Abstract
Virosomal vaccines were prepared by extracting hemagglutinin (HA) and neuraminidase from influenza virus and incorporating it in the membranes of liposomes composed of phosphatidylcholine. Two intranasal spray vaccine series were prepared: one series comprised 7.5 μg of HA of each of three strains recommended by the World Health Organization and 1 μg of Escherichia coli heat-labile toxin (HLT), and the other contained the HA without HLT. In addition, a third vaccine preparation contained 15 μg of HA and 2 μg of HLT. The parenteral virosomal vaccine contained 15 μg of HA without additional adjuvant. The immunogenicity of a single spray vaccination (15 μg of HA and 2 μg of HLT) was compared with that of two vaccinations (7.5 μg of HA with or without 1 μg of HLT) with an interval of 1 week in 60 healthy working adults. Twenty volunteers received one parenteral virosomal vaccine. Two nasal spray vaccinations with HLT-adjuvanted virosomal influenza vaccine induced a humoral immune response which was comparable to that with a single parenteral vaccination. A significantly higher induction of influenza virus-specific immunoglobulin A was noted in the saliva after two nasal applications. The immune response after a single spray vaccination was significantly lower. It could be shown that the use of HLT as a mucosal adjuvant is necessary to obtain a humoral immune response comparable to that with parenteral vaccination. All vaccines were well tolerated.
Current efforts to control the morbidity and mortality associated with yearly epidemics of influenza are based on the use of intramuscularly administered inactivated influenza vaccines (5). The efficacy of such vaccines in preventing respiratory disease and influenza complications is suboptimal and ranges from 75% in healthy adults to <50% in the elderly (1, 11, 14, 21).
Influenza viruses, like many pathogens, invade at mucosal surfaces, initially in the upper respiratory tract. Mucosal immunity constitutes the first line of defense for the host and is a major component of the immunologic response in the nasal passages and in the airways of the lower respiratory tract. Although the presently used injectable influenza vaccines stimulate serum hemagglutinin (HA)-specific immunoglobulin G (IgG) of HA inhibition antibody in the majority of healthy individuals, a significant rise in HA-specific nasal IgA antibody occurs in only a minority of vaccinated subjects (6). Strategies for developing influenza vaccines with improved immunogenicity and clinical efficacy need to target both local and systemic antibody responses.
Intranasally administered, live attenuated influenza vaccines offer improved mucosal immunity, with promising results, particularly in children (3, 18). Although this method is not new (2), nasal vaccination with so-called cold-adapted influenza viruses has so far failed to gain acceptance worldwide.
We have therefore investigated a mucosal vaccination strategy with an inactivated influenza virus preparation which augments both local and systemic immune responses. We describe here the safety and comparative immunogenicities in healthy working adults of a trivalent virosomal influenza vaccine (10, 12) with and without the mucosal adjuvant Escherichia coli heat-labile toxin (HLT) (25) given once or twice with an interval of 1 week by intranasal spray vaccination. These vaccine preparations were compared with a commercial parenteral virosomal vaccine (10).
We have chosen this vaccine for the following two reasons. First, for comparative reasons the influenza virus antigen had to be in the same physicochemical state as the mucosal preparations. Second, besides being extensively tested in clinical trials (10–12), this vaccine already has been licensed in Switzerland and other European countries for use in humans.
MATERIALS AND METHODS
Virosomal vaccine formulations.
The production of influenza virosomal vaccine has been described elsewhere (12). The H1N1 A/Singapore/6/86, H3N2 A/Wuhan/359/95, and B/Beijing/184/93 strains of influenza virus cultivated in embryonated hen eggs were supplied by the National Institute of Biological Standards and Control, London, United Kingdom. Intact virions were isolated from the chorioallantois fluid by zonal centrifugation and inactivated with β-propiolactone. Purified virions were put in a buffer containing 0.1 M octaethylene glycol mono(N-dodecyl)ether (OEG) (Nikko Chemicals) in phosphate-buffered saline–NaCl. These virions were incubated at 21°C for 20 min to allow complete disintegration of the viral components.
For the extraction of HA and neuraminidase, the mixture was centrifuged at 100,000 × g for 60 min. The supernatant, which contained HA, neuraminidase, and viral phospholipids, was used for preparing the different intranasal virosomal vaccine formulations. Phosphatidylcholine (Lipoid, Ludwigshafen, Germany) was added and solubilized. The virosomes were formed spontaneously during the removal of the OEG detergent by chromatography. Formulation A of the mucosal vaccine dose (200 μl) contained 7.5 μg of the HA of each influenza virus vaccine strain, 70 μg of lecithin, and 1.0 μg of HLT from the production strain E. coli HE22VK. Formulation B contained 7.5 μg of HA of each influenza virus strain and 70 μg of lecithin without HLT. Formulation C was composed of 15 μg of HA of each strain and 2 μg of HLT. The parenteral virosomal vaccine dose (0.5 ml) contained 15 μg of HA of each influenza virus vaccine strain and 150 μg of lecithin.
Clinical protocol.
The open, randomized clinical trial was conducted in full conformance with the principles of the Declaration of Helsinki and with the local laws and regulations concerning clinical trials. After approval of the protocol by the ethics committee of the Canton Lucerne and notification to the Swiss Federal Health Office, 80 healthy volunteers (age 18 to 64 years) gave their written informed consent to participate. Volunteers were excluded if they had evidence of acute or chronic disease at the time of immunization or if there was a simultaneous treatment with immunosuppressive drugs or a known immunodeficiency.
The intranasal vaccine formulations were given to three groups of 20 volunteers each (Table 1). Groups A and B received 100 μl of formulations A and B, respectively, in each nasal cavity on day 1 and the same doses 1 week later. Group C received 100 μl of formulation C (double-concentrated formulation A) only on day 1. Group D was vaccinated intramuscularly in the deltoid region with the parenteral formulation. Blood and saliva samples (Omnisal; Saliva Diagnostic Systems, Vancouver, Wash.) were taken immediately before and 1 month after (day 29 ± 2) the first immunization. Due to a technical problem, only the saliva probes for the first 47 subjects could be evaluated. Brush cytology of the nasal cavity was performed before immunization and 4 and 8 days and 1 month after the first immunization.
TABLE 1.
Clinical protocol
| Vaccine group | No. male (n = 20) | Mean age (yr) | No. of vaccinations (time interval) | Application | Amt (μg) for complete vaccination
|
|
|---|---|---|---|---|---|---|
| HA (per strain) | HLT | |||||
| A | 14 | 39.7 | 2 (1 wk) | Intranasal | 15 | 2 |
| B | 14 | 35.5 | 2 (1 wk) | Intranasal | 15 | 0 |
| C | 16 | 43.8 | 1 | Intranasal | 15 | 2 |
| D | 14 | 41.2 | 1 | Intramuscular | 15 | 0 |
Evaluation of the immune response.
The blood samples and saliva probes were coded for analysis.
The serum immune response to the HA vaccine component was determined by a standard hemagglutination inhibition test (11) with 4 HA units of the respective antigens. The sera were treated at 56°C for 30 min before being tested. Titers are expressed as the reciprocal of the highest dilution of serum which completely inhibited hemagglutination. A titer of ≥1:40 was considered protective.
Total and influenza virus-specific IgA antibodies were determined by previously described enzyme-linked immunosorbent assay methods (24). The virus-specific IgA values are expressed as enzyme-linked immunosorbent assay units of specific IgA per microgram of total IgA.
The nasal epithelial cells were harvested exclusively from the maxillary turbinates of both nasal cavities in each subject with the same type of small nylon brush employed in cytopathologic examinations during bronchoscopy (13). Sampling was performed under rhinoscopic control with a rotary and translational movement along the inferior turbinate attachment. The cells were transferred to a glass slide and fixed instantly in a solution containing 200 ml of ethanol, 100 ml of acetone, and 6 drops of trichloracetic acid. The Papanicolaou-stained slides were examined by trained cytopathologists at the Institute of Pathology, Cantonal Hospital Lucerne, who were blinded to the vaccination status. Average numbers of ciliated cells, goblet cells, lymphocytes, centroblasts, neutrophils, eosinophils, and squamous epithelial cells were determined in 25 representative fields per slide at a magnification of ×100.
Statistical analysis.
The significance of differences between baseline and postimmunization titers was determined by the paired t test. Differences in the abilities of the four vaccination regimens to elicit protective anti-HA antibodies in the study group were determined by the χ2 test.
Adverse events.
All adverse events encountered during the clinical trial were reported. An adverse event was defined as any adverse change from the baseline (prevaccination) condition of the subjects, irrespective of whether the event was considered to be related to the vaccination. Any adverse event (local or systemic reaction) which occurred after the immunization was recorded by the clinician on a special adverse-event report form. The baseline adverse-event rate was evaluated prior to immunization.
RESULTS
Characteristics of volunteers and adverse reactions.
Eighty persons (mean age of 40 years) of comparable social status were recruited for the trial. A total of 27.5% of the participants were female. All three nasal vaccination preparations as well as the parenteral vaccines were well tolerated. There were no significant differences between the three nasal vaccine groups. In isolated individual cases, the following possible related reactions were reported: fever, fatigue, nausea, rhinitis, stuffy nose, and rhinopharyngitis.
Humoral immune response.
The serological immune response is shown in Table 2. Significant increases in titer were measured in group A (two nasal vaccinations, 7 days apart), group C (one nasal vaccination, double dosage) and group D (parenteral vaccination against all three virus strains). The highest geometric mean antibody titers (GMTs) were found in groups A and D. Group D significantly had the highest GMTs against the H1N1 strain (P ≤ 0.05). In the case of the H3N2 strain, there were no significant differences between groups A and D. These groups responded significantly better than groups B and C. For the B strain, there were no significant differences between groups A, C, and D. However, these groups had significantly higher titers than group B. The seroconversion rates were highest in groups A and D; generally, they were significantly higher than the rates in groups B and C and, for all three strains, met the serological requirements for parenteral influenza vaccination recommended by the European Community (7).
TABLE 2.
Humoral (anti-HA) antibody response following immunization with intranasal or intramuscular vaccine preparations
| Vaccine group | GMTa
|
No. with a ≥4-fold rise/total (%)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| H1N1 A/Singapore
|
H3N2 A/Wuhan
|
B/Beijing
|
H1N1 A/Singapore | H3N2 A/Wuhan | B/Beijing | ||||
| Preimmunization | Postimmunization | Preimmunization | Postimmunization | Preimmunization | Postimmunization | ||||
| A | 50 (10–320) | 94* (<10–3,840) | 31 (10–160) | 150* (20–640) | 11 (<10–20) | 33* (10–80) | 8/20 (40) | 17/20 (85)*** | 12/20 (60)*** |
| B | 59 (10–640) | 83 (20–2,560) | 35 (10–640) | 40 (10–640) | 10 (<10–40) | 16 (<10–80) | 5/20 (25) | 0/20 (0) | 4/20 (20) |
| C | 49 (10–320) | 95* (<10–5,120) | 43 (10–320) | 86* (<10–640) | 19 (<10–160) | 37* (10–320) | 7/20 (35) | 7/20 (35)** | 4/20 (20) |
| D | 45 (10–320) | 169* (20–2,560) | 39 (20–320) | 163* (20–5,120) | 20 (<10–40) | 44* (10–1,280) | 12/20 (60)** | 12/20 (60)** | 9/20 (45)*** |
*, significant (P ≤ 0.05) compared with baseline value; **, significant (P ≤ 0.05) compared with value for group B; ***, significant (P ≤ 0.05) compared with values for groups B and C.
Specific IgA response in saliva.
The mucosal immune response (in saliva) is shown in Table 3. The largest increase in IgA titer was measured in group A, where results were significantly better than those for the other groups. The GMTs were also highest in group A, taking the total IgA into consideration. The mucoconversion rate (quadruple increase in IgA titer) was once again clearly highest in group A. In the case of intramuscular vaccination, there were only very low mucoconversion rates.
TABLE 3.
Mucosal (IgA) antibody (saliva) response following immunization with intranasal or intramuscular vaccine preparations
| Vaccine group | Geometric mean IgAa (U/μg of total IgA)
|
% with a rise ofa:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1N1 A/Singapore
|
H3N2 A/Wuhan
|
B/Beijing
|
≥4-fold
|
≥2-fold
|
||||||||
| Pre | Post | Pre | Post | Pre | Post | H1N1 | H3N2 | B | H1N1 | H3N2 | B | |
| A | 11.8 | 67.1* | 8.1 | 43.1* | 6.6 | 40.5* | 73*** | 73** | 80**** | 91*** | 82*** | 91*** |
| B | 11.9 | 15.0 | 9.7 | 11.2 | 9.6 | 6.3 | 14 | 14 | 0 | 29 | 14 | 33 |
| C | 9.8 | 28.8 | 7.9 | 23.3 | 6.9 | 14.4 | 58 | 39 | 33 | 69 | 62 | 58 |
| D | 18.9 | 49.3 | 15.2 | 26.9 | 14.5 | 19.7 | 25 | 38 | 27 | 50 | 38 | 47 |
*, significant (P ≤ 0.05) compared with baseline value; **, significant (P ≤ 0.05) compared with value for group B; ***, significant (P ≤ 0.05) compared with values for groups B and D; ****, significant (P ≤ 0.05) compared with values for groups B, C, and D. Pre, preimmunization; Post, postimmunization.
Cytological events after nasal spray vaccination.
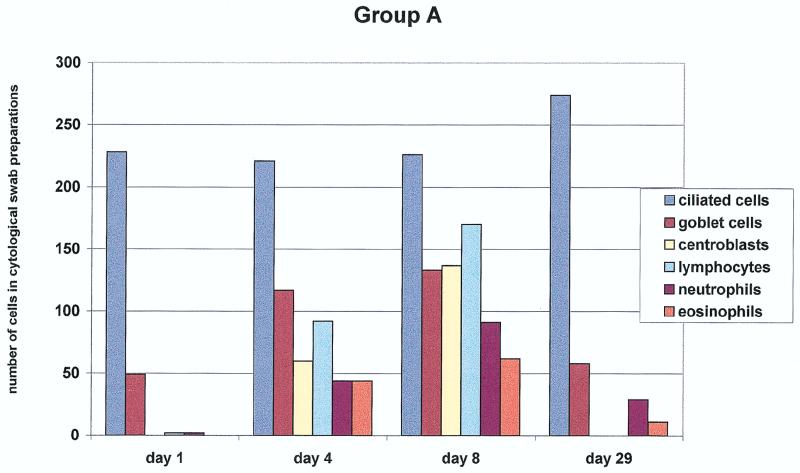
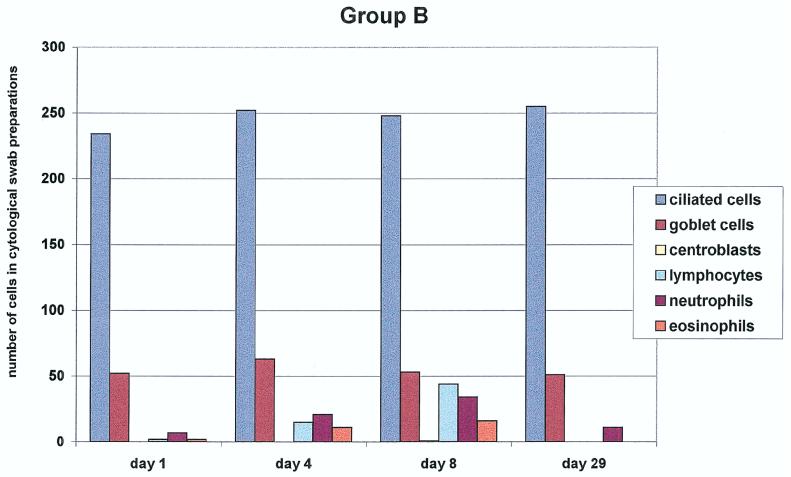
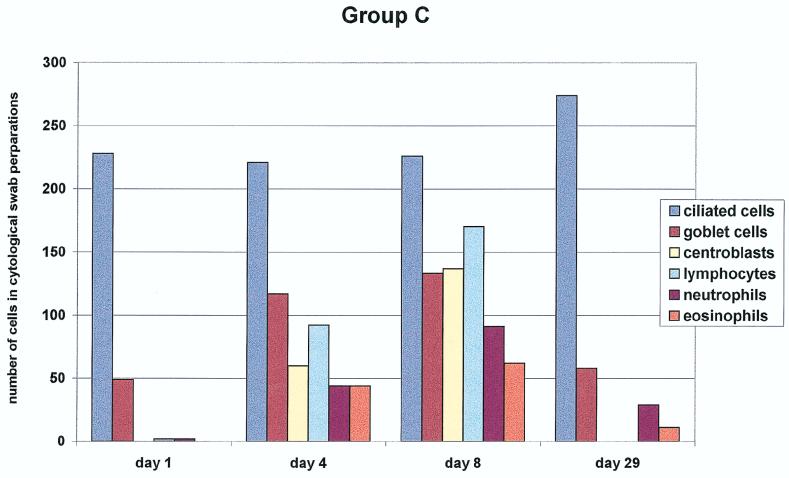
Brush cytology of the nasal mucosa was performed for groups A, B, and C. The results are summarized in Fig. 1. We assessed the cell counts of the nasal mucous membrane epithelium (ciliated and nonciliated columnar cells, goblet cells, and squamous epithelial cells) and myelo/mono- and lymphopoietic cells (lymphocytes, eosinophils, neutrophils, and centroblasts). In group A, clear goblet cell hyperplasia was seen on days 4 and 8 after the first vaccination. In addition, we observed a strong increase in lymphocytes and centroblasts on the same days (with mitotic figures) (Fig. 2). In addition, an increase in eosinophils and neutrophils was observed on day 8 after the initial vaccination. The number of columnar cells remained unchanged.
FIG. 1.
Nasal cytology. Quantification of the different cell populations obtained from nasal swabs of the subjects (20 in each group) up to 29 days after three different modes of intranasal vaccination is shown. Note the marked increase of centroblasts as a sign of a local immune response in group A, in contrast to the significantly lesser increase in groups B and C (P < 0.005).
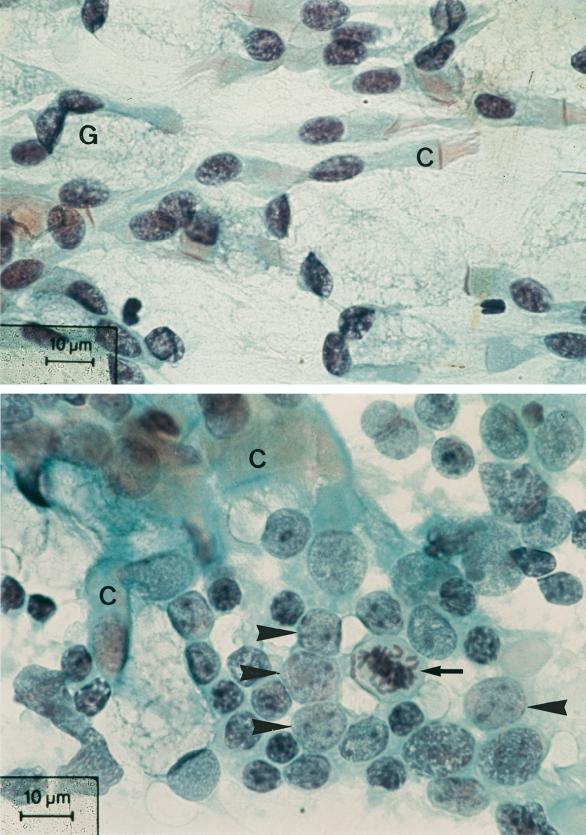
FIG. 2.
Photomicrograph of the nasal cytology before (top) and after (bottom) nasal vaccination of a subject in group A with an accumulation of activated lymphocytes and centroblasts (arrowheads) with a mitotic figure (arrow) and ciliated epithelial cells (C). Papanicolaou staining was used.
In group B, there was only a slight increase in lymphocytes, neutrophils, and eosinophils. There was no evidence of activated lymphocytes in this group.
In group C, an even clearer goblet cell hyperplasia than in group A was noted. In addition, eosinophils and neutrophils had increased most in this group on days 4 and 8. The increase in lymphoblasts was less in this group than in group A (P ≤ 0.05). One month after first vaccination, the cellular composition had returned to prevaccination status in all groups.
DISCUSSION
It was demonstrated in this study that after two nasal vaccinations with an HLT-adjuvanted virosomal influenza vaccine, it was possible to induce a humoral immune response that was comparable to that after a single parenteral vaccination with the same total influenza virus HA content. In addition, a significantly higher induction of influenza virus-specific IgA was noted in the saliva after nasal spray vaccination. This supports the results of investigations with nasal lavage fluid, where clearly increased specific IgA was also observed (unpublished observations). Our investigations showed that two nasal applications were significantly better than one application with double antigen and adjuvant doses. This applies to both the humoral and the mucosal IgA immune responses (8).
Most of our knowledge of the mucosa-associated immune system (MALT) is based on data from animal experiments and on investigations of the human gastrointestinal tract (17). The essentials of this immune system are the capability for local antigen absorption, intramucosal antigen processing, specific lymphocytic stimulation, and generalized seeding of primed lymphocytes in mucosal sites of different organs (respiratory, gastrointestinal, and urogenital tracts; lactating breast; oropharynx; and lacrimal and salivary glands). In particular, the immune response expressed in mucosal tissues is typified by secretory IgA, the predominant Ig class in human exogenous secretions and the best-known entity in providing specific immune protection for mucosal tissues (17). Secretory IgA is the first immunological barrier to influenza viruses and other pathogens at epithelial surfaces. Resistance to virus infection has been correlated with the presence of antiviral IgA antibody in mucous secretions (22, 27).
It is conceivable that the local stimulation and priming of the nasal MALT results in a generalized immunization of the entire respiratory mucosa as well as the systemic immune system. Unfortunately, little is known about the human nasal MALT, although it is immediately accessible for investigation, representing a sentinel of the respiratory tract for airborne antigens (4, 19).
In our study, we were able to clearly demonstrate signs of a local immune response in the nasal mucosa following nasal vaccination. We found typical blastic transformation of B lymphocytes into centroblasts (germinal center cells of lymphoid follicles) in cytological swabs from the nasal mucosa as evidence of local lymphocyte activation (16). The rise of specific IgA antibody titers in the saliva is further evidence of the assumed local immune reaction of the nasal MALT in response to local vaccination (20).
On the basis of our cytological results, we were able to confirm the process of the identification and activation phase of specific immunity (different sizes of lymphocytes with lymphoblasts and mitotic figures of lymphoblasts). Our results on the nasal mucosal cytology are also consistent with the results of a previous investigation in which the cytokine profile was determined from the nasal lavage after influenza virus-induced rhinitis. The proinflammatory cytokines identified were typically derived from cells whose presence we had identified in the nasal swabs (16, 26).
The strongest immune response with respect to lymphocyte activation (blast formation and IgA production) following vaccination was elicited by vaccine combined with the adjuvant HLT.
Besides the local immune response, we saw epithelial alterations with goblet cell hyperplasia in cytological smears. In subjects receiving the HLT-adjuvanted virosome formulation, this could be interpreted as an exogenous irritation caused by the vaccine’s adjuvant or as endogenous stimulation of the local immune response, but it cannot be explained by our findings alone. Goblet cells have a protective function for the mucous layer, and their response to irritation such as viral infection of the epithelium is manifested by hyperplasia (23). Local immune reactions may be a cause of goblet cell differentiation in the gut. This might occur during the process of the local immune response in the nose following vaccination and could explain the local goblet cell hyperplasia.
The number of ciliated cells in the cytological preparations did not change during the 30 days postvaccination. Epithelial damage was not observed, and the tested mucociliary transport capacity (saccharin test) was constant.
These findings demonstrate the safety, humoral immunogenicity, and superior mucosal immunogenicity of a new trivalent adjuvanted virosomal vaccine in healthy working adults. Additional studies to investigate this new spray influenza vaccine in high-risk groups such as elderly nursing home residents, infants at risk (9, 15), and asthmatic individuals are under evaluation. This new vaccine could play an important part in preventing morbidity and mortality associated with influenza among the entire population due to its simplicity of application. It is expected that this new method of vaccine administration may considerably increase the acceptance of influenza vaccination.
ACKNOWLEDGMENTS
We thank Robert Mischler for the help in preparing the vaccine, Emil Fürer for providing the HLT, Béatrice Finkel for doing the tests of specific IgG and IgA, Alois Lang for doing the tests of total IgA, Christian Herzog for helping with the clinical protocol, Bernhard Wegmüller for carrying out the statistical analysis, and Christine Lanzrein for preparing the manuscript.
REFERENCES
- 1.Ahmed A E H, Nicholson K G, Nguyen-Van Tam J S. Reduction in mortality associated with influenza vaccine during 1989–1990 epidemic. Lancet. 1995;346:591–595. doi: 10.1016/s0140-6736(95)91434-x. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrova G, Smorodintsev A. Obtaining of an additionally attenuated vaccinating cryophilic influenza strain. Rev Roum Inframicrobiol. 1965;2:179–189. [Google Scholar]
- 3.Belshe R B, Mendelmann P M, Treanor J, King J, Gruber W C, Piedra P, Bernstein D I, Hayden F G, Kotloff K, Zangwill K, Iacuzio D, Wolff M. The efficacy of live-attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P. Immune functions of human nasal mucosa and tonsils in health and disease. In: Bienenstock J, editor. Immunology of the lung and upper respiratory tract. New York, N.Y: McGraw-Hill; 1984. pp. 28–95. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Prevention and control of influenza. Morbid Mortal Weekly Rep. 1995;44:1–22. [Google Scholar]
- 6.Clements M L, Betts R F, Tierney E L, Murphy B R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild type virus. J Clin Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commission of the European Community. Rules governing medicinal products in the European Community. Harmonisation of requirements for influenza vaccines. Luxembourg, Luxembourg: European Community Publication Office; 1992. pp. 93–98. [Google Scholar]
- 8.De Haan A, Wilschut J. Liposomes and antiviral mucosal immunity. In: Shek P N, editor. Liposomes in biomedical applications. London, United Kingdom: Harwood Academic Publishers; 1995. pp. 69–83. [Google Scholar]
- 9.Glezen W P, Paredes A, Taber L H. Influenza in children: relationship to other respiratory agents. JAMA. 1980;243:1345–1349. doi: 10.1001/jama.243.13.1345. [DOI] [PubMed] [Google Scholar]
- 10.Glück R, Mischler R, Finkel B, Que J U, Scarpa B, Cryz S J., Jr Immunogenicity of new virosome influenza vaccine in the elderly people. Lancet. 1994;344:160–163. doi: 10.1016/s0140-6736(94)92758-8. [DOI] [PubMed] [Google Scholar]
- 11.Glück R. Wirksamkeit der Grippe-Schutzimpfung bei gesunden, arbeitenden Erwachsenen. Schweiz Med Wochenschr. 1996;126:1579. [Google Scholar]
- 12.Glück R, Wegmann A. Liposomal presentation of influenza antigens. In: Nicholson K G, Webster R G, Hay A J, editors. Textbook of influenza. London, United Kingdom: Blackwell; 1998. pp. 400–409. [Google Scholar]
- 13.Glück U, Gebbers J-O. Nasal cytopathology in smokers: a possible biomarker of air-pollution? Am J Rhinol. 1996;10:55–57. [Google Scholar]
- 14.Govaert T M E, Thijs C T M C N, Masurel N, Sprenger M J W, Dinant G J, Knottnerus J A. The efficacy of influenza vaccination in elderly individuals. JAMA. 1994;272:1661–1665. [PubMed] [Google Scholar]
- 15.Groothuis J R, Levin M J, Rabalais G P, Meiklejohn G, Lauer B A. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics. 1991;87:823–828. [PubMed] [Google Scholar]
- 16.Ikuta K, Uchica N, Friedman J, Weissman I L. Lymphocyte development from stem cells. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- 17.Kagnoff M F, Kiyono H. Essentials of mucosal immunity. San Diego, Calif: Academic Press; 1996. [Google Scholar]
- 18.Keitel W A, Piedra P A. Live cold-adapted, reassortant influenza vaccines (USA) In: Nicholson K G, Webster R G, Hay A J, editors. Textbook of influenza. London, United Kingdom: Blackwell; 1998. pp. 373–398. [Google Scholar]
- 19.Kuper C F, Koornstra P J, Hameleers D N M, Biewenga J. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 20.McGhee J R, Mestecky J, Elson C O, Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989;9:175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- 21.Nichol K L, Margolis K L, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994;331:778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 22.Ogra P L, Cumella J C, Welliver R C. Immune responses to viruses. In: Bienstock J, editor. Immunology of the lung and upper respiratory tract. New York, N.Y: McGraw-Hill; 1984. pp. 242–263. [Google Scholar]
- 23.Pedersen M, Sakakura J, Winther B, Brofeldt S, Mygind N. Nasal mucociliary transport. Number of ciliated cells, and beating pattern in naturally acquired common cold. Eur J Respir Dis. 1983;64(Suppl. 128):355–365. [PubMed] [Google Scholar]
- 24.Tamura S I, Ito Y, Asanuma H. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992;149:981–987. [PubMed] [Google Scholar]
- 25.Vogel F R, Powell M F. A compendium of vaccine adjuvents and excipients. In: Powell M F, Newmann M J, editors. Vaccine design. The subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 182–192. [Google Scholar]
- 26.Winther B, Gwaltney J M, Jr, Mygind N, Hendley J O. Viral-induced rhinitis. Am J Rhinol. 1998;12:17–20. doi: 10.2500/105065898782102954. [DOI] [PubMed] [Google Scholar]
- 27.Wright P F, Murphy B R, Kervina M, Lawrence E M, Phelan M A, Karzon D T. Secretory immunologic response after intranasal inactivated influenza A virus vaccination: evidence for immunoglobulin A memory. Infect Immun. 1983;40:1092–1095. doi: 10.1128/iai.40.3.1092-1095.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]