Abstract
We present assessment of chest pain patients by multiparametric dobutamine stress echocardiography to differentiate inducible ischemia with obstructive coronary artery disease and with no obstructive coronary artery disease. In addition to the classical regional wall motion abnormality, we illustrate how coronary flow velocity reserve by Doppler echocardiography assists diagnosing coronary microvascular dysfunction. (Level of Difficulty: Advanced.)
Key Words: coronary flow velocity reserve, coronary microvascular dysfunction, Doppler echocardiography, INOCA, IOCA, multiparametric stress echocardiography
Central Illustration
In this case series, we demonstrate the value of multiparametric stress echocardiography in differentiating the causes of chest pain owing to obstructive coronary artery disease (CAD) and ischemia with nonobstructive CAD (INOCA). Pharmacological stress echocardiography is a powerful functional test to detect inducible ischemia predominantly by assessing inducible regional wall motion abnormality (RWMA). Ultrasound-enhancing agents improved its diagnostic and predictive accuracy as well as increases investigators confidence in reporting.1, 2, 3, 4, 5 Recent Multi-Societal North American guidelines for evaluation and diagnosing patients with persistent stable chest pain and nonobstructive CAD, stated that stress echocardiography with the addition of coronary blood flow velocity reserve (CFVR) measurement may be reasonable to improve diagnosis of coronary microvascular dysfunction (CMD) and for estimating risk of major adverse cardiovascular events.6 The learning curve for assessing CFVR by Doppler echocardiography is onerous; however, once skills are acquired it provides a noninvasive, cost-efficient bedside test to the wider chest pain population. We demonstrate the added benefit of assessing RWMA combined with Doppler measurement of CFVR during contrast-enhanced stress echocardiography (in all our cases dobutamine stress echocardiography [DSE]) in diagnosing and designing management plan for patients with INOCA. For our cases, a General Electric-E9 platform was used with Sonovue ultrasound enhancing agent and a very low mechanical index.
Learning Objectives
-
•
To understand the role of contrast enhanced stress echocardiography in the detection of regional wall motion abnormality as a marker of inducible ischemia owing to occlusive and nonocclusive coronary artery disease.
-
•
To introduce the Doppler echocardiography–derived coronary flow velocity measurement technique as a noninvasive tool to diagnose coronary microvascular dysfunction.
-
•
To describe the subtypes of ischemia with nonobstructive coronary artery disease based on multiparametric stress echocardiography using ultrasound enhancing agents to detect new regional wall motion abnormality and measuring coronary blood flow velocity reserve by Doppler echocardiography.
Case Series
Patient 1
A 66-year-old man with few months history of effort angina was referred for DSE. He was on antihypertensive medication and aspirin. DSE was terminated at the end of protocol at 91% of age predicted maximal heart rate owing to progressive angina that resolved after 1 mg of intravenous metoprolol (40 μg/kg/min and 0.25 mg atropine in addition to handgrip exercise; rate–pressure product: 22,983 mm Hg·beats/min). The resting echocardiogram showed no RWMA; however, the CFV in the distal left anterior descending coronary artery (LAD) was very slow (10 cm/s), indicating severe proximal LAD stenosis. All segments of the left ventricle augmented well during low-dose but during peak stress the 5 apical segments became thin and dyskinetic (Figures 1 and 2, Video 1). Invasive coronary angiography (ICA) confirmed severe proximal LAD disease with TIMI flow grade 2 distally, as well as moderate proximal left circumflex coronary artery (LCX) and right coronary artery disease (Figure 3). The instantaneous percutaneous coronary intervention (PCI) to the LAD rendered the patient symptom free. The patient was recommended secondary prevention therapy.
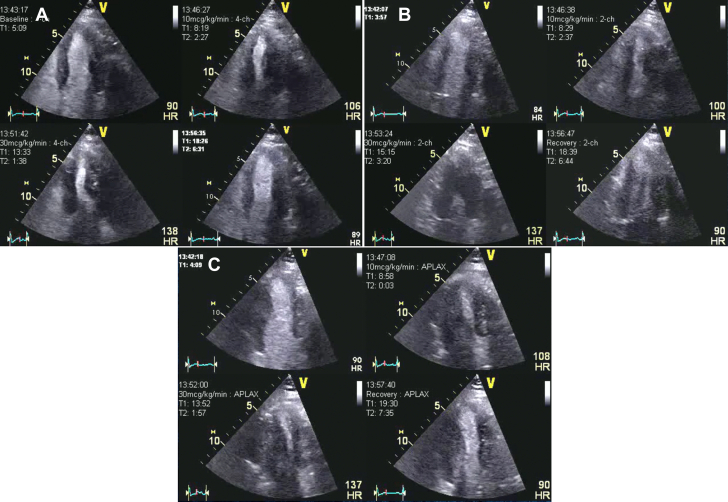
Figure 1.
Patient 1: Dobutamine Stress Echocardiography
Dobutamine stress echocardiography with ultrasound-enhancing agent. End-systolic frames at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent resting, low-dose, peak stress, and recovery stages of the protocol. The yellow arrows indicate extensive area of apical thinning and dyskinesis at peak stress in all the views (5/17 segments ischemia).
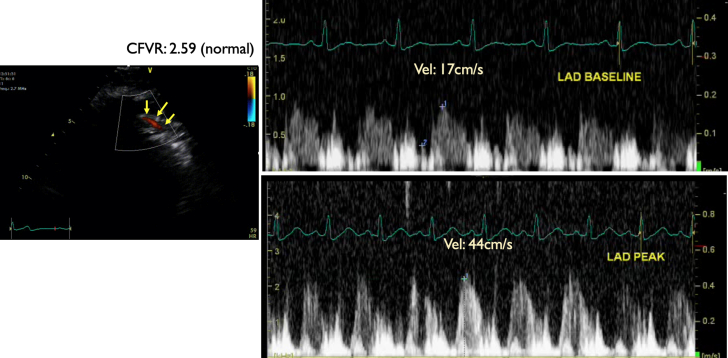
Figure 2.
Patient 1: Coronary Flow Velocity by Doppler Echocardiography
(A) Coronary flow velocity (CFV) by color Doppler echocardiography of the distal left anterior descending coronary artery. Yellow arrows indicate distal left anterior descending coronary artery color Doppler flow in diastole. (B) Doppler velocity was 10 cm/s at baseline, indicating very low distal flow, consequently severe proximal stenosis of the left anterior descending coronary artery.
Figure 3.
Patient 1: Invasive Coronary Angiography
Invasive coronary angiography showing severe proximal left anterior descending stenosis (yellow arrow) and moderate proximal left circumflex coronary artery disease (A) and moderate nonocclusive right coronary arteries (B).
Follow-up
One year after the successful LAD PCI, patient 1 was re-referred for sequential DSE to assess the bystander proximal LCX and right coronary artery disease. DSE was terminated at the end of protocol at 93% of age-predicted maximal heart rate with no symptoms (30 μg/kg/min and 0.10 mg atropine in addition to handgrip exercise; rate–pressure product, 16,159 mm Hg·beats/min). The resting echocardiogram showed no RWMA, and all segments of the left ventricle augmented well during low dose and remained hyperdynamic at peak. The CFV in the distal LAD was 17 cm/s and 44 cm/s at baseline and peak, respectively, in keeping with normal CFVR (2.59) (Figure 4, Figure 5, Figure 6, Video 2). The patient continued with secondary preventive therapy. A low major adverse cardiovascular event risk was concluded.
Figure 4.
Patient 1 Follow-Up: Dobutamine Stress Echocardiography
Dobutamine stress echocardiography with an ultrasound enhancing agent, end-systolic frames at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. One year after percutaneous coronary intervention to proximal the left anterior descending coronary artery with symptomatic improvement to assess bystander left circumflex coronary artery and right coronary artery stenosis.
Figure 5.
Patient 1 Follow-Up: Coronary Flow Velocity by Doppler Echocardiography
One year after percutaneous coronary intervention to proximal left anterior descending coronary artery (LAD) with symptomatic improvement to assess bystander left circumflex coronary artery and right coronary artery stenosis. Coronary flow velocity by Doppler echocardiography in the distal LAD 12 cm/s, indication very low distal flow, consequently severe proximal stenosis. Yellow arrows indicate distal LAD color Doppler flow in diastole. Coronary flow reserve of 44 cm/s/17 cm/s = 2.59 (normal).
Figure 6.
Patient 1 Follow-Up: Invasive Coronary Angiography
The patient underwent percutaneous coronary intervention to his proximal left anterior descending coronary artery with good results and symptomatic improvement (A). The bystander moderate proximal left circumflex coronary artery and right coronary artery disease (B) were treated with tight secondary preventative medications.
Patient 2
A 53-year-old man with history of LCX angioplasty for angina was referred for recurrence of typical chest pain for DSE. He was treated for hypertension and dyslipidemia with valsartan, rosuvastatin, and ezetimibe and was taking aspirin. His stress test was terminated at the end of protocol at 86% of age-predicted maximal heart rate owing to progressive angina that resolved after 2 mg of intravenous metoprolol (30 μg/kg/min and 0.75 mg atropine in addition to handgrip exercise; rate–pressure product, 24,215 mm Hg·beats/min). The resting echocardiogram showed no RWMA, and all segments of the left ventricle augmented well during low dose. During peak stress, the apical segment became thin and dyskinetic. The CFV in the distal LAD was 21 cm/s and 37 cm/s at baseline and peak, respectively, in keeping with a reduced CFVR (1.76) (Figures 7 and 8, Video 3). The ICA showed a patent LCX stent and nonocclusive coronary arteries (Figure 9). The patient was recommended secondary prevention and symptoms control with antianginal medication with calcium channel blockers and ranolazine for CMD.
Figure 7.
Patient 2: Dobutamine Stress Echocardiography
Dobutamine stress echocardiography with ultrasound-enhancing agent, end-systolic frames at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. The yellow arrows indicate small area of apical thinning and dyskinesis at peak stress in all the views.
Figure 8.
Patient 2: Coronary Flow Velocity by Doppler Echocardiography
Coronary flow velocity by Doppler echocardiography in the distal left anterior descending coronary artery (LAD) (yellow arrows indicate distal LAD color Doppler flow in diastole). LAD flow velocity reserve (CFVR) = baseline CFV/peak CFV: 37/21 = 1.76 (abnormal).
Figure 9.
Patient 2: Invasive Coronary Angiography
Invasive coronary angiography showing nonocclusive left (A) and right (B) coronary arteries with patent left circumflex coronary artery stent (yellow arrow).
Patient 3
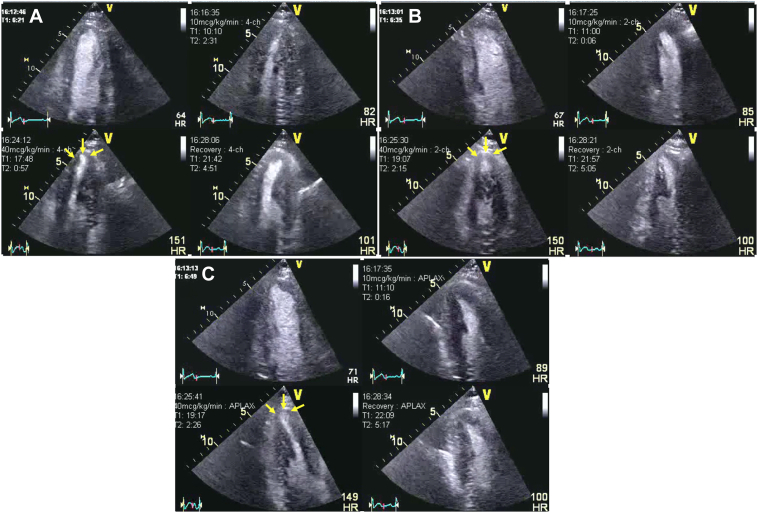
A 42-year-old man with a known family history of premature CAD and a current smoker was referred for ongoing typical chest pain for DSE after his ICA showed nonobstructive CAD (Figure 10). His DSE was terminated at the end of protocol at 85% of age-predicted maximal heart rate (at the end of 40 μg/kg/min and 1 mg atropine in addition to handgrip exercise). The rate-pressure product was 26,727 mm Hg·beats/min. At the peak test, the patient developed nonlimiting chest pain that was quick to resolve spontaneously in the recovery. The resting echocardiogram showed no RWMA, and all segments of the left ventricle augmented well during low dose. During peak stress the apical segment became thin and dyskinetic associated with the chest pain. The CFV in the distal LAD was 33 cm/s and 77 cm/s at baseline and peak, respectively, in keeping with a normal CFVR (3.35) (Figures 11 and 12, Video 4). The patient was recommended lifestyle modification and guideline-directed medical therapy. A diagnosis of coronary vasospasm was entertained.
Figure 10.
Patient 3: Dobutamine Stress Echocardiography
Dobutamine stress echocardiography with ultrasound enhancing agent, end-systolic frames at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. The yellow arrow indicates small area of apical thinning and dyskinesis at peak stress in all the views. The changes in apical kinesis can be more appreciated if the same regions are compared between low dose and peak stress.
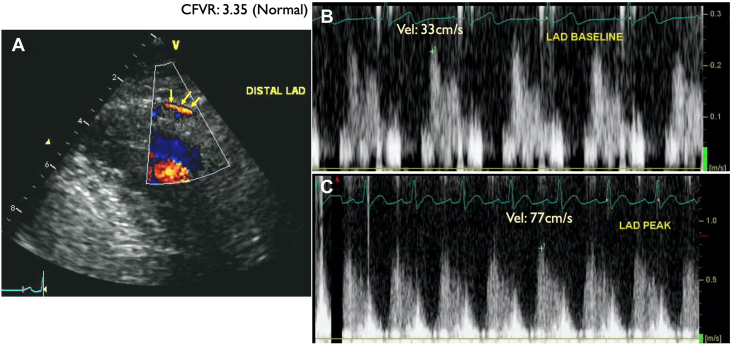
Figure 11.
Patient 3: Coronary Flow Velocity by Doppler Echocardiography
Coronary flow velocity by Doppler echocardiography in the distal left anterior descending coronary artery (LAD) (yellow arrows indicate distal LAD color Doppler flow in diastole). LAD flow velocity reserve (CFVR) = baseline CFV/peak CFV: 77/33 = 3.35 (normal).
Figure 12.
Patient 3: Invasive Coronary Angiography
Invasive coronary angiography showing nonocclusive left (A) and right (B) coronary arteries.
Patient 4
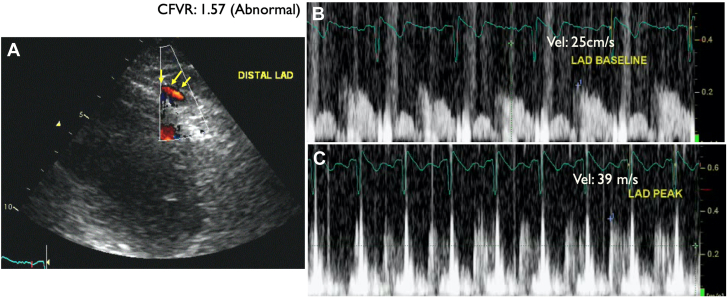
A 64-year-old woman with coronary calcification on chest computed tomography scan at the time of COVID-19 infection was referred for elective DSE. She had atypical chest pain and was on metoprolol for occasional palpitation. The DSE was terminated at the end of protocol at 92% of age-predicted maximal heart rate with no symptoms (30 μg/kg/min and 0.10 mg atropine in addition to handgrip exercise; rate-pressure product, 19,140 mm Hg·beats/min). The resting echocardiogram showed no RWMA, and all segments of the left ventricle augmented well during low dose and remained hyperdynamic at peak. The CFV in the distal LAD was 23 cm/s and 36 cm/s at baseline and peak, respectively, in keeping with reduced CFVR (1.57) (Figures 13 and 14, Video 5). After the DSE, the patient was recommended ICA, but she refused it. The patient was recommended risk factor management and symptom control with antianginal medication for CMD.
Figure 13.
Patient 4: Dobutamine Stress Echocardiography
Dobutamine stress echocardiography (DSE) with ultrasound enhancing agent, end-systolic frames at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. No regional wall motion abnormalities were detected.
Figure 14.
Patient 4: Coronary Flow Velocity by Doppler Echocardiography
Coronary flow velocity by Doppler echocardiography in the distal left anterior descending coronary artery (LAD) (yellow arrows indicate distal LAD color Doppler flow in diastole). LAD flow velocity reserve (CFVR) = baseline CFV/peak CFV: 39/25 = 1.57 (abnormal).
Discussion
Stress echocardiography has been a cost-effective bedside test in the diagnosis of myocardial ischemia over >50 years. Irrespective of the type of the stress modality, it relies on the detection of new RWMA. The outcome of patients with the normal and abnormal stress test had been well-documented.4,5 Patients with moderate and high pretest probability stress echocardiography are recommended for diagnosing myocardial ischemia.6 Coronary flow reserve is an integrated measure of flow through both the large epicardial arteries and the coronary microcirculation. Once obstructive disease of the epicardial arteries is ruled out, reduced coronary flow reserve is a marker of CMD. CFV can be evaluated at baseline and during hyperemia by pulsed-wave Doppler echocardiography, with the sample volume placed on the color signal in the mid or distal part of the LAD. The peak diastolic velocity is measured.7 Doppler-derived CFV has been validated with positron emission tomography, as well as invasive measurements, with good reproducibility.8,9 The limitation of this technique is the ability to assess predominantly the LAD. Ultrasound-enhancing contrast agents have ameliorated the CFV measurements (technical description in the Supplemental Appendix). Comparative description of noninvasive assessment of coronary flow reserve has been summarized in the expert consensus document on INOCA.10
As in our patient 1 with typical effort angina, DSE has shown extensive LAD territory ischemia with high-risk features. Interestingly, the reduced resting CFV (10 cm/s) raised the suspicion of severe proximal LAD stenosis already at baseline. Successful PCI to LAD has rendered the patient symptom free. The complimentary power of the RWMA and CFVR assessment was eloquently demonstrated in this same patient (labelled as Patient 1 follow-up), who 1 year after his LAD PCI was re-referred for a sequential DSE and CFVR assessment to address the bystander LCX and right coronary artery lesions. Post-PCI stress echocardiography showed now no inducible ischemia and the CFVR was normal at 2.59.
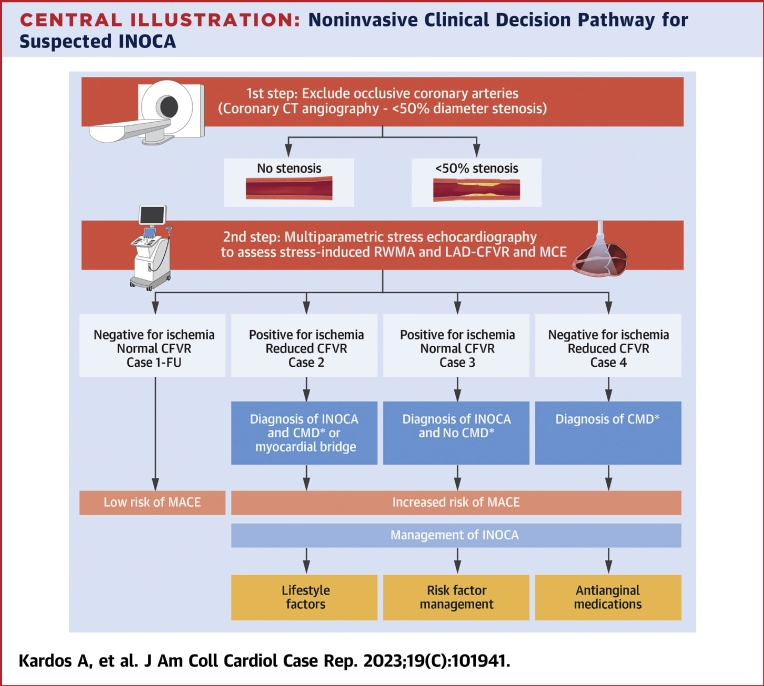
Remarkably, 70% of patients with angina with inducible ischemia have been found to have nonobstructive coronary arteries on anatomical imaging (INOCA).7 These patients are underdiagnosed and undertreated with significant associated morbidity and mortality.10 Beside inducible ischemia by stress echocardiography, CFVR can establish the mechanism and implement guideline-directed medical therapy. To measure CFVR by Doppler echocardiography as a marker of CMD (as a ratio between the CFV at peak stress and at rest, with a cut-point of <2) in patients with INOCA has been endorsed by the current Multi-Societal guideline with a Class of Recommendation of 2b.6 The alternative tests to be able to assess CMD are either very expensive or not readily available or are invasive with inherent risks.6 Our Patients 2, 3 and 4 demonstrate the interaction between stress echocardiography induced RWMA and CFVR. We clustered these patients within the INOCA population using the guidelines recommendation (Central Illustration) as a noninvasive clinical decision pathway for suspected INOCA. All the 3 phenotypes (inducible ischemia with reduced CFVR, inducible ischemia with normal CFVR, and abnormal CFVR and no inducible ischemia) represent increased risk of major adverse cardiovascular event and management option should be tailored accordingly. We could not rule out coronary vasoreactivity (endothelial dependent or independent vasospasm) in patient 3 with normal CFVR despite of inducible ischemia as the possible mechanism of their symptoms. Similar to the other INOCA phenotypes this patient would also benefit from lifestyle factor modification, cardiovascular risk factor management and appropriate antianginal therapy addressing vasospastic angina (i.e., calcium channel blocker, long-acting nitrate, or nicorandil). In patients 2 and 4 where CMD had been confirmed the same management plan and the tailored antianginals by either betablocker, calcium channel blocker, nicorandil, ranolazine, ivabradine or Trimetazidine are recommended10. Dobutamine is a beta and to a smaller degree alpha agonist agent with a very low prevalence of causing coronary vasospasm. Indeed, Dobutamine induced coronary vasospasm has been reported to be between 0.14%-0.40%11. They usually occur in the recovery phase and after betablocker administration, assumed owing to imbalance of the blockade of the beta-receptors and exposing the alpha receptors. We believe that in our case the probability that either of our patients had coronary vasospasm during stress echocardiography is very unlikely.
Central Illustration.
Noninvasive Clinical Decision Pathway for Suspected INOCA
Focus on stress echocardiography. ∗Macrovascular or microvascular vasoreactivity had not been assessed and cannot be ruled out. CFVR = coronary flow velocity reserve; CMD = coronary microvascular dysfunction; CT = computed tomography; FU = follow-up; INOCA = ischemia with no obstructive coronary artery disease; MACE = major adverse cardiovascular event.
Conclusions
With our case series, we would like to raise awareness of the existence of INOCA and to offer a diagnostic pathway for such patients to provide appropriate treatment to improve quality of life and survival. We propose a simple, entirely noninvasive pathway to assess coronary artery anatomy by cardiac computed tomography angiography and, once occlusive CAD was ruled out, refer for ischemia testing and CFVR measurement by multiparametric contrast-enhanced stress echocardiography to investigate CMD.12 We acknowledge that the macrovascular and microvascular vasospastic angina will not be differentiated from the genuine CMD. There will be a need to develop a noninvasive coronary vascular reactivity assessment tool to be implemented in the stress echocardiography protocol. We also recognize that the learning curve for CFVR assessment is steep, but perhaps with a simple standardized protocol it can be successfully rolled out into any stress echo laboratories.13,14
Funding Support and Author Disclosures
Dr Kardos has received speaker fees from TomTec Imaging Systems Ltd. Dr Becher has received speaker fee for Bracco Imaging; and consults for Lantheus. Dr Soulis has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental appendix including the technical description, please see the online version of this paper.
Appendix
Patient 1: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. The yellow arrows indicate extensive area of apical thinning and dyskinesis at peak stress in all the views (5/17 segments ischemia).
Patient 1 Follow-Up: DSE
One year after percutaneous coronary intervention to proximal left anterior descending coronary artery (LAD) with symptomatic improvement to assess bystander left circumflex coronary artery (LCX) and right coronary artery lesions. Dobutamine Stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. Normal regional wall motions of all left ventricular wall segment at each stage.
Patient 2: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress and recovery stages of the protocol. The yellow arrows indicate a small area of apical thinning and dyskinesis at peak stress in all the views.
Patient 3: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress and recovery stages of the protocol. The yellow arrows indicate a small area of apical thinning and dyskinesis at peak stress in all the views. The changes in apical kinesis can be more appreciated if the same regions are compared between low-dose and peak stress.
Patient 4: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. No regional wall motion abnormalities were detected.
References
- 1.Porter T.R., Mulvagh S.L., Abdelmoneim S.S., et al. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 ASE guidelines update. J Am Soc Echocardiogr. 2018;3:241–274. doi: 10.1016/j.echo.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Sicari R., Nihoyannopoulos P., Arturo Evangelista, et al. on behalf of the European Association of Echocardiography. Stress echocardiography expert consensus statement: EACVI. Eur Heart J. 2009;3:278–289. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 3.Pellikka P., Arruda-Olson A., Farooq A., et al. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: from the ASE. J Am Soc Echocardiogr. 2020;33:1–41. doi: 10.1016/j.echo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Shah B.N., Balaji G., Alhajiri A., et al. Incremental diagnostic and prognostic value of contemporary stress echocardiography in a chest pain unit: mortality and morbidity outcomes from a real-world setting. Circ Cardiovasc Imaging. 2013;6:202–209. doi: 10.1161/CIRCIMAGING.112.980797. [DOI] [PubMed] [Google Scholar]
- 5.Woodward W., Dockerill C., McCourt A., et al. Real-world performance and accuracy of stress echocardiography: the EVAREST observational multi-centre study. Eur Heart J Cardiovasc Imaging. 2021;21:1–10. doi: 10.1093/ehjci/jeab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulati M., Levy P.D., Mukherjee D., et al. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the ACC/AHA Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 7.Hozumi T., Yoshida K., Akasaka T., et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32:1251–1259. doi: 10.1016/s0735-1097(98)00389-1. [DOI] [PubMed] [Google Scholar]
- 8.Michelsen M.M., Mygind N.D., Pena A., et al. Transthoracic Doppler echocardiography compared with positron emission tomography for assessment of coronary microvascular dysfunction: the iPOWER study. Int J Cardiol. 2017;228:435–443. doi: 10.1016/j.ijcard.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Caiati C., Montaldo C., Zedda N., et al. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol. 1999;34:1193–1200. doi: 10.1016/s0735-1097(99)00342-3. [DOI] [PubMed] [Google Scholar]
- 10.Kunadian V., Chieffo A., Camici P.G., et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansencal N., El Hajjaji I., El Mahmoud R., et al. Prevalence of coronary artery spasm during dobutamine stress echocardiography. Am J Cardiol. 2012;4(109):800–8004. doi: 10.1016/j.amjcard.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Carbone A., D’Andrea A., Sperlongano S., et al. Echocardiographic assessment of coronary microvascular dysfunction: basic concepts, technical aspects, and clinical Settings. Echocardiography. 2021;38:993–1001. doi: 10.1111/echo.15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krzanowski M., Bodzoń W., Dimitrow P.P. Imaging of all three coronary arteries by transthoracic echocardiography. Cardiovasc Ultrasound. 2003;16:1–51. doi: 10.1186/1476-7120-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigo F., Murer B., Giovanni Ossena G., et al. Transthoracic echocardiographic imaging of coronary arteries: tips, traps, and pitfalls. Cardiovasc Ultrasound. 2008;6:11–20. doi: 10.1186/1476-7120-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient 1: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. The yellow arrows indicate extensive area of apical thinning and dyskinesis at peak stress in all the views (5/17 segments ischemia).
Patient 1 Follow-Up: DSE
One year after percutaneous coronary intervention to proximal left anterior descending coronary artery (LAD) with symptomatic improvement to assess bystander left circumflex coronary artery (LCX) and right coronary artery lesions. Dobutamine Stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. Normal regional wall motions of all left ventricular wall segment at each stage.
Patient 2: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress and recovery stages of the protocol. The yellow arrows indicate a small area of apical thinning and dyskinesis at peak stress in all the views.
Patient 3: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress and recovery stages of the protocol. The yellow arrows indicate a small area of apical thinning and dyskinesis at peak stress in all the views. The changes in apical kinesis can be more appreciated if the same regions are compared between low-dose and peak stress.
Patient 4: DSE
Dobutamine stress echocardiography (DSE) at different views and stages. (A) Apical 4-chamber view. (B) Apical 2-chamber view. (C) Apical 3-chamber view. The quad images represent the resting, low-dose, peak stress, and recovery stages of the protocol. No regional wall motion abnormalities were detected.