Abstract
Three monoclonal antibodies (MAbs) generated against rainbow trout gonad cells (RTG-2) have been selected for their ability to protect cells from the viral hemorrhagic septicemia virus (VHSV) infection, a salmonid rhabdovirus. Protection from infection was restricted to the salmonid-derived cell lines indicating species specificity of the blocking MAbs. Surprisingly, the blocking activity of these MAbs was also effective against other nonantigenically related fish rhabdoviruses. Indirect immunofluorescence and immunoelectron microscopy observations demonstrated that the three MAbs were all directed against an abundant cell plasma membrane component, and immunoprecipitation studies indicated that the target consisted of a heterodimeric complex with molecular masses of 200 and 44 kDa. Biochemical data provided the following evidence that fibronectin is part of this complex and that it could represent the main receptor for fish rhabdoviruses. (i) An antiserum generated against the 200-kDa protein reacted against the recombinant rainbow trout fibronectin expressed in Escherichia coli. (ii) The purified rainbow trout fibronectin was able to bind specifically to VHSV. To our knowledge, this is the first identification of a cellular component acting as a primary receptor for a virus replicating in lower vertebrates and, more interestingly, for viruses belonging to the Rhabdoviridae family.
Viral hemorrhagic septicemia virus (VHSV) is a serious salmonid fish pathogen belonging to the rhabdovirus family which leads to an acute to chronic viscerotropic disease, mostly but not exclusively in fingerling to yearling rainbow trouts, and causes significant mortality. Similar to the structures in mammalian rhabdoviruses, the VHSV virion structure is composed of a 12-kb negative single-stranded RNA tightly associated with a nucleoprotein N, a polymerase-associated protein P and an RNA-dependant RNA polymerase L, and a matrix protein M and a glycoprotein G which induces neutralizing antibodies and possesses a fusion region (14, 22). VHSV has been adapted to grow in tissue culture in various fish cell lines, including those derived from salmonid fish (RTG-2, RTH, and CHSE-214) and from other fish species, such as cyprinids (EPC).
Transmission of VHSV occurs mainly by shedding from infected fish, and the disease is probably spread by waterborne contact. Vertical transmission of the virus has not been demonstrated. It has been postulated that gills could be the prime portal of entry of VHSV or infectious hematopoietic necrosis virus (IHNV) in fish, since 2 or 3 days after virus challenge, virus can be observed through electron microscopy in gill cells (9). Recent studies on the infection route in rainbow trout for IHNV (18) indicate that the esophagus-cardiac stomach region and, more particularly, the mucus-secreting serous cardiac glands are the early targets for the virus and so far could be the possible portals of entry.
The initial step in the replication cycle of a virus is its attachment to a cell surface receptor, the expression pattern of which influences host range and tissue tropism. For the mammalian rhabdoviruses, many studies, mainly carried out on vesicular stomatitis virus (VSV) and rabies virus, have attempted to identify the cellular receptors for these viruses. For instance, the cellular receptor for the VSV has been identified as a phospholipid, the phosphatidylserine (31). For rabies virus, the nature of the target on the cell surface is still elusive, since several different cell surface components have been shown to bind rabies virus. Many studies demonstrated that the nicotinic acetylcholine receptor (nAChR) was probably one of the cell targets for rabies virus (5, 16, 20, 21); however, rabies virus can infect neurons which do not express nAChR. Thus, it has been postulated that other molecules may act as viral receptors. For instance, phospho- or glycolipids (36, 43), sialic acid (10), and a fibronectin-like protein complex (6) have been tentatively postulated to play that role. Recently, the neural cell adhesion molecule CD56 has been shown to be a receptor for rabies virus (37). Tuffereau et al. (39, 40), in developing a sophisticated strategy based on the use of a soluble form of the rabies virus glycoprotein as a ligand, successfully identified the low-affinity nerve growth factor as a receptor for rabies virus.
One of the approaches used in the past for several virus families to identify the cell receptor (2, 17) consisted of generating monoclonal antibodies (MAbs) against the cell surface that are able to block the viral entry and thus potentially directed against the receptor. Using this strategy, we have generated MAbs against rainbow trout cells which exhibit the expected properties for receptor-directed antibodies. Blocking activity of MAbs M45, G35, and Q3 was shown for a large spectrum of fish rhabdoviruses, independently of the fish species origin, but was restricted to the salmonid-derived cells. These MAbs were directed against an abundant cell surface protein complex for which the heavy chain was identified as the fibronectin. Moreover, direct interaction between VHSV and fibronectin was demonstrated by an in vitro binding assay. The demonstration that fibronectin acts as an initial cell molecule target for VHSV and other fish rhabdoviruses opens new insights for viral propagation in fish.
MATERIALS AND METHODS
Cells and viruses.
The following cell lines were used: rainbow trout gonad (RTG-2), Chinook salmon embryo (CHSE-214), rainbow trout hepatoma (RTH), epithelioma papulosum cyprini (EPC), brown bullhead (BB), and bluegill fibroblast (BF2) cell lines. All cell lines were maintained in Eagle’s minimal essential medium supplemented with 10% fetal bovine serum, 0.3% tryptose phosphate broth, and 2 mM l-glutamine, Tris buffered at 20°C. Viruses used in this study were propagated in EPC cells as previously described (15).
Membrane preparation.
Confluent monolayers of RTG-2 cells were washed with cold phosphate-buffered saline (PBS), scraped with a rubber policeman, and pelleted at 2,000 rpm for 10 min. The pellet was resuspended in buffer A {20 mM K-MES [2-(N-morpholino)ethanesulfonic acid buffered to pH 6.7 with KOH], 250 mM sucrose, 1 mM EDTA}, incubated on ice for 15 min, and disrupted by Dounce homogenization. Nuclei and cell debris were pelleted at 2,000 rpm for 10 min. The supernatant was mixed with 2.55 M sucrose (final concentration, 1.4 M), loaded onto an 0.8 to 2 M discontinuous sucrose gradient, and centrifuged for 2 h at 25,000 rpm in a Beckman SW28 rotor at 4°C. The interface between 0.8 and 1.2 M sucrose fractions was collected and diluted fivefold in distilled water, and the membranes were pelleted at 100,000 rpm for 15 min. The membrane pellet was resuspended at 2 mg/ml in distilled water and stored at −70°C.
Production and selection of protective MAbs.
BALB/c mice were immunized three times every 2 weeks by intraperitoneal injection of 5 × 107 intact cells in PBS without adjuvant. Mice were bled, and aliquots of sera were tested for their protective effect (see below) against VHSV infection of RTG-2 cells. One of the positive mice was boosted with 200 μg of purified RTG-2 cell membranes. Three days following the last boost, immunized mice spleen lymphocytes were fused with SP2O myeloma cells and hybridomas were selected in hypoxanthine-aminopterin-thymidine medium.
For the selection of protective MAbs, RTG-2 cells were plated in 96-well tissue culture plates (8 × 104/well), incubated for 1 h at room temperature with 100 μl of hybridoma supernatants, and then washed; a dose of VHSV was added that yielded a total cytopathic effect in 3 days at 14°C (about 500 PFU/well). Wells were stained with crystal violet to visualize VHSV-induced cytopathic effect, measured by spectrophotometer at 495 nm, and scored positive when protected from infection. Hybridomas scoring positive were subcloned and rescreened (see above) with a dilution of hybridoma supernatants. Positive hybridomas were injected into mice to produce ascitic fluids.
Plaque reduction assay.
RTG-2 cells grown in 24-well plates were incubated with MAbs (dilution, 1/1,000) for 1 h at room temperature. The medium was then removed, and cells were infected with VHSV or IHNV. After 1 h of adsorption, cells were overlaid with 0.3% agarose. Plaques were counted 3 days later, after fixation with 10% Formol and crystal violet staining. The control consisted of cells preincubated with irrelevant MAbs.
Indirect immunofluorescence and immunoelectron microscopy.
RTG-2 cells grown in six-well plates were rinsed with PBS and covered with mouse ascitic fluids diluted (1:1,000) in PBS containing 3% bovine serum albumin (PBSA). After incubation for 1 h at room temperature, cells were extensively washed with PBS and incubated for 1 h with a fluorescein-conjugated anti-mouse immunoglobulin (BIOSYS) diluted (1:800) in PBSA. After washing, cells were examined for staining with a UV-light microscope (Leizt Westlar, Leika, Rueil Malmaison, France). Immunomarking was performed directly on RTG-2 cell monolayers according to a preembedding technique. Briefly, live RTG-2 cells were incubated for 45 min at room temperature with MAbs (dilution, 1/1,000), washed with PBS, and labeled with a 10-nm gold-labeled goat anti-mouse immunoglobulin G (IgG) antibody (Biocell Research Laboratories). After washing, cells were fixed in 1.25% glutaraldehyde, postfixed in 1% OsO4, embedded in Epon by using conventional techniques, and processed for electron microscopy.
Immunoprecipitation.
Confluent RTG-2 monolayer cells were washed in PBS and scraped in 50 mM Tris-HCl–40 mM EDTA (pH 8) (24). Cells were lysed for 2 h at 4°C in the same buffer, containing 0.5% Nonidet P-40, 0.5% deoxycholate, and protease inhibitors. After clarification at 10,000 × g, lysates were immunoprecipitated with MAbs diluted to 1:100. Immune complexes were recovered by adding protein A-Sepharose (Pharmacia) and separated by electrophoresis on a sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gel. The same procedure was employed when starting from rainbow trout organs. Aliquots (roughly 0.2 g per immunoprecipitation) of frozen tissues were first homogenized in 50 mM Tris-HCl–40 mM EDTA with a potter and then lysed as they were for RTG-2 cells.
Mouse antiserum production against the 200-kDa protein.
Large-scale preparation of the 200-kDa protein was performed by immunoprecipitation of rainbow trout muscle following separation on preparative acrylamide gel electrophoresis. The protein band of interest was cut and electroeluted in 40 mM Tris-HCl (pH 8)–2 mM EDTA–20 mM sodium acetate–0.1% SDS and then was extensively dialyzed against PBS. Purified protein was injected into mice in complete Freund adjuvant. Mice were bled 1 month later, and serum was tested by Western blot analysis.
Reactivity of the mouse anti-200K antiserum.
Gelatin-purified fibronectin (see below) and immunoprecipitation of rainbow trout muscle were separated on an SDS–7.5% polyacrylamide gel and electrotransferred onto a ProBlott membrane (Applied Biosystems) in CAPS buffer (3-[cyclohexylamino]-1-propanesulfonic acid) containing 10% methanol. The membrane was blocked overnight in PBSA (3% bovine serum albumin in PBS) and then incubated with anti-200K antiserum diluted to 1:100 in PBSA containing 0.05% Tween 20. The membrane was washed and incubated with an alkaline phosphatase-conjugated anti-mouse immunoglobulin (BIOSYS). Detection of bound antibodies was accomplished with the Gibco-BRL NBT/BCIP detection kit.
Fibronectin purification and in vitro virus binding assay.
The ability of fibronectin to bind gelatin was used to purify it from rainbow trout muscle. Homogenized tissue was lysed in immunoprecipitation buffer and incubated overnight with gelatin-cellufine (Touzard et Matignon, Les Ullis, France). Following washes, either the beads were resuspended in Laemmli’s buffer, boiled, and loaded on an SDS-polyacrylamide gel or the absorbed proteins were eluted from the beads in 1 M NaCl–6 M urea and dialyzed overnight against PBS. For virus binding assays, purified fibronectin and viruses were mixed in 200 μl of Tris-buffered Eagle’s medium and incubated for 1 h at room temperature. Mixtures were layered on top of a cushion of 400 μl of 10% glycerol in culture medium and centrifuged for 30 min at 100,000 × g in a Beckman TL100.2 rotor. Pellets were resuspended in Laemmli’s buffer, boiled, and separated on an SDS-polyacrylamide gel.
Expression of recombinant rainbow trout fibronectin.
From an RTG-2 λ ZAP cDNA library (unpublished results), a 1.2-kb-long DNA fragment was amplified by PCR with a pair of primers derived from the Danio rerio (zebra fish) fibronectin sequence (accession no. AF081128): for nucleotide position 5967, 5′CATATGACAATTGCTGGTCTGGAG3′, and for nucleotide position 7117, 5′CATATGTGGCACCATTTGGATGAA3′ (additional NdeI restriction site is italized). The PCR product was gel purified, digested with NdeI restriction enzyme, and subcloned into the NdeI-digested pET-14b procaryotic expression vector (Novagen) leading to the pFIB1 plasmid. Correct in-frame insertion of the rainbow trout fibronectin DNA insert into the pET-14b vector was verified by partial nucleotide sequencing of pFIB1 with the universal T7 primer. pFIB1 was transferred into Escherichia coli BL21 (DE3), and expression of the recombinant protein was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) into log-phase growing cultures.
Aliquots of induced E. coli cell lysates expressing the recombinant rainbow trout fibronectin and the immunoprecipitated 200-kDa protein were separated on an SDS–7.5% polyacrylamide gel and visualized after Coomassie blue staining. Western blot analyses were performed as described above.
RESULTS
Isolation of MAbs which protect cells from VHSV infection.
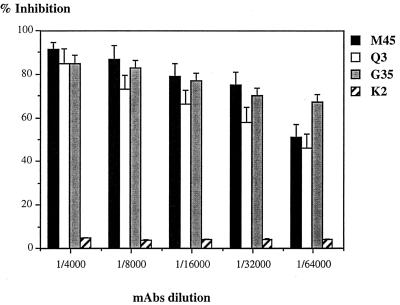
A number of cell receptors for a variety of viruses have been successfully identified by using receptor-directed MAbs that are able to block the viral infection. We used this strategy to identify the cell receptor for VHSV infection. Three MAbs were isolated, M45, G35, and Q3, that inhibited virus infection of RTG-2 cells. At a 1:100 dilution, 90% of the RTG-2 cells were protected from VHSV infection. All three MAbs belonged to the IgG1 subclass. Blocking activity of the M45, G35, and Q3 MAbs was evaluated as shown in Fig. 1; all three were found to have similar titers. When MAbs were applied to the cells in dilutions ranging from 1:100 to 1:64,000, the cells were 95% and 50 to 70%, respectively, protected from viral infection, whereas incubation of cells with irrelevant MAb K2 did not affect the VHSV replication process. But even when a mixture of the three MAbs was used, the cells were never totally protected, probably indicating an alternative way for viral entry (not shown).
FIG. 1.
Cell protection assays with selected anti-RTG-2 MAbs. RTG-2 cells grown in 96-well plates were incubated for 1 h with indicated MAb dilutions, and cells were washed and infected. The cytopathic effect was evaluated 3 days postinfection, following fixation with crystal violet, and measured with a spectrophotometer at 495 nm. The absorbance for uninfected cells was set at 100%, and the absorbance for infected cells was set at 0%. Results are expressed as the means of three experiments. K2 is an irrelevant MAb.
It has been shown that rhabdovirus particles contain several cell-derived membrane components, either phospholipids (25) or proteins, such as ezrin-radixin-moesin family proteins (29). Thus, to ascertain that MAbs were not directed against a cell-derived viral component and did not act as viral neutralizing antibodies, two kinds of experiment were conducted. In a first assay, VHSV was incubated for 1 h with MAb M45 (dilution, 1:1,000) and then the mixture was added to the cells. Three days later, the cytopathic effect was comparable to that of cells incubated with virus alone (data not shown). This result indicated that MAb M45 was not directed against a viral component. When the MAb protection experiments were performed with non-salmonid-derived cell lines (Table 1), MAbs exhibited no protecting activity against virus infection. Altogether, these results suggest that the three selected MAbs were directed against a cellular component and were specific for the salmonid-derived cell species.
TABLE 1.
Cell species specificity of the blocking MAbsa
| Cell line | Protection |
|---|---|
| RTG-2 | + |
| CHSE-214 | + |
| RTH | + |
| EPC | − |
| BF2 | − |
| BB | − |
Various cell lines were incubated with MAbs diluted to 1:1,000 and then washed and infected with VHSV. The cytopathic effect was evaluated 3 days later. Protection was estimated positive (+) when more than 90% of the cell monolayer was unaffected. Salmonid-derived cell lines: RTG-2, rainbow trout gonads; CHSE-214, Chinook salmon embryo; RTH, rainbow trout hepatoma cell lines. Non-salmonid-derived cell lines: EPC, epithelioma papulosum cyprini; BF2, bluegill fibroblast; BB, brown bullhead cell lines.
Protective MAbs recognize a cell membrane component.
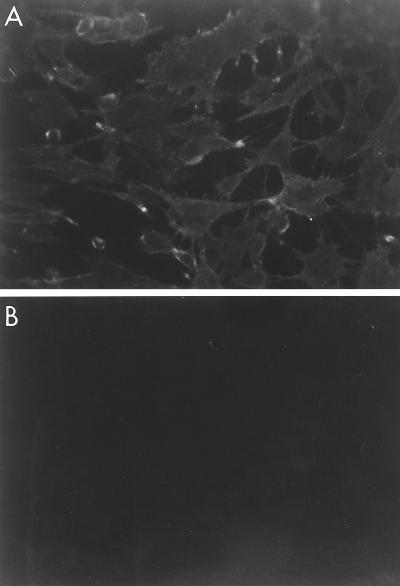
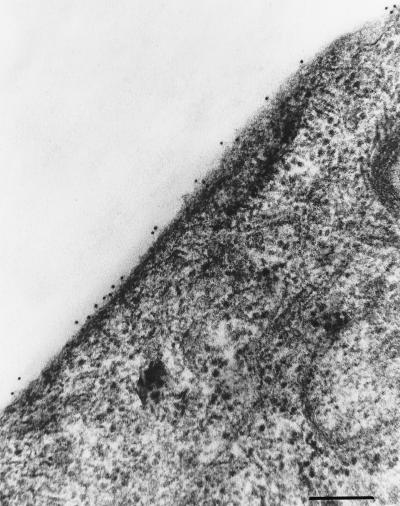
To verify the membrane localization of the molecule recognized by the blocking MAbs, live or acetone-fixed RTG-2 cells were incubated with MAb M45 and stained with a fluorescein-conjugated anti-mouse immunoglobulin. As shown in Fig. 2, immunofluorescence staining is visible on the RTG-2 cell surface when the cells are not fixed (panel A), whereas there is a total absence of fluorescence on fixed RTG-2 cells (panel B). Control experiments with live RTG-2 cells incubated with a primary irrelevant MAb did not present any reactivity (data not shown). The molecule recognized by the MAbs was thought to be quantitatively represented on the cell surface, since MAb M45 at dilution 1:64,000 still produced visible immunofluorescence staining on RTG-2 cells that were not fixed (data not shown). Immunoelectron microscopy with gold-labeled MAbs produced electron micrographs of cells with gold particles evenly distributed along the cell surface (Fig. 3). At that point, we thought it highly probable that the three MAbs were directed against a cell plasma membrane component essential during the first step of the cell-virus interaction.
FIG. 2.
Indirect immunofluorescence assays with blocking MAbs. Live (A) or methanol-acetone-fixed (B) RTG-2 cell monolayers were incubated with MAb M45 (1 μg/ml) for 1 h at room temperature, washed, and stained with fluorescein isothiocyanate-labeled anti-mouse immunoglobulins.
FIG. 3.
Immunogold staining of RTG-2 cells. Immunomarking was performed directly on RTG-2 cell monolayers according to a preembedding technique. Live cells were incubated for 45 min at room temperature with MAb M45 (dilution, 1/1,000), rinsed with PBS, and labeled with a 10-nm colloidal gold-conjugated goat anti-mouse IgG antibody. After washing, cells were fixed in 1.25% glutaraldehyde, postfixed in 1% OsO4, and embedded in Epon by using conventional techniques. Bar, 0.15 mm; Original magnification, ×45,000.
Cell MAb protection from viral infection is not restricted to VHSV.
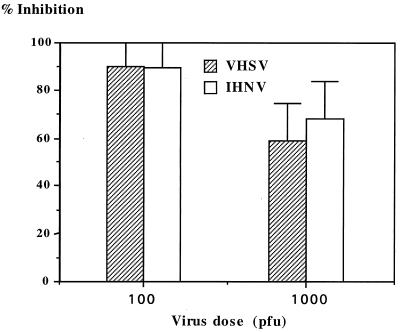
A number of fish rhabdoviruses have been isolated and adapted to tissue culture (for a review, see reference 42). Two of them, IHNV and VHSV, have been studied intensively because of their impact on aquacultured fish. IHNV and VHSV are structurally similar but do not cross-react antigenically. It was of interest to study the ability of the MAbs to protect RTG-2 cells from IHNV infection as well. Cell protection assays were conducted by using a plaque formation assay with two different infectious virus doses (Fig. 4). Cells were protected from infection with VHSV and IHNV, even when high infectious viral doses were used. This result prompted us to broaden the study to include a panel of various fish rhabdoviruses and other fish viruses. The titers of all the viruses used were calibrated in order to have a total cytopathic effect in 3 days, as for VHSV. The results presented in Table 2 indicate that the MAbs are able to protect cells from infection by all the rhabdoviruses tested but not from infection by enveloped or nonenveloped viruses belonging to other viral families. This result ruled out the possibility of cell protection by a nonspecific masking of the cell surface. Thus, contrary to the case for mammalian rhabdoviruses, these results suggest that the fish rhabdoviruses use a common target to bind to the cells.
FIG. 4.
Plaque inhibition formation for two rainbow trout rhabdoviruses. RTG-2 cells grown in 24-well plates were incubated with MAb M45 (dilution, 1/1,000) for 1 h at room temperature, the medium was removed, and cells were infected with IHNV or VHSV. After 1 h of adsorption, cells were overlaid with 0.3% agarose. Plaques were counted 3 days later. The control, set at 100% absorbance, was cells preincubated with irrelevant MAb.
TABLE 2.
Blocking activity of the MAbs of various fish virusesa
| Viruses and cell line | Inhibition |
|---|---|
| Fish rhabdoviruses | |
| PFR | + |
| SVC | + |
| C30 | + |
| Perch rhabdovirus | + |
| VHSV | + |
| IHNV | + |
| Other fish viruses | |
| IPNV | − |
| EHNV | − |
| OMV | − |
All viruses were grown in RTG-2 cells, and titers were calibrated in order to induce a total cytopathic effect 3 days postinfection. The inhibition was scored positive (+) when the cell monolayer was protected by MAbs from virus infection and negative (−) when cells were not protected. PFR, pike fry rhabdovirus; SVC, spring viremia of carp; C30, eel rhabdovirus; IPNV, infectious pancreatic necrosis virus (birnavirus); EHNV, epizootic hematopoietic necrosis virus (iridovirus); OMV, oncorhyncus masou virus (herpesvirus). All four serotypes of VHSV were used.
Biochemical characterization of the VHSV cell receptor.
To investigate the nature of the VHSV cell receptor, a dot blot assay was carried out to test MAb reactivity to total purified RTG-2 cell membranes and to total membrane lipids from the same membrane preparation (8). Positive signals appeared only when the total membrane fraction was spotted, suggesting that MAbs were not directed against a lipid component (data not shown). Western blot analysis of total RTG-2 cell lysates or purified cell membranes did not reveal any reacting protein, even with high MAb concentrations. This lack of reactivity for all three MAbs suggested that the MAbs were directed against conformational epitopes and thus that the protein of interest could be identified only in its native form. RTG-2 cells were radiolabeled, and lysates were subjected to immunoprecipitation. A broad spectrum of lysis buffers and immunoprecipitation conditions was tested, including various mixtures of detergents, pH ranges, and salt concentrations. Finally, the lysis buffer composition described by Opstelten et al. (24) was used successfully to selectively immunoprecipitate a heterodimeric protein complex of roughly 200 and 47 kDa (Fig. 5, lane 1). Due to the apparent abundance observed by immunofluorescence of the reactive cell surface protein, we immunoprecipitated an RTG-2 cell lysate followed by a direct visualization on a Coomassie blue-stained gel. A similar protein complex was immunoprecipitated when any of the three MAbs (M45, G35, or Q3) was used (data not shown). To evaluate the relative abundance of this protein complex on various rainbow trout organs, immunoprecipitations of organ homogenates were performed with MAb M45 (Table 3). The immunorecognized protein appeared to be very abundant in the muscle tissue. This tissue was chosen as the source of material to purify the VHSV cell receptor for the generation of an antireceptor mouse polyclonal antiserum (see Materials and Methods).
FIG. 5.
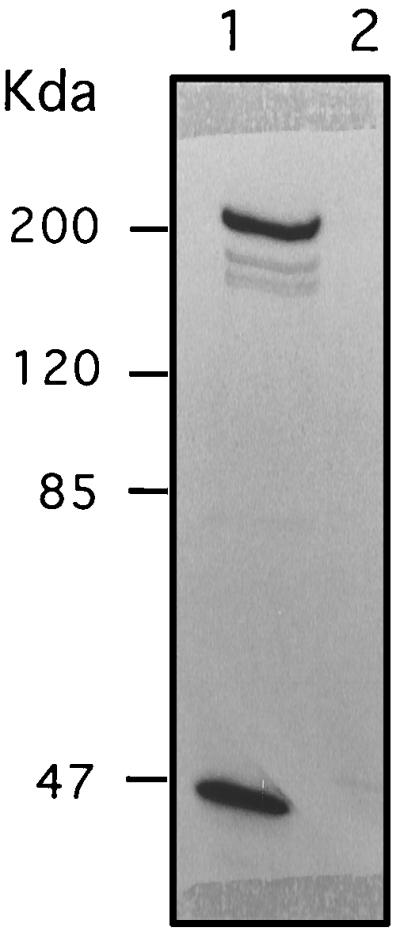
Immunoprecipitation of VHSV receptor from radiolabeled RTG-2 cells. RTG-2 cells were labeled with [35S]methionine for 3 days, lysed, and immunoprecipitated with MAb M45 (lane 1) or irrelevant MAb K2 (lane 2). Products were separated on an SDS–7.5% polyacrylamide gel and subjected to autoradiography. Protein molecular mass standards are indicated on the left.
TABLE 3.
Relative abundance of VHSV cell target in various rainbow trout organs
| Organ(s)a | Immunoprecipitationb |
|---|---|
| Muscle | ++ |
| Heart | ++ |
| Front kidney | − |
| Rear kidney | − |
| Brain | + |
| Spleen | ± |
| Intestine | − |
| Gills | − |
A 0.2-g aliquot of each organ was washed, sliced, and lysed overnight in immunoprecipitation lysis buffer. Lysates were clarified, and the supernatants were subjected to immunoprecipitation.
The relative abundance of the immunoprecipitated product was visually estimated on Coomassie blue-stained SDS-polyacrylamide gels. ++, very abundant; +, fairly abundant; ±, hardly visible; −, not detectable.
Dithiothreitol (DTT) treatment.
To analyze in more detail the nature of the VHSV binding factor, we attempted to characterize the 200-kDa heavy chain of the complex. A migration on SDS-polyacrylamide gels in nonreducing conditions allowed us to show that the 200-kDa heavy chain is a dimer of two similar disulfide-linked chains (data not shown). The monomeric 200-kDa protein was shown to be glycosylated with an Immun-Blot kit for glycoprotein detection (Bio-Rad). Mostly based on the apparent molecular mass observed in SDS-polyacrylamide gel electrophoresis under reducing and nonreducing conditions, we postulated that the 200-kDa protein could be the fibronectin. To investigate this hypothesis, we carried out several experiments. Taking into account knowledge of the disulfide bond-linked fibronectin chains and the extreme sensitivity to conformational changes of the receptor recognized by the blocking MAbs, we reasoned that if we could reduce the fibronectin complex on the cell surface, cell entry of VHSV and recognition by the blocking MAbs should be abolished.
RTG-2 cell monolayers were briefly treated with DTT and then carefully washed and infected with VHSV. The cells were 90 to 95% protected from viral infection, demonstrating that DTT treatment possibly affected the entry of VHSV into the cells. To ascertain that DTT treatment acted on the virus cell binding and not on the subsequent steps of the viral replication, a similar experiment was performed and cells were examined by immunofluorescence staining for the presence of neosynthetized viral antigens. One day postinfection, no VHSV antigens could be detected in VHSV-infected DTT-treated cells. Moreover, reactivity of mock-infected DTT-treated cells with blocking MAbs was also totally abolished. Altogether, these data support the hypothesis that the fibronectin may represent the VHSV receptor recognized by the blocking MAbs.
Direct evidence of fibronectin as the main receptor for fish rhabdovirus.
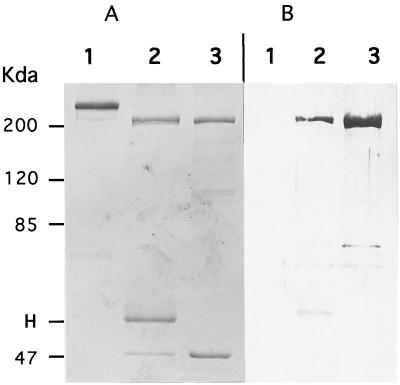
It is well documented (26) that fibronectin has several functional domains, one of those being a collagen-gelatin binding domain. This property to bind gelatin is routinely used to purify fibronectin from plasma. Starting from rainbow trout muscle homogenized and lysed in the immunoprecipitation lysis buffer, we have in parallel proceeded to immunoprecipitation and gelatin affinity purification. Analysis on an SDS-polyacrylamide gel (Fig. 6A) showed the exact comigration of material eluted from both procedures of affinity purification (protein A-Sepharose and gelatin-cellufine). To further confirm the relationship between both purified products, the gel was transferred onto a polyvinylidene difluoride (PVDF) membrane and subjected to reaction with the mouse anti-200K polyclonal antiserum (Fig. 6B). The cross-reaction observed demonstrates that fibronectin was the protein recognized by the blocking MAbs.
FIG. 6.
VHSV cell target is related to rainbow trout fibronectin. Rainbow trout muscle (0.2 g/assay) was subjected to Dounce homogenization, clarified at low-speed centrifugation, and lysed in immunoprecipitation buffer. Lysate was either immunoprecipitated with MAb M45 or incubated with gelatin-cellufine (see Materials and Methods). Samples were boiled in Laemmli’s buffer and separated on an SDS–7.5% polyacrylamide gel. The gel was then stained with Coomassie blue (A) or transferred onto PVDF membranes (B) for Western blot analysis. The immunoblot was reacted with mouse anti-200K antiserum. Lanes 1, purified human plasma fibronectin; lanes 2, immunoprecipitation of rainbow trout muscle lysate; lanes 3, gelatin affinity-purified fibronectin from rainbow trout muscle. Protein molecular mass standards are indicated on the left. H, immunoglobulin heavy chain.
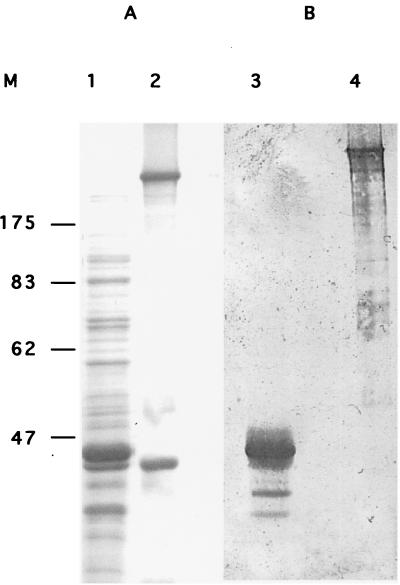
Definitive confirmation that the 200-kDa protein is the fibronectin came with the expression in E. coli of part of rainbow trout fibronectin. Western blot analysis of the expected 43-kDa recombinant fibronectin expressed by using the pET-14b expression vector shows the reactivity of the anti-200K antiserum (Fig. 7). Interestingly, a multialignment of pFIB1 and some of the fibronectin amino acid sequences available in data banks showed that rainbow trout fibronectin is distantly related to other vertebrates’ fibronectin, since sequence identity ranged from 50 to 67%, respectively, for human and D. rerio fibronectin (data not shown).
FIG. 7.
Western blot analysis of E. coli expressed rainbow trout fibronectin. The partial rainbow trout fibronectin produced in E. coli and immunoprecipitated fibronectin were separated on an SDS–10% polyacrylamide gel. The gel was stained with Coomassie blue (A), transferred onto a PVDF membrane (B), and incubated with mouse anti-200K antiserum (dilution, 1:100). Lanes 1 and 3, recombinant E. coli fibronectin; lanes 2 and 4, rainbow trout fibronectin. Molecular mass markers (M) (in kilodaltons) are indicated on the left.
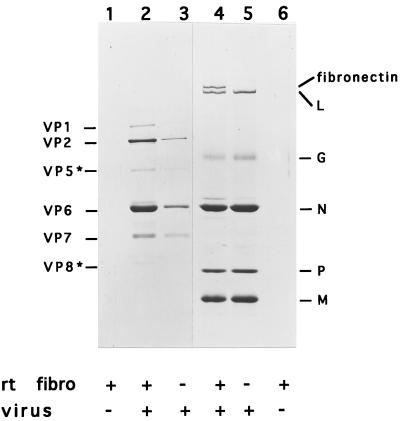
A direct association between VHSV and fibronectin was demonstrated in mixing gelatin affinity-purified fibronectin and VHSV, and the mixtures were pelleted through a glycerol cushion. The analysis of the resulting pellets on SDS-polyacrylamide gels evidenced the specific binding of VHSV to fibronectin (Fig. 8, lane 4).
FIG. 8.
In vitro binding of rainbow trout fibronectin and VHSV. Rainbow trout fibronectin (rt fibro) was purified from muscle by the gelatin affinity procedure, eluted, and extensively dialyzed. Purified fibronectin was mixed with VHSV (lane 4) or bovine rotavirus (lane 2) and pelleted through a glycerol cushion. Pellets were resuspended in Laemmli’s buffer, boiled, and analyzed on a Coomassie blue-stained SDS–10% polyacrylamide gel. Viral polypeptides are indicated on the left (rotavirus) and on the right (VHSV).
DISCUSSION
In this study, we describe the generation of three MAbs which exhibit the expected properties for antibodies directed against a viral cell receptor. These MAbs were shown to prevent cells from VHSV infection, even when very small amounts of antibody (less than 2 ng/9 × 104 cells) were used. These MAbs were shown to be fish cell species-specific since they protect the cells from viral infection exclusively on salmonid-derived cell lines (Table 1). The finding that MAbs M45, G35, and Q3 were able to block cell infection for a large panel of antigenically unrelated fish rhabdoviruses (Table 2) was unexpected and emphasized the unsatisfactory taxonomic classification adopted for fish rhabdoviruses. Indeed, like for their mammalian counterparts, the rhabdoviruses infecting lower vertebrates have been divided into two genera, Vesiculovirus (prototype, VSV) and Lyssavirus (prototype, rabiesvirus), and thus were supposed to interact with different cell surface molecules in the first step of infection. Morzunov et al. (23) and Bjorklund et al. (4) previously raised that point when they showed, by gene sequencing of various fish rhabdovirus isolates, that most of them were more closely related to each other than to mammalian vesiculovirus or lyssavirus. They proposed to adopt a new genus for fish rhabdovirus termed Piscivirus or Aquarhabdovirus, the latter being reminiscent of Aquareovirus, which was proposed by Samal et al. (30) for the fish reovirus. Recently, the classification for fish rhabdovirus has been modified and the genus of Novirhabdovirus has been adopted.
We provide several lines of evidence that fibronectin, an abundant glycoprotein of the extracellular matrix, is the major cell component allowing fish rhabdovirus to bind to the cells. It has to be pointed out that in one previous report (6) a fibronectin-like complex has been shown to interact with rabies virus. Other reports (5, 16, 20, 21) have identified the nAChR as the initial rabies virus cell target. Interestingly, extracellular matrix molecules, including fibronectin, codistribute with nAChR clusters (12, 13). Thus, fibronectin should be considered as part and parcel of the VHSV and other fish rhabdovirus cell receptor complexes, playing a role in the initial binding step, which is probably followed by a secondary binding, allowing the virus to enter into the cell by fusion or by endocytosis (19). Various pathogens have previously been shown to bind to fibronectin (for a review, see reference 26), including viruses such as cytomegalovirus (1), hepatitis B virus (7), hepatitis A virus (34), human immunodeficiency virus (38), and Rous sarcoma virus (35). Numerous other pathogenic microorganisms have been shown to interact with fibronectin (41) in a first step of binding to the cell. For instance, the ability to bind fibronectin is a property conserved among many, if not all, species of mycobacteria (27). Several mycobacterial fibronectin binding proteins have been identified (19, 28, 32, 33). These homologous proteins have been termed fibronectin attachment proteins (FAP). Interestingly, alignment of amino acid sequences of mycobacterial FAP and VHSV viral attachment protein G exhibits significant similarities between both protein families (Fig. 9). A motif L-X-KVTGP-X7-P appeared to be well conserved between G and FAP, but the biological meaning of that needs to be clarified.
FIG. 9.
Alignment of FAP and VHSV glycoprotein amino acid sequences. FAP from Mycobacterium avium (30) is 381 amino acids long, and GVHS is the VHSV (French strain 07-7) (3) glycoprotein amino acid sequence. GVHS starts at amino acid 105 and ends at amino acid 507. Alignment was done using Multalin software (11). Identical or conservative amino acids are in bold.
The finding that fibronectin specifically interacts with fish rhabdovirus and the demonstrated very high abundance of fibronectin in the rainbow trout muscle allowed us to hypothetize that rhabdoviruses infect fish following a two-step outline: (i) passive entry of rhabdovirus into fish across the skin mucus and (ii) direct interaction to fibronectin of the superficial muscle, which is in close contact with the skin.
ACKNOWLEDGMENTS
We are grateful to Daniele Monge (INRA, Jouy-en-Josas Cedex, France) for providing the rainbow trout organs used in this study.
This work has been supported in part by a grant from the French Ministère de la Recherche (ACCSV-6).
REFERENCES
- 1.Agbanyo F R, Wasi S. Human cytomegalovirus interaction with platelets and adhesive glycoproteins: significance in viral pathogenesis. J Infect Dis. 1994;170:1120–1127. doi: 10.1093/infdis/170.5.1120. [DOI] [PubMed] [Google Scholar]
- 2.Bass D M, Greenberg H B. Strategies for the identification of icosahedral virus receptors. J Clin Investig. 1992;89:3–9. doi: 10.1172/JCI115575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmansour A, Basurco B, Monnier A F, Vende P, Winton J R, de Kinkelin P. Sequence variation of the glycoprotein gene identifies three distinct lineages within field isolates of viral haemorrhagic septicaemia virus, a fish rhabdovirus. J Gen Virol. 1997;78:2837–2846. doi: 10.1099/0022-1317-78-11-2837. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund H V, Higman K H, Kurath G. The glycoprotein genes and gene junctions of the fish rhabdoviruses spring viremia of carp virus and hirame rhabdovirus: analysis of relationships with other rhabdoviruses. Virus Res. 1996;42:65–80. doi: 10.1016/0168-1702(96)01300-7. [DOI] [PubMed] [Google Scholar]
- 5.Bracci L, Antoni G, Cusi M G, Lozzi L, Niccolai N, Petreni S, Rustici M, Santucci A, Soldani P, Valensin P E. Antipeptide monoclonal antibodies inhibit the binding of rabies virus glycoprotein and alpha-bungarotoxin to the nicotinic acetylcholine receptor. Mol Immunol. 1988;25:881–888. doi: 10.1016/0161-5890(88)90125-3. [DOI] [PubMed] [Google Scholar]
- 6.Broughan J H, Wunner W H. Characterization of protein involvement in rabies virus binding to BHK-21 cells. Arch Virol. 1995;140:75–93. doi: 10.1007/BF01309725. [DOI] [PubMed] [Google Scholar]
- 7.Budkowska A, Bedossa P, Groh F, Louise A, Pillot J. Fibronectin of human liver sinusoids binds hepatitis B virus: identification by an anti-idiotypic antibody bearing the internal image of the pre-S2 domain. J Virol. 1995;69:840–848. doi: 10.1128/jvi.69.2.840-848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabraoui F, Derrington E A, Mallie-Didier F, Confavreux C, Quincy C, Caudie C. Dot-blot immunodetection of antibodies against GM1 and other gangliosides on PVDF-P membranes. J Immunol Methods. 1993;165:225–230. doi: 10.1016/0022-1759(93)90348-b. [DOI] [PubMed] [Google Scholar]
- 9.Chilmonczyk S, Monge D. Rainbow trout gill pillar cells: demonstration of inert particle phagocytosis and involvement in viral infection. J Reticuloendothel Soc. 1980;28:327–332. [PubMed] [Google Scholar]
- 10.Conti C, Superti F, Tsiang H. Membrane carbohydrate requirement for rabies virus binding to chicken embryo related cells. Intervirology. 1986;26:164–168. doi: 10.1159/000149696. [DOI] [PubMed] [Google Scholar]
- 11.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dmytrenko G M, Scher M G, Poiana G, Baetscher M, Bloch R J. Extracellular glycoproteins at acetylcholine receptor clusters of rat myotubes are organized into domains. Exp Cell Res. 1990;189:41–50. doi: 10.1016/0014-4827(90)90254-8. [DOI] [PubMed] [Google Scholar]
- 13.Dmytrenko G M, Bloch R J. Evidence for transmembrane anchoring of extracellular matrix at acetylcholine receptor clusters. Exp Cell Res. 1993;206:323–334. doi: 10.1006/excr.1993.1153. [DOI] [PubMed] [Google Scholar]
- 14.Estepa A, Coll J M. Pepscan mapping and fusion related properties of the major phosphatidylserine-binding domain of the glycoprotein of VHSV, a salmonid rhabdovirus. Virology. 1996;216:60–70. doi: 10.1006/viro.1996.0034. [DOI] [PubMed] [Google Scholar]
- 15.Fijan N, Sulimanovic M, Bearzotti M, Muzinic D, Zwillenberg L O, Chilmonczyk S, Vautherot J F, de Kinkelin P. Some properties of the Epithelioma papulosum cyprini EPC cell line from carp Cyprinus carpio. Ann Inst Pasteur Virol. 1983;134E:207–220. [Google Scholar]
- 16.Gastka M, Horvath J, Lentz T L. Rabies virus binding to the nicotinic acetylcholine receptor alpha subunit demonstrated by virus overlay protein binding assay. J Gen Virol. 1996;77:2437–2440. doi: 10.1099/0022-1317-77-10-2437. [DOI] [PubMed] [Google Scholar]
- 17.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmick C M, Bailey J F, LaPatra S, Ristow S. The esophagus/cardiac stomach region: site of attachment and internalization of infectious hematopoietic necrosis virus in challenged juvenile rainbow trout Oncorhynchus mykiss and coho salmon O. kisutch. Dis Aquat Org. 1995;23:189–199. [Google Scholar]
- 19.Korhonen T K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 20.Lentz T L, Benson J J, Klimowicz D, Wilson P T, Hawrot E. Binding of rabies virus to purified Torpedo acetylcholine receptor. Mol Brain Res. 1986;1:211–219. doi: 10.1016/0169-328x(86)90027-6. [DOI] [PubMed] [Google Scholar]
- 21.Lentz T L, Burrage T G, Smith A L, Crick J, Tignor G H. Is the acetylcholine receptor a rabies receptor? Science. 1982;215:182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzen N, Olesen N J, Jorgensen P E. Neutralization of Egtved virus pathogenicity to cell cultures and fish by monoclonal antibodies to the viral G protein. J Gen Virol. 1990;71:561–567. doi: 10.1099/0022-1317-71-3-561. [DOI] [PubMed] [Google Scholar]
- 23.Morzunov S P, Winton J R, Nichol S T. The complete genome structure and phylogenetic relationship of infectious hematopoietic necrosis virus. Virus Res. 1995;38:175–192. doi: 10.1016/0168-1702(95)00056-v. [DOI] [PubMed] [Google Scholar]
- 24.Opstelten D J, Raamsman M J, Wolfs K, Horzinek M C, Rottier P J. Envelope glycoprotein interactions in coronavirus assembly. J Cell Biol. 1995;131:339–349. doi: 10.1083/jcb.131.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal R, Barenholz Y, Wagner R R. Vesicular stomatitis virus membrane proteins and their interaction with lipid bilayers. Biochim Biophys Acta. 1987;906:175–193. doi: 10.1016/0304-4157(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 26.Proctor R A. Fibronectin: a brief overview of its structure, function, and physiology. Rev Infect Dis. 1987;9(Suppl. 4):S317–S321. doi: 10.1093/clinids/9.supplement_4.s317. [DOI] [PubMed] [Google Scholar]
- 27.Ratliff T L, McGarr J A, Abou Zeid C, Rook G A, Stanford J L, Aslanzadeh J, Brown E J. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988;134:1307–1313. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- 28.Ratliff T L, McCarthy R, Telle W B, Brown E J. Purification of a mycobacterial adhesin for fibronectin. Infect Immun. 1993;61:1889–1894. doi: 10.1128/iai.61.5.1889-1894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagara J, Tsukita S, Yonemura S, Tsukita S, Kawai A. Cellular actin-binding ezrin-radixin-moesin (ERM) family proteins are incorporated into the rabies virion and closely associated with viral envelope proteins in the cell. Virology. 1995;206:485–494. doi: 10.1016/s0042-6822(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 30.Samal S K, Dopazo C P, McPhillips T H, Baya A, Mohanty S B, Hetrick F M. Molecular characterization of a rotaviruslike virus isolated from striped bass (Morone saxatilis) J Virol. 1990;64:5235–5240. doi: 10.1128/jvi.64.11.5235-5240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlegel R, Tralka T S, Willingham M C, Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983;32:639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- 32.Schorey J S, Li Q, McCourt D W, Bong-Mastek M, Clark-Curtiss J E, Ratliff T L, Brown E J. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect Immun. 1995;63:2652–2657. doi: 10.1128/iai.63.7.2652-2657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schorey J S, Holsti M A, Ratliff T L, Allen P M, Brown E J. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol Microbiol. 1996;21:321–329. doi: 10.1046/j.1365-2958.1996.6381353.x. [DOI] [PubMed] [Google Scholar]
- 34.Seelig R, Pott G, Seelig H P, Liehr H, Metzger P, Waldherr R. Virus-binding activity of fibronectin: masking of hepatitis A virus. J Virol Methods. 1984;8:335–347. doi: 10.1016/0166-0934(84)90071-5. [DOI] [PubMed] [Google Scholar]
- 35.Stanislawski L. Attachment of Rous sarcoma virus to the fibronectin of infected chick embryo fibroblasts. J Ultrastruct Res. 1983;82:134–142. doi: 10.1016/s0022-5320(83)90048-5. [DOI] [PubMed] [Google Scholar]
- 36.Superti F, Seganti L, Tsiang H, Orsi N. Role of phospholipids in rhabdovirus attachment to CER cells. Brief report. Arch Virol. 1984;81:321–328. doi: 10.1007/BF01310002. [DOI] [PubMed] [Google Scholar]
- 37.Thoulouze M I, Lafage M, Schachner M, Hartmann U, Cremer H, Lafon M. The neural cell adhesion molecule is a receptor for rabies virus. J Virol. 1998;72:7181–7190. doi: 10.1128/jvi.72.9.7181-7190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torre D, Pugliese A, Ferrario G, Marietti G, Forno B, Zeroli C. Interaction of human plasma fibronectin with viral proteins of human immunodeficiency virus. FEMS Immunol Med Microbiol. 1994;8:127–131. doi: 10.1111/j.1574-695X.1994.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 39.Tuffereau C, Benejean J, Alfonso A M, Flamand A, Fishman M C. Neuronal cell surface molecules mediate specific binding to rabies virus glycoprotein expressed by a recombinant baculovirus on the surfaces of lepidopteran cells. J Virol. 1998;72:1085–1091. doi: 10.1128/jvi.72.2.1085-1091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuffereau C, Benejean J, Blondel D, Kieffer B, Flamand A. Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus. EMBO J. 1998;17:7250–7259. doi: 10.1093/emboj/17.24.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadström T, Switalski L M, Speziale P, Rubin K, Ryden C, Fröman G, Faris A, Lindberg M, Höök M. Binding of microbial pathogen to connective tissue fibronectin: an early step in localized and invasive infections. In: Jackson G J, editor. Pathogenesis of infection. Berlin, Germany: Springer-Verlag KG; 1985. pp. 193–200. [Google Scholar]
- 42.Wolf K. Fish viruses and fish viral diseases. Ithaca, N.Y: Comstock Publishing Associates, Cornell University Press; 1988. [Google Scholar]
- 43.Wunner W H, Reagan K J, Koprowski H. Characterization of saturable binding sites for rabies virus. J Virol. 1984;50:691–697. doi: 10.1128/jvi.50.3.691-697.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]