Abstract
Background
This study aims to comparatively analyze clinical features, treatment, and patient outcomes between the previous and the 2022 mpox (monkeypox) outbreaks.
Methods
Five bibliographic databases were searched for studies reporting clinical features, management, and patient outcomes of mpox. Systematic review and meta-analysis were performed.
Results
In total, 73 studies were included in the systematic review, of which 33 studies were subjected to meta-analysis. Previous outbreaks substantially affected children, whereas the 2022 outbreak primarily affected male adults, of which 94.66% (95% confidence interval [CI], 88.03–98.95) were men who have sex with men. Furthermore, 72.47% (95% CI, 51.04–89.71) reported high-risk sexual activity and the overall human immunodeficiency virus (HIV) prevalence was 37.65% (95% CI, 30.09–45.50). Skin lesions remain the typical symptom; however, their anatomic distribution differed. Systemic manifestations were common, but rectal pain was unique to the 2022 outbreak. The estimated overall fatality during past outbreaks in Africa was 4.61% (95% CI, 2.39%–7.35%), whereas 6.34% (95% CI, 3.35%–10.10%) of patients from the 2022 outbreak required hospitalization. Antiviral treatment, in particular tecovirimat, has been prescribed for a subset of patients, but the efficacy remains inconclusive.
Conclusions
These findings are important for better understanding the disease and guiding adequate response to mpox outbreaks.
Keywords: mpox, outcomes, sexual activity, symptoms, treatment
Mpox mainly causes skin injuries, but severe complications and even death can occur in vulnerable individuals. This systematic review and meta-analysis revealed distinct epidemiological and clinical features as well as patient outcomes between the previous and the 2022 mpox outbreaks.
As a member of the Orthopoxvirus genus, mpox (monkeypox) virus (MPXV) infection can cause smallpox-like illness, with classical symptoms such as skin lesions [1]. Since the first identification in human in 1970, mpox outbreaks have occurred periodically in Africa and it has become endemic in West and Central Africa [2]. In general, MPXV is confined to tropical regions within African countries. It was accidently carried to the United States in imported small mammals, which infected nearby housed pet prairie dogs, and the virus was then transmitted to human hosts [3]. This eventually resulted in 47 infection cases in 2003 [4]. Since then, sporadic cases have been occasionally reported in America, Europe, and Asia, but all these cases can be traced back to African origins [5]. Unexpectedly, the world is currently facing widespread mpox outbreaks across many nonendemic countries, starting in May 2022 [6]. This is the first time that sustained person-to-person transmission of MPXV without travel history to any endemic countries in Africa has been recognized. MPXV is thought to not easily spread between people, but spreads through close contact, for example, with skin lesions, body fluids, respiratory droplets, and contaminated materials [7, 8]. However, this assumption fails to explain why cases rose rapidly in the initial phase of the outbreak and now are declining, and more strikingly, the vast majority of cases identified so far have been men who have sex with men (MSM) [9].
To adequately respond and contain the 2022 outbreak, timely deployment of both nonpharmacological and pharmacological interventions is essential. Smallpox vaccination is postulated to provide cross-immunity against MPXV, and a third-generation vaccine, named Jynneos, has been approved for preventing smallpox and mpox [10]. But the efficacy of this vaccine in people remains inconclusive due to the lack of smallpox cases [11]. In recent decades, smallpox vaccinations have only been implemented in small-scale populations, such as US military recruits and specific scientific researchers, and thus the majority of the global population are expected to be susceptible to MPXV infection. Antiviral drugs, including tecovirimat and brincidofovir, have been approved by the Food and Drug Administration (FDA) for treating smallpox and the related poxviruses, but efficacy against MPXV infection was only demonstrated in experimental models [12]. Despite empirical use in some infected cases, their clinical efficacies in treating mpox remain unknown.
Given the lack of knowledge on the disease, this study first aims first to systematically review the existing clinical data by retrieving studies published over the past 5 decades. Second, we comparatively analyzed the clinical features, treatment strategies, and patient outcomes between the previous and the 2022 outbreaks.
METHODS
We conducted a systematic search through 5 databases, that is Medline, Embase, Web of Science, Cochrane Central, and Google Scholar. Databases were searched with restrictions to only English language and studies from inception until 10 January 2023, using search terms relating to epidemiological and clinical features of monkeypox/mpox. Studies with original data were included in terms of the following criteria: studies containing mpox cases that were confirmed by laboratory diagnostics, and studies containing mpox cases with a clinical diagnostic definition. Studies with mpox cases but failed to report any information about clinical symptoms, treatments, or outcomes were excluded. The full search strategies and selection criteria are provided in the Supplementary Material 1 and 2. Three reviewers (P. L., J. L., and I. A.) worked independently to determine whether a study met inclusion criteria. A fourth reviewer (Q. P.) functioned as an arbiter when there was discrepancy in determining whether to include or exclude a study. This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [13].
Evaluation of Study Quality and Data Extraction
Eligible studies were further divided into 2 sections: mpox outbreaks/cases before 2022, and mpox outbreak/cases (outside of Africa) since May 2022. Two reviewers (P. L. and J. L.) extracted the following information: first author, publication year, outbreak identification year, country, age, sex, clinical symptom, skin lesion distribution, clinical management, outcome, number of deaths, and total number of cases. A third reviewer (I. A.) checked and corrected the extracted information. Studies describing human immunodeficiency virus (HIV) infections, high-risk sexual activities (condomless sex/multiple sexual partners), clinical symptoms, skin lesions distributions, and clinical outcomes (hospitalization and death) were processed for meta-analysis. The quality of these studies were assessed according to the Joanna Briggs Institute checklist [14]. Studies were classified as high quality (score 7–9 points), moderate quality (4–6 points), and low quality (1–3 points). We here only included studies with moderate to high quality for meta-analysis (Supplementary Table 1).
Statistical Analysis
We estimated the pooled prevalence rate by operating the “Meta” package in R version 3.5.3 statistical software. Briefly, Freeman-Tukey double arcsine transformation was used for analysis. Wilson score method was used to calculate the 95% confidence interval (95% CI), and Cochran Q statistics and I2 statistics were used to judge heterogeneity. I2 with 25%–50%, 50%–75%, and >75% values were considered as mild, moderate, and severe heterogeneity. Funnel plot and Egger test were used to assess the potential publication bias.
RESULTS
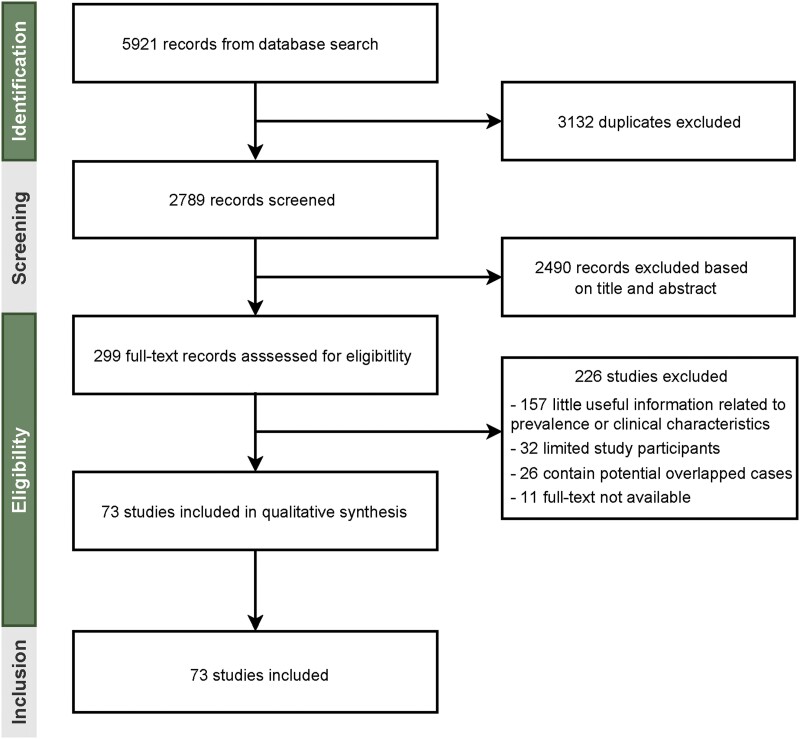
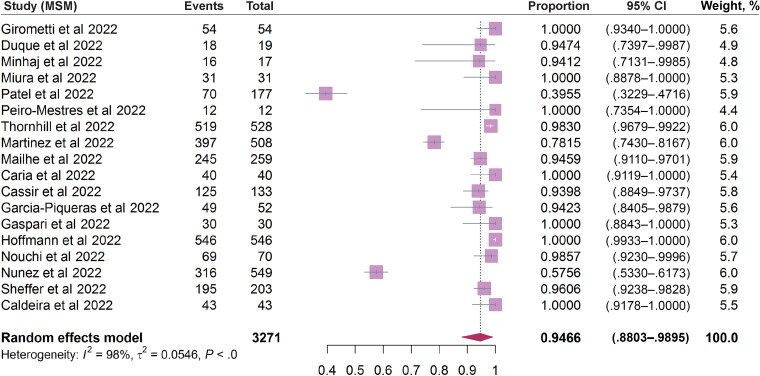
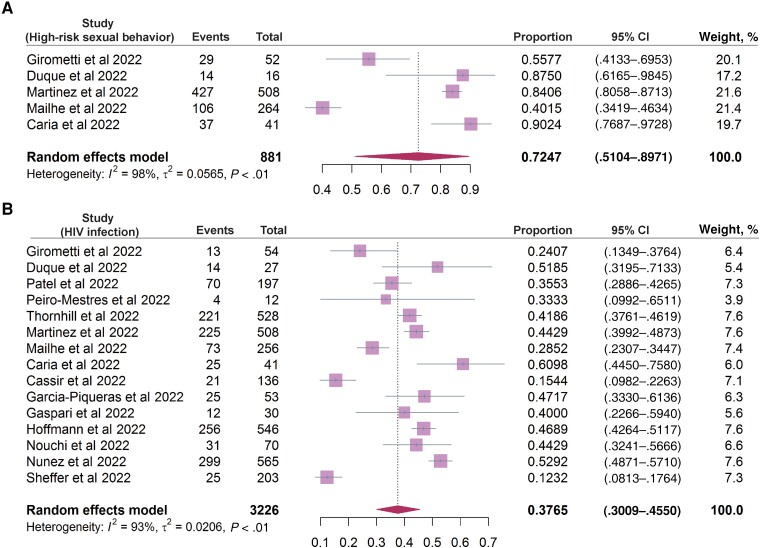
Systematic searching of literature databases identified a total of 2789 studies. Of these, 73 studies met the inclusion criteria for analyzing epidemiological and/or clinical features of mpox (Figure 1). By pooling documented cases from these 73 studies, we found distinct patterns of sex distribution between the previous and the 2022 outbreaks. A total of 1465 cases from 31 studies of the outbreaks during 1970–2021 included information on sex. The percentage of men was only slightly higher than that of women (55.7% vs 44.3%). In contrast, the vast majority of patients in the included studies from the 2022 outbreak were men (99.0%). Next, the age distribution was comparatively analyzed; 1117 cases during 1970–2021 were divided into 5 age groups, with the highest percentage of cases belonged to the 0–9 years age group (38.9%), followed by 10–19 (27.8%), 20–29 (17.2%), 30–39 (10.1%), and older than 40 years of age (6.0%) (Supplementary Table 2). In contrast, the majority of patients in the 2022 outbreak were 30–39 years of age (46.7%), followed by 40–49 (23.2%), 20–29 (21.7%), 50–59 (6.5%), older than 60 (1.1%), and younger than 20 years (0.8%). We then further analyzed the prevalence of MSM, high-risk sexual activity, as well as HIV infection status in patients of the 2022 outbreak. By pooling data related to sexual activity, 94.66% (95% CI, 88.03%–98.95%; I2 = 98%) of patients are estimated to have MSM activity (Figure 2), and 72.47% (95% CI, 51.04%–89.71%; I2 = 98%) have high-risk sexual activities (condomless sex or multiple sexual partners) (Figure 3A). Based on data from 3226 patients describing HIV infection status, 37.65% (95% CI, 30.09%–45.50%; I2 = 93%) were HIV positive (Figure 3B).
Figure 1.
Study selection.
Figure 2.
The incidence rate of MSM in 18 studies included in the analysis. Abbreviations: CI, confidence interval; MSM, men who have sex with men.Complete reference information for the following references Duque et al (2022), Minhaj et al (2022), Miura et al (2022), Patel et al (2022), Peiro-Mestres et al (2022), Martinez et al (2022), Mailhe et al (2022), Caria et al (2022), Cassir et al (2022), Garcia-Piqueras et al (2022), Gaspari et al (2022), Hoffmann et al (2022), Nouchi et al (2022), Nunez et al (2022), Sheffer et al (2022), Caldeira et al (2022) cited in Figure 2 are listed in the Supplementary Material.
Figure 3.
The incidence rate of (A) high-risk sexual activities (condomless/multipartner sexual activity; 5 studies included) and (B) human immunodeficiency virus (HIV) positivity rate (15 studies included) in patients of the 2022 mpox outbreak.Complete reference information for the following references Duque et al (2022), Patel et al (2022), Peiro-Mestres et al (2022), Martinez et al (2022), Mailhe et al (2022), Caria et al (2022), Cassir et al (2022), Garcia-Piqueras et al (2022), Gaspari et al (2022), Hoffmann et al (2022), Nouchi et al (2022), Nunez et al (2022), and Sheffer et al (2022) cited in Figure 3 are listed in the Supplementary Material.
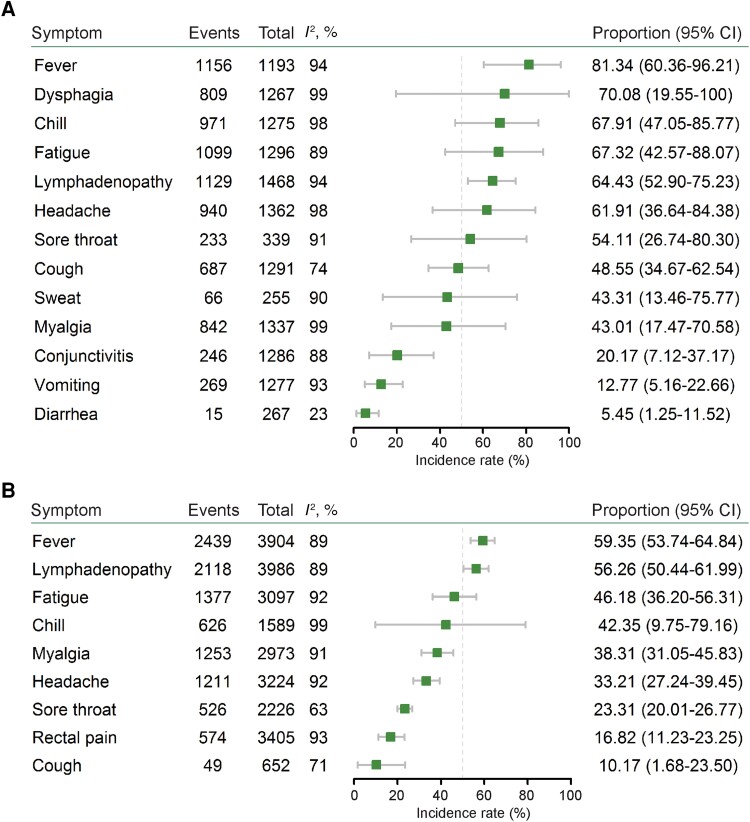
We next estimated the incidence rate of common symptoms of MPXV infection. During the outbreaks before 2022, apart from skin lesions, the most prevalent symptom was fever (81.34%; 95% CI, 60.36%–96.21%; I2 = 94%), followed by dysphagia (70.08%; 95% CI, 19.55%–100%; I2 = 99%), chills (67.91%; 95% CI, 47.05%–85.77%; I2 = 98%), fatigue (67.32%; 95% CI, 42.57%–88.07%; I2 = 89%), lymphadenopathy (64.43%; 95% CI, 52.90%–75.23%; I2 = 94%), headache (61.91%; 95% CI, 36.64%–84.38%; I2 = 98%), sore throat (54.11%; 95% CI, 26.74%–80.30%; I2 = 91%), cough (48.55%; 95% CI, 34.67%–62.54%; I2 = 74%), sweat (43.31%; 95% CI, 13.46%–75.77%; I2 = 90%), myalgia (43.01%; 95% CI, 17.47%–70.58%; I2 = 99%), conjunctivitis (20.17%; 95% CI, 7.12%–37.17%; I2 = 88%), vomiting (12.77%; 95% CI, 5.16%–22.66%; I2 = 93%), and diarrhea (5.45%; 95% CI, 1.25%–11.52%; I2 = 23%) (Figure 4A and Supplementary Figures 1–13). For the included patients from nonendemic countries in the 2022 outbreak, apart from skin lesions, the most frequent symptom was fever (59.35%; 95% CI, 53.74%–64.84%; I2 = 89%), followed by lymphadenopathy (56.26%; 95% CI, 50.44%–61.99%; I2 = 89%), fatigue (46.18%; 95% CI, 36.20%–56.31%; I2 = 92%), chills (42.35%; 95% CI, 9.75%–79.16%; I2 = 99%), myalgia (38.31%; 95% CI, 31.05%–45.83%; I2 = 91%), headache (33.21%; 95% CI, 27.24%–39.45%; I2 = 92%), sore throat (23.31%; 95% CI, 20.01%–26.77%; I2 = 63%), rectal pain (16.82%; 95% CI, 11.23%–23.25%; I2 = 93%), and cough (10.17%; 95% CI, 1.68%–23.50%; I2 = 71%) (Figure 4B and Supplementary Figures 14–22).
Figure 4.
Comparison of clinical symptoms of patients between (A) the previous (9 studies included) and (B) the 2022 (16 studies included) mpox outbreaks.
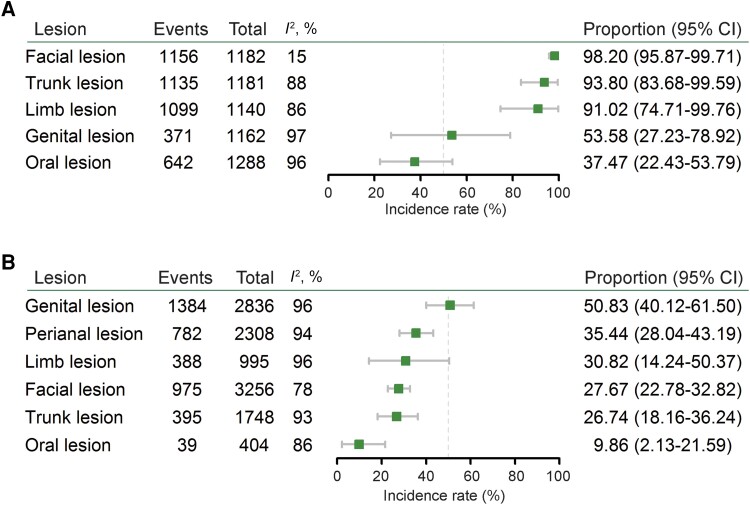
Although skin lesions was the most common presentation, the anatomical distribution of lesions varied considerably among individuals. We thus estimated the body distributions of skin lesions in patients of past outbreaks before 2022 by extracting data from 5 studies. We estimated that 98.2% of infected patients showed facial lesions (95% CI, 95.87%–99.71%; I2 = 15%), followed by 93.8% with trunk lesions (95% CI, 83.68%–99.59%; I2 = 88%), 91.02% with limb lesions (95% CI, 74.47%–99.76%; I2 = 86%), 53.58% with genital lesions (95% CI, 27.23%–78.92%; I2 = 97%), and 37.47% with oral lesions (95% CI, 22.43%–53.79%; I2 = 96%) (Figure 5A and Supplementary Figures 23–27). In contrast, patients from the 2022 outbreak showed distinct patterns of skin lesion distribution. Genital lesions were most common (50.83%; 95% CI, 40.12%–61.50%; I2 = 96%), followed by perianal lesions (35.44%; 95% CI, 28.04%–43.19%; I2 = 94%), limb lesions (30.82%; 95% CI, 14.24%–50.37%; I2 = 96%), facial lesions (27.67%; 95% CI, 22.78%–32.82%; I2 = 78%), trunk lesions (26.74%; 95% CI, 18.16%–36.24%; I2 = 93%), and oral lesions (9.86%; 95% CI, 2.13%–21.59%; I2 = 86%) (Figure 5B and Supplementary Figures 28–33).
Figure 5.
Distribution of skin lesions in patients of the previous and the 2022 outbreaks. A, The distribution of skin lesions in patients from the previous outbreaks (5 studies included). B, The distribution of skin lesions in patients from the 2022 outbreaks (14 studies included).
The presentation and progression of mpox disease range from mild to severe, and even death. Thus, pharmacological interventions have been empirically explored in patient management. We identified 17 studies describing treatment of infected cases (Table 1 [4, 15–30]). Antibiotics were prescribed to 19 hospitalized patients aiming at preventing secondary infection of bacteria. Antiviral drugs (acyclovir, valacyclovir, tecovirimat, and brincidofovir) were administrated to 63 patients. Combination use of tecovirimat with intravenous vaccinia immune globulin was given to a severe case. Because of the severe pain and accompanied sleeping trouble due to skin lesions, 3 cases received either acetaminophen, analgesic, or morphine to relieve these symptoms. Other symptomatic therapeutics, such as paracetamol, diphenhydramine, and steroids, were administrated in several cases, for relieving fever and treating inflammation (Table 1).
Table 1.
Treatment and Patient Outcomes During the Previous and the 2022 Mpox Outbreaks
| Study | Admission Year, Country | No. of Patients | Therapy | Outcome | Remark |
|---|---|---|---|---|---|
| Cases 1982–2021 | |||||
| Janseghers et al 1984 [15] | 1982, Congo | 1 | Penicillin and bronchial dilatation; then penicillin was withdrawn, chloramphenicol and corticosteroids were given | Died | Developed into pneumonia despite penicillin therapy |
| Eltvedt et al 2020 [16] | 2016, Congo | 1 | Started on IV amoxicillin-clavulanic acid, retinol tablets, paracetamol, diluted plumpynut, and IV maintenance fluids; changed to IV ceftriaxone and morphine | Died | Severe skin and oral manifestations |
| Berthet et al 2011 [17] | 2010, CAR | 1 | Cloxacillin for treating a possible staphylococcal skin infection | Discharged | … |
| McCollum et al 2015 [18] | 2012, Congo | 1 | Treated with erythromycin, acetaminophen, promethazine, and vitamin C for 7 d; then transferred to another hospital and treated with gentamicin, cloxacillin, narcotic analgesics, and a warm bath of permanganate | … | The first 7 d treatment did not relieve any symptoms |
| Froeschl et al 2015 [19] | 2012, CAR | 1 | Cloxacillin, ibuprofen, and paracetamol | … | … |
| Anderson et al 2003 [20] | 2003, United States | 1 | Intravenous diphenhydramine, lorazepam, and morphine | Discharged | … |
| Reed et al 2004 [4] | 2003, United States | 11 | 9 received antibiotics (ciprofloxacin or doxycycline); 1 received acyclovir; 2 received valacyclovir | … | … |
| Adler et al 2022 [21] | 2018–2021, United Kingdom | 7 | 3 patients were treated with brincidofovir (orally received 200 mg once/ wk); 1 patient was orally treated with tecovirimat (600 mg twice daily for 2 wk) | Discharged | Patients treated with brincidofovir showed elevated liver enzymes resulting in cessation of therapy; patient with tecovirimat treatment had a shorter duration of viral shedding and illness than other 6 patients, no obvious adverse effects |
| Cases since 2022 | |||||
| Hammerschlag et al 2022 [22] | 2022, Australia | 1 | Treated with intramuscular ceftriaxone and oral doxycycline | Discharged | … |
| Noe et al 2022 [23] | 2022, Germany | 2 | Topical zinc oxide suspension | … | … |
| Lucar et al 2022 [24] | 2022, United States | 2 | Both patients received oral tecovirimat with 600 mg twice daily | Relived pain within 48 h, and no new skin lesions formed | 1 patient reported mild, transient fatigue after tecovirimat treatment |
| Desai et al 2022 [25] | 2022, United States | 25 | All patients received oral treatment with tecovirimat (1 treated for 21 d, the remainder treated 14 d) | Complete resolution of lesions in 10 patients on day 7 of therapy; 23 had resolution of lesions and pain by day 21 | Reported adverse events on day 7 of therapy: fatigue in 7 patients, headache in 5, nausea in 4, itching in 2 and diarrhea in 2 patients |
| Matias et al 2022 [26] | 2022, United States | 3 | All 3 patients received oral tecovirimat at a dose of 600 mg twice daily | 1 patient presented no new skin lesions formed by 4 d treatment, and all 3 patients reported near-complete resolution of rash/skin lesions | Patient 1 had mild nonfocal headache after first dose; patient 2 reported 1–2 loose bowel movements a few hours after each dose |
| Thornhill et al 2022 [27] | 2022, Multiple countries | 528 | 5% of the 528 patients received mpox-specific treatment, include intravenous/topical cidofovir (12 patients), tecovirimat (8 patients), vaccinia immune globulin (1 patient), and other treatment (2 patients) | … | The effect of treatment was not commented |
| Rai et al 2022 [28] | 2022, United States | 1 | This patient was given tecovirimat 600 mg twice daily, artificial tears were given every 4 h, and erythromycin ointment 4 times daily | Conjunctival and caruncular lesions and eye irritation resolved after 4 d treatment; no new lesions developed; discharged on day 6 of treatment without adverse effects | … |
| Thet et al 2022 [29] | 2022, United States | 1 | First 2 wk 600 mg 2 times/d of oral tecovirimat; at week 3 600 mg 2 times/d of IV tecovirimat; then single dose of 414 000 U (6000 U/kg) of VIGIV and 2 wk of IV tecovirimat | 1 wk after VIGIV administration, the patient significantly improved, and no new lesions developed | This patient had medical history of polysubstance abuse and AIDS |
| Girometti et al 2022 [30] | 2022, United Kingdom | 5 | Antibiotic treatment (2 received IV ceftriaxone and oral doxycycline; 1 IV ceftriaxone and oral metronidazole, 1 oral doxycycline and antiviral tecoviramat) and analgesia | 4 discharged, 1 remained admitted at the time of reporting | … |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CAR, Central African Republic; IV, intravenous; VIGIV, vaccinia immune globulin intravenous.
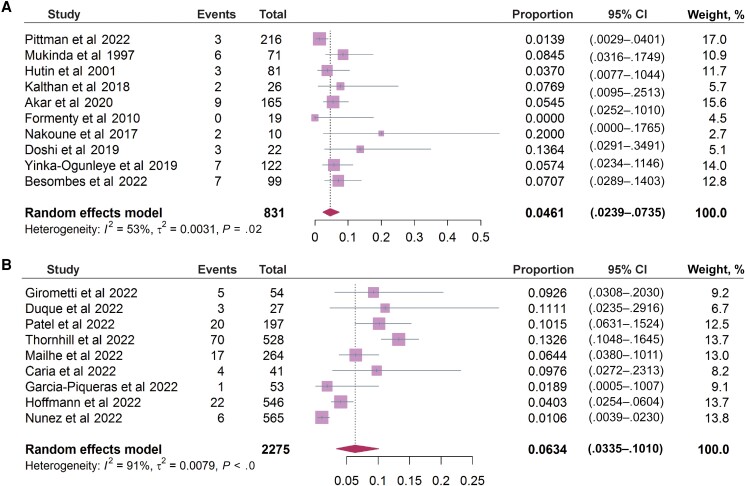
Finally, we estimated the fatality rate based on 831 cases in 10 included studies, mainly from Central African Republic, Nigeria, and Republic of the Congo. The estimated overall fatality rate was 4.61% (95% CI, 2.39%–7.35%; I2 = 53%) in these African patients (Figure 6A). No death was reported from the included studies of the 2022 outbreak. By including 2275 mpox patients from 9 studies, we estimated a hospitalization rate of 6.34% (95% CI, 3.35%–10.10%; I2 = 91%), and the major reason for hospitalization was for relieving pain of the lesions (Figure 6B).
Figure 6.
Clinical outcomes in patients during the previous and the 2022 outbreaks. A, The fatality rate of patients from major endemic African countries in previous mpox outbreaks (10 studies included). B, The rate of hospitalization in the current outbreak (9 studies included).Complete reference information for the following references Pittman et al (2022), Mukinda et al (1997), Hutin et al (2001), Kalthan et al (2018), Akar et al (2020), Formenty et al (2010), Nakoune et al (2017), Doshi et al (2019), Yinka-Ogunleye et al (2019), Besombes et al (2022), Duque et al (2022), Patel et al (2022), Mailhe et al (2022), Caria et al (2022), Garcia-Piqueras et al (2022), Hoffmann et al (2022), Nunez et al (2022) cited in Figure 6 are listed in the Supplementary Material.
DISCUSSION
Through systematic review and comparative analysis, this study identified several distinct epidemiological and clinical features of MPXV infection between the previous and the 2022 outbreaks. We found children younger than 9 years of age constitute the highest proportion of cases during 1970–2021, whereas the included cases from the 2022 outbreak were predominantly adults. It has been reported that young children but not adults are more prone to develop severe symptoms and account for the majority of lethal cases [31]. This disparity in age distribution may partially explain the favorable clinical outcomes of patients in the 2022 outbreak. The 2 distinct clades of MPXV are thought to be an important factor in disease severity. Infection caused by clade I MPXV can cause up to 10% fatality, whereas less than 1% of fatal cases have been reported for the clade II MPXV. Viral genome sequenced isolates from patient in the 2022 outbreak indicated that the circulating strains belong to the clade II virus [32]. All MPXV strains from the 2022 outbreak sequenced so far tightly cluster together, suggesting a single origin [33]. In addition, the distinct availabilities of health care service for patients from the previous outbreaks (mainly low-income countries/regions) and from the 2022 outbreaks (mainly European and American countries) also likely contributed to the different clinical outcomes.
Nevertheless, we should not underestimate the clinical burden of the 2022 outbreak. As the virus is spreading, it has reached vulnerable populations such as young children, elderly people, and immunocompromised patients [34, 35]. Previous evidence has indicated that pregnant women are highly vulnerable to both mpox and smallpox, causing maternal mortality and morbidity [36]. Furthermore, mpox can be transmitted to an unborn fetus via the placenta, leading to congenital infection and abortion [37]. Guidelines for clinical management of pregnant women with suspected MPXV exposure have recently been proposed [36]. Although deaths have not been reported in the 2022 outbreak in our included studies, 106 confirmed deaths have been recorded in public database, of which 92 cases were from nonendemic regions, whereas 14 were in locations that have historically reported mpox [38]. Thus, health care providers and public health professionals should be aware that severe morbidity and mortality associated with mpox have been observed in several countries, including Spain, the United States, Peru, and Brazil during the 2022 outbreak, particularly among highly immunocompromised patients [39]. Although for some cases it remains unclear whether mpox was a causal or contributing factor, several infected patients have died of complications associated with encephalitis [34, 40], which requires specific attention.
A striking feature of the 2022 outbreak is that a large number of cases identified in nonendemic countries were MSM [30]. We estimated that approximately 94% of patients have MSM activity, and 72% of patients have high-risk sexual behaviors, including condomless sex and multiple sexual partners, in the 2022 outbreak. Recently, sexual transmission has been proposed as a novel route of MPXV infection. Changing sexual behaviors in MSM has been shown to reduce mpox cases, which in turn supports sex-related transmission of this disease [41]. Cases during 1970–2021 mainly presented with lesions on the face, but the majority of cases in the 2022 outbreak have not shown this commonly reported symptom. Instead, the lesions presented in patients from the 2022 outbreak are more common in genital and perianal areas. Given the major lesions are present in genital-perianal areas, direct physical contact with lesions during sexual contact is likely the route of MPXV transmission. Notably, a substantial proportion of MPXV-infected cases in the 2022 outbreak are people with HIV and receiving antiretroviral therapy. Whether compromised immune systems have played any role in viral evolution and transmission is interesting to investigated [30]. With the wide spread of the outbreak, the affected populations will inevitably diversify.
MPXV infection has characteristic lesions and is spread through close contact, as the virus primarily infects skin and injures skin mucosa [42]. Interestingly, we estimated approximately 48% and 10% incidence rate of cough in infected patients from previous and the 2022 mpox outbreaks, respectively. This may indicate that MPXV also infects the human airway respiratory tract, but whether this is sufficient to support the hypothesis of possible transmission through direct contact with respiratory secretions remains to be investigated [43]. We also identified a proportion of gastrointestinal symptoms including vomiting and diarrhea, and thus it is interesting to investigate whether the intestine is susceptible to MPXV infection. If this is the case, vomiting and diarrhea could discharge virus particles to the environment, providing new routes of transmission such as fecal-oral transmission. In line with recent reports that some patients were hospitalized because of severe rectal pain, we estimated that approximately 17% of patients had rectal pain and proctitis in the 2022 outbreak. This suggests that MPXV may directly infect the rectum and cause severe inflammation, requiring further investigations and the development of specific treatment strategies.
An important lesson learned from the coronavirus disease 2019 (COVID-19) pandemic is that specific populations, such as health care workers and household members of infected cases, are at high risk of infection [44]. Ring vaccination is recommended to protect these at-risk/vulnerable populations. A third-generation smallpox vaccine has been authorized for use against mpox. Recent evidence indicates reduced risk for mpox in people after receiving 1 or 2 doses of this vaccine [45]. Two types of antiviral drugs, tecovirimat and brincidofovir (an oral lipid conjugate derivative of cidofovir), have been approved by the FDA for treating smallpox virus infection [46]. Tecovirimat inhibits the production of infectious orthopoxvirus by disrupting a major envelope wrapping protein, VP37 [47]. Brincidofovir and cidofovir are viral DNA polymerase inhibitors to suppress viral replication [48]. These antivirals have shown promise for treating MPXV infection based on experimental data (Supplementary Table 3). In cell culture models infected with MPXV isolates obtained from several patients of the 2022 outbreak, the 50% inhibitory concentrations of tecovirimat, cidofovir, and brincidofovir were within the range of therapeutic concentrations observed in patient plasma, suggesting that the currently circulating MPXV strains are likely sensitive to these antiviral drugs [49]. However, so far, only a small proportion of patients with MPXV infection have been treated with these antiviral drugs. Thus, the clinical efficacy remains far from conclusive (Table 1), and well-designed clinical trials are urgently required to confirm the efficacy for treating MPXV infection. Given the complementary mechanism of actions, we postulate that a combination of these 2 types of antiviral drugs may result in synergistic effects, and deserves to be further investigated.
Of note, there are several limitations to this study. Firstly, the overall number of qualified studies was small. In particular, studies related to treatment were very limited and mainly descriptive of segmented cases. This will likely cause bias and prevent us from drawing firm conclusions. Secondly, there was a relatively large heterogeneity in studies reporting different symptoms, which may be attributed to the huge diversity in sample size among different studies. Some analyses with heterogeneity exceeding 95% may pose interpretive challenges to the results [50]. Because of the limited number of eligible studies, we included all studies having over 10 cases for meta-analysis. We assessed publication bias by funnel plot and Egger's test, which indicated the presence of potential bias in the included studies (Supplementary Figure 34). Finally, we only included studies in English language, and thus may have missed some (small) studies published in local journals in other languages. However, we think this will not affect the overall analysis and the main conclusions of our study.
CONCLUSIONS
In summary, this study revealed distinct epidemiological and clinical features as well as patient outcomes of the mpox outbreaks during 1970–2021 and 2022. The current use of antiviral treatment is mainly based on experimental evidence and thus well-designed clinical trials are urgently needed to confirm their efficacy. These findings are important for better understanding the disease and guiding an adequate response to mpox outbreaks.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Pengfei Li, Department of Gastroenterology and Hepatology, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
Jiajing Li, Department of Gastroenterology and Hepatology, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
Ibrahim Ayada, Department of Gastroenterology and Hepatology, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
Amine Avan, Department of Gastroenterology and Hepatology, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
Qinyue Zheng, School of International Affairs and Public Administration, Ocean University of China, Qingdao, China.
Maikel P Peppelenbosch, Department of Gastroenterology and Hepatology, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
Annemarie C de Vries, Department of Gastroenterology and Hepatology, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
Qiuwei Pan, Department of Gastroenterology and Hepatology, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
Notes
Acknowledgments . The authors thank Dr Wichor Bramer and Dr Maarten Engel (biomedical information specialists, Erasmus MC Rotterdam) for assisting with the database search for this study.
Disclaimer . The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial support . This work was supported by the Netherlands Organization for Scientific Research (grant number 91719300 Vidi grant to Q. P.); and the Dutch Cancer Society (grant number 10140 young investigator grant to Q. P.). Funding to pay the Open Access publication charges for this article was provided by Netherlands Organization for Scientific Research.
References
- 1. Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis 2005; 41:1765–71. [DOI] [PubMed] [Google Scholar]
- 2. Mauldin MR, McCollum AM, Nakazawa YJ, et al. Exportation of monkeypox virus from the African continent. J Infect Dis 2022; 225:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Past U.S. cases and outbreaks. https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html#:∼:text=2003%20Outbreak%20from%20Imported%20Mammals,contact%20with%20pet%20prairie%20dogs. Accessed 17 February 2023.
- 4. Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western hemisphere. N Engl J Med 2004; 350:342–50. [DOI] [PubMed] [Google Scholar]
- 5. Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis 2022; 16:e0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thakur V, Thakur P, Srivastava S, Kumar P. Monkeypox virus (MPX) in humans a concern: trespassing the global boundaries—correspondence. Int J Surg 2022; 104:106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nolen LD, Osadebe L, Katomba J, et al. Extended human-to-human transmission during a monkeypox outbreak in the democratic Republic of the Congo. Emerg Infect Dis 2016; 22:1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doshi RH, Alfonso VH, Morier D, et al. Monkeypox rash severity and animal exposures in the democratic Republic of the Congo. Ecohealth 2020; 17:64–73. [DOI] [PubMed] [Google Scholar]
- 9. Zumla A, Valdoleiros SR, Haider N, et al. Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet Infect Dis 2022; 22:929–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaeck LM, Lamers MM, Verstrepen BE, et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med 2023; 29:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirby T. WHO celebrates 40 years since eradication of smallpox. Lancet Infect Dis 2020; 20:174. [DOI] [PubMed] [Google Scholar]
- 12. Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med 2018; 379:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. JBI . Critical appraisal tools. https://jbi.global/critical-appraisal-tools. Accessed 17 February 2023.
- 15. Janseghers L, Matamba M, Colaert J, Vandepitte J, Desmyter J. Fatal monkeypox in a child in Kikwit, Zaire. Ann Soc Belg Med Trop 1984; 64:295–8. [PubMed] [Google Scholar]
- 16. Eltvedt AK, Christiansen M, Poulsen A. A case report of monkeypox in a 4-year-old boy from the DR Congo: challenges of diagnosis and management. Case Rep Pediatr 2020; 2020:8572596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berthet N, Nakoune E, Whist E, et al. Maculopapular lesions in the Central African Republic. Lancet 2011; 378:1354. [DOI] [PubMed] [Google Scholar]
- 18. McCollum AM, Nakazawa Y, Ndongala GM, et al. Human monkeypox in the Kivus, a conflict region of the democratic Republic of the Congo. Am J Trop Med Hyg 2015; 93:718–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Froeschl G, Kayembe PK. Pox-like lesions and haemorrhagic fever in two concurrent cases in the Central African Republic: case investigation and management in difficult circumstances. Pan Afr Med J 2015; 22:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson MG, Frenkel LD, Homann S, Guffey J. A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. Pediatr Infect Dis J 2003; 22:1093–6. [DOI] [PubMed] [Google Scholar]
- 21. Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis 2022; 22:1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammerschlag Y, MacLeod G, Papadakis G, et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill 2022; 27:2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noe S, Zange S, Seilmaier M, et al. Clinical and virological features of first human monkeypox cases in Germany. Infection 2022; 51:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lucar J, Roberts A, Saardi KM, Yee R, Siegel MO, Palmore TN. Monkeypox virus-associated severe proctitis treated with oral tecovirimat: a report of two cases. Ann Intern Med 2022; 175:1626–7. [DOI] [PubMed] [Google Scholar]
- 25. Desai AN, Thompson GR 3rd, Neumeister SM, Arutyunova AM, Trigg K, Cohen SH. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA 2022; 328:1348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matias WR, Koshy JM, Nagami EH, et al. Tecovirimat for the treatment of human monkeypox: an initial series from Massachusetts, United States. Open Forum Infect Dis 2022; 9:ofac377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med 2022; 387:679–91. [DOI] [PubMed] [Google Scholar]
- 28. Rai RS, Kahan E, Hirsch B, Udell I, Hymowitz M. Ocular pox lesions in a male patient with monkeypox treated with tecovirimat. JAMA Ophthalmol 2022; 140:1244–6. [DOI] [PubMed] [Google Scholar]
- 29. Thet AK, Kelly PJ, Kasule SN, et al. The use of vaccinia immune globulin in the treatment of severe mpox virus infection in HIV/AIDS. Clin Infect Dis 2023; 76:1671–3. [DOI] [PubMed] [Google Scholar]
- 30. Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis 2022; 22:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sklenovska N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health 2018; 6:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Velavan TP, Meyer CG. Monkeypox 2022 outbreak: an update. Trop Med Int Health 2022; 27:604–5. [DOI] [PubMed] [Google Scholar]
- 33. Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med 2022; 28:1569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aguilera-Alonso D, Alonso-Cadenas JA, Roguera-Sopena M, Lorusso N, Miguel LGS, Calvo C. Monkeypox virus infections in children in Spain during the first months of the 2022 outbreak. Lancet Child Adolesc Health 2022; 6:e22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Titanji B. Protect the vulnerable from monkeypox. Science 2022; 377:1129. [DOI] [PubMed] [Google Scholar]
- 36. Dashraath P, Nielsen-Saines K, Mattar C, Musso D, Tambyah P, Baud D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet 2022; 400:21–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishiura H. Smallpox during pregnancy and maternal outcomes. Emerg Infect Dis 2006; 12:1119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention . 2022 Mpox outbreak global map. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html. Accessed 22 January 2023.
- 39. Miller MJ, Cash-Goldwasser S, Marx GE, et al. Severe monkeypox in hospitalized patients—United States, August 10-October 10, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pastula DM, Copeland MJ, Hannan MC, et al. Two cases of monkeypox-associated encephalomyelitis—Colorado and the District of Columbia, July-August 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spicknall IH, Pollock ED, Clay PA, et al. Modeling the impact of sexual networks in the transmission of monkeypox virus among gay, bisexual, and other men who have sex with men—United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis 1987; 156:293–8. [DOI] [PubMed] [Google Scholar]
- 43. Xiao SY, Sbrana E, Watts DM, Siirin M, da Rosa AP, Tesh RB. Experimental infection of prairie dogs with monkeypox virus. Emerg Infect Dis 2005; 11:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021; 385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Payne AB, Ray LC, Cole MM, et al. Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons—43 U.S. jurisdictions, July 31-October 1, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan-Tack K, Harrington P, Bensman T, et al. Benefit-risk assessment for brincidofovir for the treatment of smallpox: U.S. Food and Drug Administration's evaluation. Antiviral Res 2021; 195:105182. [DOI] [PubMed] [Google Scholar]
- 47. Yang G, Pevear DC, Davies MH, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol 2005; 79:13139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siegrist EA, Sassine J. Antivirals with activity against mpox: a clinically oriented review. Clin Infect Dis 2023; 76:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bojkova D, Bechtel M, Rothenburger T, et al. Drug sensitivity of currently circulating mpox viruses. N Engl J Med 2023; 388:279–81. [DOI] [PubMed] [Google Scholar]
- 50. Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open 2020; 3:e1919325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.