Abstract
Ibrutinib is a first-line drug for the treatment of B-cell malignancies. BTKC481S mutation has led to drug resistance during clinical application. Herein, a novel BTK-targeting PROTAC molecule with better solubility and bioavailability was developed. Compound 15-271 has better solubility than ibrutinib and some reported BTK PROTACs. 15-271 has better liver microsomal stability than its analogues in multiple species. More importantly, 15-271 has a longer half-life and better bioavailability in vivo. The development strategy of compound 15-271 can be a general procedure for the optimization of other PROTACs.
Development of a novel PROTAC molecule targeting BTK protein with better PK properties in vivo.
Introduction
Non-Hodgkin's lymphoma (NHL) is the most common hematological cancer among older adults.1 In 2020, there were 544 352 new cases of non-Hodgkin's lymphoma with 259 793 deaths worldwide. China has a large number of NHL cases, accounting for approximately 17.1% of global cases, followed by the United States at 13.5%.2 Bruton's tyrosine kinase (BTK) is a cytoplasmic non-tyrosine kinase expressed only in hematopoietic cells, with the exception of natural killer cells and T cells. BTK participates in various signalling pathways, especially in the B cell receptor (BCR) pathway. Moreover, BTK proteins play a crucial role in oncogenic signalling and are critical for the proliferation and survival of leukemia cells in many B-cell malignancies.3–6
The FDA approved ibrutinib for treatment of mantle cell lymphoma, chronic lymphocytic leukemia, Waldenstrom's macroglobulinemia, and several other indications. When the first patient appeared with Waldenstrom's macroglobulinemia in 2004, there were few drug options. Ibrutinib generates about $5 billion a year globally.7 However, clinical resistance to ibrutinib has appeared, and the main mechanism is the mutation of BTKC481S.8,9 Also, ibrutinib has a variety of serious side effects.10 Therefore, a strategy is urgently needed to overcome the shortcomings of ibrutinib.
In the past decade, PROTAC strategy has achieved milestones in either academia or industry.11,12 However, clinical application of PROTAC is severely limited by the low solubility, short half-life, and low bioavailability.13 Recently, we for the first time reported the BTKC481S degraders P13I and L18I.14,15 PROTACs can successfully overcome the drug resistance and side effects of ibrutinib. Although L18I has potent bioactivity against drug-resistant tumors in vitro and in vivo, L18I has a short half-life and moderate solubility. Therefore, it is urgent to develop a novel BTK degrader with good bioavailability for further clinical application.
In this study, we developed a novel BTK degrader 15-271 with a rigid linker (Fig. 1). Solubility of 15-271 is better than ibrutinib, L18I and some other BTK degraders. 15-271 has a shorter synthetic route than L18I. Also, 15-271 has good hepatic microsomal stability in multiple species. Compared with its analogues, 15-271 has a longer half-life and better bioavailability in vivo. In terms of PD, 15-271 has almost the same bioactivity as that of L18I. The design strategy of compound 15-271 can be a general procedure for the development of PROTAC molecules of other targets.
Fig. 1. Development of novel PROTACs targeting BTK protein with better PK properties. Schematic representation and breakthrough of compound 15-271.
Results and discussion
The screening of novel BTK degraders
According to the structure, L18I has a lengthy linker with numerous rotatable bonds,15 which is contrary to Lipinski's “Rule of Five”. In order to optimize the PK properties and solubility of BTK degraders, we replace the polyethylene glycol linker to the strctures in Table 1. Ibrutinib was used as the BTK ligand and pomalidomide was used as the CRBN ligand (Table 1). B-cell malignancy RAMOS cells were used to evaluate the BTK degradation activity of the novel PROTAC molecules. The results showed that compound 15-271 has strong degrading efficiency at a concentration of 30 nM (Fig. 2). Compared with GBD-9, 15-271 has no off-target to GSPT-1 protein. As mentioned, the first-generation of BTK degrader L18I has a potent effect in inhibition of drug-resistant tumor in vitro and in vivo, so we tested the BTK degradation ability of 15-271 and L18I in DOHH2 cells. The results showed that the bioactivity of compound 15-271 was similar to that of L18I (Fig. 3). Therefore, compound 15-271 was used for further solubility and PK tests.
Chemical structure and bioactivity of novel BTK degraders and reference compound.

| |||||
|---|---|---|---|---|---|
| Compound | Linker | Y | Position of linker relative to Y | Relative BTK levela (%) | Relative BTK level (%) |
| 15-271 |

|
CO | meta | 20.7b (30 nM) | 18.3 (100 nM) |
| 15-59 |
|
CO | meta | 18.2 (100 nM) | 20.1 (300 nM) |
| 15-81 |

|
CH2 | meta | 6.9 (100 nM) | 12.0 (300 nM) |
| 15-83-1 |

|
CO | meta | 78.4 (100 nM) | 79.7 (300 nM) |
| 15-83-2 |

|
CO | meta | 23.1 (100 nM) | 24.5 (300 nM) |
| 15-155 |

|
CO | meta | — | ∼100 (500 nM) |
| 15-177 |

|
CO | meta | 38.4 (250 nM) | 38.8 (500 nM) |
| L18I |

|
CH2 | ortho | 28.5 (100 nM) | — |
Degradation efficiency of PROTACs in B-cell malignancy RAMOS cells. Immunoblotting analysis of BTK protein and β-actin protein from RAMOS cells treated with compounds for 24 h. In 12-well plates, 3 × 105 cells were incubated in each well at 37 °C. Grayscale analysis data was generated by ImageJ for the calculation of relative level of BTK protein.
Concentration of compounds.
Fig. 2. Degradation efficiency of novel PROTACs and reference compounds in RAMOS cells. Immunoblotting analysis of BTK protein, GSPT-1 protein and β-actin protein from RAMOS cells treated with compounds for 24 h. In 12-well plates, 3 × 105 cells were incubated in each well at 37 °C. Grayscale analysis data was generated by ImageJ for the calculation of relative level of BTK protein.
Fig. 3. Degradation efficiency of novel PROTACs and L18I in DOHH2 cells. Immunoblotting analysis of BTK protein and β-actin protein from DOHH2 cells treated with compounds for 24 h. In 12-well plates, 3 × 105 cells were incubated in each well at 37 °C.
The synthesis of 15-271 and L18I
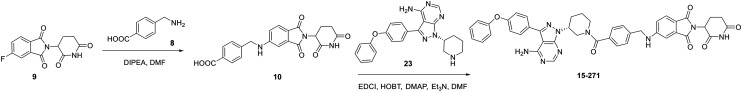
For the synthesis of compound 15-271 (Scheme 1), the substitution reaction was conducted with compound 8 and 9 as substrate to prepare compound 10. After condensation reaction, compound 15-271 was generated. The synthetic route has only two steps. L18I were prepared as before,15 and the synthetic route has six steps (Scheme 2). Therefore, compared with L18I, the synthesis of 15-271 is more convenient.
Scheme 1. Synthesis of compound 15-271.
Scheme 2. Synthesis of compound L18I.
The solubility test of novel BTK degraders
In order to explore the properties of compound 15-271, we compared the solubility of novel BTK PROTACs. The solvent was DMSO : castor oil polyoxyethylene ether : 1× PBS mixture (1 : 1 : 8). hsk-17# is an analogue of BTK degrader in clinical trials and it has been published in patent. According to the result, the solubility of compound 15-271 is higher than that of L18I or hsk-17#, indicating that the 4-aminomethylbenzoyl linker can effectively improve the solubility of PROTAC molecule. After one week, 15-271 (10 mg kg−1) remained stable in the solvent at room temperature, while the solvent of ibrutinib (10 mg kg−1) become turbid (Table 2). Therefore, compound 15-271 may be more suitable for administration in vivo.
Chemical structure and solubility of 15-271 compared with reference compounds.

| |||||
|---|---|---|---|---|---|
| Compound | Linker | Y | Position of linker relative to Y | Solubilitya | Solubility in 7 daysb |
| 15-271 |

|
CO | meta | Y | Y |
| ibrutinib |

|
— | — | Y | N |
| hsk-17# |

|
CO | meta | N | N |
| L18I |

|
CH2 | ortho | N | N |
Compound was dissolved in DMSO: Cremophor EL: 1× PBS = 1/1/8 (v/v/v) with 10 mg mL−1. Y: the solution was clear and transparent. N: the liquid was suspension.
The liquid with compound was kept at room temperature for 7 days.
The PK properties of novel BTK degraders in vitro and in vivo
Next, permeability of compound 15-271 and its analogues (structures of compound 15-293 and compound 15-295 were shown in the ESI†) were tested using Madin–Darby canine kidney type II (MDCKII) cells. Compounds were diluted with the transport buffer (HBSS with 10 mM Hepes, pH = 7.40) to a concentration of 10 μM. Concentration of test compounds were determined by LC-MS/MS based on the peak area ratio of analyte/internal standard. As shown in Table 3, Papp of all tested compounds was low, indicating that all tested compounds were classified as having low permeability.
Permeability results on MDCK monolayer.
| Test article | P app (×10−6 cm s−1) | %Recovery | Classification | ||
|---|---|---|---|---|---|
| A→B (n = 2) | B→A (n = 2) | A→B (n = 2) | B→A (n = 2) | ||
| Atenolol | 0.85 | 0.87 | 94.36 | 99.49 | Low |
| Metoprolol | 21.05 | 11.35 | 97.07 | 95.89 | High |
| 15-271 | 0.016 | 0.0099 | 110.75 | 116.12 | Low |
| 15-293 | 0.00088 | 0.071 | 107.28 | 100.54 | Low |
| 15-295 | 0.0033 | 0.25 | 98.49 | 101.38 | Low |
For liver stability tests, microsomes from different species (mice, rabbits, dogs, monkeys or humans) were utilized for the tests of compound 15-271 and its analogues (Table 4). The results showed that compound 15-271 had the best liver microsome stability and lowest hepatic clearance in all tested species, indicating that the 4-aminomethylbenzoyl group has better liver microsomal stability than the 4-aminophenylacetyl group in the case of PROTAC molecules.
Liver microsome stability test.
| Species | Mouse | Rat | Dog | Monkey | Human | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | T 1/2 (minute) | CLint, mic (μL min−1 mg−1 protein) | T 1/2 (minute) | CLint, mic (μL min−1 mg−1 protein) | T 1/2 (minute) | CLint, mic (μL min−1 mg−1 protein) | T 1/2 (minute) | CLint, mic (μL min−1 mg−1 protein) | T 1/2 (minute) | CLint, mic (μL min−1 mg−1 protein) |
| 15-271 | 135.78 | 10.21 | 180.99 | 7.66 | 254.21 | 5.45 | 306.56 | 4.52 | 141.00 | 9.83 |
| 15-293 | 12.08 | 114.79 | 14.60 | 94.96 | 22.97 | 60.34 | 10.00 | 138.68 | 14.41 | 96.19 |
| 15-295 | 12.98 | 106.81 | 24.82 | 55.86 | 28.48 | 48.67 | 8.91 | 155.67 | 9.46 | 146.55 |
To test the PK properties of the novel BTK degraders in vivo, BALB/c mice were used for oral administration. The plasma was separated after centrifugation (Table 5). The result showed that compound 15-271 had the best bioavailability (%F = 4.78). 15-293 and 15-295 had pretty low bioavailability, which was consistent with the results in vitro.
Pharmacokinetic parameters of the tested compounds.
| Compound | Route | Dose (mg kg−1) | T 1/2 (h) | CL (mL h−1 kg−1) | AUClast (h × ng mL−1) | Vz (mL kg−1) | C max (ng mL−1) | T max (h) | %F |
|---|---|---|---|---|---|---|---|---|---|
| 15-271 | IV | 2 | 0.98 | 709.56 | 2811.61 | 998.32 | |||
| PO | 5 | 0.52 | 336.28 | 251.7 | 0.5 | 4.78 | |||
| 15-293 | IV | 2 | 5.15 | 310.41 | 6404.33 | 2306.51 | |||
| PO | 5 | 1.14 | 356.83 | 364.0 | 0.5 | 2.23 | |||
| 15-295 | IV | 2 | 4.93 | 244.93 | 8116.92 | 1740.73 | |||
| PO | 5 | 1.45 | 102.81 | 83.2 | 0.5 | 0.51 |
Conclusions
In summary, a novel BTK degrader 15-271 with high efficiency and bioavailability was developed. Compound 15-271 with rigid linker has better solubility than ibrutinib, L18I or hsk-17#. Compared with the first generation of BTK degrader L18I, 15-271 has fewer rotatable bonds and a simple synthetic route. Moreover, 15-271 has almost the same bioactivity as that of L18I, whose anti-cancer activity has been confirmed in vivo. More importantly, PK results indicated that compound 15-271 had the best liver microsome stability and lowest hepatic clearance in all tested species. Among the analogues, compound 15-271 had the best bioavailability in vivo. Therefore, our results indicated a general strategy for further optimization of PK properties of PROTACs.
Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Zhejiang University of Technology and approved by the Animal Ethics Committee of Zhejiang University of Technology.
Author contributions
The project and experiments were designed by Y. S., D. L., and Y. R. The chemistry was performed by Y. S., Z. Y., Z. L. and X. L. The biological research was carried out by Y. S., Z. Z., L. G., and H. P. All authors contributed to the manuscript writing and review process.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (#82125034), and the National Key R&D Program of China (#2020YFE0202200, #2021YFA1300200, and #2021YFA1302100).
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3md00216k
Notes and references
- Cheson B. D. Leonard J. P. N. Engl. J. Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L. Dominguez-Sola D. Chiarenza A. Fabbri G. Grunn A. Trifonov V. Kasper L. H. Lerach S. Tang H. Ma J. Rossi D. Chadburn A. Murty V. V. Mullighan C. G. Gaidano G. Rabadan R. Brindle P. K. Dalla-Favera R. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton L. M. Wang S. S. Devesa S. S. Hartge P. Weisenburger D. D. Linet M. S. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. C. Furman R. R. Coutre S. E. Flinn I. W. Burger J. A. Blum K. A. Grant B. Sharman J. P. Coleman M. Wierda W. G. Jones J. A. Zhao W. Heerema N. A. Johnson A. J. Sukbuntherng J. Chang B. Y. Clow F. Hedrick E. Buggy J. J. James D. F. O'Brien S. N. Engl. J. Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg L. A. Smitha A. M. Sirisawada M. Verner E. Loury D. Chang B. Li S. Pan Z. Thamm D. H. Miller R. A. Buggy J. J. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. H. Young R. M. Schmitz R. Yang Y. Pittaluga S. Wright G. Lih C. Williams P. M. Shaffer A. L. Gerecitano J. de Vos S. Goy A. Kenkre V. P. Barr P. M. Blum K. A. Shustov A. Advani R. Fowler N. H. Vose J. M. Elstrom R. L. Habermann T. M. Barrientos J. C. McGreivy J. Fardis M. Chang B. Y. Clow F. Munneke B. Moussa D. Beaupre D. M. Staudt L. M. Nat. Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. Liu X. Chen X. Nat. Rev. Clin. Oncol. 2020;17:589–590. doi: 10.1038/s41571-020-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyach J. A. Furman R. R. Liu T. Ozer H. G. Zapatka M. Ruppert A. S. Xue L. Li D. H. Steggerda S. M. Versele M. Dave S. S. Zhang J. Yilmaz A. S. Jaglowski S. M. Blum K. A. Lozanski A. Lozanski G. James D. F. Barrientos J. C. Lichter P. Stilgenbauer S. Buggy J. J. Chang B. Y. Johnson A. J. Byrd J. C. N. Engl. J. Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman R. R. Cheng S. Lu P. Setty M. Perez A. R. Guo A. Racchumi J. Xu G. Wu H. Ma J. Steggerda S. M. Coleman M. Leslie C. Wang Y. L. N. Engl. J. Med. 2014;370:2352–2354. doi: 10.1056/NEJMc1402716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. C. Harrington B. O'Brien S. Jones J. A. Schuh A. Devereux S. Chaves J. Wierda W. G. Awan F. T. Brown J. R. Hillmen P. Stephens D. M. Ghia P. Barrientos J. C. Pagel J. M. Woyach J. Johnson D. Huang J. Wang X. Kaptein A. Lannutti B. J. Covey T. Fardis M. McGreivy J. Hamdy A. Rothbaum W. Izumi R. Diacovo T. G. Johnson A. J. Furman R. R. N. Engl. J. Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K. M. Kim K. B. Kumagai A. Mercurio F. Crews C. M. Deshaies R. J. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G. E. Buckley D. L. Paulk J. Roberts J. M. Souza A. Dhe-Paganon S. Bradner J. E. Science. 2015;348:1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Song Y. J. Hematol. Oncol. 2020;13:50. doi: 10.1186/s13045-020-00885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Zhao X. Ding N. Gao H. Wu Y. Yang Y. Zhao M. Hwang J. Song Y. Liu W. Rao Y. Cell Res. 2018;28:779–781. doi: 10.1038/s41422-018-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Ding N. Song Y. Yang Z. Liu W. Zhu J. Rao Y. Leukemia. 2019;33:2105–2110. doi: 10.1038/s41375-019-0440-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.