Abstract
Deletion of the γ34.5 gene coding for virulence markedly reduces cytotoxicity mediated by herpes simplex virus type 1 (HSV-1) (J. M. Markert et al., Neurosurgery 32:597–603, 1993; N. S. Markovitz et al., J. Virol. 71:5560–5569, 1997). To target lytic virulence to tumors, we have created a novel HSV-1 mutant, designated Myb34.5. This viral mutant is characterized by a deletion of the gene for infected cell polypeptide 6 (ICP6; also known as UL39 or ribonucleotide reductase) and of the two endogenous copies of the γ34.5 gene (RL1) and by reintroduction of one copy of γ34.5 under control of the E2F-responsive, cellular B-myb promoter. On direct intracerebral inoculation in BALB/c mice, the 50% lethal dose (LD50) for Myb34.5 was 2.7 × 107 PFU while that for HSVs with mutations in the γ34.5 gene could not be technically achieved with available viral stocks and it was estimated as >1 × 107 PFU. The LD50 for an HSV with a single defect in ICP6 function was 1.3 × 106 PFU. Conversely, Myb34.5’s oncolytic efficacy against a variety of human glioma cells in culture and in vivo was enhanced compared to that of HSVs with γ34.5 mutations, and in fact, it was comparable to that of the wild-type F strain and of viral mutants that possess a wild-type γ34.5 gene. The characteristic shutoff of host protein synthesis, occurring after infection of human SK-N-SH neuroblastoma cells by γ34.5 mutant viruses (J. Chou and B. Roizman, Proc. Natl. Acad. Sci. USA 89:3266–3270, 1992), was not present after infection with Myb34.5. There was an increase of almost 3 logarithmic units in the production of progeny virus in arrested fibroblasts compared to that in cycling fibroblasts infected with Myb34.5. These results suggest that transcriptional regulation of γ34.5 by cell cycle-regulated promoters can be used to target HSV-1 virulence toward tumors while maintaining the desirable neuroattenuated phenotype of a γ34.5 mutant.
A recent development in cancer gene therapy has revolved around the use of genetically engineered, replication-conditional (oncolytic) viruses to deliver cytotoxic genes to tumor cells as well as destroy them directly via lytic infection (14, 32). The use of replication-conditional herpes simplex virus type 1 (HSV-1) mutants appears promising for both purposes, as its intraneoplastic replication should allow enhanced anatomic spread of anticancer effects throughout an inoculated tumor mass and augmentation of this effect by delivery of anticancer genes (4, 38). This might circumvent the limited anatomic spread observed with the inoculation of replication-defective vectors and/or producer cells into human tumors (43, 47).
Two broad types of replication-conditional HSV mutants in a single gene have been studied to date. The first consists of viral mutants with defects in the function of a viral gene needed for nucleic acid metabolism, such as thymidine kinase (32), ribonucleotide reductase (RR) (5, 6, 18, 34), or uracyl N-glycosylase (44). The second consists of viral mutants with defects in the function of the γ34.5 gene (8), which functions as a virulence factor by markedly enhancing the viral burst size of infected cells through suppression of the shutoff of host protein synthesis (11, 13). The single-mutant strains have certain inherent limitations, including resistance to ganciclovir for mutants in thymidine kinase (34), the risk of reversion to wild-type by a single recombination event with wild-type virus, and reduced oncolytic efficacy for γ34.5 mutants, at least in certain tumor cell lines (26, 37, 51).
In an effort to decrease the risk of wild-type recombination, HSV viruses that are multiply mutated have been developed. These include mutants G207 (35) and MGH1 (26), which possess deletions of both copies of γ34.5 and an insertional mutation of RR and the γ34.5–uracil N-glycosylase mutant strain 3616UB (45). These double-mutant strains demonstrate markedly reduced neurovirulence upon direct intracranial injection, retain sensitivity to ganciclovir, and show relatively selective replication in tumor cells compared to normal tissues. Such double-mutant HSV strains retain the defective γ34.5 gene, thus demonstrating little virulence toward normal tissues. Although, they clearly demonstrate oncolytic effects against tumor cells, such effects are less than those observed in mutants with intact γ34.5 genes (26, 46). However, the toxicity exhibited by an intact γ34.5 gene might reduce the potential application of the latter viruses as oncolytic agents.
We reasoned that transcriptional retargeting of γ34.5 might provide a means to achieve selective virulence for tumors while retaining attenuated virulence for normal tissues. In this report, we have reintroduced the γ34.5 gene into an RR-γ34.5 double-mutant strain (MGH1) under transcriptional control of the cell-cycle regulated, cellular B-myb promoter. We show that this novel oncolytic virus (Myb34.5) remains as oncolytic as a single RR mutant virus that possesses a wild-type γ34.5 gene yet retains a favorable toxicity profile upon intracerebral inoculation in mice, in that its 50% lethal dose (LD50) is >107 PFU, a value comparable to that observed with a γ34.5 deletion mutant. These findings thus show that transcriptional retargeting of a viral gene responsible for preventing the shutoff of protein synthesis of infected cells can provide an avenue for achieving selective viral oncolysis.
MATERIALS AND METHODS
Plasmids and viruses.
HSV strain F (wild type) was acquired through the American Type Culture Collection (Manassas, Va.). Mutant virus R3616 (containing 1,000-bp BstEII-StuI deletions within both γ34.5 loci) was kindly provided by B. Roizman, University of Chicago. Mutant virus hrR3 was kindly provided by S. Weller, University of Connecticut. This mutant virus contains an Escherichia coli lacZ cDNA inserted into the UL39 locus. The mutant virus MGH1 is characterized by insertion of the E. coli lacZ cDNA into the UL39 locus and deletions of both γ34.5 loci, and it was constructed by recombination of the infected cell polypeptide 6 (ICP6)-lacZ region of hrR3 into the viral mutant R3616 (26). Plasmid pKX-BG3, which contains the lacZ gene within a 2.3-kb XhoI region of ICP6 (KOS origin; see reference 19), was kindly provided by S. Weller. Plasmid pKpX2, which contains 2.3 kb of the ICP6 (UL39) gene was also provided by S. Weller. Plasmid pBGL34.5, which contains the entire γ34.5 coding sequence, was a gift from Xandra Breakefield and Peter Pechan (Massachusetts General Hospital, Charlestown, Mass.). Plasmid pBGL2myb was kindly provided by R. Watson (Ludwig Institute for Cancer Research, St. Mary’s Hospital, London, United Kingdom), and it contains the promoter for B-myb.
Engineering of Myb34.5 and of a Myb34.5 revertant.
The plasmid used for the engineering of Myb34.5 by homologous recombination into MGH1 was designed to replace the lacZ cDNA in MGH1 in its entirety and delete an additional 888 nucleotides of ICP6 (UL39) sequence. Specifically, the recombining plasmid (pKpX2-myb34.5) was engineered as follows. The full-length γ34.5 cDNA was excised as an NcoI-SacI fragment from pBGL34.5, it was blunt-ended, and then it was subcloned into pBSKII (Stratagene, La Jolla, Calif.) to generate plasmid pBS34.5. The B-myb promoter was excised as a KpnI-HindIII fragment from pBGL2myb and directionally cloned upstream of γ34.5 in pBS34.5. The resulting expression cassette, containing the B-myb promoter upstream of the γ34.5 cDNA, was excised as a KpnI-XbaI fragment, was blunt-ended, and was then subcloned into the NruI sites of pKpX2. Through this process, the intervening NruI-NruI fragment within UL39 was deleted. The resulting plasmid, pKpX2-myb34.5, was then linearized with ScaI and cotransfected with MGH1 viral DNA into Vero cells at various molar ratios with Lipofectamine (Gibco, Gaithersburg Md.). Virus progeny was harvested 5 to 7 days following transfection when cytopathic effects were evident. This progeny was released from cells through three cycles of freeze-thawing, and it was then plated onto a monolayer of Vero cells. After overlayering the monolayer with agarose, incubation at 37°C in an atmosphere containing 5% carbon dioxide was performed. Plaques were then stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Colorless plaques were selected as potential recombinants. These isolates underwent three rounds of plaque purification before having their genetic identity tested by Southern blot analysis. A Myb34.5 revertant (MybRevt) was engineered by using Myb34.5 as the parental strain and pKX-BG3 as the plasmid for homologous recombination of the lacZ cDNA back into the ICP6 locus and deletion of the B-myb–γ34.5 expression cassette.
Southern blot analysis.
Viral DNAs were isolated after lysis of infected Vero cells with SDS-proteinase K, repeated phenol-chloroform extraction, and ethanol precipitation. DNA was digested with appropriate restriction endonucleases (New England Biolabs, Beverly, Mass.), separated by agarose electrophoresis, and transferred to a nylon membrane (Amersham Corp., Arlington Heights, Illinois). Probes included the HindIII-XbaI ICP6 fragment from pKpX2, the BstEII-BbsI fragment from pBSKγ34.5, and a BbsI fragment of lacZ from pKX-BG3. Probe labeling and hybridizations were performed with the ECL system (Amersham), used according to the manufacturer’s protocol.
Cell culture studies.
All cells were cultured at 37°C in an atmosphere containing 5% carbon dioxide in Dulbecco’s modified essential medium supplemented with 10% fetal calf serum, 100 U of pencillin/ml, and 10 μg of streptomycin/ml. Host protein synthesis shutoff studies were performed by infecting cells with viral strains for 16 h. Cells were then placed in methionine-free medium for 10 min, before adding [35S]methionine (New England Nuclear, Boston, Mass.) for 90 min. Cells were then washed with 10 mM sodium phosphate in 0.9% sodium chloride (phosphate-buffered saline), pH 7; solubilized; subjected to SDS-polyacrylamide gel electrophoresis; transferred to a nitrocellulose membrane; and subjected to autoradiography. Protein concentrations were calculated with a commercially available kit (Bio-Rad, Hercules, Calif.). ICPs were labeled as previously published (39). Human glioblastoma cell lines U87, U373, T98G, and U343; rat gliosarcoma 9L cells; human neuroblastoma SKNSH cells; and Vero (African green monkey) cells were obtained from the American Type Culture Collection and cultured with Dulbecco’s minimal essential medium or minimal alpha essential medium (Gibco) supplemented with 10% serum and antibiotics. Primary mouse fetal striatal neurons (embryonic stage 18) (kindly provided by M. Schwarzchild, Massachusetts General Hospital) were isolated from brains by using published procedures (49).
Animal studies.
Nude (nu/nu) mice were obtained from the Cox 7 breeding facility, Massachusetts General Hospital. BALB/c mice were obtained from Charles River Laboratories, (Wilmington, Mass.). Subcutaneous tumors were obtained by injection of 2 × 105 cells into the flanks of athymic mice (five animals per group for 9L gliosarcoma cells and six animals per group for human U87ΔEGFR glioma cells). Fourteen (for 9L) or ten (for U87ΔEGFR) days after tumor implantation, animals with similar tumor volumes were randomly divided, and various viral strains were injected intratumorally at 5 × 107 PFU/dose in 100-μl volumes on days 1, 3, 5, and 7. Animals were euthanatized at day 33 (9L) or day 34 (U87ΔEGFR). Tumor volumes were measured with external calipers as previously described (53). For neurotoxicity experiments, BALB/c mice were stereotactically injected in the right frontal lobe (depth, 3 mm) with 10-μl volumes of virus at different dilutions, up to the highest stock titers obtainable. Animals were checked daily for 28 days. All animal studies were performed in accordance with guidelines issued by the Massachusetts General Hospital Subcommittee on Animal Care. Viral inoculation and care of animals harboring viruses were performed in approved viral vector rooms.
RESULTS
Engineering of Myb34.5.
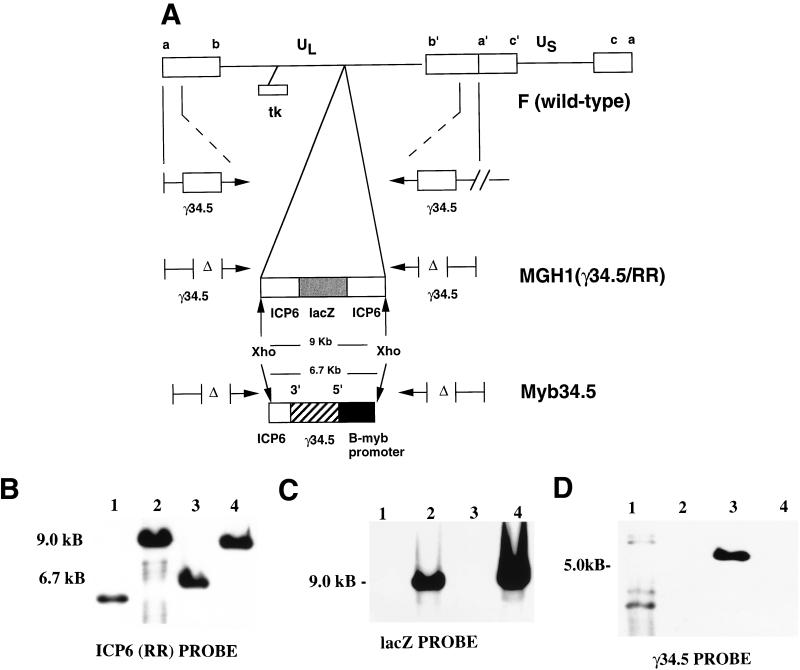
The multiply mutated virus Myb34.5 was constructed by recombining a B-myb promoter–γ34.5 construct into the UL39 (also known as ICP6 or RR) locus of MGH1. MGH1 (26) was generated by recombining a lacZ cDNA into the ICP6 locus of the γ34.5 deletion mutant R3616 (11). Figure 1A provides a schematic of the DNA structure of Myb34.5. This structure was confirmed by restriction endonuclease mapping (data not shown), Southern blot hybridization (Fig. 1B to D), and sequence analysis (data not shown) of the junctions between UL39 and the B-myb promoter–γ34.5 expression cassette. To show the deletion of lacZ in Myb34.5, XhoI-digested Myb34.5 DNA was hybridized to probes containing either ICP6 (Fig. 1B) or lacZ sequence (Fig. 1C). As expected, the parental virus, MGH1, contained a 9.0-kb XhoI ICP6-lacZ fragment (26) that hybridized to an ICP6 probe (Fig. 1B). Homologous recombination led to the deletion of lacZ and additional ICP6 sequence and insertion of the B-myb promoter–γ34.5 sequence. This is evident by hybridization of the ICP6 probe to a 6.7-kb fragment in Myb34.5 DNA (Fig. 1B). Hybridization with a LacZ probe revealed the absence of hybridizing fragments in digested DNA from Myb34.5 and the presence of the expected 9.0-kb hybridizing fragment in digested DNA from MGH1 (Fig. 1C). To confirm that Myb34.5 possessed a reintroduction of the γ34.5 gene, BamHI-digested viral DNA was hybridized with a BstEII-Bbs γ34.5 fragment (internal to the deleted regions). This demonstrated a ladder of hybridizing bands that is typically observed with the wild-type F strain (11, 35). As discussed in the work of Chou et al. (1991), γ34.5 maps in BamHI S and SP fragments, forming a characteristic ladder of bands at 500-bp increments, which are a consequence of a variable number of a sequences in the repeats flanking the unique sequences of the long component. This ladder is observed in the Southern blot for the F strain (lane 1 of Fig. 1D), where the top hybridizing bands represent the BamHI SP fragment, formed by the fusion of the terminal BamHI S fragment with BamHI P, while the lower hybridizing bands represent the BamHI S fragment (11). In R3616 and its derived viruses, MGH1, Myb34.5, and Myb34.5Revt, a similar ladder of hybridizing bands whose molecular size was decreased by approximately 1 kb (the size of the internal deletion of γ34.5 in R3616) would be expected if a full-length γ34.5 cDNA probe were employed for hybridization. In fact, in the work of Chou et al. (11) this pattern of hybridization is evident for R3616, and in the work of Kramm et al. (26) this pattern of hybridization is evident for MGH1. However, for the Southern analysis shown in Fig. 1D a BstEII-Bbs γ34.5 fragment that is internal to the 1-kb deleted fragment of the γ34.5 gene of R3616, MGH1, Myb34.5, and Myb34.5Revt was employed as a probe. Therefore, no hybridizing bands are observed for MGH1 (Fig. 1D, lane 2) and Myb34.5Revt (Fig. 1D, lane 4), while a single 5.3-kb hybridizing fragment is observed for Myb34.5 (Fig. 1D, lane 3), corresponding to the γ34.5 gene, reintroduced into the ICP6 locus.
FIG. 1.
(A) Schematic maps of HSV strain F (wild-type), MGH1 (RR [ICP6]-γ34.5 mutant), and Myb34.5. All strains contain the typical HSV genome with two unique segments one long and one short (UL and US, respectively), each flanked by inverted repeat elements (ab and ca, respectively) (33). Depending on their localization in either unique or repeat segments, the HSV genes are present in one or two copies. The locations of the diploid γ34.5 genes and of the thymidine kinase gene (tk) are shown in the top construct, representing wild-type F strain HSV. In the middle construct, the insertion of a lacZ cDNA (gray box) into a BamHI site within ICP6 (19) and the deletions (Δ) within γ34.5 are shown for MGH1 (26). The lower construct shows the site of recombination of the B-myb promoter (black box)-γ34.5 (hatched box) expression cassette into ICP6, giving rise to the novel virus Myb34.5. The approximate sizes of the XhoI fragment from MGH1 and from Myb 34.5 are provided. (B) Characterization of HSV mutant Myb34.5 by Southern blot analysis. Hybridization of XhoI-digested viral DNA to a full-length probe for ICP6 reveals the expected 9.0-kb fragment sizes for the ICP6 gene with a full-length lacZ insertion in MGH1 (lane 2) and MybRevt (lane 4). In Myb34.5 (lane 3) there is replacement by the B-myb promoter-γ34.5 cassette and further deletion of ICP6 to give a 6.7-kb band. DNA from the wild-type F strain is in lane 1, hybridizing to a fragment of approximately 5 kb (size not indicated in figure). (C) Hybridization with a lacZ probe reveals hybridization to 9.0-kb fragments for XhoI-digested MGH1 (lane 2) and MybRevt DNAs (lane 4), with no hybridization to Myb34.5 (lane 3) or F strain DNAs (lane 1). (D) A BstEII-Bbs fragment of γ34.5, internal to the deleted regions of R3616 and MGH1, reveals a 5.3-kb fragment in BamHI-digested Myb34.5 DNA (lane 3) and several bands in F (lane 1) but fails to hybridize to either MGH1 (lane 2) or MybRevt (lane 4).
In order to demonstrate that the altered phenotype of Myb34.5 was the result of the B-myb–γ34.5 insertion, a revertant (marker-rescued) virus, designated MybRevt, was also engineered. This was achieved by homologous recombination, with Myb34.5 as the parental strain and linearized pKX2-BG3 as the recombining plasmid. This plasmid contains the lacZ-ICP6 insertion and was used to create hrR3, the source of the ICP6::LacZ fusion region in MGH1 (19, 26). The MybRevt revertant demonstrated a pattern of hybridization to the ICP6 (Fig. 1B), LacZ (Fig. 1C), and γ34.5 (Fig. 1D) probes upon Southern analysis that was identical to that shown by MGH1, the parent strain of Myb34.5. A list of the mutant viruses employed in this study is provided in Table 1.
TABLE 1.
Viral mutants employed in this study
| Mutant (reference) | Strain | Genotype | Phenotype | Parental virus |
|---|---|---|---|---|
| HrR3 (18) | KOS | UL39−lacZ+ | ICP6::LacZ fusion | KOS |
| R3616 (11) | F | γ34.5− | γ34.5− | F |
| MGH1 (26) | F | γ34.5− UL39−lacZ+ | γ34.5− ICP6::LacZ fusion | R3616 |
| Myb34.5 | F | UL39− B-myb promoter+-γ34.5+ | Conditional γ34.5+ | MGH1 |
| MybRevt | F | γ34.5− UL39−lacZ+ | γ34.5− ICP6::LacZ fusion | Myb34.5 |
Functional expression of γ34.5.
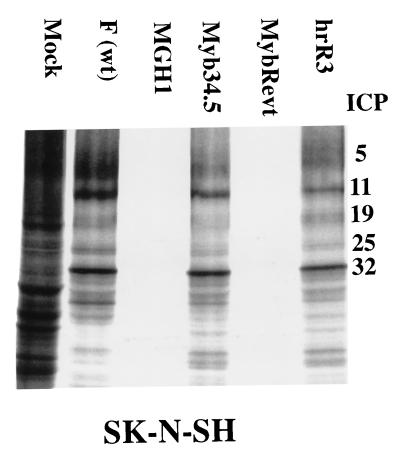
To confirm that Myb34.5 produced functional γ34.5 protein, human SKNSH neuroblastoma cells were infected with a variety of viral strains. Fig. 2 shows that, as expected, MGH1 and the revertant virus (MybRevt) failed to prevent the infected-cell response consisting of shutoff of protein synthesis which is characteristic of intact γ34.5 function (13). However, Myb34.5 and other strains with intact γ34.5 (wild-type F and hrR3) prevented the infected-cell response (shutoff of protein synthesis), thus leading to viral protein production.
FIG. 2.
Autoradiographic image of electrophoretically separated lysates of infected cells demonstrating inhibition of host protein synthesis shutoff by mutant viral strains. Human neuroblastoma cells (SK-N-SH) were plated at 106 cells/100-mm-diameter dish. Twenty-four hours later, cells were infected at an MOI of 3.0. Fifteen hours following viral infection, cells were briefly washed with methionine-free medium and then incubated for 90 min with medium containing 60 μCi of [S35]methionine. After labeling, cells were harvested, solubilized in a buffer containing SDS, separated by electrophoresis on 10% polyacrylamide gels, transferred to nitrocellulose, and subjected to autoradiography. ICPs were designated according to the method in reference 39. wt, wild type.
Additional evidence that Myb34.5 expressed functional γ34.5 protein was provided by assessment of viral replication in the U373 glioblastoma cell line which has been previously noted to restrict replication of γ34.5 mutant HSV strains (37). After infecting 5 × 105 cells at a multiplicity of infection (MOI) of 1.0 and harvesting viral output 48 h later, Myb34.5 yields were 1.1 × 107 PFU, while those for the F strain were 5.0 × 107 PFU. In contrast, yields of MGH1 (3.3 × 104 PFU) or MybRevt (3.4 × 104 PFU) (averages of triplicate experiments) were significantly less. The ability of Myb34.5 to efficiently replicate in this nonpermissive line, in contrast to that of MGH1 or MybRevt, indicates that the encoded γ34.5 in Myb34.5 was functional.
Neurotoxicity studies.
One important characteristic of any replication-competent HSV strain is its level of neurovirulence, which can be assessed both in vitro and in vivo. The ability of the Myb34.5 virus to replicate in primary neuronal cultures was measured in comparison to the F, hrR3, MGH1, and MybRevt strains. Murine fetal striatal neurons were infected, and viral yields were assessed by plaque assay on Vero cells (which do not require γ34.5 for efficient viral replication). While the wild-type F strain demonstrated vigorous replication in neurons, all the mutant strains, including Myb34.5, demonstrated minimal viral replication (Table 2).
TABLE 2.
Viral replication in primary neuronal culturea
| HSV strain | Mean (SEM) viral output (PFU) |
|---|---|
| F | 1.0 × 107 (1.4 × 106) |
| hrR3 | 4.1 × 103 (4.2 × 102) |
| MGH1 | 1.4 × 103 (73) |
| Myb34.5 | 6.0 × 103 (4.3 × 102) |
| MybRevt | 1.5 × 103 (74) |
A total of 2.5 × 105 murine fetal (day 18) striatal neurons were harvested and plated as previously described (9). Two days after plating, cells were infected at an MOI of 0.1 with wild-type (strain F) and mutant viral strains in duplicate experiments. Twenty-four hours after infection, cells and supernatant were harvested and subjected to freeze-thaw cycles to liberate viral particles. Viral output was determined by plaque assay on Vero cells. Viral outputs of the four mutant viral strains (hrR3, MGH1, Myb34.5, and MybRevt) were not statistically different between groups (P > 0.3 [t test]).
We then tested the neurovirulence of Myb34.5 in the cerebrums of BALB/c mice. Table 3 shows that Myb34.5’s LD50 was 2.7 × 107 PFU, at least 3 logarithmic units higher than that of F strain (wild-type). The γ34.5 mutants (MGH1, R3616, and MybRevt) do not grow well, and achievement of titers of >109 pfu/ml is prohibitive without large-scale production. This limited our ability to estimate accurately the LD50 for these mutants in these experiments. For comparative purposes, one of six mice perished when inoculated intracerebrally with 107 PFU of Myb34.5, while zero of six mice perished when inoculated with the same amount of MGH1. These findings confirmed that reintroduction of γ34.5 under the control of the B-myb promoter produced minimal neurovirulence.
TABLE 3.
Ability of wild-type and mutant viral strains to cause death after intracerebral inoculation into micea
| Virus in inoculum | Description | LD50 (PFU) |
|---|---|---|
| HSV-1 (F) | Wild-type | 1 × 104 |
| hrR3 | lacZ insertion in RR | 1.3 × 106 |
| R3616 | γ34.5 mutant | >1 × 107b |
| MGH1 | γ34.5-RR double mutant | >1 × 107b |
| Myb34.5 | RR-myb34.5 | 2.7 × 107c |
Serial dilutions of virus in serum-free medium (volume, 10 μl) were stereotactically injected into the right hemisphere of 3-week-old female BALB/c mice (six animals per PFU level; Charles River Laboratories). Animals were inspected daily for 28 days for mortality and the LD50 was calculated by using a proportional distance model.
No deaths (zero deaths per six animals) occurred at the listed dose, which was the highest attainable for these strains in the given injection volume. This is because titers of >109 PFU/ml could not be technically achieved with these mutants in growing Vero cells without industrial-scale production and intracerebral injections were limited to a maximum volume of 10 μl.
In parallel experiments, one death per six animals occurred at a dose of 107 PFU. Since titers of 5 × 109 to 1 × 1010 PFU/ml could be achieved with Myb34.5 in rapidly dividing Vero or tumor cells, a more accurate LD50 could be measured.
Regulation of γ34.5 gene expression in arrested and cycling cells.
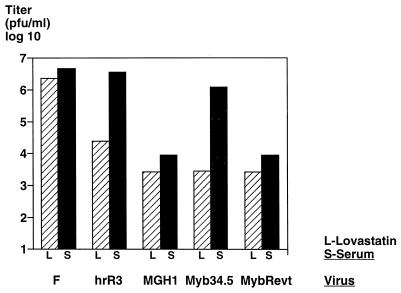
In order to further assess the behavior of Myb34.5 in quiescent versus cycling cells, primary human fibroblasts were plated and cell-cycle arrested with 20 μM lovastatin, which has been shown not to interfere with herpes virus replication (48). In arrested primary fibroblasts, MGH1, Myb34.5, and MybRevt demonstrated decreased viral replication relative to the F strain, at levels 1 logarithmic unit (PFU) lower than the single RR mutant hrR3 (Fig. 3). In contrast, in the presence of serum, hrR3 and Myb34.5 demonstrated marked induction of replication, while MGH1 and MybRevt did not. These results suggested that (i) the γ34.5 gene is required for efficient replication in this nontransformed cell type and (ii) the B-myb promoter functions to allow efficient replication of Myb34.5 in cycling cells. It is also notable that Myb34.5 exhibited a greater differential in the production of viral progeny in quiescent versus cycling cells than that of hrR3 (3 versus 2 logaritmic units) and that its viral production in quiescent cells was the same as that of the γ34.5 mutant viruses.
FIG. 3.
Viral replication in arrested and cycling cells. Human embryo-derived primary fibroblasts (CRL 7706) were plated at 105 cells/60-mm-diameter dish. Forty-eight hours after plating, the medium was replaced with DMEM containing 20 μM lovastatin for 36 h (hatched bars). Triplicate plates were counted and infected at an MOI of 1.0 with various mutant strains (triplicate experiments). Forty-eight hours after infection, cells and supernatants were harvested and virus was liberated by freeze-thaw cycles and ultrasonication. Parallel experiments were performed with cells allowed to remain in medium containing 10% fetal bovine serum (solid bars). Viral output was determined by plaque assay on Vero cells and is represented as log10 PFU/105 input virions (values reflect averages of triplicate experiments). Outputs of cells with lovastatin were statistically lower than those obtained with serum for the tested viruses (F, P = 0.027; hrR3, P = 0.001; MGH1, P = 0.011; Myb34.5, P = 0.001; MybRevt, P = 0.003 [Students’ t test]).
Comparative studies on oncolytic effects.
To determine the oncolytic efficiency of Myb34.5, five different glioma cell lines (9L, U87, U87ΔEGFR, T98G, and U343) were mock infected (no virus) or infected with a panel of viral strains, including F, MGH1, Myb34.5, and MybRevt. Surviving cells were counted 48 h later and expressed as a percentage of cells surviving on mock-treated plates. In published studies of rat 9L gliosarcoma cells (26) and in unpublished experiments on human glioma cells U343 and T98 (46), we had preliminarily shown that tumor cell killing by MGH1 was similar to that by R3616 for two human glioma lines (Table 4), and thus the latter mutant was excluded from further analysis. In all tumor cell lines tested, Myb34.5 demonstrated greater oncolytic efficiency in vitro than did the parental strain MGH1, and for some tumor cell lines its oncolytic efficacy approached that of the wild-type virus (Table 4). These findings thus showed that the oncolytic effect of Myb34.5 was greater than that of the γ34.5 mutant viruses (MGH1, MybRevt, and R3616, whose killing efficacy closely replicates that of MGH1).
TABLE 4.
In vitro glioma cell killing by wild-type and mutant HSV strainsa
| Cell line | % Survival (SEM) after infection with HSV strain
|
|||
|---|---|---|---|---|
| F | MGH1b | Myb34.5 | MybRevt | |
| U87 | 2.9 (0.7) | 52 (2.0) | 19.5 (1.5) | 51 (2.5) |
| 9L | 23.7 (1.7) | 60 (1.2) | 29 (1.4) | 59.5 (1.4) |
| U343 | 19 (1.1) | 35.7 (1.6) | 26.3 (1.1) | 32.3 (3.0) |
| T98G | 26.8 (0.8) | 38.5 (1.0) | 29.3 (1.7) | 40 (1.2) |
| U87ΔEGFR | 15 (0.4) | 48 (0.3) | 10 (0.6) | 50 (0.7) |
Human (U87MG, U343, T98G, and U98ΔEGFR) and rat (9L) glioma-derived cell lines were plated at 5 × 105 cells/60-mm-diameter plate and we subsequently infected with the strains noted at an MOI of 0.1. Oncolytic effect is reflected by cell survival at 48 h postinfection, expressed as a percentage of the number of surviving cells from triplicate, noninfected control plates. Values represent averages of triplicate experiments. Differences in killing of tumor cells by Myb34.5 versus MGH1 or MybRevt were statistically significant. For example, for U87 glioma cells P was <0.001 (one-way analysis of variance with Tukey pairwise multiple comparison procedures).
Killing for R3616 was not included in this particular set of experiments because it is relatively similar to that observed with MGH1. This was shown by Kramm et al. (26) for rat 9L gliosarcoma cells as well as in a number of previously unpublished experiments (46) performed at a different time from the present experiment. For example, for human U343 glioma cells infected with MGH1 or R3616 cells at an MOI of 0.1, survival rates were 54 and 50%, respectively, at 2 days. For human T98 cells infected with MGH1 or R3616 at an MOI of 0.1, survival rates were 40 and 38%, respectively.
In vivo anticancer effects.
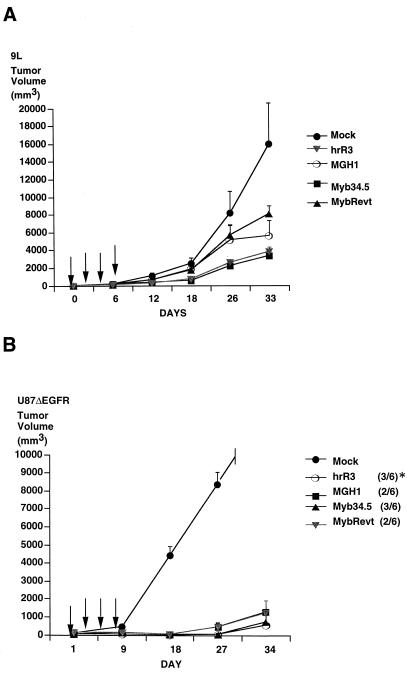
We then sought to determine the in vivo anticancer effects of Myb34.5. After establishing rat 9L gliosarcoma (Fig. 4A) or human U87dEGFR glioma (Fig. 4B) tumors in the flanks of athymic mice, intraneoplastic inoculation with each virus was performed. 9L tumor growth, as assessed by mean tumor volumes, was significantly reduced by Myb34.5 treatment compared to control animals, with inhibition quantitatively similar to that after hrR3 treatment (Fig. 4A). For U87ΔEGFR, a human glioma which expresses a common truncated epidermal growth factor receptor (22, 40, 41), all strains significantly inhibited growth, with hrR3 and Myb34.5 producing 3 of 6 complete regressions, while MGH1 and MybRevt produced two of six regressions (to no visible tumor [Fig. 4B]).
FIG. 4.
In vivo growth inhibition by Myb34.5. In similar experiments, rat gliosarcoma 9L (A) and human U87ΔEGFR glioma (B) cells were implanted subcutaneously into the flanks of nude mice. Beginning 14 (9L) and 10 (U87) days later (day 1), vehicle or mutant viral strains were inoculated into tumors. Arrows indicate the times of viral injection (days 1, 3, 5, and 7), while values are the averages for five (9L) and six (U87ΔEGFR) mice per group. (A) Differences in tumor volumes were significant at the 12-, 18-, and 33-day time points (P < 0.05 [one-way repeated measures of variance]). (B) Differences in tumor volumes were significant at the 18-, 27-, and 34-day time point (P < 0.05 [one-way repeated measures of variance] for day 34 alone, as shown in Table 5, P < 0.005). The asterisk denotes the number of complete tumor regressions.
Although a cursory analysis of Fig. 4B may lead to the conclusion that there were no significant differences in the growth of human U87ΔEGFR tumors treated with the γ34.5 mutants (MGH1 and MybRevt) versus the γ34.5+ viruses (hrR3 and Myb34.5), review of the actual tumor volumes at the 34-day point does reveal the presence of a significant difference (Table 5). These results thus showed that Myb34.5’s in vivo oncolytic effects paralleled those of the single RR mutant and were superior to those of the γ34.5 mutants.
TABLE 5.
Volumes of human U87ΔEGFR tumors after treatmenta
| Animal no. | Tumor vol (mm3) after:
|
||||
|---|---|---|---|---|---|
| Mock infection | Infection with viral mutant
|
||||
| HrR3 | MGH1 | Myb34.5 | MybRevt | ||
| 1 | 11,880 | 0 | 3,960 | 4,000 | 221 |
| 2 | 19,530 | 32 | 0 | 0 | 561 |
| 3 | 15,592 | 15 | 0 | 132 | 0 |
| 4 | 10,626 | 0 | 1,300 | 0 | 3,420 |
| 5 | 10,584 | 0 | 1,914 | 0 | 3,344 |
| 6 | 15,620 | 3,366 | 900 | 405 | 0 |
| Medianb | 13,736 | 7.5 | 1,100 | 66 | 391 |
| Confidence range (25–75%) | 10,626–15,620 | 0–32 | 0–1,914 | 0–405 | 0–3,344 |
Details of experimental procedures are provided in the legend to Fig. 4. Results are from the 34-day time point.
The differences in the median values among the treatment groups were statistically different (P = 0.004 [Kruskal-Wallis one-way analysis of variance on ranks]).
DISCUSSION
The objective of this report was to show that the newly engineered, targeted double-mutant HSV strain Myb34.5 effectively kills malignant glioma cells both in vitro and in vivo, while retaining a high degree of safety in terms of neurotoxicity on direct intracranial inoculation in mice. This strain also exhibited minimal replication in primary arrested fibroblasts and marked induction of replication in cycling cells similar to the induction seen with an HSV characterized by a single RR mutation. In tumor cells, it also replicated vigorously, with improved oncolytic efficacy compared to the parental, double RR-γ34.5 mutant, MGH1. Perhaps most importantly, Myb34.5, like MGH1 or other γ34.5 mutants, demonstrated little pathogenicity in mice at intracerebral doses of 107 PFU, remaining neuroattenuated. Therefore, Myb34.5 exhibits improved oncolytic efficacy compared to the parental mutant MGH1, the marker-rescued revertant MybRevt, and MGH1’s parental mutant R3616, while maintaining characteristics of neuroattenuation, with an LD50 of >107 PFU. This value is qualitatively similar to those observed with γ34.5 mutants, although strict quantitative comparisons were limited by the technical inability to accurately determine an LD50 for the latter group of mutants.
A major objective in cancer gene therapy is to identify viral mutants that provide significant anticancer effects while at the same time demonstrating minimal side effects and toxicity towards normal cells and tissues. This feat has been accomplished by engineering replication-defective viral vectors, which should display minimal toxicity toward infected normal and tumor cells, and endowing them with the ability to express anticancer genes to achieve biologic effects (38). When applied as inocula to large human tumor masses, such vectors do not diffuse well due to their size, thus limiting anticancer effects to cells located in proximity to the injection tract (3, 43). One potential solution to this problem consists of the use of replication-conditional (oncolytic, replication-restricted) viral mutants that maintain the ability to replicate in a relatively selective fashion in tumor or mitotic cell while being restricted in their ability to replicate in normal cells. Such viral mutants would thus propagate from initially infected tumor cells to surrounding tumor cells, thus achieving a larger volume of distribution and enhanced anticancer effects. However, one might expect increased toxicity with these mutants. In order to minimize the potential toxicities associated with replication-conditional HSV-1, deletion of the endogenous γ34.5 genes has been shown to significantly limit or eliminate the risk of encephalitis or meningitis upon intracerebral injection in rodents (11, 29, 30) and Aotus monkeys (35). Further, in a phase I clinical trial in humans afflicted with malignant brain tumors, this type of mutant has not shown evidence of ill effects (31).
We have been concerned that complete elimination of endogenous γ34.5 function would also limit the anticancer effect of HSV. In published experiments, this limitation was shown for rat 9L gliosarcoma cells both in vitro and in vivo (26). In the present study, we also tested killing mediated by HSV mutants with deletions of the γ34.5 function (MGH1 and MybRevt) against a panel of five human glioma cell lines (Table 3). As expected, we found that these mutants were relatively limited in their oncolytic efficacy, compared to the wild-type F strain. Similar findings were also observed with R3616, MGH1’s parental strain (46). However, the oncolytic efficacy of MGH1 was restored to levels that were closer to those observed with the wild-type F strain upon insertion of a single γ34.5 gene under B-myb promoter control.
The significance of this result is that inoculation of Myb34.5 into tumors is expected to produce more extensive oncolysis than inoculation of MGH1, R3616, or other γ34.5 mutants into tumors. In fact, the antitumor efficacy of Myb34.5 in vivo was found to be quantitatively similar to that of a single RR mutant (hrR3), suggesting that, in tumor cells, active expression of the γ34.5 product reverts Myb34.5 to a γ34.5-positive phenotype. However, because hrR3 is a simple insertional mutant, Myb34.5 may offer the theoretical advantage of being less prone to recombinatorial repair to wild-type in the presence of latent preexisting or subsequent HSV infection. Even in the unlikely event that repair of the ICP6 locus via homologous recombination might occur, the B-myb promoter–γ34.5 insert would be excised and return Myb34.5 to the γ34.5 deletion genotype of R3616, the parental virus for MGH1.
Published results have demonstrated that at least one function of γ34.5 is to preclude the host cell’s response to viral infection, namely, the triggering of host protein synthesis shutoff in an apoptosis-like response (10, 11, 13). A similar function is widespread among pathogenic viruses (16, 17, 23, 50). While γ34.5 is nonessential for viral growth in culture in Vero cells, it enables the virus to spread in the mouse central nervous system (24, 25, 30) and maps to a region of the HSV genome previously implicated in CNS replication (7, 30). This may be due to the fact that the γ34.5-encoded protein inhibits the double-stranded RNA-dependent kinase. On exposure to double-stranded RNA molecules, as seen commonly with viral infection, RNA-dependent kinase phosphorylates the alpha subunit of elongation initiation factor 2, resulting in inhibition of protein synthesis (11–13). Infection of cells of neuronal origin with mutants incapable of expressing γ34.5 results in shutoff of cellular protein synthesis, with the resultant limitation of viral production. One critical aspect of the present project was to show restoration of γ34.5 function by B-myb promoter transcriptional control. This was done by demonstrating that infection of cells with MGH1 and MybRevt resulted in suppression of protein synthesis, while protein synthesis was restored when Myb34.5 was the infecting virus. Further evidence for the novel B-myb transcriptional dependence of γ34.5 function to the cell cycle was provided by the lovastatin arrest experiments. These clearly showed that under growth arrest conditions, when B-myb transcriptional activity is minimal, titers of Myb34.5 were similar to those of MGH1 and MybRevt and dissimilar from those of the wild-type F strain and hrR3. However, when cells were serum stimulated, titers of Myb34.5 increased by 3 orders of magnitude, approaching titers observed with strains F and hrR3, while titers of the γ34.5 mutants (MGH1 and MybRevt) increased only slightly. It was notable that the basal level of replication of Myb34.5 was lower than that of hrR3 in quiescent cells and was more quantitatively similar to that of the RR-γ34.5 mutant MGH1. Myb34.5 demonstrated a higher-fold induction of replication in cycling cells than hrR3, while MGH1 and MybRevt showed minimal induction, suggesting that the effects of the Myb34.5 construct exceed the effect of simple complementation of ribonucleotide reductase. These results thus appear to confirm our initial hypothesis that the B-myb promoter restricts viral replication in quiescent cells and redirects viral replication to cycling cells. In the context of brain tumor therapy, Myb34.5 would thus replicate at relatively low levels (similar to the levels observed with MGH1) in cells that are quiescent, but infection of dividing brain tumor cells would produce a significant increase in viral titers, thus providing a therapeutic advantage over MGH1 or other γ34.5 mutants. Infection of normal brain cells can occur with γ34.5 mutants (24, 25, 30), and one would expect Myb34.5 action to mimic that of these mutants in quiescent infected neural cells. In fact, our in vitro and in vivo studies do show that Myb34.5 replication in cultured neurons and in the brains of mice is similar to that of the γ34.5 mutant viruses (MGH1, MybRevt, and R3616) and dissimilar from that of wild-type F strain and hrR3.
An alternative explanation may be that observed effects of Myb34.5 are due to the replacement of the two endogenous γ34.5 genes by a single γ34.5 gene and by use of a promoter (B-myb) that is weaker than the endogenous HSV γ34.5 promoter and whose characteristics of strict cell cycle regulation are disrupted in the context of the HSV genome. Although formal exclusion of this possibility would require extensive additional experimentation with mutant B-myb promoters, we believe that it remains unlikely in view of the current experimental data. If this explanation were true, then one would predict (i) Myb34.5 replication in quiescent cells to be higher than that of γ34.5 deletion mutants because of low level expression of the γ34.5 gene by a deregulated B-myb promoter in the former mutants, (ii) the inhibition of host protein synthesis shutoff observed in neuroblastoma cells infected with Myb34.5 (Fig. 2) to be less pronounced than that observed in cells infected with F or hrR3 because of lower expression of the single γ34.5 gene with a weaker promoter compared to the robust expression achieved by the two γ34.5 genes driven by the endogenous HSV promoter, (iii) the replication of Myb34.5 in U373 cells, known to severely restrict replication of γ34.5 mutants (37), to also be somewhat restricted by the weaker expression of the γ34.5 gene in Myb34.5 compared to that of F or hrR3, and (iv) the differential in replication between quiescent and cycling cells to be higher for the hrR3 or F strain (expressing two copies of γ34.5 driven by the endogenous HSV promoter) than that for Myb34.5 (expressing one copy of γ34.5 driven by a deregulated B-myb promoter).
The experimental data in this report does not agree with the aforementioned predictions. The most likely explanation for the observed results is that the B-myb promoter remains regulated in a relatively tight fashion even in the context of the HSV genome, thus leading to minimal, if any, expression of γ34.5 in quiescent cells and to levels of expression in cycling and tumor cells that appear functionally similar to those observed with viral strains with intact γ34.5 function.
We have recently shown that HSV mutants can be engineered to function as vectors. This allows them to express not only viral oncolytic functions but also additional anticancer effects, thereby increasing their therapeutic efficacy (9). Clearly, Myb34.5 may also provide a suitable backbone for the addition of anticancer genes, such as those that activate prodrugs. As tumor-selective promoters are identified, it would be relatively easy to use these to control expression of the γ34.5 gene or other virulence genes in order to further restrict viral production to tumor versus normal cells. The approach described in this report may also be used to restrict virulence to specific cell types in a tissue, by employing cell-specific promoters.
The B-myb promoter contains a consensus E2F binding site, is strictly regulated in cycling cells, and is in fact repressed in G0 (1, 27, 28). A replication-defective adenovirus containing an E2F-responsive promoter has been used to demonstrate tumor-specific gene expression, relative not only to quiescent neuronal tissue but also to nontransformed normal cycling cells (42). Alteration of some portion of the cell cycle-regulatory p16-retinoblastoma-cdk4 pathway, which regulates E2F, appears to be a near-universal event in human gliomas as well as many other tumor types and provides an excellent substrate for targeting tumor specific expression of viral gene products (21, 52). Another HSV-1 mutant in which an albumin promoter was employed to regulate the expression of ICP4 toward hepatocytes has been described (36). The primary difference from the strategy described in the present report is the use of a promoter that may be considered tumor or cell cycle specific instead of hepatocyte specific and the use of an HSV gene that is directly related to virulence (γ34.5) rather than an essential transcription factor, such as ICP4. In contrast to many viral vectors under investigation for treatment of glioblastoma, Myb34.5 is replication competent, and targeted virulence is obtained by regulating viral replication and direct oncolysis. In this respect, Myb34.5 represents a novel, targeted oncolytic herpesvirus and adds to recently described tumor-selective, oncolytic adeno- and reoviruses (2, 15). The E1B-defective adenovirus ONYX-015 is thought to depend on alterations of the p53 tumor suppressor pathway for efficient replication in tumor cells, although recent evidence has called into question this mechanism (20). A reovirus strain has been shown to selectively replicate in cells with an activated ras pathway (15). Myb34.5 may take advantage of alterations of the p16-cdk4-RB-E2F pathway and adds to the possibility that multiple tumor genetic alterations may be targeted by different viral treatment strategies. The strategy of using cell-specific or tumor-specific promoters to drive expression of the γ34.5 gene may also be suitable as a means to eliminate selected cell populations in vivo. Finally, the finding of increased safety combined with potent antitumor efficacy suggests that Myb34.5 should be further studied as a treatment agent for malignant tumors.
ACKNOWLEDGMENTS
R.Y.C. is a Gloria Rogers fellow of the American Brain Tumor Association. This work was supported by the National Cancer Institute (CA6924602).
REFERENCES
- 1.Bennett J D, Farlie P G, Watson R J. E2F binding is required but not sufficient for repression of B-myb transcription in quiescent fibroblasts. Oncogene. 1996;13:1073–1082. [PubMed] [Google Scholar]
- 2.Bischoff J R, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 3.Bobo R H, Laske D W, Akbasak A, Morrison P F, Dedrick R L, Oldfield E H. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boviatsis E J, Chase M, Wei M X, Tamiya T, Hurford R K, Jr, Kowall N W, Tepper R I, Breakefield X O, Chiocca E A. Gene transfer into experimental brain tumors mediated by adenovirus, herpes simplex virus, and retrovirus vectors. Hum Gene Ther. 1994;5:183–191. doi: 10.1089/hum.1994.5.2-183. [DOI] [PubMed] [Google Scholar]
- 5.Boviatsis E J, Park J S, Sena-Esteves M, Kramm C M, Chase M, Efird J T, Wei M X, Breakefield X O, Chiocca E A. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994;54:5745–5751. [PubMed] [Google Scholar]
- 6.Boviatsis E J, Scharf J M, Chase M, Harrington K, Kowall N W, Breakefield X O, Chiocca E A. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. [PubMed] [Google Scholar]
- 7.Centifanto-Fitzgerald Y M, Yamaguchi T, Kaufman H E, Tognon M, Roizman B. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982;155:475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers R, Gillespie G Y, Soroceanu L, Andreansky S, Chatterjee S, Chou J, Roizman B, Whitley R J. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase M, Chung R Y, Chiocca E A. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16:444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 10.Chou J, Chen J J, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou J, Kern E R, Whitley R J, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 12.Chou J, Poon A P W, Johnson J, Roizman B. Differential response of human cells to deletions and stop codons in the γ134.5 gene of herpes simplex virus. J Virol. 1994;68:8304–8311. doi: 10.1128/jvi.68.12.8304-8311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou J, Roizman B. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung R Y, Chiocca E A. Gene therapy for tumors of the central nervous system. Surg Oncol Clin N Am. 1998;7:589–602. [PubMed] [Google Scholar]
- 15.Coffey M C, Strong J E, Forsyth P A, Lee P W. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 16.Cosentino G P, Venkatesan S, Serluca F C, Green S R, Mathews M B, Sonenberg N. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc Natl Acad Sci USA. 1995;92:9445–9449. doi: 10.1073/pnas.92.21.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale M, Jr, Blakely C M, Kwieciszewski B, Tan S-L, Dossett M, Tang N M, Korth M J, Polyak S J, Gretch D R, Katze M G. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein D J, Weller S K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein D J, Weller S K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988;62:2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodrum F D, Ornelles D A. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J, Allen J R, Collins V P, Allalunis-Turner M J, Godbout R, Day III R S, James C D. CDK4 amplification is an alternative mechanism to p16 gene homozygous deletion in glioma cell lines. Cancer Res. 1994;54:5804–5807. [PubMed] [Google Scholar]
- 22.Huang H S, Nagane M, Klingbeil C K, Lin H, Nishikawa R, Ji X D, Huang C M, Gill G N, Wiley H S, Cavenee W K. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 23.Katze M G. Regulation of the interferon-induced PKR: can viruses cope? Trends Microbiol. 1995;3:75–78. doi: 10.1016/s0966-842x(00)88880-0. [DOI] [PubMed] [Google Scholar]
- 24.Kesari S, Lasner T M, Balsara K R, Randazzo B P, Lee V M, Trojanowski J Q, Fraser N W. A neuroattenuated ICP34.5-deficient herpes simplex virus type 1 replicates in ependymal cells of the murine central nervous system. J Gen Virol. 1998;79:525–536. doi: 10.1099/0022-1317-79-3-525. [DOI] [PubMed] [Google Scholar]
- 25.Kesari S, Lee V M, Brown S M, Trojanowski J Q, Fraser N W. Selective vulnerability of mouse CNS neurons to latent infection with a neuroattenuated herpes simplex virus-1. J Neurosci. 1996;16:5644–5653. doi: 10.1523/JNEUROSCI.16-18-05644.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramm C M, Chase M, Herrlinger U, Jacobs A, Pechan P A, Rainov N G, Sena-Esteves M, Aghi M, Barnett F H, Chiocca E A, Breakefield X O. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997;8:2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- 27.Lam E W, Bennett J D, Watson R J. Cell-cycle regulation of human B-myb transcription. Gene. 1995;160:277–281. doi: 10.1016/0378-1119(95)00184-8. [DOI] [PubMed] [Google Scholar]
- 28.Lam E W, Watson R J. An E2F-binding site mediates cell-cycle regulated repression of mouse B-myb transcription. EMBO J. 1993;12:2705–2713. doi: 10.1002/j.1460-2075.1993.tb05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markert J M, Malick A, Coen D M, Martuza R L. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Markovitz N S, Baunoch D, Roizman B. The range and distribution of murine central nervous system cells infected with the γ134.5− mutant of herpes simplex virus 1. J Virol. 1997;71:5560–5569. doi: 10.1128/jvi.71.7.5560-5569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martuza, R. L. Personal communication.
- 32.Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 33.McGeoch D J, Cunningham C, McIntyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 34.Mineta T, Rabkin S D, Martuza R L. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 35.Mineta T, Rabkin S D, Yazaki T, Hunter W D, Martuza R L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 36.Miyatake S, Iyer A, Martuza R L, Rabkin S D. Transcriptional targeting of herpes simplex virus for cell-specific replication. J Virol. 1997;71:5124–5132. doi: 10.1128/jvi.71.7.5124-5132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohr I, Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- 38.Moriuchi S, Oligino T, Krisky D, Marconi P, Fink D, Cohen J, Glorioso J C. Enhanced tumor cell killing in the presence of ganciclovir by herpes simplex virus type 1 vector-directed coexpression of human tumor necrosis factor-alpha and herpes simplex virus thymidine kinase. Cancer Res. 1998;58:5731–5737. [PubMed] [Google Scholar]
- 39.Morse L S, Pereira L, Roizman B, Schaffer P A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978;26:389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagane M, Coufal F, Lin H, Bogler O, Cavenee W K, Huang H J. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 41.Nishikawa R, Ji X D, Harmon R C, Lazar C S, Gill G N, Cavenee W K, Huang H J. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parr M J, Manome Y, Tanaka T, Wen P, Kufe D W, Kaelin W G, Jr, Fine H A. Tumor-selective transgene expression in vivo mediated by an E2F-responsive adenoviral vector. Nat Med. 1997;3:1145–1149. doi: 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- 43.Puumalainen A M, Vapalahti M, Agrawal R S, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- 44.Pyles R B, Thompson R L. Evidence that the herpes simplex virus type 1 uracil DNA glycosylase is required for efficient viral replication and latency in the murine nervous system. J Virol. 1994;68:4963–4972. doi: 10.1128/jvi.68.8.4963-4972.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pyles R B, Warnick R E, Chalk C L, Szanti B E, Parysek L M. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 46.Qureshi, N., and E. A. Chiocca. Unpublished data.
- 47.Ram Z, Culver K W, Oshiro E M, Viola J J, DeVroom H L, Otto E, Long Z, Chiang Y, McGarrity G J, Muul L M, Katz D, Blaese R M, Oldfield E H. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 48.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarzchild M A, Cole R L, Hyman S E. Glutamate, but not dopamine, stimulates stress-activated protein kinase and AP-1-mediated transcription in striatal neurons. J Neurosci. 1997;17:3455–3466. doi: 10.1523/JNEUROSCI.17-10-03455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharp T V, Schwemmle M, Jeffrey I, Laing K, Mellor H, Proud C G, Hilse K, Clemens M J. Comparative analysis of the regulation of the interferon-inducible protein kinase PKR by Epstein-Barr virus RNAs EBER-1 and EBER-2 and adenovirus VAI RNA. Nucleic Acids Res. 1993;21:4483–4490. doi: 10.1093/nar/21.19.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toda M, Rabkin S D, Martuza R L. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Hum Gene Ther. 1998;9:2177–2185. doi: 10.1089/hum.1998.9.15-2177. [DOI] [PubMed] [Google Scholar]
- 52.Ueki K, Ono Y, Henson J W, Efird J T, von Deimling A, Louis D N. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150–153. [PubMed] [Google Scholar]
- 53.Wei M X, Tamiya T, Chase M, Boviatsis E J, Chang T K, Kowall N W, Hochberg F H, Waxman D J, Breakefield X O, Chiocca E A. Experimental tumor therapy in mice using the cyclophosphamide-activating cytochrome P450 2B1 gene. Hum Gene Ther. 1994;5:969–978. doi: 10.1089/hum.1994.5.8-969. [DOI] [PubMed] [Google Scholar]