Abstract
Exonuclease domain mutation (EDM) in polymerase epsilon (POLE)-mutated colorectal cancer patients is characterized by specific clinical features and a very high tumor mutation burden (TMB). The therapeutic effectiveness of immune checkpoint inhibitors (ICIs) for the treatment of colorectal cancer in patients with POLE mutations is poorly defined. Our case represents a young-onset colon cancer patient who has had a continued response to programmed cell death protein 1 (PD1) blockade alongside clearance of circulating tumor DNA (ctDNA) using a tumor-informed approach. Utilizing ctDNA kinetics to assess minimal residual disease (MRD) in the context of colorectal cancer is a very important topic. Furthermore, utilizing ctDNA kinetics in response to immunotherapy is something that is relevant to all tumor types undergoing immunotherapy. Recently, several landmark articles have proposed this as a promising approach. There is, however, limited information in the literature showing the feasibility of such an approach. Our case report is going to be of value, both from a scientific as well as a clinical standpoint. This is particularly relevant given the rise of colorectal cancers in young individuals.
Keywords: metastatic colorectal cancer, pole mutation, minimal residual disease assay, cancer immunotherapy, circulating tumor dna (ctdna)
Introduction
Immunotherapy in the form of pembrolizumab (PD-1-blockade) has now been approved first-line for patients with mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC) based on the results from the Keynote-177 study [1]. PD-1 blockade when combined with a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor too has shown very promising brisk and durable responses [2]. However, dMMR/MSI-H CRC constitutes only 4% to 5% of tumors in the metastatic setting [3]. For the mismatch repair proficient/microsatellite stable (pMMR/MSS) CRC, immunotherapy, unfortunately, does not work [4-5]. The reason why immunotherapy works so well for dMMR/MSI-H CRC versus pMMR/MSS CRC is secondary to the hypermutated nature of mismatch repair-deficient tumors. In addition, this immune sensitivity is given by the continuous generation of new mutations, providing the production of neoantigens activating the immune system over time [6]. In the initial landmark study by Le and colleagues, dMMR/MSI- H CRC had, on average approximately 1782 somatic mutations compared to 73 in pMMR/MSS CRC (p-value = 0.007) [5]. While immunotherapy does not work for pMMR/MSS CRC, one rare exception to this rule is the so-called ultra-hypermutated CRC resulting from a mutation in the exonuclease domain of the catalytic subunit of the DNA polymerase epsilon (POLE) [7]. Here, we present a case of a young patient with POLE-mutated CRC with near complete ongoing response to immune checkpoint blockade.
Case presentation
We present a case of a 50-year-old male with a longstanding history of ulcerative colitis who got diagnosed with advanced unresectable colon cancer at the hepatic flexure with extension into the peri-colonic fascia in the setting of hematochezia. The patient's medical history was significant for a 50-pound weight loss in the last five months preceding the diagnosis alongside worsening abdominal pain. On imaging, there were concerns for multiple enlarged abdominal lymph nodes as well as the potential involvement of the second and third part of the duodenum by the hepatic flexure carcinoma. The cancer was considered to be unresectable and potentially metastatic; therefore, systemic therapy was recommended. A biopsy of the hepatic flexure mass came back as adenocarcinoma with squamous differentiation. Given his otherwise young age and good performance status, the patient received triplet chemotherapy with targeted therapy (FOLFOXIRI with bevacizumab). He had an excellent clinical and radiographic response allowing for conversion surgery. Final pathology came back as ypT4bN0 (0 out of 30 lymph nodes), with no evidence of metastases and grade 3 tumor regression was noted. However, there were concerns about the margins being positive on the duodenal side as well as tumor deposits noted with the specimen. The patient underwent a subtotal colectomy given his prior history of inflammatory bowel disease.
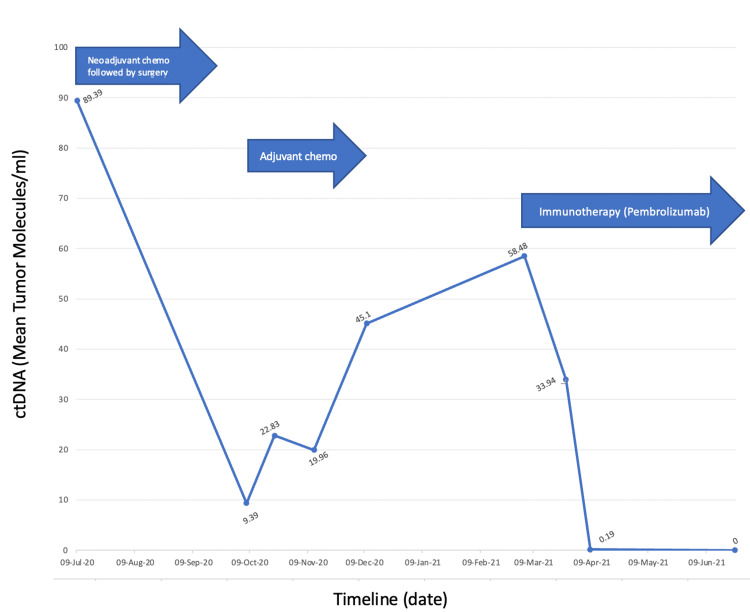
Postoperatively, the patient received adjuvant therapy consisting of the same regimen of FOLFOXIRI with the omission of the targeted therapy (bevacizumab). While radiographically, there was no evidence of disease before initiation of adjuvant chemotherapy, the patient's tumor-informed minimal residual disease (MRD) circulating tumor DNA (ctDNA) assay using a commercial platform (SignateraTM) under the expanded access program was reported positive (Figure 1). A timeline of the chemotherapy the patient received along with the ctDNA levels are reported in Figure 1. Despite the completion of the planned six months of adjuvant systemic chemotherapy, the ctDNA assay continued to remain positive and continued to rise. Follow-up imaging postcompletion of adjuvant therapy now revealed an exophytic soft tissue thickening around the duodenum upstream from the small bowel anastomosis narrowing the lumen and circumferentially encompassing the superior mesenteric vein causing high-grade stenosis. Peritoneal deposits were also visualized in the right hemipelvis adjacent to the colonic anastomosis. All these findings were suspicious of recurrence.
Figure 1. Circulating tumor DNA (ctDNA) kinetics in a patient with POLE-mutant colorectal cancer undergoing therapy using the tumor-informed approach. The patient received FOLFOXIRI and bevacizumab as neoadjuvant chemotherapy and a continued regimen of FOLFOXIRI only as adjuvant chemotherapy.
The tumor specimen was sent to commercially available next-generation sequencing (NGS) testing (FoundationONETM) given the concerns of persistently positive ctDNA assay (Figure 1). To our surprise, while the tumor was noted to be microsatellite stable (MSS), it was noted to be ultra-hypermutated with a tumor mutation burden (TMB) of 295 mutations per megabase (Muts/Mb). This is secondary to pathogenic P286R mutation in the polymerase epsilon, catalytic subunit (POLE) exonuclease domain. Given these findings, the patient was started on immunotherapy with single-agent pembrolizumab at the approved 400 mg Q6 weekly dosing with rapid clearance of ctDNA (Figure 1) and imaging consistent with a near complete response. On imaging, a significant interval decrease in the size of the mass as well as the implants in the peritoneum was reported. No new lesions were noted.
Discussion
Immunotherapy in the form of a PD-1 blockade with pembrolizumab has been approved in a tumor-agnostic fashion for any tumor with a TMB ≥10 Muts/Mb as determined by a Food and Drug Administration (FDA)-approved test on June 16, 2020 [8]. While this may be a reasonable approach for a lot of tumors, in the context of CRC, this approval is often criticized since the pMMR/MSS tumors that have a TMB ≥10 Muts/Mb do not derive benefit from immunotherapy [4]. Patients with dMMR/MSI-H CRC generally have a significantly higher TMB, which potentially leads to more expression of neo-antigens and, in turn, response to immune checkpoint blockade. However, one rare exception to this rule, as noted in our case, is the POLE-mutated CRC.
POLE-mutant CRC is a very rare finding. The POLE-gene encodes the DNA polymerase epsilon; important in correcting replication errors and is most commonly seen in endometrial cancers [9]. A cohort study, including more than 47,000 patients of all cancer types reported POLE/POLD1 mutations, with the percentage of mutations as high as 14.8% in endometrial cancer [10].
Table 1 summarizes to the best of our knowledge, all reported cases in literature for POLE-mutant colorectal cancer treated with immunotherapy. Most common mutations include but are not limited to; P286R, S297F, and V411L [11-12]. As mentioned earlier, our patient's tumor was also noted to have the P286R mutation leading to an extremely high tumor mutation burden. In the context of CRC, these tend to be more right-sided and more often seen in men, as seen in our case (Table 1) [13].
Table 1. The table shows the patient's age and sex; tumor location, POLE mutation, immunotherapy received; response to treatment; overall survival, and tumor mutational burden.
Abbreviations: NED, no evidence of disease; CR, complete response; PR, partial response
| Case no. | Age | Sex | Cancer Location | POLE Mutation | Immunotherapy received | Survival Status | Overall Survival (mos) | Tumor Mutational Burden (TMB; mutations/megabase) | Study |
| 1. | 50 | Male | Hepatic Flexure | P286 | Pembrolizumab | Alive (NED) | >24 | 295 | |
| 2. | 81 | Male | Hepatic Flexure | V411L | Pembrolizumab | Alive (CR) | 12 | 122 | Gong et al. [14] |
| 3. | >30 | Male | Rectum | P286 | Toripalimab | Alive (NED) | 12 | 453 | Wen et al. [15] |
| 4. | >70 | Male | Ascending Colon | P286 | Sintilimab | Alive (NED) | 18 | 255 | |
| 5. | >20 | Male | Sigmoid Colon | P286 | Sintilimab | Alive (NED) | 7 | 320 | |
| 6. | >30 | Male | Transverse Colon | P286 | Toripalimab | Alive (NED) | 14 | 307 | |
| 7. | 28 | Female | Transverse Colon | P286 | Ipilimumab + nivolumab | Alive (PR) | - | 198 | Keenan et al. [12] |
| 8. | 37 | Male | Ascending Colon | P286 | Pembrolizumab | Alive (Significant Response) | >24 | 168 | |
| 9. | 44 | Male | Rectum | V411L | Pembrolizumab | Alive (CR) | >28 | 200 | Silberman et al. [7] |
| 10. | 20 | Male | Sigmoid Colon | P286 | Pembrolizumab | PD | - | - | Wang et al. [16] |
| 11. | 34 | Male | Cecum | P286 | Pembrolizumab | Alive (SD) | >7 | - | |
| 12. | 82 | Male | Ascending Colon | V411L | Pembrolizumab | Alive (CR) | >12 | - | |
| 13. | 24 | Male | Descending Colon | P286 | Pembrolizumab | Alive (CR) | >48 | 126 | Durando et al. [17] |
| 14. | 55 | Male | Cecum | P436 | Pembrolizumab | Alive (CR) | >24 | >150 | Bikhchandani et al. [18] |
| 15. | 16 | Male | Transverse and Descending Colon | Ser297Cys* Lynch Syndrome | Nivolumab + anti LGA3 | Alive (CR) | >18 | 530 | Berrino et al. [19] |
| 16. | 34 | Male | Transverse Colon | P286 | Terriprizumab and bevacizumab | Alive (PR) | >18 | 120 | Xiang et al. [20] |
Our case is unique from a therapeutic standpoint since while aggressive systemic chemotherapy and surgery rendered the patient with no evidence of disease, molecularly, from an MRD or ctDNA standpoint, the patient was still positive (not cured) [21-22]. Not surprisingly, radiographic recurrence was noted a month post-completion of adjuvant therapy. What is also of value to note is the ctDNA kinetics in predicting response to immune checkpoint blockade [23]. ctDNA started going down within eight days of administration of the first dose of immunotherapy (Figure 1). It is often underestimated how quickly immunotherapy responses can be seen at the molecular level. Furthermore, the ultra-hypermutated nature of the neoplasm leading to the significantly high TMB is indeed a predictive marker of response to immunotherapy for this subset of pMMR/MSS CRC. While rare, it is important especially in the context of individuals with young onset colorectal cancer to not only have panel-based testing but also to have the TMB checked as well [13]. Responses here to immunotherapy are brisk, deep, and durable - similar to dMMR/MSI-H CRC [7,12,24-25].
Conclusions
There is a need to identify subsets of CRC that would benefit from an immunotherapy approach besides those with dMMR/MSI-H tumors. Patients with pMMR/MSS CRC that have the POLE or other polymerase mutations leading to an ultra-hypermutated phenotype are another subset that can benefit from immunotherapy. With the rise of colorectal cancers in the young, which are often diagnosed as advanced/metastatic, panel-based NGS testing, including TMB assessment should be the norm.
Acknowledgments
No grant funding was used for this report. Testing for the tumor-informed circulating tumor DNA assay was under the expanded access program. Thanks are also due to the patient for allowing us to present his findings as a case report. Written informed consent was obtained.
The authors have declared financial relationships, which are detailed in the next section.
Pashtoon M Kasi declare(s) personal fees and non-financial support from Saga Diagnostics. Pashtoon M Kasi declare(s) personal fees and non-financial support from Neogenomics. Pashtoon M Kasi declare(s) personal fees from Taiho Pharmaceutical . Pashtoon M Kasi declare(s) Travel Support from AstraZeneca. Pashtoon M Kasi declare(s) personal fees from Ipsen. Pashtoon M Kasi declare(s) personal fees and non-financial support from Eisai. Pashtoon M Kasi declare(s) personal fees and non-financial support from Do More Diagnostics AS. Pashtoon M Kasi declare(s) personal fees and non-financial support from Seattle Genetics. Pashtoon M Kasi declare(s) a grant from Merck. Pashtoon M Kasi declare(s) a grant from Agenus. Pashtoon M Kasi declare(s) a grant from Novartis. Pashtoon M Kasi declare(s) a grant from Advanced Accelerator Applications. Pashtoon M Kasi declare(s) a grant from Tersera. Pashtoon M Kasi declare(s) a grant from Boston Scientific. Pashtoon M Kasi declare(s) personal fees and non-financial support from Elicio. Pashtoon M Kasi declare(s) personal fees and non-financial support from Guardant Health. Pashtoon M Kasi declare(s) personal fees and non-financial support from Natera. Pashtoon M Kasi declare(s) personal fees and non-financial support from Foundation Medicine. Pashtoon M Kasi declare(s) personal fees and non-financial support from Illumina. Pashtoon M Kasi declare(s) personal fees and non-financial support from BostonGene. Pashtoon M Kasi declare(s) personal fees and non-financial support from Merck/MSD Oncology. Pashtoon M Kasi declare(s) personal fees and non-financial support from Tempus. Pashtoon M Kasi declare(s) personal fees and non-financial support from Bayer. Pashtoon M Kasi declare(s) personal fees and non-financial support from Lilly. Pashtoon M Kasi declare(s) personal fees and non-financial support from Delcath Systems. Pashtoon M Kasi declare(s) personal fees and non-financial support from IPBA. Pashtoon M Kasi declare(s) personal fees and non-financial support from QED Therapeutics. Pashtoon M Kasi declare(s) personal fees and non-financial support from Boston Healthcare Associates. Pashtoon M Kasi declare(s) personal fees and non-financial support from Servier. Pashtoon M Kasi declare(s) personal fees and non-financial support from Taiho Oncology. Pashtoon M Kasi declare(s) personal fees and non-financial support from Exact Sciences. Pashtoon M Kasi declare(s) personal fees and non-financial support from Daiichi Sankyo/AstraZeneca.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. André T, Shiu KK, Kim TW, et al. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 2.Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. Accessed July 19, 2023. [ Jul; 2023 ]. 2018. https://iris.unito.it/handle/2318/1669348. https://iris.unito.it/handle/2318/1669348 [DOI] [PubMed]
- 3.Colorectal cancer. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 4.Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the Canadian Cancer Trials Group Co.26 study. Chen EX, Jonker DJ, Loree JM, et al. JAMA Oncol. 2020;6:831–838. doi: 10.1001/jamaoncol.2020.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PD-1 blockade in tumors with mismatch-repair deficiency. [ Jul; 2023 ];Le DT, Uram JN, Wang H, et al. https://www.nejm.org/doi/full/10.1056/nejmoa1500596. N Engl J Med. 2015 372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targeting the DNA damage response in immuno-oncology: developments and opportunities. Chabanon RM, Rouanne M, Lord CJ, Soria JC, Pasero P, Postel-Vinay S. Nat Rev Cancer. 2021;21:701–717. doi: 10.1038/s41568-021-00386-6. [DOI] [PubMed] [Google Scholar]
- 7.Complete and prolonged response to immune checkpoint blockade in POLE-mutated colorectal cancer. Silberman R, F Steiner D, Lo AA, Gomez A, Zehnder JL, Chu G, Suarez CJ. JCO Precis Oncol. 2019;3:1–5. doi: 10.1200/PO.18.00214. [DOI] [PubMed] [Google Scholar]
- 8.FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Marcus L, Fashoyin-Aje LA, Donoghue M, et al. Clin Cancer Res. 2021;27:4685–4689. doi: 10.1158/1078-0432.CCR-21-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frequent POLE-driven hypermutation in ovarian endometrioid cancer revealed by mutational signatures in RNA sequencing. Davila JI, Chanana P, Sarangi V, et al. BMC Med Genomics. 2021;14:165. doi: 10.1186/s12920-021-01017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu ZX, Xu RH. JAMA Oncol. 2019;5:1504–1506. doi: 10.1001/jamaoncol.2019.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A panoply of errors: polymerase proofreading domain mutations in cancer. Rayner E, van Gool IC, Palles C, Kearsey SE, Bosse T, Tomlinson I, Church DN. https://www.nature.com/articles/nrc.2015.12. Nat Rev Cancer. 2016;16:71–81. doi: 10.1038/nrc.2015.12. [DOI] [PubMed] [Google Scholar]
- 12.Molecular and radiological features of microsatellite stable colorectal cancer cases with dramatic responses to immunotherapy. Keenan BP, VA Loon K, Khilnani AD, Fidelman N, Behr SC, Atreya CE, Oh DY. Anticancer Res. 2021;41:2985–2992. doi: 10.21873/anticanres.15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The somatic POLE P286R mutation defines a unique subclass of colorectal cancer featuring hypermutation, representing a potential genomic biomarker for immunotherapy. Ahn SM, Ansari AA, Kim J, et al. Oncotarget. 2016;7:68638–68649. doi: 10.18632/oncotarget.11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Response to PD-1 blockade in microsatellite stable metastatic colorectal cancer harboring a pole mutation. Gong J, Wang C, Lee PP, Chu P, Fakih M. J Natl Compr Canc Netw. 2017;15:142–147. doi: 10.6004/jnccn.2017.0016. [DOI] [PubMed] [Google Scholar]
- 15.Pathological complete response to immune checkpoint inhibitor in patients with colorectal cancer liver metastases harboring POLE exonuclease domain mutation. Wen L, Chen Z, Ji X, et al. J Immunother Cancer. 2022;10:0. doi: 10.1136/jitc-2022-004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Immune profiling of microsatellite instability-high and polymerase ε (POLE)-mutated metastatic colorectal tumors identifies predictors of response to anti-PD-1 therapy. Wang C, Gong J, Tu TY, Lee PP, Fakih M. J Gastrointest Oncol. 2018;9:404–415. doi: 10.21037/jgo.2018.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Four-year disease-free remission in a patient with POLE mutation-associated colorectal cancer treated using anti-PD-1 therapy. Durando ML, Menghani SV, Baumann JL, Robles DG, Day TA, Vaziri C, Scott AJ. J Natl Compr Canc Netw. 2022;20:218–223. doi: 10.6004/jnccn.2021.7115. [DOI] [PubMed] [Google Scholar]
- 18.POLE-mutant colon cancer treated with PD-1 blockade showing clearance of circulating tumor DNA and prolonged disease-free interval. Bikhchandani M, Amersi F, Hendifar A, et al. Genes (Basel) 2023;14:1054. doi: 10.3390/genes14051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collision of germline POLE and PMS2 variants in a young patient treated with immune checkpoint inhibitors. Berrino E, Filippi R, Visintin C, et al. NPJ Precis Oncol. 2022;6:15. doi: 10.1038/s41698-022-00258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Case report: POLE (P286R) mutation in a case of recurrent intestinal leakage and its treatment. Xiang D, Fu G, Chen Y, Chu X. Front Oncol. 2023;13:1028179. doi: 10.3389/fonc.2023.1028179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Kotani D, Oki E, Nakamura Y, et al. Nat Med. 2023;29:127–134. doi: 10.1038/s41591-022-02115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utility and debate of liquid biopsy assays in surveillance setting. ASCO Daily News. [ Aug; 2023 ]. 2023. https://dailynews.ascopubs.org/do/10.1200/ADN.23.201313/full https://dailynews.ascopubs.org/do/10.1200/ADN.23.201313/full
- 23.Current and future clinical applications of ctDNA in immuno-oncology. Stadler JC, Belloum Y, Deitert B, et al. Cancer Res. 2022;82:349–358. doi: 10.1158/0008-5472.CAN-21-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilot clinical trial of perioperative durvalumab and tremelimumab in the treatment of resectable colorectal cancer liver metastases. Kanikarla Marie P, Haymaker C, Parra ER, et al. https://aacrjournals.org/clincancerres/article/27/11/3039/671421/Pilot-Clinical-Trial-of-Perioperative-Durvalumab. Clin Cancer Res. 2021;27:3039–3049. doi: 10.1158/1078-0432.CCR-21-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A phase II study of avelumab monotherapy in patients with mismatch repair-deficient/microsatellite instability-high or POLE-mutated metastatic or unresectable colorectal cancer. Kim JH, Kim SY, Baek JY, et al. Cancer Res Treat. 2020;52:1135–1144. doi: 10.4143/crt.2020.218. [DOI] [PMC free article] [PubMed] [Google Scholar]