Abstract
Background
The gold standard treatment for Anterior Cruciate Ligament injury is reconstruction (ACL-R). Graft failure is the concern and ensuring a durable initial graft with rapid integration is crucial. Graft augmentation with implantable devices (internal brace reinforcement) is a technique purported to reduce the risk of rupture and hasten recovery. Few studies have examined these techniques, in particular when compared to non-augmented grafts. This study assesses the short-term outcome of ACL-R using augmented and non-augmented hamstring tendon autografts.
Methods
This was a retrospective cohort study comparing augmented and non-augmented ACL-R. All procedures were performed in a single centre using the same technique. The Knee injury and Osteoarthritis Outcome Score [KOOS] was used to assess patient-reported outcomes.
Results
There were 70 patients in the augmented and 111 patients in the control group. Mean graft diameter in the augmented group was 8.82 mm versus 8.44 mm in the non-augmented. Six strand graft was achievable in 73.5% of the augmented group compared to 33% in the non-augmented group. Two graft failures were reported in the non-augmented group and none in the augmented group. Patient satisfaction rates were higher in the augmented group. There was a statistically insignificant improvement in the postoperative KOOS in the augmented group compared to the non-augmented group (p 0.6). Irrespective of augmentation status, no correlation was found between the functional score and age, or femoral tunnel width.
Conclusion
No statistically significant difference was demonstrated in the short-term functional outcome of ACL reconstruction using an augmented or non-augmented hamstring graft. Augmented ACL-R may achieve superior graft diameters, failure rates and patient reported outcomes when compared to nonaugmented ACL-R. Prospective trials are needed to examine this further.
Keywords: Short-term outcomes, Anterior cruciate ligament reconstruction, Internal brace, Hamstring autograft
1. Introduction
Injuries to the anterior cruciate ligament (ACL) are common, with a rising incidence.1 Although ACL repair techniques have been introduced recently, the gold standard surgical management is ACL reconstruction (ACL-R).2 Over 300,000 ACL-R's are undertaken annually in United states, while in England the rate of ACL- R increased twelve-fold between 1997 and 2017.1 ACL-R aims to maximise knee stability, thus allowing a return to normal function and sporting activity.3,4 Successful outcome following ACL-R varies between 75 and 97% but the likelihood of a return to a preinjury level of sports is only 65%.5, 6, 7
Graft failure following ACL-R is a major concern, particularly for young patients and athletes. Current failure rates vary from 10% to 15% and are primarily due to technical errors such as nonanatomic tunnel placement, trauma and biologic causes.5,8 Revision ACL-R carries the added risk of further autograft harvest and donor site morbidity, the possible need for allograft and suboptimal outcomes when compared to primary reconstruction.5,8 The emphasis is placed therefore, on ensuring an optimally seated, strong, durable initial graft with rapid integration.
Graft augmentation with synthetic devices (Internal Bracing) has been proposed as a method of reducing graft failure and ACL-R failure rates, while simultaneously allowing accelerated rehabilitation.9 These internal brace augmentation techniques have been described extensively in other areas of orthopaedics with strong evidence supporting their use, but few have examined outcomes in ACL-R.10 We aim to investigate the short-term functional outcome of ACL-R using Infinity-Lock neoligament (Infinity-Lock Button System, Xiros, Leeds, UK) augmented, compared to non-augmented hamstring tendon auto-grafts.
2. Materials and methods
This was a retrospective cohort study comparing augmented and non-augmented ACL grafts. All procedures were performed by or under the supervision of a single surgeon in a single centre using the same technique. Institutional research board approval was obtained for this study [reg number: S125 20/21]. Cases were retrospectively collated using electronic theatre logs [Opera system, GE Health care, Chicago, United States].
Inclusion criteria were patients with age range from 18 to 50, both gender, ACL rupture confirmed on pre-operative MRI scans and successful completion of rehabilitation. Only partial meniscectomy patients with an intact rim [less than 50% of the meniscus removed] were included if the patient had a meniscal resection along with ACL-R. An intact meniscus rim and more than 50% retained meniscus ensure its functionality and hence less chance for early arthritis.11 Nonprofessional sportspeople irrespective of type of sports included and professional and elite athletes are excluded as they often follow accelerated rehabilitation.
Exclusion criteria included age outside the 18–50 range, associated ipsilateral injury including fracture, multi-ligament injury, osteochondral fracture, previous ipsilateral ACL-R, concurrent or previous total or sub-total meniscectomy due to the risk of early arthritis11 [when more than 50% of the meniscus is removed] and concurrent meniscal repair as it changes the rehabilitation protocol. All patients were evaluated preoperatively with radiographs and magnetic resonance imaging (MRI) by specialist musculoskeletal (MSK) radiologists to exclude associated injury.
As the surgeon changed his practice from non-augmented to augmented ACL grafts from January 2019 onwards, we divided patient groups into two cohorts: the non-augmented graft cohort before January 2019 and the augmented graft group from January 2019 onwards, with no differences in patient selection criteria. All patients were followed up at 12 months in the face-to-face clinic and later with a KOOS questionnaire. As it was a retrospective study, we could not blind patients or surgeon; however, the independent researcher who did the outcome analysis was blinded to patient demography and the type of graft used. Graft failure was defined as persistent clinical instability resulting from ruptured, lax, or otherwise incompetent graft confirmed on MRI reported by a specialist MSK radiologist.
2.1. Surgical technique
Each patient received an examination under anaesthesia to identify any missed associated injury. Following harvest, a diagnostic arthroscopy and ACL-R using a three-portal technique was undertaken.
Graft Harvest and Preparation: A mix of 1 in 200,000 adrenaline and 1% xylocaine was infiltrated to the graft incision site. Ipsilateral gracilis and semitendinosus harvest was undertaken using a Pigtail Hamstring Tendon Stripper (Arthrex GmbH, Munich, Germany). The graft was stripped of debris, placed in a vancomycin-soaked, damp swab, and tensioned at 15 pounds, while diagnostic arthroscopy and/or partial meniscectomy is undertaken. Heavy, braided non-absorbable suture (FiberWire and TigerWire; Arthrex GmbH, Munich, Germany) was used to secure the graft ends. For additional traction and the creation of 6 bundle graft, fibre wire was attached to the graft ends [Fig. 1]. The graft was looped to 6 strands through the Endobutton Fixation System (Smith & Nephew, London, UK) which is used as the conduit for traction and fixation [Fig. 1]. We used 20 mm Endobutton for every patient.
Fig. 1.
Augmented graft preparation. Image 1, six strand hamstring graft (A), Infinity-Lock neoligament (B) and fibre tape.
For the augmented group, an Infinity-Lock neoligament (Infinity-Lock Button System, Xiros, Leeds, UK) was then looped through the Endobutton, after which the construct was streamlined using a 2.0 undyed vicryl suture to ensure full contact of the neoligament to the graft and host tissue [Fig. 1]. The surgical technique for the non-augmented group was identical but excluded the use of any internal brace. Here the autografts were looped through the endobuton to create a six-strand construct which was then immediately streamlined with a 2.0 vicryl suture.
ACL-R: The three-portal technique (standard anterolateral, anteromedial, and high medial portals) was used for femoral and tibial preparation. The standard anteromedial and anterolateral portals to start with, and then the scope switched to the high medial portal for visualisation of the lateral wall and anteromedial portal used for instrumentation. A Guidewire was passed to the ACL femoral footprint, followed by over-drilling with a cannulated 4.5 mm drill to facilitate passage of the endo button. Further drilling of the femoral tunnel about the size of the graft diameter stopped 10–15 cm from the femur far cortex to flip the endobutton. A wire is drilled into the ACL tibial footprint from the anteromedial tibia through the graft harvest wound using the AcuFix guide at 50–70° (ACUFEX™ Director Drill Guide, Smith & Nephew, London, UK), and the tibial tunnel was drilled in keeping with the graft size. The graft was then pulled through the tibial tunnel and held at the femoral outer cortex with the flipped endo button and supplementally fixed at the aperture by bone wedge technique [anterior to the femoral tunnel with the graft, a divot is made with a small osteotome leaving a bony bridge and a bioabsorbable shim wedge is tapped into this divot for aperture fixation (Megashim™, Biovision GmbH, Ilmenau, Germany). The bony bridge between the shim and graft ensures no damage caused to the graft. The graft was cycled ten times and then fixed at the tibial tunnel with an interference screw (Biosure, Regenesorb, Smith & Nephew, London, UK). A further EUA is completed before closure ensuring stability.
Post-operative care: Thromboprophylaxis consisted of Aspirin for two weeks. The post-operative rehabilitation programme did not differ between groups and was a dedicated ACL-R regimen under the care of a specialist physiotherapist. Rehabilitation milestones were checked ta 2 weeks, 6 weeks, 12 weeks, and 6 months for all patients.
All the cases are planned day-case surgeries. On discharge, we aim for 90° knee flexion, full knee extension, independently mobile with 2x elbow crutches and independently ascend/descend the stairs as required. In the first two weeks, we will focus on stage 1 exercises and open chain knee exercises are avoided [ankle range of movements, static quadriceps strengthening, static gluteal exercises, closed chain knee exercises, straight leg raise to 6 inches from the bed, patella mobilisations, hamstring, and calf muscle stretch exercises], weight-bearing allowed as tolerated with two elbow crutches. At two weeks, we aim to achieve full knee hyperextension (compared to contralateral leg), ≥90° knee flexion, full weight bearing unaided, and straight leg raise with no lag. Between 2 and 6 weeks, stage 2 exercises will be started with open chain exercises [controlled single leg drops, half wall sits, half lunges and step-ups, single leg balance and standing heel raise], gradually discontinue crutches, and return to nonmanual work and driving. At six weeks, we aim to achieve independently mobile unaided, full range of hip, knee, and ankle movements, SLR without lag, hyperextension comparable to the opposite knee and double leg half squats with equal weight bearing. From 6 to 12 weeks, we will start stage 3 exercises [single leg dips, single leg sits to stand, full lunges, drop jumps and hamstring strengthening. Beyond 12 weeks, stage 4 exercises [through range quadriceps, dynamic lunges and jumping or hoping, squat jumps, lunge jumps, resisted hamstring exercises and running], return to manual work. Sports are allowed from 6 to 9 months.
2.2. Patient reported outcomes
Once cases were identified, both groups of patients were sent a Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire, which has been validated for use in ACLR.11 At final face to face follow up for all patients an overall satisfaction rating was taken and graded as excellent, good, fair, or poor. KOOS completion was done at or following final follow up depending on patients’ availability or preference.
2.3. Statistical analysis
Results were collated and analysed using Microsoft Excel (Microsoft 365, 2019) and statistical analysis was conducted using Statistica 13.1 software. Statistical significance was set as P value less than 0.05. Descriptive statistics of categorical data were summarised by total number and missing data number, whilst for continuous variables mean, median, standard deviation (SD), minimum, maximum, lower, and upper interquartile range (IQR) were presented. The data distribution was assessed with Shapiro-Wild test. Categorical variables were compared with Chi-square test and the continuous variables were compared with Mann Whitney-U Test with continuity correction. Spearman rank correlation was used for correlation assessment. Demographics were compared using the unpaired T-test.
3. Results
All cases were operated between January 2016 and March 2020. Seventy (n70) cases were included in the augmented group, of which 18 were females (26%) and 52 were males (74%). The mean age was 34.5 years (range 19–50). The average body mass index (BMI) was 27.7 (range 21–42.6). Mean follow-up was 18 months (range 14–25 months). A total of 111 cases were included in the non-augmented group, of which with 23 were female (21%) and 88 were male (79%). The mean age was 30.4 (range 18–55) and the mean BMI was 28.5 (range 20.1–43.9). The mean follow-up was 20 months (range 16–25 months).
Data on the number of strands the graft achieved was not available in four of the augmented cases. However, a graft diameter over 8 mm was achieved in both groups and augmented grafts were on average 0.4 mm thicker and achieved a higher rate of six strands when compared to non-augmented grafts (p = 0.001) [Table 1]. The femoral tunnel width, an indirect measure of the graft width, was larger in the augmented group (mean 8.8 versus 8.4; p = 0.0006) [Table 2].
Table 1.
Graft characteristics.
| Augmented | Non-Augmented | |
|---|---|---|
| 4 Strands | 5 [7.4%] | 44 [39.6%] |
| 5 Strands | 11 [16.2%] | 30 [27%] |
| 6 Strands | 50 [73.5%] | 37 [33.3%] |
| Average diameter (mm) | 8.82 [range 7–11] | 8.44 [range 6.5–10] |
Table 2.
Mann-Whitney U test demonstrating femoral tunnel width. Femoral tunnel width (FTW).
| Augmented | Non-augmented | Rank Sum Augmented | Rank Sum Non-augmented |
U | P value | |
|---|---|---|---|---|---|---|
| FTW | 111 | 70 | 10,606 | 8309 | 3103 | 0.0006 |
Post-operative complications included graft failure (n2, both of which were in the non-augmented group), persistent instability, chronic pain (>3 months duration), and hematoma [Table 3]. All failures resulted from poor compliance with physiotherapy (early return to sport) and were revised using augmented hamstring graft from the contralateral knee. The hematoma in the non-augmented group was found at the graft donor site and required washout. Cases with persistent instability excluding graft failure (defined as lack of confidence and fear of subluxation or patient reported mechanical symptoms) were treated with rehabilitation. The complication rates grossly favoured augmented group (2 versus 14 cases). This was not statistically significant for total complications (p = 0.416) or failure rate (p = 0.258), possibly due to a low sample size [Table 3].
Table 3.
Number of post-operative complications.
| Augmented | Non-Augmented | |
|---|---|---|
| Post-operative instability | 1 [1.4%] | 7 [6.3%] |
| Pain | 1 [1.4%] | 4 [3.6%] |
| Hematoma | 0 | 1 [0.90%] |
| Graft failures | 0 | 2 [1.8%] |
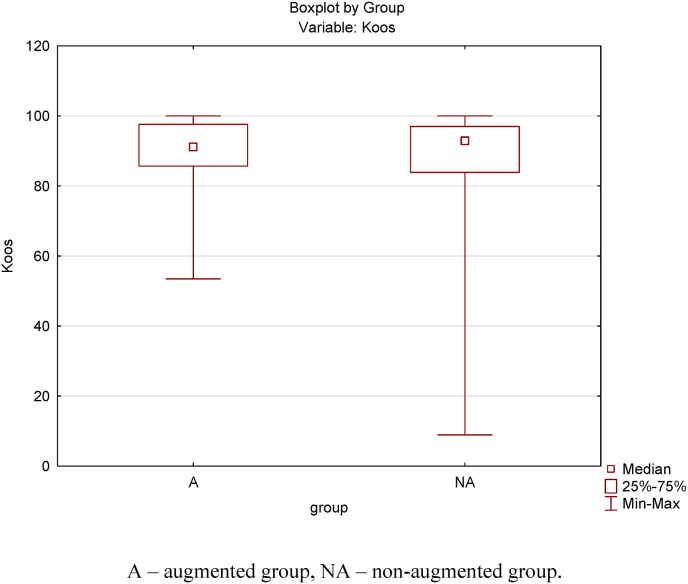
In total 44 patients (63%) in the augmented group and 47 (42%) in the nonaugmented group responded to the KOOS questionnaire. All completed the questionnaire within 4 weeks of receipt. Patient satisfaction were better in the Augmented group, with a larger percentage of responders rating their satisfaction as good or excellent [Table 4]. The average KOOS (five subcategories scored 0 to 100; where 0 represents major problems and 100 represents no problems) in the augmented group was 90.7 versus 85.9 in the non-augmented group [Fig. 2]; this was not statistically significant (p = 0.623). A Box plot analysis demonstrated comparable functional score in both groups [Fig. 3]. No correlation between KOOS and age, or femoral tunnel width (an indirect measure of graft size) was found [Table 5].
Table 4.
Patient satisfaction rate.
| Augmented | Non-Augmented | |
|---|---|---|
| Excellent | 49 [70%] | 57 [51.3%] |
| Good | 20 [28.6%] | 41 [36.9%] |
| Fair | 01 [1.4%] | 09 [8.1%] |
| Poor | 00 | 04 [3.6%] |
Fig. 2.
Bar diagram demonstrating mean KOOS. QOL = Quality of life.
Fig. 3.
Boxplot diagram representing KOOS comparison. Note that the IQR (25–75%) and the median for both groups are.
Table 5.
Spearman Rank Order Correlation between KOOS and other variables.
| Variable 1 | Variable 2 | r Cumulative | r Augmented | r Non-Augmented |
|---|---|---|---|---|
| KOOS | Age | 0.03 | −0.13 | 0.11 |
| KOOS | FTW | −0.14 | −0.04 | −0.26 |
4. Discussion
Anterior cruciate ligament tears are common injuries.1 Optimal treatment consists of dedicated physiotherapy and reconstruction, however some patients suffer ruptures of the graft and inconsistent return to sports.5,12, 13, 14, 15, 16 Failure rates are up to 18% in high-risk activities and as high as 25% in patients 21 years old and younger.13,14 Early return to sports, family history of ACL injury and the use of allografts are other factors associated with re-rupture.14 Revision ACL-R is carried out on a third of these patients and is associated with poorer outcomes, higher complication rates, donor site morbidity including contralateral knee insult, the use of allograft and higher rates of associated soft tissue knee trauma.5,8
Strategies to reduce the risk of re-rupture are directed at the patient and the surgeon. Improper or non-compliance with rehabilitation and premature return to activity are common problems. Graft strength reduces during the revascularisation phase of healing, hence early rehabilitation should be guarded to reduce the risk of graft laxity, instability and failure.17, 18, 19 Delaying return to sport for up to 2 years significantly reduces the risk of reinjury, improves graft incorporation, functional outcome and neuromuscular control.14,19 This is especially beneficial in adolescents and with the use of hamstring grafts where the graft remodelling phase occurs between 12 and 24 months compared to the 6 and 12 months seen with bone-patella-bone grafts.19 However, a delay in return to sport is undesirable for the typical ACL injury demographic and in the professional athlete where livelihoods are at stake.
Multiple ACL-R techniques exist and are varied in terms of graft choice and graft augmentation with synthetic devices.20, 21, 22 Autografts have a lower failure rate, superior outcomes and an earlier return to sports when compared to allografts and do not carry the risk of disease transmission.6 Technical factors related to graft failure include tunnel malposition, suboptimal graft tensioning, poor fixation, mechanical overload due to a missed concomitant knee injury and graft attrition.20, 21, 22 Extraarticular procedures such as anterolateral ligament reconstruction have evolving indications but are gaining in popularity and aim to improve failure rates.23 Graft diameter of 8 mm or more is a crucial consideration in failed ACL-R. The risk of attrition failure is reduced by a factor of seven or more when quadruple grafts achieve this threshold and is especially noted in the younger patient.24 Methods to increase graft diameter include achieving higher number of bundles by harvesting multiple tendons with maximal length and graft augmentation.20, 21, 22 These have included augmentation using synthetics, biologic materials including allograft and additional autograft.25, 26, 27, 28
Following their introduction in the 1980s, isolated synthetic grafts were found to be associated with effusion, high failure rates, pain and often required explantation.10,29 In knee surgery, earlier studies reported synovitis and high failure rates which limited the use of synthetics in the field.29 However, the use of synthetics in conjugation with biological material elsewhere in orthopaedics demonstrated superior outcomes, which has maintained their use in clinical practice.9,30,31 Gilmer et al. noted the comparable outcome of suture tape augmentation to allograft reconstruction and its superior results than isolated ligament repair.32 Similarly, Yoo et al. studied suture tape augmentation of anterior talofibular and calcaneofibular ligament reconstruction and found excellent results, with better function and return to sports.33
The arguments in favour of graft augmentation vary from an improvement in diameter, the promotion of ligamentisation, improved biomechanics including greater tensile strength, and better on table handling and manoeuvrability. Graft diameters rapidly reduce within the first two weeks post-operatively further adding to the importance of maximising graft size.34 In our study, graft diameter was increased by 0.4 mm on average in the augmented group, with sizes consistently above 8 mm. Recent studies reported improved biomechanical parameters in augmented grafts including higher load and energy to failure and greater graft stiffness.35
LARS (Ligament Augmentation and Reconstruction System) augmented hamstring grafts have been reported with improved functional scores, patient satisfaction and laxity measurements however it is associated with persistent effusion and synovitis.9,10,28,30,31 Ebert et al. reported 2-year follow-up study with high mean patient reported outcome measures (PROM) and satisfaction scores using LARS augmentation.28 They reported 100% patient satisfaction, 76% return to preinjury sports in a year and 86% returned to sports in 2 years. Our study reports 1.8% graft failures and 6.3% instability in the non-augmented group versus no graft failures and 1.4% risk of instability in the augmented group, none of which required revision. Hence, we utilised the benefits of LARS without causing synovitis or effusion. Furthermore, our results show excellent patent perception and PROMS at short term follow up. The potential clinical advantages of ACL augmentation has also been demonstrated with allografts and ACL repair techniques in paediatric setting.36
Concerns with augmentation relate to expense, indications for use, complexity of revision and the lack of data on the natural history of ligamentisation in absorbable or non-absorbable augments. Neoligament devices function as scaffold for ligamentisation which is crucial for the maturation and incorporation of the graft, however there is controversy as to the effects of these augments over time. Animal models have shown that tissue ingrowth is accelerated by the scaffolding properties of the graft and a total coverage of the neoligament is achieved at 24 weeks.37 However, areas of an augment devoid of direct contact with native tissue are subject to significant tearing and global weakening at 1 year postoperatively increasing the chances of catastrophic failure.38 This may be especially true for woven nonabsorbable terephthalic polythene polyester augments such as LARS and the Infinity-Lock, which initially promote tissue integration, but may block ligamentisation at its later stages.39 Perfecting the technique to ensure total contact of the augment to host tissue is therefore critical [Fig. 1].
There are no publications to our knowledge that compare absorbable augments or scaffold devices with non-absorbable devices in ACL-R. It may be that absorbable augments maximise the initial gains in tissue integration while allowing complete ligamentisation. The scaffold properties of these devices are not only ideal for host on growth, but also bacterial colonisation. There are reports of chronic indolent infection and chronic immune responses to the chemical composition of these devices.40 Thorough surgical antisepsis, the use of topical antibiotics and systemic prophylaxis in addition to careful preoperative planning including an immune history must be considered by the surgeon. Another area of controversy relates to the indications of augmented ACL-R. The lack of laxity of neoligament, introduced to improve graft characteristics during the healing and integration phase, may be an area of concern in the paediatric setting where the potential for skeletal growth must be considered. Some have therefore advocated the use of augments only in the adult population, in particular the elderly and in those seeking a rapid recovery.41
Ours is among the few studies which investigate the outcome of implantable device augmentation in hamstring ACL grafts. The limitations of this study include the lack of randomisation and its retrospective nature. Other limitations include the short follow up of patients, and low rates of KOOS completion. Nonetheless, this study adds to the evidence in support of graft augmentation in ACL-R.
5. Conclusion
No statistically significant difference was demonstrated in the short-term functional outcome of ACL reconstruction using an augmented or non-augmented hamstring graft. Augmented ACL-R may achieve superior graft diameters, less failure rates, stability and patient satisfaction compared to non-augmented ACL-R. Prospective trials are needed to examine this further.
Conflict of interest
None.
Funding/sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Institutional ethical committee approval
Obtained.
Author statement
Rahul Mohan: Conceptualization, Methodology, formal analysis, investigation, Writing- original draft, review and editing, visualisation, and project administration. Tariq Kwaees: Methodology, formal analysis, and Writing-review & editing. Terin Thomas: Methodology, formal analysis, investigation, Visualisation, Writing-review & editing. Ravi Pydisetty: Conceptualization, Methodology, formal analysis, Writing- Reviewing and Editing. Conflict of interest: Each author certifies that he or she has no associations that might pose a conflict of interest in connection with the submitted article.
Acknowledgements
None.
References
- 1.Abram S.G.F., Price A.J., Judge A., Beard D.J. Anterior cruciate ligament (ACL) reconstruction and meniscal repair rates have both increased in the past 20 years in England: hospital statistics from 1997 to 2017. Br J Sports Med. 2020;54(5):286–291. doi: 10.1136/BJSPORTS-2018-100195. [DOI] [PubMed] [Google Scholar]
- 2.van der List J.P., Vermeijden H.D., Sierevelt I.N., et al. Repair versus reconstruction for proximal anterior cruciate ligament tears: a study protocol for a prospective multicenter randomized controlled trial. BMC Muscoskel Disord. 2021;22(1):1–10. doi: 10.1186/S12891-021-04280-Y/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber-Westin S.D., Noyes F.R. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(12):1697–1705. doi: 10.1016/J.ARTHRO.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Ardern C.L., Taylor N.F., Feller J.A., Webster K.E. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. doi: 10.1136/BJSPORTS-2013-093398. [DOI] [PubMed] [Google Scholar]
- 5.Bach B.R. Revision anterior cruciate ligament surgery. Arthrosc J Arthrosc Relat Surg. 2003;19(10 SUPPL. 1):14–29. doi: 10.1016/j.arthro.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 6.Baer G.S., Harner C.D. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661–681. doi: 10.1016/J.CSM.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Duchman K.R., Lynch T.S., Spindler K.P. Graft selection in anterior cruciate ligament surgery. Clin Sports Med. 2017;36(1):25–33. doi: 10.1016/j.csm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 8.George M.S., Dunn W.R., Spindler K.P. Current concepts review: revision anterior cruciate ligament reconstruction. Am J Sports Med. 2006;34(12):2026–2037. doi: 10.1177/0363546506295026. [DOI] [PubMed] [Google Scholar]
- 9.Falconer T.M., Tusak L., Breidahl W.H., Annear P.T. The LARS augmented 4-TUNNEL hamstring “hybrid” ACLR graft construction allows accelerated rehabilitation without knee laxity — case series of 111 patients after 2 years. J Musculoskelet Res. 2015;18(4) doi: 10.1142/S0218957715500207. [DOI] [Google Scholar]
- 10.Kumar K., Maffulli N. The ligament augmentation device: an historical perspective. Arthrosc J Arthrosc Relat Surg. 1999;15(4):422–432. doi: 10.1016/S0749-8063(99)70061-7. [DOI] [PubMed] [Google Scholar]
- 11.Roos E.M., Lohmander L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcome. 2003;1 doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardern C.L., Webster K.E., Taylor N.F., Feller J.A. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45(7):596–606. doi: 10.1136/bjsm.2010.076364. [DOI] [PubMed] [Google Scholar]
- 13.Nagelli C.V., Hewett T.E. Should return to sport be delayed until 2 Years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Med. 2017;47(2):221–232. doi: 10.1007/S40279-016-0584-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekker T.J., Godin J.A., Dale K.M., Garrett W.E., Taylor D.C., Riboh J.C. Return to sport after pediatric anterior cruciate ligament reconstruction and its effect on subsequent anterior cruciate ligament injury. J Bone Joint Surg Am. 2017;99(11):897–904. doi: 10.2106/JBJS.16.00758. [DOI] [PubMed] [Google Scholar]
- 15.Snaebjörnsson T., Hamrin-Senorski E., Svantesson E., et al. Graft diameter and graft type as predictors of anterior cruciate ligament revision. J Bone Jt Surg. 2019;101(20):1812–1820. doi: 10.2106/JBJS.18.01467. [DOI] [PubMed] [Google Scholar]
- 16.Paterno M.V., Rauh M.J., Schmitt L.C., Ford K.R., Hewett T.E. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. doi: 10.1097/JSM.0B013E318246EF9E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkerson L.A. Martial arts injuries. J Am Osteopath Assoc. 1997;97(4):221–226. doi: 10.7556/JAOA.1997.97.4.221. [DOI] [PubMed] [Google Scholar]
- 18.Pauzenberger L., Syré S., Schurz M. “Ligamentization” in hamstring tendon grafts after anterior cruciate ligament reconstruction: a systematic review of the literature and a glimpse into the future. Arthroscopy. 2013;29(10):1712–1721. doi: 10.1016/J.ARTHRO.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Vaquette C., Viateau V., Guérard S., et al. The effect of polystyrene sodium sulfonate grafting on polyethylene terephthalate artificial ligaments on in vitro mineralisation and in vivo bone tissue integration. Biomaterials. 2013;34(29):7048–7063. doi: 10.1016/J.BIOMATERIALS.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H.S., Seon J.K., Jo A.R. Current trends in anterior cruciate ligament reconstruction. Knee Surg Relat Res. 2013;25(4):165–173. doi: 10.5792/KSRR.2013.25.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paschos N.K., Howell S.M. Anterior cruciate ligament reconstruction: principles of treatment. EFORT Open Rev. 2016;1(11):398. doi: 10.1302/2058-5241.1.160032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahapatra P., Horriat S., Anand B.S. Anterior cruciate ligament repair - past, present and future. J Exp Orthop. 2018;5(1) doi: 10.1186/S40634-018-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DePhillipo N.N., Cinque M.E., Chahla J., Geeslin A.G., LaPrade R.F. Anterolateral ligament reconstruction techniques, biomechanics, and clinical outcomes: a systematic review. Arthroscopy. 2017;33(8):1575–1583. doi: 10.1016/J.ARTHRO.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Alkhalaf F.N.A., Hanna S., Alkhaldi M.S.H., Alenezi F., Khaja A. Autograft diameter in ACL reconstruction: size does matter. SICOT-J. 2021;7:16. doi: 10.1051/sicotj/2021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kdolsky R.K., Gibbons D.F., Kwasny O., Schabus R., Plenk H. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1–10. doi: 10.1002/JOR.1100150102. [DOI] [PubMed] [Google Scholar]
- 26.Berdis A.S., Veale K., Fleissner P.R. Outcomes of anterior cruciate ligament reconstruction using biologic augmentation in patients 21 Years of age and younger. Arthroscopy. 2019;35(11):3107–3113. doi: 10.1016/J.ARTHRO.2019.05.047. [DOI] [PubMed] [Google Scholar]
- 27.Dahlstedt L., Dalén N., Jonsson U. Goretex prosthetic ligament vs. Kennedy ligament augmentation device in anterior cruciate ligament reconstruction. A prospective randomized 3-year follow-up of 41 cases. Acta Orthop Scand. 1990;61(3):217–224. doi: 10.3109/17453679008993504. [DOI] [PubMed] [Google Scholar]
- 28.Ebert J.R., Annear P.T. ACL reconstruction using autologous hamstrings augmented with the ligament augmentation and reconstruction system provides good clinical scores, high levels of satisfaction and return to sport, and a low retear rate at 2 years. Orthop J Sport Med. 2019;7(10) doi: 10.1177/2325967119879079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batty L.M., Norsworthy C.J., Lash N.J., Wasiak J., Richmond A.K., Feller J.A. Synthetic devices for reconstructive surgery of the cruciate ligaments: a systematic review. Arthroscopy. 2015;31(5):957–968. doi: 10.1016/J.ARTHRO.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Lavoie P., Fletcher J., Duval N. Patient satisfaction needs as related to knee stability and objective findings after ACL reconstruction using the LARS artificial ligament. Knee. 2000;7(3):157–163. doi: 10.1016/S0968-0160(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 31.Hamido F., Al Harran H., Al Misfer A.R., et al. Augmented short undersized hamstring tendon graft with LARS® artificial ligament versus four-strand hamstring tendon in anterior cruciate ligament reconstruction: preliminary results. Orthop Traumatol Surg Res. 2015;101(5):535–538. doi: 10.1016/J.OTSR.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Lubowitz J.H., MacKay G., Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505–e508. doi: 10.1016/J.EATS.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo J.-S., Yang E.-A. Clinical results of an arthroscopic modified Brostrom operation with and without an internal brace. J Orthop Traumatol. 2016;17(4):353–360. doi: 10.1007/s10195-016-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astur DC, Pires D, Parente T, et al. Short Term Evaluation of the Hamstring Graft Diameter after ACL Reconstruction. doi:10.32098/mltj.01.2019.01.
- 35.Massey P., Parker D., McClary K., Robinson J., Barton R.S., Solitro G.F. vol. 77. Clin Biomech; Bristol, Avon): 2020. (Biomechanical Comparison of Anterior Cruciate Ligament Repair with Internal Brace Augmentation versus Anterior Cruciate Ligament Repair without Augmentation). [DOI] [PubMed] [Google Scholar]
- 36.Smith J.O., Yasen S.K., Palmer H.C., Lord B.R., Britton E.M., Wilson A.J. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845–1851. doi: 10.1007/S00167-016-4150-X. [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Ji G., Wang X., Kang H., Wang F. Biological and biomechanical evaluation of autologous tendon combined with ligament advanced reinforcement system artificial ligament in a rabbit model of anterior cruciate ligament reconstruction. Orthop Surg. 2018;10(2):144–151. doi: 10.1111/os.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viateau V., Manassero M., Anagnostou F., Guérard S., Mitton D., Migonney V. Biological and biomechanical evaluation of the ligament advanced reinforcement system (LARS AC) in a sheep model of anterior cruciate ligament replacement: a 3-month and 12-month study. Arthroscopy. 2013;29(6):1079–1088. doi: 10.1016/J.ARTHRO.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Chen T., Jiang J., Chen S. Status and headway of the clinical application of artificial ligaments. Asia-Pacific J Sport Med Arthrosc Rehabil Technol. 2015;2(1):15. doi: 10.1016/J.ASMART.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J., Xu J., Wang A., Zheng M. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expet Rev Med Dev. 2009;6(1):61–73. doi: 10.1586/17434440.6.1.61. [DOI] [PubMed] [Google Scholar]
- 41.Di Benedetto P., Giardini P., Beltrame A., Mancuso F., Gisonni R., Causero A. Histological analysis of ACL reconstruction failures due to synthetic-ACL (LARS) ruptures. Acta Biomed. 2020;91(4-S):136–145. doi: 10.23750/ABM.V91I4-S.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]