Abstract
Background
The opioid crisis continues in full force, as physicians and caregivers are desperate for resources to help patients with opioid use and chronic pain disorders find safer and more accessible non-opioid tools.
Main body
The purpose of this article is to review the current state of the opioid epidemic; the shifting picture of cannabinoids; and the research, policy, and current events that make opioid risk reduction an urgent public health challenge. The provided table contains an evidence-based clinical framework for the utilization of cannabinoids to treat patients with chronic pain who are dependent on opioids, seeking alternatives to opioids, and tapering opioids.
Conclusion
Based on a comprehensive review of the literature and epidemiological evidence to date, cannabinoids stand to be one of the most interesting, safe, and accessible tools available to attenuate the devastation resulting from the misuse and abuse of opioid narcotics. Considering the urgency of the opioid epidemic and broadening of cannabinoid accessibility amidst absent prescribing guidelines, the authors recommend use of this clinical framework in the contexts of both clinical research continuity and patient care.
Keywords: Cannabinoids, Chronic pain, Opioids
Background
Healthcare systems continue to struggle in the face of narcotic overuse and are largely uninformed in the use of cannabinoid-based medicines for harm-reduction in the opioid crisis. There have been many calls to explore the utility of cannabinoids for pain management and to alleviate the opioid epidemic [1, 2]. These calls emphasized the urgency of the opioid epidemic, the utility of cannabinoids, and the growing opportunities presented by increased legalization.
Through the present work, The Board of Medicine, a 501(c)3 nonprofit medical board, aims to answer these calls to action. We work to continuously evaluate all available peer-reviewed data to date on cannabinoids and other currently unregulated natural medicines. In this paper, we will review the history of the opioid epidemic and the evidence surrounding the use of cannabinoids for harm reduction in the opioid crisis. Then, considering the context of broadening legalization, absent provider recommendations, and research limitations, we provide one of the first evidence-based clinical frameworks for the utilization of cannabinoids to treat patients with chronic pain who are on opioids, seeking alternatives to opioids, and tapering opioids. It is also our hope that this framework will be helpful to standardize interventions to support much needed systematic reviews and meta-analyses of cannabinoids for pain.
Main text
Introduction
Despite noble intentions, many physicians feel their hands are tied when it comes to pain management and prescribing narcotics [3]. The World Health Organization (WHO) estimates that there are an annual 0.5 million worldwide deaths attributable to drug use and 70% are attributed to opioids [4]. An estimated 2 million Americans (0.7%) aged 12 years and older meet criteria for opioid use disorder [5], as we remain amid a historic shift in responsibility for the opioid epidemic, from patients to physicians to pharmaceutical companies. Purdue Pharma filed for Chapter 11 bankruptcy in 2019 [6], and it is now widely accepted that they intentionally deceived physicians and the medical community by advertising OxyContin® (oxycodone hydrochloride) as less prone to abuse than existing alternatives to increase profits [7]. In the 1960s, over 80% of people entering treatment for heroin addiction started their habit on heroin [8]. Now, about 80% of people who use heroin misused prescription opioids first [9] and most opioid overdose deaths involve synthetic opioids [10]. COVID-19 has further aggravated the opioid epidemic [11, 12], with the US reaching the grim milestone of over 100,000 overdose deaths in a 12 month period in 2021 [13]. Before COVID-19, the estimated economic burden of opioid misuse in the US was roughly $78.5 billion annually [14], but by 2022 overdoses alone were estimated to cost over $1 trillion [15].
21% of US adults experience chronic pain [16], and when opioids are used for chronic pain, evidence demonstrates that desensitization and tolerance develop quickly [17]. The need for escalating doses often causes opioid-induced hyperalgesia, making pain nearly impossible to treat, a problem that is absent with cannabinoids [18]. Further, endocannabinoid system disruptions have been linked to post-traumatic stress disorder (PTSD) [19], which is highly comorbid in patients with opioid addiction [20] and chronic pain [21], and evidence suggests cannabinoids may be therapeutic in this condition as well [22].
Despite the facts that 91% of Americans support cannabis legalization [23], there is legal medical cannabis in 38 US states [24], and cannabis is federally legal in Canada, the medical community’s utilization of cannabinoids has been limited despite a worsening opioid crisis. In February 2022, the CDC issued the agency’s first revised guidelines on opioid prescribing since 2016, which strongly urged providers to “first turn to ‘non-opioid therapies’ for both chronic and acute pain” [25]. Given the lack of educational resources and guidance around effective and minimal-risk non-opioid alternatives, this begs the question: is the medical community positioned to effectively address the changing tides of public opinion and critical public health needs when there could not be a more relevant Hippocratic sentiment to remember than “first do no harm”?
Evidence for the utility of cannabinoids
Can cannabinoids alleviate the opioid crisis?
The debate over whether cannabinoids decrease opioid demand is longstanding and has compelling evidence in the US [26–31] and Canada [32–34], despite heterogeneous data that are largely an effect of legal restrictions on sourcing cannabis for research.
A 2018 longitudinal analysis showed that US prescriptions for all opioids fell by 14.4% when medical cannabis dispensaries opened, particularly for hydrocodone and morphine, but also for benzodiazepines, stimulants, and many other medications known to be over-prescribed and addictive [35]. This expeditious reduction in prescriptions was previously thought to be unachievable and the trend has extended to fentanyl-related overdose deaths [31, 34], which represent the greatest driver of the current epidemic. Questions remain about the longevity and causality of this effect after a 2014 study showed medical cannabis laws slowed increases in opioid mortality by an astonishing 24.8% [36], but a 2019 follow-up study showed trend reversal [37] and a systematic review and meta-analysis showed small non-significant reductions in opioid prescriptions and overdose mortality in states with operational marijuana dispensaries [38]. These studies highlight the importance of not using ecological correlations to draw causal conclusions, but the question of cannabis’ ability to alleviate the opioid crisis has remained open and been studied in diverse settings.
Observational research has explored the role that adult cannabis use plays for patients in pain management, substance use and mental health treatment, and harm reduction [39–41], especially among people who inject drugs (PWID) [42, 43]. This harm reduction role has also been confirmed in studies with vulnerable young people experiencing street entrenchment in Canada, who have a prevalence of cannabis use estimated as high as 98% [44, 45], with nearly 20% having sold cannabis in the past 6 months [46]. Research on patterns of use in these street-involved youth has observed associations with lower rates of initiation of injection drug use [47], the role of cannabis to be considered medicinal rather than recreational [48], harm-reduction from other more deadly substances [49], and transitioning away from more harmful forms of substance abuse [50].
Research among opioid users who are financially stable enough to frequent dispensaries has also been promising. A survey of 2897 medical cannabis patients found that, of the 34% who used opioid-based pain medication in the prior six months, 97% decreased their opioid consumption with medical cannabis and 81% said cannabis alone was more effective than cannabis plus opioids [43]. A retrospective cross-sectional survey of 1513 dispensary members indicated that 76.7% of regular opioid-using respondents reduced their use after starting medical cannabis, an effect that extended to use of alcohol (42.0%) and psychoactive medications for anxiety (71.8%), migraine (66.7%), sleep (65.2%), and depression (37.6%). When these participants were asked what they like most about cannabis, the most common response was that it helped with pain [51].
While the data supporting cannabinoids for pain are compelling, it is also conflicting and heterogeneous. For example, some data indicate that cannabis reduces self-efficacy in the frequently co-morbid conditions of depression and anxiety [52]. A four-year longitudinal observational study of cannabis use for cancer-related pain showed no opioid-sparing effect or reduction in pain severity, but only 6.5% of the 1514 patients used cannabis regularly 21–31 days per month, quality and type of cannabis was not assessed, and all patients were using black-market cannabis due to illegality during the study period [53]. For over 50 years, prospective studies of cannabis in the USA could only source plant material from one university until 2021 when the Drug Enforcement Agency (DEA) expanded access in response to quality issues [54]. This makes available data with interventional and longitudinal trials on the popular use of cannabinoids heterogeneous and extremely difficult to filter for systematic reviews and meta-analyses [55, 56].
Pure cannabinoid drugs such as oral synthetic tetrahydrocannabinol (THC) (dronabinol or Marinol®) and oral CBD (Epidiolex®) represent the most conventional prescription cannabinoid-based medicines. Dronabinol was FDA-approved in 1985 for nausea and vomiting associated with chemotherapy. In 1992, this product was approved to treat cachexia in AIDS patients [57]. In 2018 and after much public outcry, Epidiolex® was approved for the treatment of two rare seizure disorders (Lennox-Gastaut Syndrome and Dravet Syndrome) [58, 59]. Despite the growing availability of these cannabinoid-containing mainstream pharmaceuticals, there remains a significant disconnect between provider prescribing habits and the popular utilization of cannabis, which can be largely attributed to absent prescribing guidelines and unavailable standards for over-the-counter phytocannabinoid products.
Cannabinoid safety and utilization despite low provider knowledge
The safety of high-quality CBD has been well-established [60, 61]. Compared to most drugs for pain, psychiatric, or mood disorders, side effects of cannabis when misused are mild and can occasionally include sleepiness, diarrhea, changes in appetite/weight, cognitive effects, hyperemesis, nausea, sedation, and addiction/dependence [61, 62]. Recent research has shown a role for CBD in treating cannabis use disorder [63]. THC ingestion by children and young adults has also been associated with an increased risk of psychosis [64] and schizophrenia [65], though new research has shed light on the complexity of this correlation with shared genetic susceptibility [66]. Comparing the number needed to treat and the number needed to harm (NNH) for opiates and cannabinoids is challenging as the NNH rarely accounts for the long-term risk of opioid use disorder (OUD). However, the number needed to kill after 2.6 years of opioid therapy is 550 [67], while deaths secondary to CBD and THC have not been reported [68].
While the evidence to date suggests that cannabis is likely safe and potentially impactful, prescribers are largely uninformed about the use of cannabis-based medicines and the endocannabinoid system [69], leaving patients to self-medicate with cannabis in experimental ways [70]. A survey of 489 dispensary customers in Quebec, Canada showed that 74% had spent over one year self-medicating with cannabis, 56% were taking prescription medications, and 39% never consulted a resource on cannabis. 47% said they sometimes or never declared cannabis use to physicians and 80% said they would like to access advice from healthcare professionals about cannabis [71]. A study of 628 cannabis for therapeutic purposes (CTP) users in Canada demonstrated that barriers exist due to stigma, specifically related to patients’ perception of their providers’ attitude toward them. 48% wanted to discuss CTP with a physician but did not, and the most frequent reason (62%) for not discussing it despite a desire to do so was “don’t feel comfortable” [72]. A 2018 study in the Journal of Clinical Oncology shed light on critical gaps in research, education, and policy, with its survey of 400 medical oncologists that found, while 46% recommended medical cannabis and almost 80% discussed it, only 30% of oncologists felt sufficiently informed to recommend medical cannabis [69].
Clinical framework for prescribing cannabinoids in clinical practice and research
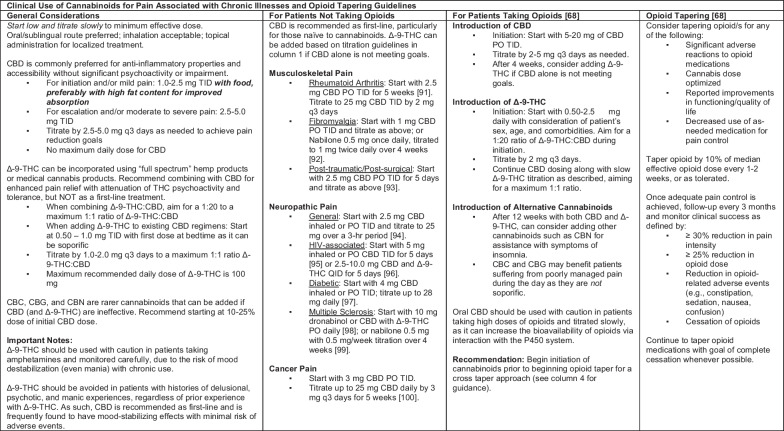
The Board of Medicine aims to address the lack of provider knowledge of cannabis, heterogenous research interventions, and untapped harm-reduction potential with an evidence-based clinical framework for utilizing cannabinoids to treat chronic pain in patients who are on opioids, seeking alternatives to opioids, and tapering opioids. The expertise of the board members was called on for review and discussion of the available evidence to generate the clinical framework found in Table 1. Board members met regularly to discuss cannabinoid dosing and opioid tapering regimens, until the final framework was agreed upon.
Table 1.
Evidence-based guidelines for the clinical use of cannabinoids for management of chronic pain in the absence of opioids, in the presence of opioids, and for those attempting to taper opioids
Best Practices for Product Recommendation Since CBD is derived from the hemp plant, multiple formulations are available on the market and are not subject to FDA regulation. We recommend only products that meet the following qualifications: manufactured in a clean and certified facility (ideally GMP and CO2 or ethanol extraction for consistency), full panel testing of the end product (for primary 8 cannabinoids and terpenes), available for consumer review, analysis verifying minimum acceptable levels of contaminants per California cannabis standards (i.e., herbicides, pesticides, fungicides), no heavy metals, no harmful solvents, no harmful or toxic filters, cannabinoid content should match label claims within a 10% variance. Considerations based on provider and patient preference include the presence of sub-cannabinoids and terpenes with maximum allowable THC content (full spectrum products are generally recommended), no synthetic or genetically engineered ingredients, and cultivation in compliance with organic farming practices. Based on current product market availability, CBD manufacturers that meet this high standard of quality and consistency for clinical use that are nationally available can be found in the following reference [101]. Guidelines and recommendations are subject to change based on new evidence
The open-access framework includes opioid tapering recommendations that are in accordance with the CDC’s newest clinical practice guidelines for managing opioids for pain. The new CDC guideline recommends the following opioids tapers: “Tapers of approximately 10% per month or slower are likely to be better tolerated than more rapid tapers when patients have been taking opioids for longer durations (e.g., ≥ 1 year). When patients have taken opioids for shorter durations (e.g., weeks to months rather than years), a decrease of 10% of the original dose per week or slower (until approximately 30% of the original dose is reached, followed by a weekly decrease of approximately 10% of the remaining dose)” [73]. We invite any clinician and researcher to access this framework to help our medical community expand our toolbox to combat the opioid epidemic and other complex public health crises with less risky non-opioid tools.
Due to the resulting low quality of available research, the International Association for the Study of Pain Presidential Task Force could not endorse the general use of cannabis and cannabinoids for pain relief [74]. Further, new CDC guidelines on prescribing opioids for pain cites the evidence base supporting cannabis for pain management as limited [73]. While these authors agree with the CDC that more research is needed to understand how to best use cannabinoids in clinical care, the gravity of the opioid epidemic, the rapid expansion of recreational cannabinoid use, and the lack of clinician education in this domain suggest an urgent need for general guidelines focused around patient safety that can be utilized in research and clinical practice, particularly for our most vulnerable patients currently dependent on opioids.
Conclusion
Moving forward
As opioid deaths continue to be a global problem, patients are increasingly self-medicating with cannabis [71, 75] while researchers struggle to standardize protocols and providers feel uncomfortable recommending cannabinoids amidst absent prescribing guidelines [69]. If we consider cannabis as a harm reduction tool that patients are already using without medical guidance, we can realign our focus to supporting researchers and providers with a clinical framework for standardizing research and recommending cannabinoids more informatively as safe, effective, accessible tools for assisting in the management of chronic pain. To our knowledge, this is one of the first comprehensive evidence-based peer-reviewed clinical frameworks for the safe use of cannabinoid products for chronic pain and OUD.
We believe that there is a precedent for the safe use of cannabinoids as risk-reduction tools in the clinical settings described, and that healthcare systems can integrate cannabinoids into viable treatment options as well as areas of clinical inquiry. However, there are many issues to confront. One question regards understanding the difference between phytocannabinoids compared to isolated and synthetic cannabinoids in treatment for pain. Another question regards whether the entourage effect plays a role in the action, tolerance, and side effect profile of cannabis. Most research on the cannabis plant’s molecular constituents has focused entirely on CBD and delta-9-THC, despite hundreds of known biologically active molecules present. New studies of novel phytocannabinoids suggest benefits from acidic cannabinoids [76, 77], cannabinol (CBN) [78, 79], cannabigerol (CBG) [80–87], and cannabichromene (CBC) [83, 88–90] among others.
Indeed, if phytocannabinoids prove to be non-inferior in safety and efficacy, then dissemination of this knowledge becomes a matter of social justice, because it could offer individuals of low socioeconomic status dramatically improved access to non-opioid pain management options. Furthermore, clinical adoption of cannabis-based therapies could justify insurance coverage of cannabis, which may be critical for lower income patients [72]. A foreseeable regulatory challenge regards the self-production of cannabis, and the resources patients may need (land, power, etc.) or legal obstacles they could face based on their state or regional location. Finally, until there is a federal consensus on the legality of medical cannabis, safeguards for cannabis-using patients may be limited including, for example, cannabis possession-related child endangerment laws.
Resistance to cannabis-based medicines for the opioid epidemic may have many origins, particularly the stigma associated with recreational cannabis use. That said, the evidence to date suggests that it is time for a sea change in the clinical approach to cannabis for pain management and OUD. Throughout the history of science and clinical medicine, there have been transformative changes that were initially considered heretical: hand hygiene as a means to prevent infection prior to germ theory, therapy for H. pylori to combat peptic ulcer disease, and even the genetic basis of cancer were all dismissed by their era’s established medical communities. Similarly, we face great resistance to the implementation of CBD and other cannabinoids for treatment-resistant chronic illnesses, despite the compelling evidence, strong overall safety profile, and urgent need. Many of our patients have already begun their own self-guided journey into pain management with cannabinoids and the burden is now on providers to consolidate the information available, conduct more rigorous research, form best practices, and implement guidelines that will inform both the field and those we care for without stigma.
“To study the phenomena of disease without books is to sail an uncharted sea, while to study books without patients is not to go to sea at all.”—William Osler
Acknowledgements
The authors would like to acknowledge Dr. Rachael Grazioplene PhD for her assistance with thoughtful review and refinement of this manuscript.
Abbreviations
- CBD
Cannabidiol
- CDC
Centers for Disease Control and Prevention
- PTSD
Post-traumatic stress disorder
- PCPs
Primary care physicians
- PWID
People who inject drugs
- THC
Tetrahydrocannabinol
- OUD
Opioid use disorder
- NNH
Number needed to harm
Biographies
Krista Hammaker
is a third-year medical student at Northeast Ohio Medical University’s M.D. program, who has been involved in clinical research since 2008. At age 17 and under the mentorship of Dr. Rebecca Bascom, MD, MPH, she began drafting permanent impairment ratings for patients with occupational lung disease and assisted with the creation of a tissue repository for research. Under the mentorship of Dr. David Campbell, MD, she completed a cohort study of medical versus surgical management of massive pulmonary embolism (PE), which led to a revision of Penn State Milton S. Hershey Medical Center’s PE treatment protocol. She presented at the American Association of Ophthalmic Oncologists & Pathologists (AAOOP) conference at age 21. Before starting medical school, she studied naturopathic medicine at Bastyr University, where she learned about herbal medicine and supplement research, sourcing, and manufacturing. While studying naturopathic medicine, she worked with Dr. Laurie Mischley, ND, PhD on a qualitative study of the effectiveness of cannabis in the palliation of Parkinson’s disease; with Dr. Leanna Standish, ND, PhD, on a retrospective matched control review of breast cancer survival in women treated with integrative medicine. With Dr. Standish, KH helped achieve the first FDA approval for human research with ayahuasca—a Phase 1 safety and dose-finding study of ayahuasca tea in healthy adults (IND 131217). She has earned a Certificate in Clinical Research from the University of Pittsburgh School of Medicine, and other notable research efforts include a study of long-term outcomes of neoadjuvant chemoradiation and esophagectomy, as well as employed research positions funded by the Patient Centered Outcomes Research Institute (PCORI) and Sleep and Behavioral Neuroscience Center at the University of Pittsburgh.
Nathaniel Weathington
M.D., PhD is a physician-scientist specializing in pulmonary and critical care medicine. His laboratory studies the biology of inflammation and pharmacology at the University of Pittsburgh Medical Center, where he is an assistant professor in the Division of Pulmonary, Allergy, and Critical Care Medicine. His research is funded by grants from foundations and the National Institutes of Health to investigate the chemical signals, molecular pathways, cellular responses, and drug actions that contribute to acute and chronic inflammatory diseases. He has authored over 30 articles in journals, including Nature Medicine, Science Translational Medicine, the Journal of Clinical Investigation, and many others.
Joseph Maroon
M.D. is a clinical professor and vice chairman of the Department of Neurological Surgery and Heindl Scholar in Neuroscience at the University of Pittsburgh Medical Center. In addition to being a renowned neurosurgeon, he is a sports medicine expert, health and nutrition expert, and Ironman triathlete. He is regarded as a premiere specialist in the surgical treatment of injuries and diseases of the brain and spine, specializing in minimally invasive procedures. Consistently listed in America’s Best Doctors for the past 20 years, he has an international referral base, including numerous professional athletes and celebrities. He obtained his medical and neurosurgical training at Indiana University, Georgetown University, Oxford University in England, and the University of Vermont. He conducted extensive research into neurotrauma, brain tumors, and diseases of the spine, which has led to many innovative techniques for diagnosing and treating these disorders. His research efforts garnered him numerous awards from various national and international neurological societies. JM is the author of six books and co-author of 40 book chapters and over 270 published scientific papers. He has given more than 150 presentations at national and international conferences and is often invited as visiting professor and keynote speaker. He has served on the editorial boards of eight medical and neurological journals and is currently associate editor of Surgical Neurology and editorial board member of Neurological Research and The Physician and Sports Medicine journals. He is also past president of the Congress of Neurological Surgeons, the largest society of neurosurgeons in the world. JM has been the team neurosurgeon for the Pittsburgh Steelers since 1981 and is medical director of the World Wrestling Entertainment (WWE). He has successfully performed surgery on numerous professional football players and other elite athletes with potentially career-ending neck and spine injuries. Notably, he safely returned most to their high level of athletic performance. He is highly invested in the prevention and treatment of concussions in high school, college, and professional sports, specifically in football. While working with the Steelers in the early 1990’s, the lack of an objective, reliable instrument to evaluate concussion symptoms became very apparent to JM. To fill this void, he and Dr. Mark Lovell, PhD developed ImPACT™ (Immediate Post-Concussion Assessment and Cognitive Testing), an easy-to-administer, 20-min-long test to assess presence and severity of concussion symptoms. ImPACT™ has become the world-wide standard tool to assess sports-related concussions and has been used in over 4.5 million athletes. In 1994, JM joined the National Football League’s (NFL) Mild Traumatic Brain Injury Committee as a concussion expert. This committee was renamed NFL’s Head, Neck and Spine Committee in 2007 and is still in place. For his expertise in sports medicine and concussions, JM is frequently interviewed and quoted by the media, including the New York Times, USA Today, Associated Press, ESPN, Sports Illustrated, and ABC News Nightline.
Lawton W. Tang
M.D. is trained in general surgery and trauma critical care and is fellowship trained in aesthetics, breast, and body contouring. He further specialized in plastic and reconstructive surgery. He served as a clinical instructor at the University of Pittsburgh Department of Plastic and Reconstructive Surgery and is currently involved in education by teaching surgeons in training from Huntington Memorial Hospital and the University of Southern California. He is published in many top plastic surgery journals and has received prestigious federal research grants to fund his regenerative medicine research.
Brian Donohue
M.D. is a cardiologist who has been practicing at the University of Pittsburgh Medical Center Heart and Vascular Institute for twenty years. His clinical interests include heart disease, primary and secondary prevention of heart disease, and heart disease in women. His professional interests include the athletic heart. His clinical research has been published in leading peer-reviewed journals and he has been invited to present and speak on his work both nationally and internationally.
Rachel Yehuda
PhD is a professor of psychiatry and neuroscience, vice chair for Veterans Affairs in the Department of Psychiatry, and director of the Traumatic Stress Studies Division at Mount Sinai School of Medicine. She also leads the PTSD clinical research program at the neurochemistry and neuroendocrinology laboratory at James J. Peters VA Medical Center. In 2020, she became director of the Center for Psychedelic Psychotherapy and Trauma Research at Mount Sinai. She received her PhD in psychology and neurochemistry and her M.S. in biological psychology from the University of Massachusetts at Amherst and completed her postdoctoral training in biological psychiatry in the Department of Psychiatry at Yale School of Medicine. In 2019, she was elected to the National Academy of Medicine. RY has authored more than 500 published papers, chapters, and books in the field of traumatic stress and the neurobiology of PTSD. Her interests include the study of risk and resilience factors, psychological and biological predictors of treatment response in PTSD, genetic and epigenetic studies of PTSD, and the intergenerational transmission of trauma and PTSD.
Kenneth M. Ford
PhD is founder and chief executive officer of the Florida Institute for Human and Machine Cognition (IHMC)—a not-for-profit research institute located in Pensacola, Florida. IHMC has grown into one of the nation’s premier research organizations with world-class scientists and engineers investigating a broad range of topics related to building technological systems aimed at amplifying and extending human cognition, perception, locomotion, and resilience. Richard Florida has described IHMC as “a new model for interdisciplinary research institutes that strive to be both entrepreneurial and academic, firmly grounded and inspiringly ambitious.” IHMC headquarters are in Pensacola with a branch research facility in Ocala, Florida. KF is the author of hundreds of scientific papers and six books. His research interests include artificial intelligence, cognitive science, human-centered computing, and entrepreneurship in government and academia. He received his PhD in computer science from Tulane University. He is emeritus editor-in-chief of the Association for the Advancement of Artificial Intelligence (AAAI)/MIT Press and has been involved in the editing of several journals. KF is a fellow of AAAI, a charter fellow of the National Academy of Inventors, a member of the Association for Computing Machinery, a member of the IEEE Computer Society, and a member of the National Association of Scholars. He has received many awards and honors, including a Doctor Honoris Causa from the University of Bordeaux in 2005 and the 2008 Robert S. Englemore Memorial Award for his work in artificial intelligence (AI). In 2012 Tulane University named KF its Outstanding Alumnus in the School of Science and Engineering. In 2015, AAAI named KF the recipient of the 2015 Distinguished Service Award. Also in 2015, KF was elected a fellow of the American Association for the Advancement of Science (AAAS). In 2017 KF was inducted into the Florida Inventors Hall of Fame. In January 1997, KF was asked by NASA to develop and direct its new Center of Excellence in Information Technology at the Ames Research Center in Silicon Valley. He served as associate center director and director of NASA’s Center of Excellence in Information Technology. In July 1999, KF was awarded the NASA Outstanding Leadership Medal. That same year, he returned to private life and to IHMC. In October 2002, President George W. Bush nominated Dr. Ford to serve on the National Science Board (NSB) and the United States Senate confirmed his nomination in March of 2003. The NSB is the governing board of the National Science Foundation (NSF) and plays an important role in advising the President and Congress on science policy issues. In 2005, KF was appointed and sworn in as a member of the Air Force Science Advisory Board. In 2007, he became a member of the NASA Advisory Council and on October 16, 2008, KF was named chairman, a capacity in which he served until October 2011. In August 2010, KF was awarded NASA’s Distinguished Public Service Medal—the agency’s highest honor. In February of 2012, KF was named to a two-year term on the Defense Science Board (DSB) and in 2013, he became a member of the Advanced Technology Board (ATB) which supports the Office of the Director of National Intelligence (ODNI). In 2018, KF was appointed to the National Security Commission on Artificial Intelligence.
Myro Figura
M.D. is an entrepreneur, researcher, and academic faculty anesthesiologist at UCLA. MF has co-founded several ventures ranging from emergency university medical services and consumer devices startup to a nutraceutical supplements company. He is a published researcher with a number of articles and book chapters on various topics in anesthesiology, pain management, and medical innovation. He is also a frequent lecturer for medical students and a clinical educator for resident physicians at UCLA. MF has deep research and innovation experience at several powerhouse research institutions, has designed and run clinical trials, and has been a part of the development team for a novel intraoperative nasal CPAP device, which he has extensively studied and presented nationally. He is also a co-founder of HealFast, Inc., an innovative physician-led company that develops clinical-grade evidence-based nutraceutical products for healthcare and consumer use. MF brings deep industry experience in supplement development from ideation to product, research, sourcing, manufacturing, certifications, and all aspects of commercialization.
Ben Kelmendi
M.D. is a psychiatrist and an associate professor in the Department of Psychiatry at Yale University School of Medicine. His primary clinical expertise is in PTSD, depression, OCD, and the endocannabinoid system. He is co-founder of the Yale Psychedelic Science Group. He leads a research program focused on the therapeutic potential of psychedelic medicines across a range of psychiatric diagnoses. He is currently investigating the effects of psilocybin on the neurocircuitry implicated in the development, maintenance, and treatment of patients with OCD. He is also exploring the effects of MDMA on brain activation and neural network organization in PTSD to understand the relationship between MDMA-induced neural changes and the acute cognitive and behavioral effects of the drug.
Belinda Tan
M.D., PhD is a board-certified dermatologist and dermatopathologist who spent her formative professional training years in Los Angeles where she currently resides. Early in her career, she began applying high impact technology ideas to clinical practice with the purpose of providing broader access to quality care. Today she is a physician-scientist-entrepreneur recognized as one of the LA’s top 100 technology leaders. BT is a member of the early founding team and continuing advisor to DirectDerm, the national leader delivering teledermatology services and improving health outcomes among underserved populations. She most recently co-founded and served as chief medical officer of Science 37, a pioneering market leader in virtual clinical trials that raised more than $100M in venture capital and led several groundbreaking first-ever FDA-regulated studies. The company’s focus on bringing clinical research to people in their homes significantly improved diversity among clinical study participants from an average of 5–40%. BT is a continuing student of life, a listener, and a mentor. As a spouse, mother, daughter, and sister, her first-generation immigrant journey thus far has enriched her appreciation of belonging to a global community. Her personal and professional pursuits are guided by promoting inclusivity and access. Having personally seen the healing impact of complementary and alternative medicines, she is focused on advocating for access to culturally competent care for these therapies. Inspired by the needs of people seeking healthcare within the LA County public health system where she trained, BT continues to mentor medical students and residents as an assistant clinical professor of medicine at Harbor-UCLA Medical Center. She also provides expertise to Venice Family Clinic, LA’s leading community health center. BT’s multiple advisory roles include the LA Mayor’s WiSTEM initiative, Seed Health, and VisualDx. As an immunologist, she has published several peer-reviewed studies describing mechanisms of how the human innate immune system combats challenging infections like Mycobacterium tuberculosis and Mycobacterium leprae. She earned her MD and PhD from UCLA and a BS from MIT, with additional clinical training at the Memorial Sloan-Kettering Cancer Center and Cornell-New York Presbyterian Hospital.
Matthew W. Cook
M.D. is a board-certified anesthesiologist with over 20 years of experience in practicing medicine. He graduated from the University of Washington School of Medicine and completed his residency in anesthesiology at the University of California San Francisco. MC has also completed a fellowship in functional medicine and has a Doctorate in Traditional Chinese Medicine. His early career as an anesthesiologist and medical director of an outpatient surgery center that specialized in sports medicine and orthopedic procedures provided invaluable training in the skills needed to become a leader in the emerging field of regenerative medicine. Currently, MC is president and founder of BioReset™ Medical and BioReset™ International. His practice provides treatments for conditions ranging from pain and complex illness to anti-aging and wellness. MC’s approach is to use the most non-invasive, natural, and integrative treatment methods possible.
Steven D. Factor
M.D. is a graduate of Northwestern University, where he completed a double major in philosophy and economics. After a very brief stint with the bulls and bears of Chicago’s finance markets, he earned a medical degree from Loyola Stritch School of Medicine in Chicago. He then completed an internship and neurological residency at the University of Pittsburgh Medical Center, followed by a fellowship in clinical neurophysiology at Vanderbilt University Medical Center, where his clinical research activities included multiple investigational protocols for epilepsy treatment. SF is currently triple-board certified in neurology, clinical neurophysiology, and epilepsy. He is also experienced in the fields of intraoperative monitoring and headache treatment and always strives to match plant-based medicinal options to symptomatology when such alternatives match patient goals.
Laura Lagano
M.S., R.D.N., C.D.N., A.F.M.C.P. is a registered dietitian nutritionist and author of The CBD Oil Miracle (St. Martin’s Press, 2019). She is the co-founder and education director of the Holistic Cannabis Academy. As one of a handful of RDNs who has completed the prestigious Institute of Functional Medicine program, LL integrates her expertise in nutrition, functional medicine, and the endocannabinoid system in her private practice. She speaks frequently at conferences about plant medicine, consults with cannabis-related companies, and advises several cannabis companies. One of the first dietitians to work in the media, LL writes for The Fresh Toast, Kitchen Toke, and Holistic Primary Care magazines, and has written for or been quoted in the New York Times, Forbes, HuffPost, Stitcher, MerryJane, The Daily Beast, and numerous other media outlets. She was awarded an OZY Rising Star Award for her pioneering work shining a light on the endocannabinoid system and the healing benefits of the cannabis plant. LL’s core value system is about nurturing relationships through collaboration to eliminate the global stigma around cannabis and plant medicines.
Henry Patrick Driscoll
M.D. is a pediatric, adolescent, and adult psychiatrist with a focus on substance use disorders and psychoanalysis. Early in his training, HPD started his journey in medicine and healing with a deep interest in holistic approaches to health and wellness. A student of the mind and thinking, he studied philosophy as an undergraduate which led to his choice of neuroscience and psychiatry as ways to most positively impact the health and well-being of patients in a career in medicine. His early research in the biology of aging at the National Institute on Aging included several peer-reviewed papers from the Laboratory of Molecular Gerontology under Drs. Robert Brosh, PhD and Wilhelm Bohr, MD, PhD. He completed his medical training as a dean’s merit scholar at the University of Pittsburgh School of Medicine, where he also completed a master’s degree in clinical research with Dr. Charles Reynolds in his laboratory for the study of sleep, pharmacotherapy, and treatment-resistant late-life depression. He completed his psychiatric residency at Western Psychiatric Institute and Clinic at the University of Pittsburgh Medical Center, including a fellowship in child and adolescent psychiatry and a clinical focus in addiction medicine. This broad clinical experience with patients across the lifespan positions him well to communicate about our most current knowledge of how, to paraphrase Hippocrates, to use our food as remedies and make remedies of our foods. He has also contributed to several books and training manuals on doctor-patient engagement, including co-authoring Humanizing Addiction Treatment with Antoine Douaihy, MD. HPD found his calling in clinical work with young people and their families, which is the primary focus of his current practice in Pittsburgh. As a dedicated educator who has earned top teaching awards from the University of Pittsburgh School of Medicine and the University of Pittsburgh Medical Center, he continues his work in the use of collaborative therapeutic inquiry into all layers of thought and experience within the doctor-patient relationship as an advanced candidate at the Pittsburgh Psychoanalytic Center.
Adam S. Howe
M.D. attended Union College, where he majored in biochemistry. After volunteering with local hospice and working as a research technician in an infectious disease laboratory, he went on to receive his medical degree at Albany Medical College. He then specialized in general surgery at the University of Louisville. AH then sub-specialized in urology at The Ohio State University, followed by a fellowship in pediatric urology at Cohen Children’s Medical Center of New York. He lives in upstate New York and currently holds a position as an assistant professor of surgery at Albany Medical College and pediatric urologist at Albany Medical Center in Albany, NY. AH is board-certified in urology and has multiple published peer-reviewed manuscripts. AH’s work has included investigations of minimally-invasive treatments, such as the use of transcutaneous electric nerve stimulation (TENS) for children with nocturnal enuresis, and he started the Pediatric Onco-Fertility program at Albany Medical Center.
EunBit G. Cho
M.D is a resident physician in psychiatry at Einstein Medical Center in Philadelphia, PA. Previously, EGC completed a year of internal medicine as a resident physician at The University of Tennessee Health Science Center (UTHSC) in Memphis, TN during the onset of the global COVID-19 pandemic, where she/they witnessed the dire need for a biopsychosocial-spiritual approach to critically ill patients with comorbid medical and psychiatric conditions. After receiving her M.D. at UTHSC, EGC worked in both public and private sectors as a psycholinguist and clinical researcher, respectively, prior to resuming their graduate medical education. As a polyglot and psycholinguist, EGC has served as a federal consultant to the US Army throughout the course of their education and training. EGC’s academic interests are expansive and include complex PTSD, culturally sensitive trauma-informed care, psychodynamic psychopharmacology, as well as the relationships between chronic stress, neuroplasticity, and spirituality and their impact on biometric data. She has a keen interest in the study of using psychedelics as clinical tools for the treatment of psychiatric conditions, such as depression and PTSD, especially in the psychotherapeutic setting. Her current research aims to study the effects of the Apollo™ device on the remission of PTSD symptomatology in patients who have undergone a trial of MDMA-assisted psychotherapy sponsored by the Multidisciplinary Association of Psychedelic Studies (MAPS).
David M. Rabin
M.D., PhD is a board-certified psychiatrist, translational neuroscientist, inventor, and entrepreneur specializing in the treatment of PTSD, depression, anxiety, and substance use disorders. DR has spent the last 15 years researching treatments to combat the negative effects of chronic stress on physical and mental health. DR received his M.D. in medicine and his PhD in neuroscience from Albany Medical College and trained in psychiatry at Western Psychiatric Institute & Clinic at the University of Pittsburgh Medical Center. While conducting research at the University of Pittsburgh, DR developed the science and technology behind Apollo, a breakthrough wearable technology that uses the neuroscience of touch and vibration to combat the negative effects of stress. Apollo sends gentle waves of vibration to the body that have been demonstrated in scientific studies to bring the heart, lungs, and mind into balance, resulting in improved heart rate variability (HRV). After the first university studies of the Apollo technology demonstrated its viability for assisting with mood, sleep, and focus, DR co-founded Apollo Neuroscience, Inc. to make the discovery available to the public. In addition to his psychiatry practice and training clinic, DR is currently conducting research on the epigenetic regulation of trauma responses and recovery to elucidate the mechanism of psychedelic-assisted psychotherapy and trauma healing in collaboration with colleagues at Yale, Mt. Sinai Medical Center, University of Arizona, the University of Southern California, and MAPS. The goal is to determine the mechanisms of the dramatic therapeutic benefits observed following psychedelic-assisted psychotherapy in treatment-resistant mental illnesses such as severe PTSD. This study collected DNA samples from patients with treatment-resistant PTSD who are participating in the current MAPS-sponsored trial of MDMA-assisted psychotherapy to evaluate gene expression changes that result from the therapy and was published in 2023. DR is also the Executive Director of The Board of Medicine.
Author contributions
Conceptualization: DMR, NW, HPD. Investigation: KH, DMR, NW, MF. Writing—original draft: NW, KH, DMR. Writing—review and editing: NW, KH, DMR, EGC, JM, LWT, BD, KF, RY, MF, BK, BT, MWC, SDF, LL, HPD.
Funding
Not applicable.
Availability of data and materials
All data are available in the main text or the supplementary materials.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hurd YL. Cannabidiol: swinging the marijuana pendulum from 'weed' to medication to treat the opioid epidemic. Trends Neurosci. 2017;40(3):124–127. doi: 10.1016/j.tins.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Lucas P. Rationale for cannabis-based interventions in the opioid overdose crisis. Harm Reduct J. 2017;14(1):58. doi: 10.1186/s12954-017-0183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desveaux L, Saragosa M, Kithulegoda N, Ivers NM. Understanding the behavioural determinants of opioid prescribing among family physicians: a qualitative study. BMC Fam Pract. 2019;20(1):59. doi: 10.1186/s12875-019-0947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. Opioid Overdose: WHO Newsroom 2021. https://www.who.int/news-room/fact-sheets/detail/opioid-overdose.
- 5.Centers for Disease Control and Prevention. 2019 annual surveillance report of drug-related risks and outcomes—United States surveillance special report 2019 [June 6, 2022]. https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillancereport.pdf.
- 6.Hoffman J, Walsh M. Purdue Pharma, maker of OxyContin, files for bankruptcy The New York Times The New York Times; 2020. https://www.nytimes.com/2019/09/15/health/purdue-pharma-bankruptcy-opioids-settlement.html.
- 7.Meier B. In guilty plea, OxyContin maker to pay $600 million: The New York Times 2007 [updated May 10, 2007]. https://www.nytimes.com/2007/05/10/business/11drug-web.html.
- 8.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 9.Muhuri P, Gfroerer J, Davies M. Associations of nonmedical pain reliever use and initiation of heroin use in the United States Center for Behavioral Health Statistics and Quality: Substance Abuse and Mental Health Services Administration; 2013. https://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.pdf.
- 10.Julie K, O’Donnell P, John Halpin M, Christine L, Mattson P, Bruce A, Goldberger P, Gladden RM. Deaths involving fentanyl, fentanyl analogs, U-47700—10 states, July-December 2016. Morb Mortal Wkly Rep. 2017;66(43):1197–202. doi: 10.15585/mmwr.mm6643e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochalek TA, Cumpston KL, Wills BK, Gal TS, Moeller FG. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673–1674. doi: 10.1001/jama.2020.17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niles JK, Gudin J, Radcliff J, Kaufman HW. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020. Popul Health Manag. 2021;24(S1):S43–S51. doi: 10.1089/pop.2020.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Drug overdose deaths in the U.S. top 100,000 annually National Center for Health Statistics2021. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm.
- 14.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901–906. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trafficking CoCSO. Commission on combating synthetic opioid traffiking RAND corporations 2022. https://www.rand.org/pubs/external_publications/EP68838.html.
- 16.Nahin RL, Feinberg T, Kapos FP, Terman GW. Estimated rates of incident and persistent chronic pain among US adults, 2019–2020. JAMA Netw Open. 2023;6(5):e2313563. doi: 10.1001/jamanetworkopen.2023.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Pierre M, Russo EB, Walsh Z. No evidence of altered reactivity to experimentally induced pain among regular cannabis users. Clin J Pain. 2020;36(8):589–593. doi: 10.1097/AJP.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 19.Hill MN, Campolongo P, Yehuda R, Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43(1):80–102. doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilevicius E, Sommer JL, Asmundson GJG, El-Gabalawy R. Posttraumatic stress disorder and chronic pain are associated with opioid use disorder: results from a 2012–2013 American nationally representative survey. Drug Alcohol Depend. 2018;188:119–125. doi: 10.1016/j.drugalcdep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kind S, Otis JD. The interaction between chronic pain and PTSD. Curr Pain Headache Rep. 2019;23(12):91. doi: 10.1007/s11916-019-0828-3. [DOI] [PubMed] [Google Scholar]
- 22.Orsolini L, Chiappini S, Volpe U, Berardis D, Latini R, Papanti GD, et al. Use of medicinal cannabis and synthetic cannabinoids in post-traumatic stress disorder (PTSD): a systematic review. Medicina (Kaunas) 2019;55(9):525. doi: 10.3390/medicina55090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green TV. Americans overwhelmingly say marijuana should be legal for recreational or medical use: Pew Research Center 2021. https://www.pewresearch.org/fact-tank/2021/04/16/americans-overwhelmingly-say-marijuana-should-be-legal-for-recreational-or-medical-use/.
- 24.Medical Marijuana Laws: NORML; 2022. https://norml.org/laws/medical-laws/.
- 25.Hoffman J. CDC proposes new guidelines for treating pain, including opioid use The New York Times 2022 [updated February 10, 2022. https://www.nytimes.com/2022/02/10/health/cdc-opioid-pain-guidelines.html.
- 26.Wen H, Hockenberry JM. Association of medical and adult-use marijuana laws with opioid prescribing for medicaid enrollees. JAMA Intern Med. 2018;178(5):673–679. doi: 10.1001/jamainternmed.2018.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishida JH, Wong PO, Cohen BE, Vali M, Steigerwald S, Keyhani S. Substitution of marijuana for opioids in a national survey of US adults. PLoS ONE. 2019;14(10):e0222577. doi: 10.1371/journal.pone.0222577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah A, Hayes CJ, Lakkad M, Martin BC. Impact of medical marijuana legalization on opioid use, chronic opioid use, and high-risk opioid use. J Gen Intern Med. 2019;34(8):1419–1426. doi: 10.1007/s11606-018-4782-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford A, Bradford D. Medical marijuana laws reduce prescription medication use in medicare part D. Health Aff (Millwood) 2016;35(7):1230–1236. doi: 10.1377/hlthaff.2015.1661. [DOI] [PubMed] [Google Scholar]
- 30.Liang D, Bao Y, Wallace M, Grant I, Shi Y. Medical cannabis legalization and opioid prescriptions: evidence on US Medicaid enrollees during 1993–2014. Addiction. 2018;113(11):2060–2070. doi: 10.1111/add.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu G, Kovacs B. Association between county level cannabis dispensary counts and opioid related mortality rates in the United States: panel data study. BMJ. 2021;372:m4957. doi: 10.1136/bmj.m4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dranitsaris G, DeAngelis C, Pearson B, McDermott L, Pohlmann-Eden B. Opioid prescribing in Canada following the legalization of cannabis: a clinical and economic time-series analysis. Appl Health Econ Health Policy. 2021;19(4):537–544. doi: 10.1007/s40258-021-00638-4. [DOI] [PubMed] [Google Scholar]
- 33.Meng H, Page MG, Ajrawat P, Deshpande A, Samman B, Dominicis M, et al. Patient-reported outcomes in those consuming medical cannabis: a prospective longitudinal observational study in chronic pain patients. Can J Anaesth. 2021;68(5):633–644. doi: 10.1007/s12630-020-01903-1. [DOI] [PubMed] [Google Scholar]
- 34.Socias ME, Choi J, Lake S, Wood E, Valleriani J, Hayashi K, et al. Cannabis use is associated with reduced risk of exposure to fentanyl among people on opioid agonist therapy during a community-wide overdose crisis. Drug Alcohol Depend. 2021;219:108420. doi: 10.1016/j.drugalcdep.2020.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradford AC, Bradford WD, Abraham A, Bagwell AG. Association between US State medical cannabis laws and opioid prescribing in the medicare part D population. JAMA Intern Med. 2018;178(5):667–672. doi: 10.1001/jamainternmed.2018.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174(10):1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shover CL, Davis CS, Gordon SC, Humphreys K. Association between medical cannabis laws and opioid overdose mortality has reversed over time. Proc Natl Acad Sci U S A. 2019;116(26):12624–12626. doi: 10.1073/pnas.1903434116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chihuri S, Li G. State marijuana laws and opioid overdose mortality. Inj Epidemiol. 2019;6:38. doi: 10.1186/s40621-019-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau N, Sales P, Averill S, Murphy F, Sato SO, Murphy S. A safer alternative: cannabis substitution as harm reduction. Drug Alcohol Rev. 2015;34(6):654–659. doi: 10.1111/dar.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas P, Walsh Z, Crosby K, Callaway R, Belle-Isle L, Kay R, et al. Substituting cannabis for prescription drugs, alcohol and other substances among medical cannabis patients: the impact of contextual factors. Drug Alcohol Rev. 2016;35(3):326–333. doi: 10.1111/dar.12323. [DOI] [PubMed] [Google Scholar]
- 41.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474–2483. doi: 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- 42.Kral AH, Wenger L, Novak SP, Chu D, Corsi KF, Coffa D, et al. Is cannabis use associated with less opioid use among people who inject drugs? Drug Alcohol Depend. 2015;153:236–241. doi: 10.1016/j.drugalcdep.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res. 2017;2(1):160–166. doi: 10.1089/can.2017.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saddichha S, Linden I, Krausz MR. Physical and mental health issues among homeless youth in British Columbia, Canada: are they different from older homeless adults? J Can Acad Child Adolesc Psychiatry. 2014;23(3):200–206. [PMC free article] [PubMed] [Google Scholar]
- 45.Werb D, Kerr T, Buxton J, Shoveller J, Richardson C, Montaner J, et al. Crystal methamphetamine and initiation of injection drug use among street-involved youth in a Canadian setting. CMAJ. 2013;185(18):1569–1575. doi: 10.1503/cmaj.130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddon H, Fast D, DeBeck K, Werb D, Hayashi K, Wood E, et al. Prevalence and correlates of selling illicit cannabis among people who use drugs in Vancouver, Canada: a ten-year prospective cohort study. Int J Drug Policy. 2019;69:16–23. doi: 10.1016/j.drugpo.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddon H, DeBeck K, Socias ME, Dong H, Wood E, Montaner J, et al. Cannabis use is associated with lower rates of initiation of injection drug use among street-involved youth: a longitudinal analysis. Drug Alcohol Rev. 2018;37(3):421–428. doi: 10.1111/dar.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul B, Thulien M, Knight R, Milloy MJ, Howard B, Nelson S, et al. "Something that actually works": cannabis use among young people in the context of street entrenchment. PLoS ONE. 2020;15(7):e0236243. doi: 10.1371/journal.pone.0236243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bozinoff N, Small W, Long C, DeBeck K, Fast D. Still, "at risk": an examination of how street-involved young people understand, experience, and engage with "harm reduction" in Vancouver's inner city. Int J Drug Policy. 2017;45:33–39. doi: 10.1016/j.drugpo.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyd J, Fast D, Hobbins M, McNeil R, Small W. Social-structural factors influencing periods of injection cessation among marginalized youth who inject drugs in Vancouver, Canada: an ethno-epidemiological study. Harm Reduct J. 2017;14(1):31. doi: 10.1186/s12954-017-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piper B, DeKeuster R, Beals ML, Cobb CM, Burchman CA, Perkinson L, et al. Substitution of medical cannabis for pharmaceutical agents for pain, anxiety and sleep. J Psychopharmacol. 2017;31(5):569–575. doi: 10.1177/0269881117699616. [DOI] [PubMed] [Google Scholar]
- 52.Wilson M, Gogulski HY, Cuttler C, Bigand TL, Oluwoye O, Barbosa-Leiker C, et al. Cannabis use moderates the relationship between pain and negative affect in adults with opioid use disorder. Addict Behav. 2018;77:225–231. doi: 10.1016/j.addbeh.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Campbell G, Hall WD, Peacock A, Lintzeris N, Bruno R, Larance B, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health. 2018;3(7):e341–e350. doi: 10.1016/S2468-2667(18)30110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zagorski N. Experts reflect on what new law might mean for cannabis research Psychiatry Online American Psychiatric Association; 2023.
- 55.Gedin F, Blome S, Ponten M, Lalouni M, Fust J, Raquette A, et al. Placebo response and media attention in randomized clinical trials assessing cannabis-based therapies for pain: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(11):e2243848. doi: 10.1001/jamanetworkopen.2022.43848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lake S, St PM. The relationship between cannabis use and patient outcomes in medication-based treatment of opioid use disorder: a systematic review. Clin Psychol Rev. 2020;82:101939. doi: 10.1016/j.cpr.2020.101939. [DOI] [PubMed] [Google Scholar]
- 57.Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57(6):547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- 58.Kelley K. FDA approves first marijuana-derived drug NEJM Journal Watch; 2018. https://www.jwatch.org/fw114313/2018/06/26/fda-approves-first-marijuana-derived-drug. [DOI] [PubMed]
- 59.FDA approves new indication for drug containing an active ingredient derived from cannabis to treat seizures in rare genetic disease [press release]. FDA News Release, July 31, 2020.
- 60.Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2(1):139–154. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 62.McDonagh MS, Selph SS, Buckley DI, Holmes RS, Mauer K, Ramirez S, et al. Nonopioid pharmacologic treatments for chronic pain. AHRQ Comparative Effectiveness Reviews. Rockville (MD) 2020. [PubMed]
- 63.Freeman TP, Hindocha C, Baio G, Shaban NDC, Thomas EM, Astbury D, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7(10):865–874. doi: 10.1016/S2215-0366(20)30290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull. 2016;42(5):1262–1269. doi: 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Godin SL, Shehata S. Adolescent cannabis use and later development of schizophrenia: an updated systematic review of longitudinal studies. J Clin Psychol. 2022;78(7):1331–1340. doi: 10.1002/jclp.23312. [DOI] [PubMed] [Google Scholar]
- 66.Cheng W, Parker N, Karadag N, Koch E, Hindley G, Icick R, et al. The relationship between cannabis use, schizophrenia, and bipolar disorder: a genetically informed study. Lancet Psychiatry. 2023;10(6):441–451. doi: 10.1016/S2215-0366(23)00143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frieden TR, Houry D. Reducing the risks of relief-the CDC opioid-prescribing guideline. N Engl J Med. 2016;374(16):1501–1504. doi: 10.1056/NEJMp1515917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sihota A, Smith BK, Ahmed SA, Bell A, Blain A, Clarke H, et al. Consensus-based recommendations for titrating cannabinoids and tapering opioids for chronic pain control. Int J Clin Pract. 2021;75(8):e13871. doi: 10.1111/ijcp.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braun IM, Wright A, Peteet J, Meyer FL, Yuppa DP, Bolcic-Jankovic D, et al. Medical oncologists' beliefs, practices, and knowledge regarding marijuana used therapeutically: a nationally representative survey study. J Clin Oncol. 2018;36(19):1957–1962. doi: 10.1200/JCO.2017.76.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiGrande S. Medical marijuana in cancer treatment: no standards of care, and so far, no coverage. Am J Manag Care. 2018;24(12 Spec No.):SP519–SP521. [PubMed] [Google Scholar]
- 71.Asselin A, Lamarre OB, Chamberland R, McNeil SJ, Demers E, Zongo A. A description of self-medication with cannabis among adults with legal access to cannabis in Quebec, Canada. J Cannabis Res. 2022;4(1):26. doi: 10.1186/s42238-022-00135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Belle-Isle L, Walsh Z, Callaway R, Lucas P, Capler R, Kay R, et al. Barriers to access for Canadians who use cannabis for therapeutic purposes. Int J Drug Policy. 2014;25(4):691–699. doi: 10.1016/j.drugpo.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain: United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95. doi: 10.15585/mmwr.rr7103a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haroutounian S, Arendt-Nielsen L, Belton J, Blyth FM, Degenhardt L, Di Forti M, et al. International association for the study of pain presidential task force on cannabis and cannabinoid analgesia: research agenda on the use of cannabinoids, cannabis, and cannabis-based medicines for pain management. Pain. 2021;162(Suppl 1):S117–S124. doi: 10.1097/j.pain.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erridge S, Coomber R, Sodergren MH. Medical cannabis, CBD wellness products and public awareness of evolving regulations in the United Kingdom. J Cannabis Res. 2022;4(1):56. doi: 10.1186/s42238-022-00165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen LC, Yang D, Nicolaescu V, Best TJ, Gula H, Saxena D, et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci Adv. 2022;8(8):eabi6110. doi: 10.1126/sciadv.abi6110. [DOI] [PubMed] [Google Scholar]
- 77.van Breemen RB, Muchiri RN, Bates TA, Weinstein JB, Leier HC, Farley S, et al. Cannabinoids block cellular entry of SARS-CoV-2 and the emerging variants. J Nat Prod. 2022;85(1):176–184. doi: 10.1021/acs.jnatprod.1c00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lavender I, McGregor IS, Suraev A, Grunstein RR, Hoyos CM. Cannabinoids, insomnia, and other sleep disorders. Chest. 2022;162:452–465. doi: 10.1016/j.chest.2022.04.151. [DOI] [PubMed] [Google Scholar]
- 79.Corroon J. Cannabinol and sleep: separating fact from fiction. Cannabis Cannabinoid Res. 2021;6(5):366–371. doi: 10.1089/can.2021.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. 2010;159(1):129–141. doi: 10.1111/j.1476-5381.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG, Mechoulam R, et al. Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology. 2011;215(3):505–512. doi: 10.1007/s00213-010-2157-4. [DOI] [PubMed] [Google Scholar]
- 82.Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology. 2012;219(3):859–873. doi: 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- 83.Stone NL, Murphy AJ, England TJ, O'Sullivan SE. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br J Pharmacol. 2020;177(19):4330–4352. doi: 10.1111/bph.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou C, Assareh N, Arnold JC. The cannabis constituent cannabigerol does not disrupt fear memory processes or stress-induced anxiety in mice. Cannabis Cannabinoid Res. 2021;7:294–303. doi: 10.1089/can.2021.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zagzoog A, Mohamed KA, Kim HJJ, Kim ED, Frank CS, Black T, et al. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci Rep. 2020;10(1):20405. doi: 10.1038/s41598-020-77175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Navarro G, Varani K, Reyes-Resina I, Sanchez de Medina V, Rivas-Santisteban R, Sanchez-Carnerero Callado C, et al. Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1–CB2 heteroreceptor complexes. Front Pharmacol. 2018;9:632. doi: 10.3389/fphar.2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walsh KB, McKinney AE, Holmes AE. Minor cannabinoids: biosynthesis, molecular pharmacology and potential therapeutic uses. Front Pharmacol. 2021;12:777804. doi: 10.3389/fphar.2021.777804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Filipiuc LE, Ababei DC, Alexa-Stratulat T, Pricope CV, Bild V, Stefanescu R, et al. Major phytocannabinoids and their related compounds: should we only search for drugs that act on cannabinoid receptors? Pharmaceutics. 2021;13(11):1823. doi: 10.3390/pharmaceutics13111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karschner EL, Swortwood-Gates MJ, Huestis MA. Identifying and quantifying cannabinoids in biological matrices in the medical and legal cannabis era. Clin Chem. 2020;66(7):888–914. doi: 10.1093/clinchem/hvaa113. [DOI] [PubMed] [Google Scholar]
- 90.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45(1):50–52. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- 92.Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9(2):164–173. doi: 10.1016/j.jpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694–701. doi: 10.1503/cmaj.091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):136–148. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68(7):515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 96.Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34(3):672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain. 2015;16(7):616–627. doi: 10.1016/j.jpain.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329(7460):253. doi: 10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65(6):812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 100.Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438–449. doi: 10.1016/j.jpain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 101.The Board of Medicine. https://www.boardofmedicine.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the main text or the supplementary materials.