Abstract
Background
Donor site wounds of split‐thickness skin grafts can be a major cause of morbidity. Choosing the appropriate dressing for these wounds is crucial to successful healing. Various types of dressing are available, including hydrogel dressings. A review of current evidence is required to guide clinical decision‐making on the choice of dressing for the treatment of donor sites of split‐thickness skin grafts.
Objectives
To assess the effects of hydrogel dressings on donor site wounds following split‐thickness skin grafts for wound healing.
Search methods
In July 2022 we searched the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL EBSCO Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses, and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication, or study setting.
Selection criteria
Randomised controlled trials (RCTs) comparing hydrogel dressings with other types of dressing, topical treatments or no dressing, or with different types of hydrogel dressings in managing donor site wounds irrespective of language and publication status.
Data collection and analysis
Two review authors independently carried out data extraction, risk of bias assessment using the Cochrane risk of bias tool, RoB 1, and quality assessment according to GRADE methodology.
Main results
We included two studies (162 participants) in this review. One study with three arms and 101 participants (15 months' duration) was conducted in a children's hospital, and compared hydrogel dressings in the form of Sorbact with Algisite, an alginate dressing and Cuticerin, a smooth acetate gauze impregnated with water‐repellent ointment. Another study with two arms and 61 participants (19 months' duration) was conducted in three surgery departments and compared an octenidine‐containing hydrogel dressing with an identical non‐antimicrobial hydrogel dressing. We identified no studies that compared hydrogel dressings with another therapy such as a topical agent (a topical agent is a cream, an ointment or a solution that is applied directly to the wound), or no dressing, or a combination of hydrogel dressings and another therapy versus another therapy alone. Both studies were at high risk of attrition bias and the second study was also at unclear risk of selection bias.
Amorphous hydrogel dressings versus other types of dressings
Amorphous hydrogel dressings may increase time to wound healing when compared with alginate (mean difference (MD) 1.67 days, 95% confidence interval (CI) 0.56 to 2.78; 1 study, 69 participants; low‐certainty evidence) or Cuticerin dressings (MD 1.67 days, 95% CI 0.55 to 2.79; 1 study, 68 participants; low‐certainty evidence). The effect of amorphous hydrogel dressings compared with other types of dressings is uncertain for pain at the donor site and wound complications, including scarring and itching (very low‐certainty evidence). No adverse events were reported in any of the groups. The study did not report health‐related quality of life or wound infection.
Octenidine‐based hydrogel dressing versus octenidine‐free hydrogel dressing
The effect of octenidine‐based hydrogel dressings versus octenidine‐free hydrogel dressings is uncertain for time to wound healing (MD 0.40, 95% CI 0.28 to 0.52; 1 study, 41 participants) and wound infection, as the certainty of the evidence is very low. The certainty of the evidence is also very low for adverse events, with two participants in the intervention group and one participant in the comparison group reporting adverse events (risk ratio (RR) 0.58, 95% CI 0.06 to 5.89; 1 study, 41 participants). The study did not report donor site pain, health‐related quality of life, or wound complications.
Authors' conclusions
There is insufficient evidence to determine the effect of hydrogel dressings on donor site wounds of split thickness skin grafts compared with other types of dressings. There is a need for adequately powered and well‐designed RCTs, with adequate sample sizes, types of populations and subgroups, types of interventions, and outcomes, that compare hydrogel dressings with other treatment options in the treatment of donor site wounds of split‐thickness skin grafts.
Keywords: Child; Humans; Alginates; Alginates/therapeutic use; Bandages, Hydrocolloid; Hydrogels; Hydrogels/therapeutic use; Ointments; Skin Transplantation; Wound Infection
Plain language summary
How effective are hydrogel dressings for helping donor site wounds to heal after split‐thickness skin grafts?
Key messages
‐ We do not know if hydrogel dressings (designed to keep wounds moist) are better than other dressings for helping donor site wounds to heal after split‐thickness skin grafts (skin taken from another part of the body).
‐ We did not find any studies comparing hydrogel dressings with creams, ointments, or other solutions applied directly to the wound.
‐ More and better designed studies are needed to answer this question.
What are donor site wounds and split‐thickness skin grafts?
Donor site wounds result from removing part of the skin from a healthy, unaffected region and transferring it to help heal areas with damaged or lost skin. These wounds are a result of a standard surgical procedure called skin grafting. A partial or split‐thickness skin graft is a thin layer of skin shaved from an area such as the thigh and buttocks, that usually heals well in about two weeks.
Which dressings are used on donor site wounds?
Dressings to cover donor site wounds vary considerably regarding how they work, their cost, and ease of application. Hydrogel dressings are made of natural or synthetic materials that allow oxygen and nutrients to pass through them. They provide a moist environment for healing, which may prevent the dressing from sticking to the wound, causing further damage. They do not need to be changed as frequently as other dressings and are often used on wounds that are slow to heal or that require a lot of moisture to promote healing, such as burns. There are many other types of dressings including paraffin gauze, absorbent dressings, hydrocolloid, and antimicrobial impregnated dressings.
What did we want to find out?
We wanted to find out if hydrogel dressings effectively help heal donor site wounds after partial skin grafts. We were particularly interested in investigating the effects of different types of dressings on donor site wounds of split thickness skin grafts.
What did we do?
We searched for studies that compared hydrogel dressings with other treatments for healing donor site wounds after partial skin grafts. We analysed the results and rated our confidence in the combined evidence based on the size and quality of the included studies.
What did we find?
We found two studies with 162 participants.
‐ One study with 101 participants was conducted at a children’s burns unit and compared a hydrogel dressing (gauze mesh coated with hydrogel) with an alginate (algae‐ or seaweed‐based) dressing or Cuticerin (a smooth acetate gauze impregnated with water‐repellent ointment).
‐ The second study, with 61 participants, took place in three surgery departments and compared a hydrogel dressing that contained an antiseptic (octenidine ‐ to reduce the chance of infection) with a dressing with no octenidine.
We did not find any studies that compared hydrogel dressings with treatments directly applied to the wound (like creams or ointments).
People in the studies were chosen randomly to receive treatment with hydrogel dressings or another dressing.
Main results
Hydrogel dressings versus other types of dressings
Hydrogel dressings may increase time to wound healing by about 1.7 days compared with alginate (1 study, 69 participants) or Cuticerin dressings (1 study 68 participants). We are not sure about the results for hydrogel dressings compared with other dressings for pain at the donor site and wound complications, including scarring and itching. The study did not report health‐related quality of life or wound infection and did not report any unwanted effects of the dressings.
Octenidine‐based hydrogel dressing versus octenidine‐free hydrogel dressing
We are unsure of the effect of octenidine‐based hydrogel dressings versus octenidine‐free hydrogel dressings for time to wound healing, wound infection and unwanted effects. The study did not report donor site pain, health‐related quality of life, or wound complications.
Limitations of the evidence
Our confidence in the evidence is limited because there are not enough studies to be certain about the results of our outcomes. The studies were of poor quality and did not provide data about everything that we were interested in.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to 20 July 2022.
Summary of findings
Summary of findings 1. Summary of findings 1. Amorphous hydrogel dressings versus other types of dressings.
| Amorphous hydrogel dressings versus other types of dressings | ||||||
|
Patient or population: children with skin graft donor sites Settings: outpatient Intervention: amorphous hydrogel dressing (a gauze mesh coated with a dialkyl carbamoyl chloride and amorphous hydrogel (Sorbact)) Comparison: other dressings (Algisite and Cuticerin) |
||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other dressings | Amorphous hydrogel | |||||
|
Time to complete wound healing (re‐epithelialisation) (days) Measured using photographs |
Alginate dressing (Algisite) | Amorphous hydrogel (Sorbact) | ||||
| Mean days to re‐epithelialisation was 7.66 days | Mean days to re‐epithelialisation in the intervention groups was 1.67 days higher (0.56 higher to 2.78 higher) | MD 1.67 (0.56, 2.78) | 69 (1 RCT) |
⊕⊕⊝⊝ Lowa |
Amorphous hydrogel may result in increased time to complete re‐epithelialisation compared with alginate dressing | |
| Cuticerin dressing | Amorphous hydrogel (Sorbact) | |||||
| Mean days to re‐epithelialisation was 7.66 days | Mean days to re‐epithelialisation in the intervention groups was 1.67 days higher (0.55 higher to 2.79 higher) | MD 1.67 (0.55, 2.79) | 68 (1 RCT) |
⊕⊕⊝⊝ Lowa |
Amorphous hydrogel may result in increased time to complete re‐epithelialisation compared with Cuticerin dressing | |
|
Donor site pain Measured with NRS, FLACC; dichotomised as 'no pain' and 'some pain' Follow‐up: 4 and 24 hours |
NRS done at 4 hours: 61/90, 67.8% no pain; and 24 hours: 71/89, 78.9% no pain FLACC scores at 4 hours: 69/91, 85.2% no pain There was no difference in pain scores at 4 and 24 hours (data not shown) Children over 3 years of age (N = 24) were asked to score their pain directly using the FPS‐R, but study authors did not report results as the number of participants is too small |
‐ | 91 (1 RCT) |
⊕⊝⊝⊝ Very lowb |
The evidence is very uncertain about the effect of amorphous hydrogel on donor site pain | |
| Health‐related quality of life | Outcome not measured or reported | |||||
| Wound infection | Outcome not measured or reported | |||||
|
Wound complications: scarring Measured with POSAS, dichotomised to ‘less than 10’ and ‘10 or more’ Follow‐up: 3 months |
For 33/57 (38%) of participants score was less than 10. Overall participant scores on the POSAS scale (1 to 10) were not associated with any of the trial dressings (P = 0.3172; data not shown) | ‐ | 57 (1 RCT) |
⊕⊝⊝⊝ Very lowb |
The evidence is very uncertain about the effect of amorphous hydrogel on scarring at 3 months' follow‐up | |
|
Wound complications: scarring Measured with POSAS, dichotomised to ‘less than 10’ and ‘10 or more’ Follow‐up: 6 months |
For 51/66 (77.3%) of participants score was less than 10. There was no difference across dressing type when assessed by a trained clinical observer (P = 0.075) or by the participants or parents themselves (P = 0.355) (data not shown) | ‐ | 66 (1 RCT) |
⊕⊝⊝⊝ Very lowb |
The evidence is very uncertain about the effect of amorphous hydrogel on scarring at 6 months' follow‐up | |
|
Wound complications: itching Measured with Itch Man Scale; dichotomised as no itch (score = 0) or some itch (score = 1 to 4) Follow‐up: 4 and 24 hours |
Only 7/91 (7.7%) participants or parents reported any itch at 4 hours, and only 10/91 (11%) reported any itch at 24 hours (results per group not reported). The remaining participants had scores of 0 for both time points There were no differences in itch across the 3 dressings at each time point (P = 0.835 at 4 hours, P = 0.311 at 24 hours) |
‐ | 91 (1 RCT) |
⊕⊝⊝⊝ Very lowb |
The evidence is very uncertain about the effect of amorphous hydrogel on itching at up to 24 hours' follow‐up | |
|
Wound complications: itching Measured with itching component of POSAS scale, NRS 1 to 10; dichotomised scores (details not reported) Follow‐up: 3 and 6 months |
There was no difference in dichotomised itch scores using this component of the POSAS at the 3‐ and 6‐month reviews of participants (P = 0.125 at 3 months, P = 0.845 at 6 months) | ‐ | Not reported (1 RCT) |
⊕⊝⊝⊝ Very lowb |
The evidence is very uncertain about the effect of amorphous hydrogel on itching and 3‐ and 6‐month follow‐up | |
| Adverse events | There were no adverse effects related to the donor site wounds dressing with any participant | 101 (1 RCT) |
⊕⊝⊝⊝ Very lowb |
The evidence is very uncertain about the effect of amorphous hydrogel on adverse events | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FLACC: face, legs, activity, cry, consolability scale; FPS‐R: revised faces pain scale; MD: mean difference; NRS: numeric rating scale; POSAS: Patient and Observer Scar Assessment Scale; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 1 level for risk of bias (incomplete outcome data) and 1 level for imprecision (small sample size). bDowngraded 2 levels for risk of bias (incomplete outcome data, poor reporting of results (data per group not shown), and performance and attrition bias due to lack of blinding) and 1 level for imprecision (small sample size).
Summary of findings 2. Summary of findings 2. Octenidine‐dihydrochloride (OCT) hydrogel dressings versus OCT‐free placebo hydrogel dressings.
| Octenidine‐dihydrochloride (OCT) hydrogel dressings versus OCT‐free hydrogel dressings | ||||||
|
Patient or population: adults with skin graft donor sites Settings: outpatient Intervention: octenidine‐dihydrochloride (OCT) hydrogel dressing Comparison: OCT‐free hydrogel dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| OCT‐free hydrogel dressing | OCT‐based hydrogel dressing | |||||
|
Time to complete wound healing of skin graft donor sites (days) Documented using a digital camera + standardised measuring scale and assessed by 2 independent observers Follow‐up: up to 14 days |
The mean time to complete healing across control groups was 6.9 days | The mean time to complete healing was 7.3 days (0.4 days higher, 0.28 higher to 0.52 higher) | MD 0.40 (95% CI 0.28 to 0.52) | 41 (1 RCT) | ⊕⊝⊝⊝ Very lowa | The evidence is very uncertain about the effect of OCT‐based hydrogel dressings compared with placebo on time to wound healing |
| Donor site pain | Outcome not measured or reported | |||||
| Health‐related quality of life | Outcome not measured or reported | |||||
|
Wound infection (bacterial colonisation) Measured using: contact cultures Follow‐up: days 4 and 6 |
At day 4 significantly more skin graft donor site wounds in the treatment group showed bacterial colonisation (P = 0.014) At day 6 (3 days after using non‐antimicrobial wound dressings), bacterial colonies in both groups were comparable (P = 0.363) |
41 (1 RCT) |
⊕⊝⊝⊝ Very lowb | The evidence is very uncertain about the effect of OCT‐based hydrogel compared with placebo on wound infection | ||
| Wound complications | Outcome not measured or reported | |||||
|
Adverse events Follow‐up: up to 14 days |
91 per 1000 | 53 per 1000 (5 to 535) |
RR 0.58 (0.06 to 5.89) | 41 (1 RCT) | ⊕⊝⊝⊝ Very lowc | The evidence is very uncertain about the effect of OCT‐based hydrogel compared with placebo on adverse events (AE). In the ITT population, 8 participants presented with 11 AEs (data per group not reported). In the PP population, 4 AEs were reported in 3 participants (2 in the intervention group and 1 in the placebo group) |
| *The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; ITT: intention to treat; MD: mean difference; OCT: octenidine; PP: per protocol; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 1 level due to risk of bias (unclear risk of bias due to sequence generation and allocation concealment and high risk of bias due to incomplete outcome data (reasons for missing data were not reported)) and 2 levels due to imprecision (very small sample size). bDowngraded 1 level due to risk of bias (unclear risk of bias due to sequence generation and allocation concealment and high risk of bias due to incomplete outcome data (reasons for missing data were not reported)), 1 level due to indirectness (colonisation is only indirectly relevant to infection), and 2 levels due to imprecision (very small sample size). cDowngraded 1 level due to risk of bias (unclear risk of bias due to sequence generation and allocation concealment and high risk of bias due to incomplete outcome data (reasons for missing data were not reported)) and 2 levels due to imprecision (few events and small sample size, and a large confidence interval that incorporates the possibility of benefit and harm).
Background
Description of the condition
Skin grafting is a common surgical procedure for managing wounds to facilitate healing (Humrich 2018; Nanchahal 1992). The process involves removing a tissue section from one part of the body, called the donor site, which is then used to cover healthy granulating surfaces of skin defects, such as ulcers or burns, that cannot be apposed by bringing the two edges together. A skin graft comprises two layers of the skin, the epidermis (the outermost layer of skin) and the dermis (the layer beneath the epidermis containing connective tissue, hair follicles and sweat glands). Split‐thickness skin grafts are obtained by harvesting a piece of the epidermis and part of the dermis and transplanting it to the area of skin defect after the removal of tissue debris. There are many donor sites for split‐thickness skin grafts, such as the upper and anterior thigh, buttocks, upper medial arm, back and scalp, depending on the recipient site (the area where the split‐thickness skin grafts will be transplanted to) and the thickness and texture of potential donor site skin (Francis 1998; Ratner 2003). Donor site wounds regenerate by re‐epithelialisation (restoration of the thin tissue forming the outer layer of the body’s surface) from the periphery of the wound to its centre and the epithelium of the underlying sweat glands and hair follicles in the remaining dermis (Francis 1998; Ratner 2003). Re‐epithelialisation usually takes one to two weeks depending on factors such as age and general health (e.g. smoking, diabetes and autoimmune diseases can slow the process; Francis 1998; Rakel 1998; Ratner 2003). People with donor site wounds have a significant risk of morbidity due to complications from their wounds. These complications can significantly impact their quality of life and increase treatment costs (Humrich 2018).
Description of the intervention
Different types of dressings are available for managing donor site wounds. These dressings also come in varying forms (Voineskos 2009; Wiechula 2003). The British National Formulary for wound management products classifies dressings into basic, advanced, antimicrobial and specialised dressings (BNF 2017; Appendix 1). The concept of optimal wound healing conditions was first introduced by Winter, who found faster re‐epithelialisation rates of experimental animal wounds covered with occlusive (air and water‐tight) dressings than wounds exposed to air (Winter 1962). The optimal healing environment for donor site wounds is achieved by keeping the wound covered and moist until complete healing occurs. Hydrogel dressings are classed as advanced wound dressings. They are thought to provide a moist environment for wound healing as they contain hydrophilic polymers (large, chain‐like molecules that contain polar or charged groups, rendering them soluble in water) with the ability to retain water in up to 90% of their content (Caló 2015; Jones 2005; Peppas 1993; Wichterle 1960). Hydrogels are available in two forms: flat sheets (e.g. ActiFormCool (Activa)) or amorphous hydrogel (e.g. Aquaflo (Covidien)). These dressings are usually applied by healthcare professionals (BNF 2017).
How the intervention might work
The ideal dressing should provide a moist environment for healing, protect against bacterial invasion, absorb exudate, and provide permeability to gases and oxygen, be comfortable for patients, elicit no (or little) pain, be readily available, easy to handle, and cheap (Gupta 2010; Lars 2013). As the donor site wound of split‐thickness skin grafts is at risk of infection, fluid exudate and scarring, its management requires special care (Wiechula 2003). Hydrogels absorb wound exudate and promote moisture and oxygenation. Hydrogel dressings may also reduce pain, either by the process of cooling or providing analgesia to the wound (Trudgian 2000). Hydrogel dressings may be more effective than basic wound dressings in treating foot ulcers in people with diabetes (Dumville 2013).
Why it is important to do this review
The World Health Organization (WHO) recognises wounds as a source of significant global morbidity; 5 to 7 million chronic or complex wounds occur annually in North America (Macdonald 2010). Split‐thickness skin grafts are the most common surgical procedures used to cover skin defects (Kanapathy 2017). Dressing choice in the management of donor site wounds is crucial. Many dressings are used in wound management, including dry dressings, alginates, hydrocolloids and hydrogels (Voineskos 2009). However, there is a lack of evidence to support the efficacy and advantages of one dressing type over another in managing donor site wounds (Uraloğlu 2012). This review aims to assess the benefits and harms of hydrogel dressings compared with other types of dressing in managing donor site wounds of split‐thickness skin grafts.
Objectives
To assess the effects of hydrogel dressings on donor site wounds following split‐thickness skin grafts for wound healing.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) irrespective of language and publication status. Cluster‐RCTs and split‐body designs, where treatment options are randomly assigned to different wound sites, were also eligible for inclusion. We included abstracts of eligible trial reports regardless of the type or availability of data. We excluded quasi‐randomised, cross‐over and split‐wound trials as we anticipated a high risk of carry‐over effect given the nature of the condition and interventions.
Types of participants
We included RCTs that included people of any age with one or more donor site wounds who had had split‐thickness skin grafts. This included donor site wounds created during an emergency or an elective surgical procedure. Trials involving other types of wounds were eligible only if the results for people with donor site wounds were presented separately, randomisation was stratified by wound type, or if the majority of wounds in the trial (75% or more) were donor site wounds.
Types of interventions
The intervention of interest was any hydrogel dressing.
We anticipated comparisons for this review to be:
hydrogel dressings compared with no dressing;
hydrogel dressings compared with other types of dressings;
different types of hydrogel dressings compared with each other;
hydrogel dressings compared with another therapy such as a topical agent (a topical agent is a cream, an ointment or a solution that is applied directly to the wound);
hydrogel and another therapy versus other therapy alone.
In cases where different trade names were used, we resorted to the generic and active ingredients and the type of dressing (flat sheets, amorphous hydrogel). We used the generic names of dressings to facilitate easy identification.
Types of outcome measures
If a trial was eligible for inclusion (i.e. it had the right design, population, and intervention(s)) but did not report one of the study outcomes, the review team contacted the study authors to request study protocols. For studies where authors responded, we assessed the report for eligibility, and the potential for ‘selective outcome reporting’ was evaluated and discussed with an arbitrator when reporting was unclear. However, such studies were included in the review regardless of the study authors' responses.
Some of the outcomes listed below may be recorded at multiple time points. We planned to group outcomes into specified intervals:
short‐term: from 0 to 30 days;
medium‐term: more than 30 days to 6 months;
long‐term: more than 6 months.
Primary outcomes
Complete wound healing measured using 'time to event' (wound healing) or the time (in days) from donor site wound creation until re‐epithelialisation, as defined by study authors
Donor site pain (measured using any validated instrument, e.g. visual analogue scale (Hawker 2011))
Secondary outcomes
Health‐related quality of life (measured using any validated outcome measure such as the World Health Organization Quality of Life (WHOQOL‐BREF; Kim 2014), 36‐Item Short‐Form Health Survey (SF‐36; Lins 2016), European Quality of Life 5 dimensions (EQ‐5D; Herdman 2011) or 12‐item Short‐Form Health Survey (SF‐12; Ware 1996), measured at the completion of the study
Number of people with wound infection (we accepted the study authors' definition of an infected wound)
Cost of treatment (measured at the completion of the study)
Number of people with the following wound complications: itching, over‐granulation, skin discolouration, and problematic scar formation. We accepted the study authors' definitions of these complications.
Number of people with adverse events (non‐serious and serious), where the study provided a clear methodology for collecting adverse event data. We documented whether events were reported at the participant level or if multiple events per person were reported, that an appropriate adjustment was made for data clustering. This outcome does not include individual types of adverse events, such as pain or infection, which require specific assessment. Rather, it covers the generic assessment of any event classed as adverse by the participant or health professional during the trial.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
Cochrane Wounds Specialised Register (searched 20 July 2022);
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 6) in the Cochrane Library (searched 20 July 2022);
MEDLINE Ovid including In‐Process & Other Non‐Indexed Citations (1946 to 20 July 2022);
Embase Ovid (1974 to 20 July 2022);
CINAHL Plus EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 20 July 2022).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid and CINAHL Plus EBSCO can be found in Appendix 2. In MEDLINE Ovid, we combined the subject‐specific strategy with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (2008 revision; Lefebvre 2022). We combined the Embase Ovid search with the Ovid Embase filter developed by Cochrane UK (Lefebvre 2022). We combined the CINAHL Plus EBSCO search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication, or study setting.
We also searched the following clinical trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 20 July 2022);
World Health Organization International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform; searched 20 July 2022).
The search strategies for clinical trial registries are in Appendix 2.
Searching other resources
Searching reference lists of included trials and relevant reviews
We searched the reference lists of included trials, relevant systematic reviews, meta‐analyses and health technology assessment reports to identify additional studies.
Searching by contacting individuals or organisations
We contacted authors of key papers and abstracts to request further information about their trials when necessary. We contacted experts in the field and manufacturers of dressings used to treat donor site wounds to ask for information relevant to this review.
Adverse effects
We did not perform a separate search for adverse effects of interventions; we only considered adverse effects described in included studies.
Data collection and analysis
We collected and analysed data according to the methods stated in the published protocol (Younis 2020), which was based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Selection of studies
Two review authors worked independently to screen the titles and abstracts of the studies identified from the search strategy against the inclusion criteria and exclude irrelevant reports. We retrieved the full texts of potentially eligible studies that appeared to fulfil the inclusion criteria and assessed them independently for inclusion. We resolved disagreements by discussion with a third review author. We contacted study authors to request missing information when the eligibility of the study was unclear. If we found more than one publication linked to the same study, we included all the papers, and marked one as the primary source of information.
We completed a PRISMA flowchart to summarise this process (Liberati 2009). We recorded all reasons for excluding studies for which we obtained full‐text copies in the Characteristics of excluded studies table.
Data extraction and management
Two review authors independently extracted data from eligible studies using an offline electronic form that we piloted. We resolved discrepancies through discussion and consensus, and when there was disagreement, we consulted a third review author. We tried to obtain missing or unclear data by contacting the study authors. Where a study with more than two intervention arms was included, we only extracted data from relevant intervention and control groups that met the eligibility criteria. We entered data into Review Manager 5 (RevMan 5) software (Review Manager 2020) and checked them for accuracy. The data in RevMan 5 were later transferred to Review Manager Web (RevMan Web; RevMan Web 2023).
In accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Li 2022), we extracted the following information.
Study ID and year of publication
-
Methods
Study design
Total study duration
Country of origin
Study setting
Unit of investigation (per person): single donor site wounds or multiple donor site wounds on the same person
Duration of follow‐up
-
Participants
Inclusion and exclusion criteria
Total number
Participant demographic data (gender, age, ethnicity); relevant history (such as diabetes, vascular disease, etc.)
Donor site wound size and site
Recipient site
-
Intervention
Number of participants randomised to each treatment group
Details of the dressing/treatment regimen received by each group
Details of any co‐interventions
-
Outcomes
Outcomes and time points (i) collected; (ii) reported
Primary and secondary outcome(s) (with definitions)
Unit of measurement (if relevant)
Unit of analysis (participant or wound)
For scales: upper and lower limits, and whether a high or low score is favourable
-
Results
Number of participants allocated to each intervention group
For each outcome: sample size; missing participants; summary data for each intervention group (e.g. 2 × 2 tables with proportions for dichotomous data; means and standard deviations (SDs) for continuous data); the number of withdrawals (by group, with reasons)
-
Notes
Source of funding
Key conclusions of the study authors
Citation and contact details
Assessment of risk of bias in included studies
Two review authors independently assessed the methodological quality of included studies using the Cochrane risk of bias tool, RoB 1, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any discrepancies by discussion; if consensus was not achieved, we resolved disagreements by consulting a third review author. RoB 1 includes the following domains:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessors;
incomplete outcome data;
selective outcome reporting;
other bias, e.g. incorrect study analysis for the unit of analysis issues.
See Appendix 3 for a detailed description of the criteria for a judgement of ‘low risk’, ‘high risk’ or ‘unclear risk’ of bias. We would have evaluated additional risk of bias domains: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability for cluster trials, but none were identified (Higgins 2022; see Appendix 4).
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data.
Continuous data
We calculated the mean difference (MD) with 95% confidence intervals (CIs) for continuous data if outcomes were measured using similar outcome measures between studies. We used the standardised mean difference (SMD) to combine studies that measured the same outcome but used different scales.
For data on wound healing, we recorded 'time to event (wound healing)' as the time (in days) from donor site wound creation until re‐epithelialisation, as defined by each study. We planned to express time‐to‐event data (e.g. time to complete wound healing) as hazard ratios (HRs) where possible, following the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022).
Unit of analysis issues
We would have considered the participant as the unit of analysis for instances of clustered data where a proportion of trial participants had outcome data collected and reported on multiple wounds. In such situations, not all participants will have multiple wounds, and it would not be a cluster‐randomised trial per se but rather a trial that incorrectly includes a mixture of individual and clustered data. No such trials were identified; hence, data extraction, presentation and issues related to the risk of bias assessment did not apply.
We planned to incorporate well‐conducted cluster‐randomised trials that provided complete reports of the randomisation process and performed the meta‐analyses adequately. If a cluster‐randomised trial had been conducted but incorrectly analysed, we would have recorded this in the risk of bias assessment. If possible, by following the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), and approximate estimates of the correct analyses, we assessed risk of bias using the information on:
the number of clusters randomised to each intervention or the mean size of each cluster;
outcome data ignoring cluster design for the total number of individuals; and
an estimate of the intra‐cluster correlation coefficient (ICC).
In studies with multiple intervention arms, we analysed only the arms related to our review topic. In the presence of multiple related arms, we created two or more equal‐sized groups from the shared group and compared them with similar groups as a control. However, we did not have to combine arms between studies to perform meta‐analyses of results.
We planned to include studies with a split‐body design where people with two similar donor site wounds were enrolled, and each wound was randomised to one of the interventions. We would have analysed these studies using paired data to reflect reduced variation in evaluating different treatments on the same person. If we had found these types of studies, and it was unclear whether such analysis had been undertaken, we planned to present this information on the lack of clarity in the risk of bias assessment and the notes in the 'Characteristics of included studies' table. However, no such trials were identified; hence, differences in the procedure for data extraction, presentation of findings and issues related to the risk of bias assessment did not apply. Studies where one half of a wound was randomised to one treatment and the other half to a different treatment (split‐wound), were excluded as there would have been a high carry‐across effect and diffusion of treatment effects.
Dealing with missing data
For included studies, we noted levels of attrition. Excluding participants, postrandomisation from the analysis, or ignoring those lost to follow‐up compromises the randomisation and potentially introduces bias into the trial. Where possible, we contacted named corresponding study authors to request these data if details were not provided.
Where data remained missing for the 'complete wound healing' outcome, we assumed that if randomised participants were not included in an analysis, their wounds did not heal (i.e. they were considered in the denominator but not the numerator). We tested the impact of this assumption by performing sensitivity analysis and assumed that those with missing outcome data had the outcome of interest; that is, they were included in both the numerator and the denominator (see Sensitivity analysis).
For continuous variables (e.g. change in wound area or length of hospital stay), we have presented available data from the study reports/authors and did not plan to impute missing data. Where measures of variance were missing, we planned to calculate these wherever possible. If the calculation was not possible, we planned to document this but exclude the study from any relevant meta‐analyses conducted.
Assessment of heterogeneity
We planned to explore clinical or methodological heterogeneity by examining the following factors: care setting, participant characteristics, methods, interventions and outcomes of studies. We planned to supplement this assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity. We planned to inspect forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We planned to assess statistical heterogeneity in each meta‐analysis using Tau², the I² statistic (Higgins 2003), and the Chi² statistic. For the Chi² test, we planned to consider a significance level of P < 0.10. For the I² test, we used the following thresholds: 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity (Deeks 2022). Collectively, we planned to regard heterogeneity as substantial if Tau² was greater than zero and either I² statistic was 50% or greater or the P value in the Chi² test for heterogeneity is less than 0.1.
Where there was evidence of considerable heterogeneity, we planned to explore this further (see Data synthesis and Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the nature and direction of results influence the dissemination of research findings. Publication bias is a possible cause of ‘small‐study effects’, that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small‐study effects may be present in a meta‐analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each trial’s size or precision (Page 2022). We planned to present funnel plots for meta‐analyses using RevMan 5 (Review Manager 2020) if 10 or more RCTs were available for inclusion in any single meta‐analysis.
Data synthesis
Data synthesis would have been conducted using RevMan 5 (Review Manager 2020). We planned to use a fixed‐effect meta‐analysis for combining data where the included studies had low or moderate heterogeneity. In case of substantial heterogeneity that could not be explained clinically or methodologically, we planned to use a random‐effects meta‐analysis to produce an overall summary where an average treatment effect across trials was considered clinically meaningful. We planned to treat the random‐effects summary as the average range of possible treatment effects and discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not have combined trials. If we had used random‐effects analyses, we would have presented the results as the average treatment effect with its 95% CI and the estimates of Tau2 and the I2 statistic (Deeks 2022). We planned to pool dichotomous outcomes using Mantel‐Haenszel analysis for the fixed‐effect model or DerSimonian and Laird for a random‐effects model.
We calculated the MD for continuous outcomes with corresponding 95% CIs. We planned to present a pooled MD with corresponding 95% CIs for continuous outcomes. If studies had measured the same outcome using different instruments, we planned to combine the data using an SMD. For time to healing, we planned to plot estimates of HRs with 95% CIs from study reports using the generic inverse method in RevMan 5 (Review Manager 2020) and later in RevMan Web (RevMan Web 2023).
We presented the results of the individual trials in the form of forest plots. As data were insufficient or unsuitable for meta‐analysis, we collated a summary of the results to summarise the findings narratively.
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to check the data for accuracy and assess methodological or clinical explanations of heterogeneity.
We planned to perform the following subgroup analysis for the primary outcomes if there were a minimum of 10 studies in the meta‐analysis:
• people with diabetes, both type 1 and type 2 (Dumville 2013).
We did not carry out this subgroup analysis as there were insufficient studies to include in the review.
Sensitivity analysis
We planned to perform sensitivity analysis for all outcomes by excluding studies at high risk of bias; that is, any study that we assessed as being at high risk of bias in any of the following domains:
generation of the randomisation sequence;
allocation concealment;
blinding of outcome assessor;
incomplete outcome data.
We also planned to perform sensitivity analysis if split‐body studies were included in the analysis and to remove these studies if there was evidence that the results differed substantially from those of parallel‐group studies.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Schünemann 2013), related to the following main outcomes, which are important for decision‐making:
complete wound healing;
donor site pain;
health‐related quality of life;
number of people with wound infections;
number of people with adverse events;
number of people with the following wound complications: over‐granulation, skin discolouration, problematic scar formation, and itching.
We used GRADEpro GDT to import data from RevMan 5 (Review Manager 2020), which was migrated to RevMan Web (RevMan Web 2023), to create summary of findings tables. Using the GRADE approach, we produced a summary of the intervention effect and a measure of certainty for each of the above outcomes (Schünemann 2022). This uses the following five considerations to assess the certainty of the body of evidence for each outcome: study limitations, inconsistency of effect, imprecision, indirectness, and publication bias. We downgraded the certainty of the evidence if we had concerns about each factor (from high certainty to moderate, low, or very low certainty) using the guidelines developed by Rubinstein and colleagues (Rubinstein 2013). We downgraded the certainty of the evidence if more than 25% of the participants providing data for an outcome were from studies with a high risk of bias (see Sensitivity analysis). If we identified significant heterogeneity or there were large differences between studies in magnitude or direction of effects (or both), we downgraded the level of evidence further.
Lastly, we downgraded the evidence if more than 50% of the participants were unrepresentative of the target group and if single studies included fewer than 400 participants for continuous outcomes or 300 participants for dichotomous outcomes. We downgraded the level of evidence from 'high certainty' by one level for serious limitations or by two or more levels for very serious limitations, depending on assessments for risk of bias, indirectness of evidence, inconsistency, and imprecision of effect estimates or potential publication bias (Rubinstein 2013; Schünemann 2022).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search generated 526 records and 266 trial registry records. After we removed duplicates there were 454 database records and 185 trial registry records. We retrieved 14 of these records for consideration for inclusion (Figure 1).
1.
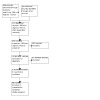
Study flow diagram.
Ongoing studies and studies awaiting classification
We did not find any ongoing studies and there are no studies awaiting classification.
Included studies
This review included two studies (Eisenbeiß 2012; McBride 2018): see Characteristics of included studies.
Eisenbeiß 2012 was a randomised, double‐blinded, controlled trial that evaluated the effect of octenidine‐dihydrochloride hydrogel on donor site wounds of split‐thickness skin grafts. The study was conducted in three centres in Germany. It randomised 61 participants who presented with burns or chronic wounds that required skin grafts. The average area that required skin graft was greater than 25 cm2, with the possibility of harvesting skin grafts from the thighs. The intervention group received octenidine hydrogel, and the usual care group received octenidine‐free hydrogel on donor site wounds. The primary outcome of the study was time to complete (100%) re‐epithelialisation of the treated skin graft donor site. The secondary outcomes of the study were the colonisation of bacteria on wounds and adverse events.
McBride 2018 was a single‐centre, three‐armed, RCT comparing Sorbact, a gauze mesh coated with a dialkyl carbamoyl chloride and amorphous hydrogel, Algisite M, and Cuticerin. The study was conducted in Australia and included 101 participants under 16 years with thigh donor site wounds. Primary outcomes were reported pain and time to complete re‐epithelialisation. Itching, donor site wound scarring, ease of dressing change, and cost were secondary outcomes.
Excluded studies
In total, we excluded 12 studies from the review. We have listed reasons for exclusion below.
Seven studies did not evaluate a hydrogel dressing (Blome‐Eberwein 2010; Dilokhuttakarn 2016; Gemberling 1976; Hickerson 1994; Johansen 1972; Poinas 2019; Solanki 2012).
Akita 2006 applied polyurethane and hydrogel dressings side‐by‐side on the same wound carrying the risk of cross‐over effect.
Li 2015 used a split‐wound design in some donor site wounds.
Vogt 2006 did not evaluate the effect of the intervention on donor site wounds.
Koivuniemi 2020 and Weingart 1993 are non‐randomised clinical trials.
Risk of bias in included studies
We considered studies as having an overall high risk of bias if we deemed one of the following domains to be at a high risk of bias: generation of randomisation sequence, allocation concealment, blinded outcome assessment, or incomplete outcome data. Based on this approach, we deemed both studies to have an overall high risk of bias due to incomplete outcome data (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated Eisenbeiß 2012 as having an unclear risk of bias for this domain, as no information regarding the generation of the randomisation sequence or allocation concealment was available for assessment.
We rated McBride 2018 as being at a low risk of bias for this domain.
Blinding
We rated both studies as being at low risk of performance bias and detection bias as the outcome assessors, personnel and participants were blinded to the dressing type until first dressing, and blinded photographs were used to assess re‐epithelialisation (Eisenbeiß 2012; McBride 2018). Although McBride 2018 had low risk of bias for time to re‐epithelialisation and adverse events, there was a high‐risk of bias for pain, itching and scarring.
Incomplete outcome data
We rated both studies as having a high risk of attrition bias due to the high percentage of loss to follow‐up.
Selective reporting
We rated both included studies as having a low risk of reporting bias because outcomes prespecified in the methods section of the protocol were reported in results. We contacted the authors and they sent us the protocols.
Other potential sources of bias
There was no evidence of other potential sources of bias. The study authors reported sources of funding and indicated conflicts of interest.
Effects of interventions
See Summary of main results; Table 1; Table 2.
Hydrogel dressings compared with no dressing
No trials compared hydrogel dressings with no dressings.
Hydrogel dressings compared with other types of dressings
Comparison 1. Amorphous hydrogel dressing versus other types of dressings
We identified one trial, McBride 2018, that compared three types of dressings in 101 children less than 16 years old. Interventions were Algisite M, an alginate dressing (Smith & Nephew, Hull, UK), Cuticerin, a smooth acetate gauze impregnated with water‐repellent ointment (petrolatum, paraffin, and Eucerite; Smith & Nephew, Hull, UK), and Sorbact, a gauze mesh coated with a dialkyl carbamoyl chloride (DACC; a synthetic fatty acid derivative) and amorphous hydrogel (Abigo Medical AB, Gothenburg, Sweden).
McBride 2018 reported differences between the intervention arms but did not report the individual statistical measures of each outcome. Therefore, we contacted the study authors to obtain the statistical data and tables.
See Table 1.
Primary outcome: time to wound healing
McBride 2018 reported on time to healing measured as the time to complete re‐epithelialisation (95% or greater). The study authors reported no difference in time to healing between interventions, with the median time for re‐epithelialisation of seven days for Algisite, Cuticerin and 10 days for Sorbact. The study authors reported that all wounds were healed.
We analysed these data by converting the median to the mean using the Wan method (Wan 2014). Amorphous hydrogel dressings may result in an increase in time to healing when compared to alginate (MD 1.67 days; 95% CI 0.56 to 2.78; 69 participants; low‐certainty evidence) or Cuticerin dressings (MD 1.67 days; 95% CI 0.55 to 2.79; 68 participants; low‐certainty evidence). We downgraded the certainty of the evidence by one level for risk of bias (due to incomplete outcome data) and one level for imprecision (small sample size).
Primary outcome: donor site pain
McBride 2018 measured pain at 4 hours and 24 hours with nursing staff assessing pain and distress using the Face, Legs, Activity, Cry, Consolability (FLACC) scale. Parents used an 11‐point (0 to 10) numeric rating scale (NRS). The study authors noted that there were no differences in pain scores between groups (data not shown). The certainty of the evidence is very low. We downgraded two levels for risk of bias (due to incomplete outcome data, poor reporting of results (data per group not shown), and performance and attrition bias due to lack of blinding) and one level for imprecision (small sample size).
The study used the Revised Faces Pain Scale (FPS‐R) for 24 children aged over three years, but the study authors noted that they did not report results as the sample size was too small to draw any conclusions.
Secondary outcome: health‐related quality of life
Outcome not measured or reported
Secondary outcome: wound infection
Outcome not measured or reported
Secondary outcome: cost of treatment
McBride 2018 reported no difference in cost between the intervention arms. Approximate prices per cm2 were USD 0.038 to USD 0.083 for Algisite M, USD 0.002 to USD 0.007 for Cuticerin, and USD 0.053 to USD 0.107 for Sorbact. There were no complications associated with any of the dressings, and therefore, no cost increases were incurred through treating complications.
Secondary outcome: wound complications
McBride 2018 reported on both itching and scarring.
McBride 2018 assessed scarring at three and six months using the Patient and Observer Scar Assessment Scale (POSAS), and dichotomised the results to 'less than 10' or 'more than 10'. At three months, 33/57 (38%) participants had a score that was less than 10. Overall participant scores on the POSAS scale (1 to 10) were not associated with any of the trial dressings (P = 0.3172; data not shown). Similarly, at six months 51/66 (77.3%) participants had a score that was less than 10. There was no difference across dressing types when assessed by a trained clinical observer (P = 0.075) or by the participants or parents themselves (P = 0.355; data not shown).
McBride 2018 assessed itching at 4 hours and 24 hours using the Itch Man scale, and dichotomised results as no itch (score = 0) and some itch (score 1 to 4). Data were available for 91 participants. Only 7/91 (7.7%) participants or parents reported any itch at 4 hours, and only 10/91 (11%) reported any itch at 24 hours (results per group not reported). The remaining participants had scores of zero for both time points. The study authors report that there were no differences in itch across the three dressings at each time point (P = 0.835 at 4 hours, P = 0.311 at 24 hours; data not shown).
McBride 2018 also assessed itching at three months and six months using the itching component of the POSAS scale, and dichotomised the results (details not reported). The study authors reported that there were no differences between the groups at three months (P = 0.125) and at six months (P = 0.845; number of participants not reported, data not shown).
The certainty of the evidence is very low. We downgraded two levels for risk of bias (due to incomplete outcome data, poor reporting of results (data per group not shown), and performance and attrition bias due to lack of blinding), and one level for imprecision (small sample size).
Secondary outcome: adverse events
The study authors report that there were no adverse events in any of the groups. The certainty of the evidence is very low. We downgraded two levels for risk of bias (due to incomplete outcome data, poor reporting of results (data per group not shown) and performance and attrition bias due to lack of blinding), and one level for imprecision (small sample size).
Different types of hydrogel dressings compared with each other
Comparison 2. Octenidine‐based hydrogel dressings versus octenidine‐free hydrogel dressings
We identified one trial (Eisenbeiß 2012), with 61 participants, that compared the effects of octenidine‐based hydrogel dressings with ordinary hydrogel dressings on donor sites of split‐thickness skin grafts. We obtained the protocol after contacting the study author.
See Table 2.
Primary outcome: time to wound healing
Eisenbeiß 2012 reported on the mean days to complete re‐epithelialisation. We are uncertain about the effects of octenidine‐based hydrogel dressings on time to wound healing because the certainty of the evidence is very low. The mean time to complete re‐epithelialisation was 7.3 days compared with 6.9 days in octenidine‐free hydrogel (MD 0.40, 95% CI 0.28 to 0.52; 41 participants; Analysis 2.1). We downgraded the certainty of the evidence one level due to risk of bias (unclear risk of bias due to sequence generation and allocation concealment and high risk of bias due to incomplete outcome data; reasons for missing data were not reported), and two levels due to imprecision (very small sample size). The study authors reported that all wounds were healed.
2.1. Analysis.

Comparison 2: Comparison 2. Octenidine (OCT)‐based hydrogel vs OCT‐free placebo hydrogel dressings, Outcome 1: Time to complete healing
Primary outcome: donor site pain
Outcome not measured or reported
Secondary outcome: health‐related quality of life
Outcome not measured or reported
Secondary outcome: wound infection
Eisenbeiß 2012 did not report on wound infection. However, the study authors noted that on day 4, more skin graft donor site wounds in the treatment group showed bacterial colonisation (P = 0.014). On day 6 (3 days after using non‐antimicrobial wound dressings), bacterial colonies in both groups were comparable (P = 0.363). The certainty of the evidence is very low. We downgraded one level due to risk of bias (unclear risk of bias due to sequence generation and allocation concealment and high risk of bias due to incomplete outcome data; reasons for missing data were not reported), one level due to indirectness (colonisation is only indirectly relevant to infection), and two levels due to imprecision (very small sample size).
Secondary outcome: cost of treatment
Outcome not measured or reported
Secondary outcome: wound complications
Outcome not measured or reported
Secondary outcome: adverse events
Eisenbeiß 2012 reported on the number of adverse events at 14 days. We do not know about the effect of octenidine‐based hydrogel on adverse events. In the ITT population, eight participants presented with 11 adverse events (data per group not reported). In the per‐protocol population, two participants in the intervention group and one participant in the comparison group reported adverse events (RR 0.58, 95% CI 0.06 to 5.89; 41 participants; Analysis 2.2). We downgraded the certainty of the evidence one level due to risk of bias (unclear risk of bias due to sequence generation and allocation concealment and high risk of bias due to incomplete outcome data; reasons for missing data were not reported), and two levels due to imprecision (few events and small sample size, and a large confidence interval that incorporates the possibility of benefit and harm).
2.2. Analysis.

Comparison 2: Comparison 2. Octenidine (OCT)‐based hydrogel vs OCT‐free placebo hydrogel dressings, Outcome 2: Adverse events
Hydrogel dressings compared with another therapy such as topical agents (a topical agent is a cream, an ointment or a solution that is applied directly to the wound)
No trials compared hydrogel dressings with another therapy.
Hydrogel and another therapy versus other therapy alone
No trials compared hydrogel in combination with another therapy versus other therapy alone.
Discussion
Summary of main results
This review included all identified RCTs that compared hydrogel dressings with each other or with other dressings in managing donor site wounds of split‐thickness skin grafts. Two RCTs (Eisenbeiß 2012; McBride 2018), involving a total of 162 participants, met the prespecified eligibility criteria.
There may be a slight increase in time to wound healing with hydrogel dressings when compared with other types of dressings (low‐certainty evidence). The effect of hydrogel dressings, when compared with other types of dressings, is uncertain for donor site pain, scarring, itching, and adverse events (one study, 101 participants; very low‐certainty evidence; McBride 2018). McBride 2018 reported no difference in the cost of treatment between hydrogel dressings and other types of dressings. McBride 2018 did not report health‐related quality of life or wound infection.
The effect of octenidine‐based hydrogel dressings compared with non‐antimicrobial hydrogel dressings (placebo without octenidine) is uncertain for time to complete wound healing, wound infection in the form of bacterial colonisation and adverse events (1 study, 61 participants; very low‐certainty evidence; Eisenbeiß 2012). Eisenbeiß 2012 did not report donor site pain, cost of treatment, wound complications or health‐related quality of life.
Overall completeness and applicability of evidence
We ran a comprehensive set of literature searches to maximise the relevant research included here. We found two RCTs that evaluated the effect of two types of hydrogel dressings, or other types of dressings on split‐thickness skin graft donor sites. Very low‐certainty evidence means we are uncertain if there is a difference between octenidine‐based hydrogel dressings and octenidine‐free hydrogel dressings in managing donor site wounds following split‐thickness skin grafts. There is low‐certainty evidence that there may be an increase in time to healing with hydrogel (Sorbact) compared with alginate dressings (Algisite M) and Cuticerin, and very low‐certainty evidence supporting no difference between hydrogel (Sorbact), alginate dressings (Algisite M), and Cuticerin in donor site pain, adverse events and wound complications. Split‐thickness skin graft donor sites that are complicated by wound infection are at risk of converting into a full‐thickness wound, requiring grafting, so we consider not reporting the outcome of wound infection to be a major flaw in McBride 2018.
Evidence from the studies included in this review was not sufficient to address the objectives. Our findings highlight a research gap in this field and the need for well‐designed studies to address this clinical question.
Quality of the evidence
We used the GRADE approach to assess the certainty of the evidence. The overall certainty of the evidence ranged from low to very low. We downgraded the certainty of the evidence largely due to the risk of bias and imprecision due to the small sample size and few events, and large confidence intervals. We could not assess inconsistency because we included only one study for each comparison.
Potential biases in the review process
Although we performed a comprehensive search process, applied filters where appropriate, and received guidance from an Information Specialist, we cannot rule out the effect of publication bias.
Agreements and disagreements with other studies or reviews
This review presents data on hydrogel dressings used to treat donor site wounds of split‐thickness skin grafts. A systematic review and meta‐analysis by Zhang 2019 included RCTs and controlled clinical trials that compared hydrogel dressings with other dressings in various types of wounds, such as burns, ulcers, surgical wounds and canine bites, but did not include donor site wounds. The review showed that hydrogel dressings caused a significant decrease in wound healing time and better pain relief compared with non‐hydrogel dressings. Weingart 1993 compared zinc oxide hydrogel with potassium/calcium chloride hydrogel on donor site wounds in a non‐randomised study and showed a better wound healing effect with zinc oxide hydrogel. Li 2015 reported improved healing time and rate in recombinant human granulocyte‐macrophage colony‐stimulating factor hydrogel compared with Vaseline gauze. Koivuniemi 2020, a non‐randomised clinical trial, compared nano‐fibrillar cellulose hydrogel dressing (NFC hydrogel dressing) with synthetic copolymer dressing to treat donor site wounds of split‐thickness skin grafts. The authors concluded that NFC hydrogel dressings achieved healing in a comparable time to the copolymer dressing with some added benefits, such as lower pain level, and that the donor site wound separates after re‐epithelialisation and does not degrade into the tissue. The study authors also reported improvement in the elasticity of the re‐epithelialised donor skin site.
Authors' conclusions
Implications for practice.
There is insufficient evidence to assess the clinical benefits and harms of hydrogel dressings on donor‐site wounds of split‐thickness skin grafts compared with other dressings. There is low‐certainty evidence that there may be an increase in time to wound healing with hydrogel dressings compared with other dressings. The evidence was of very low certainty for pain, adverse events and wound complications.
Implications for research.
There is a need for adequately powered and well‐designed randomised controlled trials with adequate sample sizes, types of populations and subgroups, types of interventions to be compared, and outcomes to be measured. Trials should compare hydrogel dressings with other treatment options in treating donor site wounds of split‐thickness skin grafts so that the evidence gathered could be useful for future meta‐analyses in order to provide evidence to inform clinical practice when managing such wounds.
History
Protocol first published: Issue 4, 2020
Acknowledgements
The review authors would like to thank the following peer reviewers: Andrew B Jull, Michael Gallagher and Amanda Roberts, and Jessica Sharp for copy editing the protocol of this review.
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Robert Boyle, National Heart & Lung Institute, Section of Inflammation and Repair, Imperial College London
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Luisa M Fernandez Mauleffinch, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Denise Mitchell, Senior Production Editor, Cochrane Central Executive Team
Peer‐reviewers (provided comments and recommended an editorial decision): Jennifer Hilgart, Cochrane (methods); Steve McDonald, Cochrane Australia (search); Amanda Roberts (consumer); Eduardo Gus, MD Staff Plastic Surgeon, The Hospital for Sick Children Assistant Professor, University of Toronto Project Investigator, SickKids Research Institute (clinical); Beryl De Souza, Hon Senior Clinical Lecturer in Plastic Surgery Imperial College, Asst Prof of Surgery St George's International Medical School, Grenada (clinical); and Devi Prasad Mohapatra, JIPMER (clinical).
Appendices
Appendix 1. British National Formulary Wound Dressings Classification, based on BNF 2017
Basic wound contact dressings
Low‐adherence dressings and wound contact materials
These dressings are usually cotton pads that are placed directly in contact with the wound. They can be either non‐medicated (e.g. paraffin gauze dressing) or medicated (e.g. containing povidone iodine or chlorhexidine). Examples include paraffin gauze dressing, BP 1993 and Xeroform (Covidien) dressing ‐ a non‐adherent petrolatum blend with 3% bismuth tribromophenate on fine mesh gauze.
Absorbent dressings
These dressings are applied directly to the wound and may be used as secondary absorbent layers in the management of heavily exuding wounds. Examples include Primapore (Smith & Nephew), Megapore (Molnlycke) and absorbent cotton gauze (BP 1988).
Advanced wound dressings
Hydrogel sheet and amorphous dressings
These dressings consist of a starch polymer and up to 96% water. They can absorb wound exudate or rehydrate a wound, depending on the wound moisture levels. They are supplied in either flat sheets or amorphous hydrogel. Examples of hydrogel sheet dressings include: Actiformcool (Activa) and Aquaflo (Covidien). Examples of amorphous hydrogel dressings include: Purilon Gel (Coloplast) and NuGel (Systagenix).
Films: permeable film and membrane dressings
These dressings are permeable to water vapour and oxygen, but not to liquid water or micro‐organisms. Examples include Tegaderm (3M); and Opsite (Smith & Nephew).
Soft polymer dressings
These dressings are composed of a soft silicone polymer held in a non‐adherent layer. They are moderately absorbent. Examples include: Mepitel (Molnlyckye) and Urgotul (Urgo).
Hydrocolloid dressings
These dressings are usually composed of an absorbent hydrocolloid matrix on a vapour‐permeable film or foam backing. Examples include: Granuflex (Conva Tec) and NU DERM (Systagenix). Fibrous alternatives have been developed that resemble alginates and are not occlusive: Aquacel (Conva Tec).
Foam dressings
These dressings contain hydrophilic polyurethane foam and are designed to absorb wound exudate and maintain a moist wound surface. There are various versions; some include additional absorbent materials, such as viscose and acrylate fibres, or particles of superabsorbent polyacrylate, while some are silicone‐coated for non‐traumatic removal. Examples include: Allevyn (Smith & Nephew); Biatain (Coloplast); and Tegaderm (3M).
Alginate dressings
These dressings are highly absorbent and consist of calcium alginate or calcium sodium alginate, which can be combined with collagen. The alginate forms a gel when in contact with wound surface. This gel can be lifted off at dressing removal, or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency. Examples include: Curasorb (Covidien); SeaSorb (Coloplast); and Sorbsan (Unomedical).
Capillary‐action dressings
These dressings consist of an absorbent core of hydrophilic fibres held between two low‐adherent contact layers. Examples include: Advadraw (Advancis); and Vacutx (Protex).
Odour‐absorbent dressings
These dressings contain charcoal and are used to absorb wound odour. Often this type of dressing is used in conjunction with a secondary dressing to improve absorbency. Examples include: CarboFLEX (Conva Tec).
Antimicrobial dressings
Honey‐impregnated dressings
These dressings contain medical‐grade honey, which is supposed to have antimicrobial and anti‐inflammatory properties, and can be used for acute or chronic wounds. Examples include: Medihoney (Medihoney) and Activon Tulle (Advancis).
Iodine‐impregnated dressings
These dressings release free iodine, which is thought to act as a wound antiseptic, when exposed to wound exudate. An example is Iodozyme (Insense).
Silver‐impregnated dressings
These dressings are used to treat infected wounds, as silver ions are thought to have antimicrobial properties. Silver versions of most dressing types are available (e.g. silver foam, silver hydrocolloid, etc). Examples include: Acticoat (Smith & Nephew) and Urgosorb Silver (Urgo).
Other antimicrobial dressings
These dressings are composed of a gauze or low‐adherent dressing impregnated with an ointment thought to have antimicrobial properties. Examples include: chlorhexidine gauze dressing (Smith & Nephew) and Cutimed Sorbact (BSN Medical).
Specialist dressings
Protease‐modulating matrix dressings
These dressings alter the activity of proteolytic (protein‐digesting) enzymes in chronic wounds. Examples include: Promogran (Systagenix) and Sorbion (H & R).
Silicone keloid dressing
These dressings reduce or prevent hypertrophic or keloid scarring. Examples include: Cica‐Care (Smith & Nephew) and Ciltech (Sumed).
Appendix 2. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Skin Transplantation EXPLODE ALL AND INREGISTER
2 MESH DESCRIPTOR Transplantation, Autologous EXPLODE ALL AND INREGISTER
3 MESH DESCRIPTOR Transplant Donor Site EXPLODE ALL AND INREGISTER
4 (((split next thick*) or split‐thick* or "split skin" or split‐skin or "partial dermal" or partial‐dermal or (partial next thick*) or partial‐thick*) near3 graft*) AND INREGISTER
5 ((skin or derm*) next transplant*) AND INREGISTER
6 STSG AND INREGISTER
7 donor next site* AND INREGISTER
8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 AND INREGISTER
9 MESH DESCRIPTOR Hydrogels EXPLODE ALL AND INREGISTER
10 MESH DESCRIPTOR Bandages EXPLODE ALL AND INREGISTER
11 hydrogel* AND INREGISTER
12 ("Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Aquaform or Askina or Cutimed or Granugel or Intrasite or "Nu Gel" or "Nu‐Gel" or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Hydrosorb or Iodozyme or Kerralite or Novogel or Oxyzyme or Hyiodine or Flexigran or Purilon or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curafil or dermagran or duoderm or hypergel or normlgel or "suprasorb gel" or hypligel or "elasto‐ gel" or tegagel or curasol or curate) AND INREGISTER
13 #9 OR #10 OR #11 OR #12 AND INREGISTER
14 #8 AND #13 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Skin Transplantation] explode all trees
#2 MeSH descriptor: [Transplantation, Autologous] explode all trees
#3 MeSH descriptor: [Transplant Donor Site] explode all trees
#4 (((split next thick*) or split‐thick* or "split skin" or split‐skin or "partial dermal" or partial‐dermal or (partial next thick*) or partial‐thick*) near/3 graft*):ti,ab,kw
#5 ((skin or derm*) next transplant*):ti,ab,kw
#6 STSG:ti,ab,kw
#7 "donor site*":ti,ab,kw
#8 {or #1‐#7}
#9 MeSH descriptor: [Hydrogels] explode all trees
#10 MeSH descriptor: [Bandages] explode all trees
#11 hydrogel*:ti,ab,kw
#12 ("Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Aquaform or Askina or Cutimed or Granugel or Intrasite or "Nu Gel" or "Nu‐Gel" or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Hydrosorb or Iodozyme or Kerralite or Novogel or Oxyzyme or Hyiodine or Flexigran or Purilon or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curafil or dermagran or duoderm or hypergel or normlgel or "suprasorb gel" or hypligel or "elasto‐ gel" or tegagel or curasol or curate):ti,ab,kw
#13 {or #9‐#12}
#14 #8 and #13 in Trials
Trial Registry specific search of The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) via Cochrane Register of Studies
1 MESH DESCRIPTOR Skin Transplantation EXPLODE ALL AND CENTRAL:TARGET
2 MESH DESCRIPTOR Transplantation, Autologous EXPLODE ALL AND CENTRAL:TARGET
3 MESH DESCRIPTOR Transplant Donor Site EXPLODE ALL AND CENTRAL:TARGET
4 (((split next thick*) or split‐thick* or "split skin" or split‐skin or "partial dermal" or partial‐dermal or (partial next thick*) or partial‐thick*) near3 graft*) AND CENTRAL:TARGET
5 ((skin or derm*) next transplant*) AND CENTRAL:TARGET
6 STSG AND CENTRAL:TARGET
7 donor next site* AND CENTRAL:TARGET
8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 AND CENTRAL:TARGET
9 MESH DESCRIPTOR Hydrogels EXPLODE ALL AND CENTRAL:TARGET
10 MESH DESCRIPTOR Bandages EXPLODE ALL AND CENTRAL:TARGET
11 hydrogel* AND CENTRAL:TARGET
12 ("Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Aquaform or Askina or Cutimed or Granugel or Intrasite or "Nu Gel" or "Nu‐Gel" or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Hydrosorb or Iodozyme or Kerralite or Novogel or Oxyzyme or Hyiodine or Flexigran or Purilon or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curafil or dermagran or duoderm or hypergel or normlgel or "suprasorb gel" or hypligel or "elasto‐ gel" or tegagel or curasol or curate) AND CENTRAL:TARGET
13 #9 OR #10 OR #11 OR #12 AND CENTRAL:TARGET
14 #8 AND #13 AND CENTRAL:TARGET
15 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET
16 http*:SO AND CENTRAL:TARGET
17 #15 OR #16 AND CENTRAL:TARGET
18 #14 AND #17
Ovid MEDLINE
1 exp Skin Transplantation/
2 exp Transplantation, Autologous/
3 exp Transplant Donor Site/
4 (((split adj thick*) or split‐thick* or "split skin" or split‐skin or "partial dermal" or partial‐dermal or (partial adj thick*) or partial‐thick*) adj3 graft*).ab,ti.
5 ((skin or derm*) adj transplant*).ab,ti.
6 STSG.ti,ab.
7 (donor adj site*).ti,ab.
8 or/1‐7
9 exp Hydrogels/
10 exp Bandages/
11 hydrogel*.ab,ti.
12 ("Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Aquaform or Askina or Cutimed or Granugel or Intrasite or "Nu Gel" or "Nu‐Gel" or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Hydrosorb or Iodozyme or Kerralite or Novogel or Oxyzyme or Hyiodine or Flexigran or Purilon or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curafil or dermagran or duoderm or hypergel or normlgel or "suprasorb gel" or hypligel or "elasto‐ gel" or tegagel or curasol or curate).ab,ti. 885
13 or/9‐12
14 8 and 13
15 randomized controlled trial.pt.
16 controlled clinical trial.pt.
17 randomi?ed.ab.
18 placebo.ab.
19 clinical trials as topic.sh.
20 randomly.ab.
21 trial.ti.
22 or/15‐21
23 exp animals/ not humans.sh.
24 22 not 23
25 14 and 24
Ovid Embase
1 exp skin transplantation/
2 exp autotransplantation/
3 exp donor site/
4 (((split adj thick*) or split‐thick* or "split skin" or split‐skin or "partial dermal" or partial‐dermal or (partial adj thick*) or partial‐thick*) adj3 graft*).ab,ti.
5 ((skin or derm*) adj transplant*).ab,ti.
6 STSG.ti,ab.
7 (donor adj site*).ti,ab.
8 or/1‐7
9 hydrogel/
10 exp bandage/
11 hydrogel*.ab,ti.
12 ("Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Aquaform or Askina or Cutimed or Granugel or Intrasite or "Nu Gel" or "Nu‐Gel" or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Hydrosorb or Iodozyme or Kerralite or Novogel or Oxyzyme or Hyiodine or Flexigran or Purilon or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curafil or dermagran or duoderm or hypergel or normlgel or "suprasorb gel" or hypligel or "elasto‐ gel" or tegagel or curasol or curate).ab,ti.
13 or/9‐12
14 8 and 13
15 Randomized controlled trial/
16 Controlled clinical study/
17 Random$.ti,ab.
18 randomization/
19 intermethod comparison/
20 placebo.ti,ab.
21 (compare or compared or comparison).ti.
22 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
23 (open adj label).ti,ab.
24 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
25 double blind procedure/
26 parallel group$1.ti,ab.
27 (crossover or cross over).ti,ab.
28 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
29 (assigned or allocated).ti,ab.
30 (controlled adj7 (study or design or trial)).ti,ab.
31 (volunteer or volunteers).ti,ab.
32 human experiment/
33 trial.ti.
34 or/15‐33
35 (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
36 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
37 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
38 (Systematic review not (trial or study)).ti.
39 (nonrandom$ not random$).ti,ab.
40 Random field$.ti,ab.
41 (random cluster adj3 sampl$).ti,ab.
42 (review.ab. and review.pt.) not trial.ti.
43 we searched.ab. and (review.ti. or review.pt.)
44 update review.ab.
45 (databases adj4 searched).ab.
46 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
47 Animal experiment/ not (human experiment/ or human/)
48 or/35‐47
49 34 not 48
50 14 and 49
EBSCO CINAHL Plus
S37 S13 AND S36
S36 S35 NOT S34
S35 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28
S34 S32 NOT S33
S33 MH (human)
S32 S29 OR S30 OR S31
S31 TI (animal model*)
S30 MH (animal studies)
S29 MH animals+
S28 AB (cluster W3 RCT)
S27 MH (crossover design) OR MH (comparative studies)
S26 AB (control W5 group)
S25 PT (randomized controlled trial)
S24 MH (placebos)
S23 MH (sample size) AND AB (assigned OR allocated OR control)
S22 TI (trial)
S21 AB (random*)
S20 TI (randomised OR randomized)
S19 MH cluster sample
S18 MH pretest‐posttest design
S17 MH random assignment
S16 MH single‐blind studies
S15 MH double‐blind studies
S14 MH randomized controlled trials
S13 S8 AND S12
S12 S9 OR S10 OR S11
S11 TI ( ("Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Aquaform or Askina or Cutimed or Granugel or Intrasite or "Nu Gel" or "Nu‐Gel" or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Hydrosorb or Iodozyme or Kerralite or Novogel or Oxyzyme or Hyiodine or Flexigran or Purilon or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curafil or dermagran or duoderm or hypergel or normlgel or "suprasorb gel" or hypligel or "elasto‐ gel" or tegagel or curasol or curate) ) OR AB ( ("Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Aquaform or Askina or Cutimed or Granugel or Intrasite or "Nu Gel" or "Nu‐Gel" or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Hydrosorb or Iodozyme or Kerralite or Novogel or Oxyzyme or Hyiodine or Flexigran or Purilon or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curafil or dermagran or duoderm or hypergel or normlgel or "suprasorb gel" or hypligel or "elasto‐ gel" or tegagel or curasol or curate) )
S10 TI hydrogel* OR AB hydrogel*
S9 (MH "Bandages and Dressings+")
S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7
S7 TI (donor site*) OR AB (donor site*)
S6 TI STSG OR AB STSG
S5 TI ( ((skin or derm*) N1 transplant*) ) OR AB ( ((skin or derm*) N1 transplant*) )
S4 TI ( ((split thick* or split‐thick* or "split skin" or split‐skin or "partial dermal" or partial‐dermal or partial thick* or partial‐thick*) N3 graft*) ) OR AB ( ((split thick* or split‐thick* or "split skin" or split‐skin or "partial dermal" or partial‐dermal or partial thick* or partial‐thick*) N3 graft*) )
S3 (MH "Transplant Donor Site")
S2 (MH "Autografts+")
S1 (MH "Skin Transplantation")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
Hydrogel OR bandage OR dressing OR Askina OR Cutimed OR Intrasite OR Xtrasorb OR ActivHeal OR Aquaform OR Cutimed OR Granugel OR Intrasite OR Nu Gel OR Prontosan OR Octenillin OR Actiform OR Hydrosorb OR Iodozyme OR Kerralite OR Novogel | split thickness OR split‐thickness skin graft OR skin graft OR donor site OR donor site complication OR STSG OR autologous transplantation OR skin transplant OR autotransplantation
Oxyzyme OR Hyiodine OR Flexigran OR Purilon OR Aquaflo OR Coolie OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curafil OR dermagran OR duoderm OR hypergel OR normlgel OR suprasorb OR hypligel OR elasto‐ gel OR tegagel OR curasol OR curate | split thickness OR split‐thickness skin graft OR skin graft OR donor site OR donor site complication OR STSG OR autologous transplantation OR skin transplant OR autotransplantation
World Health Organization International Clinical Trials Registry Platform
Hydrogel OR bandage OR dressing OR Askina OR Cutimed OR Intrasite OR Xtrasorb OR ActivHeal OR Aquaform OR Cutimed OR Granugel OR Intrasite OR Nu Gel OR Prontosan OR Octenillin OR Actiform OR Hydrosorb OR Iodozyme OR Kerralite OR Novogel | split thickness OR split‐thickness skin graft OR skin graft OR donor site OR donor site complication OR STSG OR autologous transplantation OR skin transplant OR autotransplantation [Title]
Hydrogel OR bandage OR dressing OR Askina OR Cutimed OR Intrasite OR Xtrasorb OR ActivHeal OR Aquaform OR Cutimed OR Granugel OR Intrasite OR Nu Gel OR Prontosan OR Octenillin OR Actiform OR Hydrosorb OR Iodozyme OR Kerralite OR Novogel | split thickness OR split‐thickness skin graft OR skin graft OR donor site OR donor site complication OR STSG OR autologous transplantation OR skin transplant OR autotransplantation [Condition]
Oxyzyme OR Hyiodine OR Flexigran OR Purilon OR Aquaflo OR Coolie OR Geliperm OR SanoSkin OR Vacunet OR curafil OR dermagran OR duoderm OR hypergel OR normlgel OR suprasorb OR hypligel OR elasto‐ gel OR tegagel OR curasol OR curate | split thickness OR split‐thickness skin graft OR skin graft OR donor site OR donor site complication OR STSG OR autologous transplantation OR skin transplant OR autotransplantation [Title]
Oxyzyme OR Hyiodine OR Flexigran OR Purilon OR Aquaflo OR Coolie OR Geliperm OR SanoSkin OR Vacunet OR curafil OR dermagran OR duoderm OR hypergel OR normlgel OR suprasorb OR hypligel OR elasto‐ gel OR tegagel OR curasol OR curate | split thickness OR split‐thickness skin graft OR skin graft OR donor site OR donor site complication OR STSG OR autologous transplantation OR skin transplant OR autotransplantation [Condition]
Appendix 3. The Cochrane tool for assessing risk of bias (RoB 1)
1) Random sequence generation (checking for possible selection bias)
We will describe the method used to generate the allocation sequence in each study in sufficient detail to allow an assessment of whether it should produce comparable groups.
Low risk of bias: the investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias: the investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear risk of bias: insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We will assess the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear risk of bias (insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement).
3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes. We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes. We will assess methods used to blind outcome assessment as:
low risk of other bias;
Either of the following. • No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. • Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
high risk of other bias;
Either of the following. • No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. • Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
unclear risk of bias;
• Insufficient information available to permit a judgement of 'low risk' or 'high risk'.
4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses. We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; ≦ 20% participants missing);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation; more than 20% participants missing);
unclear risk of bias.
5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias. We will assess the methods as being at:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We will consider other risk of bias issues as follows: comparability of treatment groups in relation to donor site wound surface area; choice of analysis where participant(s) with multiple donor site wounds are studied; and choice of analysis in cluster‐randomised trials. For trials using cluster randomisation we planned to assess the risk of bias using the following domains: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised trials (Higgins 2022). We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
The study appears to be free of other sources of bias.
high risk of other bias;
There is at least one important risk of bias. For example, the study:
• has extreme baseline imbalance; or • had a potential source of bias related to the specific study design used; • had an inappropriate influence of funders due to industry initiated protocols; • has been claimed to have been fraudulent; or • had some other problem.
Or in cluster‐randomised trials there is: • recruitment bias (differential participant recruitment in clusters for different interventions); • baseline imbalance; or • loss of clusters.
unclear whether there is risk of other bias;
There may be a risk of bias, but there is either: • insufficient information to assess whether an important risk of bias exists; or • insufficient rationale or evidence that an identified problem will introduce bias.
7) Overall risk of bias
We will make explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2022). With reference to 1 to 6 above, we will assess the likely magnitude and direction of the bias and whether we will consider it likely to impact the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses.
We will present our assessment of the risk of bias using a 'Risk of bias' summary figure which will present the judgements in cross‐tabulation. This display of internal validity indicates the weight the reader may give to the results of each study.
Appendix 4. Risk of bias assessment for cluster‐randomised trials
In cluster‐randomised trials, particular biases to consider include: recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; comparability with individually randomised trials.randomisation
Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomised, as knowledge of whether each cluster is an 'intervention' or 'control' cluster could affect the types of participants recruited.
Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
Occasionally, complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials.
Many cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a 'unit of analysis error' and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
5) In a meta‐analysis including both cluster‐randomised and individually randomised trials, or including cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than if the vaccine was applied to only half of the people. Another example is provided by a Cochrane Review of hip protectors (Santesso 2014). The cluster trials showed large positive effect, whereas individually randomised trials did not show any clear benefit. One possibility is that there was a 'herd effect' in the cluster‐randomised trials (many of which were performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such 'contamination' would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and 'herd effects' may be different for different types of cluster.
Data and analyses
Comparison 1. Comparison 1. Amorphous hydrogel versus other types of dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Time to complete re‐epithilisation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Amorphous hydrogel vs alginate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 Amorphous hydrogel vs Cuticerin | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: Comparison 1. Amorphous hydrogel versus other types of dressings, Outcome 1: Time to complete re‐epithilisation (days)
Comparison 2. Comparison 2. Octenidine (OCT)‐based hydrogel vs OCT‐free placebo hydrogel dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Time to complete healing | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eisenbeiß 2012.
| Study characteristics | ||
| Methods | Multi‐centred, prospective, double‐blinded, clinical RCT Study participants were recruited from 3 centres in Germany Duration of follow‐up was a maximum of 14 days |
|
| Participants | 61 participants with DSWs
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Group A: treated with the OCT‐based hydrogel (Octenilin wound gel, Schuelke & Mayr GmbH, Norderstedt/Germany) Group B: received treatment with an OCT‐free identical hydrogel (placebo) |
|
| Outcomes |
Primary objective: time for complete (100%) epithelialisation of treated skin graft donor site Secondary objectives:
|
|
| Notes | Trial registration: not reported Source of funding: the study was funded by Schuelke & Mayr, Norderstedt Conflict of interest: the study authors have no financial or other conflict of interest Contact details: ojan.assadian@meduniwien.ac.at |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: “participants (n=61) were double blinded, randomly divided into two groups: one group (n=31) was treated with the OCT‐based hydrogel (Octenilin® wound gel, Schuelke & Mayr GmbH, Norderstedt/Germany); the second group (n=30) received treatment with an OCT‐free identical hydrogel (placebo).” Comment: method of generation of random schedule was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quotation: no direct quotation Comment: not stated if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotation: "skin graft donor site wounds treated with an OCT‐based hydrogel compared to an identical non‐antimicrobial hydrogel (placebo without OCT)" Comment: method of blinding stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotation: "The study was designed as a prospective, double‐blinded randomised controlled clinical trial." "The time needed to achieve complete (100%) epithelialization was documented by two independent observers." Comment: method of blinding stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotation: "20 participants lost follow‐up". Comment: > 20% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes prespecified in the methods section of the protocol were reported in results |
| Other bias | Low risk | The study appears to be free of other sources of bias |
McBride 2018.
| Study characteristics | ||
| Methods | Single‐centre, 3‐arm, parallel‐group, prospective RCT 101 participants |
|
| Participants |
Inclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome measures:
Secondary outcome measures:
|
|
| Notes | Trial registration: Australia and New Zealand Clinical Trials Register (ACTRN12614000380695) Source of funding: "This trial was partially supported by a grant from Abigo Medical AB (Research Management number RM2013002823). This company had no part in the trial design, conduct, analysis, or preparation for publication." Conflict of interest: "None of the authors have any financial ties to Abigo Medical AB. None of the authors have received honoraria, travel, or accommodation from the company. None of the authors have undertaken speaking engagements at the request of the company. RMK and CAM have both accepted invitations, with attendant travel and accommodation, from Smith & Nephew to speak at meetings and conferences regarding the treatment of paediatric burns and the use of negative pressure wound therapy in children. RMK has provided medico‐legal opinion for Smith & Nephew. Smith & Nephew had no involvement in the conduct of this trial. The authors declare that they have no competing interests." Correspondence: cmcbride@paedsurgery.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "The randomization sequence was generated using an online program (http://stattrek.com/), with unrestricted simple randomisation in a 1:1:1 ratio." |
| Allocation concealment (selection bias) | Low risk | Quotation: "Allocation concealment was via the use of sequentially numbered, sealed opaque envelopes pre‐prepared by a third party. Envelopes were only opened in the operating theatre after the STSG had been harvested and immediately before the application of the DSW dressing." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotation: "At the first dressing change, the trial dressing was revealed, since all have a distinctive appearance on the removal of the secondary dressing. From that point blinding to pain scores was not possible in the clinic setting, as we were not able to use different staff for each dressing change. Partial blinding was possible regarding the other primary outcome of re‐epithelialization" Low risk of bias for time to re‐epithelialisation and adverse events and high‐risk of bias for pain, itching and scarring |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotation: "Researchers not present in the theatre assessed outcome measures until the first dressing change. Participants and care providers, as well as assessors, were therefore blinded to dressing." Low risk of bias for time to re‐epithelialisation and adverse events, and high risk of bias for pain, itching and scarring |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: loss of > 20% for most outcomes and time points. In addition, raw data for pain, itching, scarring and adverse events are not reported Low risk of bias for time to healing; and high risk of bias for all other outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes prespecified in the protocol were reported in the results |
| Other bias | Low risk | The study appears to be free of other sources of bias |
DSW: donor site wound; OCT: octenidine; RCT: randomised controlled trial; STSG: split‐thickness skin graft
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akita 2006 | Applied polyurethane and hydrogel dressings side by side on the same wound carrying risk of cross‐over effect |
| Blome‐Eberwein 2010 | Did not evaluate a hydrogel dressing |
| Dilokhuttakarn 2016 | Did not evaluate a hydrogel dressing |
| Gemberling 1976 | Did not evaluate a hydrogel dressing |
| Hickerson 1994 | Did not evaluate a hydrogel dressing |
| Johansen 1972 | Did not evaluate a hydrogel dressing |
| Koivuniemi 2020 | Non‐RCT |
| Li 2015 | Some DSWs were split‐wound design |
| Poinas 2019 | Did not evaluate a hydrogel dressing |
| Solanki 2012 | Did not evaluate a hydrogel dressing |
| Vogt 2006 | Did not study donor sites |
| Weingart 1993 | Non‐RCT |
DSW: donor site wound; RCT: randomised controlled trial
Differences between protocol and review
We did not find any cluster‐randomised trials to include in the review.
The included studies in the review did not report the outcome, health‐related quality of life.
As the review included only two studies, we could not perform subgroup or sensitivity analyses.
We did not perform a meta‐analysis as the data from the two included studies could not be combined.
Contributions of authors
Ahmed S Younis: conceived the review; designed the review; co‐ordinated the review; extracted data; analysed or interpreted data; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; approved final review prior to submission; is guarantor of the review.
Ibrahim M Abdelmonem: conceived the review; designed the review; co‐ordinated the review; extracted data; analysed or interpreted data; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; approved final review prior to submission.
Moheeb Gadullah: designed the review; extracted data; analysed or interpreted data; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; approved final review prior to submission.
Hamdy E Alnaggar: co‐ordinated the review; extracted data; analysed or interpreted data; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; approved final review prior to submission.
Yasser R Mohamed: conceived the review; designed the review; extracted data; analysed or interpreted data; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; approved final review prior to submission.
GemmaVillanueva: designed the review; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; provided data; approved final review prior to submission.
Jacqueline Y Thompson: produced the first draft of the review; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Carlos Areia: produced the first draft of the review; contributed to writing or editing the review; advised on the review; approved final review prior to submission.
Ashraf F Nabhan: designed the review; checked quality of data extraction; checked quality assessment; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; approved final review prior to submission; provided standard author training.
Contributions of the editorial base
Jo Dumville (Joint Co‐ordinating Editor): edited the protocol; advised on methodology, interpretation and content; approved the final version of the protocol prior to submission.
Gill Norman (Editor): edited the review; advised on methodology, interpretation and content.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process for the protocol; advised on content; edited the protocol and the review.
Sophie Bishop (Information Specialist): designed the search strategy, ran the search and edited the search methods section.
Tom Patterson (Editorial Assistant): edited the reference sections and the plain language summary.
Sources of support
Internal sources
-
None, Other
None
External sources
-
Egyptian Center for Evidence Based Medicine, Egypt
Author training
-
National Institute for Health and Care Research (NIHR), UK
This project was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to Cochrane Wounds until 31st March 2023. The views and opinions expressed are those of the authors and not necessarily those of the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Declarations of interest
Ahmed S Younis: none known.
Ibrahim M Abdelmonem: works as a health professional.
Moheeb Gadullah: none known.
Hamdy E Alnaggar: works as a health professional.
Yasser R Mohamed: works as a health professional.
Gemma Villanueva: none known.
Jacqueline Thompson: none known.
Carlos Areia: none known.
Ashraf F Nabhan: works as a health professional and is a member of the Editorial Board of Cochrane Pregnancy and Childbirth. He was not involved in the editorial process.
New
References
References to studies included in this review
Eisenbeiß 2012 {published data only}
- Eisenbeiß W, Siemers F, Amtsberg G, Hinz P, Hartmann B, Kohlmann T, et al. Prospective, double-blinded, randomised controlled trial assessing the effect of an octenidine-based hydrogel on bacterial colonisation and epithelialization of skin graft wounds in burn patients. International Journal of Burns and Trauma 2012;2(2):71-9. [PMC free article] [PubMed] [Google Scholar]
McBride 2018 {published data only}
- McBride CA, Kimble RM, Stockton KA. Prospective randomised controlled trial of Algisite™ M, Cuticerin™, and Sorbact® as donor site dressings in paediatric split-thickness skin grafts. Burns & Trauma 2018;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akita 2006 {published data only}
- Akita S, Akino K, Imaizumi T, Tanaka K, Anraku K, Yano H, et al. A polyurethane dressing is beneficial for split-thickness skin-graft donor wound healing. Burns 2006;32(4):447-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Blome‐Eberwein 2010 {published data only}
- Blome-Eberwein S, Johnson RM, Miller SF, Caruso DM, Jordan MH, Milner S, et al. Hydrofiber dressing with silver for the management of split-thickness donor sites: a randomized evaluation of two protocols of care. Burns 2010;36(5):665-72. [DOI: 10.1016/j.burns.2009.06.193] [CN-00768178] [DOI] [PubMed] [Google Scholar]
Dilokhuttakarn 2016 {published data only}
- Dilokhuttakarn T, Vilai P, Rungsinaporn V. The efficacy of chitosan dressing in reducing blood loss for harvest site in split thickness skin graft: a randomized control trial. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2016;99 Suppl 8:S19-24. [CN-01616203] [PubMed] [Google Scholar]
Gemberling 1976 {published data only}
- Gemberling RM, Miller TA, Caffee H, Zawacki BE. Dressing comparison in the healing of donor sites. Journal of Trauma 1976;16(10):812-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hickerson 1994 {published data only}
- Hickerson WL, Kealey GP, Smith DJ Jr, Thomson PD. A prospective comparison of a new, synthetic donor site dressing versus an impregnated gauze dressing. Journal of Burn Care & Rehabilitation 1994;15(4):359-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johansen 1972 {published data only}
- Johansen AM, Sorensen B. Treatment of donor sites. A controlled trial with fucidin gauze. Scandinavian Journal of Plastic and Reconstructive Surgery 1972;6(1):47-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Koivuniemi 2020 {published data only}
- Koivuniemi R, Hakkarainen T, Kiiskinen J, Kosonen M, Vuola J, Valtonen J, et al. Clinical study of nanofibrillar cellulose hydrogel dressing for skin graft donor site treatment. Advances in Wound Care 2020;9(4):199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li C, Li S-J, Li Y-T, Fu Z-Y, Ren C-Y. External use of recombinant human granulocyte-macrophage colony stimulating factor hydrogel to repair thick skin graft donor sites. Chinese Journal of Tissue Engineering Research 2015;19(34):5458-62. [DOI: 10.3969/j.issn.2095-4344.2015.34.010] [CN-01210697] [DOI] [Google Scholar]
Poinas 2019 {published data only}
- Poinas A, Perrot P, Lorant J, Nerrière O, Nguyen JM, Saiagh S, et al. CICAFAST: comparison of a biological dressing composed of fetal fibroblasts and keratinocytes on a split-thickness skin graft donor site versus a traditional dressing: a randomized controlled trial. Trials 2019;20(1):612. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solanki 2012 {published data only}
- Solanki NS, Mackie IP, Greenwood JE. A randomised prospective study of split skin graft donor site dressings: AWBAT-D™ vs. Duoderm. Burns 2012;38(6):889-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vogt 2006 {published data only}
- Vogt PM, Reimer K, Hauser J, Rossbach O, Steinau HU, Bosse B, et al. PVP-iodine in hydrosomes and hydrogel--a novel concept in wound therapy leads to enhanced epithelialization and reduced loss of skin grafts. Burns 2006;32(6):698-705. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weingart 1993 {published data only}
- Weingart D, Stoll P. The epithelialization of split skin graft donor sites - a test model for the efficacy of topical wound therapeutic agents. European Journal of Plastic Surgery 1993;16(1):22-5. [Google Scholar]
Additional references
BNF 2017
- British National Formulary, British Medical Association, British Royal Pharmaceutical Society of Great Britain. British National Formulary. 74th edition. London (UK): British Medical Association, 2017. [Google Scholar]
Caló 2015
- Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. 50 Years of European Polymer Journal 2015;65:252-67. [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Dumville 2013
- Dumville JC, O'Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD009101. [DOI: 10.1002/14651858.CD009101.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 1998
- Francis A. Nursing management of skin graft sites. Nursing Standard 1998;12(33):41-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 23 July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2022. Available at gradepro.org.
Gupta 2010
- Gupta B, Agarwal R, Alam MS. Textile-based smart wound dressings. Indian Journal of Fibre and Textile Research 2010;35:174-87. [DOI: 10.3238/arztebl.2008.0239] [DOI] [Google Scholar]
Hawker 2011
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care & Research 2011;63 Suppl 11:S240-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 2011;20(10):1727-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2001;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Higgins 2022
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Humrich 2018
- Humrich M, Goepel L, Gutknecht M, Lohrberg D, Blessmann M, Bruning G, et al. Health-related quality of life and patient burden in patients with split-thickness skin graft donor site wounds. International Wound Journal 2018;15(2):266-73. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones A, Vaughan D. Hydrogel dressings in the management of a variety of wound types: a review. Journal of Orthopaedic Nursing 2005;9:1-11. [DOI: 10.1016/S1361-3111(05)80001-9] [DOI] [Google Scholar]
Kanapathy 2017
- Kanapathy M, Hachach-Haram N, Bystrzonowski N, Connelly JT, O'Toole EA, Becker DL, et al. Epidermal grafting for wound healing: a review on the harvesting systems, the ultrastructure of the graft and the mechanism of wound healing. International Wound Journal 2017;14(1):16-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014
- Kim S. World Health Organization Quality of Life (WHOQOL) Assessment. In: Michalos AC, editors(s). Encyclopedia of Quality of Life and Well-Being Research. Dordrecht (Netherlands): Springer, 2014:7260-1. [Google Scholar]
Lars 2013
- Lars PK, Giretzlehner M, Trop M, Parvizi D, Spendel S, Schintler M, et al. The properties of the "ideal" donor site dressing: results of a worldwide online survey. Annals of Burns and Fire Disasters 2013;26(3):136-41. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Li 2022
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;6:339:b2700. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lins 2016
- Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Medicine 2016;4:4. [DOI: 10.1177/2050312116671725] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macdonald 2010
- Macdonald JM, Geyer MJ, World Health Organization. Wound and lymphoedema management. Available at apps.who.int/iris/bitstream/handle/10665/44279/9789241599139_eng.pdf.
Nanchahal 1992
- Nanchahal J, Ward CM. New grafts for old? A review of alternatives to autologous skin. British Journal of Plastic Surgery 1992;45(5):354-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Peppas 1993
- Peppas N, Khare AR. Preparation, structure and diffusional behavior of hydrogels in controlled release. Modern Hydrogel Delivery Systems 1993;11(1):1-35. [Google Scholar]
Rakel 1998
- Rakel BA, Bermel MA, Abbott LI, Baumler SK, Burger MR, Dawson CJ, et al. Split-thickness skin graft donor site care: a quantitative synthesis of the research. ANR 1998;11(4):174-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ratner 2003
- Ratner D. Skin grafting. Seminars in Cutaneous Medicine and Surgery 2003;22(4):295-305. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web 2023 [Computer program]
- Review Manager Web (RevMan Web). Version 5.5.0. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Rubinstein 2013
- Rubinstein SM, Terwee CB, Assendelft WJ, De Boer MR, Van Tulder MW. Spinal manipulative therapy for acute low back pain: an update of the Cochrane Review. Spine 2013;38(3):E158-77. [DOI] [PubMed] [Google Scholar]
Santesso 2014
- Santesso N, Carrasco‐Labra A, Brignardello‐Petersen R. Hip protectors for preventing hip fractures in older people. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD001255. [DOI: 10.1002/14651858.CD001255.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Trudgian 2000
- Trudgian J. Investigating the use of aquaform hydrogel in wound management. British Journal of Nursing 2000;9(14):943-8. [DOI: 10.12968/bjon.2000.9.14.943] [DOI] [PubMed] [Google Scholar]
Uraloğlu 2012
- Uraloğlu M, Livaoğlu M, Agdoğan Ö, Mungan S, Alhan E, Karaçal N. An evaluation of five different dressing materials on split-thickness skin graft donor site and full-thickness cutaneous wounds: an experimental study. International Wound Journal 2012;11(1):85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voineskos 2009
- Voineskos SH, Ayeni OA, McKnight L, Thoma A. Systematic review of skin graft donor-site dressings. Plastic and Reconstructive Surgery 2009;124(1):298-306. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wichterle 1960
- Wichterle O, Lim D. Hydrophilic gels for biological use. Nature 1960;185:117-8. [DOI: 10.1038/185117a0] [DOI] [Google Scholar]
Wiechula 2003
- Wiechula R. The use of moist wound-healing dressings in the management of split-thickness skin graft donor sites: a systematic review. International Journal of Nursing Practice 2003;9(2):S9-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Winter 1962
- Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193(4812):293-4. [DOI] [PubMed] [Google Scholar]
Zhang 2019
- Zhang L, Yin H, Lei X, Lau JN, Yuan M, Wang X. A systematic review and meta-analysis of clinical effectiveness and safety of hydrogel dressings in the management of skin wounds. Frontiers in Bioengineering and Biotechnology 2019;7:342. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Younis 2020
- Younis AS, Abdelmonem IM, Mohamed YR, Alnaggar HE, Villanueva G, Thompson JY, et al. Hydrogel dressings for donor sites of split-thickness skin grafts. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013570. [DOI: 10.1002/14651858.CD013570] [DOI] [PMC free article] [PubMed] [Google Scholar]


