Summary
The tumor microenvironment is essential for mediating drug resistance and tumor progression. Here, we present a coculture system, which enables drug testing of colorectal cancer organoids and fibroblasts without additional matrix components such as Matrigel or basement membrane extracts. First, we describe steps to use a readout for high-throughput drug testing using a luminescence-based viability assay. Second, we detail a readout that uses flow cytometry to distinguish toxic effects on either colorectal cancer organoids or fibroblasts.
Subject areas: Cell Biology, Cell Culture, Cancer, Organoids
Graphical abstract
Highlights
-
•
Protocol for the coculture of PDOs with fibroblasts
-
•
Coculture without additional matrix component such as Matrigel
-
•
Coculture with simple culture medium without additional growth factors
-
•
High-throughput drug testing using a luminescence-based viability assay
-
•
Distinction of toxic effect on PDOs or fibroblasts using flow cytometry
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The tumor microenvironment is essential for mediating drug resistance and tumor progression. Here, we present a coculture system, which enables drug testing of colorectal cancer organoids and fibroblasts without additional matrix components such as Matrigel or basement membrane extracts. First, we describe steps to use a readout for high-throughput drug testing using a luminescence-based viability assay. Second, we detail a readout that uses flow cytometry to distinguish toxic effects on either colorectal cancer organoids or fibroblasts.
Before you begin
The protocol below describes specific steps for using the fibroblast cell line CCD-18Co in the coculture with organoids derived from colorectal cancer patients. However, we have also used this protocol using primary fibroblasts. Ensure you have received the appropriate institutional permission to work with human samples before proceeding with this protocol.
Institutional permissions
Primary colorectal cancer samples were obtained from patients who underwent surgery at the Robert-Bosch-Hospital, Stuttgart. The study was approved by the Ethical Committee at the University Tübingen and written informed consent was obtained. Residual tissue samples, which were not used for pathological routine examination, were transferred to the laboratory for cell isolation within a maximum of 8 h after surgery.
Preparation of reagents and solutions
Timing: 1 day
-
1.Bovine serum albumin (BSA, 10%, Roth, Cat.: 8076).
-
a.Dissolve 50 g BSA powder in 500 mL sterile H2O and mix carefully.
-
b.Filter aseptically and aliquot 1 mL/low protein binding tube.
-
c.Store at −20°C.
-
a.
Note: They can be thawed multiple times. Pipette tips and low protein binding tubes should be coated with BSA prior every step working with organoids.
-
2.Epidermal growth factor (EGF, stock: 100 μg/mL, Peprotech, Cat.: AF-100-15).
-
a.Dissolve 100 μg powder in 1 mL sterile H2O.
-
b.Aliquot 25 μL per tube.
-
c.Store at −20°C.
-
a.
-
3.Fetal calf serum (FCS, Pan Biotech, Cat.: P30-3031).
-
a.Inactivate proteins by incubating FCS for 1 h at 56°C.
-
b.Aliquot 50 mL per tube.
-
c.Store at −20°C.
-
a.
-
4.2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES, stock: 1 M, Roth, Cat.: 9105.3).
-
a.Dissolve 238.3 g in 1 L H2O.
-
b.Filter aseptically.
-
c.Store at 20°C–25°C.
-
a.
-
5.N-Acetyl-L-cysteine (NAC, stock: 500 mM, SIGMA-Aldrich, Cat.: A7250).
-
a.Dissolve 80 mg in 1 mL H2O at 37°C.
-
b.Sterile filter.
-
a.
Note: NAC should be freshly prepared.
-
6.Phosphate buffered saline (1× PBS; Lonza, Cat: BE17-517Q).
-
a.Dilute 1:9 (v/v) in sterile H2O.
-
b.Store at 20°C–25°C.
-
a.
-
7.Rho kinase inhibitor Y-27632 2HCI (stock: 10 mM; Selleck Chemicals, Cat.: S1049).
-
a.Dissolve 10 mg in 3,122 mL sterile H2O.
-
b.Aliquot 50 μL.
-
c.Store at −20°C.
-
a.
-
8.TGF-ß1 (2 μg/mL, Peprotech, Cat.: 100-21).
-
a.Prepare 10 mM citric acid.
-
i.Dissolve 21 mg citric acid monohydrate in 10 mL H2O.
-
ii.Filter aseptically.
-
i.
-
b.Dissolve 2 μg recombinant TGF-ß1 in 20 μL 10 mM citric acid.
-
c.Dilute in 980 μL PBS with 0.1% BSA.
-
d.Store at −20°C for up to 12 months.
-
a.
-
9.Matrigel (Corning, Cat.: 356231).
-
a.Thaw Matrigel on ice for 12 h at 4°C.
-
b.Mix the Matrigel by swirling multiple times.
-
c.Aliquot 250 μL or 500 μL per tube on ice.
-
d.Store at −20°C.
-
a.
Note: Pipette tips and low protein binding tubes should also be pre-cooled on ice. Freeze-thaw cycles should be avoided as much as possible.
CRITICAL: Everything in contact with Matrigel has to be pre-cooled and kept cold the whole time working with the Matrigel.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Annexin V-APC (1:200 dilution) | ImmunoTools | 31490016x2 |
| Anti-human EpCAM (REA764; (1:50 dilution) | Miltenyi Biotec | 130-111-004 |
| Biological samples | ||
| Patient-derived organoids (PDO) | Robert-Bosch-Hospital, Stuttgart | |
| Chemicals, peptides, and recombinant proteins | ||
| Advanced Dulbecco’s modified Eagle’s medium (DMEM)/F12 | Fisher Scientific | 12634028 |
| Amphotericin B solution | Merck | A2942-100ML |
| Animal-free recombinant human EGF | PeproTech | AF-100-15 |
| B-27 ser.-free supplement | Fisher Scientific | 17504-044 |
| Bovine serum albumin (BSA) albumin fraction V | Roth | 8076 |
| CaCl2x2H2O | Roth | 5239.1 |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D2438-5X10ML |
| Dulbecco’s modified Eagle’s medium (DMEM) | LGC | ATCC® 30−2002™ |
| Fetal calf serum (FCS) | PanBiotech | P30-3031 |
| GlutaMAX™ supplement | Fisher Scientific | 35050061 |
| 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | Roth | 9105.3 |
| L-Glutamine | Merck | K0283 |
| N2 supplement | Fisher Scientific | 17502-048 |
| N-Acetyl-L-cysteine | Sigma-Aldrich | A7250-50G |
| NaCl | Sigma-Aldrich | 1.06404.1000 |
| Non-essential amino acids (100×) | Sigma-Aldrich | K0293 |
| Matrigel®, phenol red-free, LDEV-free | Corning | 356231 |
| Penicillin-streptomycin | Merck | P4333−20ML |
| Phosphat-buffered saline (PBS) | Lonza | BE17-517Q |
| Propidium iodide (1:40 dilution) | Sigma | P4170 |
| Recombinant human FGF-basic | PeproTech | 100-18B |
| Recombinant human TGF-β1 (HEK293 derived) | PeproTech | 100-21 |
| RHO/ROCK pathway inhibitor (Y-27632) | Selleck Chemicals | S1049 |
| RPMI 1640, without glutamine | Sigma (Biochrome) | F1215 |
| Trypan blue | Biochrom | L6323 |
| TrypLE™ Express enzyme (1×), no phenol red | Thermo Fisher Scientific | 12604013 |
| Trypsin-EDTA (0.25%), phenol red | Thermo Fisher Scientific | 25200056 |
| Critical commercial assays | ||
| CellTiter-Glo® three-dimensional cell viability | Promega | G9682 |
| Experimental models: Cell lines | ||
| CCD-18Co (ATCC® CRL-1459™) | ATCC | CRL-1459 |
| Other | ||
| Centrifuge universal 320 | Hettich Zentrifugen | Typ1406 |
| Counting chamber, Fuchs-Rosenthal | Glaswarenfabrik Karl Hecht | 40449001 |
| Counting chamber, Neubauer-improved | Paul Marienfeld | 0640010 |
| FACS Lyrics™ flow cytometer | BD | 659180 |
| Falcon® round-bottom polystyrene tubes | Thermo Fisher Scientific | 10100151 |
| Incubator | New Brunswick | Galaxy 170R |
| Low protein binding tubes | Thermo Fisher Scientific | 15342617 |
| Microtome | Leica | RM225 |
| Milli-Q | Merck Millipore | Integral 10 |
| Multimode plate reader | PerkinElmer | Enspire |
| Pipet boy | Integra Bioscience | 155 017 |
| Pipette 0.1–2 | Eppendorf | 3123000012 |
| Pipette 2–20 | Eppendorf | 3123000098 |
| Pipette 10–100 | Eppendorf | 3123000047 |
| Pipette 20–200 | Eppendorf | 3123000055 |
| Pipette 100–1,000 | Eppendorf | 3123000063 |
| Pipette multichannel | Mettler Toledo | 17013793 |
| Pipette multichannel | Mettler Toledo | 17013796 |
| Polypropylene tubes, 15 mL | Sarstedt | 62.554.502 |
| Slide scanner | Olympus | BX61VS |
| Sterile bench | BDK | |
| Thermo-Shaker | Kisker Biotech | TS-100 |
| Vortex | Heidolph | Reax Top |
| Water bath | Julabo | MB (V.2) |
Materials and equipment
Coculture and fibroblast medium
| Reagent | Final concentration | Amount |
|---|---|---|
| RPMI 1640 | N/A | 435 mL |
| FCS | 10% | 50 mL |
| L-Glutamine 100× | 1× | 5 mL |
| Non-essential amino acids 100× | 1× | 5 mL |
| Penicillin-streptomycin 100× | 1× | 5 mL |
| Total | N/A | 500 mL |
Note: Can be prepared in advance and stored up to 1 month at 4°C
Organoid – Basal medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Advanced DMEM/F12 | N/A | 48.4 mL |
| GlutaMAX 100× | 1× | 0.5 mL |
| HEPES 1 M | 10 mM | 0.5 mL |
| N-Acetyl-L-cysteine 100 mg/mL | 200 μg/mL | 0.1 mL |
| Penicillin-streptomycin 100× | 1× | 0.5 mL |
| Total | N/A | 50 mL |
Note: Can be prepared in advance and stored up to 6 months at −20°C
Organoid – Tumor organoid medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Basal medium | N/A | 48.2 mL |
| Amphotericin 250 μg/mL | 5 μg/mL | 250 μL |
| B27 supplement 50× | 1× | 1 mL |
| EGF 100 μg/mL | 50 ng/mL | 25 μL |
| N2 supplement 100× | 1× | 0.5 mL |
| Y27632 10 mM | 10 μM | 50 μL |
| Total | N/A | 50 mL |
Note: Can be prepared in advance and stored up to 2 weeks at 4°C
Annexin Binding Buffer (10×)
| Reagent | Final concentration | Amount |
|---|---|---|
| Sterile H20 | N/A | 450 mL |
| HEPES 1 M | 0.1 M | 50 mL |
| NaCl | 1.4 M | 40.9 g |
| CaCl2 | 25 mM | 1.38 g |
| Total | N/A | 500 mL |
Note: Can be prepared in advance and stored up to 1 year at 4°C. Dilute 1:9 (v/v) in distilled H2O.
Step-by-step method details
Culturing patient-derived organoids
Timing: ∼ 5 h/week
Patient derived organoids were formed and maintained according to already published protocols.1,2,3 For detailed step-by-step protocol see Pleguezuelos-Manzano et al.4 and Schuhmacher et al.5 This section details the maintenance of long-term organoid culture by weekly passaging.
-
1.
Culture organoids in 15 μL domes consisting of 75% Matrigel and 25% tumor organoid (TORG) medium in 48 well plate and overlay with 300 μL TORG medium. Check organoids daily (Monday to Friday) and change medium or split organoids when necessary.
CRITICAL: Use BSA coated low protein binding tubes and pipet tips whenever possible to prevent organoids from sticking to the surfaces. Matrigel should always be kept and thawed on ice. Only thaw amount of Matrigel that will be used that day.
-
2.Passage organoid cultures every 3–7 days, depending on growth and maintenance of Matrigel domes. Split the cells depending on density, usually in the ratio of 1:2 to 1:4.
 CRITICAL: Pre-warm 48 well plates in the incubator at 37°C. Do not remove the medium of all wells at once to prevent organoids from getting too dry or cold. Start with 1–6 wells.
CRITICAL: Pre-warm 48 well plates in the incubator at 37°C. Do not remove the medium of all wells at once to prevent organoids from getting too dry or cold. Start with 1–6 wells.-
a.Carefully remove and discard the medium in the wells without disrupting the Matrigel domes.
-
b.Add 1 mL ice-cold PBS into 1 well.
-
c.Use a fresh pipette tip and transfer 10% BSA solution from a low-binding tube into a new low protein binding tube (now the empty tube and the pipette tip are BSA coated) and use the same pipette tip to pool the organoids in the next steps.
-
d.Use the pipette tip (now BSA coated) and resuspend Matrigel dome with organoids with PBS added in step 2b.
-
e.Pool maximum of 6 wells and transfer into the BSA coated low protein binding tube (prepared in step c.).
-
f.Use a fresh pipette tip and wash wells with 500 μL ice cold PBS and add to the organoid suspension.
-
g.Centrifuge the tube at 2,370 g at 23°C for 50 s.
-
h.Carefully discard the supernatant with the dissolved Matrigel without disrupting the cell pellet.
-
i.Add 1 mL ice cold PBS to the tube and resuspend the cell pellet with a BSA coated pipette tip.
-
j.Centrifuge at 2,370 g at 23°C for 50 s and discard the supernatant.
-
k.Resuspend the cell pellet in 100 μL TrypLe with a Pipette 10–100 to enzymatically and mechanically digest organoids.
-
l.Incubate the cell pellet at 37°C roughly 5 min with resuspension by pipetting up and down every 1–2 min.Note: Mechanical disruption works best using a 10–100 μL pipette due to the narrow tip.
 CRITICAL: Some organoids may need more than 5 min to split up due to donor-to-donor variabilities. They might be incubated up to 30 min without damage. Be careful to resuspend and check regularly to stop the splitting as early as possible. As an alternative use a Thermo Shaker at 1 g at 37°C and check the state of dissociation every 3 min.
CRITICAL: Some organoids may need more than 5 min to split up due to donor-to-donor variabilities. They might be incubated up to 30 min without damage. Be careful to resuspend and check regularly to stop the splitting as early as possible. As an alternative use a Thermo Shaker at 1 g at 37°C and check the state of dissociation every 3 min. -
m.Monitor disruption microscopically and stop the “digestion” when organoids break up into small cell clumps.
-
n.Add 1 mL advanced DMEM to stop digestion and mix gently.
-
o.Store organoids on ice for 15 min without moving the tube in order to get rid of debris accumulating at the top.
-
p.Carefully remove 100 μL–300 μL of the supernatant without discarding organoids.
 CRITICAL: The volume of medium that is removed can be adjusted depending on state and amount of cells.
CRITICAL: The volume of medium that is removed can be adjusted depending on state and amount of cells. -
q.Spin down organoids at 2,370 g for 50 s and discard the supernatant.Note: Depending on the doubling time and amount of organoids, splitting ratio can vary between 1:2–1:4.
-
r.For each well 10 μL Matrigel are necessary. For the TORG medium volume, calculate 1/3 of the Matrigel volume (e.g., 10 wells would need 100 μL of Matrigel and 33.3 μL of TORG medium). Calculate:Matrigel volume:TORG medium volume:
-
s.Add the cold TORG medium to the organoids. Store the organoids on ice and resuspend carefully. Add the Matrigel and mix again carefully avoiding bubbles.
 CRITICAL: Avoid bubbles in the Matrigel mixture. Make sure to work on ice.
CRITICAL: Avoid bubbles in the Matrigel mixture. Make sure to work on ice. -
t.Dispense a 15 μL dome in the middle of each well (use 48 well plates prewarmed at 37°C).
-
u.Incubate the Matrigel domes containing the organoids upside down for 15 min in the incubator at 37°C.
-
v.Overlay each dome with 300 μL TORG medium.
-
a.
Culturing fibroblast cell line CCD-18Co
Timing: ∼ 1 h/week
This section details the maintenance of long-term CCD-18Co cell culture by weekly passaging.
-
3.
Fibroblasts are cultured in T75 flasks with 15 mL fibroblast medium. Medium is changed every 3–5 days.
CRITICAL: The utilized CCD-18Co fibroblasts are not sensitive in growing too dense, but are sensitive if too sparsely seeded.
-
4.Split fibroblast when they are 100% confluent, usually at a ratio of 1:2.
-
a.Aspirate the medium and wash fibroblasts with 10 mL warm PBS.
-
b.Aspirate the PBS and add 1 mL 0.25% trypsin. Move flask gently to cover all fibroblasts with trypsin.
-
c.Incubate the fibroblasts in the incubator for 5–10 min.
-
d.Check fibroblasts under microscope. If the cells detach, resuspend the cells with 10 mL fibroblast medium. Use a pipet boy to mix gently and transfer into a 15 mL polypropylene tube.
-
e.Centrifuge for 5 min at 180 g at 23°C and discard the supernatant.
-
f.Resuspend the cell pellet in 10 mL fibroblast medium and transfer 5 mL cell suspension to each T75 flask. Top up to 15 mL by adding 10 mL fibroblast medium to each T75 flask.
-
g.Move flasks gently to distribute the cells evenly and let the cells attach in the incubator at 37°C.
-
a.
Coculture of fibroblast cell line CCD-18Co with PDOs
Timing: ∼ 5 h/week
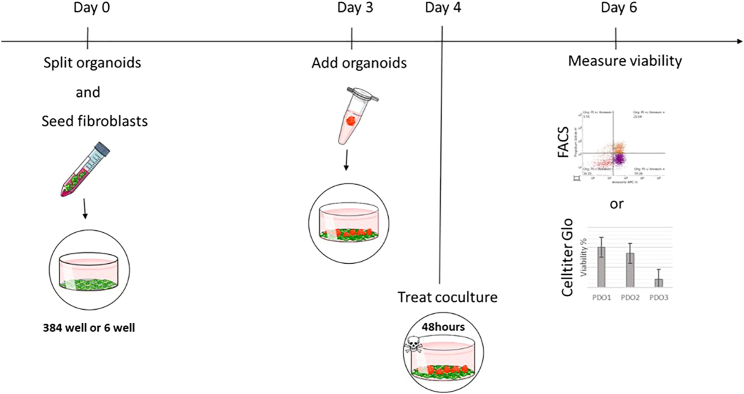
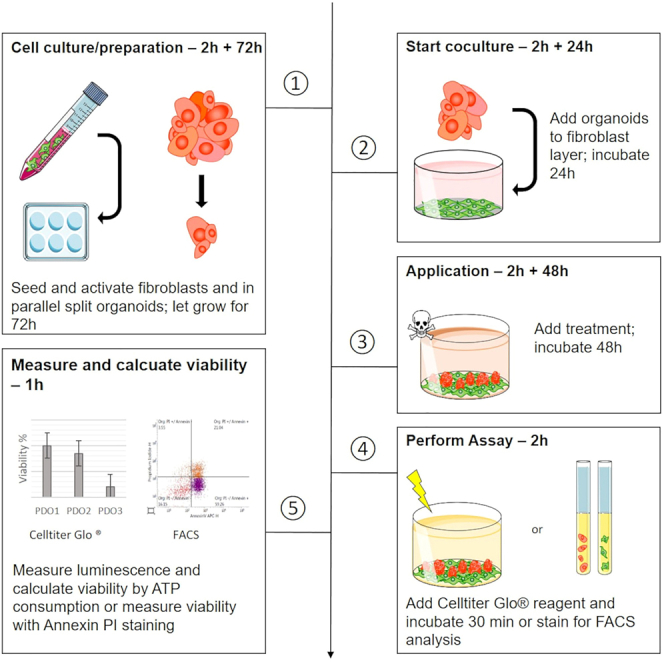
Here we describe the procedure of coculturing the fibroblast cell line CCD-18Co with patient-derived organoids. We describe two coculture setups depending on the readout - in the first protocol, viability of cells can be analyzed separately using FACS while in the second approach the coculture will be analyzed using CellTiter Glo® (Figure 1).
-
5.Assessment of viability using FACS.
-
a.Seeding fibroblasts.
-
i.Harvest fibroblasts in step 4a–4e and resuspend in 10 mL fibroblast medium.
-
ii.Count viable cells in a Neubauer counting chamber. Therefore, mix 20 μL cell suspension with 20 μL trypan blue. Count 4 large corner squares and calculate the cell number:(a) dilution factor of trypan blue.(b) correction factor converting counting chamber volume into.
-
iii.Calculate 100,000 fibroblasts per well.
-
iv.Resuspend fibroblasts in 3 mL fibroblast medium and 15 μL TGF-ß1 (stock 2 μg/mL) per 100,000 fibroblasts.
-
v.Seed the cells in a 6 well plate.
-
vi.Cultivate for 3 days before conducting the next step.
-
i.
-
b.Seeding organoids.
-
i.Harvest organoids as described in step 2.
-
ii.Discard the supernatant and resuspend the pellet in 1 mL fibroblast medium using BSA coated low-binding tips.
-
iii.Transfer 20 μL cell suspension to the Fuchs-Rosenthal cell counting chamber and count the squares. Calculate the cell number:
-
iv.Calculate 10,000 organoids per well.
-
v.Resuspend organoids in 3 mL fibroblast medium per 10,000 organoids.Note: Always use BSA pre-coated 15 mL Falcons when working with organoids.
-
vi.Aspirate the medium from the fibroblasts in the 6 wells.
-
vii.Add 3 mL organoid cell suspension with 10,000 organoids per well.
-
viii.Cultivate for one day.
-
ix.Add treatment (optional).
-
x.Incubate for 2 days in the incubator until day 6.
-
xi.Harvest and stain for FACS.
-
i.
-
c.FACS staining.
-
i.Prepare falcon® round-bottom polystyrene tubes:Label falcon® round-bottom polystyrene tubes and coat tubes with BSA.
-
ii.To ensure that cells loosely connected to the well will be analyzed as well, harvest supernatant.
-
iii.Centrifuge for 5 min at 180 g at 23°C.
-
iv.Discard the supernatant and store the tubes with the cell pellets on ice.
-
v.Wash the wells with PBS and aspirate PBS.
-
vi.Add 300 μL 0.25% trypsin to each well.
-
vii.Check cells microscopically and resuspend every 5 min until cells detach.Note: Be sure to dissociate organoids into a single cell suspension.
-
viii.Add 1 mL fibroblast medium and transfer into the falcon® round-bottom polystyrene tubes where the cells are mixed with the pellet from the supernatant (Step 5c.).
-
ix.Centrifuge for 5 min at 180 g at 4°C and discard the supernatant.
-
x.Add 1 mL 1× Annexin Binding Buffer (ABB) and centrifuge again for 5 min at 180 g at 4°C.
-
xi.Discard the supernatant.
-
xii.Prepare the EpCAM – bv421 staining solution:For each sample calculate 2 μL of EpCAM – bv421 antibody and 98 μL of 1× ABB.
-
xiii.Add 100 μL of the staining solution to the tubes.
-
xiv.Vortex and incubate on ice in the refrigerator for 30 min.
-
xv.Add 1 mL 1× ABB, vortex and centrifuge for 5 min at 180 g at 4°C and discard the supernatant.
-
xvi.Prepare the Annexin/Propidium Iodide (Annexin/PI) staining solution.For each sample prepare 300 μL staining solution with 1:200 AnnexinV-APC and 1:40 PI in 1× ABB. For one sample e.g., 1.5 μL AnnexinV-APC, 7.5 μL PI and 291 μL 1× ABB are mixed.
-
xvii.Add 300 μL to each sample and incubate for 10 min on ice.
-
xviii.Measure cell viability using a flow cytometer.Note: See Figure 2 for an example FACS gating strategy. Prepare the appropriate FACS compensation controls of unstained cells and single stained cells.
-
i.
-
a.
-
6.Assessment of viability using CellTiter-Glo.
-
a.Seeding fibroblasts.
-
i.Harvest and count fibroblasts as described in Steps 5a.
-
ii.Calculate 2,000 fibroblasts per well.
-
iii.Resuspend 8 μL fibroblast medium with 10 ng/mL TGF-ß1 per 2,000 fibroblasts.Note: Prepare at least 10 wells additionally to make sure there is enough cell suspension. For example 80 wells (plus 10 wells backup): calculate 190,000 fibroblasts in 720 μL fibroblast medium with 3.6 μL TGF-ß1 (2 ng/μL).
-
iv.Dispense 8 μL with 2,000 fibroblasts in each well of a 384 clear bottom plate.Note: Make sure to fill the outer row of the plate with water to prevent the wells from drying out.
-
v.Incubate in the incubator at 37°C for three days.
-
vi.Add organoids on day 4.
-
i.
-
b.Seeding organoids.
-
i.Harvest and count organoids as described in Steps 5b.
-
ii.Calculate 200 organoids per well.
-
iii.Resuspend in 32 μL fibroblast medium per 200 organoids.Note: Make sure to calculate more wells than needed to have enough cell suspension.
-
iv.Seed organoids on top of the fibroblasts and incubate 1 day at 37°C.
-
v.Add treatment in a volume of 20 μL (optional, otherwise add 20 μL of fibroblast medium).
-
vi.Incubate for 2 days in the incubator until day 6.
-
i.
-
c.CellTiter Glo®.
-
i.Thaw CellTiter Glo® reagent and incubate 30 min in waterbath at 37°C.
-
ii.Add 1:1 (v/v) CellTiter Glo® reagent to each well.
-
iii.Incubate plate for 30 min in the incubator at 37°C.
-
iv.Measure luminescence.
 CRITICAL: Be sure to exclude wells with bubbles or other inconsistencies.
CRITICAL: Be sure to exclude wells with bubbles or other inconsistencies. -
v.Calculate viability of treated coculture relative to control.
-
i.
-
a.
Figure 1.
Timeline of fibroblast - organoid coculture
Depending on the readout - either FACS analysis or CellTiter Glo – fibroblasts and organoids are seeded in 6 well plates or 384 well plates, respectively. On day 0, fibroblasts are seeded and organoids are split. After 3 days, organoids are seeded on top of fibroblasts. After 1 day of coculture, cells can be treated for 2 days and analyzed using either FACS or CellTiter Glo.
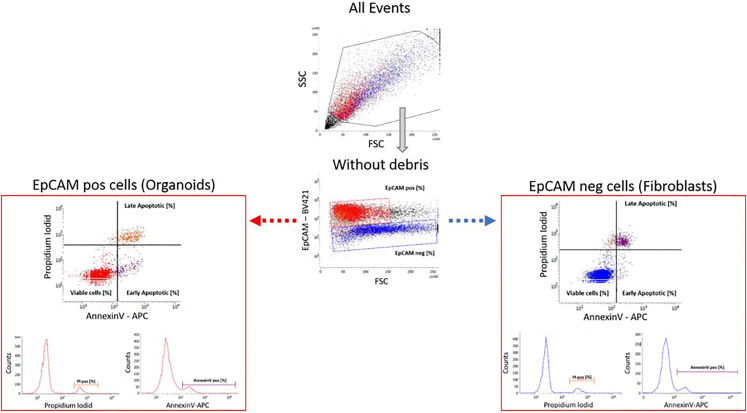
Figure 2.
Gating strategy analyzing viability of EpCAM positive organoids and EpCAM negative fibroblasts
A single cell suspension was stained with EpCAM – bv421, AnnexinV – APC and Propidium Iodide. Debris was removed by their scatter properties (FSC x SSC) and EpCAM positive (organoids) and EpCAM negative (fibroblasts) were analyzed on the Annexin V – APC versus PI scatter for live (bottom left), early (bottom right) and late apoptosis (upper right).
Expected outcomes
The protocol provides a consistent and robust method to coculture fibroblast cell line CCD-18Co with organoids from colorectal cancer patients. Cocultured cells can be analyzed using FACS and luminescence based assays like CellTiter-Glo. This Coculture protocol can be utilized to investigate fibroblast mediated drug resistance and underlying mechanism to provide a basis for personalized medicine.
Limitations
CCD-18Co
Normal colon fibroblast cells are activated with TGF-ß1 for organoid coculture experiments.6 Nevertheless, the fibroblasts are not the matched primary CAFs and therefore it might be possible that the culture models only part of the tumor microenvironmental interactions. As a proof of concept the model could be validated with autologous primary organoids and primary CAFs.
Tumor microenvironment
The tumor microenvironment consists of different cells like stromal, immune cells, endothelial cells and several other components which are not recapitulated in this model.
Troubleshooting
Problem 1
Organoids are dissociated into single cell suspension instead of small clumps. (Related to passaging Organoids in step 2k).
Potential solution
Due to donor-to-donor variations in organoid cohesion, organoids may dissociate into single cells using enzymatic and mechanic digestion. Here, PDOs should be digested only mechanically in the absence of TrypLE. Be careful to resuspend and check regularly to stop the splitting as early as possible.
Problem 2
Difficulty processing PDO to single cells. (Related to FACS staining in step 5c).
Potential solution
Extend Trypsin incubation time and volume and increase trituration frequency and intensity.
Problem 3
Due to donor-to-donor variabilities there are differences in doubling time, growth and form of each patient-derived organoid (PDO). (Related to setting up the coculture in step 5 and 6).
Potential solution
In order to reduce donor-to-donor variations in doubling time we established an experimental setting with low culture periods. Here, PDO has been split into small cell cluster containing 3–10 cells and subsequently grown for 3 days before harvesting and seeding into the coculture. Upon three days of culture, cell cluster have grown to small organoids with low variation in size between donors ranging from (100 μM–200 μM).
Problem 4
There is no valid device for counting organoids of all sizes. (Related to seeding correct numbers of organoids in step 5 and 6).
Potential solution
We used a Fuchs-Rosenthal counting chamber with a depth of 200 μM. Due to our protocol we are seeding small PDOs, with only 3 days growth time, which are smaller than 200 μM and can be counted in the counting chamber.
Problem 5
Organoids grow in 2D. (Related to setting up the coculture in step 5 and 6).
Potential solution
In the setting of organoids cultured in Matrigel domes – ensure that the Matrigel concentration is correct. Furthermore incubate the Matrigel domes containing the organoids upside down to avoid organoids sink down to the bottom of the well. In the setting of organoids cocultured with fibroblasts- ensure that fibroblasts are confluent before adding organoids with fibroblasts.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nicole Janssen (Nicole.janssen@ikp-stuttgart.de ).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by the Robert Bosch Stiftung (Stuttgart, Germany), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy EXC 2180–390900677, and the Interfaculty Centre for Pharmacogenomics and Pharma Research (ICEPHA) Graduate Program, University of Tübingen (Tübingen, Germany). We gratefully acknowledge Kathleen Siegel for excellent technical assistance.
Author contributions
S.W. developed and validated the protocol with input from N.J. and T.E.M. S.W. and N.J. wrote the manuscript and prepared figures, and J.M. and T.E.M. provided input on manuscript preparation. S.K.N. and L.D. were involved in the proof reading. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate/analyze datasets/code.
References
- 1.Regan J.L. Immunofluorescence staining of colorectal cancer patient-derived organoids. Methods Cell Biol. 2022;171:163–171. doi: 10.1016/bs.mcb.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Sato T., Stange D.E., Ferrante M., Vries R.G.J., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 3.Schütte M., Risch T., Abdavi-Azar N., Boehnke K., Schumacher D., Keil M., Yildiriman R., Jandrasits C., Borodina T., Amstislavskiy V., et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat. Commun. 2017;8 doi: 10.1038/ncomms14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pleguezuelos-Manzano C., Puschhof J., van den Brink S., Geurts V., Beumer J., Clevers H. Establishment and culture of human intestinal organoids derived from adult stem cells. Curr. Protoc. Immunol. 2020;130:e106. doi: 10.1002/cpim.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumacher D., Regan J.L., Przybilla D., Schäfer R. Generation of patient-derived colorectal cancer organoids for RAS studies. In Ras Activity and Signaling. Methods Mol. Biol. 2021;2262:349–360. doi: 10.1007/978-1-0716-1190-6_22. [DOI] [PubMed] [Google Scholar]
- 6.Bauer J., Emon M.A.B., Staudacher J.J., Thomas A.L., Zessner-Spitzenberg J., Mancinelli G., Krett N., Saif M.T., Jung B. Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci. Rep. 2020;10:50. doi: 10.1038/s41598-019-55687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets/code.