Abstract
Background
Ticks are known to transmit a wide range of diseases, including those caused by bacteria, viruses, and protozoa. The expansion of tick habitats has been intensified in recent years due to various factors such as global warming, alterations in microclimate, and human activities. Consequently, the probability of human exposure to diseases transmitted by ticks has increased, leading to a higher degree of risk associated with such diseases.
Methods
In this study, we conducted a comprehensive review of domestic and international literature databases to determine the current distribution of tick species in Inner Mongolia. Next, we employed the MaxEnt model to analyze vital climatic and environmental factors influencing dominant tick distribution. Subsequently, we predicted the potential suitability areas of these dominant tick species under the near current conditions and the BCC-CSM2.MR model SSP245 scenario for the future periods of 2021–2040, 2041–2060, 2061–2080, and 2081–2100.
Results
Our study revealed the presence of 23 tick species from six genera in Inner Mongolia, including four dominant tick species (Dermacentor nuttalli, Ixodes persulcatus, Dermacentor silvarum, and Hyalomma asiaticum). Dermacentor nuttalli, D. silvarum, and I. persulcatus are predominantly found in regions such as Xilin Gol and Hulunbuir. Temperature seasonality (Bio4), elevation (elev), and precipitation seasonality (Bio15) were the primary variables impacting the distribution of three tick species. In contrast, H. asiaticum is mainly distributed in Alxa and Bayannur and demonstrates heightened sensitivity to precipitation and other climatic factors. Our modeling results suggested that the potential suitability areas of these tick species would experience fluctuations over the four future periods (2021–2040, 2041–2060, 2061–2080, and 2081–2100). Specifically, by 2081–2100, the centroid of suitable habitat for D. nuttalli, H. asiaticum, and I. persulcatus was predicted to shift westward, with new suitability areas emerging in regions such as Chifeng and Xilin Gol. The centroid of suitable habitat for H. asiaticum will move northeastward, and new suitability areas are likely to appear in areas such as Ordos and Bayannur.
Conclusions
This study provided a comprehensive overview of the tick species distribution patterns in Inner Mongolia. Our research has revealed a significant diversity of tick species in the region, exhibiting a wide distribution but with notable regional disparities. Our modeling results suggested that the dominant tick species’ suitable habitats will significantly expand in the future compared to their existing distribution under the near current conditions. Temperature and precipitation are the primary variables influencing these shifts in distribution. These findings can provide a valuable reference for future research on tick distribution and the surveillance of tick-borne diseases in the region.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-023-05870-6.
Keywords: Tick, MaxEnt model, Suitability area, Environmental factor, Inner Mongolia
Background
Ticks are hematophagous arthropods that parasitize various vertebrate hosts, including livestock and wildlife [1]. Approximately 900 species of ticks worldwide are classified into three families and 18 genera. The reported presence of 125 tick species in China, consisting of 111 hard ticks (Ixodidae) and 14 soft ticks (Argasidae), serves as compelling evidence of the striking heterogeneity of tick diversity within the country [2]. Ticks not only are significant ectoparasites of animals but also serve as vectors and reservoirs for a wide range of important pathogens affecting humans and animals [3]. Ticks require blood meals to complete their life cycle, but this process can also cause localized infection, edema, acute inflammation, and secondary infections in the host. Additionally, ticks can transmit various tick-borne pathogens, including bacteria, viruses, and protozoans, which pose a significant threat to human and animal health [4, 5]. With global climate change and the expansion of human activities, tick bite incidents and associated infections have become a significant public health threat with increasingly severe consequences.
Due to the unique physiological habits of ticks, they spend most of their life cycle in natural environments outside their hosts. Consequently, ecological conditions are closely related to tick distribution patterns and population abundance. Conversely, climatic factors and host diversity can be used to predict areas at risk for tick-borne diseases [6]. Increased temperatures can positively impact ticks’ subsistence and reproduction during their non-parasitic life stages, promoting population growth and the possibility of expansion, establishment, and survival in new geographical areas [7]. Global climate change also indirectly affects tick-borne disease transmission, such as influencing vegetation distribution, the abundance of pathogen reservoir hosts, human behavior, and land use, which affect tick abundance and pathogen transmission [8]. Therefore, under the increasing risk of tick-borne diseases with climate change, understanding the distribution of dominant tick species under current and future climate conditions is profoundly significant for the prevention and control of tick-borne disease [9].
Several models have been developed to predict the distribution of species, including the maximum entropy (MaxEnt) model, classification and regression tree (CART) model, generalized linear model (GLM), habitat model (HABITAT), genetic algorithm for rule-set prediction (GARP) model, and bioclimatic model (BIOCLIM) [10, 11]. Among these, the MaxEnt model is based on the theory of species ecological niches and uses the location information of species occurrence and environmental background data to fit the probability distribution with the MaxEnt value, estimating the potential species distribution. Under the premise of known partial information, the most reasonable inference about the unknown distribution is the least specific or most random inference that conforms to the known information. The MaxEnt model has several advantages, such as using existing incomplete, small-sample, and discrete distribution data to predict species’ suitable distribution with high accuracy, stability, and easy interpretation [12, 13]. Therefore, it is widely used to predict ticks’ suitable habitat distribution.
Inner Mongolia, the widest province in China by latitude, is located along the northern border and is currently a high-incidence area for tick-borne diseases. Various tick-borne diseases, such as rickettsiosis, Crimean-Congo hemorrhagic fever, and brucellosis, have been reported in the region [14–16]. Inner Mongolia's unique natural climate, nomadic lifestyle, and ecotones between forests and grasslands provide favorable conditions for tick subsistence and reproduction. The rapidly developing livestock industry has significantly altered the interactions between animals and their habitats, making the relationship between ticks and host animals more complex. Moreover, multiple border ports in Inner Mongolia increase the risk of tick expansion and cross-border transmission of pathogens due to human activities and international trade. This study predicted the distribution of dominant tick species in Inner Mongolia based on the MaxEnt model and the ArcGIS spatial technology platform. This predictive data can provide research data for further studies on the distribution of tick populations in Inner Mongolia and the prevention and control of tick-borne diseases. Additionally, in-depth research on the current distribution status and trends of ticks in Inner Mongolia is of great significance for the monitoring and risk warning of tick-borne diseases in the region and the entire country.
Methods
Collection of tick geographical distribution data
A literature search was conducted in the CNKI (China National Knowledge Infrastructure), Wanfang, Baidu Scholar, PubMed, and Google Scholar databases using the keywords “Inner Mongolia” and “tick” for articles published between January 1, 1960, and November 30, 2022. By reading the titles, abstracts, and full texts of the articles, two reviewers filtered literature containing geographical distribution information of tick species in Inner Mongolia and extracted their geographical location information and distribution site coordinates. If the latitude and longitude were not indicated in the text, we determined the distribution site coordinates using the coordinate picker function on Google Maps based on the geographical location specified in the text. Based on a comprehensive review of existing literature reports, a summary of tick species distribution in Inner Mongolia has been compiled. The dominant tick species have been unequivocally determined through a thorough analysis of the number of distribution sites, ecological environments, records, and frequencies. To avoid overfitting due to overly concentrated distribution sites, ArcGIS 10.4 software was used to set a buffer zone with a radius of 10 km (consistent with the 10 km resolution of environmental climate data) for each acquired distribution point. Only one distribution site was retained in each 10 × 10 km grid.
Selection of environmental factors
Environmental factors such as bioclimatic variables and geographical data were used in our study. We used the 19 bioclimatic variables from the WorldClim database [17]. The resolution of the 19 bioclimatic variable layers was 5 arc minutes (approximately 10 × 10 km pixels near the equator). To avoid overfitting multicollinearity, the 19 climate factors (Bio1–Bio19) were resampled using the sampling function in ArcGIS 10.4 software (Esri ArcMap; Esri, Redlands, CA, USA). The data were imported into SPSS 22.0 software (IBM Corporation, Armonk, NY), and Pearson's matrix was used for correlation analysis. Highly correlated variables were defined as those with a correlation coefficient |r|> 0.9 and were screened based on the contribution rate of environmental factors. Basic map data are derived from the Department of Natural Resources Standard Map Service System (https://www.webmap.cn/). Geographical data, including slope, aspect, and elevation data for China, were downloaded from the Geospatial Data Cloud (www.gscloud.cn), and slope and aspect variables were calculated using ArcGIS 10.4 software.
Construction of the maximum entropy model
For the selected dominant tick species, the MaxEnt model was used to determine the key factors influencing tick distribution in Inner Mongolia. We randomly selected 75% of tick distribution sites as the training set and the remaining 25% as the test set to validate the model’s accuracy. The convergence domain limit was 10−5, the maximum number of iterations was 500, and 10 bootstrap calculations were performed for the current dominant tick species in Inner Mongolia using the MaxEnt model. The screened species distribution data and climate data were imported into the model. The jackknife method was selected in the environmental parameter settings to evaluate the weight of each ecological factor. We established univariate response curves for the distribution probability and environmental factors to determine the suitable range of environmental variable values.
Next, the accuracy of the model’s prediction results was validated using the receiver operating characteristic (ROC) curve in MaxEnt software. The area under the ROC curve (AUC value) was used to measure the model's predictive accuracy. Evaluation criteria were as follows: AUC values of 0.5–0.6 indicate failure, 0.6–0.7 indicate poor, 0.7–0.8 indicate fair, 0.8–0.9 indicate good, and 0.9–1.0 indicate excellent. The closer the AUC value was to 1, the more accurate the model's prediction results and the more significant the correlation between environmental factors and species distribution. The Jenks natural breaks classification method in ArcGIS 10.4 MaxEnt software was used to classify the habitat suitability levels of the four selected dominant tick species in Inner Mongolia.
Construction of potential suitability areas model for dominant tick species
The future climate data were derived from the high-resolution climate model BCC-CSM2.MR in the Sixth Coupled Model Intercomparison Project (CMIP6) [18]. The BCC-CSM2-MR model included four components: atmosphere, land surface, ocean, and sea ice. Following the literature, the SSP245 scenario under the BCC-CSM2-MR model was selected [19]. Four future periods were used in the study: 2021–2040, 2041–2060, 2061–2080, and 2081–2100; SSP245 represents an upgrade of the RCP4.5 scenario based on SSP2, a medium forcing scenario. From the perspective of future greenhouse gas emissions and concentration trends, the RCP 4.5 scenario peaked in 2040 and stabilized by 2080. This greenhouse gas emission trend is consistent with China's future development trend and conforms to China's national conditions [20]. Therefore, we used the climate factors of the four periods under the SSP245 climate scenario as future environmental factors, along with the current geographical factors and distribution data for dominant tick species. These data were imported into MaxEnt software and run again to predict the habitat suitability of dominant tick species in Inner Mongolia under future environmental conditions.
Changes in the centroid of suitable habitat of dominant species
In this study, we used the suitability areas of dominant tick species to investigate the spatial changes in the overall habitat suitability by examining the changes in their centroid from the near current and 2081–2100 time period. To obtain the centroid coordinates, we performed binary conversion on the distribution maps of the MaxEnt model’s habitat suitability using the ArcGIS 10.4 software. We revealed the changes in direction and distance of the suitable habitat for the dominant tick species by connecting the centroids of suitable habitat under various climate conditions.
Results
Tick species distribution in Inner Mongolia
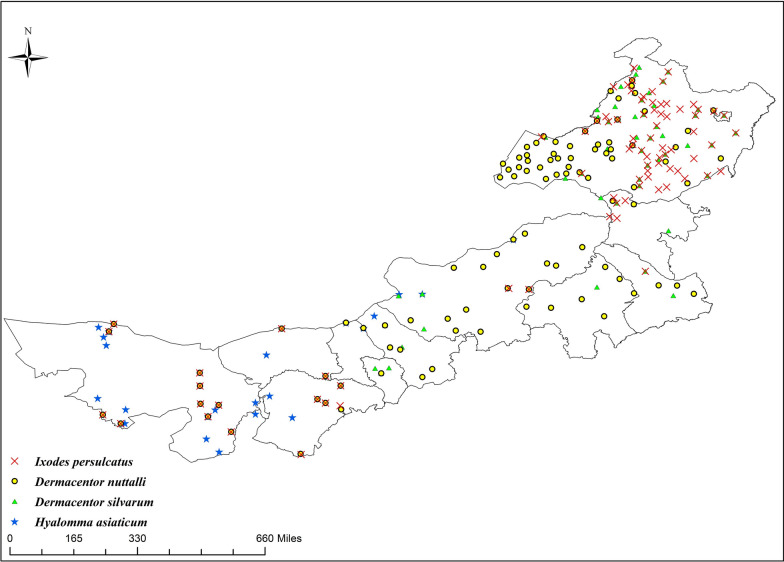
In this study, a total of 5109 articles were retrieved, including 2483 Chinese articles (1740 from CNKI, 253 from Wanfang, 490 from Baidu Scholar) and 2626 English articles (1629 from PubMed, 997 from Google Scholar). After removing duplicates and articles with unclear distribution information that did not meet the criteria, 102 Chinese and 49 English articles were included (Additional file 1: Figure S1). The results showed that 23 tick species belonging to six genera were currently distributed in Inner Mongolia (Table 1). Considering the distribution of different tick species in various ecological environments and the available collection of distribution sites, we selected the four dominant tick species for subsequent modeling analysis. Among the four species, 39 articles and 172 distribution sites were included for Dermacentor nuttalli; 41 articles and 140 distribution sites for Ixodes persulcatus; 35 articles and 128 distribution sites for Dermacentor silvarum; and 23 articles and 38 distribution sites for Hyalomma asiaticum. We set a 10-km-radius buffer zone for the extracted tick distribution sites, and only one distribution site was retained in each buffer zone. Finally, 104 D. nuttalli distribution sites, 85 I. persulcatus distribution sites, 82 D. silvarum distribution sites, and 23 H. asiaticum distribution sites were determined (Fig. 1). The distribution sites of the two subspecies (Hyalomma asiaticum asiaticum and Hyalomma asiaticum kozlovi) were combined to establish the model.
Table 1.
Distribution records of ticks in Inner Mongolia by references
| Genus | Species | References |
|---|---|---|
| Dermacentor | D. nuttalli | [14, 15, 21–58] |
| D. silvarum | [23, 26, 57, 59–92] | |
| D. marginatus | [22, 23, 93, 94] | |
| D. niveus | [28, 95] | |
| D. sinicus | [96] | |
| Hyalomma | H. asiaticum kozlovi | [36, 38, 39, 45, 87, 88, 95, 97–102] |
| H. asiaticum asiaticum | [16, 22, 23, 27, 41, 94, 103–106] | |
| H. marginatum | [54] | |
| H. detritum | [88, 107] | |
| H. dromedarii | [22, 23] | |
| H. rufipes | [108, 109] | |
| Haemaphysalis | H. concinna | [26, 54, 58, 62–64, 67, 68, 74–79, 82, 84, 86, 91, 92, 110–115] |
| H. verticalis | [45, 87, 95, 96, 116–119] | |
| H. japonica | [54, 84, 92] | |
| H. longicornis | [57, 93, 120, 121] | |
| H. bispinosa | [93] | |
| Rhipicephalus | R. turanicus | [54, 94] |
| R. pumilio | [88] | |
| R. sanguineus | [93] | |
| Ixodes | I. persulcatus | [15, 29, 32, 37, 46, 48, 50, 55, 57, 58, 62–64, 67, 68, 75–77, 79, 81–84, 86, 89, 91, 92, 96, 110, 112, 118, 122–135] |
| I. crenulatus | [121, 136] | |
| Argas | A. persicus | [137–139] |
| A. japonicus | [140] |
Fig. 1.
Distribution map of primary tick species in Inner Mongolia
Key variables that influence tick distribution
To construct the MaxEnt model for tick distribution, 19 bioclimatic variables were screened, and six variables were selected for modeling analysis (Additional file 3: Table S1). We used the jackknife analysis to evaluate the impact of various environmental factors on the potential suitability areas for dominant ticks. The top five main variables and their contribution rates are shown in Table 2. The distribution of D. nuttalli, I. persulcatus, and D. silvarum is primarily influenced by temperature seasonality (Bio4), elevation (elev), and precipitation seasonality (Bio15), with contribution rates exceeding 10%. In contrast, the distribution of H. asiaticum was mainly affected by precipitation.
Table 2.
Key variables contributing to tick distribution
| Tick species | ||||
|---|---|---|---|---|
| Key variables (contribution rate %) | D. nuttalli | I. persulcatus | D. silvarum | H. asiaticum |
| 1st | Temperature seasonality = Bio4 (44.0) | Mean temperature of coldest quarter = Bio11 (29.9) | Temperature seasonality = Bio4 (43.2) | Annual precipitation = Bio12 (21.6) |
| 2nd | Elevation = Elev (16.6) | Temperature seasonality = Bio4 (22.9) | Precipitation seasonality = Bio15 (14.6) | Elevation = Elev (18.1) |
| 3rd | Precipitation seasonality = Bio15 (14.6) | Precipitation seasonality = Bio15 (16.0) | Elevation = Elev (11.4) | Precipitation of driest month = Bio14 (10.0) |
| 4th | Slope = Slop (5.1) | Elevation = Elev (11.6) | Mean diurnal range = Bio2 (4.9) | Precipitation of warmest quarter = Bio18 (9.5) |
| 5th | Precipitation of coldest quarter = Bio19 (4.2) | Mean temperature of driest quarter = Bio9 (3.9) | Mean temperature of coldest quarter = Bio11 (4.2) | Aspect = Aspe (9.0) |
Inferring potential suitability areas of ticks under near current and future conditions
The average AUC values of the ROC curve for the four tick species in 10 runs were 0.960, 0.964, 0.956, and 0.986, indicating that the model prediction accuracy is excellent in this study.
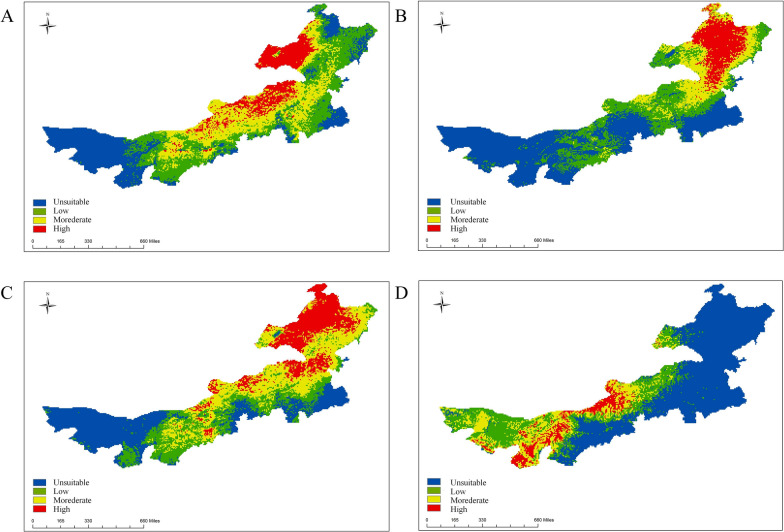
MaxEnt software was used to obtain the potential suitability areas for the four dominant tick species in Inner Mongolia. The potential suitability areas were divided into four levels: high-suitability area, medium-suitability area, low-suitability area, and unsuitable area (Fig. 2). The results showed that the potential suitability areas for D. nuttalli were mainly distributed in Hulunbuir, Xilin Gol, and Ulanqab. In contrast, the unsuitable areas were located in Alxa, Tongliao, and Wuhai. Under future climate scenarios, the proportion of potential suitability and the high-suitability regions for D. nuttalli in Inner Mongolia increased (Fig. 3). Compared to the near current condition, the potential suitability area for D. nuttalli in Inner Mongolia increased by 168,900 km2, with new potential suitability areas distributed in Hulunbuir, Hinggan League, and Chifeng in 2081–2100. The potential suitability area decreased by 34,000 km2, and the loss of potential suitability areas mainly occurred in the northeastern part of Hulunbuir (Additional file 2: Figure S2A).
Fig. 2.
Predicted spatial distribution of tick suitability areas in Inner Mongolia. A D. nuttalli; B I. persulcatus; C D. silvarum; D H. asiaticum
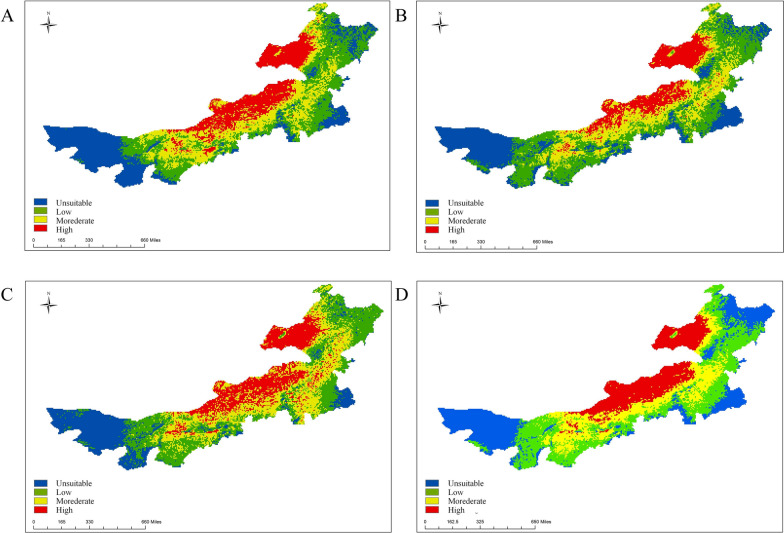
Fig. 3.
Predicted spatial distribution of D. nuttalli suitability areas in Inner Mongolia under future periods: A 2021–2040; B 2041–2060; C 2061–2080; D 2081–2100
The potential suitability areas for I. persulcatus were mainly distributed in Hulunbuir, Hinggan League, and Xilin Gol, while the unsuitable areas were located in Alxa, Tongliao, and Ulanqab. Under future climate scenarios, the proportion of potential high-suitability areas for I. persulcatus in Inner Mongolia decreased and then increased (Fig. 4). Compared to the near current climate scenario, the potential suitability area for I. persulcatus in Inner Mongolia increased by 156,300 km2, with new potential suitability areas distributed in Chifeng, Xilin Gol, and Ordos in the 2081–2100 time period. The potential suitability area decreased by 34,000 km2, and the loss of potential suitability areas were mainly located in Ulanqab (Additional file 2: Figure S2B).
Fig. 4.
Predicted spatial distribution of I. persulcatus in Inner Mongolia under future periods: A 2021–2040; B 2041–2060; C 2061–2080; D 2081–2100
The potential suitability areas for D. silvarum were found in Hulunbuir, Xilin Gol, and Baotou, while the unsuitable areas were mainly located in Tongliao and Alxa. Under future climate scenarios, the proportion of potential suitability areas for D. silvarum in Inner Mongolia increases in all time periods, except for 2021–2040 (Fig. 5). Compared to the near current, the potential suitability area for D. silvarum in Inner Mongolia increased by 135,300 km2, with new potential suitability areas distributed in Chifeng, Xilin Gol, and Bayan Nur in 2081–2100. The potential suitability area decreased by 24,800 km2, and the loss of potential suitability areas were mainly located in Hulunbuir and Tongliao (Additional file 2: Figure S2C) in the future climate scenarios.
Fig. 5.
Predicted spatial distribution of D. silvarum in Inner Mongolia under future periods: A 2021–2040; B 2041–2060; C 2061–2080; D 2081–2100
The potential suitability areas for H. asiaticum were mainly distributed in Alxa, Bayan Nur, and Baotou, while the unsuitable areas were primarily located in Hulunbuir, Chifeng, and Hinggan League. Under future climate scenarios, the proportion of potential high-suitability areas for H. asiaticum in Inner Mongolia increased (Fig. 6). Compared to the near current scenario, the potential suitability area for H. asiaticum in Inner Mongolia increased by 54,100 km2, with new potential suitability areas distributed in Ordos and Bayan Nur. The potential suitability area decreased by 9300 km2, and the loss of potential suitability areas were mainly located in Xilin Gol (Additional file 2: Figure S2D) in the future climate scenarios.
Fig. 6.
Predicted spatial distribution of H. asiaticum in Inner Mongolia under future periods: A 2021–2040; B 2041–2060; C 2061–2080; D 2081–2100
Changes in the centroid of the potential suitability areas for dominant tick species in the near current and future period 2081–2100
The changes in the centroid of the potential suitability areas for dominant tick species in the near current and 2081–2100 time periods are shown in Fig. 7. By 2081–2100, the centroid of the potential suitability area for D. nuttalli shifted 156.89 km to the west-southwest direction, with a latitude change of approximately 0.9° and a longitude change of approximately 1.4°. There was also a westward expansion trend in the overall potential suitability area. By 2081–2100, the centroid of the potential suitability area for I. persulcatus shifted 156.89 km to the west-southwest direction, with a latitude change of approximately 0.9° and a longitude change of approximately 1.4°. The overall potential suitability area presented a westward expansion trend. By 2081–2100, the centroid of the potential suitability area for D. silvarum shifted about 133.65 km towards the west-southwest, with a latitude change of approximately 0.63° and a longitude shift of approximately 1.25°. The overall potential suitability area exhibited a westward expansion trend as well. By 2081–2100, the centroid of the potential suitability area for H. asiaticum moved about 28.75 km towards the east, with little change in latitude and a longitude change of approximately 0.3°. The overall potential suitability area demonstrated an eastward expansion trend.
Fig. 7.
Changes in the centroid of the potential suitability areas for the dominant tick species in Inner Mongolia
Discussion
The MaxEnt model for tick distribution was first reported in 2006 [10] and has since become widely used for predicting tick habitat. In this study, we used the latest version of climate factors and tick distribution data in Inner Mongolia to generate new insights into the potential environmental factors and spatial patterns for tick distribution in Inner Mongolia. Through a comprehensive literature review, we identified 23 species from six genera of ticks reported in Inner Mongolia over the past six decades. The most dominant tick species are D. nuttalli, I. persulcatus, D. silvarum, and H. asiaticum. Using the MaxEnt model, we predicted the potential suitability areas for these four dominant tick species across different ecological environments in Inner Mongolia.
The distribution results for different tick species indicate that species of the genus Dermacentor, including D. nuttalli and D. silvarum, are mainly distributed in arid grassland areas suitable for grazing cattle and sheep, such as Hulunbuir and Xilin Gol within Inner Mongolia. Hulunbuir is an important pastoral area in Inner Mongolia, known for its vast grasslands, making it a significant livestock production area and an essential habitat for Dermacentor and Ixodes genera. Several tick-borne pathogens have been reported in the Hulunbuir region, such as a high Brucella positivity rate of 4.00–87.80% in tick samples in 2020 [14] and a high tick infection rate of Rickettsia up to 85.14% in 2021 [141], posing a severe threat to human and animal health. Moreover, the grassland areas of Hulunbuir and Xilin Gol are home to a diverse and abundant population of rodents and grazing animals, which can support a high density of ticks by serving as natural hosts. Moreover, local herders typically adopt grazing prohibition, rest, and rotation systems [142], which results in large-scale mobility of local wildlife and livestock. The migration of animal populations can lead to the passive movement of ticks that feed on them, thus expanding the potential suitability areas and distribution ranges of these tick species.
In contrast, I. persulcatus is mainly distributed in the Greater Khingan Range in northeastern China, adjacent to Russia. This region is characterized by high vegetation coverage and is one of China's important forestry and animal husbandry bases [54]. The forest–grassland transition zone of the Greater Khingan Range is a typical large-scale community-interlaced area in northern China and a key area where the Greater Khingan Range forest borders the Hulunbuir grassland. The transition zone has high biodiversity, frequent material flow, and strong spatio-temporal dynamic changes, which facilitates the development and reproduction of ticks [143]. Haemaphysalis asiaticum is mainly distributed in arid desert and desert environments in Inner Mongolia, such as Alxa and Bayan Nur. Alxa is located in the western part of the Inner Mongolia Autonomous Region and has a temperate continental climate. The geographical area in question exhibits high temperatures, a limited amount of rainfall, and arid conditions. This environment promotes significant evaporation, and noteworthy fluctuations in temperature are common throughout the day. Additionally, sand and dust storms frequently occur, providing favorable conditions for the development and reproduction of H. asiaticum [144]. In addition, H. asiaticum parasitizes multiple hosts, with larvae feeding on mice, rabbits, hedgehogs, and other small animals typically found in arid areas [145]. In contrast, nymphs and adults usually parasitize large animals such as camels, cattle, horses, sheep, and wild boars [146]. Therefore, host distribution is also a crucial factor for the survival of H. asiaticum in arid environments.
Based on the analysis of the primary climatic and environmental variables, it is evident that temperature seasonality standard deviation (Bio4), elevation (elev), and precipitation seasonality (Bio15) significantly influence the distribution of D. nuttalli, I. persulcatus, and D. silvarum. Meanwhile, we also found considerable overlap in their suitable habitat ranges among these species. The probability of these tick species' distribution reaches its maximum when the temperature seasonality standard deviation (Bio4) is 1719.62, 1939.03, and 1891.02, respectively. It can be inferred that these species are more sensitive to temperature. This finding is consistent with the results of Yang et al., which indicated that the probability of D. nuttalli distribution is more susceptible to temperature than other tick species (Ixodes granulatus, Haemaphysalis longicornis, and Dermacentor marginatus) [147]. The probabilities of the three tick species’ distribution are within the high-suitability range when elevations are 530.84–848.75 m, 581.65–907.59 m, and 493.56–961.61 m, respectively. This demonstrates that D. silvarum has a lower elevation requirement for subsistence and reproduction, which is consistent with our model proposing that the high-suitability area for D. silvarum is larger than that for the other three tick species. In the near current scenario, the high-suitability area for D. silvarum accounts for 19.13% of the total area of Inner Mongolia.
Notably, when Bio15 (precipitation seasonality) is approximately 118 mm, the suitability for three tick species is the highest. This could be a key reason for the overlapping distribution of these tick species in arid grassland regions. Wang et al. revealed the significant impact of Bio15 on D. nuttalli distribution [148]. In contrast to the three ticks mentioned earlier, H. asiaticum is primarily affected by precipitation-related climatic factors. A study on H. asiaticum distribution in Xinjiang showed that a longer summer and shorter winter is an ideal habitat for the species. A lower precipitation variability level corresponds to higher suitability, as stable precipitation helps maintain relatively stable air humidity [149]. Under these conditions, H. asiaticum can absorb water vapor from the air during their host-seeking period, maintaining hydrological balance for subsistence.
Considering the overall increase and decrease in the potential suitability areas inferred for the four tick species, their suitability areas are expected to expand in the near current and the future period of 2081–2100. There are several studies that are consistent with our model. For instance, Yang et al. revealed that the northeastern forest region would become warmer and more suitable for D. nuttalli due to global warming and land-use changes [147]. Additionally, Ma et al. indicated that suitable areas for I. persulcatus will increase in Inner Mongolia by 2070 [150]. Thus, it is evident that the combined effects of climate change, human activities, land use, and vector population growth will lead to the expansion of suitable habitat areas for the dominant tick species in Inner Mongolia. During 2081–2100, the centroid of suitable habitats for D. nuttalli, I. persulcatus, and D. silvarum is expected to shift westward, with local expansion in parts of Alxa, Bayannur, and Ordos. The centroid of suitable habitat areas for H. asiaticum will migrate towards the east-northeast, with newly suitable habitat areas emerging in parts of Ordos and Bayannur. This shift may be related to the reforestation, forest protection, and afforestation projects undertaken in desert areas such as Alxa, as well as the establishment of forest ecosystem benefit compensation systems and afforestation subsidy pilot projects, which have reduced the unused land area in sandy, Gobi, and desert regions [151].
The present study has utilized a comprehensive dataset gathered from various literature sources spanning a broad temporal and geographical range. The majority of reports included in the study were based on either morphological or molecular identification of tick species, and the MaxEnt distribution modeling was performed based on these data. Although there is the possibility of prediction distribution biases due to repeated sampling. By removing duplicate records at the same location and ensuring a minimum distance between sampling records, this study has effectively corrected the geographical sampling bias in the tick distribution dataset. However, it is important to note that the model only provides potential areas where a given species may survive, which may not necessarily represent actual distributions or species abundance. Our model considers only abiotic factors and does not consider the influence of hosts and other biotic factors. For example, the analysis does not include influential factors such as the distribution of animal hosts and human social activities, which may result in tick distribution found in unsuitable habitat areas. Future investigations could therefore incorporate tick-host analysis and further combine multiple modeling and evaluation methods to improve the limitations of the current MaxEnt model.
Conclusion
In summary, the present study has unveiled the extant distribution of tick species in Inner Mongolia through analysis of available data sources. Moreover, utilizing the MaxEnt and ArcGIS spatial technology platforms and taking into account the tick distribution site data and pertinent bioclimatic variable data, we have projected the distribution of the four dominant tick species under the near current and future periods. These findings are important for tick research and monitoring the spread of tick-borne diseases in the region.
Supplementary Information
Additional file 1: Figure S1 Flow diagram of literature search and inclusion.
Additional file 2: Figure S2 Changes in the potential suitability areas for the four dominant tick species under the near current and 2081–2100.
Additional file 3: Table S1 Environmental and bioclimatic variables for the four dominant tick species distribution models by MaxEnt.
Acknowledgements
Not applicable.
Author contributions
XYF and WH conceived the study and contributed the original idea. RM, CFL, HQT, and ZY performed the experiments. RM, JL, and XYF wrote the initial draft of the paper. XYF and WH contributed to the revision of the manuscript, and the final version was reviewed by WH. All authors approved the final manuscript.
Funding
This work was supported by the Inner Mongolia Autonomous Region Science and Technology leading talent team: Zoonotic disease prevention and Control Technology innovation team (2022SLJRC0023); Key Technology Project of Inner Mongolia Science and Technology Department (2021GG0171); State Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock (2020ZD0008); Study on pathogen spectrum, temporal and spatial distribution and transmission features of the important emerging and re-emerging zoonosis in Inner Mongolia Autonomous Region (U22A20526).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All participants consented to have their data published.
Competing interests
We declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinyu Feng, Email: fengxinyu2013@163.com.
Jian Li, Email: leejianshin@163.com.
Wei Hu, Email: huw@imu.edu.cn.
References
- 1.Wikel SK. Ticks and tick-borne infections: complex ecology, agents, and host interactions. Vet Sci. 2018;5:60. doi: 10.3390/vetsci5020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang YK, Zhang XY, Liu JZ. Ticks (Acari: Ixodoidea) in China: geographical distribution, host diversity, and specificity. Arch Insect Biochem Physiol. 2019;102:e21544. doi: 10.1002/arch.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li T. Dermacor population ecology and physiological adaptation in Tibet. Shijiazhuang: Hebei Normal University; 2018. [Google Scholar]
- 4.Kazimírová M, Štibrániová I. Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front Cell Infect Microbiol. 2013;3:43. doi: 10.3389/fcimb.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingry L, et al. Targeted metagenomics for clinical detection and discovery of bacterial tick-borne pathogens. J Clin Microbiol. 2020;58:e00147–e220. doi: 10.1128/JCM.00147-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sukumaran B, et al. An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J Exp Med. 2006;203:1507–1517. doi: 10.1084/jem.20060208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capligina V, et al. Large-scale countrywide screening for tick-borne pathogens in field-collected ticks in Latvia during 2017–2019. Parasites Vectors. 2020;13:1–12. doi: 10.1186/s13071-020-04219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray J, et al. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009 doi: 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madison-Antenucci S, et al. Emerging tick-borne diseases. Clin Microbiol Rev. 2020;33:e00083–e118. doi: 10.1128/CMR.00083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elith J, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 11.Hernandez PA, et al. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography. 2006;29:773–785. [Google Scholar]
- 12.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 13.Elith J, et al. A statistical explanation of MaxEnt for ecologists. Divers Distrib. 2011;17:43–57. [Google Scholar]
- 14.Huang T, et al. A novel arthropod host of brucellosis in the arid steppe ecosystem. Front Vet Sci. 2020;7:566253. doi: 10.3389/fvets.2020.566253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao J, et al. Identification of tick-borne pathogens by metagenomic next-generation sequencing in Dermacentor nuttalli and Ixodes persulcatus in Inner Mongolia, China. Parasites Vectors. 2021;14:1–11. doi: 10.1186/s13071-021-04740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, et al. Seroepidemiological investigation of Crimean-Congo hemorrhagic fever virus in sheep and camels of Inner Mongolia of China. Vector-Borne Zoonotic Dis. 2020;20:461–467. doi: 10.1089/vbz.2019.2529. [DOI] [PubMed] [Google Scholar]
- 17.Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017;37:4302–4315. [Google Scholar]
- 18.Yang G, Pei Y, Song M. Evaluation and prediction of precipitation distribution in Southwest China by CMIP6 model. Open J Nat Sci. 2021;9:11. [Google Scholar]
- 19.Hurtt G, 2016. Harmonization of global land-use change and management for the period 850–2100. Geosci Model Dev. [DOI]
- 20.Zhang L, Huang D, Yang B. Future population exposure to high temperature in China under RCP4. 5 scenario. Geogr Res. 2016;35:2238–2248. [Google Scholar]
- 21.Gui Z, et al. Genetic diversity analysis of Dermacentor nuttalli within Inner Mongolia, China. Parasites Vectors. 2021;14:1–12. doi: 10.1186/s13071-021-04625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong Y, et al. Phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in inner Mongolia, China. Ticks Tick-Borne Dis. 2022;13:101856. doi: 10.1016/j.ttbdis.2021.101856. [DOI] [PubMed] [Google Scholar]
- 23.Kong Y, et al. Metatranscriptomics reveals the diversity of the tick virome in Northwest China. Microbiol Spectr. 2022;10:e01115–e1122. doi: 10.1128/spectrum.01115-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gui Z, et al. Identification and genetic diversity analysis of Rickettsia in Dermacentor nuttalli within inner Mongolia, China. Parasites Vectors. 2022;15:286. doi: 10.1186/s13071-022-05387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan M, et al. Rickettsial and serologic evidence for prevalent spotted fever rickettsiosis in inner Mongolia. Am J Trop Med Hyg. 1987;36:615–620. doi: 10.4269/ajtmh.1987.36.615. [DOI] [PubMed] [Google Scholar]
- 26.Tian J, et al. The diversity and evolutionary relationships of ticks and tick-borne bacteria collected in China. Parasites Vectors. 2022;15:1–14. doi: 10.1186/s13071-022-05485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q-H, et al. Evidence for a high prevalence of spotted fever group rickettsial infections in diverse ecologic zones of Inner Mongolia. Epidemiol Infect. 1995;115:177–183. doi: 10.1017/s0950268800058246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao G-F, et al. Detection of tick and tick-borne pathogen in some ports of Inner Mongolia. Zhonghua liu Xing Bing xue za zhi= Zhonghua Liuxingbingxue Zazhi. 2009;30:365–367. [PubMed] [Google Scholar]
- 29.Wu L, et al. Microbial Diversity of Ticks And A Novel Tyhpus Group Rickettsia Species (R. bacterium Ac37b) Detected In Inner Mongolia, China. 2021.
- 30.Lv J, et al. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida) Parasites Vectors. 2014;7:1–11. doi: 10.1186/1756-3305-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gui, Z., J. Yu, and L. Mu, DNA detection genotype distribution of spotted fever group Rickettsiae carried by dermpor steppe in some areas of Inner Mongolia Journal of Jilin, University. Journal of Jilin University(Medicine Edition), 2021. 47(01).
- 32.Jiang L, Lian-gui X, Jin-guo Z. Kirksiella bayeri was detected from dermacor steppe at Erguna port for the first time. Chin J Front Health Quar. 2017;40:3. [Google Scholar]
- 33.Zhu J, et al. Tick investigation at Erguna port and adjacent forest area in 2015. Chin J Front Health Quar. 2016;39:3. [Google Scholar]
- 34.Jin Y, et al. Isolation and identification of Rickettsia Ha-91 strain from Inner Mongolia-Amun spotted fever group. Chin J Zoonoses. 1993;9:3. [Google Scholar]
- 35.Niu L-C, et al. Geographical epidemiological survey of rickettsiae of spotted fever group in forest steppe of Inner Mongolia. Vet Orientat. 1993;1:8. [Google Scholar]
- 36.Wu C, Chen X. Rickettsia Q was isolated from tick adults in central Inner Mongolia for the first time. Chin J Zoonoses. 1990;6:3. [Google Scholar]
- 37.Zhang, J. and Q.e. Zhang, Chinese researchers found Lyme disease natural focus in Inner Mongolia. People's Military Surgeon, 1990(10).
- 38.Zhao H, Li Q. Rickettsia Q was isolated from Inner Mongolia for the first time and its serological investigation. Inner Mong Med J. 1990;10:2. [Google Scholar]
- 39.Jiang Z, Morphological study of D. nuttalli and D. silvarum nymphes. Journal of Beijing Normal University (Natural Science), 1987; (01): p. 68–75.
- 40.Jiang Z, Morphological study of D. nuttalli and D. silvarum larvae. Journal of Beijing Normal University (Natural Science), 1985; (01): p. 60–69.
- 41.Li Y, et al. Investigation on dominant species of ticks and pathogen carrying in central and western port areas of Inner Mongolia Autonomous Region in 2020. Chin J Vector Biol Control. 2022;33:5. [Google Scholar]
- 42.Huang T, et al. Isolation and identification of Brucella sheep from parasitic ticks on bovine body surface in Hulunbuir area. Sci Agric Sinica. 2022;002:055. [Google Scholar]
- 43.Kong Y, et al. Pigeon circovirus has been detected from parasitic ticks in sheep and camels in Inner Mongolia. Chin J Vet Sci. 2021;41:6. [Google Scholar]
- 44.Wu L. et al. Detection and evolution analysis of Dermacentor nuttalli carrier anaplasmosis in grassland of Inner Mongolia. Chinese J Zoonoses. 2021;37(12):1084–1090
- 45.Yang C, et al. Investigation on ticks and pathogens carried by them in western port area of Inner Mongolia in 2019. Chin J Front Health Quar. 2021;44:3. [Google Scholar]
- 46.Sheng Y, et al. A survey of spotted fever group rickettsia carried by ticks in some areas of Inner Mongolia from 2019 to 2020. Chin J Front Health Quar. 2021;44:3. [Google Scholar]
- 47.He H, et al. Isolation and identification of an Inner Mongolia strain of tick-derived alkali-producing Bacillus faecalis. Chin J Vet Sci. 2018;38:6. [Google Scholar]
- 48.Yang Y, et al. Investigation on pathogens carried by ticks in Manzhouli port area of Inner Mongolia from 2012 to 2014. Chin J Vector Biol Control. 2018;29:5. [Google Scholar]
- 49.Huang T, et al. Identification and gene polymorphism analysis of dominant tick species in Hulunbuir, Inner Mongolia. Chin J Vet Sci. 2017;37:6. [Google Scholar]
- 50.Ji C. Investigation on the foci of tick-borne lyme disease in Alatanheli area of Bogda Mountain. Electron J Clin Med Lit. 2017;5:2. [Google Scholar]
- 51.Zhaomu L, et al. First isolation and identification of a tick-derived strain Oceanobacillus oncorhynchi IMH with salt and alkali tolerance. Acta Microbiologica Sinica. 2019;59(05):841–850. 10.13343/j.cnki.wsxb.20180323.
- 52.Huang F, et al. Dominant tick species and their genetic diversity in Xilin Gol league, Inner Mongolia. Chin J Vet Sci. 2019;39:7. [Google Scholar]
- 53.Fan Y. Detection of rickettsia in Dermacentor nuttalli and its effect on host genetic diversity. Shijiazhuang: Hebei Normal University; 2019. [Google Scholar]
- 54.Liu D, et al. Population distribution and pathogen diversity of different parasitic ticks in Inner Mongolia from 2015 to 2019. Modern Prev Med. 2021;08:1345–1349. [Google Scholar]
- 55.Deng H, et al. Nucleic acid detection and sequence analysis of spotted fever group rickettsiae carried by ticks at Manzhouli Port. Chin J Front Health Quar. 2014;5:3. [Google Scholar]
- 56.Robinson KA, Saldanha IJ, Na M. Development of a framework to identify research gaps from systematic reviews. J Clin Epidemiol. 2011 doi: 10.1016/j.jclinepi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Li X. Isolation and identification of tick-derived lymphocytic choriomeningitis virus and forest encephalitis virus. Changchun: Jilin Agricultural University; 2021. [Google Scholar]
- 58.Sheng Y. Molecular epidemiological characteristics of tick-borne spotted fever group Rickettsia in Inner Mongolia. Chengde: Chengde Medical College; 2021. [Google Scholar]
- 59.Li L-F, et al. Dermacentor silvarum, a medically important tick, may not be a competent vector to transmit Jingmen tick virus. Vector-Borne Zoonotic Dis. 2022;22:402–407. doi: 10.1089/vbz.2021.0092. [DOI] [PubMed] [Google Scholar]
- 60.Ruan Q, et al. Investigation of ticks at Erguna port and adjacent forest areas in 2015. Acta Parasitol Et Med Entomol Sinica. 2019;26:65–68. [Google Scholar]
- 61.Yu H, et al. Tick species survey in Inner Mongolia border port area in 2019. Chin J Vector Biol Control. 2022;33:289–292. [Google Scholar]
- 62.Zhou L, et al. Investigation of spotted fever Rickettsia carried by ticks in Qigan, Inner Mongolia. Chin J Front Health Quar. 2017;40:96–99. [Google Scholar]
- 63.Tang F, et al. Investigation on Borrelia burgdorferi carried by ticks in Qigan, Inner Mongolia. Infect Dis Inf. 2018;31:31–33. [Google Scholar]
- 64.Gao D, et al. Detection of Ehrlichia human granulocyte 16SrRNA gene in ticks from northern China. Acta Parasitol Et Med Entomol Sinica. 2000;02:103–108. [Google Scholar]
- 65.Qin W, Li Y, Song C. Epidemiological investigation of Erich disease in the population in the Yakesite area. Inner Mong Med J. 2006;01:65–66. [Google Scholar]
- 66.Yang H, et al. Risk assessment and management strategy of vector-borne disease in Zhurihe training base. Chin J Hyg Insect Equip. 2017;23:511–515. [Google Scholar]
- 67.Ruan Q, et al. Ticks in Inner Mongolia port areas on Sino-Russia border. Acta Parasitol et Med Entomol Sinica. 2019;26:65–68. [Google Scholar]
- 68.Zhou L. Investigation on pathogens carried by ticks in Inner Mongolia forest area. Beijing: PLA Academy of Military Medical Sciences; 2017. [Google Scholar]
- 69.Sun Y, et al. Experimental study on infection of forest dermatitis and steppe dermatitis and transmission of Lyme disease spirochetes during menstrual period. Acta Entomol Sin. 2002;05:578–582. [Google Scholar]
- 70.Yao W, G Chen, Relationship between life history and temperature and humidity of dermacorph forest. Acta Entomologica Sinica. 1981; (02): p. 233–236.
- 71.Wang M, et al. Tissue localization and variation of major symbionts in Haemaphysalis longicornis, Rhipicephalus haemaphysaloides, and Dermacentor silvarum in China. Appl Environ Microbiol. 2018;84:e00029–e118. doi: 10.1128/AEM.00029-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen B, Cao W, Pan H. Ehrlichiae and ehrlichial diseases in China. Ann N Y Acad Sci. 2003;990:45–53. doi: 10.1111/j.1749-6632.2003.tb07335.x. [DOI] [PubMed] [Google Scholar]
- 73.Guo W-B, et al. Distribution of Dermacentor silvarum and associated pathogens: meta-analysis of global published data and a field survey in China. Int J Environ Res Public Health. 2021;18:4430. doi: 10.3390/ijerph18094430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Y, Xu R. Ability of Ixodes persulcatus, Haemaphysalis concinna and Dermacentor silvarum ticks to acquire and transstadially transmit Borrelia garinii. Exp Appl Acarol. 2003;31:151–160. doi: 10.1023/b:appa.0000005119.30172.43. [DOI] [PubMed] [Google Scholar]
- 75.Chu C, et al. Genotyping of Borrelia lyme in ticks and mice in the Greater Khingan Mountains. Chinese Journal of Epidemiology. 2006; (08): p. 681–684.
- 76.Qin W, Li Y, Song C. Tick fluctuation in Yakeshi area, Inner Mongolia. Chin J Zoonoses. 2005;11:11. [Google Scholar]
- 77.Sun Y, et al. Isolation and identification of Spirochaeta lyme disease from some common ticks in China. Acta Parasitologica Et Medica Entomologica Sinica. 2002;02:114–119. [Google Scholar]
- 78.Yan D, et al. Forest encephalitis virus was isolated for the first time in the greater Khingan Mountains of Inner Mongolia Inner Mongolia. Med J. 1996;02:65–67. [Google Scholar]
- 79.Ren G, et al. Three strains of forest encephalitis virus were isolated from Ixodes holosulcus for the first time. J Med Pest Control. 1994;02:71–73. [Google Scholar]
- 80.Zhu J, et al. Vector investigation of Lyme disease in greater Khingan Mountains of Inner Mongolia. Chin J Public Health. 1993;03:109. [Google Scholar]
- 81.Yan D, et al. Ixodes holosulis is the main biological vector of Spirochaeta lyme disease in the Greater Khingan Mountains of Inner Mongolia. Chin J Vector Biol Control. 1991;04:270–272. [Google Scholar]
- 82.Lu J, et al. Investigation of Lyme disease in Hulunbuir league forest area. Inner Mong Med J. 1992;03:30–31. [Google Scholar]
- 83.Duan L, et al. Detection and genotyping of Borrelia burgdorferi carried by free ticks in Alshan, Inner Mongolia. Chin J Vector Biol Control. 2022;33:642–647. [Google Scholar]
- 84.Liu D, et al. Investigation on tick species and tick-borne infection in Yakeshi member of greater Khingan Mountains, Inner Mongolia. Chin J Zoonoses. 2021;37:845–850. [Google Scholar]
- 85.Sheng Y, et al. Investigation of spotted fever group rickettsia carried by ticks in Inner Mongolia from 2019 to 2020. Chin J Front Health Quar. 2021;44:168–170. [Google Scholar]
- 86.Zhu J, et al. Tick investigation at Erguna port and adjacent forest area in 2015. Chin J Front Health Quar. 2016;39:336–338. [Google Scholar]
- 87.Cao X, et al. Investigation on the species of parasitic ticks and tick-borne pathogens in sheep at Erenhot port. Chin J Hyg Insect Equip. 2014;20:421–423. [Google Scholar]
- 88.Hao G, et al. Detection of main ticks and tick-borne pathogens at some border ports in Inner Mongolia. Chin J Epidemiol. 2009;4:365–367. [Google Scholar]
- 89.Tian X, et al. Tick population survey in Erguna National Nature Reserve, Inner Mongolia. Acta Parasitologica Et Medica Entomologica Sinica. 2019;2:2. [Google Scholar]
- 90.Guo W. Epidemiological investigation of pathogens carried by dermacor forest. Beijing: Academy of Military Medical Sciences; 2021. [Google Scholar]
- 91.Wang, S., et al., Investigation on natural foci of forest encephalitis in greater Khingan Mountains, Inner Mongolia. Bulletin of Disease Control & Prevention. 1995; (02): p. 72–74
- 92.Cui Y, et al. Surveillance of rickettsia-associated bacteria in the greater Khingan Mountains of Inner Mongolia from 2016 to 2019. Modern Prev Med. 2021;48:2850–2856. [Google Scholar]
- 93.Ulan T, et al. Diversity and genotype analysis of tick-borne pathogens in central and western grasslands of Inner Mongolia. Chin J Parasitol Parasitic Dis. 2021;39:27–34. [Google Scholar]
- 94.Yin X, et al. Spotted fever group rickettsiae in Inner Mongolia, China, 2015–2016. Emerg Infect Dis. 2018;24:2105. doi: 10.3201/eid2411.162094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao X, et al. Investigation on the pathogen of parasitic tick infection in rats at Erenhot port. Chin J Vector Biol Control. 2014;25:127–130. [Google Scholar]
- 96.Guo W, Gao Y, Tian F. Investigation of vector-borne pathogens in Manzhouli port area in 2016. Chin J Front Health Quar. 2017;40:410–412. [Google Scholar]
- 97.Chen Z, et al. The life cycle of Hyalomma asiaticum kozlovi Olenev, 1931 (Acari: Ixodidae) under laboratory conditions. Vet Parasitol. 2009;160:134–137. doi: 10.1016/j.vetpar.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 98.Yang Y, et al. Investigation on pathogens carried by ticks at Ganqidi Maodu port in China-Mongolia border area from 2012 to 2013. Chin J Front Health Quar. 2016;39:330–332. [Google Scholar]
- 99.Yang Y, et al. Investigation on pathogens carried by ticks at Ganqidi Maodu port in China and Mongolia in 2010. Chin J Front Health Quar. 2011;34:339–342. [Google Scholar]
- 100.Yao W. Effects of temperature and humidity on oviposition of Hyalomonas Yadonensis. Acta Entomol Sin. 1985;02:173–180. [Google Scholar]
- 101.Chen Z. Systematic classification of ticks in China and analysis of biological characteristics of two Ixodes. Shijiazhuang: Hebei Normal University; 2010. [Google Scholar]
- 102.Zhao H, et al. Epidemiological investigation of Lyme disease in cattle and sheep in Xilin Gol area, Inner Mongolia. Prog Vet Med. 2002;02:72–74. [Google Scholar]
- 103.Wang Y, Huang T, Geri L. Identification and gene polymorphism analysis of Hyalomonas asiatica from Ejin banner, Inner Mongolia. Chin J Vet Sci. 2020;40:2337–2341. [Google Scholar]
- 104.Cai Y, et al. Virology analysis of parasitic ticks in camels and sheep in Inner Mongolia. Chin J Virol. 2021;37:1148–1157. [Google Scholar]
- 105.Liu C. Morphological comparison and gene polymorphism analysis of Hyalomma asiaticum from different regions of China. Shijiazhuang: Henan Agricultural University; 2016. [Google Scholar]
- 106.Batu N, et al. Molecular epidemiology of Rickettsia sp. and Coxiella burnetii collected from Hyalomma asiaticum in Bactrian camels (Camelus bactrianus) in Inner Mongolia of China. Ticks Tick-Borne Dis. 2020;11:101548. doi: 10.1016/j.ttbdis.2020.101548. [DOI] [PubMed] [Google Scholar]
- 107.Na K-H, et al. Artificial cultivation of Hyalomonas fragilis and experimental transmission to Tyleria cylindrica. Chin Vet Sci. 2002;06:27–28. [Google Scholar]
- 108.Zheng P. Cloning, prokaryotic expression and functional analysis of the ferritin gene from Hyalomonas matricorosa and Haemaphysalis obtusis. Shijiazhuang: Hebei Normal University; 2020. [Google Scholar]
- 109.Gao Z, et al. The molecular and functional characterization of ferritins in the hard tick Hyalomma rufipes. Parasites Vectors. 2022;15:368. doi: 10.1186/s13071-022-05515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guosong R, et al. Investigation on the seasonal fluctuation of Ixodes holosulis in the natural foci of Lyme disease in greater Khingan Mountains, Inner Mongolia. J Med Pest Control. 1992;02:107–109. [Google Scholar]
- 111.Men H. Study on the population ecology of Hemophila and control of American Ticks. Shijiazhuang: Hebei Normal University; 2015. [Google Scholar]
- 112.Wang H. Isolation, detection and genotyping of Spirochaeta lyme from ticks in China. Shanghai: Academy of Military Medical Sciences; 2005. [Google Scholar]
- 113.Meng H, et al. Abundance and seasonal activity of Haemaphysalis concinna (Acari: Ixodidae) at the border between China and Russia in Northern Inner Mongolia, China. Parasites Vectors. 2016;9:1–7. doi: 10.1186/s13071-015-1291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meng H, et al. The life cycle and occurrence of Haemaphysalis concinna (Acari: Ixodidae) under field conditions. Ticks Tick-Borne Dis. 2014;5:887–891. doi: 10.1016/j.ttbdis.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 115.Kasama K, et al. Genomic features of Rickettsia heilongjiangensis revealed by intraspecies comparison and detailed comparison with Rickettsia japonica. Front Microbiol. 2019;10:2787. doi: 10.3389/fmicb.2019.02787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Temu Q, et al. Overwintering investigation of steppe haemaphysalis in desert steppe of northern Inner Mongolia. Neimenggu Prev Med. 1995;02:82. [Google Scholar]
- 117.Zhao H, Su J. Investigation of lyme disease foci on grassland in Xilin Gol league. Neimenggu Prev Med. 2003;01:6–7. [Google Scholar]
- 118.Zhang F, et al. Detection of Francisella tularensis in ticks and identification of their genotypes using multiple-locus variable-number tandem repeat analysis. BMC Microbiol. 2008;8:1–5. doi: 10.1186/1471-2180-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shuai L-Y, et al. Ecological correlates of ectoparasite load in a rodent: complex roles of seasonality. Int J Parasitol Parasites Wildlife. 2022;18:244–248. doi: 10.1016/j.ijppaw.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao Y, et al. First detection of Borrelia miyamotoi infections in ticks and humans from the northeast of Inner Mongolia. China Acta Tropica. 2021;217:105857. doi: 10.1016/j.actatropica.2021.105857. [DOI] [PubMed] [Google Scholar]
- 121.Ma J, et al. Identification of a new orthonairovirus associated with human febrile illness in China. Nat Med. 2021;27:434–439. doi: 10.1038/s41591-020-01228-y. [DOI] [PubMed] [Google Scholar]
- 122.Sato K, et al. Surveillance of Borrelia miyamotoi-carrying ticks and genomic analysis of isolates in Inner Mongolia, China. Parasites Vectors. 2021;14:1–8. doi: 10.1186/s13071-021-04809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu L, et al. Investigations on rickettsia in ticks at the Sino-Russian and Sino-Mongolian borders, China. Vector-Borne Zoonotic Dis. 2015;15:785–789. doi: 10.1089/vbz.2014.1732. [DOI] [PubMed] [Google Scholar]
- 124.Li X, et al. Molecular detection and phylogenetic analysis of tick-borne encephalitis virus in ticks in northeastern China. J Med Virol. 2022;94:507–513. doi: 10.1002/jmv.27303. [DOI] [PubMed] [Google Scholar]
- 125.Chu C-Y, et al. Genetic diversity of Borrelia burgdorferi sensu lato isolates from Northeastern China. Vector-Borne Zoonotic Dis. 2011;11:877–882. [PubMed] [Google Scholar]
- 126.Lu X, et al. Molecular survey of hard ticks in endemic areas of tick-borne diseases in China. Ticks Tick-Borne Dis. 2013;4:288–296. doi: 10.1016/j.ttbdis.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Shi M, et al. Meta-transcriptional Detection of Rickettsia canadensis from Ixodes persulcatus in China. 2022.
- 128.Takada N, et al. Lyme disease spirochetes in ticks from northeastern China. J Parasitol. 1998 doi: 10.2307/3284712. [DOI] [PubMed] [Google Scholar]
- 129.Lu M, Qin X. Study on Ixodes holofurrow carrying rickettsia in Yakeshi city, Inner Mongolia Autonomous Region. Dis Surveill. 2021;36:276–279. [Google Scholar]
- 130.Li S, et al. Bacterial community structure and diversity of Ixodes holosulcus in Inner Mongolia. Chin J Vector Biol Control. 2019;30:607–612. [Google Scholar]
- 131.Deng H, et al. Investigation of medical vectors and their pathogens in Manzhouli Port area. Chin J Front Health Quar. 2015;38:263–265. [Google Scholar]
- 132.Zhao Q, et al. Study on compound infection of three tick-borne infectious diseases in vector ticks and mice. Chin J Epidemiol. 2005;01:12–16. [Google Scholar]
- 133.Zhu Z, et al. Preliminary observation and study on perifeeding membrane of Ixodes holosulcus. Chin J Animal Infect Dis. 2002;04:1–4. [Google Scholar]
- 134.Ji C. Investigation on the foci of tick-borne forest encephalitis in Alatanheli area of Bogeda Mountain. Electron J Clin Med Lit. 2017;5:2. [Google Scholar]
- 135.Xu Q. Population ecology and tick-borne pathogens of Ixodes holosulcus. Shijiazhuang: Hebei Normal University; 2015. [Google Scholar]
- 136.Quan, L., et al., Identification of a new chuvirus associated with febrile illness in China. 2020.
- 137.Liu M, et al. Preliminary investigation on tick flora in nine provinces. J Chang Med Coll. 2005;04:249–250. [Google Scholar]
- 138.Hao Y, Xu Z, Ma C. Human invasion by the Persian sharp edge tick. Chin J Parasitol Parasitic Dis. 1995;02:63. [Google Scholar]
- 139.Danone D, et al. Experimental study of iodinophenol repellent against periscerus gallinis. Jiangxi J Animal Husb Vet Medi. 2002;02:8. [Google Scholar]
- 140.Yan P. Study on the microbial community of arystick japonicus. Shijiazhuang: Hebei Normal University; 2018. [Google Scholar]
- 141.Gui Z. Genetic diversity analysis of dermacor and identification of rickettsia-carrying species in Inner Mongolia grassland. Hohhot: Inner Mongolia Medical University; 2021. [Google Scholar]
- 142.Chen Y, Wang J. We will comprehensively implement the system of balancing grass and livestock to promote sustained ecological improvement in grasslands. Inner Mong For. 2021;09:4–5. [Google Scholar]
- 143.Duan X. Dynamic changes of typical plant communities and their influencing factors in the transition zone of forest steppe in the Greater Khingan Mountains. Changchun: Northeast Normal University; 2022. [Google Scholar]
- 144.Wang Y. Identification of tick species and isolation of pathogen from Bactrian camel in Alashan, Inner Mongolia. Hohhot: Inner Mongolia Agricultural University; 2020. [Google Scholar]
- 145.Snow K. The life-history of Hyalomma anatolicum anatolicum Koch, 1844 (Ixodoidea, Ixodidae) under laboratory conditions. Parasitology. 1969;59:105–122. doi: 10.1017/s0031182000069869. [DOI] [PubMed] [Google Scholar]
- 146.Apanaskevich DA, Horak IG. The genus Hyalomma. XI. Redescription of all parasitic stages of H. (Euhyalomma) asiaticum (Acari: Ixodidae) and notes on its biology. Exp Appl Acarol. 2010;52:207–220. doi: 10.1007/s10493-010-9361-0. [DOI] [PubMed] [Google Scholar]
- 147.Yang X, et al. Projecting the potential distribution of ticks in China under climate and land use change. Int J Parasitol. 2021;51:749–759. doi: 10.1016/j.ijpara.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 148.Wang F, et al. Species delimitation of the Dermacentor ticks based on phylogenetic clustering and niche modeling. PeerJ. 2019;7:e6911. doi: 10.7717/peerj.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu E, et al. Distribution prediction of Hyalomma asiaticum (Acari: Ixodidae) in a Localized Region in Northwestern China. J Parasitol. 2022;108:330–336. doi: 10.1645/21-96. [DOI] [PubMed] [Google Scholar]
- 150.Ma B, et al. Prediction of suitable area of Ixodes holosulcus in China. Chin J Schistosomiasis Control. 2021;33:169–176. doi: 10.16250/j.32.1374.2020244. [DOI] [PubMed] [Google Scholar]
- 151.Zhang R, et al. Land use change and its impact on ecosystem service value in Alxa. Agric Technol. 2022;42:71–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hurtt G, 2016. Harmonization of global land-use change and management for the period 850–2100. Geosci Model Dev. [DOI]
Supplementary Materials
Additional file 1: Figure S1 Flow diagram of literature search and inclusion.
Additional file 2: Figure S2 Changes in the potential suitability areas for the four dominant tick species under the near current and 2081–2100.
Additional file 3: Table S1 Environmental and bioclimatic variables for the four dominant tick species distribution models by MaxEnt.
Data Availability Statement
Not applicable.