Abstract
Background
Tonsil surgery causes significant and challenging postoperative pain. The Analgesia Nociception Index (ANI) and videopupillometry are two techniques of interest to monitor nociception in adults and may predict postoperative morphine requirements. We hypothesised that these techniques could predict the need for morphine after tonsillectomy in children. The main objective was to assess the prognostic significance of ANI and videopupillometry, measured at the end of surgery, on morphine consumption determined by a Face, Legs, Activity, Cry, Consolability (FLACC) scale score >3 in the Post Anesthesia Care Unit (PACU).
Methods
A single-centre, prospective, interventional study evaluating children between 2 and 7 yr old undergoing tonsil surgery was performed. ANI and videopupillometry with tetanic stimulation were measured under general anaesthesia 4 min after the end of the surgical procedure. Each child was evaluated every 10 min by a nurse using the FLACC scale in the PACU and blinded to the measurements performed in the operating theatre.
Results
Eighty-nine children were analysed and 39 (44%) received morphine in the PACU. Neither ANI values nor videopupillometry values were predictive of postoperative morphine consumption (areas under the receiver operating characteristic curve 0.54, 95% confidence interval [CI; 0.42–0.65], and P=0.57; and 0.52, 95% CI [0.41–0.63], and P=0.69, respectively). Neither ANI values nor videopupillometry values were correlated to the maximum FLACC scale score in the PACU with ρ=0.04 (P=0.71) and ρ=0.06 (P=0.57), respectively.
Conclusions
Neither ANI nor videopupillometry performed at the end of surgery can predict morphine consumption in the PACU in children undergoing tonsillectomy.
Keywords: analgesia, Analgesia Nociception Index, FLACC scale, paediatric anaesthesia, tonsillectomy, videopupillometry
Tonsillectomy is one of the most common surgical procedures in children1 and remains associated with significant postoperative morbidity.2 Postoperative pain after this surgery remains challenging and undertreated,3 resulting in increased health service requirements.4 In contrast, inappropriate use of morphine increases the risk of postoperative hypoxaemia.5 Despite standardised scales, pain assessment in children remains challenging with a high heterogeneity depending on age and brain maturation.6 Moreover, treatments are more limited compared with adults and mainly include non-opioid drugs followed by opioids, as codeine is contraindicated for analgesia in young children.7,8
Tools developed to monitor the depth of analgesia intraoperatively have shown potential interest in the assessment or prediction of postoperative pain.9,10 The Analgesia Nociception Index (ANI) analyses the balance between nociception and anti-nociception according to the heart rate variability with the respiratory cycle,11 whereas videopupillometer uses the change in pupil diameter in response to a nociceptive stimulus.12 However, none of these devices has been studied to predict the need for postoperative analgesia in children. We hypothesised that ANI measurement or videopupillometry at the end of surgery predicts postoperative nociceptive status.
Thus, the aim of the study was to assess the prognostic significance of ANI and videopupillometry to predict the need for morphine requirements after tonsil surgery in children. The secondary objectives were to assess the correlation between the values of ANI and the pupillometer and the highest Face, Legs, Activity, Cry, Consolability (FLACC) scale score recorded in the PACU.
Methods
In this single-centre interventional study performed between December 2018 and December 2019, each patient was included for 24 h after written informed consent of the parents. The study was approved by a French ethics committee (Comité de Protection des Personnes Ile de France IV no. 2018/61; trial registration NCT 03698565).
All children from 2 to 7 yr old scheduled for tonsil surgery and affiliated to or receiving Social Security were eligible. Whenever ANI and videopupillometer measurements were not possible, children were excluded from the study (i.e. neurological disease, eye disease, arrhythmia, chronic treatment with opioids, and contraindication to NSAIDs or any treatment affecting the autonomic nervous system [atropine, neostigmine, beta blocker, and droperidol]).
Children were secondarily excluded from the study after parents gave informed consent if their skin impedance was too high, which precluded analysis using a videopupillometer, or when they received one of the following drugs before ANI or videopupillometer measurements: atropine, neostigmine, beta blocker, antipsychotic, and morphine.
Protocol
The pre-inclusion visit took place during the anaesthesia consultation. The anaesthesiologist checked the eligibility criteria and provided oral and written information to the parents and to the child in a manner appropriate for their age. Children were included during the preanaesthetic visit. The surgical techniques of tonsillectomy or tonsillotomy were at the discretion of the surgeon. Anaesthetic induction was by inhalation of sevoflurane 6% and nitrous oxide 50%, followed by i.v. injection of propofol 2 mg kg−1 and alfentanil 20 μg kg−1. After orotracheal intubation, anaesthesia was maintained with inhaled sevoflurane targeted at a 1.3 minimal alveolar concentration (MAC). Ketamine 0.2 mg kg−1, paracetamol 15 mg kg−1, and ketoprofen 1 mg kg−1 were administered to provide analgesia before the end of surgery. All children received dexamethasone 150 μg kg−1 as anti-emetic prophylaxis. Further doses of alfentanil were at the discretion of the anaesthesiologist.
Post-surgical measurements
At the end of surgery, general anaesthesia was maintained for 4 min without stimulating the child to obtain an averaged measurement of ANI (MetroDoloris Medical Systems, Lille, France). At the end of 4 min, the mean ANI (mANI) was collected. Afterwards, the Pupillary Pain Index (PPI) in response to a tetanic stimulation of the ulnar nerve was recorded with videopupillometry (AlgiScan, IDMed, Marseille, France). PPI measures the changes in pupillary dilation in response to a continuously increasing electric stimulus of 1 s from 10 to 60 mA until 13% of pupillary variation is reached. The stimulus is then stopped, and the PPI score is calculated by the device. Scores vary from 1 (when pupillary dilation is <5% despite maximal tetanic stimulation intensity) to 10 (when pupillary dilation rises above 13% with 10 mA).13
The FLACC scale score14 was assessed blinded to the ANI and pupillometry values every 10 min for an hour by an independent nurse in the PACU. Morphine was administered at the dose of 0.1 mg kg−1 if the FLACC scale score was >3, followed by 0.025 mg kg−1 boluses every 5 min as required. Adverse events were recorded for 24 h after surgery.
Statistics
We estimated that 30% of patients would receive morphine in the PACU (FLACC score >3) based on previous observations in our patients. Thus, considering an alpha risk of 5%, a beta risk of 20%, and an expected area under the receiver operating characteristic (ROC) curve of 0.8, at least 74 patients should be analysed. We estimated the proportion of subjects that could not be analysed at 20%, such that 93 patients would be required to be included in the study.
Categorical variables were expressed as numbers and percentages and were compared using the χ2 test. Continuous variables were expressed as medians with their 25th and 75th percentiles and compared using the Mann–Whitney test.
The area under the ROC curve with its 95% confidence intervals (CIs) of the morphine requirement in the PACU was calculated according to the values of the mANI and PPI score collected 4 min after the end of surgery.
Correlations between the maximum FLACC scale score, mANI, and the PPI values were calculated with the Pearson or Spearman correlation coefficient, as appropriate. Statistical significance was defined as P<0.05.
Analyses were performed using SAS (version 7.1; SAS Institute, Cary, NC, USA) and JASP (version 0.16; University of Amsterdam, Amsterdam, The Netherlands).
Results
Study population
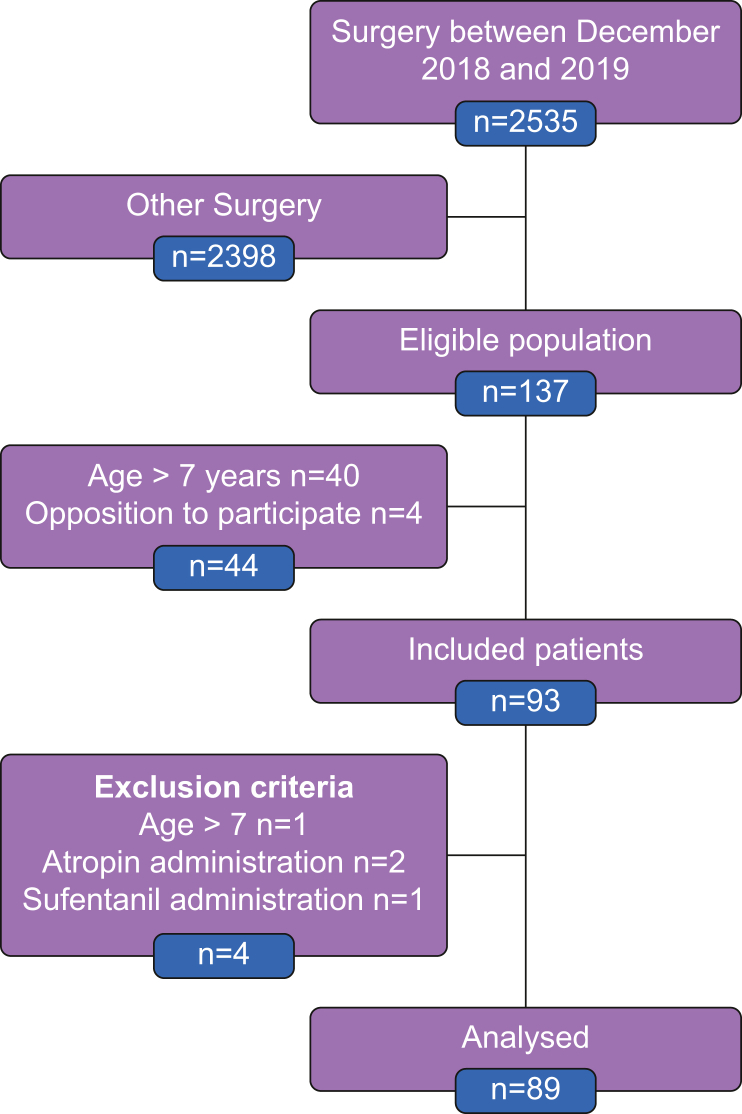
Between December 2018 and December 2019, 2535 children were managed in the operating theatres of the Limoges University Hospital for all types of surgery (Fig. 1). Among these patients, 137 underwent tonsil surgery for the first time. Forty children were over 7 yr, and parents denied consent for four. Finally, 93 children with a median age of 5 [4–6] yr were included (Fig. 1; Table 1). Four were subsequently excluded from the analysis: three received a therapy contraindicated in the study protocol and one was older than 7 yr. Forty-seven (53%) were male, median height of 110 [101–120] cm, and weight of 18 [16–22.0] kg. Sixty-five (73%) were scored ASA 1 and 24 (27%) ASA 2 and 3 (Table 1). Medical history included sleep apnoea syndrome in 38 children (43%), obstructive respiratory disorder in 15 (17%), and passive smoking in 10 (11%). There were similar numbers of tonsillotomy and tonsillectomy procedures (Table 1).
Fig 1.
Flow chart of the study.
Table 1.
Patient characteristics at inclusion and post-surgical complications. ∗Median [25th–75th percentiles], n (%).
| Patient characteristics | n=89∗ |
|---|---|
| Age | 5 [4–6] yr |
| Male | 47 (53%) |
| ASA score | |
| 1 | 65 (73%) |
| 2–3 | 24 (27%) |
| Size | 110 [101–120] cm |
| Weight | 18 [16–22] kg |
| History and related diseases, n (%) | |
| Sleep apnoea | 38 (43) |
| Obesity | 1 (1) |
| Passive smoking | 10 (11) |
| Asthma | 15 (17) |
| Premedication | |
| Midazolam | 79 (89%) |
| Dose | 0.1 [0.1–0.1] mg kg−1 |
| Surgical techniques, n (%) | |
| Tonsillectomy by coblation | 45 (50) |
| Tonsillectomy by electrocautery | 44 (50) |
| Postoperative complications, n (%) | |
| Bleeding | 3 (3) |
| Transfusion | 0 (0) |
| Surgical revision | 1 (1) |
| Laryngospasm | 0 (0) |
| Postoperative nausea/vomiting | 14 (15) |
Fifty-eight patients (65%) received a single injection of alfentanil, whereas 31 (35%) needed a second dose. The median time elapsed between videopupillometry or ANI assessment and the last alfentanil dose was 29 [23–38] min in patients with a single dose of alfentanil and 16 [11–20] min in patients requiring a second injection (Table 2).
Table 2.
Anaesthesiology protocol and assessment by the Analgesia Nociception Index (ANI) and videopupillometry. ∗Median [25th–75th percentiles], n (%).
| Drugs | n=89∗ |
|---|---|
| Acetaminophen | 89 (100%) |
| Ketoprofen | 89 (100%) |
| Ketamine | 89 (100%) |
| Alfentanil | 89 (100%) |
| Alfentanil dose | 20 [19–21] μg kg−1 |
| Alfentanil second injection | 31 (35%) |
| Alfentanil second injection dose | 10 [8–11] μg kg−1 |
| Time between ANI and videopupillometer assessments and last alfentanil injection | 24 [17–34] min |
| Time between ANI and videopupillometer assessments and alfentanil injection in patients without second injection | 29 [23–38] min |
| Time between ANI and videopupillometer assessments and second alfentanil injection |
16 [11–20] min |
| Total anaesthesia duration |
30 [24–36] min |
| ANI assessment | |
| Patients with ANI assessment | 81 (91%) |
| Mean ANI at 4 min | 55 [48–66] |
| Instant ANI at 4 min | 59 [49–70] |
| Pupillometry assessment | |
| Patients with videopupillometry assessment | 87 (98%) |
| Pupillary Pain Index score at 4 min | 2 [1–2] |
| Pupillary diameter variations at 4 min | 6 [2–10]% |
Pain management in the PACU
The maximum median FLACC scale score measured in the PACU was 1 [0–5] (Table 3). Thirty-nine patients (44%) received morphine in the PACU, whereas the median total dose of morphine administrated in this subset of patients was 0.11 [0.11–0.13] mg kg−1. The first dose of morphine was injected with a median time of 30 [20–40] min after PACU admission. The proportion of children receiving morphine was not dependent on the surgical technique, by coblation or electrocautery (40% vs 47%; P=0.5), and nor was the maximum FLACC scale score (0 [0–5] vs 3 [0–5]; P=0.2). Two patients had a FLACC scale score ≤3 but received morphine, and two patients had a FLACC scale score >3 and did not receive morphine. Postoperative bleeding occurred in three patients, including one requiring surgical intervention (Table 1). The proportion of patients developing postoperative nausea/vomiting was not statistically different between those who did not receive morphine compared with those who did (six patients [12%] vs 8 [21%]; P=0.2).
Table 3.
Pain assessment and morphine use in the PACU. ∗Time of admission in the PACU. FLACC, Face, Legs, Activity, Cry, Consolability; ∗∗ Number of patients after admission in PACU at different time point.
| H∗+0 min, n=89∗∗ | H+10 min, n=89∗∗ | H+20 min, n=86∗∗ | H+30 min, n=85∗∗ | H+40 min, n=84∗∗ | H+50 min, n=66∗∗ | H+60 min, n=47∗∗ | |
|---|---|---|---|---|---|---|---|
| FLACC scale score (/10) | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–3] | 0 [0–4] | 0 [0–3] | 0 [0–0] |
| Patients with morphine administration, n (%) | 1 (1) | 3 (3) | 11 (13) | 18 (22) | 23 (27) | 15 (23) | 4 (9) |
| Heart rate (beats min−1) | 111 [100–123] | 116 [107–130] | 119 [107–130] | 115 [105–130] | 112 [105–125] | 112 [105–124] | 110 [98–120] |
| Mean BP (mm Hg) | 60 [56–68] | 68 [60–75] | 74 [64–81] | 75 [69–83] | 80 [69–87] | 73.5 [66–81] | 73 [69–78] |
| Ventilatory frequency (cycles min−1) | 25 [20–33] | 25 [21–34] | 25 [20–34] | 24 [20–36] | 23.5 [20–31] | 22 [18–27] | 24 [18–31] |
Diagnostic value of ANI and videopupillometry for postoperative morphine consumption
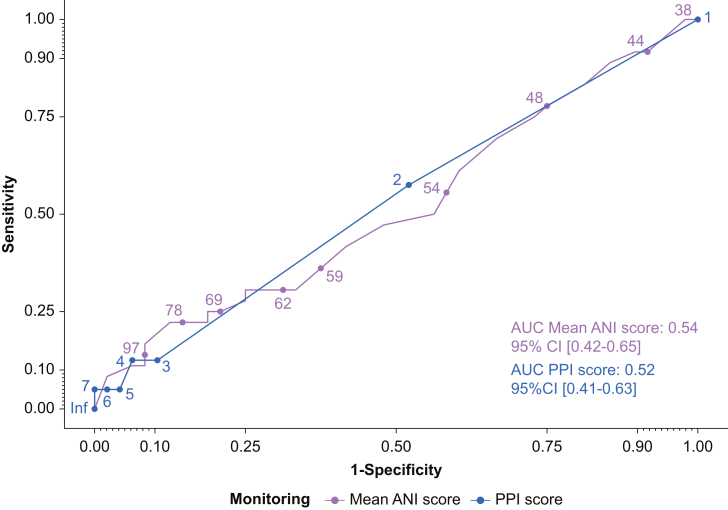
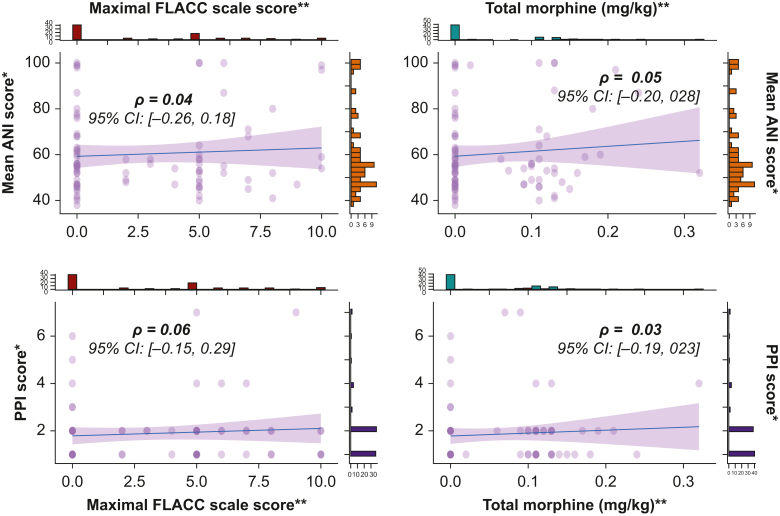
Neither the mANI nor the PPI score was predictive of morphine requirements in the PACU (mANI AUC=0.54, 95% CI [0.42–0.65], and P=0.57; PPI score AUC=0.52, 95% CI [0.41–0.63], and P=0.69) (Fig. 2). There was no correlation between the maximum FLACC scale score assessed in the PACU and the mANI, or the PPI measured at the end of surgery (ρ=0.04, 95% CI [–0.26/0.18], and P=0.71; and ρ=0.06, 95% CI [–0.15/0.29], and P=0.57, respectively) (Fig. 3). Similarly, there was no correlation between the dose of morphine and the other variables assessed with the ANI and the videopupillometer (Fig. 3). Similar results were obtained when considering only patients receiving a single dose of alfentanil (Supplementary Fig 1).
Fig 2.
Comparison of the discriminatory power of the mean Analgesia Nociception Index (mANI) vs the Pain Pupillary Index (PPI) of videopupillometry with regard to the PACU morphine prescription. AUC, area under the curve; CI, confidence interval.
Fig 3.
Scatter plots with histogram between the mean Analgesia Nociception Index (mANI), the Pupillary Pain Index (PPI), the maximal Face, Legs, Activity, Cry, Consolability (FLACC) scale score, and the total morphine consumption in the postoperative care unit. Correlation coefficients are given with 95% confidence intervals (CIs). ∗Measurement performed in the operating theatre after the end of the surgery. ∗∗Measurement performed in the PACU.
Discussion
In this pragmatic interventional single-centre study conducted in children, neither the ANI nor videopupillometry immediately after surgery predicted morphine consumption in the PACU. None of the values obtained with the different devices at the end of surgery was correlated with the maximum FLACC scale score assessed or the morphine consumption in the PACU.
The ANI monitor is a noninvasive tool collecting reproducible values based on the analysis of the nociception/anti-nociception balance through the analysis of changes in heart rate.11 In adults, ANI measured immediately before tracheal extubation has been correlated with the numerical rating scale of pain obtained within 10 min after PACU admission, with an ANI threshold below 50, over which morphine is needed, with a sensitivity and a specificity of 86%.9 In children, mANI has shown a good predictive value for postoperative opioid requirements when measured at the same time of the FLACC scale assessment, whereas the instant ANI analysis was not correlated with the FLACC scale score.10 In our study, mANI was measured whilst the children remained under general anaesthesia with sevoflurane at the same concentration as during the surgery. As sevoflurane decreases the activity of the sympathetic nervous system and modulates the baroreflex, it may have resulted in underestimation of nociception after awakening.15
The variation in pupil diameter when applying a nociceptive stimulus using the videopupillometer has been validated during surgery, reflecting opioid infusion during general anaesthesia.16,17 In our study, the use of the videopupillometer could not predict the postoperative need for morphine. Increasing the concentration of sevoflurane can indeed decrease pupil dilatation for the same nociceptive stimulation and with constant opioid concentration.18 However, such a decrease appears from 1.9 MAC sevoflurane in prepubertal children, which is higher than the targeted MAC in the present study.19 In the same way, alfentanil use may have had a significant impact on the measurements made by videopupillometry, as it stimulates the parasympathetic nucleus resulting in miosis.20 The median time lag between the last alfentanil dose and the videopupillometry measurement was 24 min, which is less than the half-life of alfentanil and might have influenced the videopupillometry values. As videopupillometry requires tetanic stimulus, it cannot be done in awake children. Ly-Liu and Reinoso-Barbero21 performed the assessment just before extubating the patients with a MAC between 0.3 and 0.5, with a good correlation with the Llanto, Actitud, Normorrespiración, Tono Postural y Observación Facial scale score performed 10 min after tracheal extubation. Because a painful stimulus applied just before extubation increases the risk of laryngospasm,22 we decided to perform the measurement when the children were still under general anaesthesia. Neice and colleagues12 showed a moderate correlation between the magnitude of pupillary unrest under ambient light and the response to opioid therapy in adults. However, it did not predict the final consumption of opioids in PACU. Finally, most of the children in our study had a low PPI score, potentially explained by a combined effect of drugs on pupil reflex.18, 19, 20 Accordingly, videopupillometry does not seem useful under general anaesthesia to predict morphine consumption after surgery.
Our study had several limitations. First, different surgical techniques were used, which might have created a bias. Specifically, coblation tonsillectomy was associated with less postoperative pain.23 However, the proportion of children receiving postoperative opioids in our study was unaffected by the surgical technique. Second, assessment of the FLACC scale might have been overestimated by its anxiety component, which is not taken into account by this scale. Anxiety and fear seem to increase the FLACC scale score, as they modify children's facial expression and behaviour.24 Babl and colleagues25 showed that children had a high FLACC scale score before undergoing an anxiety-provoking invasive procedure. A combined use of different self-assessment scales, such as the Wong–Baker FACES® Pain Rating Scale, could have helped to better discriminate anxious children from those who suffered physical pain.26
Conclusions
Analgesia monitoring techniques using ANI and videopupillometry at the end of tonsillectomy surgery were not able to predict postoperative morphine consumption and pain status of children.
Authors' contributions
Study design: BE, CL, PS, JC.
Patient inclusion: CH, CC, BY, FG, MD, PS, NN-D.
Statistical analysis: AL, LM.
Drafting of article: BE, CL, PS, JC.
Critical review of article: CH, CC, BY, FG, MD, PS, NN-D.
Approval of final version of article: all authors.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Limoges University Hospital
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2022.100024.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cullen K.A., Hall M.J., Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11:1–25. [PubMed] [Google Scholar]
- 2.Mitchell R.B., Archer S.M., Ishman S.L., et al. Clinical practice guideline: tonsillectomy in children (update)—executive summary. Otolaryngol Head Neck Surg. 2019;160:187–205. doi: 10.1177/0194599818807917. [DOI] [PubMed] [Google Scholar]
- 3.Guntinas-Lichius O., Geißler K., Komann M., Schlattmann P., Meissner W. Inter-hospital variability of postoperative pain after tonsillectomy: prospective registry-based multicentre cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warnock F.F., Lander J. Pain progression, intensity and outcomes following tonsillectomy. Pain. 1998;75:37–45. doi: 10.1016/S0304-3959(97)00202-9. [DOI] [PubMed] [Google Scholar]
- 5.Kelly L.E., Sommer D.D., Ramakrishna J., et al. Morphine or ibuprofen for post-tonsillectomy analgesia: a randomized trial. Pediatrics. 2015;135:307–313. doi: 10.1542/peds.2014-1906. [DOI] [PubMed] [Google Scholar]
- 6.Cohen L.L., La Greca A.M., Blount R.L., Kazak A.E., Holmbeck G.N., Lemanek K.L. Introduction to special issue: evidence-based assessment in pediatric psychology. J Pediatr Psychol. 2008;33:911–915. doi: 10.1093/jpepsy/jsj115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldamluji N., Burgess A., Pogatzki-Zahn E., Raeder J., Beloeil H., PROSPECT Working Group collaborators PROSPECT guideline for tonsillectomy: systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2021;76:947–961. doi: 10.1111/anae.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly L.E., Rieder M., van den Anker J., et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129:e1343–e1347. doi: 10.1542/peds.2011-2538. [DOI] [PubMed] [Google Scholar]
- 9.Boselli E., Bouvet L., Begou G., et al. Prediction of immediate postoperative pain using the analgesia/nociception index: a prospective observational study. Br J Anaesth. 2014;112:715–721. doi: 10.1093/bja/aet407. [DOI] [PubMed] [Google Scholar]
- 10.Gall O., Champigneulle B., Schweitzer B., et al. Postoperative pain assessment in children: a pilot study of the usefulness of the analgesia nociception index. Br J Anaesth. 2015;115:890–895. doi: 10.1093/bja/aev361. [DOI] [PubMed] [Google Scholar]
- 11.Jeanne M., Clément C., De Jonckheere J., Logier R., Tavernier B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput. 2012;26:289–294. doi: 10.1007/s10877-012-9354-0. [DOI] [PubMed] [Google Scholar]
- 12.Neice A.E., Behrends M., Bokoch M.P., Seligman K.M., Conrad N.M., Larson M.D. Prediction of opioid analgesic efficacy by measurement of pupillary unrest. Anesth Analg. 2017;124:915–921. doi: 10.1213/ANE.0000000000001728. [DOI] [PubMed] [Google Scholar]
- 13.Sabourdin N., Diarra C., Wolk R., Piat V., Louvet N., Constant I. Pupillary Pain Index changes after a standardized bolus of alfentanil under sevoflurane anesthesia: first evaluation of a new pupillometric index to assess the level of analgesia during general anesthesia. Anesth Analg. 2019;128:467–474. doi: 10.1213/ANE.0000000000003681. [DOI] [PubMed] [Google Scholar]
- 14.Merkel S.I., Voepel-Lewis T., Shayevitz J.R., Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297. [PubMed] [Google Scholar]
- 15.Nagasaki G., Tanaka M., Nishikawa T. The recovery profile of baroreflex control of heart rate after isoflurane or sevoflurane anesthesia in humans. Anesth Analg. 2001;93:1127–1131. doi: 10.1097/00000539-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Barvais L. Effect site concentrations of remifentanil and pupil response to noxious stimulation. Br J Anaesth. 2003;91:347–352. doi: 10.1093/bja/aeg178. [DOI] [PubMed] [Google Scholar]
- 17.Chapman C.R., Oka S., Bradshaw D.H., Jacobson R.C., Donaldson G.W. Phasic pupil dilation response to noxious stimulation in normal volunteers: relationship to brain evoked potentials and pain report. Psychophysiology. 1999;36:44–52. doi: 10.1017/s0048577299970373. [DOI] [PubMed] [Google Scholar]
- 18.Sabourdin N., Peretout J.-B., Khalil E., Guye M.-L., Louvet N., Constant I. Influence of depth of hypnosis on pupillary reactivity to a standardized tetanic stimulus in patients under propofol-remifentanil target-controlled infusion: a crossover randomized pilot study. Anesth Analg. 2018;126:70–77. doi: 10.1213/ANE.0000000000001802. [DOI] [PubMed] [Google Scholar]
- 19.Bourgeois E., Sabourdin N., Louvet N., Donette F.X., Guye M.L., Constant I. Minimal alveolar concentration of sevoflurane inhibiting the reflex pupillary dilatation after noxious stimulation in children and young adults. Br J Anaesth. 2012;108:648–654. doi: 10.1093/bja/aer459. [DOI] [PubMed] [Google Scholar]
- 20.Fedder I.L., Vlasses P.H., Mojaverian P., et al. Relationship of morphine-induced miosis to plasma concentration in normal subjects. J Pharm Sci. 1984;73:1496–1497. doi: 10.1002/jps.2600731047. [DOI] [PubMed] [Google Scholar]
- 21.Ly-Liu D., Reinoso-Barbero F. Immediate postoperative pain can also be predicted by pupillary pain index in children. Br J Anaesth. 2015;114:345–346. doi: 10.1093/bja/aeu473. [DOI] [PubMed] [Google Scholar]
- 22.Al-alami A.A., Zestos M.M., Baraka A.S. Pediatric laryngospasm: prevention and treatment. Curr Opin Anaesthesiol. 2009;22:388–395. doi: 10.1097/aco.0b013e32832972f3. [DOI] [PubMed] [Google Scholar]
- 23.Pynnonen M., Brinkmeier J.V., Thorne M.C., Chong L.Y., Burton M.J. Coblation versus other surgical techniques for tonsillectomy. Cochrane Database Syst Rev. 2017;8:CD004619. doi: 10.1002/14651858.CD004619.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crellin D.J., Harrison D., Santamaria N., Babl F.E. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain. 2015;156:2132–2151. doi: 10.1097/j.pain.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 25.Babl F.E., Crellin D., Cheng J., Sullivan T.P., O’Sullivan R., Hutchinson A. The use of the Faces, Legs, Activity, Cry and Consolability scale to assess procedural pain and distress in young children. Pediatr Emerg Care. 2012;28:1281–1296. doi: 10.1097/PEC.0b013e3182767d66. [DOI] [PubMed] [Google Scholar]
- 26.Garra G., Singer A.J., Domingo A., Thode H.C. The Wong-Baker pain FACES scale measures pain, not fear. Pediatr Emerg Care. 2013;29:17–20. doi: 10.1097/PEC.0b013e31827b2299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.