Abstract
Background
Hypofibrinogenaemia is associated with excessive bleeding after cardiac surgery. Our aim was to compare the efficacy and safety of weight-adjusted vs empiric dosing of fibrinogen replacement in cardiac surgery.
Methods
In the Fibrinogen Replenishment in Cardiac Surgery (FIBRES) RCT, patients (n=735) received fibrinogen concentrate (4 g) or cryoprecipitate (10 units). In this post-hoc analysis, patients were grouped into quartiles based on increasing weight-adjusted dosing. Generalised estimating equations were used to account for hospital site, age, sex, surgical complexity, urgency, and critical preoperative status. The primary outcome was the number of units of red blood cells transfused within 24 h of cardiopulmonary bypass. Secondary outcomes included allogeneic blood components within 24 h, tamponade or major bleeding, and thromboembolic complications, ischaemic complications, or both within 28 days of cardiopulmonary bypass.
Results
The median weight-adjusted doses were 52 mg kg−1 of fibrinogen concentrate (inter-quartile range [IQR], 45–61; n=372) and 1.30 units per 10 kg of cryoprecipitate (IQR, 1.11–1.54; n=363). When patients were divided into quartiles of lowest to highest weight-adjusted dosing, no differences were seen in the primary outcome of red blood cell units transfused within 24 h of cardiopulmonary bypass between the lowest and highest quartiles in either the fibrinogen group (adjusted relative risk [RR]=0.90; 95% confidence interval [CI], 0.71–1.13; P=0.36) or the cryoprecipitate group (adjusted RR=1.04; 95% CI, 0.76–1.43; P=0.80). Results were similar for all secondary outcomes.
Conclusion
Outcomes for the lowest and highest weight-adjusted doses of fibrinogen replacement were comparable. Weight-adjusted dosing does not appear to offer advantages over empiric dosing in this context.
Clinical trial registration
Keywords: cardiac surgery, coagulopathy, fibrinogen, haemostasis, transfusion
Clinically significant bleeding is a common perioperative complication of cardiac surgery, and coagulopathy has been associated with the use of cardiopulmonary bypass (CPB).1 Major surgical or coagulopathic bleeding increases the need for allogeneic blood component transfusion, and is independently associated with increased morbidity and mortality.2,3 Effective treatment of coagulopathic bleeding has been shown to reduce allogeneic transfusion, which itself is associated with increased morbidity and mortality.4, 5, 6, 7
A common cause of coagulopathic bleeding after CPB is acquired hypofibrinogenaemia.8, 9, 10, 11 Plasma fibrinogen levels decrease from baseline by 34–58% during CPB.1,12,13 Fibrinogen is often the first coagulation factor to be depleted to critically low concentrations in ongoing bleeding. In addition, CPB impairs fibrin formation to a greater extent than thrombin generation or platelet function.13,14 The decrease in plasma fibrinogen is multifactorial, but it is often related to the pre-surgery level, haemodilution, increased consumption, and increased degradation.1,11,15,16
There is significant evidence supporting fibrinogen supplementation as a first-line treatment for post-CPB coagulopathic bleeding related to hypofibrinogenaemia.7,17 The main products used for fibrinogen replacement in surgical bleeding are cryoprecipitate and fibrinogen concentrate. Cryoprecipitate was used as first-line therapy for hypofibrinogenaemia before the development of factor concentrates.18 Fibrinogen concentrate is a newer product approved for use in many countries, including Canada. The type of product used, the dose, and the transfusion protocol varies by centre, and few studies have compared these issues directly.7,17
Dosage recommendations have been made for fibrinogen concentrate and cryoprecipitate based on formulae that account for plasma fibrinogen concentration and patient weight19 but, particularly in the context of cardiac surgery, empiric doses of 4 g per administration of fibrinogen concentrate and 10 units of cryoprecipitate are often used and have been demonstrated to be efficacious and to show a favourable safety profile.7,20, 21, 22, 23 However, larger patients have larger circulating blood volumes,24,25 and fixed dosing of fibrinogen-containing products may be suboptimal in some patients. Larger patients who are relatively under-dosed may receive inadequate treatment of acquired hypofibrinogenaemia with potentially increased transfusion requirements. Smaller patients may be relatively overdosed, which may in turn impact clinical outcomes.26
Our goal was to evaluate whether a body weight-adjusted dosing strategy has clinical advantages over a fixed empiric dosing strategy in the clinical setting of bleeding cardiac surgical patients. We performed a post-hoc analysis of the Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery (FIBRES) RCT7 to examine the association between the weight-adjusted doses of fibrinogen concentrate and cryoprecipitate administered with transfusion requirements, return to the operating room for tamponade or bleeding, and thromboembolic complications.
Methods
Study design and aims
This was a post-hoc analysis of prospectively collected data from the FIBRES RCT,7 which included patients undergoing a cardiac surgical procedure with CPB between 10 February 2017 and 1 November 2018, at one of 11 centres across Canada (ClinicalTrials.gov identifier: NCT03037424). Approval for this substudy was obtained from the University Health Network Research Ethics Board (REB Study Number 16–5636.16), and results are reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.27
Recruited patients received fibrinogen replacement for clinically significant bleeding deemed related to hypofibrinogenaemia (plasma fibrinogen <2.0 g L−1 by Clauss method or fibrin-based thromboelastometry [FIBTEM], or clinical suspicion where fibrinogen levels were unavailable).7 Exclusion criteria were as detailed in the original FIBRES study.7
In FIBRES, patients were randomly allocated to receive a fixed dose of 4 g fibrinogen concentrate per administration infused over approximately 10 min, or 10 units of cryoprecipitate infused according to local practice. The individual dose for each product was fixed. This empirical dosing strategy is consistent with prior published literature and guidelines.7 Fibrinogen concentrate was only received for 24 h after CPB, after which cryoprecipitate was to be used for subsequent doses in both groups. No other alterations to patient care were made.5,28 The aim of this analysis was to evaluate the association between the dose of fibrinogen concentrate or cryoprecipitate administered, as adjusted for patient weight, and postoperative outcomes. Our primary analysis included all patients in the modified intention-to-treat analysis of FIBRES. We additionally examined the subpopulation who only received one dose of product in a separate analysis to more reliably estimate differences in plasma fibrinogen concentration between groups after a single administration.
Primary predictor
Each 4 g dose of fibrinogen concentrate was adjusted for total patient body weight according to product monograph and weight-based guidelines, which typically recommend 50–70 mg kg−1 fibrinogen per individual administration.29,30 Cryoprecipitate dosing at 10 units was similarly adjusted for total body weight where recommendations suggest 1 unit per 5–10 kg.30,31 In order to compare outcomes between patients receiving lower doses of fibrinogen replacement as adjusted for body weight compared with those receiving higher amounts, quartiles were created distinguishing groups of patients treated with lower amounts of weight-based product dosing (Quartile 1, lowest 25% of weight-based dosing) compared with higher weight-based doses up to Quartile 4 (highest 25% of weight-based dosing). Quartiles were generated to enable separation by clinically meaningful differences in fibrinogen product dosing while still retaining enough patients within each group to enable statistical comparisons.
Outcomes
The primary outcome was the number of units of packed red blood cells transfused within 24 h of CPB.8,32, 33, 34 The secondary outcomes included the total number of individual allogeneic blood components transfused within 24 h after CPB (packed red cells, platelets, frozen plasma [FP]), re-exploration because of tamponade or major bleeding, and the incidence of thromboembolic complications, ischaemic complications, or both (defined as stroke, myocardial infarction, intra-abdominal or limb ischaemia, and pulmonary embolism or other venous thrombosis) within 28 days of CPB.
Statistical analyses
Continuous variables were handled as medians and inter-quartile ranges (IQRs). Categorical variables were handled as frequencies or percentages. Descriptive analyses were conducted examining variability in weight-based dosing. The change in plasma fibrinogen concentration after a single product dose was examined with scatterplots. Box plots and histograms were used to visually examine the change in plasma fibrinogen concentration after a single dose of product.
For the primary and secondary outcomes, unadjusted and adjusted associations between increasing quartiles of weight-based product doses and counts of RBC or total allogeneic transfusions were examined. Generalised estimating equations with specification of a Poisson distribution were used for count data, whereas a logistic distribution was specified for binary outcomes. Adjustment variables were chosen a priori based on the literature.35,36 Models were adjusted for surgical complexity, urgency, critical preoperative status, sex, and age. The absence of higher level clustering beyond the site level was assumed, and values of the intraclass correlation coefficient were examined in relation to appropriate specification of the model covariance matrix.37 Global measures of model fit based on information criterion statistics (QIC statistics) for a multilevel data structure were used to assess individual models.38 Unadjusted and adjusted odds ratios or risk ratios were calculated where appropriate, with 95% confidence intervals (95% CIs). A P-value ≤0.05 was considered significant. SAS Studio (SAS Institute, Inc., Cary, NC, USA) was used to perform the statistical analyses.
Results
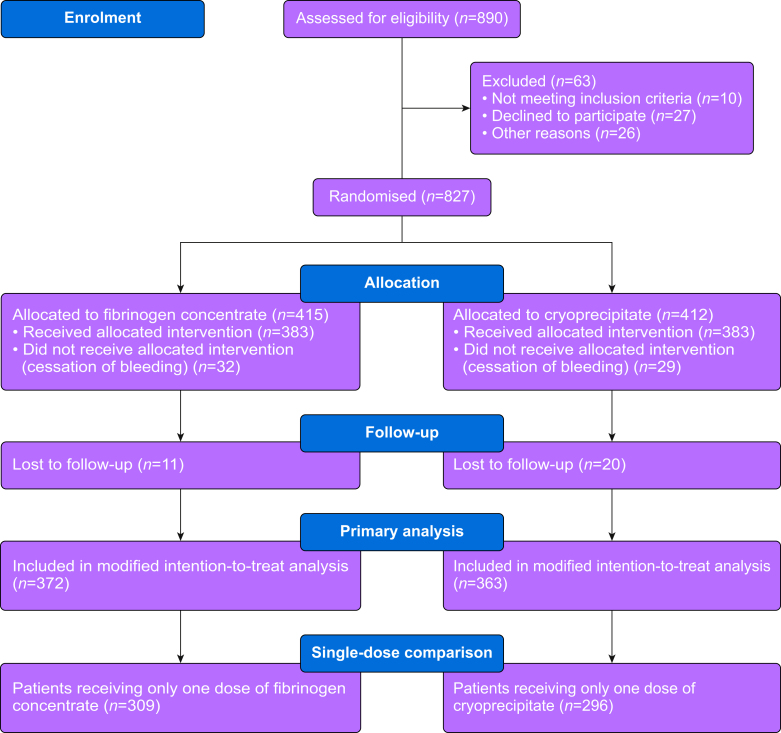
Of the 827 patients included in FIBRES, 372 patients from the fibrinogen concentrate group and 363 from the cryoprecipitate group were included in the modified intention-to-treat analysis and comprised the population of interest for this study (Fig 1). Patient characteristics are presented in Table 1. For analyses including patients who received only one dose of fibrinogen replacement, 309 patients in the fibrinogen concentrate and 296 patients in the cryoprecipitate group were eligible. Of these patients, 252 (82%) in the fibrinogen concentrate group and 225 (76%) in the cryoprecipitate group had plasma Clauss fibrinogen values available before and after product administration.
Fig. 1.
Participant identification and selection flow diagram.
Table 1.
Descriptive characteristics of the study population. IQR, inter-quartile range; sd, standard deviation.
| Fibrinogen arm (n=372 | Cryoprecipitate arm (n=363) | |
|---|---|---|
| Patient characteristics | ||
| Age, yr, median (IQR) | 65 (54, 72) | 64 (53, 72) |
| Sex Female, n (%) Male, n (%) |
113 (30%) 259 (70%) |
105 (29%) 258 (71%) |
| Height, cm, median (IQR) | 170 (161, 178) | 170 (163, 177) |
| Weight, kg, median (IQR) | 76 (65, 87) | 77 (65, 90) |
| BMI, kg m−2, median (IQR) | 22 (20, 25) | 23 (20, 26) |
| Creatinine clearance, ml min−1, median (IQR) | 73 (51, 95) | 74 (50, 96) |
| Diabetes mellitus, n (%) | 80 (22%) | 74 (20%) |
| Chronic lung disease, n (%) | 53 (14%) | 37 (11%) |
| Cerebrovascular disease, n (%) | 46 (12%) | 49 (14%) |
| Left ventricular ejection fraction, n (%) >50% 31–50% 21–31% <21% |
256 (72%) 51 (14%) 28 (8%) 22 (6%) |
263 (76%) 57 (16%) 12 (3%) 15 (4%) |
| Surgical characteristics | ||
| Complex surgery, n (%) | 267 (72%) | 260 (72%) |
| Urgent surgery, n (%) | 141 (38%) | 128 (35%) |
| Critical preoperative Status, n (%) | 64 (17%) | 37 (11%) |
| Cardiopulmonary bypass time, min, median (IQR) | 143 (102, 209) | 134 (99, 200) |
| Perioperative fibrinogen details | ||
| Typical product amount administered per individual dose, g | ||
| Mean (sd) Median (IQR) |
4 (0.23) 4 (4, 4) |
10 (0.13) 10 (10, 10) |
| Weight-adjusted dose, mg kg−1 (Fibrinogen Concentrate) or units per 10 kg (Cryoprecipitate) | ||
| Mean (sd) Median (IQR) |
54 (13) 52 (45, 61) |
1.34 (0.31) 1.30 (1.11, 1.54) |
| Fibrinogen concentration before product administration, g L−1 | ||
| All patients, median (IQR)Patients receiving only one fibrinogen or cryoprecipitate dose, median (IQR) | 1.6 (1.3, 1.9) [n=352] 1.60 (1.33, 1.90) [n=293] |
1.6 (1.3, 1.9) [n=346] 1.66 (1.35, 1.92) [n=287] |
| Fibrinogen concentration after product administration, g L−1 | ||
| All patients, median (IQR)Patients receiving only one fibrinogen or cryoprecipitate dose, median (IQR) | 2.5 (2.1, 2.9) [n=324] 2.58 (2.23, 2.96) [n=267] |
2.3 (2.0, 2.6) [n=296] 2.40 (2.11, 2.71) [n=233] |
| Plasma fibrinogen concentration change after product administration, g L−1 | ||
| All patients, median (IQR)Patients receiving only one fibrinogen or cryoprecipitate dose, Median (IQR) | 0.90 (0.6, 1.2) [n=306] 0.96 (0.74, 1.28) [n=252] |
0.70 (0.5, 1.0) [n=280] 0.78 (0.52, 1.00) [n=225] |
| Bleeding and transfusion within 24 h of separation from cardiopulmonary bypass | ||
| Packed red blood cells, units, median (IQR) | 2 (0, 5) | 2 (1, 5) |
| Platelets, units, median (IQR) | 2 (1, 3) | 2 (1, 3) |
| Frozen plasma, units, median (IQR) | 2 (0, 4) | 2 (0, 4) |
| 24 h chest tube output, ml, median (IQR) | 800 (500, 1350) | 830 (540, 1350) |
Dose variability in empiric vs weight-adjusted strategies
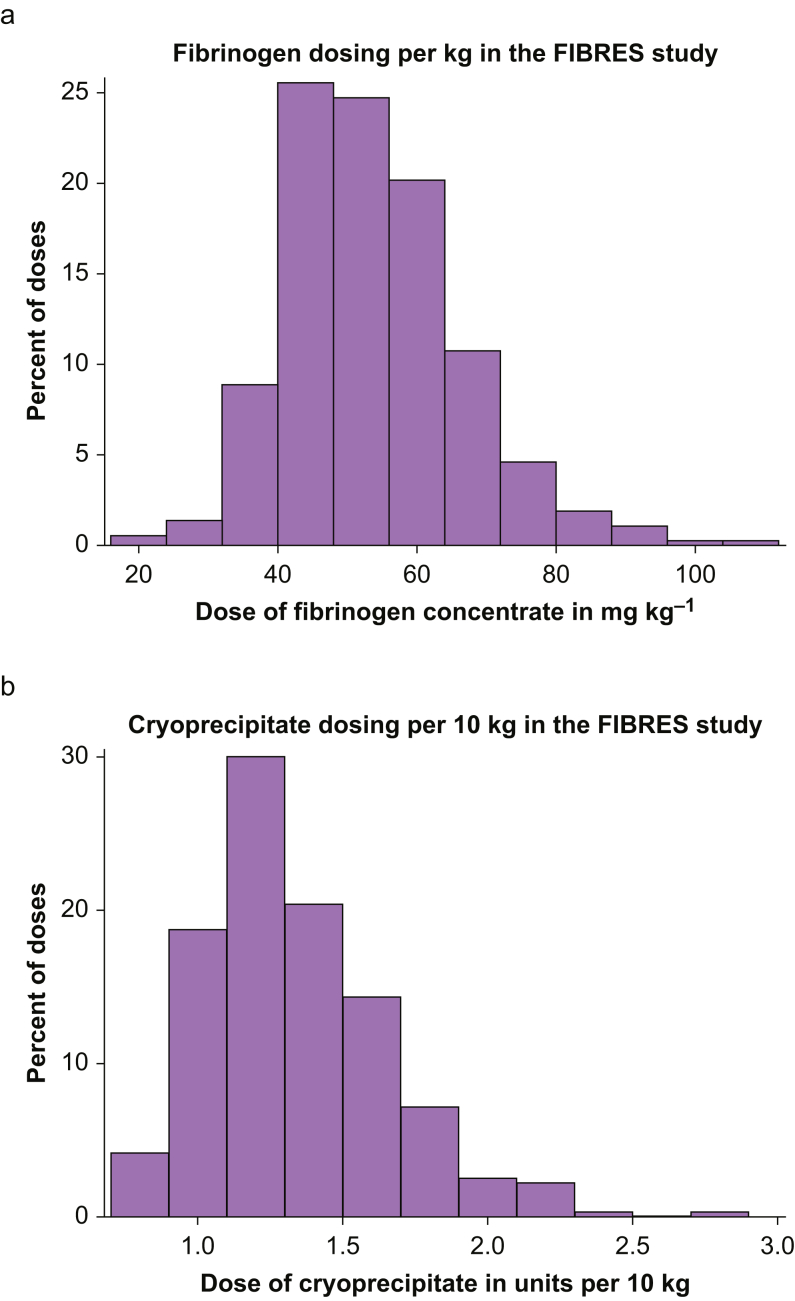
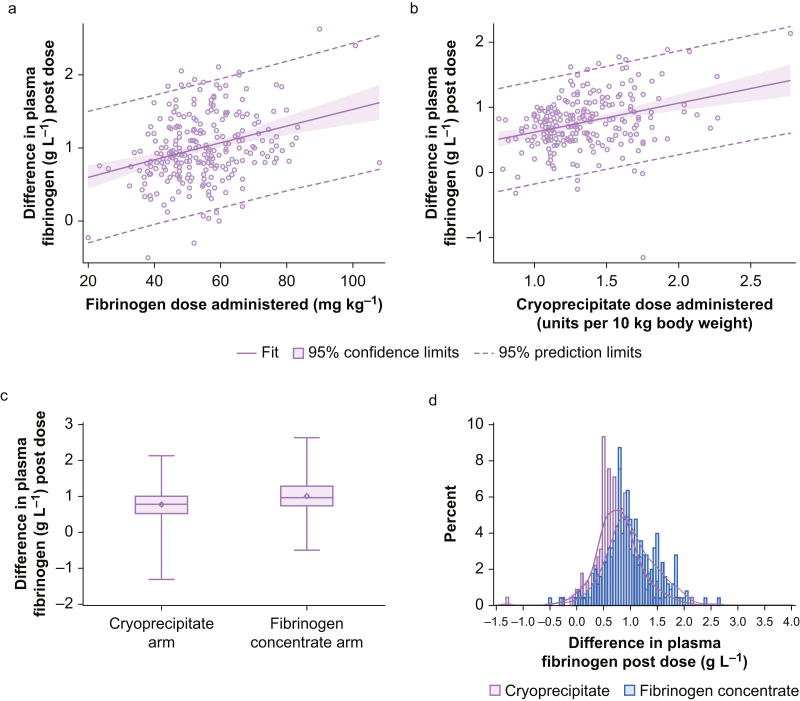
Variability in the empiric doses of fibrinogen concentrate or cryoprecipitate received was low (fibrinogen concentrate median dose=4.0 g; IQR, 4.0–4.0; cryoprecipitate median dose=10 units; IQR, 10–10). When adjusted for individual patient weight, however, more variability per dose was observed. This translated to a median dose of 52 mg kg−1 (IQR, 45–61) for fibrinogen concentrate and 1.30 units per 10 kg (IQR, 1.11–1.54) for cryoprecipitate. Variability in the dose of each product adjusted for weight is shown in Fig 2. The plasma fibrinogen concentration increased proportionately with increases in weight-adjusted dosing of both fibrinogen concentrate and cryoprecipitate (Fig 3), with significant inter-patient variability in dose response.
Fig. 2.
Variability in individual dose of product adjusted for weight. (a) Variability in fibrinogen concentrate dose adjusted for total body weight (kg) (n=372). (b) Variability in cryoprecipitate dose adjusted for total body weight (per 10 kg) (n=363). FIBRES, Fibrinogen Replenishment in Cardiac Surgery.
Fig. 3.
Plasma fibrinogen change after a single administration of fibrinogen concentrate or cryoprecipitate. Cryoprecipitate, n=225; fibrinogen concentrate, n=252. (a) Change in plasma fibrinogen concentration after a single dose of fibrinogen concentrate, adjusted for total body weight (kg) (n=252). Parameter estimate for fibrinogen concentrate per mg kg−1: 0.01 (95% confidence interval [CI], 0.007–0.016); P<0.0001. (b) Change in plasma fibrinogen concentration after a single dose of cryoprecipitate, adjusted for total body weight (per 10 kg) (n=225). Parameter estimate for cryoprecipitate per 10 kg: 0.45 (95% CI, 0.28–0.62); P<0.0001. (c) Box plot of change in plasma fibrinogen concentration after a single product dose. Wilcoxon signed-rank test P-value <0.0001. (d) Histogram of individual plasma fibrinogen changes after a single product dose. Superimposed data distributions are shown using kernel density estimates.
In all included patients where Clauss plasma fibrinogen was measured before and after a product dose, the median change in plasma fibrinogen concentration after either a single or repeated dose of fibrinogen concentrate (n=324, 87%) was 0.90 g L−1 (IQR, 0.6–1.2), and after a single or repeated dose of cryoprecipitate (n=296, 82%) it was 0.70 g L−1 (IQR, 0.5–1.0). Examining only patients where Clauss fibrinogen concentration was measured before or after a single dose of fibrinogen concentrate (n=252, 82%) or cryoprecipitate (n=225, 76%), the median increase in plasma fibrinogen concentration in the fibrinogen concentrate group was 0.96 g L−1 (IQR, 0.74–1.28), and in the cryoprecipitate group it was 0.78 g L−1 (IQR, 0.52–1.00), with a median difference between groups of –0.18 g L−1 (95% CI, –0.28 to –0.08; P=0.0002) (Fig 3). Detailed information regarding the change in plasma fibrinogen level and the absolute value of the plasma fibrinogen concentration after a single dose is available in Supplementary Appendix Tables S1 and S2.
Association of weight-adjusted fibrinogen concentrate or cryoprecipitate dose with outcomes
Patients in the Fibrinogen Concentrate group were divided into quartiles of weight-based fibrinogen dosing, with those in the lowest quartile receiving a median fibrinogen concentrate dose of 41 mg kg−1 (IQR, 38–43) and those in the highest quartile receiving a median fibrinogen concentrate dose of 67 mg kg−1 (IQR, 64–75) (P<0.001; Table 2). With Quartile 1 as the reference quartile, there was no consistently observed difference in unadjusted or adjusted models for the outcomes of units of RBC transfused within 24 h of CPB, number of allogeneic products transfused (RBC, platelets, FP) within 24 h of CPB, return to the operating room for bleeding or tamponade, or thromboembolic or ischaemic complications (Table 3, Supplementary Fig. S1). The median numbers of units of RBCs transfused per quartile with corresponding median weights are shown in Supplementary Table S3.
Table 2.
Quartiles of product dosing when adjusted for total body weight. IQR, inter-quartile range; sd, standard deviation.
| Group | Fibrinogen arm (n=372) (mg kg−1) |
Cryoprecipitate arm (n=363) (units per 10 kg) |
||||
|---|---|---|---|---|---|---|
| Number (%) | Mean (sd) | Median (IQR) | Number (%) | Mean (sd) | Median (IQR) | |
| Quartile 1Lowest 25% | 94 (25%) | 40 (5) | 41 (38, 43) | 94 (26%) | 1.01 (0.09) | 1.02 (0.94, 1.09) |
| Quartile 2 | 92 (25%) | 49 (2) | 48 (47, 51) | 87 (24%) | 1.21 (0.06) | 1.22 (1.15, 1.25) |
| Quartile 3 | 93 (25%) | 57 (2) | 56 (55, 59) | 91 (25%) | 1.39 (0.06) | 1.39 (1.33, 1.44) |
| Quartile 4Highest 25% | 93 (25%) | 71 (10) | 67 (64, 75) | 91 (25%) | 1.78 (0.22) | 1.69 (1.63, 1.86) |
Table 3.
Results of unadjusted and adjusted regression models for outcomes. ∗Adjusted model results accounted for surgical complexity (complex procedures were those other than coronary artery bypass surgery only, single valve only, or repair of atrial septal defect only), urgency (non-elective vs elective status), critical preoperative status (determined by blinded adjudication on patients who underwent emergency surgery and had any of the following conditions: (1) ventricular tachycardia or fibrillation or cardiac arrest; (2) preoperative cardiac massage; (3) preoperative ventilation before anaesthetic room; (4) haemodynamic support requiring preoperative inotropes or ventricular assist devices; (5) preoperative acute renal failure [anuria or oliguria]), sex, and age. CI, confidence interval.
| Outcome 1: Number of packed red blood cells transfused within 24 h of cardiopulmonary bypass Poisson regression accounting for clustering by study site | ||||||
|---|---|---|---|---|---|---|
| Fibrinogen arm (n=372) | Cryoprecipitate arm (n=363) | |||||
| Unadjusted results | ||||||
| Quartile | Number of red blood cells transfused | Relative risk (95% CI) | P-value | Number of red blood cells transfused | Relative risk (95% CI) | P-value |
| 1 2 3 4 |
349 336 272 277 |
Reference 1.03 (0.67–1.58) 0.83 (0.66–1.05) 0.84 (0.65–1.10) |
0.90 0.12 0.21 |
345 232 350 274 |
Reference 0.72 (0.47–1.12) 1.04 (0.73–1.48) 0.81 (0.60–1.08) |
0.14 0.84 0.15 |
| Adjusted results∗ | ||||||
| Quartile | Number of red blood cells transfused | Relative risk (95% CI) | P-value | Number of red blood cells transfused | Relative risk (95% CI) | P-value |
| 1 2 3 4 |
349 336 272 277 |
Reference 1.04 (0.77–1.40) 0.89 (0.70–1.15) 0.90 (0.71–1.13) |
0.81 0.38 0.36 |
345 232 350 274 |
Reference 0.82 (0.52–1.27) 1.17 (0.85–1.60) 1.04 (0.76–1.43) |
0.37 0.33 0.80 |
|
Outcome 2: number of all allogeneic product transfusions within 24 h of cardiopulmonary bypass (red blood cells, frozen plasma, platelets) Poisson regression accounting for clustering by study site | ||||||
|---|---|---|---|---|---|---|
| Fibrinogen arm (n=372) | Cryoprecipitate arm (n=363) | |||||
| Unadjusted results | ||||||
| Quartile | Number of blood products transfused | Relative risk (95% CI) | P-value | Number of blood products transfused | Relative risk (95% CI) | P-value |
| 1 2 3 4 |
1003 927 677 771 |
Reference 1.01 (0.77–1.31) 0.75 (0.63–0.89) 0.83 (0.69–1.00) |
0.97 0.0009 0.05 |
1083 671 902 721 |
Reference 0.69 (0.49–0.96) 0.89 (0.64–1.25) 0.73 (0.59–0.90) |
0.03 0.51 0.003 |
| Adjusted results∗ | ||||||
| Quartile | Number of blood products transfused | Relative risk (95% CI) | P-value | Number of blood products transfused | Relative risk (95% CI) | P-value |
| 1 2 3 4 |
1003 927 677 771 |
Reference 1.02 (0.87–1.20) 0.83 (0.69–1.00) 0.90 (0.78–1.04) |
0.81 0.05 0.16 |
1083 671 902 721 |
Reference 0.78 (0.55–1.11) 0.99 (0.73–1.34) 0.92 (0.70–1.21) |
0.17 0.95 0.56 |
|
Outcome 3: return to the operating room for re-exploration because of bleeding or tamponade Logistic regression accounting for clustering by study site | ||||||
|---|---|---|---|---|---|---|
| Fibrinogen arm (n=372) | Cryoprecipitate arm (n=363) | |||||
| Unadjusted results | ||||||
| Quartile | Number of events | Odds ratio (95% CI) | P-value | Number of events | Odds ratio (95% CI) | P-value |
| 1 2 3 4 |
13 12 17 8 |
Reference 0.93 (0.55–1.59) 1.39 (0.82–2.36) 0.59 (0.32–1.09) |
0.79 0.22 0.09 |
9 13 11 14 |
Reference 1.66 (0.65–4.23) 1.30 (0.59–2.89) 1.73 (0.67–4.49) |
0.29 0.51 0.26 |
| Adjusted results∗ | ||||||
| Quartile | Number of events | Odds ratio (95% CI) | P-value | Number of events | Odds ratio (95% CI) | P-value |
| 1 2 3 4 |
13 12 17 8 |
Reference 0.89 (0.46–1.70) 1.40 (0.80–2.44) 0.49 (0.23–1.04) |
0.72 0.24 0.06 |
9 13 11 14 |
Reference 1.29 (0.45–3.72) 1.20 (0.41–3.48) 1.80 (0.49–6.58) |
0.63 0.74 0.37 |
|
Outcome 4: thromboembolic and ischaemic complications (stroke, myocardial infarction, intra-abdominal or limb ischaemia, pulmonary embolism, and venous thrombosis) Logistic regression accounting for clustering by study site | ||||||
|---|---|---|---|---|---|---|
| Fibrinogen arm (n=372) | Cryoprecipitate arm (n=363) | |||||
| Unadjusted results | ||||||
| Quartile | Number of events | Odds ratio (95% CI) | P-value | Number of events | Odds ratio (95% CI) | P-value |
| 1 2 3 4 |
8 8 12 9 |
Reference 1.04 (0.41–2.62) 1.58 (0.67–3.75) 1.17 (0.47–2.92) |
0.93 0.30 0.74 |
9 9 13 14 |
Reference 1.10 (0.37–3.30) 1.59 (0.71–3.57) 1.70 (0.68–4.22) |
0.86 0.26 0.25 |
| Adjusted results∗ | ||||||
| Quartile | Number of events | Odds ratio (95% CI) | P-value | Number of events | Odds ratio (95% CI) | P-value |
| 1 2 3 4 |
8 8 12 9 |
Reference 0.94 (0.38–2.34) 1.67 (0.64–4.40) 0.92 (0.40–2.11) |
0.90 0.30 0.85 |
9 9 13 14 |
Reference 1.37 (0.42–4.46) 1.79 (0.70–4.59) 1.98 (0.73–5.36) |
0.60 0.22 0.18 |
Similarly, patients in the Cryoprecipitate group were divided into quartiles of weight-based dosing, with those in the lowest quartile receiving a median cryoprecipitate dose of 1.02 units per 10 kg (IQR, 0.94–1.09), and those in the highest quartile receiving a median cryoprecipitate dose of 1.69 units per 10 kg (IQR, 1.63–1.86; P<0.001). There was no consistently observed difference between quartiles in the unadjusted or adjusted models for the outcomes of RBC transfused within 24 h of CPB, any allogeneic transfusion within 24 h of CPB, return to the operating room for bleeding or tamponade, or thromboembolic or ischaemic complications (Table 3, Supplementary Fig. S2).
Discussion
In this post-hoc analysis of the FIBRES RCT, we examined the association between weight-adjusted dosing with plasma fibrinogen concentration response to product administration and clinical outcomes, including allogeneic blood product transfusion, major postoperative bleeding, and thromboembolic or ischaemic events. Although plasma fibrinogen concentration increased more with higher weight-based dosing, there was no observed difference in our pre-specified clinical outcomes when comparing patients who received lower or higher weight-adjusted doses of either fibrinogen concentrate or cryoprecipitate.
We observed a range of doses of both fibrinogen concentrate and cryoprecipitate when adjusted for weight, but the observed variability appears to be limited in its clinical importance given that only a small proportion of patients had a post-supplementation fibrinogen concentration less than 2 g L−1 (Supplementary Table S2: Fibrinogen Concentrate group, 8%; Cryoprecipitate group, 15%). When the plasma fibrinogen level is unknown or unreliable (such as during ongoing haemorrhage), the recommended weight-based dose of fibrinogen concentrate is 70 mg kg−1, whereas cryoprecipitate is generally dosed at 1 unit per 7–10 kg.39 When analysed retrospectively, many patients within the FIBRES trial were not dosed within this recommended weight-based range, yet we did not observe an increase in transfusion requirements or return to the operating room for re-exploration in patients receiving lower weight-adjusted doses. In addition, despite the variability in the actual weight-adjusted dose received, post-transfusion plasma fibrinogen concentrations were 2.5 g L−1 (IQR, 2.1–2.9) in the fibrinogen concentrate group and 2.3 g L−1 (IQR, 2.0–2.6) in the cryoprecipitate group, suggesting adequacy of fibrinogen replacement in both groups.7 In addition, it is unlikely that increasing the plasma fibrinogen concentration above that recommended by international guidelines will have any additional haemostatic effect.5
Similarly, we did not observe an association between higher weight-adjusted fibrinogen concentrate or cryoprecipitate dosing and thromboembolic or ischaemic events. The risk of venous thromboembolism increases with plasma fibrinogen concentrations >4.6 g L−1, with the thrombosis risk increasing almost fourfold when compared with the general population at plasma concentrations >5 g L−1.28,40 Such high concentrations during active bleeding are rare and were not observed in FIBRES. Therefore, an empiric fixed dosing strategy of 4 g for fibrinogen concentrate and 10 units for cryoprecipitate is logistically simpler to implement in clinical practice for actively bleeding patients, and when utilised properly appears unlikely to be harmful to patients.
The results of this analysis support the use of a standardised fixed dosing regimen for fibrinogen concentrate and cryoprecipitate in treating post-CPB acquired hypofibrinogenaemic bleeding in patients with complex cardiac surgery as demonstrated in the FIBRES trial. Support for the efficacy of fixed dosing of fibrinogen concentrate in adult patients is important to establish in the context of the many practical and safety benefits of fibrinogen concentrates over cryoprecipitate.41 Although cryoprecipitate does contain other pro-haemostatic factors (von Willebrand factor, factor XIII, and fibronectin), this substudy was unable to detect an additional benefit to haemostasis in this patient population when compared with fibrinogen concentrate. Our data suggest that fibrinogen concentrate is a more reliable product for fibrinogen replacement, with a significantly higher increase in median plasma fibrinogen concentration observed after a single dose compared with cryoprecipitate (fibrinogen concentrate=0.96 g L−1 [IQR, 0.74–1.28]; cryoprecipitate=0.78 g L−1 [IQR, 0.52–1.00]; P<0.0001). This may be a result of the known variability in fibrinogen content between doses of cryoprecipitate, given that it is a plasma-derived product. Before the publication of the FIBRES trial, variability in clotting factor levels amongst blood donors was known to contribute to variability in the fibrinogen concentration in cryoprecipitate.42 In contrast, fibrinogen concentrate products have greater standardisation of fibrinogen content. Despite this variability, clinical efficacy overall appears to be similar.
Limitations of this post-hoc analysis relate to the relatively small sample size when examining rare clinical outcomes such as return to the operating room for bleeding or thromboembolic events. In addition, higher BMI is associated with greater circulating blood volume and higher baseline fibrinogen concentration, and so may be protective from transfusion and clinically significant bleeding events.24,25 This may in part explain why no increase in bleeding complications or transfusion was seen with increases in body weight, despite relative under-dosing of product.24,30,39,43 In addition, although our study does not measure every endpoint which may capture bleeding (e.g. delayed chest closure indicative of ongoing microvascular bleeding), we did examine endpoints highly associated with morbidity (all allogeneic blood component transfusions and the incidence of return to the operating room for re-exploration because of bleeding). There were no differences found in these analyses, suggesting that clinically important bleeding is unlikely to be different between groups.
Conclusion
Our analysis shows no statistically significant differences in blood product usage, return to the operating room for bleeding or tamponade, or thromboembolic events when comparing quartiles of patients receiving the lowest weight-based doses to patients receiving the highest weight-based doses of the individual fibrinogen sources. Fibrinogen concentrate was shown in our results to provide a larger single dose increase in plasma fibrinogen concentration compared with cryoprecipitate; however, the majority of patients had fibrinogen levels greater than 2 g L−1 after a single dose of either product. When considering the above in the context of fibrinogen concentrate's logistical and safety advantages over cryoprecipitate, it is likely that the adoption of fibrinogen concentrates in treating bleeding associated with CPB will continue to grow.
Authors' contributions
All authors contributed to manuscript concept, content, analyses, writing and review of final paper.
Declarations of interest
Justyna Bartoszko and Keyvan Karkouti were partly supported by a merit award from the Department of Anesthesiology and Pain Medicine, University of Toronto. Justyna Bartoszko has received a travel honorarium from Octapharma. Keyvan Karkouti has also received research support, honoraria, or consultancy for speaking engagements from Octapharma, Instrumentation Laboratory, and Bayer. Jeannie Callum has received research support from Canadian Blood Services and Octapharma. The FIBRES randomized controlled trial was supported by the Canadian Institutes of Health Research and Octapharma.
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2022.100016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bartoszko J., Karkouti K. Managing the coagulopathy associated with cardiopulmonary bypass. J Thromb Haemost. 2021;19:617–632. doi: 10.1111/jth.15195. [DOI] [PubMed] [Google Scholar]
- 2.Bartoszko J., Wijeysundera D.N., Karkouti K., et al. Comparison of two major perioperative bleeding scores for cardiac surgery trials: universal definition of perioperative bleeding in cardiac surgery and European coronary artery bypass grafting bleeding severity grade. Anesthesiology. 2018;129:1092–1100. doi: 10.1097/ALN.0000000000002179. [DOI] [PubMed] [Google Scholar]
- 3.Murphy G.J., Reeves B.C., Rogers C.A., Rizvi S.I., Culliford L., Angelini G.D. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 4.Mueller M.M., Van Remoortel H., Meybohm P., et al. Patient blood management: recommendations from the 2018 frankfurt consensus conference. JAMA. 2019;321:983–997. doi: 10.1001/jama.2019.0554. [DOI] [PubMed] [Google Scholar]
- 5.Raphael J., Mazer C.D., Subramani S., et al. Society of Cardiovascular Anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Anesth Analg. 2019;129:1209–1221. doi: 10.1213/ANE.0000000000004355. [DOI] [PubMed] [Google Scholar]
- 6.Joshi R.V., Wilkey A.L., Blackwell J.-M., et al. Blood conservation and hemostasis in cardiac surgery: a survey of practice variation and adoption of evidence-based guidelines. Anesth Analg. 2021;133:104–114. doi: 10.1213/ANE.0000000000005553. [DOI] [PubMed] [Google Scholar]
- 7.Callum J., Farkouh M.E., Scales D.C., et al. Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: the FIBRES randomized clinical trial. JAMA. 2019;322:1–11. doi: 10.1001/jama.2019.17312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karkouti K., Callum J., Crowther M.A., et al. The relationship between fibrinogen levels after cardiopulmonary bypass and large volume red cell transfusion in cardiac surgery: an observational study. Anesth Analg. 2013;117:14–22. doi: 10.1213/ANE.0b013e318292efa4. [DOI] [PubMed] [Google Scholar]
- 9.Blome M., Isgro F., Kiessling A.H., et al. Relationship between factor XIII activity, fibrinogen, haemostasis screening tests and postoperative bleeding in cardiopulmonary bypass surgery. Thromb Haemost. 2005;93:1101–1107. doi: 10.1160/TH04-12-0799. [DOI] [PubMed] [Google Scholar]
- 10.Solomon C., Hagl C., Rahe-Meyer N. Time course of haemostatic effects of fibrinogen concentrate administration in aortic surgery. Br J Anaesth. 2013;110:947–956. doi: 10.1093/bja/aes576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gielen C., Dekkers O., Stijnen T., et al. The effects of pre- and postoperative fibrinogen levels on blood loss after cardiac surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2014;18:292–298. doi: 10.1093/icvts/ivt506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon C., Baryshnikova E., Tripodi A., et al. Fibrinogen measurement in cardiac surgery with cardiopulmonary bypass: analysis of repeatability and agreement of Clauss method within and between six different laboratories. Thromb Haemost. 2014;112:109–117. doi: 10.1160/TH13-12-0997. [DOI] [PubMed] [Google Scholar]
- 13.Solomon C., Rahe-Meyer N., Sørensen B. Fibrin formation is more impaired than thrombin generation and platelets immediately following cardiac surgery. Thromb Res. 2011;128:277–282. doi: 10.1016/j.thromres.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Hiippala S.T., Myllylä G.J., Vahtera E.M. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81:360–365. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Gielen C.L., Grimbergen J., Klautz R.J., Koopman J., Quax P.H. Fibrinogen reduction and coagulation in cardiac surgery: an investigational study. Blood Coagul Fibrinolysis. 2015;26:613–620. doi: 10.1097/MBC.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 16.Martini W.Z. Coagulopathy by hypothermia and acidosis: mechanisms of thrombin generation and fibrinogen availability. J Trauma. 2009;67:202–208. doi: 10.1097/TA.0b013e3181a602a7. [DOI] [PubMed] [Google Scholar]
- 17.Downey L.A., Andrews J., Hedlin H., et al. Fibrinogen concentrate as an alternative to cryoprecipitate in a postcardiopulmonary transfusion algorithm in infants undergoing cardiac surgery: a prospective randomized controlled trial. Anesth Analg. 2020;130:740–751. doi: 10.1213/ANE.0000000000004384. [DOI] [PubMed] [Google Scholar]
- 18.Callum J.L., Karkouti K., Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev. 2009;23:177–188. doi: 10.1016/j.tmrv.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Levy J.H., Goodnough L.T. How I use fibrinogen replacement therapy in acquired bleeding. Blood. 2015;125:1387–1393. doi: 10.1182/blood-2014-08-552000. [DOI] [PubMed] [Google Scholar]
- 20.Bilecen S., de Groot J.A.H., Kalkman C.J., et al. Effect of fibrinogen concentrate on intraoperative blood loss among patients with intraoperative bleeding during high-risk cardiac surgery: a randomized clinical trial. JAMA. 2017;317:738–747. doi: 10.1001/jama.2016.21037. [DOI] [PubMed] [Google Scholar]
- 21.Winearls J., Wullschleger M., Wake E., et al. Fibrinogen early in severe trauma studY (FEISTY): study protocol for a randomised controlled trial. Trials. 2017;18:241. doi: 10.1186/s13063-017-1980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nascimento B., Callum J., Tien H., et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117:775–782. doi: 10.1093/bja/aew343. [DOI] [PubMed] [Google Scholar]
- 23.Ranucci M., Baryshnikova E., Crapelli G.B., et al. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemmens H.J., Bernstein D.P., Brodsky J.B. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16:773–776. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 25.Ditschuneit H.H., Flechtner-Mors M., Adler G. Fibrinogen in obesity before and after weight reduction. Obes Res. 1995;3:43–48. doi: 10.1002/j.1550-8528.1995.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 26.Klovaite J., Nordestgaard B.G., Tybjærg-Hansen A., Benn M. Elevated fibrinogen levels are associated with risk of pulmonary embolism, but not with deep venous thrombosis. Am J Respir Crit Care Med. 2013;187:286–293. doi: 10.1164/rccm.201207-1232OC. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 28.Karkouti K., Callum J., Wijeysundera D.N., et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. 2016;134:1152–1162. doi: 10.1161/CIRCULATIONAHA.116.023956. [DOI] [PubMed] [Google Scholar]
- 29.Franchini M., Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus. 2012;10:23–27. doi: 10.2450/2011.0015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy J.H., Welsby I., Goodnough L.T. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion. 2014;54:1389–1405. doi: 10.1111/trf.12431. [DOI] [PubMed] [Google Scholar]
- 31.Storch E.K., Custer B.S., Jacobs M.R., Menitove J.E., Mintz P.D. Review of current transfusion therapy and blood banking practices. Blood Rev. 2019;38:100593. doi: 10.1016/j.blre.2019.100593. [DOI] [PubMed] [Google Scholar]
- 32.Erdoes G., Dietrich W., Stucki M.P., et al. Short-term recovery pattern of plasma fibrinogen after cardiac surgery: a prospective observational study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201647. e0201647–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdoes G., Koster A., Meesters M.I., et al. The role of fibrinogen and fibrinogen concentrate in cardiac surgery: an international consensus statement from the Haemostasis and Transfusion Scientific Subcommittee of the European Association of Cardiothoracic Anaesthesiology. Anaesthesia. 2019;74:1589–1600. doi: 10.1111/anae.14842. [DOI] [PubMed] [Google Scholar]
- 34.Karkouti K., Wijeysundera D.N., Yau T.M., et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44:1453–1462. doi: 10.1111/j.1537-2995.2004.04144.x. [DOI] [PubMed] [Google Scholar]
- 35.Ranucci M., Baryshnikova E., Castelvecchio S., Pelissero G. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96:478–485. doi: 10.1016/j.athoracsur.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Bartoszko J., Karkouti K. Can predicting transfusion in cardiac surgery help patients? Br J Anaesth. 2017;119:350–352. doi: 10.1093/bja/aex216. [DOI] [PubMed] [Google Scholar]
- 37.Zeger S.L., Liang K.Y., Albert P.S. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 38.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 39.Nascimento B., Levy J.H., Tien H., Da Luz L.T. Cryoprecipitate transfusion in bleeding patients. CJEM. 2020;22:S4–S11. doi: 10.1017/cem.2019.409. [DOI] [PubMed] [Google Scholar]
- 40.Koster T., Rosendaal F.R., Reitsma P.H., van der Velden P.A., Briët E., Vandenbroucke J.P. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case–control study of plasma levels and DNA polymorphisms—the Leiden Thrombophilia Study (LETS) Thromb Haemost. 1994;71:719–722. [PubMed] [Google Scholar]
- 41.Hensley N.B., Mazzeffi M.A. Pro-con debate: fibrinogen concentrate or cryoprecipitate for treatment of acquired hypofibrinogenemia in cardiac surgical patients. Anesth Analg. 2021;133:19–28. doi: 10.1213/ANE.0000000000005513. [DOI] [PubMed] [Google Scholar]
- 42.Wong H., Curry N. Do we need cryoprecipitate in the era of fibrinogen concentrate and other specific factor replacement options? ISBT Sci Ser. 2018;13:23–28. [Google Scholar]
- 43.Green L., Bolton-Maggs P., Beattie C., et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Brit J Haematol. 2018;181:54–67. doi: 10.1111/bjh.15167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.