Abstract
Fucoidan is a sulfated polysaccharide with various bioactivities. The application of fucoidan in cancer treatment, wound healing, and food industry has been extensively studied. However, the therapeutic value of fucoidan in cardiovascular diseases has been less explored. Increasing number of investigations in the past years have demonstrated the effects of fucoidan on cardiovascular system. In this review, we will focus on the bioactivities related to cardiovascular applications, for example, the modulation functions of fucoidan on coagulation system, inflammation, and vascular cells. Factors mediating those activities will be discussed in detail. Current therapeutic strategies and future opportunities and challenges will be provided to inspire and guide further research.
Keywords: Sulfated fucan, biological function, structure, cardiovascular disease, therapeutic strategy
1. Introduction
Fucoidan is a sulfated polysaccharide mainly isolated from the matrix of brown seaweed. It is primarily composed of sulfated L-fucose and different proportions of xylose, mannose, galactose, glucose, and uronic acid depending on the species of brown seaweeds (Cumashi et al., 2007; Jin, Zhang, Wang, & Zhang, 2013; Ushakova et al., 2009). The structure of fucoidan is heterogeneous. Two main types of fucoidan backbone have been identified as mono-(1–3)- α-L-fucopyranose and repeated alternating (1–3)- and (1–4)-α-L-fucopyranose with sulfate groups locating at C-2, C-3 and/or C-4 of fucose ring (Figure 1) (Li, Lu, Wei, & Zhao, 2008). Fucoidan was first isolated from brown algae in 1913 by Kylin, and now fucoidan has been marketed as dietary supplement in food industry. Since its discovery, fucoidan has attracted steadily increased research interest, attributed to its low toxicity and diverse bioactivities (Li, Lu, et al., 2008; Wang et al., 2019). The most extensively studied area of fucoidan is cancer treatment due to its potent antitumor activity (Oliveira, Neves, Reis, Martins, & Silva, 2020; N. E. Ustyuzhanina et al., 2014; Wu et al., 2016). In addition to the antitumor activity, fucoidan also exhibits antioxidant (Rocha de Souza et al., 2007; Wang, Zhang, Zhang, Song, & Li, 2010), anticoagulant (Irhimeh, Fitton, & Lowenthal, 2009; Mourão, 2004), anti-inflammatory (Lee et al., 2012; Lee, Ko, et al., 2013), antibacterial (Lee, Jeong, Choi, Na, & Cha, 2013) and immune regulatory effects (Itoh, Amano, Kakinuma, & Noda, 2002; Lee, Cho, Kim, & Cho, 2020). These bioactivities enable both the therapeutic and biomedical applications of fucoidan. Approaches for treating cardiovascular diseases remain the core research area in many countries. Fucoidan has been shown to alleviate atherosclerotic lesions through its anti-inflammatory and anticoagulant effects (Patil et al., 2018; Zaporozhets & Besednova, 2016). Moreover, fucoidan has been shown to suppress neointima formation and reduce adverse vascular remodeling (Hlawaty et al., 2011). These findings suggest the potential of fucoidan as therapeutic agent for cardiovascular application. Meanwhile, many studies have suggested that the activities of fucoidan appear to be significantly altered by variables related to the derivation and the resulted molecular structure, such as species of brown seaweeds, structural features and molecular weight. In this paper, we aim to provide a scientific review on the bioactivities and functions of fucoidan that are valuable for cardiovascular application. The factors affecting these activities of fucoidan will be summarized. This review will also discuss the strategies and challenges in the future development of fucoidan as cardiovascular therapeutic agents.
Figure 1.
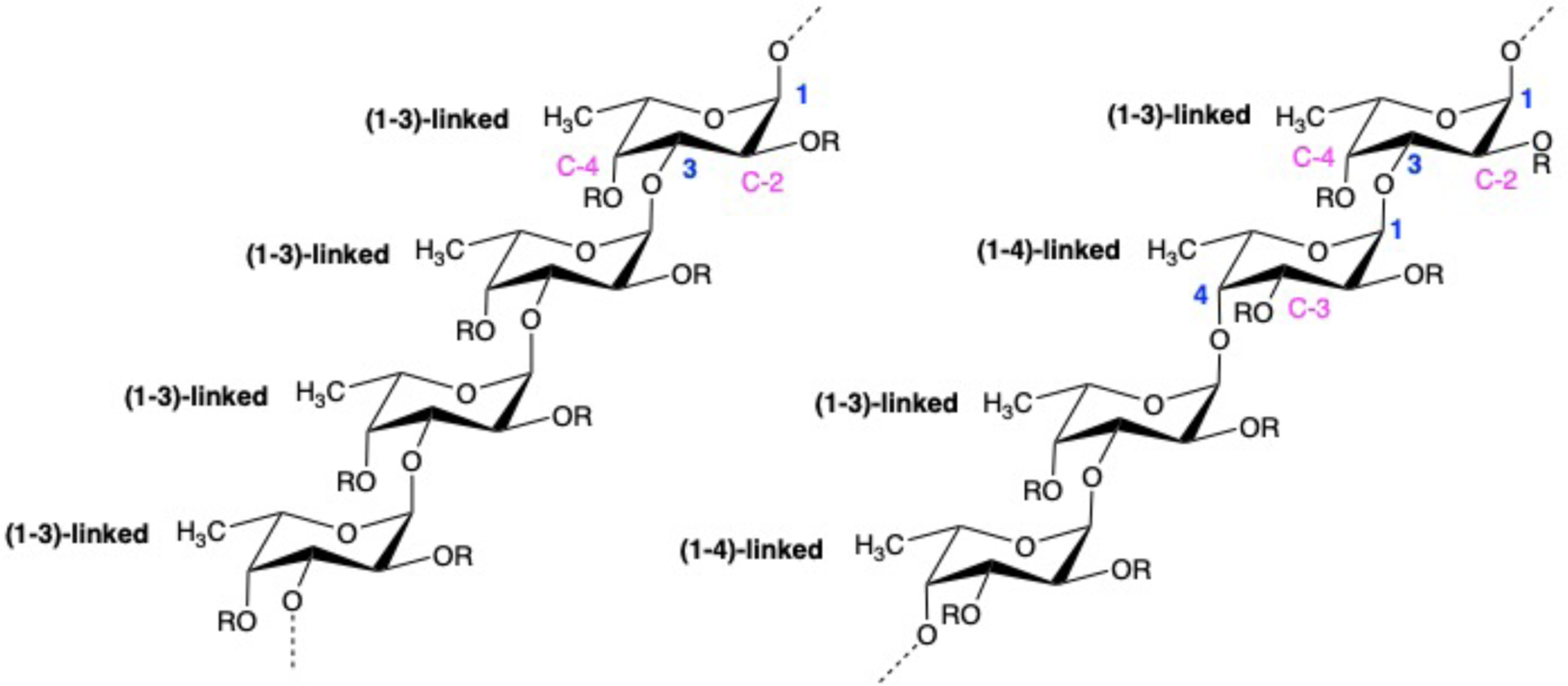
Fucoidan structure with mono-(1–3)-α-L-fucopyranose backbone and repeated (1–3)- and (1–4)-α-L-fucopyranose backbone
2. Bioactivities of fucoidan for cardiovascular application
Fucoidan has a wide range of bioactivities that will be beneficial for its potential cardiovascular applications. The therapeutic effect on atherosclerosis is one of the key functional activities of fucoidan. Fucoidan alleviates atherosclerotic lesion by reducing leukocyte and platelet adhesion on dysfunctional or damaged endothelium (Chauvet, Bienvenu, Théorêt, Latour, & Merhi, 1999), and inhibiting thrombin generation (Mourão, 2015). It also inactivates free-radical processes (Tariq et al., 2015), reduces cholesterol levels (Cuong, Thuy, Huong, Ly, & Van, 2015) and inhibits lipid accumulation (Yokota, Nagashima, Ghazizadeh, & Kawanami, 2009). Moreover, fucoidan reduces thrombin generation in microvasculature (Thorlacius, Vollmar, Seyfert, Vestweber, & Menger, 2000) and promotes angiogenesis through recruitment of endothelial progenitor cells (Zemani et al., 2005), which could attenuate myocardial infarction and stimulate revascularization in ischemic regions. These bioactivities of fucoidan for cardiovascular application can be divided into three main categories: (1) the anticoagulant and antithrombotic activity; (2) the anti-inflammatory activity; and (3) the modulation of vascular cell behaviors.
2.1. Anticoagulant and antithrombotic activity
2.1.1. Coagulation
The coagulation cascade has three different pathways: intrinsic pathway (contact activation pathway), extrinsic pathway (tissue factor pathway), and common pathway (Fig 2). Activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT) are three important parameters of coagulation activity. APTT reflects the coagulation activity through both intrinsic and common coagulation pathways; PT shows the clot formation in the extrinsic pathway, while TT measures the thrombin-induced clotting formation time in the common coagulation pathway. Fucoidan was first reported to exhibit anticoagulant activity both in vitro and in vivo in 1957 by Springer (Springer, Wurzel, McNeal, Ansell, & Doughty, 1957). They found that fucoidan fractions from Fucus vesiculosus significantly prolonged PT when injected intravenously to rabbits weighing 2–3 kg at a dose of 45–50 mg per animal. Later, similar trend was observed in baboons that fucoidan injection at a dose of 1.0 mg/kg/h has increased PT from 18 s to 65 s (Alwayn et al., 2000). The prolonging of APTT by fucoidan has been reported by many studies (Haroun-Bouhedja, Ellouali, Sinquin, & Boisson-Vidal, 2000; Kim et al., 2010; Kuznetsova et al., 2003; Wijesinghe, Athukorala, & Jeon, 2011). Shang et al. studied the anticoagulant activity of fucoidans from three sea cucumbers at a concentration ranging from 1 to 100 μg/mL, and showed that the prolonging in APTT was significant, while TT and PT were not affected (Shang et al., 2018). Fucoidan from Hizikia fusiforme has been reported to prolong APTT distinctly but delay TT slightly (Li, Zhao, & Wei, 2008). The findings, showing that fucoidan could prolong both APTT and PT, suggest that fucoidan exhibits anticoagulant activity through both intrinsic and extrinsic pathways. The main targets of fucoidan have been identified as factor Xa and thrombin.
Figure 2.
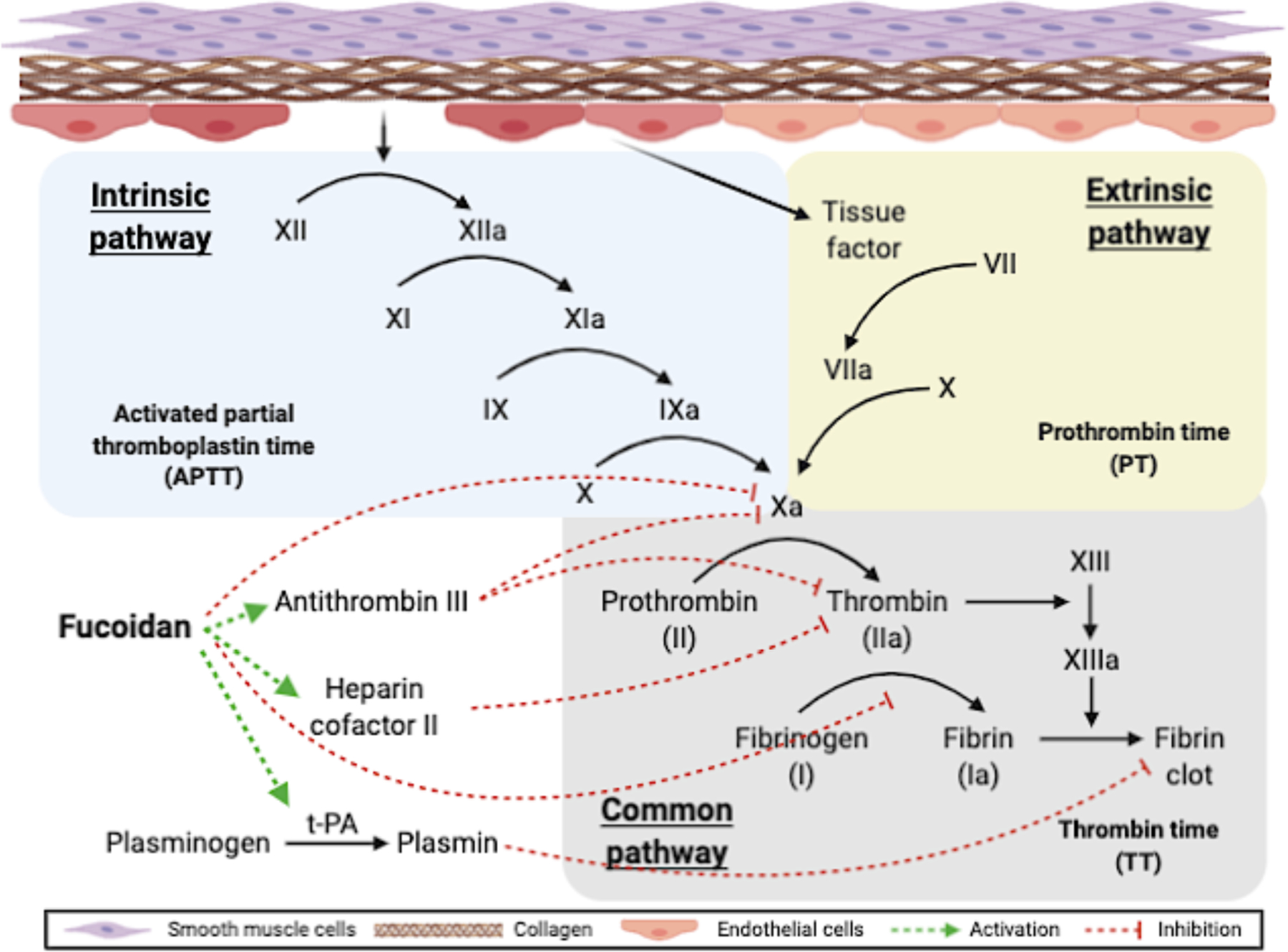
Coagulation pathways and the role of fucoidan. Fucoidan exhibits anticoagulant activity by inhibiting FXa formation in the intrinsic pathway (Drozd et al., 2006; Lapikova et al., 2008; Nishino et al., 1999). Fucoidan improves the activity of heparin cofactor II and antithrombin III in inactivating thrombin by binding to antithrombin III and heparin cofactor II (Colliec et al., 1991; Mansour et al., 2019; Minix & Doctor, 1997). Fucoidan inhibits fibrinogen-thrombin reaction by binding to the thrombin attacking site in fibrinogen (Nishino, Aizu, & Nagumo, 1991; Nishino, Ura, & Nagumo, 1995). Fucoidan also promotes fibrinolytic effect by simulating t-PA catalyzed plasminogen activation (Ghebouli et al., 2018; Soeda, Sakaguchi, Shimeno, & Nagamatsu, 1992). The figure was created with BioRender.com.
Factor Xa (FXa) is an activated form of factor X, and it can cleave prothrombin to yield active thrombin. The interaction of fucoidan with FXa is emphasized in the mechanism of the anticoagulant activity of fucoidan. Fucoidan is a potent inhibitor of FXa generation in the intrinsic pathway (Drozd et al., 2006; Lapikova et al., 2008; Nishino, Fukuda, Nagumo, Fujihara, & Kaji, 1999). Furthermore, Shang et al. recently found that fucoidan inhibited the intrinsic coagulation pathway by targeting the intrinsic coagulation factor Xase. They reported an IC50 ranging from 50 ng/mL to 200 ng/mL depending on the species, and they found that increased concentration of fucoidan resulted in complete inhibition of factor Xase (Shang et al., 2018).
The anticoagulant activity of fucoidan is highly dependent on the structure of fucoidan. Among all of the structural features, sulfate content and molecular weight appear to be the most important. A common consensus is that the anticoagulant effect is positively related to the number of sulfate groups, while negatively associated with the molecular size. More details will be discussed in Section 3.2 and 3.3.
The pro-anticoagulant potential of fucoidans has also been reported. Fucoidan was first described as a non-anticoagulant polysaccharide by McCaffrey et al. due to the inefficiency in prolonging TT (McCaffrey, Falcone, Borth, Brayton, & Weksler, 1992). Later, fucoidan was reported to accelerate the clotting time of human plasma with hemophilia A and hemophilia B and plasma with reduced factor VII level at concentrations from 4–500 nM (Liu et al., 2006). One of the suggested mechanisms of the procoagulant activity of fucoidan is acting through the inhibition of tissue factor pathway inhibitor (TFPI). TFPI is a potent inhibitor of the extrinsic coagulation pathway, released from endothelium. Fucoidan has been shown to have a TFPI-inhibiting activity with EC50 values around 0.4 μg/mL and reverse the anticoagulant action of TFPI (Zhang et al., 2015).
The balance between anticoagulant and procoagulant activities of fucoidan appears to be dose-and structure-dependent. Generally, procoagulant activity of fucoidan is observed at a low concentration, while anticoagulant activity is observed at a high concentration. In a hemostatic thrombin generation assay with hemophilia plasma, fucoidan was shown to have an onset of procoagulation at the concentration of 0.01 μg/mL and reach the optimal activity at about 1 μg/mL. However, the procoagulation was reversed at a concentration higher than 10 μg/mL (Dockal, Till, Knappe, Ehrlich, & Scheiflinger, 2011). Zhang et al. obtained a bell-shaped concentration profile for the co-existing pro- and anti-coagulant activities (Zhang et al., 2015). The profile has indicated that the procoagulant window of fucoidan may span a wide concentration range from 0.1 to 100 μg/mL. In a different study, Zhang et al. also showed that a minimal sulfation degree of 0.5 sulfates per sugar unit and minimal molecular size of 70 sugar units are required for Fucus vesiculosus fucoidan to exhibit procoagulant activity (Zhang et al., 2014).
2.1.2. Thrombin generation
Thrombin plays a crucial role in thrombosis. Fucoidan has been shown to have an antithrombotic activity by thrombin inhibition. With intravenous injection into rabbits at 1.8 mg/kg, fucoidan has been shown to inhibit thrombosis by significantly prolonging thrombin clotting time while only slightly increase bleeding (Mauray et al., 1995). In a contact-activated plasma model, fucoidan has been reported to extend the lag phase of thrombin generation at a concentration of 40–100 μg/mL (Mauray et al., 1998). Thrombus formation in microvasculature can lead to ischemia or even tissue infarction. Intravenous injection of fucoidan (25 mg/kg) also effectively slowed down the thrombus occlusion caused by endothelial denudation in both arterioles and venules in vivo (Thorlacius et al., 2000).
The thrombin inhibition activity of fucoidan is mainly mediated by heparin cofactor II- and antithrombin III-dependent pathways (Colliec et al., 1991; Mansour et al., 2019; Minix & Doctor, 1997), as shown in Fig 2. Heparin cofactor II and antithrombin III inactivate thrombin by forming complexes with thrombin, blocking the active site of thrombin. Fucoidan has a high affinity for heparin cofactor II and is potent in enhancing the reaction rate of heparin cofactor II-thrombin (Church, Meade, Treanor, & Whinna, 1989; Nishino et al., 1991). This activity of fucoidan has been shown to be more effective than that of heparin (Minix & Doctor, 1997). Fucoidan also promotes the activity of antithrombin III in inactivating thrombin, although to a less extent compared to heparin cofactor II (Colliec et al., 1991; Grauffel, Kloareg, Mabeau, Durand, & Jozefonvicz, 1989; Soeda, Ohmagari, Shimeno, & Nagamatsu, 1993; Ustyuzhanina et al., 2013).
Other mechanisms of the antithrombotic activity of fucoidan have been explored as well. Fibrin polymerization can be suppressed by fucoidan (Soeda et al., 1992). Fucoidan is capable of binding to fibrinogen, presenting a steric hindrance, thereby inhibiting fibrinogen clotting by thrombin (Nishino et al., 1991; Nishino et al., 1995). Plasminogen, a serine protease in blood, lyses fibrin once activated and converted to plasmin. Tissue plasminogen activator (t-PA) is the primary activator of plasminogen. Fucoidan can simulate t-PA catalyzed plasminogen activation (Soeda et al., 1993; Soeda et al., 1992). A recent study has also demonstrated that mixing fucoidan into recombinant t-PA (rt-PA) yielded a greater fibrinolytic effect than rt-PA alone (Ghebouli et al., 2018). Richard et al. looked into the impact of fucoidan on protease nexin-I (PN-I), which has an inhibitory effect on thrombin (Richard et al., 2006). They have observed that fucoidan bound to thrombin, PN-I, and PN-I/thrombin complex. The resulting kinetic curve showed that the fucoidan accelerated thrombin inhibition by PN-I.
Among all the studies on the antithrombotic activity of fucoidan, little evidence showed that fucoidan has a direct inhibitory effect on thrombin. Ustyuzhanina et al. reported that a high concentration of fucoidan and a minimum of 16 saccharide units might be required to achieve a direct inhibition of thrombin (Ustyuzhanina et al., 2013).
2.1.3. Platelets
In addition to the anticoagulant and antithrombotic activities, fucoidan also affects platelet adhesion, aggregation, and activation. Fucoidan has been shown to inhibit thrombin-induced and adenosine diphosphate (ADP)-induced platelet aggregation (Cumashi et al., 2007; Jeong, Yoon, & Kim, 2009). Intravenous administration of fucoidan in baboons at a dose of 0.5 to 1.0 mg/kg/h has been shown to cause complete inhibition of platelet aggregation (Alwayn et al., 2000). Furthermore, the administration of fucoidan in a pig angioplasty model has showed inhibition of platelet adhesion to the damaged arterial surfaces (Chauvet et al., 1999). Fucoidan has also been shown to inhibit platelet activation by reducing platelet aggregation. Chen et al. showed that fucoidan from Saccharina japonica inhibited platelet activation indirectly by reducing the concentration of endothelial microparticles and von Willebrand factor (vWF). Additionally, fucoidan has been shown to directly inhibit platelet activation by decreasing the intracellular Ca2+ influx (Chen, Zhang, Shi, Zhao, & Yan, 2016). Several studies using fucoidan as functional modification molecules have also shown that fucoidan reduces or does not increase platelet adhesion on synthetic materials (Su et al., 2017; Yao, Zaw, Anderson, Hinds, & Yim, 2020; Ye et al., 2016; Zhang et al., 2017).
However, fucoidan from Fucus vesiculosus was previously reported to induce irreversible platelet aggregation and cause platelet activation (Dürig et al., 1997; Manne et al., 2013), although low molecular weight fucoidan exhibited less severe effects. Fucoidan has been previously suggested to induce platelet activation through Src family kinase signaling pathways, which are critical initiators of platelet response to vascular injury (Senis, Mazharian, & Mori, 2014). Also, fucoidan from Fucus vesiculosus causes human platelet aggregation via platelet endothelial aggregation receptor-1 and platelet glycoprotein Ib, facilitating the initial platelet adhesion at the vascular injury site. However, when being used as a functional molecule on synthetic vascular graft, fucoidan from Fucus vesiculosus did not cause obvious platelet aggregation nor activation (Yao et al., 2020).
The contradictory results could be explained by the dose and molecular weight differences of the fucoidans used in different studies. The irreversible induction of platelet aggregation by fucoidan from Fucus vesiculosus has been shown to only appear at high concentrations (0.5–5 mg/mL). Additionally, low molecular weight fractions of fucoidan (15.2 kDa) has been shown to induce lower platelet aggregation compared to high molecular weight fucoidans (170 kDa and 117 kDa) (Carvalho G. de Azevedo et al., 2009). Other studies have also found that high molecular weight fucoidan from Saccharina japonica caused pro-aggregation response of platelets, while low molecular weight fucoidan inhibited thrombin-induced platelet aggregation (Zhao et al., 2012; Zhu et al., 2010).
2.2. Anti-inflammation
Inflammation has been highlighted and evidenced to play a significant role in cardiovascular pathogenesis. Fucoidan has been identified as a promising anti-inflammatory agent to treat early-stage atherosclerosis (Patil et al., 2018). The anti-inflammatory effect of fucoidan is achieved by inhibiting complement system, binding to selectins, and suppressing the activities of several inflammatory enzymes (Zaporozhets & Besednova, 2016).
2.2.1. Complement
The complement system is a surface-mediated and calcium-dependent reaction of innate immunity (Carroll, 2008). Complement activation has three pathways: the classical pathway, the mannan-binding lectin (MBL) pathway, and the alternative pathway (Sarma & Ward, 2011). The three pathways result in the formation of C3-convertases through different mechanisms, as shown in Fig 3. The classical pathway is triggered by activating the C1 complex component, including C1q, C1r, and C1s. C1q binds to the pathogen surfaces, followed by the cleavage of C2 and C4 and the formation of C3-convertase, C4b2a (Walport, 2001). MBL pathway is activated by the interaction of MBL or ficolin with pathogen surfaces, leading to the cleavage of C2 and C4, forming C4b2a (Petersen, Thiel, & Jensenius, 2001). The alternative pathway involves the constant hydrolysis of C3 to form C3b and occurs directly by contacting the carbohydrates, lipids, and proteins on the pathogen surfaces (Harboe & Mollnes, 2008). Complement activation has been suggested to contribute to the development of atherosclerosis, thrombosis, and ischemic heart disease (Bjerre, Hansen, & Flyvbjerg, 2008; Carter, 2012; Oksjoki, 2007). Undesired complement activation can also occur either in blood or on the surface of a cardiovascular implant through the classical pathway and alternative pathway, contributing towards thrombus formation (Bruins et al., 1997; Engberg et al., 2011).
Figure 3.
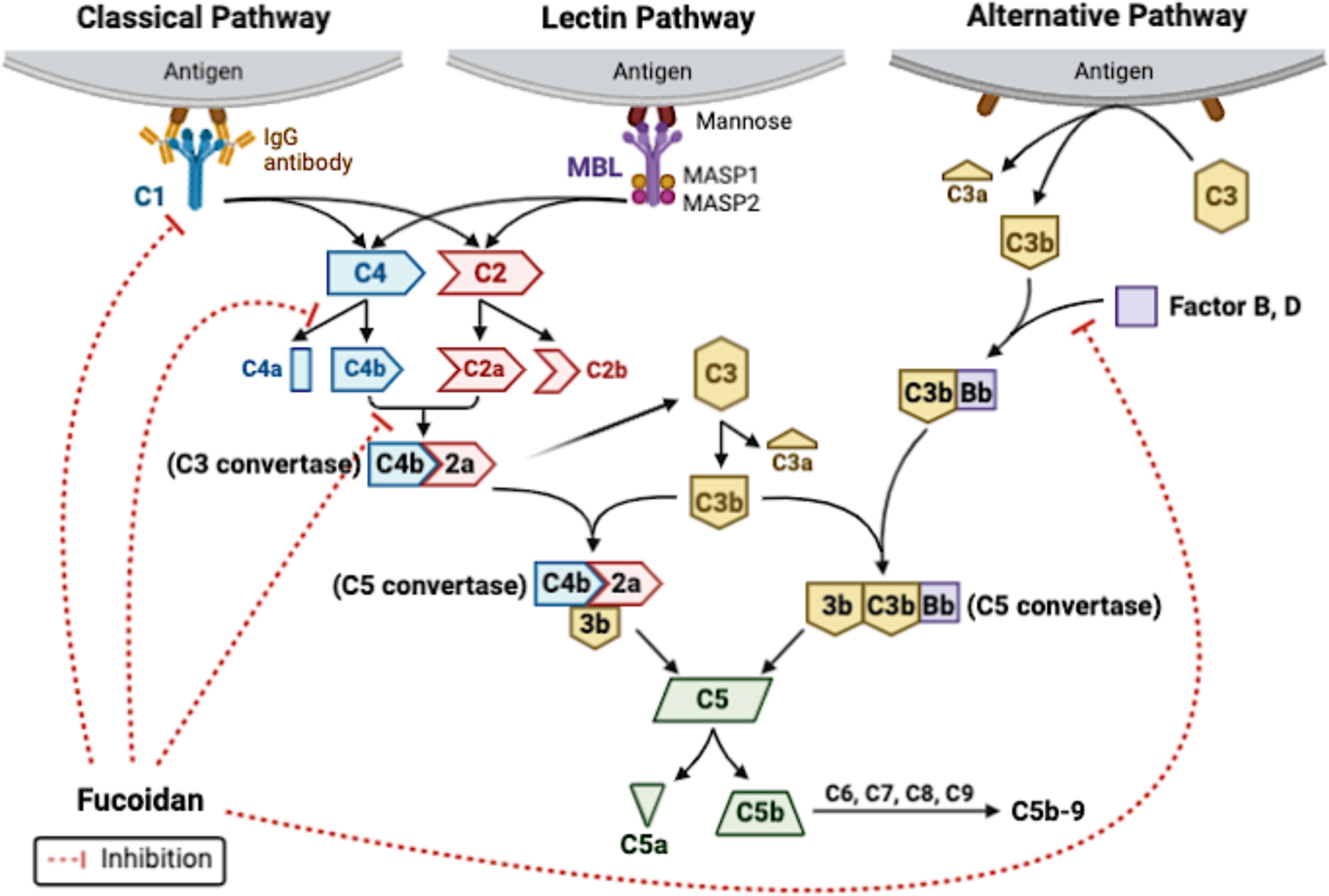
Three complement activation pathways and the role of fucoidan in complement activation. Fucoidan inhibits complement activation through the classical pathway by preventing active C1 formation, inhibiting C4 activity by forming a complex with C4, and preventing the binding of C4b and C2a (Blondin et al., 1994; Tissot, Montdargent, et al., 2003). Fucoidan also inhibits complement activation through the alternative pathway by preventing the binding of factor B to C3b (Blondin et al., 1994). The figure was created with BioRender.com.
Fucoidan was first described as a potent inhibitor of complement activation by Blondin et al. in 1994 (Blondin, Chaubet, Nardella, Sinquin, & Jozefonvicz, 1996; Blondin, Fischer, Boisson-Vidal, Kazatchkine, & Jozefonvicz, 1994). Later, Zvyagintseva et al. showed that fucoidans from Laminaria cichorioides, Saccharina japonica, Fucus evanescens caused 50% inhibition of alternative complement activation at a concentration from 0.5 to 20 mg/mL, depending on the sources (Zvyagintseva et al., 2000). As shown in Fig 3, fucoidan inhibits complement activation mainly through the classical pathway and to a lesser extent through the alternative pathway. Fucoidan binds to C1q and interfere with the association of the C1r2-C1s2 subunit, thus inhibiting the full activation of C1 (Tissot, Daniel, & Place, 2003; Tissot et al., 2005; Tissot, Montdargent, et al., 2003). Fucoidan has been hypothesized to prevent C4 cleavage by forming a complex with C4 (Tissot, Montdargent, et al., 2003). The binding of fucoidan to C4 was later confirmed by nuclear magnetic resonance (NMR) characterization using fucoidan oligosaccharides (Clément et al., 2010). In addition, fucoidan directly inhibits the formation of C3 convertase. By interfering with the interaction between C4b and C2, fucoidan inhibits the formation of C4b2a. Also, fucoidan suppresses the generation of C3bBb by decreasing the number of binding sites on factor B for C3b (Blondin et al., 1994).
Recent studies have shown that the anticomplementary effect of fucoidan is dependent on its structural features (Kopplin et al., 2018; Liu, Wang, Zhang, & Zhang, 2018). The reducing end and methyl groups on fucoidan are suggested to be responsible for the interaction between fucoidan and C4. Fucoidan with branched structure appears to be more beneficial for its anticomplementary activity (Clément et al., 2010). Sulfated and benzoylated derivatives of fucoidan exhibit a better anti-complementary effect than native fucoidan, and high molecular weight derivatives cause the most substantial effect (Liu et al., 2018).
2.2.2. Selectin
Selectins are a group of glycoproteins, playing a vital role in the leukocyte recruitment and accumulation at the sites of inflammation and tissue damage. P-selectin is a cell adhesion molecule expressed by both endothelial cells and platelets. It has a high-affinity ligand, P-selectin glycoprotein ligand-1 (PSGL-1), which is expressed by leukocytes. Upon the activation of endothelial cells and platelets, P-selectin relocates to the surfaces of activated endothelial cells and platelets, which triggers the leukocyte recruitment into the inflammatory sites and contributes to thrombus formation (Lorant et al., 1993). Fucoidan is another ligand of P-selectin; it attenuates the interaction of P-selectin with leukocytes by binding to its carbohydrate recognition domain (Preobrazhenskaya et al., 1997a, 1997b; Usov, Smirnova, Bilan, & Shashkov, 1998). L-selectin, expressed by most circulating leukocytes, can recognize endothelial mucin-like ligands and regulate leukocyte tethering, rolling, and activation. Fucoidan is also a ligand of L-selectin and can reduce leukocyte rolling and accumulation (Mebius & Watson, 1993; Ruehl et al., 2002).
By binding to the selectins, fucoidan inhibits leukocyte, neutrophil, and platelet adhesion to damaged arterial surfaces (Chauvet et al., 1999; Månsson, Zhang, Jeppsson, Johnell, & Thorlacius, 2000; Thorlacius et al., 2000). In different animal models, injection of fucoidan reduces leukocyte rolling and neutrophil migration (Gaboury & Kubes, 1994; Omata, Matsui, Inomata, & Ohno, 1997; Preobrazhenskaya et al., 1997a). Cumashi et al. compared the inhibition of neutrophil extravasation by fucoidans from different species (Cumashi et al., 2007). However, no conclusive relationship has been seen between the structural parameters and the effects on neutrophil extravasation.
The ability of fucoidan to bind with selectins allows for diverse biomedical applications of fucoidan. Fucoidan can bind to activated platelets by targeting the P-selectin on the surfaces of activated platelets. Juenet et al. reported a nanomolar affinity of fucoidan for the P-selectin expressed by activated platelets in thrombus, which suggests that fucoidan can be used as carriers for targeting thrombosis (Juenet et al., 2018). Fucoidan functionalized microcapsules and microparticles has been shown to bind to activated platelet aggregates and explicitly locate at the abdominal aortic aneurysm thrombotic wall in a rat model (Bonnard, Yang, et al., 2014; Li et al., 2017). The ability of fucoidan to locate activated platelets in thrombus enables fucoidan to work as a promising molecular imaging agent. Furthermore, fucoidan also shows therapeutic potential in attenuating myocardial infarction by reducing selectin-dependent adhesion of platelets (Barrabés et al., 2005; Tanaka et al., 2010).
2.2.3. Enzyme activity
Recent studies have found out that in addition to the inhibition of complement and selectins, fucoidan also suppresses the activity of several inflammatory enzymes. Dietary intake of low molecular weight fucoidan from Saccharina japonica at a dose of 200 mg/kg/day markedly downregulates the matrix metalloproteinase (MMP) level, reduces expression of interleukin 1β, tumor necrosis factor-α (TNF-α) and monocyte chemotactic protein-1, and limits the growing of abdominal aortic aneurysm (AAA) (Zhou et al., 2018). MMP-2 is responsible for intimal hyperplasia (Kishi et al., 2007; Rotmans et al., 2004). Fucoidan from Fucus vesiculosus inhibits MMP-2 activity and reduces the narrowing of the lumen of the damaged vessels (Fujimura et al., 2002). Administration of fucoidan at 100 mg/kg/d has also been shown to attenuate MMP activities by suppressing the activation of nuclear factor κB and c-Jun N-terminal kinase (JNK) (Tsai et al., 2018).
2.3. Directing vascular cell behaviors
2.3.1. Endothelial cells and angiogenesis
Vascular endothelial cells (ECs) serve as a barrier between blood and tissues and participates actively in maintaining homeostasis. Endothelial dysfunction is a significant contributor of cardiovascular diseases, such as atherosclerosis, hypertension, and diabetes (Haybar, Shahrabi, Rezaeeyan, Shirzad, & Saki, 2019). Endothelial denudation is the primary factor that causes restenosis after surgical interventions to treat cardiovascular diseases (Verrier & Boyle, 1996). Several studies have shown that fucoidan may have a protective function for ECs. Chen et al. investigated the EC protective activity of fucoidan by measuring the vWF level and the number of endothelial microparticles, which are important indexes of endothelial injury (Chen et al., 2016). vWF level was significantly suppressed by fucoidan from Saccharina japonica in vitro and in vivo in an endothelial injury rat model. The fucoidan also substantially decreases the number of endothelial microparticles stimulated by TNF-α. Chen et al. later investigated the relationship between the structural features and EC protective activity of fucoidan from Saccharina japonica (Chen et al., 2017). They found that low molecular weight fucoidan (3.2 kDa and 7.4 kDa) was more effective than medium molecular weight fucoidan (28 kDa and 34 kDa) in protecting ECs. Furthermore, highly sulfated fucoidan (30%) has been demonstrated to be more effective at inducing EC proliferation in the presence of fibroblast growth factors (FGFs). A recent study using dyslipidemia and atherosclerosis mouse models indicates that the EC protective activity of fucoidan may be related to its ability to reduce the expression of endothelial dysfunction marker, endothelin-1, and proinflammatory cytokines, TNF-α and interferon-γ (IFNγ) (Kuznetsova, Ivanushko, Persiyanova, Ermakova, & Besednova, 2019). Other studies employing fucoidan as functional molecules have also suggested that fucoidan can enhance endothelialization on synthetic materials or decellularized scaffolds (Marinval et al., 2018; Wang, Ye, et al., 2016; Yao et al., 2020).
Angiogenesis is essential for normal physiological process. Therapeutic angiogenesis can improve revascularization and myocardial function, and is one of the most attractive approaches to treat cardiovascular diseases (Deveza, Choi, & Yang, 2012). However, pathological angiogenesis has been suggested to contribute to atherosclerosis progression and neointimal growth (Khurana, Simons, Martin, & Zachary, 2005). Fucoidan exhibits both pro-and anti-angiogenesis activities. The pro-angiogenesis activity of fucoidan has been observed in several studies, but mainly on low molecular weight fucoidan. Fucoidan from Ascophyllum nodosum with molecular weight around 16kDa stimulates FGF-1-mediated EC proliferation and migration, while inhibiting FGF-2-induced endothelial proliferation and migration (Giraux et al., 1998). However, later studies found that fucoidan could enhance angiogenesis, EC proliferation, and migration induced by FGF-2 (Chabut, Fischer, Helley, & Colliec, 2004; Kim, Park, Kang, Kim, & Lee, 2014; Matou, Helley, Chabut, Bros, & Fischer, 2002). The pro-angiogenesis activity of fucoidan with the presence of FGF-2 is related to the modulation of integrin α6 expression (Chabut et al., 2004; Matou et al., 2002) and protein kinase B (AKT)/MMP-2 signaling (Kim, Park, et al., 2014). Moreover, low molecular weight fucoidan has been found to increase the circulating levels of endothelial progenitor cells (EPCs) (Roux et al., 2012) and enhance the proangiogenic phenotype of EPCs (Zemani et al., 2005).
The anti-angiogenesis potential of fucoidan attracts broad attention in cancer treatment. The anti-angiogenesis activity of fucoidan contributes to its antitumor effect, as angiogenesis is one of the essential process for cancer progression. Fucoidan has been shown to alter the morphology of cultured endothelial monolayer at a concentration of 10 mg/mL, inducing a dramatic disruption of endothelial monolayers (Glabe, Yednock, & Rosen, 1983). Later, fucoidan has also been reported to inhibit the thrombospondin-induced EC spreading (Taraboletti, Roberts, Liotta, & Giavazzi, 1990). Moreover, fucoidan inhibits or disrupts tube formation of ECs when being added in cell culture media at a concentration of 0.5 mg/mL, and fucoidan significantly decreases the number of blood vessels when being injected into chicken eggs at a concentration of 0.5 mg/mL (Oliveira et al., 2019). Treatment of human umbilical vein endothelial cells (HUVECs) with fucoidan at a concentration of 400 μg/mL in vitro has been shown to inhibit cell proliferation and migration and reduce the formation of tube and vascular network. In addition, treatment with fucoidan at a concentration of 100 μg/mL ex vivo causes a substantial reduction of microvessel outgrowth (Liu et al., 2012). The anti-angiogenesis of fucoidan has been elucidated to be attributed to its ability to reduce the expression of FGF (Croci et al., 2011), vascular endothelial growth factor (VEGF) (Dithmer et al., 2014) and platelet-derived growth factor (PDGF) (Oliveira et al., 2019).
The contradictory functions of fucoidan on angiogenesis are hypothesized to be attributed to the structural differences of tested fucoidans. Matsubara et al. prepared fucoidans with different molecular weight. They found that anti-angiogenesis effects were shown in fucoidan with molecular weight of around 30kDa, while fucoidan with lower molecular weight enhanced EC migration without affecting their tubulogenesis (Matsubara et al., 2005). Other structural features, including degree and pattern of sulfation (Soeda, Shibata, & Shimeno, 1997) and type of branches (Cumashi et al., 2007) also affect the activity of fucoidan on angiogenesis. More details and comparison on anti-angiogenesis and pro-angiogenesis of fucoidan has been reviewed by Ustyuzhanina et al. (N. E. Ustyuzhanina et al., 2014).
2.3.2. Smooth muscle cells
Vascular smooth muscle cells (SMCs) play significant roles in atherosclerosis, hypertension, and intimal hyperplasia formation. SMCs are in a quiescent, differentiated, and contractile state in normal blood vessels. Upon the damage or dysfunction of ECs, SMCs change phenotype to a proliferative state and migrate from media to intima. This causes intimal thickening, atherosclerosis plaques, and stenosis of vascular lumens (Li, Qian, Kyler, & Xu, 2018).
Adding fucoidan in cell culture media has been shown to be potent in inhibiting SMC proliferation (Logeart, Letourneur, Jozefonvicz, & Kern, 1996; Vischer & Buddecke, 1991). McCaffrey et al. compared the antiproliferative effect of fucoidan on SMCs with that of heparin, and found that fucoidan had more potent inhibition on SMC proliferation than heparin (McCaffrey et al., 1992). In a later study, they reported that fucoidan suppressed SMC proliferation by protecting transforming growth factor- β1(TGF-β1) from plasmin degradation and increasing the TGF-β1 activity in SMCs (McCaffrey et al., 1994). Fucoidan is an effective inhibitor of DNA synthesis stimulated by fetal calf serum, thrombospondin-1, and PDGF BB (Patel, Mulloy, Gallagher, O’Brien, & Hughes, 2002), contributing to its anti-proliferative effect on SMCs. Fucoidan has also been reported to inhibit phosphorylation and nuclear translocation of mitogen-activated protein kinase (MAPK) in SMCs (Religa et al., 2000). The antiproliferative effect of fucoidan is dependent on its structural features. Sulfation (Vischer & Buddecke, 1991) and a minimum of 30 saccharide units (Logeart et al., 1997) may be necessary to demonstrate the antiproliferative activity of fucoidan on SMCs.
In addition to inhibiting SMC proliferation, fucoidan suppresses SMC migration. Low molecular weight fucoidan suppresses MMP-2 expression in SMCs and reduces SMC migration by 40 ± 3% (Hlawaty et al., 2011). In a recent study in a pulmonary arterial hypertension mouse model, injection of fucoidan at a dose of 25 mg/kg has been shown to inhibit hypoxia and growth factor-stimulated SMC proliferation and migration (Novoyatleva et al., 2019). Fucoidan also substantially reduces the incorporation of methionine into fibronectin and increases the synthesis of thrombospondin (Vischer & Buddecke, 1991), which may contribute to the inhibition effect of fucoidan on SMC migration.
Several studies have demonstrated that fucoidan is capable of mitigating the formation of atherosclerotic lesions and suppressing intimal hyperplasia formation (Deux et al., 2009; Kim et al., 2015; Roux et al., 2012; Xu et al., 2019). Fucoidan coating on stent has been shown to inhibit SMC proliferation and yield a smaller histopathological restenosis area (Kim et al., 2015). Xu et al. studied the mitigation effect of a low molecular weight fucoidan on atherosclerosis in a mouse model. They found that fucoidan ameliorated atherosclerosis lesions by reducing SMC proliferation and migration, and inhibiting macrophage formation and differentiation (Xu et al., 2019). Our recent study developed a fucoidan modified small diameter vascular graft (Fig 4A) (Yao et al., 2020). The fucoidan modification significantly improves EC adhesion (Fig 4B). The fucoidan modified vascular grafts yields a lower stenosis percentage (Fig 4C) than the unmodified grafts, which could be due to reduced SMC proliferation and increased EC adhesion.
Figure 4.
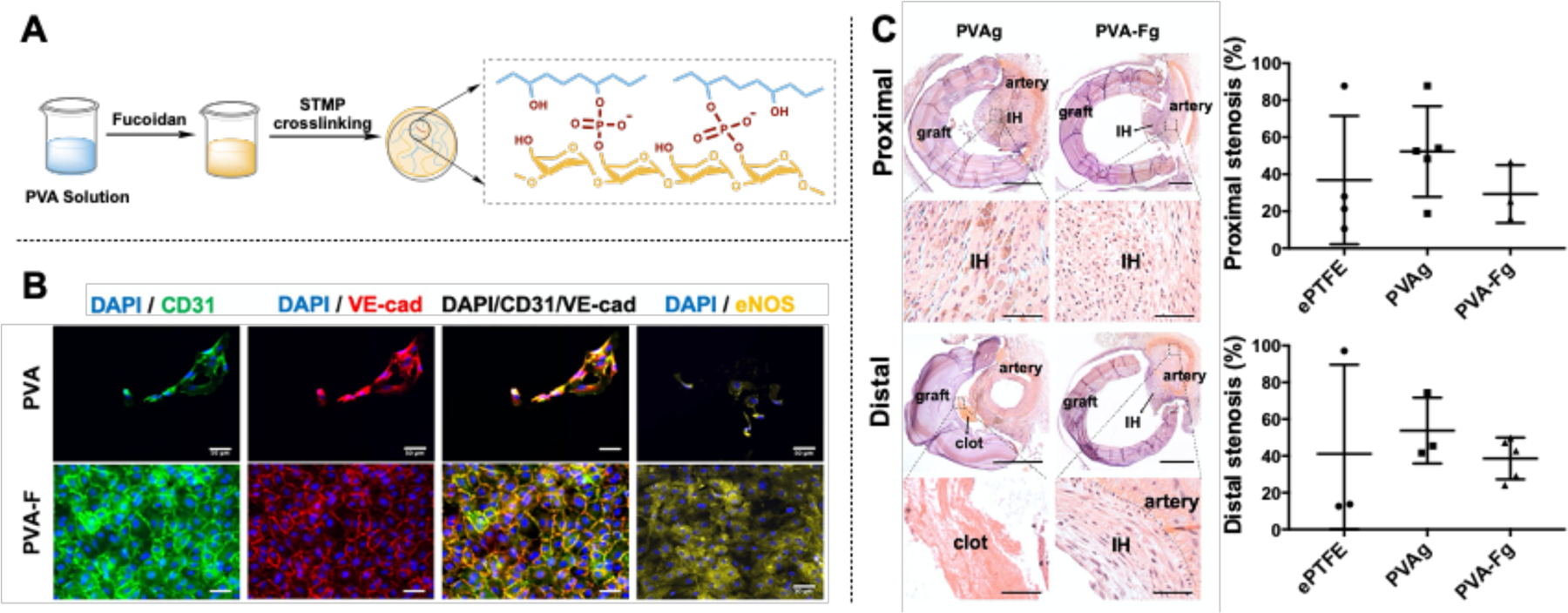
The use of fucoidan as functional molecules for polyvinyl alcohol (PVA) small diameter vascular grafts. (A) Modification of PVA by fucoidan. (B) Fucoidan (PVA-F) significantly improved endothelial cell adhesion on PVA. (C) Fucoidan modified PVA vascular grafts (PVA-Fg) yielded a lower re-stenosis percentage. Reproduced with permission from Ref (Yao et al., 2020).
The interactions between polysaccharides with target proteins have been recognized to mediate many biological processes (Williams & Davies, 2001). Fucoidan has been shown to form complexes with different proteins, which is responsible for its various bioactivities. In addition to the above-mentioned proteins and molecules, fucoidan also binds to membrane receptors, such as scavenger receptor and C-type lectin receptor, integrins, growth factors, and extracellular matrix proteins. The binding affinity also exhibited a structural-dependent manner. The interaction of fucoidan with those molecules was summarized and reviewed by Lin et al. (Lin et al., 2020).
3. Variables mediating the application of fucoidan in cardiovascular area
While previous studies in bioactivities of fucoidan have demonstrated the potential of fucoidan for cardiovascular applications, several variables have been shown to affect the bioactivities of fucoidan substantially. These variables include the sources of fucoidan, molecular weight, and structural features.
3.1. Sources of fucoidan
The main sources of fucoidan are seaweeds and echinoderms, specifically sea cucumber and sea urchin. Fucoidans from different sources exhibit different bioactivities. Table 1 (Supplementary Table 1 with additional details) summarizes the key findings on the bioactivities of fucoidan from representative sources. These differences in bioactivities of fucoidan are attributed to varied structural characteristics, molecular weight, and monosaccharide composition.
Table 1.
Bioactivities of fucoidan from different species *.
| Activity | Species | Experiment setting | Key findings | Refs |
|---|---|---|---|---|
| Anti-coagulant and Anti-thrombotic | Fucus vesiculosus; Undaria pinnatifida; Fucus evanescens | immobilized on plasma treated PET; in vitro anticoagulant assay | prolonged TT and APTT | (Kim et al., 2010; Kuznetsova et al., 2003; Ozaltin et al., 2019; Zhang et al., 2015) |
| Laminaria hyperborean | lepirudin-based human whole blood model in vitro anticoagulant assay; in | inhibited coagulation above 100 μg/mL; stimulated coagulation at 10 μg/mL slowed thrombosis formation, increased | (Kopplin et al., 2018) | |
| Saccharina japonica | vivo arterial thrombosis rat model; oral administration in human | TFPI; prolonged APTT and TT; inhibited thrombin-induced platelet aggregation; shortened lysis time of thrombus | (Ren et al., 2013; Zhang et al., 2015; Zhao et al., 2012; Zhao et al., 2016) | |
| Ecklonia maxima | in vitro anticoagulant assay | increased APTT | (Zhang et al., 2015) | |
| Ecklonia cava | in vitro anticoagulant assay | prolonged bleeding time | (Wijesinghe et al., 2011) | |
| Hizikia fusiforme | in vitro anticoagulant assay | prolonged APTT distinctly, but delayed TT little | (Li, Zhao, et al., 2008) | |
| Ascophyllum nodosum | in vitro anticoagulant assay | bound to antithrombin in a 1:1 stoichiometry | (Varenne, Gareil, Colliec-Jouault, & Daniel, 2003) | |
| Turbinaria conoides | in vitro anticoagulant assay | prolonged both APTT and PT | (Ganapathy et al., 2019) | |
| Holothuria edulis; Ludwigothurea grisea | in vitro anticoagulant assay | prolonged APTT, but not PT nor TT; inhibited thrombin and factor Xa | (Wu et al., 2015) | |
| Holothuria polii | in vitro anticoagulant assay | inhibited thrombin with the presence of heparin cofactor II and antithrombin III | (Mansour et al., 2019) | |
| Isostichopus badionotus | in vitro anticoagulant assay | improved antithrombin activity on thrombin and factor Xa | (Chen et al., 2012) | |
| Platelet | Fucus vesiculosus | in vitro platelet rich plasma | induced irreversible platelet aggregation and activation through SFK-dependent pathways; increased CD62p and CD63 positive platelets | (Carvalho G. de Azevedo et al., 2009; Dürig et al., 1997; Kardeby et al., 2019; Manne et al., 2013) |
| Saccharina japonica | in vivo intravenous injection of LMWF at 10 mg/kg | inhibit activation of platelets by reducing platelet aggregation | (Chen et al., 2016; Zhu et al., 2010) | |
| Holothuria edulis; Ludwigothurea grisea | in vitro citrated human platelet rich plasma; | did not induce platelet aggregation at various concentrations (7.5–30 μg/mL) | (Wu et al., 2015) | |
| Anti-complement | Saccharina japonica; Sargassum thunbergii | in vitro | anticomplement activity | (Jin, Liu, Zhong, Sun, & Zhang, 2017; Liu et al., 2018) |
| Laminaria hyperborea | lepirudin-based human whole blood model | sulfation and high molecular weight are important in anticomplement activity | (Kopplin et al., 2018) | |
| Saccharina latissimi; Fucus vesiculosus | in vitro | reduced IL-8 and C5a-induced calcium release by binding to IL-8 and C5a in a concentration-dependent manner | (Liewert, Ehrig, & Alban, 2017) | |
| Ascophyllum nodosum | MALDI-TOF mass spectroscopy; in vitro hemolytic assay in whole serum | bound to C1q; blocked the dissociation of C2 and C4, to a lesser extent of C3 | (Clément et al., 2010; Tissot, Daniel, et al., 2003; Tissot et al., 2005; Tissot, Montdargent, et al., 2003) | |
| Laminaria cichorioides; fucus evanescens | in vitro assay with human serum | caused 50% inhibition of alternative pathway of complement | (Zvyagintseva et al., 2000) | |
| Inhibit selectin | Sargassum fusiforme | in vitro static and flow chamber | blocked P-selectin mediated adhesion of neutrophil | (Wu et al., 2019) |
| Saccharina japonica | diabetic nephropathy rat | decreased the level of P-selectin and selectin-dependent inflammatory cytokines | (Xu, Zhang, Luo, Wang, & Duan, 2016) | |
| Sacharina latissima | in vitro binding activity | inhibited L- and P-selectin | (Ushakova et al., 2005) | |
| Endothelial cells | Fucus vesiculosus; Ascophyllum nodosom | layer-by-layer stent coating with laminin; polyvinyl alcohol hydrogels; decellularized pulmonary heart valve | promoted endothelial cell adhesion and proliferation | (Marinval et al., 2018; Yao et al., 2020; Ye et al., 2016) |
| Fucus evanescens | dyslipidemia mice model | reduced levels of endothelin-1 and TNFα, IFNγ in blood serum. | (Kuznetsova et al., 2019) | |
| Ascophyllum nodosom | EPC proliferation and adhesion; in vitro tube formation assay | promoted proangiogenic phenotype, proliferation and migration of EPCs | (Roux et al., 2012) | |
| Undaria piniatifida | HUVEC proliferation, migration, and tube formation | reduced HUVEC proliferation and migration; inhibited tube formation | (Liu et al., 2012) | |
| Anti-proliferation | Fucus vesiculosus | in vitro cell culture; in vivo injection; fucoidan coated stent | inhibited SMC proliferation and migration; interacted with TGF-β1; reduced restenosis | (Kim et al., 2015; McCaffrey et al., 1992; Novoyatleva et al., 2019; Religa et al., 2000) |
| Saccharina japonica | in vitro cell culture; in vivo in ApoE (−/−) mouse model | inhibited SMC proliferation and migration; reduced atherosclerosis | (Xu et al., 2019) | |
| Undaria pinnatifida | in vivo in eNOS inhibition-induced hypertensive rat model | reduced media thickening and SMC proliferation | (Li et al., 2016) | |
| Ascophyllum nodosum | in vitro cell culture; balloon-induced thoracic aorta injury rat model | inhibited SMC growth and migration; inhibited collagen synthesis; reduced intimal hyperplasia | (Hlawaty et al., 2011; Logeart et al., 1996) |
The extended version of the table is provided in the Supplementary Table 1
Fucose is the primary monosaccharide in fucoidan; other monosaccharides, namely xylose, mannose, galactose, glucose, rhamnose, and uronic acid, as shown in Fig 5, also exist in fucoidan. Several studies have summarized the correlation between monosaccharide content and the bioactivities of fucoidan. A higher fucose content is generally believed to be beneficial for a wide range of bioactivities of fucoidan (Hwang, Yan, Kuo, Phan, & Lin, 2017; Jin et al., 2019; Pozharitskaya, Obluchinskaya, & Shikov, 2020). Fucoidan with lower xylose content has shown greater anticoagulant activity (Drozd et al., 2011), while higher galactose content has enhanced anticoagulant activity (Jin et al., 2013). Uronic acid has also been shown to contribute indirectly to the anticoagulant activity of fucoidan by altering the flexibility of fucoidan chains (Li, Zhao, et al., 2008). The anticomplement activity of fucoidan increased with the increment of galactose and uronic acid content (Blondin et al., 1996), and the authors suggested that those residues might be essential for full anticomplementary activity.
Figure 5.

(A) Structures of monosaccharides. (B) Structures of fucoidan, reproduced with permission from Ref (Oliveira et al., 2020).
The backbone structure of fucoidan also varies depending on the species of the fucoidan source. Fucoidan has both linear and branched backbones, as shown in Fig 5B. Mono (1–3)-α-L-fucopyranose and the repeated alternating (1–3)-α-L-fucopyranose and (1–4)-α-L-fucopyranose are the two main types of linear backbones. Branched fucoidans are usually obtained from certain species, such as Saccharina latissima. The branches are generally oligomers located at C-2 or C-4 (Ale & Meyer, 2013). The structural differences of fucoidans result in significantly varied bioactivities. In the study by Cumashi et al., the anti-inflammatory, anticoagulant, anti-angiogenetic and anti-adhesive activities of fucoidans from 9 seaweeds were compared. Fucoidans from Sacharina latissima and Fucus evanescens appeared to be the most versatile due to their high efficiency in anti-inflammatory, anticoagulant, and antiangiogenetic activities (Cumashi et al., 2007). The anticoagulant activity of fucoidan from Undaria pinnatifida was found to be twice that of fucoidans from Saccharina japonica, Fucus vesiculosus, and Ecklonia maxima (Zhang et al., 2015). The presence of branches is believed to increase the chain flexibility, which is beneficial for fucoidans to maintain an effective conformation to inhibit thrombin generation.
In contrast to fucoidans from seaweeds, fucoidans from sea cucumber and sea urchins were shown to have a more regular structure with a well-defined repetitive sequence (Alves, Mulloy, Diniz, & Mourão, 1997; Mulloy, Ribeiro, Alves, Vieira, & Mourão, 1994; Yu et al., 2014). Mulloy et al. first reported that fucoidans from sea cucumber and sea urchin have a linear backbone of mono (1–3)-α-L-fucopyranose or mono (1–4)-α-L-fucopyranose with sulfate substitution at C-2 or C-2,4 position. The group later compared the structure and the anticoagulant activity of three types of linear fucoidans from echinoderms with the linear and branched fucoidans from brown seaweeds (Pereira, Mulloy, & Mourão, 1999). Among the three fucoidans from echinoderms, only the fucoidan with 4-O-sulfated fucose unit exhibited significant anticoagulant actions. Also, the branched fucoidan from brown seaweed, but not linear fucoidan, from echinoderms appeared to be a direct thrombin inhibitor. The mechanism of the anticoagulant activity of fucoidan from echinoderms, in particular sea cucumber, was suggested to be acting through the inhibition of the intrinsic coagulation pathway, by targeting the intrinsic coagulation Factor Xa (Shang et al., 2018). Branched fucoidan has also been isolated from certain sea cucumbers recently. Fucoidan from sea cucumber Pattalus Mollis has a (1–4)-α-L-fucopyranose backbone. Along this backbone, every trisaccharide unit has a branched sulfate fucose, which has been shown to be beneficial for the anticoagulant and anti-FXase activities (Zheng, Zhou, et al., 2019). Meanwhile, fucoidan from sea cucumber Holothuria polii has a branched backbone with glucuronic acid and tetrafucose repeating unit. This fucoidan from Holothuria polii has high sulfate content and abundant disulfated residues, thus exhibiting a strong anticoagulant effect in the presence of heparin cofactor II with an IC50 of 0.16 μg/mL (Mansour et al., 2019).
The relationship between the activities and species of fucoidan is hard to conclude. Fucoidans from different species seem to be distinguishable in various aspects. Thus, the selection of fucoidan should be made depending on the intended application of fucoidan.
3.2. Molecular weight of fucoidan
Molecular weight is a crucial determinant for the activities of fucoidans. Native fucoidans usually have a large molecular weight, and crude products of fucoidan typically have a wide molecular weight distribution due to the presence of fucoidan fractions with different molecular weight range. These fucoidan fractions can be classified into high molecular weight fucoidan (HMWF), medium molecular weight fucoidan (MMWF), and low molecular weight fucoidan (LMWF). The differences in molecular weight of fucoidan could generate different efficiency of bioactivities and even opposite effects.
Molecular weight affects the anticoagulant and antithrombotic activities of fucoidan from multiple aspects. On one hand, LMWF is better at inhibiting platelet aggregation than HMWF (Dürig et al., 1997; Zhu et al., 2010); on the other hand, HMWF is more effective in promoting antithrombin III- and heparin cofactor II-thrombin reaction. HMWF has been reported to have a pro-aggregation response, while LMWF inhibits thrombin-induced platelet aggregation (Zhu et al., 2010). The ability of fucoidan to stimulate t-PA has been shown to be decreased with a decrease in the molecular weight (Soeda et al., 1993). Pomin et al. degraded fucoidan by acid, and the obtained LMWF did not show any anticoagulant activity (Pomin et al., 2004). They proposed that the molecular weight of fucoidan will need to be larger than that of mammalian dermatan sulfate to improve heparin cofactor-mediated thrombin inactivation (Pomin et al., 2004). Moreover, fucoidan fractions with different molecular weight may exhibit antithrombotic activities by different mechanisms. In the study of Zhao et al., the antithrombotic activities of MMWF (28 kDa and 35 kDa) and LMWF (3.9 kDa and 7.6 kDa) were compared. MMWF substantially prolonged the time-to-occlusion in an electrical induced arterial thrombosis model, while LMWF showed significant inhibition on thrombin-induced platelet aggregation (Zhao et al., 2012).
Molecular weight is also a leading contributor to the chain conformation of fucoidan. Fucoidan with high molecular weight exhibits random coil conformation, while those with low molecular weight exists as spheres in solution (Xu, Xue, Chang, & Liu, 2017). This could possibly explain the beneficial effect of high molecular weight fucoidan for reduced thrombin generation since a more flexible conformation is required for fucoidan to bind to thrombin (Li, Lu, et al., 2008). Different from anticoagulant activity, a more consistent trend on the favorable anticomplementary activity of high molecular weight fucoidan has been found (Kopplin et al., 2018; Liu et al., 2018). The anticomplementary activity of fucoidan has been shown to increase with increasing molecular weight until reaching a plateau of Mw 40000 and 13500 for complement-mediated hemolysis of sheep and rabbit erythrocytes, respectively (Blondin et al., 1996). Fucoidans with different molecular weight also have different reactions with inflammatory proteins. HMWF has been reported to increase plasma concentration of both MMP-9 and stromal cell-derived factor-1 (SDF-1), while LMWF only increased stromal cell-derived factor-1 concentration but not MMP-9 (Luyt et al., 2003).
Oligo-fucoidan has been developed by enzymatic degradation of fucoidan for better oral absorption. However, oligo-fucoidan has yet to demonstrate satisfactory activities for cardiovascular application. Kim et al. showed that oligo-fucoidan was 3–20 times less effective in prolonging APTT and TT (Kim et al., 2010). Oligo-fucoidan also seems to have no antithrombotic activity in the presence of antithrombin III. The possible reason was proposed to be the lack of sufficient chain length to form a complex with antithrombin III (Ustyuzhanina et al., 2014).
3.3. Structure modification
3.3.1. Sulfation
Sulfate groups in the saccharide unit, as shown in Fig 1, is the key structural feature of fucoidan and play a vital role in maintaining or mediating the bioactivities of fucoidan. The effects of sulfate groups involve two aspects: the sulfate content and the location of sulfate groups.
Native fucoidans from different species have a wide range of sulfate content, varying from 10% to nearly 40% (by weight). Various activities of fucoidan were shown to be positively correlated to sulfate content. Fucoidan with a higher degree of sulfate inhibits coagulation, complement, and cytokines more effectively (Kopplin et al., 2018). More sulfate groups have shown to be beneficial for prolonged prothrombin time of normal citrated human plasma (Qiu, Amarasekara, & Doctor, 2006). However, the increment from the sulfate groups has an upper limit. The antithrombotic and anticoagulant activities of oversulfated fucoidans were reported to increase with the increase of sulfate content and then reach a plateau when the sulfate/sugar ratio rose to 1 (Nishino & Nagumo, 1992). Similarly, APTT and TT have been shown to increase with the increase of sulfate content in fucoidan from Undaria pinnatifida sporophyll, with an optimum sulfate reported to be around 24% (Park, Cho, In, Kim, & Chae, 2012).
Sulfate groups usually locate at C-2 or C-4 and to a lesser extent at C-3, or occupy C-2,3 or C-2,4 as disulfate residues (Li, Lu, et al., 2008). Fucoidan, enriched with 2,4-disulfated residues, has been demonstrated to bind to antithrombin III more effectively and possess a more potent inhibition on clotting formation (Ustyuzhanina et al., 2014). Furthermore, the location of the 2,4-disulfated fucose unit also affects the reaction pathways of fucoidan on coagulation and thrombosis. In a study by Fonseca et al., the fucosylated chondroitin sulfate (FCS) with 2,4-disulfated fucose unit located in branches and sulfated fucan (SF) with 2,4-disulfated fucose unit located in the linear chain were compared (Fonseca, Santos, & Mourão, 2009). Although both molecules exhibited anticoagulant and antithrombic activities, the inhibitory effect of FCS on thrombin was mediated by heparin cofactor II, while that of SF was mediated by both antithrombin III and heparin cofactor II.
To further verify the importance of sulfate groups in fucoidan bioactivities, desulfation of fucoidan was investigated. By comparing native fucoidan and desulfated fucoidan from Sargassum fusiforme, Li et al. found that sulfate groups are vital to achieve the anticoagulant effect of fucoidan (Li, Zhao, et al., 2008). Sulfation was demonstrated to be essential in suppressing the proliferation of SMCs, as the activity was denuded by desulfation of fucoidan (Vischer & Buddecke, 1991). Oversulfation of fucoidan has been developed to enhance its bioactivities. Oversulfated fucoidan has exhibited more potent inhibition in thrombin-induced fibrinogen clotting (Nishino et al., 1995) and tube formation by ECs (Soeda, Kozako, Iwata, & Shimeno, 2000; Soeda et al., 1997). The desulfation and oversulfation of fucoidan have been performed in different approaches. Techniques involved in these processes were reviewed and summarized by Fernando et al. (Fernando, Kim, Nah, & Jeon, 2019) and Wang et al. (Wang & Zhang, 2017).
3.3.2. Acetylation
Fucoidan also contains acetyl groups predominantly at C-4 and to a lesser extent at other random locations (Bilan et al., 2007; Mourão, 2004; Nagaoka et al., 1999; Synytsya et al., 2010). The acetyl groups in fucoidan are responsible for the anti-oxidant activity of fucoidan (Wang, Liu, et al., 2009). Another impact of the acetyl group is on the immunomodulatory activity of fucoidan. Teruya et al. showed that fucoidan with high acetylation from Cladosiphon okamuranus induced macrophage activation (Teruya, Tatemoto, Konishi, & Tako, 2009). Meanwhile, the sulfate and acetyl groups in fucoidan from fucus evanescens have been reported to stimulate the release of inflammatory cytokines by bone marrow-derived immune cells (Khil’chenko et al., 2011). However, few studies showed direct effect of acetyl groups on the anticoagulant and antithrombotic activities of fucoidan. For example, studies on deacetylation of fucoidan from Fucus evanescens showed that the ability of fucoidan to inhibit thrombin and Factor Xa was not changed by the deacetylation (Chizhov et al., 1999; Lapikova et al., 2008).
3.3.3. Amination
The addition of aminated groups to fucoidan has been studied due to its ability to increase anti-coagulant property. Aminated derivative of fucoidan from Fucus vesiculosus has been shown to be more effective in promoting t-PA-induced plasma clot lysis. It also exhibited 2.3 times more potent efficiency in accelerating heparin cofactor II-mediated thrombin inhibition than native fucoidan (Soeda, Ohmagari, Shimeno, & Nagamatsu, 1994). Wang et al. reported that the beneficial effect of aminated fucoidan on anticoagulant activity was acting through the intrinsic pathway, and the authors suggested that the change in charge density of fucoidan was the potential reason (Wang, Zhang, Zhang, Hou, & Zhang, 2011).
Due to the heterogeneous nature of fucoidan, direct amination of fucoidan through hydroxyl groups on the fucose ring is challenging. Several methods have been developed to aminate fucoidan. Epichlorohydrin has been commonly used as a space molecule before the amination reaction of fucoidan with ammonia water (Soeda et al., 1994). Element analysis (Soeda et al., 1994) and Fourier-transform infrared spectroscopy (FTIR) (Wang, Zhang, Zhang, Zhang, & Li, 2009) proved the success of the reaction. Amination using diaminopropane at the reducing end of fucoidan has also shown success (Bachelet-Violette et al., 2014).
Other structural modifications of fucoidan, such as methylation, esterification, and phosphorylation, have not presented obvious alteration in fucoidan bioactivities for cardiovascular application. The details have been covered in a recent review by Fernando et al. (Fernando et al., 2019).
4. Therapeutic strategies and challenges
4.1. Therapeutic strategies
4.1.1. Oral and intravenous administration
The diverse bioactivities of fucoidan have enabled its therapeutic applications in the treatment of several cardiovascular diseases. Extensive studies have demonstrated that fucoidan is capable of mitigating atherosclerosis lesions (Kuznetsova et al., 2019; Patil et al., 2018; Wang, Pei, et al., 2016), which is mainly attributed to its anti-inflammatory, antithrombotic and anticoagulant activity. Fucoidan also attenuates AAA in vivo in various animal models (Alsac et al., 2013; Tsai et al., 2018; Zhou et al., 2018). The non-anticoagulant fucoidans have also been developed to treat hemophilia (Liu et al., 2006; Prasad et al., 2008; Zhang et al., 2015). These therapeutic effects of fucoidan are mainly achieved by administering fucoidan through intravenous (IV) injection and oral administration.
IV administration of fucoidan has proved successful therapeutic activity in various animal models (Alwayn et al., 2000; Kwak et al., 2010; Luyt et al., 2003; Mauray et al., 1995). The effectiveness of IV administrated fucoidan has shown to be in a dose-dependent manner. In a non-human primate baboon model, no significant alteration on platelet aggregation or coagulation profile was seen with a low dose (0.5 mg/kg) of fucoidan. However, complete inhibition of platelet aggregation was achieved when fucoidan was administered at a dose of 1.0 mg/kg (Alwayn et al., 2000). The clearance of IV-administered fucoidan was studied in a rabbit model (Zhang et al., 2016). Low molecular weight fucoidan (7.1kDa) injected at a dose of 50 mg/kg was shown to reach a peak serum concentration at 5 min, having a mean residence time of 109 min. Despite all the success in animal models, the IV administration of fucoidan as therapeutic drugs has not been studied in human clinical trials.
Several groups have studied the absorption and bioavailability of oral administrated fucoidan. Zhao et al. showed that the content of LMWF from Saccharina japonica in the plasma and urine peaked at 15 hours after oral administration in rats. The LMWF was found to have much better absorption and bioavailability than MMWF (Zhao et al., 2016). Pharmacokinetic and tissue distribution studies of orally administered fucoidan from Fucus vesiculosus showed that fucoidan preferentially accumulated in kidney, spleen and liver, with an extended mean residence time in blood (Pozharitskaya et al., 2018). Although many studies have proved the biological effects of fucoidan after oral ingestion, only a few studies have elucidated the mechanism of intestinal absorption of fucoidan. Nagamine et al. examined the absorption of fucoidan from Cladosiphon okamuranus after oral administration in a rat model (Nagamine, Nakazato, Tomioka, Iha, & Nakajima, 2014). They demonstrated that absorption of fucoidan was through small intestine. By fluorescently labeling fucoidan, they observed that fucoidan was taken up by intestinal macrophages and Kupffer cells. The scavenger receptors were speculated to be responsible for the uptake. A more recent study also showed that fucoidan could be absorbed by small intestine, majorly in the jejunum, ileum, and duodenum (Zhang et al., 2018). Inhibitors of clathrin-mediated endocytosis, chlorpromazine, dynasore and NH4Cl, were used to explore the mechanism of fucoidan absorption. The addition of these inhibitors reduced the absorption of fucoidan significantly, indicating that absorption of fucoidan involves the clathrin endocytic pathway (Bai et al., 2020; Zhang et al., 2018)
4.1.2. Fucoidan-based drug delivery system
Fucoidan is negatively charged due to the presence of sulfate groups in the main structure, allowing it to complex easily with other molecules. This advantage of fucoidan enables researchers to incorporate fucoidan into delivery systems to improve efficacy and achieve targeted drug delivery (Citkowska, Szekalska, & Winnicka, 2019; Sezer & Cevher, 2011).
A number of studies have reported that fucoidan-based delivery systems have better anticoagulant and antithrombotic activities and more potent inhibition of platelet aggregation than fucoidan alone. A glutaraldehyde-crosslinked chitosan nanoparticle system loaded with fucoidan demonstrated 60% fucoidan release within 24 hours, and exhibited increased anticoagulant activities than fucoidan alone (Da Silva et al., 2018). Nanoparticles prepared by fucoidan and red ginseng extracts significantly decreased platelet aggregation in vitro and ex vivo (Kim, Lee, & Lee, 2016). In another study, nanoparticles coated with fucoidan showed a suppressive effect on monocyte recruitment under non-uniform shear stress (Matuszak et al., 2018).
The ability of fucoidan to bind specifically to activated platelets also enables the design of fucoidan-based targeted delivery systems. Core-shell polymer nanoparticles functionalized with fucoidan have been designed for targeted delivery of rt-PA to the thrombus site (Fig 6A) (Juenet et al., 2018). The fucoidan functionalized nanoparticles have shown significantly higher accumulation onto activated platelets than nanoparticles without fucoidan. Similarly, core-shell microcapsules with fucoidan present on the surface localized mainly in the rat abdominal aortic aneurysm thrombotic wall instead of healthy vessel walls (Li et al., 2017).
Figure 6.
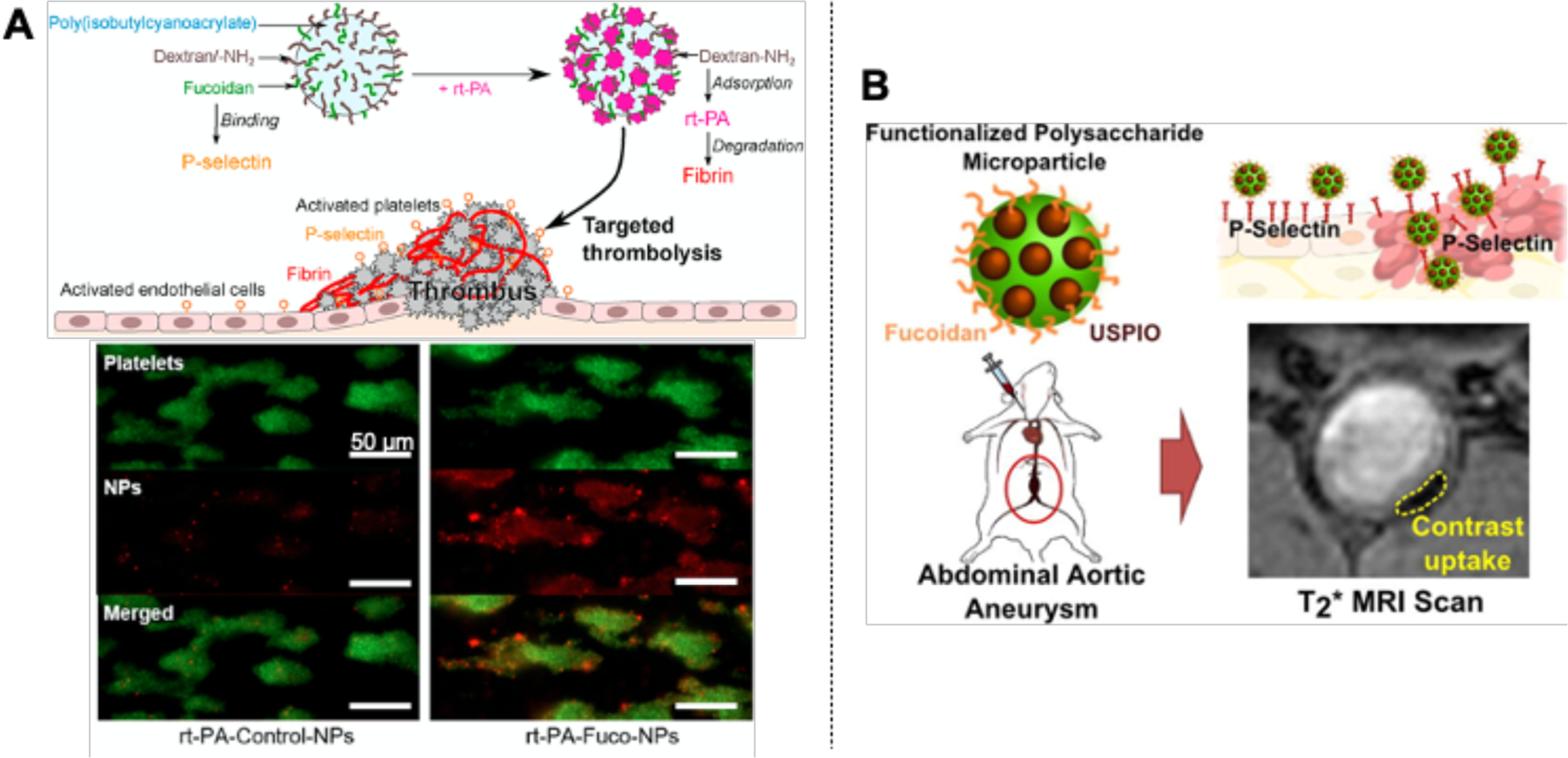
The use of fucoidan as a targeted drug delivery system and imaging agent. (A) Nanoparticles functionalized with fucoidan showed targeted delivery of recombinant tissue plasminogen activator (rt-PA) to the thrombus site. Reproduced with permission from Ref (Juenet et al., 2018). (B) Ultrasmall particles of iron oxide (USPIOs) functionalized with fucoidan localized inside the aneurysm wall, presenting a significant contrast enhancement on magnetic resonance imaging (MRI) of abdominal aortic aneurysm (AAA). Adapted with permission from Ref (Bonnard, Serfaty, et al., 2014).
4.1.3. Imaging agent
In addition to targeted drug delivery, the binding ability of fucoidan to selectins and activated platelets also enables its use as imaging agents. Magnetic nanoparticles functionalized with fucoidan have been shown to be localized at intraluminal thrombus and activated nanoparticles, improving the visualization of thrombus using magnetic resonance imaging (MRI) (Bachelet-Violette et al., 2014; Suzuki et al., 2015). Similarly, fucoidan/peptide nanoparticles have also been developed as MRI agents for inflamed endothelial cells and activated platelets through P-selectin binding (Cheng et al., 2020). Fucoidan functionalized microparticles combined with a fluorescent dye and a MRI contrasting agent can also facilitate AAA imaging (Bonnard, Serfaty, et al., 2014). Through intravenous injection, the microparticles have shown to exhibit 20 times higher adhesion on activated endothelium, accumulated inside the lumen of the aneurysm wall, and provided a significant contrast enhancement on AAA imaging (Fig 6B).
The first clinical study using a radiolabeled fucoidan as a diagnostic agent for P-selectin imaging was done by Zheng et al. (Zheng, Kaiser, et al., 2019). The radiolabeled microparticles did not exhibit any drug-related adverse effects in all participants, and the microparticles were cleared rapidly after 24 hours. These studies suggested the potential and safety of fucoidan as an imaging agent.
4.1.4. Functional molecules for cardiovascular devices and implants
The promising findings on fucoidan as therapeutic drugs inspired the researchers to explore the application of fucoidan for biomedical devices. Fucoidan modification is capable of improving the hemocompatibility of blood-contacting biomaterials and devices due to its antithrombotic activities. Synthetic biomaterials, including polyethylene terephthalate (Ozaltin et al., 2019), polyvinyl alcohol (Yao et al., 2020) and polydopamine (Su et al., 2017) have been functionalized with fucoidan through various strategies. The resulting materials have exhibited substantially improved hemocompatibility, endothelialization, and reduced thrombus and intimal hyperplasia formation. Fucoidan-coated stent also has been shown to yield higher patency than bare metal stent (Kim et al., 2015). Meanwhile, fucoidan has been shown to improve the efficiency of other functional molecules. A decellularized pulmonary heart valve has been shown to have enhanced re-endothelialization in vitro and in vivo after being modified with fucoidan and VEGF (Marinval et al., 2018). Similarly, in another study, fucoidan/laminin films assembled layer-by-layer have been shown to promote the adhesion of HUVECs with laminin as the outermost layer and reduced platelet adhesion with fucoidan as the outermost layer (Wang, Ye, et al., 2016). Adding fucoidan into a chitosan/alginate scaffold could prolong FGF release and modulate the migration of fibroblasts (Zeng & Huang, 2018).
These findings have demonstrated the potential of fucoidan as a functional molecule in biomedical devices and implants, and the actual efficacy will need to be further validated and tested in animal studies and clinical trials.
4.2. Challenges
Despite all the positive results and progress of fucoidan studies in both research and clinical trials, several challenges confine the actual application of fucoidan.
4.2.1. Complexity in structure and composition
Among all the challenges, structural variation remains the primary obstacle. Many techniques have been employed to reveal the chemical structure of fucoidan. Using gas chromatography-mass spectrometry (GC-MS), Pankter et al. first identified that fucoidan from Fucus vesiculosus has a (1–3)-α-L-fucopyranose structure with sulfate substituted at the C-4 location (Patankar, Oehninger, Barnett, Williams, & Clark, 1993). Later, the presence of repeated (1–3)- and (1–4)-α-L-fucopyranose has also been evidenced using nuclear magnetic resonance (NMR) (Bilan et al., 2004). However, native fucoidan typically has a large molecular weight, which hinders the in-depth characterization of fucoidan using the majority of currently available techniques. To overcome the problem, researchers have explored approaches to degrade or depolymerize fucoidan. The most common method is enzymatic degradation using fucoidanase, which degrades fucoidan without destroying the sulfate group (Kitamura, Matsuo, & Tsuneo, 1992; Silchenko et al., 2013). In addition, degradation in aqueous solution either at a high temperature (121°C) (Yang et al., 2008) or at 37 °C for prolonged periods (M. I. Bilan, Grachev, Shashkov, Nifantiev, & Usov, 2006) also generates LMWF while retaining the sulfate groups.
As more and more studies reveal the main structure of different fucoidans, the structural complexity is also evident. The effects of molecular weight, sulfate content and pattern, conformation, and monosaccharide composition on the bioactivities of fucoidan have all been brought to attention. Although many researchers have tried to correlate these structural features to different bioactivities, as discussed in section 3, these studies used fucoidans from various sources and applied in different animal models. Thus, no consensus has been reached.
To take advantage of the bioactivities of fucoidan and bypass the structural complications, several studies simplified the structure of fucoidan by synthesizing a fucoidan-mimetic glycopolymer with a regular sulfation pattern (Fan et al., 2018). The glycopolymer exhibited similar platelet modulation behaviors as fucoidan (Tengdelius et al., 2017; Tengdelius et al., 2014), providing a new approach to investigate the molecular and cellular responses of human blood system to fucoidan.
4.2.2. Seasonal variation of fucoidan harvest
The distinguishable structural features and varied bioactivities of fucoidans from different species of the source have been elucidated by numerous studies, as discussed in Section 3.1. In addition to the inherent heterogeneity, fucoidan is also subjected to seasonal and monthly variations of the fucoidan harvest. The harvest season of the source that fucoidan was extracted from affects both the yield and composition of the resulting fucoidan products. The yield of fucoidan from seaweeds increases as the plants mature (Fletcher, Biller, Ross, & Adams, 2017; Kim, Rioux, & Turgeon, 2014; Qu et al., 2019). Fucus serratus, Fucus vesiculosus, and Ascophyllum nodosum were shown to have the highest yield of fucoidan in autumn and the lowest in spring (Fletcher et al., 2017). The monosaccharide composition of fucoidan changes with the harvest season, and fucoidan collected in July was shown to be predominantly mono-(1–3)-α-L-fucopyranose (Anastyuk et al., 2010). From March to July, an increase of sulfate content (from 0.37% to 1.62%) and fucose proportion (from 32.15% to 60.85%) and a decrease of galactose (from 21.89% to 9.03%) were reported (Qu et al., 2019). This seasonal change decreased the consistency of fucoidan product from the same species of the source, which affects detrimentally to the repeatability of experimental results and adds to the complications of fucoidan research and application.
4.2.3. Lack of universal production protocol
Although fucoidan is commercially available in the market, the primary production process of fucoidan is still relying on the extraction from seaweeds. Commonly adopted conventional extraction methods include hot water extraction, acid extraction using hydrochloric acid or sulphuric acid, and salt extraction using calcium chloride. However, these extraction methods have yielded fucoidans with distinguishable differences. For example, hot water-extracted fucoidan contained a much higher fucose composition than salt-extracted ones, while acid extracted fucoidan had the highest level of uronic acid contamination (January, Naidoo, Kirby-McCullough, & Bauer, 2019). Novel techniques, such as ultrasonication-, microwave- and enzyme-assisted extractions, have been developed to facilitate fucoidan extraction. Hanjabam et al. have compared the fucoidans from Sargassum wightii produced using hot water and ultrasonication extraction (Hanjabam et al., 2019). The generated fucoidan products had different fucose concentration and thermal transition temperature. Ale et al. (Ale, Mikkelsen, & Meyer, 2011) and Dobrinčić et al. (Dobrinčić et al., 2020) reviewed both conventional and advanced technologies for extracting fucoidan and discussed the influence of these extraction techniques on the chemical structures and biological activities of the yielded fucoidans. The generation and development of these techniques have facilitated the commercial production of fucoidan; however, the structure and composition variations among different techniques are inevitable, and a standardized extraction approach is yet to be established.
Furthermore, extracted fucoidan also contains contaminants, such as alginate, proteins, and ashes. These impurities influence the bioactivities of fucoidan and hinder the in-depth research on the pharmacological and biochemical mechanism behind the structure-activity relationship. Unfortunately, most studies testing pharmacological activities of fucoidan still used crude or partially purified products, which is likely due to the complexity and high cost of isolation and purification techniques. Many studies have focused on developing novel purification approaches of fucoidan, as reviewed by Dobrinčić et al. (Dobrinčić et al., 2020); however, the difficulty and complexity remain the problem. A universal technique and a standard isolation protocol are yet to be developed.
5. Conclusion
Numerous and increasing evidence has proven that fucoidan is promising for application in treating cardiovascular diseases. The contribution of fucoidan to this area is versatile due to the various bioactivities, and the employment of fucoidan in this area can be achieved through different approaches. However, the fact that those bioactivities are highly affected by multiple parameters, such as species of source, structural differences, and molecular weight range, cannot be neglected. This alteration offers both opportunities and challenges. A more potent activity could be obtained by selecting the species, preparing fucoidan with specific molecular size, and modifying fucoidan by adding or removing functional groups. However, we have to note that this variation also increases the complexity and inconsistency in practice. Although a general trend of influences from individual parameters on various bioactivities has been found, the underlying mechanism is unclear. The combination of different parameters, impurities, and different experimental settings increased the difficulty of getting a consensual conclusion. To facilitate future research on the structure-activity relationship, the use of purified or fractioned fucoidan will be more favorable than crude products to eliminate impurity contamination. A standard and universal production protocol for fucoidan is in urgent need to get consistent quality control. Furthermore, the application of fucoidan in treating cardiovascular diseases is exciting but young compared to cancer treatment. In-depth research on the molecular and cellular mechanism are yet to be done. Clinical trials on both healthy volunteers and patients to prove the safety and efficiency will be necessary to move forward.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health [NIH RO1 HL130274–01A1] and NSERC-CREATE Training in Global Biomedical Technology Research and Innovation at the University of Waterloo [CREATE-509950–2018]. The authors would thank Ms. YeJin Jeong and Ms. Dency David for their help with proofreading and editing.
References
- Ale MT, Mikkelsen JD, & Meyer AS (2011). Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Marine drugs, 9(10), 2106–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsac JM, Delbosc S, Rouer M, Journé C, Louedec L, Meilhac O, & Michel JB (2013). Fucoidan interferes with Porphyromonas gingivalis-induced aneurysm enlargement by decreasing neutrophil activation. Journal of Vascular Surgery, 57(3), 796–805. [DOI] [PubMed] [Google Scholar]
- Alves A-P, Mulloy B, Diniz JA, & Mourão PAS (1997). Sulfated polysaccharides from the egg jelly layer are species-specific inducers of acrosomal Reaction in sperms of sea urchins. Journal of Biological Chemistry, 272(11), 6965–6971. [DOI] [PubMed] [Google Scholar]
- Alwayn IPJ, Appel Iii JZ, Goepfert C, Buhler L, Cooper DKC, & Robson SC (2000). Inhibition of platelet aggregation in baboons: therapeutic implications for xenotransplantation. Xenotransplantation, 7(4), 247–257. [DOI] [PubMed] [Google Scholar]
- Anastyuk SD, Shevchenko NM, Nazarenko EL, Imbs TI, Gorbach VI, Dmitrenok PS, & Zvyagintseva TN (2010). Structural analysis of a highly sulfated fucan from the brown alga Laminaria cichorioides by tandem MALDI and ESI mass spectrometry. Carbohydrate Research, 345(15), 2206–2212. [DOI] [PubMed] [Google Scholar]
- Bachelet-Violette L, Silva AKA, Maire M, Michel A, Brinza O, Ou P, … Chaubet F (2014). Strong and specific interaction of ultra small superparamagnetic iron oxide nanoparticles and human activated platelets mediated by fucoidan coating. RSC Advances, 4(10), 4864–4871. [Google Scholar]
- Bai X, Zhang E, Hu B, Liang H, Song S, & Ji A (2020). Study on absorption mechanism and tissue distribution of fucoidan. Molecules, 25(5), 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrabés JA, Garcia-Dorado D, Mirabet M, Inserte J, Agulló L, Soriano B, … Soler-Soler J (2005). Antagonism of selectin function attenuates microvascular platelet deposition and platelet-mediated myocardial injury after transient ischemia. Journal of the American College of Cardiology, 45(2), 293. [DOI] [PubMed] [Google Scholar]
- Bilan, Grachev AA, Ustuzhanina NE, Shashkov AS, Nifantiev NE, & Usov AI (2004). A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydrate Research, 339(3), 511–517. [DOI] [PubMed] [Google Scholar]
- Bilan, Zakharova AN, Grachev AA, Shashkov AS, Nifant’ev NE, & Usov AI (2007). Polysaccharides of algae: 60. Fucoidan from the Pacific brown alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Bioorganicheskaia khimiia, 33(1), 44–53. [DOI] [PubMed] [Google Scholar]
- Bilan MI, Grachev AA, Shashkov AS, Nifantiev NE, & Usov AI (2006). Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydrate Research, 341(2), 238–245. [DOI] [PubMed] [Google Scholar]
- Bjerre M, Hansen TK, & Flyvbjerg A (2008). Complement activation and cardiovascular disease. Hormone and Metabolic Research, 40(9), 626–634. [DOI] [PubMed] [Google Scholar]
- Blondin C, Chaubet F, Nardella A, Sinquin C, & Jozefonvicz J (1996). Relationships between chemical characteristics and anticomplementary activity of fucans. Biomaterials, 17(6), 597–603. [DOI] [PubMed] [Google Scholar]
- Blondin C, Fischer E, Boisson-Vidal C, Kazatchkine MD, & Jozefonvicz J (1994). Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Molecular Immunology, 31(4), 247–253. [DOI] [PubMed] [Google Scholar]
- Bonnard, Serfaty JM, Journé C, Ho Tin Noe B, Arnaud D, Louedec L, … Le Visage C (2014). Leukocyte mimetic polysaccharide microparticles tracked in vivo on activated endothelium and in abdominal aortic aneurysm. Acta Biomaterialia, 10(8), 3535–3545. [DOI] [PubMed] [Google Scholar]
- Bonnard, Yang G, Petiet A, Ollivier V, Haddad O, Arnaud D, … Le Visage C (2014). Abdominal aortic aneurysms targeted by functionalized polysaccharide microparticles: a new tool for SPECT imaging. Theranostics, 4(6), 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins P, Velthuis Henk t., Yazdanbakhsh Aria P, Jansen Piet GM, van Hardevelt Fred WJ, de Beaumont Eddy MFH, … Hack CE (1997). Activation of the complement system during and after cardiopulmonary bypass surgery. Circulation, 96(10), 3542–3548. [DOI] [PubMed] [Google Scholar]
- Carroll MC (2008). Complement and humoral immunity. Vaccine, 26 Suppl 8(0 8), I28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM (2012). Complement activation: an emerging player in the pathogenesis of cardiovascular disease. Scientifica, 2012, 402783–402783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho G de Azevedo T, Bezerra MEB, Santos M. d. G. d. L., Souza LA, Marques CT, Benevides NMB, & Leite EL (2009). Heparinoids algal and their anticoagulant, hemorrhagic activities and platelet aggregation. Biomedicine & Pharmacotherapy, 63(7), 477–483. [DOI] [PubMed] [Google Scholar]
- Chabut D, Fischer AM, Helley D, & Colliec S (2004). Low molecular weight fucoidan promotes FGF-2-induced vascular tube formation by human endothelial cells, with decreased PAI-1 release and ICAM-1 downregulation. Thrombosis Research, 113(1), 93–95. [DOI] [PubMed] [Google Scholar]
- Chauvet P, Bienvenu J-G, Théorêt J-F, Latour J-G, & Merhi Y (1999). Inhibition of platelet-neutrophil interactions by fucoidan reduces adhesion and vasoconstriction after acute arterial injury by angioplasty in pigs. Journal of Cardiovascular Pharmacology, 34(4), 597–603. [DOI] [PubMed] [Google Scholar]
- Chen, Hu Y, Ye X, Li G, Yu G, Xue C, & Chai W (2012). Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochimica et Biophysica Acta (BBA) - General Subjects, 1820(7), 989–1000. [DOI] [PubMed] [Google Scholar]
- Chen A, Lan Y, Liu J, Zhang F, Zhang L, Li B, & Zhao X (2017). The structure property and endothelial protective activity of fucoidan from Laminaria japonica. International Journal of Biological Macromolecules, 105, 1421–1429. [DOI] [PubMed] [Google Scholar]
- Chen A, Zhang F, Shi J, & Zhao X (2012). Study on antithrombotic and antiplatelet activities of low molecular weight fucoidan from Laminaria japonica. Journal of Ocean University of China, 11(2), 236–240. [Google Scholar]
- Chen A, Zhang F, Shi J, Zhao X, & Yan M (2016). Mechanism study of endothelial protection and inhibits platelet activation of low molecular weight fucoidan from Laminaria japonica. Journal of Ocean University of China, 15(5), 918–922. [Google Scholar]
- Cheng T-M, Li R, Kao Y-CJ, Hsu C-H, Chu H-L, Lu K-Y, … Mi F-L (2020). Synthesis and characterization of Gd-DTPA/fucoidan/peptide complex nanoparticle and in vitro magnetic resonance imaging of inflamed endothelial cells. Materials Science and Engineering: C, 114, 111064. [DOI] [PubMed] [Google Scholar]
- Chizhov AO, Dell A, Morris HR, Haslam SM, McDowell RA, Shashkov AS, … Usov AI (1999). A study of fucoidan from the brown seaweed Chorda filum. Carbohydrate Research, 320(1), 108–119. [DOI] [PubMed] [Google Scholar]
- Church FC, Meade JB, Treanor RE, & Whinna HC (1989). Antithrombin activity of fucoidan. The interaction of fucoidan with heparin cofactor II, antithrombin III, and thrombin. Journal of Biological Chemistry, 264(6), 3618–3623. [PubMed] [Google Scholar]
- Citkowska A, Szekalska M, & Winnicka K (2019). Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Marine drugs, 17(8), 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément M-J, Tissot B, Chevolot L, Adjadj E, Du Y, Curmi PA, & Daniel R (2010). NMR characterization and molecular modeling of fucoidan showing the importance of oligosaccharide branching in its anticomplementary activity. Glycobiology, 20(7), 883–894. [DOI] [PubMed] [Google Scholar]
- Colliec S, Fischer AM, Tapon-Bretaudiere J, Boisson C, Durand P, & Jozefonvicz J (1991). Anticoagulant properties of a fucoidan fraction. Thrombosis Research, 64(2), 143–154. [DOI] [PubMed] [Google Scholar]
- Croci DO, Cumashi A, Ushakova NA, Preobrazhenskaya ME, Piccoli A, Totani L, … on behalf of the Consorzio Interuniversitario Nazionale per la Bio-Oncologia, I. (2011). Fucans, but not fucomannoglucuronans, determine the biological activities of sulfated polysaccharides from Laminaria saccharina brown seaweed. PLOS ONE, 6(2), e17283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, … and on behalf of the Consorzio Interuniversitario Nazionale per la Bio-Oncologia, I. (2007). A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology, 17(5), 541–552. [DOI] [PubMed] [Google Scholar]
- Cuong HD, Thuy TTT, Huong TT, Ly BM, & Van TTT (2015). Structure and hypolipidaemic activity of fucoidan extracted from brown seaweed Sargassum henslowianum. Natural Product Research, 29(5), 411–415. [DOI] [PubMed] [Google Scholar]
- Da Silva LCRP, Todaro V, Do Carmo FA, Frattani FS, De Sousa VP, Rodrigues CR, … Cabral LM (2018). A promising oral fucoidan-based antithrombotic nanosystem: Development, activity and safety. Nanotechnology, 29(16), 165102. [DOI] [PubMed] [Google Scholar]
- Deux J-F, Meddahi-Pellé A, Bree F, Bataille I, Michel J-B, & Letourneur D (2009). Comparative studies on the mechanisms of action of four polysaccharides on arterial restenosis. Journal of Biomaterials Science, Polymer Edition, 20(5–6), 689–702. [DOI] [PubMed] [Google Scholar]
- Deveza L, Choi J, & Yang F (2012). Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics, 2(8), 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dithmer M, Fuchs S, Shi Y, Schmidt H, Richert E, Roider J, & Klettner A (2014). Fucoidan reduces secretion and expression of vascular endothelial growth factor in the retinal pigment epithelium and reduces angiogenesis in vitro. PLOS ONE, 9(2), e89150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinčić A, Balbino S, Zorić Z, Pedisić S, Bursać Kovačević D, Elez Garofulić I, & Dragović-Uzelac V (2020). Advanced technologies for the extraction of marine brown algal polysaccharides. Marine drugs, 18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockal M, Till S, Knappe S, Ehrlich HJ, & Scheiflinger F (2011). Anticoagulant activity and mechanism of non-anticoagulant sulfated polysaccharides. Blood, 118(21), 1208–1208.21562040 [Google Scholar]
- Drozd NN, Shevchenko NM, Ermakova SP, Lapikova ES, Makarov VA, & Zvyagintseva TN (2011). Effects of the structural characteristics of fucoidans from brown seaweeds on anticoagulant activity and the electrophoretic mobility of complexes with protamine sulfate. Pharmaceutical Chemistry Journal, 45(1), 56–61. [Google Scholar]
- Drozd NN, Tolstenkov AS, Makarov VA, Kuznetsova TA, Besednova NN, Shevchenko NM, & Zvyagintseva TN (2006). Pharmacodynamic parameters of anticoagulants based on sulfated polysaccharides from marine algae. Bulletin of Experimental Biology and Medicine, 142(5), 591–593. [DOI] [PubMed] [Google Scholar]
- Dürig J, Bruhn T, Zurborn K-H, Gutensohn K, Bruhn HD, & Béress L (1997). Anticoagulant fucoidan fractions from Fucus vesiculosus induce platelet activation in vitro. Thrombosis Research, 85(6), 479–491. [DOI] [PubMed] [Google Scholar]
- Engberg AE, Rosengren-Holmberg JP, Chen H, Nilsson B, Lambris JD, Nicholls IA, & Ekdahl KN (2011). Blood protein-polymer adsorption: Implications for understanding complement-mediated hemoincompatibility. Journal of Biomedical Materials Research Part A, 97A(1), 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Cai C, Wang W, Gao L, Li J, Li J, … Yu G (2018). Synthesis of fucoidan-mimetic glycopolymers with well-defined sulfation patterns via emulsion ring-opening metathesis polymerization. ACS Macro Letters, 7(3), 330–335. [DOI] [PubMed] [Google Scholar]
- Fernando IPS, Kim D, Nah J-W, & Jeon Y-J (2019). Advances in functionalizing fucoidans and alginates (bio)polymers by structural modifications: A review. Chemical Engineering Journal, 355, 33–48. [Google Scholar]
- Fletcher HR, Biller P, Ross AB, & Adams JMM (2017). The seasonal variation of fucoidan within three species of brown macroalgae. Algal Research, 22, 79–86. [Google Scholar]
- Fonseca RJC, Santos GRC, & Mourão PAS (2009). Effects of polysaccharides enriched in 2,4-disulfated fucose units on coagulation, thrombosis and bleeding: Practical and conceptual implications. Thrombosis and Haemostasis, 102(5), 829–836. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Tsukahara K, Moriwaki S, Kitahara T, Sano T, & Takema Y (2002). Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties. Journal of cosmetic science, 53(1), 1–9. [PubMed] [Google Scholar]
- Gaboury JP, & Kubes P (1994). Reductions in physiologic shear rates lead to CD11/CD18-dependent, selectin-independent leukocyte rolling in vivo. Blood, 83(2), 345–350. [PubMed] [Google Scholar]
- Ganapathy S, Lingappa S, Naidu K, Selvaraj U, Ramachandiran S, Ponnusamy S, & Somasundaram ST (2019). Isolation and bioactive potential of fucoidan from marine macroalgae Turbinaria conoides. ChemistrySelect, 4(48), 14114–14119. [Google Scholar]
- Ghebouli R, Loyau S, Maire M, Saboural P, Collet JP, Jandrot-Perrus M, … Michel JB (2018). Amino-fucoidan as a vector for rtPA-induced fibrinolysis in experimental thrombotic events. Thrombosis and Haemostasis, 118(1), 42–53. [DOI] [PubMed] [Google Scholar]
- Giraux J-L, Matou S, Bros A, Tapon-Bretaudière J, Letourneur D, & Fischer A-M (1998). Modulation of human endothelial cell proliferation and migration by fucoidan and heparin. European Journal of Cell Biology, 77(4), 352–359. [DOI] [PubMed] [Google Scholar]
- Glabe CG, Yednock T, & Rosen SD (1983). Reversible disruption of cultured endothelial monolayers by sulphated fucans. Journal of Cell Science, 61, 475–490. [DOI] [PubMed] [Google Scholar]
- Grauffel V, Kloareg B, Mabeau S, Durand P, & Jozefonvicz J (1989). New natural polysaccharides with potent antithrombic activity: fucans from brown algae. Biomaterials, 10(6), 363–368. [DOI] [PubMed] [Google Scholar]
- Hanjabam MD, Kumar A, Tejpal CS, Krishnamoorthy E, Kishore P, & Ashok Kumar K (2019). Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioactive Carbohydrates and Dietary Fibre, 20, 100200. [Google Scholar]
- Harboe M, & Mollnes TE (2008). The alternative complement pathway revisited. Journal of cellular and molecular medicine, 12(4), 1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun-Bouhedja F, Ellouali M, Sinquin C, & Boisson-Vidal C (2000). Relationship between sulfate groups and biological activities of fucans. Thrombosis Research, 100(5), 453–459. [DOI] [PubMed] [Google Scholar]
- Haybar H, Shahrabi S, Rezaeeyan H, Shirzad R, & Saki N (2019). Endothelial cells: from dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovascular Toxicology, 19(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Hlawaty H, Suffee N, Sutton A, Oudar O, Haddad O, Ollivier V, … Charnaux N (2011). Low molecular weight fucoidan prevents intimal hyperplasia in rat injured thoracic aorta through the modulation of matrix metalloproteinase-2 expression. Biochemical Pharmacology, 81(2), 233–243. [DOI] [PubMed] [Google Scholar]
- Hwang P-A, Yan M-D, Kuo K-L, Phan NN, & Lin Y-C (2017). A mechanism of low molecular weight fucoidans degraded by enzymatic and acidic hydrolysis for the prevention of UVB damage. Journal of Applied Phycology, 29(1), 521–529. [Google Scholar]
- Irhimeh MR, Fitton JH, & Lowenthal RM (2009). Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagulation and Fibrinolysis, 20(7), 607–610. [DOI] [PubMed] [Google Scholar]
- Itoh H, Amano H, Kakinuma M, & Noda H (2002). Antitumor activities and immunological studies with algal polysaccharides. Fisheries science, 68(sup2), 1453–1456. [Google Scholar]
- January GG, Naidoo RK, Kirby-McCullough B, & Bauer R (2019). Assessing methodologies for fucoidan extraction from South African brown algae. Algal Research, 40, 101517. [Google Scholar]
- Jeong ES, Yoon YH, & Kim JK (2009). Contrasting correlation in the inhibition response of ADP-induced platelet aggregation and the anti-coagulant activities of algal fucoidans derived from Eisenia bicyclis and Undaria pinnatifida sporophylls (Mekabu). Fisheries and Aquatic Sciences, 12(3), 194–202. [Google Scholar]
- Jin W, Liu G, Zhong W, Sun C, & Zhang Q (2017). Polysaccharides from Sargassum thunbergii: Monthly variations and anti-complement and anti-tumour activities. International Journal of Biological Macromolecules, 105, 1526–1531. [DOI] [PubMed] [Google Scholar]
- Jin W, Wu W, Tang H, Wei B, Wang H, Sun J, … Zhong W (2019). Structure analysis and anti-tumor and anti-angiogenic activities of sulfated galactofucan extracted from Sargassum thunbergii. Marine drugs, 17(1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Zhang Q, Wang J, & Zhang W (2013). A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydrate Polymers, 91(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Juenet M, Aid-Launais R, Li B, Berger A, Aerts J, Ollivier V, … Chauvierre C (2018). Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials, 156, 204–216. [DOI] [PubMed] [Google Scholar]
- Kardeby C, Fälker K, Haining EJ, Criel M, Lindkvist M, Barroso R, … Grenegård M (2019). Synthetic glycopolymers and natural fucoidans cause human platelet aggregation via PEAR1 and GPIba. Blood Advances, 3(3), 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khil’chenko SR, Zaporozhets TS, Shevchenko NM, Zvyagintseva TN, Vogel U, Seeberger P, & Lepenies B (2011). Immunostimulatory activity of fucoidan from the brown alga Fucus evanescens: role of sulfates and acetates. Journal of Carbohydrate Chemistry, 30(4–6), 291–305. [Google Scholar]
- Khurana R, Simons M, Martin FJ, & Zachary CI (2005). Role of angiogenesis in cardiovascular disease. Circulation, 112(12), 1813–1824. [DOI] [PubMed] [Google Scholar]
- Kim, Bae I-H, Lim KS, Park J-K, Park DS, Lee S-Y, … Jeong MH (2015). A method for coating fucoidan onto bare metal stent and in vivo evaluation. Progress in Organic Coatings, 78, 348–356. [Google Scholar]
- Kim, Koo Y-K, Jung M-K, Moon HR, Kim SM, Synytsya A, … Park YI (2010). Anticoagulating activities of low-molecular weight fuco-oligosaccharides prepared by enzymatic digestion of fucoidan from the sporophyll of Korean Undaria pinnatifida. Archives of Pharmacal Research, 33(1), 125–131. [DOI] [PubMed] [Google Scholar]
- Kim, Lee JS, & Lee HG (2016). Nanoencapsulation of red ginseng extracts using chitosan with polyglutamic acid or fucoidan for improving antithrombotic activities. Journal of Agricultural and Food Chemistry, 64(23), 4765–4771. [DOI] [PubMed] [Google Scholar]
- Kim, Park J-Y, Kang H-J, Kim H-J, & Lee J (2014). Fucoidan/FGF-2 induces angiogenesis through JNK- and p38-mediated activation of AKT/MMP-2 signalling. Biochemical and Biophysical Research Communications, 450(4), 1333–1338. [DOI] [PubMed] [Google Scholar]
- Kim, Rioux L-E, & Turgeon SL (2014). Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry, 98, 27–33. [DOI] [PubMed] [Google Scholar]
- Kishi K, Muramatsu M, Jin D, Furubayashi K, Takai S, Tamai H, & Miyazaki M (2007). The effects of Chymase on matrix metalloproteinase-2 activation in neointimal hyperplasia after balloon injury in dogs. Hypertension Research, 30(1), 77–83. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Matsuo M, & Tsuneo Y (1992). Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Bioscience, Biotechnology, and Biochemistry, 56(3), 490–494. [DOI] [PubMed] [Google Scholar]
- Kopplin G, Rokstad AM, Mélida H, Bulone V, Skjåk-Bræk G, & Aachmann FL (2018). Structural characterization of fucoidan from Laminaria hyperborea: assessment of coagulation and inflammatory properties and their structure-function relationship. ACS Applied Bio Materials, 1(6), 1880–1892. [DOI] [PubMed] [Google Scholar]
- Kuznetsova TA, Besednova NN, Mamaev AN, Momot AP, Shevchenko NM, & Zvyagintseva TN (2003). Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bulletin of Experimental Biology and Medicine, 136(5), 471–473. [DOI] [PubMed] [Google Scholar]
- Kuznetsova TA, Ivanushko LA, Persiyanova EV, Ermakova SP, & Besednova NN (2019). Markers of systemic inflammation in experimental dyslipidemia induced by P-407: Modulation with fucoidan from brown alga Fucus evanescens. Bulletin of Experimental Biology and Medicine, 166(6), 766–769. [DOI] [PubMed] [Google Scholar]
- Kwak K-W, Cho K-S, Hahn O-J, Lee K-H, Lee B-Y, Ko J-J, & Chung K-H (2010). Biological effects of fucoidan isolated from Fucus vesiculosus on thrombosis and vascular cells. Korean J Hematol, 45(1), 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapikova ES, Drozd NN, Tolstenkov AS, Makarov VA, Zvyagintseva TN, Shevchenko NM, … Kuznetsova TA (2008). Inhibition of thrombin and factor Xa by Fucus evanescens fucoidan and its modified analogs. Bulletin of Experimental Biology and Medicine, 146(3), 328–333. [DOI] [PubMed] [Google Scholar]
- Lee, Cho Y, Kim GH, & Cho H (2020). Undaria pinnatifida fucoidan-rich extract recovers immunity of immunosuppressed mice. Journal of microbiology and biotechnology, 30(3), 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Jeong M-R, Choi S-M, Na S-S, & Cha J-D (2013). Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Archives of Oral Biology, 58(5), 482–492. [DOI] [PubMed] [Google Scholar]
- Lee, Ko C-I, Ahn G, You S, Kim J-S, Heu MS, … Jeon Y-J (2012). Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydrate Polymers, 89(2), 599–606. [DOI] [PubMed] [Google Scholar]
- Lee, Ko C-I, Jee Y, Jeong Y, Kim M, Kim J-S, & Jeon Y-J (2013). Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydrate Polymers, 92(1), 84–89. [DOI] [PubMed] [Google Scholar]
- Li, Juenet M, Aid-Launais R, Maire M, Ollivier V, Letourneur D, & Chauvierre C (2017). Development of polymer microcapsules functionalized with fucoidan to target P-selectin overexpressed in cardiovascular diseases. Advanced Healthcare Materials, 6(4), 1601200. [DOI] [PubMed] [Google Scholar]
- Li, Li J, Li Z, Sang Y, Niu Y, Zhang Q, … Yin S (2016). Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the l-NAME-induced hypertensive rat model. Food & Function, 7(5), 2398–2408. [DOI] [PubMed] [Google Scholar]
- Li, Lu F, Wei X, & Zhao R (2008). Fucoidan: structure and bioactivity. Molecules, 13(8), 1671–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Qian M, Kyler K, & Xu J (2018). Endothelial–vascular smooth muscle cells interactions in atherosclerosis. Frontiers in Cardiovascular Medicine, 5(151), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Zhao RX, & Wei XJ (2008). Anticoagulant activity of fucoidan from Hizikia fusiforme. Agro Food Industry Hi-Tech, 19(1), 22–24. [Google Scholar]
- Liewert I, Ehrig K, & Alban S (2017). Effects of fucoidans and heparin on reactions of neutrophils induced by IL-8 and C5a. Carbohydrate Polymers, 165, 462–469. [DOI] [PubMed] [Google Scholar]
- Lin Z, Tan X, Zhang Y, Li F, Luo P, & Liu H (2020). Molecular targets and related biologic activities of fucoidan: a review. Marine drugs, 18(8), 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Scallan CD, Broze GJ Jr, Patarroyo-White S, Pierce GF, & Johnson KW (2006). Improved coagulation in bleeding disorders by Non-Anticoagulant Sulfated Polysaccharides (NASP). Thrombosis and Haemostasis, 95(1), 68–76. [PubMed] [Google Scholar]
- Liu, Wang J, Chang AK, Liu B, Yang L, Li Q, … Zou X (2012). Fucoidan extract derived from Undaria pinnatifida inhibits angiogenesis by human umbilical vein endothelial cells. Phytomedicine, 19(8), 797–803. [DOI] [PubMed] [Google Scholar]
- Liu, Wang J, Zhang Q, & Zhang H (2018). The effect of different substitute groups and molecular weights of fucoidan on neuroprotective and anticomplement activity. International Journal of Biological Macromolecules, 113, 82–89. [DOI] [PubMed] [Google Scholar]
- Logeart D, Letourneur D, Jozefonvicz J, & Kern P (1996). Collagen synthesis by vascular smooth muscle cells in the presence of antiproliferative polysaccharides. Journal of Biomedical Materials Research, 30(4), 501–508. [DOI] [PubMed] [Google Scholar]
- Logeart D, Prigent-Richard S, Boisson-Vidal C, Chaubet F, Durand P, Jozefonvicz J, & Letourneur D (1997). Fucans, sulfated polysaccharides extracted from brown seaweeds, inhibit vascular smooth muscle cell proliferation. II. Degradation and molecular weight effect. European Journal of Cell Biology, 74(4), 385–390. [PubMed] [Google Scholar]
- Lorant DE, Topham MK, Whatley RE, McEver RP, McIntyre TM, Prescott SM, & Zimmerman GA (1993). Inflammatory roles of P-selectin. The Journal of clinical investigation, 92(2), 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt C-E, Meddahi-Pellé A, Ho-Tin-Noe B, Colliec-Jouault S, Guezennec J, Louedec L, … Michel J-B (2003). Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. Journal of Pharmacology and Experimental Therapeutics, 305(1), 24–30. [DOI] [PubMed] [Google Scholar]
- Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, Naik UP, … Kunapuli SP (2013). Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2). Journal of Biological Chemistry, 288(11), 7717–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour MB, Balti R, Yacoubi L, Ollivier V, Chaubet F, & Maaroufi RM (2019). Primary structure and anticoagulant activity of fucoidan from the sea cucumber Holothuria polii. International Journal of Biological Macromolecules, 121, 1145–1153. [DOI] [PubMed] [Google Scholar]
- Månsson P, Zhang XW, Jeppsson B, Johnell O, & Thorlacius H (2000). Critical role of P-selectin-dependent rolling in tumor necrosis factor-α-induced leukocyte adhesion and extravascular recruitment in vivo. Naunyn-Schmiedeberg’s Archives of Pharmacology, 362(2), 190–196. [DOI] [PubMed] [Google Scholar]
- Marinval N, Morenc M, Labour MN, Samotus A, Mzyk A, Ollivier V, … Hlawaty H (2018). Fucoidan/VEGF-based surface modification of decellularized pulmonary heart valve improves the antithrombotic and re-endothelialization potential of bioprostheses. Biomaterials, 172, 14–29. [DOI] [PubMed] [Google Scholar]
- Matou S, Helley D, Chabut D, Bros A, & Fischer A-M (2002). Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thrombosis Research, 106(4), 213–221. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Xue C, Zhao X, Mori M, Sugawara T, & Hirata T (2005). Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. International journal of molecular medicine, 15, 695–699. [PubMed] [Google Scholar]
- Matuszak J, Dörfler P, Lyer S, Unterweger H, Juenet M, Chauvierre C, … Cicha I (2018). Comparative analysis of nanosystems’ effects on human endothelial and monocytic cell functions. Nanotoxicology, 12(9), 957–974. [DOI] [PubMed] [Google Scholar]
- Mauray S, De Raucourt E, Chaubet F, MaÏGa-Revel O, Sternberg C, & Fischer AM (1998). Comparative anticoagulant activity and influence on thrombin generation of dextran derivatives and of a fucoidan fraction. Journal of Biomaterials Science, Polymer Edition, 9(4), 373–387. [DOI] [PubMed] [Google Scholar]
- Mauray S, Sternberg C, Theveniaux J, Millet J, Sinquin C, Tapon-Bretaudiere J, & Fischer AM (1995). Venous antithrombotic and anticoagulant activities of a fucoidan fraction. Thrombosis and Haemostasis, 74(5), 1280–1285. [PubMed] [Google Scholar]
- McCaffrey TA, Falcone DJ, Borth W, Brayton CF, & Weksler BB (1992). Fucoidan is a non-anticoagulant inhibitor of intimal hyperplasia. Biochemical and Biophysical Research Communications, 184(2), 773–781. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Falcone DJ, Vicente D, Du B, Consigli S, & Borth W (1994). Protection of transforming growth factor β activity by heparin and fucoidan. Journal of Cellular Physiology, 159(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Mebius RE, & Watson SR (1993). L- and E-selectin can recognize the same naturally occurring ligands on high endothelial venules. Journal of Immunology, 151(6), 3252–3260. [PubMed] [Google Scholar]
- Minix R, & Doctor VM (1997). Interaction of fucoidan with proteases and inhibitors of coagulation and fibrinolysis. Thrombosis Research, 87(5), 419–429. [DOI] [PubMed] [Google Scholar]
- Mourão. (2004). Use of sulfated fucans as anticoagulant and antithrombotic agents: Future perspectives. Current Pharmaceutical Design, 10(9), 967–981. [DOI] [PubMed] [Google Scholar]
- Mourão. (2015). Perspective on the use of sulfated polysaccharides from marine organisms as a source of new antithrombotic drugs. Marine drugs, 13(5), 2770–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloy B, Ribeiro AC, Alves AP, Vieira RP, & Mourão PAS (1994). Sulfated fucans from echinoderms have a regular tetrasaccharide repeating unit defined by specific patterns of sulfation at the 0–2 and 0–4 positions. Journal of Biological Chemistry, 269(35), 22113–22123. [PubMed] [Google Scholar]
- Nagamine T, Nakazato K, Tomioka S, Iha M, & Nakajima K (2014). Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Marine drugs, 13(1), 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka M, Shibata H, Kimura-Takagi I, Hashimoto S, Kimura K, Makino T, … Yokokura T (1999). Structural study of fucoidan from Cladosiphon okamuranus tokida. Glycoconjugate Journal, 16(1), 19–26. [DOI] [PubMed] [Google Scholar]
- Nishino T, Aizu Y, & Nagumo T (1991). Antithrombin activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Thrombosis Research, 62(6), 765–773. [DOI] [PubMed] [Google Scholar]
- Nishino T, Fukuda A, Nagumo T, Fujihara M, & Kaji E (1999). Inhibition of the generation of thrombin and factor Xa by a fucoidan from the brown seaweed Ecklonia kurome. Thrombosis Research, 96(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Nishino T, & Nagumo T (1992). Anticoagulant and antithrombin activities of oversulfated fucans. Carbohydrate Research, 229(2), 355–362. [DOI] [PubMed] [Google Scholar]
- Nishino T, Ura H, & Nagumo T (1995). The relationship Between the sulfate content and the antithrombin activity of an α(1→2)-fucoidan purified from a commercial fucoidan fraction. Botanica Marina, 38(1–6), 187–194. [Google Scholar]
- Novoyatleva T, Kojonazarov B, Owczarek A, Veeroju S, Rai N, Henneke I, … Schermuly RT (2019). Evidence for the fucoidan/p-selectin axis as a therapeutic target in hypoxia-induced pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine, 199(11), 1407–1420. [DOI] [PubMed] [Google Scholar]
- Oksjoki R (2007). Function and regulation of the complement system in cardiovascular diseases. Frontiers in Bioscience, 12, 4696. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Granja S, Neves NM, Reis RL, Baltazar F, Silva TH, & Martins A (2019). Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydrate Polymers, 223, 115034. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Neves NM, Reis RL, Martins A, & Silva TH (2020). A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydrate Polymers, 239. [DOI] [PubMed] [Google Scholar]
- Omata M, Matsui N, Inomata N, & Ohno T (1997). Protective effects of polysaccharide fucoidin on myocardial ischemia- reperfusion injury in rats. Journal of Cardiovascular Pharmacology, 30(6), 717–724. [DOI] [PubMed] [Google Scholar]
- Ozaltin K, Lehocky M, Humpolicek P, Pelkova J, Di Martino A, Karakurt I, & Saha P (2019). Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers, 11(5), 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Cho E, In M-J, Kim DC, & Chae HJ (2012). Physicochemical properties and bioactivity of brown seaweed fucoidan prepared by ultra high pressure-assisted enzyme treatment. Korean Journal of Chemical Engineering, 29(2), 221–227. [Google Scholar]
- Patankar MS, Oehninger S, Barnett T, Williams RL, & Clark GF (1993). A revised structure for fucoidan may explain some of its biological activities. Journal of Biological Chemistry, 268(29), 21770–21776. [PubMed] [Google Scholar]
- Patel MK, Mulloy B, Gallagher KL, O’Brien L, & Hughes AD (2002). The antimitogenic action of the sulphated polysaccharide fucoidan differs from heparin in human vascular smooth muscle cells. Thrombosis and Haemostasis, 87(1), 149–154. [PubMed] [Google Scholar]
- Patil NP, Le V, Sligar AD, Mei L, Chavarria D, Yang EY, & Baker AB (2018). Algal polysaccharides as therapeutic agents for atherosclerosis. Frontiers in Cardiovascular Medicine, 5(153). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MS, Mulloy B, & Mourão PAS (1999). Structure and anticoagulant activity of sulfated fucans: comparison between the regular, repetitive, and linear fucans from Echinoderms with the more heterogeneous branched polymers from brown algae. Journal of Biological Chemistry, 274(12), 7656–7667. [DOI] [PubMed] [Google Scholar]
- Petersen VS, Thiel S, & Jensenius JC (2001). The mannan-binding lectin pathway of complement activation: biology and disease association. Molecular Immunology, 38(2), 133–149. [DOI] [PubMed] [Google Scholar]
- Pomin VH, Pereira MS, Valente A-P, Tollefsen DM, Pavão MSG, & Mourão PAS (2004). Selective cleavage and anticoagulant activity of a sulfated fucan: stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II–dependent anticoagulant activity. Glycobiology, 15(4), 369–381. [DOI] [PubMed] [Google Scholar]
- Pozharitskaya ON, Obluchinskaya ED, & Shikov AN (2020). Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Marine drugs, 18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozharitskaya ON, Shikov AN, Faustova NM, Obluchinskaya ED, Kosman VM, Vuorela H, & Makarov VG (2018). Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Marine drugs, 16(4), 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Lillicrap D, Labelle A, Knappe S, Keller T, Burnett E, … Johnson KW (2008). Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood, 111(2), 672–679. [DOI] [PubMed] [Google Scholar]
- Preobrazhenskaya ME, Berman AE, Mikhailov VI, Ushakova NA, Mazurov AV, Semenov AV, … Bovin NV (1997a). Fucoidan inhibits leukocyte recruitment in a model peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochemistry and Molecular Biology International, 43(2), 443–451. [DOI] [PubMed] [Google Scholar]
- Preobrazhenskaya ME, Berman AE, Mikhailov VI, Ushakova NA, Mazurov AV, Semenov AV, … Bovin NV (1997b). Fucoidan inhibits leukocyte recruitment in a model peritonial inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. IUBMB Life, 43(2), 443–451. [DOI] [PubMed] [Google Scholar]
- Qiu X, Amarasekara A, & Doctor V (2006). Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydrate Polymers, 63(2), 224–228. [Google Scholar]
- Qu Y, Cao Z, Wang W, Wang N, Li X, & Pan J (2019). Monthly variations of fucoidan content and its composition in the farmed brown alga Saccharina sculpera (Laminariales, Phaeophyceae). Journal of Applied Phycology, 31(4), 2623–2628. [Google Scholar]
- Religa P, Kazi M, Thyberg J, Gaciong Z, Swedenborg J, & Hedin U (2000). Fucoidan inhibits smooth muscle cell proliferation and reduces mitogen-activated protein kinase activity. European Journal of Vascular and Endovascular Surgery, 20(5), 419–426. [DOI] [PubMed] [Google Scholar]
- Ren R, Azuma Y, Ojima T, Hashimoto T, Mizuno M, Nishitani Y, … Kanazawa K (2013). Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. British Journal of Nutrition, 110(5), 880–890. [DOI] [PubMed] [Google Scholar]
- Richard B, Bouton MC, Loyau S, Lavigne D, Letourneur D, Jandrot-Perrus M, & Arocas V (2006). Modulation of protease nexin-I activity by polysaccharides. Thrombosis and Haemostasis, 95(2), 229–235. [DOI] [PubMed] [Google Scholar]
- Rocha de Souza MC, Marques CT, Guerra Dore CM, Ferreira da Silva FR, Oliveira Rocha HA, & Leite EL (2007). Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. Journal of Applied Phycology, 19(2), 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotmans JI, Velema E, Verhagen HJM, Blankensteijn JD, de Kleijn DPV, Stroes ESG, & Pasterkamp G (2004). Matrix metalloproteinase inhibition reduces intimal hyperplasia in a porcine arteriovenous-graft model. Journal of Vascular Surgery, 39(2), 432–439. [DOI] [PubMed] [Google Scholar]
- Roux N, Brakenhielm E, Freguin-Bouillant C, Lallemand F, Henry JP, Boyer O, … Plissonnier D (2012). Progenitor cell mobilizing treatments prevent experimental transplant arteriosclerosis. Journal of Surgical Research, 176(2), 657–665. [DOI] [PubMed] [Google Scholar]
- Ruehl ML, Orozco JA, Stoker MB, McDonagh PF, Coull BM, & Ritter LS (2002). Protective effects of inhibiting both blood and vascular selectins after stroke and reperfusion. Neurological Research, 24(3), 226–232. [DOI] [PubMed] [Google Scholar]
- Sarma JV, & Ward PA (2011). The complement system. Cell and Tissue Research, 343(1), 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senis YA, Mazharian A, & Mori J (2014). Src family kinases: at the forefront of platelet activation. Blood, 124(13), 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezer AD, & Cevher E (2011). Fucoidan: A Versatile Biopolymer for Biomedical Applications. In Zilberman M (Ed.), Active implants and scaffolds for tissue regeneration (pp. 377–406). Berlin, Heidelberg: Springer Berlin Heidelberg [Google Scholar]
- Shang F, Mou R, Zhang Z, Gao N, Lin L, Li Z, … Zhao J (2018). Structural analysis and anticoagulant activities of three highly regular fucan sulfates as novel intrinsic factor Xase inhibitors. Carbohydrate Polymers, 195, 257–266. [DOI] [PubMed] [Google Scholar]
- Silchenko AS, Kusaykin MI, Kurilenko VV, Zakharenko AM, Isakov VV, Zaporozhets TS, … Zvyagintseva TN (2013). Hydrolysis of fucoidan by fucoidanase isolated from the marine bacterium, Formosa algae. Marine drugs, 11(7), 2413–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda S, Kozako T, Iwata K, & Shimeno H (2000). Oversulfated fucoidan inhibits the basic fibroblast growth factor-induced tube formation by human umbilical vein endothelial cells: Its possible mechanism of action. Biochimica et Biophysica Acta - Molecular Cell Research, 1497(1), 127–134. [DOI] [PubMed] [Google Scholar]
- Soeda S, Ohmagari Y, Shimeno H, & Nagamatsu A (1993). Preparation of oversulfated fucoidan fragments and evaluation of their antithrombotic activities. Thrombosis Research, 72(3), 247–256. [DOI] [PubMed] [Google Scholar]
- Soeda S, Ohmagari Y, Shimeno H, & Nagamatsu A (1994). Preparation of aminated fucoidan and its evaluation as an antithrombotic and antilipemic agent. Biological & Pharmaceutical Bulletin, 17(6), 784–788. [DOI] [PubMed] [Google Scholar]
- Soeda S, Sakaguchi S, Shimeno H, & Nagamatsu A (1992). Fibrinolytic and anticoagulant activities of highly sulfated fucoidan. Biochemical Pharmacology, 43(8), 1853–1858. [DOI] [PubMed] [Google Scholar]
- Soeda S, Shibata Y, & Shimeno H (1997). Inhibitory effect of oversulfated fucoidan on tube formation by human vascular endothelial cells. Biological and Pharmaceutical Bulletin, 20(11), 1131–1135. [DOI] [PubMed] [Google Scholar]
- Springer GF, Wurzel HA, McNeal GM, Ansell NJ, & Doughty MF (1957). Isolation of anticoagulant fractions from crude fucoidin. Proceedings of the Society for Experimental Biology and Medicine, 94(2), 404–409. [DOI] [PubMed] [Google Scholar]
- Su H, Xue G, Ye C, Wang Y, Zhao A, Huang N, & Li J (2017). The effect of anti-CD133/fucoidan bio-coatings on hemocompatibility and EPC capture. Journal of Biomaterials Science, Polymer Edition, 28(17), 2066–2081. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Bachelet-Violette L, Rouzet F, Beilvert A, Autret G, Maire M, … Letourneur D (2015). Ultrasmall superparamagnetic iron oxide nanoparticles coated with fucoidan for molecular MRI of intraluminal thrombus. Nanomedicine, 10(1), 73–87. [DOI] [PubMed] [Google Scholar]
- Synytsya A, Kim W-J, Kim S-M, Pohl R, Synytsya A, Kvasnička F, … Il Park Y (2010). Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydrate Polymers, 81(1), 41–48. [Google Scholar]
- Tanaka K, Ito M, Kodama M, Tomita M, Kimura S, Hoyano M, … Aizawa Y (2010). Sulfated polysaccharide fucoidan ameliorates experimental autoimmune myocarditis in rats. Journal of Cardiovascular Pharmacology and Therapeutics, 16(1), 79–86. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, Roberts D, Liotta LA, & Giavazzi R (1990). Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: A potential angiogenesis regulatory factor. Journal of Cell Biology, 111(2), 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq A, Athar M, Ara J, Sultana V, Ehteshamul-Haque S, & Ahmad M (2015). Biochemical evaluation of antioxidant activity and polysaccharides fractions in seaweeds. Global Journal of Environmental Science and Management, 1(1), 47–62. [Google Scholar]
- Tengdelius M, Kardeby C, Fälker K, Griffith M, Påhlsson P, Konradsson P, & Grenegård M (2017). Fucoidan-mimetic glycopolymers as tools for studying molecular and cellular responses in human blood platelets. Macromolecular Bioscience, 17(2), 1600257. [DOI] [PubMed] [Google Scholar]
- Tengdelius M, Lee C-J, Grenegård M, Griffith M, Påhlsson P, & Konradsson P (2014). Synthesis and biological evaluation of fucoidan-mimetic glycopolymers through cyanoxyl-mediated free-radical polymerization. Biomacromolecules, 15(7), 2359–2368. [DOI] [PubMed] [Google Scholar]
- Teruya T, Tatemoto H, Konishi T, & Tako M (2009). Structural characteristics and in vitro macrophage activation of acetyl fucoidan from Cladosiphon okamuranus. Glycoconjugate Journal, 26(8), 1019–1028. [DOI] [PubMed] [Google Scholar]
- Thorlacius, Vollmar, Seyfert, Vestweber, & Menger. (2000). The polysaccharide fucoidan inhibits microvascular thrombus formation independently from P- and l-selectin function in vivo. European Journal of Clinical Investigation, 30(9), 804–810. [DOI] [PubMed] [Google Scholar]
- Tissot B, Daniel R, & Place C (2003). Interaction of the C1 complex of Complement with sulfated polysaccharide and DNA probed by single molecule fluorescence microscopy. European Journal of Biochemistry, 270(23), 4714–4720. [DOI] [PubMed] [Google Scholar]
- Tissot B, Gonnet F, Iborra A, Berthou C, Thielens N, Arlaud GJ, & Daniel R (2005). Mass spectrometry analysis of the oligomeric C1q protein reveals the B chain as the target of trypsin cleavage and interaction with fucoidan. Biochemistry, 44(7), 2602–2609. [DOI] [PubMed] [Google Scholar]
- Tissot B, Montdargent B, Chevolot L, Varenne A, Descroix S, Gareil P, & Daniel R (2003). Interaction of fucoidan with the proteins of the complement classical pathway. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1651(1), 5–16. [DOI] [PubMed] [Google Scholar]
- Tsai SH, Wang JC, Liao WI, Hsu YJ, Lin CY, Liao MT, … Lin SJ (2018). Fucoidan attenuates angiotensin II-induced abdominal aortic aneurysms through the inhibition of c-Jun N-terminal kinase and nuclear factor κB activation. Journal of Vascular Surgery, 68(6), 72S–81S.e71. [DOI] [PubMed] [Google Scholar]
- Ushakova NA, Morozevich GE, Ustyuzhanina NE, Bilan MI, Usov AI, Nifantiev NE, & Preobrazhenskaya ME (2009). Anticoagulant activity of fucoidans from brown algae. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 3(1), 77–83. [Google Scholar]
- Ushakova NA, Preobrazhenskaya ME, Bird MI, Priest R, Semenov AV, Mazurov AV, … Bovin NV (2005). Monomeric and multimeric blockers of Selectins: Comparison of in vitro and in vivo activity. Biochemistry (Moscow), 70(4), 432–439. [DOI] [PubMed] [Google Scholar]
- Usov AI, Smirnova GP, Bilan MI, & Shashkov AS (1998). Polysaccharides of algae: 53. Brown alga Laminaria saccharina (L.) Lam. as a source of fucoidan. Russian Journal of Bioorganic Chemistry, 24(6), 382–389. [Google Scholar]
- Ustyuzhanina, Natalia AU, Marina EP, Maria IB, Eugenia AT, Vadim BK, … Nikolay EN (2014). Fucoidans as a platform for new anticoagulant drugs discovery. Pure and Applied Chemistry, 86(9), 1365–1375. [Google Scholar]
- Ustyuzhanina, Ushakova NA, Zyuzina KA, Bilan MI, Elizarova AL, Somonova OV, … Nifantiev NE (2013). Influence of fucoidans on hemostatic system. Marine drugs, 11(7), 2444–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustyuzhanina NE, Bilan MI, Ushakova NA, Usov AI, Kiselevskiy MV, & Nifantiev NE (2014). Fucoidans: Pro- or antiangiogenic agents? Glycobiology, 24(12), 1265–1274. [DOI] [PubMed] [Google Scholar]
- Varenne A, Gareil P, Colliec-Jouault S, & Daniel R (2003). Capillary electrophoresis determination of the binding affinity of bioactive sulfated polysaccharides to proteins: study of the binding properties of fucoidan to antithrombin. Analytical Biochemistry, 315(2), 152–159. [DOI] [PubMed] [Google Scholar]
- Verrier ED, & Boyle EM (1996). Endothelial cell injury in cardiovascular surgery. The Annals of Thoracic Surgery, 62(3), 915–922. [DOI] [PubMed] [Google Scholar]
- Vischer P, & Buddecke E (1991). Different action of heparin and fucoidan on arterial smooth muscle cell proliferation and thrombospondin and fibronectin metabolism. European Journal of Cell Biology, 56(2), 407–414. [PubMed] [Google Scholar]
- Walport MJ (2001). Complement. First of two parts. N Engl J Med, 344(14), 1058–1066. [DOI] [PubMed] [Google Scholar]
- Wang, Liu L, Zhang Q, Zhang Z, Qi H, & Li P (2009). Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chemistry, 114(4), 1285–1290. [Google Scholar]
- Wang, Pei L, Liu H, Qv K, Xian W, Liu J, & Zhang G (2016). Fucoidan attenuates atherosclerosis in LDLR−/− mice through inhibition of inflammation and oxidative stress. International Journal of Clinical and Experimental Pathology, 9(7), 6896–6904. [Google Scholar]
- Wang, Xing M, Cao Q, Ji A, Liang H, & Song S (2019). Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Marine drugs, 17(3), 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Ye C, Su H, Wang J, Wang Y, Wang H, … Huang N (2016). Layer-by-layer self-assembled laminin/fucoidan films: Towards better hemocompatibility and endothelialization. RSC Advances, 6(61), 56048–56055. [Google Scholar]
- Wang, & Zhang Q (2017). Chemical modification of fucoidan and their application. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications (pp. 157–173) [Google Scholar]
- Wang, Zhang Q, Zhang Z, Hou Y, & Zhang H (2011). In-vitro anticoagulant activity of fucoidan derivatives from brown seaweed Laminaria japonica. Chinese Journal of Oceanology and Limnology, 29(3), 679–685. [Google Scholar]
- Wang, Zhang Q, Zhang Z, Song H, & Li P (2010). Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. International Journal of Biological Macromolecules, 46(1), 6–12. [DOI] [PubMed] [Google Scholar]
- Wang, Zhang Q, Zhang Z, Zhang J, & Li P (2009). Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. International Journal of Biological Macromolecules, 44(2), 170–174. [DOI] [PubMed] [Google Scholar]
- Wijesinghe WAJP, Athukorala Y, & Jeon YJ (2011). Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydrate Polymers, 86(2), 917–921. [Google Scholar]
- Williams SJ, & Davies GJ (2001). Protein–carbohydrate interactions: learning lessons from nature. Trends in Biotechnology, 19(9), 356–362. [DOI] [PubMed] [Google Scholar]
- Wu, Sun J, Su X, Yu Q, Yu Q, & Zhang P (2016). A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydrate Polymers, 154, 96–111. [DOI] [PubMed] [Google Scholar]
- Wu, Xu L, Zhao L, Xiao C, Gao N, Luo L, … Zhao J (2015). Structural analysis and anticoagulant activities of the novel sulfated fucan possessing a regular well-defined repeating unit from sea cucumber. Marine drugs, 13(4), 2063–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Zhang X, Liu J, Song J, Yu P, Chen P, … Tong H (2019). Physicochemical characterization of Sargassum fusiforme fucoidan fractions and their antagonistic effect against P-selectin-mediated cell adhesion. International Journal of Biological Macromolecules, 133, 656–662. [DOI] [PubMed] [Google Scholar]
- Xu, Xue C, Chang Y, & Liu G (2017). Chain conformation, rheological and charge properties of fucoidan extracted from sea cucumber Thelenota ananas: A semi-flexible coil negative polyelectrolyte. Food Chemistry, 237, 511–515. [DOI] [PubMed] [Google Scholar]
- Xu, Zhang Q, Luo D, Wang J, & Duan D (2016). Low molecular weight fucoidan modulates P-selectin and alleviates diabetic nephropathy. International Journal of Biological Macromolecules, 91, 233–240. [DOI] [PubMed] [Google Scholar]
- Xu, Zhu W, Wang T, Jin L, Liu T, Li X, … Guo Y (2019). Low molecule weight fucoidan mitigates atherosclerosis in ApoE (−/−) mouse model through activating multiple signal pathway. Carbohydrate Polymers, 206, 110–120. [DOI] [PubMed] [Google Scholar]
- Yang C, Chung D, Shin I-S, Lee H, Kim J, Lee Y, & You S (2008). Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. International Journal of Biological Macromolecules, 43(5), 433–437. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zaw AM, Anderson DEJ, Hinds MT, & Yim EKF (2020). Fucoidan functionalization on poly(vinyl alcohol) hydrogels for improved endothelialization and hemocompatibility. Biomaterials, 249, 120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C, Wang Y, Su H, Yang P, Huang N, F. Maitz M, & Zhao A (2016). Construction of a fucoidan/laminin functional multilayer to direction vascular cell fate and promotion hemocompatibility. Materials Science and Engineering: C, 64, 236–242. [DOI] [PubMed] [Google Scholar]
- Yokota T, Nagashima M, Ghazizadeh M, & Kawanami O (2009). Increased effect of fucoidan on lipoprotein lipase secretion in adipocytes. Life Sciences, 84(15), 523–529. [DOI] [PubMed] [Google Scholar]
- Yu L, Ge L, Xue C, Chang Y, Zhang C, Xu X, & Wang Y (2014). Structural study of fucoidan from sea cucumber Acaudina molpadioides: A fucoidan containing novel tetrafucose repeating unit. Food Chemistry, 142, 197–200. [DOI] [PubMed] [Google Scholar]
- Zaporozhets T, & Besednova N (2016). Prospects for the therapeutic application of sulfated polysaccharides of brown algae in diseases of the cardiovascular system: review. Pharmaceutical Biology, 54(12), 3126–3135. [DOI] [PubMed] [Google Scholar]
- Zemani F, Benisvy D, Galy-Fauroux I, Lokajczyk A, Colliec-Jouault S, Uzan G, … Boisson-Vidal C (2005). Low-molecular-weight fucoidan enhances the proangiogenic phenotype of endothelial progenitor cells. Biochemical Pharmacology, 70(8), 1167–1175. [DOI] [PubMed] [Google Scholar]
- Zeng HY, & Huang YC (2018). Basic fibroblast growth factor released from fucoidan-modified chitosan/alginate scaffolds for promoting fibroblasts migration. Journal of Polymer Research, 25(3), 83. [Google Scholar]
- Zhang, Chu F, Xu L, Liang H, Song S, & Ji A (2018). Use of fluorescein isothiocyanate isomer I to study the mechanism of intestinal absorption of fucoidan sulfate in vivo and in vitro. Biopharmaceutics & Drug Disposition, 39(6), 298–307. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhao X, Jin W, Wang J, & Zhang Q (2016). Microanalysis and preliminary pharmacokinetic studies of a sulfated polysaccharide from Laminaria japonica. Chinese Journal of Oceanology and Limnology, 34(1), 177–185. [Google Scholar]
- Zhang S, Jiang C, Knappe S, Reutterer S, Scheiflinger F, … Dockal M (2014). Structure-activity relationship of the pro- and anticoagulant effects of Fucus vesiculosus fucoidan. Thromb Haemost, 111(3), 429–437. [DOI] [PubMed] [Google Scholar]
- Zhang S, Knappe S, Quinn C, Catarello J, Ray GJ, … Dockal M (2015). Screening of complex fucoidans from four brown algae species as procoagulant agents. Carbohydrate Polymers, 115, 677–685. [DOI] [PubMed] [Google Scholar]
- Zhang, Zhao L, Ma J, Wang X, Wang Y, Ran F, … Yu S (2017). Electrospinning of fucoidan/chitosan/poly(vinyl alcohol) scaffolds for vascular tissue engineering. Fibers and Polymers, 18(5), 922–932. [Google Scholar]
- Zhao, Dong S, Wang J, Li F, Chen A, & Li B (2012). A comparative study of antithrombotic and antiplatelet activities of different fucoidans from Laminaria japonica. Thrombosis Research, 129(6), 771–778. [DOI] [PubMed] [Google Scholar]
- Zhao, Guo F, Hu J, Zhang L, Xue C, Zhang Z, & Li B (2016). Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thrombosis Research, 144, 46–52. [DOI] [PubMed] [Google Scholar]
- Zheng, Kaiser Y, Poel E, Verberne H, Aerts J, Rouzet F, … Chauvierre C (2019). 99Mtc-fucoidan as diagnostic agent For P-Selectin imaging: first-in-human evaluation (phase I). Atherosclerosis, 287, e143. [Google Scholar]
- Zheng, Zhou L, Lin L, Cai Y, Sun H, Zhao L, … Zhao J (2019). Physicochemical characteristics and anticoagulant activities of the polysaccharides from sea cucumber Pattalus mollis. Marine drugs, 17(4), 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Ding Y, Cai L, Wang Y, Lin C, & Shi Z (2018). Low molecular weight fucoidan attenuates experimental abdominal aortic aneurysm through interfering the leukocyte-endothelial cells interaction. Molecular Medicine Reports, 17(5), 7089–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zhang Q, Chen L, Ren S, Xu P, Tang Y, & Luo D (2010). Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thrombosis Research, 125(5), 419–426. [DOI] [PubMed] [Google Scholar]
- Zvyagintseva TN, Shevchenko NM, Nazarova IV, Scobun AS, Luk’yanov PA, & Elyakova LA (2000). Inhibition of complement activation by water-soluble polysaccharides of some far-eastern brown seaweeds. Comparative Biochemistry and Physiology - C Pharmacology Toxicology and Endocrinology, 126(3), 209–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


