Abstract
Focused Ultrasound (FUS) is emerging as a promising primary and adjunct therapy for the treatment of cancer. This includes histotripsy, which is a non-invasive, non-ionizing, non-thermal ultrasound guided ablation modality. As histotripsy has progressed from bench-to-bedside, it has become evident that this therapy has benefits beyond local tumor ablation. Specifically, histotripsy has the potential to shift the local tumor microenvironment from immunologically “cold” to “hot”. This is associated with the production of damage associated molecular patterns, the release of a selection of proinflammatory mediators, and the induction of inflammatory forms of cell death in cells just outside of the treatment zone. In addition to the induction of this innate immune response, histotripsy can also improve engagement of the adaptive immune system and promote systemic anti-tumor immunity targeting distal tumors and metastatic lesions. These tantalizing observations suggest that, in settings of widely metastatic disease burden, selective histotripsy of a limited number of accessible tumors could be a means of maximizing responsiveness to systemic immunotherapy. More work is certainly needed to optimize treatment strategies that best synergize histotripsy parameters with innate and adaptive immune responses. Likewise, rigorous clinical studies are still necessary to verify the presence and repeatability of these phenomena in human patients. As this technology nears regulatory approval for clinical use, it is our expectation that the insights and immunomodulatory mechanisms summarized in this review will serve as directional guides for rational clinical studies to validate and optimize the potential immunotherapeutic role of histotripsy tumor ablation.
Keywords: immunology, abscopal effect, cancer, innate immunity, adaptive immunity, immunotherapy, focused ultrasound
HISTROTRIPSY:
Focused Ultrasound (FUS) is a non-invasive technology that is currently in development as a primary or adjunct therapy for the treatment of cancer. In FUS procedures, sound waves are non-invasively applied by an external transducer to a precise focal region within a targeted tumor in order to elicit a desired bioeffect. FUS procedures currently in development for the treatment of cancer include low intensity focused ultrasound (LIFU), high intensity focused ultrasound (HIFU), and histotripsy. These FUS methods are capable of inducing a large range of different bioeffects ranging from reversible tissue modulation (LIFU) to complete ablation of the targeted tumors (HIFU, histotripsy). In LIFU procedures, low intensity FUS is applied to a targeted tumor in order to induce bioeffects such as mild heating (1-2°C) to enhance circulation or activate cellular pathways, mechanical tissue disruption to enhance tissue permeability and drug delivery, or direct cell stimulation in order to induce cell death (1–3). LIFU procedures can also be combined with microbubbles or acoustically-active nanoparticles for opening the blood brain barrier and enhancing drug delivery(4–6), inducing sonodynamic therapy (7, 8), or enabling targeted ablation (9–11). In contrast to LIFU, thermal HIFU and histotripsy procedures utilize higher intensity FUS in order to directly induce cell death within the targeted tumor through either thermal or mechanical mechanisms. Thermal ablation by HIFU has shown promise for the treatment of tumors and other tissues by non-invasively generating thermal necrosis within the targeted tissue (12–17). While thermal HIFU has shown success for certain applications, some limitations include relatively long treatment times, reliance on MRI thermometry for treatment feedback, and the potential for inconsistent ablation in highly perfused tissues (18–22). To address these challenges, histotripsy has been developed as a non-thermal FUS ablation method.
As a non-thermal ablation method, histotripsy non-invasively disintegrates and effectively removes tissue through the control of acoustic cavitation or boiling, without inducing thermal necrosis (23–26). Histotripsy utilizes short, high amplitude acoustic pulses to generate cavitation “bubble clouds” within the targeted tissue, resulting in the complete breakdown of the tissue into an acellular homogenate (23, 24, 27–30). Due to the non-thermal mechanism of action, histotripsy has been demonstrated to be capable of producing extremely precise ablation guided by real-time imaging using ultrasound or MRI (27, 31–38). Studies have also demonstrated that histotripsy can be used to selectively ablate tissues and tumors while preserving critical tissue structures including vessels, nerves, bile ducts, and bone due to their higher mechanical strength (11, 29, 38–42). Furthermore, multiple studies have demonstrated that the tissue ablated by histotripsy is rapidly resorbed and removed by the body after treatment (43–45), as opposed to a necrotic mass that may remain for months or years after thermal ablation procedures (46, 47).
Although the term histotripsy is often used to describe all cavitation-based non-thermal FUS ablation, it is worth noting that there are multiple types of histotripsy that are used to generate the cavitation bubble clouds. The three most commonly used approaches for generating histotripsy are intrinsic-threshold histotripsy, shock-scattering histotripsy, and boiling histotripsy. In intrinsic-threshold histotripsy, bubble clouds are generated using single cycle acoustic pulses with a sufficient peak negative pressure, p-, (>25-30MPa) to form cavitation directly from cavitation nuclei that are intrinsic to the tissue. Shock-scattering histotripsy procedures use longer pulses of 3-20 cycles in duration at slightly lower pressures (p- ~10-25 MPa) to generate cavitation bubble clouds through a multi-step shock scattering process in which initial bubbles scatter the incident shock waves geometrically in order to grow a dense bubble cloud over the course of multiple pulses (42, 48). Finally, boiling histotripsy uses much longer pulses (1-20 ms in duration) at lower pressures (p- ~6-15MPa) to generate large boiling bubbles at the transducer focus through a process of shock-enhanced heating (49, 50). For all forms of histotripsy, studies have shown that that dominant mechanism of tissue ablation is non-thermal and due to cavitation activity, resulting in the breakdown of tissue into an acellular tissue homogenate with no apparent thermal necrosis to cells within the treated tissues. The description of histotripsy as a non-thermal ablation method has been well-established in the literature and refers to the type of tissue damage induced by histotripsy. More specifically, the histotripsy-induced tissue damage is a result of the mechanical forces applied by the expanding/collapsing bubble cloud, which results in the mechanical breakdown of the tissue into acellular debris. In contrast to thermal HIFU, histological analysis of histotripsy-treated tissues shows no signs of thermal necrosis, with the tissue damage being due to the non-thermal (cavitation) mechanism. Although it is likely that some mild temperature rises are observed after each pulse, the low duty cycle used in histotripsy allows sufficient cooling to occur between pulses, minimizing any thermal buildup in the tissue over the course of the treatment, with the resulting ablation being induced through purely non-thermal bioeffects.
Due to the unique features of histotripsy described above, a large number of studies have been conducted in order to investigate histotripsy for the treatment of cancer in both preclinical (11, 33, 42, 45, 51) and early clinical studies (52). Results from preclinical studies have demonstrated that histotripsy can be used to produce precise and tissue-selective tumor ablation for a large number of potential applications discussed in more detail throughout this review, including tumors of the liver, pancreas, breast, kidney, muscle, bone, skin, and brain. In addition to these preclinical studies, recent results from a Phase I clinical trial showed histotripsy was capable of achieving precise and complete ablation of liver tumors in 8 patients with either primary or metastatic liver cancer, with follow up imaging showing rapid resorption of the ablation zones matching preclinical studies(52). This study further showed that histotripsy was generally well-tolerated with no significant device-related adverse events, suggesting that histotripsy has the potential to be developed further for the treatment of liver tumors and other cancers in humans.
Intriguingly, in 2 of the 8 patients treated, a significant reduction in the volume of the nontreated tumor lesions in the liver were also observed suggesting a histotripsy mediated abscopal effect (53). The abscopal effect is a hypothesized systemic anti-tumor immune response induced by various tumor ablation modalities. This effect has been observed in various pre-clinical animal models and sporadically observed in human patients with a number of cancer types. Here, we review the ability of histotripsy to (1) shift the local anti-inflammatory tumor microenvironment from immunologically cold to immunologically hot; (2) enhance innate immune signaling and recognition of damage associated molecular patterns (DAMPs); and (3) improve systemic anti-tumor adaptive immune system activation in a variety of cancers. Together, each of these functions converge and ultimate contribute to both local and systemic anti-tumor immune responses that are predicted to improve patient outcomes.
SHIFTING THE TUMOR MICROENVIRONMENT FROM COLD TO HOT:
The immunosuppressive nature and lack of immune cell infiltration in major subsets of patients with advanced solid tumors presents a significant therapeutic limitation. These tumors are colloquially termed “cold” tumors and are reflective of biological processes that are associated with poor patient outcomes. This immunosuppressive tumor microenvironment (TME) is directly associated with more aggressive disease progression, poor responses to many of the most promising immunotherapeutic strategies, and contributes to metastatic disease. Thus, there is significant interest in shifting this pro-tumor, cold immunosuppressive microenvironment to one that is more immunologically “hot” and pro-inflammatory. The TME consists of tumor cells, stromal cells, the extracellular matrix surrounding the tumor mass, and anti-inflammatory immune cells. Each of these components contributes to the immunosuppressive TME (54). Focusing on the immune cell niche, tumors commonly recruit immune cells to the TME and through various methods, such as selective cytokine and chemokine secretion or decoy surface receptor expression, differentiate or polarize these immune cells into pro-tumor phenotypes (55–57). In the tumors described later in this review, these immunosuppressive cells include regulatory T cells, myeloid derived suppressor cells, tumor associated neutrophils, and tumor associated macrophages (56, 58). Together, these cells secrete a variety of anti-inflammatory mediators, including cytokines and signaling molecules that cloak the tumor or create an impregnable chemical gradient around the tumor to restrict pro-inflammatory, anti-tumor immune cell access (Figure 1). While the TME is, by definition, a local phenotype, the immunosuppressive nature of the tumor has significant impacts on both local and systemic immune responses that must be overcome for effective therapeutic strategies.
Figure 1: Schematic of Histotripsy Mediated Immune System Modulation.
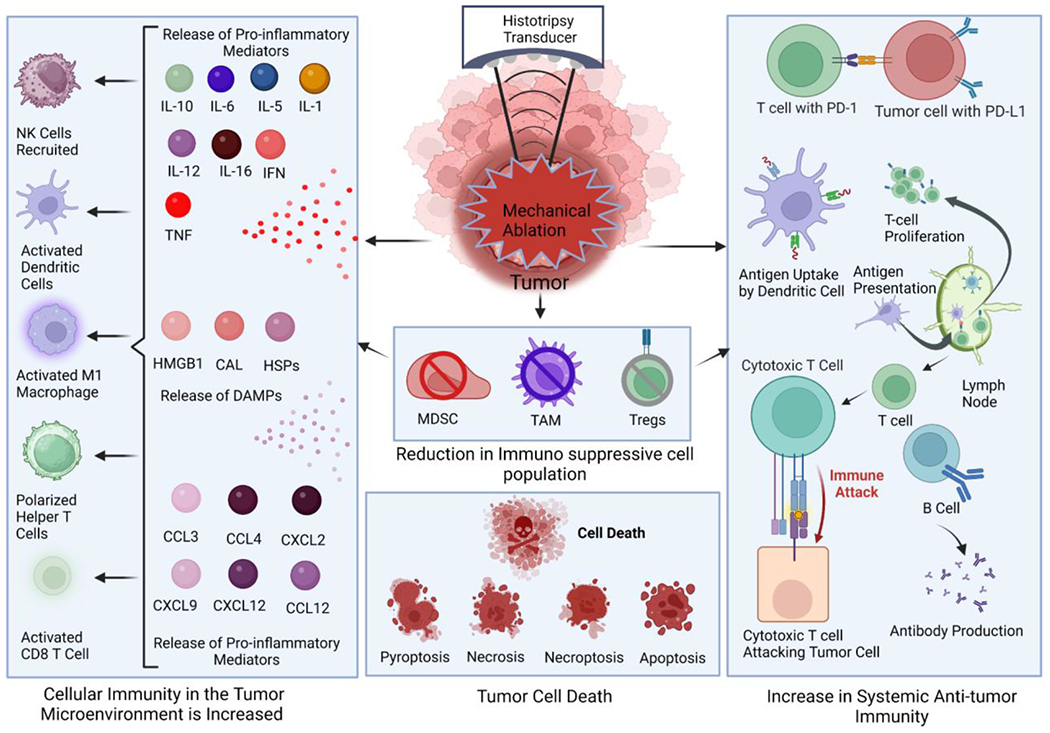
Histotripsy significantly alters the tumor microenvironment through the elimination of immunosuppressive cells in the ablation zone. Likewise, cells adjacent to the ablation zone that are damaged may undergo a range of cell death processes, including inflammatory cell death and apoptosis. The reduction in immunosuppression appears to be countered by an increase in a selection of damage associated molecular patterns and the production of inflammatory mediators, including cytokines and chemokines. Together, these changes shift the immunologically “cold” tumor microenvironment towards one that is more pro-inflammatory and “hot”. This is characterized by an influx of inflammatory cells into the tumor microenvironment. It has been postulated that histotripsy treatment generates higher quality and quantity antigens. The improved antigen presentation has been suggested to augment the systemic, anti-tumor immune response, which has been characterized by an increase in cytotoxic T cell recruitment to local and distal tumors and reduced metastatic burden. Ultimately, these changes may improve immunotherapy responsiveness.
Histotripsy has shown significant potential in shifting the immune cell population in the TME (Figure 1). As discussed above, histotripsy is effective at ablating cancer cells, both in vitro and in vivo/in situ, which significantly debulks the targeted tumors and reduces the production of immunomodulatory factors such as cytokines and chemokines (59, 60). In addition to the cancer cells, immunosuppressive immune cells in the treatment zone are also effectively ablated, which directly impacts the anti-inflammatory mediator milieu protecting the remaining tumor cells. Flow cytometry assessments post-histotripsy have found significant reductions in regulatory T cells, myeloid-derived suppressor cells (MDSCs), and tumor associated macrophage populations (61–63) (Figure 1). Consistent with this, a significant shift has been observed from immunosuppressive cytokines to pro-inflammatory cytokines in the TME, including IL-1, IL-6, IL-10 and TNF (61, 63, 64). (Figure 1). In addition to impacting the immune niche, histotripsy has also been shown to significantly alter the stromal cell niche and extracellular matrix (65). Reducing the density of each of these components of the TME improves immune cell access to the remaining cancer cells to enhance recognition, killing, and clearance.
INNATE IMMUNE SENSING OF THE CHAOS FOLLOWING ABLATION:
As discussed above, histotripsy results in the complete breakdown of tumor tissue into an acellular homogenate within the ablation zone. This acellular homogenate contains a rich mixture of damage associated molecular patterns (DAMPs) that can be immunostimulatory and further promote the shift from the “cold” TME to a “hot” TME. DAMPs are recognized by both extracellular and intracellular pattern recognition receptors, including Toll-like receptors (TLRs) and Nod-like receptors (NLRs) (66, 67) (Figure 1). A variety of DAMPs have been identified both locally and systemically following histotripsy, including extracellular/extranuclear DNA, HMGB1, and ATP and HMGB1(59, 68) (Figure 1). DNA outside of either the nucleus or the cell is a potent DAMP that is sensed by a range of pattern recognition receptors, including AIM2 and NLRP3 (68–71). HMGB1 is a protein typically found in the nucleus of healthy cells. However, HMGB1 is a potent DAMP that is also sensed by pattern recognition receptors, including NLRP3, whenever it is located outside of the cell nucleus (72, 73). ATP is critical for cell function and bioenergetics, among other biological functions. In addition, during cellular stress or following damage, excess ATP is released from injured cells and serves as a potent DAMP, which is also sensed by pattern recognition receptors, including NLRP3 (74). While there are many other potential DAMPs, these three have been reported locally following histotripsy, and HMGB1 has been found both locally and systemically increased in serum (75, 76). DAMP recognition by pattern recognition receptors on both immune cells and stromal cells facilitates a robust immune response through the activation of intracellular signaling networks, which can include NF-κB signaling, that eventually results in pro-inflammatory cytokine production and the recruitment of additional pro-inflammatory immune cells (60, 72, 73) (Figure 1). This cytokine response effectively creates a local Th1/Th17 microenvironment, characterized by the production of a combination of IL-1β, IL-18, IL-6, TNF, and IFNγ, depending on tumor type (52, 75, 77) (Figure 1).
In addition to the DAMPs generated following histotripsy, the type of cell death induced by the ablation in cells that are injured, but not destroyed, is also a critical aspect of immune system activation. Programmed cell death would be expected to occur in cells at the margins of the ablation zone. Cell death can proceed through a variety of pathways following histotripsy and each pathway can be further regulated by genetic, epigenetic, and regulatory factors depending on the cell type and tissue (78). During tumor ablation, the primary types of cell death commonly observed includes apoptosis, necrosis, pyroptosis, and necroptosis (79). In general terms, apoptosis is non-inflammatory and is characterized by limited DAMP release and minimal cytokine responses (78). Markers of apoptosis include effector caspases like caspase 3, annexin V, and phosphatidylserine which is exposed on the outer surface of the plasma membrane that serves as a signal for phagocytic cells during apoptosis (78). Conversely, necrosis is moderately inflammatory and is characterized by spontaneous cell lysis or rapid cell death and the release of DAMPs (79). Markers of necrosis include RIP-1 activation, increased NF-κB signaling, and upregulation of TNF signaling (78, 80). Similarly, necroptosis is pro-inflammatory and is commonly described as programmed lysis (79). Compared to necrosis, necroptosis is a slower process that results in the release of RAMPs, cytokines, and is also associated with enhanced antigen presentation (78, 79). Markers of necroptosis include RIPK3 and MLKL activation (78). Finally, pyroptosis is a highly inflammatory form of cell death that is characterized by the release of DAMPs, pro-inflammatory cytokine production, and enhanced antigen presentation (78). Markers of pyroptosis include cleaved caspase-1/−11, cleaved gasdermin D, and inflammasome activation (78).
The non-thermal, mechanical, nature of histotripsy has also been suggested to improve antigen generation and presentation following tumor ablation (76). Previous studies comparing thermal ablation, cryoablation, IRE, and histotripsy demonstrated that the non-thermal modalities appear to generate increased quantities of antigens that are significantly better at driving predictable and effective antigen presentation compared to the thermal approaches (59, 81, 82). It has been postulated that the antigens generated by histotripsy are in a more natural conformation compared to those exposed to heat (76). These antigens are also more accessible to the antigen presenting cells, which are recruited in higher numbers to the site of the ablation due to the pro-inflammatory shift in the tumor microenvironment (76) The breakdown in the tumor stroma has also been hypothesized to improve antigen presenting cell accessibility. This has been demonstrated in vivo in studies using the B16F10 mouse melanoma cell line and a genetically modified variant of the cell that expressed the lymphocytic choriomeningitis virus antigen GP33 (60). Here, animals with tumors engrafted from the GP33 cell line and treated with histotripsy generated robust CD8+ T cells that generated IFNγ after stimulation with IL-2 and brefeldin A to stimulate memory CD8+ T cells (60) These data demonstrate that the GP33 antigen remains stimulatory and viable following histotripsy and that the adaptive immune response is activated following ablation. Complementing the CD8+ T cell studies, the activation and migration of the antigen presenting dendritic cells and the proliferation of CD4+ T cells have also been suggested to be impacted by histotripsy (76). It is important to note that the differences in field size between mouse models and humans can indeed influence the observed outcomes, such as inflammation, immune response, and cell death mechanisms. The larger field size in mice relative to their size may contribute to more extensive tissue damage and subsequent immune reactions. This can be a factor in the substantial changes in inflammation and immunogenic cell death (ICD) observed in mouse models. It is also important to note that while mouse models provide valuable insights and preclinical data, translating the findings to human patients requires careful consideration of the anatomical and physiological differences. The scale and response to treatments can vary significantly, and additional studies in human subjects are necessary to further evaluate the clinical applicability of histotripsy. This is in large part rationale for why large animal tumor models and healthy large animal clinical trials are also being used to investigate off target damage, inflammation, and recovery after ablation (83, 84). We believe that the combined use of murine models, as well as, large animal models and veterinary clinical studies for the development of histotripsy will reduce the gap in translation from preclinical animal studies to clinical trials.
ENGAGING THE SYSTEMIC ANTI-TUMOR ADAPTIVE IMMUNE RESPONSE
The changes described above in the local tumor microenvironment and the improved antigen presentation likely contribute to the more robust adaptive immune system response reported following histotripsy. This has been illustrated primarily in preclinical animal models. For example, CD8+ cytotoxic T cells have been found systemically in distal lymph nodes and in the spleen following histotripsy treatment and these cells can be activated following re-challenge with the original tumor cells (60, 85) Functionally, the activation of the systemic anti-tumor immune response has been best observed through tumor killing in contralateral tumor engraftment studies and the reduction of metastatic lesions. For example, in studies using murine melanoma, tumors treated with histotripsy and surgically removed two days post-treatment showed significant improvement in metastatic burden compared to those not treated (86). Similar effects have also been reported in subcutaneous models of pancreatic cancer in mice (59). In these studies, decreases in metastasis appear to be correlated to systemic immune system activation and imply an abscopal response. Indeed, multiple studies have reported increased CD8+ cytotoxic T cells in both the treated and contralateral tumors that increase over time (60, 77). This effect was significantly increased compared to other tumor ablation modalities, including RFA, radiation therapy, and sham-control treated animals (60) However, it should be noted in all studies to date, while there is a reduction in metastasis commonly reported, the established contralateral tumors only demonstrate attenuated growth. This implies that the systemic immune response is effective, but not potent enough to fully prevent tumor growth or be curative without other combination therapeutic approaches or treatment strategies.
The most promising combination therapeutic approaches for pairing with histotripsy include immunotherapeutics. Specifically, the use of checkpoint inhibitors is emerging as a promising approach to pair with histotripsy. Many tumors are immunologically resistant to current checkpoint inhibitor strategies, due in part to the “cold” and generally immunosuppressive tumor microenvironment. However, the ability of histotripsy to shift this tumor microenvironment from “cold” to immunologically “hot” has the potential to significantly improve therapeutic response. The increase in DAMPs, pro-inflammatory mediators, influx of immune cells into the tumor microenvironment, and increased systemic anti-tumor immune response all have the potential to significantly improve checkpoint inhibitor functions. Likewise, it should be noted that many of the immune checkpoint molecules, such as PD-L1, are inducible. Thus, a tumor that is negative for such ligand before treatment could upregulate the ligand during or after therapy. This has been shown for histotripsy, whereby the release of IFN-γ following treatment can upregulate PD-L1 by some tumor cell types (54, 87). This upregulation appears to be temporal, tumor cell type, and tissue specific (87). Thus, a better understanding of which checkpoints are up-regulated following histotripsy will be critical for pairing with appropriate checkpoint inhibitor strategies.
CLINICAL PERSPECTIVES AND COMBINATIONAL THERAPY
Emerging insights into the mechanistic underpinnings of histotripsy immune stimulation shed light on potential clinical applications and combinatorial strategies that could be developed for clinical application. As early as 2019, investigators in South Korea and the United States began observing evidence of pro-inflammatory immunogenic cell death (ICD) when boiling histotripsy was applied to experimental models of breast cancer and renal cell carcinoma (77, 88). In 2020, Qu and co-authors used immunocompetent murine models of melanoma and hepatocellular carcinoma to demonstrate that, in contrast to thermal modes of tissue ablation, non-thermal histotripsy was capable of liberating immunogenically-intact tumor antigens from treated tumors. The ability of histotripsy ability to promote ICD as evidenced by the concurrent release of immunogenic antigens and pro-inflammatory damage associated molecular patterns (DAMPs) gave rise to an early local influx of inflammatory innate immune cells followed by progressive intratumoral infiltration of CD8+ T cells and natural killer cells (60). In 2021, Hendricks-Wenger and colleagues used a murine model of pancreatic adenocarcinoma to demonstrate local release of nucleotides, proteins, and DAMPs with upregulation of inflammatory signaling pathways following histotripsy ablation (59).
These demonstrations of post-histotripsy ICD suggest that histotripsy is not merely capable of mechanically killing cells, but of triggering specific programmed pathways of cellular suicide to create a local pro-inflammatory milieu that favors the genesis of tumor-directed immune responses. This unique capacity implies that histotripsy could be a means to sensitize tumors to checkpoint inhibition immunotherapy. Indeed, Qu observed that the immunostimulatory effects of histotripsy powerfully enhanced the efficacy of checkpoint inhibition immunotherapy against melanoma and hepatocellular carcinoma (60). In 2021, Singh and colleagues employed a combinatorial strategy of boiling histotripsy with agonistic anti-CD40 mAb costimulation to induce favorable T cell and macrophage polarization that augmented the effects of checkpoint inhibition against melanoma (89). In 2023, Pepple and co-authors observed that histotripsy induced significant transcriptomic changes among tumor cell and immune cell populations consistent with inflammatory induction of ICD and innate immune cell activation (90). They observed that tumor cells within and just outside the histotripsy ablation zone underwent necroptosis. Moreover, they showed that activated CD8+ T cells primed by histotripsy to infiltrate into distant tumors were uniquely capable of inducing a specific pathway of cancer ICD called ferroptosis – an oxidative cell death pathway that has recently been shown to be the critical mechanism by which checkpoint inhibition immunotherapy-primed CD8+ T cells kill their cancer cell targets (91). This shared mechanism of cytotoxicity may explain the therapeutic cooperativity between histotripsy and checkpoint inhibition; indeed, the combination of histotripsy and checkpoint inhibition appeared to cause a synergistic increase in cancer cell ferroptotic death (90).
Another observation that has been made in the preclinical and clinical settings is the apparent ability of histotripsy to trigger abscopal inhibition of distant tumors. Qu observed that the systemic tumor antigen-specific T cell responses generated by local histotripsy tumor ablation were strong enough to induce abscopal inhibition of distant, non-ablated tumors(60). In comparing the immune landscape of treated versus distant tumors, Pepple noted that, whereas the treated tumor underwent a biphasic pattern of early inflammatory innate immune cell infiltration followed by delayed but progressive adaptive immune cell infiltration, distant tumors exhibited no early inflammatory response to histotripsy but did exhibit a delayed adaptive immune response that was proteomically indistinguishable from that seen in ablated tumors (90). The combination of boiling histotripsy plus CD40 costimulation and checkpoint inhibition also triggered abscopal inhibition of distant, non-ablated murine melanoma tumors (89). In 2022, Worlikar and her team used an aggressive rat model of hepatocellular carcinoma to show that early partial histotripsy not only caused eventual regression of the non-ablated tumor remnant, but abrogated the onset of intrahepatic metastases that were universally observed in the absence of histotripsy treatment (92). Interestingly, these experimental findings were not dissimilar from a clinical report reported by Vidal-Jove and partners in 2021, who observed a case of a patient with widely metastatic colon adenocarcinoma who exhibited durable regression of distant, non-ablated liver metastases following partial histotripsy tumor ablation on a clinical trial (53). These tantalizing observations suggest that, in settings of widely metastatic disease burden, selective histotripsy of a limited number of accessible tumors could be a means of maximizing responsiveness to systemic immunotherapy.
COMPARISON OF IMMUNE MODULATION BETWEEN HISTOTRIPSY AND HIFU
In contrast to the relative recency of publications exploring the immunostimulatory effects of histotripsy, there is a larger and older body of work demonstrating the immune effects of high intensity focused ultrasound (HIFU). In 2005, Hu and colleagues demonstrated liberation of DAMPs from murine colon adenocarcinoma cells treated with ex vivo HIFU, with antigen presenting cell activation seen in response to co-culture with HIFU-generated tumor homogenates (93). They drew in vivo parallels to this observation in 2007, when they identified dendritic cell accumulation in tumor-draining lymph nodes and evidence of tumor-reactive T cells in the peripheral blood of mice bearing colon adenocarcinoma tumors treated with HIFU (94). In 2008, Xing and colleagues used a murine melanoma tumor model to demonstrate abscopal inhibition of distant tumors following HIFU tumor ablation (94), and Zhang and co-authors used HIFU to generate immunoprotective vaccines in a murine hepatocellular carcinoma model in 2010 (95).
To date, there have been no direct comparisons of the immunostimulatory effects of histotripsy and HIFU in a clinical setting. However, unlike histotripsy, HIFU has yielded only modest evidence of anti-tumor immune responses when used as monotherapy. The strongest observations of ICD, antigen presenting cell activation, and T cell activation following HIFU have been seen using combinatorial strategies with immune adjuvants like the TLR9 agonist cytosine guanine dinucleotide (CpG) and checkpoint inhibitors (96–100). One study showed that HIFU combined with immune checkpoint inhibitors like αCTLA-4 and αPD-L1 leads to abscopal effects and shows evidence of systemic antitumor effects by upregulating DC, tumor-infiltrating T-cells, effector memory T-cells, proinflammatory cytokines, and DAMPs while downregulating Foxp3, IL10 and VEGF-α which are pro-tumorigenic regulators thereby increasing long term survival of mice with neuroblastoma tumors (97). Another study with pulsed focused ultrasound and immune checkpoint inhibitors (anti-CTLA-4/PD-1 antibodies) in murine pancreatic cancer showed increasing pro-immune cells infiltration and decreasing regulatory T cells and MDSCs in the tumors to basal levels (101).
One potential limitation of HIFU is its thermal nature, as the potentially denaturing effects of heat might negatively impact the integrity of tumor antigens or DAMPs. Qu observed that the ability to mobilize intratumoral CD8+ T cell infiltration in vivo and to release immunogenic tumor antigens was far stronger for histotripsy than for thermal ablation (60). Hendricks-Wenger similarly observed that histotripsy released higher levels of tumor-derived nucleotide and protein than thermal ablation (59). Indeed, two recent comparisons between mechanical and thermal modes of HIFU identified more potent antigen presenting cell and T cell responses with combinatorial strategies that employed mechanical HIFU (99, 100).
LIMITATIONS AND FUTURE ASPECTS
The landscape of cancer therapy has been transformed by immunotherapy. With the advent of checkpoint inhibitor therapy, diseases like advanced melanoma have gone from having no meaningful therapeutic options to being potentially curable. However, a sizable majority of cancers remain refractory to checkpoint inhibition, and this shortcoming has sparked great interest in the immunostimulatory effects of tumor-directed therapies like histotripsy focused ultrasound ablation. There is an abundance of experimental evidence indicating that histotripsy is capable of inducing ICD of treated cancer cells, leading to systemic anti-tumor immune responses. The great promise of these data is the possibility that histotripsy ablation could be used in conjunction with contemporary and future immunotherapies to extend their transformative benefits to more patients and more cancer types. Of course, all of this will remain in the domain of unrealized expectations until rigorous clinical studies verify the presence and repeatability of these phenomena in patients. As the technology nears regulatory approval for clinical use, the insights and immunomodulatory mechanisms summarized in this review will serve as directional guides for rational clinical studies to validate and optimize the potential immunotherapeutic role of histotripsy tumor ablation.
ACKNOWLEDGEMENTS:
This work was funded by the National Institutes of Health, National Cancer Institute R01CA269811 (ICA) and the National Institute of Biomedical Imaging and Bioengineering R21EB027979 (ICA) and R01CA262474 (EV) and the Department of Veterans Affairs 1I01BX001619 (CSC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Department of Veterans Affairs. This work was also supported by the Virginia Maryland College of Veterinary Medicine, the Virginia Tech Center for Engineered Health, the Virginia Tech Institute for Critical Technology and Applied Science, and the Virginia Tech Center for Drug Discovery, and the University of Michigan Rogel Cancer Center and Michigan Medicine-Peking University Joint Institute.
DECLARATION OF COMPETING INTERESTS:
C.S.C. is a consultant co-Principal Investigator for the HistoSonics Hope4Liver multicenter clinical trial of histotripsy liver tumor ablation. I.C.A. and E.V. have consulted and received research support from HistoSonics, Inc. The authors declare no additional conflicting financial interests or any other personal relationships that could have influenced this article.
REFERENCES
- 1.Joiner JB, Kren NP, Durham PG, McRee AJ, Dayton PA, Pylayeva-Gupta Y. Low-Intensity Focused Ultrasound Produces Immune Response in Pancreatic Cancer. Ultrasound in Medicine & Biology. 2022;48(11):2344–53. [DOI] [PubMed] [Google Scholar]
- 2.Mungur R, Zheng J, Wang B, Chen X, Zhan R, Tong Y. Low-intensity focused ultrasound technique in glioblastoma multiforme treatment. Frontiers in Oncology. 2022;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schibber E, Mittelstein D, Gharib M, Shapiro M, Lee P, Ortiz M. A dynamical model of oncotripsy by mechanical cell fatigue: selective cancer cell ablation by low-intensity pulsed ultrasound. Proceedings of the Royal Society A. 2020;476(2236):20190692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan JJ, Myers R, Coviello CM, Graham SM, Shah AR, Stride E, et al. Ultrasound‐propelled nanocups for drug delivery. small. 2015;11(39):5305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood–brain barrier with focused ultrasound. Journal of Controlled Release. 2015;219:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yildirim A, Blum NT, Goodwin AP. Colloids, nanoparticles, and materials for imaging, delivery, ablation, and theranostics by focused ultrasound (FUS). Theranostics. 2019;9(9):2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mess G, Anderson T, Kapoor S, Thombre R, Liang R, Derin E, et al. Sonodynamic Therapy for the Treatment of Glioblastoma Multiforme in a Mouse Model Using a Portable Benchtop Focused Ultrasound System. JoVE (Journal of Visualized Experiments). 2023(192):e65114. [DOI] [PubMed] [Google Scholar]
- 8.Wallace G 4th, Haar CP, Vandergrift WA, 3rd, Giglio P, Dixon-Mah YN, Varma AK, Ray SK, Patel SJ, Banik NL, Das A. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J Neurooncol. 2013;114(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edsall C, Khan ZM, Mancia L, Hall S, Mustafa W, Johnsen E, et al. Bubble cloud behavior and ablation capacity for histotripsy generated from intrinsic or artificial cavitation nuclei. Ultrasound in medicine & biology. 2021;47(3):620–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghi-Goughari M, Jeon S, Kwon H-J. Enhancing thermal effect of focused ultrasound therapy using gold nanoparticles. IEEE Transactions on NanoBioscience. 2019;18(4):661–8. [DOI] [PubMed] [Google Scholar]
- 11.Vlaisavljevich E, Durmaz YY, Maxwell A, ElSayed M, Xu Z. Nanodroplet-mediated histotripsy for image-guided targeted ultrasound cell ablation. Theranostics. 2013;3(11):851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. Therapeutic Ultrasound. 2016:83–95. [DOI] [PubMed] [Google Scholar]
- 13.ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. International journal of hyperthermia. 2007;23(2):89–104. [DOI] [PubMed] [Google Scholar]
- 14.Chang I, Hwang KJ, Choi HJ, Yoon HJ, Lee ES, Choi SY. HIFU: effects and clinical effectiveness of non-surgical therapy for uterine fibroids. Journal of Menopausal Medicine. 2016;22(2):59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji Y, Hu K, Zhang Y, Gu L, Zhu J, Zhu L, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Archives of gynecology and obstetrics. 2017;296:1181–8. [DOI] [PubMed] [Google Scholar]
- 16.Lessick J, Abadi S, Agmon Y, Keidar Z, Carasso S, Aronson D, et al. Multidetector computed tomography predictors of late ventricular remodeling and function after acute myocardial infarction. European journal of radiology. 2012;81(10):2648–57. [DOI] [PubMed] [Google Scholar]
- 17.Ruhnke H, Eckey T, Bohlmann M, Beldoch M, Neumann A, Agic A, et al. , editors. MR-guided HIFU treatment of symptomatic uterine fibroids using novel feedback-regulated volumetric ablation: effectiveness and clinical practice. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren; 2013: © Georg Thieme Verlag KG. [Google Scholar]
- 18.Zibari GB, Riche A, Zizzi HC, McMillan RW. Surgical and nonsurgical management of primary and metastatic liver tumors. The American surgeon. 1998;64(3):211. [PubMed] [Google Scholar]
- 19.McGahan J, Dodd G. Radiofrequency ablation of malignant liver tumours. Am J Roentgenol. 2001;176:3–16. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda H, Ito R, Ohto M, Sakamoto A, Karasawa E, Yamaguchi T, et al. Treatment of small hepatocellular carcinomas with US-guided high-intensity focused ultrasound. Ultrasound in medicine & biology. 2011;37(8):1222–9. [DOI] [PubMed] [Google Scholar]
- 21.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. American Journal of Roentgenology. 2005;185(1):64–71. [DOI] [PubMed] [Google Scholar]
- 22.McClure TD, Chow DS, Tan N, Sayre JA, Pantuck AJ, Raman SS. Intermediate outcomes and predictors of efficacy in the radiofrequency ablation of 100 pathologically proven renal cell carcinomas. Journal of Vascular and Interventional Radiology. 2014;25(11):1682–8. [DOI] [PubMed] [Google Scholar]
- 23.Vlaisavljevich E, Kim Y, Allen S, Owens G, Pelletier S, Cain C, et al. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound in medicine & biology. 2013;39(8):1398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlaisavljevich E, Maxwell A, Mancia L, Johnsen E, Cain C, Xu Z. Visualizing the histotripsy process: Bubble cloud–cancer cell interactions in a tissue-mimicking environment. Ultrasound in medicine & biology. 2016;42(10):2466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bader KB, Vlaisavljevich E, Maxwell AD. For whom the bubble grows: physical principles of bubble nucleation and dynamics in histotripsy ultrasound therapy. Ultrasound in medicine & biology. 2019;45(5):1056–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Hall TL, Vlaisavljevich E, Lee FT Jr. Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. International Journal of Hyperthermia. 2021;38(1):561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall TL, Hempel CR, Wojno K, Xu Z, Cain CA, Roberts WW. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009;74(4):932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Hall TL, Fowlkes JB, Cain CA. Optical and acoustic monitoring of bubble cloud dynamics at a tissue-fluid interface in ultrasound tissue erosion. The Journal of the acoustical Society of America. 2007;121(4):2421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lake A, Xu Z, Wilkinson J, Cain C, Roberts W. Renal ablation by histotripsy—Does it spare the collecting system? The Journal of urology. 2008;179(3):1150–4. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, Raghavan M, Hall TL, Chang C-W, Mycek M-A, Fowlkes JB, et al. High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy-histotripsy. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2007;54(10):2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD Jr, Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. Journal of vascular and interventional radiology. 2011;22(3):369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlaisavljevich E, Kim Y, Owens G, Roberts W, Cain C, Xu Z. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Physics in Medicine & Biology. 2013;59(2):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlaisavljevich E, Owens G, Lundt J, Teofilovic D, Ives K, Duryea A, et al. Non-invasive liver ablation using histotripsy: preclinical safety study in an in vivo porcine model. Ultrasound in Medicine & Biology. 2017;43(6):1237–51. [DOI] [PubMed] [Google Scholar]
- 34.Allen SP, Roberts WW, Hall TL, Cain CA, Hernandez-Garcia L, editors. Characterization of the in vivo histotripsy lesion using high field MRI. Proceedings, International Society for Magnetic Resonance in Medicine 20th Annual Meeting and Exhibition; 2012. [Google Scholar]
- 35.Allen SP, Hall TL, Cain CA, Hernandez‐Garcia L. Controlling cavitation‐based image contrast in focused ultrasound histotripsy surgery. Magnetic resonance in medicine. 2015;73(1):204–13. [DOI] [PubMed] [Google Scholar]
- 36.Wang T-Y, Hall TL, Xu Z, Fowlkes JB, Cain CA. Imaging feedback of histotripsy treatments using ultrasound shear wave elastography. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2012;59(6):1167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T-Y, Xu Z, Winterroth F, Hall TL, Fowlkes JB, Rothman ED, et al. Quantitative ultrasound backscatter for pulsed cavitational ultrasound therapy-histotripsy. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2009;56(5):995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macoskey JJ, Zhang X, Hall TL, Shi J, Beig SA, Johnsen E, et al. Bubble-induced color Doppler feedback correlates with histotripsy-induced destruction of structural components in liver tissue. Ultrasound in medicine & biology. 2018;44(3):602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancia L, Rodriguez M, Sukovich J, Xu Z, Johnsen E. Single–bubble dynamics in histotripsy and high–amplitude ultrasound: Modeling and validation. Physics in Medicine & Biology. 2020;65(22):225014. [DOI] [PubMed] [Google Scholar]
- 40.Styn N, Hall TL, Fowlkes JB, Cain CA, Roberts WW. Histotripsy homogenization of the prostate: thresholds for cavitation damage of periprostatic structures. Journal of endourology. 2011;25(9):1531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold L, Hendricks-Wenger A, Coutermarsh-Ott S, Gannon J, Hay AN, Dervisis N, et al. Histotripsy ablation of bone tumors: Feasibility study in excised canine osteosarcoma tumors. Ultrasound in medicine & biology. 2021;47(12):3435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlaisavljevich E, Maxwell A, Warnez M, Johnsen E, Cain CA, Xu Z. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2014;61(2):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts WW, Teofilovic D, Jahnke RC, Patri J, Risdahl JM, Bertolina JA. Histotripsy of the prostate using a commercial system in a canine model. The Journal of urology. 2014;191(3):860–5. [DOI] [PubMed] [Google Scholar]
- 44.Smolock AR, Cristescu MM, Vlaisavljevich E, Gendron-Fitzpatrick A, Green C, Cannata J, et al. Robotically assisted sonic therapy as a noninvasive nonthermal ablation modality: proof of concept in a porcine liver model. Radiology. 2018;287(2):485–93. [DOI] [PubMed] [Google Scholar]
- 45.Vlaisavljevich E, Greve J, Cheng X, Ives K, Shi J, Jin L, et al. Non-invasive ultrasound liver ablation using histotripsy: chronic study in an in vivo rodent model. Ultrasound in medicine & biology. 2016;42(8):1890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance‐guided focused ultrasound surgery for uterine myomas: 24‐month follow‐up. Ultrasound in Obstetrics and Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2009;34(5):584–9. [DOI] [PubMed] [Google Scholar]
- 47.Tamura N, Kurabayashi T, Nagata H, Matsushita H, Yahata T, Tanaka K. Effects of testosterone on cancellous bone, marrow adipocytes, and ovarian phenotype in a young female rat model of polycystic ovary syndrome. Fertility and sterility. 2005;84:1277–84. [DOI] [PubMed] [Google Scholar]
- 48.Maxwell AD, Wang T-Y, Cain CA, Fowlkes JB, Sapozhnikov OA, Bailey MR, et al. Cavitation clouds created by shock scattering from bubbles during histotripsy. The Journal of the Acoustical Society of America. 2011;130(4):1888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maxwell AD, Yuldashev PV, Kreider W, Khokhlova TD, Schade GR, Hall TL, et al. A prototype therapy system for transcutaneous application of boiling histotripsy. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2017;64(10):1542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y-N, Khokhlova T, Bailey M, Hwang JH, Khokhlova V. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound in medicine & biology. 2013;39(3):424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin K-W, Kim Y, Maxwell AD, Wang T-Y, Hall TL, Xu Z, et al. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: microtripsy. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2014;61(2):251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vidal-Jove J, Serres X, Vlaisavljevich E, Cannata J, Duryea A, Miller R, et al. First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study. International Journal of Hyperthermia. 2022;39(1):1115–23. [DOI] [PubMed] [Google Scholar]
- 53.Vidal-Jové J, Serres-Créixams X, Ziemlewicz TJ, Cannata JM. Liver histotripsy mediated abscopal effect—Case report. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2021;68(9):3001–5. [DOI] [PubMed] [Google Scholar]
- 54.Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends in cancer. 2020;6(7):605–18. [DOI] [PubMed] [Google Scholar]
- 55.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. The Journal of experimental medicine. 2006;203(7):1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer cell. 2012;21(6):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer cell. 2014;26(5):638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boland PM, Ma WW. Immunotherapy for colorectal cancer. Cancers. 2017;9(5):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hendricks-Wenger A, Sereno J, Gannon J, Zeher A, Brock RM, Beitel-White N, et al. Histotripsy ablation alters the tumor microenvironment and promotes immune system activation in a subcutaneous model of pancreatic cancer. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2021;68(9):2987–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu S, Worlikar T, Felsted AE, Ganguly A, Beems MV, Hubbard R, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. Journal for immunotherapy of cancer. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kindlund B, Sjöling Å, Yakkala C, Adamsson J, Janzon A, Hansson L-E, et al. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer. 2017;20:116–25. [DOI] [PubMed] [Google Scholar]
- 62.Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-β in the differentiation and effector function of T regulatory cells. International archives of allergy and immunology. 2002;129(4):263–76. [DOI] [PubMed] [Google Scholar]
- 63.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher Frequencies of GARP+ CTLA-4+ Foxp3+ T Regulatory Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients Are Associated with Impaired T-Cell FunctionalityHigh Frequency of GARP+ Tregs and MDSC in HCC Patients. Cancer research. 2013;73(8):2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Dea LSL, MacDougall J, Alexander VJ, Digenio A, Hubbard B, Arca M, et al. Differentiating familial chylomicronemia syndrome from multifactorial severe hypertriglyceridemia by clinical profiles. Journal of the Endocrine Society. 2019;3(12):2397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu X, Zhu L, Wang T, Chen J. Immune microenvironment of cholangiocarcinoma: Biological concepts and treatment strategies. Frontiers in Immunology. 2023;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune network. 2018;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage‐associated molecular pattern molecules. Journal of leukocyte biology. 2007;81(1):28–37. [DOI] [PubMed] [Google Scholar]
- 68.Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern recognition receptors and the host cell death molecular machinery. Frontiers in immunology. 2018;9:2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCall KD, Muccioli M, Benencia F. Toll-like receptors signaling in the tumor microenvironment. Tumor Microenvironment: Signaling Pathways–Part A. 2020:81–97. [DOI] [PubMed] [Google Scholar]
- 70.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140(6):798–804. [DOI] [PubMed] [Google Scholar]
- 72.Kate S, Jurg T. The inflammasomes. Cell. 2010;140(6):821–32. [DOI] [PubMed] [Google Scholar]
- 73.Tattoli I, Carneiro LA, Jehanno M, Magalhaes JG, Shu Y, Philpott DJ, et al. NLRX1 is a mitochondrial NOD‐like receptor that amplifies NF‐κB and JNK pathways by inducing reactive oxygen species production. EMBO reports. 2008;9(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grazioli S, Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Frontiers in immunology. 2018;9:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nam GH, Pahk KJ, Jeon S, Park HJ, Kim GB, Oh SJ, et al. Investigation of the potential immunological effects of boiling histotripsy for cancer treatment. Advanced Therapeutics. 2020;3(8):1900214. [Google Scholar]
- 76.Hendricks-Wenger A, Hutchison R, Vlaisavljevich E, Allen IC. Immunological effects of histotripsy for cancer therapy. Frontiers in oncology. 2021;11:681629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schade GR, Wang Y-N, D’Andrea S, Hwang JH, Liles WC, Khokhlova TD. Boiling histotripsy ablation of renal cell carcinoma in the Eker rat promotes a systemic inflammatory response. Ultrasound in medicine & biology. 2019;45(1):137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brock RM, Beitel-White N, Davalos RV, Allen IC. Starting a fire without flame: The induction of cell death and inflammation in electroporation-based tumor ablation strategies. Frontiers in Oncology. 2020;10:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ringel-Scaia VM, Beitel-White N, Lorenzo MF, Brock RM, Huie KE, Coutermarsh-Ott S, et al. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. EBioMedicine. 2019;44:112–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weinlich R, Green DR. The two faces of receptor interacting protein kinase-1. Molecular cell. 2014;56(4):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alinezhadbalalami N, Graybill PM, Imran KM, Verbridge SS, Allen IC, Davalos RV. Generation of Tumor-activated T cells Using Electroporation. Bioelectrochemistry. 2021;142:107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shao Q, O’Flanagan S, Lam T, Roy P, Pelaez F, Burbach BJ, et al. Engineering T cell response to cancer antigens by choice of focal therapeutic conditions. International Journal of Hyperthermia. 2019;36(1):130–8. [DOI] [PubMed] [Google Scholar]
- 83.Hendricks-Wenger A, Arnold L, Gannon J, Simon A, Singh N, Sheppard H, et al. Histotripsy ablation in preclinical animal models of cancer and spontaneous tumors in veterinary patients: a review. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2021;69(1):5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hendricks-Wenger A, Nagai-Singer MA, Uh K, Vlaisavljevich E, Lee K, Allen IC. Employing Novel Porcine Models of Subcutaneous Pancreatic Cancer to Evaluate Oncological Therapies. Biomedical Engineering Technologies: Springer; 2022. p. 883–95. [DOI] [PubMed] [Google Scholar]
- 85.Huang X, Yuan F, Liang M, Lo H-W, Shinohara ML, Robertson C, et al. M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PloS one. 2012;7(7):e41632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xing Y, Lu X, Pua EC, Zhong P. The effect of high intensity focused ultrasound treatment on metastases in a murine melanoma model. Biochemical and biophysical research communications. 2008;375(4):645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qian J, Wang C, Wang B, Yang J, Wang Y, Luo F, et al. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. Journal of neuroinflammation. 2018;15(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pahk KJ, Shin C-H, Bae IY, Yang Y, Kim S-H, Pahk K, et al. Boiling histotripsy-induced partial mechanical ablation modulates tumour microenvironment by promoting immunogenic cell death of cancers. Scientific reports. 2019;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh MP, Sethuraman SN, Miller C, Malayer J, Ranjan A. Boiling histotripsy and in-situ CD40 stimulation improve the checkpoint blockade therapy of poorly immunogenic tumors. Theranostics. 2021;11(2):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pepple AL, Guy JL, McGinnis R, Felsted AE, Song B, Hubbard R, et al. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Frontiers in Immunology. 2023;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Worlikar T, Zhang M, Ganguly A, Hall TL, Shi J, Zhao L, et al. Impact of histotripsy on development of intrahepatic metastases in a rodent liver tumor model. Cancers. 2022;14(7):1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Z, Yang XY, Liu Y, Morse MA, Lyerly HK, Clay TM, et al. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochemical and biophysical research communications. 2005;335(1):124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. Journal of translational medicine. 2007;5(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Deng J, Feng J, Wu F. Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World journal of gastroenterology: WJG. 2010;16(28):3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chavez M, Silvestrini MT, Ingham ES, Fite BZ, Mahakian LM, Tam SM, et al. Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics. 2018;8(13):3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eranki A, Srinivasan P, Ries M, Kim A, Lazarski CA, Rossi CT, et al. High-Intensity Focused Ultrasound (HIFU) Triggers Immune Sensitization of Refractory Murine Neuroblastoma to Checkpoint Inhibitor TherapyHIFU with Immunotherapy Cure Refractory Murine Neuroblastoma. Clinical Cancer Research. 2020;26(5):1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fite BZ, Wang J, Kare AJ, Ilovitsh A, Chavez M, Ilovitsh T, et al. Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Scientific reports. 2021;11(1):927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van den Bijgaart RJ, Mekers VE, Schuurmans F, Raaijmakers TK, Wassink M, Veltien A, et al. Mechanical high-intensity focused ultrasound creates unique tumor debris enhancing dendritic cell-induced T cell activation. Frontiers in Immunology. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abe S, Nagata H, Crosby EJ, Inoue Y, Kaneko K, Liu C-X, et al. Combination of ultrasound-based mechanical disruption of tumor with immune checkpoint blockade modifies tumor microenvironment and augments systemic antitumor immunity. Journal for Immunotherapy of Cancer. 2022;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mouratidis PX, Costa M, Rivens I, Repasky EE, Ter Haar G. Pulsed focused ultrasound can improve the anti-cancer effects of immune checkpoint inhibitors in murine pancreatic cancer. Journal of the Royal Society Interface. 2021;18(180):20210266. [DOI] [PMC free article] [PubMed] [Google Scholar]


