B., Gong F., et al. Enhancing Electrocatalytic NO Reduction to NH 3 by the CoS Nanosheet with Sulfur Vacancies. Inorg. Chem. 2022;61:8096–8102. doi: 10.1021/acs.inorgchem.2c01112. [DOI] [PubMed] [Google Scholar]
- 108.Li Z., Zhou Q., Liang J., Zhang L., Fan X., Zhao D., Cai Z., Li J., Zheng D., He X., et al. Defective TiO 2− x for High-Performance Electrocatalytic NO Reduction toward Ambient NH 3 Production. Small. 2023;19:2300291. doi: 10.1002/smll.202300291. [DOI] [PubMed] [Google Scholar]
- 109.Li X., Zhang G., Shen P., Zhao X., Chu K. A defect engineered p-block SnS 2− x catalyst for efficient electrocatalytic NO reduction to NH 3. Inorg. Chem. Front. 2023;10:280–287. doi: 10.1039/D2QI02118H. [DOI] [Google Scholar]
- 110.Meng G., Jin M., Wei T., Liu Q., Zhang S., Peng X., Luo J., Liu X. MoC nanocrystals confined in N-doped carbon nanosheets toward highly selective electrocatalytic nitric oxide reduction to ammonia. Nano Res. 2022;15:8890–8896. doi: 10.1007/s12274-022-4747-y. [DOI] [Google Scholar]
- 111.Qi D., Lv F., Wei T., Jin M., Meng G., Zhang S., Liu Q., Liu W., Ma D., Hamdy M.S., et al. High-efficiency electrocatalytic NO reduction to NH 3 by nanoporous VN. Nano Res. Energy. 2022;1:e9120022. doi: 10.26599/NRE.2022.9120022. [DOI] [Google Scholar]
- 112.Chen K., Tian Y., Li Y., Liu Y., Chu K. Amorphous NiB 2 for electroreduction of NO to NH 3. J. Mater. Chem. 2023;11:7409–7414. doi: 10.1039/D3TA00394A. [DOI] [Google Scholar]
- 113.Kim D., Shin D., Heo J., Lim H., Lim J.A., Jeong H.M., Kim B.S., Heo I., Oh I., Lee B., et al. Unveiling Electrode-Electrolyte Design-Based NO Reduction for NH3Synthesis. ACS Energy Lett. 2020;5:3647–3656. doi: 10.1021/acsenergylett.0c02082. [DOI] [Google Scholar]
- 114.De Vooys A.C.A., Beltramo G.L., Van Riet B., Van Veen J.A.R., Koper M.T.M. Mechanisms of electrochemical reduction and oxidation of nitric oxide. Electrochim. Acta. 2004;49:1307–1314. doi: 10.1016/j.electacta.2003.07.020. [DOI] [Google Scholar]
- 115.Rabiee H., Ge L., Zhang X., Hu S., Li M., Yuan Z. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review. Energy Environ. Sci. 2021;14:1959–2008. doi: 10.1039/D0EE03756G. [DOI] [Google Scholar]
- 116.Cheon S., Kim W.J., Kim D.Y., Kwon Y., Han J.-I. Electro-synthesis of Ammonia from Dilute Nitric Oxide on a Gas Diffusion Electrode. ACS Energy Lett. 2022;7:958–965. doi: 10.1021/acsenergylett.1c02552. [DOI] [Google Scholar]
- 117.Meng G., Wei T., Liu W., Li W., Zhang S., Liu W., Liu Q., Bao H., Luo J., Liu X. NiFe layered double hydroxide nanosheet array for high-efficiency electrocatalytic reduction of nitric oxide to ammonia. Chem. Commun. 2022;58:8097–8100. doi: 10.1039/D2CC02463B. [DOI] [PubMed] [Google Scholar]
- 118.Zhang W., Qin X., Wei T., Liu Q., Luo J., Liu X. Single atomic cerium sites anchored on nitrogen-doped hollow carbon spheres for highly selective electroreduction of nitric oxide to ammonia. J. Colloid Interface Sci. 2023;638:650–657. doi: 10.1016/j.jcis.2023.02.026. [DOI] [PubMed] [Google Scholar]
- 119.Liang J., Hu W.-F., Song B., Mou T., Zhang L., Luo Y., Liu Q., Alshehri A.A., Hamdy M.S., Yang L.-M., Sun X. Efficient nitric oxide electroreduction toward ambient ammonia synthesis catalyzed by a CoP nanoarray. Inorg. Chem. Front. 2022;9:1366–1372. doi: 10.1039/D2QI00002D. [DOI] [Google Scholar]
- 120.Wang D., Chen Z.-W., Gu K., Chen C., Liu Y., Wei X., Singh C.V., Wang S. Hexagonal Cobalt Nanosheets for High-Performance Electrocatalytic NO Reduction to NH 3. J. Am. Chem. Soc. 2023;145:6899–6904. doi: 10.1021/jacs.3c00276. [DOI] [PubMed] [Google Scholar]