Abstract
Like other eukaryotes, fungi use MAP kinase (MAPK) pathways to mediate cellular changes responding to external stimuli. In the past two decades, three well-conserved MAP kinase pathways have been characterized in various plant pathogenic fungi for regulating responses and adaptations to a variety of biotic and abiotic stresses encountered during plant infection or survival in nature. The invasive growth (IG) pathway is homologous to the yeast pheromone response and filamentation pathways. In plant pathogens, the IG pathway often is essential for pathogenesis by regulating infection-related morphogenesis, such as appressorium formation, penetration, and invasive growth. The cell wall integrity (CWI) pathway also is important for plant infection although the infection processes it regulates vary among fungal pathogens. Besides its universal function in cell wall integrity, it often plays a minor role in responses to oxidative and cell wall stresses. Both the IG and CWI pathways are involved in regulating known virulence factors as well as effector genes during plant infection and mediating defenses against mycoviruses, bacteria, and other fungi. In contrast, the high osmolarity growth (HOG) pathway is dispensable for virulence in some fungi although it is essential for plant infection in others. It regulates osmoregulation in hyphae and is dispensable for appressorium turgor generation. The HOG pathway also plays a major role for responding to oxidative, heat, and other environmental stresses and is overstimulated by phenylpyrrole fungicides. Moreover, these three MAPK pathways crosstalk and coordinately regulate responses to various biotic and abiotic stresses. The IG and CWI pathways, particularly the latter, also are involved in responding to abiotic stresses to various degrees in different fungal pathogens, and the HOG pathway also plays a role in interactions with other microbes or fungi. Furthermore, some infection processes or stress responses are co-regulated by MAPK pathways with cAMP or Ca2+/CaM signaling. Overall, functions of individual MAP kinase pathways in pathogenesis and stress responses have been well characterized in a number of fungal pathogens, showing the conserved genetic elements with diverged functions, likely by rewiring transcriptional regulatory networks. In the near future, applications of genomics and proteomics approaches will likely lead to better understanding of crosstalk among the MAPKs and with other signaling pathways as well as roles of MAPKs in defense against other microbes (biotic interactions).
Keywords: Fungal pathogens, Signal transduction, Pathogenesis, Virulence, Oxidative stress, Osmotic stress, Fungicide resistance, Bacterial-fungal interactions
Introduction
Plant pathogenic fungi are exposed to a variety of abiotic and biotic stresses in their natural habitats as well as during infectious growth, such as hyperosmolarity, extreme pH or temperature, mycoparasitic organisms, oxidative burst, and phytoalexins. Effective stress perception and responses are necessary for adaptation, survival, and overcoming plant defense. Like other eukaryotic organisms, fungi have the well-conserved mitogen-activated protein (MAP) kinase pathways that play important roles in responding to external stimuli (Chen and Thorner 2007; Brewster and Gustin 2014). In the past two decades, MAP kinase (MAPK) pathways have been functionally characterized in various plant pathogenic fungi that differ in dispersal, survival, host ranges, and infection mechanisms. In addition to their roles in infection and developmental processes, MAPKs in fungal pathogens are important for regulating responses to various environmental stresses and interactions with other microbes (Hamel et al. 2012; Braunsdorf et al. 2016; Jiang et al. 2018).
The typical MAP kinase pathway comprises a protein kinase cascade consisting of a MAP kinase (MAPK), a MAPK kinase (MEK), and a MEK kinase (MEKK). The sequential activation of these protein kinases in response to extracellular signals results in the activation of MAPKs, which then phosphorylate downstream target proteins and transcription factors (TFs) to regulate transcriptional and cellular changes. The budding yeast Saccharomyces cerevisiae, a model organism in which MAP kinase pathways are best characterized, have five MAPKs (Fus3, Kss1, Slt2, Hog1, and Smk1) that regulate pheromone response, filamentation/invasiveness, cell wall integrity, high osmolarity growth, and ascospore cell assembly (Schwartz and Madhani 2004; Chen and Thorner 2007). However, most plant pathogenic ascomycetes, the focus of this review, normally have only three MAPKs that are orthologous to yeast Fus3/Kss1, Slt2, and Hog1, and three corresponding upstream MEKs and MEKKs (Chen and Thorner 2007; Li et al. 2012), with only a few exceptions such as two HOG1 MAPKs in Verticillium dahlia and two BCK1 MEKKs in Fusarium oxysporum. These three well conserved MAPK pathways have both common and distinct biological functions in pathogenesis, differentiation, and stress responses in phytopathogenic fungi (Jiang et al. 2018). In this review, we will summarize general functions of fungal MAPKs in pathogenesis, but the emphasis will be on their roles in regulating responses to abiotic and biotic stresses.
Roles of MAPK pathways in fungal pathogenesis
Considering all the physical barriers and possible defense responses, the host plant is likely a ‘hostile’ habitat for fungal pathogens, which often use MAPK signaling to regulate infection-related morphogenesis and overcome plant immunity. The rice blast fungus Magnaporthe oryzae, a model for studying fungal-plant interactions, is the first fungal pathogen with all three MAPK pathways functionally characterized (Hamel et al. 2012; Jiang et al. 2018). Therefore, we organized this section based on the M. oryzae MAPKs (Fig. 1) and their homologs in S. cerevisiae (Table 1). To date, all three MAPK pathways have been characterized in a number of plant pathogenic ascomycetes, including Alternaria brassicicola, Bipolaris sorokiniana, Botrytis cinerea, Cryphonectria parasitica, Fusarium graminearum, F. oxysporum, and Zymoseptoria tritici (Leng and Zhong 2015; So and Kim, 2017; Jiang et al. 2018; Francisco et al. 2020). In general, at least two of the MAPK pathways play critical roles in pathogenesis. In some fungi such as F. graminearum, all three are important for plant infection (Wang et al. 2011). However, the exact infection-related functions of each MAP kinase pathway may vary significantly among different fungal pathogens.
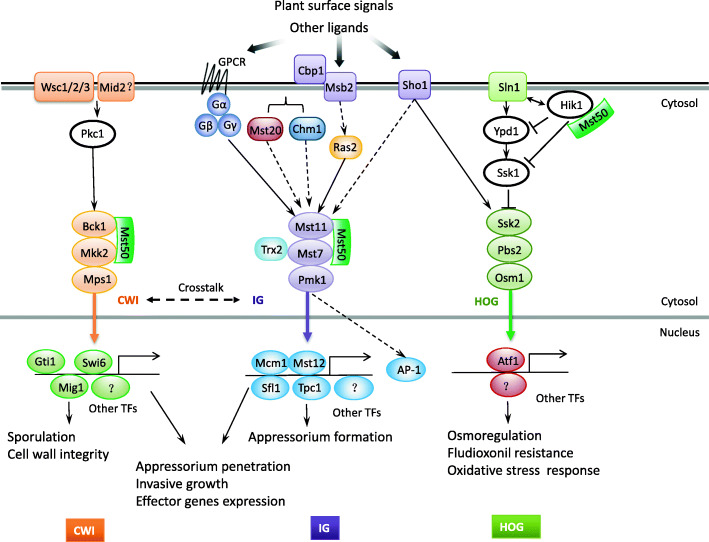
Fig. 1.
Functions of three MAPK pathways in the model plant pathogenic fungus Magnaporthe oryzae. Plant surface (chemical and physical) signals and other ligands of infected plant tissues are recognized by Sho1, two putative mucins (Msb2 and Cbp1), GPCR (such as Pth11), or other uncharacterized receptors to activate the downstream Pmk1 IG MAPK pathway possibly via Gα-GTP, freed Gβγ, PAK kinases (Mst20/Chm1), and Ras2. Mst50 is an adaptor protein that is also involved in the other two MAPK pathways. Thioredoxin Trx2 affects the activation of Mst7, which interacts with Pmk1 at the docking sites. Activated Pmk1 regulates the expression of genes important for appressorium formation, penetration, and invasive growth by Mst12, Mcm1, Sfl1, Tpc1, and possibly other transcription factors. (Mst12 interacts with Mcm1 and Tpc1, and Sfl1 directly interacts with Pmk1). For the Mps1 CWI pathway that functions downstream from PKC for regulating appressorium penetration, sporulation, and invasive growth, all the yeast cell wall integrity sensors are conserved in plant pathogenic fungi (Table 1). Transcription factors regulated by the Mps1 MAPK cascade include Mig1, Swi6, and Gti1, with the latter as a regulator of many effector genes. Both IG and CWI MAPK pathways are important for invasive growth and disease development. Unlike the other two MAPKs, the Osm1 MAPK is dispensable for plant infection although it has the conserved role in osmoregulation and sensitivity to fludioxonil. It is also important for responses to oxidative stress through transcription factor Atf1, which may be activated by other MAPKs or protein kinases because the atf1 deletion mutant is defective in plant infection. Although Sho1 is an osmosensor in M. oryzae, it is also involved in activating the IG MAPK together with Msb2
Table 1.
Key components of the IG, CWI, and HOG MAPK pathways in M. oryzae and F. graminearum, and their orthologs in S. cerevisiae
| S. cerevisiae | M. oryzae | F. graminearum | Functions | |
|---|---|---|---|---|
| Kss1/Pmk1 IG | Ste2 | Ste2b | Pre2 | GPCR |
| Ste3 | Ste3b | Pre1 | GPCR | |
| Msb2 | MoMsb2 | FGSG_01127a | Mucin | |
| – | Cbp1 | – | GPCR | |
| – | Pth11 | FGSG_08408 | GPCR | |
| – | – | Giv1 | GPCR | |
| Gpa1 | MagA | FgGpa1 | G-alpha | |
| Ste4 | Mgb1 | FgGpb1 | G-beta | |
| Ste18 | MGG1 | FgGpg1 | G-gamma | |
| Ras2 | MoRAS2 | FgRAS2 | GTPase | |
| Ste20 | Mst20b | FgSte20 | PAK | |
| Cla4 | Chm1b | FgCla4 | PAK | |
| Ste5 | – | – | Scaffold | |
| Ste50c | Mst50 | FgSte50 | Adaptor | |
| Ste11 | Mst11 | FgSte11 | MEKK | |
| Ste7 | Mst7 | FgSte7 | MEK | |
| Fus3/Kss1 | Pmk1 | Gpmk1 (Fmk1) | MAPK | |
| Ste12 | Mst12 | FgSte12 | TF | |
| Mcm1 | MoMcm1 | FgMcm1 | TF | |
| Sfl1 | MoSfl1 | FgSfl1 | TF | |
| – | Tpc1 | FGSG_08769 | TF | |
| Slt2 CWI | Wsc1 | MGG_04325 | FgWsc1 | Sensor-transducer |
| Wsc2 | MGG_02754 | FgWsc2/2B | ||
| Wsc3 | MGG_01466 | FgWsc3 | ||
| Mid2 | MGG_12606 | FGSG_08788 | Sensor | |
| Mtl1 | – | – | Sensor | |
| Rho1 | MGG_07176 | FGSG_04400 | Rho | |
| Pkc1 | MoPkc1 | FgPkc1 | PKC | |
| Bck1 | Mck1 | FgBck1 | MEKK | |
| Mkk1/Mkk2 | Mkk2 | FgMkk1 | MEK | |
| Slt2 | Mps1 | Mgv1 | MAPK | |
| Swi6 | MoSwi6 | FgSwi6 | TF | |
| Swi4 | MGG_ | FGSG_04220 | TF | |
| Rlm1 | Mig1 | FgRlm1 | TF | |
| Wor1 | MoGti1 | Fgp1 | TF | |
| Hog1 HOG | Sln1 | MoSln1 | FgSln1 | Histidine kinase |
| Sho1d | MoSho1 | FgSho1 | Osmosensor | |
| – | MoHik1 | FGSG_07118 | Histidine kinase | |
| Ypd1 | MoYpd1 | FGSG_04363 | Phosphotransfer protein | |
| Ssk1 | MoSsk1 | FGSG_08948 | MEKK | |
| Ssk2/Ssk22 | MoSssk2 | FgSsk2 | MEKK | |
| Pbs2 | MoPbs2 | FgPbs2 | MEK | |
| Hog1 | Osm1 | FgHog1 | MAPK | |
| Skn7 | MoSkn7 | FgSkn7 | TF | |
| Atf1 | MoAtf1 | FgAtf1 | TF |
- No homolog
aPredicted gene number (Homolog are present but not characterized)
bHomologs not important for appressorium formation
c Ste50 is functionally related to multiple MAPKs in S. cerevisiae and M. oryzae
d Sho1 functions mainly in the HOG pathway as an osmosensor in the budding yeast although it ortholog play an important role together with Msb2 to regulate the IG pathway in fungal pathogens
The Kss1/Pmk1 invasive growth (IG) MAPK pathway
In S. cerevisiae, Kss1 and Fus3 are two partially redundant MAPKs that have overlapping functions in pheromone response and filamentation or invasive growth into agar (Morillon et al. 2000). Most filamentous ascomycetes have only one MAPK that is orthologous to Fus3/Kss1 (Table 1). In plant pathogenic fungi, this conserved MAPK cascade is generally required for plant infection and best characterized in M. oryzae and the corn smut fungus Ustilago maydis (Muller et al. 2003; Li et al. 2012). Pmk1, the first MAPK gene characterized in plant pathogens, is essential for appressorium formations in M. oryzae. It also plays a critical role in appressorium penetration and invasive growth (IG) or cell-to-cell spreading in planta (Xu and Hamer 1996; Sakulkoo et al. 2018). Pmk1 is activated by its upstream Mst7 MEK and Mst11 MEKK (Fig. 1), but M. oryzae, like other fungi, lacks a distinct homolog of yeast scaffold protein Ste5. Instead, Mst50 functions as an adaptor protein that interacts with Mst11 and Mst7 as well as Ras proteins (Zhao and Xu 2007; Zhou et al. 2014). Neither MST20 nor CHM1, two PAK kinases in M. oryzae, is required for appressorium formation. RAS2 is an essential gene and Ras2 likely functions upstream from both the cAMP-PKA and MAPK pathways (Zhou et al. 2012; Qi et al. 2015). In M. oryzae, trimeric G-proteins and G protein-coupled receptor (GPCR) Pth11 also are involved in regulating appressorium formation and plant infection, mainly via cAMP signaling (DeZwaan et al. 1999; Nishimura et al. 2003). Msb2 mucin and Sho1 act upstream from the Pmk1 cascade as sensors for plant surface chemicals such as primary alcohols (Liu et al. 2011). For physical signals, a putative chitin-binding protein may play a role in sensing a hydrophobic surface for appressorium formation (Kamakura et al. 2002). MoEnd3 mediated receptor endocytosis for Pth11 and MoSho1 and transduce signals into intercellular compartment in M. oryzae (Li et al. 2017a, 2017b). For downstream targets, besides Mst12, Mcm1 and Sfl1 are other two transcription factors that may be regulated by Pmk1 for appressorium penetration and invasive growth (Park et al. 2002; Li et al. 2011; Zhou et al. 2011). MST12 is essential for pathogenicity and likely regulates septin-mediated cytoskeleton reorganizations in appressoria for penetration. Pmk1 is also essential for hyphopodium formation and root infection as well as the development of appressorium-like structures at hyphal tips (Kong et al. 2013).
In U. maydis, a basidiomycete, Kpp2 and Kpp6 are MAPKs with overlapping functions in plant infection and the kpp2 kpp6 double mutants are nonpathogenic. Kpp6 plays a more important role than Kpp2 in appressorium penetration, but both kpp2 and kpp6 deletion mutants are reduced in virulence (Muller et al. 2003; Hu et al. 2007). Interestingly, transcription factor Prf1 appears to function downstream from both the cAMP-PKA and MAPK pathways for regulating plant infection processes (Kaffarnik et al. 2003). In M. oryzae, which lacks a Prf1 homolog, Sfl1 also may be coregulated by the Pmk1 MAPK and cAMP signaling pathways (Li et al. 2011; Li et al. 2017a, 2017b). For upstream sensors, Msb2 and Sho1 also are important for appressorium development in U. maydis (Lanver et al. 2010). Their homologs in B. cinerea and F. oxysporum play critical roles in plant infection as well (Leroch et al. 2015; Perez-Nadales and Di Pietro 2015). It is likely that these two sensors are conserved in other fungal pathogens for recognizing plant surface chemical signals to activate the IG MAPK cascade for regulating infection-related development. In fact, in all the appressorium-forming pathogens, including B. sorokiniana, Colletotrichum gloeosporioides, C. lagenarium, and C. heterostrophus, the IG MAPK pathway is required for appressorium formation (Li et al. 2012; Leng and Zhong 2015; Liang et al. 2019). Furthermore, expression of the PMK1 orthologs from C. lagenarium and Puccinia striiformis rescues the defect of pmk1 in appressorium formation, indicating the well-conserved nature of this MAPK (Jiang et al. 2018).
PMK1 orthologs also are important for plant infection in various fungal pathogens that do not form appressoria, including vascular wilt pathogens F. oxysporum and V. dahliae, the wheat scab fungus F. graminearum, corn stalk and ear rot pathogen F. verticillioides, canker pathogen C. parasitica, and biotrophic pathogen Claviceps purpurea (Hamel et al. 2012; Li et al. 2012; Jiang et al. 2018). In Mycosphaerella graminicola and Stagonospora nodorum, the Pmk1 ortholog is important for invasion through stomata and growth in mesophyll tissues (Solomon et al. 2005; Cousin et al. 2006). Genes of diverse functions are found to be regulated by the IG MAPK pathway, such as PTH11 GPCR, GAS2/GAS2, and MoHOX7 homeobox TF in M. oryzae, cell wall degrading enzyme (CWDE) genes in F. oxysporum, F. graminearum, and Valsa mali, and pheromone precursors in C. parasitica (Kim et al. 2009; Li et al. 2012; Wu et al. 2017). Interestingly, this MAPK pathway also regulates the biosynthesis of deoxynivalenol (DON) in F. graminearum and fumonisins in F. verticillioides (Wang et al. 2011; Zhang et al. 2011). These mycotoxins also are toxic to plant cells and DON is a critical virulence factor in the wheat scab fungus.
In summary, the IG MAPK is well conserved for regulating plant penetration and invasive growth in phytopathogenic fungi. Although the exact developmental and infection processes that it regulates vary among different fungi, one common theme is that this MAPK regulates the arrest of germ tube or hyphal tip growth before re-establishing polarized growth for penetrating and invading plant tissues. In addition, this MAPK pathway may regulate the expression of stage-specific genes during disease development, likely in response to plant signals recognized at different infection stages. Besides surface cues, other possible plant signals known to activate the IG MAPK cascade include ethylene in C. gloeosporioides (Kim et al. 2000), secreted class III peroxidases in F. oxysporum (Turrà et al. 2015), and wheat floral tissue extract in F. graminearum (Jiang et al. 2019).
The Slt2/Mps1 Cell Wall integrity (CWI) pathway
The CWI pathway is required for remodeling of the fungal cell wall during growth, development, and for responding to environmental stimuli. In S. cerevisiae, cell wall stresses activate the small G protein Rho1, which then activates the Slt2 MAPK cascade (Table 1) via Pkc1 to regulate gene expression by transcription factors Rlm1 and Swi6 (Jiménez-Gutiérrez et al. 2020). In M. oryzae, the mps1 mutant forms melanized appressoria but is defective in penetration, infectious growth, and sporulation (Xu et al. 1998). Deletion of its upstream MEKK Mck1 results in similar defects in cell wall integrity and plant infection (Jeon et al. 2008). Similar to mps1 and bck1 mutants, the mig1 (MoRlm1) deletion mutant is nonpathogenic and defective in the differentiation and growth of invasive hyphae, likely due to defects in overcoming defense responses (Mehrabi et al. 2008). The Moswi6 mutant has defects in appressorium turgor generation and forms small specks, but not typical blast lesions, on rice leaves (Qi et al. 2012). Mps1 also controls MoGti1, a transcription factor important for penetration peg formation and expression of several effector genes in M. oryzae (Li et al. 2016) (Fig. 1).
The CWI MAPK pathway also has been characterized in a number of plant pathogenic fungi, including B. cinerea, C. parasitica, C. purpurea, F. graminearum, M. graminicola, and Sclerotinia sclerotiorum (Sanz et al. 2017; Jiang et al. 2018). In general, mutants deleted of the Slt2 ortholog and its upstream MEK or MEKK are significantly reduced in virulence or are non-pathogenic, indicating a conserved role of the CWI MAPK pathway during plant infection. However, infection processes regulated by this pathway differ among fungal pathogens. For example, unlike in M. oryzae, the Slt2 ortholog is important for appressorium development in C. lagenarium and C. gloeosporioides (Kojima et al. 2002; Yong et al., 2013). Whereas the MgSlt2 mutant is normal in stomata penetration but defective in developing invasive hyphae in M. graminicola (Mehrabi et al. 2006), SMK3 is important for initial infection and sclerotium formation but not for lesion expansion in S. sclerotiorum (Bashi et al. 2016).
In M. oryzae, the mps1 mutant has a normal growth rate but produces only limited aerial hyphae. Its orthologs also are dispensable for normal growth rate in Colletotrichum species. However, mutants deleted of the Slt2 ortholog have severe growth defects in other fungi, such as F. graminearum, B. cinerea, and C. parasitica (Hou et al. 2002; Rui and Hahn 2007; So and Kim, 2017). Interestingly, the Cpslt2 and Cpbck1 mutants of C. parasitica often produce spontaneous suppressors that have a faster growth rate but are still defective in plant infection (So and Kim, 2017), suggesting different roles of the CWI MAPK pathway during vegetative and infectious growth. In F. graminearum, deletion of FgHOG1 partially rescues the growth defect of the mgv1 mutant but not its defect in pathogenesis (Ren et al. 2019).
The Hog1/Osm1 high-Osmolarity glycerol (HOG) MAPK pathway
Whereas the other two fungal MAPKs have the TEY dual phosphorylation site, Hog1 and its orthologs have the TGY motif, which is similar to p38 stress activated MAP kinases (SAPKs) in animals. In yeast, the Ssk2/Ssk22-Pbs2-Hog1 MAPK cascade is activated by Sln1 and Sho1 (Table 1), two partially redundant but mechanistically distinct sensors, to mainly regulate responses to hyperosmotic stress (Brewster and Gustin 2014). In M. oryzae, the osm1 deletion mutant is defective in osmoregulation in hyphae but normal in appressorium turgor generation and plant infection (Dixon et al. 1999). Its upstream sensor histidine kinases MoSln1 and MoHik1, phosphotransfer protein MoYpd1p, and response regulator MoSsk1 MEKK (Table 1) also are important for osmoregulation but dispensable for pathogenesis (Jacob et al. 2016) (Fig. 1). However, the Sho1 homolog plays an important role in activating the IG MAPK pathway together with Msb2 for plant infection than for osmoregulation in M. oryzae (Liu et al. 2011).
Like in M. oryzae, the Hog1/Osm1 ortholog is dispensable for plant infection in several fungal pathogens such as Cochliobolus orbiculare and Bipolaris oryzae (Moriwaki et al. 2006; Jiang et al. 2018). However, the HOG MAPK pathway is important for plant infection in other plant pathogenic fungi (Jiang et al. 2018). For example, the hog1 mutant is nonpathogenic and defective in the switch to hyphal growth in M. graminicola (Mehrabi et al. 2006). In B. cinerea, Sak1 is important for appressorium development and penetration of plant epidermal cells (Liu et al. 2008). In F. graminearum, the Fghog1 mutant is defective in plant infection and DON production (Zheng et al. 2012). In Ustilaginoidea virens, UvHog1 regulates the production of secondary metabolites that are toxic to plant cells (Zheng et al. 2016). These observations indicate that the HOG pathway likely plays a species-specific role during plant infection in fungal pathogens. Interestingly, the C. sativus Cshog1 mutant is normal in root infection but significantly reduced in virulence on barley leaves (Leng and Zhong 2015). In C. parasitica, whereas the other two MEK kinase genes are important, the Cpkk3 MEK (CpPbs2) is dispensable for pathogenesis on chestnut (Moretti et al. 2014). However, the Cpmk1 MAPK (CpHog1) mutant is slightly reduced in virulence in a different C. parasitica strain (Park et al. 2004). Therefore, the HOG MAPK pathway may have strain-specific and tissue-specific roles during plant infection as well.
The best characterized downstream target of the HOG MAPK in plant pathogens is the Atf1 (a CREB-like) bZIP transcription factor. In F. graminearum, Atf1 interacts with FgOs2 (FgHog1) in the nucleus under osmotic stress, and constitutive expression of FgATF1 partially complements the defects of Fgos-2 mutant in osmoregulation and pathogenesis (Van Nguyen et al. 2013). Atf1 orthologs also are important for virulence in F. verticillioides, M. oryzae, V. dahlia, and other fungal pathogens (Jiang et al. 2018; Szabó et al. 2020; Tang et al. 2020; Liu et al. 2020). However, in these fungi, ATF1 orthologs mainly regulate response to oxidative stress instead of hyperosmotic stress. Furthermore, responses to oxidative stress often involve the CWI as well as HOG MAPK pathways as described below.
MAPK signaling in regulating abiotic stress responses
In S. cerevisiae, Slt2 and Hog1 mainly regulate responses to cell wall and hyperosmotic stresses, respectively, although these MAPKs, particularly Hog1, also are involved in other stress responses (Ikner and Shiozaki 2005; Serrano et al. 2006; Brewster and Gustin 2014). In comparison, MAPK pathways in phytopathogenic fungi generally play more important roles in response to various environmental stresses (Fig. 2), including antifungal chemicals, reactive oxidative species (ROS), elevated temperatures, UV irradiation, and plant defense compounds (Lee et al. 2017; Dunayevich et al. 2018; Yang et al. 2020).
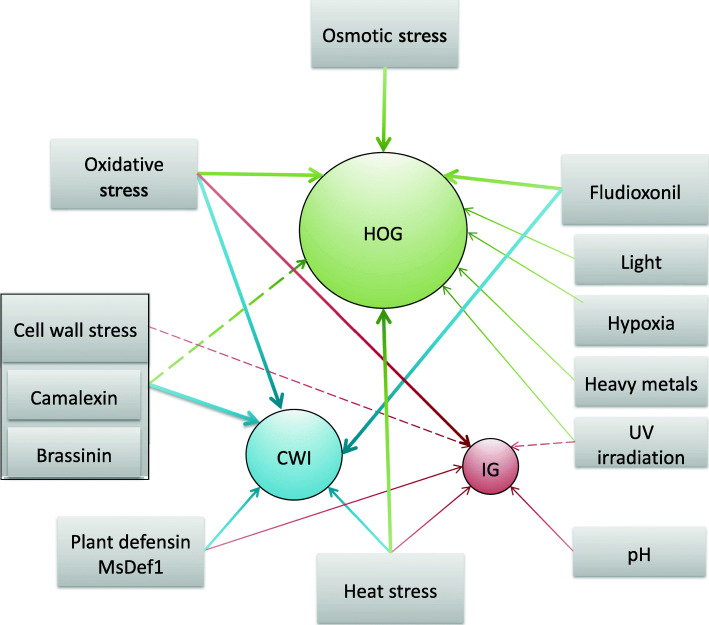
Fig. 2.
Regulation of responses to abiotic stresses by MAPK Signaling. In general, the HOG pathway plays a major role in regulating responses to osmotic and oxidative stresses in fungi. It is also a major regulator for responses to various environmental stresses, such as UV irradiation, heavy metals, and heat stress in many fungal pathogens. The CWI MAPK pathway mainly regulates responses to cell wall stresses caused by lytic enzymes, cell wall disturbing chemicals, and camalexin and brassinin phytoalexins. In some plant pathogenic fungi, the CWI and HOG MAPKs crosstalk to regulate responses to cell wall and oxidative stresses. In comparison with the other two MAPKs, the IG MAPK generally plays less important roles in regulating stress responses in fungal pathogens although it may be involved in the co-regulation of responses to heat, pH, and other environmental stresses together with the HOG or CWI pathway. Nevertheless, in some fungi, the IG and CWI MAPKs regulate melanin synthesis and melanization of cell wall confer resistance to various stresses
Cell wall stress
The fungal cell wall is not only important for maintaining morphology but also for protecting against environmental stresses. Like in S. cerevisiae, the CWI MAPK pathway plays a critical role in regulating responses to cell wall stress in plant pathogenic fungi, and mutants deleted of the Slt2/Mps1 ortholog have increased sensitivities to cell wall lytic enzymes and cell wall stressors such as Congo Red (CR) or Calcofluor White (CFW) (Hamel et al. 2012; Jiang et al. 2018). Autolysis of aerial hyphae is observed in old cultures of the mps1 and bck1 mutants in M. oryzae and Aspergillus flavus (Xu et al. 1998; Jeon et al. 2008; Zhang et al. 2020; Feng et al. 2021). In S. cerevisiae, cell stressors, such as CR and Caspofungin, are sensed by the Mid2 and Wsc1 sensors (Jin et al. 2013). Similar sensors appear to be involved in sensing these cell wall stressors in fungal pathogens, such as U. maydis and F. graminearum (Carbó and Pérez-Martín 2010; Xu et al. 2019). In yeast, cell wall damage caused by β-1,3-glucanase and protease activities is sensed by Sho1 and mucin Hkr1 (not Msb2) to activate Hog1, which in turn activates Slt2 (Rodríguez-Peña et al. 2013). Although whether mediated by the CWI MAPK or not is not clear, the involvement of the HOG pathway in responding to cell wall stress also has been observed in plant pathogenic fungi such as F. graminearum and S. sclerotiorum (Zheng et al. 2012; Duan et al. 2013). In A. brassicicola, both CWI and HOG pathways are involved in response to cell wall stress caused by camalexin and brassinin, two indolic phytoalexins produced by Brassica species (Joubert et al. 2011).
In some plant pathogenic fungi, the IG MAPK pathway also plays a role in cell wall stress responses. However, its importance and regulatory functions vary among different fungal pathogens. For example, deletion of ChMK1 results in hypersensitivity to CFW and CR in C. higginsianum (Wei et al. 2016), but the Cfpmk1 mutant has increased tolerance against CR and SDS in C. fructicola (Liang et al. 2019). Whereas the CcPmk1 mutant is hypersensitive to cell wall stress and cell wall lytic enzymes in Cytospora chrysosperma (Yu et al. 2019), the chk1 mutant has the hyphal autolysis and aerial hyphal growth defects in C. heterostrophus (Lev et al. 1999). In F. graminearum, both Gpmk1 and Mgv1 MAPKs are involved in regulating basal resistance to plant defensin MsDef1 but not MtDef4 (Ramamoorthy et al. 2007). In M. oryzae, deletion of a Pmk1-interacting gene PIC5 results in increased sensitivity to cell wall lytic enzyme (Zhang et al. 2011). In F. oxysporum, deletion of FMK1 in the wild type has no obvious effect on sensitivity to cell wall stress but increases the sensitivity of the mpk1 (Slt2) mutant (Segorbe et al. 2017), suggesting a minor role of the IG MAPK in cell wall integrity.
Osmotic stress
Plant pathogenic fungi may face hyperosmotic stress during infection of tissues with high sugar contents and survival in desiccated plant tissues. The HOG pathway is conserved in fungal pathogens for regulating adaptive responses to hyperosmotic stress, including the synthesis and retention of compatible osmolytes such as glycerol, arabitol, and sorbitol (Dixon et al. 1999; Zheng et al. 2016; Li et al. 2020). Deletion of HOG1 orthologs results in increased sensitivity to hyperosmotic stress in all the plant pathogenic fungi studied. Like in yeast, Hog1 orthologs are rapidly phosphorylated in response to hyperosmotic stress in fungal pathogens such as C. heterostrophus (Yoshimi et al. 2005). For the upstream histidine kinases of the HOG pathway in M. oryzae, the Mosln1 mutant is more susceptible to salt stress, but the Mohik1 mutant is more sensitive to sugar stress (Jacob et al. 2014). Downstream transcription factors of the HOG pathway that have been shown to regulate osmoregulation in fungal pathogens include Atf1 and Skn7 (Zheng et al. 2016; Tang et al. 2020). However, these transcription factors have species-specific functions in osmoregulation. For example, Atf1 is more important in F. graminearum but Skn7 is more important in B. cinerea for regulating responses to osmotic stress (Jiang et al. 2015; Viefhues et al. 2015; Yang et al. 2015).
Interestingly, knocking down the expression of PiHOG1 in Piriformospora indica, an endophyte of rice roots, results in an increased sensitivity to osmotic stress not only in the fungus but also in rice plants colonized by the knocked down strain (Jogawat et al. 2016). The accumulation of compatible osmolyte proline is reduced in rice roots inoculated with this strain, suggesting that the endophytic fungus may confer osmotic stress tolerance to the host plant by upregulating proline production via the HOG MAPK. Although likely irrelevant to plant pathogens, it is worth noting that the HOG pathway also regulates response to hypoosmotic stress in the halophilic fungus Wallemia ichthyophaga, which has two functional Hog1 MAPKs (Konte and Plemenitas 2013).
Oxidative stress
Oxidative stress can be caused by ROS generated intracellularly or exposure to oxidants from the environment or host plants. In many plant pathogenic fungi, the HOG pathway plays a critical role in oxidative stress response. Mutants deleted of the Hog1 MAPK or other key components of this pathway have increased sensitivity to oxidative stress, and some are defective in plant infection as described above. The Atf1 ortholog is one major TF functioning downstream from the HOG MAPK to regulate genes important for oxidative responses in fungal pathogens (Guo et al. 2011; Tang et al. 2020). Homologs of the response regulator Skn7, another component of the HOG pathway, also is important for oxidative stress response in fungal pathogens such as A. alternata (Chen et al. 2012), likely by regulating the expression of oxidative stress-induced genes, including those encoding catalases and superoxide dismutase (Fassler and West 2011). Nevertheless, in some fungi such as M. oryzae, the Skn7 ortholog is dispensable for response to oxidative stress and pathogenesis (Motoyama et al. 2008).
Mutants deleted of key components of the CWI MAPK pathway also have increased sensitivities to oxidative stress in some fungal pathogens such as B. cinerea (Yin et al. 2018) and F. verticillioides (Zhang et al. 2015). However, it is not clear whether this MAPK regulates stress response genes directly or by crosstalk with the HOG pathway in these fungi. In yeast, under low or moderate oxidative stress conditions, the highly O-mannosylated Mtl1 protein acts together with Wsc1 or its paralog Mid2 as the sensors of the CWI pathway to activate Slt2, which results in the translocation and degradation of cyclin C, a negative regulator of genes involved in stress responses (Vilella et al. 2005; Jin et al. 2013). Homologs of Mtl1 and Mid2 are present in the genomes of fungal pathogens but none of them have been functionally characterized.
AP1 is another b-ZIP transcription factor (conserved from yeast to human) that acts as a redox-responsive regulator for regulating oxidative stress-related genes. The C-terminal domain of Yap1 contains cysteine residues that can form an intramolecular disulfide bridge under oxidizing conditions, which enable its localization to the nucleus for activating its target genes. Although AP1 orthologs are involved in oxidative stress responses in general, their importance for plant infection vary among fungal pathogens. For example, the ChAP1 deletion mutant of C. heterostrophus is increased in sensitivity to H2O2 but has no obvious defect in virulence (Lev et al. 2005). However, mutants deleted of the AP1 ortholog are hypersensitive to oxidative stress and defective in plant infection in A. alternata, C. gloeosporioides, and M. oryzae (Lin et al. 2010; Guo et al. 2011; Sun et al. 2016). Nevertheless, the functional relationship between AP1 and MAPKs remains to be clarified in these pathogens. In the budding or fission yeast, Yap1 is a redox-sensitive transcription factor that is not known to be a direct substrate of Hog1/Sty1 MAPK, suggesting an indirect relationship. In M. oryzae, Mst7 interacts with thioredoxin Trx2, which is a target of MoAP1 and important for response to H2O2 or diamide (Zhang et al. 2016; Wang et al. 2017), suggesting a possible indirect relationship between of Pmk1 and MoAP1. The Tpc1 transcription factor that regulates NOXD expression in M. oryzae interacts with Mst12 (Galhano et al. 2017), further implicating the involvement of the IG MAPK pathway in responses to oxidative stress.
Antifungal chemicals
Treatments with fungicides or antifungals with different modes of actions likely cause target-specific stresses in fungi, which may trigger MAPK-mediated responses. One well-characterized example is the roles of multiple MAPKs in responding to cell wall stress caused by Caspofungin, CR, and CFW described above (2.1). Another example is the over-stimulation of the HOG pathway by phenylpyrrole fungicides fludioxonil and fenpiclonil. First discovered in Neurospora crassa, treatments with fludioxonil results in the accumulation of intracellular glycerol and cell burst, and null mutations in OS-2 (HOG1) confer resistance against these fungicides (Zhang et al. 2002). Fludioxonil also stimulates cell burst and Hog1 overactivation in C. lagenarium (Kojima et al. 2004), and mutants deleted of the Hog1 ortholog are resistant to fludioxonil in yeast and other plant pathogenic fungi (Jiang et al. 2018). Deletion of the HOG1 ortholog or upstream components of the HOG pathway in fungal pathogens, including homologs of yeast PBS2, SSK2/SSK2, SLN1, YPD1, and HIK1 also confer resistance or tolerance to fludioxonil. However, none of these HOG components are the direct target of fludioxonil. A recent study suggested that fludioxonil may target and inhibit triosephosphate isomerase, resulting in elevated cytosolic methyglyoxal, which in turn changes a Sln1-like group III histidine kinase into a phosphatase to constitutively activate the downstream Hog1 MAPK cascade (Brandhorst et al. 2019). Remarkably, fludioxonil and fenpiclonil have been applied to control foliar pathogens for over 30 years, but field isolates with complete resistance against these phenylpyrrole fungicides have not emerged and spread widely in crop fields (Kilani and Fillinger 2016), which may be related to the defects of HOG mutants in stress response and survival in nature.
Interestingly, the os-2, os-5 (PBS2), and ssk22 deletion mutants in N. crassa are also resistant to dicarboximide fungicides vinclozolin and iprodione (Zhang et al. 2002; Fujimura et al. 2003). Resistance against dicarboximide fungicides also has been observed in mutants defective in the HOG pathway in plant pathogens (Jiang et al. 2018). For example, in Alternaria alternata, the hog1, ssk1, skn7, and hsk1 deletion mutants all express increased tolerance at various degrees against fludioxonil and vinclozolin, with the hsk1 mutant having the highest level of tolerance (Yu et al. 2016). However, although stimulation of glycerol accumulation has been reported (Ochiai et al. 2002), the actual target of dicarboximide fungicides is not certain. In some fungi, mutants in the cAMP-PKA pathway and PKC also confer resistance against dicarboximide fungicides (Ramesh et al. 2001; Mehrabi et al. 2006). In addition, the chk1 and mps1 mutants of C. heterostrophus are slightly increased in tolerance, but the F. graminearum mgv1 mutant is increased in sensitivity to fludioxonil (Degani 2015; Ren et al. 2019), suggesting that the other two MAPKs may play minor roles in fludioxonil tolerance, likely by crosstalk with the HOG pathway.
Heat stress (HS)
Fungal pathogens must tolerate elevated temperatures in the field. Although the functions of MAPK pathways in heat stress (HS) responses, particularly the CWI pathway, are well characterized in the budding yeast, such as the activation of Slt2 via Cbk1 and Bck2, and Hog1 phosphorylation due to glycerol loss at elevated temperatures (Kuravi et al. 2011; Dunayevich et al. 2018), there are only limited studies on the regulation of heat tolerance by MAPKs in plant pathogenic fungi. The mgv1 mutant of F. graminearum has increased sensitivity to elevated temperatures, which is partially suppressed by deletion of FgHog1 (Ren et al. 2019). Like other organisms, fungi produce heat shock proteins (HSPs) and chaperones to protect proteins from aggregation and degradation. In yeast, Sfl1 is a heat shock factor-like transcriptional regulator that controls the expression of Hsp30 at 42 °C (Galeote et al. 2007). In M. oryzae, MoSfl1 functions downstream from both the cAMP signaling and Pmk1 MAPK pathways and the Mosfl1 deletion mutant has increased sensitivity to elevated temperatures (Li et al. 2011; Li et al., 2017).
The MoSsb1 HSP70-like protein interacts with MoMkk1, the MEK for Mps1, and regulates its expression. MoSsb1 forms protein complexes with MoSsz1 (another member of HSP70) and MoZuo1 (a HSP40 protein) that are important for tolerance to temperature stress in M. oryzae (Yang et al. 2018). HSP90, one of the most ubiquitous chaperones, facilitates the activation of Slt2 in response to heat stress, and its client proteins that include Hog1 in S. cerevisiae and Candida albicans (Leach et al. 2012). In plant pathogens, HSP90 likely has similar functions in response to heat and other stresses via its association with MAPKs. Interestingly, some plant pathogens produce resorcyclic acid lactones such as zearalenone, which may be inhibitory to plant and fungal HSP90 proteins during their interactions with host plant (pathogenesis) or other fungi (competition).
Responses to other environmental factors
Although there are only limited studies, fungal MAPKs also have been implicated in responding to other environmental stresses. In M. oryzae, vegetative growth of the Mohik5 and Mohik9 mutants is sensitive to hypoxia-inducing NaNO2 and treatment with NaNO2 resulted in Osm1 phosphorylation, suggesting a role for the HOG pathway in response to hypoxia (Jacob et al. 2014). In Trichoderma atroviride, silencing of ThHOG1 (TMK3) results in a minor increase in sensitivity to 40 mM CuSO4 (heavy metal stress) that stimulates ThHog1 phosphorylation (Delgado-Jarana et al. 2006). The HOG pathway is also involved in regulating DNA repair in T. atroviride because the tmk3 and pbs2 mutants are highly sensitive to UV irradiation when incubated in dark (Esquivel-Naranjo et al. 2016). Furthermore, light stimulates Tmk3 phosphorylation, and deletion of TMK3 affects the expression of genes induced by blue light (Esquivel-Naranjo et al. 2016). The HOG pathway is known to be involved in light response and circadian rhythm in the model filamentous ascomycetes N. crassa and Aspergillus nidulans (Bennett et al. 2013; Yu et al. 2021).
In F. oxysporum, alkaline pH induces the rapid phosphorylation of Fmk1 MAPK, whereas shifting to acidic pH (5.0) results in its dephosphorylation (Masachis et al. 2016). Because rapid alkalization or acidification of infected tissues may be parts of plant defense responses, it is possible that fungal pathogens use changes in pH to trigger the expression of certain effectors or virulence factors via the IG MAPK pathway. The biosynthesis of melanin, an excellent photoprotectant, is also known to be regulated by the IG MAPK pathway in plant pathogenic fungi, such as C. gloeosporioides, C. heterostrophus, and V. dahliae (Lev et al. 1999; Rauyaree et al. 2005; He et al. 2017). Melanization of the cell wall provides protection against various stresses, including ionizing and UV irradiation, host defensive compounds, elevated ROS, and fungivores. In fact, many fungi that live in extreme habitats such as extreme cold are melanized (Gessler et al. 2014). Regulation of melanin synthesis by the IG MAPK pathway suggests its indirect involvement in response to various environmental stresses, likely together with the HOG and/or CWI MAPKs. In C. heterostrophus, both Chk1 and Mps1 MAPKs are involved in the regulation of CMR1 and melanin synthesis (Eliahu et al. 2007).
Roles of MAPK signaling in biotic stress responses
Besides infection of their hosts, plant pathogenic fungi also have to compete with other microbes in the environment or on plant surface for survival and propagation. In comparison with their roles in pathogenesis and response to abiotic stresses, functions of MAPKs in the interactions of fungal pathogens with mycoviruses, bacteria, and other fungi are under-investigated (Fig. 3).
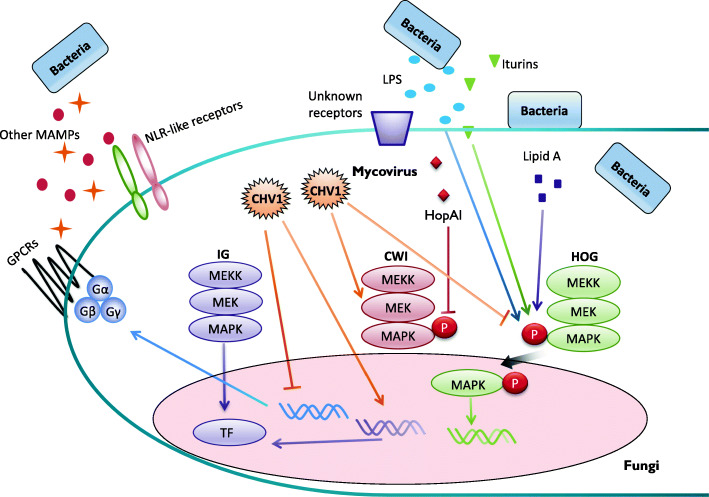
Fig. 3.
Roles of MAPK signaling in fungal interactions with viruses and bacteria. Although with only limited studies, the HOG and CWI pathways have been implicated in bacteria-fungal interactions (BFIs). For examples, iturins, a special class of pore-forming lipopeptides secreted by B. amyloliquefaciens, and E. coli lipopolysaccharide (LPS) or endotoxin lipid A stimulate the phosphorylation of Hog1. Activated Hog1 MAPK localizes to the nucleus and regulates the expression of GPD1 and other downstream targets. Whereas the recognition of MAMPs (microbe-associated molecular pattern) such as LPS by GPCR and NLR-like receptors in fungal pathogens may lead to the activation of MAPK pathways, bacteria may secrete effector proteins to suppress the activation of MAPKs involved in BFIs. In M. oryzae, expression of the bacterial effector HopAI significantly reduces the phosphorylation of Mps1 and therefore inactivates the CWI signaling. Similarly, fungal pathogens likely utilize MAPKs to regulate defense against mycoviruses but mycovirus infection may directly or indirectly interfere with MAPK signaling in fungal pathogens. In C. parasitica, CHV1 infection reduces the expression of trimeric G-proteins and downstream Ste12 transcription factor of the IG MAPK pathway. CHV1 infection also affects the phosphorylation of the MEK (positively) of the CWI pathway and Hog1 MAPK (negatively) of the HOG pathway
Interactions with bacteria
Fungal and bacteria can form a range of physical associations, and bacteria-fungal interactions (BFI) can affect the ecosystems in which they coexist or their associations with host plants or animals (Deveau et al. 2018). Whereas ‘fungiphile’ bacteria are preferentially associated with fungi and mainly live in the ‘hyphosphere’ habitat surrounding hyphae, fungal endobacteria live inside hyphal cells as symbionts. Some bacteria are parasites that degrade fungal cells but others simply use hyphae for bacterial transport, such as the utilization of F. oxysporum hyphae by Rahnella aquatilis (Palmieri et al. 2020). In these diverse and dynamic physical interactions, both bacteria and fungi are active in signaling and signal recognition for responding appropriately to their partners. Fungal quorum sensing molecules (such as farnesol and tyrosol) and bacterium-secreted metabolites (such as quinolones and homoserine lactones) have been implicated in inter-kindom signaling (Chatterjee et al. 2020; Sharma et al. 2020). However, the roles of fungal MAP kinase pathways in the establishment of BFI or BFI networks have not been well studied. In S. cerevisiae, treatments with Escherichia coli lipopolysaccharide (LPS) and endotoxically active lipid A stimulate the phosphorylation of Hog1 and its translocation to the nucleus, as well as the expression of its downstream target GPD1 (Marques et al. 2006). In V. dahliae, treatment with iturins, pore-forming lipopeptides, produced by Bacillus amyloliquefaciens, activates Hog1 and causes cell wall integrity defects (Han et al. 2015). During symbiotic establishment with Mycetohabitans endobacteria, several components of the HOG pathway and its downstream targets were upregulated in Rhizopus microsporus (Lastovetsky et al. 2016). These observations indicate a role of the HOG MAPK pathway in fungal-bacteria interactions. Furthermore, the TOR kinase targeted by rifamycin functions upstream from MAP kinase cascades in S. cerevisiae and filamentous fungi (Loewith and Hall 2011; Inoue and Nomura 2018), suggesting indirect inhibition of fungal MAPKs by antibiotics produced by bacteria.
Recognition of microbe-associated molecular patterns (MAMPs) by Nod-like immune receptors (NLRs) leads to the activation of downstream MAPKs for regulating defense responses in plants and animals. Secreted effectors of some bacterial pathogens are known to interfere with host MAPK signaling (Shan et al. 2007; Krachler et al. 2011). NLR-like receptor genes are widely distributed in filamentous fungi, and some of them may be involved in MAMP recognition (Uehling et al. 2017) to activate fungal MAPKs for defense response against bacteria. On the other hand, fungal MAPKs may be targeted by bacterial effectors for inhibition. Consistent with this hypothesis, expression of the Pseudomonas syringae effector HopAI, a MAPK inhibitor, significantly reduces the phosphorylation level of Mps1 and Mgv1 MAPKs in M. oryzae and F. graminearum (Zhang et al. 2017). Along the same line, some of the bacterial species used for biocontrol against fungal pathogens may target fungal MAPKs for interfering with their pathogenesis-related functions. To test these hypotheses and better understand the roles of fungal MAPKs in BFI, it will be helpful to have a model plant pathogenic fungus for studying fungal-bacterial interactions during plant infection.
Fungal-mycovirus interactions
Like plants and animals, fungi are also susceptible to infections by mycoviruses. The best characterized mycovirus is Cryphonectria hypovirus 1 (CHV1) that causes hypovirulence and defects in sexual/asexual development in C. parasitica. CHV1 infection interferes with the expression levels of the trimeric G-proteins that function upstream from both cAMP signaling and MAPK pathways (Dawe and Nuss 2013). Although the exact mechanisms are not clear, all three MAPKs in C. parasitica are involved in its interactions with CHV1. The first MAPK found to be affected by mycovirus infection is CpMK1 (Hog1). The phosphorylation of CpMK1 under hyperosmotic conditions is reduced in the virus-infected hypovirulent strain, which has increased osmotic sensitivity (Park et al. 2004), indicating the suppression of CpMK1 activation by CHV1.
Unlike CpMK1, the level of CpMK2 (Pmk1) phosphorylation is not affected but its downstream TF CpSte12 is down-regulated by CHV1 infection (Choi et al. 2005; Deng et al. 2007). Furthermore, only the CpKK2 (CpSte7) deletion mutant, not mutants deleted of the two other MEK genes, could not be infected with CHV1 virus or transformed with infectious CHV1 cDNA via protoplasts (Turina et al. 2016), suggesting a role of this MAPK pathway in mycovirus infection and replication. In C. parasitica, CpPK1, a Cot-1 homolog, is transcriptionally upregulated by CHV1 and overexpressing CpPK1 reproduces some of the viral symptoms (Kim et al. 2004). CpPK1 may be functionally related to the IG MAPK pathway because spontaneous suppressor mutations in this pathway can partially rescue the cot-1 mutant, and the scaffold protein Hym1 for the Cot-1 NDR kinase complex is essential for the activation of this MAPK in N. crassa (Maerz et al. 2008; Dettmann et al. 2012).
For the CWI pathway, CHV1 partially represses the dephosphorylation of CpKK1 MEK, resulting in its elevated phosphorylation and likely the hyperactivation of its downstream MAPK (Turina et al. 2016). Furthermore, studies in other fungi such as F. graminearum and N. crassa have showed that the CWI MAPK is essential for hyphal fusion (Hou et al. 2002; Fischer and Glass, 2019). Because cell death triggered by fusion between vegetative incompatible strains protects against the spreading of mycoviruses in fungal hyphae (Dawe and Nuss 2013), the CWI MAPK pathway likely plays an indirect but important role in defense against mycoviruses in plant pathogenic fungi in general.
Fungal-fungal interactions
Many fungi also co-exist or compete against each other in nature. Whereas some interactions are mediated via antagonistic/inhibitory compounds or metabolites released into the environment, some involve direct hyphal-hyphal contacts, leading to hyphal fusion and mycoparasitism or heterokaryosis. Although signaling compounds and their receptors remain to be identified, two MAP kinase pathways and the Striatin-Interacting protein Phosphatase And Kinase (STRIPAK) complex have been implicated in regulating chemotropism and hyphal fusion, an integrated process of colonial growth in the saprophytic model fungus N. crassa and Sordaria macrospora (Reschka et al. 2018; Fischer and Glass, 2019). In N. crassa, the Soft protein functions as a scaffold for the upstream components of the CWI MAPK pathway and the Soft complexes undergo oscillations of assembly and disassembly (4-min intervals) at hyphal tips during chemotropic interactions. Ham-5 functions as the scaffold protein for the Mak-2 (Kss1) cascade, and the Ham-5 complexes mirror the Soft complexes in 4-min interval oscillations of assembly and disassembly at hyphal tips in interacting hyphae. Mirroring oscillations of the Soft and Ham-5 complexes may be related to their functions in signal secretion and reception, respectively (Goryachev et al. 2012). The STRIPAK complex (Kück et al. 2016) also localizes to the hyphal tip and cross-talks with Mak1 and Mak-2 pathways to regulate hyphal fusion. Besides their physical associations detected by mass spectrometry analysis, key components of the Soft, Ham-5, and STRIPAK complexes are required for full phosphorylation of Mak-1 and Mak-2 MAPK (Dettmann et al. 2014; Fischer and Glass, 2019). Furthermore, PP-1 (Ste12) of the Mak-2 pathway directly activates the expression of Adv-1 that functions downstream from Mak-1 for regulating genes important for hyphal fusion (Fischer et al. 2018). However, the functions of these three complexes in hyphal fusion may be not well conserved in fungal pathogens. In F. graminearum, Mgv1 MAPK is essential for hyphal fusion and heterokaryon formation (Hou et al. 2002) but hyphal fusion still occurs in the Fgso (Fgsoft) and Gpmk1 deletion mutants (Zheng et al. 2013).
Hyphal fusion can occur between hyphae of the same strain, different strains of the same species, or different species. In N. crassa, the doc genes regulate non-self recognition before hyphal fusion (Heller et al. 2016), but the Het genes control heterokaryon incompatibility after hyphal fusion (Glass and Kaneko 2003). Whereas fusion between incompatible strains leads to cell death in heterokaryotic cells, heterokaryons are formed between compatible strains, which may lead to parasexual reproduction and somatic recombination to increase genetic variation in asexual fungal pathogens (Clutterbuck 1996; Daskalov et al. 2017). Although studies are lacking, MAPKs may also play regulatory roles in parasexual reproduction processes after hyphal fusion and heterokaryon formation.
Mycoparasitism also involves recognition and hyphal attachment but results in the killing and degradation of host hyphae by the mycoparasite after hyphal fusion or penetration. Mycoparasitic fungi are often facultative parasites that switch from saprophytic to parasitic growth when triggered by host-derived signals such as oligopeptides or oligochitosaccharides and secondary metabolites (Druzhinina et al. 2011; Holzlechner et al. 2016). The best characterized mycoparasitic fungi are Trichoderma species, such as T. atroviride and T. virens that are used for biocontrol of fungal diseases. However, biocontrol involves interactions with the host plant, and some Trichoderma species can stimulate plant immune responses. Therefore, whereas the roles of MAPKs in hyphal-hyphal interactions may be conserved during mycoparasitism, their functions in biocontrol may involve species- or strain-specific stimulation of plant immunity.
All three MAPKs named as Tmk1/TmkA (Kss1), Tmk2/TmkB (Slt2), and Tmk3/TmkC (Hog1) have been characterized in Trichoderma species. In T. virens, a gliovirin producer, the tmkA and tmkB deletion mutants both are defective in vegetative growth and reduced in mycoparasitism against S. rolfsii but normal against Rhizoctonia solani, indicating overlapping functions of these two MAPKs (Mukherjee et al. 2003; Mukherjee et al. 2012; Kück et al. 2016). Whereas TmkB is important for cell wall integrity, Tmk1 regulates mycoparasitism-relatedt genes, showing their distinct functions. In T. atroviride, the tmk1 deletion mutant is reduced in mycoparasitic ability to overgrow host fungi but increased in antifungal activities and ability to protect against R. solani infection in bean (Reithner et al. 2007), and TaSte12 is involved in regulating hyphal interactions (coiling) and mycoparasitic activity (Gruber and Zeilinger 2014). These results suggest that the Tmk1/TmkA MAPK has a conserved role in mycoparasitism by regulating the expression of mycoparasitism-related genes (such as chitinase genes and genes responsible for synthesis of antifungal metabolites) and mycoparasitic hyphal interactions. Consistent with these observations, deletion of the TMK1 ortholog in Clonostachys chloroleuca, another mycoparasitic fungus, also affects the expression of cell wall lytic enzymes, mycoparasitic ability, and biocontrol (Sun et al. 2020). However, deletion of this MAPK gene in a gliovirin-deficient mutant of T. virens increases mycoparasitic activity against R. solani (Mendoza-Mendoza et al. 2003), which is contradictory to the regulation of mycoparasitic hyphal-hyphal interactions by this MAPK pathway.
As expected, Tmk3/ThHog1 regulates responses to hyperosmotic and oxidative stresses in T. atroviride and T. harzianum (Delgado-Jarana et al. 2006; Esquivel-Naranjo et al. 2016). In T. harzianum, deletion of ThHOG1 reduces its antagonistic activity against host fungi Phoma betae and C. acutatum. ThHog1 likely plays a role in mycoparasitism by regulating responses to toxic compounds produced by host fungi. Although studies are lacking, the host fungi may also use Hog1 or other MAPKs to regulate defense or antagonistic responses against mycoparasitic fungi or other predators. Interestingly, Tmk3 is involved in the repression of subsets of secondary metabolism (SM) genes that are stimulated by mechanical wounding but suppressed by Drosophila larvae (Atriztán-Hernández et al. 2019). In comparison with the wild type, Drosophila larvae prefer feeding on hyphae of the tmk3 mutant but have a higher mortality rate, suggesting a role of this MAPK in interactions with fungivorous insects.
Perspectives
In phytopathogenic fungi, the well-conserved IG, CWI, and HOG MAPKs have both conserved and species-specific functions in regulating plant infection, stress responses, growth, and sexual or asexual development (Hamel et al. 2012; Jiang et al. 2018). However, it is worth mentioning that, to date, most of the MAPK studies in plant pathogenic fungi deal with ascomycetous pathogens. Based on studies in U. maydis, the components and functions of MAPK pathways in basidiomycetes are likely different from those in phytopathogenic ascomycetes. Also, most of these MAPK studies in fungal pathogens are based on targeted gene knockout mutants. Proteomics studies in yeast have shown that MAPKs often are hubs in the protein-protein networks (Chen and Snyder 2010). Therefore, some of the phenotypes observed with mutants disrupted in MAPK signaling may be due to indirect effects via protein-protein interaction networks. It will be important to determine the effects of transient inhibition with ATP analog PP-1 on MAPKs with appropriate mutants at the ATP binding site (Sakulkoo et al. 2018) for comparisons.
For all the known upstream sensors or receptors of yeast MAPK pathways, their homologs are present in fungal pathogens, including GPCRs, Sho1, Msb1, Wsc1–3, Mst11, Mid2, and Sln1 (Jendretzki et al. 2011; Alvaro and Thorner 2016; Vázquez-Ibarra et al. 2020). However, in comparison with other sensors, GPCRs are significantly expanded in fungal pathogens. In the budding yeast, three typical GPCR genes encode two pheromone receptors and one glucose sensor. In M. oryzae, there are over 40 putative GPCRs, and at least one of them with the CFEM domain, PTH11, has been convincingly shown to be important for appressorium formation and virulence (Kulkarni et al. 2005). F. graminearum has over 100 putative GPCRs, including 12 CFEM GPCRs and a subfamily of infection-related or specific GPCRs (Jiang et al. 2019). These GPCRs may be involved in sensing different host and environmental signals to activate downstream MAPKs. Some of them may be involved in sensing their own signals (quorum sensing) or ligands from other microbes during fungal-fungal or fungal-bacterial interactions.
Unlike their roles in pathogenesis or mycoparasitism, the roles of fungal MAPK pathways in defensive interactions against viruses, bacteria, and other fungi have not been well studied. Nevertheless, several publications have shown that microbes have the capacity to activate fungal MAPK signaling, possibly by the recognition of microbial signals/ MAMPs by membrane receptors. The presence of putative NLRs in fungal pathogens suggest that MAPK signaling may function downstream from some of those NLRs involved in MAMP recognition. For genes regulated by MAPKs for defense responses, there are only very limited studies. Fungi are known to secrete enzymes, toxins, and other secondary metabolites to compete with other microbes in the environment or on the plant surface. Genes encoding defense-related enzymes or proteins may be regulated by multiple MAPKs, and the activation of multiple MAPK pathways can alter the composition of the surrounding microbiota. Therefore, to better understand the role of fungal MAPKs in biotic interactions, the single, double, or triple MAPK mutants should be comparatively studied for changes in fungal-bacterial or fungal-fungal interactions.
Another important area is to further characterize the functional relationships among these MAPK pathways. A considerable amount of evidence shows the crosstalk between HOG and CWI, CWI and IG, or IG and HOG pathways in fungal pathogens in responses to biotic and abiotic stresses. Nevertheless, fungal pathogens must utilize all these signaling pathways to coordinately regulate responses to a variety of stresses encountered during plant infection or survival in nature. Therefore, it will be helpful to systematically characterize the functional relationships among all three MAPK cascades in responses to different stresses or during different infection and developmental stages. These MAPKs may share some common downstream targets and upstream components or regulators/sensors. Furthermore, all three MAPKs are likely involved in crosstalk with other signaling pathways. For example, the IG MAPK is known to interact with the cAMP-PKA pathways in regulating infection structure formation and invasive growth in M. oryzae and other fungi (Jiang et al. 2018). Crosstalk between calcium signaling and CWI and other MAPKs also occur in plant pathogens (Wurzinger et al. 2011). It will be important to use transcriptomics and proteomics approaches to systematically characterize the crosstalk and functional relationships among these well-conserved signal transduction pathways.
Acknowledgements
We thank Dr. Huiquan Liu for discussions and assistance during the preparation of this manuscript. We also thank Dr. Larry Dunkle for his assistance during the preparation of this manuscript. This work is supported by grants from National Natural Science Foundation of China (no. 31772114) to JC, the Natural Science Basic Research Plan in Shaanxi Province of China (no. 2020JQ-252) to XZ and grants from USWBSI and NSF to JX.
Code availability (software application or custom code)
Not applicable.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Authors’ contributions
JX XZ and CJ write the manuscript. XZ CJ and ZW draw the figures. The author(s) read and approved the final manuscript.
Funding
This work is supported by grants from National Natural Science Foundation of China (no. 31772114) to JC, the Natural Science Basic Research Plan in Shaanxi Province of China (no. 2020JQ-252) to XZ and grants from USWBSI CI and NSF to JX.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
All authors consent to participate.
Consent for publication
All authors consent for publication.
Competing interests
Author JX is a member of the Editorial Board and was not involved in the journal's review of, or decisions related to, this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alvaro CG, Thorner J. Heterotrimeric G protein-coupled receptor signaling in yeast mating pheromone response. J Biol Chem. 2016;291:7788–7795. doi: 10.1074/jbc.r116.714980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atriztán-Hernández K, Moreno-Pedraza A, Winkler R, Markow T, Herrera-Estrella A (2019) Trichoderma atroviride from predator to prey: Role of the mitogen-activated protein kinase Tmk3 in fungal chemical defense against fungivory by Drosophila melanogaster larvae. Appl Environ Microbiol 85:e01825–e01818. 10.1128/aem.01825-18 [DOI] [PMC free article] [PubMed]
- Bashi ZD, Gyawali S, Bekkaoui D, Coutu C, Lee L, Poon J et al (2016) The Sclerotinia sclerotiorum Slt2 mitogen-activated protein kinase ortholog, SMK3, is required for infection initiation but not lesion expansion. Can J Microbiol 62:836–850. 10.1139/cjm-2016-0091 [DOI] [PubMed]
- Bennett LD, Beremand P, Thomas TL, Bell-Pedersen D (2013) Circadian activation of the mitogen-activated protein kinase MAK-1 facilitates rhythms in clock-controlled genes in Neurospora crassa. Eukaryot Cell 12:59–69 10.1128/ec.00207-12 [DOI] [PMC free article] [PubMed]
- Brandhorst TT, Kean IRL, Lawry SM, Wiesner DL, Klein BS. Phenylpyrrole fungicides act on triosephosphate isomerase to induce methylglyoxal stress and alter hybrid histidine kinase activity. Sci Rep. 2019;9:5047. doi: 10.1038/s41598-019-41564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunsdorf C, Mailänder-Sánchez D, Schaller M. Fungal sensing of host environment. Cell Microbiol. 2016;18:1188–1200. doi: 10.1111/cmi.12610. [DOI] [PubMed] [Google Scholar]
- Brewster JL, Gustin MC. Hog1: 20 years of discovery and impact. Sci Signal. 2014;7:re7. doi: 10.1126/scisignal.2005458. [DOI] [PubMed] [Google Scholar]
- Carbó N, Pérez-Martín J (2010) Activation of the cell wall integrity pathway promotes escape from G2 in the fungus Ustilago maydis. Plos Genet 6:e1001009. 10.1371/journal.pgen.1001009 [DOI] [PMC free article] [PubMed]
- Chatterjee P, Sass G, Swietnicki W, Stevens DA (2020) Review of potential Pseudomonas weaponry, relevant to the Pseudomonas-Aspergillus interplay, for the mycology community. J Fungi 6:81. 10.3390/jof6020081 [DOI] [PMC free article] [PubMed]
- Chen LH, Lin CH, Chung KR (2012) Roles for SKN7 response regulator in stress resistance, conidiation and virulence in the citrus pathogen Alternaria alternata. Fungal Genet Biol 49:802–813. 10.1016/j.fgb.2012.07.006 [DOI] [PubMed]
- Chen R, Snyder M. Yeast proteomics and protein microarrays. J Proteomics. 2010;73:2147–2157. doi: 10.1016/j.jprot.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RE, Thorner J (2007) Function and regulation in MAPK signaling pathways: Lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta Mol Cell Res 1773:1311–1340. 10.1016/j.bbamcr.2007.05.003 [DOI] [PMC free article] [PubMed]
- Choi ES, Chung HJ, Kim MJ, Park SM, Cha BJ, Yang MS, Kim DH (2005) Characterization of the ERK homologue CpMK2 from the chestnut blight fungus Cryphonectria parasitica. Microbiology 151:1349–1358. 10.1099/mic.0.27796-0 [DOI] [PubMed]
- Clutterbuck AJ. Parasexual recombination in fungi. J Genet. 1996;75:281–286. doi: 10.1007/bf02966308. [DOI] [Google Scholar]
- Cousin A, Mehrabi R, Guilleroux M, Dufresne M, TVDL, Waalwijk C et al (2006) The MAP kinase-encoding gene MgFus3 of the non-appressorium phytopathogen Mycosphaerella graminicola is required for penetration and in vitro pycnidia formation. Mol Plant Pathol 7:269–278. 10.1111/j.1364-3703.2006.00337.x [DOI] [PubMed]
- Daskalov A, Heller J, Herzog S, Fleißner A, Glass NL (2017) Molecular mechanisms regulating cell fusion and heterokaryon formation in filamentous fungi. Microbiol Spectr 5: FUNK-0015-2016. 10.1128/microbiolspec.funk-0015-2016 [DOI] [PMC free article] [PubMed]
- Dawe AL, Nuss DL. Hypovirus molecular biology: from Koch’s postulates to host self-recognition genes that restrict virus transmission. Adv Virus Res. 2013;86:109–147. doi: 10.1016/B978-0-12-394315-6.00005-2. [DOI] [PubMed] [Google Scholar]
- Degani O (2015) Mediation of fludioxonil fungicide activity and resistance through Cochliobolus heterostrophus G-protein and MAPK signaling pathways. Phytoparasitica 43:215–228. 10.1007/s12600-014-0434-1
- Delgado-Jarana J, Sousa S, González F, Rey M, Llobell A (2006) ThHog1 controls the hyperosmotic stress response in Trichoderma harzianum. Microbiology 152:1687–1700. 10.1099/mic.0.28729-0 [DOI] [PubMed]
- Deng F, Allen TD, Hillman BI, Nuss DL (2007) Comparative analysis of alterations in host phenotype and transcript accumulation following hypovirus and mycoreovirus infections of the chestnut blight fungus Cryphonectria parasitica. Eukaryot Cell 6:1286–1298. 10.1128/ec.00166-07 [DOI] [PMC free article] [PubMed]
- Dettmann A, Heilig Y, Valerius O, Ludwig S, Seiler S. Fungal communication requires the MAK-2 pathway elements STE-20 and RAS-2, the NRC-1 adapter STE-50 and the MAP kinase scaffold HAM-5. Plos Genet. 2014;10:e1004762. doi: 10.1371/journal.pgen.1004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmann A, Illgen J, März S, Schürg T, Fleissner A, Seiler S (2012) The NDR kinase scaffold HYM1/MO25 is essential for MAK2 map kinase signaling in Neurospora crassa. Plos Genet 8:e1002950. 10.1371/journal.pgen.1002950 [DOI] [PMC free article] [PubMed]
- Deveau A, Bonito G, Uehling J, Paoletti M, Becker M, Bindschedler S, et al. Bacterial-fungal interactions: ecology, mechanisms and challenges. FEMS Microbiol Rev. 2018;42:335–352. doi: 10.1093/femsre/fuy008. [DOI] [PubMed] [Google Scholar]
- DeZwaan TM, Carroll AM, Valent B, Sweigard JA (1999) Magnaporthe grisea Pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell 11:2013–2030. 10.2307/3871094 [DOI] [PMC free article] [PubMed]
- Dixon KP, Xu JR, Smirnoff N, Talbot NJ (1999) Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11:2045–2058 [DOI] [PMC free article] [PubMed]
- Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E et al (2011) Trichoderma: the genomics of opportunistic success. Nat Rev Microbiol 9:749–759. 10.1038/nrmicro2637 [DOI] [PubMed]
- Duan Y, Ge C, Liu S, Wang J, Zhou M (2013) A two-component histidine kinase Shk1 controls stress response, sclerotial formation and fungicide resistance in Sclerotinia sclerotiorum. Mol Plant Pathol 14:708–718. 10.1111/mpp.12041 [DOI] [PMC free article] [PubMed]
- Dunayevich P, Baltanás R, Clemente JA, Couto A, Sapochnik D, Vasen G, Colman-Lerner A. Heat-stress triggers MAPK crosstalk to turn on the hyperosmotic response pathway. Sci Rep. 2018;8:15168. doi: 10.1038/s41598-018-33203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliahu N, Igbaria A, Rose MS, Horwitz BA, Lev S (2007) Melanin biosynthesis in the maize pathogen Cochliobolus heterostrophus depends on two mitogen-activated protein kinases, Chk1 and Mps1, and the transcription factor Cmr1. Eukaryot Cell 6:421–429. 10.1128/ec.00264-06 [DOI] [PMC free article] [PubMed]
- Esquivel-Naranjo EU, García-Esquivel M, Medina-Castellanos E, Correa-Pérez VA, Parra-Arriaga JL, Landeros-Jaime F et al (2016) A Trichoderma atroviride stress-activated MAPK pathway integrates stress and light signals. Mol Microbiol 100:860–876. 10.1111/mmi.13355 [DOI] [PubMed]
- Fassler JS, West AH. Fungal Skn7 stress responses and their relationship to virulence. Eukaryot Cell. 2011;10:156–167. doi: 10.1128/ec.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Yin Z, Wu H, Liu P, Liu X, Liu M et al (2021) Balancing of the mitotic exit network and cell wall integrity signaling governs the development and pathogenicity in Magnaporthe oryzae. Plos Pathog 17:e1009080. 10.1371/journal.ppat.1009080 [DOI] [PMC free article] [PubMed]
- Fischer MS, Glass NL (2019) Communicate and fuse: How filamentous fungi establish and maintain an interconnected mycelial network. Front Microbiol 10:619. 10.3389/fmicb.2019.00619 [DOI] [PMC free article] [PubMed]
- Fischer MS, Wu VW, Lee JE, O’Malley RC, Glass NL (2018) Regulation of cell-to-cell communication and cell wall integrity by a network of map kinase pathways and transcription factors in Neurospora crassa. Genetics 209:489–506. 10.1534/genetics.118.300904 [DOI] [PMC free article] [PubMed]
- Francisco CS, Zwyssig MM, Palma-Guerrero J (2020) The role of vegetative cell fusions in the development and asexual reproduction of the wheat fungal pathogen Zymoseptoria tritici. BMC Biol 18:99. 10.1186/s12915-020-00838-9 [DOI] [PMC free article] [PubMed]
- Fujimura M, Ochiai N, Oshima M, Motoyama T, Ichiishi A, Usami R, et al. Putative homologs of SSK22 MAPKK kinase and PBS2 MAPK kinase of Saccharomyces cerevisiae encoded by os-4 and os-5 genes for osmotic sensitivity and fungicide resistance in Neurospora crassa. Biosci Biotechnol Biochem. 2003;67:186–191. doi: 10.1271/bbb.67.186. [DOI] [PubMed] [Google Scholar]
- Galeote VA, Alexandre H, Bach B, Delobel P, Dequin S, Blondin B. Sfl1p acts as an activator of the HSP30 gene in Saccharomyces cerevisiae. Curr Genet. 2007;52:55–63. doi: 10.1007/s00294-007-0136-z. [DOI] [PubMed] [Google Scholar]
- Galhano R, Illana A, Ryder LS, Rodríguez-Romero J, Demuez M, Badaruddin M, et al. Tpc1 is an important Zn (II)2Cys6 transcriptional regulator required for polarized growth and virulence in the rice blast fungus. Plos Pathog. 2017;13:e1006516–e1006516. doi: 10.1371/journal.ppat.1006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler NN, Egorova AS, Belozerskaia TA. Melanin pigments of fungi under extreme environmental conditions. Appl Biochem Micriobiol. 2014;50:105–113. doi: 10.7868/s0555109914020093. [DOI] [PubMed] [Google Scholar]
- Glass NL, Kaneko I. Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot Cell. 2003;2:1–8. doi: 10.1128/ec.2.1.1-8.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryachev AB, Lichius A, Wright GD, Read ND. Excitable behavior can explain the “ping-pong” mode of communication between cells using the same chemoattractant. Bioessays. 2012;34:259–266. doi: 10.1002/bies.201100135. [DOI] [PubMed] [Google Scholar]
- Gruber S, Zeilinger S. The transcription factor Ste12 mediates the regulatory role of the Tmk1 MAP kinase in mycoparasitism and vegetative hyphal fusion in the filamentous fungus Trichoderma atroviride. Plos One. 2014;9:e111636. doi: 10.1371/journal.pone.0111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Chen Y, Du Y, Dong Y, Guo W, Zhai S, et al. The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae. Plos Pathog. 2011;7:e1001302. doi: 10.1371/journal.ppat.1001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel LP, Nicole MC, Duplessis S, Ellis BE. Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell. 2012;24:1327–1351. doi: 10.1105/tpc.112.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Wu F, Wang X, Qi H, Shi L, Ren A, et al. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ Microbiol. 2015;17:1166–1188. doi: 10.1111/1462-2920.12538. [DOI] [PubMed] [Google Scholar]
- He P, Wang Y, Wang X, Zhang X, Tian C. The mitogen-activated protein kinase CgMK1 governs appressorium formation, melanin synthesis, and plant infection of Colletotrichum gloeosporioides. Front Microbiol. 2017;8:2216. doi: 10.3389/fmicb.2017.02216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J, Zhao J, Rosenfield G, Kowbel DJ, Gladieux P, Glass NL. Characterization of greenbeard genes involved in long-distance kind discrimination in a microbial eukaryote. Plos Biol. 2016;14:e1002431. doi: 10.1371/journal.pbio.1002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzlechner M, Reitschmidt S, Gruber S, Zeilinger S, Marchetti-Deschmann M. Visualizing fungal metabolites during mycoparasitic interaction by MALDI mass spectrometry imaging. Proteomics. 2016;16:1742–1746. doi: 10.1002/pmic.201500510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Xue C, Peng Y, Katan T, Kistler HC, Xu JR. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant-Microbe Interact. 2002;15:1119–1127. doi: 10.1094/mpmi.2002.15.11.1119. [DOI] [PubMed] [Google Scholar]
- Hu G, Kamp A, Linning R, Naik S, Bakkeren G. Complementation of Ustilago maydis MAPK mutants by a wheat leaf rust, Puccinia triticina homolog: potential for functional analyses of rust genes. Mol Plant-Microbe Interact. 2007;20:637–647. doi: 10.1094/mpmi-20-6-0637. [DOI] [PubMed] [Google Scholar]
- Ikner A, Shiozaki K. Yeast signaling pathways in the oxidative stress response. Mutat Res. 2005;569:13–27. doi: 10.1016/j.mrfmmm.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Nomura W (2018) TOR signaling in budding yeast. In: Abdulkhair WMH (ed) The yeast role in medical applications. IntechOpen, Rijeka. 10.5772/intechopen.70784
- Jacob S, Foster AJ, Yemelin A, Thines E. Histidine kinases mediate differentiation, stress response, and pathogenicity in Magnaporthe oryzae. MicrobiologyOpen. 2014;3:668–687. doi: 10.1002/mbo3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Schüffler A, Thines E. Hog1p activation by marasmic acid through inhibition of the histidine kinase Sln1p. Pest Manag Sci. 2016;72:1268–1274. doi: 10.1002/ps.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendretzki A, Wittland J, Wilk S, Straede A, Heinisch JJ. How do I begin? Sensing extracellular stress to maintain yeast cell wall integrity. Eur J Cell Biol. 2011;90:740–744. doi: 10.1016/j.ejcb.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Jeon J, Goh J, Yoo S, Chi MH, Choi J, Rho HS, et al. A putative MAP kinase kinase kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Mol Plant-Microbe Interact. 2008;21:525–534. doi: 10.1094/mpmi-21-5-0525. [DOI] [PubMed] [Google Scholar]
- Jiang C, Cao S, Wang Z, Xu H, Liang J, Liu H, et al. An expanded subfamily of G-protein-coupled receptor genes in Fusarium graminearum required for wheat infection. Nat Microbiol. 2019;4:1582–1591. doi: 10.1038/s41564-019-0468-8. [DOI] [PubMed] [Google Scholar]
- Jiang C, Zhang S, Zhang Q, Tao Y, Wang C, Xu JR. FgSKN7 and FgATF1 have overlapping functions in ascosporogenesis, pathogenesis and stress responses in Fusarium graminearum. Environ Microbiol. 2015;17:1245–1260. doi: 10.1111/1462-2920.12561. [DOI] [PubMed] [Google Scholar]
- Jiang C, Zhang X, Liu H, Xu JR. Mitogen-activated protein kinase signaling in plant pathogenic fungi. Plos Pathog. 2018;14:e1006875. doi: 10.1371/journal.ppat.1006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Gutiérrez E, Alegría-Carrasco E, Sellers-Moya Á, Molina M, Martín H. Not just the wall: the other ways to turn the yeast CWI pathway on. Int Microbiol. 2020;23:107–119. doi: 10.1007/s10123-019-00092-2. [DOI] [PubMed] [Google Scholar]
- Jin C, Parshin AV, Daly I, Strich R, Cooper KF. The cell wall sensors Mtl1, Wsc1, and Mid2 are required for stress-induced nuclear to cytoplasmic translocation of cyclin C and programmed cell death in yeast. Oxid Med Cell Longev. 2013;2013:1–15. doi: 10.1155/2013/320823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogawat A, Vadassery J, Verma N, Oelmüller R, Dua M, Nevo E, Johri AK. PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Sci Rep. 2016;6:36765. doi: 10.1038/srep36765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert A, Bataille-Simoneau N, Campion C, Guillemette T, Hudhomme P, Iacomi-Vasilescu B, et al. Cell wall integrity and high osmolarity glycerol pathways are required for adaptation of Alternaria brassicicola to cell wall stress caused by brassicaceous indolic phytoalexins. Cell Microbiol. 2011;13:62–80. doi: 10.1111/j.1462-5822.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- Kaffarnik F, Muller P, Leibundgut M, Kahmann R, Feldbrugge M. PKA and MAPK phosphorylation of Prf1 allows promoter discrimination in Ustilago maydis. EMBO J. 2003;22:5817–5826. doi: 10.1093/emboj/cdg554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura T, Yamaguchi S, Saitoh K, Teraoka T, Yamaguchi I. A novel gene, CBP1, encoding a putative extracellular chitin-binding protein, may play an important role in the hydrophobic surface sensing of Magnaporthe grisea during appressorium differentiation. Mol Plant-Microbe Interact. 2002;15:437–444. doi: 10.1094/mpmi.2002.15.5.437. [DOI] [PubMed] [Google Scholar]
- Kilani J, Fillinger S. Phenylpyrroles: 30 years, two molecules and (nearly) no resistance. Front Microbiol. 2016;7:2014. doi: 10.3389/fmicb.2016.02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Park SM, Kim YH, Cha BJ, Yang MS, Kim DH. Deletion of a hypoviral-regulated Cppk1 gene in a chestnut blight fungus, Cryphonectria parasitica, results in microcolonies. Fungal Genet Biol. 2004;41:482–492. doi: 10.1016/j.fgb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Kim S, Park SY, Kim KS, Rho HS, Chi MH, Choi J, et al. Homeobox transcription factors are required for conidiation and appressorium development in the rice blast fungus Magnaporthe oryzae. Plos Genet. 2009;5:e1000757. doi: 10.1371/journal.pgen.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Kawano T, Li D, Kolattukudy PE. A mitogen-activated protein kinase kinase required for induction of cytokinesis and appressorium formation by host signals in the conidia of Colletotrichum gloeosporioides. Plant Cell. 2000;12:1331–1343. doi: 10.2307/3871133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Kikuchi T, Takano Y, Oshiro E, Okuno T. The mitogen-activated protein kinase gene MAF1 is essential for the early differentiation phase of appressorium formation in Colletotrichum lagenarium. Mol Plant-Microbe Interact. 2002;15:1268–1276. doi: 10.1094/mpmi.2002.15.12.1268. [DOI] [PubMed] [Google Scholar]
- Kojima K, Takano Y, Yoshimi A, Tanaka C, Kikuchi T, Okuno T. Fungicide activity through activation of a fungal signalling pathway. Mol Microbiol. 2004;53:1785–1796. doi: 10.1111/j.1365-2958.2004.04244.x. [DOI] [PubMed] [Google Scholar]
- Kong LA, Li GT, Liu Y, Liu M, Zhang SJ, Yang J, et al. Differences between appressoria formed by germ tubes and appressorium-like structures developed by hyphal tips in Magnaporthe oryzae. Fungal Genet Biol. 2013;56:33–41. doi: 10.1016/j.fgb.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Konte T, Plemenitas A. The HOG signal transduction pathway in the halophilic fungus Wallemia ichthyophaga: identification and characterisation of MAP kinases WiHog1A and WiHog1B. Extremophiles. 2013;17:623–636. doi: 10.1007/s00792-013-0546-4. [DOI] [PubMed] [Google Scholar]
- Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol. 2011;195:1083–1092. doi: 10.1083/jcb.201107132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kück U, Beier AM, Teichert I. The composition and function of the striatin-interacting phosphatases and kinases (STRIPAK) complex in fungi. Fungal Genet Biol. 2016;90:31–38. doi: 10.1016/j.fgb.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kulkarni RD, Thon MR, Pan H, Dean RA. Novel G-protein-coupled receptor-like proteins in the plant pathogenic fungus Magnaporthe grisea. Genome Biol. 2005;6:R24. doi: 10.1186/gb-2005-6-3-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuravi VK, Kurischko C, Puri M, Luca FC. Cbk1 kinase and Bck2 control MAP kinase activation and inactivation during heat shock. Mol Biol Cell. 2011;22:4892–4907. doi: 10.1091/mbc.e11-04-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanver D, Mendoza-Mendoza A, Brachmann A, Kahmann R. Sho1 and Msb2-related proteins regulate appressorium development in the smut fungus Ustilago maydis. Plant Cell. 2010;22:2085–2101. doi: 10.1105/tpc.109.073734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastovetsky OA, Gaspar ML, Mondo SJ, LaButti KM, Sandor L, Grigoriev IV, et al. Lipid metabolic changes in an early divergent fungus govern the establishment of a mutualistic symbiosis with endobacteria. Proc Natl Acad Sci U S A. 2016;113:15102–15107. doi: 10.1073/pnas.1615148113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MD, Budge S, Walker L, Munro C, Cowen LE, Brown AJ. Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast. Plos Pathog. 2012;8:e1003069. doi: 10.1371/journal.ppat.1003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim E, An J, Lee Y, Choi E, Choi W, et al. Dissection of the HOG pathway activated by hydrogen peroxide in Saccharomyces cerevisiae. Environ Microbiol. 2017;19:584–597. doi: 10.1111/1462-2920.13499. [DOI] [PubMed] [Google Scholar]
- Leng Y, Zhong S. The Role of Mitogen-Activated Protein (MAP) kinase signaling components in the fungal development, stress response and virulence of the fungal cereal pathogen Bipolaris sorokiniana. Plos One. 2015;10:e0128291. doi: 10.1371/journal.pone.0128291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroch M, Mueller N, Hinsenkamp I, Hahn M. The signalling mucin Msb2 regulates surface sensing and host penetration via BMP1 MAP kinase signalling in Botrytis cinerea. Mol Plant Pathol. 2015;16:787–798. doi: 10.1111/mpp.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Hadar R, Amedeo P, Baker SE, Yoder OC, Horwitz BA. Activation of an AP1-like transcription factor of the maize pathogen Cochliobolus heterostrophus in response to oxidative stress and plant signals. Eukaryot Cell. 2005;4:443–454. doi: 10.1128/ec.4.2.443-454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Sharon A, Hadar R, Ma H, Horwitz BA. A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc Natl Acad Sci U S A. 1999;96:13542–13547. doi: 10.1073/pnas.96.23.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhou X, Kong L, Wang Y, Zhang H, Zhu H, et al. MoSfl1 is important for virulence and heat tolerance in Magnaporthe oryzae. Plos One. 2011;6:e19951. doi: 10.1371/journal.pone.0019951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhou X, Xu JR. Genetic control of infection-related development in Magnaporthe oryzae. Curr Opin Microbiol. 2012;15:678–684. doi: 10.1016/j.mib.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Li X, Gao C, Li L, Liu M, Yin Z, Zhang H, et al. MoEnd3 regulates appressorium formation and virulence through mediating endocytosis in rice blast fungus Magnaporthe oryzae. Plos Pathog. 2017;13:e1006449. doi: 10.1371/journal.ppat.1006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He P, Tian C, Wang Y. CgHog1 controls the adaptation to both sorbitol and fludioxonil in Colletotrichum gloeosporioides. Fungal Genet Biol. 2020;135:103289. doi: 10.1016/j.fgb.2019.103289. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang G, Xu JR, Jiang C. Penetration peg formation and invasive hyphae development require stage-specific activation of MoGTI1 in Magnaporthe oryzae. Mol Plant-Microbe Interact. 2016;29:36–45. doi: 10.1094/mpmi-06-15-0142-r. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang X, Hu S, Liu H, Xu JR. PKA activity is essential for relieving the suppression of hyphal growth and appressorium formation by MoSfl1 in Magnaporthe oryzae. Plos Genet. 2017;13:e1006954. doi: 10.1371/journal.pgen.1006954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Wei T, Cao M, Zhang X, Liu W, Kong Y, et al. The MAP kinase CfPMK1 is a key regulator of pathogenesis, development, and stress tolerance of Colletotrichum fructicola. Front Microbiol. 2019;10:1070. doi: 10.3389/fmicb.2019.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yang SL, Wang NY, Chung KR. The FUS3 MAPK signaling pathway of the citrus pathogen Alternaria alternata functions independently or cooperatively with the fungal redox-responsive AP1 regulator for diverse developmental, physiological and pathogenic processes. Fungal Genet Biol. 2010;47:381–391. doi: 10.1016/j.fgb.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Liu W, Leroux P, Fillinger S. The HOG1-like MAP kinase Sak1 of Botrytis cinerea is negatively regulated by the upstream histidine kinase Bos1 and is not involved in dicarboximide- and phenylpyrrole-resistance. Fungal Genet Biol. 2008;45:1062–1074. doi: 10.1016/j.fgb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhou X, Li G, Li L, Kong L, Wang C, et al. Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. Plos Pathog. 2011;7:e1001261. doi: 10.1371/journal.ppat.1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhou Q, Guo Z, Liu P, Shen L, Chai N, et al. A self-balancing circuit centered on MoOsm1 kinase governs adaptive responses to host-derived ROS in Magnaporthe oryzae. Elife. 2020;9:e61605. doi: 10.7554/elife.61605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerz S, Ziv C, Vogt N, Helmstaedt K, Cohen N, Gorovits R, et al. The nuclear Dbf2-related kinase COT1 and the mitogen-activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa. Genetics. 2008;179:1313–1325. doi: 10.1534/genetics.108.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JM, Rodrigues RJ, de Magalhães-Sant’ana AC, Gonçalves T. Saccharomyces cerevisiae Hog1 protein phosphorylation upon exposure to bacterial endotoxin. J Biol Chem. 2006;281:24687–24694. doi: 10.1074/jbc.m603753200. [DOI] [PubMed] [Google Scholar]
- Masachis S, Segorbe D, Turrà D, Leon-Ruiz M, Fürst U, El Ghalid M, et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat Microbiol. 2016;1:16043. doi: 10.1038/nmicrobiol.2016.43. [DOI] [PubMed] [Google Scholar]
- Mehrabi R, Ding S, Xu JR. MADS-box transcription factor Mig1 is required for infectious growth in Magnaporthe grisea. Eukaryot Cell. 2008;7:791–799. doi: 10.1128/ec.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabi R, Van der Lee T, Waalwijk C, Gert HJ. MgSlt2, a cellular integrity MAP kinase gene of the fungal wheat pathogen Mycosphaerella graminicola, is dispensable for penetration but essential for invasive growth. Mol Plant-Microbe Interact. 2006;19:389–398. doi: 10.1094/mpmi-19-0389. [DOI] [PubMed] [Google Scholar]
- Mendoza-Mendoza A, Pozo MJ, Grzegorski D, Martínez P, García JM, Olmedo-Monfil V, et al. Enhanced biocontrol activity of Trichoderma through inactivation of a mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2003;100:15965–15970. doi: 10.1073/pnas.2136716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M, Rossi M, Ciuffo M, Turina M. Functional characterization of the three mitogen-activated protein kinase kinases (MAP 2Ks) present in the Cryphonectria parasitica genome reveals the necessity of Cpkk1 and Cpkk2, but not Cpkk3, for pathogenesis on chestnut (Castanea spp.) Mol Plant Pathol. 2014;15:500–512. doi: 10.1111/mpp.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon A, Springer M, Lesage P. Activation of the Kss1 invasive-filamentous growth pathway induces Ty1 transcription and retrotransposition in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:5766–5776. doi: 10.1128/mcb.20.15.5766-5776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki A, Kubo E, Arase S, Kihara J. Disruption of SRM1, a mitogen-activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett. 2006;257:253–261. doi: 10.1111/j.1574-6968.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Motoyama T, Ochiai N, Morita M, Iida Y, Usami R, Kudo T. Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr Genet. 2008;54:185–195. doi: 10.1007/s00294-008-0211-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee M, Mukherjee PK, Horwitz BA, Zachow C, Berg G, Zeilinger S. Trichoderma-plant-pathogen interactions: advances in genetics of biological control. Indian J Microbiol. 2012;52:522–529. doi: 10.1007/s12088-012-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Latha J, Hadar R, Horwitz BA. TmkA, a mitogen-activated protein kinase of Trichoderma virens, is involved in biocontrol properties and repression of conidiation in the dark. Eukaryot Cell. 2003;2:446–455. doi: 10.1128/ec.2.3.446-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Weinzierl G, Brachmann A, Feldbrugge M, Kahmann R. Mating and pathogenic development of the Smut fungus Ustilago maydis are regulated by one mitogen-activated protein kinase cascade. Eukaryot Cell. 2003;2:1187–1199. doi: 10.1128/ec.2.6.1187-1199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Park G, Xu JR. The G-beta subunit MGB1 is involved in regulating multiple steps of infection-related morphogenesis in Magnaporthe grisea. Mol Microbiol. 2003;50:231–243. doi: 10.1046/j.1365-2958.2003.03676.x. [DOI] [PubMed] [Google Scholar]
- Ochiai N, Fujimura M, Oshima M, Motoyama T, Ichiishi A, Yamada-Okabe H, Yamaguchi I. Effects of iprodione and fludioxonil on glycerol synthesis and hyphal development in Candida albicans. Biosci Biotechnol Biochem. 2002;66:2209–2215. doi: 10.1271/bbb.66.2209. [DOI] [PubMed] [Google Scholar]
- Palmieri D, Vitale S, Lima G, Di Pietro A, Turrà D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat Commun. 2020;11:5264. doi: 10.1038/s41467-020-18994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G, Xue C, Zheng L, Lam S, Xu JR. MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol Plant-Microbe Interact. 2002;15:183–192. doi: 10.1094/mpmi.2002.15.3.183. [DOI] [PubMed] [Google Scholar]
- Park SM, Choi ES, Kim MJ, Cha BJ, Yang MS, Kim DH. Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus-mediated perturbation of its phosphorylation in response to hypertonic stress. Mol Microbiol. 2004;51:1267–1277. doi: 10.1111/j.1365-2958.2004.03919.x. [DOI] [PubMed] [Google Scholar]
- Perez-Nadales E, Di Pietro A. The transmembrane protein Sho1 cooperates with the mucin Msb2 to regulate invasive growth and plant infection in Fusarium oxysporum. Mol Plant Pathol. 2015;16:593–603. doi: 10.1111/mpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Kim Y, Jiang C, Li Y, Peng Y, Xu JR. Activation of Mst11 and feedback inhibition of germ tube growth in Magnaporthe oryzae. Mol Plant-Microbe Interact. 2015;28:881–891. doi: 10.1094/mpmi-12-14-0391-r. [DOI] [PubMed] [Google Scholar]
- Qi Z, Wang Q, Dou X, Wang W, Zhao Q, Lv R, et al. MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol Plant Pathol. 2012;13:677–689. doi: 10.1111/j.1364-3703.2011.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy V, Zhao X, Snyder AK, Xu JR, Shah DM. Two mitogen-activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell Microbiol. 2007;9:1491–1506. doi: 10.1111/j.1462-5822.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- Ramesh MA, Laidlaw RD, Dürrenberger F, Orth AB, Kronstad JW. The cAMP signal transduction pathway mediates resistance to dicarboximide and aromatic hydrocarbon fungicides in Ustilago maydis. Fungal Genet Biol. 2001;32:183–193. doi: 10.1006/fgbi.2001.1258. [DOI] [PubMed] [Google Scholar]
- Rauyaree P, Ospina-Giraldo MD, Kang S, Bhat RG, Subbarao KV, Grant SJ, Dobinson KF. Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr Genet. 2005;48:109–116. doi: 10.1007/s00294-005-0586-0. [DOI] [PubMed] [Google Scholar]
- Reithner B, Schuhmacher R, Stoppacher N, Pucher M, Brunner K, Zeilinger S. Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk 1 differentially affects mycoparasitism and plant protection. Fungal Genet Biol. 2007;44:1123–1133. doi: 10.1016/j.fgb.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Li C, Gao C, Xu JR, Jiang C, Wang G. Deletion of FgHOG1 is suppressive to the mgv1 mutant by stimulating Gpmk1 activation and avoiding intracellular turgor elevation in Fusarium graminearum. Front Microbiol. 2019;10:1073. doi: 10.3389/fmicb.2019.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschka EJ, Nordzieke S, Valerius O, Braus GH, Pöggeler S. A novel STRIPAK complex component mediates hyphal fusion and fruiting-body development in filamentous fungi. Mol Microbiol. 2018;110:513–532. doi: 10.1111/mmi.14106. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Peña JM, Díez-Muñiz S, Bermejo C, Nombela C, Arroyo J. Activation of the yeast cell wall integrity MAPK pathway by zymolyase depends on protease and glucanase activities and requires the mucin-like protein Hkr1 but not Msb2. FEBS Lett. 2013;587:3675–3680. doi: 10.1016/j.febslet.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Rui O, Hahn M. The Slt2-type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol Plant Pathol. 2007;8:173–184. doi: 10.1111/j.1364-3703.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Sakulkoo W, Osés-Ruiz M, Oliveira Garcia E, Soanes DM, Littlejohn GR, Hacker C, et al. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science. 2018;359:1399–1403. doi: 10.1126/science.aaq0892. [DOI] [PubMed] [Google Scholar]
- Sanz AB, García R, Rodríguez-Peña JM, Arroyo J. The CWI pathway: Regulation of the transcriptional adaptive response to cell wall stress in Yeast. J Fungi (Basel) 2017;4:1. doi: 10.3390/jof4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Madhani HD. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu Rev Genet. 2004;38:725–748. doi: 10.1146/annurev.genet.39.073003.112634. [DOI] [PubMed] [Google Scholar]
- Segorbe D, Di Pietro A, Pérez-Nadales E, Turrà D. Three Fusarium oxysporum mitogen-activated protein kinases (MAPKs) have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol Plant Pathol. 2017;18:912–924. doi: 10.1111/mpp.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R, Martín H, Casamayor A, Ariño J. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J Biol Chem. 2006;281:39785–39,795. doi: 10.1074/jbc.m604497200. [DOI] [PubMed] [Google Scholar]
- Shan L, He P, Sheen J. Intercepting host MAPK signaling cascades by bacterial type III effectors. Cell Host Microbe. 2007;1:167–174. doi: 10.1016/j.chom.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Sharma A, Singh P, Sarmah BK, Nandi SP (2020) Quorum sensing: its role in microbial social networking. Res Microbiol 171:159–164. 10.1016/j.resmic.2020.06.003 [DOI] [PubMed]
- So KK, Kim DH (2017) Role of MAPK signaling pathways in regulating the hydrophobin cryparin in the chestnut blight fungus Cryphonectria parasitica. Mycology 45:362–369. 10.5941/myco.2017.45.4.362 [DOI] [PMC free article] [PubMed]
- Solomon PS, Waters OD, Simmonds J, Cooper RM, Oliver RP. The Mak2 MAP kinase signal transduction pathway is required for pathogenicity in Stagonospora nodorum. Curr Genet. 2005;48:60–68. doi: 10.1007/s00294-005-0588-y. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Y, Tian C. bZIP transcription factor CgAP1 is essential for oxidative stress tolerance and full virulence of the poplar anthracnose fungus Colletotrichum gloeosporioides. Fungal Genet Biol. 2016;95:58–66. doi: 10.1016/j.fgb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Sun ZB, Wang Q, Sun MH, Li SD. The mitogen-activated protein kinase gene CrMapk is involved in Clonostachys chloroleuca Mycoparasitism. Mol Plant-Microbe Interact. 2020;33:902–910. doi: 10.1094/mpmi-03-20-0062-r. [DOI] [PubMed] [Google Scholar]
- Szabó Z, Pákozdi K, Murvai K, Pusztahelyi T, Kecskeméti Á, Gáspár A, et al. FvAtfA regulates growth, stress tolerance as well as mycotoxin and pigment productions in Fusarium verticillioides. Appl Microbiol Biotechnol. 2020;104:7879–7899. doi: 10.1007/s00253-020-10717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Li T, Klosterman SJ, Tian C, Wang Y. The bZIP transcription factor VdAtf1 regulates virulence by mediating nitrogen metabolism in Verticillium dahliae. New Phytol. 2020;226:1461–1479. doi: 10.1111/nph.16481. [DOI] [PubMed] [Google Scholar]
- Turina M, Rossi M, Moretti M. Investigation on the partial resistance of Cpkk2 knock out strain of Cryphonectria parasitica to Cryphonectria hypovirus 1 infection in presence of geneticin and geneticin resistance gene. Virus Res. 2016;219:58–61. doi: 10.1016/j.virusres.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Turrà D, El Ghalid M, Rossi F, Di Pietro A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature. 2015;527:521–524. doi: 10.1038/nature15516. [DOI] [PubMed] [Google Scholar]
- Uehling J, Deveau A, Paoletti M. Do fungi have an innate immune response? An NLR-based comparison to plant and animal immune systems. Plos Pathog. 2017;13:e1006578. doi: 10.1371/journal.ppat.1006578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nguyen T, Kröger C, Bönnighausen J, Schäfer W, Bormann J. The ATF/CREB transcription factor Atf1 is essential for full virulence, deoxynivalenol production, and stress tolerance in the cereal pathogen Fusarium graminearum. Mol Plant-Microbe Interact. 2013;26:1378–1394. doi: 10.1094/mpmi-04-13-0125-r. [DOI] [PubMed] [Google Scholar]
- Vázquez-Ibarra A, Rodríguez-Martínez G, Guerrero-Serrano G, Kawasaki L, Ongay-Larios L, Coria R. Negative feedback-loop mechanisms regulating HOG- and pheromone-MAPK signaling in yeast. Curr Genet. 2020;66:867–880. doi: 10.1007/s00294-020-01089-5. [DOI] [PubMed] [Google Scholar]
- Viefhues A, Schlathoelter I, Simon A, Viaud M, Tudzynski P. Unraveling the function of the response regulator BcSkn7 in the stress signaling network of Botrytis cinerea. Eukaryot Cell. 2015;14:636–651. doi: 10.1128/ec.00043-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella F, Herrero E, Torres J, de la Torre-Ruiz MA. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J Biol Chem. 2005;280:9149–9159. doi: 10.1074/jbc.m411062200. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang S, Hou R, Zhao Z, Zheng Q, Xu Q, et al. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. Plos Pathog. 2011;7:e1002460. doi: 10.1371/journal.ppat.1002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yin Z, Tang W, Cai X, Gao C, Zhang H, et al. The thioredoxin MoTrx2 protein mediates reactive oxygen species (ROS) balance and controls pathogenicity as a target of the transcription factor MoAP1 in Magnaporthe oryzae. Mol Plant Pathol. 2017;18:1199–1209. doi: 10.1111/mpp.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhu W, Cheng J, Xie J, Jiang D, Li G, et al. Nox complex signal and MAPK cascade pathway are cross-linked and essential for pathogenicity and conidiation of mycoparasite Coniothyrium minitans. Sci Rep. 2016;6:24325. doi: 10.1038/srep24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xu L, Liu J, Yin Z, Gao X, Feng H, Huang L. A mitogen-activated protein kinase gene (VmPmk1) regulates virulence and cell wall degrading enzyme expression in Valsa mali. Microb Pathog. 2017;111:298–306. doi: 10.1016/j.micpath.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Wurzinger B, Mair A, Pfister B, Teige M. Cross-talk of calcium-dependent protein kinase and MAP kinase signaling. Plant Signal Behav. 2011;6:8–12. doi: 10.4161/psb.6.1.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JR, Hamer JE. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 1996;10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- Xu JR, Staiger CJ, Hamer JE. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc Natl Acad Sci U S A. 1998;95:12713–12718. doi: 10.1073/pnas.95.21.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wang M, Tang G, Ma Z, Shao W. The endocytic cargo adaptor complex is required for cell-wall integrity via interacting with the sensor FgWsc2B in Fusarium graminearum. Curr Genet. 2019;65:1071–1080. doi: 10.1007/s00294-019-00961-3. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu M, Liu X, Yin Z, Sun Y, Zhang H, et al. Heat-shock proteins MoSsb1, MoSsz1, and MoZuo1 attenuate MoMkk1-mediated cell-wall integrity signaling and are important for growth and pathogenicity of Magnaporthe oryzae. Mol Plant-Microbe Interact. 2018;31:1211–1221. doi: 10.1094/mpmi-02-18-0052-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Song L, Miao Z, Su M, Liang W, He Y. Acetylation of BcHpt lysine 161 regulates Botrytis cinerea sensitivity to fungicides, multistress adaptation and virulence. Front Microbiol. 2020;10:2965–2965. doi: 10.3389/fmicb.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Yin D, Yin Y, Cao Y, Ma Z. The response regulator BcSkn7 is required for vegetative differentiation and adaptation to oxidative and osmotic stresses in Botrytis cinerea. Mol Plant Pathol. 2015;16:276–287. doi: 10.1111/mpp.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin WX, Adnan M, Shang Y, Lin Y, Luo CX (2018) Sensitivity of Botrytis cinerea from nectarine/cherry in China to six fungicides and characterization of resistant isolates. Plant Dis 102:2578–2585. 10.1094/pdis-02-18-0244-re [DOI] [PubMed]
- Yong HY, Bakar FD, Illias RM, Mahadi NM, Murad AM (2013) Cgl-SLT2 is required for appressorium formation, sporulation and pathogenicity in Colletotrichum gloeosporioides. Braz J Microbiol 44:1241–1250. 10.1590/s1517-83822013000400031 [DOI] [PMC free article] [PubMed]
- Yoshimi A, Kojima K, Takano Y, Tanaka C. Group III histidine kinase is a positive regulator of Hog1-type mitogen-activated protein kinase in filamentous fungi. Eukaryot Cell. 2005;4:1820–1828. doi: 10.1128/ec.4.11.1820-1828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Xiong D, Han Z, Liang Y, Tian C. The mitogen-activated protein kinase gene CcPmk1 is required for fungal growth, cell wall integrity and pathogenicity in Cytospora chrysosperma. Fungal Genet Biol. 2019;128:1–13. doi: 10.1016/j.fgb.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Yu PL, Chen LH, Chung KR. How the pathogenic fungus Alternaria alternata copes with stress via the response regulators SSK1 and SHO1. Plos One. 2016;11:e0149153. doi: 10.1371/journal.pone.0149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Gao J, Igbalajobi O, Skoneczny M, Sieńko M, Maciejewska AM, et al. The sulfur metabolism regulator MetR is a global regulator controlling phytochrome-dependent light responses in Aspergillus nidulans. Sci Bull. 2021;66:592–602. doi: 10.1016/j.scib.2020.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang J, Tao H, Dang X, Wang Y, Chen M, et al. FvBck1, a component of cell wall integrity MAP kinase pathway, is required for virulence and oxidative stress response in sugarcane Pokkah Boeng pathogen. Front Microbiol. 2015;6:1096. doi: 10.3389/fmicb.2015.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Geng L, Deng J, Huang L, Zhong H, Xin S, et al. The MAP kinase AflSlt2 modulates aflatoxin biosynthesis and peanut infection in the fungus Aspergillus flavus. Int J Food Microbiol. 2020;322:108576. doi: 10.1016/j.ijfoodmicro.2020.108576. [DOI] [PubMed] [Google Scholar]
- Zhang S, Jiang C, Zhang Q, Qi L, Li C, Xu JR. Thioredoxins are involved in the activation of the PMK1 MAP kinase pathway during appressorium penetration and invasive growth in Magnaporthe oryzae. Environ Microbiol. 2016;18:3768–3784. doi: 10.1111/1462-2920.13315. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu W, Li Y, Li G, Xu JR. Expression of HopAI interferes with MAP kinase signalling in Magnaporthe oryzae. Environ Microbiol. 2017;19:4190–4204. doi: 10.1111/1462-2920.13884. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Choi YE, Zou X, Xu JR. The FvMK1 mitogen-activated protein kinase gene regulates conidiation, pathogenesis, and fumonisin production in Fusarium verticillioides. Fungal Genet Biol. 2011;48:71–79. doi: 10.1016/j.fgb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lamm R, Pillonel C, Lam S, Xu JR. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl Environ Microbiol. 2002;68:532–538. doi: 10.1128/aem.68.2.532-538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Xu JR. A highly conserved MAPK-docking site in Mst7 is essential for Pmk1 activation in Magnaporthe grisea. Mol Microbiol. 2007;63:881–894. doi: 10.1111/j.1365-2958.2006.05548.x. [DOI] [PubMed] [Google Scholar]
- Zheng D, Wang Y, Han Y, Xu JR, Wang C. UvHOG1 is important for hyphal growth and stress responses in the rice false smut fungus Ustilaginoidea virens. Sci Rep. 2016;6:24824. doi: 10.1038/srep24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Zhang S, Zhou X, Wang C, Xiang P, Zheng Q, Xu JR. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. Plos One. 2012;7:e49495. doi: 10.1371/journal.pone.0049495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Hou R, Juanyu Z, Ma J, Wu Z, et al. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. Plos One. 2013;8:e66980. doi: 10.1371/journal.pone.0066980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Liu W, Wang C, Xu Q, Wang Y, Ding S, Xu JR. A MADS-box transcription factor MoMcm1 is required for male fertility, microconidium production and virulence in Magnaporthe oryzae. Mol Microbiol. 2011;80:33–53. doi: 10.1111/j.1365-2958.2011.07556.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang H, Li G, Shaw B, Xu JR. The Cyclase-associated protein Cap1 is important for proper regulation of infection-related morphogenesis in Magnaporthe oryzae. Plos Pathog. 2012;8:e1002911. doi: 10.1371/journal.ppat.1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhao X, Xue C, Dai Y, Xu JR. Bypassing both surface attachment and surface recognition requirements for appressorium formation by overactive ras signaling in Magnaporthe oryzae. Mol Plant-Microbe Interact. 2014;27:996–1004. doi: 10.1094/mpmi-02-14-0052-r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.