Abstract
Due to its superior mechanical properties and chemical stability, Polyetheretherketone (PEEK) has emerged as an alternative to conventional metal implants. However, the bio-inertness of PEEK's surface has limited its applications. Ambient sulfonation has been adopted to enhance bioactivity, but its nanoscale topographic changes are insufficient for implant-bone interlock. To further improve bone-implant interlock, this study employs CO2 laser machining to create sub-millimeter (0.5 mm) grooves on PEEK's surface, aiming to encourage bone ingrowth and strengthen the implant-bone interface. This research investigated the physical and chemical properties and bio-interaction of PEEK surface modified by sulfonation (SPEEK), laser machining (L-PEEK), and combination of both technique (L-SPEEK). X-ray photoelectron spectroscopy (XPS) spectra revealed that sulfonation compensates for the surface chemical shift instigated by laser ablation, aligning the surface chemistry of L-SPEEK with that of SPEEK. Furthermore, L-PEEK surfaces presented pores with sizes ranging from 1 to 600 μm, while SPEEK surfaces exhibited pores between 5 and 700 nm. All tested samples demonstrated non-cytotoxicity, with L-SPEEK exhibiting the highest mineralization and ALP activity as 2 and 2.1 times that of intrinsic PEEK, after 21 days of incubation. Microscopic imaging reveals a notably higher extracellular content on L-SPEEK compared to the other groups. This study underscores the potential of combining sub-millimeter laser machining with sulfonation in enhancing early osteogenic markers, providing a promising pathway for future PEEK-based orthopedic applications.
Keywords: Polyetheretherketone, Surface modification, Sulfonation, Laser etching, Osseointegration
Graphical abstract
Highlights
-
•
·Investigated the effect of combining sulfonation and laser machining on Polyetheretherketone (PEEK) surface.
-
•
XPS confirmed that sulfonation counteracts chemical shift caused by laser ablation and confirmed successful sulfonation.
-
•
L-SPEEK surface exhibited non-cytotoxic behavior in viability tests.
-
•
Synergistic effect of sulfonic functional group and laser-created grooves promotes early osteogenic markers.
1. Introduction
Since the revelation of its biocompatibility in late 1980s [1], Polyetheretherketone (PEEK) has gradually became one of the thermoplastics of choice in orthopedic devices, however its applications are still limited due to its low surface bioactivity [[2], [3], [4]]. Despite its advantages over its metal counterpart, for instance, having lower risk of wear particles release, minimum subsidence, and being radio translucence, yet there are reports on post operative complications due to PEEK's relatively lower osseointegration [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. Numerous studies have been focusing on the technique to improve surface cellular response, particularly on the osseointegration [3,[15], [16], [17], [18], [19]]. Currently, a quest to enhance bioactivities on PEEK sulfonation could be classified briefly by physical and chemical means. The physical approaches alter surface topography in micro scale, for instance, increasing roughness by oxygen plasma [20,21] or introducing of micro grooves by laser ablation [22,23]. For the chemical approach, surface treatment and functionalization are evolving around surface activation by sulfuric acid, due to PEEK's impeccable chemical stability. Sulfuric acid etching has recently gained attention as an approach to improve surface bioactivity in orthopedic and dental applications owing to its simplicity and the fact that it does not alter the bulk properties of PEEK.
When exposed to concentrated sulfuric acid, sulfonation occurs on the outermost layer of PEEK substrates, it is a chemical reaction involving protonation and electrophilic substitution. In sulfonation of PEEK, sulfuric attacks benzene ring of the polymer, replacing hydrogen with sulfonic functional group [24,25]. As a result, sulfonated PEEK becomes more hydrophilic, more chemically active, and, in case of PEEK substrates, porosity structure are introduced to the surface immersed in the acid [26]. Several studies have explored biocompatibility and cellular surface adhesion of sulfonated PEEK surface [[27], [28], [29], [30], [31]]. Ying Zhao et al. had successfully implanted a small rod of surface sulfonated PEEK in vivo. The assessment of both cytocompatibility and adhesion of the implants to surrounded bone after 8 weeks demonstrated promising superior osseointegration, compared to PEEK [32].
Laser machining is a pragmatic approach to tailor physical of PEEK surface, specifically for CO2 laser, as the machine is commonly used in industrial, and the operation is done in ambient conditions. It also controls the dimensions of the customized feature precisely, allowing physical alterations to fulfill the application's specifications [33]. D Cordero et al. have examined cells interaction to the surface exposed to laser ablation and established its non-cytotoxicity [34]. Combination of laser and other surface treatment has also been investigated, a study by Yanyan Zheng et al. has shown the improvement of MC3T3-E1 cells proliferation on PEEK surface undergone laser micro-grooving, followed by plasma polymerization [35]. Though it has been established as non-cytotoxic, there has been no literature on clinical applications of PEEK's surface modifications by laser up until now.
While sulfonation at ambient condition is proven to enhance the surface bioactivity, reality of orthopedic applications often requires the ability to withstand mechanical loads and torsion [[36], [37], [38]]. Therefore, combining of both surface modifications techniques could address such challenge by introducing stronger interlock at the interface between PEEK and bone. This work has studied the effects of sulfonation to the laser machined PEEK surface on its physical and chemical properties, along with pre-osteoblast interactions to the surface and osteoconductivity. The objective of this study is to determine the feasibility of combining sulfonation and laser machining as an alternative low energy and simple approach to enhance surface osseointegration of PEEK.
2. Materials and methods
2.1. Sample preparation
Two different dimensions of medical grade PEEK rods, TECAPEEK MT CLASSIX white, were purchased from Ensinger Group, Germany. The PEEK rods were in stock shapes with dimension of 20 mm and 8 mm. The rods with dimension of 8 mm were machined to small disks with dimension of 6 mm and thickness of 2.5 mm to fit in 96-Well plate, while the rods with 20 mm dimension were machined to the thickness of 2.5 mm disks. Surface exposed to blade was polished by sandpapers. All disks were washed in acetone, ethanol, and water in ultrasonic bath at room temperature, consequently, to remove dirt residuals from machining processes. Sulfonation was performed by immersing PEEK disks in sulfuric acid 98% (Loba Chemie, India), with one face submerged in acid, in an ultrasonic bath at room temperature for various time durations. The sulfonation length studied were 10, 50, 90, and 180 s (Labeled as SPEEK10, SPEEK50, SPEEK90 and SPEEK180, respectively). Subsequently, disks were transferred to DI water in the ultrasonic bath at room temperature for 5 min to stop sulfonation. All sulfonated samples (SPEEK) undergone subsequently a post treatment in a different container of new DI water, in ultrasonic bath at 60 °C, to discard leftover sulfuric residual. Lines of 0.5 mm width and 0.5 mm depth, with 0.5 mm space between each line, were engraved on untreated surface of PEEK disks by CO2 laser machine (Epilog, USA), with laser energy at 80 W and pulse frequency of 2500 Hz (L-PEEK) in air. Samples with combination of both surface modifications techniques (L-SPEEK), were subjected to laser engraving prior to sulfonation. All the drying steps took place in air at room temperature for 24 h.
2.2. Surface characterization
Surface roughness of samples from each group was analyzed by an optical microscope (Huvitz HRM-300, Republic of Korea), in which the values were measured and calculated by Panasis software, an official supplement software package of the microscope. Based on surface 3D profile of samples, the software calculated two different roughness parameters: Arithmetical mean deviation of the profile (Ra) and Maximum peak to valley height of the profile (Rz). The wettability of surface was investigated by water contact angle measurements using a goniometer (DSA 10-MK2, Kruss, Germany). A droplet of 100 μl deionized water was placed on each PEEK disk with 20 mm diameter at 5 distinctive locations. Functional groups on surface of samples of all group were observed by ATR-FTIR (Nicolet FTIR, Thermofisher scientific, USA). The scan was performed in absorbance mode at the resolution of 4 cm−1 with steps spanning from vibration frequency of 0–4000 cm−1. The scan was performed with Happ-Genzel apodization at scanning rate of 40 scans per spectrum.
Topography and elemental composition of the sample surface was assessed using field emission scanning electron microscope (FE-SEM) (FEI Quanta FEG 250, Thermo Fisher Scientific, USA) equipped with Energy Dispersive Spectroscopy Systems (EDS) (Aztec, Oxford Instruments, UK). Imaging for samples without cells were conducted without gold coating at low vacuum and at 1.00 kV. Comparison between surface porous structure of L-PEEK and SPEEK were conducted by ImageJ, in which 7 SEM images from different L-PEEK samples and 6 SEM images from L-SPEEK samples were subjected the pore-size distribution analysis. X-ray photoelectron spectroscopy (XPS) (Axis Ultra, Kratos Analytical Ltd, Japan) was employed to analyze the shift of chemical states as the result of each surface modification approach. Type of source was monochromatic aluminum with emission current of 10 mA and anode voltage of 15.0 kV.
2.3. In vitro studies
For cell in vitro studies, MC3T3-E1 cells (ATCC CRL-2594) were used, cells were maintained in low glucose-Minimum Essential Medium α (MEM- α, Gibco), in which the complete medium was supplemented with 10% Fetal Bovine Serum and 1% Antibiotic-Antimycotic (100×) (Anti-Anti, Gibco). Incubation conditions were 37 °C and 5% CO2. The growth medium was replaced three times per week. Prior to cells seeding, sample of all groups (PEEK, SPEEK, L-PEEK, L-SPEEK) were autoclaved at 121 °C under the pressure of 15 psi for 15 min.
2.3.1. Cell viability and adhesion
Cytotoxicity was assessed following ISO10993-5 guideline for direct test. Cells were seeded in 12-Well plate with the density of 3 × 105 cells/ml/well and left to adhere to the tissue culture polystyrene substrate (TCPS) for 24 h. Afterward, samples with dimension of 6 mm diameter (5 repetitive) were placed in each well. CCK-8 assay (96992, Sigma Aldrich, USA) was conducted at 24 h after immersion of samples in tissue culture wells. 5 samples of the same dimension from all groups were placed in a 96-Well plate where MTT assay was employed to assess the viability of cells seeded on surface modified PEEK samples. Cells were seeded with the density of 1 × 104 cells/well. MTT assay was performed after 1, 3 and 7 days of incubation. To study cells’ adhesion and morphology, SEM imaging was conducted on samples from 1, 3 and 7 days timepoints. Samples were rinsed 3 times with PBS before undergoing cells fixation with 2.5% glutaraldehyde and dehydration by immersing in ethanol with gradient concentration and Hexamethyldisilane (HMDS). Following the dehydration process, samples were left to dry completely in a desiccator before sputter-coated by gold.
2.3.2. Cell proliferation
In the same manner as the previous section, cells were seeded on the surface of PEEK samples in a 96-Well plate, with the density of 1 × 104 cells/well. At 1, 3 and 7 days timepoints, samples were rinsed with PBS before fixation with 4.7% formaldehyde in Hanks' Balanced Salt Solution (HBSS). Cells cytosol was stained with Calcein AM (Invitrogen, USA) while nuclei were stained with DAPI (Invitrogen, USA). Fluorescent imaging was performed by Axio Scope A1 (Carl Zeiss AG, Germany). Fluorescent images were processed and merged by ZEN 3.5 software.
2.3.3. Alkaline phosphatase (ALP) activity
PEEK substrates of all groups in dimension of 20 mm diameter and 2.5 mm thickness were placed in 12-Well plates (4 repetitive) with cells seeding density of 1.5 × 105 per well. Alkaline phosphatase activity was determined at the timepoints of 14 and 21 days by Alkaline Phosphatase Assay Kit Ab83369 (Abcam, UK). The assay was performed according to the manufacturer's protocol. In brief, cells were detached using Trypsin following by cells lysis with RIPA buffer by 1× protease inhibitor in fridge for 5 min. Cells suspensions were then centrifuged at 15,000×g for 8 min to obtain lysate solution. Lysate solution from 14 days was stored at −70 °C until 21st day after incubation in which the lysate from both 14 days and 21 days were then reacted with pNPP solution. ALP standard curves were also constructed following the manufacture's protocol.
2.3.4. Mineralization
Three samples from each group of surface-modified 20 mm diameter PEEK substrates were incubated in 12-Well plates for mineralization study. Calcium secretion from MC3T3-E1 cells was examined by Alizarin red S (ARS) Ab146374 (Abcam, UK) staining at 14 and 21 days. At each timepoint, cells were fixed with 3.75% formaldehyde in PBS, rinsed three times with PBS and were permeabilized with Triton X-100, respectively. Sequentially, samples were incubated with ARS for 45 min at 37 °C then rinsed with PBS three times before imaging. For quantification of calcium ions, each sample was treated with Cetylpyridinium chloride (CPC) (Sigma, USA) in Sodium Dihydrogen Orthophosphate solvent (Ajax Finechem, Australia) to extract calcium. Microplate reader scanned the optical density of extraction at the absorbance of 540 nm.
2.4. Statistical analysis
All in vitro studies data and surface roughness were subjected to one-way ANOVA statistical analysis with significant level at P-value less than 0.05. Charts are represented as mean averages with error bars representing standard deviation ranges.
3. Result and discussion
3.1. Duration of sulfonation
Microscopic images and surface 3D profiles (Fig. 1) reveal the effect of sulfonation length to surface topography of PEEK samples. Fig. 1B suggests that 10 s exposure to sulfuric acid is sufficient to modify the surface by introducing nanopores, while also increasing the wettability. The size of surface macropores increases with the longer sulfonation time (10, 50, and 90 s), however for SPEEK180, this trend was absent. This is in accordance with roughness, specifically Rz, in which SPEEK180 exhibits lower surface Rz than SPEEK90. On the other hand, effect on contact angle exhibits different trend, there is no significant variation among average surface contact angle of SPEEK10, SPEEK50 and SPEEK90, however, mean contact angle of SPEEK180 was subsequently higher than the pristine PEEK surface. The subverted trend in contact angle and wettability of SPEEK180 is due to the dissolution of PEEK, which also arose simultaneously during sulfonation [39], causing the loss in polymer mass on the surface thus reducing the surface micropores (Fig. 1E). Measurement of surface roughness post sulfonation confirms that roughness and wettability of PEEK surface could be improved by sulfonation only at some extend, excessive sulfonation length reverts the trend hence decreases hydrophilicity. Since SPEEK90 displays the optimal roughness and wettability (surface contact angle of 80.31° ± 10), duration of sulfonation in this study was set to be 90 s.
Fig. 1.
(A–E) 3D profile of PEEK and sulfonated PEEK with sulfonation duration of 10s, 50s, 90s and 180s, respectively. Upper right corner is water droplet from contact angle test. Adjacent are the images from optical microscope. (F) Is Arithmetical mean deviation of the profile (Ra) and (G) is Maximum peak to valley height of the profile (Rz) (n = 5), (H) is surface contact angle (5 different spot from 3 samples of each group) (*p < 0.05 for all charts).
3.2. Surface characteristics
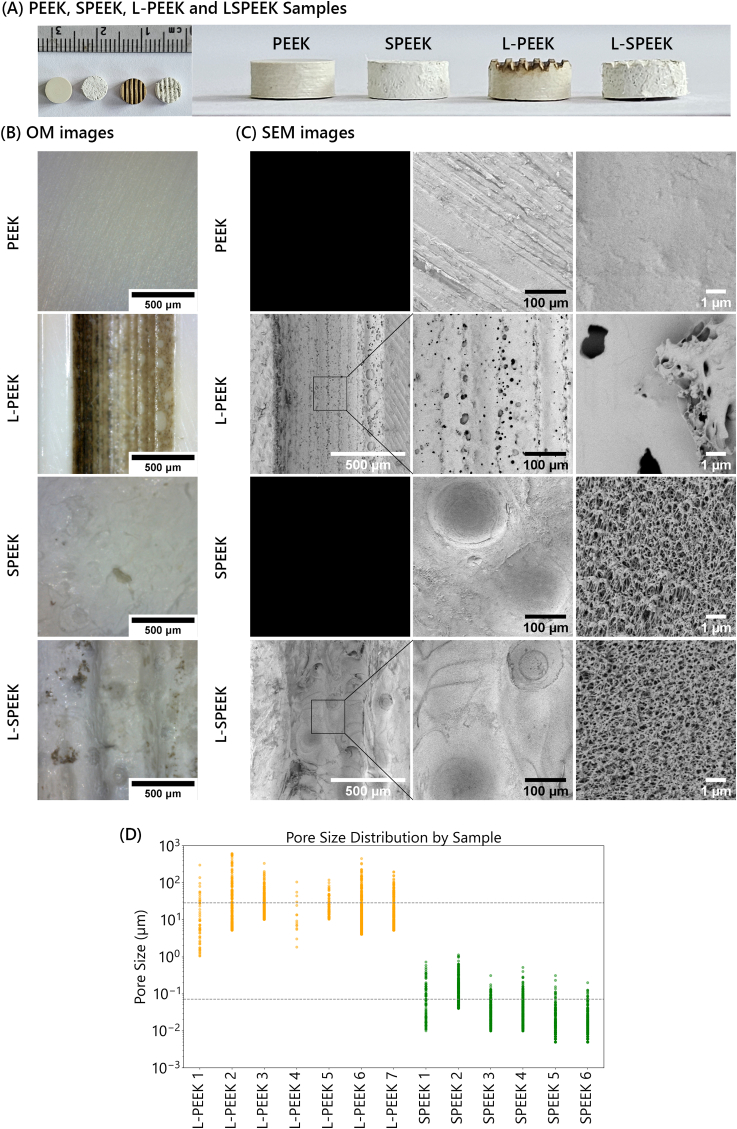
From FTIR spectra (Fig. 3a), L-PEEK shows additional peaks at around 1707 cm−1 (C O), 1450 cm−1 (C–H bending), and 965 cm−1 (C C bending), comparing to PEEK, indicating scissoring of PEEK chain by laser energy which potentially broke ketone and aromatic moieties. SPEEK spectrum displays comparable pattern as PEEK but with the sharper peak at 1488 cm−1 (Benzene ring) and 926 cm−1 (C O stretching). Peak at 1218 cm−1 appeared to be taller than peaks at 1157 cm−1 and 1099 cm−1 for sulfonated PEEK comparing the non-sulfonated PEEK which three peaks show similar intensity, nevertheless it is inconclusive whether which functional group caused the shift of these peaks as the range covers both S O stretching and C–O stretching. L-SPEEK spectrum is analogous to SPEEK, implying the sulfuric etching discarded the thin surface layer, which was the effect of laser energy. SEM images provide visual evidence of successful surface modification in L-PEEK, and L-SPEEK, comparing to the plain surface of PEEK. The image of L-SPEEK from area both with and without laser engraving (Fig. 2A) demonstrate distributed microporous network similar to images from the other studies of sulfonated PEEK substrates [26,32], whereas laser ablation alone left pores in the larger scale on the surface. The difference in porous characteristic between the two surface modification techniques is significant. The average size of pores created by laser ablation is 25 μm, with the range of 1–600 μm, whilst the size distribution of sulfonated porous structure is rather in the nanometer scale, ranging between 5 nm and 700nm with the average value of 70 nm. Since this area was not exposed to laser ablation, it also represents samples of SPEEK group. Nodule structures could be the result of conversion from crystalline to amorphous after exposing to the thermal energy as per study by Hartwig et al. [22]. EDS spectra do not show significant change to the surface composition, thus, further evaluation from the other characterization techniques is required to provide better understanding of the shift in elemental composition. Incorporation of sulfur was also confirmed by the presence of S peak at around 2.307 KeV, though the peak is subtle and could only be detected at high kV beam (Fig. 3c).
Fig. 3.
(a) FTIR spectra, (b–c) EDS spectra and, (d) XPS wide scan spectra of PEEK, L-PEEK, SPEEK and L-SPEEK.
Fig. 2.
(A) Photographs of PEEK, SPEEK, L-PEEK and L-SPEEK samples. (B) Images from optical microscope (C) Micro-topography of samples as captured by SEM, noting that high magnification of L-PEEK and L-SPEEK were done in the bottom of the grooves, where the surface was blasted by laser. (D) Dots plot representing pore size distribution on L-PEEK (in the area exposed to laser ablation) and SPEEK surface.
Greater detail of surface chemistry deviance caused by both surface modification techniques was assessed by XPS studies. Wide scan spectrum shows characteristic peaks of PEEK, which C1s, O1s and O (KLL) orbital are prominent. Appearance of S2s and S2p peaks (Fig. 3d) are consistent with EDS analysis, which affirms sulfonation on SPEEK and L-SPEEK. C1s, O1s and S2p spectra from narrow scan offers information about change in orbital levels portions (Table 1). Both sulfonated groups exhibit the similar percent change of peaks at binding energy of 284.70 ± 0.04 eV, 286.26 ± 0.06 eV, 287.59 ± 0.39 eV and 291.78 ± 0.51 eV, corresponding to C–C/C–H, C–O, C O, and aromatic π bonds respectively (Fig. 4a–d). One notable aspect is the increasing of percentage at 286.26 ± 0.06 eV, this energy level was addressed as C–O for PEEK and L-PEEK, however its peak was enhanced in sulfonated groups. In sulfonation, the chemical reaction is first degree, where sulfonic groups attack the benzene rings adjacent by ketone [40,41], inferring ketone groups are not affected by sulfonic substitution. Hence, significant rising at 286.26 ± 0.06 eV was not contributed by multiplying of C–O, but rather because of the additional of C–S bond which exhibits the same energy level. The similar augmentation of peak at C–O and C–S energy level, was also observed in the other study on sulfonated graphene [42]. On the other hand, atomic percentage of all C1s orbital levels on L-PEEK surface was consistent with PEEK, deeming that laser ablation has negligible effect on surface chemistry despite the optical quality which laser treated PEEK has brownish color (Fig. 2B). The curve convolution of O1s prevails addition of two peaks at 530.9 ± 1 eV and 532.0 ± 1 eV in the sulfonated groups, denoting O–H and O–S bonds of the sulfonic moiety. While in non-sulfonated samples, O1s spectrums comprise of three peaks at 531.44 ± 0.18 eV, 533.33 ± 0.01 eV, and 534.68 ± 0.31 eV, representing O C/O S, O–C and O–H bond respectively (Fig. 4e–f). As per XPS characteristic study of M.M. Nasef et al. on grafted sulfonic acid membrane, O S bonding exhibits binding energy around 531.5 eV [29], this value agrees with another overlap energy peak at 531.44 ± 0.18 in this study. Therefore, shift in the peaks at 530.88 ± 0.02 eV, 531.44 ± 0.18 eV, and 531.99 ± 0.01 eV is correlated to sulfonic incorporation, noting that 530.88 ± 0.02 eV is of O–H on sulfonic group whereas 534.68 ± 0.31 in all groups is hydroxy group at polymer chain's terminals. S2p spectra of SPEEK and L-SPEEK (Fig. 4i and j) assure integration of sulfonic acid on the polymer chain, as the two highest atomic portions are of SO3− at S2p3/2 (167.19 ± 0.11 eV) and S2p1/2 (168.07 ± 0.04 eV) and the third highest peak is of S–C bond at 170.52 ± 0.02 eV. The congruity between SPEEK and L-SPEEK atomic percentage in S2p orbital also confirms that PEEK surface which underwent laser machining following by sulfonation (L-SPEEK) demonstrated the same surface chemistry as SPEEK. The difference in cells interaction and level of bioactivities between SPEEK and L-SPEEK is thus stemmed from the sub-millimeter topographical feature, rather than the surface chemistry.
Table 1.
Atomic composition of C1s, O1s and S2p orbital.
| Sample Group |
BE [eV±SD] | |||||
|---|---|---|---|---|---|---|
| PEEK | SPEEK | L-PEEK | L-SPEEK | |||
| C1s | C–C/C–H | 79.17% | 72.26% | 80.00% | 73.31% | 284.70 ± 0.04 |
| C–O/C–S | 16.35% | 22.94% | 16.22% | 21.09% | 286.26 ± 0.06 | |
| C O | 2.82% | 1.64% | 2.05% | 2.74% | 287.59 ± 0.39 | |
| π | 1.65% | 3.15% | 1.73% | 2.86% | 291.78 ± 0.51 | |
| O1s | O–H | – | 4.84% | – | 4.29% | 530.88 ± 0.02 |
| O C/O S | 35.56% | 16.85% | 35.30% | 20.56% | 531.44 ± 0.18 | |
| O–S | – | 15.96% | – | 14.42% | 531.99 ± 0.01 | |
| O–C | 59.89% | 55.44% | 61.49% | 54.27% | 533.33 ± 0.01 | |
| O–H | 4.55% | 6.92% | 3.21% | 6.46% | 534.68 ± 0.31 | |
| S2p | S2p3/2 (S–C) | – | 3.38% | – | 2.18% | 170.52 ± 0.02 |
| S2p3/2 (SO3−) | – | 12.88% | – | 13.80% | 167.19 ± 0.11 | |
| S2p1/2 (SO3−) | – | 52.63% | – | 54.20% | 168.07 ± 0.04 | |
| S2p3/2(SO42−) | – | 31.11% | – | 29.83% | 169.19 ± 0.04 | |
Fig. 4.
XPS high resolution spectra and their peak convolutions of (a–d) C1s orbital, and (e–h) O1s of all groups. (i–j) S2p orbital of SPEEK and L-SPEEK.
3.3. In vitro studies
3.3.1. Biocompatibility
Viability assessment after 24 h direct test incubation indicated the non-cytotoxicity of all sample groups (Fig. 5d), in which the level of cell viability among groups shows no substantial difference. In contrary, cells adhesion and proliferation were significantly affected by the surface treatment, however samples with both laser ablation and sulfonation exhibit lower MTT absorbance than laser machining or sulfonation alone. It can be concluded that both laser and sulfonation improve cell proliferation, though with a different mechanism. SEM and micro fluorescence images were used to evaluate the influence of physical and chemical changes on the behavior of MC3T3-E1 cells. The morphology of cells adhering on the PEEK surface appears flat. In Fig. 5a, it is ambiguous whether the flat layer covering the PEEK surface represents the entire cell cytosol or not, as the layer could also be formed by ECM molecules secreted from cells. Cell morphology of MC3T3-E1 differs on the SPEEK surface, and the edge of the cytoplasm could be identified by filopodium protruding out to attach to the SPEEK surface. Rod-like structures were found to be disseminated all over the surface of both groups of sulfonated PEEK after 7 days of incubation, indicating that such structures are likely ECM secreted from MC3T3-E1 cells. EDS analysis did not detect significant signal of mineral elements, implying that the structure of interest might be organic (e.g., Protein). It is suggestive that sulfonic group promotes this ECM release, though the presence of this structure could also be the result of enhanced protein adsorption on sulfonated surface. Further study should be conducted to confirm which kind of protein is present and whether it has a positive effect on osteogenic differentiation. Among all sample groups, cells on L-PEEK surface had the longest filopodium and the smallest cytoplast size. The altered surface chemistry is the cause of variation in cell morphology between these two sample groups. Greater polar functional groups (O–H and O S) on SPEEK and L-SPEEK increase hydrophilicity and improve protein adsorption, whilst L-PEEK surface chemistry retains PEEK's nonpolar characteristics. Nonetheless, laser-induced micropores and micronodules give a superior physical adhering side for cells, resulting in prominent filopodia.
Fig. 5.
(a) SEM images of PEEK, SPEEK, L-PEEK and L-SPEEK surface, 24 h after incubation with MC3T3-E1 cells seeded on surface. (b) SEM image of rod structure assumed to be ECM secreted from cells, this image is from SPEEK with MC3T3-E1 cells incubation for 24 h. (c) Comparison between Calcein AM fluorescence versus PEEK's autofluorescence after 24 h incubation. (d) Viability as tested following ISO10993-5 guideline and (e) proliferation of MC3T3-E1 cultured on surface of PEEK, SPEEK, L-PEEK, L-SPEEK and TCPS for 1, 3, 7 days. (*p < 0.05, n = 5).
In Fig. 6, cell nuclei were stained with DAPI (Invitrogen,USA) and the cytosol was stained with Calcein AM (Invitrogen, USA). Cells in all sample group successfully proliferated to fully cover the PEEK surface within the third day of incubation. The dark area in L-SPEEK sample at 7 days of incubation could be ECM as observed from SEM images of L-SPEEK. The auto-fluorescence properties of PEEK itself could be useful to explain such phenomena [43]. In Fig. 5c, the bright background in autofluorescence images is the PEEK substrate as excited by photons in the range around 500 nm, while opaque masses are other compounds foreign to the intrinsic PEEK substrate. Together with Calcein AM staining images, which small bright green dots represent cells body, the images suggest deposition of ECM molecules on the surface of samples. Although MTT assay suggests the highest metabolic activity in L-PEEK samples, it is not explicit that laser machining yields better osteoconductivity since there are other factors affecting the osteogenic behavior further than proliferation rate. MC3T3-E1 cells proliferating on L-PEEK surfaces exhibit significant smaller cytosol size compared to cells on PEEK or TCPS, thereby the higher absorbance of MTT assay in L-PEEK is rather due to the higher number of cells. Nonetheless, both SEM and fluorescence images reveal that sulfonation stimulated ECM secretion while the addition of sub-millimeter grooves on the surface, from laser ablation, promoted ECM deposition in the grooves.
Fig. 6.
Fluorescence images of sample surface after 1, 3, 7 days of incubation, green is MC3T3-E1 cytosol stained by Calcein AM and blue is nucleus stained by DAPI. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3.2. Osteogenic differentiation
The evaluation of osteogenic phenotype was conducted on MC3T3-E1 cells without any osteogenic supplements, in order to study the correlation between the intrinsic properties of the modified PEEK surfaces and osteogenic differentiation. Cells in both studies were of the subculture passage lower than 26th in order to maintain their osteogenic differentiation behavior as suggested by W.J. Peterson et al. [44] and Xiang-Zhen Yan et al. [45]. Red nodules resulted from alizarin red staining were apparent in all group at the 14th day (Fig. 7a). The density of such red nodules was visually higher in samples incubated for 21 days, though the visual evidence does not wholly suggest which group promotes greater calcium deposition. Results from absorbance at 540 nm show that L-SPEEK has the highest concentration of Alizarin red extracts following by L-PEEK, SPEEK and PEEK, respectively (Fig. 7b). ALP activity in all groups was detected at 14th day and increased by the 21st day (Fig. 7c), parallel to a study from L. Darryl Quarles et al., in which MC3T3-E1 cells supplemented by Ascorbate and β-glycerol phosphate displayed initial elevation of ALP activity around 15th day [46]. Enhancement of these two osteogenic markers in SPEEK comparing to PEEK is likely the result of both increased hydrophilicity and nano porosity. It has been established in literature that such pores promote the formation of a filopodia structure, which in turn encourages cells to interact with their environment and secrete ECM components [47,48]. In case of L-PEEK, the significant higher release of calcium and ALP activity, in spite of the non-functional surface chemistry, was instigated by the presence of sub-millimeter grooves, micro pores and micro nodules on the surface. Roughness has been acknowledged to influence osteogenesis in both human bone mesenchymal stem cells and pre-osteoblast cell lines. Specifically, roughness in the micrometer-scale enhances cellular response and osteogenic gene expression [[49], [50], [51]]. As a result, L-SPEEK exhibits the highest capacity to stimulate mineralization and ALP activity, which is attributed to the combination of nanopores and enhanced protein adsorption from sulfonation, as well as the sub-millimeter groove topography introduced by laser machining. The data from both studies display a consistent trend among the four groups, thereby validating the synergistic effect of sulfonation and topography alteration using a CO2 laser on the early osteogenic markers of MC3T3-E1 cells.
Fig. 7.
(a) Photograph of sample surface of all groups as stained by Alizarin red after 14 and 21 days of MC3T3-E1 cells culture. (b) Optical absorbance of Alizarin red as extracted from samples (n = 3) and (c) Measurement of ALP activity (p < 0.05 for all groups, n = 4). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Conclusions
This study assesses the effects of combining sulfonation and CO2 laser machining to create sub-millimeter grooves on the PEEK surface, considering the physical and chemical properties, and cell-surface interaction using mouse pre-osteoblast MC3T3-E1 cells. Sulfonation introduces –SO3 functional groups and interconnected nanopores to the surface, thereby altering the surface chemistry and nanotopography of the PEEK substrate. In contrast, laser machining provides controllable sub-millimeter topological features, leaving micropores and micro-nodules on the areas subjected to laser ablation and thus modifying the surface's sub-millimeter and micro-topography. Despite variations in optical quality, PEEK surfaces exposed to laser ablation are non-cytotoxic. Independently, both surface modification techniques improve cell adhesion and proliferation on the PEEK surface. When these techniques are combined, L-SPEEK exhibits the highest early osteogenic markers. SEM and fluorescence imaging reveal a significant amount of extracellular matrix secretion on the L-SPEEK surface. This approach to modifying the PEEK surface could be a practical option for the industry to manufacture orthopedic devices with enhanced osseointegration due to its simplicity. However, successful implantation requires further investigation into the adhesive strength between the implant and the bone, as well as the body's long-term response to the implant. Thus, the next crucial step for advancing this surface modification technique towards practical application is an in-depth in vivo study.
CRediT authorship contribution statement
Slila Chayanun: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization, Project administration. Theerapat Chanamuangkon: Formal analysis, Investigation, Data curation. Budsaraporn Boonsuth: Investigation. Boonrat Lohwongwatana: Methodology, Validation, Resources, Writing – review & editing, Supervision, Funding acquisition. Aldo R. Boccaccini: Validation, Resources, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
This work was funded by the Royal Golden Jubilee (RGJ) Ph.D. Programme of The Thailand Research Fund, Thailand [grant numberPHD/0250/2560] with fund receiver code of 1.BE.CU/60/D.1.N.XX
Contributor Information
Slila Chayanun, Email: slila.chayanun@gmail.com.
Theerapat Chanamuangkon, Email: teera.pat.n61@gmail.com.
Budsaraporn Boonsuth, Email: smindbudsaraporn@gmail.com.
Aldo R. Boccaccini, Email: aldo.boccaccini@fau.de.
Boonrat Lohwongwatana, Email: boonrat.l@chula.ac.th.
Data availability
Data will be made available on request.
References
- 1.Williams D.F., McNamara A., Turner R.M. Potential of polyetheretherketone (PEEK) and carbon-fibre-reinforced PEEK in medical applications. J. Mater. Sci. Lett. 1987;6(2):188–190. [Google Scholar]
- 2.de Ruiter L., Janssen D., Briscoe A., Verdonschot N. Fixation strength of a polyetheretherketone femoral component in total knee arthroplasty. Med. Eng. Phys. 2017;49:157–162. doi: 10.1016/j.medengphy.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Torstrick F.B., et al. Getting PEEK to stick to bone: the development of porous PEEK for interbody fusion devices. Tech. Orthop. 2017;32(3):158–166. doi: 10.1097/BTO.0000000000000242. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieminen T., Kallela I., Wuolijoki E., Kainulainen H., Hiidenheimo I., Rantala I. Amorphous and crystalline polyetheretherketone: mechanical properties and tissue reactions during a 3-year follow-up. J. Biomed. Mater. Res., Part A. 2008;84A(2):377–383. doi: 10.1002/jbm.a.31310. Feb. [DOI] [PubMed] [Google Scholar]
- 5.Punchak M., et al. Outcomes following polyetheretherketone (PEEK) cranioplasty: systematic review and meta-analysis. J. Clin. Neurosci. 2017;41:30–35. doi: 10.1016/j.jocn.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Polacek M., Nyegaard C.P., Høien F. Day-case opening wedge high tibial osteotomy with intraosseous PEEK implant. Arthrosc. Sport. Med. Rehabil. 2020;2(2):e145–e151. doi: 10.1016/j.asmr.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Z., et al. 2020. Articular Cartilage and Bone Changes Following Treatment of Focal Osteochondral Defects in the Femoral Head with Polyether Ether Ketone Implants versus Cobalt Chromium Molybdenum Alloy Implants: Assessment in a Goat Model. [Google Scholar]
- 8.Sharma N., et al. Quantitative assessment of point-of-care 3D-printed patient-specific polyetheretherketone (PEEK) cranial implants. Int. J. Mol. Sci. 2021;22(16) doi: 10.3390/ijms22168521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomidis S., Ashqar G., Kaulhausen T., Herren C., Siewe J., Sobottke R. Clinical experiences with a PEEK-based dynamic instrumentation device in lumbar spinal surgery: 2 years and no more. J. Orthop. Surg. Res. 2018;13(1):196. doi: 10.1186/s13018-018-0905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan K., Hogan J.A., Assem Y., Mobbs R.J. PEEK-Halo effect in interbody fusion. J. Clin. Neurosci. 2016;24:138–140. doi: 10.1016/j.jocn.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Lindtner R.A., Schmid R., Nydegger T., Konschake M., Schmoelz W. Pedicle screw anchorage of carbon fiber-reinforced PEEK screws under cyclic loading. Eur. Spine J. 2018;27(8):1775–1784. doi: 10.1007/s00586-018-5538-8. [DOI] [PubMed] [Google Scholar]
- 12.Hevesi M., et al. High tibial osteotomy with modern PEEK implants is safe and leads to lower hardware removal rates when compared to conventional metal fixation: a multi-center comparison study. Knee Surg. Sports Traumatol. Arthrosc. 2019;27(4):1280–1290. doi: 10.1007/s00167-018-5329-0. [DOI] [PubMed] [Google Scholar]
- 13.Behrbalk E., Uri O., Parks R.M., Musson R., Soh R.C.C., Boszczyk B.M. Fusion and subsidence rate of stand alone anterior lumbar interbody fusion using PEEK cage with recombinant human bone morphogenetic protein-2. Eur. Spine J. 2013;22(12):2869–2875. doi: 10.1007/s00586-013-2948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atef M., Mounir M., Shawky M., Mounir S., Gibaly A. Polyetheretherketone patient-specific implants (PPSI) for the reconstruction of two different mandibular contour deformities. Oral Maxillofac. Surg. 2022;26(2):299–309. doi: 10.1007/s10006-021-00984-6. [DOI] [PubMed] [Google Scholar]
- 15.Goharian A., Abdullah M.R., Kadir M.R.A. Elsevier Inc.; 2017. Bioinert Polymers (Polyetheretherketone) [Google Scholar]
- 16.Almasi D., Iqbal N., Sadeghi M., Sudin I., Abdul Kadir M.R., Kamarul T. Preparation methods for improving PEEK's bioactivity for orthopedic and dental application: a review. Int. J. Biomater. 2016;2016 doi: 10.1155/2016/8202653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H., Goel V.K., Bhaduri S.B. A fast route to modify biopolymer surface: a study on polyetheretherketone (PEEK) Mater. Lett. 2014;125:96–98. [Google Scholar]
- 18.Hahn B.D., et al. Osteoconductive hydroxyapatite coated PEEK for spinal fusion surgery. Appl. Surf. Sci. 2013;283:6–11. [Google Scholar]
- 19.Ouyang L., et al. Influence of sulfur content on bone formation and antibacterial ability of sulfonated PEEK. Biomaterials. 2016;83:115–126. doi: 10.1016/j.biomaterials.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki N., Tasaka S., Horiuchi T., Suyama R. Surface modification of poly (aryl ether ether ketone) film by remote oxygen plasma. J. Appl. Polym. Sci. 1998;68(2):271–279. [Google Scholar]
- 21.Ha S.W., Hauert R., Ernst K.H., Wintermantel E. Surface analysis of chemically-etched and plasma-treated polyetheretherketone (PEEK) for biomedical applications. Surf. Coating. Technol. 1997;96(2–3):293–299. [Google Scholar]
- 22.Hartwig A., Hunnekuhl J., Vitr G., Dieckhoff S., Vohwinkel F., Hennemann O.D. Influence of CO2 laser radiation on the surface properties of poly (ether ether ketone) J. Appl. Polym. Sci. 1997;64(6):1091–1096. [Google Scholar]
- 23.Laurens P., Sadras B., Decobert F., Arefi-Khonsari F., Amouroux J. Enhancement of the adhesive bonding properties of PEEK by excimer laser treatment. Int. J. Adhesion Adhes. 1998;18(1):19–27. [Google Scholar]
- 24.Jin X., Bishop M.T., Ellis T.S., Karaszt F.E. A Sulphonated Poly (ary1 Ether Ketone) 1985;17(1):4–10. [Google Scholar]
- 25.Shibuya N., Porter R.S. Kinetics of PEEK sulfonation in concentrated sulfuric acid. Macromolecules. 1992;25(24):6495–6499. [Google Scholar]
- 26.Wang W., Luo C.J., Huang J., Edirisinghe M. PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications. J. R. Soc. Interface. 2019;16 doi: 10.1098/rsif.2018.0955. 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brum R.S., et al. On the sulphonated PEEK for implant dentistry: biological and physicochemical assessment. Mater. Chem. Phys. 2019;223:542–547. November 2018. [Google Scholar]
- 28.Sun Z., Ouyang L., Ma X., Qiao Y., Liu X. Controllable and durable release of BMP-2-loaded 3D porous sulfonated polyetheretherketone (PEEK) for osteogenic activity enhancement. Colloids Surf. B Biointerfaces. 2018;171(August):668–674. doi: 10.1016/j.colsurfb.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Nasef M.M., Saidi H. Surface studies of radiation grafted sulfonic acid membranes: XPS and SEM analysis. Appl. Surf. Sci. 2006;252(8):3073–3084. [Google Scholar]
- 30.Li Y., et al. Bending Properties, compression Properties, biocompatibility and bioactivity of sulfonated carbon Fibers/PEEK composites with graphene oxide coating. Appl. Surf. Sci. 2022;575 October 2021. [Google Scholar]
- 31.Jeeranun P., Chalongkuakul N., Arksornnukit M., Silthampitag P., Chaijareenont P. Sulfonated PEEK characteristic after various surface cleaning techniques. J. Int. Dent. Med. Res. 2022;15(1):131–139. [Google Scholar]
- 32.Zhao Y., et al. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials. 2013;34(37):9264–9277. doi: 10.1016/j.biomaterials.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 33.Riveiro A., et al. Laser surface modification of PEEK. Appl. Surf. Sci. 2012;258(23):9437–9442. [Google Scholar]
- 34.Cordero D., López-Álvarez M., Rodríguez-Valencia C., Serra J., Chiussi S., González P. In vitro response of pre-osteoblastic cells to laser microgrooved PEEK. Biomed. Mater. 2013;8(5) doi: 10.1088/1748-6041/8/5/055006. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y., Xiong C., Wang Z., Li X., Zhang L. A combination of CO 2 laser and plasma surface modification of poly(etheretherketone) to enhance osteoblast response. Appl. Surf. Sci. 2015;344:79–88. [Google Scholar]
- 36.Pokorný D., Fulín P., Slouf M., Jahoda D., Landor I., Sosna A. [Polyetheretherketone (PEEK). Part II: application in clinical practice] Acta Chir. Orthop. Traumatol. Cech. 2010;77(6):470–478. [PubMed] [Google Scholar]
- 37.Kurtz S.M. Elsevier Inc.; 2012. Applications of Polyaryletheretherketone in Spinal Implants: Fusion and Motion Preservation. [Google Scholar]
- 38.Kurtz S.M., Devine J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailly C., Williams D.J., Karasz F.E., MacKnight W.J. The sodium salts of sulphonated poly(aryl-ether-ether-ketone) (PEEK): preparation and characterization. Polymer (Guildf). 1987;28(6):1009–1016. [Google Scholar]
- 40.Shibuya N., Porter R.S. A kinetic study of PEEK sulfonation in concentrated sulfuric acid by ultraviolet-visible spectroscopy. Polymer (Guildf). 1994;35(15):3237–3242. [Google Scholar]
- 41.Huang R.Y.M., Shao P., Burns C.M., Feng X. Sulfonation of poly(ether ether ketone)(PEEK): kinetic study and characterization. J. Appl. Polym. Sci. 2001;82(11):2651–2660. [Google Scholar]
- 42.Li C., et al. Sulfonic acid functionalized graphene oxide paper sandwiched in sulfonated poly(ether ether ketone): a proton exchange membrane with high performance for semi-passive direct methanol fuel cells. Int. J. Hydrogen Energy. 2017;42(26):16731–16740. [Google Scholar]
- 43.Althaus J., Padeste C., Köser J., Pieles U., Peters K., Müller B. Nanostructuring polyetheretherketone for medical implants. Eur. J. Nanomed. 2012;4(1):7–15. [Google Scholar]
- 44.Peterson W.J., Tachiki K.H., Yamaguchi D.T. Serial passage of MC3T3-E1 cells down-regulates proliferation during osteogenesis in vitro. Cell Prolif. 2004;37(5):325–336. doi: 10.1111/j.1365-2184.2004.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan X.Z., Yang W., Yang F., Kersten-Niessen M., Jansen J.A., Both S.K. Effects of continuous passaging on mineralization of MC3T3-E1 cells with improved osteogenic culture protocol. Tissue Eng. C Methods. 2014;20(3):198–204. doi: 10.1089/ten.tec.2012.0412. [DOI] [PubMed] [Google Scholar]
- 46.Quarles L.D., Yohay D.A., Lever L.W., Caton R., Wenstrup R.J. Distinct proliferative and differentiated stages of murine MC3T3‐E1 cells in culture: an in vitro model of osteoblast development. J. Bone Miner. Res. 1992;7(6):683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- 47.Guadarrama Bello D., Fouillen A., Badia A., Nanci A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017;60:339–349. doi: 10.1016/j.actbio.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Yu W., Jiang X., Zhang F., Xu L. The effect of anatase TiO2 nanotube layers on MC3T3-E1 preosteoblast adhesion, proliferation, and differentiation. J. Biomed. Mater. Res., Part A. 2010;94A(4):1012–1022. doi: 10.1002/jbm.a.32687. Sep. [DOI] [PubMed] [Google Scholar]
- 49.Faia-Torres A.B., et al. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014;35(33):9023–9032. doi: 10.1016/j.biomaterials.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 50.Hatano K., et al. Effect of surface roughness on proliferation and alkaline phosphatase expression of rat calvarial cells cultured on polystyrene. Bone. 1999;25(4):439–445. doi: 10.1016/s8756-3282(99)00192-1. [DOI] [PubMed] [Google Scholar]
- 51.Gittens R.A., et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32(13):3395–3403. doi: 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.