Abstract
Although, there is a high rate of good prognosis in early stage head and neck tumors, about half of these tumors are detected in advanced stages with poor prognosis. A combination of chemotherapy, radiotherapy, and surgery is the treatment option in head and neck cancer (HNC) patients. Although, cisplatin (CDDP) as the first-line drug has a significant role in the treatment of HNC patients, CDDP resistance can be observed in a large number of these patients. Therefore, identification of the molecular mechanisms involved in CDDP resistance can help to reduce the side effects and also provides a better therapeutic management. MicroRNAs (miRNAs) as the post-transcriptional regulators play an important role in drug resistance. Therefore, in the present review we investigated the role of miRNAs in CDDP response of head and neck tumors. It has been reported that the miRNAs exerted their roles in CDDP response by regulation of signaling pathways such as WNT, NOTCH, PI3K/AKT, TGF-β, and NF-kB as well as apoptosis, autophagy, and EMT process. The present review paves the way to suggest a non-invasive miRNA based panel marker for the prediction of CDDP response among HNC patients. Therefore, such diagnostic miRNA based panel marker reduces the CDDP side effects and improves the clinical outcomes of these patients following an efficient therapeutic management.
Keywords: Head and neck cancer, MicroRNAs, Cisplatin, Drug resistance, Diagnosis, Panel marker
Background
Head and neck cancers (HNCs) are a heterogeneous group of malignancies originating from the epithelium of the upper aero-digestive tracts [1, 2]. HNCs are the sixth most frequent malignancies globally that account for 5.3% of all cancers [3]. HNC incidence has been growing during the past decade in which almost 880,000 newly diagnosed cases and 450,000 mortalities have been reported annually worldwide [4]. Lip and oral cavity cancers are the most common types of HNC with 377,713 new cases and 177,757 deaths, followed by larynx cancer with 184,615 new cases and 99,840 deaths in 2020 [5, 6]. Surgery and chemo radiotherapy are the most common therapeutic methods in HNC patients. Surgery or radiotherapy alone has been shown to be sufficient for treating and enhancing survival rates of nearly one-third of the early-stage HNC cases. However, most of the advanced-stage HNC cases require chemotherapy alone or in combination with radiotherapy, which have favorable results in improving the patient’s survivals [7, 8]. Head and neck squamous cell carcinoma (HNSCC) is the most frequent pathological HNC type [9]. The locally advanced HNSCC is a poor prognostic cancer with the 5-year survival rate of 40–60%. Metastatic HNSCC or loco regional recurrence of tumor cells has a poor response to the surgery or radiation therapy [10, 11]. Cisplatin (CDDP), docetaxel, paclitaxel, and 5-fluorouracil (5-FU) are the first line chemotherapeutic regimens that are typically used for HNC patients [12, 13]. Cisplatin exerts its cytotoxic effects via DNA adducts which results in induction of DNA damage response and apoptosis [14]. It can also interact with different cytoplasmic molecules resulting in the formation of reactive oxygen species which further promotes DNA damage [15]. Cisplatin has a wide spectrum of the side effects in HNC patients including dermatitis, dysphagia, kidney injury, anemia, and hearing loss [16, 17]. Chemo-radiotherapy is the main clinical approach for nasopharyngeal carcinoma (NPC). However, despite the considerable advances in treatment methods, the 5-year survival rate remains significantly low because of the distant metastasis and drug resistance. Cisplatin (CDDP) is used as the first-line drug for the NPC treatment. Due to CDDP resistance and dose-related cytotoxicity, which are key barriers for the successful NPC treatment, the efficiency of CDDP-based chemotherapy is limited [18]. Oral squamous cell carcinoma (OSCC) is the most common HNC that contains almost 90% of oral cancers [19]. A combination of the surgery, chemotherapy, and radiotherapy is recommended as the common treatment option for the OSCC [20]. Cisplatin is the first-line therapeutic method in OSCC [21, 22]. However, cisplatin resistance is commonly observed among the OSCC patients with tumor relapse who have poor clinical outcomes [23]. Cisplatin is widely used to treat advanced-stage laryngeal carcinoma, however there is a poor prognosis due to cisplatin resistance among laryngeal cancer patients [24, 25]. Neoadjuvant systemic therapy is one of the therapeutic options to reduce death rate in advanced tongue cancer. On the other hand, chemotherapy has some limitations due to the intrinsic or acquired drug-resistance. A considerable number of tongue cancers are found to be resistant to chemotherapy, which leads to a more aggressive phenotype and poor prognosis [26, 27].
Regarding the dose-related cytotoxicity and side effects of CDDP in normal tissues and organs, it is necessary to predict the CDDP response in head and neck tumors. Therefore, the molecular mechanisms of CDDP resistance should be clarified in these tumors to reduce the side effects and introduce the most efficient therapeutic methods. CDDP resistance can be associated with a variety of cellular processes, including drug efflux, enhanced DNA repair, and stimulation of drug-detoxifying systems [28]. Mismatch repair (MMR) is a DNA repair mechanism involved in single-strand DNA (ssDNA) errors repair. MMR pathway is accomplished by MutSα (MSH2-MSH6) and MutSβ (MSH2-MSH3) [29]. It has been shown that aberrant MMR can be associated with CDDP resistance and poor prognosis. MMR has a key role to maintain CDDP interstrand DNA cross-links that promotes cellular sensitivity. MSH2-MSH6 complex is required for the CDDP sensitivity [30]. MicroRNAs (miRNAs) function as the key post-transcriptional regulators through the inhibition of mRNA translation or mRNA degradation [28]. About one third of human miRNAs are organized in clusters that can be transcribed in a single transcriptional unit. Although, majority of the miRNAs are intronic, a minor group of miRNAs are located in repetitive sequences. The miRNA biogenesis begins with the pri-miRNA transcription by RNA polymerase II that can be processed to pre-miRNA by Drosha/DGCR8 complex. Subsequently, pre-miRNA is exported to the cytoplasm to convert to the mature duplex miRNA by Dicer [31]. MiRNAs have pivotal roles in regulation of cell proliferation, cell death, migration, and drug resistance as oncogenes or tumor suppressors [14, 32]. They have higher stabilities in body fluids compared with mRNAs enabling to suggest them as the non-invasive diagnostic biomarkers for cancer patients [33]. Since, miRNAs have key roles in regulation of CDDP response in head and neck tumors [34, 35]; in the present review we discussed the cell and molecular mechanisms by which miRNAs affect the CDDP response in these tumors (Table 1).
Table 1.
All of the miRNAs associated with Cisplatin (CDDP) resistance in head and neck tumors
| STUDY | YEAR | TUMOR TYPE | GENE | TARGET | SAMPLES | RESULTS |
|---|---|---|---|---|---|---|
| Song (35) | 2021 | LSCC | miR-497-5p | SEPT2 |
38 patients Hep2, TU212, TU686, SCC-2 and 16HBE cell lines |
Increased CDDP sensitivity |
| Lin (46) | 2016 | OSCC | miR-203 | PIK3CA |
10 patients Tca8113 cell line |
Increased CDDP Sensitivity |
| Wang (50) | 2017 | NPC | ANRIL | let-7a |
35 patients CNE1, CNE2, S18, HONE1, and 5–8 F cell lines |
Increased CDDP resistance |
| Lin (51) | 2020 | NPC | miR-454-3p | c-Met |
96 patients C666-1 and HNE1 cell lines |
Increased CDDP sensitivity |
| Zheng (53) | 2015 | TSCC | miR-24 | PTEN |
79 patients NHOK, UMI, UM2, Cal27, SCCI, SCC2, SCC9, SCC15 and SCC25 cell lines |
Increased CDDP resistance |
| Sheng (54) | 2022 | HNSCC | miR-21 | PTEN |
30 patients UMSCC-1, UMSCC-10 A, UMSCC-22B, Cal33, UPCI-4B, UPCI-15B, 1483, and 686LN cell lines |
Increased CDDP resistance |
| Zhen (56) | 2017 | NPC | miR-374a | CCND1 |
239 patients SUNE1, 5 − 8 F, shPDCD4-SUNE1, shPDCD4-HONE1 and PDCD4-overexpressed 5 − 8 F cell lines |
Increased CDDP sensitivity |
| Liu (57) | 2017 | OSCC | miR-21 | PTEN and PDCD4 | HSC-3 and SCC-9 cell lines | Increased CDDP resistance |
| Shi (62) | 2016 | NPC | miR-26b | JAG1 |
66 patients CNE2, HNE1, HNE1/DDP and CNE2/DDP cell lines |
Increased CDDP sensitivity |
| Yuan (66) | 2018 | laryngeal carcinoma | miR-320a | RBPJ |
24 patients HEp-2 cell line |
Increased CDDP sensitivity |
| Zeng (72) | 2021 | OSCC | miR-4786-3p | SELENBP1 | Cal27, HSC3, SCC25, HOK and SCC9 cell lines | Increased CDDP resistance |
| Zhuang (75) | 2017 | oral cancer | miR-218 | PPP2R5A |
61 patients UM1, UM2, Cal27, MD1386Ln and Tca8113 cell lines |
Increased CDDP resistance |
| Wang (77) | 2017 | NPC | miR-183 | MTA1 |
29 patients C666-1, CNE1, CNE2, HONE1, and 5-8 F cell lines |
Increased CDDP sensitivity. |
| Shibata (78) | 2022 | HNSCC | miR-766-3p | NR3C2 |
12 patients CAL27 and FaDu cell lines |
Increased CDDP resistance |
| Yuan (80) | 2019 | laryngeal carcinoma | miR-425-5p | PTCH1 |
24 patients Hep-2 Hep-2/R Cell lines |
Increased CDDP sensitivity |
| Bissey (96) | 2020 | NPC | miR-34c | SOX4 |
246 patients C666–1, NP69, NP460, and HEK 293T cell lines |
Increased CDDP sensitivity |
| Chen (97) | 2020 | OSCC | miR-132 | TGF-β1 |
37 patients SCC-9 and CAL-27 cell lines |
Increased CDDP sensitivity |
| Gu (100) | 2018 | TSCC | miR-22 | KAT6B |
28 patients CAL27, SCC9, and HCT 116 cell lines |
Increased CDDP sensitivity |
| Lin (102) | 2021 | NPC | miR-515-5p | IL-25 |
138 patients HK-1 and CNE-1 cell lines |
Increased CDDP sensitivity |
| Liu (109) | 2015 | ACC | miR-101-3p | Pim-1 |
30 patients SACC-LM and SACC-83 cell lines |
Increased CDDP sensitivity |
| Wang (110) | 2019 | OSCC | miR-214-3p | PIM-1 |
31 patients NHOK, OSCC, TSCCA, CAL-27, SCC-9, and Tca8113 cell lines |
Increased CDDP sensitivity |
| Tian (116) | 2017 | laryngeal cancer | miR-26b | ATF2 | Hep-2 and Hep-2/R cell lines | Increased CDDP sensitivity. |
| Fan (123) | 2015 | TSCC | miR-483-5p | FIS1 |
108 patients CAL27 and SCC9 cell lines |
Increased CDDP resistance |
| Chen (130) | 2015 | NPC | miR-125a and miR-125b | p53 mRNA |
10 patients TW03, CNE-1, CNE-2 and NP69 |
Increased CDDP resistance |
| Wang (134) | 2020 | OSCC | miR-421 | MEIS2 |
45 patients NHOK, CAL-27, Tca8113, SCC-9, and TSCCA |
Increased CDDP sensitivity |
| Liu (138) | 2016 | laryngeal carcinoma | miR-125a | HAX-1 |
30 patients Hep-2 cell line |
Increased CDDP sensitivity. |
| Lin (140) | 2020 | LSCC | miR-936 | GPR78 |
25 patients Hep-2, 16HBE, HEK293T and KB-3-1 cell lines |
Increased CDDP sensitivity |
| Chen (148) | 2021 | LSCC | miR-107 | HMGB1 | 30 patients | Increased CDDP sensitivity |
| Zhao (149) | 2020 | NPC | miR-1278 | ATG2B |
90 patients CNE-1, CNE-2, C666-1, 5–8 F and HONE-1 cell lines |
Increased CDDP sensitivity |
| Feng (150) | 2021 | laryngeal carcinoma | miR-376a | ATG2A |
30 patients SNU46 and M4E cell lines |
Increased CDDP sensitivity |
| Hao (155) | 2020 | NPC | miR-205 | HER3 | CNE1, CNE2, SUNE1 and HK1 cell lines | Increased CDDP sensitivity |
| Zhang (160) | 2019 | NPC | miR-205-5p | PTEN | HNE1 and HNE1/DDP cell lines | Increased CDDP resistance |
| Peng (162) | 2015 | TSCC | miR-23a | Twist1 | SCC-4 and Tca8113 cell lines | Increased CDDP resistance |
| Wang (163) | 2017 | TSCC | miR-15b | TRIM14 | SCC25 and SCC25-res cell lines | Increased CDDP sensitivity |
| Li (167) | 2021 | NPC | miR-98 | PBX3 |
40 patients NP-69 and 5-8 F cell lines |
Increased CDDP sensitivity |
| Sun (170) | 2012 | TSCC | miR-200b and miR-15b | BMI1 | CAL27, SCC25, CAL27-res and SCC25-res cell lines | Increased CDDP sensitivity |
| Yang (174) | 2020 | NPC | miR-200c | c-myc |
149 patients CNE1, CNE2, 5–8 F cell lines |
Increased CDDP sensitivity |
| Yang (178) | 2021 | HNC | miR-136-5p | ROCK1 | FaDu and FD-LSC-1 cell lines | Increased CDDP sensitivity |
| Zhang (181) | 2018 | TSCC | miR-211-5p | Ezrin |
102 patients CAL27, SCC9, CAL27-res and SCC9-res cell lines |
Increased CDDP sensitivity. |
| Cao (182) | 2020 | NPC | miR-218-5p | GDPD5 |
CNE1, SUNE1, HNE1, and 5-8 F Cell lines |
Increased CDDP sensitivity |
| Shan (186) | 2015 | TSCC | miR-338 | HIF-1α |
5 patients NP69, CNE2, CNE1, 5-8 F and 6-10B cell lines |
Increased CDDP sensitivity |
| Yuan (188) | 2021 | NPC | miR-454 | USP47 |
50 patients 5-8 F and SUNE-1 cell lines |
Increased CDDP sensitivity |
| Song (190) | 2021 | OSCC | miR-619-5p | ATXN3 |
40 patients HOK, Leuk-1, HN4, HN6, CAL27, and UMSCC38 cell lines |
Increased CDDP sensitivity |
| Yuan (195) | 2017 | NPC | miR-125b | Bcl-2 and MDR1 | CNE2 cell line | Increased CDDP sensitivity |
| Gobin (196) | 2023 | LSCC | miR-9 | ABCC1 | UM-SCC-12 and UM-SCC-10 A cell lines | Increased CDDP sensitivity |
| Gao (197) | 2022 | OSCC | miR-188-3p | ABCB1 |
60 patients SCC-4, SCC-9, CAL-27, UM1, and UM2 cell lines |
Increased CDDP sensitivity |
Role of miRNAs in pathology of head and neck cancers
Despite recent progresses in therapeutic methods, there is still a poor prognosis in advanced HNC. Regarding the heterogeneity of HNSCC, there is a need for the early diagnosis. Tumor metastasis is a complex process, including the tumor cells dissemination from the primary tumor, intravasation, extravasation, and secondary colonization. MiRNAs have a key role in tumor metastasis by regulation of EMT, invasion, and tumor cells self-renewal. EMT enhances tumor cell invasion, anoikis resistance, CSC features, and drug resistance in HNC tumors [36, 37]. MiRNAs have both oncogenic and tumor suppressor roles during HNC progression. OncomiRs are associated with malignant transformation and metastasis via the regulation of cell migration, proliferation, and angiogenesis [38, 39]. MiRNAs not only target a single gene, but they can also regulate an entire signaling pathway that shows the complexity of the intracellular interactions during the pathogenesis of LSCC [40]. Aberrant miRNAs expressions have been associated with chemo-radio resistance that introduce miRNAs as diagnostic and prognostic markers for HNC patients [41]. HNC cells with deregulation of miRNAs have different metastatic capabilities via EMT, CSC features, and aniokis. Therefore, evaluation of miRNAs in these processes uncovers the molecular mechanisms of HNC progression to introduce novel therapeutic and diagnostic markers for the HNC patients. Liquid biopsy is a key non-invasive diagnostic approach for the HNC cancers that can be done by the miRNA analysis in peripheral blood and saliva. This method improves the screening programs and early diagnosis for the real-time tumor monitoring in personalized medicine [42].
Role of miRNAs in cDDP reponse in HNC cells by regulation of PI3K/AKT signaling pathway
Phosphatidylinositol 3-kinase (PI3K) is a serine/threonine kinase that transfers the extracellular signals into the cells to mediate a variety of cellular processes. Protein kinase B (AKT) is the main effector of PI3K pathway that promotes cell growth and metabolism while inhibits the apoptosis [43]. Deregulation of PI3K/AKT is an important process that modulates multi-drug resistance (MDR) [44]. PIK3CA as the catalytic component of the PI3K complex is involved in tumor progression [45]. MiRNAs are involved in CDDP response in head and neck tumors by regulation of PI3K/AKT pathway (Fig. 1). It was found that miR-203 promoted cisplatin-induced apoptosis by inhibiting the self-renewal of cancer stem cells. MiR-203 was down regulated subsequent to the cisplatin treatment in tumor tissues. MiR-203 was associated with CDDP resistance through PIK3CA targeting in tongue squamous cancer [46]. C-Met as an activator of the PI3K/AKT pathway can induce mTOR and MDM2 while suppress BAD and GSK3 to promote cell growth and apoptosis resistance [47, 48]. Hence, the PI3K/AKT/mTOR axis and its downstream signaling cascade can promote tumor cell invasion and drug resistance through increasing cell cycle and inhibiting cell apoptosis [49, 50]. It has been investigated that HOXA11-AS silencing promoted apoptosis and CDDP-sensitivity in NPC cells by suppressing the Met/Akt/mTOR pathway through up-regulation of miR-454-3p [51]. IGF1R is a receptor tyrosine kinase that promotes the PI3K/AKT pathway. Circ_0005033 increased CDDP resistance in LSCC through miR-107/IGF1R axis [52]. PETN is an inhibitor of PI3K/AKT pathway. It was shown that miR-24 targeted PTEN/AKT pathway to promote cell viability and CDDP resistance in TSCC cells [53]. MiR-21 enhanced cell proliferation and CDDP resistance through PTEN targeting in HNSCC cells [54]. PDCD4 is a cytoplasmic tumor suppressor that inhibits PI3K/AKT/c-JUN pathway and cell cycle regulators including CCND1 and c-MYC that suppress cell cycle progression [55]. It is a tumor suppressor that inhibits tumor growth by interacting with eIF4A and eIF4G to suppress mRNA translation. It has been observed that miR-374a inhibited NPC cell growth and invasion, while enhanced CDDP sensitivity. MiR-374a was negatively associated with CCND1, PI3K/AKT, and c-JUN. CCND1 was the direct target of miR-347a and reduced the miR-374a mediated cell growth suppression, metastasis, and chemo resistance. C-JUN down regulated miR-374a and increased the levels of CCND1 expressions. PDCD4 up regulated the miR-374a via inhibition of PI3K/AKT/c-JUN signaling pathway [56]. MiR-21 promoted the CDDP resistance in OSCC cells by PTEN and PDCD4 targeting [57].
Fig. 1.
Role of miRNAs in CDDP response through the regulation of PI3K/AKT pathway in head and neck tumor cells. HOXA11-AS silencing promoted apoptosis and CDDP-sensitivity in NPC cells by suppressing the Met/Akt/mTOR pathway through up-regulation of miR-454-3p. MiR-21 enhanced cell proliferation and CDDP resistance through PTEN targeting in HNSCC cells. MiR-374a inhibited NPC cell growth and invasion, while enhanced CDDP sensitivity. PDCD4 up regulated the miR-374a via inhibition of PI3K/AKT/c-JUN signaling pathway. MiR-203 was associated with CDDP resistance through PIK3CA targeting in tongue squamous cancer. (Created with BioRender.com)
Role of miRNAs in cDDP reponse in HNC cells by regulation of NOTCH, WNT, and Shh signaling pathways
NOTCH is a developmental cell-cell adhesion dependent signaling pathway that is orchestrated by the ligand (JAG and DLL) binding to NOTCH receptors. Ligand binding induces the cleavage of NOTCH intracellular domain that enters to the nucleus where it regulates the expression of target genes by the CSL/MAML transcriptional complex [58]. NOTCH pathway has a crucial role in metastasis and chemo resistance of tumor cells [59, 60]. MiRNAs are involved in CDDP response of head and neck tumors by regulation of NOTCH pathway (Fig. 2). FOXC2 promoted chemo resistance of NPCs through EMT induction [61]. There was significant miR-26b down-regulation in CDDP resistant NPC compared with sensitive patients. FOXD3 regulated the miR-26b expression that subsequently targeted the JAG1 in NPC cells [62]. RBPJ is a pivotal transcriptional factor associated with NOTCH signaling [63, 64]. RBPJ binds to the NOTCH and serves as a transcriptional activator. RBPJ also suppresses gene expression in cooperation with co-repressors [65]. AFAP1-AS1 increased laryngeal tumor cell chemo resistance and self-renewal via sponging miR-320a and RBPJ up regulation [66]. SELENBP1 is a member of the selenium-binding protein family that is widely expressed in organs such as kidney, liver, heart, and lung [67]. SELENBP1 inhibits the malignant behaviors of cancer cells such as cell proliferation, migration, and EMT [68–70]. It suppresses tumor angiogenesis by binding and inhibiting the DLL4 in NOTCH pathway [71]. SELENBP1 was reported to inhibit chemo resistance in OSCC cells by functioning as a KEAP1 transcriptional activator, resulting in ubiquitination and degradation of NRF2. Down-regulation of SELENBP1 was associated with poor prognosis, increased tumor growth, and recurrence in OSCC patients. SELENBP1 down-regulation increased 5-FU and cisplatin resistance in OSCC cells. SELENBP1 targeting by miR-4786-3p promoted chemo-resistance in OSCC through modulation of KEAP1–NRF2 axis [72].
Fig. 2.
Role of miRNAs in CDDP response through the regulation of NOTCH and WNT pathways in head and neck tumor cells. FOXD3 regulated the miR-26b expression that subsequently targeted the JAG1 in NPC cells. AFAP1-AS1 increased laryngeal tumor cell chemo resistance and self-renewal via sponging miR-320a and RBPJ up regulation. MiR-183 increased CDDP sensitivity through MTA1 targeting in NPC cells. (Created with BioRender.com)
WNT is also a developmental signaling pathway involved in tumor progression and chemo resistance. It is orchestrated by binding of the WNT ligands to the FZD receptor that result in β-catenin activation. Then β-catenin enters into the nucleus to regulate WNT target genes by TCF/LEF transcriptional complex [73]. MiRNAs have critical roles in CDDP response of head and neck tumors by regulation of WNT pathway (Fig. 2). Protein Phosphatase 2 Regulatory Subunit B’Alpha (PPP2R5A) has been shown to regulate cell proliferation via the WNT pathway [74]. There was miR-218 up regulation in CDDP-resistant oral tumor cells and tissues. MiR-218 down regulation suppressed WNT signaling in oral tumor cells and promoted CDDP-mediated apoptosis through PPP2R5A targeting. MiR-218 up regulated the β-catenin and GSK3β while down regulated PPP2R5A [75]. Metastasis-associated protein 1 (MTA1) is a regulator of WNT1 signaling [76]. It has been shown that miR-183 increased CDDP sensitivity through MTA1 targeting in NPC cells [77]. Inhibition of miR-766-3p increased the CDDP sensitivity of HNSCC cells via NR3C2 targeting that induced β-catenin/c-Myc axis [78].
Sonic hedgehog (Shh) is a pivotal signaling pathway involved in cell proliferation and differentiation that functions by PTCH1 binding to the Hedgehog ligands and activation of smoothened (SMO) receptor following the SUFU release. Subsequently, the binding of PTCH1 to hedgehog activates GLI which in turn induces expression of GLI and PTCH1. GLI is involved in the transcriptional control of Sox2 and Nanog [79]. It has been shown that LINC-PINT decreased both cancer cell self-renewal and chemo resistance in laryngeal tumor cells. Deregulation of the LINC-PINT resulted in miR-425p up regulation and subsequent PTCH1 down regulation. Silencing of PTCH1 inhibited the GLI and its downstream targets such as Sox2 and Nanog [80]. Hyaluronan (HA) is a crucial extracellular matrix molecule in mammalians [81]. CD44 is a trans-membrane glycoprotein that is expressed in a wide variety of cells and tissues [82]. All of the CD44 isoforms have a HA binding site in their extracellular domain, rendering them a key HA cell surface receptor [83]. Nanog is a developmental transcription factor associated with self-renewal of stem cells [84]. It has been observed that the HA-CD44 interaction increased sphere formation, and self-renewal in CD44v3highALDH1high head and neck squamous cell tumor cells. HA-CD44v3 interaction also activated the Oct4, Sox2, and Nanog in these cells that along with miR-302 cluster activity were considered as the targets to overcome CDDP resistance in HNC tumors [85].
Role of miRNAs in cDDP reponse in HNC cells by regulation of transforming growth factor β (TGF-β), NF-kb, and JNK signaling pathways
Transforming growth factor-β (TGF-β) is a pivotal intracellular signaling pathway in regulation of cell proliferation, cell adhesion, apoptosis, EMT, and drug resistance [86, 87]. TGFβ1 up-regulates the SOX4 during tumor progression [88–90]. SOX4 deregulation plays a pivotal role in cell cycle, apoptosis, chemo radiation response, and EMT [91–95]. It has been observed that TGFβ1 up regulated the SOX4 following the miR-34c down-regulation, which resulted in EMT induction and CDDP resistance in NPC cells [96]. MiR-132 decreased cell proliferation and invasion, while induced CDDP sensitivity in OSCC cells via TGF-β1 inhibition [97].
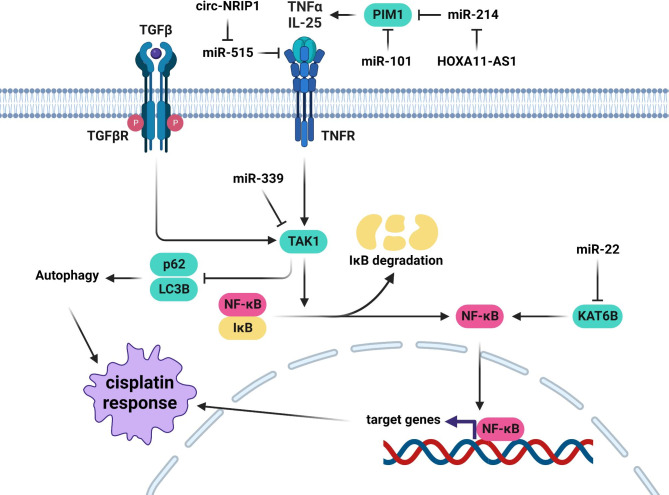
NF-κB is a key signaling pathway involved in tumor progression by regulation of cell proliferation and angiogenesis [98]. MiRNAs are the key players in CDDP response of head and neck tumors by regulation of NF-κB pathway (Fig. 3). KAT6B is a Histone acetyltransferase involved in regulation of cell cycle, DNA repair, and signal transduction [99]. There was a significant correlation between miR-22 expression and CDDP sensitivity in tongue cancer patients. It was suggested that the enhanced chemo sensitivity in tongue cancer cells could be achieved by miR-22-mediated down regulation of KAT6B, which results in decreased NF-kB activity and increased cell death in response to chemotherapy [100]. IL-25 has been shown to promote chemo resistance in tumor cells by activating the NF-κB signaling [101]. There was a significant circ-NRIP1 up-regulation in the serum samples of CDDP-resistant NPC compared with sensitive patients. Circ-NRIP1 down regulation decreased the CDDP-resistance of NPC cells by regulating the miR-515-5p/IL-25 axis [102]. Adenoid cystic carcinoma (ACC) is an uncommon neoplasm of salivary glands with neural and vessel invasion and poor long-term survival rate due to a high risk of distant metastasis [103, 104]. A third of ACC patients with distant metastasis are likely to die within two years [105]. Tumor cells commonly acquire MDR subsequent to the administration of a single chemotherapy drug, which accounts for the majority of cancer-related mortality [106]. PIM1 belongs to the active serine/threonine kinase family that promotes tumor progression by regulation of cell cycle, cell death, and signaling pathways [107]. It activates NF-kB signaling following the TNF-α induction by RelA/p65 recruitment [108]. There was a significant miR-101-3p down regulation in ACC tissues compared with normal parotid glands. MiR-101-3p inhibited cell proliferation, invasion, and colony formation, while promoted apoptosis and CDDP sensitivity in ACC cells through Pim-1 targeting [109]. There was HOXA11-AS up-regulation in CDDP-resistant OSCC cells. It promoted CDDP-resistance by modulating the miR-214-3p/PIM1 axis [110].
Fig. 3.
Role of miRNAs in CDDP response through the regulation of NF-kB pathway in head and neck tumor cells. Enhanced chemo sensitivity in tongue cancer cells could be achieved by miR-22-mediated down regulation of KAT6B. Circ-NRIP1 down regulation decreased the CDDP-resistance of NPC cells by regulating the miR-515-5p/IL-25 axis. MiR-101-3p promoted apoptosis and CDDP sensitivity in ACC cells through Pim1 targeting. HOXA11-AS promoted CDDP-resistance by modulating the miR-214-3p/PIM1 axis. MiR-339-5p suppressed autophagy to increase CDDP sensitivity in laryngeal carcinoma cells through TAK1 targeting. (Created with BioRender.com)
C-Jun N-terminal kinases (JNKs) are the members of the MAP kinases that are involved in stress response, cell death, and senescence [111]. ATF2 is a cAMP response element binding family member which is activated by JNK [112, 113]. DNA damage causes ATF2 phosphorylation via activating the JNK pathway. Phosphorylated ATF2 subsequently promotes DNA repair via targeting the factors involved in cell survival. Therefore, phosphorylation of ATF2 results in CDDP resistance through promoting the DNA-repair [114, 115]. MiR-26b reduced CDDP resistance through ATF2 targeting in laryngeal tumor cells [116].
Role of miRNAs in cDDP reponse in HNC cells by regulation of apoptosis and autophagy
CDDP forms DNA adducts that can be finally results in apoptosis induction. Therefore, miRNAs can also affect the CDDP response by regulation of apoptosis pathway in head and neck tumors (Fig. 4). Mitochondrial fission triggers the apoptosis by the release of pro-apoptotic factors that activate caspase proteins. Abnormal mitochondrial dynamics are implicated in the regulation of apoptosis and have been associated with a variety of disorders [117–119]. Different proteins, such as DRP1, FIS1, and MFF in mammalian cells, mediate mitochondrial fission. DRP1 is required for mitochondrial fission, and its inhibition leads to decreased cell death in various tumors [120]. FIS1 acts as a DRP1 receptor, allowing DRP1 to enter mitochondria and carry out mitochondrial fission and cell death [121, 122]. It has been reported that miR-483-5p suppressed mitochondrial fission and CDDP sensitivity via FIS1 targeting in tongue squamous cell carcinoma (TSCC) cells [123]. The importance of the tumor microenvironment in tumor development and chemo resistance has been the subject of numerous studies [124]. Cancer-associated fibroblasts (CAFs) have a pivotal role in HNC progression through extracellular matrix remodeling, growth factors secretion, and therapeutic resistance induction [124–126]. ING5 proteins regulate the expression of many genes, including p53 target genes BAX and p21 [127]. It was observed that HNC-derived CAFs were intrinsically resistant to cisplatin and that CAF-CM could promote HNC cell proliferation and survival following cisplatin administration. CAF-derived exosomes carrying miR-196a were shown to be associated with cisplatin resistance in HNC cells. MiR-196a increased cell proliferation, while decreased cell death in HNC subsequent to cisplatin therapy. After being transported from CAFs to HNC cells, exosomal miR-196a modulated cell proliferation and apoptosis via targeting CDKN1B and ING5. MiR-196a up-regulation was also associated with CDDP resistance in HNC cells [128]. There was GAS5 down regulation in CDDP-resistant OSCC cells and tissues that were correlated with survival rates. GAS5 recovered the CDDP sensitivity in OSCC cells by miR-196a sponging [129]. There were miR-125 up regulations in CDDP-resistant NPC tissues in comparison with normal tissues which were associated with p53 targeting [130]. SF1 RNA-binding protein is involved in the formation of spliceosomes [131]. There was a significant UCA1 up regulation in OSCC tissues compared with normal samples. UCA1 increased OSCC cells proliferation and CDDP-resistance through miR-184 sponging and SF1 up regulation. UCA1 down-regulation could be associated with BAX up regulation, caspase-3 activity, and BCL2 inhibition [132]. Zinc finger antisense 1 (ZFAS1) is involved in regulation of cell death, cell proliferation, and invasion [133]. ZFAS1 up regulation was correlated with increased cell proliferation and CDDP resistance via miR-421 sponging and MEIS1 up regulation in OSCC cells. ZFAS1 had an anti-apoptotic effect via modulating CASP-3, BAX, and BCL2 expression in CDDP resistant OSCC cells [134]. HAX-1 suppresses the mitochondrial apoptosis pathway by reducing the accumulation of BAX. Hence, HAX-1 protects cancer cells against drug-induced apoptosis [135–137]. MiR-125a promoted CDDP-induced apoptosis through HAX-1 targeting in laryngeal cancer stem cells [138]. GPR78 is a G-protein coupled receptor that serves as a death receptor to activate cell death [139]. There was miR-936 down regulation in Laryngeal Squamous Cell Carcinoma (LSCC) compared with normal tissues that was associated with poor clinical outcomes. MiR-936 suppressed LSCC cell proliferation, migration, and CDDP resistance by GPR78 targeting [140].
Fig. 4.
Role of miRNAs in regulation of CDDP mediated apoptosis in head and neck tumor cells. MiR-483-5p suppressed mitochondrial fission and CDDP sensitivity via FIS1 targeting in TSCC cells. MiR-196a modulated cell proliferation and apoptosis via targeting ING5. There were miR-125 up regulations in CDDP-resistant NPC tissues in comparison with normal tissues which were associated with p53 targeting. MiR-125a promoted CDDP-induced apoptosis through HAX1 targeting in laryngeal cancer stem cells. (Created with BioRender.com)
Autophagy is a cell survival mechanism that includes self-degradation through the transportation of cytoplasmic ligands to the lysosome to maintain body equilibrium due stressful conditions such as hypoxia and nutritional deficiency [141]. Autophagy has been shown to significantly reduce the accumulation of damaged proteins and organelles, thereby inhibiting tumorigenesis [142]. It was reported that autophagy was commonly induced in tumor cells through both chemotherapy and radiation, preserving the tumor cells from the antitumor therapy [143]. Drug resistance and tumor growth have been reported to be frequently promoted through the autophagic flux in advanced malignancies via ensuring tumor cell survival by maintaining essential energy production [144, 145]. High mobility group box-1 protein (HMGB1) is involved in DNA repair, cell proliferation, and apoptosis [146]. HMGB1 was also associated with therapeutic tolerance in some cancers as a key autophagy regulator [147]. H19 up-regulation was correlated with autophagy-mediated drug in LSCC. H19 increased CDDP sensitivity by miR-107/HMGB1 targeting in LSCC [148]. There was a significant miR-1278 down regulation in NPC tissues that was correlated with poor survival and chemotherapy response. MiR-1278 increased DDP sensitivity in NPC cells and decreased autophagy through ATG2B targeting [149]. It was found that circPGAM1-mediated drug resistance was associated with miR-376a in laryngocarcinoma. CircPGAM1 enhanced CDDP resistance by miR-376a/ATG2A targeting in laryngocarcinoma [150]. Circ-PKD2 induced Atg13-mediated autophagy through miR-646 sponging to promote the CDDP sensitivity in oral squamous cell carcinomas [151]. The increased LC3-II/LC3-I ratio is commonly linked to autophagy [152]. TAK1 is an important key regulator of signal transduction, that has an essential role in TGF-β-mediated EMT and apoptosis through controlling the JNK and p38 pathway [153]. TAK1 is also able to enhance tumor cell proliferation, metastasis, and invasion [154]. MiR-339-5p suppressed autophagy to increase CDDP sensitivity in laryngeal carcinoma cells through TAK1 targeting. MiR-339-5p could efficiently reduce the LC3-II/LC3-I ratio in CDDP-resistant laryngeal carcinoma cells [35]. There was a significant miR-205 down-regulation in nasopharyngeal carcinoma cells. MiR-205 inhibited the cell proliferation and invasion, while enhanced CDDP sensitivity in CNE1 cells. MiR-205 also increased autophagy through LC3B II up-regulation and p62 down-regulation in the nasopharyngeal carcinoma cells [155].
Role of miRNAs in cDDP reponse in HNC cells by regulation of epithelial-mesenchymal transition
Epithelial to mesenchymal transition (EMT) is a cellular mechanism in which epithelial cells obtain the mesenchymal phenotype via down regulation of the epithelial markers like CDH1, as well as up-regulation of mesenchymal markers like Vimentin and CDH2 [156–158]. EMT is defined as the loss of polarity, cell-cell adhesion, and cytoskeletal components in epithelial cell layers [159]. There was a considerable miR-205-5p up-regulation in the CDDP-resistant cells in comparison with the parental cells. MMP-2 and MMP-9 were down regulated via miR-205-5p in HNE1 cells. MiR-205-5p down regulated CDH1 while up regulated the Vimentin and, CDH2, Slug, and SNAI1 in HNE1 cells. MiR-205-5p enhanced the EMT through PTEN inhibition in CDDP-resistant NPC cells [160]. Twist1 is also an EMT specific transcription factor that can be activated by different signaling pathways [161]. It has been shown that miR-23a induced CDDP resistance via Twist1 targeting in TSCC cells [162]. Yes Associated Protein (YAP) is the main nuclear effector in Hippo pathway that is involved in tumor growth. MiR-15b induced mesenchymal-epithelial transition and CDDP sensitivity in SCC25 cells by TRIM14 targeting. TRIM14 significantly up regulated YAP in the SCC25 cells [163]. PBX3 is a transcription factor involved in EMT process and tumor progression [164–166]. Inhibition of HOXA11AS promoted the CDDP sensitivity of NPC cells via miR-98/PBX3 axis [167].
BMI1 belongs to the polycomb proteins and suppresses CDH1 transcription via PRC1/PRC2- related chromatin remodeling to promote EMT [168]. It can stabilize Snail via the regulation of PI3K/AKT/GSK-3β pathway [169]. MiR-200b and miR-15b down regulations were implicated in chemotherapy-induced EMT and chemo-resistance in TSCC cells. They reversed mesenchymal characteristics and inhibit tumor invasion in chemo resistant TSCC cells through BMI1 targeting. Down regulation of miR-200b and miR-15b were also contributed with lymph node involvement [170]. C-Myc transcription factor has been shown to regulate cell proliferation, apoptosis, metabolism, and genomic stability [171–173]. C-Myc expression was associated with miR-200c suppression through directly binding to the miR-200c promoter in primary NPC tumors. MiR-200c down regulated the CDH1 and up regulated Vimentin through ZEB2 targeting. BMI1, Suz12, and Sox2 were also down regulated by miR-200c or c-Myc suppression. Therefore, C-Myc/miR-200c axis was found to be a negative regulatory feedback loop that has a pivotal role in the EMT, chemotherapy resistance, and CSC phenotypes in nasopharyngeal cancer [174].
ROCK is an effector of Rho A that interacts with actin cytoskeleton to induce the generation of focal adhesion and tumor cell invasion [175, 176]. It is also involved in regulation of cell proliferation and migration, and EMT. It plays a critical role in TGF-induced EMT by activating RhoA-dependent pathways [177]. MiR-136-5p suppressed LSCC and HPSCC cells migration while promoted CDDP sensitivity via ROCK1 targeting. It up regulated CDH1 and down regulated the CDH2 and vimentin. Over expression of miR-136-5p in combination with CDDP down regulated the p62 and suppressed the Akt/mTOR pathway [178]. EZR is a linkage protein between the membrane proteins and actin cytoskeleton [179]. It has also a significant role in chemo-resistance [180]. KCNQ1OT1 up regulation in CDDP resistant TSCC samples were indicated to be associated with poor prognosis. KCNQ1OT1 promoted cell proliferation and CDDP resistance through controlling the EZR/FAK/SRC axis via miR-211-5p [181]. Increased levels of MAGI2-AS3 were reported to be associated with enhanced cell proliferation, migration, and EMT by miR-218-5p/GDPD5/SEC61A1 axis in NPC. MAGI2-AS3 increased CDDP resistance through GDPD5 regulation [182].
Hypoxia has been identified as a characteristic of the variety of malignant tumor microenvironments [183, 184]. Hypoxia promotes tumor cells invasiveness and chemo resistance and leads to the poor clinical outcomes. Hypoxia-inducible factor 1-alpha (HIF-1a) is the crucial regulator of angiogenesis and hypoxia that is used as a prognostic marker in NPC [185]. It has been found that down regulation of miR-338-3p suppressed tumor proliferation via HIF-1α targeting. MiR-338-3p inhibited the CNE2 cell proliferation and migration and reversed hypoxia-induced CDDP resistance and EMT [186]. USP47 is an ubiquitin peptidase involved in hypoxia-induced EMT by SNAI1 deubiquitination and stabilization [187]. KCNQ1OT1 knockdown significantly inhibited NPC cell viability, while induced CDDP sensitivity via miR-454/USP47 axis [188]. ATXN3 is a deubiquitinase involved in cell homeostasis and tumor progression. It promoted the lung cancer through KLF4 deubiquitinating [189]. It has been found that miR-619-5p reduced OSCC cells migration via PI3K/AKT pathway. MiR-619-5p increased CDDP sensitivity in OSCC cells by ATXN3 targeting [190].
Role of miRNAs in cDDP reponse in HNC cells by regulation of transporters
MDR is a significant challenge during the cancer treatment that is mainly associated with the efflux of drugs through the ATP-binding cassette (ABC) transporters [191]. MDR1 belongs to the ABC transporter protein family that confer MDR by keeping the intracellular concentration of hydrophobic chemicals below a cell-killing threshold through an active transport mechanism [192]. MRP1 is also an ABC transporter, promoting MDR in tumor cells through reduced anticancer medication absorption [193]. It was observed that the up-regulation of circ_0004507 was associated with tumor stage, lymph node metastasis, and CDDP resistance in laryngeal cancer tissues. MRP1 and MDR1 protein levels were reduced subsequent to the Circ_0004507 down-regulation. Circ_0004507 enhanced the tumor progression and CDDP resistance of laryngeal cancer cells through miR-873 sponging [194]. There was a significant miR-125b down regulation in CNE2/DDP resistant cells. MiR-125b increased apoptosis and CDDP-sensitivity of tumor cells via Bcl-2 and MDR1 targeting [195]. MiR-9 reduced cell proliferation and migration while promoted the CDDP sensitivity via ABCC1 targeting in laryngeal tumor cells [196]. Circ_0109291 induced CDDP resistance by regulation of miR-188-3p/ABCB1 axis in of OSCC cells [197].
Conclusions
Head and neck tumors are recognized as a global health challenge due to their digestive and nutritional problems for the patients. Although, these tumors have a high chance of treatment and good prognosis, a significant proportion of these tumors are diagnosed in the advanced stages with a poor prognosis. CDDP as a first-line treatment has a critical role in the treatment of head and neck cancer patients. However, CDDP resistance can be observed in a significant rate of patients. We investigated the role of miRNAs in CDDP response of head and neck cancers. It has been reported that miRNAs affect the CDDP response in head and neck tumors by regulating signaling pathways, autophagy, apoptosis, and membrane transporters. Since, miRNAs has a higher stability in body fluids in comparison with the mRNAs; they can be suggested as non-invasive markers to predict the CDDP response in head and neck tumors. Therefore, CDDP response prediction by miRNA based panel markers can reduce the CDDP side effects and helps to define the most efficient therapeutic modality based on the personalized medicine for these cancer patients. MiRNA-based therapy can be associated with the miRNA function by the promotion of tumor suppressor miRNAs (mimics) while suppression of oncogenic miRNAs (antagomiRs) in tumor cells. Therefore, antagomiRs or mimics can be used to overcome the CDDP resistance in HNC patients. However, the cytoplasmic miRNA degradation and their side effects in normal tissues are the main therapeutic challenges in miRNA-based treatments. Therefore, the site-specific miRNA delivery can reduce the concentrations of antagomiRs or mimics that reduce the probable side effects in normal tissues.
Acknowledgements
Not applicable.
List of Abbreviations
- CDDP
Cisplatin
- miRNAs
MicroRNAs
- HNCs
Head and neck cancers
- HNSCC
Head and neck squamous cell carcinoma
- 5-FU
5-fluorouracil
- NPC
Nasopharyngeal carcinoma
- OSCC
Oral squamous cell carcinoma
- PI3K
Phosphatidylinositol 3-kinase
- MDR
Multi-drug resistance
- PPP2R5A
Protein Phosphatase 2 Regulatory Subunit B’Alpha
- MTA1
Metastasis-associated protein 1
- Shh
Sonic hedgehog
- SMO
Smoothened
- HA
Hyaluronan
- TGFβ
Transforming growth factorβ
- ACC
Adenoid cystic carcinoma
- JNKs
C-Jun N-terminal kinases
- CAFs
Cancer-associated fibroblasts
- OSCC
Oral squamous cell carcinoma
- ZFAS1
Zinc finger antisense 1
- LSCC
Laryngeal Squamous Cell Carcinoma
- HMGB1
High mobility group box-1 protein
- EMT
Epithelial to mesenchymal transition
- YAP
Yes Associated Protein
- HIF-1a
Hypoxia-inducible factor 1-alpha
- ABC
ATP-binding cassette
Authors’ contributions
FTG, AM, ASZ, and AZ were involved in search strategy, drafting, and graphical illustrations. MM supervised the project and revised and edited the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data Availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zandberg DP, Bhargava R, Badin S, Cullen KJ. The role of human papillomavirus in nongenital cancers. CA Cancer J Clin. 2013;63(1):57–81. doi: 10.3322/caac.21167. [DOI] [PubMed] [Google Scholar]
- 2.Gupta B, Johnson NW, Kumar N. Global epidemiology of Head and Neck Cancers: a Continuing Challenge. Oncology. 2016;91(1):13–23. doi: 10.1159/000446117. [DOI] [PubMed] [Google Scholar]
- 3.Chen SMY, Krinsky AL, Woolaver RA, Wang X, Chen Z, Wang JH. Tumor immune microenvironment in head and neck cancers. Mol Carcinog. 2020;59(7):766–74. doi: 10.1002/mc.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Aupérin A. Epidemiology of head and neck cancers: an update. Curr Opin Oncol. 2020;32(3):178–86. doi: 10.1097/CCO.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 7.Chow LQM. Head and Neck Cancer. N Engl J Med. 2020;382(1):60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 8.Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, et al. Head and neck cancers, Version 2.2014. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(10):1454–87. doi: 10.6004/jnccn.2014.0142. [DOI] [PubMed] [Google Scholar]
- 9.Chan JYK, Zhen G, Agrawal N. The role of tumor DNA as a diagnostic tool for head and neck squamous cell carcinoma. Semin Cancer Biol. 2019;55:1–7. doi: 10.1016/j.semcancer.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol. 2018;52(Pt 2):228–40. doi: 10.1016/j.semcancer.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Anderson G, Ebadi M, Vo K, Novak J, Govindarajan A, Amini A. An updated review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers (Basel) 2021;13:19. doi: 10.3390/cancers13194912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rambeau A, Licaj I, Gery B, Gervais R, Florescu C, Babin E, et al. Platinum rechallenge in recurrent head and neck squamous cell carcinoma after primary chemoradiation. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(4):257–61. doi: 10.1016/j.anorl.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Zangouei AS, Moghbeli M. MicroRNAs as the critical regulators of cisplatin resistance in gastric tumor cells. Genes and environment: the official journal of the Japanese Environmental Mutagen Society. 2021;43(1):21. doi: 10.1186/s41021-021-00192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–83. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 16.Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Low-dose vs. high-dose cisplatin: Lessons learned from 59 chemoradiotherapy trials in Head and Neck Cancer. Front Oncol. 2019;9:86. doi: 10.3389/fonc.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuette A, Lander DP, Kallogjeri D, Collopy C, Goddu S, Wildes TM, et al. Predicting hearing loss after Radiotherapy and Cisplatin Chemotherapy in patients with Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg. 2020;146(2):106–12. doi: 10.1001/jamaoto.2019.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–35. doi: 10.1056/NEJMoa1905287. [DOI] [PubMed] [Google Scholar]
- 19.Gasche JA, Goel A. Epigenetic mechanisms in oral carcinogenesis. Future Oncol. 2012;8(11):1407–25. doi: 10.2217/fon.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai Y, Sha J, Kanno T. The role of carcinogenesis-related biomarkers in the wnt pathway and their Effects on epithelial-mesenchymal transition (EMT) in oral squamous cell carcinoma. Cancers (Basel). 2020;12(3). [DOI] [PMC free article] [PubMed]
- 21.Karabajakian A, Gau M, Reverdy T, Neidhardt EM, Fayette J. Induction chemotherapy in Head and Neck squamous cell carcinoma: a question of belief. Cancers (Basel). 2018;11(1). [DOI] [PMC free article] [PubMed]
- 22.de Castro G, Jr, Alves GV, Castro AF, Chaves ALF, De Marchi P, de Oliveira TB, et al. Criteria for eligibility to cisplatin in the curative treatment of head and neck cancer: Consensus opinion from a panel of experts. Crit Rev Oncol Hematol. 2018;131:30–4. doi: 10.1016/j.critrevonc.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Hu H, Li B, Wang J, Tan Y, Xu M, Xu W, et al. New advances into cisplatin resistance in head and neck squamous carcinoma: mechanisms and therapeutic aspects. Biomed Pharmacother. 2023;163:114778. doi: 10.1016/j.biopha.2023.114778. [DOI] [PubMed] [Google Scholar]
- 24.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen X, Gao X, Li H, Gu Y, Wang J. TIMP-3 increases the chemosensitivity of laryngeal carcinoma to cisplatin via facilitating mitochondria-dependent apoptosis. Oncol Res. 2018;27(1):73. doi: 10.3727/096504018X15201099883047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun K, Tan JY, Thomson PJ, Choi SW. Influence of time between surgery and adjuvant radiotherapy on prognosis for patients with head and neck squamous cell carcinoma: a systematic review. Head Neck. 2023;45(8):2108–19. doi: 10.1002/hed.27401. [DOI] [PubMed] [Google Scholar]
- 27.Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk A, Epidemiology. Risk factors, and Prevention of Head and Neck squamous cell carcinoma. Med Sci. 2023;11(2). [DOI] [PMC free article] [PubMed]
- 28.Moghbeli M. MicroRNAs as the critical regulators of cisplatin resistance in ovarian cancer cells. J ovarian Res. 2021;14(1):127. doi: 10.1186/s13048-021-00882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha CRR, Silva MM, Quinet A, Cabral-Neto J, Menck CFM. DNA repair pathways and cisplatin resistance: an intimate relationship. Clinics. 2018;73(suppl 1):e478s. doi: 10.6061/clinics/2018/e478s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawant A, Kothandapani A, Zhitkovich A, Sobol RW, Patrick SM. Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair. 2015;35:126–36. doi: 10.1016/j.dnarep.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zangouei AS, Alimardani M, Moghbeli M. MicroRNAs as the critical regulators of doxorubicin resistance in breast tumor cells. Cancer Cell Int. 2021;21(1):213. doi: 10.1186/s12935-021-01873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghbeli M, Zangouei AS, Nasrpour Navaii Z, Taghehchian N. Molecular mechanisms of the microRNA-132 during tumor progressions. Cancer Cell Int. 2021;21(1):439. doi: 10.1186/s12935-021-02149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Cheng N, Luo J. Downregulation of lncRNA ANRIL represses tumorigenicity and enhances cisplatin-induced cytotoxicity via regulating microRNA let‐7a in nasopharyngeal carcinoma. J Biochem Mol Toxicol. 2017;31(7):e21904. doi: 10.1002/jbt.21904. [DOI] [PubMed] [Google Scholar]
- 35.Song K, Yu P, Zhang C, Yuan Z, Zhang H. The LncRNA FGD5-AS1/miR‐497‐5p axis regulates septin 2 (SEPT2) to accelerate cancer progression and increase cisplatin‐resistance in laryngeal squamous cell carcinoma. Mol Carcinog. 2021;60(7):469–80. doi: 10.1002/mc.23305. [DOI] [PubMed] [Google Scholar]
- 36.Bhat MY, Advani J, Rajagopalan P, Patel K, Nanjappa V, Solanki HS, et al. Cigarette smoke and chewing tobacco alter expression of different sets of miRNAs in oral keratinocytes. Sci Rep. 2018;8(1):7040. doi: 10.1038/s41598-018-25498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Z, Hu W, Xu J, Kaufmann AM, Albers AE. MicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitro. Int J Oncol. 2015;47(4):1339–50. doi: 10.3892/ijo.2015.3142. [DOI] [PubMed] [Google Scholar]
- 38.Hammond SM. RNAi, microRNAs, and human disease. Cancer Chemother Pharmacol. 2006;58(Suppl 1):63–8. doi: 10.1007/s00280-006-0318-2. [DOI] [PubMed] [Google Scholar]
- 39.Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2015;54(Suppl 1):E148–61. doi: 10.1002/mc.22221. [DOI] [PubMed] [Google Scholar]
- 40.Geng J, Liu Y, Jin Y, Tai J, Zhang J, Xiao X, et al. MicroRNA-365a-3p promotes tumor growth and metastasis in laryngeal squamous cell carcinoma. Oncol Rep. 2016;35(4):2017–26. doi: 10.3892/or.2016.4617. [DOI] [PubMed] [Google Scholar]
- 41.Yang CX, Sedhom W, Song J, Lu SL. The role of MicroRNAs in recurrence and metastasis of Head and Neck squamous cell carcinoma. Cancers (Basel). 2019;11(3). [DOI] [PMC free article] [PubMed]
- 42.Gattuso G, Crimi S, Lavoro A, Rizzo R, Musumarra G, Gallo S et al. Liquid Biopsy and circulating biomarkers for the diagnosis of precancerous and cancerous oral lesions. Noncoding RNA. 2022;8(4). [DOI] [PMC free article] [PubMed]
- 43.Moghbeli M, Makhdoumi Y, Soltani Delgosha M, Aarabi A, Dadkhah E, Memar B, et al. ErbB1 and ErbB3 co-over expression as a prognostic factor in gastric cancer. Biol Res. 2019;52(1):2. doi: 10.1186/s40659-018-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z, et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11(9):797. doi: 10.1038/s41419-020-02998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3(10):1221–4. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, Lin Y, Fan L, Kuang W, Zheng L, Wu J, et al. miR-203 inhibits cell proliferation and promotes cisplatin induced cell death in tongue squamous cancer. Biochem Biophys Res Commun. 2016;473(2):382–7. doi: 10.1016/j.bbrc.2016.02.105. [DOI] [PubMed] [Google Scholar]
- 47.Dai C, Xie Y, Zhuang X, Yuan Z. MiR-206 inhibits epithelial ovarian cancer cells growth and invasion via blocking c-Met/AKT/mTOR signaling pathway. Biomed Pharmacother. 2018;104:763–70. doi: 10.1016/j.biopha.2018.05.077. [DOI] [PubMed] [Google Scholar]
- 48.Hung CM, Kuo DH, Chou CH, Su YC, Ho CT, Way TD. Osthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food Chem. 2011;59(17):9683–90. doi: 10.1021/jf2021489. [DOI] [PubMed] [Google Scholar]
- 49.Vadlakonda L, Pasupuleti M, Pallu R. Role of PI3K-AKT-mTOR and wnt signaling pathways in transition of G1-S phase of cell cycle in Cancer cells. Front Oncol. 2013;3:85. doi: 10.3389/fonc.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Cheng N, Luo J. Downregulation of lncRNA ANRIL represses tumorigenicity and enhances cisplatin-induced cytotoxicity via regulating microRNA let-7a in nasopharyngeal carcinoma. J Biochem Mol Toxicol. 2017;31(7). [DOI] [PubMed]
- 51.Lin FJ, Lin XD, Xu LY, Zhu SQ, Long Noncoding RNA HOXA11-AS modulates the resistance of nasopharyngeal carcinoma cells to Cisplatin via miR-454-3p/c-Met. Mol Cells. 2020;43(10):856–69. doi: 10.14348/molcells.2020.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong L, Chen J, Jiang X. Circ_0005033 is an oncogene in laryngeal squamous cell carcinoma and regulates cell progression and cisplatin sensitivity via miR-107/IGF1R axis. Anticancer Drugs. 2022;33(3):245–56. doi: 10.1097/CAD.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 53.Zheng X, Li J, Peng C, Zhao J, Chi J, Meng X, et al. MicroRNA-24 induces cisplatin resistance by targeting PTEN in human tongue squamous cell carcinoma. Oral Oncol. 2015;51(11):998–1003. doi: 10.1016/j.oraloncology.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Sheng S, Su W, Mao D, Li C, Hu X, Deng W, et al. MicroRNA-21 induces cisplatin resistance in head and neck squamous cell carcinoma. PLoS ONE. 2022;17(4):e0267017. doi: 10.1371/journal.pone.0267017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, et al. Tumor suppressor PDCD4 modulates mir-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013;4(10):e872. doi: 10.1038/cddis.2013.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhen Y, Fang W, Zhao M, Luo R, Liu Y, Fu Q, et al. miR-374a-CCND1-pPI3K/AKT-c-JUN feedback loop modulated by PDCD4 suppresses cell growth, metastasis, and sensitizes nasopharyngeal carcinoma to cisplatin. Oncogene. 2017;36(2):275–85. doi: 10.1038/onc.2016.201. [DOI] [PubMed] [Google Scholar]
- 57.Liu T, Chen G, Sun D, Lei M, Li Y, Zhou C, et al. Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma. Acta Biochim Biophys Sin (Shanghai) 2017;49(9):808–16. doi: 10.1093/abbs/gmx078. [DOI] [PubMed] [Google Scholar]
- 58.Moghbeli M, Mosannen Mozaffari H, Memar B, Forghanifard MM, Gholamin M, Abbaszadegan MR. Role of MAML1 in targeted therapy against the esophageal cancer stem cells. J translational Med. 2019;17(1):126. doi: 10.1186/s12967-019-1876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19(2):192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee S, Kong D, et al. Targeting notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta. 2010;1806(2):258–67. doi: 10.1016/j.bbcan.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Z, Zhang L, Xie B, Wang X, Yang X, Ding N, et al. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015;363(2):137–45. doi: 10.1016/j.canlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Shi L, Yin W, Zhang Z, Shi G. Down-regulation of miR-26b induces cisplatin resistance in nasopharyngeal carcinoma by repressing JAG1. FEBS Open Bio. 2016;6(12):1211–9. doi: 10.1002/2211-5463.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by notch signaling status. Genes Dev. 2013;27(9):1059–71. doi: 10.1101/gad.211912.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lake RJ, Tsai PF, Choi I, Won KJ, Fan HY. RBPJ, the major transcriptional effector of notch signaling, remains associated with chromatin throughout mitosis, suggesting a role in mitotic bookmarking. PLoS Genet. 2014;10(3):e1004204. doi: 10.1371/journal.pgen.1004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou S, Hayward SD. Nuclear localization of CBF1 is regulated by interactions with the SMRT corepressor complex. Mol Cell Biol. 2001;21(18):6222–32. doi: 10.1128/MCB.21.18.6222-6232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan Z, Xiu C, Song K, Pei R, Miao S, Mao X, et al. Long non-coding RNA AFAP1-AS1/miR-320a/RBPJ axis regulates laryngeal carcinoma cell stemness and chemoresistance. J Cell Mol Med. 2018;22(9):4253–62. doi: 10.1111/jcmm.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang PW, Tsui SK, Cc L, Cy L, Waye MM. Fung Kp. Isolation, characterization, and chromosomal mapping of a novel cDNA clone encoding human selenium binding protein. J Cell Biochem. 1997;64(2):217–24. doi: 10.1002/(sici)1097-4644(199702)64:2<217::aid-jcb5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 68.Feng S, Wang S, Wang Y, Yang Q, Wang D, Li H. Identification and expression of carbonic anhydrase 2, myosin regulatory light chain 2 and selenium-binding protein 1 in zebrafish Danio rerio: implication for age-related biomarkers. Gene Expr Patterns. 2018;29:47–58. doi: 10.1016/j.gep.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Schott M, de Jel MM, Engelmann JC, Renner P, Geissler EK, Bosserhoff AK, et al. Selenium-binding protein 1 is down-regulated in malignant melanoma. Oncotarget. 2018;9(12):10445. doi: 10.18632/oncotarget.23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elhodaky M, Diamond AM. Selenium-binding protein 1 in human health and disease. Int J Mol Sci. 2018;19(11):3437. doi: 10.3390/ijms19113437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Hong R, Bei L, Yang J, Zhao X, Hu Z, et al. Selenium binding protein 1 inhibits tumor angiogenesis in colorectal cancers by blocking the Delta-like ligand 4/Notch1 signaling pathway. Translational Oncol. 2022;18:101365. doi: 10.1016/j.tranon.2022.101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeng H, Zhao X, Tang C. Downregulation of SELENBP1 enhances oral squamous cell carcinoma chemoresistance through KEAP1–NRF2 signaling. Cancer Chemother Pharmacol. 2021;88(2):223–33. doi: 10.1007/s00280-021-04284-4. [DOI] [PubMed] [Google Scholar]
- 73.Duchartre Y, Kim YM, Kahn M. The wnt signaling pathway in cancer. Crit Rev Oncol/Hematol. 2016;99:141–9. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wlodarchak N, Xing Y. PP2A as a master regulator of the cell cycle. Crit Rev Biochem Mol Biol. 2016;51(3):162–84. doi: 10.3109/10409238.2016.1143913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuang Z, Hu F, Hu J, Wang C, Hou J, Yu Z, et al. MicroRNA-218 promotes cisplatin resistance in oral cancer via the PPP2R5A/Wnt signaling pathway. Oncol Rep. 2017;38(4):2051–61. doi: 10.3892/or.2017.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu B, Qiao J, Hu J, Fan M, Zhao Y, Su H, et al. Leptin promotes endothelial dysfunction in chronic kidney disease by modulating the MTA1-mediated WNT/beta-catenin pathway. Mol Cell Biochem. 2020;473(1–2):155–66. doi: 10.1007/s11010-020-03816-5. [DOI] [PubMed] [Google Scholar]
- 77.Wang G, Wang S, Li C. MiR-183 overexpression inhibits tumorigenesis and enhances DDP-induced cytotoxicity by targeting MTA1 in nasopharyngeal carcinoma. Tumor Biology. 2017;39(6):1010428317703825. doi: 10.1177/1010428317703825. [DOI] [PubMed] [Google Scholar]
- 78.Shibata T, Cao DY, Dar TB, Ahmed F, Bhat SA, Veiras LC et al. miR766-3p and miR124-3p Dictate Drug Resistance and Clinical Outcome in HNSCC. Cancers (Basel). 2022;14(21). [DOI] [PMC free article] [PubMed]
- 79.Chung JH, Bunz F. A loss-of-function mutation in PTCH1 suggests a role for autocrine hedgehog signaling in colorectal tumorigenesis. Oncotarget. 2013;4(12):2208–11. doi: 10.18632/oncotarget.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan Z, Xiu C, Liu D, Zhou G, Yang H, Pei R, et al. Long noncoding RNA LINC-PINT regulates laryngeal carcinoma cell stemness and chemoresistance through miR-425-5p/PTCH1/SHH axis. J Cell Physiol. 2019;234(12):23111–22. doi: 10.1002/jcp.28874. [DOI] [PubMed] [Google Scholar]
- 81.Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12(5):581–6. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 82.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Underhill C. CD44: the hyaluronan receptor. J Cell Sci. 1992;103(Pt 2):293–8. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- 84.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–42. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 85.Bourguignon LY, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287(39):32800–24. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Massagué J, Xi Q. TGF-β control of stem cell differentiation genes. FEBS Lett. 2012;586(14):1953–8. doi: 10.1016/j.febslet.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oshimori N, Oristian D, Fuchs E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160(5):963–76. doi: 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ikushima H, Todo T, Ino Y, Takahashi M, Saito N, Miyazawa K, et al. Glioma-initiating cells retain their tumorigenicity through integration of the Sox axis and Oct4 protein. J Biol Chem. 2011;286(48):41434–41. doi: 10.1074/jbc.M111.300863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weina K, Wu H, Knappe N, Orouji E, Novak D, Bernhardt M, et al. TGF-β induces SOX 2 expression in a time‐dependent manner in human melanoma cells. Pigment Cell & Melanoma Research. 2016;29(4):453–8. doi: 10.1111/pcmr.12483. [DOI] [PubMed] [Google Scholar]
- 90.Liu Z, Kuang W, Zhou Q, Zhang Y. TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int J Mol Med. 2018;42(6):3395–403. doi: 10.3892/ijmm.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bilir B, Osunkoya AO, Wiles WG, Sannigrahi S, Lefebvre V, Metzger D, et al. SOX4 is essential for prostate tumorigenesis initiated by PTEN ablation. Cancer Res. 2016;76(5):1112–21. doi: 10.1158/0008-5472.CAN-15-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun R, Jiang B, Qi H, Zhang X, Yang J, Duan J, et al. SOX4 contributes to the progression of cervical cancer and the resistance to the chemotherapeutic drug through ABCG2. Cell Death Dis. 2015;6(11):e1990–e. doi: 10.1038/cddis.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23(6):768–83. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 94.Yoon TM, Kim S, Cho WS, Lee DH, Lee JK, Park Y-L, et al. SOX4 expression is associated with treatment failure and chemoradioresistance in oral squamous cell carcinoma. BMC Cancer. 2015;15(1):1–10. doi: 10.1186/s12885-015-1875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao X, et al. SOX4 induces epithelial–mesenchymal transition and contributes to breast cancer progression. Cancer Res. 2012;72(17):4597–608. doi: 10.1158/0008-5472.CAN-12-1045. [DOI] [PubMed] [Google Scholar]
- 96.Bissey P-A, Teng M, Law JH, Shi W, Bruce JP, Petit V, et al. MiR-34c downregulation leads to SOX4 overexpression and cisplatin resistance in nasopharyngeal carcinoma. BMC Cancer. 2020;20(1):1–13. doi: 10.1186/s12885-020-07081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen L, Zhu Q, Lu L, Liu Y. MiR-132 inhibits migration and invasion and increases chemosensitivity of cisplatin-resistant oral squamous cell carcinoma cells via targeting TGF-β1. Bioengineered. 2020;11(1):91–102. doi: 10.1080/21655979.2019.1710925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discovery. 2009;8(1):33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohta K, Ohigashi M, Naganawa A, Ikeda H, Sakai M, Nishikawa J-i, et al. Histone acetyltransferase MOZ acts as a co-activator of Nrf2–MafK and induces tumour marker gene expression during hepatocarcinogenesis. Biochem J. 2007;402(3):559–66. doi: 10.1042/BJ20061194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu Y, Liu H, Kong F, Ye J, Jia X, Zhang Z, et al. miR-22/KAT6B axis is a chemotherapeutic determiner via regulation of PI3k-Akt-NF-kB pathway in tongue squamous cell carcinoma. J Experimental Clin Cancer Res. 2018;37(1):1–14. doi: 10.1186/s13046-018-0834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen W, Qiu Y, Li J, Wu C, Liu Z, Zhang X, et al. IL-25 promotes cisplatin resistance of lung cancer cells by activating NF‐κB signaling pathway to increase of major vault protein. Cancer Med. 2019;8(7):3491–501. doi: 10.1002/cam4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin J, Qin H, Han Y, Li X, Zhao Y, Zhai G. circNRIP1 modulates the miR-515-5p/IL-25 axis to control 5-Fu and cisplatin resistance in nasopharyngeal carcinoma. Drug Des Devel Ther. 2021;15:323. doi: 10.2147/DDDT.S292180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ellington CL, Goodman M, Kono SA, Grist W, Wadsworth T, Chen AY, et al. Adenoid cystic carcinoma of the head and neck: incidence and survival trends based on 1973-2007 surveillance, epidemiology, and end results data. Cancer. 2012;118(18):4444–51. doi: 10.1002/cncr.27408. [DOI] [PubMed] [Google Scholar]
- 104.Chau N, Hotte S, Chen E, Chin S, Turner S, Wang L, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23(6):1562–70. doi: 10.1093/annonc/mdr522. [DOI] [PubMed] [Google Scholar]
- 105.Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12(8):815–24. doi: 10.1016/S1470-2045(10)70245-X. [DOI] [PubMed] [Google Scholar]
- 106.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discovery. 2006;5(3):219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 107.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer. 2011;11(1):23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 108.Nihira K, Ando Y, Yamaguchi T, Kagami Y, Miki Y, Yoshida K. Pim-1 controls NF-kappaB signalling by stabilizing RelA/p65. Cell Death Differ. 2010;17(4):689–98. doi: 10.1038/cdd.2009.174. [DOI] [PubMed] [Google Scholar]
- 109.Liu X-Y, Liu Z-J, He H, Zhang C, Wang Y-L. MicroRNA-101-3p suppresses cell proliferation, invasion and enhances chemotherapeutic sensitivity in salivary gland adenoid cystic carcinoma by targeting Pim-1. Am J cancer Res. 2015;5(10):3015. [PMC free article] [PubMed] [Google Scholar]
- 110.Wang X, Li H, Shi J. LncRNA HOXA11-AS promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by suppression of miR-214-3p expression. BioMed Research International. 2019;2019. [DOI] [PMC free article] [PubMed]
- 111.Yarza R, Vela S, Solas M, Ramirez MJ. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s Disease. Front Pharmacol. 2015;6:321. doi: 10.3389/fphar.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gupta S, Campbell D, Dérijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267(5196):389–93. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 113.Lopez-Bergami P, Lau E, Ronai Z. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer. 2010;10(1):65–76. doi: 10.1038/nrc2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V. The role of ATF-2 in oncogenesis. BioEssays. 2008;30(4):314–27. doi: 10.1002/bies.20734. [DOI] [PubMed] [Google Scholar]
- 115.Hayakawa J, Depatie C, Ohmichi M, Mercola D. The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J Biol Chem. 2003;278(23):20582–92. doi: 10.1074/jbc.M210992200. [DOI] [PubMed] [Google Scholar]
- 116.Tian L, Zhang J, Ren X, Liu X, Gao W, Zhang C, et al. Overexpression of miR-26b decreases the cisplatin-resistance in laryngeal cancer by targeting ATF2. Oncotarget. 2017;8(45):79023–33. doi: 10.18632/oncotarget.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suen D-F, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15(10):634–46. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brooks C, Wei Q, Cho S-G, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Investig. 2009;119(5):1275–85. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qian W, Wang J, Van Houten B. The role of dynamin-related protein 1 in cancer growth: a promising therapeutic target? Expert Opin Ther Targets. 2013;17(9):997–1001. doi: 10.1517/14728222.2013.823160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee Y-j, Jeong S-Y, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15(11):5001–11. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12(6):565–73. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fan S, Chen W-X, Lv X-B, Tang Q-L, Sun L-J, Liu B-D, et al. Mir-483-5p determines mitochondrial fission and cisplatin sensitivity in tongue squamous cell carcinoma by targeting FIS1. Cancer Lett. 2015;362(2):183–91. doi: 10.1016/j.canlet.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 124.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qin X, Yan M, Wang X, Xu Q, Wang X, Zhu X, et al. Cancer-associated fibroblast-derived IL-6 promotes head and neck cancer progression via the osteopontin-NF-kappa B signaling pathway. Theranostics. 2018;8(4):921. doi: 10.7150/thno.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv Z, et al. TGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci Rep. 2016;6(1):1–15. doi: 10.1038/srep20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gunduz M, Gunduz E, Rivera RS, Nagatsuka H. The inhibitor of growth (ING) gene family: potential role in cancer therapy. Curr Cancer Drug Targets. 2008;8(4):275–84. doi: 10.2174/156800908784533454. [DOI] [PubMed] [Google Scholar]
- 128.Qin X, Guo H, Wang X, Zhu X, Yan M, Wang X, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20(1):1–21. doi: 10.1186/s13059-018-1604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan X, Jing Y, Guang M, Zhu J, Wang J, Wang Y, et al. GAS5 alleviates cisplatin drug resistance in oral squamous cell carcinoma by sponging miR-196a. J Int Med Res. 2022;50(10):3000605221132456. doi: 10.1177/03000605221132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen JJ, Liu SX, Chen MZ, Zhao ZY. Has–miR–125a and 125b are induced by treatment with cisplatin in nasopharyngeal carcinoma and inhibit apoptosis in a p53–dependent manner by targeting p53 mRNA. Mol Med Rep. 2015;12(3):3569–74. doi: 10.3892/mmr.2015.3863. [DOI] [PubMed] [Google Scholar]
- 131.Tanackovic G, Kramer A. Human splicing factor SF3a, but not SF1, is essential for pre-mRNA splicing in vivo. Mol Biol Cell. 2005;16(3):1366–77. doi: 10.1091/mbc.E04-11-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fang Z, Zhao J, Xie W, Sun Q, Wang H, Qiao B. LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression. Cancer Med. 2017;6(12):2897–908. doi: 10.1002/cam4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang X, Yang Z, Li Z. Zinc finger antisense 1: a long noncoding RNA with complex roles in human cancers. Gene. 2019;688:26–33. doi: 10.1016/j.gene.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 134.Wang X, Hao R, Wang F, Wang F. ZFAS1 promotes cisplatin resistance via suppressing miR-421 expression in oral squamous cell carcinoma. Cancer Manage Res. 2020;12:7251. doi: 10.2147/CMAR.S248869. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Trebinska A, Högstrand K, Grandien A, Grzybowska EA, Fadeel B. Exploring the anti-apoptotic role of HAX-1 versus BCL-XL in cytokine-dependent bone marrow-derived cells from mice. FEBS Lett. 2014;588(17):2921–7. doi: 10.1016/j.febslet.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 136.Trebinska A, Rembiszewska A, Ciosek K, Ptaszynski K, Rowinski S, Kupryjanczyk J, et al. HAX-1 overexpression, splicing and cellular localization in tumors. BMC Cancer. 2010;10:76. doi: 10.1186/1471-2407-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan J, Ma C, Cheng J, Li Z, Liu C. HAX-1 inhibits apoptosis in prostate cancer through the suppression of caspase-9 activation. Oncol Rep. 2015;34(5):2776–81. doi: 10.3892/or.2015.4202. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Tang Q, Li S, Yang X. Inhibition of HAX-1 by miR-125a reverses cisplatin resistance in laryngeal cancer stem cells. Oncotarget. 2016;7(52):86446–56. doi: 10.18632/oncotarget.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ge R, Kao C. Cell surface GRP78 as a death receptor and an Anticancer Drug Target. Cancers. 2019;11(11). [DOI] [PMC free article] [PubMed]
- 140.Lin XJ, Liu H, Li P, Wang HF, Yang AK, Di JM, et al. miR-936 suppresses cell proliferation, Invasion, and Drug Resistance of laryngeal squamous cell carcinoma and targets GPR78. Front Oncol. 2020;10:60. doi: 10.3389/fonc.2020.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mijaljica D, Nazarko TY, Brumell JH, Huang W-P, Komatsu M, Prescott M, et al. Receptor protein complexes are in control of autophagy. Autophagy. 2012;8(11):1701–5. doi: 10.4161/auto.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Singh SS, Vats S, Chia AY-Q, Tan TZ, Deng S, Ong MS, et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37(9):1142–58. doi: 10.1038/s41388-017-0046-6. [DOI] [PubMed] [Google Scholar]
- 143.Thorburn A, Thamm DH, Gustafson DL. Autophagy and cancer therapy. Mol Pharmacol. 2014;85(6):830–8. doi: 10.1124/mol.114.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10(9):1533–41. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–42. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Di X, He G, Chen H, Zhu C, Qin Q, Yan J, et al. High-mobility group box 1 protein modulated proliferation and radioresistance in esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2019;34(4):728–35. doi: 10.1111/jgh.14371. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y, Lin C, Liu Y, Jiang Y. HMGB1 promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2. Tumor Biology. 2016;37(4):4399–408. doi: 10.1007/s13277-015-4049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen L, Xu Z, Zhao J, Zhai X, Li J, Zhang Y, et al. H19/miR-107/HMGB1 axis sensitizes laryngeal squamous cell carcinoma to cisplatin by suppressing autophagy in vitro and in vivo. Cell Biol Int. 2021;45(3):674–85. doi: 10.1002/cbin.11520. [DOI] [PubMed] [Google Scholar]
- 149.Zhao Y, Wang P, Wu Q. miR-1278 sensitizes nasopharyngeal carcinoma cells to cisplatin and suppresses autophagy via targeting ATG2B. Mol Cell Probes. 2020;53:101597. doi: 10.1016/j.mcp.2020.101597. [DOI] [PubMed] [Google Scholar]
- 150.Feng B, Chen K, Zhang W, Zheng Q, He Y. circPGAM1 enhances autophagy signaling during laryngocarcinoma drug resistance by regulating miR-376a. Biochem Biophys Res Commun. 2021;534:966–72. doi: 10.1016/j.bbrc.2020.10.063. [DOI] [PubMed] [Google Scholar]
- 151.Gao L, Zhang Q, Li S, Zheng J, Ren W, Zhi K. Circ-PKD2 promotes Atg13-mediated autophagy by inhibiting miR-646 to increase the sensitivity of cisplatin in oral squamous cell carcinomas. Cell Death Dis. 2022;13(2):192. doi: 10.1038/s41419-021-04497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang T-X, Zou J-B, Zhu Q-Q, Liu CH, Wang G-F, Du T-T et al. SIP/CacyBP promotes autophagy by regulating levels of BRUCE/Apollon, which stimulates LC3-I degradation. Proceedings of the National Academy of Sciences. 2019;116(27):13404-13. [DOI] [PMC free article] [PubMed]
- 153.Hekmat M, Schaalan MF, Rahmo RM, Farag DB, Khedr LH. Implications of miRNAs on TGF-β/TAK1/mTOR pathway in mediating the renoprotective effects of pentoxifylline against cisplatin-induced nephrotoxicity in rats. Toxicol Appl Pharmcol. 2020;404:115184. doi: 10.1016/j.taap.2020.115184. [DOI] [PubMed] [Google Scholar]
- 154.Takahashi H, Jin C, Rajabi H, Pitroda S, Alam M, Ahmad R, et al. MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene. 2015;34(40):5187–97. doi: 10.1038/onc.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hao Y, Li J, Zhang H, Guan G, Guo Y. MicroRNA-205 targets HER3 and suppresses the growth, chemosensitivity and metastasis of human nasopharyngeal carcinoma cells. J BUON. 2020;25(1):350–6. [PubMed] [Google Scholar]
- 156.Gulei D, Magdo L, Jurj A, Raduly L, Cojocneanu-Petric R, Moldovan A, et al. The silent healer: mir-205-5p up-regulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly up-regulating E-cadherin expression. Cell Death Dis. 2018;9(2):1–16. doi: 10.1038/s41419-017-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rahmani Z, Mojarrad M, Moghbeli M. Long non-coding RNAs as the critical factors during tumor progressions among iranian population: an overview. Cell Biosci. 2020;10:6. doi: 10.1186/s13578-020-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hamidi AA, Khalili-Tanha G, Nasrpour Navaei Z, Moghbeli M. Long non-coding RNAs as the critical regulators of epithelial mesenchymal transition in colorectal tumor cells: an overview. Cancer Cell Int. 2022;22(1):71. doi: 10.1186/s12935-022-02501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang P, Lu X, Shi Z, Li X, Zhang Y, Zhao S, et al. Mir-205-5p regulates epithelial-mesenchymal transition by targeting PTEN via PI3K/AKT signaling pathway in cisplatin-resistant nasopharyngeal carcinoma cells. Gene. 2019;710:103–13. doi: 10.1016/j.gene.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 161.Forghanifard MM, Rad A, Farshchian M, Khaleghizadeh M, Gholamin M, Moghbeli M, et al. TWIST1 upregulates the MAGEA4 oncogene. Mol Carcinog. 2017;56(3):877–85. doi: 10.1002/mc.22541. [DOI] [PubMed] [Google Scholar]
- 162.Peng F, Zhang H, Du Y, Tan P. miR-23a promotes cisplatin chemoresistance and protects against cisplatin-induced apoptosis in tongue squamous cell carcinoma cells through twist. Oncol Rep. 2015;33(2):942–50. doi: 10.3892/or.2014.3664. [DOI] [PubMed] [Google Scholar]
- 163.Wang X, Guo H, Yao B, Helms J. miR-15b inhibits cancer-initiating cell phenotypes and chemoresistance of cisplatin by targeting TRIM14 in oral tongue squamous cell cancer. Oncol Rep. 2017;37(5):2720–6. doi: 10.3892/or.2017.5532. [DOI] [PubMed] [Google Scholar]
- 164.Guo H, Chu Y, Wang L, Chen X, Chen Y, Cheng H, et al. PBX3 is essential for leukemia stem cell maintenance in MLL-rearranged leukemia. Int J Cancer. 2017;141(2):324–35. doi: 10.1002/ijc.30739. [DOI] [PubMed] [Google Scholar]
- 165.Wang S, Li C, Wang W, Xing C. PBX3 promotes gastric cancer invasion and metastasis by inducing epithelial-mesenchymal transition. Oncol Lett. 2016;12(5):3485–91. doi: 10.3892/ol.2016.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li H, Wang J, Xu F, Wang L, Sun G, Wang J, et al. By downregulating PBX3, miR-526b suppresses the epithelial-mesenchymal transition process in cervical cancer cells. Future Oncol. 2019;15(14):1577–91. doi: 10.2217/fon-2018-0575. [DOI] [PubMed] [Google Scholar]
- 167.Li H, Huang J, Yu S, Li H, Zhou Y, Wu Q. HOXA11-AS induces cisplatin resistance by modulating the microRNA-98/PBX3 axis in nasopharyngeal carcinoma. Oncol Lett. 2021;21(6):493. doi: 10.3892/ol.2021.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12(10):982–92. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 169.Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119(12):3626–36. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M, et al. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31(4):432–45. doi: 10.1038/onc.2011.263. [DOI] [PubMed] [Google Scholar]
- 171.Cammareri P, Scopelliti A, Todaro M, Eterno V, Francescangeli F, Moyer MP, et al. Aurora-a is essential for the tumorigenic capacity and chemoresistance of colorectal cancer stem cells. Cancer Res. 2010;70(11):4655–65. doi: 10.1158/0008-5472.CAN-09-3953. [DOI] [PubMed] [Google Scholar]
- 172.Paulson AK, Linklater ES, Berghuis BD, App CA, Oostendorp LD, Paulson JE, et al. MET and ERBB2 are coexpressed in ERBB2 + breast cancer and contribute to innate resistance. Mol Cancer Res. 2013;11(9):1112–21. doi: 10.1158/1541-7786.MCR-13-0042. [DOI] [PubMed] [Google Scholar]
- 173.Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S et al. p21CIP1 attenuates Ras-and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proceedings of the National Academy of Sciences. 2009;106(45):19035-9. [DOI] [PMC free article] [PubMed]
- 174.Yang J, Wu S-P, Wang W-J, Jin Z-R, Miao X-B, Wu Y, et al. A novel miR-200c/c-myc negative regulatory feedback loop is essential to the EMT process, CSC biology and drug sensitivity in nasopharyngeal cancer. Exp Cell Res. 2020;391(2):111817. doi: 10.1016/j.yexcr.2020.111817. [DOI] [PubMed] [Google Scholar]
- 175.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 176.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28(1–2):65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 177.Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12(1):27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang B, Zang J, Yuan W, Jiang X, Zhang F. The miR-136-5p/ROCK1 axis suppresses invasion and migration, and enhances cisplatin sensitivity in head and neck cancer cells. Exp Ther Med. 2021;21(4):317. doi: 10.3892/etm.2021.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen Q-Y, Xu W, Jiao D-M, Wu L-J, Song J, Yan J, et al. Silence of ezrin modifies migration and actin cytoskeleton rearrangements and enhances chemosensitivity of lung cancer cells in vitro. Mol Cell Biochem. 2013;377(1):207–18. doi: 10.1007/s11010-013-1586-x. [DOI] [PubMed] [Google Scholar]
- 180.Zhang L, Xiao R, Xiong J, Leng J, Ehtisham A, Hu Y, et al. Activated ERM protein plays a critical role in drug resistance of MOLT4 cells induced by CCL25. PLoS ONE. 2013;8(1):e52384. doi: 10.1371/journal.pone.0052384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang S, Ma H, Zhang D, Xie S, Wang W, Li Q, et al. LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via mir-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 2018;9(7):1–16. doi: 10.1038/s41419-018-0793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cao C, Zhou S, Hu J, editors. Long noncoding RNA MAGI2-AS3/miR‐218‐5p/GDPD5/SEC61A1 axis drives cellular proliferation and migration and confers cisplatin resistance in nasopharyngeal carcinoma. International Forum of Allergy & Rhinology; 2020. [DOI] [PubMed]
- 183.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen Y, Li X, Wu S, Xu G, Zhou Y, Gong L, et al. Expression of HIF-1α and CAIX in nasopharyngeal carcinoma and their correlation with patients’ prognosis. Med Oncol. 2014;31(12):304. doi: 10.1007/s12032-014-0304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pan WL, Wong JH, Fang EF, Chan YS, Ng TB, Cheung RC. Preferential cytotoxicity of the type I ribosome inactivating protein alpha-momorcharin on human nasopharyngeal carcinoma cells under normoxia and hypoxia. Biochem Pharmacol. 2014;89(3):329–39. doi: 10.1016/j.bcp.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shan Y, Li X, You B, Shi S, Zhang Q, You Y. MicroRNA-338 inhibits migration and proliferation by targeting hypoxia-induced factor 1α in nasopharyngeal carcinoma. Oncol Rep. 2015;34(4):1943–52. doi: 10.3892/or.2015.4195. [DOI] [PubMed] [Google Scholar]
- 187.Choi BJ, Park SA, Lee SY, Cha YN, Surh YJ. Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of snail: a potential role of Sox9. Sci Rep. 2017;7(1):15918. doi: 10.1038/s41598-017-15139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yuan F, Lou Z, Zhou Z, Yan X. Long non–coding RNA KCNQ1OT1 promotes nasopharyngeal carcinoma cell cisplatin resistance via the miR–454/USP47 axis. Int J Mol Med. 2021;47(4). [DOI] [PMC free article] [PubMed] [Retracted]
- 189.Zou H, Chen H, Zhou Z, Wan Y, Liu Z. ATXN3 promotes breast cancer metastasis by deubiquitinating KLF4. Cancer Lett. 2019;467:19–28. doi: 10.1016/j.canlet.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 190.Song A, Wu Y, Chu W, Yang X, Zhu Z, Yan E, et al. Involvement of mir-619-5p in resistance to cisplatin by regulating ATXN3 in oral squamous cell carcinoma. Int J Biol Sci. 2021;17(2):430–47. doi: 10.7150/ijbs.54014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR) Curr Drug Targets. 2006;7(7):893–909. doi: 10.2174/138945006777709520. [DOI] [PubMed] [Google Scholar]
- 192.Bossennec M, Di Roio A, Caux C, Ménétrier-Caux C. MDR1 in immunity: friend or foe? Oncoimmunology. 2018;7(12):e1499388. doi: 10.1080/2162402X.2018.1499388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sodani K, Patel A, Kathawala RJ, Chen Z-S. Multidrug resistance associated proteins in multidrug resistance. Chin J cancer. 2012;31(2):58. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yi X, Chen W, Li C, Chen X, Lin Q, Lin S, et al. Circular RNA circ_0004507 contributes to laryngeal cancer progression and cisplatin resistance by sponging miR-873 to upregulate multidrug resistance 1 and multidrug resistance protein 1. Head Neck. 2021;43(3):928–41. doi: 10.1002/hed.26549. [DOI] [PubMed] [Google Scholar]
- 195.Yuan TZ, Zhang HH, Lin XL, Yu JX, Yang QX, Liang Y, et al. microRNA-125b reverses the multidrug resistance of nasopharyngeal carcinoma cells via targeting of Bcl-2. Mol Med Rep. 2017;15(4):2223–8. doi: 10.3892/mmr.2017.6233. [DOI] [PubMed] [Google Scholar]
- 196.Gobin C, Inkabi S, Lattimore CC, Gu T, Menefee JN, Rodriguez M, et al. Investigating miR-9 as a mediator in laryngeal cancer health disparities. Front Oncol. 2023;13:1096882. doi: 10.3389/fonc.2023.1096882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gao F, Han J, Wang Y, Jia L, Luo W, Zeng Y. Circ_0109291 promotes cisplatin resistance of oral squamous cell carcinoma by sponging mir-188-3p to increase ABCB1 expression. Cancer Biother Radiopharm. 2022;37(4):233–45. doi: 10.1089/cbr.2020.3928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.