Abstract
Background
Blood lactate concentration in the postoperative period is a marker of physiological stress and a predictor of complications and mortality. Cardiopulmonary exercise testing (CPET) is a common preoperative risk stratification tool. We aimed to investigate the association between preoperative CPET results and postoperative lactate concentration with postoperative mortality after major noncardiac surgery.
Methods
We analysed data from patients undergoing major noncardiac surgery in a tertiary UK centre between 2007 and 2014 who had preoperative CPET and postoperative lactate measurements. Univariate and multivariate analyses were performed to assess the association between lactate concentration, CPET results, or both and mortality.
Results
We analysed data from 1075 patients. A mean lactate concentration >2 mM in the first 12, 24, and 48 h after surgery was associated with odds ratios (ORs) and 95% confidence intervals (CIs) for 30-day mortality of 3.9 (2.1–7.3; P<0.005), 4.5 (2.4–8.4; P<0.005), and 6.1 (3.3–11.5; P<0.005), respectively. The dichotomous CPET variable, ventilatory equivalence for CO2 (V̇E/V̇co2; cut-off 34), was associated with increased risk of 30-day mortality (OR 2.5; 95% CI: 1.3–4.8; P<0.005). In a multivariable model, hyperlactataemia and poor V̇E/V̇co2 retained their significant associations with 30- and 90-day mortality when adjusted for age, BMI, and surgical risk. When looking at the combined effect of the dichotomous hyperlactataemia in the first 24 h (cut-off 2 mM) and preoperative V̇E/V̇co2, the OR for 30-day mortality was 11.53 (95% CI: 4.6–28.8; P≤0.005).
Conclusions
Our study suggests that postoperative hyperlactataemia and preoperative poor V̇E/V̇co2 are independently associated with an increased risk of mortality after major noncardiac surgery.
Keywords: CPET, CPEX, hyperlactataemia, lactate, mortality, perioperative, risk stratification
During critical illness and after major surgery, plasma lactate concentration is frequently measured as a physiological marker to determine severity of illness and organ dysfunction.1, 2, 3 In the postoperative setting, hyperlactataemia has been shown to be predictive of postoperative complications and mortality for a range of surgical specialties.4, 5, 6, 7, 8, 9 Hyperlactataemia is associated with the physiological stress response, the mechanism for which is thought to include both aerobic and anaerobic biochemical pathways. The aerobic pathways reflect a state of accelerated glycolysis, whereas the anaerobic pathways reflect tissue hypoperfusion and hypoxia. Impaired lactate clearance by the liver and kidneys can compound the effect to further increase lactate concentration.10, 11, 12
Cardiopulmonary exercise testing (CPET) is performed routinely to stratify risk and guide the perioperative care of patients undergoing major surgery. Individually derived CPET variables directly associated with functional capacity, such as peak oxygen uptake (V̇o2 peak), the anaerobic threshold (AT), and ventilatory efficiency index (V̇E/V̇co2), correlate with postoperative outcomes, complications, and mortality for a range of surgical procedures.13, 14, 15, 16, 17, 18
CPET testing provides an assessment of patient physiological reserve through the response to graduated exercise. The ability to withstand exercise stress is extrapolated to indicate the patient's ability to withstand the physiological stress of surgery. Therefore, if postoperative lactate concentration is a marker of the physiological stress of surgery, patients deemed to have low physiological reserve by CPET may be more likely to develop hyperlactataemia postoperatively.
Our study aimed to assess the association of variables generated during CPET and postoperative lactate concentration with increased risk of mortality after major noncardiac surgery. It aimed to explore whether a poor CPET result increases the likelihood of postoperative hyperlactataemia and whether the already-known mortality risks of both are independent in nature or a reflection of the same underlying inability to withstand the physiological stress of surgery. We hope this will aid the interpretation of postoperative hyperlactataemia and influence future clinical management, particularly in the critical care setting.
Methods
Patients undergoing major head and neck, thoracic, vascular, or abdominal surgery at our institution, referred for routine CPET preoperatively and receiving postoperative care on the critical care unit, were investigated between July 2007 and January 2014. Patients who underwent liver resection surgery were excluded. The data set was not continued post-2014 because changes to the surgical specialities at the institution in 2014 meant the cohort would not be consistent after this date and could have introduced confounding factors to the analysis.
Cardiopulmonary exercise testing
Testing equipment consisted of a cycle ergometer and gas exchange analysis system (Ultima™ CardiO2® MedGraphics (Medical Graphics, St Paul, MN, USA) linked with a BreezeSuite™ software package (Medical Graphics). Patients performed resting spirometry followed by baseline measurements of gas exchange, ECG, blood pressure, and arterial oxygen saturation. They then began a 3-min period of unloaded cycling at a rate of 55–60 rpm followed by a ramped Wasserman protocol with work rate increasing at a rate between 10 and 20 W min−1 while maintaining this speed. The test was symptom-limited or continued to peak performance. A maximal test was defined as peak heart rate >80% peak predicted or reaching a respiratory exchange ratio of >1.15. Prospectively collected CPET data included AT, V̇o2 peak, ventilatory equivalent for CO2 at (V̇E/V̇co2), and % predicted peak V̇o2 for age and gender according to the modified prediction algorithm of Wasserman and colleagues.19 BMI was recorded at the same time. Data were stored in the Manchester University NHS Foundation Trust CPET Research Database, which has previously received ethical approval.
Postoperative serum lactate
Blood lactate results were acquired by retrospective data extraction of the electronic blood gas analyser database. All blood gas analyses performed in the critical care unit from July 2007 to January 2014 were extracted. These results were then cross-referenced with the CPET patient database to extract the relevant results for the study patients. To accurately time the results relative to the end of surgery, we extracted the end of surgery times from the Anesthesia Information Management System and anaesthetic chart electronic record system (Recall).
Serum lactate analysis was undertaken by automated blood gas analysers (Radiometer Limited, Crawley, UK ABL8500/Roche Diagnostics Limited, West Sussex, UK OMNI). Both machine types were calibrated equally and underwent regular quality assurance by the organisation's point-of-care laboratory team. Only arterial blood gas samples were included, taken from an arterial catheter inserted for invasive blood pressure monitoring. Based on previous studies, normal laboratory ranges, and clinical consensus, an elevated lactate (hyperlactataemia) was defined as >2 mM. The first 12, 24, and 48 h post-surgery were considered, and the mean and peak lactate concentrations were calculated within each period. The summation of the time within each period (12, 24, and 48 h) when the lactate value went over the threshold of 2 mM was calculated and defined as hyperlactataemia time using the method detailed by Li and colleagues8; single values were excluded.
Length of stay and operation data were obtained from the hospital Patient Administration System database. Mortality for 30 and 90 days was determined with reference to the NHS Demographics Batch Service. Risk of surgery (medium/high) was determined according to local guidelines (see Supplementary Appendix 1).
Statistical analysis
Logistic regression analysis was conducted using both continuous and categorical predictor variables (IBM SPSS Statistics version 25 [Armonk, NY, USA] and R version 3.1 [R Foundation for Statistical Computing, Vienna, Austria]). CPET cut-off values as identified in other surgical populations were used to determine whether ‘sub-threshold’ patients (defined as any of V̇o2 at lactate threshold <11 ml kg−1 min−1, V̇o2 peak <15 ml kg−1 min−1, or V̇E/V̇co2 >34) had a significantly increased risk of mortality.17 All variables were first tested in an unadjusted univariable model, and then significant predictors from the latter analysis were added in a forward stepwise manner into a multivariable logistic regression after checks for normality, multicollinearity, homoscedasticity, and a Hosmer–Lemeshow goodness-of-fit test for the model. The multivariable models were constructed to use only one CPET variable and one hyperlactataemia variable at a time and adjusted for BMI and surgery risk. The ‘guideline’ of number of events per predictor in the models (5–10 cases per predictor) was obeyed for each mortality outcome. The duration of hospital stay and comparison of the model predictor variables were compared using Student's t-test or Mann–Whitney U-test as appropriate. Pearson's product-moment correlation coefficient and Spearman rank correlation coefficient were used to assess the strength of relationship of the CPET variables with the lactate measurement variables. Survival calculations of hazard ratios with 95% confidence intervals (CIs) were done using Cox regression analysis. Kaplan–Meier survival curves were constructed as appropriate.
Results
CPET was undertaken on 1075 patients undergoing surgery who went on to recover in critical care. Their median age was 70 yr (range: 26–90 yr) and median BMI was 27 kg m−2 (range: 15–61 kg m−2), and 65.3% of patients were male. The type of surgery and patient characteristics are presented in Table 1. Mortality during the study follow-up was 4% at 30 days and 6.3% at 90 days.
Table 1.
Patient characteristics and hyperlactataemia.
| Surgery type | N (M/F) | BMI, median (range) (kg m−2) | Age, median (range) (yr) | Mean lactate >2 mM first 12 h, N (%) | Mean lactate >2 mM first 24 h, N (%) | Mean lactate >2 mM first 48 h, N (%) | Critical care LOS, range (day) | Hospital LOS, range (day) |
|---|---|---|---|---|---|---|---|---|
| Thoracic | 59 (21/38) | 26 (19–40) | 68 (44–83) | 8 (14) | 4 (7) | 4 (7) | 1–18 | 2–58 |
| Head and neck | 67 (40/27) | 26 (21–36) | 68 (47–81) | 19 (28) | 13 (19) | 6 (9) | 1–31 | 3–89 |
| General | 31 (19/12) | 28 (19–49) | 67 (52–81) | 6 (19) | 4 (13) | 3 (10) | 1–15 | 2–35 |
| Upper gastrointestinal tract | 143 (94/49) | 26 (16–45) | 68 (40–89) | 11 (8) | 15 (10) | 11 (8) | 1–105 | 5–159 |
| Hepato-pancreato-biliary | 123 (77/46) | 26 (15–44) | 68 (26–84) | 34 (28) | 26 (21) | 15 (12) | 1–35 | 3–75 |
| Colorectal | 146 (91/55) | 27 (16–48) | 71 (41–87) | 32 (22) | 28 (19) | 26 (18) | 1–45 | 2–133 |
| Endocrine | 4 (4/0) | 31 (28–37) | 64 (60–70) | 1 (25) | 1 (25) | 1 (25) | 1–5 | 6–14 |
| Vascular | 282 (224/58) | 27 (16–40) | 72 (47–90) | 46 (16) | 38 (13) | 24 (9) | 1–37 | 1–75 |
| Urology | 158 (114/44) | 28 (16–46) | 68 (50–90) | 47 (30) | 36 (23) | 31 (20) | 1–37 | 1–75 |
| Gynae | 62 (0/62) | 31 (19–61) | 72 (44–85) | 14 (23) | 11 (18) | 10 (16) | 1–8 | 1–128 |
| High risk | 298 (204/94) | 26 (15–45) | 67 (26–89) | 82 (28) | 66 (22) | 40 (13) | 1–102 | 1–102 |
| Medium risk | 777 (498/279) | 27 (16–61) | 71 (41–90) | 157 (20) | 121 (16) | 97 (12) | 1–105 | 1–176 |
Groups are displayed by surgical specialty and risk category of surgery and include sex, age, BMI, and length of stay (LOS) in addition to percentage of patients with hyperlactataemia (>2 mM) in the first 12, 24, and 48 h after surgery.
Lactate measurements and mortality
The proportions of patients undergoing each type of surgery with a mean lactate concentration (mlac) >2 mM in the first 12 h (mlac 12), first 24 h (mlac 24), and first 48 h (mlac 48) after surgery are shown in Table 1. The associated hospital and critical care lengths of stay are also shown. The proportions of patients and associated risk of mortality of the dichotomous lactate variable, mlac < or >2 mM, along with the continuous variables of hyperlactataemia time and mlac are shown in Table 2. There was an association between mlac 12, mlac 24, and mlac 48 >2 mM with increased 30- and 90-day mortality. The greatest risk, with a >6-fold increased odds, was for 30-day mortality with mlac 48 >2 mM. Peak lactate concentrations were also analysed but were not better predictors of mortality than mlac.
Table 2.
Univariable analysis of both dichotomous and continuous variables of hyperlactataemia and cardiopulmonary exercise testing (CPET) for 30- and 90-day mortality.
| Lactate variable (N) |
30-day mortality |
90-day mortality |
||||
|---|---|---|---|---|---|---|
| N | OR (95% CI) | P-value | N | OR (95% CI) | P-value | |
| mlac >2 mM 0–12 h (239) | 22 | 3.9 (2.1–7.3) | <0.005 | 28 | 2.6 (1.6–4.4) | <0.005 |
| mlac >2 mM 0–24 h (187) | 20 | 4.5 (2.4–8.4) | <0.005 | 27 | 3.5 (2.1–5.8) | <0.005 |
| mlac >2 mM 0–48 h (137) | 19 | 6.1 (3.3–11.5) | <0.005 | 23 | 4.0 (2.3–6.9) | <0.005 |
| Hyperlact-time 0–12 h (335) | 24 | 1.19 (1.10–1.29) | <0.005 | 32 | 1.15 (1.08–1.23) | <0.005 |
| Hyperlact-time 0–24 h (380) | 27 | 1.12 (1.08–1.17) | <0.005 | 38 | 1.10 (1.07–1.14) | <0.005 |
| Hyperlact-time 0–48 h (401) | 28 | 1.07 (1.05–1.1) | <0.005 | 39 | 1.06 (1.04–1.08) | <0.005 |
| mlac 0–12 h continuous (990) | 43 | 1.54 (1.3–1.8) | <0.005 | 68 | 1.45 (1.26–1.7) | <0.005 |
| mlac 0–24 h continuous (1014) | 43 | 1.67 (1.39–2.0) | <0.005 | 68 | 1.54 (1.31–1.8) | <0.005 |
| mlac 0–48 h continuous (1017) | 43 | 1.78 (1.5–2.2) | <0.005 | 68 | 1.62 (1.4–1.9) | <0.005 |
| CPET variable | ||||||
| V̇o2 at LT <10.2 | 8 | 1.4 (0.71–2.8) | 0.32 | 12 | 1.2 (0.71–2.1) | 0.49 |
| V̇o2 at LT <11.0 | 20 | 1.23 (0.64–2.5) | 0.49 | 28 | 0.94 (0.55–1.6) | 0.83 |
| V̇E/V̇co2 >42 | 10 | 2.5 (1.2–5.2) | 0.02 | 14 | 2.4 (1.1–5.2) | 0.009 |
| V̇E/V̇co2 >34 | 28 | 2.5 (1.3–4.8) | 0.005 | 40 | 2.0 (1.2–3.2) | 0.009 |
| Peak V̇o2 <15 | 28 | 1.8 (0.95–3.3) | 0.074 | 40 | 1.38 (0.84–2.25) | 0.21 |
| % predicted V̇o2 <60% | 18 | 1.4 (0.8–2.6) | 0.28 | 29 | 1.47 (0.89–2.4) | 0.13 |
| % predicted V̇o2 <75% | 32 | 148 (0.74–3.0) | 0.27 | 50 | 1.42 (0.82–2.5) | 0.21 |
| V̇E/V̇co2 continuous | 43 | 1.07 (1.03–1.11) | 0.001 | 68 | 1.05 (1.01–1.08) | 0.006 |
| Other model predictors | ||||||
| Age | 43 | 1.04 (1.0–1.11) | 0.06 | 68 | 1.03 (1.0–1.106) | 0.051 |
| BMI | 43 | 0.96 (0.90–1.02) | 0.25 | 68 | 0.93 (0.89–0.99) | 0.013 |
| Risk of surgery | 3.0%/6.6% | 2.24 (1.21–4.16) | 0.01 | 5.7%/8.0% | 1.43 (0.85–2.41) | 0.18 |
| Medium vs high (73.2%/26.8%) | ||||||
CI, confidence interval; Hyperlact, hyperlactataemia; LT, lactate threshold; mlac, mean lactate concentration; OR, odds ratio.
Hyperlactataemia time for 0–12, 0–24, and 0–48 h post-surgery are also included; the highest odds ratios (ORs) were for 30-day mortality, which ranged from 1.07 (0–48) to 1.19 (0–12) h (i.e. an increase of 1 h of hyperlactataemia time was associated with a 7–19% increase in odds of 30-day mortality).
Table 2 also includes continuous mlac as predictors for increased risk of 30- and 90-day mortality. The ORs indicate that an increase in 1 mM of mlac is associated with a 54–78% increase in odds of 30-day mortality and 45–62% increase in odds of 90-day mortality. The risk was more pronounced when the increase in continuous mlac is over a longer period. Receiver operating characteristic (ROC) curves were constructed to examine the specificity and sensitivity of mlac in predicting increased risk of 30- and 90-day mortality. For mlac 24 predicting 30- and 90-day mortality, the optimum cut-off values as determined by Youden's index were 1.85 and 1.65 mM, respectively.
CPET variables and mortality
Commonly used dichotomous and continuous CPET variables and their associated 30- and 90-day mortality are shown in Table 2. Only the CPET variables V̇E/V̇co2 >34 or >42 and continuous V̇E/V̇co2 showed an increased risk of mortality. The dichotomous variables, V̇E/V̇co2 >34 and >42, both demonstrated an OR of 2.5 for 30-day mortality and ORs 2.0 and 2.4 for 90-day mortality, respectively. Continuous V̇E/V̇co2 showed an increased risk in 30-day mortality OR of 1.07, corresponding to an increase in 2 units of V̇E/V̇co2 being associated with an increase in odds of 30-day mortality of 14%. ROC curves were constructed to examine specificity and sensitivity of V̇E/V̇co2 in predicting an increased risk of 30- and 90-day mortality. For V̇E/V̇co2 predicting 30- and 90-day mortality, the optimum cut-off values as determined by Youden's index were 37.5 and 36.5, respectively.
Other predictors
An increase in BMI was significant in univariable analysis for predicting a lower risk of mortality at 90 days. Increased age was not associated with increased risk of mortality at 30 and 90 days. The type of surgery, as denoted by medium or high risk, was only significant at 30 days (Table 2).
Multivariable analysis
Significant predictors from the univariable analyses for hyperlactataemia variables (mlac 12, 24, and 48 >2 mM and hyperlactataemia time for the same time intervals) were included one at a time in a model that also included the significant CPET variables of dichotomous V̇E/V̇co2 >34 and continuous V̇E/V̇co2. All ORs were adjusted by including the significant predictors BMI and risk of surgery in the models. The multivariable models are detailed in Table 3. The model combinations show similar results for predicting increased risk of 30- and 90-day mortality regardless of whether hyperlactataemia develops in the first 12, 24, or 48 h after surgery. The only exception to this was for the prediction of 90-day mortality when combining hyperlactataemia time in the first 48 h and V̇E/V̇co2 as a continuous variable in the model; in this circumstance, V̇E/V̇co2 as a continuous variable did not retain significance. Hyperlactataemia as defined by mlac >2 mM and V̇E/V̇co2 as a dichotomous variable remained significantly associated with both 30- and 90-day mortality when adjusted for BMI and risk of surgery. Furthermore, mlac 12, 24, and 48 >2 mM indicate a >4-fold increase in risk of 30-day mortality and >3-fold increase in risk of 90-day mortality in these models. The ORs for hyperlactataemia are greater than that for V̇E/V̇co2 when considered similarly as a dichotomous variable in the models.
Table 3.
Multivariable analyses of both dichotomous and continuous variables of hyperlactataemia and cardiopulmonary exercise testing for 30- and 90-day mortality. BMI (only significant in univariable analysis for 90-day mortality and risk of surgery (Surg risk, only significant in univariable analysis for 30-day mortality) are included in the model as appropriate. CI, confidence interval; Hyperlactate, hyperlactataemia; mlac, mean lactate concentration; OR, odds ratio.
| Model | Predictors in model | 30-day mortality |
90-day mortality |
||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| 1 | mlac >2 mM first 12 hV̇E/V̇co2 >34Surg risk/BMI | 4.7 (2.4–9.0)2.2 (1.1–4.4)2.2 (1.7–4.2) (Surg risk) | <0.0050.0040.16 | 3.7 (2.2–6.3)1.7 (1.02–3.0)0.92 (0.87–0.97) (BMI) | <0.0050.040.004 |
| 2 | Hyperlactate time first 12 hV̇E/V̇co2 >34Surg risk/BMI | 1.15 (1.1–1.2)2.4 (1.2–4.6)2.0 (1.1–3.8) (Surg risk) | <0.0050.0120.03 | 1.13 (1.06–1.2)1.8 (1.07–3.0)927(0.88–0.98) (BMI) | <0.0050.0270.007 |
| 3 | Hyperlactate time first 12 hV̇E/V̇co2Surg risk/BMI | 1.15 (1.07–1.12)1.08 (1.04–1.1)2.08 (1.1–3.9) (Surg risk) | <0.005<0.0050.025 | 1.13 (1.06–1.2)1.04 (1.004–1.07)0.93 (0.88–0.98) (BMI) | <0.0050.0290.010 |
| 4 | mlac >2 mM first 24 hV̇E/V̇co2 >34Surg risk/BMI | 4.6 (2.4–8.8)2.15 (1.09–4.2)2.0 (1.0–4.0) (Surg risk) | <0.0050.0270.03 | 3.43 (2.05–5.76)1.77 (1.05–3.0)930 (0.88–0.98) (BMI) | <0.0050.0320.010 |
| 5 | Hyperlactate time first 24 hV̇E/V̇co2 >34Surg risk/BMI | 1.11 (1.06–1.15)2.3 (1.15–4.4)2.07 (1.09–3.93) (Surg risk) | <0.0050.0080.026 | 1.09 (1.06–1.13)1.8 (1.05–3.0)0.93 (0.88–0.98) (BMI) | <0.0050.0310.007 |
| 6 | Hyperlactate time first 24 hV̇E/V̇co2Surg risk/BMI | 1.11 (1.11–1.15)1.08 (1.04–1.12)2.14 (1.12–4.08) (Surg risk) | <0.005<0.0050.021 | 1.16 (1.1–1.13)1.04 (1.01–1.07)0.931 (0.881–0.983) (BMI) | <0.0050.0260.011 |
| 7 | mlac >2 mM first 48 hV̇E/V̇co2 >34Surg risk/BMI | 5.0 (2.6–9.5)2.15 (1.09–4.22)2.0 (1.1–3.8) (Surg risk) | <0.0050.0270.032 | 3.6 (2.13–6.0)1.8 (1.05–3.00.92 (0.88–0.98) (BMI) | <0.0050.0340.006 |
| 8 | Hyperlactate time first 48 hV̇E/V̇co2 >34Surg risk/BMI | 1.08 (1.06–1.1)2.9 (1.5–5.6)2.13 (1.11–4.1) (Surg risk) | <0.0050.0020.023 | 1.07 (1.05–1.09)1.8 (1.1–3.1)93 (0.88–0.98) (BMI) | <0.0050.0250.006 |
| 9 | Hyperlactate time first 48 h V̇E/V̇co2 Surg risk/BMI |
1.08 (1.06–1.1) 1.07 (1.03–1.1) 2.19 (1.13–4.20) (Surg risk) |
<0.005 <0.005 0.019 |
1.07 (1.05–1.09) Not in model 0.91 (0.86–0.97) (BMI) |
<0.005 0.001 |
Cox regression analysis
Cox regression analysis of survival using continuous variables of the most sensitive CPET variable, V̇E/V̇co2, and mlac 12, 24, and 48 values showed a similar pattern of survival risk, adjusted for BMI. V̇E/V̇co2 and mlac 12, 24, and 48 were both predictive in a multivariate model for 30-day mortality with mlac 24 having a greater OR than V̇E/V̇co2: mlac 24 OR 1.6 (95% CI: 1.45–1.75; P<0.005), V̇E/V̇co2 OR 1.07 (95% CI: 1.03–1.11; P=0.001). For 90-day mortality, there is a similar pattern when considering mlac 12 and mlac 24, but mlac 48 V̇E/V̇co2 was not retained in the model (mlac 48 OR 1.4 [95% CI: 1.24–1.6; P<0.005]).
Surgical risk
The cohort was split by surgical risk as medium/high 73.2%/26.8%. In the medium-risk group, the mortality was 3.0% (30 days) and 5.7% (90 days). In the high-risk surgical group, the mortality was 6.6% (30 days) and 8.0% (90 days). The medium-risk cohort had a median (range) lactate concentration at 0–24 h postoperative of 1.06 (0.3–4.75) mM compared with the high-risk cohort 2.3 (0.7–13.5) mM. There was no difference in the V̇E/V̇co2 continuous variable between cohorts.
Correlation of CPET variables and hyperlactataemia
There were no continuous or dichotomous CPET variables predicting increased risk of mlac >2 or hyperlactataemia time in any of the first 12, 24, or 48 h after surgery. Pearson correlation showed no association for V̇E/V̇co2 with mlac 12, 24, and 48 (R values were 0.09, 0.07, and 0.05, respectively).
Combining hyperlactataemia and abnormal CPET results
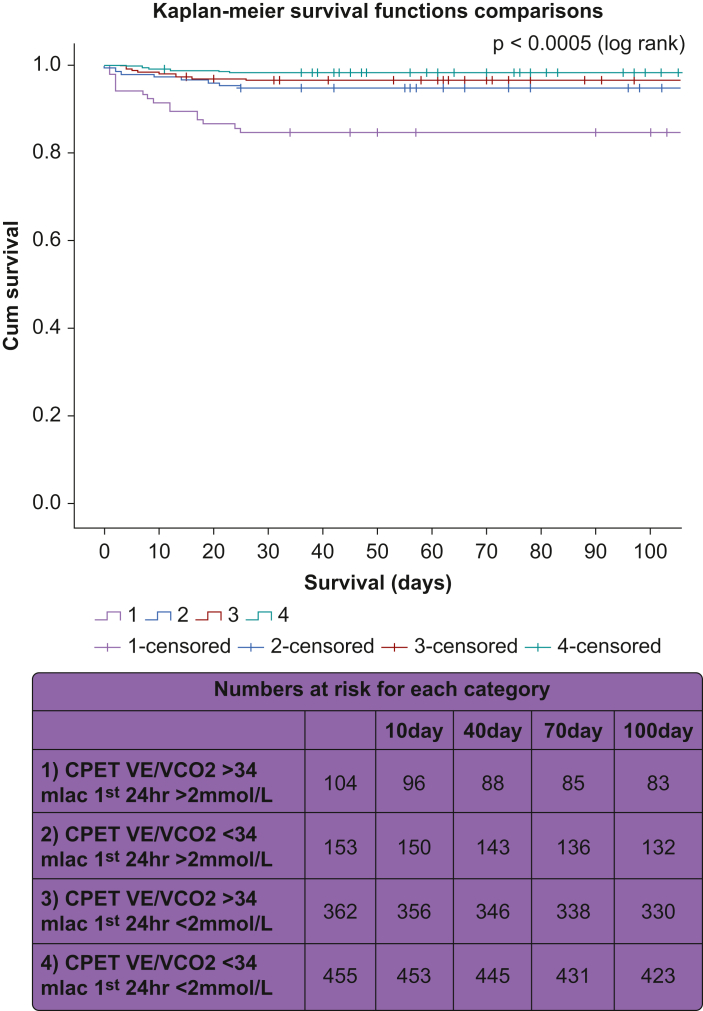
A comparison of survival using standard clinically accepted cut-offs for an abnormal CPET variable (V̇E/V̇co2 >34) and an abnormal lactate concentration (mlac 24 >2 mM) is shown in survival curves in Fig 1 and in Table 4. Both abnormal CPET (V̇E/V̇co2 >34) and abnormal lactate (>2 mM) were independent risk factors for mortality, and interaction terms were not found to be significant, so it was concluded that there is no evidence of synergy between the variables as predictors. An abnormal lactate concentration and an abnormal CPET combined as above raises the predicted risk of mortality 11.5 times at 30 days and 6.5 times at 90 days compared with a patient who had no hyperlactataemia in the first 24 h after surgery and a normal CPET. This is in comparison with 3.5 times the risk of 30-day mortality with an abnormal lactate concentration and a normal CPET.
Fig. 1.
Kaplan–Meier survival estimates. Survival curve comparison of the following groups: (1) CPET V̇E/V̇co2 >34 mlac first 24 h >2 mM (abnormal lactate; abnormal CPET), (2) CPET V̇E/V̇co2 <34 mlac first 24 h >2 mM (abnormal lactate; normal CPET), (3) CPET V̇E/V̇co2 >34 mlac first 24 h <2 mM (abnormal lactate; normal CPET), and (4) CPET V̇E/V̇co2 <34 mlac first 24 h <2 mM (normal lactate; normal CPET). CPET, cardiopulmonary exercise testing; mlac, mean lactate concentration.
Table 4.
Dichotomous predictors in combinations of CPET and lactate variable models. Odds ratios of predictors 1-3 are compared with CPET V̇E/V̇co2 <34 mlac 24 <2 mM (normal lactate and normal CPET). Sensitivity of 1) abnormal lactate and abnormal CPET was 30.2% for 30-day mortality and 22.1% for 90-day mortality. Specificity of 1) abnormal lactate and abnormal CPET was 95.0% for 30-day mortality and 95.0% for 90-day mortality.
| Dichotomous predictor | 30-day mortality, OR (95% CI) | 30-day mortality, P-value | 90-day mortality, OR (95% CI) | 90-day mortality, P-value |
|---|---|---|---|---|
| 1) CPET V̇E/V̇co2 >34 mlac first 24 h >2 mM (abnormal lactate; abnormal CPET) | 11.53 (4.6–28.8) | <0.005 | 6.50 (3.18–13.28) | <0.005 |
| 2) CPET V̇E/V̇co2 <34 mlac first 24 h >2 mM (abnormal lactate; normal CPET) | 3.52 (1.25–8.86) | 0.017 | 2.71 (1.26–5.83) | 0.011 |
| 3) CPET V̇E/V̇co2 >34 mlac first 24 h <2 mM (abnormal lactate; normal CPET) | 2.19 (0.85–5.63) | 0.103 | 1.81 (0.92–3.6) | 0.08 |
CI, confidence interval; CPET, cardiopulmonary exercise testing; mlac, mean lactate concentration; OR, odds ratio.
Discussion
Our retrospective observational study demonstrates a novel relationship between preoperative CPET results and postoperative hyperlactataemia on mortality. Both high postoperative lactate concentration and abnormal V̇E/V̇co2 were independently associated with an increased risk of postoperative mortality at 30 and 90 days in univariable and multivariable analyses. The peak lactate concentration was no more predictive than mlac for increased risk of mortality, which, along with the results seen for hyperlactataemia-time, suggests that the duration of hyperlactataemia is more important than isolated high values. In the multivariable model, hyperlactataemia and abnormal V̇E/V̇co2 both made a statistically significant contribution to the increased odds of mortality at 30 days (with hyperlactataemia giving the higher OR) and suggested that their risks are independent of each other in nature.
The upper range of normal of blood lactate concentration is usually quoted as 2 mM.20 In keeping with the results of Veličković and colleagues,21 who demonstrated that at 12 h after surgery a lactate concentration of 1.85 mM was the optimal cut-off value for in-hospital mortality, our data suggest that for predicting 30-day mortality the optimal cut-off value for mean lactate in the first 24 h is 1.85 mM. This would suggest that an otherwise normal lactate between 1.85 and 2 mM should not give false reassurance of the patient's condition.
In line with previous studies, we have found that, in a mixed surgical cohort, the dichotomous CPET marker of ventilatory equivalents of CO2 is sensitive in predicting postoperative mortality.13,22 Patients with a poor V̇E/V̇co2 demonstrate poor matching of lung ventilation and pulmonary blood flow (V/Q mismatch), which in turn can be caused by poor cardiac output. Wilson and colleagues22 suggested that a number of patients undergoing colorectal surgery with ventilatory inefficiency are likely to have a burden of undiagnosed heart failure, which would otherwise not be picked up in routine preoperative screening. It is likely that a similar picture is seen in our cohort of patients. These patients with a poor cardiorespiratory reserve are less likely to have the ability to overcome the initial physiological stress of surgery or subsequent insults in the postoperative period caused by complications.
The physiological stress response is important in both exercise and after major surgery to allow the body to produce energy substrate to overcome physiological and metabolic demands. In the types of surgery deemed to have a large physiological insult (so-called high-risk surgery), we found higher mlac in the first 12, 24, and 48 h postoperatively than those deemed medium risk. As with previous studies, we have demonstrated that postoperative hyperlactataemia is associated with increased mortality.7,8,23,24 There is expanding evidence that blood lactate concentration in both exercise and perioperatively occurs irrespective of the adequacy of tissue oxygenation and conversion of aerobic to anaerobic metabolism. Increased metabolic activity generates increased lactate, which accumulates in the cells of origin where it can be used in the intracellular lactate shuttle or released locally or remotely in the cell-to-cell lactate shuttle to serve as oxidative or gluconeogenic substrate.11,12,25, 26, 27, 28 If this theory is correct, then the postoperative hyperlactataemia seen in our patients can be interpreted as a sign of physiology under strain or stress. When assessing if less fit patients (as labelled by their CPET results) were more prone to having postoperative hyperlactataemia, none of the recognised CPET markers of poor physiological reserve correlated with high postoperative lactate concentration. Therefore, we suggest that the degree of postoperative hyperlactataemia is independent of preoperative fitness. Importantly, when analysing the relationship of both hyperlactataemia and poor V̇E/V̇co2 on mortality, hyperlactataemia seems to be independent to the effect of poor V̇E/V̇co2 (Table 4; Fig 1). The independence of these predictive factors suggests that patients with poor V̇E/V̇co2 and hyperlactataemia have the highest risk for postoperative mortality. Perhaps this is because of those patients with poor physiological reserve (i.e. poor V̇E/V̇co2) being less capable of surviving the large stress response suffered as a result of major surgery.
We believe the advantages to our study are the inclusion of a substantial number of patients with a wide range of ages, primary pathologies, comorbidities, and types of procedure undertaken. We have controlled for potential biases by controlling for multiple covariates in the multivariable model and excluding patients who would potentially bias the data (e.g. patients undergoing liver resection). By performing the study at a single tertiary centre, we hope to have controlled for differences in overall clinical postoperative care, reduced the variation in surgical technique, and negated any possible errors caused by variation in the point-of-care laboratory testing.
We recognise a number of limitations to our study. The study design was retrospective observational in nature, and therefore, aspects of data collection, such as the timings of arterial gas sampling and lactate measurements, did not follow a pre-planned, controlled protocol. Data on complications and morbidity were not available, and therefore, an unknown morbidity burden for some patients experiencing hyperlactataemia may have been missed. We know that there is an effect of catecholamines on glycolytic flux and hence lactate concentration.12,28 Some of our patients will have been receiving infusions of noradrenaline (norepinephrine), which may have increased lactate concentration, but we did not have the data to ascertain or correct for this effect. The liver and kidneys are the major sites of lactate clearance in the body; they clear and metabolise approximately 53%29 and 30%30,31 of the daily lactate production, respectively.12 Our cohort did include a number of patients who underwent single nephrectomy, but the authors considered the likely effect to be minimal. Haemofiltration and dialysis are also known to reduce lactate concentration,32 but the data required to correct for this effect were not collected. Intraoperative factors, such as duration of surgery, type of anaesthetic, intraoperative hypotension, or complications, were not examined, as they were outside the scope of the study.
We suggest future studies into this area would include a prospective study design with a controlled protocol for postoperative lactate measurement. Data on morbidity and postoperative complications should be collected and analysed for a link between CPET results, hyperlactataemia, and mortality. Data on important intraoperative factors and critical care factors, such as catecholamine infusions, use of haemofiltration/dialysis, and fluid resuscitation, should be collected prospectively to control for their effects on lactate concentration and to suggest any improvements in the postoperative care of patients with hyperlactataemia.
Our data show that in the cohort of patients with a high V̇E/V̇co2, the presence of postoperative hyperlactataemia significantly increases their risk of mortality compared with a cohort with normal CPET. We suggest that such patients are closely monitored in a critical care area (although we recognise it is likely this is already the case in most centres), they should be monitored for longer in these areas, and there should be aggressive attempts to look for postoperative complications and to correct any abnormalities in physiology. On discharge to ward-level care, we suggest they should be followed up by a member of a critical care outreach team, and there should be a low threshold for re-escalation of care if appropriate. As our results showed that the mortality risk of postoperative hyperlactataemia persisted at 90 days (Fig 1; Table 4), there should be consideration for close follow-up of these patients on discharge by multidisciplinary hospital teams and community doctors to optimise their rehabilitation and maintain vigilance for complications.
Authors’ contributions
Study design: CD, JM.
Literature search: TQ-A.
Data collection: CD, AB.
Writing of results: AB.
Statistical analysis: AB.
Writing of first draft of paper: CD, TQ-A.
Writing of final paper: CD.
Editing of final paper: CD, AB, TQ-A.
Final review/editing of paper: JM.
Acknowledgements
The authors gratefully acknowledge Julie Morris, honorary reader in medical statistics, University of Manchester, who provided advice regarding statistical analysis. The authors also thank Gareth Kitchen, a consultant in anaesthetics and critical care, Manchester University NHS Foundation Trust, who assisted in editing of paper.
Handling Editor: Phil Hopkins
Footnotes
Preliminary account in published abstract: Darwen C, Bryan A, McCaffrey K, Boardman S, Shannon S, Moore J. Post-operative serum lactate measurement and pre-operative fitness as determined by CPET as predictors of mortality after major elective abdominal surgery. Br J Anaesth 2018; 120: e14.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2023.100124.
Declarations of interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Kruse O., Grunnet N., Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74. doi: 10.1186/1757-7241-19-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreruela M., Raurich J.M., Ayestarán I., Llompart-Pou J.A. Hyperlactatemia in ICU patients: incidence, causes and associated mortality. J Crit Care. 2017;42:200–205. doi: 10.1016/j.jcrc.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 3.Chebl R.B., Tamim H., Dagher G.A., Sadat M., Enezi F.A., Arabi Y.M. Serum lactate as an independent predictor of in-hospital mortality in intensive care patients. J Intensive Care Med. 2020;35:1257–1264. doi: 10.1177/0885066619854355. [DOI] [PubMed] [Google Scholar]
- 4.Lindsay A.J., Xu M., Sessler D.I., Blackstone E.H., Bashour C.A. Lactate clearance time and concentration linked to morbidity and death in cardiac surgical patients. Ann Thorac Surg. 2013;95:486–492. doi: 10.1016/j.athoracsur.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki J., Motohashi G., Nishida K., Ubukata H., Tabuchi T. Postoperative arterial blood lactate level as a mortality marker in patients with colorectal perforation. Int J Colorectal Dis. 2014;29:51–55. doi: 10.1007/s00384-013-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal R., Coghill J.E., Guy A., Bradbury A.W., Adam D.J., Scriven J.M. Serum lactate and base deficit as predictors of mortality after ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2005;30:263–266. doi: 10.1016/j.ejvs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Wiggans M.G., Starkie T., Shahtahmassebi G., et al. Serum arterial lactate concentration predicts mortality and organ dysfunction following liver resection. Perioper Med (Lond) 2013;2:21. doi: 10.1186/2047-0525-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S., Peng K., Liu F., Yu Y., Xu T., Zhang Y. Changes in blood lactate levels after major elective abdominal surgery and the association with outcomes: a prospective observational study. J Surg Res. 2013;184:1059–1069. doi: 10.1016/j.jss.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 9.Hajjar L.A., Almeida J.P., Fukushima J.T., et al. High lactate levels are predictors of major complications after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146:455–460. doi: 10.1016/j.jtcvs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Stephens E.H., Epting C.L., Backer C.L., Wald E.L. Hyperlactatemia: an update on postoperative lactate. World J Pediatr Congenit Heart Surg. 2020;11:316–324. doi: 10.1177/2150135120903977. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Alvarez M., Marik P., Bellomo R. Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol. 2014;2:339–347. doi: 10.1016/S2213-8587(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 12.Gómez H., Mizock B. In: Critical care nephrology. 3rd Edn. Ronco C., Bellomo R., Kellum J.A., Ricci Z., editors. Elsevier; 2019. Hyperlactatemia and lactic acidosis; pp. 394–404. [Google Scholar]
- 13.Carlisle J., Swart M. Mid-term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. Br J Surg. 2007;94:966–969. doi: 10.1002/bjs.5734. [DOI] [PubMed] [Google Scholar]
- 14.Hartley R.A., Pichel A.C., Grant S.W., et al. Preoperative cardiopulmonary exercise testing and risk of early mortality following abdominal aortic aneurysm repair. Br J Surg. 2012;99:1539–1546. doi: 10.1002/bjs.8896. [DOI] [PubMed] [Google Scholar]
- 15.Older P., Hall A., Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116:355–362. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 16.Junejo M.A., Mason J.M., Sheen A.J., et al. Cardiopulmonary exercise testing for preoperative risk assessment before pancreaticoduodenectomy for cancer. Ann Surg Oncol. 2014;21:1929–1936. doi: 10.1245/s10434-014-3493-0. [DOI] [PubMed] [Google Scholar]
- 17.Benington S., Bryan A., Milne O., Alkhaffaf B. CPET and cardioesophagectomy: a single centre 10-year experience. Eur J Surg Oncol. 2019;45:2451–2456. doi: 10.1016/j.ejso.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Snowden C.P., Prentis J., Jacques B., et al. Cardiorespiratory fitness predicts mortality and hospital length of stay after major elective surgery in older people. Ann Surg. 2013;257:999–1004. doi: 10.1097/SLA.0b013e31828dbac2. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman K., Hansen J.E., Sue D.Y., et al. 5th Edn. Wolters Kluwer/Lippincott Williams & Wilkins; Alphen aan den Rijn, Netherlands: 2012. Principles of exercise testing and interpretation. [Google Scholar]
- 20.Rishu A.H., Khan R., Al-Dorzi H.M., et al. Even mild hyperlactatemia is associated with increased mortality in critically ill patients. Crit Care. 2013;17:R197. doi: 10.1186/cc12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veličković J., Palibrk I., Miličić B., et al. The association of early postoperative lactate levels with morbidity after elective major abdominal surgery. Bosn J Basic Med Sci. 2019;19:72–80. doi: 10.17305/bjbms.2018.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson R.J.T., Yates D.R.A., Walkington J.P., Davies S.J. Ventilatory inefficiency adversely affects outcomes and longer-term survival after planned colorectal cancer surgery. Br J Anaesth. 2019;123:238–245. doi: 10.1016/j.bja.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNelis J., Marini C.P., Jurkiewicz A., et al. Prolonged lactate clearance is associated with increased mortality in the surgical intensive care unit. Am J Surg. 2001;182:481–485. doi: 10.1016/s0002-9610(01)00755-3. [DOI] [PubMed] [Google Scholar]
- 24.Husain F.A., Martin M.J., Mullenix P.S., Steele S.R., Elliott D.C. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185:485–491. doi: 10.1016/s0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 25.Brooks G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Brooks G.A. The tortuous path of lactate shuttle discovery: from cinders and boards to the lab and ICU. J Sport Health Sci. 2020;9:446–460. doi: 10.1016/j.jshs.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks G.A., Arevalo J.A., Osmond A.D., Leija R.G., Curl C.C., Tovar A.P. Lactate in contemporary biology: a phoenix risen. J Physiol. 2022;600:1229–1251. doi: 10.1113/JP280955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glancy B., Kane D.A., Kavazis A.N., Goodwin M.L., Willis W.T., Gladden L.B. Mitochondrial lactate metabolism: history and implications for exercise and disease. J Physiol. 2021;599:863–888. doi: 10.1113/JP278930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowell L.B., Kraning K.K., 2nd, Evans T.O., Kennedy J.W., Blackmon J.R., Kusumi F. Splanchnic removal of lactate and pyruvate during prolonged exercise in man. J Appl Physiol. 1966;21:1773–1783. doi: 10.1152/jappl.1966.21.6.1773. [DOI] [PubMed] [Google Scholar]
- 30.Mizock B.A., Falk J.L. Lactic acidosis in critical illness. Crit Care Med. 1992;20:80–93. doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Leal-Pinto E., Park H.C., King F., MacLeod M., Pitts R.F. Metabolism of lactate by the intact functioning kidney of the dog. Am J Physiol. 1973;224:1463–1467. doi: 10.1152/ajplegacy.1973.224.6.1463. [DOI] [PubMed] [Google Scholar]
- 32.Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit Care. 2002;6:322–326. doi: 10.1186/cc1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.