Abstract
High-risk human papillomaviruses are causally associated with cervical cancer. Two viral oncogenes, E6 and E7, are expressed in most cervical cancers, and these genes cause cancer when expressed in experimental animals. The E6 protein targets the p53 tumor suppressor for degradation, while the E7 protein inactivates the retinoblastoma susceptibility protein (pRb), in part by stimulating its degradation. In contrast, expression of E7 in the absence of E6 leads to stabilization of p53. Here we show that E7 stabilizes p53 in mouse embryo fibroblasts lacking p19ARF. The stable p53 is active as a transcriptional activator, as evidenced by the increased expression of the p53-responsive mdm2 gene. Normally, MDM2 protein inhibits p53 function in an autoregulatory loop. Regulation of p53 by MDM2 is required for murine development as well as for proliferation of cultured human fibroblasts. However, E7-expressing human fibroblasts continue to divide even though E7 abrogates the ability of MDM2 and p53 to bind. Furthermore, E7-expressing cells are not more sensitive to UV light, an agent that has been reported to induce apoptosis mediated by p53. These results indicate that in addition to inhibiting the ability of MDM2 to regulate p53, E7 must block signaling steps downstream of p53 to allow cell division.
In more than 90% of cervical cancers, DNA from anogenital human papillomaviruses (HPVs) is present (3). The anogenital HPVs are divided into two types, the high-risk types found in cervical cancers (most frequently HPV type 16 [HPV-16] and HPV-18) and the low-risk types commonly found in genital warts (HPV-6 and HPV-11). Both types of HPVs express two oncogenes, E6 and E7. However, only the high-risk viruses encode E6 oncoproteins that efficiently inactivate the tumor suppressor protein p53 (12, 56). The E7 proteins from high-risk HPVs are highly effective at inactivating another tumor suppressor, the retinoblastoma susceptibility protein (pRb), whereas E7 proteins from low-risk HPVs are less effective (26, 48). The ability of HPVs to inactivate these two tumor suppressor proteins appears to contribute to their ability to increase the risk of cancer in experimental animals (21, 37, 50).
HPVs infect squamous epithelia and replicate in differentiated keratinocytes (28). There is evidence that the functions of E6 and E7 that contribute to cancer are those that allow DNA replication to occur in postmitotic cells. For example, the E7 protein induces DNA synthesis in differentiated postmitotic human keratinocytes (10). Inactivation of pRb is part of the mechanism through which E7 promotes entry of resting cells into S phase (1). E7 liberates pRb from members of the E2F family of transcription factors, thereby allowing induction of genes encoding proteins that function during S phase (6, 47). E6 stimulates the degradation of p53, a protein required for the inhibition of DNA synthesis in senescing normal diploid human fibroblasts (NDFs) (19). The activity of p53 is also required to arrest cycling cells in the G1 phase in response to DNA damage (35). The ability of p53 to induce a G1 arrest in response to ionizing radiation is in part dependent on the induction of the p21 gene, whose product inhibits the cyclin-dependent kinases (CDKs) (14, 16). CDKs phosphorylate pRb and release it from the E2F transcription factor, allowing expression of genes whose products promote DNA synthesis. CDK activity is inhibited when p53 induces p21 expression in response to ionizing radiation (16). E6 eliminates the induction of p21 by p53, allowing CDKs to remain active in NDFs exposed to ionizing radiation (16). Thus, under certain circumstances, both E6 and E7 promote passage from G1 to S phase, a process that appears to be essential for viral replication in postmitotic keratinocytes. A lack of appropriate regulation of the transition from G1 to S may contribute to the development of cancer (reviewed in reference 58). Indeed, the functions of pRb and p53 are disrupted directly or indirectly in the majority of human cancers (58).
In cells expressing E7 but not E6, the levels of pRb protein are reduced while the levels of p53 are increased (13, 30). A decrease in pRb is consistent with the idea that E7 stimulates the transition into S phase; however, the increase in the amount of p53 is counterintuitive because p53 can induce growth arrest and apoptosis (35, 69). It is not clear whether p53 is activated by E7. On one hand, Massimi and Banks (42) used p53-responsive transcriptional reporter assays to show that p53 function is inhibited in cells expressing HPV-16 E7. In agreement with these findings, Vaziri et al. (66) found that HPV-16 E7 could inhibit transcription of a luciferase reporter gene cloned downstream from the promoter of the p53-responsive p21 gene. Other data have indicated that p21 protein levels are increased in cells expressing E7 and that the majority of this increase can be accounted for by posttranscriptional events independent of p53 (29, 31). Thus, there is not strong evidence that stabilization of p53 by E7 results in a stimulation of transcription of p21. On the other hand, Thomas and Laimins (63) showed that HPV-16 E7 expression in keratinocytes results in overexpression of the MDM2 protein, which is the product of another gene induced by p53. In keratinocytes expressing E6 with E7, MDM2 protein levels were reduced, prompting Thomas and Laimins to hypothesize that E7 upregulates MDM2 by synergizing with factors that regulate p53 activity (63). In sum, it has not been established whether p53 is activated as a transcriptional regulator by E7.
Recently, the E1A, c-myc, and E2F1 proteins have been shown to stabilize p53 and stimulate its transcriptional activation function (15, 71). Expression of E1A in mouse embryo fibroblasts (MEFs) appears to disrupt the interaction between p53 and its inhibitor, MDM2 (15). MDM2 directly blocks the ability of p53 to stimulate transcription (44, 49), and inhibition of the interaction between MDM2 and p53 results in activation of p53 (2). In addition, binding of MDM2 to p53 is required for the ability of MDM2, a ubiquitin ligase (27), to target p53 for degradation (25, 36). The ability of E1A to stabilize p53 appears to be dependent on the p19ARF protein (15), which inhibits the ability of MDM2 to ubiquitinate p53 (27). However, both c-myc and E2F1 elevate p53 levels in MEFs lacking p19ARF (71). E7 elevates p53 levels in NDFs (30) and expands the life span of these cells. In contrast, antibodies that block the interaction between MDM2 and p53 in NDFs cause an increase in p53 protein that results in arrest of cell division (2, 19). Therefore, we investigated whether E7 inhibited the regulation of p53 by MDM2 in NDFs.
MATERIALS AND METHODS
Cell culture and retroviral infections.
The normal diploid human skin fibroblast strains GM00011 and GM00037 were obtained from the NIGMS Human Genetic Mutant Cell Repository at the Coriell Institute for Medical Research and used between passages 15 and 25. Cells were grown in Dulbecco’s modification of Eagle’s medium supplemented with 20% fetal calf serum (Summit), nonessential amino acids, essential amino acids, vitamins (Gibco), penicillin, and streptomycin. Retroviral packaging lines PA317LXSN, PA31716E6, PA31716E7 (23), and PA317p53CTF (64) were obtained from Karl Munger. The p53CTF virus carries the carboxy-terminal fragment of murine p53 which acts as a dominant-negative inhibitor of wild-type p53 (57, 64). Culture supernatants containing viruses were generated as described previously (23) and stored at −80°C. For infection of NDFs, viruses were added to cells with 4 μg of hexadimethrine bromide (Sigma)/ml for 8 h. Cells were passaged 1:3 the next day and grown under selection in 1 mg of Geneticin (Calbiochem)/ml for about 10 days. Resistant colonies of cells were pooled and used for experiments within three passages. Some Geneticin-resistant colonies were infected with a second retrovirus before being pooled as described by Halbert et al. (23). MEFs were either wild type (from Arnold J. Levine) or null for p19ARF (34). MEFs were cultured in Dulbecco’s modification of Eagle’s medium supplemented with 10% fetal calf serum, penicillin, and streptomycin. The retroviruses used to infect MEFs were the vector pBabe Puro (46) and pBabe Puro E7 (31). High-titer stocks of virus were generated by transient transfection of the BOSC23 cell line as described by Pear et al. (51). Early-passage MEFs were thawed into six 6-cm-diameter culture dishes, infected the next day in triplicate, and passaged 1:3 2 days later into DMEM with 2 μg of puromycin/ml. The resistant cells were then passaged 1:6 and harvested for Western analysis after 2 days.
Exposure to UV light.
Before exposure of NDFs to UV light, the medium was removed and cells were rinsed with phosphate-buffered saline. The distance between the plates and the lamp was adjusted so that the fluence of the UV-C light source (Glo-Mark Systems, Inc.) was 0.4 or 1 J/m2/s as measured by a Blak-Ray J-225 UV meter (UV Photoproducts, San Gabriel, Calif.).
Colony-forming ability.
Two quantities of NDFs were plated in triplicate on 6-cm-diameter tissue culture dishes and allowed to attach for 24 h before being irradiated with different doses of UV light. The medium was replaced, and cells were allowed to form colonies for 2 weeks. Colonies were fixed in methanol, stained with Giemsa, and counted. Data are graphed as means ± standard errors.
S1 nuclease digestion.
RNA was isolated by using Tri reagent (Molecular Research Center, Inc.). The probe was a 488-bp XbaI-ApaLI fragment from pTA-L-mdm2 containing all of exon 1, none of exon 2, all of exon 3, and 44 bp of exon 4 (38). Following end labeling with T4 kinase (New England Biolabs), the probe was denatured and hybridized overnight to 30 μg of total RNA. The hybridization products were incubated with 100 U of S1 nuclease (Gibco) and then separated on 5% polyacrylamide gels. The positive control, corresponding to RNA transcribed from the P1 promoter of human mdm2 (containing exon 1 spliced to exon 3 [38]), was synthesized in vitro from pHDM (8). The 25-bp ladder was obtained from Gibco and end labeled with T4 kinase. Products were quantified with a Molecular Dynamics PhosphorImager.
Northern analysis.
Twenty micrograms of RNA was separated on a 1% agarose gel containing formaldehyde, transferred to a membrane (GeneScreen Plus; New England Nuclear), and hybridized sequentially with probes to human p21 (from pc-Waf1S [17]) and glyceraldehyde-3-phosphate dehydrogenase (from pBSGAPDH [65]).
Immunoprecipitation and Western analysis.
Immunoprecipitations were carried out as described previously (53). The CM5 polyclonal antibody (Calbiochem) was used to immunoprecipitate p53. For detection of human p53, the DO-1 antibody (Calbiochem) was used (at a 1:1,000 dilution). Human MDM2 was detected with either monoclonal antibody IF-2 (Calbiochem) at 1 μg/ml or 2A10 culture supernatant (8), diluted 1:50. Primary antibodies were revealed with sheep anti-mouse immunoglobulin (Ig) conjugated to horseradish peroxidase (Amersham; 1:500) and by enhanced chemiluminescence (ECL; Amersham). For controls, p53 was translated in vitro from pKS53SN (a gift from Jiandong Chen) and MDM2 was translated from pHDM1A (8). For detection of murine p53, CM5 was diluted 2,000-fold and revealed with a sheep anti-rabbit Ig conjugated to horseradish peroxidase (Amersham; 1:10,000).
Immunofluorescence.
GM00011 cells were fixed for 10 min at room temperature in 100% methanol and stored at 4°C in phosphate-buffered saline. For detection of p53, DO-1 antibody was used at a dilution of 1:1,000. For detection of MDM2, 2A10 hybridoma culture supernatant was diluted 1:500. Each primary antibody was detected with Alexa 488 goat anti-mouse IgG (Molecular Probes) at a dilution of 1:500. Images were collected with a Zeiss Axiophot microscope coupled to a Spot charge-coupled device camera (Diagnostic Instruments, Inc.).
RESULTS
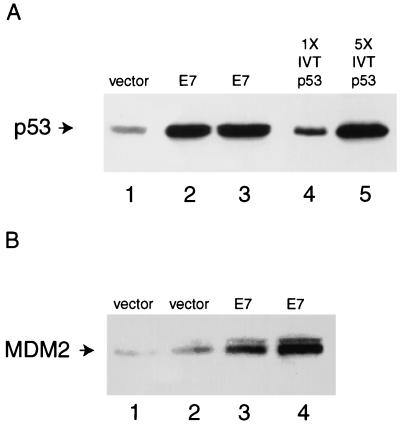
To confirm that the amount of p53 protein was increased in NDFs expressing the E7 oncoprotein of HPV-16, we generated pools of cells containing retroviruses expressing E7 and compared the level of p53 in these cells to that in cells carrying the vector alone (Fig. 1A). Western blot analysis indicated that the amount of p53 in cells expressing E7 was increased approximately fivefold over than in vector-only cells. Next, we asked whether MDM2 protein levels were increased in NDFs expressing E7, as they are in keratinocytes expressing E7 (63). Western analysis (Fig. 1B) revealed that steady-state levels of MDM2 were higher in cells expressing E7 than in cells carrying the vector alone (LXSN). Comparison of the amount of MDM2 in serial dilutions of lysates from NDFs carrying the vector or expressing E7 indicated that MDM2 was overexpressed about sixfold in the presence of E7 (52). These results indicated that although MDM2 is present at high levels in NDFs expressing E7, it may not be able to regulate p53.
FIG. 1.
Western analysis of p53 and MDM2 levels in NDFs. Proteins (150 μg) from one pool of NDFs (GM00011 cells) infected with virus carrying vector alone and from two pools of fibroblasts infected with virus expressing E7 were separated by polyacrylamide gel electrophoresis and blotted onto nitrocellulose. As a control for migration and immunoreactivity, human p53 protein was translated in a rabbit reticulocyte lysate. (A) p53 levels. Lane 1, lysate from NDFs carrying the vector alone; lanes 2 and 3, lysate from NDFs expressing E7; lane 4, 2 μl of a rabbit reticulocyte lysate programmed with RNA encoding p53; lane 5, 10 μl of a rabbit reticulocyte lysate programmed with RNA encoding p53. p53 was detected with monoclonal antibody DO-1. (B) MDM2 levels. Lanes 1 and 2, lysate from NDFs carrying the vector alone; lanes 3 and 4, lysate from NDFs expressing E7. MDM2 was detected with monoclonal antibody IF-2.
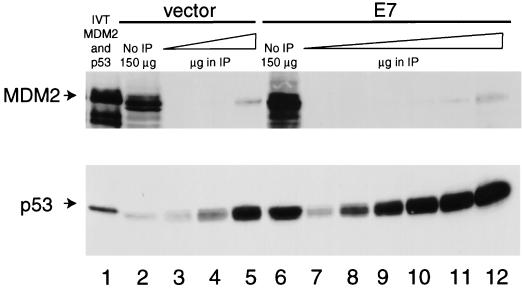
The ability of MDM2 to stimulate p53 degradation requires the domain of MDM2 that interacts with p53 (25, 36), indicating that direct binding to p53 is obligatory for MDM2 to shorten the half-life of p53. Thus, we asked whether the increased amount of p53 in E7-expressing cells was due to a decrease in the ability of MDM2 and p53 to bind. We compared the amount of MDM2 coimmunoprecipitating with equivalent amounts of p53 from cells lacking or expressing E7. To do this, we immunoprecipitated p53 from several different amounts of lysate from cells carrying the vector alone and compared it with that immunoprecipitated from various amounts of lysate from E7-expressing cells. We used the CM5 polyclonal antibody against p53 (39) because we have found that this antibody precipitates 100% of the p53 from lysates of NDFs and does not disrupt the MDM2-p53 complex (55). Comparison of the amount of p53 protein in 150 μg of each total lysate to the amount in the immunoprecipitates from 150-μg quantities of the lysates revealed that immunoprecipitation of p53 by the CM5 antibody was quantitative (Fig. 2; compare lane 2 to lane 3 and lane 6 to lane 9). Approximately equivalent amounts of p53 were immunoprecipitated from 900 μg of cells carrying the vector alone and from 150 μg of cells expressing E7 (Fig. 2; compare lanes 5 and 9), indicating that E7-expressing cells had approximately sixfold more p53 than cells containing the vector alone. We compared the amount of MDM2 coimmunoprecipitating with p53 from NDFs carrying the vector alone with that from NDFs expressing E7. MDM2 was undetectable in the immunoprecipitates of p53 from 150- and 300-μg quantities of lysate (Fig. 2, lanes 3 and 4). When 900 μg of lysate from NDFs carrying the vector alone was immunoprecipitated with the p53 antibody, MDM2 was detectable (Fig. 2, lane 5). The amount of MDM2 in the immunoprecipitate from 900 μg of lysate was less than the amount of MDM2 in 150 μg of total lysate (Fig. 2, lane 2), indicating that less than one-sixth of the MDM2 protein could be detected in a complex with p53, as had been previously reported (55). E7-expressing cells contained much more MDM2 protein than NDFs carrying the vector alone (Fig. 2, compare lanes 2 and 6), but MDM2 was still undetectable in the immunoprecipitates of p53 from 150- and 300-μg quantities of this lysate (Fig. 2, lanes 9 and 10). MDM2 was detectable in the immunoprecipitate of p53 from E7-expressing cells when 450- and 900-μg quantities of lysate were used (Fig. 2, lanes 11 and 12). The fact that the same amount of MDM2 coimmunoprecipitated with p53 in 900 μg of each lysate indicates that E7 inhibits the interaction between MDM2 and p53 by at least sixfold.
FIG. 2.
Coimmunoprecipitation of MDM2 with p53. p53 was immunoprecipitated, with the CM5 antibody, from lysates of GM00011 cells carrying the retroviral vector alone or expressing E7. Following transfer to nitrocellulose, p53 and MDM2 were detected with antibodies DO-1 and 2A10, respectively. Lane 1, 3 μl of a rabbit reticulocyte lysate programmed with p53 RNA and 3 μl of a rabbit reticulocyte lysate programmed with RNA from human mdm2; lane 2, 150 μg of total lysate from NDFs carrying the vector alone; lane 3, immunoprecipitate (IP) from 150 μg of lysate from NDFs carrying the vector alone; lane 4, immunoprecipitate from 300 μg of lysate from NDFs carrying the vector alone; lane 5, immunoprecipitate from 900 μg of lysate from NDFs carrying the vector alone; lane 6, 150 μg of total lysate from NDFs expressing E7; lane 7, immunoprecipitate from 15 μg of lysate from NDFs expressing E7; lane 8, immunoprecipitate from 75 μg of lysate from NDFs expressing E7; lane 9, immunoprecipitate from 150 μg of lysate from NDFs expressing E7; lane 10, immunoprecipitate from 300 μg of lysate from NDFs expressing E7; lane 11, immunoprecipitate from 450 μg of lysate from NDFs expressing E7; lane 12, immunoprecipitate from 900 μg of lysate from NDFs expressing E7.
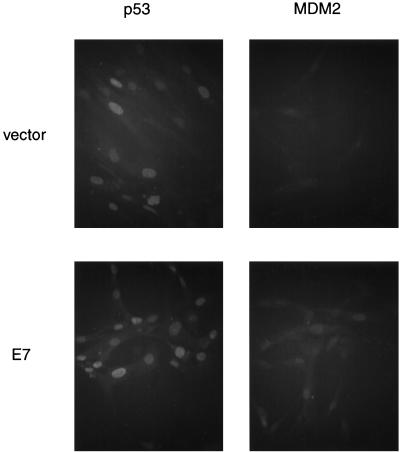
One possible explanation for the inability of MDM2 and p53 to bind is that they are not localized in the same cellular compartment. Both MDM2 and p53 are nuclear phosphoproteins, and nuclear localization of MDM2 is thought to be required for it to inhibit p53’s transcriptional activation function (9). Therefore, we investigated whether p53 or MDM2 was excluded from the nucleus in the presence of E7. Indirect-immunofluorescence analysis (Fig. 3) indicated that p53 is in the nucleus in the absence or presence of E7. MDM2 staining is faint and diffuse in the absence of E7 and is not clearly above the background staining of the secondary antibody alone (52). However, in the presence of E7, there is a clear increase in MDM2 staining in the nucleus. Therefore, in the presence of E7, both MDM2 and p53 are present in the nucleus, and localization cannot account for the inability of MDM2 and p53 to interact.
FIG. 3.
Immunofluorescence detection reveals that p53 and MDM2 are in the nucleus in cells expressing E7. Pools of NDFs (GM00011) carrying the vector alone or expressing E7 were fixed and stained with p53 antibody DO-1 or MDM2 antibody 2A10. Antibodies were revealed with a secondary antibody conjugated to a fluorescent molecule (Alexa 488).
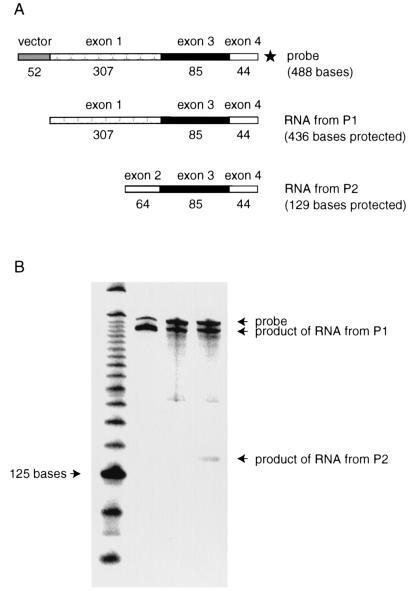
The high levels of nuclear p53 in E7-expressing cells led us to ask whether p53 was functional. If so, this might explain the elevated levels of MDM2 protein in E7-expressing cells, since p53 can induce mdm2 expression (67). Induction of mdm2 by p53 is mediated through the activity of an internal promoter, P2, within exon 2 (33, 38, 70). An upstream promoter, P1, is unaffected by p53 function (70). In the absence of functional p53, the major mdm2 transcript in many human cell lines is one, originating from the P1 promoter, in which exon 1 is directly spliced to exon 3 (38, 70). When human p53 is activated, there is an accumulation of mdm2 RNA, originating from the P2 promoter, lacking exon 1 and containing exon 2 spliced to exon 3 (70). The activity of the P2 promoter of mdm2 has been employed as a reliable indicator of the activity of p53 (5, 54). Therefore, we used an S1 nuclease digestion assay to determine whether there were changes in the levels of either or both mdm2 mRNA species in NDFs expressing E7 (Fig. 4A). The amounts of mdm2 transcript synthesized from the P1 promoter of mdm2 were the same in cells carrying the vector alone and in cells expressing E7 (Fig. 4B; compare lanes 3 and 4). However, the amount of mdm2 RNA synthesized from the P2 promoter was increased approximately ninefold in E7-expressing cells (Fig. 4B; compare lanes 3 and 4). These results indicate that E7 may stimulate p53’s function as a transcriptional activator.
FIG. 4.
S1 nuclease analysis of mdm2 transcripts in NDFs (GM00011). (A) Schematic of probe and predicted sizes of resulting products following protection by RNAs transcribed from the P1 (constitutive) and P2 (p53-responsive) promoters. The star indicates that the protected products are radiolabeled in exon 4. (B) S1 nuclease digestion pattern of mdm2 RNAs. Lane 1, 25-bp molecular size markers; lane 2, product of protection of full-length mdm2 mRNA transcribed in vitro from the P1 promoter; lane 3, RNA from NDFs carrying the vector alone; lane 4, RNA from NDFs expressing E7.
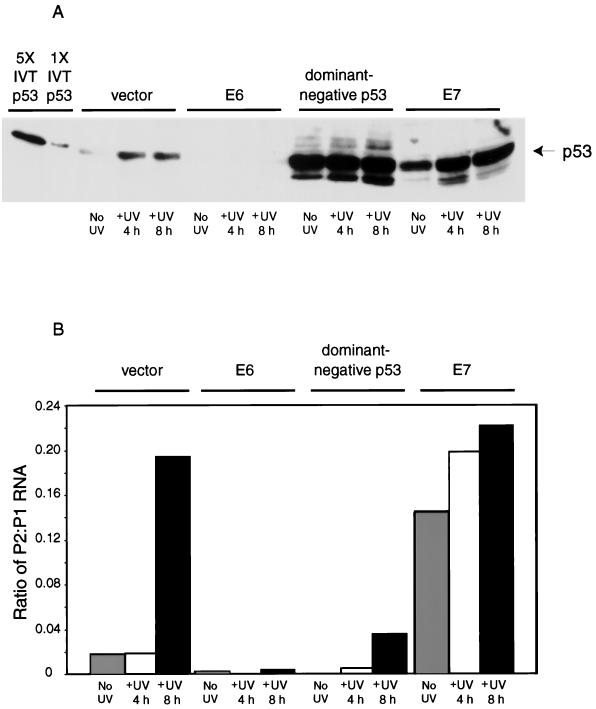
To determine whether the induction of mdm2 by E7 was dependent on p53 function, we generated pools of NDFs in which p53 activity was abrogated either by the E6 oncoprotein or by a dominant-negative p53 mutant and monitored E7’s influence on mdm2 expression in them. E6 is known to stimulate degradation of p53 and to inhibit the accumulation and activation of p53 following exposure of cells to ionizing and UV radiation (11, 16). In contrast, the dominant-negative p53 protein stabilizes wild-type p53 but inhibits sequence-specific DNA binding by wild-type p53 (57). First, we assessed the ability of retroviruses expressing E6 and dominant-negative p53 to inhibit the induction of the P2 promoter of mdm2 by p53 in NDFs. We asked whether p53 could be activated in response to UV light in pools of NDFs expressing vector alone, E6, dominant-negative p53, or E7. We measured both accumulation of p53 (Fig. 5A) and activation of the P2 promoter of mdm2 (Fig. 5B) because we had shown previously that the p53-dependent induction of murine mdm2 by UV light was mediated through the P2 promoter (54). In cells carrying the vector alone, p53 protein accumulated in response to UV exposure (Fig. 5A). In contrast, levels of p53 remained low in cells expressing E6 (Fig. 5A). The level of p53 was constitutively high in cells expressing the dominant-negative p53 mutant (Fig. 5A). Cells expressing E7 contained large amounts of p53, which accumulated to higher levels in response to DNA damage (Fig. 5A). These results indicate that the retroviruses encoding E6 and dominant-negative p53 are effective modifiers of p53 accumulation in response to UV light. S1 analysis revealed that in cells carrying the retroviral vector alone the P2 promoter of mdm2 was induced by UV light (Fig. 5B). The amount of mdm2 mRNA transcribed from the P1 promoter, which is not influenced by E7 or by p53, serves as an internal control for the S1 analysis. Therefore, the products of the S1 analysis were quantified and the ratio of mRNA transcribed from the P2 promoter to mRNA transcribed from the P1 promoter was calculated. The ratio of P2 to P1 mRNAs rose 10-fold by 8 h following exposure of NDFs carrying the vector alone to 10 J of UV light/m2. In contrast, both the basal and UV-induced activities of the P2 promoter were inhibited in NDFs expressing either E6 or dominant-negative p53 (Fig. 5B). Thus, both proteins can inhibit p53 function in NDFs. In contrast, E7 expression alone stimulated the activity of the P2 promoter, and the activity of the P2 promoter rose slightly but reproducibly in E7-expressing cells exposed to UV light. The level of p53 also rose slightly but reproducibly in E7-expressing cells exposed to UV light (Fig. 5A). In sum, these results indicate that the activity of the P2 promoter corresponds with the level of active p53.
FIG. 5.
Pools of GM00011 cells were generated carrying vector alone, E6, the dominant-negative p53 mutant, or E7. Cells were either left untreated (No UV) or exposed to UV light at a dose of 10 J/m2 and harvested 4 h (+UV 4 h) or 8 h (+UV 8 h) later. (A) Levels of p53 were determined by Western analysis as described in the legend to Fig. 1. The first lane contains 10 μl of a rabbit reticulocyte lysate programmed with p53 RNA (IVT, in vitro translated). The second lane contains 2 μl of a rabbit reticulocyte lysate programmed with p53 RNA. The next 12 lanes contain 150-μg quantities of lysates from NDFs infected with the retrovirus indicated. (B) Levels of mdm2 mRNAs were analyzed by S1 nuclease protection. The ratio of the amount of mdm2 mRNA synthesized from the P2 (p53-responsive) promoter to the amount of mdm2 mRNA synthesized from the P1 (constitutive) promoter is shown. The results are averages of data from two experiments with two independent sets of pools of infected NDFs. One of the sets of NDFs was also used to generate the data shown in Fig. 5A.
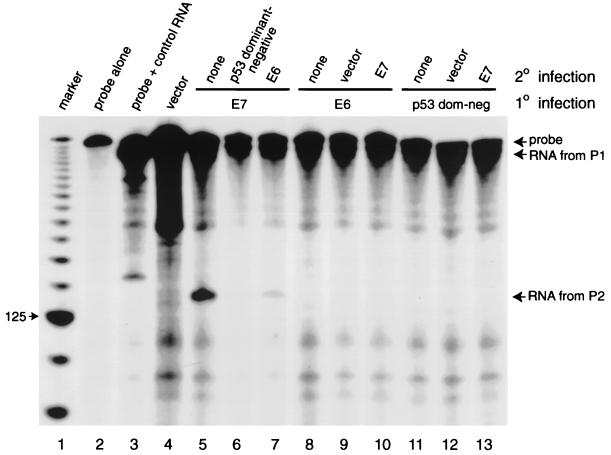
To determine whether the induction of P2 promoter activity by E7 can be inhibited when p53 function is abrogated, we coexpressed E7 with E6 and with dominant-negative p53. Reinfection of an E7-expressing population of NDFs with retroviruses expressing either the dominant-negative p53 protein or the E6 oncoprotein led to a reduction in the high levels of P2-specific mdm2 mRNA (Fig. 6; compare lane 5 to lanes 6 and 7). Low levels of expression of mdm2 from the P2 promoter were also seen when cells expressing E6 or dominant-negative p53 were reinfected with retroviruses expressing E7 (Fig. 6, lanes 8 to 10 and 11 to 13, respectively). We conclude that E7’s ability to upregulate the activity of the P2 promoter of mdm2 is dependent on an increase in p53 function.
FIG. 6.
S1 nuclease protection assay of RNA from GM00037 cells expressing E7 alone, E7 and E6, or E7 and dominant-negative p53. Lane 1, 25-bp molecular size markers; lane 2, probe alone; lane 3, product of protection of mdm2 mRNA transcribed in vitro from the P1 (constitutive) promoter; lane 4, RNA from NDFs carrying the vector alone; lane 5, NDFs expressing E7 alone; lane 6, NDFs expressing E7 reinfected with virus expressing dominant-negative p53; lane 7, NDFs expressing E7 reinfected with virus expressing E6; lane 8, NDFs expressing E6 alone; lane 9, NDFs expressing E6 reinfected with virus carrying the vector; lane 10, NDFs expressing E6 reinfected with virus expressing E7; lane 11, NDFs expressing the dominant-negative p53 mutant alone; lane 12, NDFs expressing the dominant-negative p53 mutant reinfected with virus carrying the vector; lane 13, NDFs expressing the dominant-negative p53 mutant reinfected with virus expressing E7. 1°, primary; 2°, secondary.
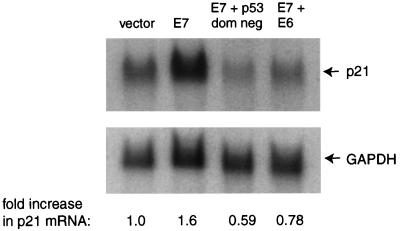
Since it has not been clear whether E7 stimulates transcription of the p21 gene in a p53-dependent manner, we performed Northern analysis on RNAs isolated from pools of NDFs expressing E7 in the presence and absence of E6 and dominant-negative p53. The results indicated that p21 mRNA was increased 1.6-fold in NDFs expressing E7, but not in those expressing both E7 and E6 or both E7 and dominant-negative p53 (Fig. 7). Thus, the small increase in transcription of p21 in response to E7 appears to be p53 dependent.
FIG. 7.
Northern analysis of p21 mRNA in GM00011 cells expressing E7 alone, E7 and E6, or E7 and dominant-negative p53. First lane from left, NDFs carrying the vector alone; second lane, NDFs expressing E7; third lane, NDFs expressing E7 and dominant-negative p53; fourth lane, NDFs expressing E7 and E6. The blot was probed with a radiolabeled cDNA to p21 and reprobed with a radiolabeled cDNA to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; a loading control). The fold increase in p21 mRNA was calculated as the ratio of the signal from p21 to the signal from GAPDH and normalized to signal from the vector control (n = 2).
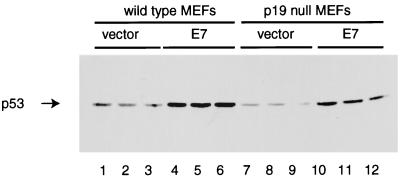
Stabilization and activation of p53 by the E1A viral oncoprotein appear to require the p19ARF protein (15). To investigate whether the increase in p53 levels mediated by E7 required p19ARF, we infected wild-type and p19-null MEFs with either a retroviral vector (pBabe Puro) or a virus expressing HPV-16 E7 (pBabe Puro E7). Western analysis of lysates from infected cells showed that E7 stimulated an increase in p53 levels independent of p19ARF gene status (Fig. 8). MDM2 protein levels were also elevated in MEFs of both genotypes (52), and this likely reflected a p19ARF-independent stimulation of p53 function.
FIG. 8.
Level of p53 in MEFs expressing E7. Early-passage wild-type or p19-null MEFs were infected in triplicate with a retroviral vector alone (pBabe Puro) or with a retrovirus expressing E7 (pBabe Puro E7). Cells were harvested after incubation for 1 week in the presence of puromycin, and 100 μg of total cell protein was analyzed by Western blotting. The amount of p53 expressed was revealed by using the CM5 antibody and goat anti-rabbit Ig conjugated to horseradish peroxidase. Lanes 1 to 3, wild-type MEFs infected with the vector pBabe Puro; lanes 4 to 6, wild-type MEFs infected with pBabe Puro E7; lanes 7 to 9, p19-null MEFs infected with the vector pBabe Puro; lanes 10 to 12, p19-null MEFs infected with pBabe Puro E7.
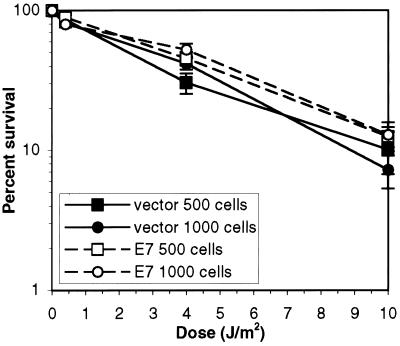
The increased activity of p53 in E7-expressing cells is likely to be due, at least in part, to the increased amount of p53 that is free of MDM2 (Fig. 2). MDM2 is induced by p53 in response to DNA-damaging agents (7, 53) and is hypothesized to regulate p53 function, in an autoregulatory loop, such that some cells recover from DNA damage (67). Indeed, overexpression of MDM2 blocks the G1 arrest mediated by p53 in response to ionizing radiation (7) and inhibits p53-mediated apoptosis in some cell types (24). Expression of E7 in the murine epidermis prolongs the elevation of p53 levels following exposure to ionizing radiation (60), perhaps because MDM2 cannot regulate p53 levels in the presence of E7. Thus, E7 expression may sensitize NDFs to p53-mediated apoptosis. Indeed, NDFs expressing E7 have an increased propensity to undergo apoptosis in response to serum deprivation (30). Since NDFs have been reported to undergo p53-mediated apoptosis in response to exposure to UV light (18), we tested whether abrogation of the interaction between MDM2 and p53 by E7 would lead to increased sensitivity to UV light. We assayed the ability of NDFs carrying the vector alone to grow into colonies following exposure to UV light and compared that to the ability of NDFs expressing E7 to do so (Fig. 9). The dose of UV light required to reduce the number of colonies of NDFs carrying the vector alone by 50% was, on average, 3.7 J/m2. The dose of UV light required to reduce the number of colonies of E7-expressing NDFs by 50% was, on average, 4.1 J/m2. Thus, E7 does not increase the sensitivity of NDFs to UV light, as measured by a colony-forming assay, even though p53 is functional as a transcription factor.
FIG. 9.
Survival of GM00011 cells following exposure to UV light. NDFs were plated 24 h prior to irradiation. Following irradiation, colonies were allowed to grow for 2 weeks. The plated cells were then fixed and stained, and colonies were counted. Closed symbols indicate the colony-forming ability of NDFs carrying the vector alone following UV exposure; open symbols indicate the colony-forming ability of E7-expressing NDFs following UV exposure. The squares represent the results obtained when 500 cells were plated on each dish; the circles represent the results obtained when 1,000 cells were plated on each dish.
DISCUSSION
Here we showed that the HPV-16 E7 protein increases the level of the p53 protein in NDFs by inhibiting the interaction of p53 and MDM2. This results in an increase in the transcriptional activation function of p53. This effect of E7 appears to be counterproductive to the well-established effect of E7 on extending the life span of NDFs, since p53 transcriptional function promotes senescence (19). However, it is clear from the data presented here that E7 expression activates p53 while inhibiting some of the downstream biological effects of p53 function. For example, NDFs expressing E7 do not exhibit an increased sensitivity to UV light, as determined by colony-forming ability, even though UV light has been reported to induce p53-mediated apoptosis in NDFs (18). Furthermore, E7-expressing NDFs continue to divide in spite of increased levels of p53, unlike NDFs in which the interaction between MDM2 and p53 has been inhibited by antibodies that block their association (2, 19). The ability of MDM2 to regulate p53 is required not only for multiplication of cultured cells but also for the development of murine embryos (32, 45). Thus, E7 promotes cell division while abrogating what is, in its absence, an essential control mechanism.
E7 expression results in a large amount of MDM2 protein that is not complexed with p53. In NDFs, transient overexpression of human MDM2 alone inhibits cell division by inducing a G1 arrest (4). Thus, E7 must change the environment of NDFs such that they can tolerate overexpression of MDM2. MDM2 has been shown to stimulate S phase in some cell types that tolerate its overexpression. For example, MDM2 allows mink lung epithelial cells to resist the G1 arrest mediated by transforming growth factor β1 (TGF-β1) (61). In these cells, MDM2 inhibits the accumulation of hypophosphorylated pRb normally caused by treatment with TGF-β1 (61). Furthermore, E2F1 levels and activity are higher in TGF-β1-treated mink lung epithelial cells that overexpress MDM2 (61). MDM2 can stimulate E2F function in other cell types (41, 68). Teoh et al. (62) showed that inhibition of MDM2 expression by antisense oligonucleotides resulted in dephosphorylation of pRb and in increased amounts of E2F1 in complexes with pRb in a human tumor cell line. Thus, in NDFs, MDM2 may be part of the mechanism by which E7 stimulates the release of E2F from pRb (47). MDM2 has been shown to directly associate with E2F1, and this interaction is postulated to allow MDM2 to stimulate E2F function, even in cells that lack pRb (41). The interaction between MDM2 and E2F1 may be enhanced in E7-expressing cells, and this may promote entry into S phase. MDM2 has been shown to promote DNA replication in differentiated mammary epithelial cells in vivo, leading to polyploidy (40). Perhaps MDM2 overexpression is part of the program through which E7 stimulates DNA synthesis in postmitotic epithelial cells (10) or tumorigenesis in the epidermis (21).
The interaction between E2F and MDM2 may be important for the stimulation of p53 function by E7. E7 liberates E2F from Rb, and overexpression of E2F1 increases the levels of both p53 and MDM2 (71). This effect of E2F1 does not require p19ARF (71). Our data indicate that E7, like E2F1, can increase the levels of both p53 and MDM2 in cells lacking p19ARF. Therefore, E7 and E2F1 may stabilize p53 through the same p19ARF-independent mechanism. One possibility for such a mechanism is that E7 interferes with the function of p300, a protein recently found to act as a platform for the stimulation of degradation of p53 by MDM2 (20). Another candidate pathway is that used by ionizing radiation, which is known to be p19ARF independent (34). Indeed, some of the kinases involved in the response to ionizing radiation have been shown to phosphorylate p53 and MDM2, resulting in an inhibition of binding of these two proteins (43, 59). E7 could physically block the interaction of MDM2 with p53 by binding either partner and hiding the necessary interaction surface. However, we have not been able to obtain evidence supporting this model; neither p53 nor MDM2 bound to an E7–glutathione S-transferase (GST) fusion protein in experiments in which pRb bound to E7-GST readily, so evidence for a direct interaction is lacking (52). Finally, our data do not eliminate the possibility that E7, like c-myc (71), activates p53 via multiple pathways, some of which are dependent on p19ARF. Delineation of the mechanism(s) used by E7 to abrogate the interaction between MDM2 and p53 may provide insight into the pathways deregulated in human tumors which contain high levels of both wild-type p53 and MDM2 (22).
It is not yet known whether E7 stimulates host cell DNA replication in postmitotic epithelial cells during a viral infection. HPVs express E6 as well as E7, and we have shown that E6 inhibits the p53-mediated induction of mdm2 transcription by E7 in NDFs. Thomas and Laimins showed that MDM2 protein levels were high in normal, proliferating human keratinocytes expressing E7 and reduced when E6 was coexpressed with E7 (63). Therefore, during a viral infection, E6 may prevent the increase in MDM2 caused by E7. It would be interesting to determine whether MDM2 is induced during an HPV infection and, if so, whether it plays a role in stimulating host cell DNA synthesis, HPV replication, or tumorigenic conversion.
ACKNOWLEDGMENTS
We thank Donna George, Hartmut Land, Vimla Band, Jiandong Chen, Bert Vogelstein, and Peggy Farnham for plasmids. We are grateful to David Baltimore and Karl Munger for retroviral packaging lines and to Arnold Levine and Martine Roussel for MEFs. We appreciate the enthusiastic encouragement of Paul Lambert, Susan Mendrysa, and Martine Roussel. We thank Paul Tannous for excellent technical assistance. Insightful comments on the manuscript by Karl Munger and members of the Perry and Lambert laboratories are gratefully acknowledged.
This work was supported by developmental funds from NCI Cancer Center support grant CA-07175 to the McArdle Laboratory for Cancer Research and by NIH grant CA-70718 to M.E.P. L.J.S. was supported by NCI predoctoral training grant CA-09135 from the National Institutes of Health.
REFERENCES
- 1.Banks L, Edmonds C, Vousden K H. Ability of the HPV16 E7 protein to bind Rb and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990;5:1383–1389. [PubMed] [Google Scholar]
- 2.Blaydes J P, Wynford-Thomas D. The proliferation of normal human fibroblasts is dependent upon negative regulation of p53 function by mdm2. Oncogene. 1998;16:3317–3322. doi: 10.1038/sj.onc.1201880. [DOI] [PubMed] [Google Scholar]
- 3.Bosch F X, Manos M M, Munoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 4.Brown D R, Thomas C A, Deb S P. The human oncoprotein MDM2 arrests the cell cycle: elimination of its cell-cycle-inhibitory function induces tumorigenesis. EMBO J. 1998;17:2513–2525. doi: 10.1093/emboj/17.9.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull E K, Chakrabarty S, Brodsky I, Haines D S. Mdm2-P2 transcript levels predict the functional activity of the p53 tumor suppressor in primary leukemic cells. Oncogene. 1998;16:2249–2257. doi: 10.1038/sj.onc.1201757. [DOI] [PubMed] [Google Scholar]
- 6.Chellappan S, Kraus V B, Kroger B, Munger K, Howley P M, Phelps W C, Nevins J R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C-Y, Oliner J D, Zhan Q, Fornace A J, Jr, Vogelstein B, Kastan M B. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA. 1994;91:2684–2688. doi: 10.1073/pnas.91.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Marechal V, Levine A J. Mapping of the p53 and mdm-2 interaction domains. Mol Cell Biol. 1993;13:4107–4114. doi: 10.1128/mcb.13.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Lin J, Levine A J. Regulation of transcription functions of the p53 tumor suppressor by the mdm2 oncogene. Mol Med. 1995;1:142–152. [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng S, Schmidt-Grimminger D-C, Murant T, Broker T R, Chow L. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9:2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- 11.Cistulli C A, Kaufmann W K. p53-dependent signaling sustains DNA replication and enhances clonogenic survival in 254 nm ultraviolet-irradiated human fibroblasts. Cancer Res. 1998;58:1993–2002. [PubMed] [Google Scholar]
- 12.Crook T, Tidy J A, Vousden K H. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- 13.Demers G W, Halbert C L, Galloway D A. Elevated wild-type p53 protein levels in human epithelial cell lines immortalized by the human papillomavirus type 16 E7 gene. Virology. 1994;198:169–174. doi: 10.1006/viro.1994.1019. [DOI] [PubMed] [Google Scholar]
- 14.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 15.de Stanchina E, McCurrach M E, Zindy F, Shieh S-Y, Ferbeyre G, Samuelson A V, Prives C, Roussel M F, Sherr C J, Lowe S W. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 17.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 18.Ford J M, Hanawalt P C. Expression of wild-type p53 is required for efficient global genomic nucleotide excision repair in UV-irradiated human fibroblasts. J Biol Chem. 1997;272:28073–28080. doi: 10.1074/jbc.272.44.28073. [DOI] [PubMed] [Google Scholar]
- 19.Gire V, Wynford-Thomas D. Reinitiation of DNA synthesis and cell division in senescent human fibroblasts by microinjection of anti-p53 antibodies. Mol Cell Biol. 1998;18:1611–1621. doi: 10.1128/mcb.18.3.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossman S R, Perez M, Kung A L, Joseph M, Mansur C, Xiao Z-X, Kumar S, Howley P M, Livingston D M. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol Cell. 1998;2:405–415. doi: 10.1016/s1097-2765(00)80140-9. [DOI] [PubMed] [Google Scholar]
- 21.Gulliver G A, Herber R L, Liem A, Lambert P F. Both conserved region 1 (CR1) and CR2 of the human papillomavirus type 16 E7 oncogene are required for induction of epidermal hyperplasia and tumor formation in transgenic mice. J Virol. 1997;71:5905–5914. doi: 10.1128/jvi.71.8.5905-5914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haines D S. The mdm2 proto-oncogene. Leukemia Lymphoma. 1997;26:227–238. doi: 10.3109/10428199709051772. [DOI] [PubMed] [Google Scholar]
- 23.Halbert C L, Demers G W, Galloway D A. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol. 1992;66:2125–2134. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haupt Y, Barak Y, Oren M. Cell type-specific inhibition of p53-mediated apoptosis by mdm2. EMBO J. 1996;15:1596–1606. [PMC free article] [PubMed] [Google Scholar]
- 25.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature (London) 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 26.Heck D V, Yee C L, Howley P M, Munger K. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc Natl Acad Sci USA. 1992;89:4442–4446. doi: 10.1073/pnas.89.10.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda R, Yasuda H. Association of p19ARF with MDM2 inhibits ubiquitin ligase activity of MDM2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howley P M. Papillomavirinae and their replication. In: Fields B N, Knipe D M, editors. Fields virology. Vol. 2. New York, N.Y: Raven Press; 1990. pp. 1625–1650. [Google Scholar]
- 29.Jian Y, Schmidt-Grimminger D-C, Chien W-M, Wu X, Broker T R, Chow L T. Post-transcriptional induction of p21cip1 protein by human papillomavirus E7 inhibits unscheduled DNA synthesis reactivated in differentiated keratinocytes. Oncogene. 1998;17:2027–2038. doi: 10.1038/sj.onc.1202142. [DOI] [PubMed] [Google Scholar]
- 30.Jones D L, Thompson D A, Munger K. Destabilization of the Rb tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997;239:97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- 31.Jones D L, Thompson D A, Suh-Burgmann E, Grace M, Munger K. Expression of the HPV E7 oncoprotein mimics but does not evoke a p53-dependent cellular DNA damage response pathway. Virology. 1999;258:406–414. doi: 10.1006/viro.1999.9733. [DOI] [PubMed] [Google Scholar]
- 32.Jones S N, Roe A E, Donehower L A, Bradley A. Rescue of embryonic lethality in mdm2-deficient mice by absence of p53. Nature (London) 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 33.Juven T, Barak Y, Zauberman A, George D L, Oren M. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene. 1993;8:3411–3416. [PubMed] [Google Scholar]
- 34.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 35.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 36.Kubbutat M H G, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature (London) 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 37.Lambert P F, Pan H, Pitot H C, Liem A, Jackson M, Griep A E. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc Natl Acad Sci USA. 1993;90:5583–5587. doi: 10.1073/pnas.90.12.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landers J E, Cassel S L, George D L. Translational enhancement of mdm2 oncogene expression in human tumor cells containing a stabilized wild-type p53 protein. Cancer Res. 1997;57:3562–3568. [PubMed] [Google Scholar]
- 39.Lane D P, Stephen C W, Midgley C A, Sparks A, Hupp T R, Daniels D A, Greaves R, Reid A, Vojtesek B, Picksley S M. Epitope analysis of the murine p53 tumor suppressor protein. Oncogene. 1996;12:2461–2466. [PubMed] [Google Scholar]
- 40.Lundgren K, Montes de Oca Luna R, McNeill Y B, Emerick E P, Spencer B, Barfield C R, Lozano G, Rosenberg M P, Finlay C A. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997;11:714–725. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
- 41.Martin K, Trouche D, Hagemeier C, Sorensen T S, La Thangue N B, Kouzarides T. Stimulation of E2F/DP1 transcriptional activity by MDM2 oncoprotein. Nature (London) 1995;375:691–694. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- 42.Massimi P, Banks L. Repression of p53 transcriptional activity by the HPV E7 proteins. Virology. 1997;227:255–259. doi: 10.1006/viro.1996.8315. [DOI] [PubMed] [Google Scholar]
- 43.Mayo L D, Turchi J J, Berberich S J. MDM2 phosphorylation by DNA-dependent protein kinase prevents interaction with p53. Cancer Res. 1997;57:5013–5016. [PubMed] [Google Scholar]
- 44.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 45.Montes de Oca Luna R, Wagner D S, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature (London) 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 46.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morozov A, Shiyanov P, Barr E, Leiden J M, Raychaudhuri P. Accumulation of human papillomavirus type 16 E7 protein bypasses G1 arrest induced by serum deprivation and by the cell cycle inhibitor p21. J Virol. 1997;71:3451–3457. doi: 10.1128/jvi.71.5.3451-3457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munger K, Werness B A, Dyson N, Phelps W C, Harlow E, Howley P M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor protein. EMBO J. 1989;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliner J D, Pietenpol J A, Thiagalingam S, Gyuris J, Kinzler K W, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumor suppressor p53. Nature (London) 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 50.Pan H, Griep A E. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994;8:1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- 51.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perry, M. E. Unpublished data.
- 53.Perry M E, Piette J, Zawadski J A, Harvey D, Levine A J. The mdm2 gene is induced in response to UV light in a p53 dependent manner. Proc Natl Acad Sci USA. 1993;90:11623–11627. doi: 10.1073/pnas.90.24.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saucedo L J, Carstens B P, Seavey S E, Albee L D, Perry M E. Regulation of transcriptional activation of mdm2 gene by p53 in response to UV radiation. Cell Growth Differ. 1998;9:119–130. [PubMed] [Google Scholar]
- 55.Saucedo L J, Myers C D, Perry M E. Multiple murine double minute gene 2 (MDM2) proteins are induced by ultraviolet light. J Biol Chem. 1999;274:8161–8168. doi: 10.1074/jbc.274.12.8161. [DOI] [PubMed] [Google Scholar]
- 56.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 57.Shaulian E, Zauberman A, Ginsberg D, Oren M. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol. 1992;12:5581–5592. doi: 10.1128/mcb.12.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherr C J. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 59.Shieh S-Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 60.Song S, Gulliver G A, Lambert P F. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci USA. 1998;95:2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun P, Dong P, Dai K, Hannon G J, Beach D. p53-independent role of MDM2 in TGF-β1 resistance. Science. 1998;282:2270–2272. doi: 10.1126/science.282.5397.2270. [DOI] [PubMed] [Google Scholar]
- 62.Teoh G, Urashima M, Ogata A, Chauhan D, DeCaprio J A, Treon S P, Schlossman R L, Anderson K C. MDM2 protein overexpression promotes proliferation and survival of multiple myeloma cells. Blood. 1997;90:1982–1992. [PubMed] [Google Scholar]
- 63.Thomas J T, Laimins L A. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J Virol. 1998;72:1131–1137. doi: 10.1128/jvi.72.2.1131-1137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson D A, Belinsky G, Chang T H-T, Jones D L, Schlegel R, Munger K. The human papillomavirus-16 E6 oncoprotein decreases the vigilance of mitotic checkpoints. Oncogene. 1997;15:3025–3035. doi: 10.1038/sj.onc.1201495. [DOI] [PubMed] [Google Scholar]
- 65.Tso J Y, Sun X-H, Kao T-h, Reece K S, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaziri H, West M D, Allsopp R C, Davison T S, Wu Y-S, Arrowsmith C H, Poirier G G, Benchimol S. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 1997;16:6018–6033. doi: 10.1093/emboj/16.19.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, Bayle J H, Olson D, Levine A J. The p53-mdm2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 68.Xiao Z-X, Chen J, Levine A J, Modjtahedl N, Xing J, Sellers W R, Livingston D M. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature (London) 1995;375:694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- 69.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukemic cells that is inhibited by interleukin-6. Nature (London) 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 70.Zauberman A, Flusberg D, Haupt Y, Barak Y, Oren M. A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Nucleic Acids Res. 1995;23:2584–2592. doi: 10.1093/nar/23.14.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]