Abstract
A 74-year-old man with prior coronary artery bypass surgery had a failing vein graft to the right coronary artery (RCA). He underwent retrograde chronic total occlusion recanalization of an ostial flush-occluded RCA facilitated by retrograde angioplasty at the aorto-ostial junction after failed retrograde electrocautery. The graft was then sacrificed. (Level of Difficulty: Advanced.)
Key Words: chronic total occlusion, coiling, electrocautery-assisted re-entry, retrograde, saphenous vein graft
Central Illustration
History of Presentation
A 74-year-old man presented with stable angina to our complex percutaneous coronary intervention (PCI) clinic in November 2022. He endorsed angina and dyspnea on exertion walking up a flight of stairs and >2 blocks on level ground, consistent with Canadian Cardiovascular Society class II angina, despite maximally tolerated medical therapy with metoprolol, isosorbide mononitrate, and amlodipine.
Learning Objectives
-
•
To recognize the role of native PCI in the setting of vein graft failure.
-
•
To understand several advanced techniques that might be necessary to achieve retrograde recanalization of complex flush occluded coronary arteries.
-
•
To understand when to sacrifice a graft after successful native coronary recanalization.
Past Medical History
The patient’s past medical history was notable for hypertension, hyperlipidemia, and moderate aortic stenosis. He had coronary artery disease, with prior coronary artery bypass grafting in 1998 with a saphenous vein graft (SVG) to the left circumflex, SVG to the diagonal, and SVG to the posterior descending artery (PDA) with a skip graft to a right posterolateral branch (RPL). The SVG-PDA had recurrent disease requiring 5 PCIs, with 2-layer in-stent restenosis both at the ostium and in the distal segment of his SVG (proximal to the anastomosis). His current symptoms started 1 month following his most recent intervention in September 2022, at which time he had repeat stenting of his ostial SVG-PDA.
Differential Diagnosis
The patient’s presentation is consistent with obstructive coronary artery disease. With prior history of recurrent vein graft interventions, the most likely culprit is the SVG-PDA. Progression of aortic stenosis could also be considered.
Investigations
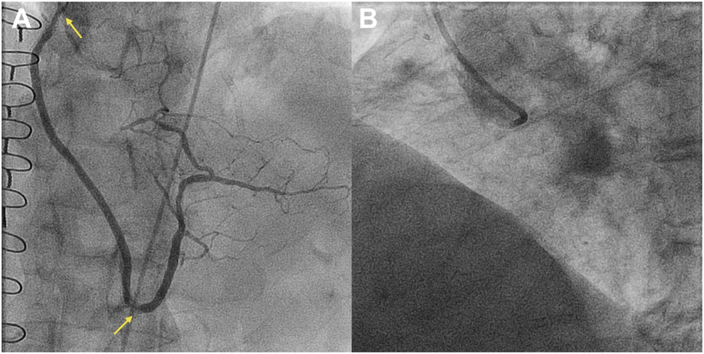
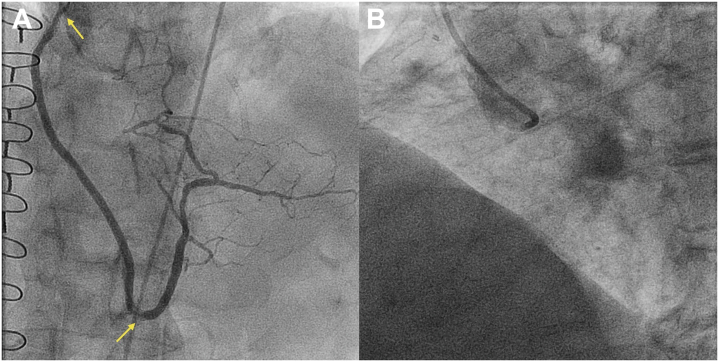
Transthoracic echocardiogram showed an ejection fraction of 65% with inferior wall hypokinesis. Aortic valve peak velocity was 2.5 m/s, mean gradient was 15 mm Hg, and aortic valve area was 1.14 cm2. Nuclear medicine stress test showed ischemia in the inferior and inferolateral wall. Angiography was notable for in-stent restenosis at the ostium and in the distal segment of the SVG-RCA (Figure 1A). The RCA was flush occluded at the ostium, which was severely calcified and had an ambiguous proximal cap; occlusion length was 120 mm and the CTO extended into both the PDA and RPL (Figure 1B).
Figure 1.
Baseline Angiography
(A) Recurrent in-stent restenosis within the saphenous vein graft–posterior descending artery (arrows). (B) Flush ostial right coronary artery chronic total occlusion.
Management
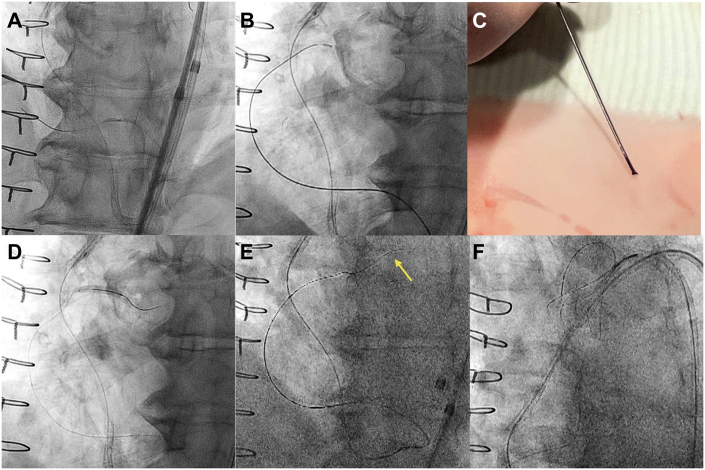
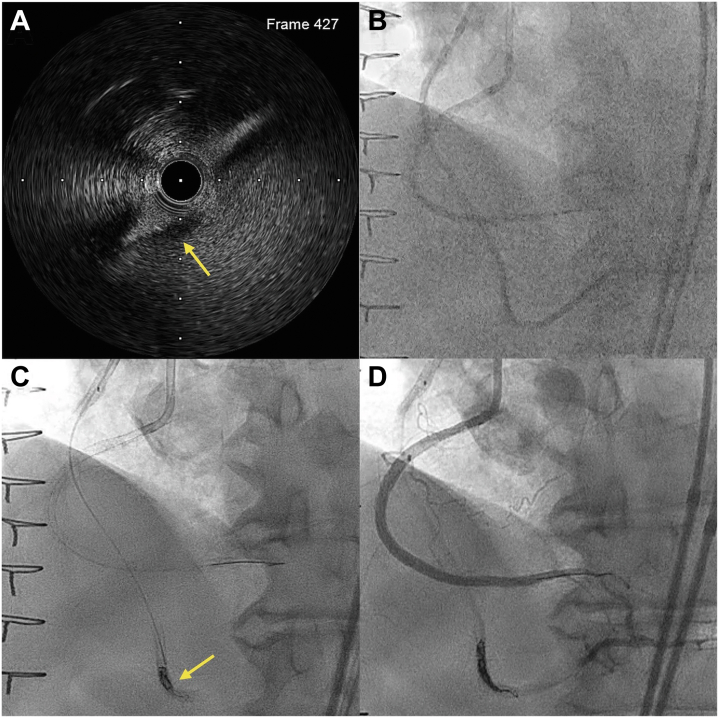
With recurrent failure of the SVG-PDA, native RCA chronic total occlusion (CTO) PCI was pursued. The flush-ostial occlusion and severe calcification of the RCA precluded an up-front antegrade approach. The SVG-PDA was engaged with a manually shortened (∼85 cm) 8-F multipurpose 1 guide catheter. Using a Pilot 200 wire (Abbott Vascular) and Corsair Pro XS microcatheter (Asahi Intecc), we obtained a retrograde position in the distal RCA. The distal cap was impenetrable and required angioplasty with a 1.5 × 20–mm balloon (Figure 2A) and exchange to a Caravel microcatheter (Asahi Intecc). The retrograde gear was advanced to the ostium of the RCA by knuckling the Pilot 200. Retrograde electrocautery to puncture into the aorta was attempted with an Astato XS 20 (Asahi Intecc) and Caravel, at 50 W for 1 second in cut mode, but this was unsuccessful (Figure 2B). The microcatheter tip was damaged in the process (Figure 2C), and the microcatheter was exchanged for a Corsair Pro XS. To facilitate controlled antegrade retrograde tracking (CART), retrograde angioplasty was performed at the ostial RCA with a Trek over-the-wire 3.0 × 20–mm balloon (Abbott Vascular), which has a 145-cm shaft length (Figure 2D). Using a 6-F internal mammary artery guide, antegrade puncture with an Astato XS 20 was unsuccessful. Subsequently, successful retrograde wiring into the aorta could be performed with an Astato XS 20 (Figure 2E). The retrograde microcatheter was advanced into the aorta, and an R350 wire (Teleflex) was snared within a 7-F Judkins Right 4 guide catheter using an 18- to 30-mm EnSnare (Merit Medical) (Figure 2F). Intravascular ultrasound was performed to inform lesion preparation and showed an almost entirely extraplaque track. Of note, the entry point of the retrograde wire into the aorta was through the aortocoronary extraplaque space (“neo-ostium”) (Figure 3A). The vessel was predilated with a 2.5 × 40–mm semicompliant balloon. Three drug-eluting stents (4 × 48, 3.5 × 48, and 3 × 38 mm) were implanted from the ostial RCA to the RPL. Competitive flow was observed via the SVG-PDA graft (Figure 3B). Therefore, 4 Axium Prime coils (5 × 15 mm, Medtronic) were deployed in the mid-to-distal shaft of the graft (Figure 3C). Final angiography of the RCA showed TIMI flow grade 3 and no residual stenosis or dissection (Figure 3D).
Figure 2.
Retrograde Recanalization of the Native Right Coronary Artery Chronic Total Occlusion
(A) Impenetrable distal cap: dilatation with a 1.5 × 20–mm balloon. (B) Electrocautery-assisted re-entry (E-CART) at the right coronary artery ostium. (C) Damaged Caravel tip (Asahi Intecc) after E-CART. (D) Retrograde balloon angioplasty of the right coronary artery ostium with a 3.0 × 20–mm Trek over-the-wire balloon. (E) Retrograde puncture of the aorta with an Astato XS 20 (Asahi Intecc) (arrow). (F) Snaring of an R350 wire with an 18- to 30-mm EnSnare (Merit Medical).
Figure 3.
Right Coronary Artery Stenting and Saphenous Vein Graft Sacrifice
(A) Extraplaque location of the retrograde wire through the aortocoronary junction, creating a “neo-ostium.” Arrow indicates dissection flap. (B) Competitive TIMI flow grade 3 via saphenous vein graft-posterior descending artery. (C) Coiling of the saphenous vein graft-posterior descending artery with 4 Axium Prime coils (Medtronic) (arrow). (D) Final result.
Discussion
For patients undergoing coronary artery bypass graft, multiple arterial grafting is recommended over vein grafts to improve long-term cardiac outcomes.1 In the setting of failing vein grafts, PCI carries an increased risk of periprocedural myocardial infarction, mortality, and restenosis.2 Therefore, PCI of the native artery is often considered in the setting of vein graft failure. PCI of the native artery instead of PCI of the bypass graft carries a Class IIa recommendation according to current guidelines.3 In our case, the diseased SVG-PDA had required 5 prior interventions, indicating minimal likelihood of long-term patency. Importantly, none of the prior stents was placed across the graft anastomosis into the native vessel, which would have made subsequent native CTO PCI much more challenging and less likely to succeed. Therefore, we decided to recanalize the native RCA.
The hybrid algorithm is widely used in current practice to standardize the approach to CTO PCI and improve success rates and procedural efficiency.4 In the setting of proximal cap ambiguity, and particularly in the case of flush-ostial occlusion, as in our case, a retrograde approach is favored.5
Long occluded segments are usually navigated using the knuckle wire technique, and true lumen re-entry is commonly achieved with reverse CART.6 Aorto-ostial occlusions represent a particularly hostile lesion subset, with pronounced calcification hampering antegrade and retrograde guidewire penetration. In this setting, retrograde electrocautery-assisted re-entry (E-CART) into the aorta has recently been described.7 This involves the electrification of a retrograde high–tip-load guidewire to enhance its penetration power. This proved unsuccessful in our case, and a combination of retrograde ballooning of the ostium and wiring with a highly penetrative wire allowed entry into the aorta through an extraplaque route, thus creating a neo-ostium (Figure 3A).8 This highlights the need of a comprehensive skillset for a CTO operator, given that no one technique, including electrocautery, is ubiquitously successful. In cases where a neo-ostium is created, intravascular ultrasound is invaluable in confirming an extraplaque track at the ostium, and antegrade contrast injections should be avoided to prevent dissection extension into the aortic root.
Another salient point of our case was the decision to sacrifice the SVG-PDA graft. Graft occlusion (with coils or plugs) has recently been proposed following native artery CTO recanalization in select cases, as competitive graft flow can promote the disruption of laminar flow, vascular dysfunction, and hence stent restenosis and thrombosis. Dautov et al9 proposed coiling the graft if it supplies significant competitive flow (TIMI flow grade 2 or 3). This approach has demonstrated low rates of native vessel restenosis at short-term follow-up in a small study.10 However, the literature on this topic is limited by the lack of randomized trials and the fact that the evidence derives from small observational cohorts with no control group. Larger studies are needed for validation of this approach before widespread adoption.
Follow-Up
The patient had no periprocedural complications and was discharged the following day. At the 4-month follow-up, he was free of angina and without adverse cardiovascular events.
Conclusions
Aorto-ostial flush occluded vessels are challenging to recanalize and often necessitate a retrograde approach. A variety of techniques, including E-CART, retrograde angioplasty at the aorto-ostial junction, and extraplaque wiring with the creation of a neo-ostium, might be required. Graft sacrifice can be considered in the case of competitive flow to prevent reocclusion of the native artery, although further studies are needed to provide data on the long-term outcomes of this approach.
Funding Support and Author Disclosures
Dr Kearney has received consulting fees from Abiomed, Abbott Vascular, Boston Scientific, Medtronic, Teleflex, Philips, and Cardiovascular Systems, Inc. Dr Lombardi has received consulting fees from Asahi Intecc, Abiomed, Boston Scientific, Medtronic, Siemens, and Teleflex; has received royalties from Asahi Intecc; and his spouse is a Philips employee. Dr Azzalini has received consulting fees from Teleflex, Abiomed, GE Healthcare, Asahi Intecc, Philips, Abbott Vascular, Reflow Medical, and Cardiovascular Systems, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Redfors B., Généreux P., Witzenbichler B., et al. Percutaneous coronary intervention of saphenous vein graft. Circ Cardiovasc Interv. 2017;10(5) doi: 10.1161/CIRCINTERVENTIONS.117.004953. [DOI] [PubMed] [Google Scholar]
- 3.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 4.Brilakis E.S., Grantham J.A., Rinfret S., et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. J Am Coll Cardiol Intv. 2012;5(4):367–379. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Ojeda S., Luque A., Pan M., et al. Percutaneous coronary intervention in aorto-ostial coronary chronic total occlusion: outcomes and technical considerations in a multicenter registry. Rev Esp Cardiol (Engl Ed) 2020;73(12):1011–1017. doi: 10.1016/j.rec.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Tajti P., Xenogiannis I., Gargoulas F., et al. Technical and procedural outcomes of the retrograde approach to chronic total occlusion interventions. EuroIntervention. 2020;16(11):e891–e899. doi: 10.4244/EIJ-D-19-00441. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson W., Harvey J., Dhawan R. E-CART (Electrocautery-Assisted Re-entry) of an aorto-ostial right coronary artery chronic total occlusion: first-in-man. J Am Coll Cardiol Intv. 2016;9(22):2356–2358. doi: 10.1016/j.jcin.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida R., Takagi K., Morita Y., Morishima I. Right coronary artery neo-ostium created via retrograde wire crossing. J Am Coll Cardiol Intv. 2019;12(19):e163–e165. doi: 10.1016/j.jcin.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Dautov R., Manh Nguyen C., Altisent O., Gibrat C., Rinfret S. Recanalization of chronic total occlusions in patients with previous coronary bypass surgery and consideration of retrograde access via saphenous vein grafts. Circ Cardiovasc Interv. 2016;9(7) doi: 10.1161/CIRCINTERVENTIONS.115.003515. [DOI] [PubMed] [Google Scholar]
- 10.Kostantinis S., Simsek B., Karacsonyi J., et al. Saphenous vein graft occlusion following native vessel chronic total occlusion percutaneous coronary intervention. J Invasive Cardiol. 2022;34(12):E836–E840. doi: 10.25270/jic/22.00221. [DOI] [PubMed] [Google Scholar]