Abstract
Clots can form on the left atrial surface of left atrial appendage closure devices, with subsequent thromboembolization. However, we are unaware of any reports of clots forming on the devices immediately after deployment. We report a case where an acute thrombus strand formed at the tip of an occlusion device immediately after deployment. (Level of Difficulty: Intermediate.)
Key Words: atrial fibrillation, COVID-19, drug-related thrombosis, left atrial appendage closure, Watchman
Central Illustration
A woman in her 70s with persistent atrial fibrillation (AF) with CHA2DS2-VASc score 5, recent COVID-19 infection, and recurrent epistaxis presented for elective left atrial appendage (LAA) closure. The results of physical examination were unremarkable, with normal vital signs and irregularly irregular heart rhythm but normal heart sounds. Her electrocardiogram at presentation showed AF.
Learning Objectives
-
•
To review that left atrial appendage closure is an alternative to novel oral anticoagulants in patients with AF at moderate to high risk for stroke.
-
•
To emphasize that acute device-related thrombus formation on the surface of Watchman device immediately after deployment is rare.
-
•
To understand that patients with recent history of COVID-19 infection with unremarkable hypercoagulability observed during workup may nevertheless be at increased risk for blood-contacting device thrombosis.
Medical History
Two months before device implantation, the patient experienced an acute COVID-19 infection complicated by superimposed bacterial pneumonia. Laboratory tests indicated an increase in inflammatory markers: C-reactive protein of 7.2 mg/dL (reference <0.5 mg/L), erythrocyte sedimentation rate of 65 mm (reference <30 mm), and D-dimer of 1423 ng/mL (reference <225 ng/mL). Treatment with remdesivir, dexamethasone, antibiotics, and oxygen supplementation led to rapid clinical improvement, and she was discharged home.
Management
The patient had taken apixaban (Eliquis) continuously until the morning of the procedure, when 1 dose was withheld. A transesophageal echocardiogram (TEE) at the beginning of the procedure demonstrated no thrombus in the left atrium or LAA, and standard LAA orifice measurements were performed. Using a modified Seldinger technique, an 8.5-F sheath was placed in the right femoral vein and exchanged for an 8.5-F SL-1 sheath. Intravenous heparin was given as a bolus before transseptal access to achieve an activated clotting time >250 seconds. With a Brockenbrough needle, a transseptal puncture was performed with TEE and fluoroscopic guidance. A pigtail catheter was positioned in the LAA, and an angiogram was performed with injection of contrast material to assess the appendage morphology. Under TEE and fluoroscopic guidance, the occlusion device was delivered and positioned at the ostium of the LAA. Owing to a shallow bifurcation, redeployment and a change in device size were required. The second device appeared well seated, without leak and with appropriate compression. The device was then released (Figure 1, Video 1). Immediately after release, a thin thrombin strand was visualized measuring 9 mm × 1 mm attached to the core wire release point (Figure 2, Videos 2 and 3). The activated clotting time at this point was 330 seconds. Protamine was withheld. Upon extubation, apixaban was initiated, and the patient was closely monitored.
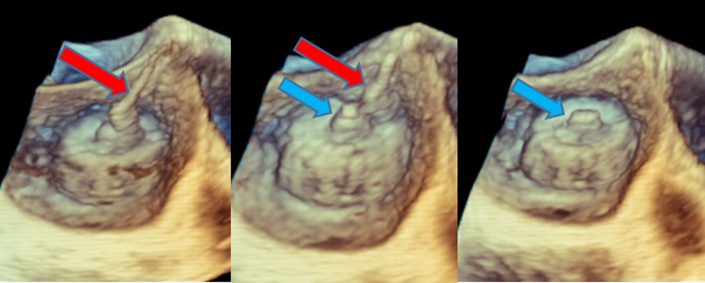
Figure 1.
3-Dimensional Transesophageal Echocardiogram
Shown is releasing of the occlusion device by detaching the delivery catheter (red arrows) from the device’s threaded insert (blue arrows).
Figure 2.
Detection of a Thrombin Strand on the Occlusion Device
A new strand was noted on the device’s threaded insert shortly after detachment of the deployment catheter. (A) 3-dimensional transesophageal echocardiogram imaging. (B) Multiplanar 2-dimensional imaging.
Discussion
Intravascular medical devices such as left atrial appendage occlusion (LAAO) devices are often used to treat cardiovascular diseases. Left atrial appendage closure (LAAC) is an alternative to oral anticoagulants in patients with AF at moderate to high risk for stroke and who are not optimal candidates for long-term anticoagulation. Because LAAC devices are composed of foreign material, they have the potential to perpetuate device-related thrombus (DRT), with potential for subsequent thromboembolization, before endothelialization.1 Therefore, it is standard practice to perform TEE 6 weeks after device implantation to ensure proper seating of the device and to assess for thrombus formation. Current published reports suggests that DRT occurs in 3% to 4% of patients after .2 In addition, DRT has most often been detected between 3 and 6 months after LAAC. However, we are unaware of any reports of clots forming on the surface of the device itself immediately after deployment.
LAAC devices offer an alternative to anticoagulation for prevention of stroke in selected patients with AF. Engineering advancements and familiarity with the implantation have reduced procedural complications and have accelerated continued growth in the use of LAAO devices. However, DRT remains a concern in the early postimplantation period. Dukkipati et al3 demonstrated that the incidence of DRT across both the PROTECT-AF and PREVAIL trials was approximately 4%. In addition, the study showed that DRT was most often detected between 3 and 6 months after LAAC.2
The mechanism underlying DRT is incompletely understood. In addition, the uniqueness of a patient’s LAA anatomy, the operator’s experience, and pharmacologic considerations make identifying risk factors for DRT formation challenging. However, some factors are known to increase coagulopathy, including stasis from left atrial dilation, implantation depth, genetic resistance to antiplatelet therapy, and hypercoagulability from systemic illness.4 The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2, has brought many unique pathologic conditions, such as coagulopathy. COVID-19–associated coagulopathy can cause various thromboembolic complications, likely resulting from endothelial injury, immobilization, and an increase in circulating prothrombotic factors.5 The clotting of intravascular access devices or extracorporeal circuits despite prophylactic-intensity anticoagulation is well documented for patients with COVID-19 infection.6,7 In addition, several cases of thrombosis involving blood-contacting devices, such as coronary stent thrombosis associated with COVID-19, have been reported.8,9 During a COVID-19 infection, cytokine storm leads to endothelial damage, activation of the platelets, and coagulation cascade. In the Watchman device, the threaded insert, which provides the mechanism for attaching the device to the threaded core wire on the delivery catheter, was the nidus for the thrombus. One can speculate that in our patient with an activated clotting time of 330 seconds, the combination of recent COVID-19 infection and an underlying genetic susceptibility to becoming procoagulable probably led to acute thrombus formation.
Follow-Up
No neurologic events occurred during close outpatient follow up. Six weeks after the procedure, TEE revealed a well-seated 24-mm Watchman device with low compression (2%) and a 1-mm residual flow around the device on the mitral side, but no thrombus on the device (Figure 3).
Figure 3.
Transesophageal Echocardiograms at 45-Day Follow-Up Visit
Previously visualized strand is no longer visible after a course of anticoagulation with apixaban. No device leak at 45-day imaging. (A) 3-dimensional transesophageal echocardiogram imaging. (B) 2-dimensional transesophageal echocardiogram imaging.
Conclusions
To our knowledge, this is the first case of DRT on the surface of an LAAC device immediately after deployment and release. The origin of the thrombus strand appears to have been the threaded insert, which is the attachment place for the delivery wire. We speculate that the patient’s recent SARS-CoV-2 infection, in addition to the prothrombotic milieu in the fibrillating left atrium, was sufficient to overcome therapeutic anticoagulation.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
3-Dimensional Transesophageal Echocardiogram
Image shows the releasing of the occlusion device by detaching the delivery catheter from the device’s threaded insert.
3-Dimensional Transesophageal Echocardiogram
Image shows a thin strand on the device’s threaded insert shortly after detachment of the deployment catheter.
Multiplanar 2-Dimensional Transesophageal Echocardiogram
Image shows a new strand on the occlusion device’s threaded insert shortly after detachment of the deployment catheter.
References
- 1.Garot P., Cormier B., Horvilleur J. Device-related thrombus after left atrial appendage closure. Interv Cardiol. 2019;14(1):42–44. doi: 10.15420/icr.2018.21.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhouli M., Busu T., Shah K., Osman M., Alqahtani F., Raybuck B. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. J Am Coll Cardiol EP. 2018;4(12):1629–1637. doi: 10.1016/j.jacep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Dukkipati S.R., Kar S., Holmes D.R., et al. Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation. 2018;138(9):874–885. doi: 10.1161/CIRCULATIONAHA.118.035090. [DOI] [PubMed] [Google Scholar]
- 4.Vukomanovic D., Unzek S., Malik K., et al. Massive device-related thrombus after LAA occlusion: intraoperative insights into mechanism. J Am Coll Cardiol Case Rep. 2022;4(21):1409–1413. doi: 10.1016/j.jaccas.2022.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo J.W., Kim D.Y., Yun N., Kim D.M. Coronavirus disease 2019-associated coagulopathy. Microorganisms. 2022;10(8):1556. doi: 10.3390/microorganisms10081556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega-Paz L., Capodanno D., Montalescot G., Angiolillo D.J. Coronavirus disease 2019-associated thrombosis and coagulopathy: review of the pathophysiological characteristics and implications for antithrombotic management. J Am Heart Assoc. 2021;10(3) doi: 10.1161/JAHA.120.019650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shekar K., Badulak J., Peek G., et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66(7):707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skorupski W.J., Grygier M., Lesiak M., Kałużna-Oleksy M. Coronary stent thrombosis in COVID-19 patients: a systematic review of cases reported worldwide. Viruses. 2022;14(2):260. doi: 10.3390/v14020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkholy K.O., Khizar A., Khan A., et al. Subacute stent thrombosis in a patient with COVID-19 despite adherence to antiplatelets. Cureus. 2021;13(2) doi: 10.7759/cureus.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3-Dimensional Transesophageal Echocardiogram
Image shows the releasing of the occlusion device by detaching the delivery catheter from the device’s threaded insert.
3-Dimensional Transesophageal Echocardiogram
Image shows a thin strand on the device’s threaded insert shortly after detachment of the deployment catheter.
Multiplanar 2-Dimensional Transesophageal Echocardiogram
Image shows a new strand on the occlusion device’s threaded insert shortly after detachment of the deployment catheter.