Abstract
Background
The coronavirus disease 2019 (COVID‐19) pandemic has impacted healthcare systems worldwide. Multiple reports on thromboembolic complications related to COVID‐19 have been published, and researchers have described that people with COVID‐19 are at high risk for developing venous thromboembolism (VTE). Anticoagulants have been used as pharmacological interventions to prevent arterial and venous thrombosis, and their use in the outpatient setting could potentially reduce the prevalence of vascular thrombosis and associated mortality in people with COVID‐19. However, even lower doses used for a prophylactic purpose may result in adverse events such as bleeding. It is important to consider the evidence for anticoagulant use in non‐hospitalised people with COVID‐19.
Objectives
To evaluate the benefits and harms of prophylactic anticoagulants versus active comparators, placebo or no intervention, or non‐pharmacological interventions in non‐hospitalised people with COVID‐19.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 18 April 2022.
Selection criteria
We included randomised controlled trials (RCTs) comparing prophylactic anticoagulants with placebo or no treatment, another active comparator, or non‐pharmacological interventions in non‐hospitalised people with COVID‐19. We included studies that compared anticoagulants with a different dose of the same anticoagulant. We excluded studies with a duration of under two weeks.
Data collection and analysis
We used standard Cochrane methodological procedures. Our primary outcomes were all‐cause mortality, VTE (deep vein thrombosis (DVT) or pulmonary embolism (PE)), and major bleeding. Our secondary outcomes were DVT, PE, need for hospitalisation, minor bleeding, adverse events, and quality of life. We used GRADE to assess the certainty of the evidence.
Main results
We included five RCTs with up to 90 days of follow‐up (short term). Data were available for meta‐analysis from 1777 participants.
Anticoagulant compared to placebo or no treatment
Five studies compared anticoagulants with placebo or no treatment and provided data for three of our outcomes of interest (all‐cause mortality, major bleeding, and adverse events). The evidence suggests that prophylactic anticoagulants may lead to little or no difference in all‐cause mortality (risk ratio (RR) 0.36, 95% confidence interval (CI) 0.04 to 3.61; 5 studies; 1777 participants; low‐certainty evidence) and probably reduce VTE from 3% in the placebo group to 1% in the anticoagulant group (RR 0.36, 95% CI 0.16 to 0.85; 4 studies; 1259 participants; number needed to treat for an additional beneficial outcome (NNTB) = 50; moderate‐certainty evidence). There may be little to no difference in major bleeding (RR 0.36, 95% CI 0.01 to 8.78; 5 studies; 1777 participants; low‐certainty evidence). Anticoagulants probably result in little or no difference in DVT (RR 1.02, 95% CI 0.30 to 3.46; 3 studies; 1009 participants; moderate‐certainty evidence), but probably reduce the risk of PE from 2.7% in the placebo group to 0.7% in the anticoagulant group (RR 0.25, 95% CI 0.08 to 0.79; 3 studies; 1009 participants; NNTB 50; moderate‐certainty evidence). Anticoagulants probably lead to little or no difference in reducing hospitalisation (RR 1.01, 95% CI 0.59 to 1.75; 4 studies; 1459 participants; moderate‐certainty evidence) and may lead to little or no difference in adverse events (minor bleeding, RR 2.46, 95% CI 0.90 to 6.72; 5 studies, 1777 participants; low‐certainty evidence).
Anticoagulant compared to a different dose of the same anticoagulant
One study compared anticoagulant (higher‐dose apixaban) with a different (standard) dose of the same anticoagulant and reported five relevant outcomes. No cases of all‐cause mortality, VTE, or major bleeding occurred in either group during the 45‐day follow‐up (moderate‐certainty evidence). Higher‐dose apixaban compared to standard‐dose apixaban may lead to little or no difference in reducing the need for hospitalisation (RR 1.89, 95% CI 0.17 to 20.58; 1 study; 278 participants; low‐certainty evidence) or in the number of adverse events (minor bleeding, RR 0.47, 95% CI 0.09 to 2.54; 1 study; 278 participants; low‐certainty evidence).
Anticoagulant compared to antiplatelet agent
One study compared anticoagulant (apixaban) with antiplatelet agent (aspirin) and reported five relevant outcomes. No cases of all‐cause mortality or major bleeding occurred during the 45‐day follow‐up (moderate‐certainty evidence). Apixaban may lead to little or no difference in VTE (RR 0.36, 95% CI 0.01 to 8.65; 1 study; 279 participants; low‐certainty evidence), need for hospitalisation (RR 3.20, 95% CI 0.13 to 77.85; 1 study; 279 participants; low‐certainty evidence), or adverse events (minor bleeding, RR 2.13, 95% CI 0.40 to 11.46; 1 study; 279 participants; low‐certainty evidence).
No included studies reported on quality of life or investigated anticoagulants compared to a different anticoagulant, or anticoagulants compared to non‐pharmacological interventions.
Authors' conclusions
We found low‐ to moderate‐certainty evidence from five RCTs that prophylactic anticoagulants result in little or no difference in major bleeding, DVT, need for hospitalisation, or adverse events when compared with placebo or no treatment in non‐hospitalised people with COVID‐19. Low‐certainty evidence indicates that prophylactic anticoagulants may result in little or no difference in all‐cause mortality when compared with placebo or no treatment, but moderate‐certainty evidence indicates that prophylactic anticoagulants probably reduce the incidence of VTE and PE.
Low‐certainty evidence suggests that comparing different doses of the same prophylactic anticoagulant may result in little or no difference in need for hospitalisation or adverse events. Prophylactic anticoagulants may result in little or no difference in risk of VTE, hospitalisation, or adverse events when compared with antiplatelet agents (low‐certainty evidence). Given that there were only short‐term data from one study, these results should be interpreted with caution.
Additional trials of sufficient duration are needed to clearly determine any effect on clinical outcomes.
Keywords: Humans, Anticoagulants, Anticoagulants/adverse effects, Aspirin, COVID-19, Platelet Aggregation Inhibitors, Pulmonary Embolism, Pulmonary Embolism/prevention & control, Venous Thromboembolism, Venous Thromboembolism/prevention & control
Plain language summary
Prophylactic blood thinners for the prevention of death and venous thromboembolism in COVID‐19 outpatients
Key messages
‐ When used in the outpatient setting, anticoagulants (blood thinners) probably reduce venous thromboembolism (VTE) and pulmonary embolism (PE) when compared with placebo or no treatment in people with COVID‐19. However, these drugs seem to have little or no effect in reducing death, major bleeding, need for hospitalisation, or adverse events.
What is VTE?
Venous thromboembolism, which includes both deep vein thrombosis (DVT) and PE, is a condition where a blood clot forms in a vein and may migrate to another location (e.g. the lung). DVT occurs when a blood clot forms inside a deep vein and blocks the blood flow. PE occurs when (part of) a blood clot detaches from the deep vein and ends up in the lung blood vessels, blocking the blood supply of the lungs.
How are COVID‐19 and VTE related?
COVID‐19 typically affects the lungs and airways; however, in addition to respiratory problems, people with COVID‐19 can also experience problems with their blood vessels, leading to blood clots forming in the veins and lungs.
How is VTE treated and how can VTE be prevented in people who are at risk?
The initial treatment includes drugs such as anticoagulants to prevent the formation of further new blood clots. Patients may also receive compression stockings and clinical care (e.g. physical exercise, skin hydration, and physical therapy). Anticoagulants such as rivaroxaban and apixaban act by inhibiting the blood elements involved in the formation of blood clots. For this reason, they are also used to prevent blood clots from forming in people who are considered to be at risk, such as people with COVID‐19. This is known as prophylactic treatment. However, the use of anticoagulants can cause side effects such as bleeding.
What did we want to find out?
We wanted to find out whether giving anticoagulants to non‐hospitalised people with COVID‐19 reduced the number of deaths or new blood clots compared to people who received placebo (an identical‐seeming medicine but with no active properties) or no intervention; a different dose or formulation of the same anticoagulant; antiplatelet agents (medications that prevent blood clots from forming); or non‐drug treatments. We also wanted to know the effects of anticoagulants on the need for hospitalisation; major bleeding or adverse events; and quality of life.
What did we do?
We searched for studies, giving preference to randomised controlled trials (studies where participants are randomly assigned to one of two or more treatment groups), that evaluated prophylactic anticoagulants given to people with COVID‐19 in the outpatient setting, compared with placebo or no treatment, a different dose of the same anticoagulant, or antiplatelet agents. We pooled the results when appropriate.
What did we find?
The results were based on five studies with a total of 1777 participants from the USA, Switzerland, Germany, Belgium, Brazil, India, South Africa, Spain, and the UK. Two large groups of participants were studied: those with COVID‐19 who did not require hospitalisation, and people with COVID‐19 who had been discharged from hospital. Five studies compared anticoagulants versus placebo or no treatment, and one study also compared a prophylactic anticoagulant with a different dose of the same anticoagulant as well as versus antiplatelet agents. Each comparison investigated the effects of anticoagulants on death, VTE, major bleeding, need for hospitalisation, and adverse events.
We have low confidence that prophylactic anticoagulants compared with placebo or no treatment for non‐hospitalised people with COVID‐19 have little or no effect on reducing the risk of death or adverse events. Prophylactic anticoagulants probably decrease the risk of VTE; 50 patients would need to be treated to avoid one VTE event.
There may be little or no difference in hospitalisation rates between people who receive prophylactic anticoagulants and those who receive a different dose of the same anticoagulant. Moreover, prophylactic anticoagulants may lead to little or no difference in reducing VTE when compared with antiplatelet agents.
What are the limitations of the evidence?
We have low confidence in the evidence due to issues with study methods and sizes. In the future, high‐quality studies may produce important data, especially regarding outcomes such as death, DVT, and PE.
How up‐to‐date is this evidence?
The evidence is current as of 18 April 2022.
Summary of findings
Summary of findings 1. Anticoagulant compared to placebo or no treatment for non‐hospitalised people with COVID‐19.
| Anticoagulant versus placebo or no treatment for non‐hospitalised people with COVID‐19 | ||||||
| Patient or population: non‐hospitalised people with COVID‐19 Setting: outpatient Intervention: anticoagulant Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with anticoagulant | |||||
|
All‐cause mortality Follow‐up: from 30 to 90 days |
Study population | RR 0.36 (0.04 to 3.61) | 1777 (5 RCTs) | ⊕⊕⊝⊝ Low a b | ||
| 6 per 1000 | 2 per 1000 (0 to 20) | |||||
|
Venous thromboembolism Follow‐up: from 30 to 90 days |
Study population | RR 0.36 (0.16 to 0.85) | 1259 (4 RCTs) | ⊕⊕⊕⊝ Moderate a c | NNTB = 50 | |
| 31 per 1000 | 11 per 1000 (5 to 26) | |||||
|
Major bleeding Follow‐up: from 30 to 90 days |
Study population | RR 0.36 (0.01 to 8.78) | 1777 (5 RCTs) | ⊕⊕⊝⊝ Low a b | ||
| 1 per 1000 | 0 per 1000 (0 to 10) | |||||
|
Deep vein thrombosis Follow‐up: from 30 to 90 days |
Study population | RR 1.02 (0.30 to 3.46) | 1009 (3 RCTs) | ⊕⊕⊕⊝ Moderate a c | ||
| 10 per 1000 | 10 per 1000 (3 to 34) | |||||
|
Pulmonary embolism Follow‐up: from 30 to 90 days |
Study population | RR 0.25 (0.08 to 0.79) | 1009 (3 RCTs) | ⊕⊕⊕⊝ Moderate a c | NNTB = 50 | |
| 27 per 1000 | 7 per 1000 (2 to 22) | |||||
|
Need for hospitalisation Follow‐up: from 30 to 90 days |
Study population | RR 1.01 (0.59 to 1.75) | 1459 (4 RCTs) | ⊕⊕⊕⊝ Moderate a c | ||
| 34 per 1000 | 34 per 1000 (20 to 59) | |||||
|
Adverse events (minor bleeding) Follow‐up: from 30 to 90 days |
Study population | RR 2.46 (0.90 to 6.72) |
1777 (5 RCTs) | ⊕⊕⊝⊝ Low a b | ||
| 6 per 1000 | 14 per 1000 (5 to 37) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aAlthough we judged some included studies as having a high risk of selection, performance, and reporting bias, the estimates did not significantly differ after sensitivity analysis, therefore we did not downgrade due to risk of bias. bDowngraded two levels due to imprecision (fewer than 300 events included in the analysis and very wide CI). cDowngraded one level due to imprecision (fewer than 300 events included in the analysis).
Summary of findings 2. Anticoagulant compared to different dose of the same anticoagulant for non‐hospitalised people with COVID‐19.
| Anticoagulant versus different dose of the same anticoagulant for non‐hospitalised people with COVID‐19 | ||||||
| Patient or population: non‐hospitalised people with COVID‐19 Setting: outpatient Intervention: higher‐dose anticoagulant Comparison: standard dose of the same anticoagulant | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard dose of the same anticoagulant | Risk with higher‐dose anticoagulant | |||||
|
All‐cause mortality Follow‐up: 45 days |
Study population | Not estimable | 278 (1 RCT) | ⊕⊕⊕⊝ Moderate a | There were no cases of mortality. | |
| ‐ | ||||||
|
Venous thromboembolism Follow‐up: 45 days |
Study population | Not estimable | 278 (1 RCT) | ⊕⊕⊕⊝ Moderate a | There were no cases of venous thromboembolism. | |
| ‐ | ||||||
|
Major bleeding Follow‐up: 45 days |
Study population | Not estimable | 278 (1 RCT) | ⊕⊕⊕⊝ Moderate a | There were no cases of major bleeding. | |
| ‐ | ||||||
| Deep vein thrombosis | This outcome was not measured. | |||||
| Pulmonary embolism | This outcome was not measured. | |||||
|
Need for hospitalisation Follow‐up: 45 days |
Study population | RR 1.89 (0.17 to 20.58) | 278 (1 RCT) | ⊕⊕⊝⊝ Low b | ||
| 7 per 1000 | 14 per 1000 1 to 152 |
|||||
|
Adverse events (minor bleeding) Follow‐up: 45 days |
Study population | RR 0.47 (0.09 to 2.54) |
278 (1 RCT) | ⊕⊕⊝⊝ Low b | ||
| 30 per 1000 | 14 per 1000 3 to 75 |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to imprecision (fewer than 300 events included in the analysis). bDowngraded two levels due to imprecision (fewer than 300 events included in the analysis and very wide CI).
Summary of findings 3. Anticoagulant compared to antiplatelet agent for non‐hospitalised people with COVID‐19.
| Anticoagulant versus antiplatelet agent for non‐hospitalised people with COVID‐19 | ||||||
| Patient or population: non‐hospitalised people with COVID‐19 Setting: outpatient setting Intervention: anticoagulant Comparison: antiplatelet agent | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with antiplatelet agents (short term) | Risk with anticoagulant | |||||
|
All‐cause mortality Follow‐up: 45 days |
Study population | Not estimable | 279 (1 RCT) | ⊕⊕⊕⊝ Moderate a | There were no cases of mortality. | |
| ‐ | ||||||
|
Venous thromboembolism Follow‐up: 45 days |
Study population | RR 0.36 (0.01 to 8.65) | 279 (1 RCT) | ⊕⊕⊝⊝ Low b | ||
| 7 per 1000 | 2 per 1000 (0 to 60) | |||||
|
Major bleeding Follow‐up: 45 days |
Study population | Not estimable | 279 (1 RCT) | ⊕⊕⊕⊝ Moderate a | There were no cases of major bleeding. | |
| ‐ | ||||||
| Deep vein thrombosis | This outcome was not measured. | |||||
| Pulmonary embolism | This outcome was not measured. | |||||
|
Need for hospitalisation Follow‐up: 45 days |
Study population | RR 3.20 (0.13 to 77.85) | 279 (1 RCT) | ⊕⊕⊝⊝ Low b | ||
| 0 out of 144 | 1 out of 135 | |||||
|
Adverse events (minor bleeding) Follow‐up: 45 days |
Study population | RR 2.13 (0.40 to 11.46) |
279 (1 RCT) | ⊕⊕⊝⊝ Low b | ||
| 14 per 1000 | 30 per 1000 (6 to 159) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to imprecision (fewer than 300 events included in the analysis). bDowngraded two levels due to imprecision (fewer than 300 events included in the analysis and very wide CI).
Background
Description of the condition
Venous thromboembolism (VTE) describes both the formation of a thrombus (blood clot) in the deep veins, most commonly in the legs (deep vein thrombosis (DVT)), or the subsequent embolisation of all or part of the thrombus to the pulmonary circulation (pulmonary embolism (PE)) (Cogo 1993; Kakkos 2021). DVT of the lower limbs may be associated with localised pain, swelling and erythema, as well as the development of pulmonary emboli, and the later occurrence of post‐thrombotic syndrome (persistent swelling, erythema, and ulceration), regardless of the treatment (Broderick 2021; Flumignan 2015; Flumignan 2022a; Flumignan 2023; Hirsh 1986). PE presents acutely, with shortness of breath, pain on inspiration, tachycardia, and right heart overload. If left untreated, it can lead to circulatory collapse and death (Stein 1991). It can also cause chronic post‐thrombotic pulmonary hypertension in the longer term. In the era of more liberal central venous catheterisation, DVT may increasingly involve the upper extremities (Verso 2003). Rarely, other parts of the venous circulation such as the cerebral, portal, and mesenteric veins can be affected (Acosta 2008; Saposnik 2011; Valla 2002).
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the pathogen responsible for coronavirus disease 2019 (COVID‐19), which since 2020 has grown rapidly into a pandemic affecting people worldwide, leading to intense demand on healthcare systems (Phelan 2020). Since the beginning of the COVID‐19 pandemic, multiple reports of thromboembolic complications related to COVID‐19 have been published worldwide (Lodigiani 2020; Tang 2020), and people with COVID‐19 have been described as being at high risk for the development of VTE (COVIDSurg 2022a; Flumignan 2021; Flumignan 2022b; Klok 2020a; Middeldorp 2020). Recent data show that the most frequently reported thrombotic events directly related to morbidity and mortality are DVT and PE (COVIDSurg 2022a; Hanff 2020). The incidence of thromboembolic events has been reported to range from 20% to 30% in people hospitalised due to COVID‐19 (Flumignan 2021; Flumignan 2022b; Klok 2020b; Middeldorp 2020). Recent studies have described hypercoagulability and endothelial dysfunction as hallmarks of COVID‐19 (Kelliher 2022), which could be explained by the association of SARS‐CoV‐2 infection with changes in the host’s coagulation profile (Zehra 2022). This phenomenon is still unclear, but could be related to high levels of inflammatory mediators that cause damage to both arterial and venous walls, leading to platelet aggregation and, consequently, causing coagulation and thrombosis (Matos 2011; Varga 2020). Another theory is that the entry of SARS‐CoV‐2 through the angiotensin‐converting enzyme 2 (ACE‐2) receptor on cells can trigger a secondary increase in tissue factor, ultimately leading to endothelial dysregulation and thrombosis (Bautista‐Vargas 2020).
VTE, which can present as DVT or PE (or both), can occur spontaneously. However, there are many risk factors for VTE, including periods of inactivity or being confined to bed, dehydration, hospitalisation, trauma, clotting disorders and previous superficial or deep vein thrombosis, pregnancy, oral combined hormonal contraceptives, malignancy, obesity, smoking, and age (Anderson 2003; Barbar 2010; Kakkos 2021; Kearon 2016; NICE 2019; Spyropoulos 2011). Regarding people with COVID‐19, the main risk factors described are immobilisation, hypoxia, endothelial cell activation or damage, and acute inflammation (Ortega‐Paz 2021; Tsaplin 2021).
Data regarding non‐hospitalised people with COVID‐19 are limited; some reports show that the incidence of thromboembolic events in this group of people is of possible concern and presents a higher thrombotic risk than has been acknowledged (Benzakoun 2020; Giannis 2021).
Description of the intervention
Prophylactic anticoagulation strategies in those deemed to be at risk (such as those undergoing surgical procedures or prolonged hospital inpatient stays) are recommended by national guidelines, such as those published by the National Institute for Health and Care Excellence (NICE) in the UK and the American College of Chest Physicians in the USA (Guyatt 2012; Kahn 2014; NICE 2019; NICE 2020). These include the use of both mechanical methods such as compression stockings and intermittent pneumatic compression devices (IPC), and pharmacological methods, including parenteral anticoagulation (e.g. low‐molecular‐weight heparin (LMWH)) (Alikhan 2014; Kakkos 2022; Sachdeva 2014).
The most used pharmacological interventions for preventing arterial and venous thrombosis are anticoagulants such as heparin, pentasaccharides, vitamin K antagonists, and direct oral anticoagulants (Amaral 2022; Biagioni 2020; Flumignan 2021; Flumignan 2022b; Flumignan 2023; Righini 2006). Because there is a high prevalence of vascular thrombosis and associated mortality in people with COVID‐19, physicians prescribe prophylactic anticoagulants untimely – for example, during the prehospital or ambulatory phase of COVID‐19 (Hippensteel 2020; Spyropoulos 2022). When used prophylactically, anticoagulant doses are usually one‐third or one‐half of those given for therapeutic purposes. Nevertheless, adverse events such as bleeding may have a significant impact on patient care (Flumignan 2021; Flumignan 2022b; Paranjpe 2020).
How the intervention might work
The COVID‐19 pandemic remains a global health issue. However, non‐transmissible circulatory diseases remain the leading cause of disease burden worldwide (Logue 2021). The risk of thromboembolic events in people with COVID‐19, even if they are receiving prophylactic anticoagulation, can reach 69% in severely ill hospitalised people when screening strategies are implemented, exceeding that observed in clinically ill people (0.42%) (Llitjos 2020; Spyropoulos 2020). Lodigiani 2020 found a cumulative rate of VTE of 21% in 388 severely ill people with COVID‐19, and half of the VTE events were diagnosed upon hospital admission, suggesting that these events developed in the early symptomatic phase, before clinical deterioration. Optimising measures to prevent vascular thrombosis is therefore essential in the management of people with COVID‐19 (Hippensteel 2020). The high prevalence of thrombosis in severely ill people with COVID‐19 led the American Society of Hematology, the International Society on Thrombosis and Haemostasis, and the American College of Chest Physicians to recommend that all people hospitalised with COVID‐19 should receive prophylactic anticoagulation (Cuker 2021; Moores 2020; Spyropoulos 2020). However, there is no consensus and there are no recommendations regarding outpatient prevention of thrombosis. Research on the prevention of vascular thrombosis has mainly concentrated on pharmacological interventions (Spyropoulos 2018). It is possible that starting anticoagulants earlier may have a role in this setting during the pre‐hospitalised phase in people with COVID‐19 (Barco 2020; Capell 2021).
Current recommendations regarding thromboprophylaxis while treating people with COVID‐19 are based on expert consensus, and the majority of scientific societies suggest that if there are no contraindications and after a careful evaluation of bleeding risk, adults hospitalised with COVID‐19 should receive thromboprophylaxis (Bikdeli 2020; Moores 2020; Spyropoulos 2020). However, there is no consensus concerning thromboprophylaxis in the outpatient setting. Some studies suggest that physicians should stratify the risk for thrombotic and haemorrhagic events individually, but the topic is still under discussion (Emert 2020; Sobreira 2020).
Why it is important to do this review
Research has shown an increase in cases of VTE during the COVID‐19 quarantine period (Tomidokoro 2021). Other studies have shown late arterial and venous thrombosis in people with COVID‐19. Prophylactic measures such as anticoagulation can reduce these effects (Benson 2021). However, there is no consensus about the impact of these interventions in managing outpatients with COVID‐19, and the effects of prophylactic anticoagulants in people with COVID‐19 in an ambulatory setting are still under discussion. Identifying strategies to prevent coagulopathy will be crucial to reduce COVID‐19 hospitalisation rates and related outcomes such as VTE and death. There is an urgent need for evidence to inform healthcare decision‐making during the COVID‐19 pandemic. Randomised controlled trials (RCTs) analysing the use of anticoagulants in outpatients with COVID‐19 are ongoing (Barco 2020; Capell 2021; NCT04542408). If performed appropriately, RCTs provide the best evidence for experimental therapies in highly controlled therapeutic settings. Non‐randomised studies (NRS) of interventions can be developed faster and may represent the only available evidence to guide decision‐making at this point. To ensure that we captured all relevant evidence, we planned to include RCTs and NRS, as we do not expect to find adequate RCT evidence for some time (Reeves 2021). In this Cochrane Review, we aimed to identify and synthesise the available evidence on the effectiveness and safety of prophylactic anticoagulants in non‐hospitalised people with COVID‐19, and so aid decision‐making for clinicians and their patients.
Objectives
To evaluate the benefits and harms of prophylactic anticoagulants versus active comparators, placebo or no intervention, or non‐pharmacological interventions in non‐hospitalised people with COVID‐19.
Methods
Criteria for considering studies for this review
Types of studies
To ensure that we captured all relevant study types, we planned to consider a broad range of empirical studies of any size that provided a quantitative measure of impact (Reeves 2021). To assess the effects of prophylactic anticoagulants on non‐hospitalised people with COVID‐19, we included RCTs (parallel, cluster, individual, or cross‐over design). In the case of insufficient evidence (very low‐certainty evidence or no evidence) available from RCTs to address the objective of this review, we planned to include quasi‐RCTs (e.g. assignment to treatment by alternation, medical register, or by date of birth) and prospective controlled cohort studies of interventions (non‐randomised studies (NRS)). As we identified sufficient RCTs (at least 400 participants), we did not include quasi‐RCTs or NRS. Details on planned methods for accessing non‐RCTs can be found in our protocol (Santos 2022). We only considered studies with a minimum duration of two weeks.
Types of participants
We included non‐hospitalised participants of both sexes and any age with a COVID‐19 diagnosis. COVID‐19 infection was confirmed by reverse transcription polymerase chain reaction (RT‐PCR) (WHO 2020). We excluded people receiving treatment for current VTE because they were receiving an anticoagulant regimen. We included participants with a previous diagnosis of VTE who had finished VTE treatment, regardless of the time of the VTE diagnosis compared with the COVID‐19 diagnosis. We considered participants with a previous history of hospitalisation, amputation, or any other outcome of interest for inclusion in the review. We excluded studies involving hospitalised participants with COVID‐19, as these participants are covered in another Cochrane Review (Flumignan 2022b).
When we found studies with mixed populations (e.g. hospitalised and non‐hospitalised participants), and only a subset of the participants met our inclusion criteria, we attempted to obtain data for the subgroup of interest from the study authors to permit inclusion in the review. If we were not able to obtain separate data for the subgroup of interest from a mixed population, but at least 50% of the study population were of interest, we included all participants in our analysis. We planned to explore the effect of this decision in a sensitivity analysis if needed. We excluded studies with mixed populations in which less than 50% of the population was of interest and data for the subgroup of interest were not available.
Types of interventions
We included studies that compared prophylactic anticoagulants with either placebo, no treatment, a pharmacological (active) comparator, or a non‐pharmacological comparator. We included studies with any combination of interventions providing the co‐treatments were balanced between the treatment and control arms. We allowed other potential interventions (e.g. antiplatelet agents, elastic stockings, intermittent pneumatic compression) as comparators or additional interventions.
We planned to undertake the following comparisons in the review.
Anticoagulant versus placebo or no treatment (we planned to pool all anticoagulants together, i.e. heparin, heparinoids, vitamin K antagonists, direct anticoagulants, etc., if possible).
Anticoagulant versus a different anticoagulant.
Anticoagulant versus a different dose, formulation, or schedule of the same anticoagulant.
Anticoagulant versus other pharmacological interventions such as antiplatelet agents.
Anticoagulant versus non‐pharmacological interventions.
We considered the following pharmacological interventions:
both unfractionated heparin (UFH) and low‐molecular‐weight heparin (LMWH), heparinoids and pentasaccharides (synthetic and selective anticoagulant drugs);
vitamin K antagonists; and
direct anticoagulants, including factor Xa inhibitors and direct thrombin inhibitors, i.e. direct oral anticoagulants and non‐oral direct anticoagulants (e.g. bivalirudin).
We included studies comparing different formulations, doses, and schedules of the same intervention (e.g. heparinoids).
Some commonly applicable prophylactic doses of the interventions of interest are LMWH, such as enoxaparin 30 mg twice a day or 40 mg daily, and UFH 5000 international units three times a day. However, all doses of anticoagulants were eligible for our review, when they were used for primary or secondary prophylaxis of thromboembolism (e.g. previous VTE event, high risk of a new event, presence of active cancer and thrombophilia) (Fernandes 2019; Weitz 2017).
Types of outcome measures
We evaluated core outcomes as predefined by the Core Outcome Measures in Effectiveness Trials Initiative for people with COVID‐19 (COMET 2021). We presented the outcomes at two different time points following the start of the intervention, if data were available:
short‐term outcomes (at 90 days or less after the start of the intervention); and
long‐term outcomes (more than 90 days after the start of the intervention).
The short‐term time points include the time frame from the start of the intervention up to 90 days, and the long‐term time points include the time frame after this period. We included studies in the review irrespective of whether the measured outcome data had been reported in a useable way.
Primary outcomes
All‐cause mortality.
VTE: DVT or PE, symptomatic or asymptomatic, first episode or recurrent, and fatal or non‐fatal. The diagnosis had to be confirmed by clinical examination and at least one additional objective diagnostic test. We accepted ultrasonography or angiography (e.g. by computed tomography (CT), magnetic resonance imaging (MRI), or digital subtraction) for the DVT diagnosis from any site (e.g. lower limbs, upper limbs, abdomen). We accepted angiography by any described method and ventilation‐perfusion scan for confirmation of PE. We also considered postmortem examination as an objective confirmation of DVT and PE. If the participant had both DVT and PE events, we counted this as one unique event of VTE in our analysis.
Major bleeding: defined by a haemoglobin concentration decrease of 2 g/dL or more, a retroperitoneal or intracranial bleed, a transfusion of two or more units of blood, or fatal haemorrhagic events, as defined by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2010).
Secondary outcomes
DVT: symptomatic or asymptomatic, and first episode or recurrent. The diagnosis had to be confirmed by ultrasonography or angiography (e.g. by CT, MRI, or digital subtraction) from any site (e.g. lower limbs, upper limbs, abdomen).
PE: symptomatic or asymptomatic, first episode or recurrent, and fatal or non‐fatal. The diagnosis had to be confirmed by angiography (e.g. by CT, MRI, or digital subtraction) and ventilation‐perfusion scan, or both. We also considered postmortem examination as an objective confirmation of DVT and PE.
Need for hospitalisation (yes or no).
Adverse events (AE): minor bleeding/clinically relevant non‐major bleeding defined as an acute or subacute clinically overt bleed that does not meet the criteria for a major bleed but prompts a clinical response, in that it leads to at least one of the following: a hospital admission for bleeding, or a physician‐guided medical or surgical treatment for bleeding, or a change in antithrombotic therapy (including interruption or discontinuation of study drug). In addition, we considered bleeding events that led to participant’s discomfort and impairment of activities of daily life as clinically relevant non‐major bleeding.
AE: all possible AEs separately, as individual outcomes, such as thrombocytopenia, gastrointestinal adverse effects (e.g. nausea, vomiting, diarrhoea, abdominal pain), allergic reactions, renal failure, acute limb ischaemia, need for surgical peripheral revascularisation, and amputations. We only considered the AEs described in the included studies.
Quality of life based on the participant's subjective perception of improvement (yes or no) as reported by the study authors or using any validated scoring system such as the 36‐Item Short Form Health Survey (SF‐36) (Ware 1992).
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year, or publication status restrictions:
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web);
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 4) via the Cochrane Register of Studies Online (CRSO);
Cochrane COVID‐19 Study Register via the CRSO;
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE);
Embase Ovid;
CINAHL (Cumulative Index to Nursing and Allied Health Literature); and
LILACS (Latin American and Caribbean Health Science Information database) (via Virtual Health Library).
We developed search strategies for other databases based on the search strategy designed for MEDLINE. Where appropriate, we combined these strategies with adaptations of the Highly Sensitive Search Strategy designed by the Cochrane to identify RCTs and controlled clinical trials (as described in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions) (Lefebvre 2022). Search strategies for the major databases are provided in Appendix 1.
We searched the following trial registries:
World Health Organization International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform); and
ClinicalTrials.gov (clinicaltrials.gov).
The most recent searches were carried out on 18 April 2022.
Searching other resources
We checked the reference lists of all included studies and any relevant systematic reviews identified for additional references to studies. We examined any relevant retraction statements and errata for the included studies. We contacted the authors of the included studies for any possible unpublished data. We contacted field specialists to enquire about relevant ongoing or unpublished studies.
Data collection and analysis
Selection of studies
We considered abstracts and full texts in all languages for inclusion in the review. All potentially eligible non‐English language abstracts progressed to full‐text review, with the methods translated for eligibility assessment and the full text translated for data extraction.
Two review authors (BCS and LCUN) independently screened the titles and abstracts of all articles identified as a result of the search; we coded these as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve' (non‐relevant) using the Covidence tool (Covidence). In the case of disagreement, we asked a third review author to arbitrate (RLGF). We retrieved the full‐text study reports/publications, and two review authors (BCS and VTC) independently screened the full texts and identified studies for inclusion, and identified and recorded the reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (RLGF) if required. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We illustrated the study selection process in a PRISMA flow diagram (Liberati 2009). We listed all articles excluded after full‐text assessment in a 'Characteristics of excluded studies' table and provided the reasons for their exclusion. We considered studies reported as full text, those published as abstract only, and unpublished data. We considered abstracts and conference proceedings if they were eligible and had useable data.
Data extraction and management
We managed and synthesised the available data using Review Manager Web (RevMan Web 2022). If there was a conflict between data reported across multiple sources for a single study (e.g. between a published article and a trial registry record), we used the article published for numerical analysis, and we reported the differences and considered any impact on the certainty of evidence (Schünemann 2021a). We used a data collection form that had been piloted on at least one study in the review for study characteristics and outcome data. Two review authors (BCS and VTC) extracted data from the included studies. Any disagreements were resolved by discussion. We extracted the following study characteristics.
Methods: study design, duration of the study, number of study centres and location, study setting, and date of the study.
Participants: comorbidities, pregnancy, number randomised, exclusions postrandomisation, number lost to follow‐up/withdrawn, number analysed, number of interest, mean age, age range, gender, severity of the condition, inclusion criteria, and exclusion criteria.
Interventions: intervention and comparison characteristics (e.g. manufacturer, dosage, additional procedures, method of administration), concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected (e.g. how outcomes were measured) and time points reported.
Funding for the trial, conflicts of interest of study authors, and registration number.
One review author (BCS) transferred data into Review Manager Web (RevMan Web 2022). We double‐checked that the data had been entered correctly by comparing the data presented in the systematic review with the data extraction form. Two review authors (RLGF and LCUN) spot‐checked the study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (BCS and VTC) assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions for RCTs (RoB 1) (Higgins 2017). Any disagreements were resolved by discussion within the review team. We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting; and
other bias.
We graded each potential source of bias as low, high, or unclear and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where there was information on risk of bias relating to unpublished data or correspondence with a study author, we noted this in the risk of bias table.
When evaluating treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
We based the overall bias judgement of included RCTs on the following three domains of RoB 1, namely:
adequate sequence generation;
blinding of outcome assessors; and
selective outcome reporting.
We labelled an RCT at low risk in all of these domains as a low‐risk study. We labelled an RCT at high risk in one of these domains as a high‐risk study. We indicated that the risk of bias in the study was unclear if there was no clear information on risk of bias for one or more key domains, but the RCT was not at high risk for any key domain.
Details on how we planned to assess risk of bias in cluster‐randomised trials, quasi‐RCTs, and NRS can be found in the protocol (Santos 2022).
Measures of treatment effect
Dichotomous data
We calculated the risk ratio (RR) and 95% confidence intervals (CIs) for dichotomous variables.
Continuous data
We calculated the mean differences (MD) and 95% CIs between treatment groups when studies reported the same outcomes for continuous data. When studies reported similar outcomes on different scales, we calculated the standardised mean difference (SMD) and 95% CIs. If standard deviations (SDs) or standard errors (SEs) were not available, we attempted to extract P values from the available data. We estimated the MD using the method reported by Wan 2014 to convert the median and interquartile range into MD and CI. When this was not possible, we narratively described skewed data reported as medians and interquartile ranges. To interpret SMD, we used the following thresholds, as recommended in Section 15.5.3.1 of the Cochrane Handbook for Systematic Reviews of Intervention (Schünemann 2021b):
SMD < 0.2 = trivial or no effect;
SMD ≥ 0.2 and < 0.5 = small effect;
SMD ≥ 0.5 and < 0.8 = medium effect; and
SMD ≥ 0.8 = large effect.
We also calculated the number needed to treat for an additional beneficial outcome (NNTB) for the primary outcomes (all‐cause mortality and VTE), using NNTB = 1/risk difference (RD). We also calculated the number needed to treat for an additional harmful outcome (NNTH) for the primary outcome major bleeding, using NNTH = 1/RD. We calculated the RD using Review Manager Web (RevMan Web 2022). We expressed the NNTB and NNTH to indicate the direction of effect, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021a).
Unit of analysis issues
We considered each participant as the unit of analysis for the outcomes all‐cause mortality, VTE, major bleeding, DVT, PE, need for hospitalisation, AEs (e.g. minor bleeding, thrombocytopenia, gastrointestinal AE, allergic reactions, renal failure), and quality of life. We considered each limb as the unit of analysis for AEs such as amputation rate. If trials included multi‐arm interventions, we considered only the arms relevant to the scope of our review.
We did not include any cross‐over or cluster‐randomised trials in the review. Details of how we planned to address any unit of analysis issues can be found in the protocol (Santos 2022).
Dealing with missing data
We included all available data from the included studies. We described missing data for each study in the 'Characteristics of included studies' table and risk of bias table, and discussed the extent to which the missing data could alter the results of the review. We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where possible, we used the Review Manager Web calculator to calculate missing SDs by using other data from the trial, such as CIs (RevMan Web 2022). We estimated the MD using the method reported by Wan 2014 to convert the median and interquartile range into MD and CI. When data were only reported in graphs, we extracted the data of interest (such as mean, SD or SE) using Graphreader software (Graphreader 2022). We identified translators for foreign languages with which we were unfamiliar (e.g. Chinese and Japanese). When translation was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results using a sensitivity analysis. For all outcomes, we followed the intention‐to‐treat (ITT) principle to the greatest degree possible, that is we analysed participants in the group to which they had been randomised regardless of what intervention they actually received. We used available‐case data for the denominator if ITT data were not available. In trials with a large proportion of missing data (more than 20%), we assessed the impact of this with sensitivity analysis, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Assessment of heterogeneity
We visually inspected forest plots to consider the direction and magnitude of effects and the degree of overlap between CIs. We used the I2 statistic to measure heterogeneity among the trials in each analysis; we acknowledge that there is substantial uncertainty in the value of I2 when there is only a small number of studies. If we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis. As strict thresholds for the interpretation of I2 are not recommended, we used the rough guide to interpretation provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021), as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
When I2 was in an area of overlap between two categories (e.g. between 50% and 60%), we considered differences in participants and interventions among the trials contributing data to the analysis (Deeks 2021).
Assessment of reporting biases
We performed searches in multiple sources to reduce the chance of reporting biases. We planned to assess the presence of publication bias and other reporting bias using funnel plots if we identified a sufficient number of studies (i.e. more than 10) for inclusion in the meta‐analysis (Sterne 2017). If asymmetry was present, we would explore possible causes, including publication bias, poor methodological quality, and true heterogeneity (Sterne 2017). We also planned to perform additional statistical analysis for continuous outcomes with intervention effects measured as MD to assess reporting biases, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions; however, as the meta‐analysis included fewer than 10 studies, it was not possible to perform this analysis (Sterne 2017).
Data synthesis
We synthesised the data by using Review Manager Web (RevMan Web 2022). We undertook meta‐analysis only when this was meaningful, that is if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. If we were confident that the trials had estimated the same underlying treatment effect (i.e. that the population, interventions, comparators, and outcome characteristics of the included studies were homogenous), we used a fixed‐effect meta‐analysis model. If clinical heterogeneity was sufficient to expect that underlying treatment effects differed between trials, or if we identified at least substantial heterogeneity, we used a random‐effects meta‐analysis model. We planned that if there was substantial clinical, methodological, or statistical heterogeneity across trials that precluded the pooling of data, we would use a narrative approach for data synthesis (Deeks 2021).
We addressed all outcomes listed in the Types of outcome measures section, in the order in which they were shown, in the Effects of interventions section of the Results. In addition, we presented one summary of findings table for each comparison, in which we summarised the main outcomes. We included the results of individual studies and any statistical summary of these in the Data and analyses tables in the review.
In preparation for synthesis (either meta‐analyses or synthesis without meta‐analysis), we assessed how much data were available for each of our comparisons using the following method:
table to compare PICO elements/study design features;
conversion of numerical data for meta‐analysis;
forest plots;
qualitative synthesis; and
synthesis without meta‐analysis.
We performed a pooled analysis for RCTs and undertook sensitivity analysis if sufficient data were available. When possible, we summarised effect estimates graphically using forest plots (McKenzie 2021).
Subgroup analysis and investigation of heterogeneity
We did not have sufficient data to undertake the planned analyses. We plan to perform the following subgroup analyses for all outcomes if sufficient data become available:
type of anticoagulants (e.g. heparin, heparinoids, vitamin K antagonists, direct anticoagulants);
antiplatelet therapy (yes or no);
duration of prophylaxis (e.g. up to 30 days after the start of intervention or more);
time of starting prophylaxis (e.g. days since positive COVID‐19 diagnosis);
age (e.g. children less than 18 years, adults (18 to 74 years), and seniors (75 years and older));
comorbidities, i.e. we assessed participants with previous risk factors for outcomes of interest for this review separately (e.g. previous VTE, cardiovascular comorbidities, and thrombophilia);
illness severity (e.g. symptomatic versus asymptomatic or minimally symptomatic); and
different doses of drugs.
We performed additional subgroup analysis to investigate if there was a difference in effect between participants who had never been hospitalised and those who started treatment after discharge from hospital (COVID‐19‐related hospitalisation).
We used the formal test for subgroup differences in Review Manager Web (RevMan Web 2022) and based our interpretation on the results.
Sensitivity analysis
As we identified sufficient RCTs, we only undertook preplanned sensitivity analyses relevant to these. See Santos 2022 for details on planned sensitivity analyses relevant to NRS. We planned to carry out the following sensitivity analyses to test whether critical methodological factors or decisions affected the main result.
Only including studies with a low overall risk of bias by RoB 1 (see Assessment of risk of bias in included studies).
We planned to examine both the fixed‐effect model and random‐effects model meta‐analyses, and explore the differences between the two estimates. However, we only used random‐effects model meta‐analyses because of clinical heterogeneity.
We planned to explore the decision to include all participants when at least 50% were of interest in a study with a mixed population. However, this was not necessary because there were no mixed populations.
We planned to explore the impact of including studies with missing data (proportion of more than 20%) in the overall assessment of results. If we identified studies with missing data that were unobtainable, we would repeat the analyses excluding these studies in order to determine their impact on the primary analyses. However, this sensitivity analysis was unnecessary because no studies had more than 20% of data missing.
We planned to present these results and compare them with the overall findings; however, this was not possible given the available data. We planned to justify in the final report any post hoc sensitivity analyses that arose during the review process. We did not undertake any unplanned sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables using GRADEpro GDT software (GRADEpro GDT), and presented the main findings of the review for the short time point (90 days or less) (Atkins 2004). The population consisted of non‐hospitalised people with COVID‐19, and we compared the effects of prophylactic anticoagulants versus active comparator, placebo, or no intervention on the most clinically relevant outcomes for these participants. We created one table for each separate comparison, in order of importance for decision makers: Anticoagulant compared to placebo or no treatment for non‐hospitalised people with COVID‐19; Anticoagulant compared to a different dose of the same anticoagulant for non‐hospitalised people with COVID‐19; and Anticoagulant compared to antiplatelet agents for non‐hospitalised people with COVID‐19. We included the following outcomes in each table.
All‐cause mortality
VTE
Major bleeding
DVT
Pulmonary embolism
Need for hospitalisation
Adverse events: minor bleeding
We evaluated the certainty of the evidence using the GRADE approach (Atkins 2004; Schünemann 2021a). We assigned one of four levels of certainty: high, moderate, low, or very low, based on the overall risk of bias, directness of the evidence, inconsistency of results, precision of the estimates, and risk of publication bias, as described in the Assessment of risk of bias in included studies section (Atkins 2004; Schünemann 2021a).
Reaching conclusions
We based our conclusions only on findings from the quantitative synthesis of studies included in this review. We avoided making any recommendations for practice, suggested priorities for future research, and outlined what the remaining uncertainties are in the area.
Results
Description of studies
Results of the search
Our searches of the databases identified 15,339 records. After de‐duplication, 9347 records were screened in Covidence (Covidence). We assessed 9304 of these records as not relevant for this review for different reasons (e.g. inadequate population of interest, inadequate study design, inadequate condition, etc.). We assessed the remaining 43 records (30 studies) by full text for eligibility. We identified five RCTs (13 records) that met our inclusion criteria (Characteristics of included studies). As a result, we did not include NRS as planned in our protocol. We excluded 10 studies (11 records); the reasons for their exclusion are summarised in Characteristics of excluded studies. We further assessed eight studies (eight reports) as irrelevant at full‐text review. We assessed nine studies (11 records) as ongoing (Characteristics of ongoing studies). See Figure 1.
1.
PRISMA flow diagram.
We contacted authors of all the included studies, as well as pharmaceutical companies and authors of the ongoing studies, in an attempt to locate any ongoing studies or data for inclusion in the review. When we received a response, it was negative for new data.
Included studies
See Characteristics of included studies.
Design and setting
We found five studies (20 records) with 1777 participants eligible for inclusion in our analysis (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022). All five included studies were parallel RCTs.
Ananworanich 2021 evaluated a group with prophylactic rivaroxaban and a group that received placebo, but the study authors did not assess VTE, DVT, or PE as outcomes.
Barco 2022 evaluated a group receiving prophylactic enoxaparin and a group that received standard care (no treatment).
Connors 2021 evaluated people with COVID‐19 diagnosed by polymerase chain reaction (PCR) or antigen test in the outpatient setting who received either apixaban at different doses, aspirin, or placebo; however, the authors did not provide separate data for the groups with DVT and PE.
The ETHIC trial evaluated a group of COVID‐19 patients in the outpatient setting who received prophylactic enoxaparin and a group who received standard care, but the study authors did not provide information on DVT and PE participants separately (Cools 2022).
Ramacciotti 2022 evaluated participants receiving prophylactic rivaroxaban compared with participants receiving no treatment. The study did not report on the need for hospitalisation.
The five included studies provided data for three different comparisons:
anticoagulant versus placebo or no treatment (1777 participants; Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022);
anticoagulant versus a different dose of the same anticoagulant (278 participants; Connors 2021); and
anticoagulant versus antiplatelet agents (279 participants; Connors 2021).
Ananworanich 2021 reported in their study protocol that the recruitment of participants began in September 2020 and concluded in March 2021, and was performed in 47 USA states. Barco 2022 also provided information about the study duration (between June 2020 and April 2022), with participants recruited from eight centres in Switzerland and Germany. Connors 2021 conducted their study between September 2020 and August 2021 and enrolled participants from 52 centres in the USA. In the ETHIC trial (Cools 2022), the study lasted from October 2020 to November 2021, with recruitment performed in 15 centres in six countries (Belgium, Brazil, India, South Africa, Spain, and the UK). Ramacciotti 2022 provided information about study duration (October 2020 to June 2021) and indicated that participants were enrolled from 14 medical centres in Brazil.
Participants
We included a total of 1777 participants in our analysis. All participants analysed in this review had been diagnosed with COVID‐19 confirmed by an objective investigation such as PCR or an antigen test. Currently hospitalised patients or those with a history of current active pathological bleeding were excluded from the studies. All studies included men and women, age 18 years or older, with the majority older than 50 years. Two studies had a majority of female participants (60% in Ananworanich 2021 and 59.1% in Connors 2021). The three remaining studies had more male participants (54% in Barco 2022, 60% in Ramacciotti 2022, and 55% in Cools 2022).
Ananworanich 2021 reported the enrolment of participants with mild COVID‐19 at screening and high risk for severe COVID‐19. Barco 2022 reported that outpatients with acute COVID‐19 were eligible if they presented with respiratory symptoms or body temperature higher than 37.5 °C. Connors 2021 considered newly diagnosed symptomatic participants with SARS‐CoV‐2 infection with positive PCR or antigen test results. Cools 2022 described the enrolment of participants who had not received a COVID‐19 vaccine and had symptomatically confirmed COVID‐19 (i.e. with a positive SARS‐CoV‐2 reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR)) in the outpatient setting plus at least one risk factor for severe disease. In the initial protocol, they planned to enrol patients aged at least 55 years and with at least two predefined risk factors: older age (≥ 70 years), a body mass index greater than 25 kg/m², chronic lung disease, diabetes, cardiovascular disease, or corticosteroid use. Ramacciotti 2022 included postdischarge patients who had been hospitalised with COVID‐19 for at least three days.
All studies reported that patients with severe renal impairment, thrombocytopenia, or any clinical condition that prohibited anticoagulation were excluded from participation.
Only one of the five included studies replied to our request for more information, but they were unable to provide any additional data. No trialist provided additional data beyond the data already published.
Interventions and co‐treatments
All included studies evaluated prophylactic anticoagulants in the intervention group, with some differences between protocols. Ananworanich 2021 and Connors 2021 compared prophylactic anticoagulants versus placebo. Barco 2022, Cools 2022, and Ramacciotti 2022 compared prophylactic anticoagulants versus no treatment. Connors 2021 made two additional comparisons: prophylactic anticoagulants versus the same anticoagulant at a different dose, and prophylactic anticoagulants versus antiplatelet agents.
Ananworanich 2021 described prophylaxis with anticoagulants for participants with COVID‐19 in the outpatient setting. The 497 participants were randomised into two groups:
rivaroxaban (one 10 mg tablet) once a day for 21 consecutive days; or
placebo equivalent (multivitamin, one tablet) orally daily for 21 consecutive days.
Barco 2022 described prophylaxis with anticoagulant agents for participants with a positive test for SARS‐CoV‐2 eligible for ambulatory treatment, with the presence of respiratory symptoms or a body temperature greater than 35.7 °C. They randomised 472 participants into two groups:
enoxaparin 40 mg/0.4 mL daily subcutaneously for 14 days; or
standard care (no thromboprophylaxis).
Connors 2021 evaluated 657 participants with newly diagnosed COVID‐19 with positive PCR or antigen test results. Participants were randomised into four groups:
apixaban 2.5 mg orally twice a day for 45 days;
apixaban 5.0 mg orally twice a day for 45 days;
aspirin 81 mg orally twice a day for 45 days; or
placebo orally twice a day for 45 days.
Cools 2022 enrolled 219 participants who had not received a COVID‐19 vaccine and had symptomatically COVID‐19 confirmed with positive PCR in the outpatient setting associated with at least one risk factor for severe disease. Participants were randomised into two groups:
enoxaparin for 21 days (40 mg once daily if weight < 100 kg; 40 mg twice daily if weight ≥ 100 kg); or
standard care (without thromboprophylaxis).
Ramacciotti 2022 considered participants at discharge who were hospitalised with COVID‐19 (confirmed by reverse transcription polymerase chain reaction (RT‐PCR), antigen, or immunoglobulin M (IgM) tests) for at least three days. They randomised 320 participants into two groups:
rivaroxaban 10 mg/day for 35 days; or
standard care (no thromboprophylaxis) for 35 days.
Sample size calculations
We were able to include participants from all studies in our analysis (see the numbers above) (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022). Ananworanich 2021 described a sample size calculation with 80% power to detect a relative risk reduction (RRR) of 35% in favour of anticoagulation compared with placebo. Barco 2022 calculated that 920 patients would be required for 80% power to show superiority of enoxaparin versus standard of care (no thromboprophylaxis) with a two‐sided significance level of 5%. Connors 2021 estimated 80% to 90% power to detect an RRR between 33% and 50% in the primary outcome between each active drug (apixaban or aspirin) and placebo, with an alpha error of 2.5% (one‐sided). Cools 2022 calculated the sample size based on an alpha level of 5% and an event rate of 25% in the standard care group, based on 80% power. Ramacciotti 2022 assumed 80% power with an RRR of 67%. Only Ramacciotti 2022 reached the calculated sample size. The remaining four studies did not reach the calculated sample size due to recommendations from the Independent Data Monitoring Committee to stop enrolment because the event rate was lower than anticipated (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022).
Length of follow‐up
Ananworanich 2021 randomised participants with acute COVID‐19 stratified by site and symptom duration (< 6 days versus ≥ 6 days). The study authors performed 12 telemedicine visits (days 1, 4, 6, 8, 10, 12, 14, 18, 21, 24, 28, and 35), and AEs and bleeding events based on standardised definitions were recorded by the investigator.
Barco 2022 performed block‐stratified randomisation (by age group 50 to 70 versus > 70 years and by study centre). Follow‐up was performed through telephone follow‐up visits by trained personnel 3, 7, 14, 30, and 90 days following randomisation.
Connors 2021 studied participants with newly diagnosed (by PCR or antigen test) symptomatic COVID‐19, and participants were contacted weekly using text links to the REDCap survey or by the Research Communication Center staff telephone calls during the 45‐day randomised treatment period to capture relevant clinical outcomes and again after a subsequent 30‐day safety follow‐up.
Cools 2022 evaluated symptomatic, PCR‐confirmed COVID‐19 participants, and data were collected at 21, 50, and 90 days following randomisation by the treating physician using an electronic case report form designed by the Thrombosis Research Institute.
Ramacciotti 2022 analysed participants at discharge after hospitalisation with COVID‐19. The first follow‐up was on day 7 after randomisation either at an outpatient clinic or by telephone. The second follow‐up was performed on day 35 at an outpatient clinic or hospital.
Outcomes
Ananworanich 2021 presented data on the frequency of AEs resulting in discontinuation; serious AEs and hypersensitivity; major bleeding events; the proportion of participants who progressed to a moderate or severe disease category; the incidence of hospitalisation and the proportion of participants with disease progression; disease resolution; and time to disease resolution. The study reported no deaths during the follow‐up period; however, there were no data regarding the number of participants diagnosed with VTE events such as DVT and PE. Despite our attempts to contact the study authors, we did not receive any additional data that could be analysed in the review.
Barco 2022 described the majority of our outcomes of interest. The primary outcome reported was a composite of any untoward hospitalisation and all‐cause death within the 30 days following randomisation. They also reported cardiovascular events including DVT, PE, myocardial infarction or myocarditis, peripheral arterial ischaemic events, acute splanchnic vein thrombosis, and ischaemic stroke. They reported information regarding major bleeding and non‐major clinically relevant bleeding and serious AEs. The authors reported no deaths within the 30 days following randomisation.
Connors 2021 described some of our outcomes of interest. The primary outcome was the composite of symptomatic DVT, PE, arterial thromboembolism, myocardial infarction, ischaemic stroke, hospitalisation for cardiovascular or pulmonary events, and all‐cause mortality. They also reported data on the individual components of the primary study endpoint, mortality without antecedent hospitalisation, major bleeding, clinically relevant non‐major bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH) criteria, and any events of disseminated intravascular coagulation. However, the authors did not provide separate data regarding DVT and PE events (combined data only reported). Despite our attempts to contact the study authors, we did not receive any additional data that could be analysed in the review. There were no deaths during the follow‐up period.
Cools 2022 presented data on all‐cause death and hospitalisation; they also reported data regarding diagnosis of VTE and bleeding events. The study authors did not provide separate data for DVT and PE during the complete follow‐up period (90 days). It was only possible to collect information at the first time point of 21 days.
Ramacciotti 2022 evaluated several relevant outcomes for this review: all‐cause mortality, VTE, DVT, PE, major bleeding, and AEs. However, the study authors did not provide data on the need for hospitalisation. Despite our attempts to contact the study authors, we did not receive any additional data regarding this outcome. They also reported data on myocardial infarction, non‐haemorrhagic stroke, and major adverse limb events.
Excluded studies
See Characteristics of excluded studies. We excluded a total of 10 studies (Aghamohammadi 2020; Borghi 2021; ChiCTR2000034796; JPRN‐UMIN000042489; Kuno 2022; Lisker 2021; Rivera‐Caravaca 2021; Sharma 2021; Spyropoulos 2021; Vergori 2021).
We excluded three studies because the participants were hospitalised patients (Sharma 2021; Spyropoulos 2021; Vergori 2021). As we had sufficient information from RCTs, we excluded seven studies that were not randomised (Aghamohammadi 2020; Borghi 2021; ChiCTR2000034796; JPRN‐UMIN000042489; Kuno 2022; Lisker 2021; Rivera‐Caravaca 2021).
Ongoing studies
We identified nine ongoing RCTs that met our inclusion criteria (Capell 2021; EUCTR2020‐005884‐29‐IT; NCT04542408; NCT04650087; NCT04715295; NCT04746339; NCT04757857; Ramos‐Peñafiel 2020; RBR‐7nzwkpg). The RCTs plan to evaluate 8197 participants in total. Seven studies are comparing prophylactic anticoagulants versus placebo or no treatment (Capell 2021; NCT04542408; NCT04650087; NCT04746339; NCT04757857; Ramos‐Peñafiel 2020; RBR‐7nzwkpg); one is comparing a prophylactic anticoagulant versus a different dose of the same anticoagulant (EUCTR2020‐005884‐29‐IT); and one is comparing prophylactic anticoagulants versus other pharmacological intervention (NCT04715295). We contacted the study authors and also searched by study registration number and title of the study on all databases of interest for this review. However, we did not receive any response, and there are no additional data from the ongoing studies. For further details, see Characteristics of ongoing studies.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We used RoB 1 to assess risk of bias as all included studies were RCTs. We judged the overall risk of bias as high for Cools 2022. We judged Ananworanich 2021 and Barco 2022 as at unclear risk of bias, and Connors 2021 and Ramacciotti 2022 as at low risk of bias.
Allocation
Ananworanich 2021 did not provide details on how the randomisation sequence was generated. The authors stated that participants were randomised with a 1:1 proportion to receive either rivaroxaban or placebo equivalent. We therefore assessed this study as at unclear risk of bias for random sequence generation, and low risk of bias for allocation concealment.
We assessed Barco 2022 as at low risk for selection bias. The study authors described randomisation by a computer‐generated approach and integrated into the electronic data capture software REDCap (Vanderbilt University, version 9.1.24), and the participants underwent block‐stratified randomisation in a 1:1 ratio.
We assessed Connors 2021 as at low risk of selection bias. The study authors reported that randomisation code lists were computer generated, and participants were randomised in a 1:1:1:1 proportion to receive aspirin with matching placebo, prophylactic‐dose apixaban, apixaban at therapeutic dose, or placebo.
We assessed Ramacciotti 2022 as at low risk of selection bias. The study authors stated that randomisation was carried out using a central, concealed, web‐based, automated randomisation system, and participants were randomly allocated in a 1:1 ratio to receive either thromboprophylaxis with rivaroxaban or regular follow‐up.
Although Cools 2022 described that the randomisation sequence was established using a prespecified, secure, central, web‐based randomisation system, the study authors stated that the study was unblinded and that no allocation concealment was applied. We therefore assessed the study as at high risk of selection bias due to lack of allocation concealment.
Blinding
Ananworanich 2021 divided participants into a treatment group (rivaroxaban) and a control group (placebo). They reported that participants and personnel were blinded, so we judged this study as at low risk for both performance and detection bias.
Despite the fact that an independent data and safety monitoring board monitored the trials by Barco 2022, Cools 2022, and Ramacciotti 2022, they were classified as open‐label trials, in which participants and study personnel were aware of treatment allocation, but not of the allocation sequence. We assessed all three studies as at high risk of performance bias because personnel and participants were not blinded (Barco 2022; Cools 2022; Ramacciotti 2022). However, we assessed Cools 2022 and Ramacciotti 2022 as at low risk of detection bias since they used an independent data evaluating or monitoring committee. We judged Barco 2022 as at unclear risk of detection bias because insufficient details were provided.
Connors 2021 stated that participants were randomly assigned (double‐blind), and all tablets in the four groups were taken two times a day. In addition, personnel were unaware of randomised drug assignment. We therefore assessed this study as at low risk of both performance and detection bias.
Incomplete outcome data
We assessed Ananworanich 2021, Barco 2022, and Cools 2022 as having a low risk of attrition bias because the study authors described dropouts, and numbers were similar between groups. The study authors used an ITT approach, reported and analysed AEs.
We assessed Connors 2021 as at low risk of attrition bias because the authors described dropouts, which were similar between groups, and AEs.
We assessed Ramacciotti 2022 as at low risk of attrition bias. The study authors described all losses/exclusions, and all participants were analysed by an ITT approach.
Selective reporting
The protocols of Ananworanich 2021, Barco 2022, and Connors 2021 were available, and all prespecified (primary and secondary) outcomes of interest in the review were reported as prespecified. We therefore assessed these studies as at low risk of reporting bias.
We assessed Cools 2022 as at high risk of reporting bias because although the protocol was available, the study authors made adjustments to the inclusion criteria during the course of the study because enrolment was slower than expected. Moreover, they reported that "due to the very low number of events and the study's early termination, only the primary efficacy outcome was tested for statistical significance".
We judged the study by Ramacciotti 2022 as at low risk of reporting bias because the full protocol was available, and all prespecified (primary and secondary) outcomes of interest in the review were reported.
Other potential sources of bias
We assessed all five included studies as at low risk of other bias as we identified no other reasons for bias (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022).
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1; Table 2; Table 3.
The included studies provided sufficient short‐term (90 days or less) follow‐up data. There were no available data regarding long‐term follow‐up (> 90 days). We calculated number needed to treat for an additional beneficial outcome (NNTB) for outcomes with clinically important differences. None of the included studies compared anticoagulant versus a different anticoagulant, or anticoagulant versus non‐pharmacological interventions.
Anticoagulant versus placebo or no treatment
Five studies with a follow‐up duration ranging from 21 to 90 days reported this comparison (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022). Ananworanich 2021 compared a group that received prophylactic rivaroxaban (10 mg daily) with placebo; Barco 2022 compared enoxaparin 40 mg daily with standard care (no treatment); Connors 2021 compared apixaban 2.5 mg twice a day with placebo; Cools 2022 compared enoxaparin 40 mg once daily if body weight was < 100 kg and 40 mg twice daily if body weight was ≥ 100 kg with standard care (no treatment); and Ramacciotti 2022 compared rivaroxaban 10 mg daily with no treatment.
Primary outcomes
All‐cause mortality
All studies reported this outcome (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022). Ananworanich 2021, Barco 2022, and Connors 2021 reported no deaths during the follow‐up period. Combining the data, overall there was little or no difference in all‐cause mortality between anticoagulants and either placebo or no treatment (risk ratio (RR) 0.36, 95% confidence interval (CI) 0.04 to 3.61; 5 studies; 1777 participants; P = 0.39; low‐certainty evidence) (Analysis 1.1). We detected no important heterogeneity (I2 = 24%). We used a random‐effects model for this analysis because of clinical heterogeneity among the included studies.
1.1. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 1: All‐cause mortality
The test for subgroup differences suggests that starting prophylaxis as an outpatient versus after discharge does not have a modifying effect on all‐cause mortality (P = 0.27) (Analysis 1.1).
Sensitivity analyses including only trials at low risk of bias did not change the effect estimate substantially (RR 0.11, 95% CI 0.01 to 2.05) (Figure 4).
4.
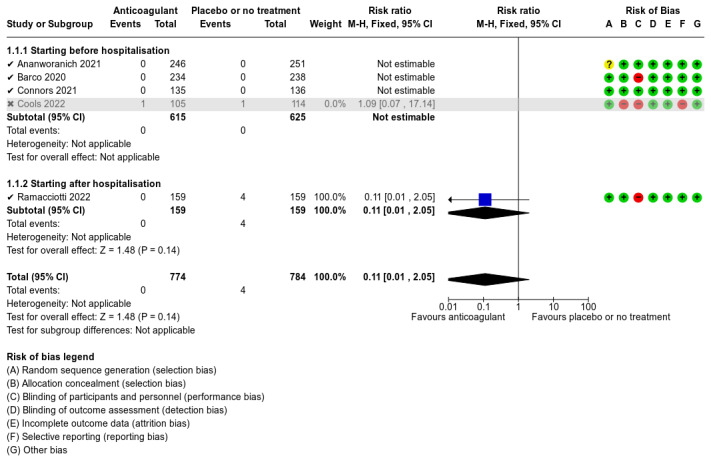
Sensitivity analysis (All‐cause mortality): only trials at low risk of bias.
Venous thromboembolism (VTE)
Ananworanich 2021 did not report data on VTE events, therefore four studies provided data for this outcome (Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022). Connors 2021 reported no VTE during the follow‐up period. Barco 2022; Cools 2022, and Ramacciotti 2022 reported VTE data. Overall, our analysis shows that anticoagulants probably decrease VTE compared with placebo or no treatment (RR 0.36, 95% CI 0.16 to 0.85; 4 studies; 1259 participants; P = 0.02; NNTB = 50; moderate‐certainty evidence; Analysis 1.2). We detected no heterogeneity (I2 = 0%). We used a random‐effects model for this analysis because of clinical heterogeneity among the included studies.
1.2. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 2: Venous thromboembolism
The test for subgroup differences suggests that starting prophylaxis as an outpatient versus after discharge does not have a modifying effect on VTE (P = 0.95) (Analysis 1.2).
Sensitivity analyses including only trials at low risk of bias did not change the effect estimate substantially (RR 0.34, 95% CI 0.14 to 0.83) (Figure 5).
5.
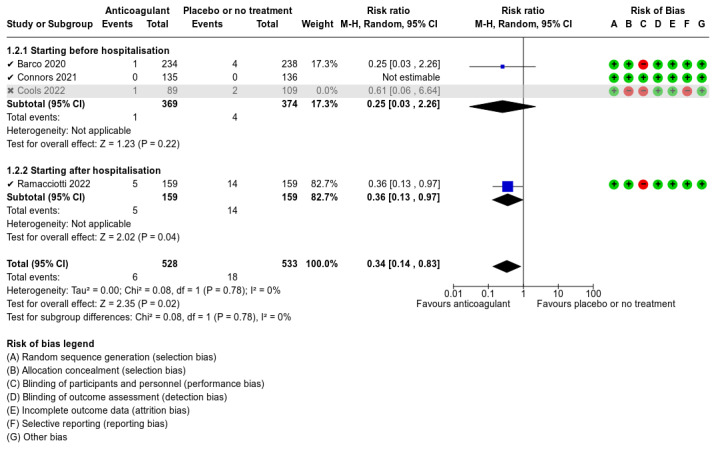
Sensitivity analysis (Venous thromboembolism): only trials at low risk of bias.
Major bleeding
Ananworanich 2021, Barco 2022, Connors 2021, and Ramacciotti 2022 reported that no major bleeding occurred during the follow‐up period, therefore we could not estimate the effect of this outcome. Cools 2022 reported data, and overall there was little or no difference in major bleeding between anticoagulant use and placebo or no treatment (RR 0.36, 95% CI 0.01 to 8.78; 5 studies; 1777 participants; I2 = not applicable; P = 0.53; low‐certainty evidence; Analysis 1.3). We used a random‐effects model for this analysis because of clinical heterogeneity among the included studies.
1.3. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 3: Major bleeding
The test for subgroup differences was not applicable because there was no event in the subgroup 'after discharge' (Analysis 1.3). Sensitivity analyses including only trials at low risk of bias were not estimable because there was no event in any of the included studies (Figure 6).
6.
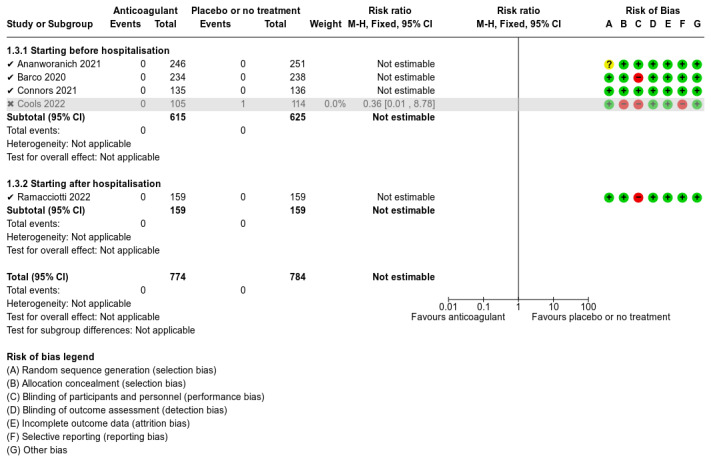
Sensitivity analysis (Major bleeding): only trials at low risk of bias.
Secondary outcomes
Deep vein thrombosis (DVT)
Ananworanich 2021 and Connors 2021 did not report this outcome. Barco 2022 reported no DVT during follow‐up. Cools 2022 and Ramacciotti 2022 reported data that overall showed little or no difference in DVT between anticoagulation use and placebo or no treatment (RR 1.02, 95% CI 0.30 to 3.46; 3 studies; 1009 participants; P = 0.98; moderate‐certainty evidence; Analysis 1.4). There was no heterogeneity among the different anticoagulant subgroups (I2 = 0). Cools 2022 reported one DVT event in the anticoagulation group at 30‐day follow‐up. The authors reported another VTE event in the standard care group at 90‐day follow‐up, but as it was not possible to establish whether it was DVT or PE, we did not include this event in our analysis. We used a random‐effects model for this analysis because of clinical heterogeneity among the included studies.
1.4. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 4: Deep vein thrombosis
The test for subgroup differences suggests that starting prophylaxis as an outpatient versus after discharge does not have a modifying effect on DVT (P = 0.96) (Analysis 1.4).
Sensitivity analyses including only trials at low risk of bias did not change the effect estimate substantially (RR 1.00, 95% CI 0.25 to 3.93) (Figure 7).
7.
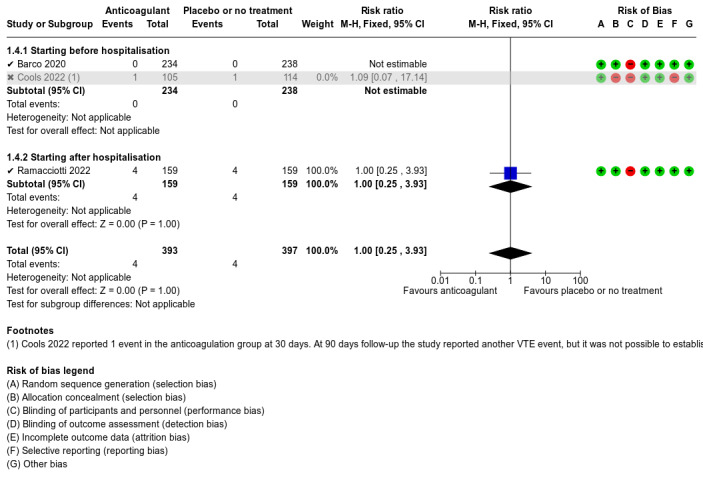
Sensitivity analysis (Deep vein thrombosis): only trials at low risk of bias.
Pulmonary embolism (PE)
Ananworanich 2021 and Connors 2021 did not report this outcome. Barco 2022, Cools 2022, and Ramacciotti 2022 reported data. Combining the data showed that anticoagulants probably decrease PE compared with placebo or no treatment (RR 0.25, 95% CI 0.08 to 0.79; 3 studies; 1009 participants; P = 0.02; NNTB = 50; moderate‐certainty evidence). We detected no heterogeneity (I2 = 0%). Cools 2022 reported one PE in the anticoagulation group at 30‐day follow‐up. The authors reported another VTE event in the standard care group at 90‐day follow‐up, but as it was not possible to establish whether it was DVT or PE, we did not include this event in our analysis. We used a random‐effects model for this analysis because of clinical heterogeneity among the included studies.
The test for subgroup differences suggests that starting prophylaxis as an outpatient versus after discharge does not have a modifying effect on PE (P = 0.84) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 5: Pulmonary embolism
Sensitivity analyses including only trials at low risk of bias did not change the effect estimate substantially (RR 0.23, 95% CI 0.07 to 0.81) (Figure 8).
8.
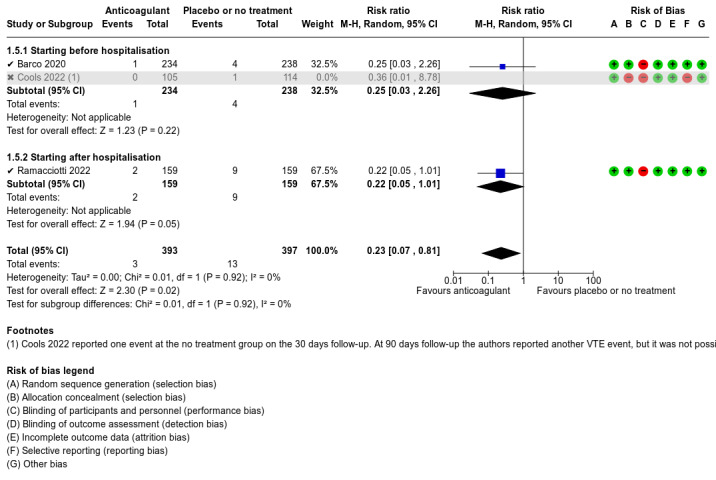
Sensitivity analysis (Pulmonary embolism): only trials at low risk of bias.
Need for hospitalisation
Ramacciotti 2022 did not report this outcome. The remaining four studies reported data (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022). Overall, there was little or no difference in need for hospitalisation with anticoagulant use compared with placebo or no treatment (RR 1.01, 95% CI 0.59 to 1.75; 4 studies; 1459 participants; P = 0.96; moderate‐certainty evidence; Analysis 1.6). There was no heterogeneity among the different anticoagulant groups (I2 = 0%). We used a random‐effects model for this analysis because of clinical heterogeneity among the included studies.
1.6. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 6: Need for hospitalisation
Lack of available data precluded subgroup analysis. Sensitivity analyses including only trials at low risk of bias did not change the effect estimate substantially (RR 0.94, 95% CI 0.43 to 2.08) (Figure 9).
9.
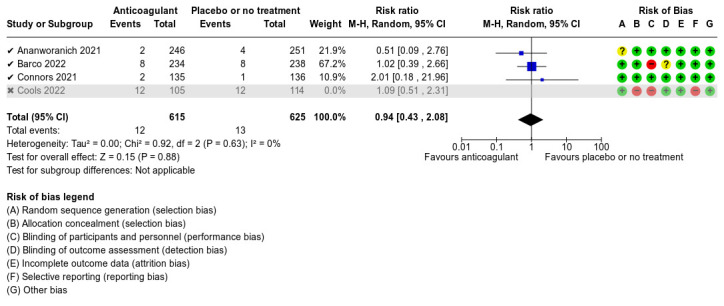
Sensitivity analysis (Need for hospitalisation): only trials at low risk of bias.
Adverse events – minor bleeding
All included studies reported this outcome. Ananworanich 2021 reported 5/246 minor bleeding events in the intervention group and 2/251 in the placebo group during the follow‐up period. Barco 2022 reported that no minor bleeding occurred during the follow‐up period. Connors 2021 reported 4/135 minor bleeding events in the anticoagulant group and 0/136 in the placebo group. Cools 2022 reported that 3/105 participants in the intervention group and 2/114 participants in the standard care group (no treatment) had minor bleeding. Ramacciotti 2022 reported 2/159 minor bleeding events in the anticoagulant group and 1/159 in the no‐treatment group. The overall effect estimate of this outcome showed little or no difference between anticoagulants and placebo or no treatment (RR 2.46, 95% CI 0.90 to 6.72; 5 studies; 1777 participants; P = 0.08; low‐certainty evidence; Analysis 1.7). We detected no heterogeneity among the studies (I2 = 0%). We used a random‐effects model for this analysis because of clinical heterogeneity among the included studies.
1.7. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 7: Adverse events (minor bleeding)
The test for subgroup differences suggests that starting prophylaxis as an outpatient versus after discharge does not have a modifying effect on minor bleeding (P = 0.85) (Analysis 1.7).
Sensitivity analyses including only trials at low risk of bias did not change the effect estimate substantially (RR 2.99, 95% CI 0.88 to 10.16) (Figure 10).
10.
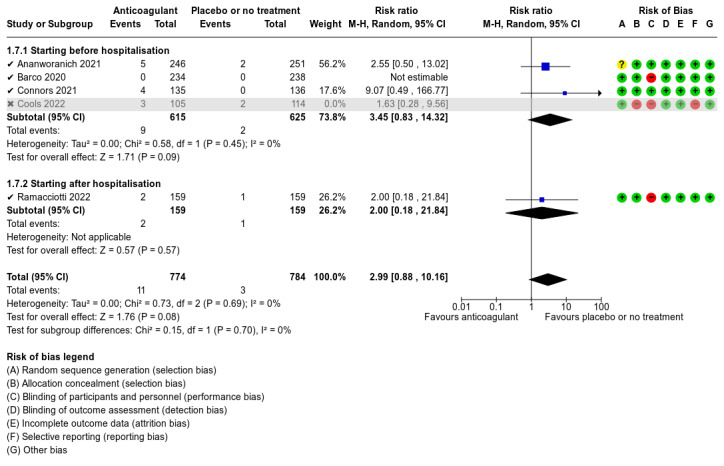
Sensitivity analysis (Adverse events (minor bleeding)): only trials at low risk of bias.
Adverse events – all
All included studies reported adverse events (AEs), but all studies reported AEs combined, therefore we could not analyse each type of adverse event separately. Ananworanich 2021 reported 35/246 AEs in the intervention group and 36/251 in the placebo group during the follow‐up period. Barco 2022 reported a total of 17 serious AEs, 8/234 in the intervention group and 9/238 in the control group. Connors 2021 reported 10/135 AEs in the anticoagulant group and 3/136 in the placebo group. Cools 2022 reported that 22/105 participants in the intervention group and 13/114 participants in the standard care group (no treatment) experienced AEs; the most common AE in both groups was COVID‐19‐related pneumonia. Ramacciotti 2022 reported 4/159 AEs in the anticoagulant group and 3/159 AEs in the no‐treatment group. The overall effect estimate of this outcome did not show a clear difference between anticoagulants and placebo or no treatment when all AEs were pooled together (RR 1.32, 95% CI 0.88 to 1.98; 5 studies; 1777 participants; P = 0.18; I2 = 25%; low‐certainty evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: Anticoagulant versus placebo or no treatment (short term), Outcome 8: Adverse events (all)
Quality of life
There were no available data for this outcome.
Higher‐dose anticoagulant versus standard dose of the same anticoagulant
Connors 2021 compared apixaban 5 mg twice daily (higher dose; intervention group) versus apixaban 2.5 mg twice daily (standard dose; control group) with a 45‐day follow‐up.
Primary outcomes
All‐cause mortality
Connors 2021 reported no deaths during the follow‐up period, therefore we could not estimate this effect with the available data (Analysis 2.1). Insufficient data precluded subgroup or sensitivity analysis.
2.1. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 1: All‐cause mortality
Venous thromboembolism (VTE)
Connors 2021 reported no VTE during the follow‐up period, therefore we could not estimate this effect (Analysis 2.2). Insufficient data precluded subgroup or sensitivity analysis.
2.2. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 2: Venous thromboembolism
Major bleeding
Connors 2021 reported no major bleeding during the follow‐up period, therefore we could not estimate this effect (Analysis 2.3). Insufficient data precluded subgroup or sensitivity analysis.
2.3. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 3: Major bleeding
Secondary outcomes
Deep vein thrombosis (DVT)
Connors 2021 reported no DVT during the follow‐up period, therefore we could not estimate this effect (Analysis 2.4). Insufficient data precluded subgroup or sensitivity analysis.
2.4. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 4: Deep vein thrombosis
Pulmonary embolism (PE)
Connors 2021 reported no PE during the follow‐up period, therefore we could not estimate this effect (Analysis 2.5). Insufficient data precluded subgroup or sensitivity analysis.
2.5. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 5: Pulmonary embolism
Need for hospitalisation
Connors 2021 reported 2/143 hospitalisations in the higher‐dose group and 1/135 hospitalisations in the standard‐dose group. There was little or no difference between higher dose and standard dose of the same anticoagulant in need for hospitalisation (RR 1.89, 95% CI 0.17 to 20.58; 1 study; 278 participants; low‐certainty evidence; Analysis 2.6). Insufficient data precluded assessment of heterogeneity or performance of subgroup or sensitivity analysis.
2.6. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 6: Need for hospitalisation
Adverse events – minor bleeding
Connors 2021 reported 2/143 events in the higher‐dose group and 4/135 events in the standard‐dose group. There was little or no difference between higher dose and standard dose of the same anticoagulant in minor bleeding (RR 0.47, 95% CI 0.09 to 2.54; 1 study; 278 participants; low‐certainty evidence; Analysis 2.7). Insufficient data precluded assessment of heterogeneity or performance of subgroup or sensitivity analysis.
2.7. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 7: Adverse events (minor bleeding)
Adverse events – all
Connors 2021 also reported different AEs combined as 13/143 events in the intervention group and 9/135 events in the control group. Data were insufficient to analyse other AEs individually. The effect estimate did not show a clear difference between higher dose and standard dose of the same anticoagulant when all AEs were pooled together (RR 1.36, 95% CI 0.60 to 3.09; 1 study; 278 participants; I2 = not applicable; moderate‐certainty evidence; Analysis 2.8).
2.8. Analysis.

Comparison 2: Anticoagulant versus a different dose of the same anticoagulant (short term), Outcome 8: Adverse events (all)
Quality of life
There were no available data for this outcome.
Anticoagulant versus antiplatelet agent
Connors 2021 compared apixaban 2.5 mg twice daily (intervention group) versus aspirin 81 mg once daily (control group) with 45‐day follow‐up data.
Primary outcomes
All‐cause mortality
Connors 2021 reported no deaths during the follow‐up period, therefore we could not estimate this effect (Analysis 3.1). Insufficient data precluded assessment of heterogeneity or performance of subgroup or sensitivity analysis.
3.1. Analysis.

Comparison 3: Anticoagulant versus antiplatelet agents (short term), Outcome 1: All‐cause mortality
Venous thromboembolism (VTE)
Connors 2021 reported little or no difference in VTE between anticoagulant and antiplatelet agent (RR 0.36, 95% CI 0.01 to 8.65; 1 study; 279 participants; low‐certainty evidence; Analysis 3.2). Insufficient data precluded assessment of heterogeneity or performance of subgroup or sensitivity analysis.
3.2. Analysis.

Comparison 3: Anticoagulant versus antiplatelet agents (short term), Outcome 2: Venous thromboembolism
Major bleeding
Connors 2021 reported no major bleeding during the follow‐up period, therefore we could not estimate this effect (Analysis 3.3). Insufficient data precluded assessment of heterogeneity or performance of subgroup or sensitivity analysis.
3.3. Analysis.

Comparison 3: Anticoagulant versus antiplatelet agents (short term), Outcome 3: Major bleeding
Secondary outcomes
Deep vein thrombosis (DVT)
Connors 2021 did not report data on this outcome.
Pulmonary embolism (PE)
Connors 2021 did not report data on this outcome.
Need for hospitalisation
Data from Connors 2021 showed little or no difference in need for hospitalisation between anticoagulant and antiplatelet agent (RR 3.20, 95% CI 0.13 to 77.85; 1 study; 279 participants; low‐certainty evidence; Analysis 3.4). Insufficient data precluded assessment of heterogeneity or performance of subgroup or sensitivity analysis.
3.4. Analysis.

Comparison 3: Anticoagulant versus antiplatelet agents (short term), Outcome 4: Need for hospitalisation
Adverse events – minor bleeding
Connors 2021 reported 4/135 events in the experimental group and 2/144 events in the control group. There was no clear difference between anticoagulant and antiplatelet agent (RR 2.13, 95% CI 0.40 to 11.46; 1 study; 279 participants; low‐certainty evidence; Analysis 3.5). Insufficient data precluded assessment of heterogeneity or performance of subgroup or sensitivity analysis.
3.5. Analysis.

Comparison 3: Anticoagulant versus antiplatelet agents (short term), Outcome 5: Adverse events (minor bleeding)
Adverse events – all
Connors 2021 also reported different AEs combined as 9/135 events in the anticoagulant group and 6/144 events in the antiplatelet agent group, but did not provide details. Data were insufficient to analyse other AEs individually. The effect estimate did not show a clear difference between groups (RR 1.35, 95% CI 0.60 to 3.06; 1 study; 279 participants; moderate‐certainty evidence; Analysis 3.6).
3.6. Analysis.

Comparison 3: Anticoagulant versus antiplatelet agents (short term), Outcome 6: Adverse events (all)
Quality of life
Connors 2021 did not report data on this outcome.
Discussion
Summary of main results
Since 2020, the COVID‐19 pandemic has affected people worldwide and has led to intense demand on healthcare systems (COVER 2022; COVIDSurg 2021a; COVIDSurg 2021b; COVIDSurg 2022a; COVIDSurg 2022b; NIHR 2022; Phelan 2020). Thromboembolic complications related to COVID‐19 have been reported worldwide throughout the entire pandemic (Lodigiani 2020; Tang 2020), and it is known that people with COVID‐19 are at high risk for VTE (Correia 2022; COVIDSurg 2022a; Flumignan 2021; Flumignan 2022b; Klok 2020a; Middeldorp 2020). The most frequently reported thrombotic events directly related to morbidity and mortality have been DVT and PE (COVIDSurg 2022a; Hanff 2020).
Current data regarding non‐hospitalised people with COVID‐19 are limited, but the incidence of thromboembolic events in this group is of possible concern and likely presents a higher thrombotic risk than has been acknowledged (Benzakoun 2020; Giannis 2021). We identified five RCTs involving a total of 1777 participants that investigated the benefits and harms of prophylactic anticoagulants in non‐hospitalised people with COVID‐19. The studies provided data on three possible comparisons: 1) anticoagulant versus placebo or no treatment; 2) anticoagulant versus a different dose of the same anticoagulant; and 3) anticoagulant versus antiplatelet agents.
Anticoagulant versus placebo or no treatment
See Table 1. All five included studies compared anticoagulants with placebo or no treatment and provided data for three of our outcomes of interest (all‐cause mortality, major bleeding, and AEs) at short‐term follow‐up (90 days) (Ananworanich 2021; Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022). Data reported by Barco 2022, Cools 2022, and Ramacciotti 2022 should be interpreted with caution given that each study was at high risk of bias for at least one of seven risk of bias domains.
Meta‐analysis showed that prophylactic anticoagulants may lead to little or no difference in reducing the risk of death, but the evidence was of low certainty. We pooled data from four studies to assess VTE (Barco 2022; Connors 2021; Cools 2022; Ramacciotti 2022). The meta‐analysis showed that anticoagulants probably decrease VTE slightly compared with placebo or no treatment (moderate‐certainty evidence). Three studies reported data on DVT and PE (Barco 2022; Cools 2022; Ramacciotti 2022). Data reported by Cools 2022 and Ramacciotti 2022 showed that anticoagulants probably result in little or no difference in DVT (moderate‐certainty evidence). Pooling data from three studies suggests that prophylactic anticoagulants probably reduce PE when compared with placebo or no treatment (moderate‐certainty evidence) (Barco 2022; Cools 2022; Ramacciotti 2022).
Four studies reported no cases of major bleeding during the study period, while one study reported no clear difference between anticoagulant prophylaxis and placebo or no treatment (low‐certainty evidence). Results showed that prophylactic anticoagulants may lead to little or no difference in minor bleeding when compared to placebo or no treatment (low‐certainty evidence). Pooling data for all AEs from five studies showed little or no difference between intervention and control groups (low‐certainty evidence).
None of the included studies reported data on quality of life.
We undertook subgroup analysis to investigate effect differences between participants when prophylaxis started before or after hospitalisation. The small number of participants and studies contributing data to each subgroup meant that the analysis was unable to produce any useful findings. No difference was detected by the test for subgroup differences for any outcome.
Anticoagulant versus a different dose of the same anticoagulant
See Table 2. One study with a 45‐day follow‐up provided data for five of our outcomes of interest (all‐cause mortality, VTE, major bleeding, need for hospitalisation, and AEs) in this comparison (Connors 2021). The study authors reported no deaths, no VTE, and no major bleeding during study follow‐up in either group, therefore we could not estimate the effect on all‐cause mortality, VTE, or major bleeding. Our analysis showed that prophylactic anticoagulants probably lead to little or no difference in reducing the need for hospitalisation (low‐certainty evidence) and all AEs (moderate‐certainty evidence) when comparing apixaban 2.5 mg twice daily with a different dose of the same anticoagulant; anticoagulants may lead to little or no difference in reducing minor bleeding events (low‐certainty evidence) for this comparison.
Anticoagulant versus antiplatelet agent
See Table 3. One study with a 45‐day follow‐up provided data for five of our outcomes of interest (all‐cause mortality, VTE, major bleeding, need for hospitalisation, and AEs) in this comparison (Connors 2021). The study authors reported no deaths and no major bleeding during follow‐up, therefore we could not estimate the effects of all‐cause mortality and major bleeding. Anticoagulants may lead to little or no difference in reducing VTE or the need for hospitalisation when compared to antiplatelet agents (low‐certainty evidence). Prophylactic apixaban may lead to little or no difference in reducing minor bleeding events when compared to aspirin (low‐certainty evidence). Apixaban may lead to little or no difference in reducing all AEs when compared with aspirin (moderate‐certainty evidence).
Overall completeness and applicability of evidence
We assessed whether the use of prophylactic anticoagulants for the treatment of people with COVID‐19 in the outpatient setting could reduce all cause‐mortality, VTE, and the need for hospitalisation safely without causing major bleeding or AEs. We also planned to evaluate other relevant parameters such as quality of life, but none of the studies reported data on this outcome.
The overall evidence was based on five studies involving 1777 eligible participants from the USA, Switzerland, Germany, Belgium, Brazil, India, South Africa, Spain, and the UK. All five studies provided data for the first comparison with placebo or no treatment as the control group. All included studies evaluated at least one of our primary outcomes. However, there were no mortality events in the studies by Ananworanich 2021, Barco 2022, and Connors 2021; no VTE in the study by Connors 2021; no major bleeding in the studies by Ananworanich 2021, Barco 2022, Connors 2021, and Ramacciotti 2022; and no DVT in the study by Barco 2022. We were only able to analyse a portion of the data from the study by Cools 2022 in the review as the study authors did not provide information regarding DVT and PE separately, therefore we could not use the complete data in our subsequent analysis of DVT and PE for the entire follow‐up period. The number of studies for each possible comparison was small, ranging from one to five studies. However, the included studies had relatively large primary sample sizes (all five studies had 219 or more participants).
We did not identify any studies comparing an anticoagulant versus a different anticoagulant or an anticoagulant versus non‐pharmacological interventions.
It is noteworthy that the studies included in this review were conducted in nine different countries, most of which (55%) were high‐income countries. Social and cultural aspects of these regions related to the evaluated interventions can also interfere with their acceptability and effectiveness for the treatment of people non‐hospitalised with COVID‐19. The external validity of the overall evidence presented in this review should therefore be interpreted with caution.
The key limitations of this review are presented below.
Some of the included studies presented a high risk of bias (Barco 2022; Cools 2022; Ramacciotti 2022).
None of the included studies presented quality of life data.
Some data were not available. We did not have access to all data in the study by Ananworanich 2021 regarding the diagnosis of VTE, DVT, and PE, therefore we did not include data for these outcomes from this study in the review. We also did not have access to all data in Connors 2021 on the occurrence of DVT and PE, therefore we did not include data for these outcomes from this study in the review. Ramacciotti 2022 did not provide data on the need for hospitalisation, therefore we did not include data for this outcome from this study in the review. Furthermore, we did not have access to all the data of interest regarding DVT and PE during the entire follow‐up period from Cools 2022.
Follow‐up was relatively short (from 35 to 90 days).
Only Connors 2021 made comparisons between anticoagulants versus a different dose of the same anticoagulant, and anticoagulants versus antiplatelet agents.
The short follow‐up (35 to 90 days) prevented long‐term analysis and impacted data for chronic complications of COVID‐19. Recent data have shown that some patients have persistent symptoms that continue or develop after the acute SARS‐CoV‐2 infection phase; this condition is known as 'long COVID' (Lee 2021; Shah 2021). Moreover, Townsend 2021 demonstrated that these individuals have prolonged elevation of D‐dimer, with an increase in serious thromboembolic events. It would therefore be important to consider a longer follow‐up to assess these outcomes in people with long COVID.
Quality of the evidence
We created a summary of findings table for each comparison using GRADEpro GDT software (GRADEpro GDT).
For the comparison prophylactic anticoagulant versus placebo or no treatment in non‐hospitalised people with COVID‐19 (Table 1), we found moderate‐certainty evidence for the majority of outcomes of interest (VTE, DVT, PE, and need for hospitalisation), and low‐certainty evidence for three of our outcomes of interest (all‐cause mortality, major and minor bleeding). We downgraded the certainty of the evidence by one either one level or two levels due to imprecision, as fewer than 300 events were included in the analysis and confidence intervals were wide. We identified high risk of bias due to a lack of allocation concealment and blinding in three studies (Barco 2022; Cools 2022; Ramacciotti 2022). However, we decided not to downgrade for risk of bias, as the effect estimate did not significantly differ after removing these studies in a sensitivity analysis. In addition, Cools 2022 adjusted the inclusion criteria during the study, but the estimates did not significantly differ after sensitivity analysis. Following GRADE recommendations, we did not downgrade for inconsistency and indirectness because heterogeneity among studies was low, and we did not find any differences in outcome measures.
For the comparison prophylactic anticoagulant versus a different dose of the same anticoagulant for non‐hospitalised people with COVID‐19 (Table 2), we found moderate‐certainty evidence for the outcomes all‐cause mortality, VTE, and major bleeding and low‐certainty evidence for need for hospitalisation and minor bleeding up to 45‐day follow‐up (short term). Following GRADE recommendations, we downgraded by one level or two levels due to imprecision, as fewer than 300 events were included in the analysis and confidence intervals were wide. The single study in this comparison demonstrated a low risk of bias (Connors 2021), therefore we did not downgrade based on risk of bias. Moreover, we did not downgrade for inconsistency because there was only one study.
For the comparison prophylactic anticoagulants versus antiplatelet agents in non‐hospitalised people with COVID‐19 (Table 3), we found moderate‐certainty evidence for the outcomes all‐cause mortality and major bleeding and low‐certainty evidence for VTE, need for hospitalisation, and minor bleeding up to 45‐day follow‐up (short term). Following GRADE recommendations, we downgraded by one level or two levels due to imprecision, as fewer than 300 events were included in the analysis and confidence intervals were wide. The study in this comparison demonstrated a low risk of bias (Connors 2021), therefore we did not downgrade based on risk of bias. Furthermore, we did not downgrade for inconsistency because there was only one study.
The risk of bias differed substantially among the included studies (Figure 2; Figure 3), and it did not affect the overall certainty of the evidence. High risk of bias in some studies was due to a lack of blinding of personnel and participants (Barco 2022; Cools 2022; Ramacciotti 2022), while in one study it was related to selective reporting (Cools 2022). We pursued a sensitivity analysis and found that excluding studies at high risk of bias did not result in changes in any of the predefined outcomes for the comparison anticoagulant versus placebo or no treatment.
Moderate–certainty evidence means that our confidence in the effect estimates is reduced because the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Including additional studies in future versions of this review will help strengthen our confidence in the findings.
Potential biases in the review process
We believe that we have identified and included all relevant RCTs by using a search methodology that included multiple sources, without restricting language, date, or status of publication. When we identified additional reports of the same study during the study selection process, we utilised the best information.
Given that we found RCTs answering our review question, we decided not to include NRS following our protocol (Santos 2022). This decision improved our evidence quality because we considered only better study designs. However, this decision may impair the estimates of rare events.
We requested additional relevant data from the study authors, but received no response to our queries. We rigorously observed the criteria described in the protocol to include or exclude participants to limit any type of non‐compliance with the protocol (Santos 2022).
We performed double data extraction to reduce bias in the review process and ensure the quality of assessment of included RCTs.
We were able to include all participants from each included study because the study authors reported the majority of our outcomes of interest.
We did not perform a funnel plot assessment because of the insufficient number of included studies (fewer than 10) in each comparison.
Agreements and disagreements with other studies or reviews
We did not find another systematic review of RCTs assessing the effects of prophylactic anticoagulants for the treatment of non‐hospitalised people with COVID‐19. Two systematic reviews assessed the effects of anticoagulant interventions for the treatment of people with COVID‐19 in the outpatient setting (Kyriakoulis 2022; Tunjungputri 2022); however, they included both NRS and RCTs, a significant difference from our review.
Kyriakoulis 2022 performed a systematic review aiming to identify published guidance reports regarding thromboprophylaxis in people with COVID‐19 in different settings (outpatients, hospitalised, and postdischarge). The authors considered only full‐text articles in English found in PubMed/Embase, and identified reports on people with COVID‐19 postdischarge (75%) and little information regarding the outpatient scenario (34%). The majority of these documents (63%) recommended thromboprophylaxis for people with a high VTE risk after hospital discharge. In the outpatient scenario, a minority of the documents (28%) recommended pharmacological thromboprophylaxis for people with a high VTE risk. However, the authors did not perform meta‐analysis and concluded that their recommendations were derived from limited evidence.
Tunjungputri 2022 aimed to provide an update on the available evidence regarding the clinical benefits and risk of oral and parenteral anticoagulants in people with COVID‐19 during hospitalisation and after discharge. They compared the use of parenteral anticoagulants with standard treatment, a comparison also made in our review. Their main outcomes included mortality and survival rates, requirement for intensive care unit and mechanical ventilation. Tunjungputri 2022 performed a systematic review searching within the Cochrane Library, EBSCO, PubMed, and Embase databases. However, they limited their search to reports in English. Of the 32 included studies, only seven were RCTs, and the population of interest was hospitalised patients with COVID‐19, which differs from our review. The study results suggest that anticoagulation may have an impact on reducing mortality in hospitalised patients (RR 0.55, 95% CI 0.43 to 0.66; P < 0.001). The other studies they considered were observational studies and case reports. Furthermore, they did not find any data regarding postdischarge COVID‐19 patients and did not include the Ramacciotti 2022 trial, one of our included studies.
It is well established that hypercoagulability is an essential hallmark of COVID‐19, and people with COVID‐19 are at higher risk of developing thromboembolic complications (Correia 2022; COVIDSurg 2022a; Kelliher 2022). In addition, several studies demonstrated that the SARS‐CoV‐2 virus causes a high level of inflammatory mediators that lead to arterial and venous wall damage, resulting in endothelial dysregulation. This process ultimately leads to coagulation and thrombosis (Goshua 2020; Matos 2011; Varga 2020).
Flumignan 2022b performed a Cochrane Review that aimed to assess the effects of prophylactic anticoagulants versus an active comparator, placebo, or no intervention, on mortality and the need for respiratory support in people hospitalised with COVID‐19. The authors stated that, when compared to a lower‐dose regimen, higher‐dose anticoagulants result in little or no difference in all‐cause mortality and increase minor bleeding in people hospitalised with COVID‐19 at 30‐day follow‐up. They also suggested that anticoagulants possibly reduce PE, but slightly increase major bleeding. However, there may be little or no difference in hospitalisation time, DVT, stroke, major adverse limb events, myocardial infarction, atrial fibrillation, and thrombocytopenia. These results in hospitalised people with COVID‐19 are similar to the results found in our analysis of non‐hospitalised people with COVID‐19.
Since the beginning of the COVID‐19 pandemic, researchers have hypothesised that anticoagulants may have a role in preventing thromboembolic events in symptomatic patients with COVID‐19 (Cuker 2021; Moores 2020; Spyropoulos 2020). The current guidelines do not recommend initiation of antiplatelet or direct oral anticoagulant therapy in non‐hospitalised symptomatic patients with COVID‐19, and also do not recommend their continuation after hospital discharge (NIH 2022; Schulman 2022). Ramacciotti 2020 recommended that VTE prophylaxis should be considered after discharge based on risk stratification for thrombotic and haemorrhagic risk for each case. Moreover, patients with an elevated risk of VTE and a low risk of bleeding should be considered for extended prophylaxis (up to 45 days). That recommendation is similar to the Italian Society on Thrombosis and Haemostasis (SISET) guidelines, which state that thromboprophylaxis should be maintained at home for 7 to 14 days postdischarge or in the pre‐hospital scenario in the case of pre‐existing or persisting VTE risk factors (Marietta 2020). Our certainty of evidence shows that prophylactic anticoagulants probably decrease the risk of VTE in non‐hospitalised people with COVID‐19 when compared with placebo or no treatment; however, the true effect may be substantially different from the estimated effect.
Authors' conclusions
Implications for practice.
We found moderate‐ to low‐certainty evidence that prophylactic anticoagulants result in little or no difference in mortality, major bleeding, deep vein thrombosis (DVT), need for hospitalisation, or adverse events (AEs) when compared with placebo or no treatment for non‐hospitalised people with COVID‐19. Moderate‐certainty evidence shows that prophylactic anticoagulants probably reduce the incidence of venous thromboembolism (VTE) and pulmonary embolism (PE) compared with placebo or no treatment.
We found moderate‐ to low‐certainty evidence that there is little or no difference between prophylactic anticoagulants and a different dose of the same anticoagulant in need for hospitalisation or AEs up to 45‐day follow‐up. Prophylactic anticoagulants may result in little or no difference in VTE, need for hospitalisation, or AEs when compared with antiplatelet agents (low‐certainty evidence). These results should be interpreted with caution given that we had only short‐term data from one study.
None of the included studies reported quality of life or compared prophylactic anticoagulant use to a different anticoagulant, or anticoagulants versus non‐pharmacological interventions. None of the included studies reported on long‐term outcomes, which could produce substantial differences in the results.
Implications for research.
Although the majority of the studies included in the review are small trials, the topic of our review is important to investigate (Handoll 2015). However, our review has limitations such as high risk of selection, performance, and reporting bias in the included studies and a very small number of less‐frequent (rare) events (e.g. VTE, DVT, PE, mortality, and AEs). To increase our confidence in the results, future trials should include the main clinical outcomes (all‐cause mortality, VTE, and major bleeding); report the outcomes separately (e.g. with DVT and PE as separate events, not VTE in general or composite scores); and be large enough to detect significant clinical outcomes. Ideally, trials should have long‐term follow‐up periods (over three years) so the long‐term effects of prophylactic anticoagulants can be investigated. This would facilitate reporting on rare outcomes, such as AEs related to anticoagulants. It could also highlight long‐term effects on chronic COVID‐19 complications because some patients have persistent symptoms that continue or develop after the acute SARS‐CoV‐2 infection phase (Lee 2021; Shah 2021). Future trials on VTE should include quality of life assessment, especially regarding long COVID, which has a major impact on quality of life.
To limit risk of performance and detection bias, the control groups should use a placebo with the same posology of the intervention. A placebo control is feasible and also improves the methodological quality of the study; we therefore hope, in the future, to include more randomised controlled trials with placebo or other active drug control.
It is of interest to differentiate the effects of anticoagulant agents in younger and older people, and in those with or without comorbidities. The use of previous antiplatelet therapy is a potential confounder and should also be reported. Large, well‐reported studies should record and report data to allow these factors to be accounted for.
History
Protocol first published: Issue 4, 2022
Notes
Parts of the Methods section of this protocol are based on a standard template established by Cochrane Vascular.
Acknowledgements
We are grateful to the following peer reviewers for their time and comments: Mark Goldin, Department of Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA; Tungki Pratama Umar, Faculty of Medicine, Universitas Sriwijaya, Indonesia.
We wish to thank Cochrane Brazil and the Division of Vascular and Endovascular Surgery of the Federal University of São Paulo, Brazil, for their methodological support.
Cochrane Vascular supported the authors in the development of this review and managed the entire editorial process from submission to acceptance. Cochrane Central Editorial Service and Cochrane Central Production Service completed the production process (Managing Editors: Helen Wakeford and Joey Kwong; Copy Editor: Lisa Winer).
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present (Date of most recent search: 18 April 2022) |
1 exp COVID‐19/ 2 COVID‐19.ti,ab. 3 COVID19.ti,ab. 4 2019 novel coronavirus infection.ti,ab. 5 coronavirus disease 19.ti,ab. 6 2019 novel coronavirus disease.ti,ab. 7 coronavirus disease 2019.ti,ab. 8 exp Coronavirus/ 9 Coronavirus*.ti,ab. 10 Deltacoronavirus*.ti,ab. 11 Delta‐coronavirus*.ti,ab. 12 or/1‐11 13 exp Anticoagulants/ 14 (anticoagul* or anti‐coagu*).ti,ab. 15 exp Heparin/ 16 heparin*.ti,ab. 17 UFH.ti,ab. 18 LMWH.ti,ab. 19 LMH.ti,ab. 20 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren).ti,ab. 21 (Clexane or klexane or lovenox).ti,ab. 22 Fragmin.ti,ab. 23 Innohep.ti,ab. 24 clivarin*.ti,ab. 25 (danaproid or danaparoid).ti,ab. 26 antixarin.ti,ab. 27 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 28 (rd 11885 or RD1185).ti,ab. 29 (Kabi‐2165 or Kabi 2165).ti,ab. 30 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169).ti,ab. 31 (fr‐860 or fr860 or cy‐216 or cy216).ti,ab. 32 (kb101 or lomoparan or orgaran).ti,ab. 33 (fluxum or lohepa or lowhepa).ti,ab. 34 (op 2123 or op2123).ti,ab. 35 (ave 5026 or ave5026).ti,ab. 36 (M118 or RO‐1).ti,ab. 37 coumar*.ti,ab. 38 (warfarin or (vitamin adj3 antagonist*)).ti,ab. 39 (VKA or coumadin* or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 40 exp Antithrombins/ 41 exp Hirudin Therapy/ 42 (thrombin adj3 inhib*).ti,ab. 43 hirudin*.ti,ab. 44 (dabigatran or Pradaxa or Rendix).ti,ab. 45 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 46 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 47 (AZD0837 or AZD‐0837).ti,ab. 48 (S35972 or S‐35972).ti,ab. 49 Factor Xa Inhibitors/ 50 Xarelto.ti,ab. 51 (Bay‐597939 or Bay597939).ti,ab. 52 PRT054021.ti,ab. 53 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 54 (DU‐176b or DU176b).ti,ab. 55 (PRT‐054021 or PRT054021).ti,ab. 56 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 57 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 58 etexilate.ti,ab. 59 agatroban.ti,ab. 60 or/13‐59 61 12 and 60 |
April 2022: 3892 |
| 2. EMBASE via Ovid (Date of most recent search: 18 April 2022) |
1 exp COVID‐19/ 2 COVID‐19.ti,ab. 3 COVID19.ti,ab. 4 2019 novel coronavirus infection.ti,ab. 5 coronavirus disease 19.ti,ab. 6 2019 novel coronavirus disease.ti,ab. 7 coronavirus disease 2019.ti,ab. 8 exp Coronavirus/ 9 Coronavirus*.ti,ab. 10 Deltacoronavirus*.ti,ab. 11 Delta‐coronavirus*.ti,ab. 12 or/1‐11 13 Anticoagulants/ 14 (anticoagul* or anti‐coagu*).ti,ab. 15 exp Heparin/ 16 heparin*.ti,ab. 17 UFH.ti,ab. 18 LMWH.ti,ab. 19 LMH.ti,ab. 20 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren).ti,ab. 21 (Clexane or klexane or lovenox).ti,ab. 22 Fragmin.ti,ab. 23 Innohep.ti,ab. 24 clivarin*.ti,ab. 25 (danaproid or danaparoid).ti,ab. 26 antixarin.ti,ab. 27 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 28 (rd 11885 or RD1185).ti,ab. 29 (Kabi‐2165 or Kabi 2165).ti,ab. 30 (emt‐966 or emt966 or emt‐967 or emt977 or pk‐10169 or pk10169).ti,ab. 31 (fr‐860 or fr860 or cy‐216 or cy216).ti,ab. 32 (kb101 or lomoparan or orgaran).ti,ab. 33 (fluxum or lohepa or lowhepa).ti,ab. 34 (op 2123 or op2123).ti,ab. 35 (ave 5026 or ave5026).ti,ab. 36 (M118 or RO‐1).ti,ab. 37 coumar*.ti,ab. 38 (warfarin or (vitamin adj2 antagonist*)).ti,ab. 39 (VKA or coumadin* or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).ti,ab. 40 exp Antithrombins/ 41 exp Hirudin Therapy/ 42 (thrombin adj3 inhib*).ti,ab. 43 hirudin*.ti,ab. 44 (dabigatran or Pradaxa or Rendix).ti,ab. 45 (BIBR‐953* or BIBR953* or BIBR‐1048 or BIBR1048).ti,ab. 46 (ximelagatran or Exanta or Exarta or melagatran).ti,ab. 47 (AZD0837 or AZD‐0837).ti,ab. 48 (S35972 or S‐35972).ti,ab. 49 Factor Xa Inhibitors/ 50 Xarelto.ti,ab. 51 (Bay‐597939 or Bay597939).ti,ab. 52 PRT054021.ti,ab. 53 (BMS‐562247 or BMS‐562247 or ELIQUIS).ti,ab. 54 (DU‐176b or DU176b).ti,ab. 55 (PRT‐054021 or PRT054021).ti,ab. 56 (YM150 or YM‐150 or LY517717 or LY‐517717 or DU‐176b or DU176*).ti,ab. 57 (GW813893 or "Tak 442" or TAK442 or PD0348292 or GSK‐813893 or GSK813893).ti,ab. 58 etexilate.ti,ab. 59 agatroban.ti,ab. 60 or/13‐59 61 12 and 60 |
April 2022: 7555 |
| 3. CINAHL via Ebsco (Date of most recent search: 18 April 2022) |
S40 S13 AND S39 S39 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 S38 TX agatroban S37 TX etexilate S36 TX Xarelto S35 TX Factor Xa Inhibitor* S34 TX dabigatran or Pradaxa or Rendix S33 TX hirudin* S32 TX thrombin inhib* S31 TX Antithrombins S30 TX VKA or coumadin* or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin S29 TX vitamin K antagonist* S28 TX warfarin S27 TX coumar* S26 TX danaproid or danaparoid S25 TX clivarin* S24 TX Innohep S23 TX Fragmin S22 TX Clexane or klexane or lovenox S21 TX Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren S20 TX LMH S19 TX LMWH S18 TX UFH S17 TX heparin* S16 (MH "Heparin+") S15 TX anticoagul* or anti‐coagu* S14 (MH "Anticoagulants+") S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S12 TX Delta‐coronavirus*. S11 TX Deltacoronavirus* S10 TX Coronavirus* S9 (MH "Coronavirus+") S8 (MH "SARS‐CoV‐2") S7 TX coronavirus disease 2019 S6 TX 2019 novel coronavirus disease S5 TX coronavirus disease 19 S4 TX 2019 novel coronavirus infection S3 TX COVID19 S2 TX COVID‐19 S1 (MH "COVID‐19") |
April 2022: 725 |
| 4. VASCULAR REGISTER IN CRSW (Date of most recent search: 18 April 2022) |
#1 COVID‐19 OR COVID19 OR 2019 novel coronavirus infection OR coronavirus disease 19 OR 2019 novel coronavirus disease OR coronavirus disease 2019 OR Coronavirus* OR Deltacoronavirus* OR Delta‐coronavirus* AND INREGISTER | April 2022: 45 |
| 5. CENTRAL via CRSO (Date of most recent search: 18 April 2022) |
#1 MESH DESCRIPTOR COVID‐19 EXPLODE ALL TREES 1004 #2 COVID‐19:TI,AB,KY 8293 #3 COVID19:TI,AB,KY 408 #4 (2019 novel coronavirus infection):TI,AB,KY 3 #5 (coronavirus disease 19):TI,AB,KY 49 #6 (2019 novel coronavirus disease):TI,AB,KY 4 #7 (coronavirus disease ):TI,AB,KY 3060 #8 MESH DESCRIPTOR Coronavirus EXPLODE ALL TREES 616 #9 Coronavirus*:TI,AB,KY 5137 #10 Deltacoronavirus*:TI,AB,KY 1 #11 Deltacoronavirus*:TI,AB,KY 1 #12 Delta‐coronavirus*:TI,AB,KY 0 #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 8923 #14 MESH DESCRIPTOR Anticoagulants EXPLODE ALL TREES 11803 #15 (anticoagul* or anti‐coagu*):TI,AB,KY 14242 #16 MESH DESCRIPTOR Heparin EXPLODE ALL TREES 4863 #17 heparin*:TI,AB,KY 12316 #18 UFH:TI,AB,KY 751 #19 LMWH:TI,AB,KY 1453 #20 LMH:TI,AB,KY 9 #21 (Ariven or Arteven or Calcilean or Hepalean or Hepathrom or Leparan or Lipo‐Hepin or Liquaemin or Liquemin or Pabyrin or Pularin or Thromboliquine or Vetren):TI,AB,KY 14 #22 (Clexane or klexane or lovenox):TI,AB,KY 177 #23 Fragmin:TI,AB,KY 224 #24 Innohep:TI,AB,KY 37 #25 clivarin*:TI,AB,KY 22 #26 (danaproid or danaparoid):TI,AB,KY 54 #27 antixarin:TI,AB,KY 2 #28 (Zibor or cy 222 or embolex or monoembolex):TI,AB,KY 38 #29 (fluxum or lohepa or lowhepa):TI,AB,KY 15 #30 coumar*:TI,AB,KY 389 #31 warfarin:TI,AB,KY 4906 #32 (vitamin k antagonist*):TI,AB,KY 1064 #33 (VKA or coumadin* or phenindione or Sinthrome or nicoumalone or phenprocoumon or Marcumar or Falithrom or AVK or phenprocoumon* or aldocumar or carfin or jantoven or kumatox or lawarin or marevan or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin):TI,AB,KY 945 #34 MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES 2289 #35 MESH DESCRIPTOR Hirudin Therapy EXPLODE ALL TREES 75 #36 (thrombin inhib*):TI,AB,KY 546 #37 hirudin*:TI,AB,KY 483 #38 (dabigatran or Pradaxa or Rendix):TI,AB,KY 1068 #39 (ximelagatran or Exanta or Exarta or melagatran):TI,AB,KY 182 #40 MESH DESCRIPTOR Factor Xa Inhibitors EXPLODE ALL TREES 1091 #41 Xarelto:TI,AB,KY 85 #42 etexilate:TI,AB,KY 327 #43 agatroban:TI,AB,KY 2 #44 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 28554 #45 #13 AND #44 279 |
April 2022: 279 |
| 6. Clinicaltrials.gov (Date of most recent search: 18 April 2022) |
COVID‐19 OR COVID19 OR 2019 novel coronavirus infection OR coronavirus disease 19 OR 2019 novel coronavirus disease OR coronavirus disease 2019 OR Coronavirus* OR Deltacoronavirus* OR Delta‐coronavirus* | Anticoagulants* OR Heparin OR LMWH OR warfarin OR Factor Xa Inhibitors OR Antithrombins OR coumarin | April 2022: 117 |
| 7. ICTRP Search Portal (Date of most recent search: 18 April 2022) |
COVID‐19 OR COVID19 OR 2019 novel coronavirus infection OR coronavirus disease 19 OR 2019 novel coronavirus disease OR coronavirus disease 2019 OR Coronavirus* OR Deltacoronavirus* OR Delta‐coronavirus* | Anticoagulants* OR Heparin OR LMWH OR warfarin OR Factor Xa Inhibitors OR Antithrombins OR coumarin | April 2022: 80 |
| 8. LILACS (Date of most recent search: 18 April 2022) |
COVID‐19 OR COVID19 OR Coronavirus OR Deltacoronavirus OR Delta‐coronavirus [Palavras] and Anticoagulants OR Heparin OR LMWH OR warfarin OR Factor Xa Inhibitors OR Antithrombins OR coumarin [Palavras] | April 2022: 75 |
| 9. COVID Cochrane Register (Date of most recent search: 18 April 2022) |
April 2022: 2571 | |
| TOTAL before de‐duplication | April 2022: 15339 | |
| TOTAL after de‐duplication | April 2022: 9347 | |
Data and analyses
Comparison 1. Anticoagulant versus placebo or no treatment (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 5 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.04, 3.61] |
| 1.1.1 As an outpatient | 4 | 1459 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.07, 17.14] |
| 1.1.2 After discharge | 1 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.05] |
| 1.2 Venous thromboembolism | 4 | 1259 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.16, 0.85] |
| 1.2.1 As an outpatient | 3 | 941 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.08, 1.90] |
| 1.2.2 After discharge | 1 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.13, 0.97] |
| 1.3 Major bleeding | 5 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.01, 8.78] |
| 1.3.1 As an outpatient | 4 | 1459 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.01, 8.78] |
| 1.3.2 After discharge | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4 Deep vein thrombosis | 3 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.46] |
| 1.4.1 As an outpatient | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.07, 17.14] |
| 1.4.2 After discharge | 1 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.25, 3.93] |
| 1.5 Pulmonary embolism | 3 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.79] |
| 1.5.1 Starting before hospitalisation | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.05, 1.72] |
| 1.5.2 Starting after hospitalisation | 1 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 1.01] |
| 1.6 Need for hospitalisation | 4 | 1459 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.59, 1.75] |
| 1.7 Adverse events (minor bleeding) | 5 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.90, 6.72] |
| 1.7.1 As an outpatient | 4 | 1459 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.85, 7.79] |
| 1.7.2 After discharge | 1 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.18, 21.84] |
| 1.8 Adverse events (all) | 5 | 1777 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.88, 1.98] |
| 1.8.1 As an outpatient | 4 | 1459 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.83, 2.20] |
| 1.8.2 After discharge | 1 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.30, 5.86] |
Comparison 2. Anticoagulant versus a different dose of the same anticoagulant (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.2 Venous thromboembolism | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.3 Major bleeding | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Deep vein thrombosis | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.5 Pulmonary embolism | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.6 Need for hospitalisation | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.17, 20.58] |
| 2.7 Adverse events (minor bleeding) | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.54] |
| 2.8 Adverse events (all) | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.60, 3.09] |
Comparison 3. Anticoagulant versus antiplatelet agents (short term).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.2 Venous thromboembolism | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.65] |
| 3.3 Major bleeding | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4 Need for hospitalisation | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.13, 77.85] |
| 3.5 Adverse events (minor bleeding) | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.40, 11.46] |
| 3.6 Adverse events (all) | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.60, 3.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ananworanich 2021.
| Study characteristics | ||
| Methods | Prospective, multicentre, 2‐armed, parallel‐assignment RCT | |
| Participants |
Number of participants: 497 Age: ≥ 18 years Gender: female (267 participants) and male (177 participants) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: rivaroxaban (one 10 mg tablet), orally daily for 21 consecutive days Control: placebo‐equivalent (multivitamin, 1 tablet), orally daily for 21 consecutive days |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Funding | Bill & Melinda Gates Medical Research Institute | |
| Declaration of interest | EG reports support from AstraZeneca, Genentech, Regeneron, TEVA, and DBV; consulting fees from P&T AscellaHealth; payment/honoraria from TEVA (Digihaler) and Genentech (omalizumab); and serving on Regeneron Pediatric Asthma Advisory Board and ACAAI Patient Advocacy Board. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation is not specified |
| Allocation concealment (selection bias) | Low risk | Quote: 'Participants were randomised 1:1, stratified by site and symptom duration (< 6 days vs ≥ 6 days), to receive either rivaroxaban (one 10‐mg tablet) or placebo equivalent (multivitamin, 1 tablet) orally daily for 21 consecutive days.' Quote: 'Participants received a box delivered to their home that contained the study drug (e.g., either rivaroxaban or placebo), thermometer, pulse oximeter, nasal swab test kit and labels, and personal protective equipment. There were 12 telemedicine visits (days 1, 4, 6, 8, 10, 12, 14, 18, 21, 24, 28, and 35)' Comment: information from study article |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: 'The bottle of medication the participants receive will not identify the treatment allocation. The active or placebo bottles will be labeled for investigational use with a randomized number to maintain blinding.' Quote: 'This randomization strategy can easily be generalized beyond 3 contemporaneously available interventions and will ensure approximately equal randomization of each intervention to control, while helping to maintain blinding and minimize possible bias.' Comment: from supplementary data of the study report. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: 'Independent data monitoring committee will be convened for this study with expertise in COVID‐19 or respiratory viruses and emerging epidemics as well as biostatistics'. Comment: data from supplementary data of the study report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: 'Between 16 August 2020 and 3 February 2021, 538 adults were screened and 497 were enrolled (246 in rivaroxaban and 251 in placebo). The numbers of participants in each analysis population were similar between treatment groups. Sixty‐four participants discontinued the study and 89 discontinued the study drug (similar between groups).' Comment: information from study article |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported as prespecified |
| Other bias | Low risk | We did not find other bias in the study. |
Barco 2022.
| Study characteristics | ||
| Methods | Prospective, 2‐armed, open‐label, parallel‐group, multicentre RCT | |
| Participants |
Number of participants: 472 Age: ≥ 50 years Gender: male (255 participants) and female (217 participants) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: enoxaparin 40 mg/0.4mL syringe daily sc for 14 days Control: standard of care (no thromboprophylaxis) |
|
| Outcomes |
Planned and reported primary outcomes
Planned and reported secondary outcomes
|
|
| Funding | National Research Programme COVID‐19, University Hospital Zurich, University of Zurich, Dr‐Ing Georg Pollert, Johanna Dürmüller‐Bol Foundation | |
| Declaration of interest | SB reports institutional research grants from Concept Medical, Bard, Bentley, Boston Scientific, INARI, Sanofi, and Bayer; and personal fees from Concept Medical, Bayer, Boston Scientific, and INARI. BG reports non‐financial support and funding for an accredited continuing medical education programme from Axonlab and Thermo Fisher Scientific; personal fees and funding for an accredited continuing medical education programme from Alnylam, Pfizer, and Sanofi; funding for an accredited continuing medical education programme from Bayer, Bristol Myers Squibb, Daiichi‐Sankyo, Takeda, Octapharma, SOBI, Janssen, Novo Nordisk, Mitsubishi Pfizer, Tanabe Pharma, outside the submitted work. SVK reports grants or contracts from Bayer AG; consulting fees from Bayer, Daiichi‐Sankyo, and Boston Scientific; and payment or honoraria from Bayer, INARI Medical, MSD, Pfizer, and Bristol Myers Squibb. SS reports research grants from Edwards Lifesciences to the institution, research grants from Medtronic to the institution, research grants from Boston Scientific to the institution, research grants from Abbott to the institution, personal fees from Boston Scientific, Teleflex, BTG ‐Boston Scientific outside the submitted work. HRE reports speaker honoraria from Daiichi‐Sankyo and Bayer. DS reports employment by Sanofi‐Aventis Switzerland. DD reports research support from German Research Foundation, CytoSorbents, Haemonetic; consulting and speaker's fees from Bayer Healthcare, Daiichi‐Sankyo, LEO Pharma, AstraZeneca, Boston Scientific, and BMS‐Pfizer. NK reports institutional research grants from Concept Medical, Bard, Bentley, Boston Scientific, INARI, Sanofi, and Bayer; and personal fees from Concept Medical, Bayer, Boston Scientific, and INARI | |
| Notes | Quote: "The funder of the study had no role in study design, data collection, management, data analysis, data interpretation, or writing of the report." Comment: information from study article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was computer‐generated and integrated into the electronic data capture software RedCAP (Vanderbild University, version 9.1.24)." Comment: information from the study article |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible participants underwent block‐stratified randomisation (by age group 50 – 70 vs > 70 years and by study centre) in a 1:1 ratio to receive either enoxaparin or standard of care (no thromboprophylaxis). " Quote: ''Participants and study personnel were aware of treatment allocation, but not of the allocation sequence." Comment: information from the study article |
| Blinding of participants and personnel (performance bias) | High risk | Quote: 'Participants and study personnel were aware of treatment allocation, but not of the allocation sequence.' Comment: information from the study article |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: 'An independent data and safety monitoring board (DSMB) composed of a vascular medicine specialist, a respiratory physician, and a clinical biostatistician monitored the trial.' Quote: 'At the beginning of the study, the sponsor planned independent monitoring of the trial in collaboration with the deputed division of the Clinical Trial Centre of the University Hospital Zurich. Monitoring was done remotely during the lockdown periods, or by visiting study sites thereafter, and consisted of a site initiation visit followed by regular visits based on the number of patients enrolled.' Comment: information from the study article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between Aug 15, 2020 and Jan, 14, 2022, from 3319 participants prescreened, 475 with acute symptomatic COVID‐19 scheduled for an ambulatory treatment were enrolled in the trial and randomly assigned to receive prophylactic‐dose enoxaparin versus standard of care (no anticoagulation). Quote: 'The final intention‐to‐treat population consisted of 472 patients: 234 received enoxaparin and 238 no thromboprophylaxis.' Comment: information from the study article |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported as prespecified |
| Other bias | Low risk | We did not find other bias in the study. |
Connors 2021.
| Study characteristics | ||
| Methods | Prospective, multicentre, double‐blind, placebo‐controlled RCT | |
| Participants |
Number of participants: 657 Age: between 40 and 80 years Gender: female (388 participants) and male (269 participants) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention 1: apixaban 2.5 mg orally twice a day for 45 days Intervention 2: apixaban 5.0 mg orally twice a day for 45 days Intervention 3: aspirin 81 mg orally twice a day for 45 days Control: placebo orally twice a day for 45 days | |
| Outcomes |
Planned primary outcome
Reported primary outcomes
Secondary outcomes
|
|
| Funding | Funded in part by NIH Agreement 1OT2HL156812‐01. Specifically, the ACTIV‐4B trial was supported by Other Transition Authorities from the NHLBI. Grantee institutions included the University of Pittsburgh; the University of Illinois Chicago; and the Brigham and Women’s Hospital. The trial drugs and matching placebo were donated by the Bristol Myers Squibb‐Pfizer Alliance. | |
| Declaration of interest | Dr Connors reported receiving personal fees from Bristol Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi, and that his institution has received research funding from CSL Behring. Dr Brooks reported receiving personal fees for data and safety monitoring board membership from Cerus Corporation. Dr Krishnan reported receiving grants from Sergey Brin Family Foundation Research in COVID. Dr Bledsoe reported receiving grants payable to his institution from the NIH for clinical trial work and receiving consulting fees from JAJ LLC. Dr Kirwan reported receiving grants from SOCAR Research SA. Dr Everett reported receiving consulting fees from Johnson & Johnson, Gilead, and Merck. Dr Hou reported receiving grants from Brigham and Women’s Hospital, NIH, Novartis, and CalciMedica. Dr Haight reported receiving grants and non‐financial support from OneFlorida. Dr Wilson reported receiving personal fees from Pfizer, Bristol Myers Squibb, Alexion, Janssen, and Paratek and receiving grants from Gilead. Dr Ridker reported receiving grants from Bristol Myers Squibb and Pfizer and serving as a consultant for work unrelated to this study for Corvidia, Novartis, Flame, Agepha, Inflazome, AstraZeneca, Janssen, Civi Biopharm, SOCAR, Novo Nordisk, Uptton, Omeicos, and Boehringer Ingelheim. No other authors reported disclosures. | |
| Notes | Quote: "The trial drugs and matching placebo were donated by the Bristol Myers Squibb‐Pfizer Alliance." Quote: "The National Heart, Lung, and Blood Institute had no role in the collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication." Comment: information from the study article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Randomisation code lists were computer generated using permuted blocks with block size equal to 4 during the process of drug labelling and then implemented electronically through the central electronic data‐capture system.' Comment: information from the study article. |
| Allocation concealment (selection bias) | Low risk | Quote: 'Participants were randomised centrally in a 1:1:1:1 ratio to receive aspirin (81 mg once daily) with matching placebo, prophylactic‐dose apixaban (2.5 mg twice daily), apixaban at therapeutic dose (5 mg twice daily), or placebo twice daily.' Quote: 'After randomisation, drug was shipped directly to the participant’s home with subsequent follow‐up conducted by the RCC central study staff.' Comment: information from the study article. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: 'To try to ensure consistency across sites in the setting of an outpatient COVID‐19 trial, all post‐randomisation participant contact was conducted by weekly electronic links to REDCap surveys or by the Research Communication Center (RCC) at the University of Illinois Chicago with live telephone calls by call centre agents and research pharmacists in Chicago and Pittsburgh. Direct shipment of study drug to participants homes, end point and safety adjudication, and 24‐hour emergency and unblinding services were provided by investigators at the Brigham and Women’s Hospital in Boston.' Comment: information from the study article. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: 'Medical records were sent to the Clinical Endpoints Committee, who adjudicated events using standardized criteria. Both the medical monitors and the end points committee were unaware of randomised drug assignment.' Comment: information from the study article. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers of participants in each analysis population were similar between treatment groups. Also, the dropouts were reported and also similar between groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported asprespecified. |
| Other bias | Low risk | We did not find other bias in the study. |
Cools 2022.
| Study characteristics | ||
| Methods | Prospective, open‐label, multicentre, multinational RCT | |
| Participants |
Number of participants: 219 Age: ≥ 50 years Gender: male (122 participants) and female (97 participants) Planned inclusion criteria
Exclusion criteria
Reported inclusion criteria
|
|
| Interventions | Intervention: enoxaparin 40 mg once daily sc if weight < 100 kg and 40 mg twice daily if weight ≥ 100 kg for 21 days Control: current standard of care (without enoxaparin) | |
| Outcomes |
Planned primary outcomes
Secondary outcomes
Reported primary outcomes
Secondary outcomes
|
|
| Funding | The Thrombosis Research Institute and Sanofi UK | |
| Declaration of interest | FC reports speaker fees from Boehringer Ingelheim Pharma, Bayer, Pfizer, and Daiichi‐Sankyo Europe, and a modest research grant from Daiichi‐Sankyo Europe. JS reports personal fees from Pfizer, AstraZeneca, Novartis, Sanofi, BMS, Dr Reddy's Laboratory, Lupin, and Abbott. RDL reports research grants from Bristol Myers Squibb, Pfizer, Amgen, GlaxoSmithKline, Medtronic, and Sanofi Aventis, and personal fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, and Bayer, outside the submitted work. BJ reports personal fees from Bayer Healthcare and Sanofi‐Aventis. JIA reports speaker fees from Sanofi, Rovi, Bayer, and Aspen. FDRH acknowledges part support as Director of the National Institute for Health and Care Research (NIHR) Applied Research Collaboration Oxford Thames Valley and Theme Lead of the NIHR Oxford University Hospital Biomedical Research Centre, and has also received occasional fees or expenses for speaking or consultancy from AstraZeneca, Boehringer Ingelheim, Bayer, BMS/Pfizer, and Novartis. HG reports personal fees from Pfizer, Bayer, and Boehringer Ingelheim. PM reports honoraria from Bayer Pharma and Portolo. SS reports speaker fees from Bayer Pharma, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi‐Sankyo, Sanofi Aventis, and Pfizer, and consultancy fees from Bayer Pharma, Boehringer Ingelheim, Daiichi‐Sankyo, Sanofi Aventis, Aspen, and Pfizer. SH reports personal fees from Bayer, Bristol Myers Squibb, Daiichi‐Sankyo, Portola, and Sanofi, outside the submitted work. AGGT reports grants from Bayer Healthcare and personal fees from Bayer Healthcare, Bristol Myers Squibb/Pfizer, Daiichi‐Sankyo, and Boehringer Ingelheim, outside the submitted work. WA reports honoraria from Bayer Pharma, Bristol Myers Squibb, Pfizer, Daiichi‐Sankyo, Portola, Aspen, Sanofi, Leo Pharma, Norgine, and Werfen. ATR reports consultancy fees from Bayer Pharma, Daiichi‐Sankyo, Sanofi, Aspen, and Pfizer. KP reports a consultancy fee from Johnson & Johnson. AKK reports research grants from Anthos, Bayer, and Sanofi and personal fees from Anthos Therapeutics, Bayer, and Sanofi. All other authors declare no competing interests. | |
| Notes | Quote: "Sanofi funded this investigator‐sponsored study and provided enoxaparin free of charge. The Thrombosis Research Institute had input in study design, data collection, data analysis, data interpretation, and writing of the report." Comment: information from the study article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'The randomisation sequence was established before enrolment of the first patient and was done by use of a prespecified, secure, central, web‐based randomisation system.' Quote: 'Randomisation was generated with a random block design (block size either 2 or 4), blocking within each site to allow equal allocation of the two treatments within each site.' Comment: information from the study article. |
| Allocation concealment (selection bias) | High risk | Quote: 'The study was unblinded and therefore no allocation concealment was applied.' Comment: information from the study article. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: 'The study was unblinded and therefore no allocation concealment was applied.' Comment: information from the study article. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: 'The Clinical Events Committee were responsible for systematically adjudicating death, hospitalisation, and the classification of bleeding in a blinded way to the treatment assignment according to predefined clinical outcome definitions.' Comment: information from the study article. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: 'Of 230 patients with COVID‐19 assessed for eligibility, 219 were randomly assigned to receive either standard of care (114 (52%) ‐ 109 (95%)) included in per‐protocol analysis) or enoxaparin (105 (48%)) ‐ 89 (85%) included in per‐protocol analysis). At 21 days, in the intention‐to‐treat analysis, the composite of all‐cause mortality and all‐cause hospitalisation was observed in 12 (11%) of 105 patients
in the enoxaparin group and in 12 (11%) of 114 patients in the standard‐of‐care group (unadjusted hazard ratio 1.09 [95% CI 0·49 – 2·43); log‐rank P = 0·83; figure 2; table 2)''. Comment: information from the study article. |
| Selective reporting (reporting bias) | High risk | Quote: 'Due to the very low number of events and the study's early termination, only the primary efficacy outcome was tested for statistical significance. Median follow‐up for the primary efficacy outcome was 21 days (IQR 21–21) for both groups.' Quote: 'Adjustments to the inclusion criteria were made during the course of the study.' Comment: information from the study article. |
| Other bias | Low risk | We did not find other bias in the study. |
Ramacciotti 2022.
| Study characteristics | ||
| Methods | Prospective, open‐label, multicentre RCT | |
| Participants |
Number of participants: 320 Age: between 18 and 90 years old Gender: male (191 participants) and female (127 participants) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: rivaroxaban 10 mg once daily for 35 days post‐hospital discharge Control: no intervention |
|
| Outcomes |
Planned and reported Primary outcomes:
Secondary outcomes:
Other outcomes:
|
|
| Funding | Bayer | |
| Declaration of interest | ER reports grants and consulting fees from Bayer and Pfizer; grants from the Brazilian Ministry of Science and Technology; and personal fees from Aspen Pharma, Biomm Pharma, and Daiichi‐Sankyo, outside of the submitted work. LBA reports grants from Bayer, Pfizer, and the Brazilian Ministry of Science and Technology. DC reports personal fees from Bayer, Janssen, Daiichi‐Sankyo, and Pfizer; and grants from Stago. ACS reports consulting fees from Janssen Research & Development, Bayer, Portola, Boehringer Ingelheim, Bristol Myers Squibb, and ATLAS group; and grants from Janssen and Boehringer Ingelheim. MLS reports personal fees from Bayer, Pfizer, and Sanofi. EEJ reports consulting and personal fees form Bayer. CD reports consulting and personal fees from Bayer, Novartis, and Daiichi‐Sankyo. SMVS reports personal fees from Bayer. RCC reports personal fees from Boehringer Ingelheim and AstraZeneca. ATaf reports personal fees from Janssen and Recovery Force and grants from Bio Tap, Idorsia, Bristol Myers Squibb, Novo Nordisk, Janssen, and Doasense. RDL reports grants and personal fees from Bristol Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and personal fees from Amgen, Bayer, and Boehringer Ingelheim, outside of the submitted work. All other authors declare no competing interests. | |
| Notes | Quote: "The study funder had no role in the planning and design of the study, data collection, analysis, and interpretation, nor writing of the manuscript." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Randomisation was done in permuted blocks of variable size, using a central, concealed, web‐based, automated randomisation system (RedCap, version 11.0.3).' Comment: information from the study article. |
| Allocation concealment (selection bias) | Low risk | Quote: 'Patients were randomly allocated in a 1:1 ratio to receive either thromboprophylaxis with rivaroxaban 10 mg/day or regular follow‐up (no anticoagulation) for 35 days.' Quote: 'Randomisation was done in permuted blocks of variable size, using a central, concealed, web‐based, automated randomisation system (RedCap, version 11.0.3).' Comment: information from the study article. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: 'The MICHELLE trial was an open‐label study, with no masking of investigators or patients to group allocation.' Comment: information from the study article. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: 'An independent clinical events classification committee, whose members were unaware of the study treatment assignment, adjudicated all venous and arterial thromboembolic and bleeding events, and causes of death. ' Comment: information from the study article. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote 'From Oct 8, 2020, to June 29, 2021, 997 patients were screened. Of these patients, 677 did not meet eligibility criteria; the remaining 320 patients were enrolled and randomly assigned to receive rivaroxaban (n = 160 (50%)) or no anticoagulation (n = 160 (50%). Thus, 159 patients per group were included in the intention‐to‐treat analysis.' |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Other bias | Low risk | We did not find other bias in the study. |
AE: adverse events BMI: body mass index CD4: cluster of differentiation 4 COPD: chronic obstructive pulmonary disease COVID‐19: coronavirus disease 2019 CYP3A: cytochrome P450 3A DOAC: direct oral anticoagulant DM: diabetes mellitus DVT: deep vein thrombosis ECG: electrocardiogram eGFR: estimated glomerular filtration rate GI: gastrointestinal GFR: glomerular filtration rate ICU: intensive care unit IMPROVE: International Medical Prevention Registry on Venous Thromboembolism ISTH: International Society on Thrombosis and Haemostasis LMWH: low‐molecular‐weight heparin NHLBI: National Heart, Lung, and Blood Institute NIH: National Institutes of Health PAD: peripheral artery disease PCI: percutaneous coronary intervention PE: pulmonary embolism P‐gp: permeability glycoprotein RCT: randomised controlled trial RT‐PCR: reverse transcription polymerase chain reaction SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 sc: subcutaneously TRI: Translational Research Institute UFH: unfractionated heparin VKA: vitamin K antagonist VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghamohammadi 2020 | Ineligible study design: case report |
| Borghi 2021 | Ineligible study design: case report |
| ChiCTR2000034796 | Ineligible study design: non‐RCT and observational study |
| JPRN‐UMIN000042489 | Ineligible study design: non‐RCT |
| Kuno 2022 | Ineligible study design: non‐RCT and observational study |
| Lisker 2021 | Ineligible study design: non‐RCT |
| Rivera‐Caravaca 2021 | Ineligible study design: non‐RCT |
| Sharma 2021 | Ineligible study design: hospitalised patients |
| Spyropoulos 2021 | Ineligible study design: hospitalised patients |
| Vergori 2021 | Ineligible study design: non‐RCT and hospitalised patients |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Capell 2021.
| Study name | A study of rivaroxaban to reduce the risk of major venous and arterial thrombotic events, hospitalisation and death in medically ill outpatients with acute, symptomatic coronavirus disease 2019 (COVID‐19) infection (PREVENT‐HD) |
| Methods | Double‐blind, placebo‐controlled, pragmatic, event‐driven RCT |
| Participants |
Number of participants: 4000 Age: ≥ 18 years Gender: male or female Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: rivaroxaban 10 mg once daily for 35 days Control: placebo once daily for 35 days |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 13 August 2020 |
| Contact information | warren.capell@cuanschutz.edu |
| Notes |
EUCTR2020‐005884‐29‐IT.
| Study name | Role of heparin in the prevention of thromboembolism in patients with COVID‐19 and respiratory failure |
| Methods | Prospective, multicentre, 2‐armed, parallel‐assignment RCT |
| Participants |
Number of participants: 652 Age: ≥ 18 years Gender: female and male Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: parnaparin, dalteparin, nadroparin, bemiparin, reviparin, or enoxaparin Control: standard LMWH prophylaxis |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 28 April 2021 |
| Contact information | mariacristina.vedovati@unipg.it |
| Notes |
NCT04542408.
| Study name | Hamburg edoxaban for anticoagulation in COVID‐19 study (HERO‐19) |
| Methods | Prospective, double‐blind RCT |
| Participants |
Number of participants: 172 Age: ≥ 18 years Gender: male and female Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: therapeutic anticoagulation using LMWH body weight‐adapted during course of hospital stay and oral anticoagulation using edoxaban according to summary of product characteristics (60 mg once a day) after being discharged from hospital/outpatient course Control: prophylactic anticoagulation using LMWH as part of standard of care while inpatient course, and placebo after discharge/outpatient course |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 12 November 2020 |
| Contact information | s.kluge@uke.de m.karakas@uke.de |
| Notes |
NCT04650087.
| Study name | COVID‐19 thrombosis prevention trials: post‐hospital thromboprophylaxis |
| Methods | Prospective, double‐blind RCT |
| Participants |
Number of participants: 1223 Age: ≥ 18 years Gender: male or female Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: apixaban 2.5 mg twice a day, once in the morning and once in the evening for 30 days Control: placebo twice a day, once in the morning and once in the evening for 30 days |
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | 15 February 2021 |
| Contact information | tracy.wang@duke.edu |
| Notes |
NCT04715295.
| Study name | Safety and efficacy of doxycycline and rivaroxaban in COVID‐19 (DOXYCOV) |
| Methods | Prospective, open‐label RCT |
| Participants |
Number of participants: 200 Age: ≥ 18 years Gender: male or female Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: doxycycline 200 mg daily for 7 days with or without rivaroxaban 15 mg tablets daily from day 1 to day 10 Control: hydroxychloroquine 400 mg daily for 5 days in combination with azithromycin 500 mg on day 1 and 250 mg daily from day 2 through day 5 Comment: the trial plan is to use a factorial design to compare anticoagulant and control group. The co‐treatment seems to be balanced across arms. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 5 October 2020 |
| Contact information | sobngwieugene@yahoo.fr |
| Notes |
NCT04746339.
| Study name | Apixaban for prophylaxis of thromboembolic outcomes in COVID‐19 (APOLLO) |
| Methods | Prospective, multicentre, quadruple‐blind RCT |
| Participants |
Number of participants: 1000 Age: ≥ 18 years Gender: male and female Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: apixaban 2.5 mg twice daily for 30 days Control: placebo twice daily for 30 days |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 4 March 2021 |
| Contact information | renato.lopes@duke.edu |
| Notes |
NCT04757857.
| Study name | COVID‐19 antithrombotic rivaroxaban evaluation (CARE) |
| Methods | Prospective, pragmatic, open‐label RCT |
| Participants |
Number of participants: 660 Age: ≥ 18 years Gender: male and female Inclusion criteria
Exclusion criteria
|
| Interventions |
Interventions: rivaroxaban 10 mg orally once a day for 14 days Control: no intervention, receive best local standardised care |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 29 September 2020 |
| Contact information | dvilanova@haoc.com.br |
| Notes |
Ramos‐Peñafiel 2020.
| Study name | Effect of the use of anticoagulant therapy during hospitalisation and discharge in patients with COVID‐19 infection |
| Methods | Prospective, double‐blind RCT |
| Participants |
Number of participants: 130 Age: between 18 and 90 years old Gender: male and female Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: prophylactic enoxaparin arm 1 mg/kg/dose daily Control: enoxaparin therapeutic regimen arm at doses of 1 mg/kg/dose twice daily during in‐hospital stay Patients who are discharged will be randomised into the following 2 treatment arms: Intervention: rivaroxaban 10 mg orally every 24 hours Control: clinical follow‐up only |
| Outcomes |
Primary outcomes
|
| Starting date | 20 June 2020 |
| Contact information | christian.ramos.penafiel@gmail.com |
| Notes |
RBR‐7nzwkpg.
| Study name | Effects of treatment with oral anticoagulant associated with antibiotic therapy in COVID‐19 |
| Methods | Prospective, double‐blind RCT |
| Participants |
Number of participants: 160 Age: ≥ 18 years Gender: male and female Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: 1 non‐transparent bottle containing 28 tablets of edoxaban 60 mg. The prescription will obey the following dosage: 1 tablet, once daily, for 28 days. Control: 1 non‐transparent bottle containing 28 non‐transparent capsules. The capsules will contain standard placebo formulation, consisting of 0.5% magnesium stearate, 1.0% Aerosil, 30% pharmaceutical talc + starch q.s.p. The prescription will obey the following dosage: 1 capsule, once daily, for 28 days. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 24 January 2021 |
| Contact information | anna.piovezan@unisul.br |
| Notes |
ALT: alanine transaminase aPTT: activated partial thromboplastin clotting time BMI: body mass index CAD: coronary artery disease CNS: central nervous system COPD: chronic obstructive pulmonary disease CYP3A4: cytochrome P450 3A4 DVT: deep vein thrombosis ECG: electrocardiogram ECMO: extracorporeal membrane oxygenation EMR: electronic medical record ER: emergency room eGFR: estimated glomerular filtration rate FIO2: fraction of inspired oxygen G6PD: glucose‐6‐phosphate dehydrogenase GFR: glomerular filtration rate HFOV: high‐frequency oscillatory ventilation ICU: intensive care unit IgM: immunoglobulin M IMP: investigational medicinal product INR: international normalised ratio ISTH: International Society of Thrombosis and Haemostasis LMWH: low‐molecular‐weight heparin MACE: major cardiovascular events MI: myocardial infarction MVP: mechanical valve prosthesis N: normal NYHA: New York Heart Association PAD: peripheral artery disease PaO2: partial pressure of arterial oxygen PE: pulmonary embolism P‐gp: P‐glycoprotein PCR: polymerase chain reaction QTc: corrected QT interval RCT: randomised controlled trial RT‐PCR: reverse transcription polymerase chain reaction ULN: upper limit of normal VTE: venous thromboembolism WHO: World Health Organization
Differences between protocol and review
In our protocol (Santos 2022), we planned the objectives of this review as: to assess the benefits and harms of prophylactic anticoagulants versus active comparator, placebo, or no intervention in non‐hospitalised people with COVID‐19. However, in the review we amended the objective as follows: to assess the benefits and harms of prophylactic anticoagulants versus active comparator, placebo or no intervention, or non‐pharmacological interventions in non‐hospitalised people with COVID‐19, because it better reflects what we performed.
In our protocol we planned to perform subgroup analyses for all outcomes comparing different doses of drugs. At the review stage, we decided to perform an isolated comparison for this purpose because we considered it a clinical relevant comparison, and the included studies compared different doses of drugs in different groups. In our protocol we planned to perform subgroup analyses for 'days since positive COVID‐19 diagnosis'; however, at the review stage we performed subgroup analysis for 'starting prophylaxis before hospitalisation and postdischarge' because we considered this to be clinically relevant.
In our protocol we planned to include additional study types to randomised controlled trials (RCTs) if we did not identify sufficient RCT data (e.g. non‐randomised studies, cohort, retrospective, etc.). Given that we found five RCTs with more than 400 participants, we did not include any non‐RCTs.
In our protocol we planned to include number needed to treat for an additional beneficial outcome (NNTB) for the primary outcomes (all‐cause mortality, venous thromboembolism, and major bleeding), but at the review stage we reported all clinically important differences using NNTB.
We planned to report all adverse events separately; however, this was not possible due to how these were reported by the included studies. We have now reported minor bleeding as a separate outcome, as well as all other adverse events combined.
Contributions of authors
BCS: drafting of protocol, study selection, data extraction, risk of bias assessment, data analysis, and drafting of final review RLGF: drafting of protocol, study selection, data extraction, data analysis, drafting of final review, and acting as guarantor of the review VTC: drafting of protocol, data extraction, risk of bias assessment, data analysis, and drafting of final review ANA: drafting of protocol, data analysis, and drafting of final review LCUN: drafting of protocol, study selection, data extraction, data analysis, and drafting of final review
Sources of support
Internal sources
-
Division of Vascular and Endovascular Surgery, Universidade Federal de Sao Paulo, Brazil
This project received methodological support from a collaboration between Cochrane Brazil and the Division of Vascular and Endovascular Surgery.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
BCS: none known; BCS has declared that she works as a private practice vascular surgeon, dealing with both venous and arterial diseases. RLGF: none known; RLGF has declared he is professor of vascular surgery at Universidade Federal de São Paulo, Brazil. VTC: none known ANA: none known; ANA has declared he is director of Cochrane Brazil. LCUN: none known
New
References
References to studies included in this review
Ananworanich 2021 {published data only (unpublished sought but not used)}
- Ananworanich J, Mogg R, Dunne MW, Bassyouni M, David CV, Gonzalez E, et al. Randomized study of rivaroxaban vs placebo on disease progression and symptoms resolution in high-risk adults with mild COVID-19. Clinical Infectious Diseases 2021;75(1):e473-e481. [DOI: 10.1093/cid/ciab813] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04504032. A trial to evaluate safety and efficacy of rivaroxaban (COVID-19). clinicaltrials.gov/ct2/show/NCT04504032 2020. [CLINICALTRIALS.GOV: NCT04504032]
Barco 2022 {published data only (unpublished sought but not used)}
- Barco S, Bingisser R, Colucci G, Frenk A, Gerber B, Held U, et al. Enoxaparin for primary thromboprophylaxis in ambulatory patients with coronavirus disease-2019 (the OVID study): a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):770. [DOI: 10.1186/s13063-020-04678-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco S, Voci D, Bingisser R, Colucci G, Frenk A, Gerber B, et al. Enoxaparin for primary thromboprophylaxis in ambulatory patients with COVID-19: the multicentre randomized controlled investigatorinitiated OVID trial. Study design, status or enrolment and patients overview. Vasa - European Journal of Vascular Medicine 2021;50(Suppl 107):15. [Google Scholar]
- Barco S, Voci D, Bingisser R, Colucci G, Frenk A, Gerber B, et al. Enoxaparin for primary thromboprophylaxis in ambulatory patients with COVID-19: the multicentre randomized controlled investigatorinitiated OVID trial. Study design, status or enrolment and patients overview. Vasa - European Journal of Vascular Medicine 2021;50(Suppl 107):15. [DOI: 10.1024/0301-1526/a000973] [DOI] [Google Scholar]
- Barco S, Voci D, Held U, Sebastian T, Bingisser R, Colucci G, et al. Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial. The Lancet Hematology 2022;9(8):e585-e593. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Connors 2021 {published data only}
- Connors JM, Brooks MM, Sciurba FC, Krishnan JA, Bledsoe JR, Kindzelski A, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA 2021;326(17):1703-12. [DOI: 10.1001/jama.2021.17272] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert JC, Krishnan JA, Heather E, Shapiro N, O'Neal S, Baucom A, et al. A novel approach to medical monitoring during the SARS-CoV-2 pandemic supporting the ACTIV 4B outpatient anticoagulation trial. American Journal of Respiratory and Critical Care Medicine 2021;203:A1749. [DOI: ] [Google Scholar]
Cools 2022 {published data only}
- Cools F, Virdone S, Sawhney J, Lopes R, Jacobson B, Arcelus J, ETHIC investigators, et al. Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematology 2022;9(8):e594-e604. [DOI: 10.1016/S2352-3026(22)00173-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04492254. ETHIC trial: early LMWH in symptomatic COVID-19 positive patients. clinicaltrials.gov/ct2/show/NCT04492254 2020. [NCT04492254]
Ramacciotti 2022 {published data only}
- NCT04662684. Medically ill hospitalized patients for COVID-19 thrombosis extended prophylaxis with rivaroxaban therapy: the MICHELLE trial (MICHELLE). clinicaltrials.gov/ct2/show/NCT04662684 2020. [CLINICALTRIALS.GOV: NCT04662684]
- Ramacciotti E, Agati LB, Calderaro D, Volpiani GG, Oliveira CC, Aguiar VCR, et al. Medically ill hospitalized patients for COVID-19 thrombosis extended prophylaxis with rivaroxaban therapy: rationale and design of the MICHELLE trial. American Heart Journal 2021;242:115-22. [DOI: 10.1016/j.ahj.2021.08.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramacciotti E, Barile Agati L, Calderaro D, Aguiar VC, Spyropoulos AC, Oliveira CC, Michelle investigators, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet 2022;399(10319):50-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aghamohammadi 2020 {published data only}
- Aghamohammadi M, Alizargar J, Hsieh NC, Wu SV. Prophylactic anticoagulant therapy for reducing the risk of stroke and other thrombotic events in COVID-19 patients. Journal of the Formosan Medical Association 2020;119(7):1230-1. [DOI: 10.1016/j.jfma.2020.05.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borghi 2021 {published data only}
- Borghi B, Borghi R, Deamicis T, Sommariva C, Pipino G. Early use of fondaparinux at therapeutic dosage in COVID-19 infection. Minerva Anestesiologica 2021;87(8):958-60. [DOI] [PubMed] [Google Scholar]
ChiCTR2000034796 {published data only}ChiCTR2000034796
- ChiCTR2000034796. The efficacy and safety of heparin in the treatment of novel coronavirus pneumonia (COVID-19): a retrospective single center clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000034796 2020.
JPRN‐UMIN000042489 {published data only}JPRN‐UMIN000042489
- JPRN-UMIN000042489. Antiinflammatory and anticoagulative agents in COVID-19 pneumonia treatment. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000042489 2020.
Kuno 2022 {published data only}
- Kuno T, Takahashi M, So M, Egorova NN. The association of anticoagulation before admission and survival of patients with COVID-19. Journal of Cardiology 2022;79(4):489-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lisker 2021 {published data only}
- Lisker G, Narasimhan M, Greenberg H, Ramdeo R, McGinn T. Ambulatory management of moderate to high risk COVID-19 patients: the coronavirus related outpatient work navigators (CROWN) protocol. Home Health Care Management and Practice 2021;33(1):49-53. [DOI: 10.1177/1084822320964196] [DOI] [Google Scholar]
Rivera‐Caravaca 2021 {published data only}
- Rivera-Caravaca JM, Buckley BJ, Harrison SL, Fazio-Eynullayeva E, Underhill P, Marin F, et al. Direct-acting oral anticoagulants use prior to COVID-19 diagnosis and associations with 30-day clinical outcomes. Thrombosis Research 2021;205:1-7. [DOI: 10.1016/j.thromres.2021.06.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2021 {published data only}
- Sharma A, Sharma C, Raina S, Singh B, Dadhwal D, Dogra V, et al. A randomized open-label trial to evaluate the efficacy and safety of triple therapy with aspirin, atorvastatin, and nicorandil in hospitalised patients with SARS Cov-2 infection: a structured summary of a study protocol for a randomized controlled trial. Trials Journal 2021;22:451. [DOI: 10.1186/s13063-021-05361-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spyropoulos 2021 {published data only}
- Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Sharifova G, et al. Treatment-dose LMWH versus prophylactic/intermediate dose heparins in high-risk COVID-19 inpatients: rationale and design of the HEP-COVID trial. Thrombosis and Haemostasis 2021;121(12):1684-95. [DOI: 10.1055/a-1475-2351] [DOI] [PubMed] [Google Scholar]
- Spyropoulos AC, Goldin M, Dimitrios G. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19. The HEP-COVID randomized clinical trial. Heart International 2021;15(2):62-4. [PMID: 10.1001/jamainternmed.2021.6203] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergori 2021 {published data only}
- Vergori A, Lorenzini P, Cozzi-Lepri A, Donno DR, Gualano G, Nicastri E, et al. Prophylactic heparin and risk of orotracheal intubation or death in patients with mild or moderate COVID-19 pneumonia. Scientific Reports 2021;11(1):11334. [DOI: 10.1038/s41598-021-90713-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Capell 2021 {published data only}
- Capell WH, Barnathan ES, Piazza G, Spyropoulos AC, Hsia J, Bull S, et al. Rationale and design for the study of rivaroxaban to reduce thrombotic events, hospitalization and death in outpatients with COVID-19: the PREVENT-HD study. American Heart Journal 2021;235:12-23. [DOI: 10.1016/j.ahj.2021.02.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04508023. A study of rivaroxaban to reduce the risk of major venous and arterial thrombotic events, hospitalization and death in medically ill outpatients with acute, symptomatic coronavirus disease 2019 (COVID-19) infection (PREVENT-HD). clinicaltrials.gov/ct2/show/NCT04508023 (first received 11 August 2020). [CLINICALTRIALS.GOV: NCT04508023]
EUCTR2020‐005884‐29‐IT {published data only}EUCTR2020‐005884‐29‐IT
- EUCTR2020-005884-29-IT. Role of heparin in the prevention of thromboembolism in patients with COVID-19 and respiratory failure. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-005884-29-IT (first received 8 June 2021).
NCT04542408 {published data only}
- NCT04542408. Hamburg edoxaban for anticoagulation in COVID-19 Study. clinicaltrials.gov/show/NCT04542408 (first received 9 September 2020). [CLINICALTRIALS.GOV: NCT04542408]
NCT04650087 {published data only}
- NCT04650087. COVID-19 thrombosis prevention trials: post-hospital thromboprophylaxis. clinicaltrials.gov/ct2/show/NCT04650087 (first received 2 December 2020). [CLINICALTRIALS.GOV: NCT04650087]
NCT04715295 {published data only}
- NCT04715295. Safety and Efficacy of Doxycycline and Rivaroxaban in COVID-19. clinicaltrials.gov/show/NCT04715295 (first received 20 January 2021).
NCT04746339 {published data only}
- NCT04746339. Apixaban for prophylaxis of thromboembolic outcomes in COVID-19. clinicaltrials.gov/show/NCT04746339 (first received 9 February 2021). [CLINICALTRIALS.GOV: NCT04746339]
NCT04757857 {published data only}
- NCT04757857. COVID-19 antithrombotic rivaroxaban evaluation. clinicaltrials.gov/ct2/show/NCT04757857 (first received 17 February 2021). [CLINICALTRIALS.GOV: NCT04757857]
Ramos‐Peñafiel 2020 {published data only}
- NCT04508439. Effect of the use of anticoagulant therapy during hospitalization and discharge in patients with COVID-19 infection. clinicaltrials.gov/show/NCT04508439 (first received 11 August 2020). [CLINICALTRIALS.GOV: NCT04508439]
- Ramos C, Madera E, Majlufc A, Cabrera A, MacEdo D, Valencia U, et al. Effect of rivaroxaban (10mg) versus observation on the risk of thrombosis at discharge in individuals with covid-19. HemaSphere 2021;5(Suppl 2):645. [EMBASE: covidwho-1393448] [Google Scholar]
RBR‐7nzwkpg {published data only}RBR‐7nzwkpg
- RBRnzwkpg. Effects of treatment with oral anticoagulant associated with antibiotics on clinical, immunological, hospitalization and death parameters in patients with suspected Covid-19 infection and coagulation disorders. trialsearch.who.int/Trial2.aspx?TrialID=RBR-7nzwkpg (first received 6 January 2021).
Additional references
Acosta 2008
- Acosta S, Alhadad A, Svensson P, Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. British Journal of Surgery 2008;95(10):1245-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Alikhan 2014
- Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD003747. [DOI: 10.1002/14651858.CD003747.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amaral 2022
- Amaral FC, Baptista-Silva JC, Nakano LC, Flumignan RL. Pharmacological interventions for preventing venous thromboembolism in people undergoing bariatric surgery. Cochrane Database of Systematic Reviews 2022, Issue 11. Art. No: CD013683. [DOI: 10.1002/14651858.CD013683.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2003
- Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 (suppl 1)):I-9. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbar 2010
- Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. Journal of Thrombosis and Haemostasis 2010;8(11):2450-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barco 2020
- Barco S, Bingisser R, Colucci G, Frenk A, Gerber B, Held U, et al. Enoxaparin for primary thromboprophylaxis in ambulatory patients with coronavirus disease-2019 (the OVID study): a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):770. [PMID: ] [DOI] [PMC free article] [PubMed]
Bautista‐Vargas 2020
- Bautista-Vargas M, Bonilla-Abadia F, Canas CA. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. Journal of Thrombosis and Thrombolysis 2020;50(3):479-83. [DOI: 10.1007/s11239-020-02172-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benson 2021
- Benson RA, Nandhra S, COVIDSurg Collaboration. Outcomes of vascular and endovascular interventions performed during the coronavirus disease 2019 (COVID-19) pandemic. Annals of Surgery 2021;273(4):630-5. [DOI: 10.1097/SLA.0000000000004722] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzakoun 2020
- Benzakoun J, Hmeydia G, Delabarde T, Hamza L, Meder JF, Ludes B, et al. Excess out-of-hospital deaths during the COVID-19 outbreak: evidence of pulmonary embolism as a main determinant. European Journal of Heart Failure 2020;22(6):1046-7. [PMID: ] [DOI] [PMC free article] [PubMed]
Biagioni 2020
- Biagioni RB, Lopes RD, Agati LB, Sacilotto R, Wolosker N, Sobreira ML, et al. Rationale and design for the study apixaban versus clopidogrel on a background of aspirin in patient undergoing infrapopliteal angioplasty for critical limb ischemia: AGRIPPA trial. American Heart Journal 2020;227:100-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bikdeli 2020
- Bikdeli B, Madhavan M, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. Journal of the American College of Cardiology 2020;75(23):2950-73. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Broderick 2021
- Broderick C, Watson L, Armon MP. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Capell 2021
- Capell WH, Barnathan ES, Piazza G, Spyropoulos AC, Hsia J, Bull S, et al. Rationale and design for the study of rivaroxaban to reduce thrombotic events, hospitalization and death in outpatients with COVID-19: the PREVENT-HD study. American Heart Journal 2021;235:12-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cogo 1993
- Cogo A, Lensing AW, Prandoni P, Hirsh J. Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Implications for simplifying the diagnostic process with compression ultrasound. Archives of Internal Medicine 1993;153(24):2777-80. [PMID: ] [PubMed] [Google Scholar]
COMET 2021
- Core outcome set developers’ response to COVID-19 (April 2021). comet-initiative.org/Studies/Details/1538 (accessed 25 May 2021).
Correia 2022
- Correia RM, Santos BC, Carvalho AA, Areias LL, Kuramoto DA, Pereda MR, et al. Vascular complications in 305 severely ill patients with COVID-19: a cohort study. Sao Paulo Medical Journal 2022;141(5):e2022171. [DOI: 10.1590/1516-3180.2022.0171.R2.17102022] [DOI] [PMC free article] [PubMed] [Google Scholar]
COVER 2022
- VERN COVER study collaborative, Benson RA. Documenting the recovery of vascular services in European centres following the initial COVID-19 pandemic peak: results from a multicentre collaborative study. EJVES Vascular Forum 2022;57:28-34. [DOI: 10.1016/j.ejvsvf.2022.10.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
COVIDSurg 2021a
- COVIDSurg Collaborative. Machine learning risk prediction of mortality for patients undergoing surgery with perioperative SARS-CoV-2: the COVIDSurg mortality score. British Journal of Surgery 2021;108(11):1274-92. [DOI: 10.1093/bjs/znab183] [DOI] [PMC free article] [PubMed] [Google Scholar]
COVIDSurg 2021b
- COVIDSurg Collaborative, GlobalSurg Collaborative. SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. British Journal of Surgery 2021;108(9):1056-63. [DOI: 10.1093/bjs/znab101] [DOI] [PMC free article] [PubMed] [Google Scholar]
COVIDSurg 2022a
- COVIDSurg Collaborative, GlobalSurg Collaborative. SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia 2022;77(1):28-39. [DOI: 10.1111/anae.15563] [DOI] [PMC free article] [PubMed] [Google Scholar]
COVIDSurg 2022b
- COVIDSurg Collaborative, GlobalSurg Collaborative. Effects of pre-operative isolation on postoperative pulmonary complications after elective surgery: an international prospective cohort study. Anaesthesia 2021;76(11):1454-64. [DOI: 10.1111/anae.15560] [DOI] [PubMed] [Google Scholar]
Cuker 2021
- Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Advances 2021;5(3):872-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Emert 2020
- Emert R, Shah P, Zampella JG. COVID-19 and hypercoagulability in the outpatient setting. Thrombosis Research 2020;192:122-3. [PMID: ] [DOI] [PMC free article] [PubMed]
Fernandes 2019
- Fernandes CJ, Calderaro D, Piloto B, Hoette S, Jardim CV, Souza R. Extended anticoagulation after venous thromboembolism: should it be done? Therapeutic Advances in Respiratory Disease 2019;13:1-13. [DOI: 10.1177/1753466619878556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2015
- Flumignan RL, Flumignan CD, Baptista-Silva JC. Angioplasty for deep venous thrombosis. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD011468. [DOI: 10.1002/14651858.CD011468] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2021
- Flumignan RL, Tinôco JD, Pascoal PI, Areias LL, Cossi MS, Fernandes MI, et al. Prophylactic anticoagulants for people hospitalized with COVID-19: systematic review. British Journal of Surgery 2021;108(9):e299-e300. [DOI: 10.1093/bjs/znab197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2022a
- Flumignan CD, Nakano LC, Baptista-Silva JC, Flumignan RL. Antiplatelet agents for the treatment of deep venous thrombosis. Cochrane Database of Systematic Reviews 2022, Issue 7. Art. No: CD012369. [DOI: 10.1002/14651858.CD012369.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2022b
- Flumignan RL, Civile VT, Tinôco JD, Pascoal PI, Areias LL, Matar CF, et al. Anticoagulants for people hospitalised with COVID‐19. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD013739. [DOI: 10.1002/14651858.CD013739.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2023
- Flumignan RL, Civile VT, Areias LL, Flumignan CD, Amorim JE, Lopes RD, et al. Stenting or angioplasty for the treatment of deep vein thrombosis: systematic review and meta-analysis of randomised controlled trials. Medicine 2023;102(22):e33924. [DOI: 10.1097/MD.0000000000033924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giannis 2021
- Giannis D, Barish MA, Goldin M, Cohen SL, Kohn N, Gianos E, et al. Incidence of venous thromboembolism and mortality in patients with initial presentation of COVID-19. Journal of Thrombosis and Thrombolysis 2021;51:1-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goshua 2020
- Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematology 2020;7(8):e575-82. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 15 March 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available from gradepro.org.
Graphreader 2022
- Larsen K. Graphreader. www.graphreader.com/ (accessed 10 April 2022).
Guyatt 2012
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141((2 Suppl)):7S-47S. [DOI: 10.1378/chest.1412S3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handoll 2015
- Handoll HH, Langhorne P. In defence of reviews of small trials: underpinning the generation of evidence to inform practice [Editorial]. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD012369. [DOI: 10.1002/14651858.CD012369.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanff 2020
- Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. American Journal of Hematology 2020;95(12):1578-89. [DOI: 10.1002/ajh.25982] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hippensteel 2020
- Hippensteel JA, LaRiviere WB, Colbert JF, Langouët-Astrié CJ, Schmidt EP. Heparin as a therapy for COVID-19: current evidence and future possibilities. American Journal of Physiology. Lung cellular and molecular physiology 2020;319(2):L211-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsh 1986
- Hirsh J, Hull RD, Raskob GE. Clinical features and diagnosis of venous thrombosis. Journal of the American College of Cardiology 1986;8(6 (suppl B)):114B-27B. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kahn 2014
- Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet 2014;383(9920):880-8. [DOI: 10.1016/S0140-6736(13)61902-9] [DOI] [PubMed] [Google Scholar]
Kakkos 2021
- Kakkos SK, Gohel M, Baekgaard N, Bauersachs R, Bellmunt-Montoya S, Black SA, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. European Journal of Vascular and Endovascular Surgery 2021;61(1):9-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kakkos 2022
- Kakkos S, Kirkilesis G, Caprini JA, Geroulakos G, Nicolaides A, Stansby G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database of Systematic Reviews 2022, Issue 1. Art. No: CD005258. [DOI: 10.1002/14651858.CD005258.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearon 2016
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149(2):315-52. [DOI] [PubMed] [Google Scholar]
Kelliher 2022
- Kelliher S, Weiss L, Cullivan S, O'Rourke E, Murphy CA, Toolan S, et al. Non-severe COVID-19 is associated with endothelial damage and hypercoagulability despite pharmacological thromboprophylaxis. Journal of Thrombosis and Haemostasis 2022;[Epub ahead of print]. [DOI: 10.1111/jth.15660] [DOI] [PMC free article] [PubMed]
Klok 2020a
- Klok FA, Kruip MJ, Meer NJ, Arbous MS, Gommers DA, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research 2020;191:145-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klok 2020b
- Klok FA, Kruip MJ, Meer NJ, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thrombosis Research 2020;191:148-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyriakoulis 2022
- Kyriakoulis KG, Kollias A, Kyriakoulis IG, Kyprianou IA, Papachrysostomou C, Makaronis P, et al. Thromboprophylaxis in patients with COVID-19: systematic review of national and international clinical guidance reports. Current Vascular Pharmacology 2022;20(1):96-110. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2021
- Lee CC, Ali K, Connell D, Mordi IR, George J, Lang EM, et al. COVID-19-associated cardiovascular complications. Diseases 2021;9(3):47. [DOI: 10.3390/diseases9030047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llitjos 2020
- Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. Journal of Thrombosis and Haemostasis 2020;18(7):1743-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lodigiani 2020
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research 2020;191:9-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Logue 2021
- Logue JK, Franko NM, McCulloch DJ. Sequelae in adults at 6 months after COVID-19 infection. JAMA Network Open 2021;4(2):e210830. [DOI: 10.1001/jamanetworkopen.2021.0830] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marietta 2020
- Marietta M, Ageno W, Artoni A, De Candia E, Gresele P, Marchetti M, et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfusion 2020;18(3):167-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matos 2011
- Matos MF, Lourenço DM, Orikaza CM, Bajerl JA, Noguti MA, Morelli VM. The role of IL-6, IL-8 and MCP-1 and their promoter polymorphisms IL-6 -174GC, IL-8 -251AT and MCP-1 -2518AG in the risk of venous thromboembolism: a case-control study. Thrombosis Research 2011;128(3):216-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Middeldorp 2020
- Middeldorp S, Coppens M, Haaps TF, Foppen M, Vlaar AP, Müller MC, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. Journal of Thrombosis and Haemostasis 2020;18(8):1995-2002. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moores 2020
- Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest 2020;158(3):1143-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04542408
- NCT04542408. Hamburg edoxaban for anticoagulation in COVID-19 study (HERO-19). clinicaltrials.gov/ct2/show/NCT04542408 (first received 6 September 2020).
NICE 2019
- National Clinical Guideline Centre. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism: NICE guideline (NG89); 2019. nice.org.uk/guidance/ng89. [PubMed]
NICE 2020
- National Clinical Guideline Centre. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. NICE guideline (NG158); 2020. nice.org.uk/guidance/ng158.
NIH 2022
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. Bethesda (MD): National Institutes of Health (US). covid19treatmentguidelines.nih.gov/ (assessed 9 November 2022). [PMID: ] [PubMed]
NIHR 2022
- NIHR Global Health Unit on Global Surgery, COVIDSurg Collaborative. Elective surgery system strengthening: development, measurement, and validation of the surgical preparedness index across 1632 hospitals in 119 countries. Lancet 2022;400(10363):1607-17. [DOI: 10.1016/S0140-6736(22)01846-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortega‐Paz 2021
- Ortega-Paz L, Capodanno D, Montalescot G, Angiolillo DJ. Coronavirus disease 2019-associated thrombosis and coagulopathy: review of the pathophysiological characteristics and implications for antithrombotic management. Journal of the American Heart Association 2021;10(3):e019650. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paranjpe 2020
- Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. Journal of the American College of Cardiology 2020;76(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phelan 2020
- Phelan AL, Katz R, Gostin LO. The novel Coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020;323(8):709-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramacciotti 2020
- Ramacciotti E, Macedo AS, Biagioni RB, Caffaro RA, Lopes RD, Guerra JC, et al. Evidence-based practical guidance for the antithrombotic management in patients with Coronavirus disease (COVID-19) in 2020. Clinical and Applied Thrombosis/Hemostasis 2020;26:1076029620936350. [DOI: 10.1177/1076029620936350] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramacciotti 2022
- Ramacciotti E, Barile Agati L, Calderaro D, Aguiar VC, Spyropoulos AC, Oliveira CC, et al. Rivaroxaban versus no anticoagulation for post-dischargethromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet 2022;399(10319):50-9. [DOI: 10.1016/S0140-6736(21)02392-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2021
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.3.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Righini 2006
- Righini M, Paris S, Le Gal G, Laroche JP, Perrier A, Bounameaux H. Clinical relevance of distal deep vein thrombosis. Review of literature data. Thrombosis and Haemostasis 2006;95(1):56-64. [PMID: ] [PubMed] [Google Scholar]
Sachdeva 2014
- Sachdeva A, Dalton M, Amaragiri SV, Lees T. Graduated compression stockings for prevention of deep vein thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD001484. [DOI: 10.1002/14651858.CD001484.pub3] [DOI] [PubMed] [Google Scholar]
Saposnik 2011
- Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42(4):1158-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulman 2010
- Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of Thrombosis and Haemostasis 2010;8(1):202-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulman 2022
- Schulman S, Sholzberg M, Spyropoulos AC, Zarychanski R, Resnick HE, Bradbury CA, et al. ISTH guidelines for antithrombotic treatment in COVID-19. Journal of Thrombosis and Haemostasis 2022;20(10):2214-25. [DOI: 10.1111/jth.15808] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Schünemann 2021b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Shah 2021
- Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ (Clinical research ed.) 2021;372:n136. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sobreira 2020
- Sobreira ML, Marques MA. Anticoagulants as panacea in COVID-19 infection. Jornal Vascular Brasileiro 2020;19:e20200063. [DOI: 10.1590/1677-5449.200063] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spyropoulos 2011
- Spyropoulos AC, Anderson FA Jr, FitzGerald G, Decousus H, Pini M, Chong BH, et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest 2011;140(3):706-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spyropoulos 2018
- Spyropoulos AC, Ageno W, Albers GW, Elliott CG, Halperin JL, Hiatt WR. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. New England Journal of Medicine 2018;379:1118-27. [DOI: 10.1056/NEJMoa1805090] [DOI] [PubMed] [Google Scholar]
Spyropoulos 2020
- Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. Journal of Thrombosis and Haemostasis 2020;18(8):1859-65. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spyropoulos 2022
- Spyropoulos AC, Connors JM, Douketis JD, Goldin M, Hunt BJ, Kotila TR, et al. Good practice statements for antithrombotic therapy in the management of COVID-19: guidance from the SSC of the ISTH. Journal of Thrombosis and Haemostasis 2022;20(10):2226-36. [DOI: 10.1111/jth.15809] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 1991
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. American Journal of Cardiology 1991;68(17):1723-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Tang 2020
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis 2020;18(4):844-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomidokoro 2021
- Tomidokoro D, Hayama H, Okazaki T, Hara H, Hiroi Y. The effect of the COVID-19 pandemic on incidence and characteristics of pulmonary embolism. Global Health and Medicine 2021;3(2):122-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsend 2021
- Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. Journal of Thrombosis and Haemostasis 2021;19(4):1064-70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsaplin 2021
- Tsaplin S, Schastlivtsev I, Zhuravlev S, Barinov V, Lobastov K, Caprini JA. The original and modified Caprini score equally predicts venous thromboembolism in COVID-19 patients. Journal of Vascular Surgery 2021;9(6):1371-81.e4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunjungputri 2022
- Tunjungputri RN, Tetrasiwi EN, Mulansari NA, Harimurti K, Nelwan EJ. Parenteral and oral anticoagulant treatment for hospitalized and post-discharge COVID-19 patients: a systematic review and meta-analysis. Acta Medica Indonesiana 2022;54(2):190-209. [PMID: ] [PubMed] [Google Scholar]
Valla 2002
- Valla DC, Condat B, Lebrec D. Spectrum of portal vein thrombosis in the west. Journal of Gastroenterology and Hepatology 2002;17(Suppl 3):S224-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Varga 2020
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395(10234):1417-8. [PMID: ] [DOI] [PMC free article] [PubMed]
Verso 2003
- Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. Journal of Clinical Oncology 2003;21(19):3665-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Weitz 2017
- Weitz JI, Lensing AW, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. New England Journal of Medicine 2017;376(13):1211-22. [DOI: 10.1056/NEJMoa1700518] [DOI] [PubMed] [Google Scholar]
WHO 2020
- World Health Organization. Protocol: real-time RT-PCR assays for the detection of SARS-CoV-2. who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2 (accessed 4 August 2021).
Zehra 2022
- Naseem Z, Khakwani MM, Ayub M, Ayaz A, Jamil B, Arshad A. SARS-CoV-2 induced coagulopathy and potential role of anticoagulation: scoping review of literature. Monaldi Archives for Chest Disease 2022;92(4):epub. [DOI: 10.4081/monaldi.2022.1958] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Santos 2022
- Santos BC, Flumignan RLG, Civile VT, Atallah ÁN, Nakano LCU. Prophylactic anticoagulants for non‐hospitalised people with COVID‐19. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD015102. [DOI: 10.1002/14651858.CD015102] [DOI] [PMC free article] [PubMed] [Google Scholar]


