Abstract
The ability to elicit protective immune responses after intranasal immunization with rotavirus particles, either with or without the attenuated Escherichia coli heat-labile enterotoxin LT(R192G) as an adjuvant, was examined in the adult mouse model. BALB/c mice were administered one or two inoculations of psoralen/UV-inactivated, triple-layered (tl) or double-layered (dl) purified rotavirus particles. Four weeks after immunization, mice were challenged with the murine rotavirus strain EDIM, and the shedding of rotavirus antigen was quantified. Rotaviruses used for immunization included EDIM and heterotypic simian (RRV), bovine (WC3), and human (89-12) strains. tl EDIM stimulated both systemic and intestinal rotavirus antibody responses and complete protection with as little as one 1-μg dose. Inclusion of LT(R192G) (10 μg) significantly increased rotavirus antibody responses and reduced antigen concentrations needed for full protection. Both dl EDIM and heterotypic dl and tl particles stimulated protection, but they did so less than tl EDIM at comparable concentrations, either with or without LT(R192G). When B-cell-deficient μMt mice were immunized with tl EDIM particles, protection was reduced to levels similar to those induced with dl EDIM and heterotypic particles in BALB/c mice. However, dl EDIM particles induced similar levels of protection in both mouse strains. The protection stimulated by tl or dl EDIM particles was not diminished by CD8 cell depletion prior to immunization in either strain of mice. These results indicate that tl EDIM induced immunity at least partially through responses to its outer capsid proteins, presumably by stimulation of serotype-specific neutralizing antibody. In contrast, the other particles stimulated protection primarily by an antibody-independent mechanism. Finally, depletion of CD8 cells had no effect on protection by either mechanism.
Rotaviruses are the primary cause of severe infantile gastroenteritis and, hence, have been targeted for vaccine development. Rotavirus vaccines evaluated to date have all been live viruses that are delivered orally to mimic the protection found after natural rotavirus infection. These vaccines have provided only partial immunity against subsequent rotavirus disease (2, 3, 5, 18, 33, 34, 36). Because intranasal (i.n.) immunization has been successful against other mucosal pathogens (19), this route of immunization should be a promising method to prevent rotavirus disease which primarily, if not solely, results from infection of the intestinal mucosa. To test this possibility, we utilized the adult mouse model developed not only to rapidly evaluate new vaccination strategies but also to identify immunological effectors of protection (37).
After oral immunization with live murine rotavirus, BALB/c mice were found to be completely protected against subsequent murine rotavirus infection as determined by the absence of viral shedding and by the lack of significant rises in serum or stool rotavirus antibody responses (28). Protection correlated with the titers of serum (24) and stool (10) rotavirus immunoglobulin A (IgA) and was found to diminish rapidly in genetically altered mice that were B cell deficient (11, 23). Not only did antibody appear to be necessary for protection, but even the resolution of rotavirus infection in immunologically normal mice correlated with the presence of CD4 cell-dependent antibody production (26). Thus, rotavirus antibody appeared to play a major role in immunity after oral inoculation of mice with live virus. CD8 cells were also found to have a major role in the resolution of rotavirus infection in mice (11, 23) and may have some role in protection as well, at least during the first weeks after oral, live-virus immunization (12). Similar to the results found in mice, CD8 cells appear to be important in the normal resolution of rotavirus infection in calves, while CD4 cells were crucial for normal antibody responses (30).
In the study reported here, we examined the protection against rotavirus infection after i.n. immunization with inactivated triple-layered (tl) and double-layered (dl) (i.e., lacking VP4 and VP7) rotavirus particles. These particles were of both homologous (murine) and heterologous (simian, bovine, and human) origin. All particles examined provided good protection, but the mechanism of this protection varied depending on the immunogen. Based on the degree of protection in BALB/c and in B-cell-deficient μMt mice stimulated by the different murine rotavirus particles, it appeared that tl murine rotaviruses protected at least partially through an antibody-dependent mechanism, while the dl particles appeared to protect through an antibody-independent mechanism.
MATERIALS AND METHODS
Mouse strains.
Two strains of mice were used in these studies. One was pathogen-free BALB/c which were purchased from Harlan-Sprague-Dawley when 6 weeks of age. No mouse had evidence of previous rotavirus infection as determined by serum rotavirus antibody titers. The other strain was genetically engineered and was unable to produce functional antibody. This strain was produced by Kitamura et al. (16) by using targeted disruption of a membrane exon of the gene encoding the μ-chain constant region (μMt mutation) in mouse embryonic stem cells. The transfected stem cell clone D3 was injected into blastocysts from C57BL/6 mice, and the derived offspring were backcrossed multiple times to C57BL/6 mice. These mice, containing a μMt mutation on a C57BL/6 background, were purchased as a breeding pair from Jackson Laboratories (Bar Harbor, Maine). Offspring of this pair were included in this study with the permission of K. Rajewsky. Experiments were conducted with adult mice (6 to 20 weeks of age). The μMt mice were found to produce no detectable rotavirus antibody.
Rotaviruses.
The murine EDIM strain of rotavirus used throughout these studies was originally obtained from M. Collins (Microbiological Associates, Bethesda, Md.) in 1980. To challenge mice after immunization, both wild-type (wt) virus from mouse stool and cell culture-adapted (passage 9) EDIM preparations were used. Because adult C57BL/6 mice were totally resistant to infection when inoculated with passage 9 of EDIM (i.e., no rotavirus shedding or immune responses were detected) but were susceptible to infection with wt EDIM based on development of rotavirus antibody, the wt strain was used to inoculate μMt mice which have a C57BL/6 background. However, since the passage 9 preparation has been used routinely for all previous studies with BALB/c mice in our laboratory since the inception of the adult mouse model for rotavirus (37), it was used to inoculate BALB/c mice. Even though adult C57BL/6 mice are more susceptible to wt EDIM, the two virus preparations were nearly equally infective in adult BALB/c mice; i.e., the doses required to infect 50% of the mice were 240 and 560 focus-forming units (FFU) for the wt and passage 9 preparations, respectively. The large number of mice required to conduct an infectivity study precluded conducting this analysis in μMt mice. Thus, the results found with μMt and BALB/c mice may not be directly comparable because of differences in the EDIM preparations used for challenge. It was observed, however, that rotavirus shedding in unimmunized μMt mice after inoculation with wt EDIM was comparable to that found in unimmunized BALB/c mice administered passage 9 of EDIM. The wt EDIM preparation used here was obtained from stools of infected neonatal mice and purified as described previously (23). The final preparation contained 107 FFU per ml. The passage 9 preparation of EDIM was obtained as previously reported (37) and contained 2 × 106 FFU/ml.
Immunization of mice was performed with inactivated particles generated from four different strains of rotavirus: the G3[P16] murine EDIM strain, the G3[P3] simian RRV strain (provided by Y. Hoshino, National Institutes of Health, Bethesda, Md., and triply plaque purified), the G6[P5] WC3 strain (provided by H. F. Clark, Children’s Hospital, Philadelphia, Pa., and triply plaque purified), and the G1[P8] human 89-12 strain (our laboratory). The EDIM strain used to generate the particles for immunization was a triply plaque purified isolate that had been passaged a total of 41 times in MA104 cells and purified by CsCl centrifugation (27). tl infectious virus (1.36 g/ml) and dl particles lacking VP4 and VP7 (1.38 g/ml) were collected from the same gradient. The dl particles were further treated with 10 mM EDTA and purified a second time in CsCl to ensure complete removal of VP4 and VP7 proteins. After purification, the virus particles were dialyzed against phosphate-buffered saline containing 20% glycerol and stored frozen at −70°C. To establish which proteins were present in the particles, each one was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described (27). Furthermore, Western blot analyses of the antibody generated after administration of these particles to mice by using methods already described (27) revealed no evidence for VP4 or VP7 in the dl particles, while the tl particles generated clear antibody responses to these proteins (results not shown). Purified tl or dl EDIM particles were inactivated by UV-psoralen treatment as described by Groene and Shaw (13). In short, the particles were dispensed into 60-mm petri dishes, and psoralen (4′-aminomethyltroxsalen HCl; Lee Biomolecular Research, Inc., San Diego, Calif.) was added at a concentration of 40 μg/ml. The virus was then placed in ice on a rotating platform at a distance of 10 cm and irradiated with high-intensity, long-wavelength UV light (365 nm, 40 min). After treatment, the viral titer was determined by a fluorescent focus assay as previously described (17). Although the infectivity of tl particles containing 8.4 × 108 FFU/ml was reduced below detectable limits (i.e., 102 FFU/ml) by 20 min of UV treatment, the irradiation time was extended to 40 min to ensure that no infectious virus remained. Evidence that these particles were not infectious was shown in two ways: no immune responses to nonstructural proteins were found after inoculation of mice with these particles by using Western blot analyses as previously described (27), and stool rotavirus IgA titers stimulated following inoculation with these particles were consistently at least 10-fold less than was found after oral infection with live EDIM (results not shown).
The other three viruses used for immunization were grown and processed in the same manner. For this study, these particles were all considered to be heterotypic relative to EDIM even though RRV and EDIM are both G3 strains and were found to share a very weak, one-way cross-neutralization response with hyperimmune antisera (38).
Properties of the LT adjuvant.
Escherichia coli heat-labile enterotoxin (LT) has been found to be an effective mucosal adjuvant with various antigens, including virus-like particles (VLPs) of rotavirus (31). wt LT is activated by protease cleavage in the intestine which simultaneously potentiates its toxigenic properties (6). A mutant LT [i.e., LT(R192G)] has been developed in which arginine 192 is replaced by glycine which eliminates the trypsin cleavage site and attenuates the protein (8). Only the R192G mutant of LT was used in this study. It was provided by John Clements and Candice Smith of Tulane University School of Medicine, New Orleans, La.
Study plan.
After collection of both blood (retroorbital capillary plexus puncture) and stool specimens (two pellets placed in 0.5 ml of Earle’s balanced salt solution [EBSS]) for rotavirus antibody measurements, groups of six or eight mice were immunized. i.n. immunization was performed under light sedation (i.e., mice were placed in a closed vessel with metafane until they could no longer stand). The immunogen was then given by gradual inoculation of the nares of mice (maximum of 25 μl/nostril). When two doses were administered, the second dose was given 2 weeks later. At 4 weeks after the final dose, blood and stool specimens were again collected for antibody measurements and 1 day later mice were orally (gavage) challenged with either 2 × 104 FFU of passage 9 EDIM (BALB/c) or 5 × 105 FFU of unpassaged EDIM (μMt). Stools were then collected for at least 7 days after challenge (two pellets/day from each mouse placed into 1 ml of EBSS) to be examined for viral shedding. At 3 weeks after challenge, blood and stool samples were again collected for antibody determinations.
To determine the effects of CD8 cell depletion prior to immunization, mice were injected intraperitoneally once per day with 1 mg of ammonium sulfate-precipitated monoclonal antibody preparation obtained from the rat hybridoma cell line 2.43 purchased from the American Type Culture Collection. Inoculations were initiated 5 days before immunization and were performed for four consecutive days and twice weekly thereafter until the end of the experiment. Depletion was monitored by using a fluorescence-activated cell sorter (FACS). This analysis was performed with mesenteric lymph node tissue collected from mice on the day of the first immunization and 21 days after EDIM challenge. Two control mice and two depleted mice were included in each analysis. Methods used for FACS analysis have been reported previously (23).
Oral immunization of mice was performed by gavage. Specimens were collected and mice were challenged by the same procedures as for i.n.-immunized mice.
Detection of rotavirus antigen in stool.
Stool pellets collected into EBSS on each day following EDIM challenge were stored frozen (−20°C). To test for rotavirus antigen, the specimens were thawed, homogenized, and analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described (26) with the following exception. Instead of basing antigen shedding directly on net optical density (A490) values, shedding was quantified from a standard curve by using CsCl-purified dl EDIM as the standard. Therefore, the quantities of rotavirus antigen shed were determined in micrograms of rotavirus protein for each stool specimen. Although rotavirus shedding was often not fully resolved in unimmunized mice until 9 days after challenge, the amount of rotavirus shed per mouse within a group was determined only during the first 7 days after challenge. Since detectable shedding in previously immunized mice was fully resolved by 7 days after challenge, the degree of protection due to immunization was somewhat greater than the values calculated. This protection was determined from the average number of micrograms of rotavirus antigen excreted for every mouse on each of the 7 days after challenge. These values were used for statistical comparison between groups of mice. In addition, the total amount of antigen shed for each group of mice was used to determine the percentage reduction in shedding relative to unimmunized control mice.
Determination of rotavirus antibody titers.
Serum rotavirus IgA and IgG and rotavirus stool IgA were measured by ELISA as previously described (23). Briefly, EIA plates (Corning Costar Co., Cambridge, Mass.) were coated overnight at 4°C with anti-rotavirus rabbit IgG. After being washed with phosphate-buffered saline plus 0.05% Tween 20, EDIM viral lysate and mock-infected cell lysate were each added to duplicate wells. Serial twofold dilutions of pooled sera from EDIM infected mice assigned concentrations of 160,000 or 10,000 U of rotavirus IgG or IgA per ml, respectively, were added to duplicate wells coated with either EDIM-infected or uninfected MA104 cell lysates to generate a standard curve. Serial 10-fold dilutions of mouse sera to be tested were also added to duplicate wells of each lysate. This was followed by a sequential addition of biotin-conjugated goat anti-mouse IgG or IgA (Sigma Chemical Co., St. Louis, Mo.), peroxidase-conjugated avidin-biotin (Vector Laboratories, Burlingame, Calif.), and o-phenylenediamine substrate (Sigma). Color development was stopped after 15 min with 1 M H2SO4, and the A490 was measured. Titers of rotavirus IgG or IgA, expressed as units per milliliter, were determined from the standard curve generated by subtraction of the average A490 values of the duplicate cell lysate wells from the average of the wells coated with EDIM lysate. The same methods were used to measure serum rotavirus IgG1 and IgG2a titers except for two modifications: (1) the biotinylated goat anti-mouse IgG used to detect bound rotavirus antibody was subtype specific (i.e., it specifically recognized mouse IgG1 or IgG2a), and (2) the concentration of rotavirus antibody was determined from a standard curve that measured rotavirus antibody in nanograms rather than in units per milliliter. To generate this curve, EIA plates were initially coated overnight with either goat anti-mouse IgG or normal goat serum. After the plates were washed, serial twofold dilutions of purified mouse IgG1 or IgG2a were added to the wells. The remaining steps were identical to those used to measure rotavirus-specific IgG1 or IgG2a.
For determination of stool rotavirus IgA, two stool pellets were collected into 0.5 ml of EBSS, homogenized, and centrifuged (1,500 × g, 5 min). Stool rotavirus IgA was then measured by the method described above.
Neutralizing antibody to EDIM was determined by using an antigen reduction ELISA assay as described previously (17).
Statistical methods.
All analyses were performed by using SAS version 6.12 (SAS Institute, Inc., Cary, N.C.). Differences in the mean quantities of rotavirus antigen shed per mouse were determined by analysis of variance since there were more than two groups being compared. The variances in the different groups were not equal. Therefore, the analyses were performed on the log transformations. The same procedure, analysis of variance, was used to determine whether there were differences in geometric mean titers of rotavirus antibody between groups of mice. Since there were numerous comparisons, an adjustment to the level of significance was made, and only when the P value is <0.01 are the differences reported.
RESULTS
Dose-dependent protection stimulated by homologous tl rotaviruses.
The initial experiment was to determine the level of protection against murine rotaviruses that could be stimulated by i.n. inoculation with the homologous strain of tl, inactivated rotavirus particles. Groups of BALB/c mice were administered one or two doses of psoralen/UV-inactivated rotavirus strain EDIM ranging from 10 to 0.1 μg/dose and challenged with live EDIM 4 weeks after the last immunization. Mice were monitored for antibody responses both after immunization and challenge and for shedding of rotavirus after challenge.
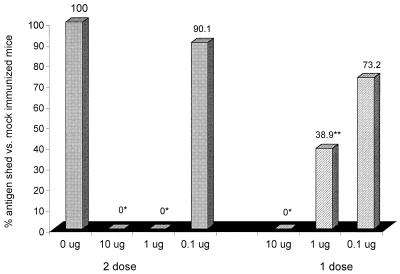
Immune responses after immunization were significantly associated with both the quantity of the immunogen and the number of doses as determined by serum rotavirus IgG and IgA and stool rotavirus IgA titers (Table 1). Only the highest concentration of immunogen administered in a two-dose regimen stimulated detectable stool rotavirus IgG or serum neutralizing antibody to EDIM. The quantity and number of doses were also significantly associated with protection against rotavirus shedding and immune responses to rotavirus after EDIM challenge. None of the mice immunized with one or two doses of 10 μg of EDIM or two doses of 1 μg shed detectable rotavirus antigen after challenge (Fig. 1), and none experienced a significant (i.e., ≥4-fold) boost in serum rotavirus IgG or IgA or stool rotavirus IgA titer. In contrast, one dose of 1 μg stimulated a partial but significant (P = 0.002) reduction in rotavirus shedding, and three of the eight mice in this group did not shed detectable rotavirus antigen or seroconvert. Finally, immunization with either one or two doses of 0.1 μg of rotavirus provided no significant protection.
TABLE 1.
Geometric mean titers (GMTs) of serum and stool rotavirus antibody after i.n. immunization with different doses of tl EDIMa
| No. of doses | Antigen per dose (μg) | GMTb
|
||||
|---|---|---|---|---|---|---|
| Serum
|
Stool
|
|||||
| IgG | IgA | NAc | IgG | IgA | ||
| 2 | 0 | <100 | <100 | <20 | <5 | <5 |
| 2 | 10 | 101,000 | 2,700 | 53 | 12 | 420 |
| 2 | 1 | 17,700 | 430 | <20 | <5 | 41 |
| 2 | 0.1 | 490 | 120 | <20 | <5 | <5 |
| 1 | 10 | 13,900 | 730 | <20 | <5 | 81 |
| 1 | 1 | 2,300 | 106 | <20 | <5 | 9 |
| 1 | 0.1 | <100 | <100 | <20 | <5 | <5 |
Groups of eight, 2-month-old female BALB/c mice were i.n. inoculated with one or two (separated by 2 weeks) doses of psoralen-inactivated, tl EDIM and orally challenged with 4 × 104 infectious EDIM (100 ID50) 4 weeks after the last immunization. Serum and stools analyzed for rotavirus antibody were collected on the day before challenge.
The limit of detection is shown by the “<” symbol, and this titer was used to determine GMTs where appropriate.
NA, neutralizing antibody to EDIM.
FIG. 1.
Dose-dependent protection after i.n. immunization with tl EDIM. Groups of mice were immunized and challenged as described in the legend to Table 1. Stools collected for the 7 days following challenge were quantified for rotavirus shedding by an ELISA. Shedding was compared to that found in mock-immunized control mice. ∗, P < 0.001 versus mock-immunized mice; ∗∗, P = 0.002 versus mock-immunized mice.
Protection stimulated by tl and dl homotypic and heterotypic rotavirus particles.
It was next determined whether protection in this model was dependent on the presence of homotypic VP4 and VP7 proteins, the outer capsid proteins of rotavirus responsible for stimulating neutralizing antibody. For this determination, either EDIM particles lacking these proteins were used for immunization (i.e., dl particles) or mice were immunized with heterotypic tl or dl rotavirus particles.
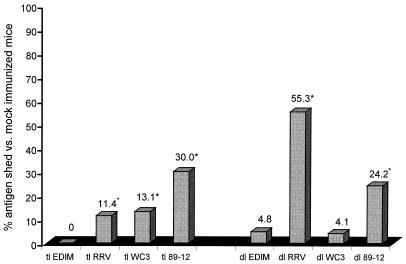
After two doses of 10 μg each of psoralen/UV-treated tl or dl EDIM or heterotypic rotavirus particles, the only rotavirus antibody response against dl particles that was significantly different from the response elicited by the same concentration of tl particles was stool IgA after WC3 inoculation (Table 2). As expected, serum neutralizing antibody to EDIM was undetectable following immunization with any particles except tl EDIM (results not shown). The level of protection stimulated by all dl particles and tl heterotypic particles was consistently less than that stimulated by tl EDIM particles as determined by antigen shedding after EDIM challenge and, in most cases, this difference was significant (P ≤ 0.01; Fig. 2). In contrast to results found after immunization with tl EDIM particles, none of the other particles (tl or dl) stimulated complete protection. Furthermore, there were no consistent differences between the protection stimulated by the two types of heterotypic particles. These results indicate that heterotypic tl and dl particles and homotypic dl EDIM particles consistently stimulate partial protection in this model, while homotypic tl EDIM particles induce complete protection.
TABLE 2.
Geometric mean titers (GMTs) of rotavirus antibodies stimulated by i.n. immunization with tl or dl particles of homotypic and heterotypic rotavirusesa
| Immunogen | GMT
|
||
|---|---|---|---|
| Serum
|
Stool (IgA) | ||
| IgG | IgA | ||
| None | <100 | <100 | <5 |
| tl EDIM | 49,900 | 1,970 | 78 |
| dl EDIM | 59,400 | 2,300 | 155 |
| tl RRV | 83,900 | 2,020 | 72 |
| dl RRV | 37,000 | 1,130 | 84 |
| tl WC3 | 78,200 | 1,780 | 37 |
| dl WC3 | 64,100 | 2,830 | 349b |
| tl 89-12 | 29,600 | 940 | 69 |
| dl 89-12 | 13,900 | 1,080 | 71 |
Groups of eight BALB/c mice immunized with two doses of 10 μg (separated by 2 weeks) of tl or dl rotavirus particles. Rotavirus antibody titers were determined in serum and stool specimens collected 4 weeks after the last immunization (i.e., 1 day before EDIM challenge).
Significantly (P < 0.001) greater than that stimulated by tl WC3.
FIG. 2.
Protection against EDIM shedding after i.n. immunization with homotypic and heterotypic dl and tl rotavirus particles. Mice immunized as stated in the legend of Table 1 were challenged with 4 × 104 infectious EDIM, and stool samples collected for the 7 days after challenge were quantified for rotavirus antigen. All particles provided significant (P < 0.001) protection from shedding relative to mock-immunized control mice. Significant (P ≤ 0.01) increases in shedding between groups relative to mice immunized with tl EDIM, are indicated by an asterisk.
Effect of E. coli LT(R192G) on immune responses and protection after i.n. immunization with rotavirus particles.
Mucosal adjuvants such as cholera toxin and E. coli heat-labile toxin (LT) have been found to stimulate increased immune responses after mucosal immunization with other antigens (9). To determine the effects of such adjuvants, mice were immunized i.n. with homotypic and heterotypic, inactivated, tl and dl particles in the presence of 10 μg of genetically attenuated E. coli LT [i.e., LT(R192G)], and both rotavirus antibody responses and protection were compared to that induced without LT(R192G). In this experiment, the concentration of tl EDIM used for immunization was reduced to 0.25 μg/dose in order to detect potential effects of LT(R192G) in protection.
Inclusion of attenuated LT(R192G) during immunization normally resulted in at least small and often significant increases in serum and stool rotavirus antibody titers (Table 3). In every case, the protection associated with inclusion of LT(R192G) was substantial but not always significant because of differences in shedding between individual mice (Table 4). This effect was most evident after tl EDIM was administered at low concentrations.
TABLE 3.
Effect of LT(192G) on rotavirus antibody responses after i.n. immunization with tl or dl particlesa
| Immunogen | GMT of rotavirus antibodies
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum IgG
|
Serum IgA
|
Stool IgA
|
|||||||
| +LT | −LT | +LT/−LT | +LT | −LT | +LT/−LT | +LT | −LT | +LT/−LT | |
| tl EDIM | 31,606 | 288 | 110b | 590 | 113 | 5.2b | 35 | 6 | 5.8b |
| dl EDIM | 345,918 | 92,292 | 3.7b | 3,673 | 3,108 | 1.2 | 561 | 280 | 2.0 |
| tl RRV | 150,315 | 74,027 | 2.0b | 2,290 | 1,497 | 1.5 | 158 | 148 | 1.1 |
| dl RRV | 209,530 | 36,551 | 5.7b | 1,668 | 1,131 | 1.5 | 265 | 84 | 3.6 |
| tl WC3 | 244,196 | 78,215 | 3.1b | 2,191 | 1,776 | 1.2 | 382 | 37 | 10.3b |
| dl WC3 | 212,488 | 64,119 | 3.3b | 3,835 | 2,831 | 1.4 | 398 | 349 | 1.1 |
| tl 89-12 | 60,146 | 29,633 | 2.0 | 1,212 | 942 | 1.3 | 58 | 69 | 0.8 |
| dl 89-12 | 92,753 | 13,940 | 6.7b | 2,116 | 1,084 | 2.0 | 255 | 71 | 3.6b |
Groups of eight BALB/c mice were given two i.n. immunizations with 10 μg/dose of each particle except for tl EDIM at 0.25 μg/dose. Titers were determined 4 weeks after the second immunization. GMT, geometric mean titer.
Significant (P ≤ 0.004) increases due to the inclusion of LT(R192G).
TABLE 4.
Effect of LT(R192G) during i.n. immunization on rotavirus shedding after challengea
| Immunogen | Rotavirus shedding (% antigen shed vs mock-immunized mice)
|
Pb | |
|---|---|---|---|
| −LT(R192G) | +LT(R192G) | ||
| tl EDIMc | 67.0 | 2.7 | <0.001 |
| dl EDIM | 4.8 | 0.6 | NS |
| tl RRV | 11.5 | 1.5 | 0.003 |
| dl RRV | 55.3 | 1.1 | <0.001 |
| tl WC3 | 13.1 | 2.1 | 0.01 |
| dl WC3 | 4.1 | 1.1 | 0.01 |
| tl 89-12 | 30.0 | 0.0 | NS |
| dl 89-12 | 24.4 | 6.1 | NS |
Mice immunized as described in the legend to Table 3 were challenged 4 weeks after the second immunization with EDIM, and the shedding of rotavirus antigen was determined by ELISA during the subsequent 7 days.
Significance of difference between mice immunized with or without LT(R192G). NS, not significant.
Groups administered tl EDIM were immunized with 0.25 μg/dose, while other groups were given 10 μg/dose.
Immune responses and protection induced by oral immunization with inactivated rotavirus particles.
Although only small volumes of immunogen were administered in these studies and mice were anesthetized during immunization, it was possible that some of the responses were due to intestinal exposure after swallowing a portion of the inoculum. To determine the possible effects of intestinal exposure, the immunogens were administered by oral gavage either with or without LT(R192G). Rotavirus antibody responses stimulated by oral immunization with rotavirus particles were almost always significantly less (P ≤ 0.004) than that found after i.n. immunization, including stool IgA (Table 5). Even so, oral immunization, even without LT(R192G), resulted in significant (P < 0.001) reductions in shedding and inclusion of LT(R192G) caused further significant (P < 0.001) decreases in shedding with all three antigens tested (Table 6). In five of six cases, the level of protection was less than after i.n. immunization under the same conditions, and in three of these cases the difference was significant (P < 0.001). These results indicate that at least some of the protection observed after i.n. immunization was not due to intestinal exposure but do not rule out the possibility that swallowing a portion of the i.n. inoculum contributed to protection.
TABLE 5.
A comparison of rotavirus antibody responses after i.n. versus oral immunizationa
| Immunogen | GMT of rotavirus antibodies
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum IgG
|
Serum IgA
|
Stool IgA
|
|||||||
| i.n. | Oral | i.n./oral | i.n. | Oral | i.n./oral | i.n. | Oral | i.n./oral | |
| tl EDIM | 71,020 | 16,707 | 4.3b | 2,311 | 485 | 4.8b | 182 | 24 | 7.6b |
| tl EDIM + LT | 362,687 | 49,905 | 7.3b | 882 | 816 | 1.1 | 131 | 97 | 1.4 |
| dl EDIM | 92,292 | 4,614 | 20.0b | 3,108 | 376 | 8.3b | 280 | 17 | 16.5b |
| dl EDIM + LT | 345,918 | 7,917 | 43.7b | 3,673 | 302 | 12.2b | 561 | 67 | 8.4b |
| tl RRV | 74,027 | 843 | 87.8b | 1,497 | 129 | 11.6b | 148 | 5 | 29.6b |
| tl RRV + LT | 150,315 | 10,861 | 13.8b | 2,290 | 439 | 5.2b | 158 | 42 | 3.8b |
Groups of eight mice were administered two doses (10 μg/dose) of each antigen either orally or by i.n. inoculation either with or without 10 μg of LT(R192G) per dose, and the rotavirus antibody responses were measured 4 weeks after the second inoculation. GMT, geometric mean titer.
Significantly (P ≤ 0.004) greater after i.n. versus oral immunization.
TABLE 6.
Protection after oral versus i.n. immunization with or without LT(R192G)a
| Immunogen | LT | Rotavirus shedding (% antigen shed vs. mock-immunized mice)
|
Pb | |
|---|---|---|---|---|
| i.n. | Oral | |||
| tl EDIM | − | 0.0c | 6.6c | <0.001 |
| tl EDIM | + | 0.0c | 0.0cd | NS |
| dl EDIM | − | 4.8c | 39.4c | <0.001 |
| dl EDIM | + | 0.6c | 1.7cd | NS |
| tl RRV | − | 11.5c | 85.1 | <0.001 |
| tl RRV | + | 1.5c | 14.6cd | NS |
Groups of eight BALB/c mice were given two oral (gavage) or i.n. immunizations (10 μg/dose) either without or with LT(R192G) (10 μg) and challenged with EDIM 4 weeks after the last dose. Shedding of rotavirus antigen was determined by ELISA during the subsequent 7 days.
Significance of difference between orally and i.n. immunized mice. NS, not significant.
Significantly (P < 0.001) less shedding than in mock-immunized mice.
Significantly (P ≤ 0.003) less shedding than in mice orally immunized without LT(R192G).
Differences in protection stimulated by tl EDIM versus other rotavirus particles is accentuated at reduced doses.
Based on the level of protection stimulated by two i.n. immunizations with 10 μg of rotavirus particles, tl EDIM was superior to the other particles. Because protection associated with tl EDIM immunization was dose dependent (Fig. 1), it was of interest to determine whether protection stimulated by other rotavirus particles also decreased when the dose was reduced 10-fold, possibly increasing the difference in the levels of protection stimulated by tl EDIM and other rotavirus particles in this model system. This possibility was tested by using dl EDIM and tl RRV as immunogens, both in the absence and in the presence of LT(R192G).
The rotavirus antibody responses stimulated with 1 μg of dl EDIM per dose were comparable to those induced with 10 μg/dose; however, those induced by the lower concentration of tl RRV were consistently less (2.1- to 12.5-fold) after immunization with the lower quantity of antigen (results not shown). Protection was consistently less with the lower dose and was particularly evident in mice immunized without LT(R192G) (Table 7). Therefore, differences in the level of protection between tl EDIM and other rotavirus particles was even more evident after two doses when the dosage was reduced to 1 μg. At this dose, tl EDIM still provided 100% protection, even without adjuvant (Fig. 1).
TABLE 7.
Comparative protection induced by 10-fold lower doses of dl EDIM and tl RRV during i.n. immunizationa
| Immunogen | LT | Rotavirus shedding (% antigen shed vs mock-immunized mice) at:
|
Pb | |
|---|---|---|---|---|
| 10 μg/dose | 1 μg/dose | |||
| dl EDIM | − | 4.8 | 31.9 | <0.001 |
| dl EDIM | + | 0.6 | 2.5 | NS |
| tl RRV | − | 11.4 | 65.5 | <0.001 |
| tl RRV | + | 1.5 | 5.5 | NS |
Groups of eight BALB/c mice were given two i.n. doses of either 1 or 10 μg/dose and challenged 4 weeks after the second dose with EDIM. Shedding of rotavirus antigen was measured during the following 7 days.
Significance of differences between mice given 1 or 10 μg/dose. NS, not significant.
Ratios of rotavirus IgG1/IgG2a stimulated by different rotavirus particles.
The degrees of protection found after i.n. immunization with rotavirus particles in the experiments already described showed distinct differences between that stimulated by homotypic tl EDIM particles versus those induced by heterotypic tl rotavirus particles and all dl particles. This suggested that the mechanisms of protection associated with the two groups of particles may be different. Likewise, inclusion of LT(R192G) as adjuvant with all particles dramatically increased their protective effects, a result possibly also due to mechanistic differences.
The processes by which protection is induced after immunization or natural infection rely on the production of immunomodulating cytokines. The induced cytokines direct the development of immune responses along specific pathways (29). Key differences in pathways often concern the relative dominance of T-helper-cell subclasses, TH1 and TH2. One outcome of TH1 induction is the eventual production of IgG2a, while TH2 responses are associated with IgG1 production. The ratios of IgG1/IgG2 have, therefore, been used in numerous studies to determine whether immunity is primarily associated with TH1 or TH2 responses. Therefore, IgG1/IgG2a ratios were measured after immunization with different rotavirus particles, either with or without LT(R192G), as surrogate markers of the relative induction of TH1 and TH2 responses.
Although differences in the IgG1/IgG2a ratios were found following immunization with the different particles, no consistent pattern was evident. In three different experiments, the ratio found after immunization with two doses of tl EDIM was consistently 0.5, and this ratio varied little with either dose number or quantity (Table 8). Likewise, i.n. immunization with dl EDIM stimulated an almost identical IgG1/IgG2a ratio. Heterotypic tl particles stimulated various IgG1/IgG2a ratios ranging from 0.2 to 2.4, but neither the ratio nor actual quantity of either IgG subclass could be associated with increased protection. Inclusion of LT(R192G) during immunization caused increases in the geometric mean titers in both IgG subclasses in every case but no consistent change in IgG1/IgG2a ratios. For example, LT(R192G) caused the ratio of IgG subclasses to decrease from 2.4 to 2.0 for tl RRV and increase from 0.2 to 0.9 for tl 89-12. Therefore, possible differences in the mechanism of protection induced by different particles or due to the inclusion of LT(R192G) could not be detected by changes in IgG1/IgG2a ratios.
TABLE 8.
Ratios of serum rotavirus IgG1/IgG2a stimulated by different rotavirus particles either with or without LT(R192G)a
| Immunogen | LT(R192G) | GMT of IgG subclasses (μg/ml)
|
||
|---|---|---|---|---|
| IgG1 | IgG2a | IgG1/IgG2ab | ||
| tl EDIM | − | 16.8 | 32.8 | 0.51 |
| dl EDIM | − | 25.8 | 45.1 | 0.57 |
| dl EDIM | + | 315.5 | 269.8 | 1.17 |
| tl RRV | − | 97.5 | 40.5 | 2.41 |
| tl RRV | + | 242.1 | 120.8 | 2.00 |
| tl WC3 | − | 46.6 | 106.4 | 0.44 |
| tl WC3 | + | 297.6 | 330.1 | 0.90 |
| tl 89-12 | − | 9.4 | 47.5 | 0.20 |
| tl 89-12 | + | 64.8 | 57.0 | 1.14 |
Mice (eight animals/group) were immunized i.n. with two doses of 10 μg/dose. Rotavirus IgG subclasses in serum were measured at 4 weeks after the second immunization. GMT, geometric mean titer.
Ratio of GMTs for the IgG subclasses.
Increased protection stimulated by tl EDIM was associated with antibody, while most protection induced by dl EDIM appeared to be independent of antibody.
Mechanistic studies on rotavirus immunity conducted to date have all suggested that rotavirus antibody plays at least some role. For example, it was found that the normal, lifelong protection against rotavirus infection in mice induced by a single oral immunization with live murine rotavirus (28) was not obtained in B-cell-deficient JHD or μMt mice (references 11 and 23 and unpublished observations). To determine the importance of antibody after i.n. immunization with inactivated rotavirus particles, shedding of rotavirus antigen was compared after EDIM challenge of immunologically normal BALB/c and B-cell-deficient μMt mice.
The total protection from shedding observed after immunization with tl EDIM, either with or without LT(R192G), in BALB/c mice was lost in μMt mice and simulated that found with dl EDIM and heterotypic tl and dl particles in BALB/c mice (Table 9; see also Table 4). In contrast, the degree of protection induced by dl EDIM particles was similar in BALB/c and μMt mice, i.e., protection after immunization in the presence of LT(R192G) was nearly identical in the two mice strains while protection was slightly but significantly (P < 0.001) less in μMt mice immunized without adjuvant. Thus, the added protection due to the presence of VP4 and VP7 proteins in EDIM particles after i.n. immunization of BALB/c mice was lost in the absence of antibody. However, protection stimulated by EDIM particles that lacked these proteins occurred in the absence of antibody.
TABLE 9.
Comparative protection stimulated by EDIM particles in BALB/c and μMT micea
| Immunogen | LT | Rotavirus shedding (% antigen shed vs mock-immunized mice)
|
Pb | |
|---|---|---|---|---|
| BALB/c | μMT | |||
| tl EDIM | − | 0.0 | 22.6 | <0.001 |
| tl EDIM | + | 0.0 | 0.5 | NS |
| dl EDIM | − | 4.8 | 23.1 | <0.001 |
| dl EDIM | + | 0.6 | 0.2 | NS |
Mice (eight animals per group for BALB/c and six animals per group for μMt) were administered two doses (10 μg/dose) of EDIM particles and challenged with EDIM 4 weeks after the second immunization. Shedding was measured during the 7 days after challenge.
Significance of differences between mice groups. NS, not significant.
CD8 cell depletion did not alter the level of protection following i.n. immunization.
The final experiments in this study were to determine the importance of CD8 T cells in protection after i.n. immunization with tl EDIM particles. BALB/c mice depleted of CD8 cells with anti-CD8 cell antibody prior to immunization and kept depleted throughout the remainder of the experiment maintained complete protection against rotavirus shedding after EDIM challenge (Table 10). Similarly, μMt mice depleted of CD8 cells were protected to the same extent against rotavirus shedding as were the nondepleted mice. This experiment was repeated using either tl or dl EDIM particles as immunogens and incorporated LT(R192G) as adjuvant during i.n. immunization of both BALB/c and μMt mice. As expected, tl particles stimulated 100% protection against EDIM shedding in BALB/c mice and nearly complete protection (i.e., >99% reduction in EDIM shedding) in μMt mice (results not shown). Also, as expected, dl particles with LT(R192G) stimulated >99% protection in both strains of mice as well. Depletion of CD8 cells beginning 5 days before challenge caused no loss in protection (results not shown). Therefore, the absence of CD8 cells in either BALB/c or B-cell-deficient μMt mice did not alter the degree of protection afforded by i.n. immunization with tl or dl EDIM.
TABLE 10.
Effect of CD8 cell depletion on protective responses stimulated by tl EDIM particlesa
| Mouse strain | Rotavirus shedding (% antigen shed vs mock-immunized controls)
|
|
|---|---|---|
| Nondepleted | CD8 cell depleted | |
| BALB/c | 0.0 | 0.0 |
| μMt | 22.6 | 22.9 |
Groups of six (μMt) or eight (BALB/c) mice either were not inoculated or were inoculated daily with anti-CD8 monoclonal antibody (2.43) by using 1 mg/dose beginning 4 days prior to immunization. On the fifth day, mice were administered 10 μg of tl EDIM i.n., and anti-CD8 injections were continued twice a week for the duration of the study. Mice were administered a second i.n. dose of tl EDIM 2 weeks after the first dose, and 4 weeks later were challenged with EDIM. Shedding of rotavirus antigen was measured during the subsequent 7 days. The levels of CD8 cell depletion were measured on the day of immunization and 21 days after challenge and were found to be >98% in the mesenteric lymph nodes of both BALB/c and μMt mice.
DISCUSSION
Rotaviruses are ubiquitous pathogens that infect the mature enterocytes on the tips of the intestinal villi, subsequently leading to gastrointestinal disease, particularly severe diarrhea. Protection in both humans and animals is believed to be due to the presence of intestinal immunological effectors and, in some instances, protection has been correlated with intestinal antibody (7, 10, 21). Therefore, immunization schemes that have the greatest probability of stimulating intestinal immune responses are the most likely to stimulate protection against rotavirus. Based on evidence for a common mucosal immune system (22, 39), i.n. immunization with rotavirus antigens may be a feasible method for protecting against rotavirus disease.
To evaluate the possible protective effects of i.n. immunization against rotavirus, we have used the adult mouse model which we developed for studies on active immunity (37). Mice have been found to be protected against reinfection with rotavirus for at least 14 months after oral immunization with live murine rotavirus (28). Therefore, we chose to immunize i.n. with inactivated rotavirus in order to avoid confounding effects of potential intestinal replication. Furthermore, we chose to anesthetize the mice during immunization to limit the quantity of antigen that was swallowed. Finally, we have evaluated the potential stimulating effects of a mucosal adjuvant [E. coli LT(R192G)] on both immune responses and protection. A similar study was reported by O’Neal et al. following immunization with VLPs of rotavirus (32).
Using these methods, we found that two i.n. immunizations with tl murine rotavirus strain EDIM, with as little as 1 μg of inactivated virus per dose, provided complete protection against rotavirus shedding after an oral challenge with a 50% infectious dose (ID50) of EDIM of ca. 100. Furthermore, when lower doses of immunogen were administered and only partial protection against shedding was obtained, the level of shedding was greatly reduced by the inclusion of LT(R192G) during immunization. Immunization with inactivated EDIM particles that lacked their outer capsid proteins (i.e., dl particles) as well as heterotypic tl and dl rotavirus particles of nonmurine origin also stimulated protection against EDIM shedding. However, the level of protection was reduced relative to tl EDIM at comparable doses. As found with tl EDIM, protection was also increased with these particles by the inclusion of LT(R192G) during immunization.
The mechanism by which LT(R192G) increases protection is unclear. Suggestions include, among others, enhanced mucosal permeability (20), selective induction of TH2-mediated antibody responses (35), induction of antigen-specific T-cell responses (15, 40), and increased antigen presentation (4). In our studies, we observed that both serum and intestinal (i.e., stool) antibody responses stimulated by i.n. immunization were enhanced by the inclusion of LT(R192G), an observation also reported by O’Neal et al. (31, 32) after immunization with VLPs with either LT, LT(R192G), or cholera toxin. However, higher titers of any specific rotavirus antibody measured could not be correlated with protection of individual mice. These findings suggested the possibility that rotavirus antibodies may only be markers of protection and not the actual effectors.
The immune responses and protection generated by immunization are, to a large extent, determined by the T-helper-cell subset that dominates the response (29). This is reflected in the titers of IgG1 and IgG2 subtypes stimulated by TH2 and TH1 responses, respectively. The increased rotavirus IgG responses and protection observed after i.n. immunization with LT(R192G), however, was not reflected in a consistent preferential increase in IgG subtype. Therefore, selective increases in either a TH1 or TH2 response could also not be associated with increased immunity due to LT(R192G). It should be noted that levels of TH1 and TH2 in serum as determined by the surrogate markers used here may not reflect the levels of these helper cells in the intestinal mucosa where rotavirus replication occurs.
The greater protection stimulated by i.n. immunization with inactivated tl EDIM relative to either dl EDIM or tl or dl heterotypic rotaviruses following a subsequent EDIM challenge indicated that immune responses to homotypic VP4 and VP7 proteins resulted in increased protection. This was not unexpected and was likely due to stimulation of serotype-specific neutralizing antibody. If this is the case, the titer of this antibody required for protection may be quite low because even serum neutralizing antibody to EDIM was detected only after immunization with two doses of the highest quantity of tl EDIM particles (i.e., 10 μg). Immunization with lesser doses or a 10-fold reduction in antigen still resulted in complete protection but no detectable serum neutralizing antibody. Although not examined in this study, intestinal neutralizing antibody concentrations were expected to be even lower based on the relative concentrations of serum and stool rotavirus IgA and IgG observed after i.n. immunization. Previous studies using oral immunization of mice with live rotaviruses revealed associations between intestinal rotavirus IgA and protection (10) but no association with neutralizing antibody (38), possibly due to its instability, low concentrations, or the insensitivity of the assay.
Protection stimulated by rotavirus particles other than tl EDIM in this study appeared to be due to a mechanism other than neutralizing antibody. dl particles and heterotypic tl particles should not stimulate neutralizing antibody to EDIM, and none was found. Furthermore, the levels of protection stimulated by these particles were comparable and somewhat less than that induced by tl EDIM immunization. Since total rotavirus IgA and IgG titers stimulated by these particles were comparable to those induced by tl EDIM following immunization with the same concentrations of antigen, differences in protection were not due to generally poorer immune responses. Finally, these differences also appeared to not be due to variations in TH1 vs. TH2 responses since IgG1/IgG2a ratios after immunization were similar for all the particles tested.
To examine the importance of rotavirus antibody in general and neutralizing antibody to EDIM in particular following i.n. immunization with different rotavirus particles, protection was measured in B-cell-deficient μMt mice. Shedding of rotavirus antigen in these mice after i.n. immunization with dl EDIM with LT(R192G) was comparable to that found in immunologically normal BALB/c mice. However, in the absence of LT(R192G), dl EDIM stimulated somewhat better protection (P < 0.001) in BALB/c than in μMt mice. This suggests the possibility that antibody may play some role in protection after inoculation with these particles but inclusion of LT(R192G) during immunization masked this role. Antibody appeared to play a more important role in protection following immunization with tl EDIM particles because the additional protection stimulated in BALB/c mice due to the presence of homotypic VP4 and VP7 proteins was lost in μMt mice. This result not only supports the suggestion that the additional protection in BALB/c mice was due to serotype-specific neutralizing antibody but indicates that protection by dl EDIM and, by extension, dl and tl heterotypic rotaviruses occurs in the absence of antibody.
Several studies with the adult mouse model have shown that CD8 cells, the major effectors of CTL activity, are involved in the resolution of rotavirus shedding in BALB/c mice (11, 12, 23). It has also been suggested that these cells may play a role in the subsequent protection against rotavirus reinfection in this model (12). Therefore, it was of interest to determine whether CD8 cells were required for protection following i.n. immunization with inactivated rotaviruses. These studies were initially performed with tl EDIM without adjuvant as the i.n. immunogen which should elicit protection by at least two separate mechanisms based on the results already discussed. No loss of protection was found in either BALB/c or μMt mice after CD8 cell depletion, even when depletion was initiated before immunization and maintained throughout the course of the experiment. Furthermore, the level of CD8 cell depletion was 98% in both strains of mice, supporting the conclusion that these cells were not needed for protection. The same conclusions were obtained during a subsequent study in which dl or tl EDIM particles with LT(R192G) were administered i.n. to either BALB/c or μMt mice. Lack of involvement of CD8 cells after i.n. immunization with inactivated rotavirus is in agreement with the proposed mechanism by which CD8 cell stimulation occurs, i.e., internal processing of antigen followed by MHC-1-associated presentation of the processed peptide to the cognate CD8 cells (14).
If B cells and CD8 cells are not required for protection following i.n. immunization with rotavirus particles, this leaves only one established immunological effector of memory-associated protection, i.e., CD4 cells. These cells are known to both produce and stimulate the production of cytokines that block rotavirus replication (1). Studies that use anti-CD4 cell depletion designed to directly determine the involvement of CD4 cells in protection after i.n. immunization are in progress. Previous results obtained with this method have shown that CD4 cells are needed for the complete normal resolution of rotavirus shedding in BALB/c mice (26). We now anticipate that they will be shown to be a major effector of protection after i.n. immunization. It should be noted that protection did not diminish when mice were challenged at 3 months rather than 1 month following i.n. immunization with either tl or dl EDIM particles plus LT(R192G) (results not shown). Therefore, if CD4 cells are responsible for protection, they must be retained for extended periods as memory cells at an inducible site in sufficient concentrations to provide the rapid and dramatic reduction in rotavirus shedding observed in this study.
The finding that protection following i.n. immunization with inactivated dl EDIM was not dependent on antibody contrasts with results found after oral immunization with live rotavirus. Immunologically normal mice are fully protected against reinfection for at least 14 months after oral inoculation with live murine rotavirus (28), whereas B-cell-deficient mice are susceptible to reinfection within weeks following this method of immunization (11, 23). Although protection could not be associated with the presence of serotype-specific neutralizing antibody following oral immunization (38), it may play some role as suggested from the results found after i.n. immunization with tl homotypic rotavirus in this study. Also of interest was the finding that oral immunization with live heterotypic rotaviruses generally provided little protection against subsequent murine rotavirus infection (38), while i.n. immunization with inactivated dl or tl heterotypic rotaviruses in the presence of LT(R192G) provided excellent protection equivalent to that induced by dl EDIM. Finally, it should be noted that inactivated tl and dl EDIM administered intramuscularly with the adjuvant (QS-21) both stimulated excellent protection against EDIM infection but that the tl particles were more effective (25). Taken together, these results indicate that inclusion of homotypic VP4 and VP7 during immunization by any of the routes examined produced increased protection, possibly due to neutralizing antibody production. However, particles lacking these homotypic proteins also stimulated excellent protection when administered with an effective adjuvant. Furthermore, protection induced by particles lacking homotypic VP4 and VP7 proteins may not depend on antibody, as shown in this study for i.n. immunization.
It should be noted that oral immunization with inactivated dl or tl EDIM and tl RRV also stimulated rotavirus antibody responses and substantial protection in this study, particularly when LT(R192G) was included during immunization. Interestingly, this protection was much greater than that found after oral immunization with larger doses of VLPs in the presence of cholera toxin even though the ID50 used to challenge mice in that study was 10-fold less than in our study (32). Reasons for these different levels of protection following oral immunization are unclear, especially since protection levels stimulated by i.n. immunization with inactivated dl particles and VLPs were very similar (reference 32 and this study). Although neither serum nor stool rotavirus antibody responses were as great after oral immunization with inactivated particles as that found after i.n. immunization with the same dosages of antigen and although protection was significantly reduced as well, oral immunization with inactivated rotavirus could have been partially responsible for the protection induced by i.n. immunization in this study. The finding that oral immunization with inactivated rotavirus results in protection also suggests the possibility that sequential i.n. and oral immunization will be an even more effective method of stimulating protection against this mucosal pathogen than either route individually. This remains to be determined.
ACKNOWLEDGMENTS
This work was funded in part by NIH NIAID contract NO1 AI-45242 to Children’s Hospital Medical Center.
The LT(R192G) was generously provided by John Clements and Candice Smith of Tulane University School of Medicine, New Orleans, La.
REFERENCES
- 1.Bass D M. Interferon gamma and interleukin 1, but not interferon alfa, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology. 1997;113:81–89. doi: 10.1016/S0016-5085(97)70083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein D I, Glass R I, Rodgers G, Davidson B L, Sack D A for the U.S. Rotavirus Vaccine Efficacy Group. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in U.S. children. J Am Med Assoc. 1995;273:1191–1196. [PubMed] [Google Scholar]
- 3.Bernstein D I, Smith V E, Sander D S, Pax K A, Schiff G M, Ward R L. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990;162:1055–1062. doi: 10.1093/infdis/162.5.1055. [DOI] [PubMed] [Google Scholar]
- 4.Bromander A K, Kjerrulf M, Holmgren J, Lycke N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand J Immunol. 1993;37:452–458. doi: 10.1111/j.1365-3083.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 5.Clark H F, Offit P A, Ellis R W, Eiden J J, Krah D, Shaw A R, Pichichero M, Treanor J J, Borian F E, Bell L M, Plotkin S A. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. J Infect Dis. 1996;174:S73–S80. doi: 10.1093/infdis/174.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 6.Clements J D, Finkelstein R A. Isolation and characterization of homogenous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979;24:760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulson, B. S., K. Grimwood, I. L. Hudson, G. L. Barnes, and R. F. Bishop. 1992. Role of coproantibody in clinical protection of children during reinfection with rotavirus. 30:1678–1684. [DOI] [PMC free article] [PubMed]
- 8.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson B L, Clements J D. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press, Inc.; 1996. pp. 73–87. [Google Scholar]
- 10.Feng N, Burns J W, Bracey L, Greenberg H B. Comparison of the mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with homologous or heterologous rotaviruses. J Virol. 1994;68:7766–7773. doi: 10.1128/jvi.68.12.7766-7773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco M A, Greenberg H B. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–7806. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco M A, Tin C, Greenberg H B. CD8+ T cells can mediate almost complete short-term and partial long-term immunity to rotavirus in mice. J Virol. 1997;71:4165–4170. doi: 10.1128/jvi.71.5.4165-4170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groene W S, Shaw R D. Psoralen preparation of antigenically intact noninfectious rotavirus particles. J Virol Methods. 1992;38:93–102. doi: 10.1016/0166-0934(92)90172-a. [DOI] [PubMed] [Google Scholar]
- 14.Hahn Y S, Yang B, Braciale T J. Regulation of antigen processing and presentation to class I MHC restricted CD8+ T lymphocytes. Immunol Rev. 1996;151:31–49. doi: 10.1111/j.1600-065x.1996.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 15.Hornquist E, Lycke N. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur J Immunol. 1993;23:2136–2143. doi: 10.1002/eji.1830230914. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 17.Knowlton D R, Spector D M, Ward R L. Development of an improved method for measuring neutralizing antibody to rotavirus. J Virol Methods. 1991;33:127–134. doi: 10.1016/0166-0934(91)90013-p. [DOI] [PubMed] [Google Scholar]
- 18.Lanata C F, Midthun K, Black R E, Butron B, Huapaya A, Penny M E, Ventura G, Gil A, Jett-Goheen M, Davidson B L. Safety, immunogenicity, and protective efficacy of one and three doses of tetravalent rhesus rotavirus vaccine in infants in Lima, Peru. J Infect Dis. 1996;174:268–275. doi: 10.1093/infdis/174.2.268. [DOI] [PubMed] [Google Scholar]
- 19.Liu M A. Vaccine developments. Nat Med. 1998;4:515–519. doi: 10.1038/nm0598supp-515. [DOI] [PubMed] [Google Scholar]
- 20.Lycke N, Karlsson U, Sjolander A, Magnusson K-E. The adjuvant effect of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991;33:691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 21.Matson D O, O’Ryan M L, Herrera I, Pickering L K, Estes M K. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993;167:577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 22.McDermott M R, Bienenstock J. Evidence for a common mucosal immunologic system. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 23.McNeal M M, Barone K S, Rae M N, Ward R L. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology. 1995;214:387–397. doi: 10.1006/viro.1995.0048. [DOI] [PubMed] [Google Scholar]
- 24.McNeal M M, Broome R L, Ward R L. Active immunity against rotavirus infection in mice is correlated with viral replication and titers of serum rotavirus IgA following vaccination. Virology. 1994;204:642–650. doi: 10.1006/viro.1994.1579. [DOI] [PubMed] [Google Scholar]
- 25.McNeal M M, Rae M N, Conner M E, Ward R L. Stimulation at local immunity and protection in mice by intramuscular immunization with triple- or double-layered rotavirus particles and QS-21. Virology. 1998;243:158–166. doi: 10.1006/viro.1998.9060. [DOI] [PubMed] [Google Scholar]
- 26.McNeal M M, Rae M N, Ward R L. Evidence that resolution of rotavirus infection in mice is due to both CD4 and CD8 cell-dependent activities. J Virol. 1997;71:8735–8742. doi: 10.1128/jvi.71.11.8735-8742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeal M M, Sheridan J F, Ward R L. Active protection against rotavirus infection of mice following intraperitoneal immunization. Virology. 1992;191:150–157. doi: 10.1016/0042-6822(92)90176-p. [DOI] [PubMed] [Google Scholar]
- 28.McNeal M M, Ward R L. Long-term production of rotavirus antibody and protection against reinfection following a single infection of neonatal mice with murine rotavirus. Virology. 1995;211:474–480. doi: 10.1006/viro.1995.1429. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretions lead to different functional properties. Annu Rev Immunol. 1989;7:145–174. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 30.Oldham G, Bridger J C, Howard C J, Parsons K R. In vivo role of lymphocyte subpopulation in the control of virus excretion and mucosa antibody responses of cattle infected with rotavirus. J Virol. 1993;67:5012–5019. doi: 10.1128/jvi.67.8.5012-5019.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Neal C M, Clements J D, Estes M K, Conner M E. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J Virol. 1998;72:3390–3393. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rennels M B, Glass R I, Dennehy P H, Bernstein D I, Pichichero M E, Zito E T, Mack M E, Davidson B L, Kapikinn A Z for the U.S. Rotavirus Vaccine Efficacy Group. Safety and efficacy of high dose rhesus-human reassortant rotavirus vaccines: Report of the national multicenter trial. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 34.Santosham M, Letson G W, Wolff M, Reid R, Gahagan S, Adams R, Callahan C, Sack R B, Kapikinn A Z. A field study of the safety and efficacy of two candidate vaccines in a Native American population. J Infect Dis. 1991;163:483–487. doi: 10.1093/infdis/163.3.483. [DOI] [PubMed] [Google Scholar]
- 35.Snider D P, Marshall J S, Perdue M H, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;53:647–657. [PubMed] [Google Scholar]
- 36.Vesikari T. Clinical trials of live, oral rotavirus vaccines: the Finnish experience. Vaccine. 1993;11:255–261. doi: 10.1016/0264-410x(93)90026-t. [DOI] [PubMed] [Google Scholar]
- 37.Ward R L, McNeal M M, Sheridan J F. Development of an adult mouse model for studies on protection against rotavirus. J Virol. 1990;64:5070–5075. doi: 10.1128/jvi.64.10.5070-5075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward R L, McNeal M M, Sheridan J F. Evidence that active protection following oral immunization of mice with live rotavirus is not dependent on neutralizing antibody. Virology. 1992;188:57–66. doi: 10.1016/0042-6822(92)90734-7. [DOI] [PubMed] [Google Scholar]
- 39.Weisz-Carrington P, Roux M E, McWilliams M, Phillips-Quagliata J M, Lamm M E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979;123:1705–1708. [PubMed] [Google Scholar]
- 40.Xu-Amano J, Jackson R J, Fujihashi K, Kiyono H, Staats H F, McGhee J R. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine. 1994;12:903–911. doi: 10.1016/0264-410x(94)90033-7. [DOI] [PubMed] [Google Scholar]