Summary
Background
Inhibiting vascular endothelial growth factor (VEGF) function can improve the efficacy of immunotherapy by modulating the tumor immune microenvironment. AK112 is the first-in-class humanized IgG1 bispecific antibody targeting programmed death-1 (PD-1) and VEGF. This study aimed to evaluate the efficacy and safety of AK112 combined with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC).
Methods
This open-label, multicenter, phase II clinical trial was conducted in 11 hospitals in China. Eligible participants were adults aged 18–75 years with locally advanced or metastatic NSCLC, an Eastern Cooperative Oncology Group performance status of 0 or 1, at least one measurable lesion, and an estimated life expectancy of at least 3 months. The participants were categorized into three cohorts based on prior therapy and functional genomic alterations. Patients in cohort 1 were previously untreated advanced NSCLC, had no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) gene modifications, and received AK112 combined with pemetrexed (500 mg/m2) for non-squamous (non-sq)-NSCLC or paclitaxel (175 mg/m2) for sq-NSCLC plus carboplatin (area under the curve of 5 mg/mL per min) for four cycles, followed by AK112 with pemetrexed for non-sq-NSCLC and AK112 alone for sq-NSCLC as maintenance therapy. The participants in cohort 2 had advanced NSCLC with EGFR-sensitive mutations, failed previous EGFR-tyrosine kinase inhibitor (TKI) therapy, and received pemetrexed plus AK112 and carboplatin for four cycles, followed by pemetrexed plus AK112 as maintenance therapy. The participants in cohort 3 had advanced NSCLC who failed systemic platinum-based chemotherapy and anti-PD-1/programmed death-ligand 1 (PD-L1) treatments and received AK112 plus docetaxel (75 mg/m2). Two dosages of AK112 (10 or 20 mg/kg) were examined in each cohort, and the drug was administered intravenously on day 1 of each 3-week treatment cycle. The primary endpoints were the investigator-assessed objective response rate (ORR) and safety. This study was registered with ClinicalTrials.gov (NCT04736823).
Findings
Eighty-three patients were enrolled from February 2021 to August 2022 and received the study treatment. Cohorts 1, 2, and 3 had 44, 19, and 20 patients, respectively. The confirmed ORR was 53.5% (23/43) [95% CI, 36.9–67.1], 68.4% (13/19) [95% CI, 43.4–87.4], and 40.0% (8/20) [95% CI, 19.1–63.9] in cohorts 1, 2, and 3, respectively. In cohort 1, the median PFS was not reached, and the 12-month PFS rate was 59.1%. In cohorts 2 and 3, the median PFS were 8.5 [95% CI, 5.5-NE] and 7.5 [95% CI, 2.3-NE] months, and the 12-month PFS rates were 35.5% and 44.5%, respectively. The most common grade ≥3 treatment-related adverse events were decreased white blood cell count [7 (8.4%)], neutropenia [5 (6.0%)], thrombocytopenia [2 (2.4%)], anemia [4 (4.8%)], and myelosuppression [2 (2.4%)].
Interpretation
AK112 plus platinum-doublet showed promising antitumor activity and safety not only in first-line treatment of advanced NSCLC patients without driver mutation but also in patients with EGFR-functional mutation who failed previous EGFR-TKI therapy and advanced NSCLC patients who failed prior systemic platinum-based chemotherapy and PD-1/PD-L1 inhibitor treatments, suggesting a valuable potential new treatment option for this patient population.
Funding
Akeso Biopharma, Inc., Zhongshan, China, and National Natural Science Foundation of China.
Keywords: AK112, Bispecific antibody, Non-small cell lung cancer, PD-1, VEGF, Anti-angiogenesis
Research in context.
Evidence before this study
We searched for articles and abstracts in PubMed and from the major oncology congresses (the American Society of Clinical Oncology, World Conference on Lung Cancer, the American Association for Cancer Research, and European Society for Medical Oncology) for studies on non-small cell lung cancer (NSCLC) and immunotherapy regimens published from database inception to June 20, 2023, using search terms of “non-small-cell lung cancer”, “chemotherapy”, “anti-PD-1”, “anti-PD-L1”, “EGFR”, “bispecific antibody”, “dual immunotherapy”, “bevacizumab” “anti-angiogenesis”, and “progressed”, without language restriction. Previous clinical trials revealed that the efficacy of PD-1 and PD-L1 inhibitors was enhanced when combined with VEGF inhibitors in non-squamous NSCLC including phase III IMpower-150 trial and TASUKI-52 trial while patients with EGFR sensitive mutation can also benefit from PD-1 and PD-L1 inhibitors combined with VEGF inhibitors after failure of targeted therapy, as shown in the subgroup analysis of IMpower-150 and phase III ORIENT-31 trial. However, no squamous-NSCLC patients were enrolled in these phase III trials and for the patients who failed first-line treatment of PD-1/L1 checkpoint inhibitor plus chemotherapy, no immune-oncology agent or combination was effective in this refractory setting, a major unmet need.
Added value of this study
AK112 is the global first-in-class humanized IgG1 bispecific antibody targeting PD-1 and VEGF, inhibiting PD-1-mediated immunosuppression, and blocking tumor angiogenesis in the tumor microenvironment. To the best of our knowledge, this was the first phase II trial evaluating the efficacy and safety of a bispecific antibody targeting PD-1 and VEGF (AK112) in combination with chemotherapy in metastatic NSCLC, including both non-squamous and squamous NSCLC. AK112 plus chemotherapy showed promising antitumor activity in advanced NSCLC patients without driver mutation as first-line treatment, in patients with EGFR-functional mutation who failed previous EGFR-TKI therapy, and in advanced NSCLC patients who failed prior systemic platinum-based chemotherapy and PD-1/L1 inhibitors.
Implications of all the available evidence
Our results supported the further evaluation of AK112 in combination with chemotherapy in treating NSCLC. AK112 may be a valuable potential new treatment option for this patient population. Based on the results of the present study, a phase III randomized control trial of AK112 plus chemotherapy in NSCLC patients with EGFR mutation who progressed with previous EGFR-TKI is now ongoing (NCT05184712).
Introduction
The current first-line therapies for advanced and metastatic non-small cell lung cancer (NSCLC) without druggable oncogenic driver mutations include anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies monotherapy for patients with high PD-L1 expression,1, 2, 3, 4 immune checkpoint inhibitors (ICI) in combination with platinum-doublet chemotherapy regardless of PD-L1 expression,5,6 and four-drug combination of bevacizumab and atezolizumab plus chemotherapy for metastatic non-squamous (non-sq)-NSCLC, based on the results of the IMpower-150 trial showed that the combination of antiangiogenic agents and ICIs significantly improved patient survival.6 Nevertheless, some patients responded poorly, and more effective options might be necessary.
Although first-line targeted therapy for NSCLC patients with epidermal growth factor receptor (EGFR) mutation is available,7, 8, 9 drug resistance is increasing, and platinum-based chemotherapy remains the main therapy after targeted therapy failure.10 In the subgroup analyses of the IMpower-150 trial, combinations of atezolizumab, bevacizumab, and chemotherapy demonstrated a trend of survival benefits in patients with EGFR mutation.11,12 Furthermore, the ORIENT-31 phase III trial showed that sintilimab (a PD-1 inhibitor) plus bevacizumab biosimilar (IBI305), cisplatin, and pemetrexed was generally effective and well tolerated in patients with EGFR-mutated NSCLC who progressed after EGFR tyrosine kinase inhibitors (TKIs).13
A major unmet need remains in patients who have failed first-line treatment of PD-1/PD-L1 inhibitors plus chemotherapy. Cabozantinib plus atezolizumab was not superior to docetaxel alone in the final OS analysis of the phase III CONTACT-01 trial.14 Ramucirumab combined with pembrolizumab showed potential benefits compared with the standard treatment in the phase II Lung-MAP S1800A trial,15 and sitravatinib plus tislelizumab demonstrated acceptable safety profile and objective responses in advanced NSCLC in a phase Ib trial.16 However, the promising results need to be confirmed with larger studies.
The vascular endothelial growth factor (VEGF) plays a central role in tumor angiogenesis,17,18 immunomodulating the tumor microenvironment (TME), and modulating multiple immune cell populations, especially myeloid cells.19,20 Given the effects of anti-angiogenesis and immune checkpoint blockade on the TME, combining ICIs and antiangiogenic agents exhibited a potential synergistic antitumor effect in preclinical and clinical studies.21, 22, 23 AK112 is the first-in-class humanized IgG1 bispecific antibody that targets PD-1 and VEGF by inhibiting PD-1-mediated immunosuppression and simultaneously blocking tumor angiogenesis in the TME. The tetravalent structure of the AK112 allows the formation of large complexes with dimeric VEGF, resulting in high avidity to PD-1 and improved function and eliciting potent antitumor activity in preclinical studies.24 AK112 showed considerable safety and significant antitumor efficacy in patients with advanced NSCLC, and AK112 monotherapy at a dosage of 20 mg/kg every 3 weeks was recommended in the phase II trial.25
Herein, we aimed to assess the safety and efficacy of AK112 combined with chemotherapy for treating advanced NSCLC in patients who were treatment naïve, patients with EGFR-activating mutations who failed previous EGFR-TKI therapies, and patients who failed systemic platinum-based chemotherapy and PD-1/L1 inhibitor treatments.
Methods
Study design and participants
This open-label, multicenter, phase II clinical study was conducted in 11 hospitals in China. The participants were categorized into three cohorts based on prior therapy and functional genomic alterations. Two dosage levels of AK112 were administered to each of the three cohorts. Cohort 1 included patients with advanced or metastatic NSCLC without EGFR or anaplastic lymphoma kinase (ALK) gene alterations and received AK112 in combination with platinum-based chemotherapy as first-line treatment. Cohort 2 included patients with advanced NSCLC with EGFR-sensitive mutations who failed first- or second-generation EGFR-TKI therapy and were negative for T790M mutation or failed third-generation EGFR-TKI therapy and received AK112 combined with platinum-based chemotherapy. Cohort 3 consisted of patients with advanced NSCLC who failed systemic platinum-based chemotherapy and PD-1/L1 inhibitor treatments and received AK112 plus docetaxel (Supplementary Fig. S1).
The main inclusion criteria were 1) 18–75 years of age, 2) Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, 3) expected survival of ≥3 months, 4) histologically or cytologically confirmed locally advanced (stage IIIB/IIIC) or metastatic (stage IV) NSCLC that could not be surgically completely resected and who could not undergo radical concurrent/sequential chemoradiotherapy, 5) prior tissue-based EGFR and ALK reports, or otherwise provided tumor tissue samples (archival or fresh, primary or metastatic) for assessment of EGFR and ALK status (either to local laboratory or to central laboratory) for non-sq-NSCLC, and 6) at least one measurable lesion as per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and appropriate for repeated accurate measurement.
The main exclusion criteria were 1) small cell carcinoma confirmed by histopathological analysis or NSCLC with ALK gene translocation, 2) tumor encircling important blood vessels, had significant necrosis and cavitation, or had central, cavitary squamous NSCLC on CT images, or patients with a risk of hemorrhage judged by the investigator, 3) other malignancies within 3 years before enrollment, or 4) metastases to the brainstem, meninges, or spinal cord, or spinal cord compression. The complete eligibility criteria are listed in the trial protocol (Supplementary File).
This study followed all applicable regulatory requirements, the Declaration of Helsinki, and good clinical practice guidelines. The study was approved by the institutional review board and ethics committee at each participating center. All patients provided written informed consent before enrollment.
Procedures
Two dosage levels of AK112 (10 mg/kg or 20 mg/kg every 3 weeks) were investigated in each cohort. Since the present study was the first study of AK112 plus chemotherapy, in order to guarantee patient safety, half of the patients in each cohort received 10 mg/kg AK112 first, and if that dose was safe and tolerable, the remaining patients received 20 mg/kg AK112 (Supplementary Fig. S1).
The participants with non-sq-NSCLC in cohort 1 received AK112 plus pemetrexed (500 mg/m2) plus carboplatin [area under the curve (AUC), 5 mg/mL per min] for four cycles, followed by maintenance therapy with AK112 plus pemetrexed (500 mg/m2). Participants with squamous (sq)-NSCLC in cohort 1 received AK112 plus paclitaxel (175 mg/m2) plus carboplatin (AUC, 5 mg/mL per min) for four cycles, followed by maintenance therapy with AK112. In cohort 2, the participants were treated with AK112 plus pemetrexed (500 mg/m2) plus carboplatin (AUC, 5 mg/mL per min) for four cycles, followed by maintenance therapy with AK112 plus pemetrexed (500 mg/m2). In cohort 3, the participants received AK112 plus docetaxel (75 mg/m2). Treatment was administered intravenously on day 1 of each 3-week treatment cycle. No dose modifications were allowed for AK112 during the treatment period, but drug administration delays were allowed for up to 12 weeks (calculated from the time of the last dosage). All patients continued to receive treatment until no longer clinical benefit as judged by the investigator, intolerable toxicity, completion of 24 months of treatment, or meeting other criteria for discontinuation of treatment in the protocol. The details of treatments were provided in the protocol (Supplementary File).
Endpoints
The two primary endpoints were the objective response rate (ORR), which was defined as the proportion of patients achieving a confirmed complete response (CR) or partial response (PR) according to RECIST v1.1 assessed by the investigator, and safety. The secondary endpoints included progression-free survival (PFS) (the time from enrollment to progression or death due to any cause), the disease control rate (DCR) (the proportion of patients achieving a confirmed CR or PR, or stable disease [SD]), duration of response (DOR) (the time from the first confirmed CR or PR to the first documentation of progressive disease [PD] or death due to any cause), overall survival (OS) (the time from enrollment to death due to any cause), and PD-L1 expression as a predictive biomarker of response. Tumor assessments were performed at baseline, then every 6 weeks (±7 days) for 48 weeks, and every 12 weeks (±7 days) thereafter. ORR was also analyzed according to PD-L1 expression level (<1%, 1–49%, and ≥50%).
The adverse events (AEs) for each patient were collected up to 30 days after the end of treatment, 90 days for serious adverse events (SAEs) and immune-related AEs (irAEs), or the initiation of other antitumor therapy. Adverse events, laboratory abnormalities, and toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, Version 5.0). Survival assessments were performed every 3 months after the last dose, and subsequent antitumor therapy information was collected after discontinuing the study drug.
Statistical analysis
The sample size was based on estimation without a statistical hypothesis to explore the efficacy and toxicity of AK112 when combined with chemotherapy. Forty patients in cohort 1 (20 non-sq-NSCLC and 20 sq-NSCLC), 20 patients in cohort 2, and 20 patients in cohort 3 were planned to be included.
Safety analysis was done in all patients who received at least one dose of the study drug (safety set; SS). Efficacy analysis was performed in all patients who received at least one dose of the study drug and had measurable disease at baseline with ≥1 efficacy assessment (full analysis set; FAS). The two-sided 95% confidence intervals (CIs) for ORR and DCR were estimated using the Clopper-Pearson method. The DOR, PFS, and OS were determined using the Kaplan–Meier method, and the corresponding 95% CIs were estimated by the Brookmeyer-Crowley method. The OS analysis was performed based on the SS. The graphical analyses included swimming plots for tumor responses at each efficacy assessment, spider plots for percentage changes in the tumor burden of the target lesions over time from the baseline, and waterfall plots for the best percentage changes in the tumor burden of the target lesions from the baseline. All statistical analyses were performed using SAS version 9.4.
This trial was registered with ClinicalTrials.gov (NCT04736823).
Role of the funding source
This study was designed by the principal investigator (LZ) and the funding agency (Akeso Biopharma Inc.). The funder provided the study drug, contributed to data collection and interpretation, funded the data analysis, and participated in the preparation and review of the manuscript in collaboration with all co-authors. All authors had full access to all the data in the study, and the corresponding author was responsible for submitting the manuscript for publication.
Results
Participants
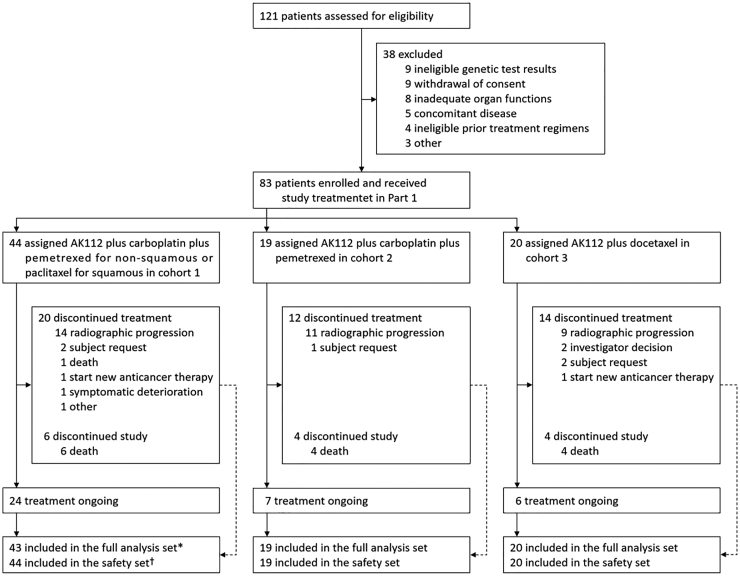
Of 121 patients screened for eligibility from February 2021 to August 2022, 38 failed to participate in this study due to withdrawal of consent (9 patients), ineligible genetic test results (9), inadequate organ functions (8), concomitant diseases (5), ineligible prior treatment regimens (4), and other reasons (3). Therefore, 83 patients were selected to receive the study treatment. Forty-four participants were in cohort 1, 19 were in cohort 2, and 20 were in cohort 3; 82 participants were included in the FAS (Fig. 1). One subject in cohort 1 refused post-baseline follow-up visits and had no efficacy assessments, and thus was not included in the FAS. The baseline demographic and disease characteristics of the patients are provided in Table 1. On August 5, 2022 (data cutoff), the median follow-up was 12.7 (95% CI, 11.6–13.8) months for cohort 1, 11.0 (95% CI, 8.5–12.9) months for cohort 2, and 10.3 (95% CI, 8.1–11.7) months for cohort 3. The median duration of drug exposure was 354.5 days (range 22–516), 259.0 days (range 44–519), and 162.5 days (range 42–438) in the three cohorts, respectively.
Fig. 1.
Trial profile. Data collection was cut off on August 5, 2022. †The safety set included all the patients who received at least one dose of study treatment. The full analysis set included patients in the safety set who had at least one efficacy assessment. ∗One subject in cohort 1 refused post-baseline follow-up visits and had no efficacy assessments, and thus was not included in the full analysis set.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristic | Cohort 1 (n = 44) | Cohort 2 (n = 19) | Cohort 3 (n = 20) |
|---|---|---|---|
| Age, years | |||
| Median (range) | 57.6 (44.3–73.0) | 60.2 (34.7–64.9) | 60.0 (31.6–73.4) |
| <65, n (%) | 35 (79.5) | 19 (100.0) | 16 (80.0) |
| ≥65, n (%) | 9 (20.5) | 0 (0.0) | 4 (20.0) |
| Sex, n (%) | |||
| Male | 105 (77.8) | 6 (31.6) | 16 (80.0) |
| Female | 30 (22.2) | 13 (68.4) | 4 (20.0) |
| ECOG performance status score, n (%) | |||
| 0 | 28 (63.6) | 5 (26.3) | 1 (5.0) |
| 1 | 16 (36.4) | 14 (73.7) | 19 (95.0) |
| Smoking status, n (%) | |||
| Current or former | 24 (54.5) | 4 (21.1) | 15 (75.0) |
| Never | 20 (45.5) | 15 (78.9) | 5 (25.0) |
| PD-L1 tumor proportion score, n (%) | |||
| <1% | 20 (45.5) | 10 (52.6) | 6 (30.0) |
| 1–49% | 16 (36.4) | 6 (31.6) | 8 (40.0) |
| ≥50% | 6 (13.6) | 3 (15.8) | 4 (20.0) |
| Unknown | 2 (4.5) | 0 (0.0) | 2 (10.0) |
| Disease stage, n (%) | |||
| III | 4 (9.1) | 0 (0.0) | 3 (15.0) |
| IV | 40 (90.9) | 19 (100.0) | 17 (85.0) |
| Brain metastases, n (%) | 11 (25.0) | 7 (36.8) | 1 (5.0) |
| Number of distant metastases (excluding lungs), n (%) | |||
| <3 | 32 (72.7) | 9 (47.4) | 15 (75.0) |
| ≥3 | 12 (27.3) | 10 (52.6) | 5 (25.0) |
| Histologic features, n (%) | |||
| Squamous | 18 (40.9) | 0 (0.0) | 7 (35.0) |
| Non-squamous | 26 (59.1) | 19 (100.0) | 13 (65.0) |
| EGFR mutation status (cohort 2), n (%) | |||
| 19del and T790M+ | 0 | 8 (42.1) | 0 |
| 19del and T790M- | 0 | 5 (26.3) | 0 |
| L858R and T790M+ | 0 | 2 (10.5) | 0 |
| L858R and T790M- | 0 | 3 (15.8) | 0 |
| G719X and T790M- | 0 | 1 (5.3) | 0 |
| Generation of previous EGFR-TKIs (cohort 2), n (%) | |||
| First or second | 0 | 5 (26.3) | 0 |
| First or second, followed by third | 0 | 14 (73.7) | 0 |
| Third as first-line | 0 | 0 | 0 |
| Using bevacizumab (cohort 3), n (%) | |||
| Yes | 0 | 0 | 4 (20.0) |
| No | 0 | 0 | 16 (80.0) |
| Lines of prior cancer therapy (cohorts 2 and 3), n (%) | |||
| 1 | 0 | 5 (26.3) | 14 (70.0) |
| ≥2 | 0 | 14 (73.7) | 6 (30.0) |
| The best curative effect of previous treatments (cohorts 2 and 3), n (%) | |||
| CR/PR | NA | 4 (21.1) | 8 (40.0) |
| SD | NA | 0 (0.0) | 7 (35.0) |
| PD | NA | 1 (5.3) | 0 (0.0) |
| Unknown | NA | 13 (68.4) | 5 (25.0) |
| Missing | NA | 1 (5.3) | 0 (0.0) |
n, number; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NA, not applicable.
Efficacy
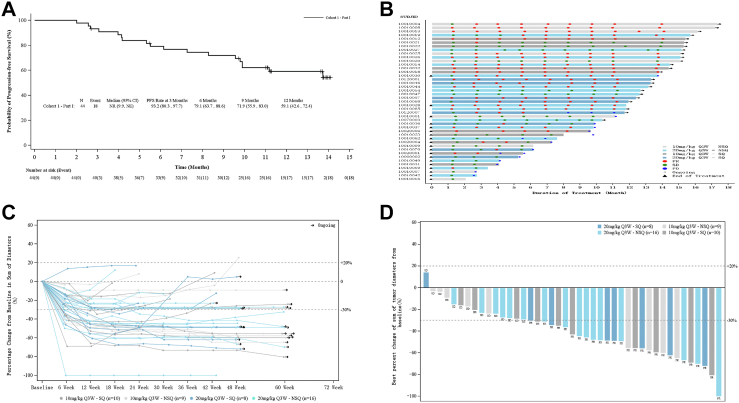
In cohort 1 (patients receiving first-line AK112 in combination with platinum-based chemotherapy), the confirmed ORR was 53.5% (23/43) [95% CI, 36.9–67.1] and DCR was 93.0% (40/43) [95% CI, 80.9–98.5] (Table 2). In non-sq-NSCLC and sq-NSCLC, the confirmed ORR was 48.0% (12/25) [95% CI, 27.8–68.7] and 61.1% (11/18) [95% CI, 35.7–82.7]. In participants with AK112 at 10 and 20 mg/kg, the ORR was 52.6% (10/19) [95% CI, 28.9–75.6] and 54.2% (13/24) [95% CI, 32.8–74.4], respectively (Table 2 and Supplementary Table S1). The median PFS was not reached (Fig. 2A). The 3-, 6-, and 12-month PFS rates were 93.2% [95% CI, 80.3–97.7], 79.1% [95% CI, 63.7–88.6], and 59.1% [95% CI, 42.6–72.4], respectively (Fig. 2A). A swimmer plot of treatment duration showed that at the data cutoff, 24 of 43 participants (55.8%) were still receiving the assigned treatment (Fig. 2B). Tumor volume changes over time were demonstrated using a spider plot, indicating that patients benefited from treatment regardless of the histological characteristics of cancer and dosage levels of AK112 (Fig. 2C). Except for one participant, the tumor of all other participants shrank to varying degrees during treatment (Fig. 2D).
Table 2.
Summary of efficacy (full analysis set, n = 82).
| Cohort 1 |
Cohort 2 |
Cohort 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 mg/kg Q3W (n = 19) | 20 mg/kg Q3W (n = 24) | Total (n = 43) | 10 mg/kg Q3W (n = 10) | 20 mg/kg Q3W (n = 9) | Total (n = 19) | 10 mg/kg Q3W (n = 10) | 20 mg/kg Q3W (n = 10) | Total (n = 20) | |
| ORR confirmed, n (%) | 10 (52.6) | 13 (54.2) | 23 (53.5) | 8 (80.0) | 5 (55.6) | 13 (68.4) | 4 (40.0) | 4 (40.0) | 8 (40.0) |
| 95% CI | [28.9–75.6] | [32.8–74.4] | [37.7–68.8] | [44.4–97.5] | [21.2–86.3] | [43.4–87.4] | 4 (40.0) | 4 (40.0) | [19.1–63.9] |
| DCR confirmed, n (%) | 17 (89.5) | 23 (95.8) | 40 (93.0) | 9 (90.0) | 9 (100.0) | 18 (94.7) | 7 (70.0) | 7 (70.0) | 14 (70.0) |
| 95% CI | [66.9–98.7] | [78.9–99.9] | [80.9–98.5] | [55.5–99.7] | [66.4–100.0] | [74.0–99.9] | [34.8–93.3] | [34.8–93.3] | [45.7–88.1] |
| DOR (months) | NR | NR | NR | 8.38 | 3.91 | 8.38 | NR | NR | NR |
| 95% CI | [2.79-NE] | [8.57-NE] | [11.07-NE] | [4.14-NE] | [2.76-NE] | [3.91-NE] | [8.54-NE] | [3.78-NE] | [3.78-NE] |
n, number; Q3W, every 3 weeks; ORR, objective response rate; DCR, disease control rate; DOR, duration of response; CI, confidence interval; NR, not reached; NE, not evaluable. Full analysis set: All subjects in the enrolled analysis set had received at least one study drug therapy and had a measurable lesion at baseline (as defined by RECIST v1.1). ORR was based on the full analysis set of subjects with at least one tumor evaluation after drug administration.
Fig. 2.
Progression-free survival and tumor response in cohort 1. (A) Kaplan–Meier curve of progression-free survival. PFS, progression-free survival; NR, not reached; NE, not evaluable. (B) Swimmer plot of treatment duration for patients grouped by histopathological characteristics and the dose of AK112 (as assessed by RECIST 1.1). Q3W, every 3 weeks; NSQ, non-squamous; SQ, squamous; PR, partial response; SD, stable disease; PD, progressive disease. (C) Spider plot of tumor volume changes over time grouped by histopathological characteristics and the dose of AK112. (D) Waterfall plot of optimal percentage variation of tumor burden in target lesion grouped by histopathological characteristics and the dose of AK112.
In cohort 2 (patients with EGFR-sensitive mutations who failed previous targeted therapy), 5 (26.3%) participants had received the first- or second-generation EGFR-TKIs, and 14 (73.7%) had received the third generation EGFR-TKIs following failure of the first- or second-generation ones. Thirteen out of 19 participants [68.4% (95% CI, 43.4–87.4)] achieved an overall response, and 18 participants [94.7% (95% CI, 74.0–99.9)] achieved disease control with AK112 plus pemetrexed and carboplatin (Table 2). Eleven disease progression events occurred for a median PFS of 8.5 months [95% CI, 5.5-NE]. The 6- and 12-month PFS rates were 63.2% [95% CI, 37.9–80.4] and 35.5% [95% CI, 12.1–60.2], respectively (Supplementary Fig. S2A). Seven out of 19 participants (36.8%) in cohort 2 were still receiving the assigned treatment at the data cutoff date (Supplementary Fig. S2B). Tumor volume was reduced in most patients during treatment, according to the spider plot (Supplementary Fig. S2C). Except for one participant in the 10-mg/kg group, the tumors of all participants shrank compared with baseline (Supplementary Fig. S2D).
In cohort 3 (patients who failed previous systemic platinum-based chemotherapy and PD-1/L1 inhibitor treatments), the ORR was 40.0% (8/20) [95% CI, 19.1–63.9] and DCR 70% (14/20) [95% CI, 45.7–88.1] with AK112 plus docetaxel therapy (Table 2). The median PFS was 7.5 months (95% CI, 2.3-NE), and the 12-month PFS rate was 44.5% [95% CI, 21.1–65.5] (Supplementary Fig. S3A). Six out of 20 participants (30.0%) in cohort 3 were still receiving the assigned treatment (Supplementary Fig. S3B). Based on the spider and waterfall plots, the tumor volume decreased in more than half of the participants (Supplementary Fig. S3C and D).
As shown in Supplementary Table S2, in all three cohorts, the ORRs appeared higher in the participants with PD-L1 expression of 1%–49% and ≥50% compared with <1% (PD-L1-negative).
Safety
Treatment-emergent adverse events (TEAEs) were reported in all 83 (100.0%) participants, with grade 3 or higher TEAEs in 43 (51.8%) participants. Any grade or grade ≥3 treatment-related adverse events (TRAEs) occurred in 76 (91.6%) and 22 (26.5%) participants, respectively. Discontinuation of study drugs due to TRAE occurred in two (2.4%) participants, and one (1.2%) participant died due to TRAE (Table 3).
Table 3.
Summary of adverse events for all patients who received treatment (safety set).
| Event | AK112 (10 mg/kg Q3W) plus chemotherapy (n = 40) |
AK112 (20 mg/kg Q3W) plus chemotherapy (n = 43) |
Total (n = 83) |
|||
|---|---|---|---|---|---|---|
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Any treatment-emergent adverse events | 40 (100.0) | 21 (52.5) | 43 (100.0) | 22 (51.2) | 83 (100.0) | 43 (51.8) |
| Adverse event leading to discontinuation of study drug | 1 (2.5) | 1 (2.3) | 2 (2.4) | |||
| Adverse event leading to death | 1 (2.5) | 4 (9.3) | 5 (6.0) | |||
| Any treatment-related adverse events | 37 (92.5) | 9 (22.5) | 39 (90.7) | 13 (30.2) | 76 (91.6) | 22 (26.5) |
| Any treatment-related adverse event leading to discontinuation of the study drug | 1 (2.5) | 1 (2.3) | 2 (2.4) | |||
| Any treatment-related adverse event leading to death | 1 (2.5) | 0 (0.0) | 1 (1.2) | |||
| Grade 1–2 any treatment-related adverse events occurring in ≥5% of patients in either group and all grade ≥3 any treatment-related adverse events | ||||||
| Hematological toxicities | ||||||
| Decreased white blood cell count | 10 (25.0) | 3 (7.5) | 5 (11.7) | 4 (9.4) | 15 (18.1) | 7 (8.4) |
| Decreased neutrophil count | 7 (17.5) | 1 (2.5) | 7 (16.3) | 4 (9.3) | 14 (16.9) | 5 (6.0) |
| Decreased platelet count | 8 (20.0) | 2 (5.0) | 2 (4.7) | 0 (0.0) | 10 (12.0) | 2 (2.4) |
| Decreased lymphocyte count | 1 (2.5) | 0 (0.0) | 3 (7.0) | 1 (2.3) | 4 (4.8) | 1 (1.2) |
| Anemia | 10 (25.0) | 2 (5.0) | 6 (13.9) | 2 (4.6) | 16 (19.3) | 4 (4.8) |
| Myelosuppression | 2 (5.0) | 2 (5.0) | 0 (0.0) | 0 (0.0) | 2 (2.4) | 2 (2.4) |
| Febrile neutropenia | 1 (2.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Non-hematological toxicities | ||||||
| Alanine aminotransferase increased | 15 (37.5) | 0 (0.0) | 5 (11.6) | 0 (0.0) | 20 (24.1) | 0 (0.0) |
| Increased amylase | 6 (15.0) | 1 (2.5) | 11 (25.6) | 0 (0.0) | 17 (20.5) | 1 (1.2) |
| Aspartate aminotransferase increased | 11 (27.5) | 0 (0.0) | 6 (14.0) | 0 (0.0) | 17 (20.5) | 0 (0.0) |
| C-reactive protein increased | 3 (7.5) | 0 (0.0) | 3 (7.0) | 0 (0.0) | 6 (7.2) | 0 (0.0) |
| Gamma-glutamyltransferase increased | 2 (5.0) | 0 (0.0) | 4 (9.3) | 1 (2.3) | 6 (7.2) | 1 (1.2) |
| Blood bilirubin increased | 4 (10.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 5 (6.0) | 0 (0.0) |
| Weight decreased | 3 (7.5) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 5 (6.0) | 0 (0.0) |
| Blood lactate dehydrogenase increased | 1 (2.5) | 0 (0.0) | 3 (7.0) | 0 (0.0) | 4 (4.8) | 0 (0.0) |
| Blood thyroid stimulating hormone increased | 2 (5.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Blood pressure increased | 2 (5.0) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 2 (2.4) | 1 (1.2) |
| Diarrhea | 3 (7.5) | 0 (0.0) | 6 (14.0) | 0 (0.0) | 9 (10.8) | 0 (0.0) |
| Constipation | 4 (10.0) | 0 (0.0) | 4 (9.3) | 0 (0.0) | 8 (9.6) | 0 (0.0) |
| Nausea | 4 (10.0) | 0 (0.0) | 4 (9.3) | 0 (0.0) | 8 (9.6) | 0 (0.0) |
| Vomiting | 2 (5.0) | 0 (0.0) | 6 (14.0) | 0 (0.0) | 8 (9.6) | 0 (0.0) |
| Toothache | 2 (5.0) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 4 (4.8) | 0 (0.0) |
| Abdominal distension | 2 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.4) | 0 (0.0) |
| Enteritis | 1 (2.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Epistaxis | 4 (10.0) | 0 (0.0) | 10 (23.3) | 0 (0.0) | 14 (16.9) | 0 (0.0) |
| Hemoptysis | 3 (7.5) | 1 (2.5) | 4 (9.3) | 0 (0.0) | 7 (8.4) | 1 (1.2) |
| Productive cough | 2 (5.0) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 4 (4.8) | 0 (0.0) |
| Cough | 0 (0.0) | 0 (0.0) | 3 (7.0) | 1 (2.3) | 3 (3.6) | 1 (1.2) |
| Dyspnoea | 2 (5.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Rash | 4 (10.0) | 0 (0.0) | 5 (11.6) | 0 (0.0) | 9 (10.8) | 0 (0.0) |
| Pruritus | 5 (12.5) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 7 (8.4) | 0 (0.0) |
| Alopecia | 0 (0.0) | 0 (0.0) | 3 (7.0) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Fatigue | 5 (12.5) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 7 (8.4) | 0 (0.0) |
| Chest discomfort | 2 (5.0) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 4 (4.8) | 0 (0.0) |
| Malaise | 2 (5.0) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 4 (4.8) | 0 (0.0) |
| Chest pain | 2 (5.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Oedema peripheral | 3 (7.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Pain | 0 (0.0) | 0 (0.0) | 2 (4.7) | 1 (2.3) | 2 (2.4) | 1 (1.2) |
| Death | 1 (2.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Proteinuria | 9 (22.5) | 0 (0.0) | 9 (20.9) | 0 (0.0) | 18 (21.7) | 0 (0.0) |
| Albuminuria | 3 (7.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Immune-related adverse events | ||||||
| Any event | 5 (12.5) | 2 (5.0) | 9 (20.9) | 0 (0.0) | 14 (16.9) | 2 (2.4) |
| Hypothyroidism | 1 (2.5) | 0 (0.0) | 4 (9.3) | 0 (0.0) | 5 (6.0) | 0 (0.0) |
| Adrenal insufficiency | 1 (2.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Hyperthyroidism | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Pruritus | 2 (5.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Rash | 1 (2.5) | 0 (0.0) | 2 (4.7) | 0 (0.0) | 3 (3.6) | 0 (0.0) |
| Amylase increased | 1 (2.5) | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Blood thyroid stimulating hormone increased | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Hyperglycaemia | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Immune-mediated pneumonitis | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
n, number.
The most common TRAEs (≥10%) were decreased white blood cell count [15 (18.1%)], neutropenia [14 (16.9%)], decreased platelet count [10 (12.0%)], anemia [16 (19.3%)], alanine aminotransferase increase [20 (24.1%)], increased amylase [17 (20.5%)], aspartate aminotransferase increase [17 (20.5%)], proteinuria [18 (21.7%)], epistaxis [14 (16.9%)], rash [9 (10.8%)], and diarrhea [9 (10.8%)]. All reported grade ≥3 TRAEs were provided (Table 3), with the most common being decreased white blood cell count [7 (8.4%)], neutropenia [5 (6.0%)], thrombocytopenia [2 (2.4%)], anemia [4 (4.8%)], and myelosuppression [2 (2.4%)]. Any-grade irAEs were reported in 14 (16.9%) participants, and those of grade 3 or worse were reported in 2 (2.4%) participants. The most common irAEs included hypothyroidism [5 (6.0%)], pruritus [3 (3.6%)], and rash [3 (3.6%)]. Two grade 3 or worse irAEs were adrenal insufficiency [1 (1.2%)], and amylase increase [1 (1.2%)] (Table 3).
Discussion
To our knowledge, it was the first phase II trial evaluating the efficacy and safety of a bispecific antibody targeting PD-1 and VEGF (AK112) in combination with chemotherapy in metastatic NSCLC. AK112 plus platinum-doublet showed promising antitumor activity not only in first-line treatment for advanced NSCLC without driver mutation but also for advanced NSCLC with EGFR-activating mutation that failed previous EGFR-TKI therapy. Besides, AK112 plus docetaxel exhibited favorable antitumor activity in patients with advanced NSCLC who failed prior systemic platinum-based chemotherapy and PD-1/L1 inhibitor treatments. Generally, AK112 was well tolerated in all patients.
Several clinical studies of solid tumors reported the clinical benefits of combining ICIs and antiangiogenic agents.26, 27, 28, 29, 30 In the phase III IMpower-150 trial that examined atezolizumab plus bevacizumab plus chemotherapy (ABCP) as first-line treatment for non-sq-NSCLC, the ORR, and PFS were 63.5% and 8.3 months, respectively. The 6- and 12-month PFS rates were 66.9% and 36.5%, respectively.6 In this study, the confirmed ORR was 53.3% in the participants who received first-line AK112 combined with chemotherapy. The median PFS was not reached, and the 6- and 12-month PFS rates were 79.1% and 59.1%, respectively. The trial with bevacizumab did not include sq-NSCLC, but the present trial included sq-NSCLC. In cohort 1, 18 of the 44 (40.9%) participants had sq-NSCLC, with an ORR of 61.1%.
Dual immunotherapies targeting PD-1 and CTLA-4 were investigated in NSCLC. In the phase III CheckMate 9LA and POSEIDON trials, both sq- and non-sq-NSCLC patients were enrolled, with proportions of 31% (113/361) and 36.7% (124/338) being sq-NSCLC, respectively, in dual immunotherapy groups.31,32 The ORR was 38.2%, the median PFS was 6.7 months, and the 12-month PFS rate was 33% in the nivolumab plus ipilimumab plus chemotherapy group, and the confirmed ORR was 38.8%, the median PFS was 6.2 months, the 12-month PFS rate was 26.6% in the tremelimumab plus durvalumab plus chemotherapy group.31,32 In the present study, AK112 showed promising efficacy as a first-line treatment in NSCLC without driver mutation. Still, because of the small sample size and of no comparator group in the present study, comparisons with previous phase II/III trials can be misleading. Although promising, the efficacy of AK112 has to be confirmed in formal phase III trials. Based on the current study, AK112 (20 mg/kg Q3W) was selected for further investigation.
Patients with NSCLC and EGFR mutations need better therapies after failure of TKI treatment. In the IMpower-150 subgroup of patients with EGFR-sensitive mutations, the ORR was 70.6%, the median PFS was 10.2 months, and the 12-month PFS rate was 42.4% in the ABCP group, while the ORR was 35.6%, the median PFS was 6.9 months, and the 12-month PFS rate was 17.5% in the ACP group, suggesting that atezolizumab combined with bevacizumab plus chemotherapy might be a new subsequent treatment option in patients with EGFR TKI treatment failure.12 Lately, in the phase III ORIENT-31 trial, the ORR was 44%, and the median PFS and 12-month PFS rate were 6.9 months and 28%, respectively, with sintilimab plus IBI305 plus cisplatin plus pemetrexed.13 In cohort 2 of the present study, the ORR was 68.4%, the PFS was 8.5 months, and the 12-month PFS rate was 35.5%. Even with a small sample size, AK112 plus chemotherapy was promising in patients with EGFR mutation who progressed with prior EGFR-TKI therapy. Based on these results, a phase III randomized control trial of AK112 20 mg/kg Q3W plus chemotherapy in these patients is ongoing (NCT05184712).
In patients who failed first-line treatment of PD-1/L1 checkpoint inhibitor plus chemotherapy, no immune-oncology agent or combination was effective. In this refractory setting, there is a major unmet need.33 The Lung-MAP S1800A trial reported that ramucirumab plus pembrolizumab exhibited potential survival benefit (median OS, 14.5 vs. 11.6 months) versus standard treatment, although it did not improve the ORR (22% vs. 28%) or median PFS (4.5 vs. 5.2 months).15 In cohort 3 of the present study, the ORR was 40.0%, the PFS was 7.5 months, and the 12-month PFS rate was 44.5%. Cohort 3 is now extended to validate the efficacy of docetaxel in combination with AK112 in the refractory setting.
This study showed that AK112 plus chemotherapy was well tolerated. This regimen had a much lower rate of grades 3–4 TRAEs compared with the ABCP group of IMpower-150 (26.5% versus 58.5%).6 Patients with sq-NSCLC were also enrolled in the present study, and there was no obvious increased risk of hemoptysis compared with the ABCP group of IMpower-150 (8.4% vs. 4.6%).6 The most common hemorrhagic event was grades 1–2 epistaxis (16.9%). In the other two clinical trials of dual immunotherapy with chemotherapy as first-line treatment of NSCLC, CheckMate 9LA, and POSEIDON, the incidence of grades 3–4 TRAEs were 47% and 51.8%, respectively.31,32 It was 26.5% in the present study. TRAEs leading to treatment discontinuation were 19% and 15.5% in the two trials mentioned above.31,32 The rate was 2.4% here. The irAEs were generally low-grade and manageable using current guidelines. No unexpected AEs were observed.
The major limitations of the present trial were the lack of statistical hypothesis, the non-randomized design, and the small sample size as an exploratory phase II study. The three groups provided preliminary data for their specific type of patients. Further, although the exploratory results suggested that positive and high PD-L1 expression may lead to a better response to AK112, as supported by previous studies,34, 35, 36 no conclusion can be drawn from the present study considering the small number of patients in each subgroup. The biomarkers required to identify a population that benefits from AK112 combination treatment remain to be revealed and confirmed by larger, prospective studies.
In conclusion, AK112, in combination with chemotherapy, showed promising antitumor activity and was safe in patients with advanced NSCLC, suggesting a valuable potential new treatment option for this patient population.
Contributors
LZ, YH, WFF, and YYZ conceived and designed this study. YYZ, GC, JHC, LZ (Li Zhuang), YYD, QTY, WZ, YQZ, MZ, WDZ, YZ, YXW, YPY, WFF, YH, and LZ (Li Zhang) enrolled patients and collected the data. All authors participated in data interpretation. The manuscript was drafted by YYZ, GC, YPY, and YH and was reviewed or revised by all authors. The final version was approved to be submitted by all authors. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. YYZ, GC, JHC, and LZ have accessed and verified the underlying data. LZ supervised the study.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of interests
LZ has received research support from Hengrui, BeiGene, Xiansheng, Eli Lilly, Novartis, Roche, Hansoh, and Bristol-Myers Squibb Pharma and consulting for MSD, Beigene, and Xiansheng Pharma. WL, WFS, ZMW, BYL and MX are employees of Akeso Biopharma. All other authors have no conflicts of interest to declare.
Acknowledgments
The study was funded by Akeso Biopharma, Inc., Zhongshan, China and the National Natural Science Foundation of China (Grants No. 82241232, 82272789, and 82072558). We thank all the patients, their families, and the institutions for supporting these studies.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102106.
Contributor Information
Yunpeng Yang, Email: yangyp@sysucc.org.cn.
Wenfeng Fang, Email: fangwf@sysucc.org.cn.
Yan Huang, Email: huangyan@sysucc.org.cn.
Li Zhang, Email: zhangli@sysucc.org.cn.
Appendix A. Supplementary data
References
- 1.Akinboro O., Larkins E., Pai-Scherf L.H., et al. FDA approval summary: pembrolizumab, atezolizumab, and cemiplimab-rwlc as single agents for first-line treatment of advanced/metastatic PD-L1-high NSCLC. Clin Cancer Res. 2022;28(11):2221–2228. doi: 10.1158/1078-0432.CCR-21-3844. [DOI] [PubMed] [Google Scholar]
- 2.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 3.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 4.Sezer A., Kilickap S., Gümüş M., et al. LBA52 EMPOWER-Lung 1: phase III first-line (1L) cemiplimab monotherapy vs platinum-doublet chemotherapy (chemo) in advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) ≥50% Ann Oncol. 2020;31:S1182–S1183. [Google Scholar]
- 5.Reck M., Remon J., Hellmann M.D. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40(6):586–597. doi: 10.1200/JCO.21.01497. [DOI] [PubMed] [Google Scholar]
- 6.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 7.Soria J.C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 8.Mok T.S., Wu Y.L., Thongprasert S., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y.L., Cheng Y., Zhou X., et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 10.NCCN clinical practice guidelines in oncology (NCCN Guidelines®) Non-small cell lung cancer. Version 1.2023. 2022. [Google Scholar]
- 11.Reck M., Mok T., Socinski M.A., et al. 1293P IMpower150: updated efficacy analysis in patients with EGFR mutations. Ann Oncol. 2020;31:S837–S838. [Google Scholar]
- 12.Reck M., Mok T.S.K., Nishio M., et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 13.Lu S., Wu L., Jian H., et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(9):1167–1179. doi: 10.1016/S1470-2045(22)00382-5. [DOI] [PubMed] [Google Scholar]
- 14.Neal J., Pavlakis N., Kim S.W., et al. 60 CONTACT-01: efficacy and safety from a phase III study of atezolizumab (atezo) + cabozantinib (cabo) vs docetaxel (doc) monotherapy in patients (pts) with metastatic NSCLC (mNSCLC) previously treated with checkpoint inhibitors and chemotherapy. J Thorac Oncol. 2023;18(4):S39–S40. [Google Scholar]
- 15.Reckamp K.L., Redman M.W., Dragnev K.H., et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-lung-MAP S1800A. J Clin Oncol. 2022;40(21):2295–2306. doi: 10.1200/JCO.22.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Yu X., Huang D., et al. SAFFRON-103: a phase 1b study of the safety and efficacy of sitravatinib combined with tislelizumab in patients with locally advanced or metastatic non-small cell lung cancer. J Immunother Cancer. 2023;11(2) doi: 10.1136/jitc-2022-006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S.H., Jeong D., Han Y.S., Baek M.J. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res. 2015;89(1):1–8. doi: 10.4174/astr.2015.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicklin D.J., Ellis L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 19.Bourhis M., Palle J., Galy-Fauroux I., Terme M. Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Yan J., Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georganaki M., van Hooren L., Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol. 2018;9:3081. doi: 10.3389/fimmu.2018.03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 23.Meder L., Schuldt P., Thelen M., et al. Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Cancer Res. 2018;78(15):4270–4281. doi: 10.1158/0008-5472.CAN-17-2176. [DOI] [PubMed] [Google Scholar]
- 24.Zhong T., Huang Z., Pang X., et al. AK112, a tetravalent bispecific antibody targeting PD-1 and VEGF, enhances binding avidity and functional activities and elicits potent anti-tumor efficacy in pre-clinical studies. J Immunother Cancer. 2022;10(Suppl 2):A546–A547. [Google Scholar]
- 25.Zhou C., Ren S., Luo Y., et al. A phase Ib/II study of AK112, a PD-1/VEGF bispecific antibody, as first- or second-line therapy for advanced non–small cell lung cancer (NSCLC) J Clin Oncol. 2022;40(16_suppl):9040. [Google Scholar]
- 26.Rini B.I., Plimack E.R., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 27.McDermott D.F., Huseni M.A., Atkins M.B., et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbst R.S., Arkenau H.T., Santana-Davila R., et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20(8):1109–1123. doi: 10.1016/S1470-2045(19)30458-9. [DOI] [PubMed] [Google Scholar]
- 29.Taylor M.H., Lee C.H., Makker V., et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol. 2020;38(11):1154–1163. doi: 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makker V., Taylor M.H., Aghajanian C., et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981–2992. doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson M.L., Cho B.C., Luft A., et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. 2023;41(6):1213–1227. doi: 10.1200/JCO.22.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld A.J., Antonia S.J., Awad M.M., et al. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann Oncol. 2021;32(12):1597–1607. doi: 10.1016/j.annonc.2021.08.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y., Wan B., Chen X., et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res. 2019;8(4):413–428. doi: 10.21037/tlcr.2019.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dantoing E., Piton N., Salaun M., Thiberville L., Guisier F. Anti-PD1/PD-L1 immunotherapy for non-small cell lung cancer with actionable oncogenic driver mutations. Int J Mol Sci. 2021;22(12):6288. doi: 10.3390/ijms22126288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorgensen J.T. PD-L1 expression and efficacy of pembrolizumab as monotherapy in NSCLC. Chin Clin Oncol. 2020;9(4):60. doi: 10.21037/cco.2020.01.03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.